Abstract
Introduction:
Rates of severe obesity in adolescents have increased at an alarming rate. Unfortunately, there are limited successful treatments for severe obesity in adolescents. Metabolic and bariatric surgery (MBS) is the most effective treatment available for adolescents with Class 2 and above severe obesity and has demonstrated variable degrees of sustained long-term weight loss which leads to resolution of multiple associated conditions and an improved quality of life.
Areas covered:
We discuss the current landscape of MBS in adolescents and evidence to support its long-term safety and efficacy. A literature search through PubMed, ResearchGate and HOLLIS Harvard Library Online Catalog was performed from the date of inception until 3/15/2021. A combination of the following keywords was used: Pediatric metabolic/bariatric surgery; long term outcomes of Pediatric metabolic/bariatric surgery, perioperative assessment, pediatric metabolic/bariatric surgery barriers; attitudes toward metabolic/bariatric surgery.
Expert opinion:
MBS is emerging as a safe and effective treatment strategy for adolescents with severe obesity, with recent studies demonstrating durable and sustainable weight loss. There remains an urgent need for longitudinal studies to assess durability of weight loss. Obesity stigma and bias, limited access to tertiary care centers, and skepticism around the treatment of obesity poses a major challenge.
Keywords: Metabolic and bariatric surgery, class 2 and 3 severe obesity, adolescent obesity, health disparities, outcomes of bariatric surgery, roux-en-y gastric bypass (rygb) and vertical sleeve gastrectomy (vsg)
1. Introduction
The epidemic of obesity affects one in every five children in the USA. In 2019, the World Health Organization (WHO) highlighted a worldwide prevalence of over 38 million children under the age 5 with overweight or obesity. With the rise in the number of children and adolescents who progress to worsening obesity, and its lifelong associated comorbidities, this disease can no longer be ignored [1,2]. In children and adolescents, obesity is defined by a body mass index (BMI) ≥ 95th percentile for age and sex above 2 years of age. Obesity is further classified by its severity into Class I, Class II, and Class III defined by BMI ≥95th to <120%, BMI ≥120% to <140%, and BMI ≥140% of 95th percentile or BMI ≥40 kg/m2, for age and sex, respectively [3].
Obesity must be tackled as early as possible to prevent debilitating comorbidities later in life. Obesity at ages as early as two years predicts a higher likelihood of transitioning into adulthood with obesity. Evidence suggests that a massive proportion, 80–85%, of pediatric obesity translates into obesity in adulthood [4]. Children with obesity also carry the burden of metabolic and cardiovascular risks [5], reproductive health concerns [6], elevated risks for malignancies [7,8] and auto-immune disorders [9-11], compromised lung health and higher asthma predisposition [12], and notable psychiatric implications [13].
In this setting, timely recognition of the severity of the risks associated with untreated obesity is important for pediatricians, and in turn they must identify individualized and effective management plans for their patients. Lifestyle interventions are the first line of treatment recommended; however, it is critical to acknowledge and understand that lifestyle interventions alone may not be effective monotherapy, especially with Class 2 and Class 3 severe obesities. Although young children with obesity may achieve a mild to moderate reduction in weight and BMI through isolated long-term behavioral interventions, clinically significant reduction in BMI among adolescents with Class 2 and Class 3 severe obesities is rarely achieved with lifestyle therapy alone [14]. A study reported an average weight loss of 3.5 kgs in patients through family-based behavioral weight control and lifestyle intervention, rendering it as an ineffective option, as this degree of weight loss is minimal in the setting of severe obesity [15]. Further, over a follow-up period of 7 months, only half of the patients maintained their reduced weight, which highlights that lifestyle intervention may be used in combination rather than as the sole management strategy in pediatric patients with Class 2 and Class 3 severe obesity [15]. It is therefore crucial to administer aggressive treatment approaches to prevent or delay obesity-associated morbidity and end organ damage [16,17]. The American Society for Metabolic and Bariatric Surgery (ASMBS) recommends metabolic and bariatric surgery (MBS) as a safe and effective treatment option for adolescents with Class 2 obesity with comorbidities or those with Class 3 obesity and higher [17].
