Abstract
Allogeneic hematopoietic cell transplantation (HCT) can cure many non-malignant conditions but concern for morbidity and mortality remains. To help physicians estimate patient-specific transplant mortality risk, the HCT comorbidity index (HCT-CI) is used. However, paediatric physicians use the HCT-CI less frequently than adult counterparts. We used the Center for International Blood and Marrow Transplant Research database to expand the HCT-CI comorbidity definitions to be more inclusive of children, adolescents and young adults (AYA) patients, adding history of mechanical ventilation, history of invasive fungal infection, assessment of chronic kidney disease (CKD) by estimated glomerular filtration rate, expanding the definition of obesity, and adding an underweight category. A total of 2,815 children and AYAs (<40yo) who received first allogeneic HCT for non-malignant diseases from 2008–2017 were included to create an expanded youth non-malignant HCT-CI (expanded ynHCT-CI) and a simplified non-malignant (simplified ynHCT-CI) HCT-CI. The expanded comorbidities occurred frequently – history of mechanical ventilation (9.6%), history of invasive fungal infection (5.9%), mild CKD (12.2%), moderate/severe CKD (2.1%), obesity (10.9%), underweight (14.5%). 39% of patients had an increase in their comorbidity score using the expanded ynHCT-CI, leading to a redistribution of scores: ynHCT-CI score 0 (35%), 1–2 (36.4%), and ≥3 (28.6%). Patients with an increase in their comorbidity score had an increased hazard of mortality compared to those whose score remained the same (HR 1.41, 95% CI 1.01–1.98). Modifications to the HCT-CI can benefit children and AYA patients with non-malignant diseases, creating a risk assessment tool that is clinically relevant and better captures comorbidity in this younger population.
Keywords: HCT-CI, comorbidities, allogeneic hematopoietic cell transplantation, paediatrics, adolescent and young adults (AYA), non-malignant diseases
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) can potentially cure many non-malignant diseases and its use has steadily increased1. With advances in donor selection, transplantation strategies, and supportive care, HCT outcomes have improved in the recent era1. However, HCT-related complications remain a concern and can result in substantial morbidity and mortality1. As the decision and timing of HCT can vary for patients with non-malignant diseases, it is imperative for physicians to be able to discuss a patient’s risk of transplant-related mortality.
To help physicians estimate patient-specific mortality risk, the HCT-Comorbidity Index (HCT-CI) is often used, incorporating 17 weighted pre-HCT comorbidities into a composite score2. Increasing HCT-CI scores, a reflection of higher comorbidity burden, are associated with inferior survival outcomes2–5. Multiple studies have validated the impact of the HCT-CI in transplantation for malignancies; our recent study from the Center for International Blood and Marrow Transplant Research (CIBMTR) also validated its use for patients with non-malignant diseases4–6. In that study, 92% of allogeneic HCT (alloHCT) recipients were younger than 40 years old and 64% had an HCT-CI score of 0, higher than encountered among older adults and those with malignancies5,6. This suggests that younger HCT recipients have unique comorbidity burdens compared to older adults, which may not be captured well enough in the HCT-CI.
The use of the HCT-CI in the paediatric transplant community has been unknown. Therefore, we executed a survey of transplant physicians that showed 58% of paediatric providers reported rarely using the HCT-CI (<5% of the time) compared to only 7% of adult providers7.
Paediatricians noted concerns about the applicability of the HCT-CI and its impact in younger patients7. This highlighted the need for a re-appraisal of the HCT-CI in this younger patient population.
To this aim, we’ve used the CIBMTR database to expand the HCT-CI comorbidities to include definitions specific to a younger patient population. We re-calculated the associations of the original HCT-CI comorbidity definitions with overall survival, and then assessed whether these adjustments allowed for better characterization of comorbidity in this younger patient population.
METHODS
Database and Patient Selection
Patients were identified from the CIBMTR database, a research affiliate of the International Bone Marrow Transplant Registry and National Marrow Donor Program. More than 450 transplant centres worldwide report baseline characteristics and longitudinal outcome data to the CIBMTR at 100 days, 6 months, and yearly after HCT for each patient. CIBMTR collects patient and transplant data for all patients undergoing alloHCT at US centres using transplant essential data forms (TED). A weighted algorithm selects patients for additional data reporting, collected on comprehensive report forms (CRF). CRFs supply more granular data on pre-transplant organ function and includes information that allows assessment of body mass index (BMI) percentile and calculation of estimated glomerular filtration rate. Compliance is monitored by on-site audits. Observational studies supported by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information is collected and maintained in the CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
We included children, adolescents, and young adult (AYA) patients that received first alloHCT from January 2008 to December 2017 and consented to their data being used for research by the CIBMTR. We used standard definitions of AYA, creating an age cut-off of 40 years old (yo) or younger at the time of HCT8. Patients had follow-up forms completed post-HCT (even if death occurred) and were transplanted at centres known to have >85% completeness in reporting (Figure 1).
Figure 1. Inclusion Criteria.
