Abstract
The treatment of choice for chronic thromboembolic pulmonary hypertension (CTEPH) is pulmonary endarterectomy (PEA). Balloon pulmonary angioplasty (BPA) is an emerging option for inoperable patients. Comparisons of the hemodynamic and functional outcome between these treatments are scarce. In this single‐center observational cohort study, we compared hemodynamics by right heart catheterization and peak oxygen consumption before and 5 months (±14 days) after either PEA or BPA. Comprehensive evaluation and selection for PEA or BPA was performed by an expert CTEPH team. Fourty‐two and fourty consecutive patients were treated with PEA or BPA, respectively. Demographics were similar between groups. Both PEA and BPA significantly reduced mean pulmonary artery pressure (from 46 ± 11 mmHg at baseline to 28 ± 13 mmHg at follow‐up; p < 0.001 and from 43 ± 12 mmHg to 31 ± 9 mmHg; p < 0.001) and pulmonary vascular resistance (from 686 ± 347 dyn s cm−5 at baseline to 281 ± 197 dyn s cm−5 at follow‐up; p < 0.001 and from 544 ± 322 dyn s cm−5 to 338 ± 180 dyn s cm−5; p < 0.001), with significantly lower reductions for both parameters in the former group. However, cardiopulmonary exercise testing revealed no significant between group differences in exercise capacity. Diffusion capacity for carbon monoxide at baseline was the only follow‐up predictor for peak VO2. In our study, PEA reduced pulmonary pressures more than BPA did, but similar improvements were observed for exercise capacity. Thus, while long term data after BPA is lacking, BPA treated CTEPH patients can expect physical gains in line with PEA.
Keywords: CTEPH, pulmonary embolism, pulmonary hypertension
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) constitutes group four of the clinical classification of pulmonary hypertension (PH) and is a potentially curable disease. 1 Incidence rates of CTEPH vary significantly between populations. The incidence has been reported to be 1.9 cases per 100,000 population per year in Japan, and 3 to 5 per 100,000 in Europe and USA. 2 CTEPH is believed to result from incomplete resolution of thromboembolic material and persistent obstruction of pulmonary vessels, 3 but the pathophysiological mechanisms are debated. 4 With concurrent microvascular remodeling and dysfunction, elevated pulmonary vascular resistance (PVR) and pulmonary artery pressure (PAP) leads to right ventricular dysfunction and failure.
If left untreated, the long‐term prognosis is poor with 3‐year survival rates of 70% or less. 5 , 6 Pulmonary endarterectomy (PEA) is the treatment of choice in operable patients, with excellent survival and maintenance of good functional status. 7 However, more than one‐third of PEA candidates are considered ineligible for surgery, 8 and PH persists or recurs in up to 50% of patients postoperatively. 9
A series of patients treated with balloon pulmonary angioplasty (BPA) was first described in 2001. 10 The method has since been refined, and studies have demonstrated improved efficacy and safety. 11 , 12 , 13 , 14 The 2022 guidelines from the European Society of Cardiology/European Respiratory Society (ECS/ERS) recommend BPA in patients with CTEPH who are technically inoperable or have residual PH after PEA. 1 Medical therapy with riociguat is recommended for similar indications.
BPA is a percutaneous, catheter‐based technique that involves dilation and reperfusion of stenotic or occluded pulmonary vessels at various segments under angiographic guidance. Several sessions are usually necessary to obtain good hemodynamic results while reducing the risks associated with too aggressive treatment in any one session. Several studies have shown good hemodynamic effects of BPA, with reductions of PVR ranging from 33% to 65%. 15 WHO functional class and 6‐min walk distance also improve significantly after BPA. 15 Functional capacity assessed by cardiopulmonary exercise testing (CPET) has been less extensively investigated, but improvements in peak oxygen uptake (peak VO2) of 17%−25% have been reported after BPA. 13 , 16
Studies comparing the effect of guideline‐recommended treatments for CTEPH are scarce. While two randomized controlled trials recently found that BPA was more effective than riociguat in reducing PVR and improving functional class after 26 weeks, 17 , 18 only two reports compare outcomes after PEA and BPA. 19 , 20
Although some patients are clearly eligible for PEA and others for BPA, there is considerable overlap in terms of localization of thrombi and risk assessment. In some patients the lesions could be targeted by both techniques. Furthermore, the indication for BPA has not been standardized, underlining the relevance of comparing results of BPA to the gold standard of PEA. The aim of this single center study was to compare hemodynamics and functional capacity after either surgery or catheter‐based intervention. We also assessed the effect of BPA in patients with residual PH after PEA.
STUDY DESIGN AND AND METHODS
Patient selection
We reviewed consecutive patients referred to our center who were treated for CTEPH between 2011 and 2021. After confirmation of the diagnosis, all patients were discussed by a multidisciplinary CTEPH team. Assessment of operability was based on the distribution and quality of thromboembolic lesions, as well as comorbidity or other determinants of increased surgical risk. Patients who were deemed operable had PEA at our center. Patients found ineligible for, or not consenting to, surgery were considered for treatment with BPA. Patients with residual PH after PEA were treated with BPA as indicated. Right heart catheterization (RHC) and CPET was performed at baseline and at 5 months (±14 days) follow‐up.
RHC
RHC was performed via the right external jugular vein, using a Swan‐Ganz catheter (Edwards Lifesciences). Right atrial, right ventricular, pulmonary artery and pulmonary arterial wedge pressures were recorded. The wedge position was identified using fluoroscopy, and by verifying a characteristic pressure wave form. Cardiac output (CO) and cardiac index was measured three times by thermodilution. Stroke volume was calculated as CO/heart rate. Pulmonary arterial compliance was calculated as stroke volume/(systolic PAP—diastolic PAP).
CPET
Exercise testing was performed using a bicycle ergometer as previously described. 13 A starting load of 20 W was increased by 10 W/min. VO2 and VCO2 were measured on a breath‐by‐breath basis. Heart rate and electrocardiogram were monitored continuously. Blood pressure was recorded at 1‐min intervals. Peak VO2 and respiratory exchange rate were measured at the last 20 s of exercise. Peak VO2 was expressed as mL kg−1min−1, and as a percentage of the expected value, adjusted for age, weight and gender.
