Abstract
Purpose
Allergic rhinitis (AR) is associated with obstructive sleep apnea (OSA) and nasal obstruction causes decreased adherence to continuous positive airway pressure (CPAP). The purpose is to evaluate the effects of antiallergic agents on CPAP adherence and sleep quality.
Methods
A longitudinal study was made of patients who use CPAP for OSA and treated with antiallergy agents for spring pollinosis. We compared the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), nasal symptoms scores (NSS), and data from CPAP before and after treatment. Then, we classified the subjects into two groups based on the baseline PSQI score: one group without a decreased sleep quality (PSQI < 6) and the other group with decreased sleep quality (PSQI ≥ 6).
Results
Of 28 subjects enrolled, 13 had good sleep quality and 15 had poor sleep quality. PSQI showed significant improvements after medication (p = 0.046). ESS showed no significant differences after AR medication (p = 0.565). Significant improvement was observed after the prescription of antiallergy agents in all items of NSS (sneezing, p < 0.05; rhinorrhea, p < 0.01; nasal obstruction, p < 0.01; QOL, p < 0.01). The percentage of days with CPAP use more than 4 h increased significantly after the administration of rhinitis medication (p = 0.022). In the intragroup comparisons of PSQI ≥ 6 group, PSQI decreased significantly (p < 0.05). For the NSS in intragroup comparisons of PSQI ≥ 6 group, all parameters showed significant improvement (sneezing, p = 0.016; rhinorrhea, p = 0.005; nasal obstruction, p < 0.005; QOL, p < 0.005).
Conclusion
The use of antiallergy agents can improve CPAP adherence and sleep quality in patients with OSA on CPAP.
Keywords: Allergic rhinitis, Obstructive sleep apnea, CPAP adherence, Antiallergic agents, Sleep quality
Introduction
Allergic rhinitis (AR) is an inflammatory disease of the nasal mucosa characterized by symptoms such as rhinorrhea and nasal obstruction [1]. The release of inflammatory mediators and activation of inflammatory cells in AR causes nasal obstruction, leading to sleep disorders followed by daytime somnolence [2, 3]. There is a correlation between the severity of AR, especially nasal obstruction, and sleep quality [4]. Seasonal AR caused by Japanese cedar pollen (JCP) is the most common disease in Japan and has been considered a national affliction. In recent years, the number of affected people has increased, and the age of the disease has decreased, and it has become known as a “national disease” [5].
AR is also associated with obstructive sleep apnea (OSA) due to nasal breathing problems. Increased nasal airway resistance causes upper airway obstruction during sleep, and it has been reported that snoring and OSA in patients with nasal breathing problems have an association not only with AR, but also chronic hypertrophic rhinitis, nasal septal deviation, and nasal polyps [6]. OSA is an upper airway breathing disorder characterized by repetitive collapse of the pharyngeal airway during sleep, leading to sleep fragmentation and oxygen desaturation [7]. The apneic episodes in OSA induce an increase in blood pressure, which further exposes the patient to cardiovascular and cerebrovascular risks. OSA is more common in patients with hypertension than in the general population, and many patients with OSA may have HTN as well [8].
As for the management of OSA in the general population, continuous positive airway pressure (CPAP) therapy improves psychological and physical impairments in patients with sleepiness, fatigue, neurocognitive impairment, and depression [9]. Furthermore, it can prevent hypertension, adverse cardiovascular events, and arrythmia [10]. However, nasal breathing disorders, such as AR, nasal septal deviation, and nasal polyps, are major factors in CPAP tolerability [11, 12]. These conditions that cause nasal breathing problems result in poor tolerability to CPAP due to their significantly high nasal resistance levels [13, 14]. Therefore, the use of CPAP therapy may be affected by symptoms of AR, especially in the spring season in Japan due to JCP. There are few reports concerning the effect of AR with nasal congestion and obstruction on OSA [15, 16]. Nakayama et al. reported that seasonal changes are present in Japanese children with clinically symptomatic OSA and differ among younger (< 6 years of age) and older (6 ≥ years) children, with global trends toward improved apnea hypopnea index (AHI) during summer in younger children [15]. They suggested that nasal obstruction may have an important role in severity of pediatric OSA. Regarding adult patients with OSA who are treated with CPAP therapy, intranasal corticosteroid therapy for AR improved objective and subjective parameters of nasal patency after acute exposure of the nasal cavity to positive pressure [16]. Therapy for JCP is commonly chosen based on the severity and symptoms at peak pollen dispersal and amounts of pollen dispersal. However, the management of nasal complaints, sleep quality, and CPAP adherence in OSA with JCP remains unclear. Thus, it is worth considering the use of antiallergy agents in JCP patients, as it may improve CPAP adherence and sleep quality. The purpose of this study was to elucidate the pathological changes in patients with OSA on CPAP treated with antiallergy agents during the JCP season.
