Dear Editor,
We read with interest the retrospective observational study investigating the short-term effect of oral anti-viral agents on United States Veterans aged 65 years and older who were at high risk of developing severe COVID-19.1 Two groups, each composed of 1370 individuals, were identified based on the presence or absence of oral antivirals treatment (either molnupiravir or nirmatrelvir plus ritonavir [NMV-r]) using propensity score matching method to balance their baseline characteristics. Compared with the group not receiving oral antivirals, elderly patients receiving NMV-r or molnupiravir had a lower risk of hospitalization or death within 30 days of diagnosis (4.75% versus 10.2%; odds ratio [OR], 0.44, 95% CI, 0.32–0.60).1 This finding was consistent with the meta-analysis of seven studies by Zheng et al.,2 in which the OR of death or hospitalization among COVID-19 patients in the NMV-r vs. control group was 0.22 (95% CI, 0.11–0.45).2 Both studies suggested the beneficial effect of oral antivirals, especially NMV-r’s effect on the short-term outcome of patients with COVID-19.1, 2 In addition to the short-term benefit, two studies further reported that NMV-r could reduce the post-acute sequelae or complications.3, 4 However, the effect of NMV-r on subacute hospitalization or mortality among COVID-19 survivors was not reviewed. Therefore, we conducted this study to assess the post-acute impact of NMV-r on COVID-19 survivors.
This retrospective cohort study was conducted using the database from the TriNetX Research Network. TriNetX is a global health-collaborative clinical-research platform, which provides real-time healthcare-associated information from more than 120 healthcare organizations (HCOs) across 19 countries.5 We conducted the patient selection and data curation on February 01, 2023. The inclusion criteria for our selected cohort comprised of: (a) patients ≥18 years old, (b) patients who tested positive for SARS-CoV-2 infection or were diagnosed with COVID-19 between March 01, 2020 and June 30, 2022, (c) patients who have visited HCOs at least twice during the time period, and (d) patients who did not decease in the first month of infection. Patients who have ever received remdesivir, molnupiravir, monoclonal antibody, or convalescent plasma were excluded. Among the eligible patients, we divided these candidates into two groups based on the use of NMV-r: a study group receiving NMV-r and a control group without the use of NMV-r. To adjust the difference for the baseline characteristics, two matched cohorts were created by propensity score with a 1:1 matching method by age, gender, race, ethnicity, and comorbid medical conditions, where a standard difference of less than 0.1 indicates good matching. The primary outcome composite endpoint of all-cause mortality or hospitalization between one and six months after the diagnosis of COVID-19. The secondary outcomes consisted of all-cause mortality, hospitalization, and critical care use individually during the same follow-up period. The hazard ratio (HR) with 95% confidence interval (95% CI) of clinical outcomes was calculated for the study group using NMV-r versus the control group. All statistical analyses were conducted using the built-in function of TriNetX network.
