Abstract
Introduction
Congenital heart disease (CHD) is the most common type of congenital defect reported to be one of the leading causes of mortality in the first year of life. Microdeletion and microduplication syndromes (MMS) are associated with cardiac malformations. Understanding which genetic factors are involved in these conditions directly impacts treatment decisions. We aimed to identify the occurrence of genetic alterations and their association with MMS in CHD pediatric patients evaluated in a reference service of Southern Brazil.
Methods
Participants were recruited during 2010 in the intensive care unit of a pediatric hospital. MMs and regions of chromosome 22 were screened by SALSA MLPA Probemix P245 Microdeletion Syndromes-1A kit for detection of copy number variations (CNVs).
Results
MMS were detected in 11 from 207 patients (5.3%). Heterozygous deletion in the 22q11.2 chromosome region was the most prevalent CNV (5 from 11 patients). Also, atypical RTDR1 deletion and 22q11.2 duplication were detected. MLPA was able to reveal microdeletions in SNRPN and NF1 genes in patients with a normal karyotype and FISH.
Conclusion
Our study reports the prevalence and variability of genomic alterations associated with MMS in CHD pediatric patients. The results by MLPA are of great help in planning and specialized care.
Key Words: Multiplex ligation-dependent probe amplification, Congenital heart disease, DNA Copy number variation, 22q11.2 deletion syndrome
Introduction
Congenital heart disease (CHD) occurs at birth and is the most common type of congenital defect, characterized by a malformation in the heart or large vessels. It is reported to be one of the leading causes of mortality in the first year of life, affecting 1% of newborns per year in the USA [Hoffman and Kaplan, 2002; Calcagni et al., 2017] and 1.3−1.7% in Brazil (Brazil Ministry of Health, 2021, http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinasc/cnv/nvuf.def.). Due to its different forms, CHD prognosis becomes difficult if it is not identified early [El Malti et al., 2016]. Understanding which genetic factors are associated with these malformations, such as microdeletion and microduplication syndromes (MMS), is important for treatment decision making, especially for severe patients who require rapid medical intervention. Few papers have been published in Latin America studying the clinical and genetic profile of these patients. Most are related to the Latin American Collaborative Study of Congenital Malformations (ECLAMC), which is a program of clinical and epidemiological investigation of risk factors in the etiology of congenital anomalies [Castilla and Orioli, 2004].
At least 20% of CHD are attributed to single or multiple gene chromosomal alterations. Still other cases are related to a combination of genetic, epigenetic, and environmental factors. Among the genetic conditions associated, MMS are commonly reported. MMS originates from submicroscopic copy number variations (CNVs) in different regions of the genome, which in turn lead to specific subchromosomal gains or losses [Deak et al., 2011]. The 22q11.2 deletion syndrome (22q11.2DS), Williams syndrome (del7p11.23), and 22q11.2 microduplication syndrome (22q11.2DupS) are frequently studied MMS, and they have a broad phenotypic spectrum that includes CHD [Ou et al., 2008; Andersen et al., 2014]. The 22q11.2DS (OMIM:188400) is the most common MMS, and about 60−80% of cases have CHD, second only to Down syndrome in the group of syndromes associated with heart diseases [Mastroiacovo et al., 2005; Simmons and Brueckner, 2017; Goldmuntz, 2020].
Chromosomal microdeletions and microduplications can be detected by MLPA (multiplex ligation-dependent probe amplification), which stands out for its high yield, low cost, and possibility of simultaneous analysis with results in up to 1 day. It allows the analysis of different regions of the genome in a single reaction (>40 sequences) with short target sequences (60-80 nt) and can identify small single gene deletions and duplications (<40 kb) that are not detected by FISH, for example. Moreover, MLPA can be used as a genetic screening method acting as a diagnostic confirmation tool, facilitating personalized care. Thus, rare and complex cases that require surgical procedures and specialized care in reference centers are diagnosed more quickly, helping to optimize the flow of care [Schouten et al., 2002; Slater et al., 2003; Lee et al., 2007]. We aimed to identify the occurrence of genetic alterations and their association with MMS in CHD pediatric patients evaluated in a reference service of Southern Brazil.
Methods
Participants were recruited from cardiac intensive care units (ICU) of the Hospital da Criança Santo Antônio (HCSA), Porto Alegre, RS, Brazil, a reference center in the treatment of patients with CHD. The patients were evaluated by clinical geneticists and classified as syndromic and nonsyndromic according to the dysmorphisms. CHDs were described based on echocardiography, cardiac catheterization, and surgical description following the classification suggested by Botto et al. [2001]. Family history was noted when present.
MLPA assay was performed using the SALSA MLPA Probemix P245 Microdeletion Syndromes-1A kit for CNVs screening in genes to different MMS and 22q11.2 chromosome region following the manufacturer‧s recommendations (MRC-Holland, Amsterdam, The Netherlands). Probe sequences are shown in Table 1. Molecular analysis was performed by ABI3130 sequencing and Coffalyser software (MRC-Holland). For each sample analyzed, commercial controls in triplicate were used.
Table 1.
