Abstract
Currently, the prevalence of gastroesophageal junction adenocarcinoma (GEJAC) is increasing in both Asian and Western countries, although the increasing rate in Asian countries is much slower than in Western countries. With these current trends, concerns regarding the surgical treatment method are also increasing among gastrointestinal surgeons. However, the surgical treatment for GEJAC has been a controversial issue for a long time due to the relative scarcity of this tumor and its characteristics from its borderline location. Recently, a large-scale prospective study of this tumor has been conducted in Japan, and the results are now available. The results of this study will be helpful for understanding this tumor. In this article, the pattern of lymph node metastasis of GEJAC is reviewed, and the extent and method of lymph node dissection for this tumor are discussed and proposed based on the review.
Keywords: Gastroesophageal junction, Adenocarcinoma, Lymph node dissection
INTRODUCTION
The worldwide incidence of gastroesophageal junction adenocarcinoma (GEJAC) has been rapidly increasing during the last two to three decades, although such an increase in the incidence in Asian countries is not as high as that in Western countries [1,2,3,4,5]. As the prevalence of this tumor is increasing, concerns regarding its surgical treatment are also increasing among gastrointestinal surgeons. However, the extent of lymph node dissection in GEJAC surgery has been controversial over the last several decades [6]. The main reasons for this long debate are the relative scarcity of this tumor compared to esophageal adenocarcinoma (EAC) in the West and gastric adenocarcinoma (GAC) in the East. Therefore, most evidence regarding GEJAC is an extrapolation of studies mainly designed for EAC or GAC. Second, the characteristics of GEJAC are derived from its borderline location between the esophagus and stomach, of which the lymphatic flow in this region can be bidirectional.
In the Siewert classification [7], GEJAC is classified into types I, II, and III; however, only type II tumors are true GEJAC. In this review, the definition and characteristics of GEJAC anatomy and lymphatic flow, the rate of lymph node metastasis, and the current opinion on the extent and method of lymphadenectomy in GEJAC surgery will be discussed, mainly focusing on Siewert type II tumors, the true GEJAC.
DEFINITION OF GEJ AND CLASSIFICATION OF GEJAC
The GEJ is where the tubular esophagus ends and the cardia of the stomach starts, but its exact location is quite complex. Most of the confusion regarding this subject may be attributed to the fact that the GEJ can be defined in several ways: anatomically, physiologically, and histologically [8]. The GEJ may be better recognized as a zone where the cardiac glands exist rather than a point. Histologically, the cardiac gland area is found to straddle the GEJ approximately 1 cm proximal and 2 cm distal to the GEJ [9]. The cardiac gland is believed to be congenitally located in the GEJ zone and secretes mucin, which is predominantly neutral, with a small amount of sialomucin. This type of mucin seems appropriate and is secreted in a transitional zone between neutral and strongly acidic environments. Endoscopically, it is recognized as the proximal end of the gastric mucosal fold in Europe and the USA and the distal end of the lower esophageal palisade vessels in Japan [10]. However, these endoscopic landmarks can be obscured, and exact localization of the GEJ may be difficult when there is a hiatal hernia or if the tumor is large and advanced [11].
GEJAC was classified by Siewert et al. [7] into three different types based on the anatomic location of the tumor epicenter or, in patients with an advanced tumor, the location of more than two-thirds of the tumor mass in relation to the GEJ: Siewert type I, located 1–5 cm above the GEJ regardless of invasion to the GEJ; Siewert type II, invading the GEJ and located within 1 cm above and 2 cm below the GEJ; and Siewert type III, invading the GEJ and located 2–5 cm below the GEJ. Type I tumor is the distal EAC and thus can be treated by an esophagectomy, like in EAC treatment, and type III tumor is GAC originating from the subcardial region and can be treated by a total gastrectomy, similar to GAC treatment. Type II tumors are true GEJ tumors that can be treated with either esophagectomy or total gastrectomy [7,12]. The main purpose of this classification was to determine the treatment strategy based on the type and extent of resection according to the tumor location. Therefore, it is important to diagnose the precise tumor location during preoperative workup. However, it is often difficult to make a precise diagnosis regarding the exact tumor location in relation to the GEJ, especially in advanced, large tumors. Several pieces of information, including the findings of endoscopy with orthograde and retroflexed view of the GEJ, CT scan, and barium contrast radiography of the entire esophagus and the GEJ, should be analyzed together to make a precise diagnosis of the location of the tumor. The diagnosis can be changed after obtaining surgical findings. In advanced tumors, barium contrast radiography findings may help establish a precise diagnosis preoperatively [7].