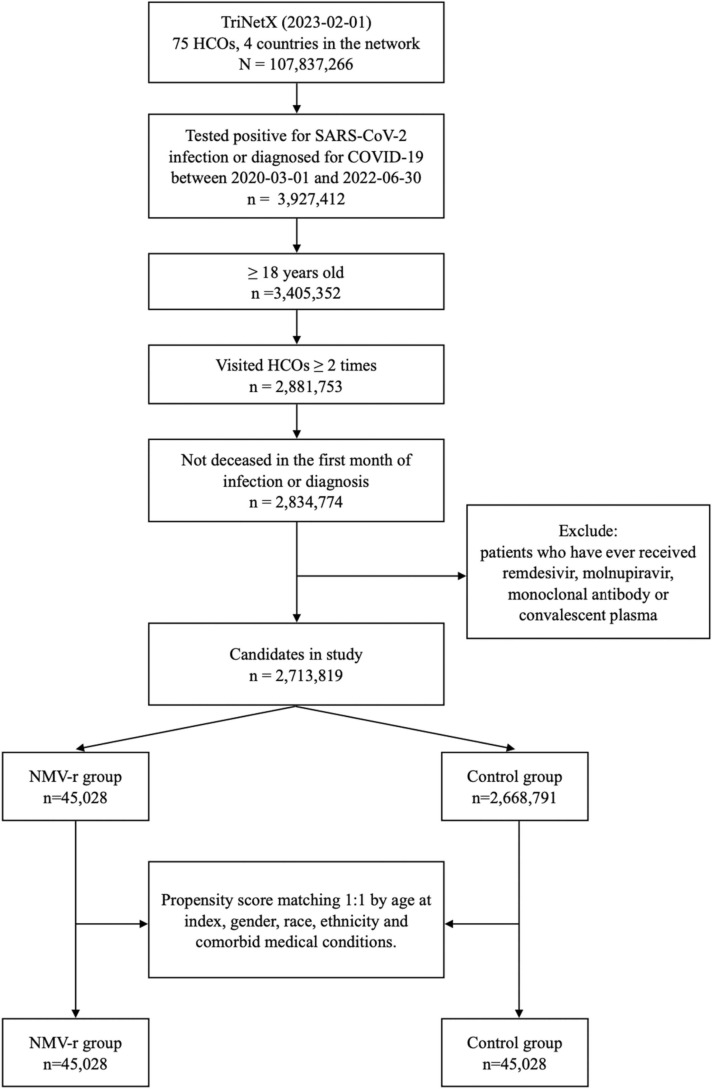
Initially, 2,713,819 patients, including 45,028 patients receiving NMV-r (study group) and 2,668,797 COVID-19 patients without NMV-r (control group) met the inclusion criteria ( Fig. 1). Compared to the control group, the study group consisted of older age and predominantly white individuals. The study group had more comorbidities, such as essential hypertension, diabetes mellitus, chronic lower respiratory tract disease, chronic kidney disease, ischemic heart disease, other forms of heart disease, and neoplasms than the control group. In addition, the study group had a higher body mass index than the control group. After propensity score matching, 45,028 cases with matched baseline characteristics were retained in each cohort ( Table 1).
Fig. 1.
The algorithm of patient selection and cohort construction.
Table 1.
Comparison of characteristics of patients receiving nirmatrelvir plus ritonavir (NMV-r) and not receiving NMV-r before and after propensity score matching.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| NMV-r group (n = 45,028) | Control group (n = 2,668,631) | Standardized difference | NMV-r group (n = 45,028) | Control group (n = 45,028) | Standardized difference | |
| Age at index (Mean±SD) | 57.0 ± 16.5 | 46.9 ± 8.3 | 0.582 | 57.0 ± 16.5 | 57.0 ± 16.8 | 0.004 |
| Gender (%) | ||||||
| Female | 27,663 (61.4) | 1572,943 (59.0) | 0.051 | 27,663 (61.4) | 27,536 (61.2) | 0.006 |
| Male | 17,347 (38.5) | 1094,047 (41.0) | 0.051 | 17,347 (38.5) | 17,483 (38.8) | 0.006 |
| Race (%) | ||||||
| White | 37,068 (82.3) | 1442,203 (54.1) | 0.637 | 37,068 (82.3) | 37,063 (82.3) | < 0.001 |
| Black or African American | 3825 (8.5) | 374,407 (14.0) | 0.176 | 3825 (8.5) | 4040 (9) | 0.017 |
| Asian | 159 (2.0) | 144,486 (2.1) | 0.016 | 159 (2.0) | 766 (1.7) | 0.001 |
| American Indian or Alaska Native | 92 (0.2) | 8812 (0.3) | 0.024 | 92 (0.2) | 102 (0.2) | 0.005 |
| Native Hawaiian or Other Pacific Islander | 22 (0.0) | 3470 (0.1) | 0.027 | 22 (0.0) | 50 (0.1) | 0.022 |
| Unknown Race | 3252 (7.2) | 787,884 (29.5) | 0.602 | 3252 (7.2) | 3001 (6.7) | 0.022 |