SALSA MLPA Probemix P245 Microdeletion Syndromes-1A probe sequences used for CNVs analysis
| Syndrome | Gene | Partial sequence (24 nt adjacent to ligation site) |
|---|---|---|
| 1p36 deletion syndrome | TNFRSF18 | CGGGTTTCTCAC-TGTGTTCCCTGG |
| TNFRSF4 | GCCGGCCAGCAA-TAGCTCGGACGC | |
| GNB1 | CTAAGATCGGAA-GATGAGTGAGCT | |
| GABRD | CGGCGACTACGT-GGGCTCCAACCT | |
|
| ||
| 2p16.1p15 microdeletion syndrome | REL | TATCACAGAACC-CGTAACAGTAAA |
| PEX13 | TGAGGATGACCA-TGTAGTTGCCAG | |
|
| ||
| 2q23.1 microdeletion/microduplication syndrome | MBD5 | CCAGCTATACAA-GTTCCTGTGGGT |
| MBD5 | CTGGAGATCTTC-CTCCTCTTGGGT | |
|
| ||
| Glass syndrome (2q32q33 microdeletion syndrome) | SATB2 | TGCCATTTATGA-CGAGATCCAACA |
| SATB2 | AGAGAAGAACAC-GCCGAGTTTGTC | |
|
| ||
| 3q29 microdeletion/microduplication syndrome | DLG1 | CTATGAAAGACA-GGATAAATGATG |
| DLG1 | CAGCTCAGAAGT-TCCATAGAACGG | |
| BDH1 | GAACTGGGCCAT-TCTAACACCCGT | |
| KIAA0226 | GCTGGAGGACAG-ATGTGCCGTCTT | |
|
| ||
| Wolf-Hirschhorn syndrome, 4p16.3 | PIGG | AAAAGCATTCAG-GCTAGATGGTGG |
| PIGG | GAGTGTGACGTA-GTCCTTCTGCTC | |
| LETM1 | CCTGTGTACACA-TCCTCCAGAGGC | |
| WHSC1 | GTGGGCATTTAT-TTTCCCTTAATG | |
|
| ||
| Cri-du-Chat syndrome, 5p15 | CCDC127 | ACGCCATGATCT-CAGAAAATCGGC |
| PDCD6 | AGGTGTCGTACG- AACAGTACCTGT | |
| TERT | TCTTTCTTTTAT-GTCACGGAGACC | |
| SEMA5A | ACTTGGGCTGGA-GTGCCCACGTGG | |
|
| ||
| Sotos syndrome, 5q35.3 | NSD1 | ACCCACCCACTG-TTATGCAGAACA |
| NSD1 | GGAAAGACTGTT-TGCAAATGTGGA | |
|
| ||
| Williams-Beuren duplication syndrome, 7q11.23 | ELN | TTTCCCGGCTTT-GGTGTCGGAGTC |
| ELN | ACCTCATCAACG-TTGGTGCTACTG | |
|
| ||
| Langer-Giedion syndrome, 8q24.11q24.13 | TRPS1 | |
| CTCIIIIIIGGT-GCTGCTGGIIIC | ||
| EXT1 | GGTGATAATGTT-AAACCCACTTAA | |
|
| ||
| 9q22.3 microdeletion syndrome | FANCC | GATAACTCACGA-GATCATTGGCTT |
| PTCH1 | GTTAATGACTCC-CAAGCAAATGTA | |
|
| ||
| DiGeorge syndrome-2, 10p13p14 | GATA3 | GAGCAACGCAAT-CTGACCGAGCAG |
|
| ||
| Prader-Willi/Angelman syndrome, 15q11.2 | MKRN3 | GGCTGCAGACCT-TGCACCCCATGG |
| NDN | ACACTGCTGCGA-GGGTAGTGGGCA | |
| SNRPN | ACCACCACCTGA-TGAAAGATACAC | |
| SNRPN | GATTCCTCGCTA-CTCCAATATGGC | |
| UBE3A | AGTGTTATTGGA-AGTGAGCCACCA | |
|
| ||
| Witteveen-Kolk syndrome/15q24 microdeletion syndrome | SEMA7A | TACCCACAGAGA-CCTTCCAGGTGG |
| CYP1A1 | GTCAACCTGAAT-AATAATTTCGGG | |
|
| ||
| Rubinstein-Taybi syndrome, 16p13.3 | CREBBP | AGCAGGTGAAAA-TGGCTGAGAACT |
|
| ||
| Miller-Dieker syndrome/lissencephaly-1, 17p13.3 | PAFAH1B1 | TGTAGGCACTCT-ATAGATCAAGCT |
| PAFAH1B1 | CCAGAAAAATAT-GCATTGAGTGGT | |
|
| ||
| Smith-Magenis syndrome/Potocki-Lupski syndrome, 17p11.2 | RAI1 | CCAAGGATCTCA-TCTGGCCACCGC |
| DRC3 | CGGATCTCCAAG-ATCGACTCCCTG | |
| LLGL1 | CAGCAGTCTGCA-TCTCTGGGAGAT | |
|
| ||
| NF1 microdeletion syndrome, 17q11.2 | NF1 | GGATCATGAAGA-ATTACTACGTAC |
| NF1 | TCTTGTTGTCTT-TGGGTGTATTAG | |
|
| ||
| Koolen-de Vries syndrome/17q21.31 microduplication syndrome | MAPT | GTCGCCAGTGGT-GTCTGGGGACAC |
| KANSL1 | CCGCTTCTTACA-GCTCAGTACAGG | |
|
| ||
| DiGeorge/22q11.2 duplication/distal 22q11.2 deletion syndrome | IL17RA | GCAGAGTTATCT-GTCCTGCAGCTG |
| BID | CTACTGGTGTTT-GGCTTCCTCCAA | |
| CLDN5, region AB | TTCGCCAACATT-GTCGTCCGCGAG | |
| GP1BB, region AB | CACAACCGAGCT-GGTGCTGACCGG | |
| SNAP29, region CD | GTATCCACTTAC-CTGTATCATCCA | |
| PPIL2, distal 22q11 | GAAGAGCCCTCA-ACCAGTGCCACT | |
| RTDR1, distal 22q11 | GGTGTGTCATTT-TGACGTCATCCC | |
|
| ||
| Phelan-McDermid syndrome, 22q13 | ARSA | GGAGGATCAGAT-CTCCGCTCGAGA |
| SHANK3 | AAGCGGCGAGTT-TATGCCCAGAAC | |
| RABL2B | AATACACAAGCC-GTAAAATCGAGT | |
|
| ||
| X chromosome copy number changes | DMD | AAACTCATAGAT-TACTGCAACAGT |
|
| ||
| Rett syndrome/MECP2 duplication syndrome, Xq28 | MECP2, exon 1 | CATTAATCCTTA-ACATTCAAATTC |
| MECP2, exon 3 | ACTTGTTCTGCA-GACTGGCATGTT | |
| MECP2, exon 4 | TTTCATCCTCCA-TGCCAAGGCCAA | |
High resolution GTG-banding karyotype and FISH technique for DiGeorge/velocardiofacial syndrome (VCFS/TUPLE1) were performed by Rosa et al. [2008] previously.
