Abstract
Allogeneic hematopoietic cell transplantation is a curative procedure for hematologic malignancies but is associated with a significant risk of non-relapse mortality (NRM). The Hematopoietic Cell Transplantation– Comorbidity Index (HCT-CI) is a prognostic tool that discriminates this risk in all age groups. A recent survey of transplant physicians demonstrated that 79% of pediatric providers used the HCT-CI infrequently, and most reported concerns about its applicability in the younger population. We conducted a retrospective study using the Center for International Blood and Marrow Transplant Research (CIBMTR) database to examine the impact of expanded HCT-CI definitions on NRM in pediatric and young adult patients with hematologic malignancies. We included 5,790 patients <40 years old receiving allogeneic transplant between 2008 and 2017 to examine broader definitions of comorbidities in the HCT-CI, including history of mechanical ventilation and fungal infection, estimated glomerular filtration rate (eGFR), and body mass index (BMI) percentiles. Multivariable Fine-Gray models were created to determine the effect of each HCT-CI defining comorbidity and its modification on NRM, and were utilized to develop two novel risk scores. We next developed the expanded HCT-CI for children and young adults (youth with malignancies; expanded ymHCT-CI), where 23% patients had an increased comorbidity score, compared to the HCT-CI. Comorbidities with hazard ratio (HR) <1.2 were then removed to create the simplified HCT-CI for children and young adults (youth with malignancies; simplified ymHCT-CI), which demonstrated higher scores corresponded to a greater risk of NRM (p<0.001). These novel comorbidity indices with broader definitions are more relevant to pediatric and young adult patients, and prospective studies are needed to validate these in the younger patient population. It remains to be seen if the development of these pediatric-specific and practical risk indices increases their utilization by the pediatric transplant community.
Keywords: HCT-CI; comorbidities; allogeneic hematopoietic cell transplantation; paediatrics; adolescent, and young adults (AYA); hematologic malignancies
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is potentially curative in hematologic malignancies, yet it is associated with a significant risk of non-relapse mortality (NRM) due to treatment-related complications. While pediatric and young adult patients have improved NRM when compared to older adults undergoing HCT1,2, it continues to be a concern1–4. In order to assess NRM risk pre-transplant, the HCT-comorbidity index (HCT-CI) was developed to capture comorbidity burden and predict outcomes after transplant. The HCT-CI is a composite score of seventeen comorbidities that effectively discriminates the risk of NRM in all ages5–7. However, while older adults have a significant frequency of comorbidities across the spectrum of definitions per the HCT-CI, these definitions appear to be limited in scope and often not applicable to the pediatric and young adult population. This was suggested by two studies which demonstrated scores of 0 in 50–60% patients ≤20 years old8,9. Similar findings have been shown in studies of adolescents and young adults (aged 15–39 years, as defined by the National Cancer Institute)10, in which the prevalence of most comorbidities was less than 5%, and the authors argued for the development of a simpler risk score for younger patients4,11. In addition, we recently conducted a survey of pediatric and adult transplant physicians to assess their utilization of this tool, and found that 71% of adult providers used the HCT-CI in assessment of nearly all of their patients (75–100% of the time), compared to only 12% among pediatric providers12. Of the pediatric providers who use the HCT-CI less than half the time, 34% of responders reported concerns about the impact and applicability of the HCT-CI in the younger population12.
Based on the potential limitations of the HCT-CI in younger patients and its limited utility13, there is an unmet need to modify the definitions of the comorbidities incorporated in the HCT-CI to make the index more suitable to apply in pediatric and young adult patients while also creating a tool that is simpler, more practical and utilized more often by clinicians in the real-world. Therefore, we conducted a retrospective study using the Center for International Blood and Marrow Transplant Research (CIBMTR) database to examine the impact of expanded comorbidities using broader definitions to evaluate NRM in pediatric and young adult patients undergoing HCT for hematologic malignancies and to develop a more practical risk score for this younger population.
METHODS
Patient population
Patients were identified from the CIBMTR registry, a research partner of the National Marrow Donor Program and International Bone Marrow Transplant Registry. There are greater than 450 bone marrow transplant centers across the world that submit baseline characteristics and longitudinal outcome data to CIBMTR. The CIBMTR collects data at two levels: transplant essential data forms (TED) and comprehensive report form (CRF) data. All centers contribute TED data that includes patient-, disease-, and transplant-related information. More detailed disease and pre- and post-transplant clinical data, including additional information organ function, are collected on a subset of patients selected for CRF data based on a weighted algorithm. TED and CRF level data are collected pre-transplant, at day 100, 6 months, and yearly. Compliance is monitored closely through on-site audits. Observational studies supported by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information is collected and maintained in the CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
Using the CIBMTR registry, patients <40 years of age who underwent first allogeneic HCT for hematologic malignancies in the United States between January 2008 and December 2017 were identified. Patients were excluded if they were transplanted using a syngeneic donor, at embargoed centers, at centers with <85% completeness of follow-up, had less than 100 days of follow-up, or if they were missing HCT-CI comorbidities or additional components to be tested in this study (Figure 1).
Figure 1.
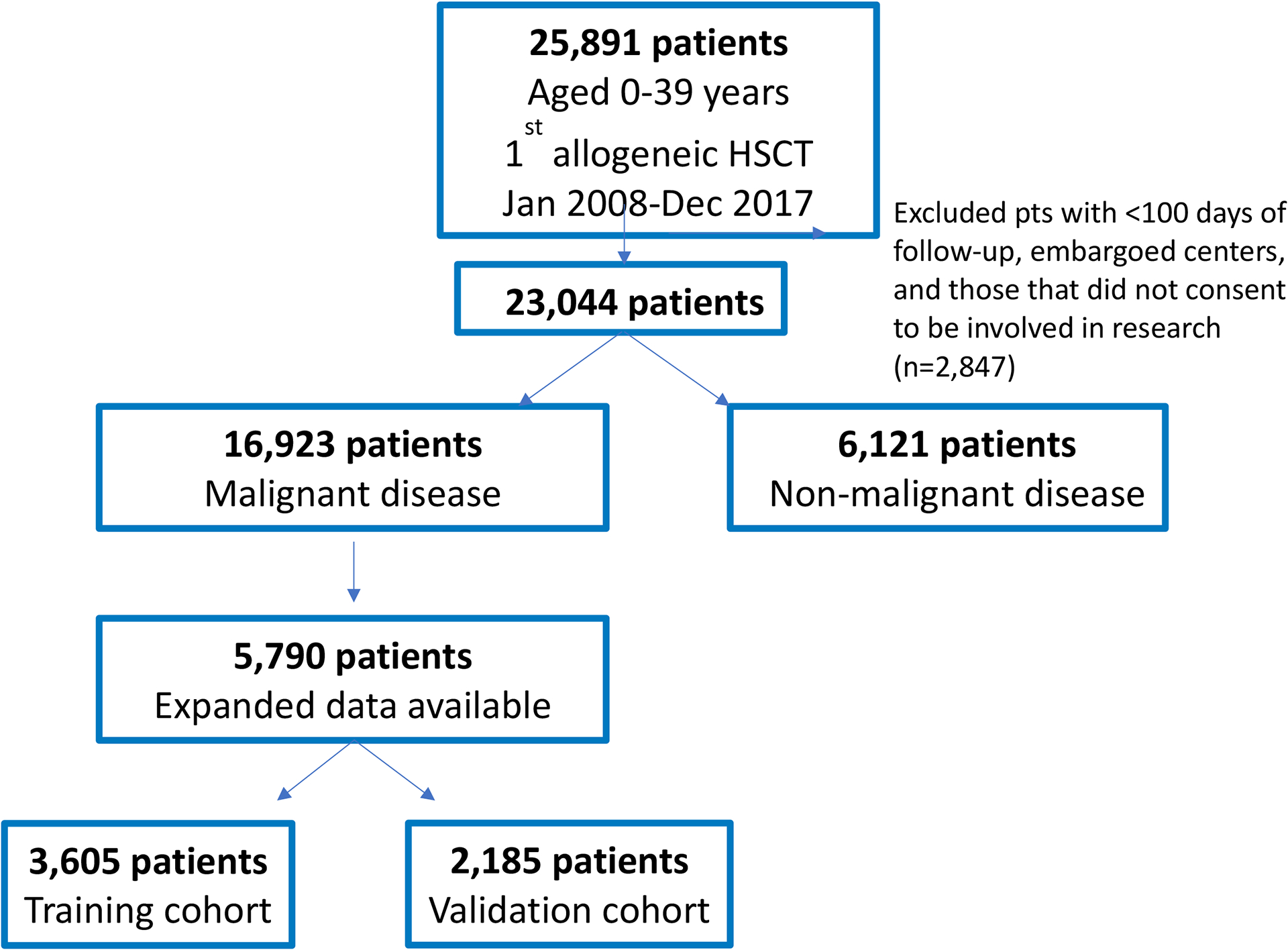
Schema of study design
Study outcomes and definitions
The primary outcome for this study was NRM. NRM was defined as death after HCT, without evidence of relapse or disease progression. Disease relapse or progression was the competing risk for NRM. The secondary outcome for these patients was overall survival (OS), defined as death from any cause.
