Abstract
Context
The safety and efficacy of probiotics during severe illness has been a subject of ongoing interest. The impact of probiotics can worsen nutritional status, which could potentially result in a deterioration of the patient’s overall life-threatening status.
Objective
This systematic review and meta-analysis evaluated the safety and efficacy of probiotics in reducing intensive care unit (ICU)–acquired infections in adult critically ill patients.
Data Sources
PubMed and Cochrane library databases for the period 2011–2020 were searched.
Data Extraction
Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) methodology was used to search for randomized controlled trials that evaluated the use of probiotics among critically ill patients.
Data Analysis
No significant difference was observed between probiotics and control groups in terms of the mortality rate (risk ratio 1.13, 95% confidence interval .82 to 1.55, P = .46). Probiotics, however, provided a significant reduction in ICU-acquired infections (risk ratio .73, 95% confidence interval .58 to .93, P = .01).
Conclusion
The use of probiotics seems to play a role in decreasing the incidence of ICU-acquired infections. Also, a potential reduction in terms of the incidence of diarrhea has been reported, with no examples of adverse incidents, suggesting probiotics are safe.
Keywords: critically ill, ICU, intensive care, critical care, prebiotics, probiotics, synbiotics, Lactobacillus, Bifido-bacterium
INTRODUCTION
Malnutrition, especially undernutrition in hospitalized patients, has attracted significant attention for decades.1 Malnutrition has been defined as a lack of the nutrients required to sustain individual health, which can be caused by one or more factors such as inadequate intake, conditions leading to reduced absorption, high energy demands, and changes in the transporting and utilization of nutrients.1 The prevalence of malnutrition in hospitalized patients is between 30% and 50%, depending on the setting and the malnutrition criteria.1 Many trials have demonstrated that nutritional management of malnourished patients lowers the risks of infection complication and wound inflammation, and the mortality rate.2 Critically ill patients are often in a hypermetabolic state when they have life-threatening conditions such as trauma, burns, or systemic inflammatory response syndrome (SIRS).3 The stomach/gastrointestinal tract has an immunoregulatory role that is influenced by the microbiota, the intestinal barrier, and the intestinal immune system.4 With hyperinflammatory status, both the species composition and the numbers of the intestinal microorganisms are significantly changed, leaving the body exposed and unprotected against infections, which can lead to SIRS or multiple organ dysfunction syndrome (MODS).4 Infections such as ventilator-associated pneumonia (VAP), sepsis, and urinary tract infections remain among the major causes of worsening of the patient’s status or even death in critically ill patients.5 Critically ill patients have an altered gut microbiome. The microbiome present in them consists more of pathogenic bacteria that cause various problems than of beneficial bacteria. In one study, the gut microbiomes of patients with severe SIRS were evaluated. The study found that the mean count of beneficial bacteria was lower and the mean Pseudomonas counts was higher. This was the first study to have shown an altered gut microbiome in critically ill patients.6 Further study in SIRS patients has revealed that an altered gut microbiome results in enhanced gastrointestinal dysmotility, and that this leads to more complications and higher mortality in patients with SIRS.7 In critically ill patients, beneficial commensals belonging to Firmicutes and Bacteroidetes are replaced by pathogens belonging to the Proteobacteria phylum. The change in the microbiome is due to the treatment of these patients with a wide range of antibiotics, which results in the loss of beneficial bacteria from the gut. Providing proper enteral nutrition would thus help in promoting the health of the patients. Several such therapies are taken into consideration and include probiotics, prebiotics, synbiotics, and fecal microbiota transplantation.8 Diarrhea is identified by the frequency of three or more loose or liquid stools per day. It is a major clinical problem that can lead to undesirable outcomes, such as poor wound healing, shifting of the electrolyte balance, fluid losses, hemodynamic instability, and deficiency of nutrients.9 Critically ill patients have diarrhea that is not infectious and has been observed to be related to the length of stay in hospital. Antibiotic-associated diarrhea is very common in critically ill patients, and there is a reported incidence of 5%–30%.10 In patients who are receiving enteral feeding, it has been reported that the prevalence of diarrhea is 15% to 40%, or even higher.11 A cohort study of 3737 patients showed that there was a higher prevalence of diarrhea in patients who stayed longer in hospital.12 However, a study seeking to identify the critical factors related to the higher prevalence has shown that microbes are not the major source, indicating the non-infectious nature of the diarrhea.12
The gastrointestinal tract of mammals hosts a large number of intestinal microorganisms that are important for maintaining good health. Many of these bacteria have been isolated and used for the treatment of a variety of gastrointestinal symptoms. These bacteria are termed “probiotics.” Administered probiotics function by inhibiting the action of pathogenic bacteria, helping immunomodulation, improving the barrier function of the gut, and helping the release of neurotransmitters. Thus, probiotics help in maintaining a healthy gut–brain axis. Probiotics belonging to the Bacteroidetes and Firmicutes phyla of bacteria have been used to treat a variety of intestinal symptoms, such as irritable bowel syndrome, diarrhea, inflammatory bowel disease, and antibiotic-induced diarrhea.13–15 Randomized controlled trials (RCTs) using probiotics in critically ill patients have shown that patients receiving probiotic treatment had better immunity than placebo-treated patients. Lactobacillus, Bifidobacterium, and Streptococcus salivarius subspp. have all been used for treatment. Both Lactobacillus and Bifidobacterium are known to have beneficial effects on the gut.16 VAP is a common occurrence in critically ill patients. A meta-analysis study on the use of probiotics and the outcome in terms of various parameters in critically ill patients showed that the use of probiotic treatment leads to a significant reduction in VAP incidence, length of intensive care unit (ICU) stay, in-hospital mortality, and duration of mechanical ventilation. In the systematic review, the use of a wide variety of probiotics was represented.17 However, a contrary report has been published wherein the use of Lactobacillus rhamnosus GG for lowering VAP in critically ill patients was shown to be ineffective in reducing VAP.18 Further research is needed to determine whether the use of Lactobacillus rhamnosus GG really helps in lowering the incidence of VAP.