Results
The total sample consisted of 207 CHD patients with a median age of 73 days (1−4,934 days) and 52.7% male. Regarding origin, most patients came from countryside towns (58.9%; only 12.6% were from the state capital). The main reasons for hospitalization in the ICU were cardiac surgery (74.8%) and catheterization (9.2%). Cyanotic and complex CHD were found in 68 (32.9%) and 67 patients (32.4%), respectively. The main CHD group consisted of septal defects (33.3%) and outflow tract defects (18.8%), with 17.4% ventricular septal defects, 15.9% atrial septal defects, 11.1% tetralogy of Fallot, 10.6% aortic coarctation, and 9.7% atrioventricular septal defect; 15.5% of patients (32 cases) had a family history of CHD.
Sixty-four patients (30.9%) were classified as syndromic, based on the physical examination, and at least one major extracardiac malformation was verified in 51.7% of sample. Analysis using high-resolution karyotyping and FISH revealed chromosomal abnormalities in 29 (14.0%) and 4 (1.9%) patients, respectively. Down syndrome was detected in 11.6% of the cases (mostly full trisomy 21; only 2 patients had 21q isochromosome). All individuals with 22q11.2 microdeletion had normal karyotypes.
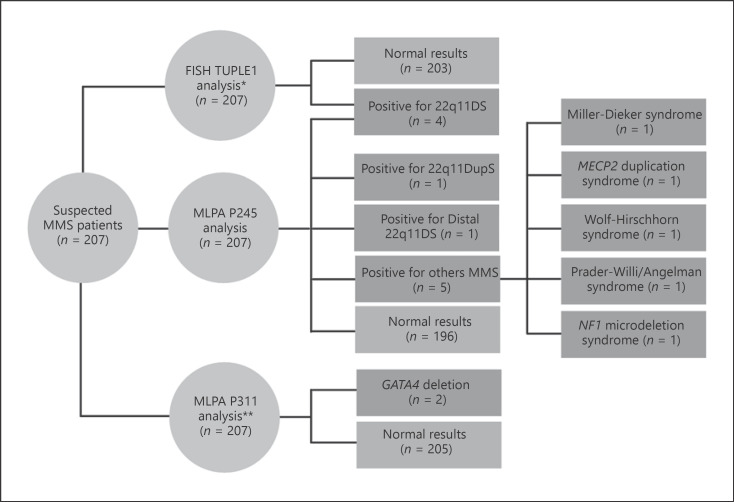
MMS were detected in 11 patients by MLPA (5.3%) (Fig. 1). Heterozygous deletion in the 22q11.2 chromosome region was the most prevalent CNV. Besides confirming the 22q11 deletions previously detected by Rosa et al. [2008] (P16, P77, P81, and P113), MLPA showed an atypical deletion in the RTDR1 gene located on chromosome 22 (P186) and one heterozygous duplication of the 22q11.2 region (22q11DupS; 0.5%) (P199), both changes not covered by FISH TUPLE1 probe. Duplication occurred in a region close to the FISH probes hybridization site (chr22:19,523,027−20,891,214) (Genome Browser GRCh38/hg38).
Fig. 1.
Flowchart of MMS diagnostic by FISH and MLPA in CHD patients. *Published data by Rosa et al. [2008]. **Published data by Floriani et al. [2021].
Additionally, MLPA was able to detect microdeletions in SNRPN (P157) and NF1 genes (P203) in patients with a normal karyotype and FISH (Fig. 2). Miller-Dieker syndrome (del17p13.3), trisomy X, and Wolf-Hirschhorn syndrome (del4p16.3) were identified in patients P35, P42, P47, respectively, findings consistent with abnormal karyotypes (Table 2). Clinical and cytogenetic findings are described in Table 2.
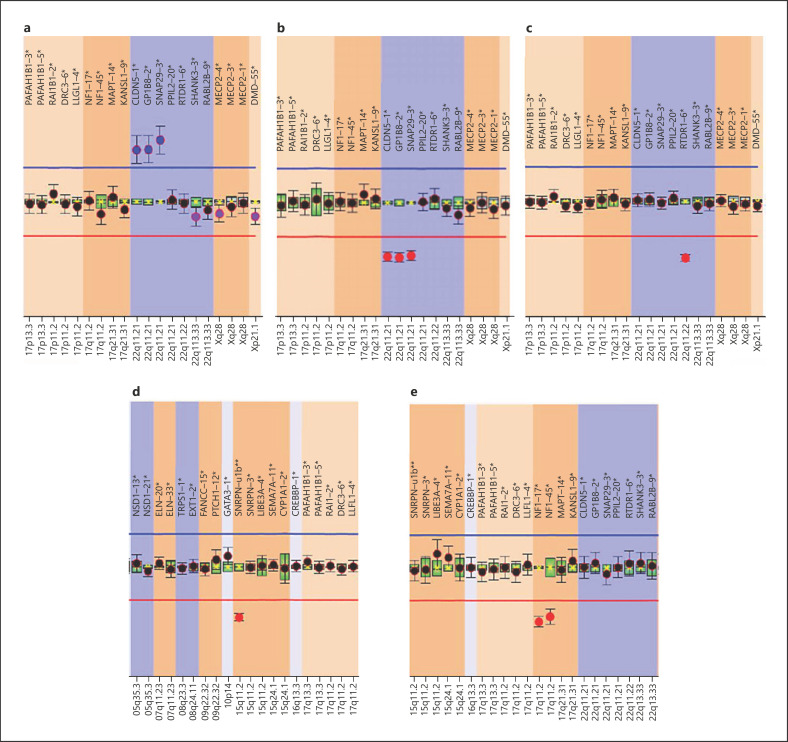
Fig. 2.
