Abstract
Objective:
To examine whether body mass index (BMI) changes modify the association between kidney donation and incident hypertension.
Summary Background Data:
Obesity increases hypertension risk in both general and living kidney donor (LKD) populations. Donation-attributable risk in the context of obesity, and whether weight change modifies that risk, is unknown.
Methods:
Nested case-control study among 1,558 adult LKDs (1976–2020) with obesity (median follow-up: 3.6 years (IQR: 2.0–9.4)) and 3,783 adults with obesity in the Coronary Artery Risk Development in Young Adults (CARDIA) and Atherosclerosis Risk in Communities (ARIC) studies (9.2 years (IQR: 5.3–15.8)). Hypertension incidence was compared by donor status using conditional logistic regression, with BMI change investigated for effect modification.
Results:
Overall, LKDs and non-donors had similar hypertension incidence (incidence rate ratio (IRR): 1.16, 95%CI: 0.94–1.43, p=0.16), even after adjusting for BMI change (IRR: 1.25, 95%CI: 0.99–1.58, p=0.05). Although LKDs and non-donors who lost >5% BMI had comparable hypertension incidence (IRR: 0.78, 95%CI: 0.46–1.34, p=0.36), there was a significant interaction between donor and >5% BMI gain (multiplicative interaction IRR: 1.62, 95%CI: 1.15–2.29, p=0.006; relative excess risk due to interaction: 0.90, 95%CI: 0.24–1.56, p=0.007), such that LKDs who gained weight had higher hypertension incidence than similar non-donors (IRR: 1.83, 95%CI: 1.32–2.53, p<0.001).
Conclusions:
Overall, LKDs and non-donors with obesity had similar hypertension incidence. Weight stability and loss were associated with similar hypertension incidence by donor status. However, LKDs who gained >5% saw increased hypertension incidence vs. similar non-donors, providing support for counseling potential LKDs with obesity on weight management post-donation.
MINI ABSTRACT
The goal of this study was to examine whether body mass index (BMI) changes modify the association between kidney donation and incident hypertension. Overall, living kidney donors and non-donors with obesity had similar hypertension incidence. However, donors who gained >5% saw increased hypertension incidence vs. similar non-donors, providing support for counseling potential LKDs with obesity on weight management post-donation.
INTRODUCTION
The link between obesity (body mass index (BMI) ≥30 kg/m2) and hypertension is well-established in the general population.1–3 Over one-third of adults in the United States with obesity are estimated to have hypertension, compared to one-fifth among individuals with normal weight.4 Weight gain independent of baseline obesity is associated with increasing blood pressure and new-onset hypertension.5–8 Most individuals with obesity remain obese or gain weight over time,9,10 and cumulative exposure to obesity increases hypertension risk, with individuals with stable11 and longer duration of obesity12 experiencing higher blood pressure and hypertension risk.
Mirroring the general population and in response to the ongoing organ shortage, obesity prevalence among living kidney donors has increased.13 Obesity is associated with increased risk of not only hypertension14 but also diabetes and end-stage kidney disease (ESKD) in living donors.15–21 Donors with obesity also experience significant weight change over time,22 which is associated with as much as a 2-fold risk of new onset hypertension.23 It remains uncertain, however, whether reduction in nephron mass resulting from donor nephrectomy further modifies obesity-related hyperfiltration23 and thus long-term risk of hypertension, a primary risk factor for ESKD. Prior studies of hypertension risk comparing living donors to non-donor controls have conflicting findings. Two studies reported increased risk in living donors compared to healthy non-donors,24,25 while others have demonstrated lower26 or similar risk.16,27 One single-center study found similar prevalence of hypertension at follow-up for donors with obesity and non-donors from the general population, leading the authors to conclude that long term hypertension risk was attributable to obesity alone and not magnified by kidney donation.28
Notably no studies have explored both weight changes and hypertension risk attributable to living donation. These gaps limit the ability of transplant providers to adequately counsel potential donors with obesity about their long-term risks of donating, particularly in the context of weight change. Therefore, our objective was to explore risk of hypertension related to kidney donation and whether changes in BMI modify this risk.
METHODS
Study Design
We utilized a nested case-control design within a dataset constructed from two ongoing multi-center retrospective cohort studies of prior living kidney donors and the ongoing, prospective Coronary Artery Risk Development in Young Adults (CARDIA) and Atherosclerosis Risk in Communities (ARIC) studies.