The American Society of Parenteral and Enteral Nutrition (ASPEN) states that using probiotics is not recommended as routine practice, enterally or parenterally.19 However, some studies have reported that probiotic use significantly reduces the infection incidence.20–22 A few studies support the administration of probiotics in adult patients with diarrhea,23,24 but no studies have recommended using probiotics as a diarrhea treatment in critically ill patients.9 The presence of many strains and species of probiotics makes the studies on probiotics more challenging, and it is important to determine whether one or a combination of two strains will have a favorable outcome.9 This review aimed to evaluate the safety and effectiveness of probiotics usage to decrease ICU-acquired infection incidence in critically ill adult patients.
METHODS
Search strategy and article selection
Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA)25 methodology was used to search for RCTs that evaluated the use of probiotics among critically ill patients. Two scientific databases, PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL), were searched independently by 2 investigators for relevant reports published between 2011 and 2020. Key words used included “critically ill” OR “ICU” OR “intensive care” OR “critical care” AND “prebiotics” OR “probiotics” OR “synbiotics” OR “Lactobacillus” OR “Bifidobacterium”.
Inclusion criteria and exclusion criteria
Table 1 shows the PICOS (Participants, Intervention, Comparators, Outcomes, and Study Design) criteria. Studies were considered in this review if they evaluated the effect of probiotics on mortality, ICU-acquired infection, and diarrhea in adult critically ill patients (ventilated or nonventilated). Only RCTs were included in this review. Systematic reviews, case studies, conference abstracts, observational studies, interventions that included synbiotics or prebiotics, and papers that were not in English were all excluded from this review.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Criteria |
|---|---|
| Population | Adult critically ill patients who are admitted to ICU |
| Intervention | Single or multi-strains of probiotics |
| Comparison | Unexposed group (no probiotics) |
| Outcome | Mortality incidences, intensive care unit–acquired infections (such as pneumonia, urinary tract infection, and bloodstream infections) and incidence of diarrhea |
| Study design | Randomized controlled trials that are published in the English language |
Quality assessment and quality of the evidence
The quality assessment was performed using the modified Cochrane Collaboration tool to assess the risk of bias of an RCT on the basis of a judgment (high, low, or unclear) regarding individual elements from 5 domains: selection, reporting, performance, attrition, and other.26
The grading of the recommendations, assessment, development, and evaluation (GRADE) was used to assess the quality of the evidence.27 Usually, RCTs start as being considered to have a high quality of evidence, which is then potentially downgraded to an assessment of having a moderate, low, or very low level of evidence, based on 5 domains (risk of bias, imprecision, heterogeneity, indirectness, and publication bias).
Statistical analysis
In this review, the meta-analysis was conducted using Review Manager (RevMan v.5.4.1, Cochrane Collaboration, Oxford, UK). The mean difference (MD), standard deviation (SD), and a random effect for the continuous variables were used to pool the data. Heterogeneity was assessed using the I2 test and was considered as “low” if I2 was ≤25%, “moderate” if I2 was >25 but <75%, or “high” if I2 was ≥75%.