LYMPH NODE METASTASIS OF GEJAC
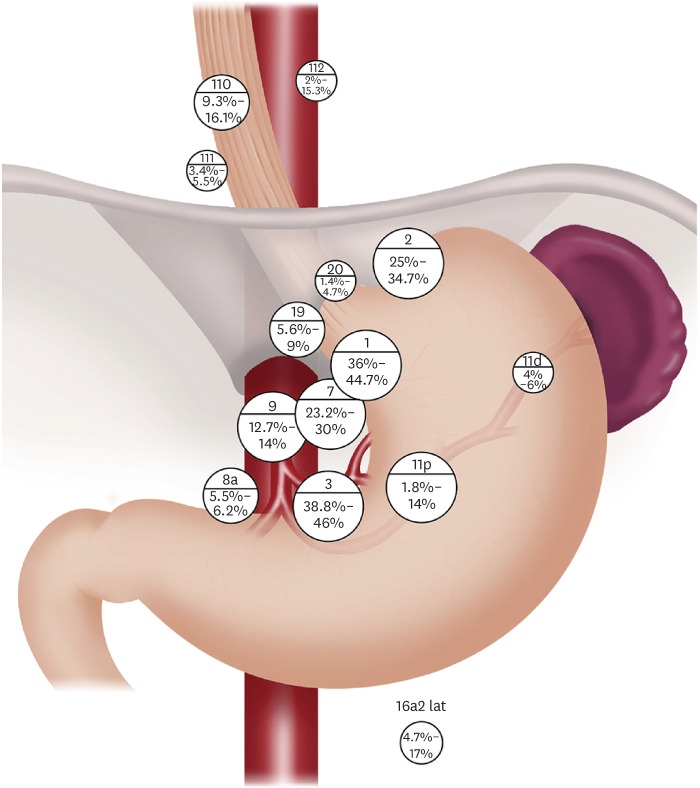
Highly frequent sites of lymph node metastasis, which are consistently observed in several large-scale studies of GEJAC [13,14,15,16], including one prospective study that included a small portion (8.5%) of squamous cell carcinoma cases and one meta-analysis, were lymph node stations 1, 2, 3, and 7. The rates of metastasis were 36%–44.7% in No. 1, 25%–34.7% in No. 2, 38.8%–46% in No. 3, and 23.2%–30% in No. 7. The metastasis rate of lymph node station No. 9 was 12.7%–14%, and the rate was also consistent among the studies, although it was slightly lower than that of Nos. 1, 2, 3, and 7. This finding correlates well with the previous observation of Aikou and Shimazu [17] that the main lymphatic flows of the cardia make their way down to the upper gastric, para-celiac, and para-aortic lymph nodes, unlike the lymphatic pathways of the lower esophagus that usually go upward and downward in their study using radioisotope lymphangiography. In the case of para-aortic lymph nodes near the left renal vein, No. 16a2lat, which was stressed by Aikou and Shimazu [17], to be dissected, the metastasis rate was inconsistent among studies: 11%–17% in three studies and 4.7% in one study. However, the number of patients who underwent para-aortic node dissection was relatively small (16.4%–62.7%) compared to the entire cohort in the former three retrospective studies. However, in one prospective study, 95.7% of the study population (313/327) underwent para-aortic node dissection. Therefore, the metastasis rate of lymph node station No. 16a2lat was not as high as previously believed. Regarding the metastasis rate of the lower mediastinal nodes, lower mediastinal nodes were treated as a whole in one study and in two studies that were treated separately by each station, and in one study, they were treated as a whole and by each station. Mine et al. [13] reported an 18% metastasis rate in the lower mediastinal nodes, and they insisted that these nodes should be considered second-tier nodes and dissected. Matsuda et al. [14] reported a 16.1% metastasis rate for No. 110, 5.5% for No. 111, and 15.3% for No. 112. In a recent meta-analysis by Chen et al. [15] including nine studies from 2003 to 2019, the metastasis rates of lower mediastinal nodes were available in three studies and the rates of the pooled data were 10% for No. 110, 3% for No. 111, 5% for No. 112, and 15% for lower mediastinal nodes. In a prospective nationwide study from Japan [16] conducted in collaboration with the Japanese Gastric Cancer Association and Japan Esophageal Society, the metastasis rates were 9% for No. 110, 3.7% for No. 111, and 1.9% for No. 112. However, the metastasis rate of No. 110 starts to increase to >10% when the length of esophageal involvement by the tumor is >2 cm. Considering that lymphatic flows from the cardia do not usually go to the lower mediastinal nodes [17], it seems that lower mediastinal node metastasis occurs after the involvement of the esophagus by the tumor originating from the cardia. In other words, the lower mediastinal node metastasis rate seems to be more closely related to the length of esophageal involvement by the tumor than to the Siewert type. The metastasis rates of the middle and upper mediastinal nodes were very low when the length of esophageal involvement by the tumor was not more than 4 cm. The metastasis rate of No. 106recR, which belongs to the upper mediastinal nodes, was 10.7%, but metastasis rates of other middle or upper mediastinal nodes were low (3.6%–7.1%) when the length of esophageal involvement was >4 cm [16]. The metastasis rates at station No. 11 were approximately 14% for No. 11p and 4%–5% for No. 11d in two studies, 1.8% for No. 11p, and 6% for No. 11d in one study. In the remaining study, the metastasis rate was not reported at each station of No. 11, and it was 16.5%. The reason for the unexpectedly low metastasis rate of No. 11p in one study is unclear. The metastasis rate of No. 11p was moderate, and that of No. 11d was consistently low among studies [14,15,16,18]. The metastasis rate of other suprapancreatic nodes including para-hiatal nodes was 5.5%–6.2% in No. 8a, 4.7%–9.7% in No. 10, 2.5%–8% in No. 12a, 5.6%–9% in No. 19, and 1.4%–4.7% in No. 20. The metastasis rates of the greater curvature and distal nodes, including No. 4sa, 4sb, 4d, 5, and 6, were consistently very low among the studies (Table 1, Fig. 1).