PEA
Median sternotomy was performed, cardiopulmonary bypass was established, and the patient gradually cooled to 20°C. An incision was made in the pulmonary artery. Deep hypothermic circulatory arrest was initiated after the proximal subintimal dissection, and continued for no more than 20 min. Subintimal dissection was performed until all available thromboembolic material was removed. Cardiopulmonary bypass was resumed, and the arteriotomy was closed. The procedure was then repeated on the contralateral side.
BPA
A detailed description of the procedure has been published previously. 13 Venous access was via a 6 French Check‐Flo 90 cm long introducer (Cook Medical) in the femoral vein. A 6 French Judkins Right guiding catheter was advanced to the pulmonary artery. Rapid exchange balloon catheters were advanced over micro guide‐wire through the stenotic or occluded pulmonary artery branch. Superselective angiography was done before balloon inflation to demonstrate stenosis, and after inflation to confirm the result and to detect possible perforation of the vessel. A maximum of three lung segments were treated per session to reduce risk of vascular injury. Repeated sessions of BPA were performed every 5–8 weeks until all available lesions were treated.
Statistical analysis
Data are presented as mean ± standard deviation or number (%). Differences in continuous, approximately normally distributed parameters were assessed using Student's t‐test. Differences in categorical variables were assessed using Fischer's exact test or χ 2 test as appropriate. The difference in the change in categorical variables between the two groups were calculated using the Mann−Whitney U test. A two‐sided p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26 (IBM Corporation).
RESULTS
Baseline characteristics and procedures
Patient characteristics at baseline are presented in Table 1. There were no statistically significant differences in demographic variables and comorbidity between patients who underwent PEA (n = 42) and patients who were primarily treated with BPA (n = 31). Patients treated with PEA had a lower CO at baseline compared to patients primarily treated with BPA (p = 0.05). Peak VO2 as percentage of the expected value was higher at baseline in the group of patients primarily treated with BPA (p = 0.03). Inoperability was due to peripheral lesion distribution (n = 22), significant comorbidity (n = 7), previous PEA (n = 9), and refusal to undergo PEA (n = 2). A mean number of 5.1 ± 2.4 BPA procedures were performed per patient, for a total of 257 BPA sessions. Each patient had a mean number of 7.8 ± 4.3 segmental arteries and 23.3 ± 15.7 subsegmental arteries dilated in the course of their treatment. Nine patients who had undergone PEA were subsequently treated with BPA due to residual or recurrent PH, with a total of 40 patients treated with BPA. Follow‐up examination was performed after a median of 5 months (IQR 4−7) and 3 months (IQR 2−4) after PEA and BPA, respectively. A median number of 28 weeks (IQR 13−38) passed between the first and last BPA sessions.
Table 1.
Population characteristics at baseline.
| Variable | PEA | BPA | p Value |
|---|---|---|---|
| Patients, n | 42 | 31 | |
| Age, years | 60 ± 17 | 63 ± 12 | 0.49 |
| Female sex | 18 (42.9) | 16 (51.6) | 0.77 |
| Body mass index, kg m−2 | 27.4 ± 5.8 | 27.9 ± 4.9 | 0.72 |
| Smokers, current or previous | 23 (54.8) | 20 (64.5) | 0.85 |
| Associated medical conditions | |||
| Previous VTE | 32 (76.2) | 24 (77.4) | 1.00 |
| Hypertension, n | 8 (19.0) | 7 (22.6) | 0.77 |
| Diabetes mellitus, n | 1 (2.4) | 2 (6.5) | 0.57 |
| Thrombophilic disorder, n | 6 (14.2) | 3 (9.7) | 0.72 |
| Pacemaker/indwelling catheter, n | 1 (2.4) | 0 (0.0) | 1.00 |
| Splenectomy, n | 1 (2.4) | 2 (6.5) | 0.57 |
| History of cancer, n | 2 (4.8) | 3 (9.7) | 0.65 |
| COPD, n | 6 (14.3) | 3 (9.7) | 0.72 |
| Blood type A/AB/B/O, % | 64/7/7/21 | 74/4/0/22 | 0.57 |
| Creatinine, µmol/L | 96.5 ± 31.1 | 95.4 ± 30.9 | 0.68 |
| NTproBNP, ng/L | 2458 ± 3662 | 1911 ± 4042 | 0.55 |
| Spirometry | |||
| FVC, % predicted | 93.4 ± 18.3 | 92.7 ± 16.0 | 0.91 |
| FEV1, % predicted | 78.9 ± 18.2 | 81.8 ± 17.2 | 0.58 |
| FEV1/FVC, % | 70.0 ± 11.2 | 71.5 ± 10.4 | 0.54 |
| DLCO, % predicted | 68.9 ± 16.4 | 65.5 ± 19.2 | 0.30 |
| PAH therapy | |||
| sGC stimulator | 2 (4.8) | 1 (3.2) | 1.00 |
| ERA | 0 (0.0) | 0 (0.0) | ‐ |
| PDE‐5 inhibitor | 1 (2.4) | 0 (0.0) | 1.00 |
| PGI | 0 (0.0) | 0 (0.0) | ‐ |
| Hemodynamics | |||
| RAP, mmHg | 9 ± 5 | 7 ± 5 | 0.04 |
| mPAP, mmHg | 46 ± 11 | 42 ± 12 | 0.14 |
| PCWP, mmHg | 10 ± 3 | 8 ± 3 | 0.07 |
| SvO2, % | 59 ± 19 | 64 ± 11 | 0.06 |
| CO, L/min | 4.8 ± 1.3 | 5.4 ± 1.5 | 0.05 |
| CI, L/min/m2 | 2.4 ± 0.6 | 2.7 ± 0.6 | 0.03 |
| Stroke volume, mL | 58 ± 20 | 68 ± 22 | 0.05 |
| PVR, dyne/sec/cm5 | 686 ± 347 | 552 ± 339 | 0.11 |
| PAC, mL/mmHg | 1.42 ± 0.89 | 1.60 ± 0.76 | 0.37 |
| HR, beats/min | 84 ± 13 | 82 ± 14 | 0.44 |
| SBP, mmHg | 128 ± 19 | 136 ± 20 | 0.11 |
| DBP, mmHg | 86 ± 10 | 20 ± 11 | 0.11 |
| CPET | |||
| Peak load, W | 83 ± 33 | 100 ± 32 | 0.06 |
| Peak load, % exp. | 56 ± 18 | 63 ± 15 | 0.15 |
| Peak heart rate | 139 ± 22 | 137 ± 21 | 0.69 |
| Peak heart rate, % exp. | 88 ± 11 | 86 ± 9 | 0.62 |
| Exercise duration, min | 6.5 ± 2.5 | 8.1 ± 3.2 | 0.03 |
| Peak VO2, mL/kg/min | 13.7 ± 4.0 | 15.1 ± 4.8 | 0.21 |
| Peak VO2, % exp. | 60 ± 19 | 70 ± 16 | 0.03 |
| Peak RER | 1.08 ± 0.10 | 1.13 ± 0.11 | 0.13 |
| VE/VCO2 slope | 43.7 ± 11.0 | 37.7 ± 9.5 | 0.07 |
| NYHA class (%, I/II/III/IV) | 0/19/69/12 | 0/29/61/10 | 0.37 |
Note: Baseline characteristics for patients treated with PEA and patients primarily treated with BPA. Data are presented as mean ± SD unless otherwise stated.
