Abstract
Research has linked executive function (EF) deficits to many of the behavioral symptoms of Attention Deficit Hyperactivity Disorder (ADHD). Evidence of the involvement of EF impairment in ADHD is corroborated by accumulating neuroimaging studies, specifically functional magnetic resonance imaging (fMRI) studies. However, in recent years functional near-infrared spectroscopy (fNIRS), has become increasingly popular in ADHD research due to its portability, high ecological validity, resistance to motion artifacts, and cost-effectiveness. While numerous studies throughout the past decade have used fNIRS to examine alterations in neural correlates of EF in ADHD, a qualitative review of the reliability of these findings compared with those reported using gold-standard fMRI measurements does not yet exist. The current review aims to fill this gap in the literature by comparing the results generated from a qualitative review of fNIRS studies (children and adolescents ages 6 to 16 years old) to a meta-analysis of comparable fMRI studies and examining the extent to which the results of these studies align in the context of EF impairment in ADHD. The qualitative analysis of fNIRS studies of ADHD shows a consistent hypoactivity in the right prefrontal cortex in multiple EF tasks. The meta-analysis of fMRI data corroborates altered activity in this region and surrounding areas during EF tasks in ADHD compared with typically developing controls. These findings indicate that fNIRS is a promising functional brain imaging technology for examining alterations in cortical activity in ADHD. We also address the disadvantages of fNIRS, including limited spatial resolution compared with fMRI.
Keywords: fNIRS, ADHD, executive functions, children, neural correlates
Introduction
Identifying neural correlates of childhood disorders provides more accurate diagnostic markers and gives practitioners and researchers a mechanistic target for novel therapy methods. Over the past few decades, many neuroimaging studies have examined alterations in neural structure and function across children with various neurogenetic and neurodevelopmental disorders. These studies have significantly improved our understanding of the neural bases underlying these disorders [1].
However, the quantification of neural differences across heterogeneous pediatric clinical populations is confounded by the limitations of the currently-utilized neuroimaging methods. The contemporaneous gold-standard for identifying functional brain markers is functional magnetic resonance imaging (fMRI). fMRI uses magnetic fields to measure blood oxygen level dependent (BOLD) response, a proxy for brain activity, in cortical and subcortical brain regions. fMRI’s ability to image subcortical regions and its high spatial resolution (millimeter) has made it the neuroimaging method of choice in investigations of brain function in pediatric populations.
However, there are multiple drawbacks of fMRI, particularly in pediatric populations. These limitations include fMRI’s high sensitivity to movement artifacts, problems with acoustic noise, low ecological validity, a dearth of studies on the potentially adverse effect of ultra-high field MRI in children [2], high costs [3], and lack of portability. These limitations make fMRI less attractive for widespread use in lower-income settings and with active, pediatric populations.
Functional near-infrared spectroscopy (fNIRS) is a relatively novel, noninvasive optical neuroimaging technology that has become increasingly popular in recent years for examining brain function in typically developing (TD) children and in clinical pediatric populations [4, 5]. fNIRS has several advantages over fMRI including portability, ecological validity, lower sensitivity to movement, and cost-effectiveness, that make fNIRS particularly useful with pediatric populations [6]. However, current fNIRS systems cannot examine brain activity below the cortical level and their signal to noise ratio and spatial resolution are lower than those of fMRI.
fNIRS systems emit near-infrared light of different wavelengths (650 – 1000nm) into the brain and measure the extent to which the light is reflected using a set of optical emitters and detectors (optodes) placed over the head [7]. Hemodynamic changes in oxygenated (HbO) and deoxygenated (HbR) hemoglobin, as measured by fNIRS, are linked to neuronal activity as explained by neurovascular coupling mechanism. Thus, fNIRS measurements can be used as a proxy for neural activity, similar to fMRI BOLD measurements. Concurrent fMRI-fNIRS studies have been utilized to examine the congruence of fMRI and fNIRS results. These studies have demonstrated strong correlations between HbO (and HbR) signals measured by fNIRS and BOLD signals from fMRI in TD populations [6, 8]. These validation data further substantiate fNIRS as an attractive tool for neurodevelopmental research.
We will provide a brief review of the current state of fNIRS research in child and adolescent psychiatry and psychology. Particularly, we will focus on ADHD as a lens to the broader use of fNIRS in child and adolescent psychiatry considering that (a) ADHD is the most prevalent neurodevelopmental disorder, and (b) there is an abundance of fNIRS studies examining alterations in brain activity during executive function (EF) tasks in children with ADHD. The latter can be explained by the idea that the core system affected in ADHD is the frontoparietal cortical network that can be readily probed using fNIRS. We will compare the aggregated fNIRS findings in children with ADHD with those reported in meta-analysis of comparable fMRI studies.
fNIRS research in child and adolescent psychiatry/psychology
Lloyd-Fox and colleagues’ review of the first decade (1998 – 2010) of fNIRS research in infants depicts the rising popularity of fNIRS in clinical pediatric research [4]. The review reported a fivefold increase of fNIRS studies in infants in the decade after the initial fNIRS study in this population. Other studies have suggested that with rapidly advancing fNIRS methodology, experiments have become increasingly complex and have shifted focus from research on TD populations to research with clinical populations [4, 5]. Indeed, recent fNIRS studies cover a wide spectrum of research in child psychology and psychiatry, including early-onset Schizophrenia, Childhood Major Depressive Disorder, and neurodevelopmental disorders such as Autism Spectrum Disorders (ASD) and ADHD [9–15].
Pediatric psychology and psychiatry research have readily taken advantage of the benefits of fNIRS, namely its portability, high ecological validity, tolerance to motion artifacts, and cost-effectiveness. Because fNIRS is portable and easy to set-up, it may be used in tandem with other neuroimaging methods such as EEG or transcranial magnetic stimulation (TMS). For example, researchers have used this benefit to investigate the effect of TMS treatment in girls with Bulimia Nervosa on brain activity, as measured by fNIRS [16].
fNIRS also allows for studies with high ecological validity, which is particularly important when investigating the neurocognitive development of pediatric populations. For example, fNIRS has been utilized for the examination of the neural correlates of social interactions in naturalistic settings in infants and children at risk of developing ASD [17].
Furthermore, fNIRS is a great alternative for more active pediatric populations or for populations that cannot readily undergo MRI measurements, including infants and other groups with health conditions (e.g. children with Cerebral Palsy or low-functioning ASD). Many childhood-onset neuropsychiatric disorders affect impulse control or social interactions and may make it difficult for participants with these disorders to comply with experimental task instructions, e.g., requirements to sit still for a long time. The frequent talking, interruptions, and movements that are often seen in individuals with these disorders make MRI a less appealing neuroimaging tool for these populations.
Finally, fNIRS is also relatively inexpensive, as compared to fMRI, and may be utilized in low-resource settings to assess a large number of individuals in a short amount of time and under conditions with ecological validity. For example, researchers have used fNIRS in rural Gambia for longitudinal investigation of the effects of nutrition on neurocognitive functions in Gambian children [18, 19].
These many benefits make fNIRS an attractive neuroimaging tool for the diagnosis and treatment of pediatric psychiatric disorders.
fNIRS Research in Children with ADHD
fNIRS is particularly useful in working with children with ADHD. This disorder is therefore one of the neuropsychiatric disorders that has been most thoroughly investigated with fNIRS. The large number of studies in this area is due, in part, to the high prevalence of ADHD in children — 11% of U.S. school-aged children were diagnosed with the disorder in 2011 [20]. Additionally, the involuntary movements of this population make fNIRS more feasible than fMRI. Furthermore, while the etiology of ADHD is still elusive, it is well understood that cortical structures, particularly the fronto-parietal networks, play an important role in this disorder [21]. These cortical structures can be readily probed by fNIRS. Thus, there has been increasing interest in using fNIRS as a cost-effective neuroimaging technique for examining cortical correlates of ADHD and their changes in response to treatments.
Given these attributes and the prevalence of fNIRS studies in children with ADHD, this study will investigate the clinical utility of fNIRS for children with ADHD, specifically its ability to reliably detect neural correlates of this disorder in comparison with gold-standard fMRI. Given the abundance of both fNIRS and fMRI research on executive dysfunction in ADHD, this paper focuses on neural correlates of executive dysfunction in ADHD in children ages six to sixteen.
