Abstract
Background and Objective
Hidradenitis suppurativa is a chronic inflammatory skin disease that can lead to a substantial reduction in quality of life. Recent studies revealed high levels of unmet care needs of patients with hidradenitis suppurativa, but their preferences in treatment decision making have scarcely been investigated. This study aimed to reveal which treatment attributes adult patients with HS in Europe consider most important in treatment decision-making.
Methods
A discrete choice experiment was conducted with adult patients with hidradenitis suppurativa in Europe to reveal which treatment attributes are most important when making treatment decisions. Participants were presented with 15 sets of two treatment options and asked for each to choose the treatment they preferred. The treatments were characterized by six attributes informed by a prior literature review and qualitative research: effectiveness, pain reduction, duration of treatment benefit, risk of mild adverse event, risk of serious infection, and mode of administration. A random parameter logit model was used to estimate patients’ preferences with additional subgroup and latent class models used to explore any differences in preferences across patient groups.
Results
Two hundred and nineteen adult patients with hidradenitis suppurativa were included in the analysis (90% women, mean age 38 years). For all six treatment attributes, significant differences were observed between levels. Given the range of levels of each attribute, the most important treatment attributes were effectiveness (47.9%), followed by pain reduction (17.3%), annual risk of a mild adverse event (14.4%), annual risk of a serious infection (10.3%), mode of administration (5.3%), and duration of treatment benefit (4.8%). Higher levels of effectiveness, namely a 75% or 100% reduction in the abscess and inflammatory nodule count, were preferred over levels of effectiveness primarily investigated in randomized clinical trials of hidradenitis suppurativa (a 50% reduction). Results were largely consistent across subgroups and three latent class groups were identified.
Conclusions
This study revealed the most important treatment characteristics for patients with hidradenitis suppurativa that can help inform joint patient-physician decision making in the management of hidradenitis suppurativa. Designing future hidradenitis suppurativa treatments according to stated preferences, namely, to offer higher levels of effectiveness and pain improvement without higher risks of adverse events, may increase patients’ treatment concordance and lead to improved disease management outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40271-022-00614-7.
Key Points for Decision Makers
| Using a multi-country preference study with 219 European patients with hidradenitis suppurativa, this study revealed that the two most important treatment attributes were effectiveness and pain reduction. |
| Higher levels of effectiveness, namely a 75% or 100% reduction in the abscess and inflammatory nodule count, were preferred over levels of effectiveness primarily investigated in current clinical trials of hidradenitis suppurativa (a 50% reduction). |
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that is characterized by recurrent nodules, tunnels, and scarring in flexural skin locations leading to a severe reduction in quality of life [1–3]. The prevalence of HS is estimated between 0.03 and 1% with an average age of onset of 22 years [4]. Low disease awareness and associated misdiagnoses as well as under-reporting by patients because of shame and embarrassment have contributed to substantial delays in diagnosis, reported to be on average between 7 and 10 years [5–7]. The course of disease is often unpredictable, which can be challenging for patients and healthcare professionals (HCPs) in the management of HS [8].
Antibacterial treatments are recommended for mild-to-moderate HS with anti-inflammatory treatments suggested for more severe HS. Surgery is commonly used to treat skin tunnels, scars, and anatomic changes that have manifested [9]. Adalimumab is currently the only approved biologic therapy in the European Union, UK, and USA for patients with moderate-to-severe HS [10]. Currently available treatment options are known to only allow one-third of treated patients to experience remission of their disease and almost half of treated patients with HS remain dissatisfied because of poor efficacy, undesirable adverse effects, inconvenience, or invasiveness [7, 11–13]. Additional treatment options are in development for HS including small-molecule or biological treatments, with bimekizumab and secukinumab (both monoclonal antibodies against interleukin-17) recently reporting positive phase III studies [10, 14–19].
As such novel therapies may offer different treatment outcomes, the understanding of patient perspectives and treatment preferences becomes more important [20]. Although recent studies began to reveal the unmet care needs and treatment desires from patients and HCPs in HS, there is a paucity of quantitative patient preference research as no published discrete choice experiment (DCE) in HS was identified at the time of this research [7, 11, 21]. Such evidence could inform future regulatory and reimbursement decision making as authorities such as the US Food and Drug Administration and the National Institute for Health and Care Excellence in England [22] by advocating the incorporation of patient preferences in the value assessment of treatments [22, 23]. Accounting for patient preferences in clinical decision making may further positively influence treatment outcomes such as treatment satisfaction and concordance, which in turn can lead to positive health and economic implications [20, 24–29]. This study was therefore designed to provide novel insights into treatment attributes patients with HS consider most important when making disease management decisions by quantifying their preferences using a DCE.
Materials and Methods
Qualitative Research for Selection of Treatment Attributes
In the absence of previously published DCEs in HS at the time of this research, qualitative interviews were conducted with adult patients diagnosed with HS (N = 12) and HCPs (N = 16) experienced in treating HS to elicit a comprehensive list of influential treatment attributes to be included in this DCE [21, 30]. All interviews were conducted online using the same semi-structured interview guide that asked participants about their unmet care needs and experiences managing the disease. Participants were subsequently asked what they liked and did not like about current and previous treatments, what the most important treatment factors are, as well as which areas of disease management future treatments should improve. The number of attributes in this DCE was targeted between 4 and 7 to be in line with previous DCEs and to be cognitively manageable for participants [26]. Based on the insights of the qualitative interviews, the following six treatment attributes were considered most relevant for this DCE (in no particular order): (a) effectiveness on reducing the number of painful inflammatory lesions, (b) reduction in pain, (c) duration of treatment benefit, (d) risk of mild side effects, (e) risk of serious infection, and (f) mode of administration. Detailed descriptions of the methodology and findings from the qualitative interviews were previously reported [21].