The comorbidities were defined pre-transplant as per HCT-CI developed by Sorror et al6. In addition to the factors needed to calculate the HCT-CI, additional pre-transplant factors were reviewed including a history of mechanical ventilation and history of invasive fungal infection (proven, suspected or documented) at any time prior to HCT per CIBMTR definitions. Further, supplemental data was collected on renal function and nutritional status. Chronic kidney disease (CKD) was assessed by calculating the estimated glomerular filtrate rate (eGFR) using the Bedside Schwartz equation for children <18 years old and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for patients 18–39 years old14,15. CKD was categorized by eGFR as normal (>90 ml/min), mild CKD (60–89 ml/min), or moderate/severe CKD (<60 ml/min)14,15. BMI percentiles were utilized to expand the definition of obesity in children based on CDC referenced percentiles (<18 years: BMI ≥95th percentile), while also assessing poor nutritional status by creating a new, underweight category (<18 years: BMI <5th percentile, ≥18 years: <18 kg/m2)16.
Conditioning intensity was described as myeloablative or reduced intensity utilizing CIBMTR consensus criteria17. Donors were classified as “well-matched” if they were human leukocyte antigen (HLA)-matched with recipient at HLA-A, -B, -C, and -DRB1 (8/8) for bone marrow or peripheral blood stem cells or matched at HLA-A, -B, and -DRB1 (6/6) for cord blood grafts. All others were partially matched or mismatched donors.
Statistical analysis
The study population was distributed randomly into a training cohort (3,605 patients) and a validation cohort (2,185 patients) using a 2/3 and 1/3 split. All HCT-CI-defining comorbidities and additional risk factors (as defined above), including history of invasive fungal infection, history of mechanical ventilation, nutritional status (obesity, underweight), or CKD by eGFR, were tested in the multivariable models. Multivariable Fine-Gray models for NRM were fit on the training data to identify the effect of each factor on NRM, after adjusting for age, conditioning intensity, disease, donor type, performance score, race, recipient CMV status, and year of HCT. From these models, broader comorbidity definitions were defined and new risk scores were built on the training dataset, and validated on an independent test sample. The expanded HCT-CI score for younger patients with malignant diseases (expanded ymHCT-CI) included all of the HCT-CI comorbidities but with expanded definitions. The simplified HCT-CI score for younger patients with malignant diseases (simplified ymHCT-CI) was a modification of the expanded ymHCT-CI, removing comorbidities with HR <1.2, as done in the development of the original HCT-CI7.
Scoring for the broader definitions used the same weights as the HCT-CI, except as noted below. For the expanded HCT-CI, history of invasive fungal infection was combined with the infection comorbidity. Similarly, BMI >95th percentile was included with the definition of obesity as a single comorbidity. History of mechanical ventilation was assessed with severe pulmonary comorbidity, primarily as a potential surrogate for patients that could not complete spirometry. Renal disease was separated into two categories: “mild disease” was characterized by HCT-CI-defined creatinine levels or history of renal transplant, with the addition of eGFR 60–89 ml/min based on similarities in HRs by multivariable models and scored as per the original HCT-CI with score of 2, and “moderate/severe disease” was defined by eGFR <60 ml/min or history of dialysis and scored as 3. The underweight category, defined by BMT <5th percentile or <18 kg/m2, was ultimately not included as a separate comorbidity as it was not shown to be associated with NRM in multivariable analysis. C-statistics were calculated to compare the performance of the new scores to the original HCT-CI. To establish the predictive value of the additional comorbidities included in the expanded ymHCT-CI, the cohort was split based on whether patients had an upgrade of their score using the expanded ymHCT-CI. This variable was then assessed in the Fine-Gray model while adjusting for patient, disease, and transplant-related factors, as well as the HCT-CI score itself. Kaplan-Meier method was used to estimate OS and Chi Square test to compare proportions.
RESULTS
Patient and donor characteristics
Based on the selection criteria, a total of 5,790 patients who received allogeneic HCT for hematologic malignancy were included in this study.
In the study cohort, the median age was 22 (0–39) years. Indications for allogeneic HCT were primarily acute myeloid leukemia (43%) and acute lymphoblastic leukemia (31%). The conditioning regimen was most frequently myeloablative (85%). The most common donor types were cord blood (35%), HLA-matched unrelated (28%) and HLA-matched sibling (18%). Donor source was peripheral blood stem cells in 41%, umbilical cord blood in 35%, and bone marrow in 24% patients. The median follow-up of survivors was 42 (3–126) months. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics for 5790 patients in the study cohort who received allogeneic transplant for hematologic malignancy between 2008 and 2017.