EF and ADHD
Deficits in EFs contribute to behavioral symptoms observed in ADHD, which can be grouped into three categories — inattention, hyperactivity, and impulsivity [22]. EFs are a collection of complex sub-processes of higher-order cognitive functions that rely on self-regulation as well as on goal-oriented behavior [23, 24]. They include working memory (WM), cognitive flexibility, response inhibition, planning, and selective and divided attention. While these subprocesses can be delineated separately to a certain extent, it must be noted that they are tightly linked with each other [25]. EF deficits have substantial impact on behavioral, educational, and social performance and, in ADHD, have been linked to poor academic functioning [26] in children and to unemployment and substance abuse in adults [27].
fMRI research on neural correlates of EF deficits in ADHD has been assessed in several reviews and meta-analyses [28, 30, 31, 32]. These studies implicate the frontal, parietal, and striatal brain regions in EF deficits observed in individuals with ADHD. Specifically, individuals with ADHD showed both hyper- and hypo-activity in the frontal regions (as compared with typically developing controls) in a range of EF tasks, including Stop-Signal, Go/NoGo, Stroop, and Oddball paradigms.
fNIRS studies have been used primarily for the investigation of the neural correlates of EF deficits in ADHD [23, 24]. Here, we qualitatively reviewed fNIRS studies of EF in children with ADHD and compared the results with those of a meta-analysis of comparable fMRI studies [28] to draw conclusions about the reliability of fNIRS findings in the context of EF in ADHD. We focused on fNIRS studies of EF in children with ADHD for multiple reasons. Primarily, this review aims to fill a gap in the literature, as there is currently no comprehensive review of fNIRS studies in EF in children with ADHD. Additionally, EF is a logical starting point because it is implicated in variety of neurodevelopmental disorders and significantly impacts children’s socioemotional behavior [29].
Methods
The following databases were searched using the keywords <NIRS> and <ADHD>: Pubmed (n = 37 studies identified initially), PsychInfo (n = 36 studies identified initially), Cochrane (n = 27 studies identified initially), Web of Science (n = 25 studies identified initially). Studies were included if they met the following criteria: (a) utilized fNIRS for functional brain imaging, (b) subjects included children with ADHD (age < 18 years) and typically developing controls, (c) reported the location of fNIRS optodes based on the international 10/20 system to facilitate comparison across studies, and (d) utilized executive function tasks (response inhibition, attention, cognitive flexibility, working memory, etc.) to elicit brain activation. Figure 1 illustrates how articles were identified.
Figure 1.
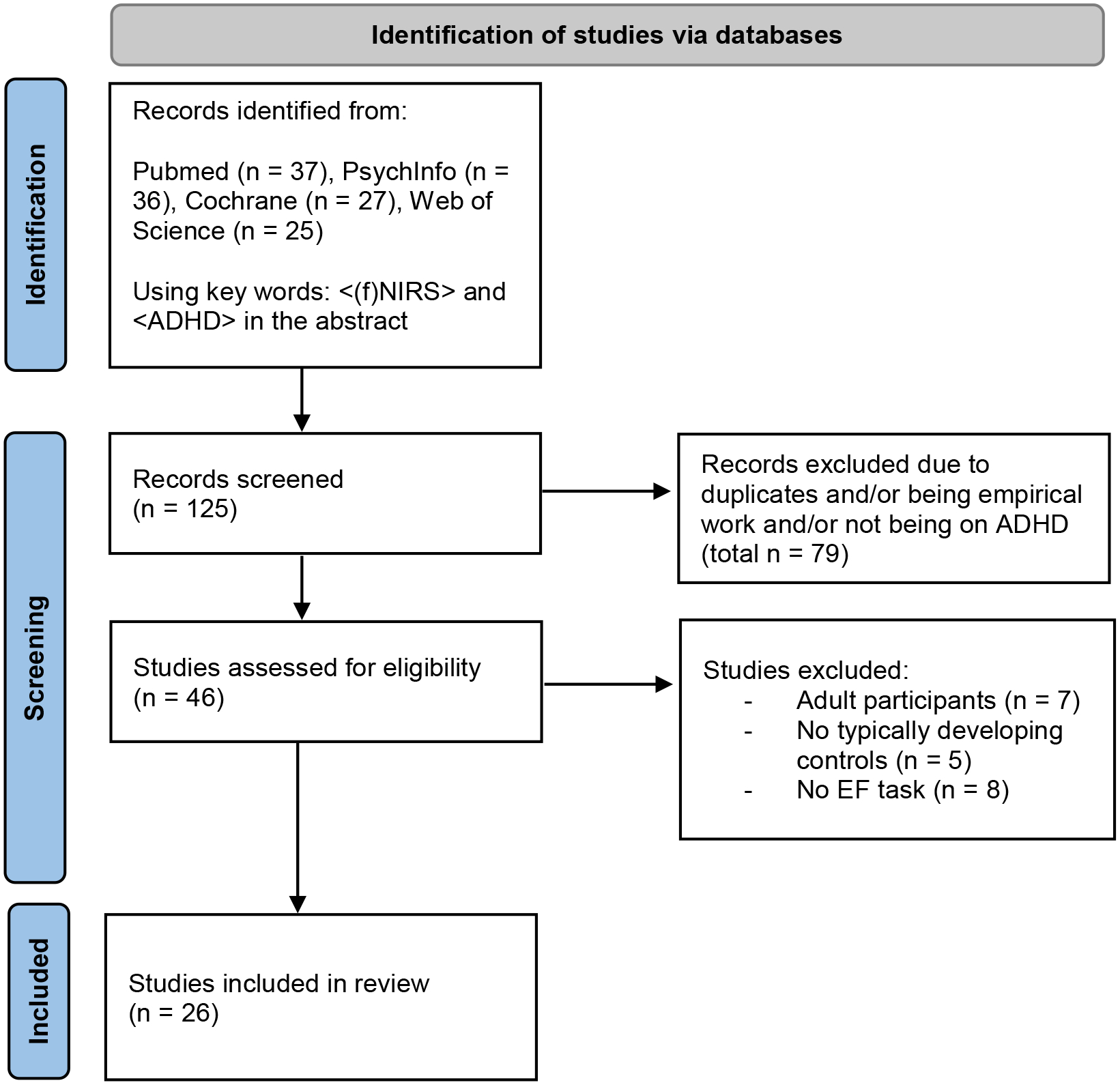
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only
Selection of studies to include in the review. *Studies were excluded based on the following criteria: a) utilized fNIRS for functional brain imaging, (b) subjects included children with ADHD (age < 18 years) and typically developing controls, (c) reported the location of fNIRS optodes based on the international 10/20 system to facilitate comparison across studies, and (d) utilized executive function tasks (response inhibition, attention, cognitive flexibility, working memory, etc.) to elicit brain activation. Figure flowchart adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71 and http://www.prisma-statement.org/
In total, 26 studies were included in the review (Table 1, Figure 1). The age ranges of participants varied across the different studies, but across all studies the subjects included were between the ages of six and sixteen. Thirteen studies investigated response inhibition [11, 33–43], seven studies examined sustained/selective attention [44–50], three studies investigated working memory [51–53], and three studies investigated cognitive interference control and cognitive flexibility [54, 55, 56]. While the studies all utilized the international 10/20 system, they used diverse fNIRS devices. NIRS systems differ substantially in terms of optode configuration/headgear design as well as source-detector separation. Also, different NIRS systems use different NIR wavelengths to probe changes in HbO and HbR [57] resulting in different signal-to-noise ratio. Thus, we also aggregated the findings for each NIRS system separately. We hope that this categorization will allow researchers to readily compare their results to those included in this review. Scholkmann et al. [57] provide a comprehensive review of most commercially used NIRS systems and their properties.
Table 1.