Population
Donors
Donors were derived from two NIH-funded cohort studies (1R01DK113980, Locke; 1R01096008, Segev) and included prior living kidney donors with BMI ≥30 at donation between September 1976 and May 2020, representing 58 US transplant centers that performed 58.3% of all living kidney donor transplants in 2019. A map depicting national representation of donors and non-donor field centers can be found in the Supplement (Figure S1) and demonstrates nearly complete overlap of geographic representation of donors and non-donors. Enrollment occurred in a hybrid design: 1) donors at collaborating centers were sent a study recruitment letter by their transplant center; 2) donors at non-participating centers were sent a recruitment letter after we obtained a waiver of authorization and consent for donor contact information from the Organ Procurement and Transplantation Network (OPTN)/Scientific Registry of Transplant Recipients (SRTR). This study also used data from the SRTR. The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. This study was reviewed and approved by the UAB Institutional Review Board (IRB-300000039; IRB-131003001). All donor data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at UAB.29
Non-donors
Non-donors were included from two ongoing, longitudinal cohort studies: the Coronary Artery Risk Development in Young Adults (CARDIA) and the Atherosclerosis Risk in Communities (ARIC) studies (designs described elsewhere).30,31 Cohort members were assessed for donor eligibility at baseline (1985–1986) and exam years 10, 15, 20, and 25 in CARDIA and at baseline (1987–1989) and visits 2 and 4 in ARIC (3 and 6 years post-baseline respectively). ARIC Visit 3 was not used for eligibility, as measures of kidney function were not captured at this visit. As such, each CARDIA/ARIC participant could contribute up to five/three eligible observations, respectively. Each non-donor participant was considered a potential living donor at each exam, with exclusions made for comorbid disease or pregnancy at time of examination, as previously described.32 (Supplemental Methods)
Inclusion Criteria and Baseline Characteristics
Donors and non-donors with confirmed BMI ≥30 kg/m2 at evaluation (donors) or any exam meeting donor eligibility criteria (non-donors) were included if there was no evidence of pre-existing hypertension. Donor pre-operative characteristics were obtained from medical record abstraction and supplemented from the SRTR living donor file. Among donors, a systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg was considered to be “white coat hypertension” if only a single blood pressure measurement was available from evaluation and was not considered as an exclusion criterion in the primary analyses. Family history of hypertension and diabetes were defined as history among first-degree relatives (parents, siblings). Metabolic syndrome (MBS) was defined as presence of ≥2 of the following in the presence of obesity: 1) systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, 2) high density lipoprotein (HDL) <40 mg/dL (males) or <50 mg/dL (females), 3) triglycerides ≥150 mg/dL, and 4) elevated fasting glucose (≥100 mg/dL, ≥5.6 mmol/L, hemoglobin A1C ≥5.6%). Study enrollment year was defined as year of donation (donors) or year of relevant eligible exam (non-donors). Cohort CONSORT diagrams are found in Figures S2–4.
Outcome Ascertainment
New-onset hypertension (cases) for all individuals was defined as presence of ≥1 of the following: two consecutive blood pressure measurements on unique days of ≥140 mmHg systolic or ≥90 mmHg diastolic, per the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) guidelines,33(the JNC7 definition was utilized given that treatment decisions for all of these cohorts would have been based on prior criteria and to be consistent with the 2017 Kidney Disease: Improving Global Outcomes (KDIGO) threshold for living donor candidates34 ), self-reported hypertension diagnosis, or self-report of medication typically indicated for hypertension. For donors only, we also used report of new hypertension in the SRTR living donor follow-up file and post-donation medical record abstraction. Given that precise time of hypertension onset could not be delineated retrospectively, individuals with evidence of hypertension were assigned a time interval, such that their left observation time was the last time they were known to be hypertension-free and their right time was the earliest of 1) date of second consecutive elevated blood pressure measurement, 2) date of first evidence of hypertension in the medical record, 3) self-reported year or age of hypertension diagnosis, 4) or survey date if self-reported year was unavailable.
Statistical Analysis
Matching
To account for differential follow-up and BMI change as a time-dependent variable, we utilized a nested case-control design in R packages Epi (ver 2.44) and Matchit (ver 4.1.0), matching each case (individual with new-onset hypertension) to up to four controls. Given that cases spent a period of time disease-free, they could also contribute records as controls. We used exact matching on sex and race (Black/non-Black) and caliper matching on age (±3 years), BMI (±2 units), and systolic/diastolic blood pressure (±5 units) at eligible study entry. Case follow-up time was assigned at the midpoint of the observation time interval (midpoint of baseline to first evidence of hypertension for those with no intervening outcome data or midpoint of last time known to be hypertension-free to first evidence of hypertension). Individuals were available to be matched if they were hypertension-free at an assigned case follow-up time (i.e., if an individual was hypertension-free until 10 years, they could serve as a control for a case whose onset time was 7.5 years). Baseline characteristics were compared for matched cases vs. controls, using medians and Wilcoxon signed-rank tests for continuous measurements and proportions and chi-square tests for categorical variables.
BMI Change (Figure S5)
Analyses incorporating BMI change included only participants with ≥2 BMI measurements. The nested case-control design allowed for time-dependent BMI for controls corresponding to the assigned onset time of the case. For those with BMI measurements on either side of hypertension onset (cases) or matched study time (controls), final BMI was interpolated from the two adjacent measurements. For those with no BMI measurements after onset/matched study time, final available BMI was used provided it was <2 years prior to onset/matched study time. Those whose final available BMI measurement occurred >2 years prior to onset/matched study time were excluded. Percent change in BMI was then calculated using matched time BMI relative to BMI from eligible study entry. Participants were grouped by BMI change using a cutpoint of >5% change in either direction, compared to those considered stable (change ≤5%), as a 5% change is a clinically meaningful threshold for tertiary prevention of hypertension in patients with obesity35 and is also recommended by The Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines as the target to decrease risk for development of obesity-related conditions and cardiovascular risk factors.36
Modeling
Using conditional logistic regression with a strata statement accounting for matched risk sets and adjusting for study enrollment year, we estimated incidence rate ratios (IRR)37 for new-onset hypertension by donor status and BMI change category among matched cases and controls. The IRR is appropriate for reporting measures of effect in the nested case-control design. Within the conditional logistic regression model, estimates for donors vs. non-donors were obtained by first estimating the effect within a risk set (“strata”) in which all participants were balanced on evaluation/eligible exam age, race, sex, BMI, and blood pressure, then averaging strata-specific effects across all strata to obtain one singular measure of association.
