Abstract
Patients with malignant diseases may develop symptoms of superior vena cava syndrome (SVCS) quickly because rapid tumor growth does not allow adequate time to develop collateral blood flow. Therefore, malignant SVCS is a medical emergency associated with neurological or pharyngeal-laryngeal signs. Recently, interventional endovascular treatment (EVT) has achieved acceptable results. We describe the case of a 55-year-old woman with pulmonary adenocarcinoma and laryngeal edema. In the first EVT, bare-metal-stent was implanted into the SVCS with intravascular ultrasound (IVUS) guidance. The IVUS showed insufficient stent-mid expansion. We did not use additional ballooning because of the risk of superior vena cava (SVC) rupture. Three months later, the SVCS recurred. A second EVT was performed, and IVUS imaging suggested tumor ingrowth into the SVC through the stent struts. We considered that the tumor ingrowth could be covered in the SVC using stent-graft. The patient showed no recurrence of SVCS for about 12 months. IVUS-guided implantation of stent for the treatment of malignant SVCS has not been reported. This case report revealed that stent therapy using IVUS for SVCS is useful.
Learning objective
Superior vena cava syndrome (SVCS) due to malignancy is not rare. Recently, endovascular treatment for SVCS has achieved acceptable results. However, SVC stenting in SVCS as having primary patency rate varies for each report. Intravascular ultrasound (IVUS) guided implantation of stent for malignant SVCS treatment has not been reported. In this case, we suspected insufficient stent expansion and tumor ingrowth as the possible cause of in-stent restenosis. Therefore, stent therapy using IVUS for malignant SVCS can be helpful.
Keywords: Recurrent superior vena cava syndrome, Stent-graft, Pulmonary adenocarcinoma, Intravascular ultrasound
Introduction
Superior vena cava syndrome (SVCS) results from any condition that leads to obstruction of blood flow through the superior vena cava (SVC). Malignant obstruction can be caused by direct invasion of a tumor into the SVC, or by external compression of the SVC by an adjacent pathologic process involving the right lung, lymph nodes, and other mediastinal structures, leading to stasis of blood flow and thrombosis [1]. It is estimated that lung cancer and non-Hodgkin lymphoma are responsible for approximately 95 % of the SVCS cases that are caused by malignancy [2]. Patients with malignant disease may develop symptoms of SVCS quickly because rapid tumor growth does not allow adequate time to develop collateral blood flow. Evidence-based guidelines for the management of SVCS are not available. A general recommendation supporting radiation therapy or stent implantation for symptomatic SVCS has been made by the National Comprehensive Cancer Network and American College of Chest Physicians for lung cancer. Malignant SVCS requires that accurate diagnosis and biopsy should precede emergent therapeutic intervention in most cases [3]. The Kishi score was developed to assist in the decision to initiate stent therapy [4]. In this case, the patient was already diagnosed with lung cancer metastasis to the lymph node. Thus, using a Kishi score of 5 as a reference, we decided on stent implantation for the SCVS. Endovascular stent implantation is a faster way of relieving symptoms compared with radiation therapy or chemotherapy. Many studies have reported the efficacy of stent implantation in patients with malignant SVCS. However, intravascular ultrasound (IVUS)-guided implantation of stent for the treatment of malignant SVCS has not been reported. Thus, we report a successful treatment of recurrent SVCS using IVUS to guide stent-graft placement for in-stent restenosis, following bare-metal-stent implantation.
Case report
A 55-year-old woman presented with dysphagia and dyspnea. Two months earlier, she started treatment for non-small-cell lung cancer (NSLC) with first-line chemotherapy, osimertinib. One month earlier, she had noticed facial and left arm edema. Vital signs on admission were: temperature, 36.8 °C; blood pressure, 116/78 mmHg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and oxygen saturation, 97 % in room air. Physical examination revealed facial edema, edema in the left arm, and jugular venous distension. Cardiac examination findings were unremarkable. Lung sounds were clear. Other examinations showed no abnormalities.