Description of studies included in the review
| Type of Statistical Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Arai et al. (2016) | Self-generated SWM task | Boys (30), Age: 8–11 y | All participants with ADHD received methylphenidate, but ceased taking all medications 24 h prior to the start of experiment | Typically developing (35) Age: 7.5 −10–5 y | 16-channel OEG-16 (Spectratech Inc., Tokyo, Japan) | Frontal array, center placed on Fpz | CH10 & CH11 | Hypo (no developmental increase in activity) | -Lateral PFC -frontal pole |
Spatial WM | -correlational analysis |
| 2 | Araki et al. (2015) | CPT*AXT study, baseline differences used only for present analysis | Children (12) Age: 6–13 y | All patients were taking ATX | Typically Developing (14) Age: 6–15y | 24-channels ETG-100 (Hitachi Medical Corp., Japan) | Frontal array, two sets of 3×3 probe holders, lowest probes in left and right medial columns positioned at Fp1/Fp2 | CH12 | Hypo | -Left VLPFC | ATT | −3-way RM-ANOVA, Post hoc analysis |
| 3 | Bell et al., (2020) | Go/NoGo | Children (20) Age: 6–13 y | Children with ADHD treated with medication were asked to stop their medication 24 h before participation | Typically Developing (27) Age: 6–13 y | 44-channels ETG-4000 (Hitachi Medical Corporation, Tokyo, Japan) | Temporal array, two 3×5 probe sets placed left and right side of the head on an EEG cap, positioned above T3/T4, angled towards eyebrows | CH17/CH22, CH1,CH5, CH6 | Hypo and broader frontal activation | -increased activation in bilateral parietal (SMG) -Hypo activity in IFG |
RI | -ANOVA -Linear mixed model -Correlation analysis |
| 4 | Güven et al. (2019) | Auditory oddball paradigm | Children (23) Age: 7–12 y | Children with ADHD were treated with methylphenidate after the baseline session. | Typically Developing (21) Age: 7–12 y | 16-channel fNIR Imager 1100 |
Frontal array (placement not specified), 4 sources 10 detectors. | N/A because unclear which NIRS system | Hypo | -right frontal cortex | ATT | -t-test |
| 5 | Inoue et al. (2012) | Go/NoGo | Children (20) Age: 6–14 y | No ADHD children had undergone pharmacotherapy before the NIRS experiment | Typically Developing (20), Age:6–14 y | 16-channel Cognoscope (NIM Inc. Philadelphia, PA, USA) | Frontal array, four light sources on Fp1-Fp2, middle of array on Fpz | CH 1–16 | Hypo | -PFC | RI | -Unpaired t-test -Correlational -mixed ANOVA |
| 6 | Ishii et al (2017) | Rock-Paper Scissor task | Group 1(8): 6–10y Group2(10): 11–16y |
5 participants with ADHD were taking MPH, but all stopped at least 24 hours before experiment | Group 1 (13): 6–10y Group2 (14): 1116y |
22-channel ETG-100 (Hitachi Medical Corp., Japan) | Frontal array, 3×5, lowest probes on Fp1-Fp2 | Group 1: CH 1–2, CH5–6 Group 2: CH 1–6, CH8–9 |
Hypo | bilateral frontal | RI | -Pearson’s correlation −1-Way ANOVA -Paired t-test -Post-hoc Tukey HSD |
| 7 | Ishii-Takahashi et al. (2015) | Stop-signal task | Children (30) Age: 7–10 y | ADHD group was divided into those who had not received any medication and those who had received chronic treatment with MPH | Typically Developing (20) Age:7–10 y | 52-channel ETG-4000 (Hitachi Medical Corp., Japan) | Frontal array, 3×11 lowest probes on T3-Fpz-T4 | Not specified | Hypo | -right IFC, (tendency towards activation in the left IFC) | RI | -Stepwise multiple regression -ANOVA |
| 8 | Moser et al. (2009) | Stroop Task | Boys (12) Age: 8–13 y | No participants with ADHD were receiving psychotropic medication | Healthy Control (12) Age: 8–13 y | 4-channel NIRO-300 (Hamamatsu Photonics K.K. Japan), | Frontal array, optodes place left and right at F3/4, F7/8, FC3/4 | FC4 | Hyper (after increased difficulty (i.e. incongruent trials/ as a response to interference) | -Right dlPFC | RI | -ANOVA -(un)paired students t-test |
| 9 | Kaga et al. (2020) | Go/NoGo | Children (20) Age: 8–11y Drug-naive | All participants with ADHD were medicaitonnaive | Typically Developing (19) Age: 8–11 y | 16-channel OEG-16; Spectratech Inc., Tokyo, Japan | 2×6 Frontal array, center placed on Fpz, bottom left and bottom right of array on F7 / F8 | CH1–3; CH4–6 | hypo | right lateral PFC | RI | -Chi-square −1-way ANOVA -Pearson’s Correlation |
| 10 | Mauri et al. (2020) | Visual performance -CPT |
Children (18) Age: 6–16 y Drug-naive | All participants with ADHD were drug-naive | Typically Developing (25) Age: 6–16 y | 14-channel DYNOT Compact 9–32 (NIRx, Berlin, Germany) | Frontal array, (6 sources, 11 detectors), array center on Fpz, along Fp1-Fp2 | ROI right prefront al (CH8–10) | Hypo | -right PFC | ATT | -Spearman Rho Correlation |
| 11 | Miao et al. (2017) | Go/NoGo | Children (14) Age: 6–9y | not available | Typically Developing(15) Age:6–9 y | 52-channel ETG-4000 (Hitachi Medical Corp., Japan) | Fronto-temporal array, center inferior optode on Fpz, orientation towards T3 /T4 | CH37 CH48 CH49 | Hypo | left frontopolar cortex | RI | N/A |
| 12 | Monden et al. (2012) | Go/NoGo | Children (16) Age: 6–13y | All ADHD participants were taking MPH, but refrained from medication for 24 hours prior to experiment | Typically Developing (16) Age: 6–13y | 44-channel ETG 4000 (Hitachi Medical Corp., Japan) | 3×5 Frontaltemporal array (placement procedure not described) | CH10 | Hypo | -right IFG -right MFG |
RI | -ROI analysis -Contrast analysis -Descriptive |
| 13 | Monden et al. (2015) | Go/NoGo | Children (30) Age: 6–15y | All ADHD subjects were pre-medicated | Typically Developing (30) Age: 6–14y | 44-channel ETG 4000 (Hitachi Medical Corp., Japan) | 2 3×5 probes on lateral prefrontal cortices and inferior parietal lobe | CH6 & CH10 | Hypo | -right PFC -right IFG -right MFG |
RI | -ROC analysis |
| 14 | Nagashima et al (2014a) | Go/NoGo | Children (16) Age: 6–14y | All ADHD subjects were taking ATX | Typically Developing (16) Age: 6–13y | 44-channel ETG 4000 (Hitachi Medical Corp., Japan) | 2 3×5 probes on lateral prefrontal cortices and inferior parietal lobe | CH10 | Hypo (only marginally sig.) | -right IFG -right MFG |
RI | -t tests |
| 15 | Nagashima et al. (2014b) | Oddball | Children (22) Age: 6–14y | All ADHD subjects were taking MPH | Typically Developing (22) Age: 6–14y | 44-channel ETG 4000 (Hitachi Medical Corp., Japan) | 2 3×5 probes on lateral prefrontal cortices and inferior parietal lobe | CH 10 | Hypo | -right IFG -right MFG |
ATT | -Channel-wise analysis -ROI based analysis |
| 16 | Nagashima et al. (2014c) | Oddball | Children (15) Age: 6–14y | All ADHD subjects were taking ATX | Typically Developing (15) Age: 7–13y | 44-channel ETG 4000 (Hitachi Medical Corp., Japan) | 2 3×5 probes on lateral prefrontal cortices and inferior parietal lobe | CH10 CH22 | Hypo | -right IFG -right MFG -right IPL |
ATT | -Channel-wise analysis -ROI based analysis |
| 17 | Nakashima et al. (2014) | Multi-source interference task (MSIT) | Boys(19) Age: Not reported | Not available | Typically Developing (14) Age: Not reported | 46-channel ETG 4000 (Hitachi Medical Corp., Japan) | Frontal-parietal cap array, 22 channels on frontal region /24 channels placed on parietal region, center on Fpz, along Fp1-Fp2 | CH6 | Hyper | -Left DLPFC | Cognitive interferen ce/flexibility | -Students t-test -Paired t-test -correlation analysis |
| 18 | Negoro et al. (2010) | Stroop task | Children (20), Age: 6–13y | All ADHD subjects were medication-naive | Typically Developing (20) Age: 6–13y | 24-channel ETG-100 (Hitachi Medical Corp., Japan) | Frontal array, lowest probes on Fp1-Fp2 | CH18, CH22 & CH 21 | Hypo | Inferior lateral PFC | RI | - t-test - correlational |
| 19 | Schecklmann (2010) | Object and spatial WM task | Children(19) Age: 10–13 y | 11 of the ADHD subjects were on MPH but stopped medication at least 1 day before experiment | Typically Developing (19) Age: 10–13 y | 52-channel ETG-4000 (Hitachi Medical Corp., Japan) | Frontal array, middle inferior optode on Fpz and the inferior row of optodes direction T3/ T4 | No differences between ADHD and controls | WM | −3-way ANOVA | ||
| 20 | Suzuki et al. (2017) | Flanker Task (arrow version) | Children (12) Age: 8 −11y | not available | Typically Developing (12) | 16-channel OEG-16 (Spectratech Inc., Tokyo, Japan) | 2×6 Frontal array, center of array on Fpz, bottom left and bottom right array corners on F7/F8 | CH 10, 11, 13, 14 | Hyper | Left SFC | Cognitive control | ANOVA |
| 21 | Tsujimoto et al. (2013) | Visuospatial WM task w & W/o distractors | Boys (16) Age: 9–13y | All ADHD subjects were chronically medicated, mostly with methylphenidate; however, nine of them had not taken medication for at least 1 day before the experiment | Typically Developing (10) Age: 8–12y | 16-channel OEG-16 (Spectratech Inc., Tokyo, Japan) | 2×6 Frontal array, center of array on Fpz, bottom left and bottom right array corners on F7/F8 | Hyper | Hyper: task related PFC Hyperactivi ty | (spatial) WM | - ANOVA, Mann-Whitney U | |