Selection of Attribute Levels
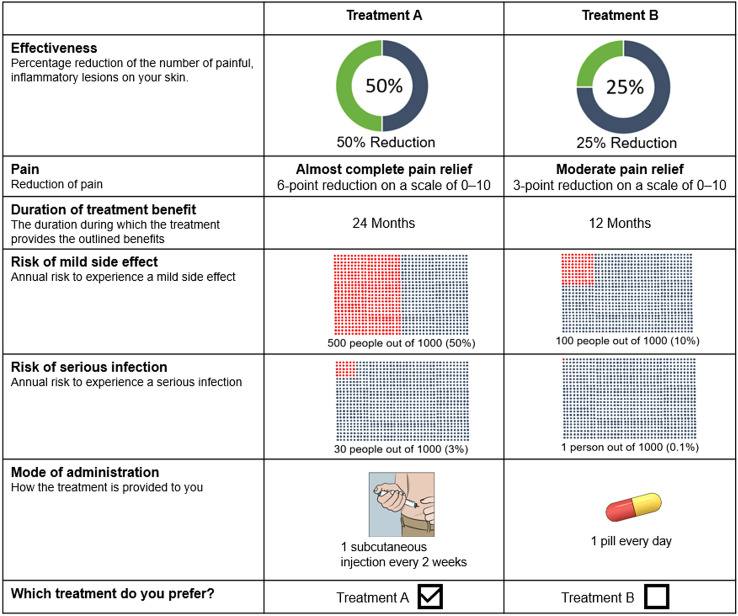
The different levels of the treatment attributes were informed by reviewing the literature and confirmed with dermatologists. Published clinical trial data on available and investigational HS treatments was deemed most appropriate to select the ranges of ‘effectiveness’ (percent reduction of the number of painful inflammatory lesions) [16, 31]. The levels of ‘pain reduction’, which was one of the most reported unmet needs in previous research, were informed by published evidence on clinically meaningful pain improvement thresholds in HS and DCEs in other chronic diseases [32–35]. The different levels of ‘duration of treatment benefit’ were based on studies of currently available treatments and recommendations of previous DCEs [36–38]. For the safety attributes ‘risk of mild adverse event’ (AE) and ‘risk of serious infection’, the levels were informed by AE data of available and investigational therapies in HS or other chronic inflammatory diseases [16, 28, 31, 39, 40]. For ‘mode of administration’, the three most common administration options of available and investigational HS treatments were selected, namely a bi-weekly subcutaneous injection, a monthly intravenous injection, or a daily oral pill [10, 16, 41]. The final attributes and levels are shown in Table 1, and an example of a choice question in the DCE is shown in Fig. 1.
Table 1.
Attributes and levels included in the discrete choice experiment questions
| Attribute | Attribute description | Attribute levels |
|---|---|---|
| Effectiveness | Percentage reduction of the number of painful inflammatory lesions on your skin |
25% 50% 75% 100% |
| Pain reduction | Reduction of pain (on a scale ranging from 0 to 10) |
Small pain relief (1 point) Moderate pain relief (3 points) Almost complete pain relief (6 points) |
| Duration of treatment benefit | The duration in which the treatment provides the proposed effectiveness and pain relief |
6 months 12 months 24 months |
| Risk of mild side effect | Annual risk of experiencing a mild side effect while taking the treatment |
100 people out of 1000 (10%) 300 people out of 1000 (30%) 500 people out of 1000 (50%) |
| Risk of serious infection | Annual risk of experiencing a serious infection while taking the treatment |
1 person out of 1000 (0.1%) 10 people out of 1000 (1%) 30 people out of 1000 (3%) |
| Mode of administration | How the treatment is provided to you |
Oral tablet, once every day Subcutaneous injection, once every 2 weeks at home or in a clinic Intravenous injection, once every 4 weeks in a clinic or hospital setting |
Fig. 1.
Example choice task
Survey Development and Conduction
The DCE was developed according to the guidelines provided by the ISPOR Good Research Practice for Conjoint Analysis Task Force and other recommendations to ensure its design was well suited to quantify the treatment preferences and trade-offs between the benefits and risks of treatments patients with HS are willing to accept [42–44]. The survey was initially developed in English by a working group that included patient preference research experts and experienced dermatologists. An introductory section explained the survey and its content, which included a description of the task prior to the presentation of the choice set questions to participants. Prior to participation, respondents read a participant information sheet and provided consent online. The survey included questions to elicit participants’ demographics, disease history, and current health status. Participants’ current health status was assessed using a pain visual analog scale, the EuroQoL 5-Dimension 5-Level Questionnaire, and the Hidradenitis Suppurativa Quality of Life (HiSQOL) Questionnaire [45–47]. The DCE experimental design was split into three different, but equally sized blocks (or versions). Each of the three blocks contained 14 different choice questions based on an efficient design using Ngene software. One additional choice question included a dominance test; in which a dominant treatment option with no difference in mode of administration was presented to allow a later exclusion of participants who preferred the dominated option, which indicated a lack of understanding of the task [48, 49]. The survey was programmed and hosted online using Qualtrics® and participants were randomly assigned to one of the three blocks with 15 choice questions to avoid ordering effects. To respect the cognitive burden of the DCE on participants, the number of choice questions was limited to 15 and complemented by graphical illustrations. The survey was made available in English, Dutch, or German with each translation verified by a native-speaking investigator. Participants were only allowed to progress in the survey if they had fully responded to all questions to avoid incomplete surveys. At the end of the survey, participants were asked to rate the difficulty of survey completion on a 0–10 scale (0 = not difficult at all to 10 = extremely difficult).
Pilot Testing
The draft survey versions including the DCE questions were sequentially pilot tested by five preference researchers, three dermatologists, and two patients with HS until finalization. The attribute descriptions for the DCE survey were confirmed to be generally well understood, and the overall survey length was considered appropriate by the test persons, who felt that the hypothetical trade-offs were relevant, well-balanced, and not overly dominant. Only minor changes to the description of the survey and attributes were made following the pilot testing. The final survey can be found in the Electronic Supplementary Material (ESM).