Methods
Patients
A longitudinal study was made of patients over 20 years old who had been receiving CPAP therapy for OSA more than 1 year and who have been treated with antiallergy agents, such as antihistamines and/or intranasal corticosteroids, for spring pollinosis, cedar and cypress, at the Department of Otorhinolaryngology, Head and Neck Surgery, Juntendo University Hospital between January 2020 and May 2020. This study was approved by the ethics committee of the Juntendo University Faculty of Medicine (E21-0231) and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by the Institutional Review Boards at each trial site. Written informed consent was obtained from all patients.
The diagnosis of AR was made based on subjective and objective symptoms from January 2020 to March 2020 according to Japanese guidelines [5]. Nasal discharge, nasal obstruction and sneeze, and objective symptoms were used for subjective symptoms. An objective assessment such as serous nasal discharge and swelling of the nasal turbinates during an intranasal examination was made by otolaryngologists at an otolaryngology outpatient clinic. The exclusion criteria were as follows: (1) perennial AR; (2) a history of nasal surgery for AR; (3) sublingual immunotherapy; (4) a history of severe lung disease; (5) neuromuscular disease; and (6) the presence of unknown, untreated neoplasms.
For evaluation of the subjective sleep symptoms, Epworth Sleepiness Scale (ESS) and Pittsburgh Sleep Quality Index (PSQI) were performed before and after treatment [11, 17]. Excessive daytime sleepiness was measured using ESS and scores of 11 and above were considered indicative of excessive daytime sleepiness. For measuring the subjective sleep quality, PSQI, a self-administered questionnaire widely used for evaluating sleep quality, was also ompleted during the previous month.
Nasal symptoms scores (NSS) were obtained for subjective nasal symptoms, which consisted of sneezing, rhinorrhea, and nasal blockage using a 5-point scale (0: none; 1: mild; 2: moderate; 3: severe; and 4: very severe) based on the Japanese guidelines for AR [5]. Problems of daily life were also assessed as a quality of life (QOL) in the NSS questionnaire for Japanese with allergic rhinitis (Table 1).
Table 1.
Nasal symptoms scores
| Types | Score (severity) | ||||
|---|---|---|---|---|---|
| 0 (none) | 1 (mild) | 2 (moderate) | 3 (severe) | 4 (very severe) | |
| Paroxysmal sneezing (average number of episodes of paroxysmal sneezing in a day) | Below score 1 | 5–1 times | 10–6 times | 20–11 times | ≥ 21 times |
| Rhinorrhea (average number of episodes of nose blowing a day) | Below score 1 | 5–1 times | 10–6 times | 20–11 times | ≥ 21 times |
| Nasal blockage | Below score 1 | Nasal blockage without oral breathing | Severe nasal blockage causing occasional oral breathing in a day | Severe nasal blockage causing prolonged oral breathing throughout the day | Completely obstructed all day |
| QOL (troubles with daily life*) | Below score 1 | Few troubles | Intermediate between score 1 and 3 | Painful and complicating daily life | Impossible |
Adapted and modified from reference #5
QOL quality of life
*Troubles with daily life: troubles with work, study, household work, sleep, going out, etc.
Data from CPAP devices were downloaded at the sleep lab before the prescription of antiallergy agents and 2 months after the medication. The following parameters from CPAP were evaluated: (1) percentage days with CPAP usage (%); (2) percentage days with CPAP usage more than 4 h (%); (3) average usage per night (min); (4) average apnea-hypopnea index (AHI) on CPAP (events/h); and (5) average CPAP pressure in 90% of usage time (cmH2O).
All parameters of PSQI, ESS, NSS, and downloaded data of CPAP were compared before and after medication with antiallergy agents in all patients. A PSQI score of 6 was used as a cutoff value based on a previous report [11]. Based upon PSQI scores before medication, patients were divided into two groups, PSQI < 6 group and PSQI ≥ 6 group. Intragroup and intergroup comparisons were performed.