Abbreviations: BPA, balloon pulmonary angioplasty; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity for carbon monoxide; ERA, endothelin receptor antagonist; FEV1, forced expiratory volume in 1 s; FVC, functional vital capacity; PAH, pulmonary arterial hypertension; PDE‐5, phosphodiesterase‐ 5; PEA, pulmonary endarterectomy; PGI, prostacyclin; SD, standard deviation; sGC, soluble guanylate cyclase; VTE, venous thromboembolism.
Medical therapy
PAH targeted therapy at baseline is shown in Table 1. At follow‐up, two PEA patients were receiving phosphodiesterase 5‐inhibitors, one patient was receiving combination treatment with phosphodiesterase 5‐inhibitor and endothelin receptor antagonist, and one patient was receiving a soluble guanylate cyclase stimulant. Among the BPA patients, one patient was on a soluble guanylate cyclase stimulant sGC and one was receiving a phosphodiesterase 5‐inhibitor at follow‐up.
Treatment response
Patient recruitment and follow‐up are shown in Figure 1. Hemodynamic variables are presented in Table 2. We observed significant reductions of mean PAP (mPAP) and PVR and a concomitant increase in CO in patients treated with PEA as well as in patients treated with BPA. The mean reduction in mPAP and PVR was significantly larger in the PEA group. If using the Bonferroni correction, only the difference in change in PVR would reach the adjusted p‐level of 0.002. The increase in CO and stroke volume was not significantly different between treatment groups. The change in CO, PVR, mPAP, and peak oxygen consumption are shown in Figure 2.
Figure 1.
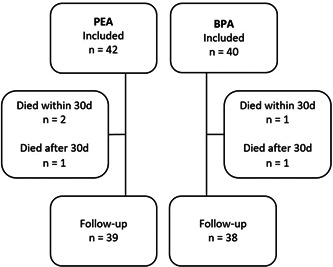
Overview of patients lost to follow‐up. BPA, balloon pulmonary angioplasty; PEA, pulmonary endarterectomy.
Table 2.
Changes in hemodynamic variables and NT‐proBNP.
| PEA (n = 42) | BPA (n = 40) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemodynamic variables | Baseline | Follow‐up | Difference | p Value | Baseline | Follow‐up | Difference | p Value | p, Δ‐comparisona |
| RAP, mmHg | 9 ± 5 | 6 ± 5 | −3 ± 5 | 0.001 | 7 ± 65 | 6 ± 3 | −2 ± 5 | 0.04 | 0.32 |
| mPAP, mmHg | 46 ± 11 | 28 ± 13 | −17 ± 12 | <0.001 | 43 ± 12 | 31 ± 9 | −11 ± 8 | <0.001 | 0.01 |
| PCWP, mmHg | 10 ± 3 | 10 ± 4 | 1 ± 4 | 0.5 | 9 ± 4 | 8 ± 3 | 0 ± 3 | 0.40 | 0.48 |
| SvO2, % | 59 ± 19 | 67 ± 7 | 8 ± 11 | <0.001 | 64 ± 11 | 68 ± 9 | 4 ± 8 | <0.001 | 0.14 |
| CO, L/min | 4.8 ± 1.3 | 5.7 ± 1.4 | 0.8 ± 1.6 | 0.003 | 5.6 ± 1.7 | 6.2 ± 1.3 | 0.5 ± 1.1 | 0.008 | 0.31 |
| CI, L/min/m2 | 2.4 ± 0.6 | 2.9 ± 0.6 | 0.4 ± 0.8 | 0.003 | 2.8 ± 0.7 | 3.1 ± 0.6 | 0.2 ± 0.6 | 0.01 | 0.33 |
| Stroke volume, mL | 58 ± 20 | 73 ± 20 | 14 ± 20 | <0.001 | 68 ± 23 | 83 ± 21 | 13 ± 15 | <0.001 | 0.81 |
| PVR, dyne/sec/cm5 | 686 ± 347 | 281 ± 197 | −387 ± 312 | <0.001 | 544 ± 322 | 338 ± 180 | −163 ± 175 | <0.001 | <0.001 |
| PAC, mL/mmHg | 1.42 ± 0.89 | 2.77 ± 1.03 | 1.25 ± 1.20 | <0.001 | 1.61 ± 0.83 | 2.45 ± 1.10 | 0.79 ± 0.95 | <0.001 | 0.08 |
| HR, beats/min | 84 ± 13 | 80 ± 14 | −4 ± 10 | 0.01 | 83 ± 14 | 76 ± 12 | −7 ± 9 | <0.001 | 0.19 |
| SBP, mmHg | 128 ± 19 | 128 ± 17 | −1 ± 15 | 0.83 | 133 ± 20 | 133 ± 17 | −2 ± 17 | 0.21 | 0.66 |
| DBP, mmHg | 86 ± 10 | 83 ± 10 | −4 ± 11 | 0.06 | 83 ± 12 | 80 ± 9 | −2 ± 10 | 0.10 | 0.51 |
| NTproBNP, ng/L | 2458 ± 3662 | 818 ± 1234 | −1287 ± 2547 | 0.005 | 1979 ± 3766 | 574 ± 1340 | −936 ± 1570 | <0.001 | 0.49 |
Note: Data are presented as mean ± SD unless otherwise stated.