Commonly used MBS approaches include vertical sleeve gastrectomy (VSG), Roux-en Y gastric bypass (RYGB), biliopancreatic diversion (BPD), and adjustable gastric band (AGB). A study which analyzed MBS trends noted that over 14,178 MBS procedures were performed in pediatric age group in the USA in the time period of 2005–2014. This study demonstrated a sharp decline in in-hospital complications from 8.8% in the year 2005 to as low as 2.0% in the year 2014 [16]. Concordantly, there has been a sharp rise in the frequency of VSG, from 10 or lower procedures in the year 2005 to as high as 1225 procedures performed in the year 2014, whereas the use of RYGB has almost halved in this time period and AGB showing a steeper decrease [16]. Overall, VSG has been identified as the most preferred choice for MBS procedure to treat obesity in adolescents [18]. Studies have noted BMI loss of up to 14.1 kg/m2, some even recording a 20 kg/m2 in patients who underwent VSG [19,20]. This is striking because of the added advantage of significantly lower complication rate compared to other MBS procedures, rendering VSG a safe and effective surgical method for treatment of obesity in adolescents [16,17].
2. Clinical and biochemical assessment of an adolescent with obesity
Based on the 2015 guidelines from the American Academy of Pediatrics (AAP) [21,22], the clinical assessment of the adolescent with obesity begins in their primary care provider’s office at the annual well visit. Universal clinical assessment for all pediatric patients includes, assessing dietary behaviors, physical activity, television viewing duration and sedentary time, and providing anticipatory guidance and obesity prevention counseling. However, in established obesity, elucidating a comprehensive microenvironment assessment including dietary constituents, physical activity, sleep, sources and levels of stress, as well as medication history with any temporal association to weight gain are tremendously crucial steps in deciding management [23]. At each visit, providers should accurately measure the adolescent’s weight and height, calculate the BMI and determine BMI percentile for age and sex. This preliminary step allows the provider to assess the severity of obesity. This is critical as it determines the aggressiveness of treatment.
For the adolescent with obesity, the clinical assessment consists of augmented, obesity-specific family history, review of systems, and physical exam to narrow the differential diagnosis of weight gain as well as to determine the presence or absence of signs, symptoms, and risk factors for cardiovascular, dermatologic, endocrine, gastrointestinal, orthopedic, neurologic, and psychological/behavioral obesity-related conditions. Providers should establish timing of the onset of weight gain to distinguish between etiologies of recent weight gain from early onset of weight gain that could be caused by single-gene defects in the leptin-melanocortin pathway or complex multisystem syndromic disorders. Additional elicited history such as the adolescent’s stature and/or height velocity has been attenuated relative to the genetic potential and pubertal stage should prompt diagnostic assessment for endocrine causes of obesity such as Cushing’s syndrome, growth hormone deficiency, hypothyroidism, Albright hereditary osteodystrophy, or pseudohypo-parathyroidism [24]. Medical and surgical history should elicit prior diagnoses of developmental delay, congenital midline defects, neurological deficits, renal defects, history of intracranial irradiation, trauma, or surgery.
An obesity-specific review of systems can help narrow the differential diagnosis of obesity as the health-care provider query symptoms and conducts a thorough physical exam to aid the diagnosis of causes of obesity and obesity-related comorbidities as outlined in Table 1.
Table 1.