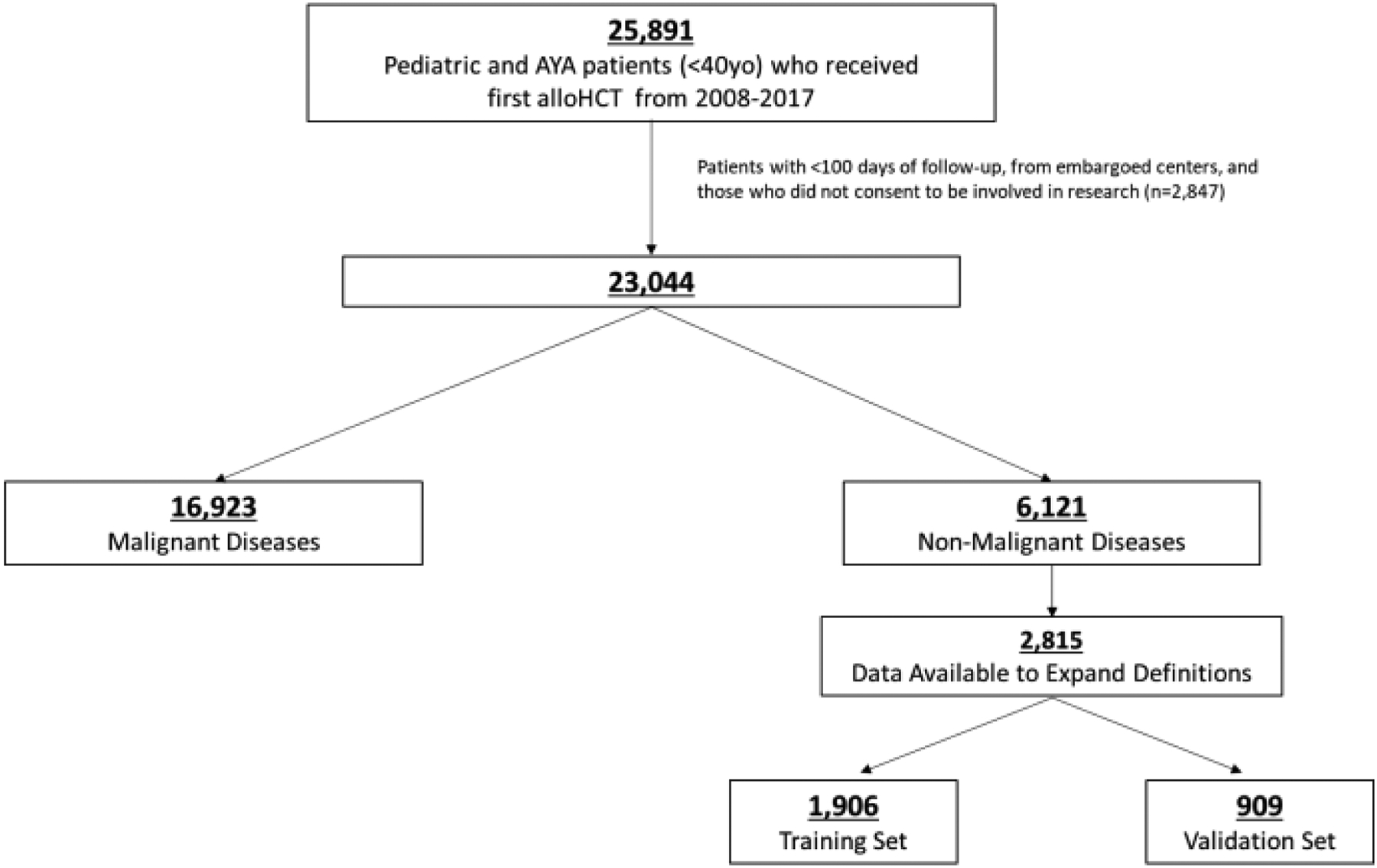
25,891 pediatric and young adult patients who received allogeneic-HCT (allo-HCT) from 2008–2017. 6,121 patients received HCT for non-malignant indications and of these, 2,815 had data available to expand comorbidity definitions.
Study Outcomes and Definitions
Comorbidities defined by Sorror and colleagues have been collected by the CIBMTR on Pre-Transplant TED (pre-TED) forms since 20072. After review of the comorbidities filed on the forms, HCT-CI scores were calculated by CIBMTR statisticians. Additional pre-transplant factors were reviewed including history of mechanical ventilation (yes/no) and history of invasive fungal infection (yes/no), which are collected on pre-TED forms.
Using CRF forms, additional information on nutritional status and renal function were calculated. Assessment of chronic kidney disease (CKD) was made by calculating the estimated glomerular filtration rate (eGFR) using the Bedside Schwartz equation for children <18yo and the CKD-EPI equation for patients 18–39yo9,10. Race was taken into consideration in the calculation of eGFR, as specified in the defined equations. CKD was categorized by eGFR as normal (>90ml/min/1.73m2), mild CKD (60–89 ml/min/1.73m2), or moderate/severe CKD (<60ml/min/1.73m2), using National Kidney Foundation guidelines11. The definition of obesity for children ≤18yo was expanded to include assessment of body mass index (BMI) with >95th CDC referenced percentile categorized as obese12–14. Additionally, an underweight category was defined as a BMI of <5th percentile in children ≤18yo or a BMI of <18kg/m2 for young adults 19–39yo13.
Patients were categorized by indication for transplant into non-malignant disease categories. Conditioning was classified as myeloablative or reduced intensity as previously described15. A subset of patients did not receive any planned conditioning. Donors were classified as matched if they were human leukocyte antigen (HLA) matched with recipient at HLA-A, B, C, and DRB1 (8/8) for bone marrow (BM) or peripheral blood (PBSC) grafts or matched at HLA-A, -B, and - DRB1 (6/6) for cord blood grafts. All others were partially matched or mismatched donors.
Statistical Analysis
Patient, transplant, and comorbidity characteristics were described using frequency (percent) for categorical variables and median (range) for continuous variables. The primary outcome of this analysis was overall survival (OS), defined as length of time alive since HCT until death from any cause. Since relapse can be difficult to define in patients with non-malignant diseases, non-relapse mortality was not assessed in this cohort.
Separate training and validation samples were created using a 2/3, 1/3 split. In the training set, Multivariable Cox regression was used to test whether each HCT-CI comorbidity, as well as additional risk factors, affected OS after adjusting for patient-, disease-, and transplant-related variables. Variables found to be significant and were adjusted for in the final multivariable model included disease, age, performance status, year of transplant, donor type, and recipient CMV status. Collectively, these additional risk factors were called “expanded comorbidities”. Proportional hazards assumption was assessed for all variables using graphical methods.
Additional information which may be more relevant in the younger patient population was used to create the “expanded comorbidities”. History of invasive fungal infection, broader obesity and underweight definitions, history of mechanical ventilation, and CKD by eGFR were all assessed independently for their effect on survival after adjusting for significant variables (disease, age, performance status, year of transplant, donor type, and recipient CMV status). History of invasive fungal infection was then incorporated in the definition of infection, which was included as the “Infection” comorbidity. Similarly, BMI >95th percentile was included within the definition of obesity and considered as a single “Obesity” comorbidity. Underweight was considered as a separate weight disturbance comorbidity. History of mechanical ventilation was assessed with pulmonary disease and added to the “Severe Pulmonary Disease” comorbidity definition, based on similarities in hazard ratios (HR) by Cox analysis. CKD categories were assessed with renal disease; moderate/severe CKD was then included in the definition of the “Moderate/Severe Renal Disease”, based on similarities in HR. Mild CKD was made an independent comorbidity. All other comorbidities were retained as in the HCT-CI and the comorbidities were weighted as per the same HR rule used for the original HCT-CI2.
Once significant predictors were obtained, two modifications to the HCT-CI were developed: 1) Using expanded comorbidities definitions that could be applied to younger patients with non-malignant diseases (expanded ynHCT-CI), and 2) Simplifying the HCT-CI by removing the comorbidity definitions with hazard ratio (HR) <1.2, creating the non-malignant HCT-CI (simplified ynHCT-CI) for pediatric and AYA patients. HR’s <1.2 would indicate minimal contribution to the predictive ability of the final model and a HR of 1.2 cut-off was used to determine inclusion of comorbidities in the original HCT-CI2. So, the simplified ynHCT-CI represents a simplified and shortened model of the expanded and original definitions of comorbidities.