Abbreviations: BPA, balloon pulmonary angioplasty; CI, cardiac index; CO, cardiac ouput; DBP, diastolic blood pressure; HR, heart rate; mPAP, mean pulmonary artery pressure; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; PAC, pulmonary arterial compliance (SV/systolic PAP–diastolic PAP); PAWP, pulmonary arterial wedge pressure; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance (systolic PAP–PAWP/CO); RAP, right atrial pressure; SBP, systolic blood pressure; SD, standard deviation; SvO2, mixed venous oxygen saturation.
p Value for the difference in change from baseline to follow‐up between the PEA group and the BPA group.
Figure 2.

(a) Boxplot showing the change in mean pulmonary artery pressure from baseline to follow‐up. (b) Boxplot showing the change in pulmonary vascular resistance. (c) Boxplot showing the change in cardiac output. (d) Boxplot showing the change in peak VO2. BPA, balloon pulmonary angioplasty; PEA, pulmonary endarterectomy.
Results of the CPET are shown in Table 3. Both in the PEA group and in the BPA group, we observed significant improvements in peak VO2, peak load, and the VE/VCO2 slope. NYHA class improved in both groups. There were no significant between‐group differences in the improvement of peak VO2, peak load, VE/VCO2 slope or NYHA class. Exercise intolerance defined as a peak VO2 lower than 80% of predicted persisted in 59% of patients treated with PEA and in 43% of patients treated with BPA (excluding those treated with BPA after initial PEA).
Table 3.
Changes in functional capacity.
| PEA (n = 42) | BPA (n = 40) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CPET | Baseline | Follow‐up | Difference | p Value | Baseline | Follow‐up | Difference | p Value | p, Δ‐comparisona |
| Peak load, W | 83 ± 33 | 114 ± 47 | 27 ± 37 | 0.001 | 98 ± 32 | 115 ± 43 | 22 ± 28 | <0.001 | 0.55 |
| Peak load, % exp. | 56 ± 18 | 79 ± 28 | 21 ± 24 | <0.001 | 62 ± 16 | 79 ± 24 | 17 ± 19 | <0.001 | 0.54 |
| Peak heart rate | 139 ± 22 | 137 ± 22 | −3 ± 26 | 0.69 | 135 ± 20 | 145 ± 22 | 10 ± 21 | 0.01 | 0.03 |
| Peak heart rate, % exp. | 88 ± 11 | 86 ± 13 | 0 ± 16 | 0.88 | 84 ± 10 | 90 ± 12 | 7 ± 14 | 0.02 | 0.08 |
| Exercise duration, min | 6.5 ± 2.5 | 7.7 ± 2.3 | 1.2 ± 2.8 | 0.03 | 8.1 ± 3.2 | 9.1 ± 3.9 | 1.4 ± 3.1 | 0.03 | 0.86 |
| Peak VO2, mL/kg/min | 13.7 ± 4.0 | 19.1 ± 6.4 | 4.9 ± 5.4 | <0.001 | 15.4 ± 4.7 | 19.2 ± 4.9 | 4.1 ± 4.3 | <0.001 | 0.51 |
| Peak VO2, % exp. | 60 ± 19 | 80 ± 25 | 22 ± 24 | <0.001 | 70 ± 20 | 83 ± 19 | 13.4 ± 19.7 | <0.001 | 0.16 |
| Peak RER | 1.08 ± 0.10 | 1.10 ± 0.12 | 0.01 ± 0.11 | 0.29 | 1.12 ± 0.11 | 1.16 ± 0.13 | 0.02 ± 0.14 | 0.51 | 0.85 |
| VE/VCO2 slope | 43.7 ± 11.0 | 34.0 ± 8.3 | −8.4 ± 10.0 | <0.001 | 37.8 + 10.4 | 31.4 ± 5.8 | −5.8 ± 7.9 | 0.003 | 0.35 |
| NYHA class (%, I/II/III/IV) | 0/19/69/12 | 33/41/26/0 | −0.95 ± 0.76 | <0.001 | 3/28/63/8 | 31/54/13/3 | −0.85 ± 0.67 | <0.001 | 0.61 |
Note: Data are presented as mean ± SD unless otherwise stated.
Abbreviations: BPA, balloon pulmonary angioplasty; HR, heart rate; NYHA, New York Heart Association; PEA, pulmonary endarterectomy; RER, respiratory exchange ratio; SD, standard deviation; VCO2, CO2 elimination; VE, ventilator equivalent; VO2, oxygen consumption; W, watts.
p Value for the difference in change from baseline to follow‐up between the PEA group and the BPA group.
Improvement in treatment techniques over time might confound results. However, a subanalysis of the first and last halves of our cohort revealed no differences in the change from baseline in mPAP, PVR, or peak VO2 for either treatment group. In an exploratory multiple linear regression analysis, diffusion capacity of carbon monoxide (DLCO) was the only baseline factor that was associated with peak VO2 at follow‐up. Gender, treatment modality (PEA vs. BPA), or baseline values of mPAP, CO, or NT‐proBNP were not associated with peak VO2 at follow‐up.