Exploration of Effect Modification
To investigate whether the magnitude of the association between donation and incident hypertension differed by BMI change, we utilized first-degree interaction terms for interaction on the multiplicative scale and relative excess risk due to the interaction (RERI) and the attributable proportion of risk due to the interaction (AP) for interaction on the additive scale.38 We produced margins around the predicted probabilities to compare donor status/BMI change.
Sensitivity Analyses
To account for donors with limited or missing post-donation medical record data, we excluded donors with only post-donation registry data. We also excluded donors we defined as having white coat hypertension. We next set the threshold for BMI change at 7.5%, such that the stable group included those whose BMI changed by <7.5%. We also re-ran the nested case-control design randomly selecting one exam per non-donor. We adjusted for additional risk factors of history of smoking and family history of hypertension in 20 imputed datasets generated using multiple imputation using chained equations. All inferences were consistent, thus we present findings from the primary analyses.
All analyses were performed in SAS 9.4 (Cary, NC), R version 4.0.2 (R Core Team, 2020), and Stata 15.1 (College Station, TX), with significance set at p <0.05.
RESULTS
Eligible cohort and follow-up time
We identified 1,558 obese donors and 3,783 obese non-donors eligible for matching cases to controls. Median follow-up was 8.1 years (IQR: 3.8–14.9; donors: 3.6 years (IQR: 2.0–9.4); non-donors: 9.2 years (IQR: 5.3–15.8)). When excluding donors with only registry data post-donation, median donor follow-up was 8.0 years (IQR: 4.0–12.2).
Demographics in the matched dataset
After matching individuals with new-onset hypertension (cases) to controls that were hypertension-free at the same time, 1,990 cases (176 unique donors; 709 unique non-donors) were matched to 6,490 controls (656 unique donors; 2,222 unique non-donors). Unique donors were compared to non-donors within matched risk sets and were found to be statistically significantly different for most variables, including systolic blood pressure, serum creatinine, family history of hypertension, and history of smoking (Table S1). Cases and controls were well-balanced, with no statistically significant differences in age, sex, diastolic blood pressure, fasting glucose, smoking history, or prevalence of MBS (Table 1). Cases and controls were significantly different with regards to race, BMI, systolic blood pressure, and serum creatinine, though differences were small. Comparisons were similar in the subset with ≥2 BMIs, and median BMI change among cases was 2.4% (IQR: −0.5, 7.1) and 1.3% (IQR: −1.2, 5.5) among matched controls (p<0.001) (Tables S2–S3).
Table 1.
Case vs. control matching, 1 case to up to 4 controls (among all matches)*
| Cases (new HTN) | Controls (no new HTN at time of matching) | p | |
|---|---|---|---|
| N= 1,990 | N= 6,490 | ||
| Age in years, median (IQR) | 49.0 (41.0–55.0) | 49.3 (42.8–55.0) | 0.13 |
| BMI in kg/m2, median (IQR) | 32.5 (31.1–34.8) | 32.3 (31.1–34.1) | 0.002 |
| WHO class, N(%) | |||
| Class I (30–34.9) | 1,518 (76.3) | 5,272 (81.2) | < 0.001 |
| Class II (35–39.9) | 362 (18.2) | 997 (15.4) | |
| Class III (40+) | 110 (5.5) | 221 (3.4) | |
| Male sex, N(%) | 758 (38.1) | 2,531 (39.0) | 0.47 |
| Black Race, N(%) | 661 (33.2) | 1,923 (29.6) | 0.002 |
| Systolic BP, median (IQR) | 118.0 (111.0–126.0) | 118.0 (110–125.0) | 0.02 |
| Diastolic BP, median (IQR) | 74.0 (69.0–79.0) | 74.0 (69.0–78.0) | 0.29 |
| MAP, median (IQR) | 88.7 (83.3–94.0) | 88.3 (83.3–93.3) | 0.08 |
| BP status, N(%) | |||
| Normotensive | 994 (50.0) | 3,410 (52.5) | 0.04 |
| Pre-hypertensivea | 969 (48.7) | 3,020 (46.5) | |
| White coat hypertensionb | 27 (1.4) | 60 (0.9) | |
| Serum creatinine, median (IQR) c | 0.73 (0.63–0.85) | 0.73 (0.63–0.88) | 0.002 |
| eGFR, median (IQR) c | 105.0 (96.4–114.0) | 103.0 (96.0–112.0) | < 0.001 |
| Fasting blood glucose, median (IQR) d | 97.3 (90.7–105.0) | 97.3 (90.5–104.6) | 0.43 |
| Impaired fasting glucose (FBG >= 100–125 or A1c >=5.6–6.9), N(%)e | 739 (42.7) | 2,378 (43.5) | 0.58 |
| Family history of HTN, N(%)f | 1,031 (57.4) | 2,917 (52.1) | < 0.001 |