Selected findings on obesity-focused history, review of systems, and physical exams that narrow the differential diagnosis of causes of weight gain to drive diagnosis of obesity-related comorbidities and preoperative diagnostic testing.
| Systems Involved |
Causal Etiologies of Obesity |
History | Review of Systems | Physical Exam | Obesity-related conditions or comorbidities |
Recommended Pre-Operative Diagnostic Testing (If Clinically Indicated)* |
|---|---|---|---|---|---|---|
| Cardio-Pulmonary | Sleep-related irregularities | Snoring Morning headaches Daytime somnolence |
Tonsil size | OSA | ECGCXREchocardiographyLipid panelDVT evaluation | |
| Endocrine | Cushing’s syndrome Growth Hormone Deficiency Hypothyroidism Albright hereditary osteodystrophy Pseudohypoparathyroidism |
Attenuated stature and/or height velocity relative to genetic potential and pubertal stage |
Proximal muscle weaknessCold intoleranceConstipationAlopecia |
Tanner staging | 1 mg overnight dexamethasone test 24-hour urinary free Cortisol 11 PM salivary Cortisol | |
| Hypothyroidism | Menstrual cycle irregularities | Dysmenorrhea | Hirsutism and acne | PCOS | TSH Total/bioavailable Testosterone DHEASDelta-4-androstenedioneBeta-HcG**Baseline measurement of lean/fat mass** | |
| Acanthosis nigricans | Insulin resistance / Diabetes mellitus | Fasting blood glucoseHbA1c (if diabetes suspected) Oral Glucose Tolerance Test** | ||||
| Gastrointestinal | Clinical nutrition evaluationIron studiesFolic acid25-vitamin DH pylori screeningLFTsGallbladder UltrasoundEndoscopy | |||||
| Neurological | Intracranial insult | Developmental delay | Headaches | Nystagmus | Genetic testing | |
| Congenital midline defects | Visual distrubances | Optic disc swelling | ||||
| Neurological deficits | Visual field deficits | |||||
| History of intracranial irradiation, trauma, or surgery | Hearing loss | |||||
| Micro/macrocephaly | ||||||
| Single gene defects in leptin-melanocortin pathway (i.e. Prader-Willi Syndrome, MC4) | Congenital midline defecits | |||||
| Musculoskeletal | Range of hip motion | SCFE / Blount | DEXA** | |||
| Psychiatric / Behavioral | Enamel erosion | Disordered eating | Psychosocial behavioral evaluation |
Based on the AACE/TOS/ASMBS Preoperative Bariatric Surgery Checklist [72], in addition to routine preoperative lab testing at the individual MBS/GI Surgery Program, which most often includes CBC, Lipid panel, kidney function, UA, PT-INR, Type & Screen
Additional recommended diagnostic testing (if clinically indicated) according to International Pediatric Endosurgery Group Standards and Safety Committee [25]
Recommended universal laboratory screening for the adolescent with BMI >95%ile starting at age 10 includes fasting glucose and/or HbA1C, fasting lipid profile, along with Alanine transaminase (ALT) and Aspartate transaminase (AST). Notably, the AAP asserts that some subspecialty pediatric weight management clinicians also recommend obtaining vitamin D levels and fasting insulin levels [21], although the 2017 Endocrine Society Clinical Practice Guideline on Pediatric Obesity recommends against this practice as their meta-analysis posits that obtaining fasting insulin levels when assessing adolescents with obesity has no diagnostic value [24]. Findings from obesity-specific history, review of systems, and physical exams which suggest particular etiologies for weight gain should prompt additional diagnosis-specific laboratory assessment as outlined in Table 1.
Another vital aspect of the assessment of the adolescent with obesity is of their health- and weight-related quality of life. Quality of Life can be assessed using validated measures including the Pediatric Quality of Life Inventory (PedsQL), the Impact of Weight on Quality of Life-Kid’s measure (IWQOL-Kids), and the 36-Item Short-Form Survey (SF36) which have been widely adopted in the adolescent obesity literature and most notably by the Teen-LABS Consortium [26].