Statistical analysis
The collected data were tabulated using Microsoft Excel for Windows, software version 2010 (Microsoft Corp., Redmond, WA, U.S.A.). Each score in the above survey items was compared by the t test for normally distributed continuous variables and Wilcoxon signed-rank test or Mann–Whitney U test for non-normally distributed continuous variables. A value of p < 0.05 was considered to be statistically significant. All values are shown as the mean ± SD or median (interquartile range).
Results
In total, 28 patients were examined. The average age of the participants was 62.9 ± 9.7 years, 26 of 28 were male, and antihistamines were used in 6 people. Internasal corticosteroids were used in 5 people, and both were used in 17 people (Table 2).
Table 2.
Patient characteristics
| Variable | Value |
|---|---|
| Age, years | 62.9 ± 9.7 |
| Sex | |
| Male:female | 26:2 |
| Complications | |
| Hypertension, n (%) | 10 (36%) |
| Diabetes, n (%) | 7 (25%) |
| Heart failure, n (%) | 3 (11%) |
| Respiratory disease, n (%) | 0 (0%) |
| Therapy for pollinosis* | |
| Second-generation antihistamine monotherapy, n (%) | 6 (21%) |
| Internasal corticosteroids monotherapy, n (%) | 4 (14%) |
| Second-generation antihistamine and internasal corticosteroids combined therapy, n (%) | 17 (61%) |
| Second-generation antihistamine and anti-LTs agent combined therapy, n (%) | 1 (4%) |
| AHI at OSA diagnosis (events/hour) | 49.0 ± 21.7 |
| Duration of CPAP use (years) | 6.1 ± 3.5 |
| PSQI score | 5.6 ± 3.2 |
| ESS score | 6.9 ± 4.0 |
N = 28. Values are expressed as mean ± SD or median
*As second-generation antihistamine agents, loratadine was administered to 1 patient, epinastine hydrochloride to 2 patients, olopatadine hydrochloride to 1 patient, fexofenadine hydrochloride to 1 patient, bepotastine besilate to 5 patients, levocetirizine hydrochloride to 3 patients, desloratadine to 1 patient, bilastine to 6 patients, rupatadine fumarate to 2 patients, and emedastine fumarate to 1 patient. As internasal corticosteroids, mometasone furoate hydrate was administered to 11 patients, fluticasone furoate to 4 patients, and dexamethasone cipecilate to 2 patients. As anti-LTs agent, pranlukast hydrate was administered to 1 patient
LTs leukotriene receptor antagonist, AHI apnea hypopnea index, CPAP continuous positive airway pressure, ESS Epworth Sleepiness Scale
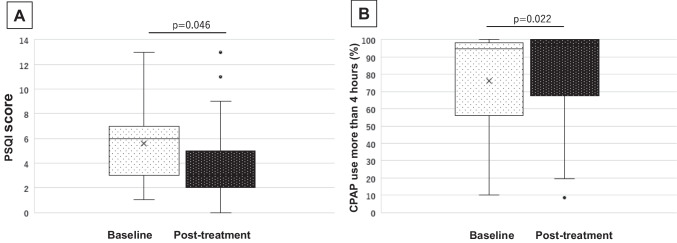
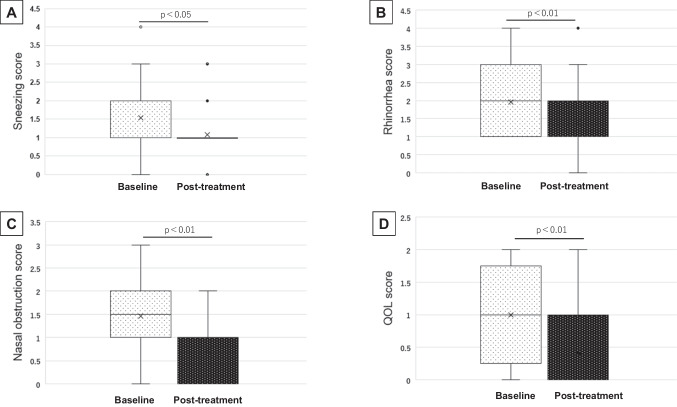
First, data from a total of 28 patients were assessed, as shown in in Table 3. PSQI for sleep quality showed significant improvements after medication (p = 0.046) (Fig. 1A). ESS, which was for subjective symptoms of daytime drowsiness, showed no significant differences after AR medication (p = 0.565). Significant improvement was observed after the prescription of antiallergy agents in all items of sneezing (p = 0.020), rhinorrhea (p = 0.007), nasal obstruction (p < 0.001), and QOL (p = 0.001) (Fig. 2). Concerning the CPAP use, percentage of days with CPAP use more than 4 h increased significantly after the administration of rhinitis medication (p = 0.022) (Fig. 1B). No other parameters in the downloaded CPAP data from CPAP showed significant differences.