Multiplex ligation dependent probe amplification (MLPA) P245 Microdeletion Syndromes-1A analysis of patients non detected/diagnosed by FISH. a Patient 199 was positive for duplication of CLDN5, GP1BB, SNAP29 (22q11DupS). b Patient P16 was positive for deletion of CLDN5, GP1BB, SNAP29 (22q11DS). c Patient 186 was positive for deletion of RTDR1 (distal 22q11.2DS). d Patient 157 was positive for deletion of SNRPN (Prader-Willi/Angelman syndrome). e Patient 203 was positive for deletion of NF1 (NF1 microdeletion syndrome).
Table 2.
Clinical and cytogenetic features of the patients with genetic alterations
| P16 | P35 | P42 | P47 | P77 | P81 | P113 | P157 | P186 | P199 | P203 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | F | F | M | M | F | M | M | M | F |
|
| |||||||||||
| Cytogenetic findings | |||||||||||
|
| |||||||||||
| FISH | Del22q11.2 | Normal | Normal | Normal | Del22q11.2 | Del22q11.2 | Del22q11.2 | Normal | Normal | Normal | Normal |
|
| |||||||||||
| MLPA | Del CLDN5, GP1BB, SNAP29 (22q11.21) | Dup PAFAH1B1, RAI1, DRC3, LLGL (17p13.3 and 11.2) | Dup MECP2 1 (Xq28) | Del LETM1, WHSC1 (4p16.3) | Del CLDN5, GP1BB, SNAP29 (22q11.21) | Del CLDN5, GP1BB, SNAP29 (22q11.21) | Del CLDN5, GP1BB, SNAP29 (22q11.21) | Del SNRPN (15q11.2) | Del RTDR1 (22q 11.22) | Dup CLDN5, GP1BB, SNAP29 (22q11.21) | Del NF1 (17q11.2) |
|
| |||||||||||
| Facial dysmorphisms | |||||||||||
|
| |||||||||||
| Dolichocephaly | + | + | + | ||||||||
|
| |||||||||||
| Macrocephaly | + | ||||||||||
|
| |||||||||||
| High forehead | + | + | + | + | |||||||
|
| |||||||||||
| Telecanthus | + | ||||||||||
|
| |||||||||||
| Epicantic folds | + | + | + | + | + | + | + | ||||
|
| |||||||||||
| Upslanting palpebral fissures | + | + | + | ||||||||
|
| |||||||||||
| High arched palate | + | + | + | ||||||||
|
| |||||||||||
| Low nasal bridge | + | + | + | ||||||||
|
| |||||||||||
| Broad nasal bridge | + | + | + | + | + | + | + | ||||
|
| |||||||||||
| High nasal bridge | + | + | + | ||||||||
|
| |||||||||||
| Anteverted nostrils | + | + | |||||||||
|
| |||||||||||
| Hypoplastic nares | + | + | |||||||||
|
| |||||||||||
| Long philtrum | + | + | |||||||||
|
| |||||||||||
| Micrognathia | + | + | + | + | + | + | |||||
|
| |||||||||||
| Prognathia | |||||||||||
|
| |||||||||||
| Posteriorly-rotated ears | + | + | |||||||||
|
| |||||||||||
| Anteflexed ears | + | ||||||||||
|
| |||||||||||
| Prominent ears | + | + | + | ||||||||
|
| |||||||||||
| Overfold helix | + | + | + | ||||||||
|
| |||||||||||
| Hypoplastic ear lobes | + | + | |||||||||
|
| |||||||||||
| Preauricular pits | + | ||||||||||
|
| |||||||||||
| CHD | |||||||||||
|
| |||||||||||
| ASD | + | + | + | + | |||||||
|
| |||||||||||
| VSD | + | + | + | + | + | + | + | + | |||
|
| |||||||||||
| PAD | + | + | |||||||||
|
| |||||||||||
| PVS | + | + | |||||||||
|
| |||||||||||
| PAA | + | ||||||||||
|
| |||||||||||
| Right aortic arch | + | ||||||||||
|
| |||||||||||
| Subvalvular aortic ring | + | ||||||||||
F, female; M, male; CHD, congential heart disease; ASD, atrial septal defect; VSD, ventricular septal defect; PAD, patent arterial duct; PVS, pulmonary valve stenosis; PAA, pulmonary artery agenesis
Discussion
Methodologies applied to genetic syndromes research are also available to investigate CHD etiology, such as comparative genomic hybridization (CGH), quantitative multiplex real-time PCR, high-resolution single nucleotide polymorphism (SNP) microarray analysis, and MLPA [Wu et al., 2017; Guo et al., 2019; Liu et al., 2019]. The MLPA technique can be easily performed in laboratories and can provide good resolution by providing approximately 98.9% sensitivity and 97.8% specificity [Benard-Slagter et al., 2017].