3. Types of metabolic and bariatric surgery and current indications/contraindications from the ASMBS/AAP
The 2019 ASMBS pediatric metabolic and bariatric surgery guidelines [17] recommends that the adolescent with class II obesity (120% BMI percentile for age and sex) along with an obesity-related comorbidity or with class III obesity (140% BMI percentile for age and sex) should be considered for MBS and referred early to a tertiary care metabolic and bariatric surgery center. The majority of MBS programs also require pre-surgical psychosocial evaluation, and the AAP recommends comprehensive pre- and post-operative evaluation by a pediatric mental health clinician who has expertise in pediatric obesity to evaluate the adolescent’s preparation for long-term post-operative management. With the exception of absolute contraindications such as active suicidality, psychosis, and substance use, elicited co-existing mental health diagnoses, disordered eating, family dysfunction, developmental delay, or syndromic obesity (such as in Prader-Willi syndrome) are not absolute contraindications to MBS, although these must be balanced by the experienced evaluator’s assessment of the adolescent’s comprehension of the procedure and their long-term commitment to post-MBS nutritional supplementation and follow-up [27]
ASMBS recommends that all adolescents who undergo MBS be routinely screened and counseled on risks of alcohol use and that smoking or vaping nicotine products should be strongly discouraged after MBS. Informed consent for adolescent patients >18 and/or adolescent assent, in addition to parental consent for adolescents <18 should be obtained.
The most common types of MBS in adolescents include Roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy (VSG), and adjustable gastric banding (AGB). RYGB is performed laparoscopically and adjoins a small proximal gastric pouch to an accompanying ‘roux’ limb of jejunum, which allows enteral content to bypass the remaining gastric remnant and proximal small bowel while preserving exposure to biliopancreatic enzymes [28]. Common complications of RYGB include subsequent intestinal ‘dumping syndrome’ and iron and Vitamin B12 deficiencies. VSG is now the most prevalent and preferred choice for MBS in adolescents, surpassing RYGB as the previous gold standard for MBS in adults and adolescents. VSG involves removing ~80% of the stomach, including the entire greater curve of the stomach, to create a resultant gastric ‘sleeve’ with capacity of 60–100 mL [28]. The VSG procedure preserves the pylorus and distal antrum and enhances postprandial satiety while avoiding the intestinal ‘dumping syndrome’ that often follows RYGB. Complications of VSG can include staple-line leak, stricture formation, and post-operative bleeding [28]. The AGB procedure places a circumferential device, constructed from a silastic belt combined with an adjustable balloon, around the proximal portion of the stomach to create a gastric ‘pseudo-pouch’ which restricts food intake and promotes early satiety [28]. Due to high failure rate and increased need for reintervention compared to VSG or RYGB, the ASMBS does not recommend AGB as preferred weight loss procedure in adolescents [28,29].
In 2020, the ASMBS also endorsed the single-anastomosis duodenoileal bypass with sleeve gastrectomy (SADI-S) procedure [30], in which the duodenum is transected and anastomosed to a loop of ileum, as an appropriate MBS for patients of all ages. The major advantages of the SADIS (considered a modified duodenal switch) over RYGB and earlier duodenal switch procedures are its relative technical ease and reduction in the rates of anastomotic complications, as well as its efficacy as a re-operative procedure after RYGB and VSG [30]
4. Bariatric surgery in adolescents: weight loss outcomes
The American Academy of Pediatrics (AAP) published a policy statement in December 2019, which made practice-changing recommendations regarding the expanded use of metabolic and bariatric surgery (MBS) in adolescent patients with obesity [29]. Much of these recommendations were founded on the data generated from a handful of multicentered studies completed over the past decade [28]. Three landmark studies, known best by their abbreviated trial names – Follow-up of Adolescent Bariatric Surgery (FABS-5+), Adolescent Morbid Obesity Surgery (AMOS), and Teen-Longitudinal Assessment of Bariatric Surgery (Teens-LABS)–were all prospective-designed trials aimed at understanding long-term weight loss outcomes at five or more years postoperatively for adolescents who underwent MBS.
The Follow-up of Adolescent Bariatric Surgery at 5 Plus Years FABS-5+ was designed as a follow-up, extension project in which 74 original patients underwent Roux-en-Y gastric bypass (RYGB) between the years 2001 and 2007. This project was designed to ascertain whether weight loss and metabolic benefits were maintained beyond 5 years postoperatively with the primary outcome being a change in BMI. Of the 58 patients who participated in this follow-up study (mean age: 17.1 years), there was a sustained reduction in BMI of 16.9 kg per meter squared (kg/m2) corresponding to a mean sustained weight loss of 49.9 kg at an average of 8.0 years post-surgery [31]. At ‘5 plus years’ of post-operative data collection, the FABS-5+ study is the longest prospective analysis to date of adolescent patients who have undergone MBS.