Table 3.
PSQI, ESS, and CPAP use before and after administration of antiallergic agents
| Variable | Baseline | Post-treatment | p-value |
|---|---|---|---|
| PSQI score | 5.6 ± 3.2 [5.5] | 3.8 ± 3.1 [3.0] | 0.046* |
| ESS score | 6.9 ± 4.0 | 7.1 ± 4.6 | 0.565 |
| Nasal symptoms score | |||
| Sneezing score | 1.5 ± 0.9 [1.0] | 1.1 ± 0.7 [1.0] | 0.020 |
| Rhinorrhea score | 2.0 ± 1.0 [2.0] | 1.4 ± 1.0 [1.0] | 0.007 |
| Nasal blockage score | 1.5 ± 0.8 [1.5] | 0.7 ± 0.7 [0.7] | < 0.001 |
| QOL (troubles of daily life) score | 1.0 ± 0.7 [1.0] | 0.4 ± 0.6 [0.4] | 0.001 |
| Downloading data from CPAP | |||
| Percent days with CPAP usage (%) | 88.4 ± 18.4 | 90.9 ± 15.5 | 0.090 |
| Percent of days with CPAP usage > 4 h (%) | 75.3 ± 29.4 | 83.3 ± 24.8 | 0.022* |
| Average usage (min) | 367 ± 99.5 [370] | 370.7 ± 101.5 [378] | 0.326 |
| Average AHI on CPAP (events/h) | 3.0 ± 3.6 [2.1] | 3.3 ± 3.6 [2.3] | 0.106 |
| Average CPAP < 90% pressure in 90% of usage time (cmH2O) | 8.4 ± 2.0 [7.8] | 8.3 ± 1.9 [8.1] | 0.513 |
Values are expressed as mean ± SD or median (interquartile range). CPAP continuous positive airway pressure, AHI apnea hypopnea index, ESS Epworth Sleepiness Scale, PSQI Pittsburgh Sleep Quality Index
*Significant values
Fig. 1.
Changes before and after medication of antiallergic agents. A PSQI showed significant improvements after the medication (p = 0.046). B CPAP use for ≥ 4 h per night increased significantly after medication (p = 0.022)
Fig. 2.
Changes of each nasal symptoms scores (NSS) before and after medication of antiallergy agents. Significant improvement was observed after the prescription of antiallergy agents in sneezing (A, p = 0.020), rhinorrhea (B, p = 0.007), nasal obstruction (C, p < 0.001), and QOL (p = 0.001)
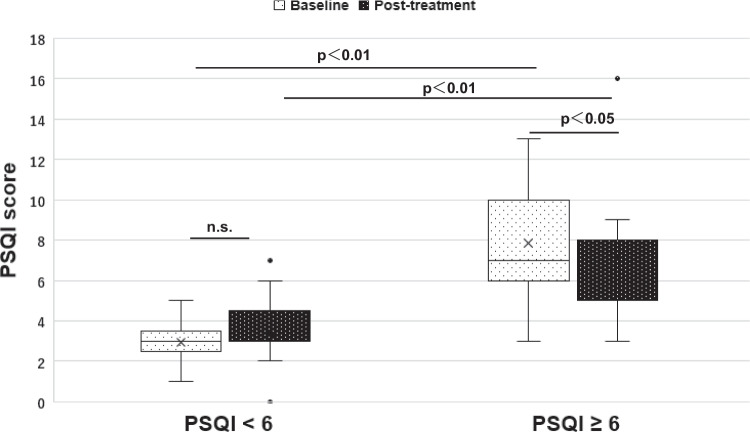
Second, patients who scored PSQI < 6 before medication with antiallergy agents were defined as “the group without decreased sleep quality,” and PSQI ≥ 6 was defined as “the group with decreased sleep quality.” Intergroup comparisons were performed between PSQI < 6 group and PSQI ≥ 6 group (Table 4). Thirteen patients were in the PSQI < 6 group and 15 patients who were considered poor sleepers were in the PSQI ≥ 6 group, who were considered poor sleepers. No significant improvement in the PSQI score was observed after medication with antiallergy agents in the PSQI < 6 group. In contrast, PSQI decreased significantly, which suggested an improvement of the sleep quality by the antiallergy agents in the PSQI ≥ 6 group (p < 0.05) (Fig. 3). ESS showed no significant differences between the intra- and intergroup comparisons. For the NSS in the intragroup comparisons of PSQI ≥ 6 group, all parameters, sneezing, rhinorrhea, nasal blockage, and QOL, showed significant improvement. On the other hand, only the sneezing score was not significantly different in the PSQI < 6 group. Both intra- and intergroup comparisons were also made for the CPAP use data. CPAP adherence had already been good before the medication of antiallergy agents even in the PSQI < 6 group. Percentage days with CPAP usage > 4 h (%) in post-treatment showed significant higher in the PSQI < 6 group for the intergroup comparisons.