BPA after PEA
Hemodynamic parameters and results of the CPET in the nine patients treated with BPA for residual PH after PEA are presented in Table 4. We observed numerical improvements in mPAP, CO and PVR that were comparable with the results obtained in the treatment‐naïve patients who received BPA. NYHA class improved significantly from 2.6 ± 0.5 to 1.8 ± 0.8 (p = 0.02). Peak load as a percentage of the expected value increased from 61 ± 21 to 83 ± 24 (p = 0.04), while the increase in peak VO2 did not reach statistical significance (p = 0.06).
Table 4.
Residual PH after PEA, results after BPA (n = 9).
| Baseline | After PEA | After BPA | p Valuea | |
|---|---|---|---|---|
| Hemodynamics | ||||
| mPAP, mmHg | 51 ± 13 | 44 ± 14 | 36 ± 9 | 0.12 |
| CO, L/min | 4.1 ± 1.2 | 6.0 ± 2.2 | 6.1 ± 1.2 | 0.84 |
| PVR, dyne/sec/cm−5 | 891 ± 443 | 516 ± 274 | 442 ± 267 | 0.36 |
| CPET results | ||||
| Peak VO2, mL/kg/min | 13.0 ± 4.7 | 16.9 ± 3.9 | 20.9 ± 6.1 | 0.06 |
| Peak VO2, % exp. | 61 ± 33 | 72 ± 34 | 82 ± 30 | 0.29 |
| Peak load, W | 80 ± 18 | 90 ± 33 | 109 ± 32 | 0.07 |
| Peak load, % exp. | 54 ± 20 | 61 ± 21 | 83 ± 35 | 0.04 |
| NYHA class (%, I/II/III/IV) | 0/11/67/22 | 11/33/56/0 | 44/33/22/0 | 0.02 |
Note: Data are presented as mean ± SD unless otherwise stated.
Abbreviations: BPA, balloon pulmonary angioplasty; CO, cardiac output; CPET, cardiopulmonary exercise testing; mPAP, mean pulmonary artery pressure; NYHA, New York Heart Association; PEA, pulmonary endarterectomy; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; SD, standard deviation; VO2, oxygen consumption.
p Value for the change from after PEA to after BPA.
Complications
The 30‐day mortality was 4.8% (n = 2) in the PEA group and 2.5% (n = 1) in the BPA group. Complications after BPA included wire perforation/vessel rupture (5.4% of procedures), hemoptysis (excluding perforations, 7.0% of procedures), lung injury (3.1% of procedures) and access site vascular complications (1.2% of procedures). Most wire perforations were minor, with no need for intervention beyond prolonged balloon inflation in the affected vessel. Most patients were free of symptoms when leaving the laboratory. Patients treated with BPA after PEA had a similar risk of complications as patients treated with primary BPA. The median stay in the intensive care unit in the PEA group was 3 days (IQR 2−6). The BPA patients were not admitted to the intensive care unit during treatment. The hospital stay (our hospital and local hospital) was significantly longer in the PEA group (sum of all sessions, median 19 days, IQR 12−25) than in the BPA group (median 10 days, IQR 7−14; p < 0.001). An overview of complications after BPA and PEA is presented in Tables 5 and 6, respectively.
Table 5.
Complications after BPA.
| Primary BPA | BPA after PEA | All patients | p Value | |
|---|---|---|---|---|
| Hemoptysis, n (%) | 14 (7.0%) | 4 (7.1%) | 18 (7.0%) | 0.74 |
| Vessel rupture/wire perforation, n (%) | 12 (6.0%) | 2 (3.5%) | 14 (5.4%) | 0.74 |
| Lung injury, n (%) | 6 (3.0%) | 2 (3.5%) | 8 (3.1%) | 0.69 |
| Access site complications, n (%) | 2 (1.0%) | 1 (1.8%) | 3 (1.2%) | 0.52 |
| Total BPA sessions, n | 201 | 56 | 257 |
Note: Complications after balloon pulmonary angioplasty (BPA) in patients selected for primary BPA and BPA on account of residual pulmonary hypertension after pulmonary endarterectomy (PEA), respectively. Data are n (%). p Value for the difference calculated by Fisher's exact test.
Table 6.
Complications after PEA.
| ICU stay, h, median (IQR) | 70 (46−103) |
| Mechanical circulatory support,a n (%) | 2 (4.8%) |
| Postoperative infection, n (%) | 4 (9.5%) |
| Postoperative bleeding,b n (%) | 5 (11.9) |
| Acute kidney injury,c n (%) | 3 (7.1%) |
| New onset atrial fibrillation, n (%) | 5 (9.5%) |
Note: Complications after pulmonary endarterectomy (PEA).
Postoperative use of temporary right ventricular assist device (RVAD, 1 patient) and veno‐arterial extracorporeal membrane oxygenation (venoarterial extracorporeal membrane oxygenation, 1 patient);
Requiring drainage or surgical intervention;
Requiring temporary dialysis.
DISCUSSION
In this single center study, we compared changes in hemodynamics and functional capacity between patients with CTEPH who were treated with PEA, and patients who were treated with BPA. We show that (i) both techniques improve hemodynamics, but with significantly larger reductions in mPAP and PVR after PEA, (ii) improvements in functional capacity measured by CPET and NYHA class did not differ between the two treatment forms, (iii) multiple regression analysis revealed baseline DLCO as the only associated factor with peak VO2 at follow‐up, and (iv) BPA in patients with residual PH after PEA improved their NYHA function class, with a trend toward improvements in hemodynamics and peak VO2.