Participants
Adult patients with a confirmed diagnosis of HS in multiple European countries (Belgium, Germany, UK, Ireland, Switzerland, Austria, The Netherlands) were recruited through patient advocacy and social media groups between January 2022 and April 2022. Optimal sample size for DCEs are challenging to predict as it depends on the true value of the parameters estimated in the DCE, which are not known prior to undertaking the research [44]. Given the number of treatment options, attributes, and levels included in the DCE, a minimum of 200 patients was targeted based on published guidance [50]. Ethics approval for this study was obtained from the Medical Ethics Committee of the Academic Hospital Maastricht and Maastricht University. Additional local ethics approvals were obtained where required.
Statistical Analyses
Participants’ demographic and disease history variables including EuroQoL 5-Dimension 5-Level Questionnaire and HiSQOL Questionnaire results were first checked for normality of variables and subsequently descriptively reported. The available patient preference data derived with the DCE were analyzed using various recommended statistical methods and carried out using Nlogit software, version 5.0 [51].
First, the choice data from the DCE were analyzed using a random parameter logit model, which allows heterogeneity to be captured by estimating the standard deviation of the parameter’s distribution. Using a random parameter logit model was consistent with good research practices and prior precedence for regulatory decision making, and provided mean coefficients as well as a measure of the distribution around the mean coefficient in the form of standard deviations [51]. The conditional relative importance of each attribute was also calculated as the coefficient difference between the attribute level with the highest preference weight and the one with the lowest preference weight, to allow for comparisons across attributes. All variables were effects coded; hence, the mean effect for each attribute was normalized at zero and the preference weight is relative to the mean effect of the different levels of the attribute. The model was estimated by using 1000 Halton draws and no interaction terms were included in the final model, as an exploratory model with an interaction term provided a similar fit and results. The sign of a coefficient reflects whether an attribute level led to an increase (positive) or a decrease (negative) on the participants’ utility, while the value of each coefficient represents the importance participants assigned to each attribute level. P values represent the statistical difference between the preference weight of the attribute levels and the mean effect of the same attribute; if the 95% confidence interval (CI) around two levels did not overlap, the differences between the preference weights were considered as statistically different [51]. A priori, it was expected that the attribute levels with large improvements such as high levels of effectiveness, pain reduction, duration of treatment benefit, and a lower risk of side effects would have a positive effect on utility (i.e., a positive sign).
Second, subgroup random parameter logit models estimating the conditional relative importance were conducted to assess whether preferences varied as a function of patient characteristics or disease history. A range of subgroups covering country of residence, age, sex, disease severity, disease duration, current level of pain, HiSQOL Questionnaire score, previous biologic therapy, and previous excisional surgery were considered based on the characteristics of the final sample. Binary subgroups for age, disease duration, current level of pain, and HiSQOL Questionnaire were created by dividing the sample by the median as conducted in previous preference research [52].
Last, a latent class model was used to determine preference classes as they allow the existence and number of classes in the population to be identified based on their treatment preferences [53]. To determine the number of latent classes, the model with the best fit based on the Akaike information criterion was selected from models with two, three, and four latent classes [51]. The association between selected patient characteristics and latent class membership was then determined using a multivariable logistic regression model. The multivariable model was considered exploratory and was limited to the variables with a different probability between latent classes. This analysis was conducted with IBM SPSS 24™.
Results
Study Sample
A total of 224 participants completed the survey, of whom 219 were included in the analysis as five participants (< 2.5%) did not pass the dominance test and were therefore excluded as pre-specified. The demographics of patients included in the DCE are reported in Table 2. Mean (standard deviation) age of participants was 38.7 (10.1) years and participants were predominantly female (90%) and of white/Caucasian ethnicity (94%). The HiSQOL Questionnaire median score (standard deviation) of 34 (16.1) and pain median score (interquartile range) of 5 (3–7) indicate HS to have a large effect on patients’ lives at the time of questionnaire completion. The difficulty of questionnaire completion was reported on a 0–10 scale at 2.8 ± 2.7 (mean ± standard deviation) by participants, which suggested that the survey completion was cognitively well manageable. Further demographics can be found in Table 2.
Table 2.