The Adolescent Morbid Obesity Surgery (AMOS) study was designed as a prospective, non-randomized, controlled study with the aim to compare weight loss outcomes for adolescents with severe obesity who underwent either surgical or non-surgical management. Of the 81 patients (mean age: 16.5 years) who underwent RYGB between the years 2006 and 2009, there was a sustained reduction in BMI of 13.1 kg/m2 corresponding to a mean sustained weight loss of 36.8 kg at an average of 5.0 years post-operation. The AMOS study was unique from these other prospective trials in that researchers included an adolescent control arm, in which matched controls for age, sex and baseline BMI received non-surgical, conventional weight reduction therapies. The conventional therapy group was identified using the Swedish Childhood Obesity Treatment Register (BORIS). The following are the baseline BMIs for the adolescent cases undergoing RYGB and the adolescent controls undergoing conventional therapy: 45.5 kg/m2 versus 42.2 kg/m2, respectively. At 5 years of follow-up, the non-surgical control group experienced a mean increase in BMI of 3.3 kg/m2, and 25% of these patients elected to undergo bariatric surgery within 5 years [32].
Lastly, the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) consortium recently published outcomes at 5-year post-operation, comparing sustained weight loss outcomes between adolescents (19 years of age and younger) and adults. Of the 161 adolescents (mean age: 17.0 years) undergoing RYGB between the years 2006 and 2012, there was a sustained reduction in BMI of 13.0 kg/m2 corresponding to a mean sustained weight loss of 36 kg at an average of 5.0 years post-operation [33]. In a separate subgroup analysis at 3-year post-operation, researchers compared weight loss outcomes in those adolescents who underwent RYGB (n = 161) with those who underwent sleeve gastrectomy (n = 670) [18]. Results indicated comparable weight reduction with a decrease in weight of 28% (95% CI, 25 to 30) versus 26% (95% CI, 22 to 30) among those who underwent RYGB versus sleeve gastrectomy, respectively. All three of the afore-mentioned studies emphasize the durable and substantial weight loss observed in adolescent patients undergoing MBS. We will next discuss those health effects that come with significant weight reduction during adolescence.
4.1. Bariatric surgery in adolescents: comorbidity outcomes
Adolescents with severe obesity are at an increased risk of having future cardiovascular disease related-events over a 30-year period [34]. Therefore, it is important to consider the benefits of MBS in terms of life-long cardiovascular disease risk reduction. In the FABS-5+ study, 88% of the adolescent patients experienced remission from diabetes, 76% experienced remission from hypertension, and 64% experienced remission from dyslipidemia at 8-year post-operation [31]. A more targeted review of cardiovascular disease risk factors at a 3-year interval follow-up of adolescents with severe obesity enrolled in the Teen-LABS study found sustainable improvements in specific markers of cardiovascular risk. Namely, there were observed improvements in impaired fasting glucose (reduced from 26% to 4%), insulin-resistance (reduced from 76% to 25%), systolic blood pressure (mean decrease by 6 mmHg), diastolic blood pressure (mean decrease by 5 mmHg), triglycerides (mean decrease by 42 mg/dL), and high-density lipoprotein (HDL-C) (mean increase by 15 mg/dL) [35]. Greater BMI reduction at the 3-year interval follow-up independently predicted normalization of certain cardiovascular risk factors, such as diabetes, hyperinsulinemia, elevated high-sensitivity C-reactive protein, blood pressure, and dyslipidemia. Interestingly, long-term outcomes were also associated with the timing of surgery and preoperative BMI, suggesting that MBS performed on adolescents with BMI levels <50 kg/m2 may have better long-term outcomes than in older adolescents with BMI >50 kg/m2 in terms of mitigating future adverse cardiovascular events.