We report an update on molecular assessment in pediatric patients with CHD. On study population recruitment, karyotype and subsequently FISH tests were performed in order to investigate 22q11.2DS, given its high prevalence in individuals with CHD and clinical characteristics of patients. Some years after the conclusion of the study by Rosa et al. [2008], MLPA has emerged as an important tool for screening and diagnosis of genetic disorders.
Studies have demonstrated the reliable contribution of MLPA results for the identification of chromosomal abnormalities in patients with CHD [Sørensen et al., 2012; Campos et al., 2015; Cowan and Ware, 2015]. It is known that chromosomal abnormalities are important causes of CHD and when 22q11.2 microdeletion is ruled out, the CHD etiology remains generally unexplained, making genetic counseling and care planning difficult [Mademont-Soler et al., 2012]. In suspicious individuals with normal karyotype or abnormal ultrasound findings during prenatal care, MLPA can be used in screening and identifying genetic alterations [Kjaergaard et al., 2010; Sørensen et al., 2012; Kuo et al., 2014].
Our results showed that alterations in chromosome 22 were prevalent, 4 individuals were diagnosed with 22q11.2DS and 2 of these had cyanotic, conotruncal, and complex CHD (P77 and P113). Classical facial dysmorphisms were observed in severely or mildly expressed phenotypes; despite this, only patient P81 was classified as syndromic according to Digilio et al. [2005]. CLDN5, GP1BB, and SNAP29 were the most frequently altered genes, with microdeletions in 4 patients and microduplication in 1. Based on the probe sequences (Table 1), these genes are located in low copy repeat (LCR) 22 A−B region (CLDN5, GP1BB, and SNAP29) and LCR22 E−F (RTDR1) but in a different genomic coordinate (chr22:19,523,024−20,891,214 and chr22:23,401,593−23,487,208; GRCh38/hg38) from that analyzed by FISH TUPLE1 [Hacıhamdioğlu et al., 2015]. In addition, GATA4 deletions were identified in 2 patients as reported by Floriani et al. [2021] (Fig. 1).
CLDN5 (OMIM*602101) is prevalent in heart and skeletal muscle [Sirotkin et al., 1997]. 22q11.2DS cases with neurological and ocular disorders have been described presenting mutations in this gene. However, CLDN5 pathways are not yet well defined to explain its association with the CHD development in individuals diagnosed with 22q11.2DS [Cordovez et al., 2014; Guo et al., 2020]. SNAP29 deficiency (OMIM*604202) is related to CEDNIK syndrome with different clinical manifestations (microcephaly, severe neurological impairment, psychomotor retardation, facial dysmorphism, palmoplantar keratoderma, and ichthyosis) [Sprecher et al., 2005]. Studies demonstrate that deletions in these genes can affect cardiovascular development [McDonald-McGinn et al., 2013; Motahari et al., 2019] as evidenced in our sample. Patients with Bernard-Soulier syndrome (BSS) and 22q11.2DS or GP1BB deletion (OMIM*138720) are particularly susceptible to bleeding and thrombocytopenia [Emanuel et al., 2001; Bartsch et al., 2011], condition present in P113 patient, while patient P81 suffered from ecchymosis. There is no doubt that at least the 3-Mb typically deleted region of chromosome 22q11.2 has a correlation with CHD development, including the CLDN5, GP1BB, and SNAP29 genes. However, the exact molecular mechanism of these CHD-associated genes still needs to be studied [Hou et al., 2020].
Karyotype analysis identified triple X, duplication 4p16.3, and deletion 17p13.3, results confirmed by MLPA proving its effectiveness in screening for common chromosomal alterations. MLPA identified MECP2 duplication syndrome (dupXq28, MECP2), Wolf-Hirschhorn syndrome (del4p16.3, PIGG, LETM1, and WHSC1), and Miller-Dieker syndrome (del17p13.3, PAFAH1B1), showing the detail of the damaged genes.
Patient P47 (del4p16.3) presented facial and foot dysmorphisms and patient P35 (del17p13.3, PAFAH1B1) has minor facial dysmorphisms (micrognathia, palpebral fissures upwards, and wide nasal bridge), dysmorphisms in hands and fingers, and hypoactivity and hypotonia that may be linked to developmental delay. Bi et al. [2016] detected 4p16.3 CNVs (deletion and duplication) prenatally and abnormal ultrasound findings such as clubfeet, heart defects, and cystic hygroma. MECP2 duplication syndrome, a rare X-linked disorder that predominantly affects males, is also known to produce symptoms that include severe motor and cognitive problems, delayed or absent speech development, autistic features, seizures, ataxia, recurrent respiratory infections, and decreased survival [D‧Mello, 2021]. The female patient (P42) identified with MECP2 duplication syndrome has characteristics not compatible with this description.
Microdeletions in SNRPN (P157) and NF1 (P203) genes, compatible with Prader-Willi/Angelman syndrome and NF1 microdeletion syndrome were identified. The patient P203 presented NF1 deletion with hypochromic spots, hair changes (head and sacrum), skeletal and hand changes (clinodactyly), in addition to facial dysmorphisms and CHD. Neurofibromatosis 1 (NF1) is an autosomal dominant disorder with a broad spectrum of clinical characteristic, as presence of multiple café-au-lait spots, neurofibromas, inguinal freckling, iris hamartomas, tumors, or skeletal abnormalities, and learning disabilities are present in 50% of the patients. Mutation detection in NF1 is still a challenge, and MLPA appears as one of the methodologies indicated in the identification of NF1 syndrome [Ishida and Gupta, 2021].