| 22 | Weber et al. (2005) | TMT | Boys (11) Age: 9–11y | ADHD subjects were drug-naive up to the time of NIRS study | Typically Developing (9), Age: 10–12y | 2-channel NIRO-300 (Hamamatsu Photonics KK, Japan) | Frontal array, two optodes were placed symmetrically on the child’s forehead, in positions between Fp1/F3 and Fp2/F4 | Right optode | Hypo | rPFC | EF (ATT) | - Wilcoxon, Mann-Whitney - Monte Carlo |
| 23 | Xiao et al. (2012) | -Go/NoGo -Stroop Task |
Children (16) Age: 8.5–11 y | not available | Typically Developing (16), Age: 8–11 y -HFA (19) Age: 8–12 y |
16 channel-JH-NIRS-BR-05 (Huazhong University of Science and Technology, China) | Frontal array | Ch7–10, CH13–16 | Hypo (during Go/NoGo) | rPFC | RI | t-tests |
| 24 | Yasamura et al. (2019) | -Reverse stroop | Children (67) Age: 6–12 y | Children taking stimulant medication for their ADHD underwent a 24-hr washout period before their assessment | Typically Developing (140) Age: 6–12 y | 16 channel OEG-16; Spectratech Inc., Tokyo, Japan | 2×6 Frontal array, center of array on Fpz, bottom left and bottom right array corners on F7/F8 | Hypo (during reverse stroop) | lPFC, mPFC, rPFC | RI | Two-way ANOVE with Bonferroni post hoc analysis | |
| 25 | Yasumura et al. (2015) | Dimensional Change Card sort | Children (22) Ag: 8–12y | Not available | Typically Developing (37) Ag: 9–12y | 16-channel OEG-16 (Spectratech Inc., Tokyo, Japan) | 2×6 Frontal array, center of array on Fpz, bottom left and bottom right array corners on F7/F8 | CH 1–2 | Hypo | rIFG | RI + cognitive shifting | - t-tests - correlational |
| 26 | Yasumura et al. (2014) | Stroop Task | Children (10), Age: 9–13 y | not available | Typically Developing(15) Age: 8–11 y -ASD (11) Age: 8–12y |
16-channel OEG-16 (Spectratech Inc., Tokyo, Japan) | 2×6 Frontal array, center of array on Fpz, bottom left and bottom right array corners on F7/F8 | CH4 | Hypo | rLPFC | RI | - ANOVA - post-hoc Tukey HSD |
Note. RI = Response Inhibition, ATT= Attention, WM= Working memory, AXT = Atomoxetine, PFC = prefrontal cortex, IFC = inferior Frontal cortex, dlPFC = dorso-lateral prefrontal cortex, vlPFC= ventrolateral prefrontal cortex, IFG = Inferior Frontal Gyrus, MFG = Middle frontal Gyrus, IPL = inferior parietal lobe, r: right, l: left, Hypo: hypoactivity, hyper: hyperactivity.
The fNIRS devices included Hitachi ETG 4000 and ETG 100 Hitachi, OEG-16, NIRO 300, Cognoscope and JH-NIRS BR05. We also aggregated the findings across studies by grouping them based on the examined EF domain when there were enough studies available. These included response inhibition, working memory, and attention. Table 1 provides a complete summary of the included studies and detailed list of tasks and probe sets used for fNIRS measurements. This table also shows the optode placement and the cortex areas covered by the fNIRS array.
The results of these fNIRS studies were then compared to Cortese et al.’s fMRI meta-analysis to examine the extent to which the outputs of these neuroimaging devices in studies with children with ADHD were in agreement.
Results
Results by NIRS Device Type
Studies were first grouped based on the type of NIRS devices used for measuring cortical activity to aggregate results across studies qualitatively. This categorization was made because NIRS devices are different with respect to headset form factors, configuration of probe sets, and the number of channels and spatial coverage. We hope that this categorization will allow researchers to readily compare their results to those included in this review.
ETG Hitachi 4000 (10 studies):
Results of EF tasks using the ETG-4000 – placed over the frontal and/or parietal regions based on the 10–20 system – coincide reasonably well. Monden et al. [37] reported hypoactivity in the right inferior and middle frontal gyrus in children with ADHD during a Go/NoGo task (probing response inhibition) as compared to typically developing controls. They replicated these findings in a larger sample and found hypoactivity in the previously reported regions as well as in the rostral prefrontal cortex in children with ADHD. In a series of fNIRS studies on attention and working memory, Nagashima and colleagues [40, 46, 47] also reported hypoactivity in the right inferior and middle frontal gyrus in children with ADHD compared with controls. In an additional study, these researchers also identified hypoactivity in the right inferior parietal lobule. A recent study by Miao and colleagues [36] also reported hypo-activity in the frontal regions in children with ADHD during the Go/NoGo task (although hypo-activity was primarily in the left frontopolar cortex). Schecklmann et al. [53] compared prefrontal activity during various working memory tasks between children with ADHD and controls but did not find any significant difference in brain activity between groups. This negative finding could be explained by the fact that more than half of children in their ADHD sample were medicated. Nakashima et al. [55] examined children’s brain activity in response to increased task difficulty during a multi-source interference task (cognitive flexibility) and found hyperactivity in the left dorsolateral prefrontal cortex in response to task load in children with ADHD. A recently published study by Bell et al. [49] also found hyperactivity in frontal regions, as well as parietal regions in children with ADHD as compared to typically developing controls. Additionally, this study found hypoactivation in the right inferior prefrontal gyrus. In sum, these studies demonstrated substantial agreement in their identification of hypoactivity in various prefrontal regions – particularly in the right inferior and middle frontal gyrus – during a variety of EF tasks in children with ADHD.
ETG-100 (3 studies).
Three studies used an older Hitachi fNIRS system, ETG-100. One study by Araki et al. reported hypoactivity in the left ventrolateral prefrontal cortex during a continuous performance task, which probed response inhibition in children with ADHD [44]. An additional study by Negoro and colleagues [39] found hypoactivity in the inferior lateral prefrontal cortex (bilaterally) during a Stroop task (probing set shifting and response inhibition) in children with ADHD, similar to the studies utilizing the ETG-4000. The third study used a rock-paper-scissor game to investigate response inhibition in ADHD and reported hypoactivity in the left inferior lateral prefrontal and left medial frontal (frontal pole) in young children with ADHD compared with controls [34]. This study also examined older children with ADHD in the same task and found similar patterns of hypoactivity among both age groups, extending bilaterally. In conclusion, these studies consistently indicated hypoactivity in the left ventrolateral prefrontal cortex in children with ADHD across EF tasks that in some cases extended bilaterally.
OEG-16 (7 studies).
Seven studies used the Spectratech OEG-16 NIRS system for measuring changes in the frontal activity in ADHD. Yasumura et al. [41, 42] and Kaga et al. [43] observed hypoactivation in the right lateral prefrontal cortex during an inhibition task (Stroop for Yamasura et al. and Go-no-Go for Kaga et al.) in children with ADHD. In a subsequent study, they reported hypoactivity in the right inferior frontal cortex during a cognitive shifting task associated with ADHD [56]. Another study examined alterations in superior frontal activity during a flanker task (probing response inhibition) and found hyperactivity in the left superior frontal cortex in children with ADHD compared to controls [54]. A fourth study compared the developmental changes in frontal activity during working memory performance in children with ADHD and controls [51]. In typically developing children, activity in the left frontal pole and bilateral prefrontal regions increased by age. No significant correlation was observed between age and frontal activity in children with ADHD. The slope of change in the left frontal pole (and superior frontal) activity over time was significantly lower in ADHD than in controls. Finally, a fifth study by Tsujimoto et al. [52] examined prefrontal activity during working memory tasks with different levels of difficulty (with and without distractions). Children with ADHD showed hyperactivation across widespread prefrontal areas only in the more difficult task. While these studies point to atypical prefrontal activity in ADHD, the results are mixed and implicate left and right prefrontal involvement irrespective of the type of task.
NIRO 300 (2 studies).
Weber and colleagues [45] performed one of the earliest NIRS studies in ADHD, using a fNIRS system with only two optodes. They reported hyperactivity in boys with ADHD in the right prefrontal cortex during an attention task. These results match, to some extent, with the findings of a second study utilizing NIRO 300 that identified hyperactivity in the right dorsolateral prefrontal cortex following increased task difficulty in patients with ADHD [35]. In sum, these findings identified hyperactivity in the right prefrontal cortex.