Table 1. Large-scale studies addressing the lymph node metastasis rate of gastroesophageal junction adenocarcinoma.
| Frequency | Mine et al. (2013) [13] | Matsuda et al. (2016) [14] | Chen et al. (2003–2019) [15]* | Kurokawa et al. (2021) [16]† |
|---|---|---|---|---|
| High frequency (≥20%) | 1, 2, 3, 7 | 1, 2, 3, 7 | 1, 2, 3, 7 | 1, 2, 3, 7 |
| MMN‡ | ||||
| Moderate frequency (≥10%, <20%) | 9, 11, 16a2 | 9, 16a2, 110, 112 | 9, 11p, 16a2, 110, 108 | 9, 11p |
| LMN | UMN‡ | LMN | ||
| Low frequency (≥5%, <10%) | 12 | 4d, 8a, 10, 19, 111 | 8a, 10, 11d, 19, 112 | 8a, 19, 110 |
| Very low frequency (<5%) | 4, 5, 6, 8, 10 | 4sa, 4sb, 4d, 5, 6, 11p, 20, 12a | 4sa, 4sb, 4d, 5, 6, 12a, 20, 111, 107, 109 | 4sa, 4sb, 4d, 5, 6, 11d, 16a2, 20, MMN, UMN |
| MMN or UMN |
MMN = middle mediastinal node; LMN = lower mediastinal node; UMN = upper mediastinal node.
*Meta-analysis; †Prospective study; ‡The unexpectedly high incidence of metastasis of MMN and UMN in Matsuda’s study is due to the low dissection rate, 23/400 in UMN and 48/400 in MMN.
Fig. 1. Schematic illustration of lymph node metastasis rate of gastroesophageal junction adenocarcinoma.
EXTENT OF LYMPHADENECTOMY IN THE SURGERY OF GEJAC
Siewert et al. [19] recommended transabdominal total gastrectomy, transhiatal resection of the distal esophagus, and lymph node dissection in the lower posterior mediastinum, in addition to D2 lymph node dissection according to the principles of gastric cancer surgery in total gastrectomy, based on the lymph node metastasis rate at each nodal station of 271 patients with GEJAC who underwent surgical resection. They also noted that the survival rate of patients who underwent extended total gastrectomy was not inferior to that of patients who underwent subtotal esophagectomy, and the postoperative mortality rate was significantly lower in patients who underwent extended total gastrectomy than in those who underwent transthoracic esophagectomy. However, unlike in the case of Siewert type III tumors that can metastasize to the greater curvature and antral nodes, the need for extended total gastrectomy has been questioned in GEJAC. In a Japanese multicenter retrospective study including 288 patients with pT2-4 GEJAC treated by R0 resection [18], in patients where the distance from the GEJ to the distal end of the tumor was ≤30 mm, the frequency of nodal involvement along the greater curvature or the antrum of the stomach was low (2.2%). In contrast, in patients with a distance of >50 mm, the incidence of nodal involvement was 20.0%. In patients with a distance was 30–50 mm incidence was intermediate (8.0%). Multivariate analyses showed that the distance from the GEJ to the distal end of the tumor was significantly related to lymph node involvement along the greater curvature or antrum (odds ratio, 3.7; 95% confidence interval [CI], 1.3–11; P=0.006). Based on these results, the authors recommended that total gastrectomy should be considered for the dissection of the greater curvature and antral nodes when the distance is >50 mm. Several studies addressing GEJAC have also reported that the incidence of metastasis to the greater curvature and antral nodes by this tumor is extremely low [14,15,16].