| Family history of diabetes, N(%)g | 595 (35.4) | 1,890 (36.7) | 0.31 |
| HDL, median (IQR)h | 46.0 (37.6–55.0) | 46.2 (38.0–55.9) | 0.22 |
| Triglycerides, median (IQR)i | 111.0 (77.0–159.5) | 107.0 (75.0–158.0) | 0.03 |
| History of high cholesterol, median (IQR)j | 126 (14.4) | 472 (15.3) | 0.50 |
| Ever smoked, N(%)k | 900 (46.0) | 2,764 (43.6) | 0.06 |
| Metabolic syndrome, N(%)l | 738 (42.7) | 2,251 (40.9) | 0.19 |
| Living donor | 322 (16.2) | 1,284 (19.8) | < 0.001 |
Observations are not unique, as a case may serve as a control if matched on a previous event-free time
Bold indicates significance at p < 0.05
Systolic blood pressure 130–139 mmHg, diastolic blood pressure 80–89 mmHg
includes individuals who had a single BP measurement that was elevated among donors
missing for 1 case and 5 controls
missing for 12.9% of donors and 15.5% of controls
missing for 9.8% of cases and 13.7% of controls
missing for 15.5% of cases and 20.7% of controls
missing for 12.3% of cases and 14.7% of controls
missing for 11.6% of cases and 14.1% of controls
missing for 55.9% of cases and 52.3% of controls
missing for 1.7% of cases and 2.2% of controls
missing for 13.1% of cases and 15.2% of controls
- exact matching on sex and race
- caliper matching on evaluation/ exam meeting donor eligibility criteria: age (+/− 3 years), BMI (+/− 2 units), systolic and diastolic BP (+/− 5 units)
Conditional logistic regression modeling
In a model among all matched cases and controls before restricting to those with ≥2 BMI measurements, donors demonstrated a similar hypertension incidence compared to non-donors (IRR: 1.16, 95%CI: 0.94–1.43, p=0.16 [Table 2]). These findings were consistent after restricting the model to those with ≥2 BMIs and adjusting for BMI change (IRR: 1.25, 95%CI: 0.99–1.58, p=0.05). Independent of donor status, individuals with a >5% decrease in BMI had a significantly lower hypertension incidence (148/790) compared to those with stable BMI (1,119/4,891, IRR: 0.74, 95%CI: 0.61–0.91, p=0.004), while those experiencing a >5% BMI gain had a significantly higher incidence of hypertension (643/2,244, IRR: 1.33, 95%CI: 1.17–1.52, p< 0.001).
Table 2.
Incidence rate ratios for new onset hypertension
| Match 1:4 on race, sex, age, BMI, BP | Model 2 dropping risk sets where the case has < 2 BMIs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | p | IRR | 95% CI | p | IRR | 95% CI | p | |
| Donor vs. non-donor | 1.16 | 0.94–1.43 | 0.16 | 1.25 | 0.99–1.58 | 0.05 | |||
| Stable (change ≤5%) | Ref | ||||||||
| Lost >5% | 0.74 | 0.61–0.91 | 0.004 | ||||||
| Gain >5% | 1.33 | 1.17–1.52 | < 0.001 | ||||||
| Enrollment year | 0.97 | 0.96–0.98 | < 0.001 | 0.97 | 0.96–0.98 | < 0.001 | 0.97 | 0.96–0.98 | < 0.001 |
| Stable donor (142/741) vs. stable non-donor (977/4,150) | 1.12 | 0.86–1.46 | 0.39 | ||||||
| Donor loss >5% (22/178) vs. stable non-donor | 0.62 | 0.38–1.03 | 0.06 | ||||||
| Donor gain >5% (101/315) vs. stable non-donor | 2.27 | 1.66–3.09 | < 0.001 | ||||||
| Non-donor loss >5% (126/612) vs. stable non-donor | 0.80 | 0.64–1.00 | 0.05 | ||||||
| Non-donor gain >5% (542/1,929) vs. stable non-donor | 1.24 | 1.08–1.43 | 0.003 | ||||||
| Donor loss >5% vs. non-donor loss >5% | 0.78 | 0.46–1.34 | 0.36 | ||||||
| Donor gain >5% vs. non-donor gain >5% | 1.83 | 1.32–2.53 | < 0.001 | ||||||
| Observations | 8,480 | 7,925 | 7,925 | ||||||
| Donors | 832 | 720 | 720 | ||||||
| Non-donors | 2,931 | 2,915 | 2,915 | ||||||
| Relative excess risk due to the interaction (RERI) | 0.90 | 0.24–1.56 | 0.007 | ||||||
| Attributable proportion | 0.40 | 0.20–0.59 | < 0.001 | ||||||
Risk attributable to donation and effect modification by BMI change
When examining the combined relationships of BMI change and risk attributable to donation, hypertension incidence did not differ significantly for donors with stable BMI or those who lost >5% BMI compared to BMI-stable non-donors (stable IRR: 1.12, 95%CI: 0.86–1.46, p=0.39; >5% loss IRR:0.62, 95%CI: 0.38–1.03, p=0.06). Donors experiencing a >5% decrease in BMI had a similar hypertension incidence (22/178) compared to non-donors with comparable BMI loss (126/612, IRR: 0.78, 95%CI: 0.46–1.34, p=0.36) (Table 2). We observed similar associations when stratifying by race (Table 2b).
Table 2b.