| Characteristic | N (%) |
|---|---|
| Age, median (range) - years | 22 (<1–39) |
| Age group, N (%) | |
| 0–2 years | 432 (8) |
| 3–10 years | 937 (16) |
| 11–18 years | 1036 (18) |
| 19–29 years | 1636 (28) |
| 30–39 years | 1749 (30) |
| Sex | |
| Male | 3246 (56) |
| Female | 2544 (44) |
| Race | |
| Caucasian | 4235 (73) |
| African-American | 701 (12) |
| Asian | 366 (6) |
| Pacific islander | 23 (<1) |
| Native American | 48 (<1) |
| Mixed race | 140 (2) |
| Missing | 277 (5) |
| Ethnicity | |
| Hispanic or Latino | 1298 (22) |
| Non-Hispanic or non-Latino | 4361 (75) |
| Non-resident of the U.S. | 38 (1) |
| Missing | 93 (2) |
| Karnofsky/Lansky performance score | |
| 90–100 | 4215 (73) |
| <90 | 1486 (26) |
| Missing | 89 (1) |
| HCT-CI | |
| 0 | 2609 (45) |
| 1–2 | 1536 (26) |
| 3+ | 1645 (29) |
| Diagnosis – no. (%) | |
| AML | 2494 (43) |
| ALL | 1807 (31) |
| MDS | 397 (7) |
| Non-Hodgkin lymphoma | 316 (6) |
| CML | 264 (5) |
| Hodgkin lymphoma | 197 (3) |
| MPN | 123 (2) |
| Other malignancy | 192 (3) |
| Leukemia/MDS disease status | |
| CR | 4226 (81) |
| PR | 12 (<1) |
| Advanced or active disease | 932 (18) |
| Missing | 71 (<1) |
| Lymphoma disease status | |
| CR | 227 (44) |
| PR | 79 (15) |
| Advanced | 206 (40) |
| Missing | 1 (<1) |
| Donor type | |
| HLA-identical sibling | 1016 (18) |
| Other related | 595 (10) |
| Well-matched unrelated | 1615 (28) |
| Cord blood | 2018 (35) |
| Mismatched unrelated | 546 (10) |
| Graft source | |
| Bone marrow | 1419 (24) |
| Peripheral blood | 2353 (41) |
| Cord blood | 2018 (35) |
| Conditioning intensity | |
| Myeloablative | 4942 (85) |
| Reduced intensity | 442 (8) |
| Non-myeloablative | 297 (5) |
| Missing | 109 (2) |
| Donor/recipient CMV serostatus | |
| +/+ | 1225 (21) |
| +/− | 478 (8) |
| −/+ | 1033 (18) |
| −/− | 998 (17) |
| CB – recipient + | 1292 (22) |
| CB – recipient − | 707 (12) |
| CB – CMV unknown | 19 (<1) |
| Missing | 38 (<1) |
| GVHD prophylaxis | |
| TAC + MTX +- other(s) (except MMF, post-CY) | 1954 (34) |
| CSA + MMF +- other(s) (except post-CY) | 1226 (21) |
| TAC + MMF +- other(s) (except post-CY) | 840 (15) |
| CSA + MTX +- other(s) (except MMF, post-CY) | 514 (9) |
| Post-CY + other(s) | 354 (6) |
| TAC + other(s) (except MMF, MTX, post-CY) | 261 (5) |
| Other regimen | 641 (10) |
| Serotherapy | |
| ATG | 1540 (27) |
| Alemtuzumab | 139 (2) |
| None | 4065 (70) |
| Missing | 46 (<1) |
| Year of transplant | |
| 2008–2010 | 2271 (39) |
| 2011–2013 | 1238 (22) |
| 2014–2017 | 2281 (39) |
| Follow-up, median (range) (months) | 42 (3–126) |
Distribution of comorbidities
The most common HCT-CI comorbidities in the cohort were pulmonary dysfunction (moderate 18%, severe 11%), psychiatric disturbance (14%), and hepatic dysfunction (mild 8.9%, moderate/severe 2.9%) (Figure 2). However, by incorporating broader age-based definitions to the original HCT-CI, the incidence of obesity increased from 8.4% to 17%. Renal dysfunction became much more frequent, increasing from 0.4% using the original HCT-CI definition, to 9.5% with the expanded definition. The incidence of infection (12.5%) and severe pulmonary comorbidity (16.4%) also increased substantially by including a history of invasive fungal infection (6.2%) and history of mechanical ventilation (6.8%), respectively. History of mechanical ventilation was reported in 12% of patients 0–2 years, 7% in 3–10 years, 8% in 11–18 years, 5% in 19–29 years, and 3% in 30–39 years. Further, 4.2% of patients were classified as underweight when using BMI percentiles.
Figure 2.
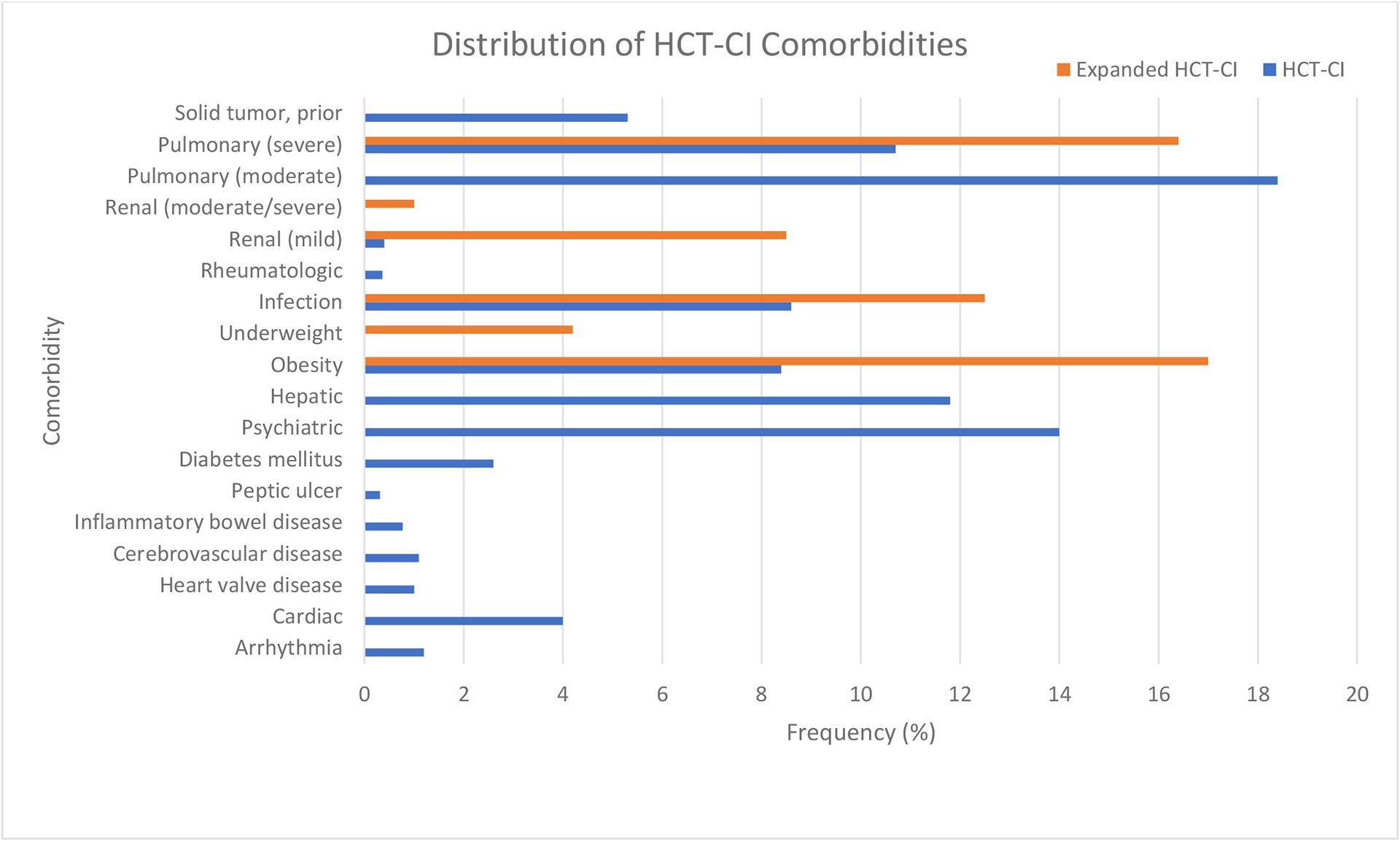
Incidence of comorbidities comparing HCT-CI vs. expanded ymHCT-CI.
To further understand the impact of the expanded definitions, we analyzed these comorbidities in more detail in pediatric (<18 years) and young adult (18–39 years) subgroups. The increase in the frequency of obesity with the expanded definitions was primarily seen in pediatric patients, as only 18.1% of patients with BMI >95th percentile met the classification of obesity based on the original HCT-CI, while 68.4% of adults fit both definitions. In contrast, the significant increase in the proportion of renal dysfunction was due to vast under-representation of this risk factor by HCT-CI definition in both age groups, as pediatric and young adult patients with GFR <90 ml/min rarely met the original HCT-CI definition of renal comorbidity (3.0% and 1.6%, respectively). Of the patients determined to have severe pulmonary comorbidity by original HCT-CI, pediatric patients were more likely to have reported a history of mechanical ventilation than young adults (21.3% vs. 9.3%).