If proximal gastrectomy is chosen for the resection of GEJAC, node dissection of the distal part of station No. 3 can be insufficient to preserve the blood supply to the distal remnant stomach [20]. However, as shown in the previous section, the metastasis rate of No. 3 in GEJAC was the highest among all the nodal stations. According to the Japanese classification of gastric cancer, 3rd English edition, station no. 3 was subdivided into no. 3a and 3b. No. 3a represents the lesser curvature nodes along the branches of the left gastric artery, and No. 3b represents the lesser curvature nodes along the second branch and distal part of the right gastric artery [21]. In a retrospective study of 2,400 patients with gastric cancer [22], the metastasis rate of No. 3a of advanced tumors in the upper third of the stomach was significantly higher than that in the middle third or lower third of the stomach (57.1% vs. 49.8%, 38.3%; P=0.043, P<0.0001). The No. 3b metastasis rates of the upper, middle, and lower third advanced tumors were 8.7%, 17.7%, and 19.6%, respectively, without a statistically significant difference; however, the therapeutic index of No. 3b nodes in the upper third of the stomach was far lower than that in the middle or lower third of the stomach (1.7 vs. 7.1, 7.0). In a subgroup analysis of upper-third tumors, the No. 3b metastasis rate of the upper-third tumors with the distal margin within the upper third was only 2%, which was significantly lower than that of the upper-third tumors, with the distal margin extending to the middle third (P<0.0001). Moreover, the therapeutic index of No. 3b node dissection in the upper third of the tumors with a distal margin within the upper third was only 1.1. The common characteristics of the tumors with No. 3b node metastasis in this subgroup were large tumors (≥45 mm), advanced T stage (≥T3), and N stage (≥N2). Many patients with GEJAC may be included in this subgroup; therefore, proximal gastrectomy and distal esophagectomy could be oncologically justified in GEJAC surgery unless the tumor is very large. In a recent Japanese prospective trial [16], total gastrectomy was recommended when the tumor size was >6 cm.
The importance of dissection of the para-aortic nodes around the left renal vein (No. 16a2lat) has been emphasized in several studies [13,14,17], including the JCOG9502 trial [23], due to the special lymphatic pathway from the GEJ to the No. 16a2lat along the left inferior phrenic artery. However, in a recent prospective Japanese trial [16], the metastasis rate of No. 16a2lat was only 4.7%. Considering that 37.7% (63/167) of the patients in the JCOG9502 trial were patients with Siewert type III tumors and the median size of the tumor was larger in the JCOG9502 trial than in the Japanese prospective trial (6.5 cm vs. 4.5 cm), prophylactic dissection of No. 16a2lat may have some meaning only in the surgery of large GEJAC or Siewert type III tumors.
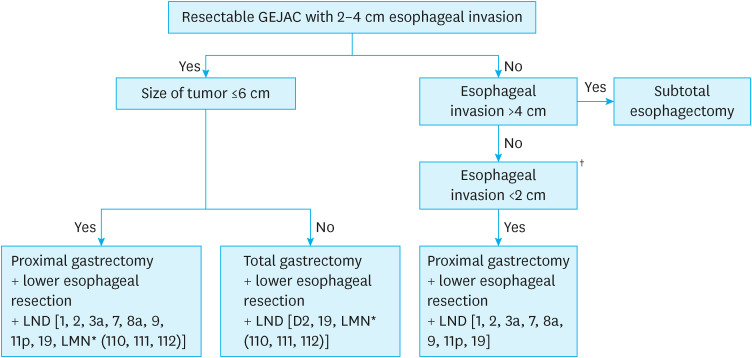
Lower mediastinal nodes have been considered second-tier nodes in GEJAC with moderate metastasis rates, and the importance of dissection of these nodes has been stressed [13]. Anatomically, the lower mediastinal nodes are composed of lower thoracic paraesophageal nodes (No. 110), supradiaphragmatic nodes (No. 111), and posterior mediastinal nodes (No. 112) [24]. However, the anatomical border among the three nodal stations of the lower mediastinal nodes cannot be clearly defined; thus, these nodes were treated as one lymph node station in the fourth English version of the Japanese Gastric Cancer Treatment Guideline [25]. Dissection of No. 110 among the lower mediastinal nodes was recommended when the length of esophageal involvement by GEJAC was more than 2 cm and not more than 4 cm in a recent Japanese prospective trial [16]. Dissections No. 111 and 112 were excluded owing to the low metastasis rate to the nodal stations in this trial. However, each nodal station of the lower mediastinum is closely located in the narrow confined lower mediastinum; thus, separate dissection is not easy. Moreover, there is a possibility of underestimation of the No. 111 and 112 metastasis rates in that study due to the difficulty in the exact classification of nodes at the back table after resection. In a retrospective study of 672 patients with GEJAC, Siewert type III, and proximal gastric cancer who underwent total gastrectomy without lower mediastinal node dissection [26], the 5-year disease-free survival of GEJAC was significantly worse than that of Siewert type III and proximal gastric cancer in advanced disease (47.9% vs. 75.4% vs. 71.8%, P<0.001). Advanced GEJAC and Siewert type III tumors had higher rates of locoregional recurrence, especially in the vicinity of the esophagojejunostomy and mediastinal nodes, compared to advanced proximal gastric cancer. Although the authors stated that they did not perform lower mediastinal node dissection in their cohorts, they stated that they tried to remove more periesophageal tissue when the tumor involved the GEJ in a personal communication. The situation in this study cannot be the same as the No. 110 dissection mentioned in the Japanese prospective trial but can be similar. From the results of this study, we may expect better locoregional control and better survival through a systematic dissection of the lower mediastinal nodes rather than a No. 110 dissection only in GEJAC surgery (Fig. 2).