Incidence rate ratios for new onset hypertension stratified by race
| Among Blacks | Among non-Blacks | ||||||
|---|---|---|---|---|---|---|---|
| IRR | 95% CI | p | IRR | 95% CI | p | ||
| Stable donor (17/82) vs. stable ND (356/1,449) | 1.34 | 0.73–2.45 | 0.35 | Stable donor (125/659) vs. stable ND (621/2,701) | 0.98 | 0.72–1.33 | 0.90 |
| Donor loss >5% (4/28) vs. stable ND | 0.77 | 0.25–3.40 | 0.65 | Donor loss >5% (18/150) vs. stable ND | 0.53 | 0.30–0.94 | 0.03 |
| Donor gain >5% (15/39) vs. stable ND | 3.08 | 1.45–6.54 | 0.003 | Donor gain >5% (86/276) vs. stable ND | 1.99 | 1.36–2.76 | < 0.001 |
| ND loss >5% (42/203) vs. stable ND | 0.77 | 0.52–1.13 | 0.18 | ND loss > 5% (84/409) vs. stable ND | 0.81 | 0.62–1.06 | 0.13 |
| ND gain >5% (219/730) vs. stable ND | 1.26 | 0.99–1.61 | 0.06 | ND gain >5% (323/1,119) vs. stable ND | 1.23 | 1.03–1.46 | 0.02 |
| Donor loss >5% vs. ND loss > 5% | 1.00 | 0.30–3.30 | 0.99 | Donor loss >5% vs. ND loss > 5% | 0.66 | 0.36–1.21 | 0.18 |
| Donor gain >5% vs. ND gain >5% | 2.44 | 1.13–5.26 | 0.02 | Donor gain >5% vs. ND gain >5% | 1.58 | 1.08–2.29 | 0.02 |
| Enrollment year | 0.96 | 0.94–0.97 | < 0.001 | Enrollment year | 0.98 | 0.97–0.99 | 0.001 |
| Observations | 2,531 | Observations | 5,394 | ||||
| Donors | 96 | Donors | 624 | ||||
| Non-donors | 1,189 | Non-donors | 1,726 | ||||
Presence of both donation and BMI gain was associated with greater incidence of hypertension compared to BMI-stable non-donors (IRR 2.27, 95%CI: 1.66–3.09, p< 0.001). There was evidence of a positive interaction on the multiplicative (IRR: 1.62, 95%CI: 1.15–2.29, p=0.006) and additive scales (RERI: 0.90, 95%CI: 0.24–1.56, p=0.007; attributable proportion=0.40, 95%CI: 0.20–0.59, p<0.001), such that donors with >5% gain in BMI saw significantly higher hypertension incidence compared to non-donors with similar gain (IRR: 1.83, 95%CI: 1.32–2.53, p<0.001 (Table 2)). The marginal probability by BMI change and donor status is presented in Figure 1. We observed similar associations when adjusting for family history and smoking in 20 imputed datasets (Table S4).
Figure 1.
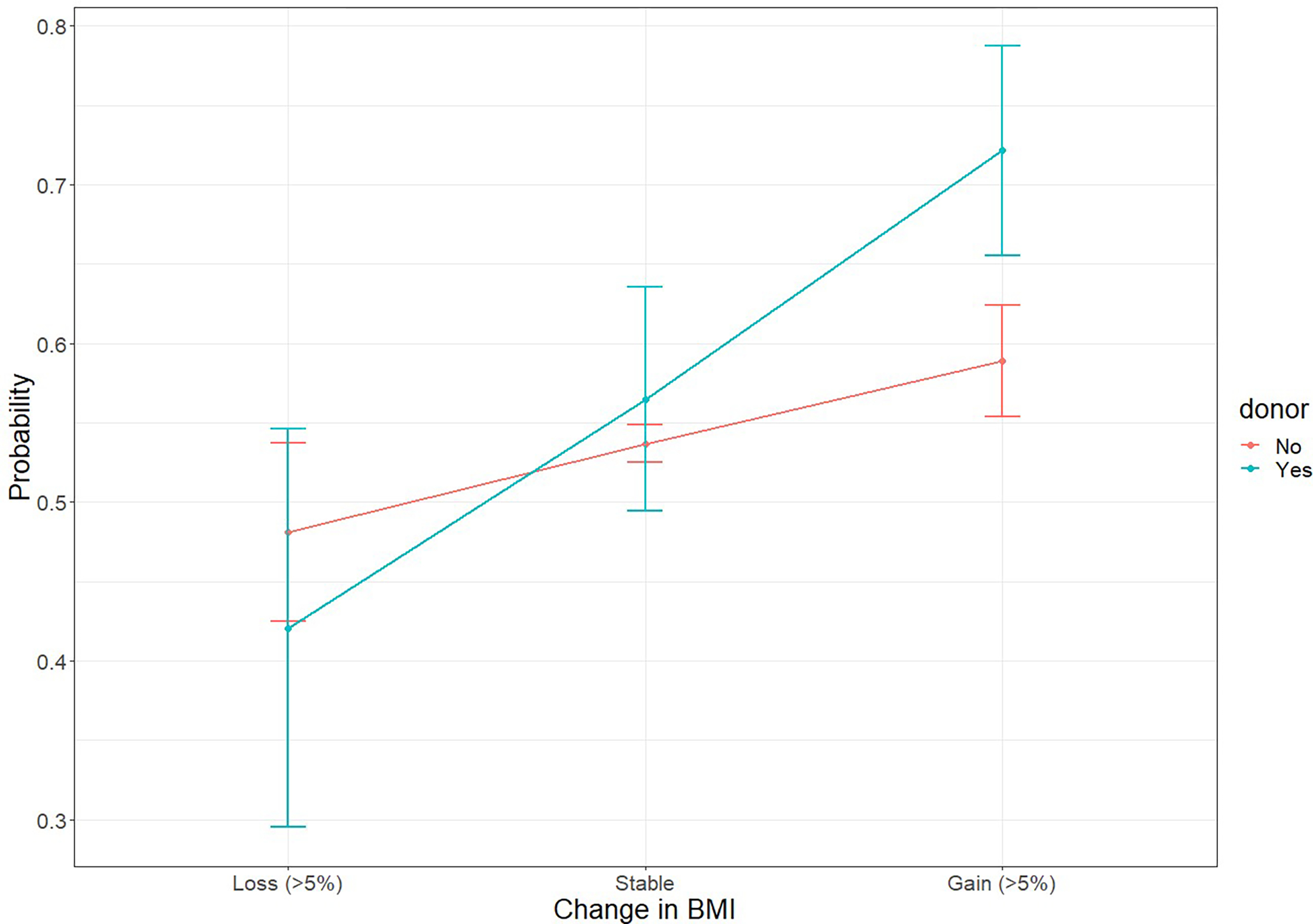
Average adjusted predicted probability from conditional logistic regression model fit
DISCUSSION
In this nationally representative, multi-center study of the largest cohort of living donors with obesity to date, we found overall donors experienced new-onset hypertension at a rate similar to non-donors. Compared to non-donors who lost >5% BMI or whose BMI remained stable, there was no significant difference in hypertension onset. However, among those experiencing a >5% gain in BMI, incidence of hypertension was greater for donors than similar non-donors.