Expanded definitions were then evaluated for their impact on NRM in a multivariate model. BMI>95th percentile (HR 1.31, 95% confidence interval [95CI] 1.07–1.59), history of invasive fungal infection (HR 1.69, 95CI 1.30–2.19), and eGFR<60 ml/min (HR 3.18, 95CI 1.82–5.54) were associated with greater NRM. History of mechanical ventilation was shown to be statistically associated with inferior outcomes using the TED-level data (HR 1.35, 95CI 1.12–1.61) (not shown). BMI<5th percentile did not affect outcomes (HR 0.99, 95CI 0.67–1.47) (Table 2).
Table 2.
Multivariable analysis demonstrating impact of HCT-CI comorbidities and expanded definitions on NRM, as well as risk scores including HCT-CI, emHCT-CI, and smHCT-CI. Models adjusted for age, conditioning regimen, disease, donor type, KPS, race, recipient CMV status, and year of transplant.
| Comorbidity | Definition | HR (95% CI) | HCT-CI | Expanded HCT-CI | smHCT-CI |
|---|---|---|---|---|---|
| Arrhythmia | Atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias | 0.88 (0.42–1.86) | 1 | 1 | 0 |
| Cardiac disease | Coronary artery disease, congestive heart failure, myocardial infarction, or EF ≤50% on most recent test | 1.40 (1.00–1.96) | 1 | 1 | 1 |
| Inflammatory bowel disease | Crohn disease or Ulcerative colitis | 0.54 (0.18–1.62) | 1 | 1 | 0 |
| Diabetes | Requiring treatment with insulin or oral hypoglycemics, but not diet alone | 1.38 (0.94–2.02) | 1 | 1 | 1 |
| Psychiatric disease | Requiring psychiatric consult or treatment in the last 4 weeks | 1.18 (0.96–1.46) | 1 | 1 | 0 |
| Cerebrovascular | Any history of transient ischemic attack, subarachnoid hemorrhage, or cerebrovascular accident | 0.76 (0.35–1.65) | 1 | 1 | 0 |
| Infection* | Requiring antimicrobial treatment OR history of invasive fungal infection | 1.51 (1.23–1.85) | 1 | 1 | 1 |
| Obesity* | BMI >35 kg/m2 OR BMI >95th percentile by CDC guidelines for (<18yo) | 1.27 (1.05–1.53) | 1 | 1 | 1 |
| Underweight* | BMI <5th percentile by CDC guidelines for (<18yo) or <18kg/m2 (≥18yo) | 0.99 (0.67–1.47) | N/A | 0 | 0 |
| Mild hepatic disease | Chronic hepatitis, bilirubin >upper limit of normal to 1.5x upper limit of normal, or AST/ALT upper limit of normal to 2.5x upper limit of normal | 0.95 (0.72–1.27) | 1 | 1 | 0 |
| Moderate/severe hepatic disease | Liver cirrhosis, bilirubin >1.5x upper limit of normal, or AST/ALT >2.5x upper limit of normal | 1.28 (0.82–1.99) | 3 | 3 | 3 |
| Moderate pulmonary disease | Corrected diffusion capacity of carbon monoxide and/or FEV1 66–80%, or dyspnea on slight activity | 0.88 (0.71–1.07) | 2 | 2 | 0 |
| Severe pulmonary disease* | Corrected diffusion capacity of carbon monoxide and/or FEV1 ≤65%, or dyspnea at rest, or requiring oxygen OR history of mechanical ventilation | 1.33 (1.10–1.60) | 3 | 3 | 3 |
| Peptic ulcer disease | Confirmed by endoscopy and requiring treatment | 0.88 (0.24–3.18) | 2 | 2 | 0 |
| Rheumatologic disease | Systemic Lupus, Rheumatoid Arthritis, Polymyositis, Mixed connective tissue disease, or polymyalgia rheumatic requiring treatment | 0.61 (0.16–2.41) | 2 | 2 | 0 |
| Mild renal disease * | Creatinine >2mg/dL or prior renal transplant OR eGFR 60–89ml/min (by Bedside Schwartz calculation for <18y, CKD-EPI calculation for ≥18yo) | 1.32 (1.04–1.70) | 2 | 2 | 2 |
| Moderate/severe renal disease* | On dialysis OR eGFR <60ml/min (by Bedside Schwartz calculation for <18y, CKD-EPI calculation for ≥18yo) | 3.18 (1.82–5.54) | N/A | 3 | 3 |
| Prior solid tumor | At any point in patient’s history, excluding non-melanoma skin cancer, leukemia, lymphoma, or multiple myeloma | 1.63 (1.23–2.16) | 3 | 3 | 3 |
| Heart valve disease | Except asymptomatic mitral valve prolapse | 1.41 (0.77–2.58) | 3 | 3 | 3 |
Comorbidities that included modified definitions. FEV1=forced expiratory volume in one second.
Assessment of HCT-CI
Based on the original HCT-CI, scores of 0, 1–2, and ≥3 were seen in 45.6%, 26.8%, and 27.6% of the patient population, respectively. Using the validation cohort, higher HCT-CI score was associated with greater risk of NRM, with scores of 1–2, and ≥3 translating to HR 0.91 (95CI 0.67–1.23) and HR 1.53 (95CI 1.16–2.01), respectively, compared to score of 0 (p=0.001). Similarly, for OS, scores of 1–2, and ≥3 translated to HR 1.02 (95CI 0.83–1.24) and HR 1.65 (95CI 1.37–1.99), respectively, compared to score of 0 (p<0.001). Three-year estimates for cumulative incidence of NRM and OS probability are shown in Table 3.
Table 3.
Prediction of non-relapse mortality (NRM), overall survival (OS) and associated hazard ratios (HRs) among the different risk scores.
| HR (95%CI), NRM | P-value | 3-year NRM | HR (95%CI), OS | P-value | 3-year OS | |
|---|---|---|---|---|---|---|
| HCT-CI | ||||||
| 0 | 1.00 | 16.8% (14.0–19.7%) | 1.00 | 62.1% (58.6–65.6%) | ||
| 1–2 | 0.91 (0.67–1.24) | 0.543 | 15.8% (12.4–19.2%) | 1.02 (0.83–1.24) | 0.874 | 60.4% (55.8–65.0%) |
| 3+ | 1.53 (1.16–2.01) | 0.002 | 25.4% (21.3–29.5%) | 1.65 (1.37–1.99) | <0.001 | 46.1% (41.5–50.7%) |
| Expanded ymHCT-CI | ||||||
| 0 | 1.00 | 15.8% (12.6–18.9%) | 1.00 | 62.0% (58.0–66.0%) | ||
| 1–2 | 1.02 (0.76–1.38) | 0.888 | 16.5% (13.2–19.7%) | 1.00 (0.82–1.22) | 0.998 | 61.3% (57.1–65.5%) |
| 3+ | 1.52 (1.16–1.99) | 0.003 | 24.1% (20.7–27.6%) | 1.51 (1.26–1.81) | <0.001 | 49.3% (45.3–53.3%) |
| Simplified ymHCT-CI | ||||||
| 0 | 1.00 | 15.0% (12.6–17.4%) | 1.00 | 62.9% (59.7–66.1%) | ||
| 1–2 | 1.41 (1.07–1.86) | 0.016 | 20.5% (16.5–24.5%) | 1.34 (1.11–1.61) | 0.002 | 53.3% (48.4–58.2%) |
| 3+ | 1.65 (1.28–2.12) | <0.001 | 24.9% (21.0–28.8%) | 1.53 (1.29–1.82) | <0.001 | 50.1% (45.7–54.6%) |
Development of novel risk scores
Adapting the HCT-CI definitions to a younger patient population
By adding pediatric-specific definitions to the HCT-CI, we developed the expanded ymHCT-CI (Table 2), with scores of 0, 1–2, and ≥3 attributed to 33.8%, 30.7%, and 35.5% of the patients, respectively. Overall, use of the expanded ymHCT-CI led to an increase in comorbidity scores for 23% of patients and fewer patients with a comorbidity score of 0, compared to the original HCT-CI (Figure 3). Utilizing the validation cohort, increased scores with the expanded ymHCT-CI corresponded to a higher risk of NRM, with scores of 1–2 and ≥3 demonstrating HR of 1.02 (95CI 0.76–1.38) and 1.52 (95CI 1.16–1.99), respectively, vs. score of 0 (p<0.002). This risk score demonstrated a similar effect on OS, with scores of 1–2 and ≥3 equating to HR 1.02 (95CI 0.76–1.38) and HR 1.52 (95CI 1.16–1.99), respectively, compared to score of 0 (p<0.001). Three-year estimate of OS probability was 62% (95CI, 58–66%) for score of 0, 61.3% (95CI, 57.1–65.5%) for score of 1–2, and 49.3% (95CI, 45.3–53.3%) for score of ≥3.