The validation cohort was used to compare predictive performance of these two modified as well as the original HCT-CI. C-statistics were computed using the ‘pec’ package at various timepoints, for both the training and validation datasets. All p-values are two-sided and significance defined as p<0.05. SAS 9.3 (SAS Inc., Cary, NC) was used for all analyses.
RESULTS
Patient Characteristics
A total of 23,044 children and AYAs received alloHCT between 2008 and 2017; 27% (n=6,121) had non-malignant diseases. Of these, 2,815 had CRF forms to assess our expanded comorbidities and were included in the final analyses (Figure 1). Patients with CRF forms were comparable to the overall population with TED forms available, except for a higher proportion of patients receiving cord blood transplants (33% versus 21%) and a shorter median follow-up time (36 months versus 44 months), Supplemental Table 1.
Patient and transplant demographics are noted in Table 1. Patients received alloHCT at a median age of 6yo, with 84% being <18yo at HCT. The most frequent indications for alloHCT were aplastic anaemia (25·8%), primary immune deficiencies (26·5%), and hemoglobinopathies (18·2%). HLA-matched unrelated donors (25·6%) and HLA-matched siblings (21.2%) were the most frequently used donors and BM was the most common graft source (54%). Conditioning intensity was myeloablative in 48·5% and reduced intensity in 47·1% of patients; 125 patients (4·4%) did not receive any planned conditioning. Most patients received serotherapy with anti-thymocyte globulin (ATG) or alemtuzumab (87·8%).
Table 1.
Patient and Transplant Characteristics of patients used to assess expanded comorbidity definitions.
| Characteristic | N (%) |
|---|---|
| Number of patients | 2815 |
| Number of centers | 135 |
| Patient-related | |
| Age, median (range), years | 6 (<1–39) |
| Age group | |
| 0–2 years | 946 (33·6) |
| 3–10 years | 908 (32·3) |
| 11–18 years | 520 (18·5) |
| 19–29 years | 326 (11·6) |
| 30–39 years | 115 (4·1) |
| Sex | |
| Male | 1708 (60·7) |
| Race | |
| Caucasian | 1734 (61·6) |
| African-American | 618 (22) |
| Asian | 159 (5·6) |
| Pacific islander | 9 (0·3) |
| Native American | 44 (1·6) |
| More than one race | 73 (2·6) |
| Missing | 178 (6·3) |
| Ethnicity | |
| Hispanic or Latino | 541 (19·2) |
| Non-Hispanic or non-Latino | 2114 (75·1) |
| Non-resident of the U.S. | 74 (2·6) |
| Missing | 86 (3·1) |
| Karnofsky/Lansky performance score | |
| < 90 | 513 (18·2) |
| Missing | 108 (3·8) |
| HCT-CI | |
| 0 | 1666 (59·2) |
| 1–2 | 586 (20·8) |
| 3+ | 563 (20) |
| Disease-related | |
| Disease | |
| Severe aplastic anemia | 726 (25·8) |
| Congenital bone marrow failure syndrome | 286 (10·2) |
| Hemoglobinopathy | 511 (18·2) |
| Primary immune deficiency/dysregulation syndrome | 745 (26·5) |
| Histiocytic disease | 201 (7·1) |
| Metabolic disease | 337 (12) |
| Autoimmune disease | 9 (0·3) |
| Transplant-related | |
| Donor type | |
| HLA-identical sibling | 598 (21·2) |
| Well-matched unrelated | 721 (25·6) |
| Other related | 290 (10·3) |
| Partially-matched or mis-matched unrelated | 250 (8·9) |
| Unrelated (matching unknown) | 6 (0·2) |
| Umbilical Cord blood | 950 (33·7) |
| Graft source | |
| Bone marrow | 1521 (54) |
| Peripheral blood | 344 (12·2) |
| Umbilical cord blood | 950 (33·7) |
| Donor sex | |
| Male | 1056 (37·5) |
| Female | 797 (28·3) |
| Cord blood – not reported | 950 (33·7) |
| Missing | 12 (0·4) |
| Donor/recipient sex match | |
| M-M | 660 (23·4) |
| M-F | 396 (14·1) |
| F-M | 458 (16·3) |
| F-F | 339 (12) |
| CB - recipient male | 580 (20·6) |
| CB - recipient female | 370 (13·1) |
| Missing | 12 (0·4) |
| Conditioning regimen intensity | |
| Myeloablative | 1365 (48·5) |
| Reduced Intensity/Non-myeloablative | 1325 (47·1) |
| No planned conditioning | 125 (4·4) |
| Donor/recipient CMV serostatus | |
| +/+ | 596 (21·2) |
| +/− | 247 (8·8) |
| −/+ | 458 (16·3) |
| −/− | 487 (17·3) |
| CB - recipient + | 437 (15·5) |
| CB - recipient − | 480 (17·1) |
| CB - recipient CMV unknown | 33 (1·2) |
| Missing | 77 (2·7) |
| GVHD prophylaxis | |
| Ex-vivo T-cell depletion/ CD34 selection | 207 (7·3) |
| Post-CY +/− other(s) | 118 (4·2) |
| TAC + MMF +/− other(s) (except post-CY) | 328 (11·7) |
| TAC + MTX +/− other(s) (except MMF, post-CY) | 451 (16) |
| TAC + other(s) (except MMF, MTX, post-CY) | 68 (2·4) |
| TAC alone | 54 (1·9) |
| CSA + MMF +− other(s) (except post-CY) | 652 (23·2) |
| CSA + MTX +− other(s) (except MMF, post-CY) | 483 (17·2) |
| CSA + other(s) (except MMF, MTX, post-CY) | 300 (10·7) |
| CSA alone | 70 (2·5) |
| Other(s)b | 64 (2·3) |
| Missing | 20 (0·7) |
| ATG/Alemtuzumab | |
| ATG alone | 1554 (55·2) |
| Alemtuzumab alone | 830 (29·5) |
| ATG + Alemtuzumab | 3 (0·1) |
| No ATG or Alemtuzumab | 355 (12·6) |
| Missing | 73 (2·6) |
| Year of transplant | |
| 2008–2012 | 1138 (40·4) |
| 2013–2017 | 1677 (59·6) |
| Median follow-up of survivors (range), months | 36 (3–125) |
Abbreviations: Cord Blood (CB), Cyclophosphamide (Cy), Tacrolimus (TAC), Mycophenolate Mofetil (MMF), Methotrexate (MTX), Cyclosporine (CSA), Anti-Thymocyte Globulin (ATG)
Most patients had a performance status of 90–100% (78%). Using the original parameters, patients were classified with HCT-CI scores of 0 in 59·2%, 1–2 in 20·8%, and ≥3 in 20%. The training and validation cohorts are described in Supplemental Table 2.