Fig. 2. A diagram of the algorithm in the surgery of GEJAC with 2–4 cm esophageal invasion.
GEJAC = gastroesophageal junction adenocarcinoma.
*Systematic dissection of the lower mediastinal nodes, including Nos. 110, 111, and 112.
†The same rule could be applied with resectable GEJAC with 2–4 cm esophageal invasion in making a decision on proximal gastrectomy vs. total gastrectomy, according to the tumor size.
Middle and upper mediastinal node dissection is not required unless the length of esophageal involvement by the tumor exceeds 4 cm, and it may be a rare occurrence for resectable GEJAC in such a situation. With regard to suprapancreatic node dissection, including para-hiatal nodes, dissection of Nos. 10, 11d, 12a, and 20 can be excluded due to the low incidence of metastasis, as mentioned in the previous section.
METHOD OF LYMPHADENECTOMY IN GEJAC
Large-scale randomized trials addressing GEJAC failed to prove the survival advantage of the transthoracic approach in GEJAC compared to the transhiatal approach, and the postoperative morbidity rate was higher with the transthoracic approach [23,27]. Although there was a trend toward improved 5-year overall survival of the transthoracic approach compared to the transhiatal approach in the Dutch trial (39% vs. 29%; 95% CI, −3 to 23), only 18% (40/220) of enrolled patients had GEJAC, pulmonary complications were significantly higher (P<0.001), and mechanical ventilator use, ICU stay, and hospital stay were all significantly longer (P<0.001) in the transthoracic approach. In the JCOG9502 trial, 56.9% (95/167) of enrolled patients had GEJAC, which was terminated early after interim analysis because the predicted probability of the left thoracoabdominal approach having a significantly better overall survival than the transhiatal approach was only 3.65%. In the intention-to-treat analysis, the 5-year overall survival was 52.3% (95% CI, 40.4–64.1) in the transhiatal approach and 37.9% (95% CI, 26.1–49.6) in the left thoracoabdominal approach. Therefore, the use of a transthoracic approach cannot be justified for the removal of lower mediastinal nodes in GEJAC surgery.
In a recent meta-analysis including nine studies dealing with the open vs. laparoscopic transhiatal approach for GEJAC [15], the laparoscopic transhiatal approach was associated with longer operation time (mean difference=31 minutes; 95% CI, 20–41; P<0.001), less blood loss (mean difference=−103 mL; 95% CI, −135 to −72; P<0.001), and harvested a similar number of lymph nodes (mean difference=0.1; 95% CI, −1.77 to 0.20; P=0.89). There were no differences in time to ambulation (mean difference=−0.79 days; 95% CI, 20–41; P=0.12) or time to first flatus (mean difference=−0.82 days; 95% CI, −1.76 to 0.11; P=0.08). However, the laparoscopic transhiatal approach was associated with a shorter postoperative hospital stay (mean difference = −1.70 days; 95% CI, −2.34 to −1.05; P<0.001). Survival data were available in two of the nine studies; unexpectedly, the 5-year overall survival rate was significantly higher with the laparoscopic transhiatal approach (risk ratio=1.43; 95% CI, 1.18–1.73). Although a randomized trial was not included in this meta-analysis, the general advantages of minimally invasive surgery over open surgery were also shown in GEJAC. Further results from large-scale randomized studies are required on this topic.