Table 4.
Intragroup and intergroup comparison of the values among the groups
| PSQI < 6 group (n = 13) | PSQI ≥ 6 group (n = 15) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-treatment | p1 | Baseline | Post-treatment | p1 | p2 | p3 | |
| PSQI | 2.8 ± 1.1 [3.0] | 3.3 ± 1.6 [3.6] | 0.156 | 8.2 ± 2.5 [7.5] | 5.9 ± 2.5 [7.0] | 0.009* | < 0.005* | < 0.05* |
| ESS | 6.6 ± 3.4 [7] | 5.8 ± 3.7 [6] | 0.551 | 7.1 ± 4.6 [6] | 7.6 ± 5.4 [6] | 0.551 | 0.963 | 0.685 |
| Nasal symptoms scores | ||||||||
| Sneezing score | 1.0 [1.0] | 1.0 [0.5] | 0.073 | 2.0 [1.0] | 1.0 [0.0] | 0.016* | 0.680 | 1.0 |
| Rhinorrhea score | 2.0 [2.0] | 1.0 [1.0] | 0.031* | 1.0 [1.0] | 1.0 [1.0] | 0.005* | 0.593 | 0.846 |
| Nasal obstruction score | 1.0 [1.0] | 1.0 [1.0] | 0.007* | 2.0 [1.0] | 1.0 [1.0] | < 0.005* | 0.813 | 1.0 |
| QOL score | 1.0 [1.5] | 0.0 [1.0] | 0.003* | 1.0 [1.0] | 0.0 [1.0] | < 0.005* | 0.302 | 0.519 |
| Downloading data from CPAP | ||||||||
| Percent days with CPAP usage (%) | 90 ± 19 | 96 ± 10 | 0.49 | 87 ± 18 | 87 ± 19 | 0.079 | 0.534 | 0.106 |
| Percent days with CPAP usage > 4 h (%) | 79.9 ± 30.0 | 95.4 ± 9.7 | 0.11 | 73.9 ± 29.0 | 70.7 ± 32.9 | 0.170 | 0.503 | 0.008 |
| Average usage (min) | 431 [226] | 430 [188] | 0.660 | 366 [122] | 323 [158] | 0.418 | 0.150 | 0.086 |
| Average AHI (events/hr) | 2.8 [2.9] | 2.3 [3.5] | 0.947 | 2.0 [1.1] | 2.0 [1.6] | 0.094 | 0.181 | 0.206 |
| CPAP pressure (cmH2O in > 90% of usage time) | 8.7 [3.2] | 8.6 [3.3] | 0.639 | 7.1 [3.0] | 7.2 [2.5] | 0.492 | 0.468 | 0.410 |
Values are expressed as mean ± SD or median (interquartile range). p1, intragroup comparison; p2, intergroup comparison before the intervention; p3, intergroup comparison after the intervention
PSQI Pittsburgh sleep quality index, ESS Epworth Sleepiness Scale, CPAP continuous positive airway pressure, AHI average apnea hypopnea index
*Significant values
1t test for normally distributed continuous variables and Wilcoxon signed-rank test for non-normally distributed continuous variables
2,3Mann-Whitney U test
Fig. 3.