She had a smoking history of 10 pack years between 20 and 40 years of age with no history of surgery. Electrocardiography revealed a normal sinus rhythm, and chest radiography findings were unremarkable. Transthoracic echocardiography revealed normal left ventricular systolic function. The ejection fraction was 60 %, and right heart overload was absent. There were no regional wall motion abnormalities or significant valvular disease.
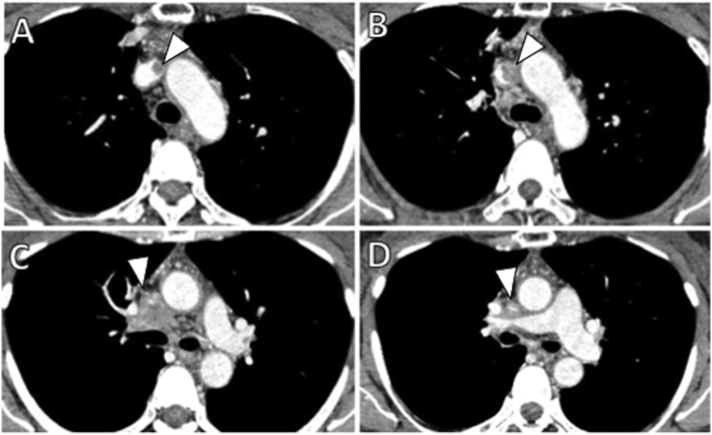
Contrast-enhanced computed tomography revealed an ill-defined infiltrating central mass with mediastinal lymphadenopathy and left brachiocephalic venous obstruction, with no evidence of pulmonary embolism (Fig. 1A–D). We diagnosed lymphadenopathy, arising from lymph node metastasis of the NSLC.
Fig. 1.
Contrast-enhanced axial computed tomography images from the upper level of the left brachiocephalic vein to the upper level of the superior vena cava. (A) shows right brachiocephalic venous thrombosis (arrowhead). (B, C, and D) show a central mass with mediastinal lymphadenopathy and left brachiocephalic venous obstruction (arrowhead).
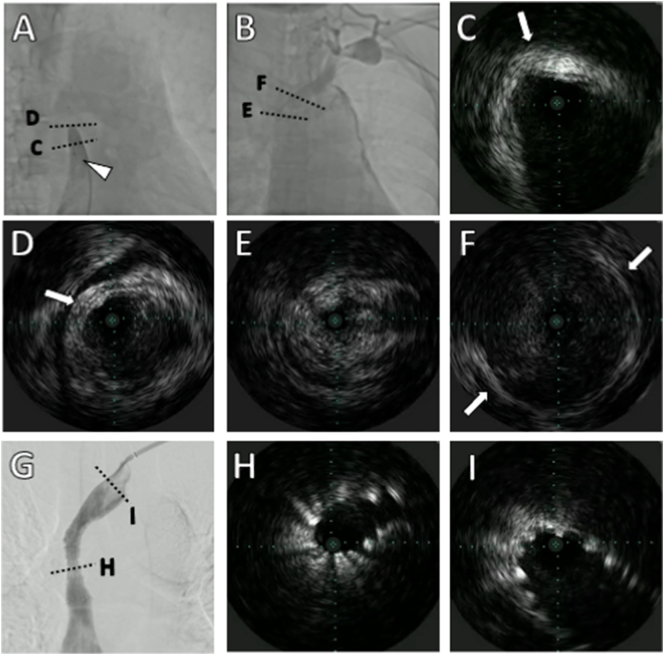
SVCS of malignant origin necessitates emergency intervention, typically with radiation therapy (RT). However, osimertinib with RT may cause interstitial pneumonia. Therefore, if the RT is performed, osimertinib should be suspended. Osimertinib is effective for primary lesions. We decided to continue the present treatment and implant a stent for the SVC lesion. A 7-French Destination EX guiding sheath (TERUMO, Tokyo, Japan) and 6-French sheath were inserted via a left upper limb vein and the right femoral vein, respectively. Angiography showed SVC occlusion with poor venous collateral development (Fig. 2A, B). Under fluoroscopic guidance, Gladius MG PV (Asahi Intecc, Aichi, Japan) was placed across the obstruction. IVUS (Visions PV .014P; PHILIPS, Amsterdam, Netherlands) was performed at a frequency of 20 MHz to observe the preservation of vascular structure. Obliteration of the circumscriptive outer membrane of a vessel was considered diagnostic of vessel invasion by tumor. However, these changes could have been indiscernible because of attenuation. If the vessel membrane was