Figure 3.
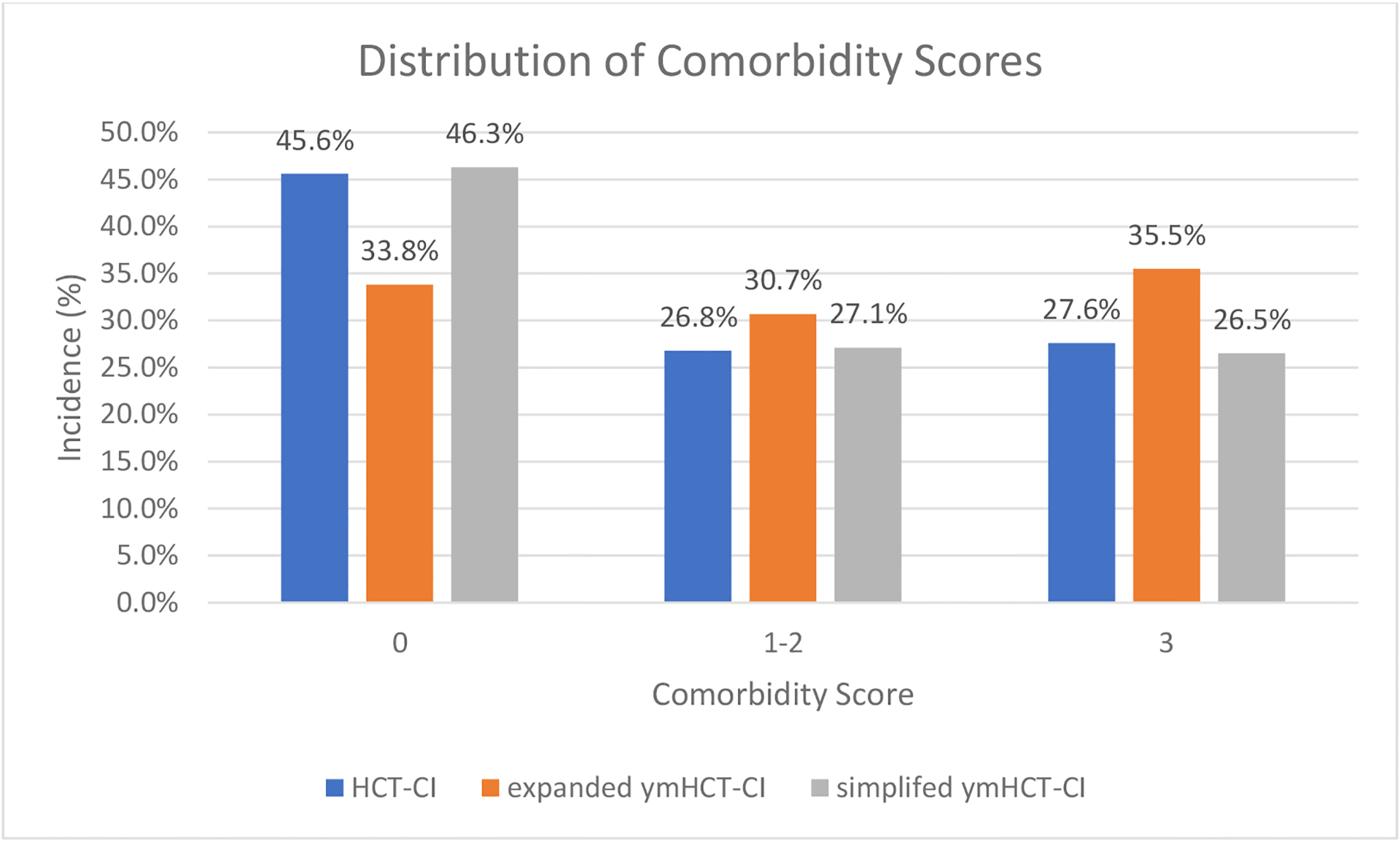
Change in comorbidity score using HCT-CI, expanded ymHCT-CI, or simplified ymHCT-CI.
In order to determine the predictive value of the modified comorbidities included in the expanded ymHCT-CI, we assessed whether patients with an increase in their comorbidity score experienced more NRM than those whose scores remained the same. An increased comorbidity score using the expanded ymHCT-CI was associated with a significant risk of NRM (HR 1.34, 95% CI 1.02–1.74), after adjusting for patient and transplant-related factors including the HCT-CI itself. Cumulative incidence of NRM is compared among the different risk scores in Figure 4, stratified by scores of 0, 1–2, and ≥3. The performance of the scores as analyzed by C-statistic, showed similar discriminatory capacity up to 24 months (HCT-CI: 65.7 vs. expanded ymHCT-CI: 66.0).
Figure 4.