Distribution of Comorbidities
The most frequent HCT-CI comorbidities seen were hepatic disease (8·8% mild, 6·2% moderate/severe), pulmonary disease (8·5% moderate, 7·2% severe), and infection (12·2%), Figure 2. A history of mechanical ventilation was present in 9·6% of patients; 47% of these were 0–2yo, 29% 2–10yo, 15% 11–18yo, and 9% 19–39yo. Eight percent of patients had a history of mechanical ventilation but were not classified as having moderate or severe pulmonary disease by HCT-CI definitions. A history of invasive fungal infection was present in 5·9% of patients. Four percent of patients had a history of invasive fungal infection that had resolved and so were not categorized as having an infection by HCT-CI definitions. Incorporating expanded CKD definitions noted above, 12·2% of patients were classified as mild and 2·1% as moderate/severe renal disease. In contrast, only 1% of patients met criteria for renal disease as specified in the HCT-CI. Using the expanded definition of obesity, 10·9% of patients were classified as obese, compared to only 1·9% using the original definition. Additionally, 14·5% were classified as underweight (Figure 2).
Figure 2. Comorbidity Distribution.
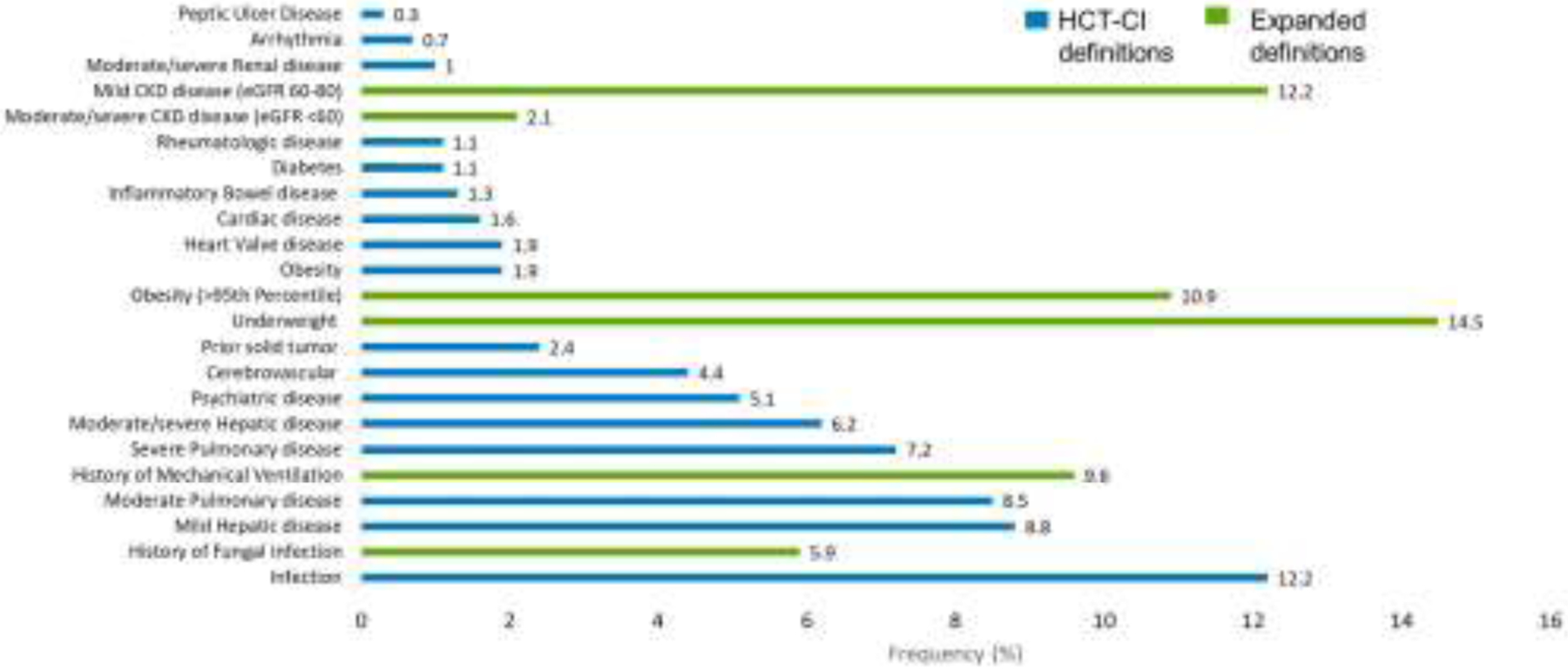
Comorbidities present in our cohort of patients with Non-Malignant Diseases.
Re-designing the HCT-CI for the Younger Patient Population
Patients with a history of mechanical ventilation had an HR for mortality of 1·85 (95% Confidence Interval (CI) 1·39–2·48), similar to severe pulmonary disease within the original HCT-CI (HR 1·91, 95% CI 1·33–2·73). Hence, both were combined to define severe pulmonary comorbidity with a final HR of 1·80 (95% CI 1·39–2·32). CKD also showed an increased hazard of death (mild CKD, HR 1·49, 95% CI 1·11–2·0; moderate/severe CKD, HR 2·04, 95% CI 1·1–3·26). Since the HR for moderate/severe CKD was similar to moderate/severe renal disease as defined in the original HCT-CI (HR 2·21, 95% CI 1·10–4·42), a single comorbidity of “moderate/severe renal disease” was created defined as creatinine >2mg/dL or eGFR of <60 mL/min, which had an HR of 1·77 (95% CI 1·09–2·87). Mild CKD was made into an independent comorbidity, defined as “mild renal disease”. Patients categorized as underweight had an HR of 1·55 (95% CI 1·18–2·04) and a new comorbidity, defined as “underweight”, was created. Table 2 shows the comorbidity definitions, results of multivariable analysis, and accompanying weighted score for the new expanded youth non-malignant HCT-CI (expanded ynHCT-CI).
Table 2.