Demographic characteristics of participants
| Parameter | N = 219 | |
|---|---|---|
| Country, n (%) | ||
| UK | 18 | (8.2%) |
| Ireland | 22 | (10.0%) |
| Germany | 71 | (32.4%) |
| Austria | 3 | (1.4%) |
| Belgium | 4 | (1.8%) |
| The Netherlands | 68 | (31.1%) |
| Denmark | 12 | (5.5%) |
| Switzerland | 16 | (7.3%) |
| Other | 5 | (2.3%) |
| Sex, n (%) | ||
| Female | 198 | (90.4%) |
| Age (years), n (%) | ||
| ≤ 30 | 49 | (22.4%) |
| 31–40 | 78 | (35.6%) |
| 41–50 | 64 | (29.2%) |
| > 50 | 28 | (12.8%) |
| Race, n (%) | ||
| White or Caucasian | 205 | (93.6%) |
| Asian | 3 | (1.4%) |
| Black or African American | 0 | – |
| Other | 11 | (5.0%) |
| Occupational status, n (%) | ||
| Full time employed | 87 | (39.7%) |
| Part time employed | 51 | (23.3%) |
| Student | 10 | (4.6%) |
| Not working or unemployed | 31 | (14.2%) |
| Retired | 40 | (18.3%) |
| Highest level of education, n (%) | ||
| Primary or elementary school | 7 | (3.2%) |
| Secondary or high school | 120 | (54.8%) |
| College or university degree | 73 | (33.3%) |
| Other | 19 | (8.7%) |
| Disease duration, (years), mean (SD) | 10.70 | (9.8) |
| Disease duration, n (%) | ||
| 0–3 | 66 | (30.1%) |
| 4–10 | 69 | (31.5%) |
| 11–20 | 51 | (23.3%) |
| > 20 | 33 | (15.1%) |
| Severity of HS (by Hurley classification) | ||
| Mild | 13 | (5.9%) |
| Moderate | 132 | (60.3%) |
| Severe | 74 | (33.8%) |
| Treatment experience | ||
| Previous biologic therapy | 65 | (29.7%) |
| Previous wide excisional surgery | 134 | (61.2%) |
| Level of pain (0–10 VAS), median (IQR) | 5 | 3-7 |
| HiSQOL score, median (SD) | ||
| Total score | 34 | (16.1) |
| Symptom subscale | 8 | (4.1) |
| Psychosocial subscale | 10 | (5.5) |
| Activities and adaptations subscale | 17 | (8.1) |
| EQ-5D-5L, mean (SD) | ||
| Mobility | 2.14 | (1) |
| Self-care | 1.50 | (0.7) |
| Usual activities | 2.21 | (0.9) |
| Pain and discomfort | 2.94 | (1) |
| Anxiety and depression | 2.58 | (1.2) |
EQ-5D-5L EuroQoL 5-Dimension-5 Level Questionnaire, HiSQOL Hidradenitis Suppurativa Quality of Life Questionnaire, HS hidradenitis suppurativa, IQR interquartile range, SD standard deviation, VAS Visual Analog Scale
Participants’ Preferences
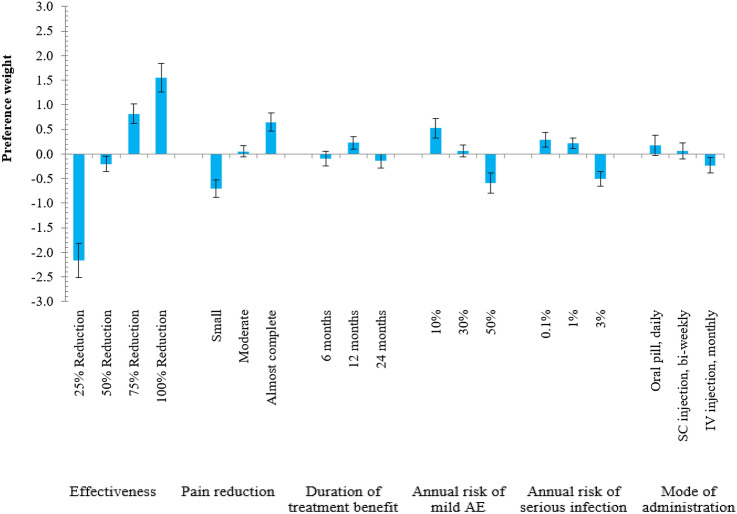
In all six treatment attributes, significant differences were observed between levels (as the 95% CI did not overlap), meaning that all attributes were important for participants as shown in Table 3. The most important treatment attribute for patients with HS was effectiveness (conditional relative importance of 47.9%) followed by pain reduction (17.3%), annual risk of mild AE (14.4%), annual risk of serious infection (10.3%), mode of administration (5.3%), and duration of treatment benefit (4.8%) as presented in Fig. 2. On average, respondents preferred treatment options with higher effectiveness, greater pain reduction, longer duration of treatment benefit, and a lower risk of mild AEs and serious infection, which are offered as a daily oral pill as can be observed from the random parameter logit model in Fig. 3. The directions of relationships were observed as expected, as the improved levels of each attribute resulted in higher coefficient values except for the duration of treatment benefit for which participants preferred 12 months over 24 months (Table 3).
Table 3.
Results from the random parameter logit model
| Attribute | Level | Coefficient estimate (95% CI)a | P value from previous level | Significant SDb |
|---|---|---|---|---|
| Effectiveness | 25% Reduction | − 2.165 (− 2.519, − 1.811) | – | – |
| 50% Reduction | − 0.206 (− 0.360, − 0.052) | 0.009 | No | |
| 75% Reduction | 0.818 (0.615, 1.020) | < 0.001 | Yes | |
| 100% Reduction | 1.553 (1.258, 1.847) | < 0.001 | Yes | |
| Pain reduction | Small | − 0.700 (− 0.875, − 0.525) | – | – |
| Moderate | 0.053 (− 0.064, 0.170) | 0.369 | No | |
| Almost complete | 0.647 (0.465, 0.830) | < 0.001 | Yes | |
| Duration of treatment benefit, months | 6 | − 0.092 (− 0.240, 0.056) | – | – |
| 12 | 0.231 (0.103, 0.352) | < 0.001 | No | |
| 24 | − 0.139 (− 0.279, 0.002) | 0.053 | Yes | |
| Annual risk of mild AE | 10% | 0.525 (0.331, 0.719) | – | – |
| 30% | 0.064 (− 0.055, 0.183) | 0.290 | No | |
| 50% | − 0.589 (− 0.797, − 0.381) | < 0.001 | Yes | |
| Annual risk of serious infection | 0.1% | 0.288 (0.138, 0.439) | – | – |
| 1% | 0.218 (0.105, 0.331) | < 0.001 | No | |
| 3% | − 0.506 (− 0.658, − 0.354) | < 0.001 | Yes | |
| Mode of administration | Oral pill, daily | 0.176 (− 0.029, 0.381) | – | – |
| SC injection, bi-weekly | 0.057 (− 0.107, 0.221) | 0.494 | Yes | |
| IV injection, monthly | − 0.233 (− 0.390, − 0.076) | < 0.001 | Yes | |
| K | 26 | |||
| LL | − 1549.7 | |||
| AIC | 3151.5 |
AE adverse event, AIC Akaike information criterion, CI confidence interval, IV intravenous, K number of parameters in the model, LL log-likelihood, SC subcutaneous, SD standard deviation
aA positive (negative) sign for a given level indicates a level has a positive (negative) effect on utility
bSignificance at 5%, SDs correspond to the random component of the model coefficients
Fig. 2.