RESULTS
Studies included
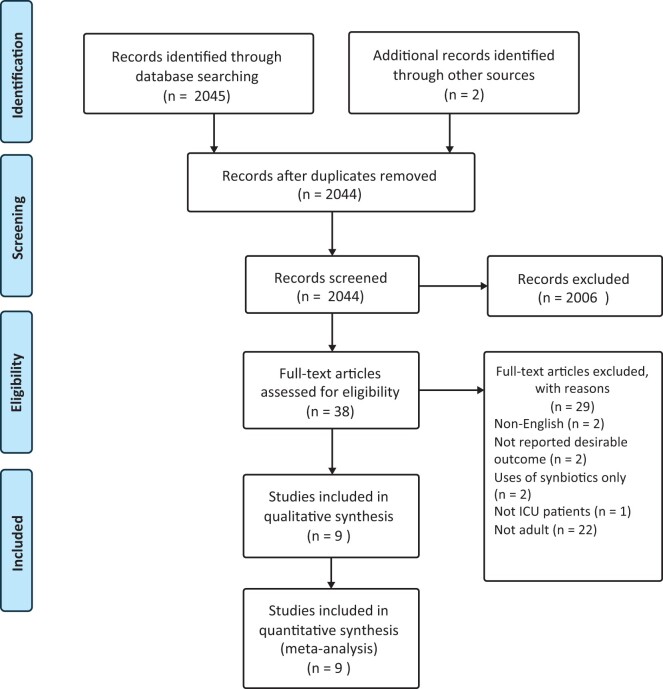
The trial flow diagram is presented in Figure 1. The initial search results revealed 2045 articles. Of these, 2006 articles were excluded due to being unrelated to the topic. An additional 29 were also excluded, for the following reasons: 2 trials were eliminated because they were not in the English language; 2 trials were eliminated due to the absence of desirable outcome; 2 trials were excluded because they only used synbiotics, and another trial was not performed on critically ill patients; finally, 22 trials were excluded because they were not performed on adult patients. A total of 9 RCTs were included in this study.
Figure 1.
PRISMA flow diagram.
Abbreviations: ICU, intensive care unit; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses (Moher et al 200925).
Study characteristics
An aggregation of 9 RCTs4,9,28–34 published between 2011 and 2020, with 860 critically ill patients from different ICUs, was evaluated. Six studies reported on the incidence of ICU mortality.4,29–32,34 Seven studies reported on the incidence of ICU-acquired infections.4,29–34 Five studies reported on the incidence of diarrhea.4,9,28,30–32Table 24,9,28–34 briefly summarizes the RCTs included in this review that evaluated the effectiveness of probiotics in critically ill patients.
Table 2.
Summary of the included studies in this review, which evaluated the effectiveness of probiotics in critically ill patients, with reporting of the clinical outcomes
| Reference | Study design | Population (n) | Probiotic strain | Age as mean ± SD (years) | Outcomes | Comments |
|---|---|---|---|---|---|---|
| Wang et al (2020)4 | A single-blind, RCT |
|
Clostridium butyricum | 81 |
|
|
| Ferrie and Daley (2010)9 | A prospective, double-blind, RCT |
|
Lactobacillus rhamnosus GG | 58.95 ± 18.45 |
|
|
| de Castro Soares et al (2017)28 | A prospective, double-blind, pilot RCT |
|
Bacillus cereus | 67.9 ± 19.4 |
|
|
| Habib, Kassem, and Ahmed (2020)29 | A double-blind, RCT |
|
Lactobacillus delbrueckii and Lactobacillus fermentum | 39.48 ± 7.69 |
|
|
| Kwon et al (2015) | A prospective, pilot RCT |
|
Lactobacillus rhamnosus GG | 62 |
|
|
| Mahmoodpoor (2018)31 | A prospective, double-blind, RCT |
|
Multistrains | 58.3 ± 13.7 |
|
|
| Rongrungruang et al. (2015)32 | A prospective, open-label, RCT |
|
Lactobacillus casei | 71.02 ± 15.8 |
|
|
| Tan et al (2011)33 | A prospective, pilot RCT |
|
Multistrains | 40.6 ± 12.9 |
|
|
| Zeng et al (2016)34 | An open-label, RCT, multicenter |
|
Bacillus subtilis and Enterococcus faecalis | 52.4 ± 18.05 |
|
|
P < .05 indicated a statistically significant difference. Abbreviations: C, control group; ICU, intensive care unit; n, number; RCT, randomized control trial; SD, standard deviation. TBI, traumatic brain injury; T, treatment group.