Studies using other fNIRS systems showed hypoactivity primarily in the prefrontal cortex in ADHD. For example, Inoue et al. [58] used a 16-channel Cognoscope fNIRS during a Go/NoGo task and reported decreased average signal over the prefrontal regions in children with ADHD compared with controls. A similar study that utilized the Go/NoGo task with the JH-NIRS-BR-05 fNIRS system reported hypoactivity in the right prefrontal cortex in children with ADHD and highlighted the involvement of this area in response inhibition [11]. These results are in line with Mauri et al. [48] study that used a 14-channel DYNOT Compact system from NIRx and found hypo-activity in the right lateral prefrontal cortex while employing the same task. Lastly, Güven et al. [50] did not state which NIRS system was used, but also noted right frontal cortex hyperactivity in ADHD compared to control during an auditory oddball paradigm.
Results by Study Task
Studies were also grouped by study tasks to aggregate results qualitatively across different domains of EF.
Response Inhibition Tasks (11 studies).
Many studies included in this review utilized either the Go/NoGo or the Stroop task, both of which test response inhibition. In the Go/NoGo task paradigm, the most common results observed were hypoactivity in the right PFC, right IFG, and right MFG [11, 37, 38, 40, 58]. Other results from studies utilizing Go/NoGo showed hypoactivity across both the left and right PFC [49, 58], in the left frontopolar cortex [36], or in the right lateral PFC [43]. Studies that utilized other response inhibition tasks also observed hypoactivity in the frontal lobe, with more results showing hypoactivity on the right side [33, 34, 56].
Interference Control and Cognitive Flexibility Tasks (6 studies).
The majority of these studies used the Stroop paradigm to test cognitive interference control and flexibility [39, 41, 42]. The majority of these studies reported hypoactivity in the prefrontal cortex associated with ADHD. These included hypoactivity in the bilateral inferior lateral PFC [39], in the right lateral PFC [41] and in the lateral, medial, and right PFC [42]. One study showed hyperactivity in the right dlPFC but that was in response to increased task difficulty [35].
Further, the two studies that utilized flanker test and multi-source interference test reported hyperactivity in the left superior frontal and left dorsolateral prefrontal cortex in children with ADHD compared to controls [54, 55].
Working Memory Tasks (3 studies).
Studies that tested working memory showed a broader range of results, including hypoactivity in the lateral PFC and frontal pole [51], no significant differences across all brain regions [53], or hyperactivity in the PFC [52].
Attention Tasks (7 studies):
Studies that tested attention showed hypoactivity in the left Ventrolateral PFC [44], or in the IFG, MFG, and IPL [46, 47, 49] or in the right PFC [45, 48, 50]. Additionally, Bell et al. [49] found increased activity during attention tasks in the bilateral parietal regions.
In summary, fNIRS studies of EF indicate a pivotal role of the prefrontal cortex (particularly atypical right prefrontal activity) during EF tasks in children with ADHD. However, it is important to examine the extent to which these results coincide with the findings across gold-standard fMRI neuroimaging studies of EF task performance in ADHD.
fMRI studies of EF network in children with ADHD
We compared the fNIRS findings with the results of a comprehensive meta-analysis of 55 fMRI studies (39 on children <18 years of age) by Cortese et al. to determine qualitatively if fMRI and fNIRS results coincide [28]. Cortese and colleagues identified multiple regions in the right cortical hemisphere with different activation patterns in participants with ADHD as compared to controls [28]. Notably, frontal regions, particularly in the right hemisphere but occasionally bilaterally, including the middle frontal gyrus, medial superior frontal gyrus and supplementary motor area were found to be hypoactive in ADHD. Hypoactivity was also observed in subcortical regions in participants with ADHD, including in the bilateral putamen. Conversely, hyperactivity was found in the occipital regions, including the right middle occipital gyrus and the right angular gyrus. Figure 2 summarizes the findings across studies and shows overlapping and distinct brain regions reported by fMRI and fNIRS studies. As expected, regions in the right middle frontal and inferior frontal gyri were reported most across fNIRS studies and were overlapping with fMRI findings.
Figure 2. Overlap between fNIRS and fMRI studies across EF tasks.
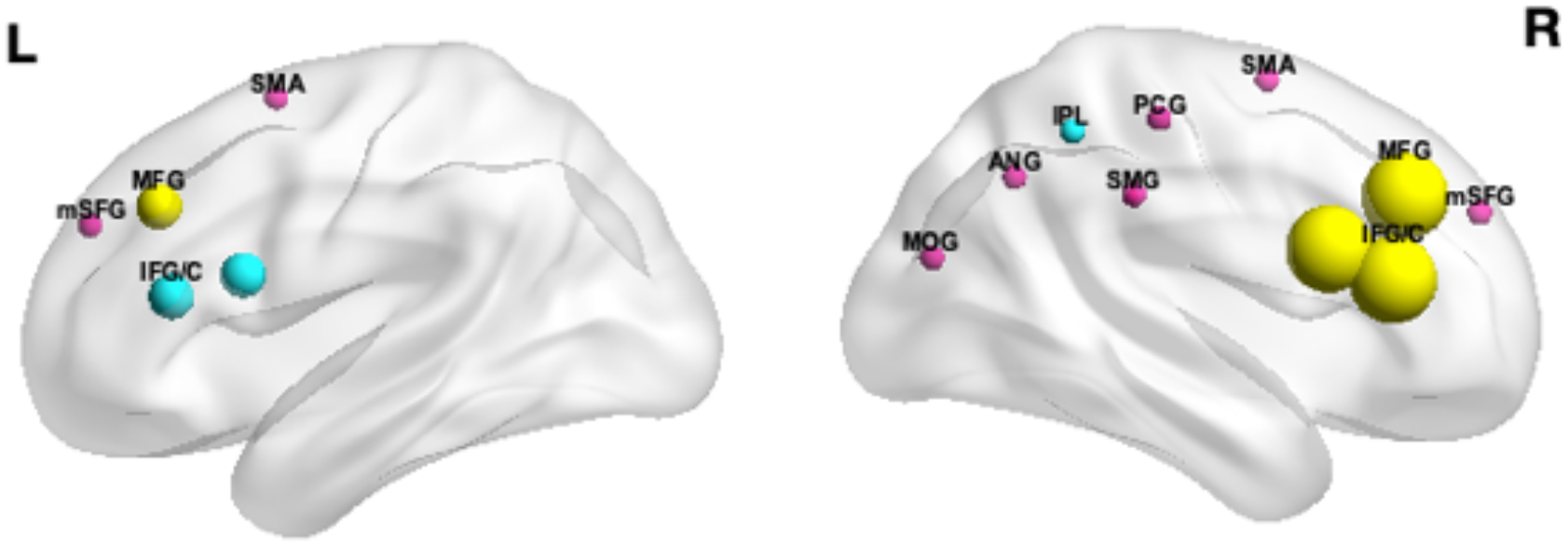
The spheres represent regions affected in ADHD during EF performance. The spheres are color-coded to differentiate regions reported only in fNIRS studies (turquoise), only in fMRI studies (pink), or those common across fNIRS and fMRI studies (yellow). The radius of the sphere corresponds to the number of fNIRS studies that reported activity in a region. This does not apply to fMRI-only studies. This figure was created using BrainNet Viewer (http://www.nitrc.org/projects/bnv/) (Xia et al., 2013).
Discussion
Our review of fNIRS studies suggests consistent hypoactivity in the right lateral prefrontal cortex across EF tasks associated with ADHD. These findings are supported by previous fMRI data that examined brain activation patterns in children with ADHD during EF tasks. These results identify fNIRS as a promising, portable, ecologically-valid, and cost-effective functional brain imaging technology with low sensitivity to motion artifacts, making it particularly well-suited for examining alterations in cortical activity in ADHD.
fNIRS-fMRI comparison
The fNIRS qualitative analysis and fMRI meta-analysis studies showed similar results, including the identification of similar regions of hypoactivity. fNIRS studies with children with ADHD demonstrated hypoactivity in the right prefrontal cortex in ADHD compared with TD, similar to the findings across fMRI studies. Areas of hypoactivation overlapped between fNIRS and fMRI studies were mainly in the right inferior and middle frontal gyrus.
There were, however, some discrepancies between the fMRI meta-analysis and this fNIRS qualitative analysis. The fMRI meta-analysis showed widespread hypo and hyper activity, while the fNIRS qualitative analysis identified more localized regions of hypo and hyper activity. This can be partially explained by the lack of spatial coverage of some of the fNIRS systems, which had only a few channels. An additional discrepancy is that most of the cortical regions identified in the fMRI studies as having atypical activity were in the medial frontal cortex, including supplementary motor area and medial superior frontal regions, while the fNIRS studies show right PFC hypoactivity. Medial frontal areas are more difficult to probe using fNIRS due to the midline fold of the brain. Additionally, fNIRS has a lower signal to noise ratio compared with fMRI and is unable to probe deep structures in the brain, leading to inability to fully capture widespread hypo and hyper activity associated with ADHD [6].