Multivariable analysis of original and expanded comorbidity definitions. Where there were multiple components of a comorbidity definition assessed, the hazard ratio for each individual component is reported, as well as when all components were combined into a single definition. The accompanying weighted score is noted for each comorbidity in the original HCT-CI, expanded youth non-malignant HCT-CI (expanded ynHCT-CI), and simplified youth non-malignant HCT-CI (simplified ynHCT-CI) for children and young adults with non-malignant Diseases (*modified comorbidities). Comorbidities were adjusted for disease, age, performance status, year of transplant, donor type, and recipient CMV.
| Comorbidity | Definition | HR (95% CI) | HCT-CI | Expanded ynHCT-CI | Simplified ynHCT-CI | |
|---|---|---|---|---|---|---|
| Arrhythmia | Atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias | 0.78 (0.19–3.17) | 1 | 1 | 0 | |
| Cardiac disease | Coronary artery disease, congestive heart failure, myocardial infarction, or EF ≤50% on most recent test | 2.32 (1.28–4.19) | 1 | 1 | 1 | |
| Inflammatory Bowel disease | Crohn disease or Ulcerative colitis | 2.64 (1.37–5.07) | 1 | 1 | 1 | |
| Diabetes | Requiring treatment with insulin or oral hypoglycemics, but not diet alone | 1.23 (0.54–2.82) | 1 | 1 | 1 | |
| Psychiatric disease | Requiring psychiatric consult or treatment in the last 4 weeks | 1.00 (0.6–1.64) | 1 | 1 | 0 | |
| Cerebrovascular | Any history of transient ischemic attack, subarachnoid hemorrhage, or cerebrovascular accident | 2.43 (1.45–4.09) | 1 | 1 | 1 | |
| Infection* | Requiring continuation of antimicrobial treatment after day 0 | 2.34 (1.77–3.07) | 1.84 (1.41–2.41) | 1 | 1 | 1 |
| History of Invasive Fungal Infection | 1.2 (0.8–1.81) | 0 | ||||
| Obesity* | >35 kg/m2 | 1.25 (0.63–2.49) | 1.04 (0.76–1.43) | 1 | 1 | 0 |
| BMI >95th percentile by CDC guidelines for (≤18yo) | 1.09 (0.79–1.52) | 0 | ||||
| Underweight * | Underweight: BMI <5th percentile by CDC guidelines for (≤18yo) or <18kg/m2 (>18yo) | 1.55 (1.18–2.04) | 0 | 1 | 1 | |
| Mild Hepatic disease | Chronic hepatitis, bilirubin >upper limit of normal to 1.5x upper limit of normal, or AST/ALT upper limit of normal to 2.5x upper limit of normal | 1.19 (0.83–1.71) | 1 | 1 | 0 | |
| Mild Renal disease * | eGFR 60–89ml/min/1.73m2 (by Bedside Schwartz calculation for <18y, CKD-EPI calculation for ≥18yo) | 1.49 (1.11–2.00) | 0 | 1 | 1 | |
| Moderate/severe Renal disease* | Creatinine >2mg/dL, on dialysis, or prior renal transplant | 2.21 (1.10–4.42) | 1.77 (1.09–2.87) | 2 | 2 | 2 |
| OR eGFR <60ml/min/1.73m2 (by Bedside Schwartz calculation for <18y, CKD-EPI calculation for ≥18yo) | 2.04 (1.18–3.53) | 0 | ||||
| Moderate Pulmonary disease | Corrected diffusion capacity of carbon monoxide and/or FEV1 66–80%, or dyspnea on slight activity | 0.84 (0.54–1.29) | 2 | 2 | 0 | |
| Peptic Ulcer Disease | Confirmed by endoscopy and requiring treatment | 2.88 (0.9–9.23) | 2 | 2 | 2 | |
| Rheumatologic disease | Systemic Lupus, Rheumatoid Arthritis, Polymyositis, Mixed connective tissue disease, or polymyalgia rheumatic requiring treatment | 1.39 (0.59–3.31) | 2 | 2 | 2 | |
| Moderate/severe Hepatic disease | Liver cirrhosis, bilirubin >1.5x upper limit of normal, or AST/ALT >2.5x upper limit of normal | 2.67 (1.88–3.78) | 3 | 3 | 3 | |
| Severe Pulmonary disease* | Corrected diffusion capacity of carbon monoxide and/or FEV1 ≤65%, dyspnea at rest or requiring oxygen | 1.91 (1.33–2.73) | 1.80 (139–2.32) | 3 | 3 | 3 |
| OR Prior history of mechanical ventilation | 1.85 (1.39–2.48) | 0 | ||||
| Prior solid tumor | At any point in patient’s history, excluding non-melanoma skin cancer, leukemia, lymphoma, or multiple myelom | 1.56 (0.9–2.71) | 3 | 3 | 3 | |
| Heart Valve disease | Except asymptomatic mitral valve prolapse | 1.41 (0.77–2.58) | 3 | 3 | 3 | |
Abbreviations: HR – Hazard Ratio; CI – confidence interval; ynHCT-CI – youth non-malignant HCT-CI; EF – ejection fraction; BMI – body mass index; eGFR – estimated glomerular filtration rate; FEV1 – forced expiratory volume in 1 second
Using the expanded definitions increased the number of patients with comorbidities pretransplant: 39% patients had an increased comorbidity score based on the expanded ynHCT-CI. Of the 59·2% of patients with an HCT-CI score of 0, 25% had an increase in their score using the ynHCT-CI, including 17·6% with an increment of 1 and 7·4% with an increase in their score by ≥2. Similarly, of the 20·8% of patients with an HCT-CI score of 1–2, 8% had an increase in their score using the ynHCT-CI and of the 20% with an HCT-CI score of ≥3, 5% had a further increase in their score with the ynHCT-CI. Patients with an increase in their comorbidity score using the ynHCT-CI had an increased hazard of mortality compared to those whose score remained the same (validation cohort HR 1·41, 95% CI 1·01–1·98, p=0.046; training cohort HR 1.34, 95% CI 1.07–1.67, p=0.011), after adjusting for covariates, including the original HCT-CI. This was true for patients with an initial HCT-CI score of 0, 1–2, and ≥3 (Figure 3). Ultimately with the ynHCT-CI, comorbidity scores were redistributed – 35% had a score of 0, 36·4% scores of 1–2, and 28·6% scores of ≥3.
Figure 3. Survival Outcomes.