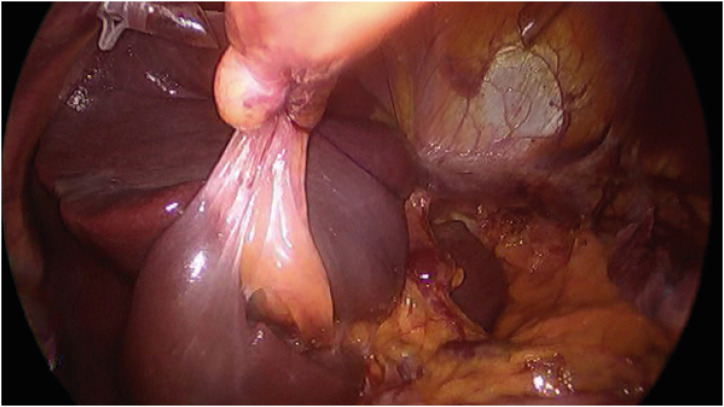
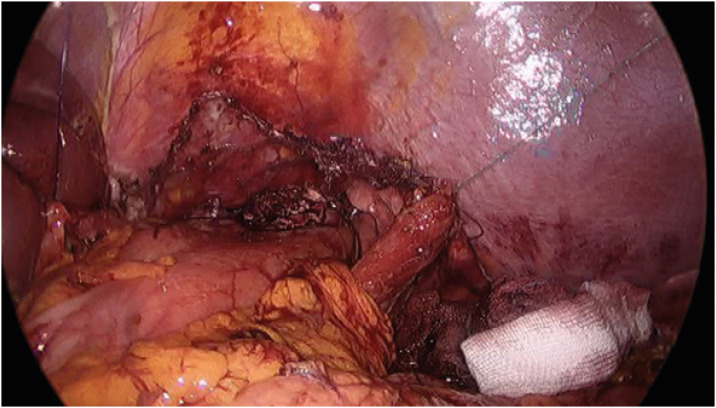
A laparoscopic transhiatal proximal gastrectomy with resection of the distal esophagus and a lower mediastinal lymphadenectomy is performed for GEJAC with 2–4 cm esophageal invasion at the author’s institution. The first step in this surgery is mobilization of the left lateral section of the liver, which is the main obstacle to a good surgical view of the lower mediastinum. All suspensory ligaments were divided, the triangular ligament was sutured and pulled from the outside of the abdomen by a thread, and a prolene hanging-up method that was previously reported [28] was performed to complete the liver retraction (Fig. 3). In the author’s opinion, this step is important for good surgical performance in the lower mediastinum and is even mandatory when the surgeon wants to perform a complex reconstruction procedure, such as the “double-flap technique” [29], in the lower mediastinum. After the distal stomach is mobilized, the stomach is cut 2–3 cm above the angle and suprapancreatic node dissection, including Nos. 8a, 9, 11p, and 19 is performed. The esophageal hiatus is widely opened anteriorly using LigaSure™ (Medtronic, Dublin, Ireland), and the bilateral diaphragmatic crus is sutured and retracted laterally by threads from the outside of the abdomen (Fig. 4). The bilateral lower pleura were resected during dissection of No. 111. Systematic lower mediastinal node dissection (Fig. 5) was performed in the cranial direction, up to the lower border of the inferior pulmonary vein (Fig. 6), and the esophagus was cut 2 cm above the upper border of the tumor. Esophagogastrostomy was performed in the lower mediastinum using a double-flap technique (Fig. 7). A closed suction drainage tube (Jackson-Pratt) was placed behind the anastomosis through a stab wound in the left 4th intercostal space. The opened hiatus was repaired to a proper size, and two to three interrupted sutures using a non-absorbable suture material were placed between the distal part of the remnant stomach and the newly formed hiatal opening. In our experience, a wide surgical view obtained by complete mobilization of the left lateral section of the liver, widely opened hiatus, and systematic lower mediastinal node dissection with resection of the bilateral lower pleura enabled the surgeon to perform a double-flap technique without any disturbance in the surgical view.
Fig. 3. The method of liver retraction in the surgery of GEJ. All suspensory ligaments of the left lateral section of the liver were divided, and the left lateral section was retracted toward the right side by a thread and sutured on the triangular ligament from the outside of the abdomen. The pars condensa of the gastrohepatic ligament was punctured and hung using #2-0 prolene.
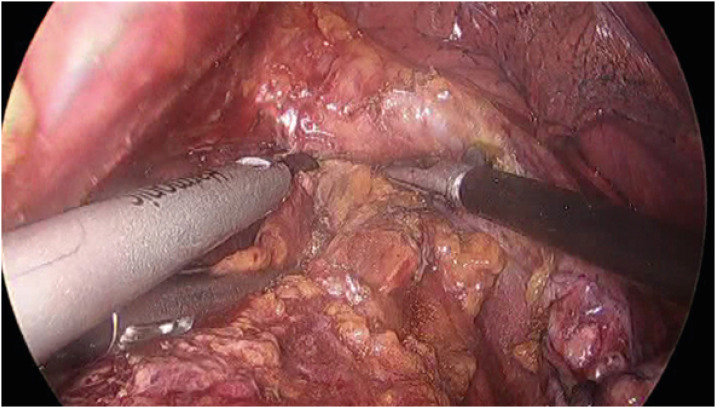
Fig. 4. Widening of the hiatus. The hiatal opening is widely opened anteriorly, and the bilateral crus is sutured and retracted laterally by threads from the outside of the abdomen.