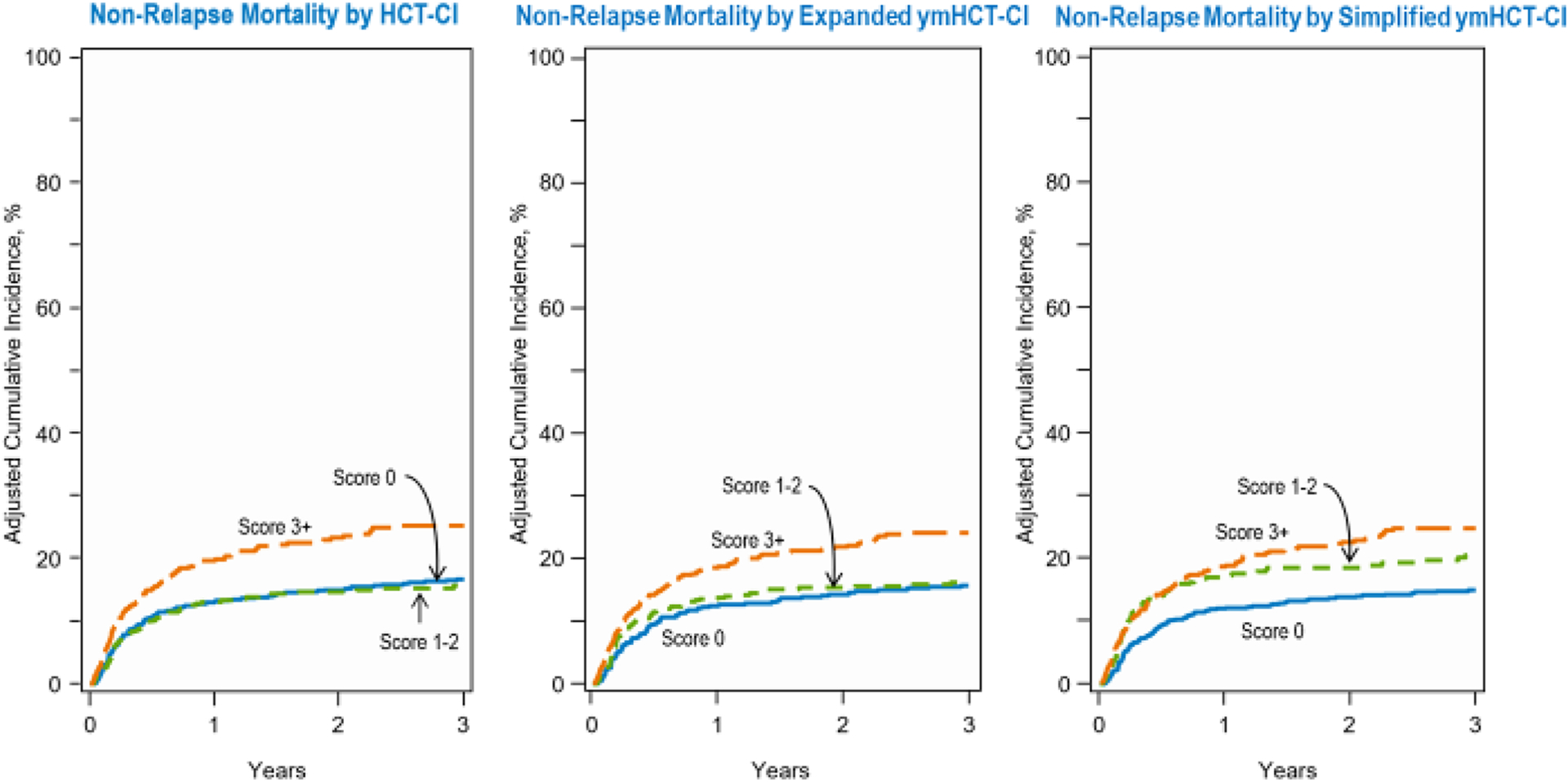
Cumulative incidence of NRM for (A) HCT-CI, (B) expanded ymHCT-CI, and (C) simplified ymHCT-CI.
Simplifying the expanded ymHCT-CI into the simplified ymHCT-CI
By removing comorbidities with HR <1.2 from the expanded ymHCT-CI model, we created the simplified ymHCT-CI, with scores of 0, 1–2, and ≥3 attributed to 46.3%, 27.1%, and 26.5% of the patients, respectively. For this younger population with hematologic malignancies, the HCT-CI categories of arrhythmia, inflammatory bowel disease, psychiatric disease, cerebrovascular disease, mild hepatic disease, moderate pulmonary disease, peptic ulcer disease, and rheumatologic disease were all removed in the simplified ymHCT-CI (Table 2). The simplified ymHCT-CI model, using the validation cohort, discriminated risk of NRM well, with scores of 1–2, and ≥3 exhibiting HRs of 1.41 (95CI 1.08–1.87) and 1.66 (95CI, 1.33–2.12), respectively, compared to score of 0 (p<0.001). Cumulative incidence of NRM is compared among the HCT-CI, expanded ymHCT-CI and simplified ymHCT-CI, stratified by score, in Figure 4. In addition, an increased comorbidity score was associated with lower OS, with scores of 1–2 and ≥3 demonstrating HRs of 1.00 (95CI 0.82–1.22) and 1.51 (95CI 1.26–1.81), respectively, vs. score of 0 (p < 0.001). Three-year estimate of OS probability was 62.9% (95CI, 59.7–66.1%) for score of 0, 53.3% (95CI, 48.4–58.2%) for score of 1–2, and 50.1% (95CI, 45.7–54.6%) for score of ≥3+. Compared to the original HCT-CI, the simplified ymHCT-CI performed similarly, as validated C-statistics at 24 months (HCT-CI: 65.7 vs. simplified ymHCT-CI: 66.0) were not significantly different.
DISCUSSION
The HCT-CI has been widely used as a predictive tool for NRM and OS for a variety of hematologic malignancies undergoing allogeneic transplantation5–7, and has been validated in the pediatric population4,8,9,11. Despite this, the HCT-CI has not been as widely accepted by the pediatric transplant community, as evidenced by our recent survey12, with many pediatric transplant physicians reporting that this tool is not pertinent to their patient population. In our study, we demonstrated that several comorbidity definitions used in the HCT-CI do not adequately capture risk assessment or are not observed with sufficiently high frequency to be relevant to this younger population. Therefore, we utilized broader comorbidity definitions to ultimately create two modified risk scores that are more applicable to pediatric and young adult patients, and can effectively discriminate the risk of NRM. By doing so, we better quantify comorbidity risk in this younger population, which will lead to not only improved recognition of pretransplant comorbidities but also the severity of the final score.
One challenge of assessing HCT-CI-defined pulmonary disease in younger patients is that spirometry cannot be reliably completed by many younger children. For this reason, pulmonary assessment is based solely on symptoms and resting pulse oximetry, which may underestimate pulmonary dysfunction13. Therefore, we tested whether a history of mechanical ventilation could act as an adjunct to conventional pulmonary function testing in the assessment of pulmonary disease. We found that a history of mechanical ventilation demonstrated an association with poor outcomes in multivariable analysis (HR 1.35, 95CI 1.12–1.61), consistent with prior studies18,19. Furthermore, of the patients less than 18-years-old in our study who had severe pulmonary dysfunction, more than 20% also reported a history of mechanical ventilation, suggesting that such history correlated with impaired pulmonary function. Among this subgroup, 28% of patients were less than 10-years-old, many of whom were likely unable to perform spirometry. Thus, we included a history of mechanical ventilation as a supplement in the expanded ymHCT-CI for patients that could not complete spirometry, thereby increasing the prevalence of significant pulmonary disease in our study from 29.1% to 34.8% (p<0.0001). While data is not available regarding the indication for mechanical ventilation in these patients, regardless of the underlying reason, mechanical ventilation can lead to significant pulmonary damage in pediatric and young adult patients, that is associated with poor outcomes20,21. We argue that a history of mechanical ventilation is a potential surrogate for prior pulmonary injury in populations that cannot perform spirometry and should be confirmed in future studies.
Similar challenges are seen with the classification of obesity in the HCT-CI, as the CDC standard definition of obesity for patients <18 years old is >95th percentile, as opposed to absolute numbers. With the addition of this classification to the definition of obesity, the prevalence of obesity increased by more than two times in our cohort (from 8.4% to 17%) and was significantly associated with greater NRM, as also shown in prior studies22–24. While patients who are underweight are not captured by the HCT-CI, recent studies have shown that these patients may also suffer worse outcomes and that this risk factor is likely underrecognized by the current reporting measures22,25,26. Therefore, in our study, we described a new “underweight” comorbidity which had a prevalence of 4%. However, this risk factor was not associated with greater NRM (HR 0.99, 95CI 0.67–1.47), though other studies have demonstrated similar findings27,28, suggesting that low BMI may only be impactful in a specific subset of patients, and requires additional investigation. Nonetheless, other markers of nutritional status including, vitamin D deficiency, hypoalbuminemia, and prediabetes have been correlated with inferior outcomes in prior publications29–31, and these factors should be studied in greater detail in larger studies.