Lee et al. [2019] reported a clinical experience with MLPA for microdeletion syndromes in prenatal diagnosis of 7,522 pregnant Korean women. A total of 124 women (1.6%) had genomic imbalances (gene loss [33.6%] and gene gain [66.4%]). Most cases with genomic imbalances showed no abnormal karyotype (64.5%). Further, Zhang et al. [2021] revealed a prevalence of 0.86% of 22q11.2 genomic imbalance in 6,034 Chinese patients with development delay and/or intellectual disability, where 71.2% had heterozygous deletions and 28.8% heterogeneous duplications. Most of these patients (65.4%) carried typical imbalance from LCR22 A to D, including CLDN5, GP1BB, and SNAP29 genes. A screening by MLPA P245 and G-band karyotyping, followed by confirmation of positive patients through MLPA P250 permitted a precise definition of the abnormal region.
Maran et al. [2020] suggested the possibility of using MLPA as a potential alternative diagnostic method in 22q11.2DS screening. Forty-two nonsyndromic Malaysians with CHD were evaluated by MLPA and confirmed the presence of deletions as detected by FISH assay in 2 patients. FISH failed to detect deletions located outside the typical deletion region (LCR22 A−B to LCR22 D−E) and deletions outside of the 22q11.2 regions as well as duplications, a region covered by MLPA.
These up-to-date studies show that MLPA identification of microdeletions and microduplication within or close to the typical deletion region may prove to be relevant mainly in relation to the identification of candidate genes and the precise extension of the region involved [Jalali et al., 2008]. Also, MLPA data can contribute to the understanding of the genes involved in the etiology of MMS phenotype. Currently, more than 300 probes sets are commercialized aiming at the investigation and diagnosis of several genetic diseases [Sørensen et al., 2012; Stuppia et al., 2012]. Studies prove the high sensitivity and specificity of the method, providing a rapid and accurate clinical evaluation for prenatal identification of common chromosomal alteration [Omrani et al., 2014]. For rare diseases, the value does not exceed USD 44.00 per test, being compatible with its use in health systems (http://www.ans.gov.br/images/ANEXO/RN/Anexo_II_DUT_Rol_2018_alterado.pdf). However, the technique also has limitations related to the inability to identify balanced rearrangements, low mosaicism, and being sensitive to the quality of the DNA used [Kozlowski et al., 2008].
Conclusion
Molecular alterations are strongly associated with MMS in pediatric patients with CHD. Results brought by the use of MLPA are of great help in planning and specialized care in referral centers, allowing the diagnosis of patients with suspected MMS. This tool allows the identification of rare and complex cases, bringing relevance to the investigation of MMS especially in patients with CHD associated with mental retardation and developmental disorders.
Statement of Ethics
Patients‧ parents agreed to participate in the study and informed consent was given and signed afterwards. The study was approved by the institutional Ethics Committee (number 2.315.917).
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
This work was funded by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS,17/2551-0,001,063-9), Programa de Extensão Universitária do Ministério da Educação e Cultura (PROEXT), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (302,931/2019-8).
This study was also supported by FAPERGS (17/2551−0001063−9), Research Support Foundation of the State of Rio Grande do Sul; PROEXT, Support Program for University Extension of MEC - Ministry of Education and Culture; and CAPES, Coordination of Superior Level Staff Improvement (001). Research productivity fellowship Brazil (CNPq) (301834/2016−4).
Author Contributions
Maiara Floriani, Andressa Santos, Bruna Diniz, and Paulo Zen performed genetic testing and prepared the manuscript. Maiara Floriani, Andressa Glaeser, and Paulo Zen supervised genetic testing. Maiara Floriani, Andressa Santos, and Rafael Rosa reviewed clinical data and edited the manuscript. Maiara Floriani, Andressa Santos, Bruna Diniz, reviewed the medical records. Maiara Floriani, Paulo Zen, and Rafael Rosa designed the study, supervised genetic tests, wrote, and edited the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding Statement
This work was funded by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS,17/2551-0,001,063-9), Programa de Extensão Universitária do Ministério da Educação e Cultura (PROEXT), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (302,931/2019-8).