Quality assessment and quality of the evidence
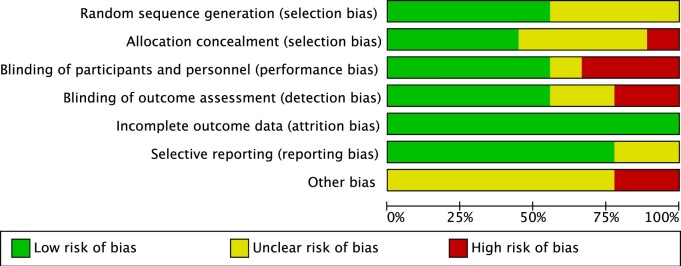
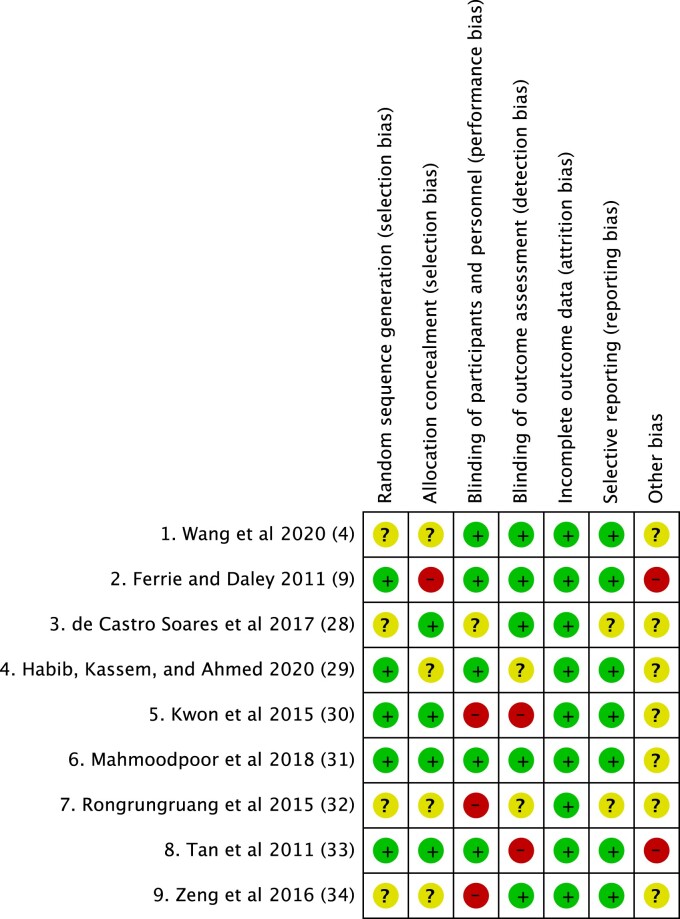
Risk of bias (high, low, or unclear) was assessed on the basis of 5 domains: selection, reporting, performance, attrition, and other. Regarding the level of the evidence, 5 studies were rated as having a “high risk of bias.”9,30,32–34 In addition, 4 studies were rated as having an ‘unclear risk of bias’.4,21,22,24 The RCTs were graded based on 5 domains: risk of bias, imprecision, heterogeneity, indirectness, and publication bias. Figure 2 shows the risk-of-bias graph, and Figure 3 shows the risk-of-bias summary of all the included studies.
Figure 2.
Risk-of-bias graph.
Review of authors’ judgments about each risk-of-bias item presented as percentages across all included studies.
Figure 3.
Risk-of-bias summary.
Review of authors’ judgments about each risk-of-bias item for each included study.
Table 3 shows the summary of the findings regarding the quality of the evidence, the risk of ICU mortality, and the risk of incidence of ICU-acquired infections. The quality of the evidence was downgraded to an assessment of a moderate level of certainty due to most of the included studies being rated as having a “high risk of bias.”
Table 3.
Summary of findings
| Probiotic efficacy compared with placebo efficacy in critically ill patients | |||||
|---|---|---|---|---|---|
| Patient or population: Critically ill patients | |||||
| Setting: ICU | |||||
| Intervention: Probiotic efficacy in critically ill patients | |||||
| Comparison: Placebo | |||||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
| Risk with placebo | Risk difference with probiotic efficacy in critically ill patients | ||||
| Mortality | 683 | ⊕⊕⊕○ | RR 1.13 (0.82 to 1.55) | 167 per 1000 | 22 more per 1000 |
| (6 RCTs) | Moderatea | ||||
| (30 fewer to 92 more) | |||||
| ICU-acquired infection | 563 | ⊕⊕⊕○ | RR 0.73 (0.58 to 0.93) | 371 per 1000 | 100 fewer per 1000 |
| (5 RCTs) | Moderatea | (156 fewer to 26 fewer) | |||
| GRADE Working Group grades of evidence | |||||
| High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. | |||||
| Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. | |||||
| Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. | |||||
| Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | |||||
Most of the included studies were rated as having a “high risk of bias.”
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI, confidence interval; RR, risk ratio.
ICU mortality
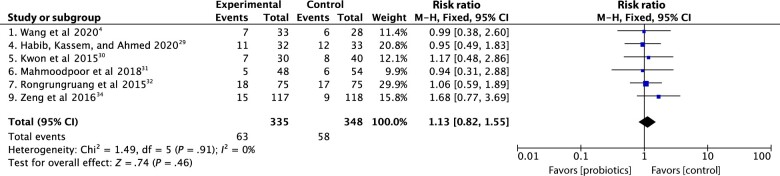
Data on ICU mortality were reported in 6 trials.4,29–32,34 No effect of probiotics on ICU mortality was detected when compared with ICU mortality for the standard fiber groups, P > .05. Rongrungruang et al32 conducted a trial on 150 critically ill patients, which did not report a significant difference between ICU mortality in the probiotic and control groups, (24% and 22.7%, respectively, P = .85). Similarly, Kwon et al30 conducted a trial on 103 critically patients, and no significant difference was observed between the probiotics and control groups in terms of death incidence (22% and 21%, respectively, P = .80). In addition, in a multicentre RCT performed by Zeng et al34 on 235 patients, there was no significant difference between the probiotic and control groups in either ICU (12.7% and 7.7%, P = .2) or hospital (10.7% and 14.8%, P = .369) mortality rates. Moreover, Mahmoodpoor et al31 carried out a double-blind RCT performed on 100 patients who were admitted to the ICU and undergoing mechanical ventilation. No significant difference in ICU mortality was observed between the probiotic and control groups (10.4% and 11.1%, respectively, P = .58). A more recent RCT published in July 2020 was conducted by Habib, Kassem, and Ahmed29 on 65 critically ill patients. No significant difference in ICU mortality was noticed between the 2 groups, (34.38% in the probiotics group and 36.36% in the control group, P = 1.0). The most recent RCT, published August 2020, was performed by Wang et al4 on 61 critically ill patients. They, too, did not report a significant difference between the probiotics and control groups (21% in each group, P = .98). There was also no significant difference between probiotic and control groups in terms of the mortality rate at 3 months, (33.3% and 34.7%, respectively, P = .86). When probiotics were given to adult critically ill patients compared with a control group, the meta-analysis found no significant reduction in mortality (risk ratio 1.13, 95% CI .82 to 1.55, P = .46, Figure 4).4,29–32,34
Figure 4.