Compared to the comorbidities mentioned above, renal disease is much less common in pediatric and young adult patients, but is likely underrecognized in this population using the definition in the HCT-CI. While often useful as a marker of renal impairment in adults, absolute serum creatinine values do not correlate well with renal function in younger patients and GFR using large-volume urine collections or nuclear medicine testing is often used to improve accuracy. When a formal GFR assessment cannot be performed, a method that provides a reasonable equivalent such as an eGFR calculation may be used, given that it accounts for age, race, sex, and body size32. We demonstrated that the inclusion of low eGFR greatly increased the incidence of renal disease in pediatric and young adult patients (from 0.8% to 9.5%), and was highly associated with NRM. In particular, patients with eGFR <60 ml/min were more than three times as likely to suffer NRM (HR 3.18, 95CI 1.82–5.54), and therefore, we created a new definition of “moderate/severe renal disease” and gave it a score of 3. Patients with eGFR 60–89 ml/min had a similar risk of NRM (HR 1.33, 95CI 1.04–1.70) as patients with creatinine >2 mg/dL (HR 1.38, 95CI 0.50–3.81), so they were both considered to have “mild renal disease” and scored as 2 per the HCT-CI. Still, the optimal method to estimate GFR in pre-transplant patients is unclear, and several studies have examined the use of alternative markers such as cystatin C that may serve as better predictors of renal injury than creatinine33–35. Further studies are needed to compare the various methodologies of assessing pre-transplant renal function, especially in pediatric and young adult patients.
Given that these more broadly-defined comorbidities were associated with inferior outcomes, we included them to create two novel risk indices for pediatric and young adult patients with hematologic malignancies. The expanded ymHCT-CI, comprised of all comorbidities, appears to better capture comorbidity risk in younger patients than the HCT-CI with 23% of patients having an increase in their comorbidity score. Further, for patients whose score increased with the utilization of the expanded ymHCT-CI, we demonstrated that such patients had a significantly higher risk of NRM. The expanded ymHCT-CI showed comparative performance to the original HCT-CI, but given that the expanded ymHCT-CI conforms to the comorbidity definitions of real-world assessments, it is more practical and relevant for use in pediatric and young adult patients. In addition, given the large number of children in our cohort, there was a substantial proportion of patients receiving umbilical cord blood as donor source (35%), which was not included in HCT-CI7. While use of umbilical cord blood was associated with greater NRM in our cohort (HR 2.59, 95CI 1.97–3.41), as our models adjusted for donor source and given our relevant patient population, we believe its inclusion enhances applicability of the risk scores in younger patients.
While the expanded ymHCT-CI captures more pediatric and young adult patients, it only discriminates risk for patients with scores of ≥3, likely because several comorbidities were rather uncommon as demonstrated in prior studies4,8,11,13. Subsequently, we excluded comorbidities with HR<1.2 and designed the simplified ymHCT-CI. This risk score included several risk factors that were quite common (prevalence >10%), including pulmonary, psychiatric, infection, and hepatic disease, similar to recent work4,11. This pattern of comorbidities could argue for a simpler model such as the proposed simplified ymHCT-CI that may be preferable, particularly for pediatric transplant physicians, given that it includes fewer comorbidities, most of which occurred frequently in this population. Further, this abbreviated model performed similarly to the expanded ymHCT-CI (c-statistic: 66.0 vs. 66.0), suggesting that the additional comorbidities included in the expanded ymHCT-CI provided no greater benefit in predicting NRM. Additionally, while scores of ≥3 discriminated risk well in both models, scores of 1–2 were significantly associated with greater NRM only in the simplified ymHCT-CI, implying that simplified ymHCT-CI may have broader applicability to the pediatric and young adult patient population. Still, as demonstrated with the original HCT-CI, scores of ≥3 on either model provided the optimal threshold to predict an elevated risk of NRM. Both models should be validated prospectively, along with study of additional biomarkers and age-related variables including pharmacokinetics, immune reconstitution, and psychosocial challenges34,36,37, in order to further improve pre-transplant risk assessment for this younger population. These scores will help guide patient counseling, influence transplant approaches, and may even prove useful as an eligibility criterion for clinical trials.
This study has several limitations inherent to a large retrospective cohort. The data that is available from the CIBMTR is supplied directly by the centers but may not always be accurate. Although CIBMTR performs audits of data that they receive, we ultimately rely on the reporting from centers to supply the data. The forms provided to centers are designed to gather information about particular patient and transplant-related variables, yet there are limited data collected on risk factors specific to pediatric and young adult patients including a comprehensive psychosocial assessment, complete medical history to provide a more detailed account including the timing of potential comorbidities, and certain biomarkers. In addition, the comprehensive research level data utilized in this study allowed for exploration of different comorbidity definitions, but is not performed on all patients undergoing HCT and therefore, may not be representative of the broader population. Further, some comorbidities were infrequent making it challenging to assess the impact of individual comorbidities on NRM. However, this study is the largest study to date to examine the use of HCT-CI in pediatric and young adult patients with hematologic malignancies, while also creating and examining the impact of broader comorbidity definitions in order to develop a more applicable risk score for a younger population.
In conclusion, while the HCT-CI has been validated in all age groups, it has not been widely adopted by pediatric transplant physicians. We carefully examined the impact of expanding the comorbidity definitions in pediatric and young adult patients with hematologic malignancies, and developed and tested two novel risk scores that are more practical and relevant for this population. Prospective studies are needed to optimally compare and validate these scores, as well as refine them by testing additional comorbidities and biomarkers specific to pediatric and young adult patients.
Supplementary Material
Highlights:
Expanded HCT-CI comorbidity definitions resulted in an increase in comorbidity score for 23% young patients (age <40 years) with hematologic malignancies in this registry study (n=5790).
Patients who had an increased comorbidity score experienced an increased non-relapse mortality risk with allogeneic transplantation, compared to those with no change in score (HR 1.34, 95% CI 1.02–1.74).
Modification to the HCT-CI specific for youth with malignancies (ymHCT) is clinically relevant and better suited to capture comorbidity for children, adolescents, and young adults.
ACKNOWLEDGMENTS (other members of the working committee)
Hisham Abdel-Azim, Allistair Abraham, Mahmoud Aljurf, Jean Yared, Siddhartha Ganguly, Eva Guinan, Shahrukh Hashmi, Seema Naik, Sunita Nathan, Sachiko Seo, Niketa C Shah
Funding Sources
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon, a Sanofi Company; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medac GmbH; Medexus Pharma; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Priothera; Sanofi; Sanofi-Aventis U.S. Inc.; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; Xenikos BV.
CONFLICT OF INTEREST
Dr. Logan reports compensation as a consultant from Blueprint Medicines.
Dr. Chhabra reports honoraria from Sanofi and GSK; and institutional research funding from Amgen, Sanofi, and Janssen.
Dr. Guilcher reports being Principal Investigator of Project Sickle Cure, a Sickle Cell Transplant Advocacy and Research Alliance (STAR) study partially funded by bluebirdbio, received support from Jazz Pharmaceuticals to attend the 2018 American Society of Hematology Meeting.
Dr. Hildebrandt reports stocks for companies with total value less than 5000 dollars but related to healthcare from Axim Biotechnologies, GW Pharmaceuticals, Cardinal Health, Clovis Oncology, Cellectis, CVS Health, Bluebird Bio, Pfizer, Charlottes Webb, AImmune Therapeutics Inc, Medical PPTYS TR Inc., Caretrust Reit Inc., Moderna Therapeutics, and Zoom; research funding from Incyte and Acerta; and advisory boards for Rapa Therapeutics.