However, fNIRS may be a much more attractive neuroimaging tool than fMRI for use in novel treatment mechanisms, including in neurofeedback settings and TMS treatments that may require cost effective, real-time localization and monitoring of functional brain activity. The portability of fNIRS and its higher tolerance to movement artifacts compared with fMRI opens new possibilities in settings such as real-world group interactions including group therapy settings, hyperscanning studies or longitudinal measurements. Longitudinal measurements are particularly beneficial when studying developmental populations such as children with ADHD.
Right prefrontal hypoactivity in ADHD
This review revealed that both fNIRS and fMRI studies have identified hypoactivity in right prefrontal brain regions of children with ADHD as compared to controls. Previous studies of typically developing individuals have demonstrated the involvement of right prefrontal brain regions in several cognitive functions affected in ADHD. These studies implicated this region primarily in response inhibition [59–63], but also found it to be relevant for target detection [64], attentional control [62], and other cognitive functions. Thus, these findings suggest that the inhibition deficits seen in individuals with ADHD may be due, in part, to hypoactivity in the right prefrontal brain region.
The involvement of the right prefrontal areas in ADHD is further corroborated by studies that were excluded from the current review, due to a lack of control group. These were mostly pharmacological studies that examined brain activation patterns with fNIRS before and after stimulant administration. One such study investigated brain activity before and after atomoxetine administration in children with ADHD [65]. They reported modulation in the activity of bilateral prefrontal areas along with improved EF performance after treatment, suggesting the potential dysfunction in these areas in ADHD populations. More specifically, there was an increase in activity in right prefrontal areas (DLPFC) and in the left ventro-lateral prefrontal cortex. This is particularly interesting as behavioral ADHD symptoms were normalized after atomoxetine administration.
Only three studies examined the correlation between performance in response inhibition and activity in the right prefrontal/IFG. Ishii et al. [34] did examine this relationship and found no significant differences at any ROI in any subject. Contrary to this Kaga et al. [43] found that oxygenation changes in the right prefrontal cortex correlated positively with Stroop task scores and with ERP amplitude during Go-NoGo tasks. This corroborates earlier findings by Monden et al. [37] that showed that increases in accuracy in certain Go-NoGo trials was associated with increased changes in oxy-hemoglobin in NIRS channels in the right prefrontal cortex.
These findings align with a major theory regarding core ADHD symptoms, which posits that these symptoms are the result of impairments in response inhibition [66]. Although deficits in self-regulation and inhibition do not underlie all behavioral deficits displayed in patients with ADHD, this theory nonetheless reflects the importance of response inhibition in modulating ADHD symptoms. Because many fNIRS studies have examined response inhibition, they may be useful in evaluating this theory. However, there is a need to investigate other domains of EF to form a more comprehensive picture of NIRS research in ADHD populations.
These results also add to the understanding of the underlying neural etiology of ADHD. The consistent identification of right prefrontal hypoactivation patterns across fMRI and fNIRS studies indicates that this activity pattern may be a possible neural marker for EF deficits in ADHD, at least in the portion of the ADHD population that exhibits executive function deficits. These findings aligned across the majority of fNIRS studies irrespective of the NIRS device used and the EF tasks involved. However, given the heterogeneity of the disorder, large-sample fNIRS studies may shed light on the clinical utility of these findings. Identification of fNIRS markers of EF deficits may also provide an opportunity for cost-effective monitoring of response to treatments. Further, fNIRS may be a much more attractive neuroimaging tool than fMRI for use in novel treatment mechanisms including in neurofeedback settings and TMS treatments that may require cost-effective, real-time monitoring of functional brain activity.
Four studies showed hyperactivity in regions of the prefrontal cortex in children with ADHD as compared to controls. Moser et al. and Nakashima et al. [35, 55] found increased activity in the right and left dorsolateral PFC, respectively; Tsujimoto et al. [52] found increased activation in the right and middle PFC; finally, Suzuki et al. [54] showed increased activity in the left SFC. Tsujimoto et al. also saw that increases in error rate positively correlated with increases in activity in right and middle PFC [52]. Most of the studies explained these results as a compensatory mechanism [52, 54, 55]. Specifically, these authors posited that there is an inefficiency in neural processing in the PFC of children with ADHD that makes the interference control particularly challenging [52, 54]. To compensate for this deficit, specific regions of the cortex, particularly those implicated in attention, must become hyperactive [54]. On the other hand, it is also possible that inefficient processing does not allow children with ADHD to target the appropriate amount of activity needed for a certain task. Thereby either producing hypo- or hyperactivity.
Other explanations articulated by the authors included small sample size [35], shorter task length allowing individuals with ADHD to maintain attention throughout the study and preventing the hypoactivity seen in the results of other studies [52], and medication effects [52, 55].
Implications for child psychiatry
We can draw several conclusions from our review of fNIRS literature for the utility of fNIRS in child psychiatry. First, the qualitative analysis and its comparison with equivalent fMRI studies highlights the many advantages of fNIRS. As previously mentioned, fNIRS is more robust to motion artifacts as compared with MRI [4, 67] and is portable, allowing for studies with high ecological validity, which is particularly important for pediatric populations. Several experiments have shown that studies conducted in an artificial environment, such as an MRI machine, where movement is constrained, produce results that differ from those conducted in a more realistic environment [68].
Given that the etiology and development of many childhood disorders is still unknown and that neuroimaging is essential in elucidating the puzzle, a system that reliably displays neural activation in a naturalistic setting and that produces meaningful results that may be used to advance treatment techniques is very attractive and can help bridge the gap between theoretical knowledge base and clinical practice.
Comparison of the studies performed in ADHD populations highlighted additional considerations. In the 21 studies that were examined, six different fNIRS devices, and consequently different probe sets, were used. Although caps and probe sets were placed using the 10/20 system in the studies, this variation in data collection may influence the results. Hence future research should therefore examine potential group differences across devices using the same task and populations. Researchers and clinicians should aim to establish a consensus about how fNIRS devices compare to each other and the suitability of each device for particular studies. Uniform guidelines will increase research collaborations and will make fNIRS literature more quantifiable [69, 70].
Limitations
Despite yielding a promising outlook towards future studies, some concessions must be made regarding the review. Current EF studies in fNIRS ADHD research were relatively biased towards response inhibition studies. Hence, there is a possibility that right prefrontal hypoactivation patterns across studies in ADHD could be a result of response inhibition tasks specifically. However, the results also found that working memory and attention tasks elicited hypoactivity in the right prefrontal region, suggesting that these results may extend beyond EF tasks. An additional limitation is that most of the fNIRS studies in ADHD probed only the frontal cortex, limiting our review to these regions. fMRI studies implicate parietal and striatal networks in EF deficits. It is important to investigate the involvement of these regions in ADHD using fNIRS. The availability of high-density NIRS arrays provides the opportunity to probe the whole cortex – including parietal regions – using fNIRS. Previous studies have shown the potential of fNIRS to infer the activity of subcortical brain regions [71]. However, there is currently no fNIRS system with the capacity to directly image subcortical activity. Further, the reviewed studies were not homogenous in terms of devices used and some devices did not correct for the confounding effects of bone and tissue [72]. Additionally, sample size and age differed across samples. It must be acknowledged that the studies included in this review and the companion fMRI review examined a mixture of medicated and unmedicated children with ADHD. Future reviews need to focus on medication-naive patients only to further our understanding of neural etiology of ADHD. Finally, Schecklmann et al.’s [53] study, which found no significant differences in brain activation between participants with ADHD and TD controls, indicates that publication bias might misrepresent the potential of fNIRS in the context of neuropsychiatric disorders. However, publication bias is present in studies of other neuroimaging techniques as well, including fMRI. Despite these differences, we found a good agreement between the fNIRS and fMRI results in the prefrontal cortex.
Implications for future research
Simultaneous fNIRS-fMRI studies in clinical populations are required to quantitatively compare the reliability of fNIRS in detecting hypoactivity and hyperactivity patterns to fMRI. Given that fNIRS is less expensive and exhibits higher ecological validity as compared to fMRI, corroborating the fNIRS findings against fMRI in psychiatric populations is quite crucial to expand fNIRS research in psychiatry. Further, considering the variety of NIRS devices (with different optical wavelengths) and configurations, it is crucial to create procedures to make research methodology more uniform with the aim of facilitating cross-study comparisons. In conclusion, the present review is one of the first steps for establishing fNIRS as an alternative to more traditional neuroimaging methods in psychiatry.
Funding:
SMH’s effort was supported in part by National Institutes of Health (K25AG050759, R61MH119289, R21AG064263 and R21MH123873).
Footnotes
Conflicts of interest/Competing interests: The authors declare that they have no competing interests.
Consent for publication: All authors have read and approved the manuscript.