Forest plot comparison: effect of probiotics on ICU mortality.
Abbreviations: CI, confidence interval; ICU, intensive care unit; M-H, mantel-heanszel fixed-effect model.
ICU-acquired infections
Six trials4,29,30,32–34 reported data regarding the infection incidence. In the Tan et al33 study, conducted on 52 traumatic brain injury patients, the aim was to assess the effect of enteral administration of probiotics on the clinical outcomes for the patients and on regulating the serum of their immune cell receptors. The authors suggested that probiotics reduced the infection risk, but no statistically significant difference was observed in terms of infections between the probiotics and control groups (34.6% and 57.7%, respectively, P = .095). Moreover, in the Kwon et al30 trial, the authors reported there was no significant difference between the probiotics and control groups P > .05. Rongrungruang et al32 and Wang et al4 concluded in their trials that there was no significant difference in terms of ICU infection, P = .46 and P = .92, respectively, between the probiotics and control groups. In contrast, Zeng et al34 and Mahmoodpoor et al31 showed a significant reduction in terms of ICU-acquired infections, particularly regarding VAP infection, P = .031 and P = .04, respectively. On the other hand, Habib, Kassem, and Ahmed29 suggested that no significant difference was seen between the probiotics and control groups (15.6% and 21.21%, respectively, P = .75) regarding VAP infection.
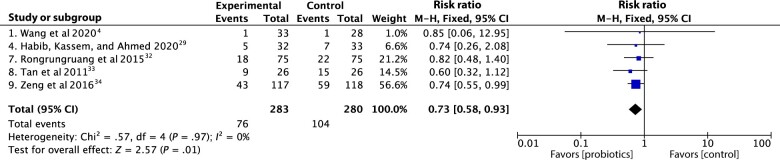
When probiotics were administered to one group and not to a control group, the meta-analysis found no significant difference in the incidence of ICU-acquired infections among adult critically ill patients (risk ratio .73, 95% CI .58 to .93, P = .01, Figure 5).4,29,30,32–34
Figure 5.
Forest plot comparison; effect of probiotics on ICU-acquired infections.
Abbreviations: CI, confidence interval; ICU, intensive care unit; M-H, mantel-heanszel fixed-effect model.
Incidence of diarrhea
Five trials reported data about the incidence of diarrhea.4,9,28 An early RCT performed by Ferrie and Daley9 was conducted on 36 critically ill patients. The result indicated that Lactobacillus rhamnosus GG increased the prevalence of daily loose stools, but this finding was not statistically significant, P = .099. No significant difference was observed in either per-protocol or intention-to-treat analyses in terms of the severity of diarrhea. In contrast, Rongrungruang et al32 concluded that there was no significant difference in the diarrhea incidence between the probiotic and control groups (25.3% and 18.7%, respectively, P = .32). A pilot study performed by de Castro Soares et al28 conducted on 58 critically ill patients concluded that critically ill patients treated with Bacillus cereusA 05 probiotic had a significantly lower number of days with diarrhea than those treated with fiber, P = .011. The Mahmoodpoor et al trial31 concluded that probiotics’ use tends to reduce the incidence of diarrhea; however, no statistically significant difference was observed between the two groups (probiotics, 14.6%, control group, 27.8%, P = .08). Furthermore, in a recent trial carried out by Wang et al,4 no significant difference was noticed between the probiotic and control groups (43% and 24%, respectively, P = .12).
DISCUSSION
This review aimed at assessing the safety and efficacy of probiotics in reducing the occurrence of ICU-acquired infections in critically ill adult patients. Nutrition support has been developed over recent years, and it is now considered a crucial part of the treatment plan for critically ill patients.35 Patients diagnosed with a systemic inflammatory disease, those with a long duration of hospitalization, and those with dysfunctions of multiple organs have a higher risk of morbidity and mortality rates due to various types of infection.35 Before any nutritional intervention, patient safety must be ensured, especially in life-threatening situations. A previous systematic review and meta-analysis of 13 RCTs published by Barraud, Bollaert, and Gibot,36 conducted on 1400 critically ill patients, reviewed a different strains of probiotics. In that study, it was concluded that probiotics did not have any beneficial effect on the ICU mortality rate, P = .29. In addition, in another systematic review and meta-analysis of 8 RCTs performed by Bo et al37 on 1083 critically ill patients, no significant difference was observed in terms of the mortality rate, P = .4. Manzanares et al38 conducted a study on 2972 ICU patients to evaluate the effect of probiotics and synbiotics on critically ill patients and concluded that no significant difference was observed between the probiotics and control groups, P = .44, in terms of mortality incidence, which is consistent with the findings of the present review.