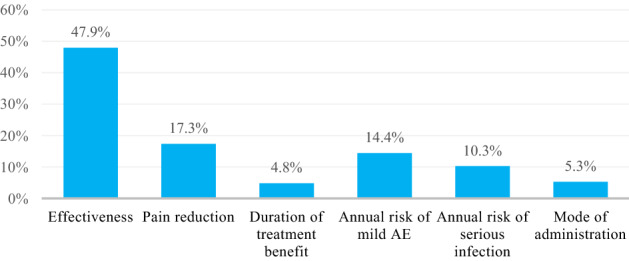
Conditional relative importance of treatment attributes. AE adverse event
Fig. 3.
Random parameter logit model estimates: preference weights (N = 219). The vertical bars around each preference weight (coefficient estimate) represent the 95% confidence interval. Within each attribute, a higher preference weight indicates that a level is more preferred, and the sum of the preference weights equals 0. AE adverse event, IV intravenous, SC subcutaneous
Subgroup Analyses
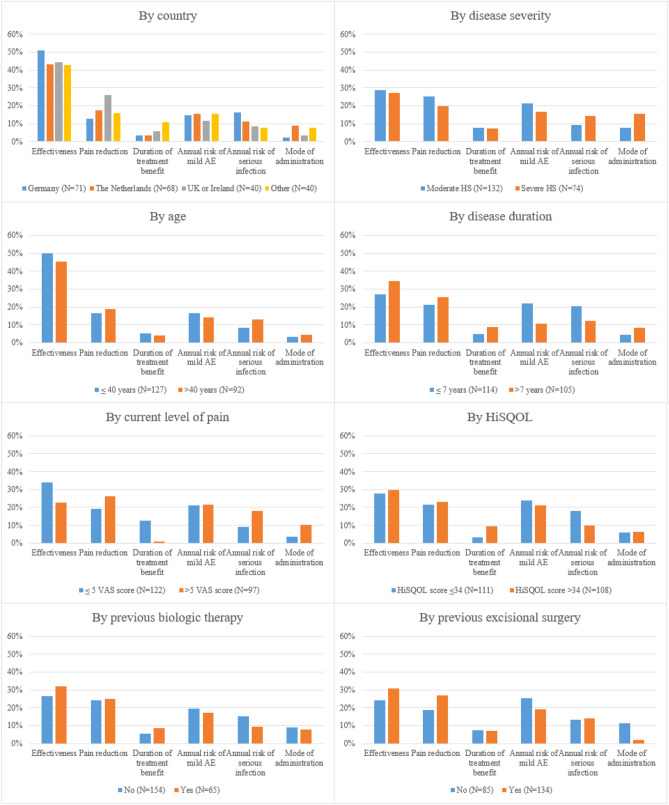
The conditional relative importance of treatment attributes was generally consistent across subgroups (Fig. 4). Patients with a longer disease duration placed greater importance on treatment effectiveness and pain reduction rather than safety-related attributes compared with patients with a shorter disease duration. Effectiveness and duration of treatment benefit were more important to patients with lower levels of pain while patients with higher levels preferred better pain improvement. No subgroup analyses for sex, race/ethnicity, and mild HS severity could be conducted because of sample size constraints.
Fig. 4.
Conditional relative importance of treatment attributes for subgroups. Disease severity defined by Hurley classification. AE adverse event, HiSQOL Hidradenitis Suppurativa Quality of Life Questionnaire, HS hidradenitis suppurativa, VAS Visual Analog Scale
Latent Class Model
The latent class analyses identified three preferences classes with class probabilities of 52%, 30%, and 18% (Table 4), which indicates that patients value treatment characteristics differently. Effectiveness (60%), annual risk of mild AE (37%), and mode of administration (36%) were the most important attributes in each latent class, respectively. The preference coefficients of the latent class analyses are presented in the ESM.
Table 4.
Latent class analyses: latent class probabilities and conditional relative importance between attributes
| Treatment attribute | Overall | Latent class 1 (52%) | Latent class 2 (30%) | Latent class 3 (18%) |
|---|---|---|---|---|
| Effectiveness | 48% | 60% | 25% | 11% |
| Pain reduction | 17% | 14% | 17% | 21% |
| Duration of treatment benefit | 5% | 7% | 4% | 6% |
| Annual risk of mild AE | 15% | 4% | 37% | 7% |
| Annual risk of serious infection | 10% | 8% | 11% | 19% |
| Mode of administration | 5% | 7% | 6% | 36% |
Akaike information criterion = 3313.8
AE adverse event
Discussion
This study aimed to reveal which treatment attributes adult patients with HS consider most important when making treatment decisions. It reported numerous novel findings by quantifying treatment attribute preferences of patients with HS in Europe using a DCE. All six selected treatment attributes (effectiveness, pain reduction, duration of treatment benefit, annual risk of mild AE, annual risk of serious infection, and mode of administration) were important for patients with HS and consistent with a priori expectations in terms of the direction and magnitude of the estimated coefficients. ‘Effectiveness’ was the most important treatment attribute for patients, which confirmed the previously reported high unmet needs regarding treatment outcomes as only one-third of patients experience remission of their disease over time with currently available treatment options [7, 11, 12, 21]. Interestingly, while previous clinical trials of HS treatments primarily investigated a 50% reduction in abscess and inflammatory nodule counts, patients in this research considered more stringent measures of treatment effectiveness, such as 75% and 100% levels of reduction in abscess and inflammatory nodule counts, to be more relevant [16, 31]. This likely reflects increasing expectations regarding treatment success in people with HS, which demonstrates that future HS clinical trials may need to consider a higher efficacy target to demonstrate treatment effectiveness. The results further highlighted the significance for patients to experience better pain control as it was the second most important treatment attribute and was also determined as relevant by the HISTORIC core outcomes set initiative and previous research [21, 54]. Patients generally preferred 12 months duration of treatment benefit over 6 months but did not prefer the benefits to last 24 months, which may indicate patients’ reluctance to commit to a therapy administered as an injection or oral pill beyond 1 year. The least preferred mode of administration was the monthly intravenous injection, which is aligned to the conclusions of a recent literature review in chronic immune system disorders that patients preferred treatment at home owing to the convenience and comfort of home treatment and the avoidance of having to attend hospital for an intravenous injection albeit less frequently administered [55].