References
- 1.Andersen TA, Troelsen KL, Larsen LA. Of mice and men: Molecular genetics of congenital heart disease. Cell Mol Life Sci. 2014;71((8)):1327–1352. doi: 10.1007/s00018-013-1430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsch I, Sandrock K, Lanza F, Nurden P, Hainmann I, Pavlova A, et al. Deletion of human GP1BB and SEPT5 is associated with Bernard-Soulier syndrome, platelet secretion defect, polymicrogyria, and developmental delay. Thromb Haemost. 2011;106((3)):475–483. doi: 10.1160/TH11-05-0305. [DOI] [PubMed] [Google Scholar]
- 3.Benard-Slagter A, Zondervan I, de Groot K, Ghazavi F, Sarhadi V, Van Vlierberghe P, et al. Digital Multiplex Ligation-Dependent Probe Amplification for Detection of Key Copy Number Alterations in T- and B-Cell Lymphoblastic Leukemia. J Mol Diag. 2017;19((5)):659–672. doi: 10.1016/j.jmoldx.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Bi W, Cheung SW, Breman AM, Bacino CA. 4p16.3 microdeletions and microduplications detected by chromosomal microarray analysis: New insights into mechanisms and critical regions. Am J Med Genet A. 2016;170((10)):2540–2550. doi: 10.1002/ajmg.a.37796. [DOI] [PubMed] [Google Scholar]
- 5.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107((3)):E32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 6.Calcagni G, Unolt M, Digilio M, Baban A, Versacci P, Tartaglia M, et al. Congenital heart disease and genetic syndromes: New insights into molecular mechanisms. Expert Rev Mol Diagn. 2017;17((9)):861–870. doi: 10.1080/14737159.2017.1360766. [DOI] [PubMed] [Google Scholar]
- 7.Campos CM, Zanardo EA, Dutra RL, Kulikowski LD, Kim CA. Investigation of Copy Number Variation in Children with Conotruncal Heart Defects. Arq Bras Cardiol. 2015;104:24–31. doi: 10.5935/abc.20140169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castilla EE, Orioli IM. ECLAMC: The Latin-American collaborative study of congenital malformations. Community Genet. 2004;7((2-3)):76–94. doi: 10.1159/000080776. [DOI] [PubMed] [Google Scholar]
- 9.Cordovez JA, Capasso J, Lingao MD, Sadagopan KA, Spaeth GL, Wasserman BN, et al. Ocular manifestations of 22q11.2 microduplication. Ophthalmology. 2014;121((1)):392–398. doi: 10.1016/j.ophtha.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 10.Cowan JR, Ware SM. Genetics and genetic testing in congenital heart disease. Clin Perinatol. 2015;42((2)):373–393. doi: 10.1016/j.clp.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Deak KL, Horn SR, Rehder CW. The evolving picture of microdeletion/microduplication syndromes in the age of microarray analysis: Variable expressivity and genomic complexity. Clin Lab Med. 2011;31((4)):543–564. doi: 10.1016/j.cll.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Digilio M, Marino B, Capolino R, Dallapiccola B. Clinical manifestations of Deletion 22q11.2 syndrome (DiGeorge/Velo-Cardio-Facial syndrome) Images Paediatr Cardiol. 2005;7((2)):23–34. [PMC free article] [PubMed] [Google Scholar]
- 13.D'Mello SR. MECP2 and the biology of MECP2 duplication syndrome. J Neurochem. 2021;159((1)):29–60. doi: 10.1111/jnc.15331. [DOI] [PubMed] [Google Scholar]
- 14.El Malti R, Liu H, Doray B, Thauvin C, Maltret A, Dauphin C, et al. A systematic variant screening in familial cases of congenital heart defects demonstrates the usefulness of molecular genetics in this field. Eur J Hum Genet. 2016;24((2)):228–236. doi: 10.1038/ejhg.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emanuel BS, McDonald-McGinn D, Saitta SC, Zackai EH. The 22q11.2 deletion syndrome. Adv Pediatr. 2001;48:39–73. [PubMed] [Google Scholar]
- 16.Floriani MA, Glaeser AB, Dorfman LE, Agnes G, Rosa RFM, Zen PRG. GATA 4 Deletions Associated with Congenital Heart Diseases in South Brazil. J Pediatr Genet. 2021;10((2)):92–97. doi: 10.1055/s-0040-1714691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldmuntz E. 22q11.2 deletion syndrome and congenital heart disease. Am J Med Genet C Semin Med Genet. 2020;184((1)):64–72. doi: 10.1002/ajmg.c.31774. [DOI] [PubMed] [Google Scholar]
- 18.Guo Z, Li B, Tian P, Li D, Zhang Y, Li Q, et al. DGCR8 expression is altered in children with congenital heart defects. Clin Chim Acta. 2019;495:25–28. doi: 10.1016/j.cca.2019.03.1619. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Singh LN, Zhu Y, Gur RE, Resnick A, Anderson SA, et al. Association of a functional Claudin-5 variant with schizophrenia in female patients with the 22q11.2 deletion syndrome. Schizophr Res. 2020;215:451–452. doi: 10.1016/j.schres.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacıhamdioğlu B, Hacıhamdioğlu D, Delil K. 22q11 deletion syndrome: Current perspective. Appl Clin Genet. 2015;8:123–132. doi: 10.2147/TACG.S82105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39((12)):1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 22.Hou HT, Chen HX, Wang XL, Yuan C, Yang Q, Liu ZG, et al. Genetic characterization of 22q11.2 variations and prevalence in patients with congenital heart disease. Arch Dis Child. 2020;105((4)):367–374. doi: 10.1136/archdischild-2018-316634. [DOI] [PubMed] [Google Scholar]
- 23.Ishida C, Gupta V. In: StatPearls. StatPearls Publishing; 2021. Genetics, Molecular Testing. [PubMed] [Google Scholar]
- 24.Jalali GR, Vorstman JAS, Errami A, Vijzelaar R, Biegel J, Shaikh T, et al. Detailed analysis of 22q11.2 with a high density MLPA probe set. Hum Mutat. 2008;29((3)):433–440. doi: 10.1002/humu.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjaergaard S, Sundberg K, Jørgensen FS, Rohde MD, Lind AM, Gerdes T, et al. Diagnostic yield by supplementing prenatal metaphase karyotyping with MLPA for microdeletion syndromes and subtelomere imbalances. Prenat Diagn. 2010;30((10)):995–999. doi: 10.1002/pd.2604. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski P, Jasinska AJ, Kwiatkowski DJ. New applications and developments in the use of multiplex ligation-dependent probe amplification. Electrophoresis. 2008;29((23)):4627–4636. doi: 10.1002/elps.200800126. [DOI] [PubMed] [Google Scholar]