The finding that overall donors with obesity had hypertension incidence similar to non-donors with obesity suggests that uninephrectomy in the setting of obesity is not in and of itself associated with increased risk of post-donation hypertension, and that a BMI of ≥30 should not be an isolated factor for determining candidacy. Moreover, we saw that both donors and non-donors whose BMI decreased by >5% had hypertension incidence similar to BMI-stable non-donors, consistent with findings from the general population that weight loss reduces risk of hypertension.39,40 However, donors who gained weight had greater hypertension incidence than similar non-donors. These findings, in conjunction with the known association between obesity-induced hyperfiltration and microalbuminuria linked to essential hypertension41 and ultimately ESKD, further motivate counseling donors on the risks of additional weight gain post-donation. While all living donors with obesity should be encouraged to maintain a healthy lifestyle, these findings are the first to quantify post-donation hypertension risk in this subset of donors and underscore the avoidance of significant increases in weight after donation.
Prior studies comparing donors and non-donors have shown similar to increased risk for hypertension, but lacked the granularity needed to explore subsets of donors at greatest risk, in particular those experiencing weight change.24,25,28 In our analyses of obese individuals accounting for BMI changes over time, we found the greatest risk of new-onset hypertension among donors experiencing clinically significant weight gain. These findings highlight the need for prospective studies of donors capturing time-updated clinical and laboratory measurements, in addition to policy changes regarding timing and frequency of post-donation engagement with living donors. Currently, donor follow-up is suboptimal, with centers required to follow donors for only two years post-donation. Moreover, there is a higher likelihood of incomplete or non-timely follow-up by donors with obesity at donation.42–44 For donors with obesity, follow-up encounters could be used for weight loss counseling and healthy lifestyle promotion. Given that donors with obesity report willingness to lose weight for donation,45 future studies could explore donor perceptions of post-donation weight loss counseling and interventions to maintain healthy weight long-term. It is also important to note no studies of weight change among donors have explored risk factors for gaining weight.
The American Association of Clinical Endocrinologists and American College of Endocrinology identified a threshold of 5–15% weight loss as necessary for tertiary prevention of hypertension in patients with obesity.35 Our findings that both donors and non-donors with obesity who lost >5% body mass had lower hypertension incidence compared with those who were weight stable was consistent with this clinical threshold and can serve as a tangible goal for transplant centers when counseling donors with obesity on weight loss. This is particularly relevant, given that obesity is one of the few risk factors that can be targeted for modification in all stages of the donation process.
Previous studies have reported increased risk of hypertension for Black donors compared to non-Black donors, irrespective of baseline BMI or weight change.24,46 In our cohort, the magnitude of the association between donor status and BMI change with hypertension onset was similar across races, suggesting a more intense level of scrutiny may be applied to Black individuals with obesity seeking to donate a kidney. Non-Hispanic Black adults have the highest age-adjusted prevalence of obesity (49.6% vs. non-Hispanic Whites at 42.2%).47 With potential donors often drawn from the same social network as the recipient48 and Blacks more often turned down for donation,48,49 this selectivity potentially exacerbates known issues in access to living donor transplantation among Black candidates. Further work to identify a donor profile that balances donor safety and autonomy without discouraging Black candidates from donating is critical.