Extensive subgroup analyses confirmed that observed differences in preferences were not explained by patient characteristics or disease history as participants’ treatment preferences were generally consistent across subgroups. Some variations in preferences were observed in patients with a longer disease duration and higher levels of pain, both placing more importance on pain reduction. The latent class analyses identified three distinct groups of respondents whose most important treatment attributes were effectiveness, annual risk of a mild AE, and mode of administration, revealing heterogeneity in preferences between patients.
The findings of this study highlight the importance of investigating individual preferences and incorporating them not only in clinical decision making but also in research, regulatory, and policy decisions. Treatments for patients with HS should offer higher levels of effectiveness than are typically reported as primary outcomes in current clinical trials, result in greater pain improvement, and minimize the risk of adverse events when possible. Treatments administered as intravenous injections are generally the least desirable mode of administration. One latent class strongly favored oral treatments, but for most patients, efficacy was the most important factor determining treatment preference. Ultimately, a variety of treatment options should be made available so that treatment can be individualized based on patient preference.
Although this study followed good research practices, was designed with experienced dermatologists and preference research experts, and underwent extensive pilot testing, some limitations are to be considered in the interpretation of the results. While most participants’ demographics are in line with recent research and were overall well balanced, no Black or African American patients participated in this study and most patients reported moderate or severe HS, with only a few patients having mild HS [13]. In addition, the sample size was targeted for the whole sample, which impaired the ability to confirm findings for every country individually. Although extensive qualitative research with patients and HCPs was conducted to select and define attributes and levels for this DCE, additional or different attributes or levels could have led to varying findings [21]. For example, costs could be an important attribute to be added in future DCEs in countries where patients have considerable out-of-pocket contributions, which was assessed not to be the case in the countries included in this research [56]. Finally, despite DCEs being widely used, they have the inherent limitation that respondents are stating their preferences on hypothetical treatments, which may differ from their preferences in real-life treatment decision making [57]. Future research can further advance the understanding of treatment preferences in HS by conducting DCEs with patients in other countries or with HCPs to allow a comparison of findings between participant groups or to explore the impact of different attributes and levels on patient preferences.
Conclusions
This research highlighted the patient perspectives surrounding the relevant benefits and risks of different HS treatments, which can help clinical, regulatory, reimbursement, and development decision making to allow future HS treatment to become better suited to the needs and preferences of patients with HS and ultimately lead to improved disease management. It was revealed patients with HS preferred treatments offering high levels of effectiveness and pain reduction.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participants for completing the survey. The authors also thank the patient organizations (Hidradenitis Patiënten Vereniging Nederland, Verein Acne Inversa SchwAIz, Irish Skin, and Patientforeningen HS Danmark) that supported this research with the invitation of participants.
Declarations
Funding
No funding was received for the conduct of this study.
Conflicts of interest/competing interests
DW is a registered PhD student at Maastricht University and an employee of UCB Pharma, UCB Pharma had no role in the design, conduct, and analysis of the study, or in the writing/reviewing of this manuscript. EH is a registered PhD student at Maastricht University and an employee of EY-Parthenon, EY-Parthenon had no role in the design, conduct, and analysis of the study, or in the writing/reviewing of this manuscript. HZ received consultancy fees from Abbvie, InflaRX, Novartis, and Insmed. CS is a speaker for Abbvie and Novartis, a consultant for Abbvie, Novartis, UCB, InflaRx, and Alcaris, has received education grants from Abbvie, and has been an investigator with fees paid to his institution for Abbvie, Novartis, Incyte, InflaRx, Chemocentryx, and UCB. CS has an unpaid position on the board of the Hidradenitis Suppurativa Foundation. JRI received a stipend as Editor-in-Chief of the British Journal of Dermatology and an authorship honorarium from UpToDate. He is a consultant for Boehringer Ingelheim, ChemoCentryx, Citryll, Novartis, and UCB Pharma and has served on advisory boards for Insmed, Kymera Therapeutics, and Viela Bio. He is co-copyright holder of the HiSQOL Questionnaire, Investigator Global Assessment, and Patient Global Assessment instruments for HS. His department receives income from copyright of the Dermatology Life Quality Instrument and related instruments. CB, SE, and MH have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Maastricht University and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethics approval was granted by the Faculty of Health, Medicine and Life Sciences of Maastricht University on 3 January, 2022 (FHML-REC/2021/115).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The submission does not include personal details or images that may lead to the identification of participants and all participants gave consent to publish their anonymized data.
Availability of data and material
Maastricht University is committed to share the data that support the findings of this study with qualified external researchers. The requests are to be made to the corresponding author and will be appraised based on their scientific merit.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by DW, LH, CB, and MH. The manuscript was written by DW and all authors provided feedback throughout the development of the manuscript and read and approved the final manuscript. All authors sufficiently contributed to this research according to ICMJE criteria to qualify as a listed author.