- 27.Kuo YL, Chen CP, Wang LK, Ko TM, Chang TY, Chern SR, et al. Prenatal diagnosis and molecular cytogenetic characterization of chromosome 22q11.2 deletion syndrome associated with congenital heart defects. Taiwan J Obstet Gynecol. 2014;53((2)):248–251. doi: 10.1016/j.tjog.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Lee C, Iafrate AJ, Brothman AR. Copy number variations and clinical cytogenetic diagnosis of constitutional disorders. Nat Genet. 2007;39((7 Suppl l)):S48–54. doi: 10.1038/ng2092. [DOI] [PubMed] [Google Scholar]
- 29.Lee D, Na S, Park S, Go S, Ma J, Yang S, et al. Clinical experience with multiplex ligation-dependent probe amplification for microdeletion syndromes in prenatal diagnosis: 7522 pregnant Korean women. Mol Cytogenet. 2019;12:10. doi: 10.1186/s13039-019-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Chang X, Glessner J, Qu H, Tian L, Li D, et al. Association of Rare Recurrent Copy Number Variants with Congenital Heart Defects Based on Next-Generation Sequencing Data from Family Trios. Front Genet. 2019;10:819. doi: 10.3389/fgene.2019.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mademont-Soler I, Morales C, Soler A, Clusellas N, Margarit E, Martínez-Barrios E, et al. MLPA: A prenatal diagnostic tool for the study of congenital heart defects? Gene. 2012;500((1)):151–154. doi: 10.1016/j.gene.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Maran S, Faten SA, Lim SHE, Lai KS, Ibrahim WPW, Ankathil R, et al. Screening of 22q11.2DS Using Multiplex Ligation-Dependent Probe Amplification as an Alternative Diagnostic Method. Biomed Res Int. 2020;2020:6945730. doi: 10.1155/2020/6945730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mastroiacovo P, Rossi P, Cancrini C, Azzari C, DiGilio M, Marino B, et al. Chromosome 22q. 11 deletion-Recommendations for Diagnosis and Treatment. IPINET: Italian Primary Immunodeficiencies Strategic Scientific Committee. 2005 [Google Scholar]
- 34.McDonald-McGinn DM, Fahiminiya S, Revil T, Nowakowska BA, Suhl J, Bailey A, et al. Hemizygous mutations in SNAP29 unmask autosomal recessive conditions and contribute to atypical findings in patients with 22q11.2DS. J Med Genet. 2013;50((2)):80–90. doi: 10.1136/jmedgenet-2012-101320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motahari Z, Moody SA, Maynard TM, LaMantia AS. In the line-up: Deleted genes associated with DiGeorge/22q11.2 deletion syndrome: are they all suspects? J Neurodev Disord. 2019;11((1)):7. doi: 10.1186/s11689-019-9267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omrani MD, Azizi F, Rajabibazl M, Safavi Naini N, Omrani S, Abbasi AM, et al. Can we rely on the multiplex ligation-dependent probe amplification method (MLPA) for prenatal diagnosis? Iran J Reprod Med. 2014;12((4)):263–268. [PMC free article] [PubMed] [Google Scholar]
- 37.Ou Z, Berg JS, Yonath H, Enciso VB, Miller DT, Picker J, et al. Microduplications of 22q11.2 are frequently inherited and are associated with variable phenotypes. Genet Med. 2008;10((4)):267–277. doi: 10.1097/GIM.0b013e31816b64c2. [DOI] [PubMed] [Google Scholar]
- 38.Rosa RFM, Pilla CB, Pereira VLB, Flores JAM, Golendziner E, Koshiyama DB, et al. 22q11.2 deletion syndrome in patients admitted to a cardiac pediatric intensive care unit in Brazil. Am J Med Genet. 2008;146A((13)):1655–1661. doi: 10.1002/ajmg.a.32378. [DOI] [PubMed] [Google Scholar]
- 39.Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30((12)):e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons MA, Brueckner M. The genetics of congenital heart disease… understanding and improving long-term outcomes in congenital heart disease: a review for the general cardiologist and primary care physician. Curr Opin Pediatr. 2017;29((5)):520–528. doi: 10.1097/MOP.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sirotkin H, Morrow B, Saint-Jore B, Puech A, Das Gupta R, Patanjali SR, et al. Identification, characterization, and precise mapping of a human gene encoding a novel membrane-spanning protein from the 22q11 region deleted in velo-cardio-facial syndrome. Genomics. 1997;42((2)):245–251. doi: 10.1006/geno.1997.4734. [DOI] [PubMed] [Google Scholar]
- 42.Slater HR, Bruno DL, Ren H, Pertile M, Schouten JP, Choo KH. Rapid, high throughput prenatal detection of aneuploidy using a novel quantitative method (MLPA) J Med Genet. 2003;40((12)):907–912. doi: 10.1136/jmg.40.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sørensen KM, El-Segaier M, Fernlund E, Errami A, Bouvagnet P, Nehme N, et al. Screening of congenital heart disease patients using multiplex ligation-dependent probe amplification: Early diagnosis of syndromic patients. Am J Med Genet. 2012;158A((4)):720–725. doi: 10.1002/ajmg.a.35214. [DOI] [PubMed] [Google Scholar]
- 44.Sprecher E, Ishida-Yamamoto A, Mizrahi-Koren M, Rapaport D, Goldsher D, Indelman M, et al. A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neurocutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am J Hum Genet. 2005;77((2)):242–251. doi: 10.1086/432556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuppia L, Antonucci I, Palka G, Gatta V. Use of the MLPA assay in the molecular diagnosis of gene copy number alterations in human genetic diseases. Int J Mol Sci. 2012;13((3)):3245–3276. doi: 10.3390/ijms13033245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu XL, Li R, Fu F, Pan M, Han J, Yang X, et al. Chromosome microarray analysis in the investigation of children with congenital heart disease. BMC Pediatr. 2017;17((1)):117. doi: 10.1186/s12887-017-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Liu X, Gao H, He R, Zhao Y. Identifying of 22q11.2 variations in Chinese patients with development delay. BMC Med Genomics. 2021;14((1)):26. doi: 10.1186/s12920-020-00849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.