VAP is linked to a longer stay in the ICU and to mechanical ventilation.39 In critically ill patients who are at a higher risk of infections, improved management and reduced risk of undesirable outcomes have been associated with the establishment and development of new approaches and interventions to evaluate the patient’s status, resulting in better quality of care.5 This review has found data supporting a significant reduction in ICU-acquired infections when probiotics are used: Manzanares et al38 reported that the overall ICU infection and VAP incidence were significantly lower in the probiotics group, P = .009, P = .002, respectively. In contrast, Barraud, Bollaert, and Gibot 36 reported that the use of probiotics did not show a beneficial effect in terms of ICU-acquired infections, P = .10. However, in a subgroup analysis of 10 trials, it was reported that there was a significant reduction in ICU-acquired pneumonia, P = .0005. In accordance with the main findings of Barraud, Bollaert, and Gibot36, Watkinson et al40 has reported that uses of both probiotics and synbiotics were not associated with any significant difference between control and experiment groups regarding the rate of nosocomial infections, especially pneumonia.
Diarrhea is one of the most common complications among critically ill patients. Many factors can induce diarrhea in critically ill patients, such as pharmaceutical (eg, antibiotics, laxatives, and enemas), bowel infections, or other non-infective causes (eg, a lack of pancreatic enzymes, and overfeeding).12 Studies have shown that diarrhea in critically ill patients is related to adverse outcomes, such as developing wound infections, lowering the actual nutritional intake, dehydration, shifting and disturbance of the electrolytes, all of which ultimately increase the risk of mortality.8 In this review, most of the included studies did not reveal any significant effect of probiotics on preventing or reducing the incidence of diarrhea. Bo et al37 reported that no significant difference was observed in terms of diarrhea incidence, P = .1. Consistent with the findings of the present review and Bo et al,37 Manzanares et al38 stated that no significant difference was observed between diarrhea incidence in probiotic and control groups, P = .74. On the other hand, Saccharomyces boulardii appears to be the most efficient fungus for preventing antibiotic-associated diarrhea, according to large single-strain meta-analyses: S. boulardii was found to have a pooled relative risk of 0.5, with 10 patients needing to be treated to prevent 1 case of antibiotic-associated diarrhea.41,42 In addition, a recent systematic review and meta-analysis included studies (performed only in China) reporting that use of probiotics results in a significant reduction in terms of gastrointestinal complications among severe stroke patients P < .00001.43
This review has some strengths, including trials with double-blind designs, which minimizes the risk of bias and reduces the placebo effect. Also, most of the included trials used both intention-to-treat and per-protocol analysis to increase the reliability. However, this review has several limitations, such as a limited number of trials being reviewed with different levels of quality, which may impact the outcomes. Most of the studies were of a small sample size or a pilot design, which only provides an initial view of the effectiveness of the probiotics. A larger trial with a higher number of participants is required. This systematic review and meta-analysis investigated the effect of probiotics that were given via the enteral route, so these findings cannot be generalized to other feeding routes. Each trial used different probiotics strains and species, so it is challenging to determine the effect of a particular strain. Also, the higher age groups presented in some of the trials are usually linked with higher mortality rates than other age groups, and this could affect the mortality rate and the infection incidence, so the results cannot be generalized across all age groups. Two of the included studies32,34 were of open-label design, impacting the clinician’s management and/or patient outcomes. The medications and antibiotics that the patients were on were either not reported (as in Rongrungruang et al32) or administered in probiotic groups more than in control groups (as in Kwon et al23), which may affect the results for both ICU-acquired infections and incidence of diarrhea. A few trials were performed in specific ICU settings, such as respiratory and trauma units, so the results cannot be generalized across all ICU settings. Additionally, the trial tests that Zeng et al34 used to confirm the VAP cases were less reliable than quantitative and microscopic examinations, which provide more accurate results. A few studies also looked at the short period of the therapy and the follow-up for probiotic therapy, which was deemed insufficient to determine the actual effect of the probiotic on mortality or the infection rate. Moreover, in the trial of de Castro Soares et al,28 the different formulas used (standard vs semi-elemental) was a factor. The semi-elemental formulas have a higher osmolality, which may induce an osmotic diarrhea,44,45 in the de Castro Soares et al,28 the participants were have one or more disease (cancer, lung diseases, heart disease, and other diseases) that affect the outcome due to the disease it self or due the medications that are used as a plan of the participants treatment plan. Finally, the absence of a pure control group tends to reduce the actual effect of the intervention.