References
- 1.Montero-Vilchez T, et al. The burden of hidradenitis suppurativa signs and symptoms in quality of life: systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(13):6709. doi: 10.3390/ijerph18136709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher S, Ziv M. Interviewing women with hidradenitis suppurativa: thematic and content analysis. Adv Skin Wound Care. 2022;35(7):381–384. doi: 10.1097/01.ASW.0000831084.75243.66. [DOI] [PubMed] [Google Scholar]
- 3.Jemec GB. Clinical practice: hidradenitis suppurativa. N Engl J Med. 2012;366(2):158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 4.Kirsten N, Petersen J, Hagenstrom K, Augustin M. Epidemiology of hidradenitis suppurativa in Germany: an observational cohort study based on a multisource approach. J Eur Acad Dermatol Venereol. 2020;34(1):174–179. doi: 10.1111/jdv.15940. [DOI] [PubMed] [Google Scholar]
- 5.Kokolakis G, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology. 2020;236(5):421–430. doi: 10.1159/000508787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunte DM, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173(6):1546–1549. doi: 10.1111/bjd.14038. [DOI] [PubMed] [Google Scholar]
- 7.Garg A, et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol. 2020;82(2):366–376. doi: 10.1016/j.jaad.2019.06.1301. [DOI] [PubMed] [Google Scholar]
- 8.Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231(2):184–190. doi: 10.1159/000431175. [DOI] [PubMed] [Google Scholar]
- 9.Zouboulis CC, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 10.Aarts P, et al. Clinical implementation of biologics and small molecules in the treatment of hidradenitis suppurativa. Drugs. 2021;81(12):1397–1410. doi: 10.1007/s40265-021-01566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingram JR, et al. Unmet clinical needs and burden of disease in hidradenitis suppurativa: real-world experience from EU5 and US. J Eur Acad Dermatol Venereol. 2022;36:1597–605. doi: 10.1111/jdv.18163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjaersgaard Andersen R, et al. Incidence and remission rates of self-reported hidradenitis suppurativa: a prospective cohort study conducted in Danish blood donors. J Eur Acad Dermatol Venereol. 2022;36(5):717–725. doi: 10.1111/jdv.17857. [DOI] [PubMed] [Google Scholar]
- 13.De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatol Treat. 2022;33(7):1–4. [DOI] [PubMed]
- 14.Markota Cagalj A, Marinovic B, Bukvic MZ. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci. 2022;23(7):3753. doi: 10.3390/ijms23073753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CH, Huang IH, Tai CC, Chi CC. Biologics and small molecule inhibitors for treating hidradenitis suppurativa: a systematic review and meta-analysis. Biomedicines. 2022;10(6):1303. doi: 10.3390/biomedicines10061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glatt S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157(11):1279–1288. doi: 10.1001/jamadermatol.2021.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zouboulis CC, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30(Suppl. 1):8–17. doi: 10.1111/exd.14338. [DOI] [PubMed] [Google Scholar]
- 18.Kimball A. Secukinumab in moderate-to-severe hidradenitis suppurativa: primary endpoint analysis from the SUNSHINE and SUNRISE phase III trials. In: Proceedings of the 31st European Academy of Dermatology and Venereology (EADV) congress, Milan, 2022. p. 7–10.
- 19.Dermatology Times. UCB announces positive phase 3 studies for bimekizumab in hidradenitis suppurativa. 2022. https://www.dermatologytimes.com/view/ucb-announces-positive-phase-3-studies-for-bimekizumab-in-hidradenitis-suppurativa. Accessed 3 Jan 2023.
- 20.Umar N, Schaarschmidt M, Schmieder A, Peitsch WK, Schollgen I, Terris DD. Matching physicians’ treatment recommendations to patients' treatment preferences is associated with improvement in treatment satisfaction. J Eur Acad Dermatol Venereol. 2013;27(6):763–770. doi: 10.1111/j.1468-3083.2012.04569.x. [DOI] [PubMed] [Google Scholar]
- 21.Willems D, Hiligsmann M, van der Zee HH, Sayed CJ, Evers S. Identifying unmet care needs and important treatment attributes in the management of hidradenitis suppurativa: a qualitative interview study. Patient. 2022;15(2):207–218. doi: 10.1007/s40271-021-00539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvy JC, Cowie L, Lovett R, Morrison D, Livingstone H, Crabb N. Use of patient preference studies in HTA decision making: a NICE perspective. Patient. 2020;13(2):145–149. doi: 10.1007/s40271-019-00408-4. [DOI] [PubMed] [Google Scholar]
- 23.Ho MP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–2993. doi: 10.1007/s00464-014-4044-2. [DOI] [PubMed] [Google Scholar]
- 24.Willems D, Charokopou M, Evers S, Hiligsmann M. Early health economic modelling for a treatment candidate in hidradenitis suppurativa. J Med Econ. 2020;23(12):1516–1524. doi: 10.1080/13696998.2020.1840181. [DOI] [PubMed] [Google Scholar]
- 25.Jevtic T, Bukumiric Z, Jankovic SM. Effects of treatment adherence on clinical and economic outcomes in patients with psoriasis. Med Glas (Zenica). 2013;10(1):106–112. [PubMed] [Google Scholar]
- 26.Sain N, Willems D, Charokopou M, Hiligsmann M. The importance of understanding patient and physician preferences for psoriasis treatment characteristics: a systematic review of discrete-choice experiments. Curr Med Res Opin. 2020;36(8):1257–1275. doi: 10.1080/03007995.2020.1776233. [DOI] [PubMed] [Google Scholar]
- 27.Gaspar K, Gergely HL, Jenei B, et al. Resource utilization, work productivity and costs in patients with hidradenitis suppurativa: a cost-of-illness study.Expert Rev Pharmacoecon Outcomes Res. 2022;22(3):1–10. [DOI] [PubMed]
- 28.Schaarschmidt ML, et al. Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol. 2013;27(2):187–198. doi: 10.1111/j.1468-3083.2011.04440.x. [DOI] [PubMed] [Google Scholar]