preserved, structures found inside the vessels were considered thrombi. Notably, thrombosis within the tumor could not be differentiated using IVUS (Fig. 2C–F; Online Video 1). We provided percutaneous cardiopulmonary support and pre-dilated the SVC occlusive lesion using a 5 × 80 mm compliant balloon (JADE, OrbusNeich; Hong Kong, China) at 20 atm and implanted a 14 × 60 mm SMART™ (Cordis, Dublin, OH, USA) stent. IVUS showed that proximal (atrial side) and distal reference vessel diameters were ~14 mm, thus 14-mm stents were selected. Using IVUS markings, we avoided inserting the stent too far proximally and instead placed it distally to cover the lesion. Post-stenting balloon dilation was performed using an 8 × 40 mm POWERFLEX® (Cordis) at 20 atm, with an excellent angiographic result (Fig. 2G). However, the IVUS showed insufficient stent expansion (Fig. 2H, I) (Online Video 2). The vessel diameters in the most stenotic areas could not be adequately evaluated through IVUS. We did not use additional ballooning because of the risk of SVC rupture. Therefore, the endpoint was defined as good proximal and distal crimping of the stent. Post-procedure, the clinical symptoms improved immediately, and she was prescribed single antiplatelet therapy with clopidogrel and anticoagulant therapy with edoxaban.
Fig. 2.
Angiography and intravascular ultrasound (IVUS) before and after bare-metal stent placement. (A and B) show diagnostic angiography. (A) Angiography through right femoral vein injection shows superior vena cava occlusion. (B) Angiography through left upper limb vein injection shows left brachiocephalic vein occlusion. The arrowhead indicates the IVUS catheter. The dotted line shows a cross-section of each IVUS. (C and D) show hyperplasia of the superior vena cava wall. Arrow shows outer membrane of the vessel. Dot to dot of the IVUS indicates an interval of 1 mm. (E) shows tissue in the superior vena cava. Outer membrane of the vessel is obscured. (F) shows insignificant thrombosis in the left brachiocephalic vein.
(G) shows the fluent passage of contrast medium via the stent. The dotted line shows the cross-section of each IVUS. (H) shows insufficient expansion at the stent midpoint. (I) shows good expansion of the stent.