| Ethnicity (%) | ||||||
| Not Hispanic or Latino | 37,231 (82.7) | 1507,413 (56.5) | 0.594 | 37,231 (82.7) | 37,190 (82.6) | 0.002 |
| Hispanic or Latino | 3138 (7.0) | 214,626 (8.0) | 0.041 | 3138 (7.0) | 3168 (7) | 0.003 |
| Other Ethnicity | 4653 (10.3) | 945,639 (35.4) | 0.626 | 4653 (10.3) | 4664 (10.4) | 0.001 |
| Comorbidities (%) | ||||||
| Essential hypertension | 18,155 (40.3) | 500,584 (18.8) | 0.486 | 18,155 (40.3) | 18,381 (40.8) | 0.01 |
| Diabetes mellitus | 8116 (18) | 251,578 (9.4) | 0.252 | 8116 (18) | 8195 (18.2) | 0.005 |
| Type 2 diabetes mellitus | 7857 (17.5) | 240,467 (9) | 0.251 | 7857 (17.5) | 7919 (17.6) | 0.004 |
| Hypertensive diseases | 18,368 (40.8) | 515,500 (19.3) | 0.482 | 18,368 (40.8) | 18,624 (41.4) | 0.012 |
| Chronic lower respiratory diseases | 7902 (17.6) | 226,554 (8.5) | 0.272 | 7902 (17.6) | 7655 (17) | 0.015 |
| Nicotine dependence | 3319 (7.4) | 128,397 (4.8) | 0.107 | 3319 (7.4) | 3282 (7.3) | 0.003 |
| Ischemic heart diseases | 4369 (9.7) | 136,952 (5.1) | 0.175 | 4369 (9.7) | 4178 (9.3) | 0.014 |
| Other forms of heart disease | 7360 (16.3) | 250,950 (9.4) | 0.208 | 7360 (16.3) | 7343 (16.3) | 0.001 |
| Hypertensive heart disease | 791 (1.8) | 31,901 (1.2) | 0.047 | 791 (1.8) | 776 (1.7) | 0.003 |
| Chronic kidney disease | 2673 (5.9) | 107,782 (4) | 0.087 | 2673 (5.9) | 2583 (5.7) | 0.009 |
| Chronic kidney disease, stage 4 | 119 (0.3) | 14,059 (0.5) | 0.042 | 119 (0.3) | 153 (0.3) | 0.014 |
| Chronic kidney disease, stage 3 | 1621 (3.6) | 53,766 (2) | 0.096 | 1621 (3.6) | 1516 (3.4) | 0.013 |
| Diseases of liver | 3003 (6.7) | 82,389 (3.1) | 0.167 | 3003 (6.7) | 2831 (6.3) | 0.016 |
| Alcoholic liver disease | 74 (0.2) | 5653 (0.2) | 0.011 | 74 (0.2) | 157 (0.3) | 0.036 |
| Hepatic failure | 60 (0.1) | 5077 (0.2) | 0.014 | 60 (0.1) | 145 (0.3) | 0.04 |
| Chronic hepatitis | 18 (0) | 830 (0) | 0.005 | 18 (0) | 22 (0) | 00.004 |
| Fibrosis and cirrhosis of liver | 332 (0.7) | 16,694 (0.6) | 0.014 | 332 (0.7) | 527 (1.2) | 0.045 |