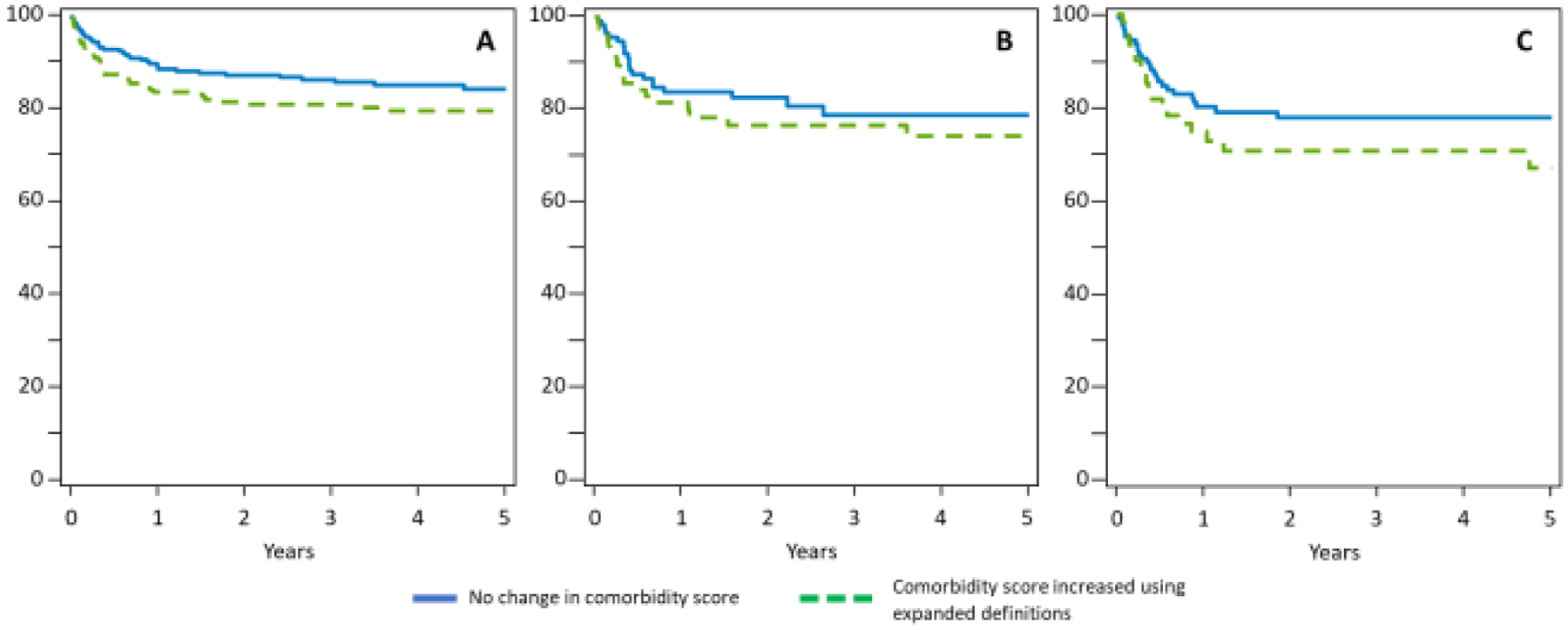
Adjusted overall survival in patients whose comorbidity score increased using expanded ynHCT-CI definitions, compared to those with expanded comorbidity score the same as initial HCT-CI score, (overall HR 1.34, 95% CI 1.07–1.67 p=0.011 in training set, HR 1.41 95% CI 1.01–1.98, p=0.046 in validation set). A) Patients with an initial HCT-CI score of 0 (p=. B) Patients with an initial HCT-CI score of 1–2. C) Patients with an initial HCT-CI score of ≥3.
In the validation cohort, ynHCT-CI scores of 1–2 and ≥3 were associated with increased HRs [HR 1·36 (95%CI 0·89–2·06) and 2·08 (95%CI 1·37–3·17), respectively] for mortality compared to score 0 on multivariable analysis. Using c-statistics estimates, both the expanded and the original HCT-CI had similar discriminatory capacity for 2-year OS (65·8 versus 64·3, respectively).
Simplifying the ynHCT-CI into the Simplified ynHCT-CI
To create the simplified ynHCT-CI, comorbidities with a HR of <1·2 were removed – arrhythmia (HR 0·78, 95%CI 0·19–3·17), psychiatric disease (HR 1·00, 95%CI 0·6–1·64), mild hepatic disease (HR 1·19, 95%CI 0·83–1·71), and moderate pulmonary disease (HR 0·84, 95%CI 0·54–1·29). When these comorbidities were removed from the score, 39% of patients were classified as having a simplified ynHCT-CI score of 0, 32% with 1–2, and 29% with ≥3.
In the validation cohort, simplified ynHCT-CI scores of 1–2 and ≥3 were associated with increased HRs [HR 1·34 (95%CI 0·89–2·02) and HR 1·97 (95%CI 1·32–2·93), respectively] for mortality compared to score 0 on multivariable analysis. The simplified ynHCT-CI showed similar discriminatory capacity to the original HCT-CI for 2-year OS (c-statistic 64.9 for simplified ynHCT-CI versus 64·3 for HCT-CI).
Subgroup Analysis by Disease Indication and Age
Subgroup analyses were performed assessing patients with aplastic anemia (n=230), hemoglobinopathies (n=154), and primary immune deficiency disorders (n=229). The frequency of patients with comorbidities, as defined in the expanded ynHCT-CI, was described for each disease cohort in Supplemental Table 3 and 4. In patients with aplastic anemia, 31% of patients increased their comorbidity scores using the expanded ynHCT-CI compared to the original HCT-CI, showing improved quantification of comorbidity in this group. These patients with an increased score had a HR of 1.46 (95% CI 0.67–3.18, p=0.34). Fourteen percent of patients with hemoglobinopathies had an increase in their comorbidity score using the expanded ynHCT-CI. Similarly, patients with hemoglobinopathies who had increased scores had inferior survival with a HR of 4.37 (95% CI 1.07–17.9, p=0.04). In patients with primary immune deficiency disorders, 54% had increase in their comorbidity scores using the expanded ynHCT-CI compared to the original HCT-CI; those with an increase in their score had a HR of 1.28 (95% CI 0.7–2.34, p=0.42). Of 748 children and adolescents 18yo and younger, 41% had an increase in their comorbidity score using the expanded ynHCT-CI. This correlated with inferior survival outcomes in those whose score increased with a HR of 1.25 (95% CI 0.86–1.8, p=0.239). For patients over the age of 18yo (n=119), 18% had an increase in comorbidity score which was associated with a HR of 3.24 (95% CI 1.37–7.7, p=0.008) compared to those whose scores stayed the same.
DISCUSSION
The introduction of the HCT-CI has impacted the clinical practice of alloHCT for adults – from counselling patients to modifying the transplant approach. However, the HCT-CI may be difficult to apply in children and AYA patients where the definition of organ dysfunction, assessment techniques, and the frequency of comorbidities often differ from adults. This was recently suggested in a survey of pediatric transplant physicians conducted by our group, where the application of the HCT-CI was less frequent than by adult counterparts7. Here, we show that expanding the definitions in the HCT-CI improved the classification of comorbidities in children and AYA patients undergoing alloHCT for non-malignant diseases. We propose two potential modified HCT-CI risk indices that are more inclusive and clinically relevant for this younger population.
The HCT-CI has been widely validated in patients receiving alloHCT with increasing scores associated with decreased survival and increased NRM2–6,16–18. However, the few studies looking at pediatric and AYA populations have highlighted challenges in completing HCT-CI assessments in these younger patients16–20. Ultimately, many pediatric transplant physicians have not adopted the HCT-CI for managing their patients, citing views that the HCT-CI is not applicable or not impactful in this younger population7.