PSQI differences before and after medication of antiallergy agents which performed intergroup and intragroup comparisons between PSQI < 6 and PSQI ≥ 6 group. In intergroup comparisons, the PSQI < 6 group had significantly lower PSQI score before and after treatment than the PSQI < 6 group. In intragroup comparisons, no significant improvement in the PSQI score was observed after medication in the PSQI < 6 group. In contrast, PSQI decreased significantly in the PSQI ≥ 6 group (p < 0.05)
Discussion
The present study revealed several novel and interesting observations relevant to the pathogenesis of OSA with AR in patients who were receiving CPAP therapy. We examined the impact of pollinosis treatment on CPAP users. First, significant improvements in PSQI and NSS were observed after medication for spring pollinosis, which suggested an improvement in sleep quality and subjective nasal symptoms. In addition, the percentage of days with CPAP use more than 4 h increased significantly after post-medication. Second, in the PSQI ≥ 6 group before treatment, the PSQI score and all NSS items decreased significantly after medication.
Many studies have reported that respiratory allergies adversely affect a patient’s QOL, causing changes in mood, a decline in cognitive function, deterioration in school and work performance, memory deficit, and inability to concentrate. Sleep disruption, sleepiness, and fatigue are often seen in patients with AR. It has been reported that a greater than normal elevation of K complex, an indicator of sleep stage NREM 2 (light sleep), appeared in EEG [12]. Furthermore, symptoms of rhinitis such as sneezing, rhinorrhea, nasal obstruction, and itching were evoked by components of the immune and inflammatory response, which may also affect sleep and daytime drowsiness [18]. Although drowsiness and fatigue are standard clinical symptoms of AR, it is controversial whether these symptoms can be explained by the side effects of antiallergy agents, especially antihisitamines [19]. As for antihistamines in this study, most are second-generation antihistamines, which have reduced central nerve system side effects such as drowsiness and anticholinergic effects compared to those of earlier generations [20]. Furthermore, since we compared between before and after antihistamine administration and the PSQI scores were reduced afterwards, it is less likely that the side effects of drowsiness were due to medication.
Inflammatory chemical mediators, such as cytokines and prostaglandin (PG), have long been reported to be involved in sleep/wake regulation in animal studies [21, 22]. These studies showed that the production of cytokines and PGs in the brain and play a role in various physiological activities, including sleep and wakefulness. Craig et al. mentioned that nasal congestion with AR might be the cause of rhinitis-related sleep disorders and that intranasal corticosteroids affect not only nasal symptoms but also sleep quality [23]. Krouse et al. showed elevated cytokines such as interleukin-1 beta (IL-1β), IL-4, IL-6, IL-10, prolonged rapid eye (REM) latency, and decreased REM sleep duration in patients with AR, and suggested that inflammatory cytokines may affect sleep/wake regulation in human [3]. Interferon-gamma (IF-γ), tumor necrosis factor-alpha (TNF-α), and other mediators are related to sleep regulation [24]. These inflammatory cytokines have also been shown to be elevated in patients with OSA patients [25, 26]. Considering that the patients with OSA in this study had disease that was moderate to severe, although their CPAP adherence had been consistent throughout the study period, there is a low probability that the OSA was a cause of the drowsiness. Inflammatory cytokines induce exacerbations of allergic rhinitis and nasal obstruction, promoting sleep disorders and OSA [27]. On the other hand, OSA-activated inflammatory chemical mediators exacerbate AR and further reduce the sleep quality. In other words, it can be said that appropriate treatment for allergic rhinitis is also important for improving sleep quality.
As for nasal obstruction due to AR, this is associated with daytime drowsiness and loss of focus during the daytime [27, 28]. Particularly, nasal congestion is considered to be one of the most bothersome and prevalent symptoms of AR [28]. Furthermore, nasal obstruction is also a major contributor to reduced CPAP adherence. It has been reported that patients with AR have higher nasal resistance in the supine position than patients without AR [29]. Hence, treatment for allergic rhinitis is important in the continuation of CPAP treatment. The use of intranasal corticosteroids for rhinitis symptoms has been reported to improve CPAP adherence [16, 28]. We also previously reported that nasal surgery for nasal obstruction improves not only nasal resistance but also CPAP adherence as well as daytime sleepiness, as evaluated by ESS [14]. In this study, CPAP adherence had been good before treatment, but the percentage of days with CPAP usage more than 4 h was significantly improved after treatment with antiallergy agents. It has been reported that the prolonged use of CPAP is beneficial for hypertension (HT), both systolic and diastolic blood pressure [10]. HT accounts for 36% (n = 10) of our cases, and it is considered that the suppression of rhinitis symptoms by antiallergic agents also contributed a significant effect on CPAP use.