References
- 1.Greene DJ, Black KJ, & Schlaggar BL (2016). Considerations for MRI study design and implementation in pediatric and clinical populations. Developmental cognitive neuroscience, 18, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslin RN, & Mehler J (2005). Near-infrared spectroscopy for functional studies of brain activity in human infants: promise, prospects, and challenges. Journal of biomedical optics, 10(1), 011009. [DOI] [PubMed] [Google Scholar]
- 3.Glover N (2014). Why Does an MRI Cost So Darn Much? Money Magazine. http://time.com/money/2995166/why-does-mri-cost-so-much/ [Google Scholar]
- 4.Lloyd-Fox S, Blasi A, & Elwell CE (2010). Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neuroscience & Biobehavioral Reviews, 34(3), 269–284. [DOI] [PubMed] [Google Scholar]
- 5.Nagamitsu S, Yamashita Y, Tanaka H, & Matsuishi T (2012). Functional near-infrared spectroscopy studies in children. BioPsychoSocial medicine, 6(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui X, Bray S, Bryant DM, Glover GH, & Reiss AL (2011). A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage, 54(4), 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutini S, & Brigadoi S (2014). Unleashing the future potential of functional near-infrared spectroscopy in brain sciences. Journal of Neuroscience Methods, 232, 152–156. [DOI] [PubMed] [Google Scholar]
- 8.Steinbrink J, Villringer A, Kempf F, Haux D, Boden S, & Obrig H (2006). Illuminating the BOLD signal: combined fMRI–fNIRS studies. Magnetic resonance imaging, 24(4), 495–505. [DOI] [PubMed] [Google Scholar]
- 9.Usami M, Iwadare Y, Kodaira M, Watanabe K, & Saito K (2014). Near infrared spectroscopy study of the frontopolar hemodynamic response and depressive mood in children with major depressive disorder: a pilot study. PloS one, 9(1), e86290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou PH, Lin WH, Lin CC, Hou PH, Li WR, Hung CC, Chan CH, et al. (2015). Duration of untreated psychosis and brain function during verbal fluency testing in first-episode schizophrenia: a near-infrared spectroscopy study. Scientific reports, 5(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao T, Xiao Z, Ke X, Hong S, Yang H, Su Y, Liu Y, et al. (2012). Response inhibition impairment in high functioning autism and attention deficit hyperactivity disorder: evidence from near-infrared spectroscopy data. PloS one, 7(10), e46569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kita Y, Gunji A, Inoue Y, Goto T, Sakihara K, Kaga M, Hosokawa T, et al. (2011). Self-face recognition in children with autism spectrum disorders: a near-infrared spectroscopy study. Brain and Development, 33(6), 494–503. [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Toda Y, Ito H, Mori T, Mori K, Goji A, Kagami S, et al. (2015). Neuroimaging in autism spectrum disorders: 1H-MRS and NIRS study. The Journal of Medical Investigation, 62(1.2), 29–36. [DOI] [PubMed] [Google Scholar]
- 14.Sutoko S, Monden Y, Tokuda T, Ikeda T, Nagashima M, Kiguchi M, Dan I, et al. (2019). Distinct methylphenidate-evoked response measured using functional near-infrared spectroscopy during go/no-go task as a supporting differential diagnostic tool between attention-deficit/hyperactivity disorder and autism spectrum disorder comorbid children. Frontiers in human neuroscience, 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda T, Tokuda T, Monden Y, Hirai M, Mizushima SG, Nagashima M, Yamagata T, et al. (2018). Hypoactivation of the right prefrontal cortex underlying motor- related inhibitory deficits in children with autism spectrum disorder: A functional near- infrared spectroscopy study. Japanese Psychological Research, 60(4), 251–264. [Google Scholar]
- 16.Sutoh C, Koga Y, Kimura H, Kanahara N, Numata N, Hirano Y, Shimizu E, et al. (2016). Repetitive transcranial magnetic stimulation changes cerebral oxygenation on the left dorsolateral prefrontal cortex in bulimia nervosa: A near-infrared spectroscopy pilot study. European Eating Disorders Review, 24(1), 83–88. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, & Roeyers H (2019). Exploring brain functions in autism spectrum disorder: A systematic review on functional near-infrared spectroscopy (fNIRS) studies. International Journal of Psychophysiology, 137, 41–53. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Fox S, Papademetriou M, Darboe MK, Everdell NL, Wegmuller R, Prentice AM, Elwell CE, et al. (2014). Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa. Scientific reports, 4, 4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begus K, Lloyd-Fox S, Halliday D, Papademetriou M, Darboe MK, Prentice AM, Elwell CE, et al. (2016). Using fNIRS to study working memory of infants in rural Africa. In Oxygen transport to tissue XXXVII (pp. 273–279). Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- 20.Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Blumberg SJ, et al. (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry, 53(1), 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu MG, Ye Z, Li QY, Liu GJ, Xie B, & Wang J (2011). Changes of brain structure and function in ADHD children. Brain topography, 24(3–4), 243–252. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 23.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological psychiatry, 57(11), 1336–1346. [DOI] [PubMed] [Google Scholar]
- 24.Chan RC, Shum D, Toulopoulou T, & Chen EY (2008). Assessment of executive functions: Review of instruments and identification of critical issues. Archives of clinical neuropsychology, 23(2), 201–216. [DOI] [PubMed] [Google Scholar]
- 25.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology, 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- 26.Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, … & Faraone SV (2004). Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. Journal of consulting and clinical psychology, 72(5), 757. [DOI] [PubMed] [Google Scholar]
- 27.Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Spencer T, et al. (2006). The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. American Journal of psychiatry, 163(4), 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, & Castellanos FX (2012). Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. American Journal of Psychiatry, 169(10), 1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wåhlstedt C, Thorell LB, & Bohlin G (2008). ADHD symptoms and executive function impairment: Early predictors of later behavioral problems. Developmental neuropsychology, 33(2), 160–178. [DOI] [PubMed] [Google Scholar]
- 30.Dickstein SG, Bannon K, Xavier Castellanos F, & Milham MP (2006). The neural correlates of attention deficit hyperactivity disorder: An ALE meta- analysis. Journal of Child Psychology and Psychiatry, 47(10), 1051–1062. [DOI] [PubMed] [Google Scholar]
- 31.Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, & Radua J (2014). Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biological psychiatry, 76(8), 616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paloyelis Y, Mehta MA, Kuntsi J, & Asherson P (2007). Functional MRI in ADHD: a systematic literature review. Expert review of neurotherapeutics, 7(10), 1337–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishii-Takahashi A, Takizawa R, Nishimura Y, Kawakubo Y, Hamada K, Okuhata S, Igarashi T, et al. (2015). Neuroimaging-aided prediction of the effect of methylphenidate in children with attention-deficit hyperactivity disorder: a randomized controlled trial. Neuropsychopharmacology, 40(12), 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii S, Kaga Y, Tando T, Aoyagi K, Sano F, Kanemura H, Aihara M, et al. (2017). Disinhibition in children with attention-deficit/hyperactivity disorder: Changes in [oxy-Hb] on near-infrared spectroscopy during “rock, paper, scissors” task. Brain and Development, 39(5), 395–402. [DOI] [PubMed] [Google Scholar]
- 35.Moser SJ, Cutini S, Weber P, & Schroeter ML (2009). Right prefrontal brain activation due to Stroop interference is altered in attention-deficit hyperactivity disorder—a functional near-infrared spectroscopy study. Psychiatry Research: Neuroimaging, 173(3), 190–195. [DOI] [PubMed] [Google Scholar]
- 36.Miao S, Han J, Gu Y, Wang X, Song W, Li D, Li X, et al. (2017). Reduced prefrontal cortex activation in children with attention-deficit/hyperactivity disorder during go/no-go task: A functional near-infrared spectroscopy study. Frontiers in neuroscience, 11, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monden Y, Dan H, Nagashima M, Dan I, Kyutoku Y, Okamoto M, Watanabe E, et al. (2012). Clinically-oriented monitoring of acute effects of methylphenidate on cerebral hemodynamics in ADHD children using fNIRS. Clinical Neurophysiology, 123(6), 1147–1157. [DOI] [PubMed] [Google Scholar]