Laboratory values and organ assessments for children and AYA patients often vary based on age or body habitus. First, assessment of obesity in children relies on CDC-defined growth curve percentiles instead of an absolute BMI. Although a previous study found obesity associated with inferior survival outcomes in patients with severe aplastic anemia, the cohort assessed was from 1990–2005 and so may not represent contemporary patient demographics and transplantation practices21. More recent studies have found no association between BMI and post-transplant outcomes in children22,23. In our study, we showed that underweight status was more common than obesity (14·5% and 10·9%, respectively) and had a greater detrimental effect on survival in this population with non-malignant diseases (HR 1·55 and 1·04, respectively). Recent studies have found similar findings in patients with malignancies, supporting the addition of “underweight” as a comorbidity in this younger population24,25. Second, renal function does not reliably correlate with absolute creatinine values in children26; for this reason, the renal disease comorbidity cannot be assessed by absolute creatinine alone, as this may underestimate renal dysfunction within this age group27. Pediatric transplant physicians often use GFR assessment as part of routine pre-transplant organ evaluation. In our study, we showed that eGFR can be used to supplement the definition of moderate/severe renal disease and create a new comorbidity category of mild renal disease. Using these new definitions, 13·3% more patients were categorized as having renal disease which was associated with worse survival outcomes in our cohort. Lastly, although pulmonary disease was commonly reported in our cohort (14·7%), many children are unable to cooperate with spirometry testing, likely resulting in under-reporting of pulmonary insufficiency28–30. As a result of this procedural limitation, pre-transplant assessment in young children relies on clinical evaluation of respiratory symptoms, exam findings, and pulse oximetry, which may also underestimate pre-transplant pulmonary disease. We attempted to improve identification of pulmonary disease by including “history of mechanical ventilation” which would suggest prior pulmonary injury and represent pulmonary impairment and was present in 9·6% of the study cohort. In addition, mechanical ventilation was more common in younger patients – 76% of patients were younger than 10yo, where completing spirometry can be most difficult. Furthermore, 8% of patients with a history of mechanical ventilation did not fit criteria for pulmonary disease by HCT-CI definitions. We suggest that a history of mechanical ventilation can potentially act as a surrogate for pulmonary disease in children.
Our report focuses on children and AYAs with non-malignant diseases, approximately 43% of HCTs performed in children and young adults, with numbers continuing to increase1. The distribution of comorbidities seen in these patients differs from patients with malignancies and, therefore, evaluation of potential adjustments to the HCT-CI that are specific to this population of patients is warranted6. The HCT-CI modifications that we propose may impact pediatric and AYA population with non-malignant diseases differently than patients with malignancies, since the former are younger and often have lower HCT-CI scores. By expanding the comorbidity definitions in the expanded ynHCT-CI, the number of patients classified with a comorbidity increased and was associated with a better ability to predict risk of posttransplant mortality in these patients.
While expanding the definitions allowed us to capture more patients, we also found that some comorbidities may not affect post-transplant survival in younger patients with non-malignant diseases, and therefore proposed a simplified risk score. This included removing arrhythmia, psychiatric disease, mild hepatic disease, and moderate pulmonary disease from the risk score. Both the expanded ynHCT-CI and simplified ynHCT-CI act similarly in predicting survival outcomes and providers can determine which to use based on the clinical situation. It is not clearly understood why these comorbidities did not predict outcomes in our cohort. Arrhythmia was infrequent in this population which may have contributed to its lack of effect on survival. Furthermore, we hypothesize that younger patients with moderate pulmonary disease may have had difficulty cooperating with spirometry leading to abnormal results. Additionally, we hypothesize that, in this population of patients with non-malignant diseases, patients may be categorized with mild hepatic diseases due to elevated bilirubin secondary to hemolysis and not primary liver disease, which can be common in patients with hemoglobinopathies, paroxysmal nocturnal hemoglobinuria, and some primary immune deficiencies. Further, prospective analysis is warranted.
One of the unique aspects of many non-malignant diseases is that there are disorders where HCT is strongly recommended for cure, while others can be survivable with supportive care. Some examples include hemoglobinopathies, chronic granulomatous disease, and some forms of immune regulatory disorders. For these diseases, the risks of HCT must be weighed against the risks of ongoing morbidity from the underlying disease. The use of a more appropriate comorbidity index for children and AYA patients can therefore better assist physicians in counselling whether the best course of therapy is to continue non-transplant therapies (possibly if high expanded or simplified ynHCT-CI), to proceed with HCT (in the case of lower scores), or to consider gene therapy if available. In addition, children and AYA patients with non-malignant diseases have comorbidities that may be augmented by their underlying disease. A validated risk score can help objectify when these comorbidities correlate with poor HCT outcomes, allowing physicians to counsel the patients to proceed to HCT earlier or to initiate interventions before these comorbidity thresholds are reached.
We acknowledge the limitations of this study. This is a retrospective study, albeit of an observational registry database, and the data available is limited to what is captured by the CIBMTR. For example, the number of patients who were unable to participate in spirometry, is unknown. Our analysis required CRF-level data, which was only available on 46% of children and AYAs who received HCT for non-malignant diseases. As the entire cohort was not included, there was the potential for selection bias. The cohorts were similar (Supplemental Table 1), but the CRF cohort used for analysis had more patients that received cord blood transplants and the follow-up was shorter. This smaller sample size may have limited our analysis of comorbidities that are seen less frequently. In addition, there may be additional comorbidities, patient-specific factors, or other socioeconomic factors that affect outcomes in pediatric and AYA patients with non-malignant diseases that are not currently collected by the CIBMTR forms16,20. These limitations notwithstanding, this study is the largest contemporary study presenting data on the impact of comorbidities in pediatric and AYA population with non-malignant diseases and highlights that the HCT-CI can be further improved and made more relevant to this population.
In conclusion, the HCT-CI has been underutilized by pediatric providers as the definitions of comorbidities are not viewed as applicable to the younger population. Modifications to the HCT-CI can benefit children and AYA patients with non-malignant diseases, creating a risk assessment tool that is clinically relevant and better captures comorbidities seen in this younger population. Although we introduce two new clinical risk scores here, the comorbidities are those commonly assessed pre-transplant for children and young adults undergoing allogeneic HCT. We anticipate the time to calculate these scores would be similar to that currently spent computing the HCT-CI. Prospective studies are needed to further compare the expanded and simplified ynHCT-CI in a larger cohort of patients. Additionally, future studies may help identify more patient- and disease-specific factors to supplement to these risk scores for patients with non-malignant diseases.
Supplementary Material
Highlights.
Expanded HCT-CI comorbidity definitions led to an increase in comorbidity score for 39% of younger patients with non-malignant diseases.