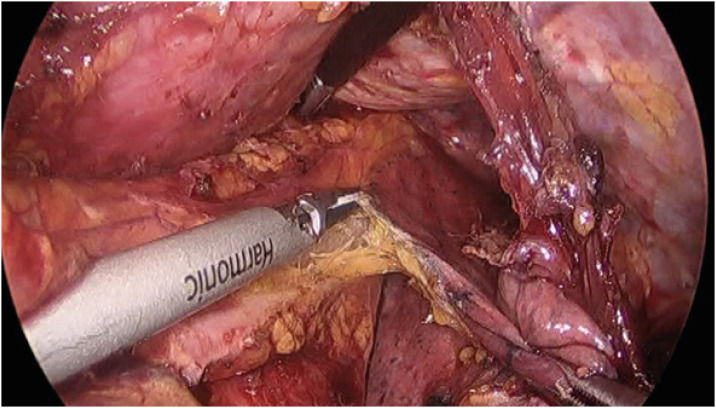
Fig. 5. The surgical view of systematic lower mediastinal node dissection. Left pulmonary ligament (node station No. 112pul) is dissected using an ultrasonic shear.
Fig. 6. The landmark of the upper border of lower mediastinal nodes. The inferior border of the left inferior pulmonary vein is dissected using an ultrasonic shear.
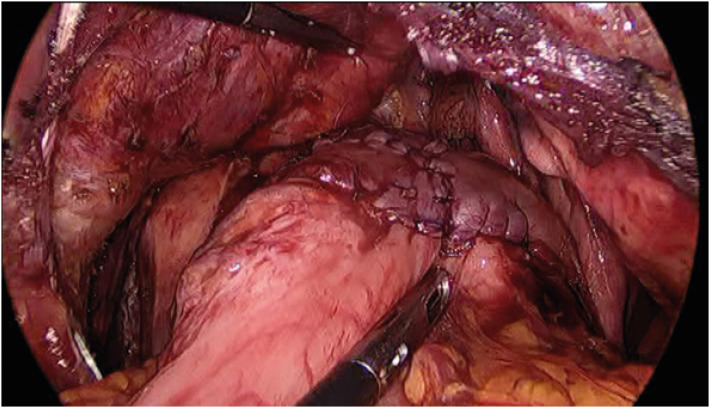
Fig. 7. The surgical view after completion of a double-flap technique in the lower mediastinum.
CONCLUSION
A systematic lower mediastinal node dissection is proposed for GEJAC with a length of esophageal involvement of 2–4 cm. Proximal gastrectomy with resection of the distal esophagus seems more suitable for this tumor when it is resectable, and a laparoscopic transhiatal approach is preferable to an open or transthoracic approach in GEJAC surgery [30]. Further randomized controlled studies are required to confirm this hypothesis.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim JJ. Epidemiology of gastroesophageal junction adenocarcinoma in Korea. J Gastric Cancer. 2018;18:328–338. doi: 10.5230/jgc.2018.18.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walther C, Zilling T, Perfekt R, Möller T. Increasing prevalence of adenocarcinoma of the oesophagus and gastro-oesophageal junction: a study of the Swedish population between 1970 and 1997. Eur J Surg. 2001;167:748–757. doi: 10.1080/11024150152707725. [DOI] [PubMed] [Google Scholar]
- 3.Matsuno K, Ishihara R, Ohmori M, Iwagami H, Shichijyo S, Maekawa A, et al. Time trends in the incidence of esophageal adenocarcinoma, gastric adenocarcinoma, and superficial esophagogastric junction adenocarcinoma. J Gastroenterol. 2019;54:784–791. doi: 10.1007/s00535-019-01577-7. [DOI] [PubMed] [Google Scholar]
- 4.Information Committee of the Korean Gastric Cancer Association. Korean Gastric Cancer Association-led nationwide survey on surgically treated gastric cancers in 2019. J Gastric Cancer. 2021;21:221–235. doi: 10.5230/jgc.2021.21.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SH, Kang MJ, Yun EH, Jung KW. Epidemiology of gastric cancer in Korea: trends in incidence and survival based on Korea Central Cancer Registry Data (1999–2019) J Gastric Cancer. 2022;22:160–168. doi: 10.5230/jgc.2022.22.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eom SS, Choi W, Eom BW, Park SH, Kim SJ, Kim YI, et al. A comprehensive and comparative review of global gastric cancer treatment guidelines. J Gastric Cancer. 2022;22:3–23. doi: 10.5230/jgc.2022.22.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siewert JR, Stein HJ. Carcinoma of the cardia: carcinoma of the gastroesophageal junction - classification, pathology and extent of resection. Dis Esophagus. 1996;9:173–182. [Google Scholar]
- 8.Wallner B. Endoscopically defined gastroesophageal junction coincides with the anatomical gastroesophageal junction. Surg Endosc. 2009;23:2155–2158. doi: 10.1007/s00464-008-0238-9. [DOI] [PubMed] [Google Scholar]
- 9.Misumi A, Murakami A, Harada K, Baba K, Akagi M. Definition of carcinoma of the gastric cardia. Langenbecks Arch Chir. 1989;374:221–226. doi: 10.1007/BF01359557. [DOI] [PubMed] [Google Scholar]
- 10.Ichihara S, Uedo N, Gotoda T. Considering the esophagogastric junction as a ‘zone’. Dig Endosc. 2017;29(Suppl 2):3–10. doi: 10.1111/den.12792. [DOI] [PubMed] [Google Scholar]
- 11.Ekmektzoglou KA, Apostolopoulos P, Samelis G, Alexandrakis G. Gastroesophageal junction and gastroesophageal junction carcinoma: a short update. Acta Gastroenterol Belg. 2016;79:471–479. [PubMed] [Google Scholar]
- 12.Hayashi T, Yoshikawa T. Optimal surgery for esophagogastric junctional cancer. Langenbecks Arch Surg. 2022;407:1399–1407. doi: 10.1007/s00423-021-02375-7. [DOI] [PubMed] [Google Scholar]