| Fatty liver | 1747 (3.9) | 37,590 (1.4) | 0.154 | 1747 (3.9) | 1393 (3.1) | 0.043 |
| Chronic passive congestion of liver | 120 (0.3) | 1884 (0.1) | 0.048 | 120 (0.3) | 53 (0.1) | 0.034 |
| Portal hypertension | 73 (0.2) | 5143 (0.2) | 0.007 | 73 (0.2) | 149 (0.3) | 0.034 |
| Other specified diseases of liver | 867 (1.9) | 14,613 (0.5) | 0.125 | 867 (1.9) | 519 (1.2) | 0.063 |
| Neoplasms | 10,852 (24.1) | 277,440 (10.4) | 0.369 | 10,852 (24.1) | 10,824 (24) | 0.001 |
| Malignant neoplasms of lymphoid, hematopoietic and related tissue | 633 (1.4) | 21,068 (0.8) | 0.059 | 633 (1.4) | 634 (1.4) | <0.001 |
| BMI (Mean±SD) | 30.2 ± 6.7 | 29.7 ± 6.9 | 0.075 | 30.2 ± 6.7 | 30.7 ± 6.6 | 0.068 |
| 25–30 kg/m2 | 9193 (20.4) | 259,692 (9.7) | 0.302 | 9193 (20.4) | 9160 (20.3) | 0.002 |
| 30–35 kg/m2 | 7113 (15.8) | 202,665 (7.6) | 0.257 | 7113 (15.8) | 7048 (15.7) | 0.004 |
| ≥35 kg/m2 | 6097 (13.5) | 172,860 (6.5) | 0.237 | 6097 (13.5) | 6149 (13.7) | 0.003 |
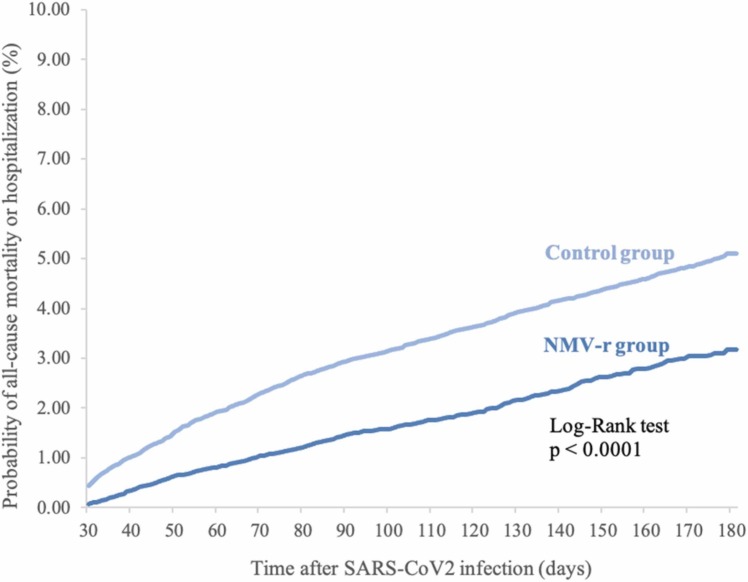
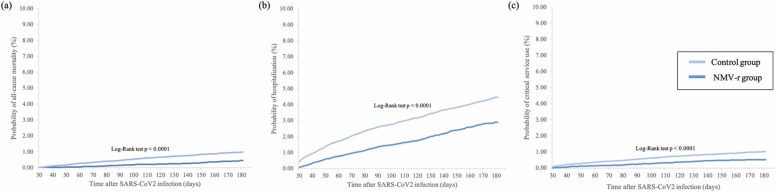
During the follow-up period of one to six months, 589 cases in the study group and 1814 cases in the control group had the composite outcomes of hospitalization or all-cause mortality. Overall, the study group had a lower risk of hospitalization or all-cause mortality within the time period (HR, 0.543; 95% CI, 0.495–0.597). Fig. 2 demonstrates the Kaplan-Meier time-to-event curves of the two groups for the composite outcome of all-cause mortality or hospitalization (log-rank p < 0.0001). Furthermore, we also found that, in comparison to the control group, the study group had a lower risk of all-cause mortality (HR, 0.392; 95% CI, 0.303–0.507), hospitalization (HR, 0.569; 95% CI, 0.515–0.628), and critical care use (HR, 0.473; 95% CI, 0.375–0.592) individually. Critical care use was defined as the need for critical care evaluation and management, ventilation assistance, or extracorporeal membrane oxygenation according to Yendewa et al.6 The three Kaplan-Meier time-to-event curves for the above-listed secondary outcomes showed similar trends (all log-rank p < 0.0001)( Fig. 3).