Patient characteristics in the PEA group and BPA group were comparable. The cohort shares similarities with those described in several other CTEPH studies. Approximately 75% of patients in both groups had a history of venous thromboembolism, and 14% and 15% respectively had an associated thrombophilic disorder. There was an over‐representation of non‐O blood types compared with the general Norwegian population. These findings are very much in line with the large patient registry in Europe and Canada. 8 Baseline hemodynamics were also similar to the average values in the Japanese Multicenter BPA registry and to the patient characteristics in the French experience with BPA in CTEPH. 21 , 22
Both therapies led to significant improvements in right atrial pressure, CO, stroke volume and mixed venous oxygen saturation. PEA results were comparable to those obtained in the large UK database, 9 and lowered mPAP and PVR to significantly lower levels than did BPA. These results contrast the only two other studies comparing hemodynamics and functional capacity after PEA or BPA, which found near‐normalization of the same parameters in Japanese patients. 19 , 20 Thus, while our hemodynamic results are comparable to the recently published French and UK experience with BPA, 9 , 14 , 22 and somewhat superior to the German results, 23 we observe less improvement in mPAP and PVR than reported from Japan. 21 This may be due to intercontinental differences in patient selection, and different approaches to complex lesions. 24 There is, however, also evidence of a difference in phenotype, with a more inflammatory profile and a higher proportion of red thrombi present in European CTEPH patients compared to Japanese CTEPH patients. 24 PEA and BPA are fundamentally different treatments. Whereas PEA is rarely performed more than once, BPA is performed in multiple sessions. This allows the operator to continually evaluate treatment response, and further tailor treatment accordingly. The aggressiveness of the operator in treating all available lesions might affect the result in BPA. Whether this accounts for some of the difference in BPA results between European and Japanese centers is unknown.
Improvements in NYHA class and functional capacity were similar after BPA and PEA, and we believe that our CPET derived data strengthen former results obtained by 6‐min walk testing. 19 , 20 Preoperative DLCO was the only predictor of postinterventional peak VO2. DLCO may be a marker of distal pulmonary vasculopathy that is inaccessible to BPA or PEA, associated with exercise intolerance and perhaps reduced survival in CTEPH. 25 Importantly, approximately 50% of our patients in both groups had exercise intolerance at follow‐up, despite improvements in functional capacity. In line with this, Ruigrok et al. reported exercise intolerance in 66% of their patients 6 months after PEA 25 Abnormal exercise hemodynamics with development of PH has been demonstrated after PEA and BPA, despite near‐normalization of resting hemodynamics, suggesting the presence of significant residual vasculopathy. 26 , 27 Hence, both treatment modalities offer partial relief of symptoms in CTEPH patients but normalization is not the rule.
In our study, the nine patients who were treated with BPA for residual PH after PEA obtained improvements that were comparable to those achieved in the treatment‐naïve patients. The differences in hemodynamic improvements were not statistically significant, but with a trend toward improved functional capacity. The subgroup was small, and the likelihood of a type two‐error is high. Reports on BPA after PEA are sparse and the results are conflicting. Improvements in hemodynamics and functional capacity have been reported in 42 patients included in three studies, 28 , 29 , 30 while a UK report observed more non‐responders with BPA after PEA than with BPA in PEA‐naïve CTEPH patients. 31 Whether these differences can be explained by more aggressive protocols and differences in the number of BPA sessions is uncertain. Larger cohorts are needed to further clarify the level of improvement attainable in patients with residual or recurrent PH after PEA.
Very few patients were on PH‐specific treatment before they were referred to our center, and we did not initiate medical therapy before BPA or PEA. Patients presenting with residual PH after PEA were considered for BPA, whereas riociguat was added in patients with mPAP > 38 mmHg and PVR > 425 dyn s cm−5 after BPA, suggestive of a poorer prognosis. 9 In the randomized RACE trial, pretreatment with riociguat resulted in a significant reduction in the rate of BPA‐related serious adverse events. 17 Mean PAP at baseline > 45 mmHg was associated with a higher risk of serious adverse events. This warrants the question whether BPA candidates with unfavorable hemodynamics at baseline should be pretreated with riociguat, or if this treatment should be offered to all patients before BPA. This study has several important limitations. It is a single center study and the patient population is small. Norway has a small population of 5.4 million, and although our center covers CTEPH treatment for all of Norway, it remains a small volume‐center by international standards. The patients were recruited prospectively, but treatment was not allocated randomly. A background of inaccessible peripheral lesions or symptomatic residual PH after surgery existed in 83% of our BPA patients, while the remaining were considered technically operable but with unacceptably high risk or refusal of surgery. According to the ESC/ERS guidelines, BPA is not a replacement for PEA, but rather an evolving treatment alternative for patients who are not eligible for PEA. A true randomized trial where the overlap between eligibility for PEA versus BPA judged by localization of lesions is evaluated, would require international collaboration between multiple centers. Follow‐up examination at 5 months could favor BPA because rehabilitation after cardiothoracic surgery might take longer. While functional capacity seems to improve from 1 to 12 months after PEA, however, insignificant improvements have been demonstrated between 3 and 12 months. 32 , 33 We did not measure DLCO after interventions. However, previous studies have observed stable DLCO values at follow‐up after both PEA and BPA, 34 , 35 , 36 suggestive of irreversible microvasculopathy.
INTERPRETATION
In conclusion, in this prospective single center study, PEA reduced pulmonary pressures to a greater degree than BPA did, but the two treatments did not differ regarding their effect on exercise capacity. BPA is an effective therapeutic option with acceptable safety in patients with CTEPH. While long‐term follow‐up results are needed, our results suggest that BPA is a good treatment alternative in selected patients with CTEPH.
AUTHOR CONTRIBUTIONS
Håvard Ravnestad takes responsibility as guarantor for the content of the manuscript. Arne K. Andreassen and Kaspar Broch conceived the project. Håvard Ravnestad, Kaspar Broch, and Arne K. Andreassen analyzed the data, created the figures and produced the initial draft of the manuscript. Arne K. Andreassen, Kaspar Broch, and John‐Peder Escobar Kvitting provided supervision. Rune Andersen, Morten Svalebjørg, Sigurd Birkeland, Per Snorre Lingaas, Einar Gude, John‐Peder Escobar Kvitting, and Lars Gullestad. helped interpret the data, and contributed substantially to revision of the manuscript. All authors have revised the manuscript, and have approved the final draft of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Patients with CTEPH who were treated with BPA or PEA were eligible for inclusion in this observational cohort study. This study has been approved by the Norwegian Regional Committee for Medical and Health Research Ethics South‐East (application number 454247). The ethics committee gave an exemption from the requirement for written consent. In accordance with the decision of the ethics committee, all patients received written information about the research project, and were given the option to decline participation. The study was conducted in accordance with the Declaration of Helsinki.