While this is the first analysis to explore hypertension incidence in the context of both kidney donation and weight change among obese individuals, this study has limitations. We were unable to explore a dose effect with baseline BMI due to small cell sizes in the strata with greater classes of obesity. The nested case-control design precluded us from estimating an overall summary of BMI change by donor type, given that the change was estimated based on assigned study time in the nested design. While all donor/non-donor estimates within the conditional logistic regression model were based on comparisons within strata balanced on age, race, sex, BMI, and blood pressure, the possibility remains for residual confounding. Cases and controls within risk strata were well-balanced on covariates not in the matching algorithm, however, thereby accounting for persistent differences between donors and non-donors in adjusted analyses. Donors had less follow-up time and thus the possibility for biased realization of outcomes, but we utilized the nested case-control design to account for and match on follow-up time. Non-donors in our analyses were drawn from large cohorts with standardized measurements at regular intervals; we utilized midpoint of time intervals for disease onset to account for this differential observation time, frequency, and outcome ascertainment. However, given that exact timing of hypertension onset was unknown and likely fell between study visits for non-donors and primary care visits for donors, this should not have created differential misclassification by donor type. Non-donors also underwent research-grade, standardized measurement of blood pressure, while we relied on primary care records for longitudinal blood pressure measurements among donors. We created a composite definition that incorporated evidence of hypertension from multiple sources, so as not to rely solely on differentially-measured blood pressure. Furthermore, this study did not distinguish between obesity type (central vs. peripheral), which contribute differentially to cardiovascular and metabolic risk. Finally, these non-donors, while selected using criteria for eligibility for kidney donation, have not undergone the same rigorous evaluation process. Our analyses underscore the need for both retrospective and prospective follow-up of individuals evaluated and approved for living donation who did not ultimately donate as the most appropriate group with which to compare donors, one of the stated goals of the Living Donor Collective,50 which is still in development.
Among this nationally representative cohort of individuals with obesity, donors and non-donors experienced similar hypertension incidence overall, but we observed increased risk among donors with clinically significant weight gain. As the transplant community continues to manage donor selection in the face of the organ shortage and disparities in access, we are reassured that overall donors with obesity did not experience a greater rate of new-onset hypertension compared to non-donors with obesity. Throughout the donation process and beyond, living donors should be counseled on the importance of weight management, with particular attention paid to the care of donors with significant weight gain post-donation.
Supplementary Material
ACKNOWLEDGEMENTS
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
FUNDING
This work was supported by the National Institutes of Health grant numbers 1R01DK113980 (PI: Locke), K23DK103918 (PI: Locke), and 1R01096008 (PI: Segev). The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI). The ARIC (Atherosclerosis Risk in Communities) study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Conflicts of Interest: J. Locke has grant funding from United Therapeutics, honoraria from Sanofi and Novartis, clinical trial with Hansa, and non-monetary relationships with the FDA and DaVita. For the remaining authors, none were declared.
REFERENCES
- 1.Wilson PW, D’Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. Sep 9 2002;162(16):1867–1872. [DOI] [PubMed] [Google Scholar]
- 2.Okosun IS, Prewitt TE, Cooper RS. Abdominal obesity in the United States: prevalence and attributable risk of hypertension. J Hum Hypertens. Jul 1999;13(7):425–430. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Brand N, Skinner JJ, Jr., et al. The relation of adiposity to blood pressure and development of hypertension. The Framingham study. Ann Intern Med. Jul 1967;67(1):48–59. [DOI] [PubMed] [Google Scholar]
- 4.Saydah S, Bullard KM, Cheng Y, et al. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999–2010. Obesity (Silver Spring). Aug 2014;22(8):1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams PT. Increases in weight and body size increase the odds for hypertension during 7 years of follow-up. Obesity (Silver Spring). Nov 2008;16(11):2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truesdale KP, Stevens J, Cai J. Effect of 3-year weight history on blood pressure: the atherosclerosis risk in communities study. Obesity (Silver Spring). May 2008;16(5):1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juhaeri Stevens J, Chambless LE, et al. Associations between weight gain and incident hypertension in a bi-ethnic cohort: the Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. Jan 2002;26(1):58–64. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa-Takata K, Ohta T, Moritaki K, et al. Obesity, weight change and risks for hypertension, diabetes and hypercholesterolemia in Japanese men. Eur J Clin Nutr. Jul 2002;56(7):601–607. [DOI] [PubMed] [Google Scholar]
- 9.Iyen B, Weng S, Vinogradova Y, et al. Long-term body mass index changes in overweight and obese adults and the risk of heart failure, cardiovascular disease and mortality: a cohort study of over 260,000 adults in the UK. BMC Public Health. Apr 15 2021;21(1):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fildes A, Charlton J, Rudisill C, et al. Probability of an Obese Person Attaining Normal Body Weight: Cohort Study Using Electronic Health Records. Am J Public Health. Sep 2015;105(9):e54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuo T, Sairenchi T, Suzuki K, et al. Long-term stable obesity increases risk of hypertension. Int J Obes (Lond). Aug 2011;35(8):1056–1062. [DOI] [PubMed] [Google Scholar]