Fig. 2.
Kaplan-Meier time-to-event curves for patients receiving nirmatrelvir/ritonavir (NMV-r) versus patients not receiving NMV-r for the composite outcome of all-cause mortality or hospitalization.
Fig. 3.
Kaplan-Meier time to event curves for patients receiving nirmatrelvir/ritonavir (NMV-r) versus patients not receiving NMV-r for the outcomes of the probabilities of (a) all-cause mortality, (b) hospitalization, and (c) critical care service use.
Based on the findings of this retrospective cohort study of 90,056 patients, NMV-r could reduce the subacute risk of mortality, hospitalization, and critical care use among COVID-19 survivors. Our findings were consistent with previous studies investigating the subacute impact of NMV-r. 3, 4 Liu et al. reported that the NMV-r cohort had a lower risk of epilepsy or seizure (HR, 0.516; 95% CI, 0.389–0.685) within one year than those without NMV-r.3 Xie et al. showed that treatment with NMV-r could be associated with a reduced risk of post-acute sequelae of SARS-CoV-2 at day 90 (HR, 0.74; 95%, CI, 0.69–0.81), including reduced risk of dysrhythmia, ischemic heart disease, deep vein thrombosis, pulmonary embolism, fatigue, liver disease, acute kidney disease, muscle pain, neurocognitive impairment, and shortness of breath.4 All these findings indicated that NMV-r can provide additional post-acute benefits for COVID-19 survivors and suggested the potential role of NMV-r in the prevention of post-acute COVID-19 complications.
Nevertheless, this study had several limitations. Although 1:1 propensity score matching was performed to exclude possible confounding variables, residual confounding factors remained. Disease severity, variants of SARS-CoV-2, and vaccination status were not discussed. Additionally, rather than reviewing COVID-19-specific mortality, hospitalization, and critical care use, our outcomes were not COVID-19-specific. Last but not least, our outcomes were limited by time constraints, where the post-acute state (one to six months after infection) was examined only. In order to assess the long-term benefits of NMV-r treatment, a longer follow-up period would be warranted.
In conclusion, on the basis of previous studies that investigated the short-term benefits of NMV-r, this study further elaborated on the potential role of NMV-r in reducing the risks of all-cause mortality, hospitalization, and critical care use in post-acute COVID-19 patients.
References
- 1.Gentry C.A., Nguyen P., Thind S.K., Kurdgelashvili G., Williams R.J. Characteristics and outcomes of US Veterans at least 65 years of age at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents. J Infect. 2023 doi: 10.1016/j.jinf.2023.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Q., Ma P., Wang M., Cheng Y., Zhou M., Ye L., et al. Efficacy and safety of Paxlovid for COVID-19:a meta-analysis. J Infect. 2023;86(1):66–117. doi: 10.1016/j.jinf.2022.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T.H., Wu J.Y., Huang P.Y., Tsai Y.W., Lai C.C. The effect of nirmatrelvir plus ritonavir on the long-term risk of epilepsy and seizure following COVID-19: a retrospective cohort study including 91,528 patients. J Infect. 2023 doi: 10.1016/j.jinf.2023.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y., Choi T, Al-Aly Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19; 2022. doi: 10.1101/2022.11.03.22281783. [DOI]
- 5.https://trinetx.com/ (Accessed January 31, 2023).
- 6.Yendewa G.A., Perez J.A., Schlick K., Tribout H., McComsey G.A. Clinical features and outcomes of coronavirus disease 2019 among people with human immunodeficiency virus in the united states: a multicenter study from a large global health research network (TriNetX) Open Forum Infect Dis. 2021;8(7) doi: 10.1093/ofid/ofab272. [DOI] [PMC free article] [PubMed] [Google Scholar]