CONCLUSION
In conclusion, probiotics are considered a safe nutritional intervention among critically ill patients. The use of probiotics demonstrated an improved outcome in reducing the overall ICU infection rate, especially VAP. The uses of Lactobacillus rhamnosus GG and Bacillus cereus A 05 reduced the incidence of diarrhea among critically ill patients. However, the effectiveness of probiotics on the incidence of diarrhea remains inconclusive and limited. Probiotic effects are strain-specific, occur through a variety of pathways and mechanisms, and might differ depending on the illness state. As a result, the optimum probiotic dosage for critically ill patients remains unknown. Further trials with appropriate patients, using higher quality and specific probiotic strains would further assess the overall effectiveness of probiotics in critically ill patients.
Acknowledgments
This article represents the original work of the authors and is not currently under consideration elsewhere, nor has it been previously published in the same or substantially similar form. No copyright for any other work was breached in the manuscript’s creation.
Author contributions. A.A. is the first author who conceived and designed the study and the search strategy, screened the titles and abstracts, and full text, assessed the quality of the included studies, extracted and analysed the data, and wrote the manuscript. F.M. helped with drafting and revising the paper. All authors reviewed and provided comment on the manuscript. All authors read and approved the final manuscript.
Funding. There was no direct funding for the review.
Declaration of interest: The authors have no relevant interests to declare.
Contributor Information
Abdulaziz Sulaiman Alsuwaylihi, is with the Department of Clinical Nutrition, King Saud Medical City, Riyadh, Saudi Arabia.
Fiona McCullough, is with the School of Biosciences Division of Food, Nutrition & Dietetics, University of Nottingham, Nottingham, UK.
REFERENCES
- 1. Mueller C, Lord LM, Marian M, et al. ; Nutrition ASFPAE. The ASPEN Adult Nutrition Support Core Curriculum. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition; 2017. [Google Scholar]
- 2.BAPEN. BAPEN Publishes Results of Biggest Malnutrition Survey Ever Undertaken (Scotland). www.bapen.org.uk. Published February 7, 2018. Available at: https://www.bapen.org.uk/media-centre/press-releases/377-bapen-publishes-results-of-biggest-malnutrition-survey-ever-undertaken-scotland. Accessed December 14, 2020.
- 3. Osooli F, Abbas S, Farsaei S, et al. Identifying critically ill patients at risk of malnutrition and underfeeding: a prospective study at an academic hospital. Adv Pharm Bull. 2019;9:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang J, Ke H, Liu KX, et al. Effects of exogenous probiotics on the gut microbiota and clinical outcomes in critically ill patients: a randomized controlled trial. Ann Palliat Med. 2021;10:1180–1190. [DOI] [PubMed] [Google Scholar]
- 5. Hranjec T, Sawyer RG.. Management of infections in critically ill patients. Surg Infect (Larchmt). 2014;15:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimizu K, Ogura H, Goto M, et al. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60:126–133. [DOI] [PubMed] [Google Scholar]
- 7. Shimizu K, Ogura H, Asahara T, et al. Gastrointestinal dysmotility is associated with altered gut flora and septic mortality in patients with severe systemic inflammatory response syndrome: a preliminary study. Neurogastroenterol Motil. 2011;23:330–335, e157. [DOI] [PubMed] [Google Scholar]
- 8. Moron R, Galvez J, Colmenero M, et al. The importance of the microbiome in critically ill patients: role of nutrition. Nutrients. 2019;11:3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrie S, Daley M.. Lactobacillus GG as treatment for diarrhea during enteral feeding in critical illness. JPEN J Parenter Enteral Nutr. 2011;35:43–49. [DOI] [PubMed] [Google Scholar]
- 10. Szymański H, Pejcz J, Jawień M, et al. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains – a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;23:247–253. [DOI] [PubMed] [Google Scholar]
- 11. Murali M, Ly C, Tirlapur N, et al. Diarrhoea in critical care is rarely infective in origin, associated with increased length of stay and higher mortality. J Intensive Care Soc. 2020;21:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tirlapur N, Puthucheary ZA, Cooper JA, et al. Diarrhoea in the critically ill is common, associated with poor outcome, and rarely due to Clostridium difficile. Sci Rep. 2016;6:24691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sánchez B, Delgado S, Blanco-Míguez A, et al. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017;61:1600240. [DOI] [PubMed] [Google Scholar]
- 14. Ranjha MMAN, Shafique B, Batool M, et al. Nutritional and health potential of probiotics: a review. Appl Sci. 2021;11:11204. [Google Scholar]
- 15. Sanjukta M, Swastik A.. A brief overview on probiotics: the health friendly microbes. Biomed Pharmacol J. 2021;14:1869–1880. [Google Scholar]
- 16. Alberda C, Gramlich L, Meddings J, et al. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85:816–823. [DOI] [PubMed] [Google Scholar]