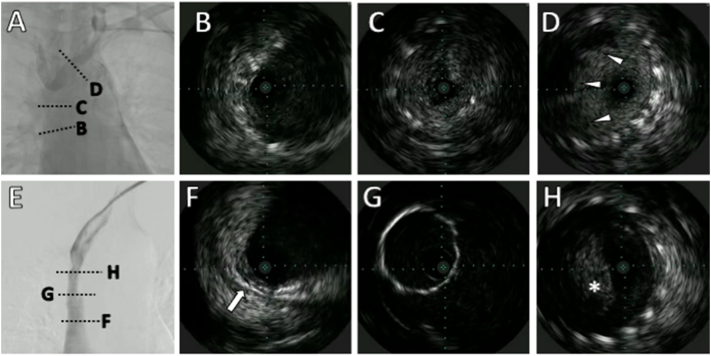
However, the same symptoms recurred three months later. Angiography showed in-stent restenosis (ISR) (Fig. 3A). The IVUS imaging suggested thrombus and tumor ingrowth into the SVC through the stent struts (Fig. 3B–D) (Online Video 3). We dilated the ISR lesion using a compliant balloon with an 8 × 40 mm POWERFLEX® at 20 atm. However, the stent easily recoiled. Therefore, we considered that the tumor in growth could be covered in the SVC using stent-graft VIABAHN VBX (Gore, Newark, DE, USA). Using IVUS, we identified the brachiocephalic vein bifurcation while also being meticulously preventing right brachiocephalic vein occlusion. An 8.0 × 29 mm VIABAHN VBX was introduced over a 0.035-in., 180-cm-stiff guidewire, which was deployed successfully across the stenosed section. The IVUS showed the tumor overhanging in the left subclavian vein. However, blood flow limitation was not evident (Fig. 3E–H) (Online Video 4). The patient showed no recurrent symptoms of SVCS for about 12 months, suggesting continued patency of the stent-graft.
Fig. 3.
Angiography and intravascular ultrasound (IVUS) of stent occlusion before and after stent-graft placement. (A) shows stenosis in part of the previously implanted stent. (B) shows a good stent expansion. (C) shows tumor ingrowth into the superior vena cava (SVC). (D) shows insignificant thrombosis (arrowhead) in the stent at the left brachiocephalic vein. (E) shows the fluent passage of contrast medium via the stent-graft. (F) shows stent-graft at the SVC side has a normal vessel wall. Arrow shows outer membrane of the vessel. (G) stent-graft with good expansion. (H) shows tumor overhang (asterisk) at the left subclavian vein.
Discussion
This case report revealed that stent therapy using IVUS for SVCS is useful not only to ascertain thrombus or tissue but also to identify the bilateral brachiocephalic vein bifurcation and to measure the vascular lumen diameter to prevent an under-sized stent from being used. The Visions PV .014P used in this case had a maximum visible diameter of 20 mm. However, a Visions PV 0.035 with a maximum visible diameter of 60 mm could also be used for more accurate evaluation of the SVC. The use of IVUS has already been suggested to be helpful in endovascular treatment for peripheral artery disease and is expected to be useful for SVCS [5]. Risk of vascular rupture can be reduced by measuring the reference vessel diameter with IVUS and selecting an appropriately sized balloon and stent. Confirming the vessel diameter with IVUS is additionally advantageous because veins are highly elastic, and the vessel size may change unexpectedly after stent placement. SVC stenting in SVCS is reportedly effective for symptomatic relief in about 99 % of patients [6]. In contrast, a review paper reported that SVC stenting in SVCS as having primary patency rates of 57–93 % and complication rates of 0–25 % [7]. The patency period varies for each report. In this case, we suspected insufficient stent expansion and tumor ingrowth as the possible cause of the ISR. Consequently, we considered stent-graft to cover the tumor ingrowth in the SVC. A prospective study of stenting to de novo SVCS found that covered stents retained patency longer than non-covered stents, with a 12-month patency rate of 94 % against 48 % for non-covered stents [8]. We did not use stent-grafts as a first choice in this case, because reduction of the tumor size could increase the possibility of stent migration, and using a stent-graft would also risk occluding the right brachiocephalic vein. Instead, self-expandable stents were our first choice because we thought that they would adapt to the highly variable vein diameter more easily than stent-grafts would. Moreover, some lesions were difficult to observe with IVUS, and we speculated that even if the self-expandable stent was too large, the risk of vessel rupture was low because it dilated slowly.
There is a lack of evidence-based guidelines for the management of SVCS. The Kishi score was developed to assist in the decision to initiate stent therapy. In this case, since the patient was already diagnosed with lung cancer metastasis to the lymph node, we used a Kishi score of 5 as a reference and considered stent implantation for the SVCS. A biopsy was not performed because of high possibility of complications. MR-venography should have been taken for qualitative evaluation of the lesion.