Ravnestad H, Andersen R, Birkeland S, Svalebjørg M, Lingaas PS, Gude E, Gullestad L, Kvitting JP, Broch K, Andreassen AK. Pulmonary endarterectomy and balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: comparison of changes in hemodynamics and functional capacity. Pulm Circ. 2023;13:e12199. 10.1002/pul2.12199
REFERENCES
- 1. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Noordegraaf AV, Delcroix M, Rosenkranz S. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–731. [DOI] [PubMed] [Google Scholar]
- 2. Gall H, Hoeper MM, Richter MJ, Cacheris W, Hinzmann B, Mayer E. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur Respir Rev. 2017;26(143):160121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lang IM, Dorfmüller P, Noordegraaf AV. The pathobiology of chronic thromboembolic pulmonary hypertension. Ann Am Thorac Soc. 2016;13(Suppl 3):S215–21. [DOI] [PubMed] [Google Scholar]
- 4. Matthews DT, Hemnes AR. Current concepts in the pathogenesis of chronic thromboembolic pulmonary hypertension. Pulm Circ. 2016;6(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow‐up of patients with pulmonary thromboembolism. Chest. 1982;81(2):151–8. [DOI] [PubMed] [Google Scholar]
- 6. Delcroix M, Lang I, Pepke‐Zaba J, Jansa P, D'Armini AM, Snijder R, Bresser P, Torbicki A, Mellemkjaer S, Lewczuk J, Simkova I, Barberà JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez‐Sanchez MA, Kovacs G, Jaïs X, Ambroz D, Treacy C, Morsolini M, Jenkins D, Lindner J, Dartevelle P, Mayer E, Simonneau G. Long‐Term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133(9):859–71. [DOI] [PubMed] [Google Scholar]
- 7. Mayer E, Jenkins D, Lindner J, D'Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke‐Zaba J, Simonneau G, Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–10. [DOI] [PubMed] [Google Scholar]
- 8. Pepke‐Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D'Armini AM, Morsolini M, Snijder R, Bresser P, Torbicki A, Kristensen B, Lewczuk J, Simkova I, Barberà JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez‐Sanchez MA, Kovacs G, Hamid AM, Jaïs X, Simonneau G. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973–81. [DOI] [PubMed] [Google Scholar]
- 9. Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, Treacy C, Ponnaberanam A, Condliffe R, Sheares K, Taboada D, Dunning J, Tsui S, Ng C, Gopalan D, Screaton N, Elliot C, Gibbs S, Howard L, Corris P, Lordan J, Johnson M, Peacock A, MacKenzie‐Ross R, Schreiber B, Coghlan G, Dimopoulos K, Wort SJ, Gaine S, Moledina S, Jenkins DP, Pepke‐Zaba J. Dynamic risk stratification of patient Long‐Term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation. 2016;133(18):1761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103(1):10–3. [DOI] [PubMed] [Google Scholar]
- 11. Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardio Interven. 2012;5(6):748–55. [DOI] [PubMed] [Google Scholar]
- 12. Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, Tamura Y, Ando M, Fukuda K, Yoshino H, Satoh T. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardio Interven. 2012;5(6):756–62. [DOI] [PubMed] [Google Scholar]
- 13. Andreassen AK, Ragnarsson A, Gude E, Geiran O, Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013;99(19):1415–20. [DOI] [PubMed] [Google Scholar]
- 14. Hoole SP, Coghlan JG, Cannon JE, Taboada D, Toshner M, Sheares K, Fletcher AJ, Martinez G, Ruggiero A, Screaton N, Jenkins D, Pepke‐Zaba J. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: the UK experience. Open Heart. 2020;7(1):e001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanabe N, Kawakami T, Satoh T, Matsubara H, Nakanishi N, Ogino H, Tamura Y, Tsujino I, Ogawa A, Sakao S, Nishizaki M, Ishida K, Ichimura Y, Yoshida M, Tatsumi K. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: a systematic review. Respir Invest. 2018;56(4):332–41. [DOI] [PubMed] [Google Scholar]
- 16. Jin Q, Luo Q, Yang T, Zeng Q, Yu X, Yan L, Zhang Y, Zhao Q, Ma X, An C, Xiong C, Zhao Z, Liu Z. Improved hemodynamics and cardiopulmonary function in patients with inoperable chronic thromboembolic pulmonary hypertension after balloon pulmonary angioplasty. Respir Res. 2019;20(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaïs X, Brenot P, Bouvaist H, Jevnikar M, Canuet M, Chabanne C, Chaouat A, Cottin V, De Groote P, Favrolt N, Horeau‐Langlard D, Magro P, Savale L, Prévot G, Renard S, Sitbon O, Parent F, Trésorier R, Tromeur C, Piedvache C, Grimaldi L, Fadel E, Montani D, Humbert M, Simonneau G. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open‐label, randomised controlled trial and ancillary follow‐up study. Lancet Respir Med. 2022;10(10):961–71. [DOI] [PubMed] [Google Scholar]
- 18. Kawakami T, Matsubara H, Shinke T, Abe K, Kohsaka S, Hosokawa K, Taniguchi Y, Shimokawahara H, Yamada Y, Kataoka M, Ogawa A, Murata M, Jinzaki M, Hirata K, Tsutsui H, Sato Y, Fukuda K. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open‐label, randomised controlled trial. Lancet Respir Med. 2022;10(10):949–60. [DOI] [PubMed] [Google Scholar]