Dr. Lazarus reports receiving compensation from BMS as a CAR-T Data Safety Monitoring Board Member, a promotional speaker and consultant for Jazz Pharmaceuticals and Seattle Genetics and received an honorarium for the non-infectious complications of hematopoietic cell transplantation talk during the Jazz Clinical Immersion Virtual Course April 2022.
Dr. Nishihori reports research support to the institution (clinical trial support by Novartis; drug supply for clinical trial by Karyopharm).
Dr. Rotz reports as a medical monitor (paid) for the RCI-BMT.
Dr. Pasquini reports research support from Novartis, Kite, BMS, Amgen (completed 2019), Consultancy (Iisted as the professional providing insight), BMS (CAR T cell Steering Committee - former as of June 2021).
Dr. Stadtmauer reports compensation from BMS, Abbvie, Amgen, Sanofi consultant; grant funding and honoraria from Sanofi, BMS, GSK, and Amgen.
Dr. Sorror reports given a talk about basics of allogeneic transplants during Jazz Clinical Immersion Virtual Course in Feb 24th, 2021; having received an honorarium; participated in Jazz Pharmaceuticals Advisory Board Meeting June 28-29, 2019; and having received an honorarium in addition to travel expenses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The results of this study were presented in a poster format at the 62nd ASH Annual Meeting on Dec 6, 2020.
DATA USE STATEMENT
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
REFERENCES
- 1.Mehta PA, Rotz SJ, Majhail NS: Unique Challenges of Hematopoietic Cell Transplantation in Adolescent and Young Adults with Hematologic Malignancies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 24 12:e11–e19, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Wood WA, Lee SJ, Brazauskas R, et al. : Survival improvements in adolescents and young adults after myeloablative allogeneic transplantation for acute lymphoblastic leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 20:829–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomizawa D, Tanaka S, Kondo T, et al. : Allogeneic Hematopoietic Stem Cell Transplantation for Adolescents and Young Adults with Acute Myeloid Leukemia. Biol Blood Marrow Transplant 23:1515–1522, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Wood W, Deal A, Whitley J, et al. : Usefulness of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in predicting outcomes for adolescents and young adults with hematologic malignancies undergoing allogeneic stem cell transplant. Pediatr Blood Cancer 57:499–505, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Raimondi R, Tosetto A, Oneto R, et al. : Validation of the Hematopoietic Cell Transplantation-Specific Comorbidity Index: a prospective, multicenter GITMO study. Blood 120:1327–1333, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Sorror ML, Logan BR, Zhu X, et al. : Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant 21:1479–87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorror ML, Maris MB, Storb R, et al. : Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106:2912–9, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AR, Majhail NS, MacMillan ML, et al. : Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood 117:2728–34, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Thakar MS, Broglie L, Logan B, et al. : The Hematopoietic Cell Transplant Comorbidity Index predicts survival after allogeneic transplant for nonmalignant diseases. Blood 133:754–762, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleyer A, Budd T, Montello M: Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer 107:1645–55, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Friend BD, Tang K, Markovic D, et al. : Identifying risk factors associated with worse outcomes in adolescents and young adults undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer 66:e27940, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Broglie L, Friend BD, Chhabra S, et al. : Differential use of the hematopoietic cell transplantation-comorbidity index among adult and pediatric transplant physicians. Leuk Lymphoma:1–4, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broglie L, Ruiz J, Jin Z, et al. : Limitations of Applying the Hematopoietic Cell Transplantation Comorbidity Index in Pediatric Patients Receiving Allogeneic Hematopoietic Cell Transplantation. Transplant Cell Ther 27:74.e1–74.e9, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. : A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Muñoz A, Schneider MF, et al. : New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogden CL, Kuczmarski RJ, Flegal KM, et al. : Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, et al. : Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15:1628–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries VA, Müller MCA, Arbous MS, et al. : Long-Term Outcome of Patients With a Hematologic Malignancy and Multiple Organ Failure Admitted at the Intensive Care. Crit Care Med 47:e120–e128, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saillard C, Blaise D, Mokart D: Critically ill allogeneic hematopoietic stem cell transplantation patients in the intensive care unit: reappraisal of actual prognosis. Bone Marrow Transplant 51:1050–61, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Fuchs H, Rossmann N, Schmid MB, et al. : Permissive hypercapnia for severe acute respiratory distress syndrome in immunocompromised children: A single center experience. PLOS ONE 12:e0179974, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kneyber MC, Zhang H, Slutsky AS: Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med 190:258–65, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doney K, McMillen K, Buono L, et al. : Impact of Body Mass Index on Outcomes of Hematopoietic Stem Cell Transplantation in Adults. Biol Blood Marrow Transplant 25:613–620, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Fuji S, Kim SW, Yoshimura K, et al. : Possible association between obesity and posttransplantation complications including infectious diseases and acute graft-versus-host disease. Biol Blood Marrow Transplant 15:73–82, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Pereira AZ, de Almeida-Pitito B, Eugenio GC, et al. : Impact of Obesity and Visceral Fat on Mortality in Hematopoietic Stem Cell Transplantation. JPEN J Parenter Enteral Nutr 45:1597–1603, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner A, Zueger N, Bargetzi A, et al. : Association of Nutritional Parameters with Clinical Outcomes in Patients with Acute Myeloid Leukemia Undergoing Haematopoietic Stem Cell Transplantation. Ann Nutr Metab 69:89–98, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Paviglianiti A, Dalle JH, Ayas M, et al. : Low Body Mass Index Is Associated with Increased Risk of Acute GVHD after Umbilical Cord Blood Transplantation in Children and Young Adults with Acute Leukemia: A Study on Behalf of Eurocord and the EBMT Pediatric Disease Working Party. Biol Blood Marrow Transplant 24:799–805, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Aplenc R, Zhang M-J, Sung L, et al. : Effect of body mass in children with hematologic malignancies undergoing allogeneic bone marrow transplantation. Blood 123:3504–3511, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White M, Murphy AJ, Hallahan A, et al. : Survival in overweight and underweight children undergoing hematopoietic stem cell transplantation. Eur J Clin Nutr 66:1120–3, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Chee L, Tacey M, Lim B, et al. : Pre-transplant ferritin, albumin and haemoglobin are predictive of survival outcome independent of disease risk index following allogeneic stem cell transplantation. Bone Marrow Transplant 52:870–877, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Hansson ME, Norlin AC, Omazic B, et al. : Vitamin d levels affect outcome in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 20:1537–43, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Verdi Schumacher M, Moreira Faulhaber GA: Nutritional status and hyperglycemia in the peritransplant period: a review of associations with parenteral nutrition and clinical outcomes. Rev Bras Hematol Hemoter 39:155–162, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Balk E, et al. : National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139:137–47, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Muto H, Ohashi K, Ando M, et al. : Cystatin C level as a marker of renal function in allogeneic hematopoietic stem cell transplantation. Int J Hematol 91:471–7, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Vignon M, Andreoli A, Dhédin N, et al. : Graft-Versus-Host Disease in Adolescents and Young Adults (15–24 Years Old) After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Leukemia in First Complete Remission. J Adolesc Young Adult Oncol 6:299–306, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Wada H, Kanda J, Akahoshi Y, et al. : Impact of estimated glomerular filtration rate based on plasma cystatin C and serum creatinine levels before allogeneic hematopoietic cell transplantation. Hematology 23:271–276, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Klein AK, Patel DD, Gooding ME, et al. : T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant 7:454–66, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Kwak M, Zebrack BJ, Meeske KA, et al. : Trajectories of psychological distress in adolescent and young adult patients with cancer: a 1-year longitudinal study. J Clin Oncol 31:2160–6, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.