Conclusion
Using IVUS to aid stent therapy for SVCS has not been reported previously. Therefore, IVUS can be helpful not only to ascertain tissue characterization but also to measure the vascular lumen diameter to prevent an improperly sized stent from being used.
The following are the supplementary data related to this article.
Diagnostic IVUS before procedure
IVUS shows hyperplasia of the superior vena cava wall. IVUS shows tissue in the superior vena cava and insignificant thrombosis in the left brachiocephalic vein.
IVUS after bare-metal stent placement
IVUS shows insufficient expansion at the stent midpoint.
IVUS of stent occlusion
IVUS shows tumor ingrowth into the SVC through the stent struts and insignificant thrombosis in the stent at the left brachiocephalic vein.
IVUS after stent-graft placement
IVUS shows stent-graft with good expansion. However, IVUS shows tumor overhang at the left subclavian vein.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgment
We appreciate the assistance of all the staff at the Nagano Municipal Hospital.
References
- 1.Drews RE, Rabkin DJ. Malignancy-related superior vena cava syndrome. [Accessed on November 06, 2021]. UpToDate, Wolters Kluwer.
- 2.Rice T.W., Rodriguez R.M., Light R.W. The superior vena cava syndrome: clinical characteristics and evolving etiology. Med (Baltimore) 2006;85:37–42. doi: 10.1097/01.md.0000198474.99876.f0. [DOI] [PubMed] [Google Scholar]
- 3.Yu J.B., Wilson L.D., Detterbeck F.C. Superior vena cava syndrome: a proposed classification system and algorithm for management. J. Thorac. Oncol. 2008;3:811–814. doi: 10.1097/JTO.0b013e3181804791. [DOI] [PubMed] [Google Scholar]
- 4.Kishi K., Sonomura T., Mitsuzane K., Nishida N., Yang R.J., Sato M., Yamada R., Shirai S., Kobayashi H. Self-expandable metallic stent therapy for superior vena cava syndrome: clinical observations. Radiology. 1993;189:531–535. doi: 10.1148/radiology.189.2.8210386. [DOI] [PubMed] [Google Scholar]
- 5.Kumakura H., Kanai H., Araki Y., Hojo Y., Iwasaki T., Ichikawa S. 15-year patency and life expectancy after primary stenting guided by intravascular ultrasound for iliac artery lesions in peripheral arterial disease. JACC Cardiovasc Interv. 2015;8:1893–1901. doi: 10.1016/j.jcin.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Straka C., Ying J., Kong F.M., Willey C.D., Kaminski J., Kim D.W.N. Review of evolving etiologies, implication and treatment strategies for the superior vena cava syndrome. SpringerPlus. 2016;5:229. doi: 10.1186/s40064-016-1900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rachapalli V., Boucher L.M. Superior vena cava syndrome: role of the interventionalist. Can. Assoc. Radiol. J. 2014;65:168–176. doi: 10.1016/j.carj.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Gwon D.I., Ko G.Y., Kim J.H., Shin J.H., Yoon H.K., Sung K.B. Malignant superior vena cava syndrome: a comparative cohort study of treatment with covered stent versus uncovered stents. Radiology. 2013;266:979–987. doi: 10.1148/radiol.12120517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnostic IVUS before procedure
IVUS shows hyperplasia of the superior vena cava wall. IVUS shows tissue in the superior vena cava and insignificant thrombosis in the left brachiocephalic vein.
IVUS after bare-metal stent placement
IVUS shows insufficient expansion at the stent midpoint.
IVUS of stent occlusion
IVUS shows tumor ingrowth into the SVC through the stent struts and insignificant thrombosis in the stent at the left brachiocephalic vein.
IVUS after stent-graft placement
IVUS shows stent-graft with good expansion. However, IVUS shows tumor overhang at the left subclavian vein.