- 38.Monden Y, Dan I, Nagashima M, Dan H, Uga M, Ikeda T, Taniguchi T, et al. (2015). Individual classification of ADHD children by right prefrontal hemodynamic responses during a go/no-go task as assessed by fNIRS. NeuroImage: Clinical, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negoro H, Sawada M, Iida J, Ota T, Tanaka S, & Kishimoto T (2010). Prefrontal dysfunction in attention-deficit/hyperactivity disorder as measured by near-infrared spectroscopy. Child Psychiatry & Human Development, 41(2), 193–203. [DOI] [PubMed] [Google Scholar]
- 40.Nagashima M, Monden Y, Dan I, Dan H, Tsuzuki D, Mizutani T, Shimoizumi H, et al. (2014a). Acute neuropharmacological effects of atomoxetine on inhibitory control in ADHD children: a fNIRS study. NeuroImage: Clinical, 6, 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasumura A, Kokubo N, Yamamoto H, Yasumura Y, Nakagawa E, Kaga M, Inagaki M, et al. (2014). Neurobehavioral and hemodynamic evaluation of Stroop and reverse Stroop interference in children with attention-deficit/hyperactivity disorder. Brain and Development, 36(2), 97–106. [DOI] [PubMed] [Google Scholar]
- 42.Yasumura A, Omori M, Fukuda A, et al. (2019). Age-related differences in frontal lobe function in children with ADHD. Brain and Development, 41(7), 577–586. [DOI] [PubMed] [Google Scholar]
- 43.Kaga Y, Ueda R, Tanaka M, et al. (2020). Executive dysfunction in medication-naïve children with ADHD: A multi-modal fNIRS and EEG study. Brain and Development. [DOI] [PubMed] [Google Scholar]
- 44.Araki A, Ikegami M, Okayama A, Matsumoto N, Takahashi S, Azuma H, & Takahashi M (2015). Improved prefrontal activity in AD/HD children treated with atomoxetine: a NIRS study. Brain and Development, 37(1), 76–87. [DOI] [PubMed] [Google Scholar]
- 45.Weber P, LÜTSCHG J, & Fahnenstich H (2005). Cerebral hemodynamic changes in response to an executive function task in children with attention-deficit hyperactivity disorder measured by near-infrared spectroscopy. Journal of Developmental & Behavioral Pediatrics, 26(2), 105–111. [DOI] [PubMed] [Google Scholar]
- 46.Nagashima M, Monden Y, Dan I, Dan H, Tsuzuki D, Mizutani T, Yamagata T, et al. (2014b). Neuropharmacological effect of methylphenidate on attention network in children with attention deficit hyperactivity disorder during oddball paradigms as assessed using functional near-infrared spectroscopy. Neurophotonics, 1(1), 015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagashima M, Monden Y, Dan I, Dan H, Mizutani T, Tsuzuki D, Shimoizumi H, et al. (2014c). Neuropharmacological effect of atomoxetine on attention network in children with attention deficit hyperactivity disorder during oddball paradigms as assessed using functional near-infrared spectroscopy. Neurophotonics, 1(2), 025007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mauri M, Grazioli S, Crippa A, et al. (2020). Hemodynamic and behavioral peculiarities in response to emotional stimuli in children with attention deficit hyperactivity disorder: An fNIRS study. Journal of Affective Disorders, 277, 671–680. [DOI] [PubMed] [Google Scholar]
- 49.Bell L, Scharke W, Reindl V, Fels J, Neuschaefer-Rube C, & Konrad K (2020). Auditory and Visual Response Inhibition in Children with Bilateral Hearing Aids and Children with ADHD. Brain Sciences, 10(5), 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Güven A, Altınkaynak M, Dolu N, et al. (2019). Combining functional near-infrared spectroscopy and EEG measurements for the diagnosis of attention-deficit hyperactivity disorder. Neural Computing and Applications, 1–14. [Google Scholar]
- 51.Arai S, Okamoto Y, Fujioka T, Inohara K, Ishitobi M, Matsumura Y, Wada Y, et al. (2016). Altered frontal pole development affects self-generated spatial working memory in ADHD. Brain and Development, 38(5), 471–480. [DOI] [PubMed] [Google Scholar]
- 52.Tsujimoto S, Yasumura A, Yamashita Y, Torii M, Kaga M, & Inagaki M (2013). Increased prefrontal oxygenation related to distractor-resistant working memory in children with attention-deficit/hyperactivity disorder (ADHD). Child Psychiatry & Human Development, 44(5), 678–688. [DOI] [PubMed] [Google Scholar]
- 53.Schecklmann M, Romanos M, Bretscher F, Plichta MM, Warnke A, & Fallgatter AJ (2010). Prefrontal oxygenation during working memory in ADHD. Journal of psychiatric research, 44(10), 621–628. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K, Okumura Y, Kita Y, Oi Y, Yamashita Y, Goto T, & Inagaki M (2017). Excessive hemodynamic activity in the superior frontal cortex during the flanker task in children with attention deficit hyperactivity disorder. Neuroreport, 28(13), 828–832. [DOI] [PubMed] [Google Scholar]
- 55.Nakashima M, Matsuo K, Hashimoto A, Nakano M, Fujii Y, Matsushige T, Sugiyama S, et al. (2014). Prefrontal abnormality in children with ADHD during cognitive interference control: a functional NIRS study. Bull Yamaguchi Med Sch, 61(3–4), 37–47. [Google Scholar]
- 56.Yasumura A, Yamamoto H, Yasumura Y, Moriguchi Y, Hiraki K, Nakagawa E, & Inagaki M (2015). Cognitive shifting in children with attention-deficit hyperactivity disorder: a near infrared spectroscopy study. Afr. J. Psychiatry, 18(196), 10–4172. [Google Scholar]
- 57.Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Pavia JM, Wolf U, & Wolf M (2014). A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage, 85, 6–27. [DOI] [PubMed] [Google Scholar]
- 58.Inoue Y, Sakihara K, Gunji A, et al. (2012). Reduced prefrontal hemodynamic response in children with ADHD during the Go/NoGo task: a NIRS study. Neuroreport, 23(2), 55–60. [DOI] [PubMed] [Google Scholar]
- 59.Aron AR, Robbins TW, & Poldrack RA (2014). Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences, 18(4), 177–185. [DOI] [PubMed] [Google Scholar]
- 60.Aron AR, Robbins TW, & Poldrack RA (2004). Inhibition and the right inferior frontal cortex. Trends in cognitive sciences, 8(4), 170–177. [DOI] [PubMed] [Google Scholar]
- 61.Morein- Zamir S, Dodds C, van Hartevelt TJ, Schwarzkopf W, Sahakian B, Müller U, & Robbins T (2014). Hypoactivation in right inferior frontal cortex is specifically associated with motor response inhibition in adult ADHD. Human brain mapping, 35(10), 5141–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hampshire A, Chamberlain SR, Monti MM, Duncan J, & Owen AM (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage, 50(3), 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubia K, Smith AB, Brammer MJ, & Taylor E (2003). Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage, 20(1), 351–358. [DOI] [PubMed] [Google Scholar]
- 64.Hampshire A, Thompson R, Duncan J, & Owen AM (2009). Selective tuning of the right inferior frontal gyrus during target detection. Cognitive, Affective, & Behavioral Neuroscience, 9(1), 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ota T, Iida J, Nakanishi Y, Sawada S, Matsuura H, Yamamuro K, Kishimoto T, et al. (2015). Increased prefrontal hemodynamic change after atomoxetine administration in pediatric attention- deficit/hyperactivity disorder as measured by near- infrared spectroscopy. Psychiatry and Clinical Neurosciences, 69(3), 161–170. [DOI] [PubMed] [Google Scholar]
- 66.Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological bulletin, 121(1), 65. [DOI] [PubMed] [Google Scholar]
- 67.Ferrari M, Mottola L, & Quaresima V (2004). Principles, techniques, and limitations of near infrared spectroscopy. Canadian journal of applied physiology, 29(4), 463–487. [DOI] [PubMed] [Google Scholar]
- 68.Okamoto M, Dan H, Shimizu K, Takeo K, Amita T, Oda I, Kohyama K, et al. (2004). Multimodal assessment of cortical activation during apple peeling by NIRS and fMRI. Neuroimage, 21(4), 1275–1288. [DOI] [PubMed] [Google Scholar]
- 69.Pinti P, Tachtsidis I, Hamilton A, Hirsch J, Aichelburg C, Gilbert S, & Burgess PW (2020a). The present and future use of functional near- infrared spectroscopy (fNIRS) for cognitive neuroscience. Annals of the New York Academy of Sciences, 1464(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinti P, Scholkmann F, Hamilton A, Burgess P, & Tachtsidis I (2019). Current status and issues regarding pre-processing of fNIRS neuroimaging data: an investigation of diverse signal filtering methods within a general linear model framework. Frontiers in human neuroscience, 12, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu N, Cui X, Bryant DM, Glover GH, & Reiss AL (2015). Inferring deep-brain activity from cortical activity using functional near-infrared spectroscopy. Biomedical optics express, 6(3), 1074–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho H, Nemoto EM, Sanders M, Fernandez K, & Yonas H (2000). Comparison of two commercially available near-infrared spectroscopy instruments for cerebral oximetry. Journal of neurosurgery, 93(2), 351–354. [DOI] [PubMed] [Google Scholar]