- 12.Norris T, Cole TJ, Bann D, et al. Duration of obesity exposure between ages 10 and 40 years and its relationship with cardiometabolic disease risk factors: A cohort study. PLoS Med. Dec 2020;17(12):e1003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naik AS, Cibrik DM, Sakhuja A, et al. Temporal trends, center-level variation, and the impact of prevalent state obesity rates on acceptance of obese living kidney donors. Am J Transplant. Mar 2018;18(3):642–649. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim HN, Murad DN, Hebert SA, et al. Intermediate Renal Outcomes, Kidney Failure, and Mortality in Obese Kidney Donors. J Am Soc Nephrol. Nov 2021;32(11):2933–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holscher CM, Bae S, Thomas AG, et al. Early Hypertension and Diabetes After Living Kidney Donation: A National Cohort Study. Transplantation. Jun 2019;103(6):1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. Jan 29 2009;360(5):459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bello RC, Bello VA, Rosa TT, et al. Male Gender and Body Mass Index Are Associated With Hypertension and Reduced Kidney Function 5 or More Years After Living Kidney Donation. Transplant Proc. Dec 2015;47(10):2816–2821. [DOI] [PubMed] [Google Scholar]
- 18.Lentine KL, Koraishy FM, Sarabu N, et al. Associations of obesity with antidiabetic medication use after living kidney donation: An analysis of linked national registry and pharmacy fill records. Clin Transplant. Oct 2019;33(10):e13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locke JE, Reed RD, Massie A, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. Mar 2017;91(3):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serrano OK, Sengupta B, Bangdiwala A, et al. Implications of excess weight on kidney donation: Long-term consequences of donor nephrectomy in obese donors. Surgery. Nov 2018;164(5):1071–1076. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim HN, Kukla A, Cordner G, et al. Diabetes after kidney donation. Am J Transplant. Feb 2010;10(2):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bugeja A, Harris S, Ernst J, et al. Changes in Body Weight Before and After Kidney Donation. Can J Kidney Health Dis. 2019;6:2054358119847203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. Apr 2001;59(4):1498–1509. [DOI] [PubMed] [Google Scholar]
- 24.Doshi MD, Goggins MO, Li L, et al. Medical outcomes in African American live kidney donors: a matched cohort study. Am J Transplant. Jan 2013;13(1):111–118. [DOI] [PubMed] [Google Scholar]
- 25.Holscher CM, Haugen CE, Jackson KR, et al. Self-Reported Incident Hypertension and Long-Term Kidney Function in Living Kidney Donors Compared with Healthy Nondonors. Clin J Am Soc Nephrol. Oct 7 2019;14(10):1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentine KL, Schnitzler MA, Garg AX, et al. Understanding antihypertensive medication use after living kidney donation through linked national registry and pharmacy claims data. Am J Nephrol. 2014;40(2):174–183. [DOI] [PubMed] [Google Scholar]
- 27.El-Agroudy AE, Sabry AA, Wafa EW, et al. Long-term follow-up of living kidney donors: a longitudinal study. BJU Int. Dec 2007;100(6):1351–1355. [DOI] [PubMed] [Google Scholar]
- 28.Tavakol MM, Vincenti FG, Assadi H, et al. Long-term renal function and cardiovascular disease risk in obese kidney donors. Clin J Am Soc Nephrol. Jul 2009;4(7):1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. Apr 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 31.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. Apr 1989;129(4):687–702. [PubMed] [Google Scholar]
- 32.Locke JE, Sawinski D, Reed RD, et al. Apolipoprotein L1 and Chronic Kidney Disease Risk in Young Potential Living Kidney Donors. Ann Surg. Jun 2018;267(6):1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. May 21 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 34.Lentine KL, Kasiske BL, Levey AS, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. Aug 2017;101(8S Suppl 1):S1–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract. Jul 2016;22 Suppl 3:1–203. [DOI] [PubMed] [Google Scholar]
- 36.American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring). Jul 2014;22 Suppl 2:S5–39. [DOI] [PubMed] [Google Scholar]
- 37.Labrecque JA, Hunink MMG, Ikram MA, et al. Do Case-Control Studies Always Estimate Odds Ratios? Am J Epidemiol. Feb 1 2021;190(2):318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanderWeele TJKM. A Tutorial on Interaction. Epidemiol. Methods. 2014. [Google Scholar]
- 39.Moore LL, Visioni AJ, Qureshi MM, et al. Weight loss in overweight adults and the long-term risk of hypertension: the Framingham study. Arch Intern Med. Jun 13 2005;165(11):1298–1303. [DOI] [PubMed] [Google Scholar]
- 40.Poorolajal J, Hooshmand E, Bahrami M, et al. How much excess weight loss can reduce the risk of hypertension? J Public Health (Oxf). Sep 1 2017;39(3):e95–e102. [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Evans JC, Meigs JB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. Mar 22 2005;111(11):1370–1376. [DOI] [PubMed] [Google Scholar]
- 42.Henderson ML, Thomas AG, Shaffer A, et al. The National Landscape of Living Kidney Donor Follow-Up in the United States. Am J Transplant. Dec 2017;17(12):3131–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed RD, Shelton BA, MacLennan PA, et al. Living Kidney Donor Phenotype and Likelihood of Postdonation Follow-up. Transplantation. Jan 2018;102(1):135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schold JD, Buccini LD, Rodrigue JR, et al. Critical Factors Associated With Missing Follow-Up Data for Living Kidney Donors in the United States. Am J Transplant. Sep 2015;15(9):2394–2403. [DOI] [PubMed] [Google Scholar]
- 45.Mustian MN, Hanaway M, Kumar V, et al. Patient Perspectives on Weight Management for Living Kidney Donation. J Surg Res. Dec 2019;244:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. Aug 19 2010;363(8):724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. Feb 2020(360):1–8. [PubMed] [Google Scholar]
- 48.Weng FL, Dhillon N, Lin Y, et al. Racial differences in outcomes of the evaluation of potential live kidney donors: a retrospective cohort study. Am J Nephrol. 2012;35(5):409–415. [DOI] [PubMed] [Google Scholar]
- 49.Kumar K, Tonascia JM, Muzaale AD, et al. Racial differences in completion of the living kidney donor evaluation process. Clin Transplant. Jul 2018;32(7):e13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasiske BL, Asrani SK, Dew MA, et al. The Living Donor Collective: A Scientific Registry for Living Donors. Am J Transplant. Dec 2017;17(12):3040–3048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