- 17. Batra P, Soni KD, Mathur P, et al. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: an updated systematic review and meta-analysis of randomized control trials. J Intensive Care. 2020;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnstone J, Meade M, Lauzier F, et al. ; Prevention of Severe Pneumonia and Endotracheal Colonization Trial (PROSPECT) Investigators and the Canadian Critical Care Trials Group. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. JAMA. 2021;326:1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McClave SA, Taylor BE, Martindale RG, et al. ; the Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) [published correction appears in JPEN J Parenter Enteral Nutr. 2016 Nov;40(8):1200]. JPEN J Parenter Enteral Nutr. 2016;40:159–211. [DOI] [PubMed] [Google Scholar]
- 20. Knight DJW, Gardiner D, Banks A, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009;35:854–861. [DOI] [PubMed] [Google Scholar]
- 21. van Santvoort HC, Besselink MG, Timmerman HM, et al. Probiotics in surgery. Surgery. 2008;143:1–7. [DOI] [PubMed] [Google Scholar]
- 22. Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation – a randomized, double-blind trial. Am J Transplant. 2005;5:125–130. [DOI] [PubMed] [Google Scholar]
- 23. Wunderlich PF, Braun L, Fumagalli I, et al. Double-blind report on the efficacy of lactic acid–producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J Int Med Res. 1989;17:333–338. [DOI] [PubMed] [Google Scholar]
- 24. Camarri E, Belvisi A, Guidoni G, et al. A double-blind comparison of two different treatments for acute enteritis in adults. Chemotherapy. 1981;27:466–470. [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. GRADEpro. GRADEpro. GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.). 2020. Available at: https://www.gradepro.org. Accessed June 18, 2021.
- 28. de Castro Soares GG, Marinho CH, Pitol R, et al. Sporulated Bacillus as alternative treatment for diarrhea of hospitalized adult patients under enteral nutrition: a pilot randomized controlled study. Clin Nutr ESPEN. 2017;22:13–18. [DOI] [PubMed] [Google Scholar]
- 29. Habib T, Kassem A, Ahmed I.. Early probiotics in preventing ventilator-associated pneumonia after multiple trauma. Asian J Pharm Clin Res. 2020;13:83–85. [Google Scholar]
- 30. Kwon JH, Bommarito KM, Reske KA, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters. Randomized controlled trial to determine the impact of probiotic administration on colonization with multidrug-resistant organisms in critically ill patients. Infect Control Hosp Epidemiol. 2015;36:1451–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahmoodpoor A, Hamishehkar H, Asghari R, et al. Effect of a probiotic preparation on ventilator-associated pneumonia in critically ill patients admitted to the intensive care unit: a prospective double-blind randomized controlled trial. Nutr Clin Pract. 2019;34:156–162. doi: 10.1002/ncp.10191 [DOI] [PubMed] [Google Scholar]
- 32. Rongrungruang Y, Krajangwittaya D, Pholtawornkulchai K, et al. Randomized controlled study of probiotics containing Lactobacillus casei (Shirota strain) for prevention of ventilator-associated pneumonia. J Med Assoc Thai. 2015;98:253–259. [PubMed] [Google Scholar]
- 33. Tan M, Zhu J-C, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain–injured patients: a prospective randomized pilot study. Crit Care. 2011;15:R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng J, Wang C-T, Zhang F-S, et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42:1018–1028. [DOI] [PubMed] [Google Scholar]
- 35. Park YE, Park SJ, Park Y, et al. Impact and outcomes of nutritional support team intervention in patients with gastrointestinal disease in the intensive care unit. Medicine (Baltimore). 2017;96:e8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barraud D, Bollaert P-E, Gibot S.. Impact of the administration of probiotics on mortality in critically ill adult patients: a meta-analysis of randomized controlled trials. Chest. 2013;143:646–655. [DOI] [PubMed] [Google Scholar]
- 37. Bo L, Li J, Tao T, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev. 2014;10:CD009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manzanares W, Lemieux M, Langlois PL, et al. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis [published correction appears in Crit Care. 2017 Feb 27;21(1):42]. Crit Care. 2016;19:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Papazian L, Klompas M, Luyt CE.. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46:888–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watkinson PJ, Barber VS, Dark P, et al. The use of pre- pro- and synbiotics in adult intensive care unit patients: systematic review. Clin Nutr. 2007;26:182–192. [DOI] [PubMed] [Google Scholar]
- 41. Szajewska H, Mrukowicz J.. Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2005;22:365–372. [DOI] [PubMed] [Google Scholar]
- 42. McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16:2202–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu X, Zhang Y, Chu J, et al. Effect of probiotics on the nutritional status of severe stroke patients with nasal feeding that receive enteral nutrition: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100:e25657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carteron L, Samain E, Winiszewski H, et al. Semi-elemental versus polymeric formula for enteral nutrition in brain-injured critically ill patients: a randomized trial. Crit Care. 2021;25:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pereira-da-Silva L, Dias MP-G, Virella D, et al. Osmolality of elemental and semi-elemental formulas supplemented with nonprotein energy supplements. J Hum Nutr Diet. 2008;21:584–590. [DOI] [PubMed] [Google Scholar]