Although ESS and PSQI were used to evaluate sleep, only PSQI after medication with antiallergy agents showed a significant improvement. In the PSQI ≥ 6 group, which experienced a deterioration of sleep quality before the treatment in spite of their consistent use of CPAP, there was a significant improvement in PSQI. In addition, significant improvements were shown in all items of NSS score sneezing, nasal obstruction, rhinorrhea, and QOL in the PSQI ≥ 6 group. There is a possibility that the improvement of nasal symptoms with antiallergy agents may affect the quality of sleep. There was no change in the CPAP adherence after treatment, even in the PSQI ≥ 6 group. Thus, nasal symptoms caused by pollinosis may have affected the quality of sleep.
In the past few years, the number of patients with spring pollinosis, especially with Japanese cedar pollinosis, has markedly increased. The number of patients with allergic rhinitis has increased between 1998 and 2019 in Japan. It is estimated that 50% of Japanese people have allergic rhinitis to some allergens, and it has come to be called a national disease. In particular, the number of patients with Japanese cedar pollinosis has increased [5]. Pollinosis adversely affects not only nasal symptoms but also daily life such as work, study, household work, sleep, and going out. It is estimated that the economic loss associated with pollinosis will reach 300 billion yen annually [30]. OSA also affects the QOL [9]. CPAP treatment is most effective in the treatment of moderate or severe OSA, but nasal symptoms such as pollinosis are factors that reduce the tolerability of CPAP [13]. The findings of the current study suggest that it is beneficial to treat CPAP users during the spring pollinosis period with antiallergy agents because not only nasal symptoms but also subjective improvement in sleep quality as well as increased CPAP use more than 4 h were observed.
Some limitations of this study should be noted. First, subjective scores such as PSQI, ESS, and NSS were mainly used in this study, so objective data from testing such as polysomnography should be performed to further our understanding of the pathophysiology. Second, the sample size was small due to the COVID-19 pandemic. No statistical sample size calculations were conducted in this study. Third, although antihistamines and/or intranasal corticosteroids were used in this treatment, the dosages were not consistent, and the starting time of administration also varied depending on the case. Hence, it is unclear if there was tachyphylaxis to H2 blockers or not. Fourth, inflammatory markers were not checked such as antigen specific and nonspecific IgE, eosinophil counts, and interleukins before and after the medication of antiallergy agents. Fifth, a control group was not employed in this study. Sixth, the present study is a pilot study and further examination is necessary.
Conclusions
The use of antiallergy agents during spring pollinosis can be expected to improve CPAP adherence and sleep quality in patients with OSA on CPAP. These possibilities should be tested in further investigations with larger sample sizes and with a single-antiallergy agent or with randomized controlled trials.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Juntendo University Faculty of Medicine) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferguson BJ. Influences of allergic rhinitis on sleep. Otolaryngol Head Neck Surg. 2004;130:617–629. doi: 10.1016/j.otohns.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Craig TJ, McCann JL, Gurevich F, Davies MJ. The correlation between allergic rhinitis and sleep disturbance. J Allergy Clin Immunol. 2004;114:139–145. doi: 10.1016/j.jaci.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 3.Krouse HJ, Davis JE, Krouse JH. Immune mediators in allergic rhinitis and sleep. Otolaryngol Head Neck Surg. 2002;126:607–613. doi: 10.1067/mhn.2002.125300. [DOI] [PubMed] [Google Scholar]
- 4.Colás C, Galera H, Añibarro B, Soler R, Navarro A, Jáuregui I, Peláez A. Disease severity impairs sleep quality in allergic rhinitis (The SOMNIAAR study) Clin Exp Allergy. 2012;42:1080–1087. doi: 10.1111/j.1365-2222.2011.03935.x. [DOI] [PubMed] [Google Scholar]
- 5.Okubo K, Kurono Y, Ichimura K, Enomoto T, Okamoto Y, Kawauchi H, Suzaki H, Fujieda S, Masuyama K, Japanese Society of Allergology Japanese guidelines for allergic rhinitis 2020. Allergol Int. 2020;69:331–345. doi: 10.1016/j.alit.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Lofaso F, Coste A, d’Ortho MP, Zerah-Lancner F, Delclaux C, Goldenberg F, Harf A. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J. 2000;16:639–643. doi: 10.1034/j.1399-3003.2000.16d12.x. [DOI] [PubMed] [Google Scholar]