Patients whose comorbidity score increased had an increased risk of death compared to an unchanged score (HR1.41 95%CI 1.01–1.98).
Modification to the HCT-CI creates a risk assessment tool that’s clinically relevant and better captures comorbidity for younger patients.
ACKNOWLEDGMENTS
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen Inc.; Astellas Pharma US Inc.; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc.; Gilead; GlaxoSmithKline; Incyte Corporation; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karyopharm Therapeutics; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend; Magenta Therapeutics; Medac GmbH; Medexus; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncopeptides, Inc.; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Priothera; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Tscan; Vertex; Vor Biopharma; Xenikos BV.
CONFLICTS OF INTEREST DISCLOSURE
Dr. Abraham reports payments to Doris Duke Charitable Foundation, American Society of Hematology, and Robert Wood Johnson Foundation, consulting fee payments from Emmes Corporation and Sangamo Therapeutics, payment support for attending meetings and/or travel from American Society of Hematology, patents pending from Children’s National Hospital, participation on a Data Safety Monitoring Board or Advisory Board of Sangamo Therapeutics, unpaid leadership or fiduciary role in other board, society, committee or advocacy group of Sickle Transplant Advocacy and research alliance.
Dr. Guinan reports grants or contracts supported by UM1AI109565 (ITN/NIAID) for work on Treg cell use in liver transplantation, occasional editorial work for Dynamed (unrelated to transplantation), Data Safety Monitoring Board for Meda and for Viatris regarding use of topical calcineurin inhibitor picrolimus for atopic dermatitis, and has small <<<<0.1% stock positions in Merck, Abbvie, Humana, Medtronic, Stryker in portfolio managed by financial firm.
Dr. Pasquini reports research support from Novartis, Kite, BMS, Amgen (completed 2019), Consultancy (Iisted as the professional providing insight), BMS (CAR T cell Steering Committee - former as of June 2021).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA USE STATEMENT
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
REFERENCES
- 1.D’Souza A, Fretham C, Lee SJ, et al. : Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 26:e177–e182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorror ML, Maris MB, Storb R, et al. : Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106:2912–9, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ElSawy M, Storer BE, Pulsipher MA, et al. : Multi-centre validation of the prognostic value of the haematopoietic cell transplantation- specific comorbidity index among recipient of allogeneic haematopoietic cell transplantation. Br J Haematol 170:574–83, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raimondi R, Tosetto A, Oneto R, et al. : Validation of the Hematopoietic Cell Transplantation-Specific Comorbidity Index: a prospective, multicenter GITMO study. Blood 120:1327–1333, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Sorror ML, Logan BR, Zhu X, et al. : Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant 21:1479–87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakar MS, Broglie L, Logan B, et al. : The Hematopoietic Cell Transplant Comorbidity Index predicts survival after allogeneic transplant for nonmalignant diseases. Blood 133:754–762, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broglie L, Friend BD, Chhabra S, et al. : Differential use of the hematopoietic cell transplantation-comorbidity index among adult and pediatric transplant physicians. Leuk Lymphoma:1–4, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Close AG, Dreyzin A, Miller KD, et al. : Adolescent and young adult oncology-past, present, and future. CA Cancer J Clin 69:485–496, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, et al. : A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Muñoz A, Schneider MF, et al. : New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens PE, Levin A: Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158:825–30, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Kit BK, et al. : Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. JAMA 307:483–490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden CL, Kuczmarski RJ, Flegal KM, et al. : Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Seidell JC, Halberstadt J: The global burden of obesity and the challenges of prevention. Ann Nutr Metab 66 Suppl 2:7–12, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Bacigalupo A, Ballen K, Rizzo D, et al. : Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15:1628–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueroa Turienzo CM, Cernadas C, Roizen M, et al. : Validation of the Hematopoietic Cell Transplantation-Specific Comorbidity Index in a retrospective cohort of children and adolescents who received an allogeneic transplantation in Argentina. Arch Argent Pediatr 114:337–42, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Smith AR, Majhail NS, MacMillan ML, et al. : Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood 117:2728–34, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Wood W, Deal A, Whitley J, et al. : Usefulness of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in predicting outcomes for adolescents and young adults with hematologic malignancies undergoing allogeneic stem cell transplant. Pediatr Blood Cancer 57:499–505, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Broglie L, Ruiz J, Jin Z, et al. : Limitations of Applying the Hematopoietic Cell Transplantation Comorbidity Index in Pediatric Patients Receiving Allogeneic Hematopoietic Cell Transplantation. Transplant Cell Ther 27:74.e1–74.e9, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Friend BD, Tang K, Markovic D, et al. : Identifying risk factors associated with worse outcomes in adolescents and young adults undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer 66:e27940, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Barker CC, Agovi MA, Logan B, et al. : Childhood obesity and outcomes after bone marrow transplantation for patients with severe aplastic anemia. Biol Blood Marrow Transplant 17:737–44, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleimer M, Li Y, Chang L, et al. : Baseline body mass index among children and adults undergoing allogeneic hematopoietic cell transplantation: clinical characteristics and outcomes. Bone Marrow Transplant 50:402–10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers LC, Sun P, Brennan LL, et al. : Effect of weight on outcomes of children undergoing hematopoietic cell transplantation. Pediatr Hematol Oncol 30:116–30, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner A, Zueger N, Bargetzi A, et al. : Association of Nutritional Parameters with Clinical Outcomes in Patients with Acute Myeloid Leukemia Undergoing Haematopoietic Stem Cell Transplantation. Ann Nutr Metab 69:89–98, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Paviglianiti A, Dalle JH, Ayas M, et al. : Low Body Mass Index Is Associated with Increased Risk of Acute GVHD after Umbilical Cord Blood Transplantation in Children and Young Adults with Acute Leukemia: A Study on Behalf of Eurocord and the EBMT Pediatric Disease Working Party. Biol Blood Marrow Transplant 24:799–805, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Mian AN, Schwartz GJ: Measurement and Estimation of Glomerular Filtration Rate in Children. Adv Chronic Kidney Dis 24:348–356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farhadfar N, Dias A, Wang T, et al. : Impact of Pretransplantation Renal Dysfunction on Outcomes after Allogeneic Hematopoietic Cell Transplantation. Transplant Cell Ther 27:410–422, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginsberg JP, Aplenc R, McDonough J, et al. : Pre-transplant lung function is predictive of survival following pediatric bone marrow transplantation. Pediatr Blood Cancer 54:454–60, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Kaya Z, Weiner DJ, Yilmaz D, et al. : Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant 15:817–26, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan A, Srinivasan S, Sunthankar S, et al. : Pre-hematopoietic stem cell transplant lung function and pulmonary complications in children. Annals of the American Thoracic Society 11:1576–1585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.