- 13.Mine S, Sano T, Hiki N, Yamada K, Nunobe S, Yamaguchi T. Lymphadenectomy around the left renal vein in Siewert type II adenocarcinoma of the oesophagogastric junction. Br J Surg. 2013;100:261–266. doi: 10.1002/bjs.8967. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda T, Kurokawa Y, Yoshikawa T, Kishi K, Misawa K, Ohi M, et al. Clinicopathological characteristics and prognostic factors of patients with Siewert type II esophagogastric junction carcinoma: a retrospective multicenter study. World J Surg. 2016;40:1672–1679. doi: 10.1007/s00268-016-3451-z. [DOI] [PubMed] [Google Scholar]
- 15.Chen XD, He FQ, Chen M, Zhao FZ. Incidence of lymph node metastasis at each station in Siewert types II/III adenocarcinoma of the esophagogastric junction: a systematic review and meta-analysis. Surg Oncol. 2020;35:62–70. doi: 10.1016/j.suronc.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Kurokawa Y, Takeuchi H, Doki Y, Mine S, Terashima M, Yasuda T, et al. Mapping of lymph node metastasis from esophagogastric junction tumors: a prospective nationwide multicenter study. Ann Surg. 2021;274:120–127. doi: 10.1097/SLA.0000000000003499. [DOI] [PubMed] [Google Scholar]
- 17.Aikou T, Shimazu H. Difference in main lymphatic pathways from the lower esophagus and gastric cardia. Jpn J Surg. 1989;19:290–295. doi: 10.1007/BF02471404. [DOI] [PubMed] [Google Scholar]
- 18.Mine S, Kurokawa Y, Takeuchi H, Kishi K, Ito Y, Ohi M, et al. Distribution of involved abdominal lymph nodes is correlated with the distance from the esophagogastric junction to the distal end of the tumor in Siewert type II tumors. Eur J Surg Oncol. 2015;41:1348–1353. doi: 10.1016/j.ejso.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katai H, Sano T, Fukagawa T, Shinohara H, Sasako M. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2003;90:850–853. doi: 10.1002/bjs.4106. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 22.Haruta S, Shinohara H, Hosogi H, Ohkura Y, Kobayashi N, Mizuno A, et al. Proximal gastrectomy with exclusion of no. 3b lesser curvature lymph node dissection could be indicated for patients with advanced upper-third gastric cancer. Gastric Cancer. 2017;20:528–535. doi: 10.1007/s10120-016-0624-2. [DOI] [PubMed] [Google Scholar]
- 23.Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7:644–651. doi: 10.1016/S1470-2045(06)70766-5. [DOI] [PubMed] [Google Scholar]
- 24.Japan Esophageal Society. Japanese classification of esophageal cancer, 11th edition: part I. Esophagus. 2017;14:1–36. doi: 10.1007/s10388-016-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee IS, Ahn JY, Yook JH, Kim BS. Mediastinal lymph node dissection and distal esophagectomy is not essential in early esophagogastric junction adenocarcinoma. World J Surg Oncol. 2017;15:28. doi: 10.1186/s12957-016-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Kim JJ, Park SM. A simple method of liver retraction for various types of laparoscopic upper gastrointestinal surgeries: the prolene hanging-up method. World J Surg. 2015;39:2362–2366. doi: 10.1007/s00268-015-3065-x. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda S, Choda Y, Otsuka S, Ueyama S, Tanaka N, Muraoka A, et al. Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as antireflux esophagogastrostomy after proximal gastrectomy (rD-FLAP Study) Ann Gastroenterol Surg. 2018;3:96–103. doi: 10.1002/ags3.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim TH, Kim IH, Kang SJ, Choi M, Kim BH, Eom BW, et al. Korean Practice Guidelines for Gastric Cancer 2022: an evidence-based, multidisciplinary approach. J Gastric Cancer. 2023;23:3–106. doi: 10.5230/jgc.2023.23.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]