- 7.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbé F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo AJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 11.Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui K, Kamei Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97:165–172 . doi: 10.1016/s0165-1781(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 12.Lavie P, Gertner R, Zomer J, Podoshin L. Breathing disorders in sleep associated with “microarousals” in patients with allergic rhinitis. Acta Otolaryngol. 1981;92:529–533. doi: 10.3109/00016488109133292. [DOI] [PubMed] [Google Scholar]
- 13.Inoue A, Chiba S, Matsuura K, Osafune H, Capasso R, Wada K. Nasal function and CPAP compliance. Auris Nasus Larynx. 2019;46:548–558. doi: 10.1016/j.anl.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Kasai M, Minekawa A, Homma H, Nakazawa A, Iizuka T, Inoshita A, Ikeda K. Nasal surgery improves continuous positive airway pressure compliance and daytime sleepiness in obstructive sleep apnea syndrome. J Otol Rhinol S. 2015;1:26–29. doi: 10.4172/2324-8785.S1-006. [DOI] [Google Scholar]
- 15.Nakayama M, Koike S, Kuriyama S, Suzuki M, Nakamura Y, Yamamoto K, Murakami S, Gozal D. Seasonal variation in a clinical referral pediatric cohort at risk for obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2013;77:266–269. doi: 10.1016/j.ijporl.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Balsalobre L, Figueiredo AB, Pezato R, Fujita RR. Effect of topical corticosteroids on nasal patency after acute positive airway pressure exposure. Braz J Otorhinolaryngol. 2021;87:326–332. doi: 10.1016/j.bjorl.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Léger D, Morin CM, Uchiyama M, Hakimi Z, Cure S, Walsh JK. Chronic insomnia, quality-of-life, and utility scores: comparison with good sleepers in a cross-sectional international survey. Sleep Med. 2012;13:43–51. doi: 10.1016/j.sleep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz H, Wilkinson CJ. Antihistamines and driving-related behavior: a review of the evidence for impairment, DOT HS 809 714. Washington: National Highway Traffic Safety Administration; 2004. pp. l1–36. [Google Scholar]
- 20.Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, Dawson DE, Dykewicz MS, Hackell JM, Han JK, Ishman SL, Krouse HJ, Malekzadeh S, Mims JW, Omole FS, Reddy WD, Wallace DV, Walsh SA, Warren BE, Wilson MN, Nnacheta LC, Guideline Otolaryngology Development Group. AAO-HNSF Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(1 Suppl):S1–43. doi: 10.1177/0194599814561600. [DOI] [PubMed] [Google Scholar]
- 21.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med Rev. 2011;15:411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Craig TJ, Sherkat A, Safaee S. Congestion and sleep impairment in allergic rhinitis. Curr Allergy Asthma Rep. 2010;10:113–121. doi: 10.1007/s11882-010-0091-5. [DOI] [PubMed] [Google Scholar]
- 24.Krueger JM, Majde JA, et al. Sleep and host defense. In: Kryger MH, et al., editors. Principles and practice of sleep medicine. 5. Philadelphia: Elsevier; 2011. pp. 281–290 . [Google Scholar]
- 25.Alberti A, Sarchielli P, Gallinella E, Floridi A, Floridi A, Mazzotta G, Gallai V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12:305–311. doi: 10.1111/j.1365-2869.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 26.Minoguchi K, Tazaki T, Yokoe T, Minoguchi H, Watanabe Y, Yamamoto M, Adachi M. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–1479. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 27.Chirakalwasan N, Ruxrungtham K. The linkage of allergic rhinitis and obstructive sleep apnea. Asian Pac J Allergy Immunol. 2014;32:276–286. [PubMed] [Google Scholar]
- 28.Storms W. Allergic rhinitis-induced nasal congestion: its impact on sleep quality. Prim Care Respir J. 2008;17:7–18. doi: 10.3132/pcrj.2008.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa M. Posture-induced nasal obstruction in patients with allergic rhinitis. Clin Otolaryngol Allied Sci. 1994;19:135–137. doi: 10.1111/j.1365-2273.1994.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamada T, Saito H, Fujieda S. Present state of Japanese cedar pollinosis: the national affliction. J Allergy Clin Immunol. 2014;133:632–639.e5. doi: 10.1016/j.jaci.2013.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.