- 29.Thorneloe RJ, Bundy C, Griffiths CE, Ashcroft DM, Cordingley L. Nonadherence to psoriasis medication as an outcome of limited coping resources and conflicting goals: findings from a qualitative interview study with people with psoriasis. Br J Dermatol. 2017;176(3):667–676. doi: 10.1111/bjd.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vass C, Rigby D, Payne K. The role of qualitative research methods in discrete choice experiments. Med Decis Mak. 2017;37(3):298–313. doi: 10.1177/0272989X16683934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimball AB, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422–434. doi: 10.1056/NEJMoa1504370. [DOI] [PubMed] [Google Scholar]
- 32.Muhlbacher AC, et al. Chronic pain patients' treatment preferences: a discrete-choice experiment. Eur J Health Econ. 2015;16(6):613–628. doi: 10.1007/s10198-014-0614-4. [DOI] [PubMed] [Google Scholar]
- 33.Sumpton D, Kelly A, Craig JC, et al. Preferences for biologic treatment in patients with psoriatic arthritis: a discrete choice experiment. Arthritis Care Res (Hoboken). 2022;74(8):1234–43. [DOI] [PubMed]
- 34.van Straalen KR, et al. The efficacy and tolerability of tetracyclines and clindamycin plus rifampicin for the treatment of hidradenitis suppurativa: results of a prospective European cohort study. J Am Acad Dermatol. 2021;85(2):369–378. doi: 10.1016/j.jaad.2020.12.089. [DOI] [PubMed] [Google Scholar]
- 35.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4(7):407–414. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 36.Copsey B, Buchanan J, Fitzpatrick R, Lamb SE, Dutton SJ, Cook JA. Duration of treatment effect should be considered in the design and interpretation of clinical trials: results of a discrete choice experiment. Med Decis Mak. 2019;39(4):461–473. doi: 10.1177/0272989X19841877. [DOI] [PubMed] [Google Scholar]
- 37.Zouboulis CC, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80(1):60–9.e2. doi: 10.1016/j.jaad.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 38.Prens LM, et al. Adalimumab and infliximab survival in patients with hidradenitis suppurativa: a daily practice cohort study. Br J Dermatol. 2021;185(1):177–184. doi: 10.1111/bjd.19863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quartuccio L, Zabotti A, Del Zotto S, Zanier L, De Vita S, Valent F. Risk of serious infection among patients receiving biologics for chronic inflammatory diseases: usefulness of administrative data. J Adv Res. 2019;15:87–93. doi: 10.1016/j.jare.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon WG, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54(8):2368–2376. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 41.Ghias MH, Johnston AD, Kutner AJ, Micheletti AG, Hosgood HD, Cohen SR. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82(5):1094–1101. doi: 10.1016/j.jaad.2019.09.071. [DOI] [PubMed] [Google Scholar]
- 42.Bridges JF, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Reed Johnson F, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 44.Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–677. doi: 10.2165/00019053-200826080-00004. [DOI] [PubMed] [Google Scholar]
- 45.Kirby JS, et al. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol. 2020;183(2):340–348. doi: 10.1111/bjd.18692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riis PT, Vinding GR, Ring HC, Jemec GB. Disutility in patients with hidradenitis suppurativa: a cross-sectional study using EuroQoL-5D. Acta Derm Venereol. 2016;96(2):222–226. doi: 10.2340/00015555-2129. [DOI] [PubMed] [Google Scholar]
- 47.Bato A, et al. The measurement performance of the EQ-5D-5L versus EQ-5D-3L in patients with hidradenitis suppurativa. Qual Life Res. 2021;30(5):1477–1490. doi: 10.1007/s11136-020-02732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tervonen T, Schmidt-Ott T, Marsh K, Bridges JFP, Quaife M, Janssen E. Assessing rationality in discrete choice experiments in health: an investigation into the use of dominance tests. Value Health. 2018;21(10):1192–1197. doi: 10.1016/j.jval.2018.04.1822. [DOI] [PubMed] [Google Scholar]
- 49.Janssen EM, Marshall DA, Hauber AB, Bridges JFP. Improving the quality of discrete-choice experiments in health: how can we assess validity and reliability? Expert Rev Pharmacoecon Outcomes Res. 2017;17(6):531–542. doi: 10.1080/14737167.2017.1389648. [DOI] [PubMed] [Google Scholar]
- 50.de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–384. doi: 10.1007/s40271-015-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hauber AB, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–315. doi: 10.1016/j.jval.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Boeri M, Sutphin J, Hauber B, et al. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatol Treat. 2022;33(3):1449–58. [DOI] [PubMed]
- 53.Zhou M, Thayer WM, Bridges JFP. Using latent class analysis to model preference heterogeneity in health: a systematic review. Pharmacoeconomics. 2018;36(2):175–187. doi: 10.1007/s40273-017-0575-4. [DOI] [PubMed] [Google Scholar]
- 54.Thorlacius L, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179(3):642–650. doi: 10.1111/bjd.16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overton PM, Shalet N, Somers F, Allen JA. Patient preferences for subcutaneous versus intravenous administration of treatment for chronic immune system disorders: a systematic review. Patient Prefer Adherence. 2021;15:811–834. doi: 10.2147/PPA.S303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somers C, Chimonas S, McIntosh E, Kaltenboeck A, Briggs A, Bach P. Using nominal group technique to identify key attributes of oncology treatments for a discrete choice experiment. MDM Policy Pract. 2019;4(1):2381468319837925. doi: 10.1177/2381468319837925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laba TL, Brien JA, Fransen M, Jan S. Patient preferences for adherence to treatment for osteoarthritis: the MEdication Decisions in Osteoarthritis Study (MEDOS) BMC Musculoskelet Disord. 2013;14:160. doi: 10.1186/1471-2474-14-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.