- 19. Inami T, Kataoka M, Ando M, Fukuda K, Yoshino H, Satoh T. A new era of therapeutic strategies for chronic thromboembolic pulmonary hypertension by two different interventional therapies; pulmonary endarterectomy and percutaneous transluminal pulmonary angioplasty. PLoS One. 2014;9(4):e94587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taniguchi Y, Miyagawa K, Nakayama K, Kinutani H, Shinke T, Okada K, Okita Y, Hirata K, Emoto N. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention. 2014;10(4):518–25. [DOI] [PubMed] [Google Scholar]
- 21. Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, Yamada N, Yao A, Ando M, Ogino H, Tanabe N, Tsujino I, Hanaoka M, Minatoya K, Ito H, Matsubara H. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017;10(11):e004029. [DOI] [PubMed] [Google Scholar]
- 22. Brenot P, Jaïs X, Taniguchi Y, Garcia Alonso C, Gerardin B, Mussot S, Mercier O, Fabre D, Parent F, Jevnikar M, Montani D, Savale L, Sitbon O, Fadel E, Humbert M, Simonneau G. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(5):1802095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olsson KM, Wiedenroth CB, Kamp J‐C, Breithecker A, Fuge J, Krombach GA, Haas M, Hamm C, Kramm T, Guth S, Ghofrani HA, Hinrichs JB, Cebotari S, Meyer K, Hoeper MM, Mayer E, Liebetrau C, Meyer BC. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J. 2017;49(6):1602409. [DOI] [PubMed] [Google Scholar]
- 24. Chausheva S, Naito A, Ogawa A, Seidl V, Winter MP, Sharma S, Sadushi‐Kolici R, Campean IA, Taghavi S, Moser B, Klepetko W, Ishida K, Matsubara H, Sakao S, Lang IM. Chronic thromboembolic pulmonary hypertension in Austria and Japan. J Thorac Cardiovasc Surg. 2019;158(2):604–614.e2. [DOI] [PubMed] [Google Scholar]
- 25. Ruigrok D, Meijboom LJ, Nossent EJ, Boonstra A, Braams NJ, van Wezenbeek J, de Man FS, Marcus JT, Vonk Noordegraaf A, Symersky P, Bogaard HJ. Persistent exercise intolerance after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2020;55(6):2000109 [DOI] [PubMed] [Google Scholar]
- 26. Waziri F, Mellemkjær S, Clemmensen TS, Hjortdal VE, Ilkjær LB, Nielsen SL, Poulsen SH. Long‐term changes of exercise hemodynamics and physical capacity in chronic thromboembolic pulmonary hypertension after pulmonary thromboendarterectomy. Int J Cardiol. 2020;317:181–7. [DOI] [PubMed] [Google Scholar]
- 27. Wiedenroth CB, Rieth AJ, Kriechbaum S, Ghofrani HA, Breithecker A, Haas M, Roller F, Richter MJ, Lankeit M, Mielzarek L, Rolf A, Hamm CW, Mayer E, Guth S, Liebetrau C. Exercise right heart catheterization before and after balloon pulmonary angioplasty in inoperable patients with chronic thromboembolic pulmonary hypertension. Pulm Circ. 2020;10(3):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimura N, Kataoka M, Inami T, Yanagisawa R, Ishiguro H, Kawakami T, Higuchi Y, Ando M, Fukuda K, Yoshino H, Satoh T. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2015;183:138–42. [DOI] [PubMed] [Google Scholar]
- 29. Araszkiewicz A, Darocha S, Pietrasik A, Pietura R, Jankiewicz S, Banaszkiewicz M, Sławek‐Szmyt S, Biederman A, Mularek‐Kubzdela T, Lesiak M, Torbicki A, Kurzyna M. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2019;278:232–7. [DOI] [PubMed] [Google Scholar]
- 30. Andersen A, Hansen JV, Dragsbaek SJ, Maeng M, Andersen MJ, Andersen G, Mellemjkaer S, Ilkjær LB, Nielsen‐Kudsk JE. Balloon pulmonary angioplasty for patients with chronic thromboembolic pulmonary hypertension previously operated by pulmonary endarterectomy. Pulm Circ. 2022;12(3):e12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hug KP, Gerry Coghlan J, Cannon J, Taboada D, Toshner M, Sheares K, Ruggiero A, Screaton N, Jenkins D, Pepke‐Zaba J, Hoole SP. Serial right heart catheter assessment between balloon pulmonary angioplasty sessions identify procedural factors that influence response to treatment. J Heart Lung Transplant. 2021;40(10):1223–34. [DOI] [PubMed] [Google Scholar]
- 32. Matsuda H, Ogino H, Minatoya K, Sasaki H, Nakanishi N, Kyotani S, Kobayashi J, Yagihara T, Kitamura S. Long‐term recovery of exercise ability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2006;82(4):1338–43, discussion 43. [DOI] [PubMed] [Google Scholar]
- 33. Zoia MC, Armini AMD, Beccaria M, Corsico A, Fulgoni P, Klersy C, Piovella F, Viganò M, Cerveri I. Mid term effects of pulmonary thromboendarterectomy on clinical and cardiopulmonary function status. Thorax. 2002;57(7):608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanabe N, Okada O, Nakagawa Y, Masuda M, Kato K, Nakajima N, Kuriyama T. The efficacy of pulmonary thromboendarterectomy on long‐term gas exchange. Eur Respir J. 1997;10(9):2066–72. [DOI] [PubMed] [Google Scholar]
- 35. Takei M, Kataoka M, Kawakami T, Kuwahira I, Fukuda K. Respiratory function and oxygenation after balloon pulmonary angioplasty. Int J Cardiol. 2016;212:190–1. [DOI] [PubMed] [Google Scholar]
- 36. Blanquez‐Nadal M, Piliero N, Guillien A, Doutreleau S, Salvat M, Thony F, Pison C, Augier C, Bouvaist H, Aguilaniu B, Degano B. Exercise hyperventilation and pulmonary gas exchange in chronic thromboembolic pulmonary hypertension: effects of balloon pulmonary angioplasty. J Heart Lung Transplant. 2022;41(1):70–9. [DOI] [PubMed] [Google Scholar]