When compared to age and racially matched patients receiving pharmacotherapy for Type 2 diabetes in the Treatment Options of Type 2 Diabetes in Adolescents and Youth (TODAY, n = 63) trial, patients in the Teen-LABS study experienced a sustained reduction in cardiovascular risk at 5-year post-surgery while patients undergoing medical therapy experienced an increase in cardiovascular risk [34]. Furthermore, physical activity has been shown to be an important adjunct to bariatric surgery to improve hypertriglyceridemia, low-density lipoprotein (LDL-C), and non-HDL-C dyslipidemias at 3-year post-surgery [36].
4.2. Comparison of outcomes after MBS: adults versus adolescents
In a large, prospective, controlled trial in adult patients with obesity (n = 2010), MBS was associated with a reduction in overall mortality when compared to more conventional management strategies (n = 2037) [37]. In a comparison of LABS (n = 1738 adults) and Teen-LABS (n = 161 adolescents), Inge and colleagues found that adult and adolescent patients undergoing MBS experienced similar overall weight loss at 5-year post-surgery [33]. This consistent trend in weight loss was also shown in the AMOS study, which prospectively compared weight outcomes between adults and adolescents. Stanford and colleagues in a third retrospective study compared short- and long-term outcomes found that adolescents experienced greater reductions in weight and BMI compared with those in adults at 4-year post-surgery [38]. Beyond weight loss, Inge and colleagues described greater rates of remission of type 2 diabetes mellitus and hypertension among adolescents compared to those rates in adults. Remission of type 2 diabetes mellitus was seen in 86% of the adolescents compared to 53% of the adults. Remission of hypertension was seen in 68% of the adolescents compared to 41% of the adults [33].
In a controlled, prospective study of adolescents with nonalcoholic steatohepatitis (NASH), researchers followed liver enzymes and pathology from liver biopsies overtime to determine the effect of bariatric surgery on liver disease. Compared to the non-surgically managed adolescents, those who underwent laparoscopic sleeve gastrectomy experienced a marked improvement in degree of liver fibrosis. Within 1-year post-operatively, 90% of the surgical recipients demonstrated a regression of liver fibrosis from stage 2 to stage 1 [39,40].
5. Complications of surgery
Complications of bariatric surgery in adolescents range from mild nutritional deficiencies to more significant adverse events that include the need for other intra-abdominal procedures and death. The FABS-5+ study described long-term adverse nutritional deficiencies in individual status post RYGB that in some cases necessitated parenteral repletion [31]. Nutritional deficiencies are well described in patients undergoing RYGB. The American Society for Metabolic and Bariatric Surgery (ASMBS) pediatric committee therefore recommends supplementation with vitamin B1 (pre- and post-operatively), as well as daily use of a multivitamin with iron, vitamin B12, calcium citrate, and vitamin D in the post-operative period [17]. The challenge with these nutritional recommendations is that they are admittedly difficult to follow and enforce. During immediate and more remote postoperative periods, the patient should be followed by a registered dietitian. Correction of micronutrient deficiencies early on is critical to prevent potential long-term complications that include hypoglycemia, protein malnutrition, and anemia [41].
Comparing the need for postoperative intra-abdominal procedures for the Teen-LABS and LABS cohorts, the incidence rate ratio between adolescent and adult patients was 2:1. The most common procedure following RYGB was cholecystectomy [33]. Likewise, in the AMOS study, 20 (25%) surgical patients underwent a total of 21 intra-abdominal procedures in the 5-year post-operation (11 for acute intestinal obstruction and 9 for symptomatic gallstones) [32]. The development of post-operative symptomatic gallstone disease is thought to be a side effect of rapid weight loss and is likely not limited to those undergoing RYGB. The prevention of gallstone disease in adolescents undergoing bariatric surgery is not well elucidated, but prior studies have investigated the use of concomitant cholecystectomy or post-operative ursodeoxycholic acid for patients who are identified as high risk [42].
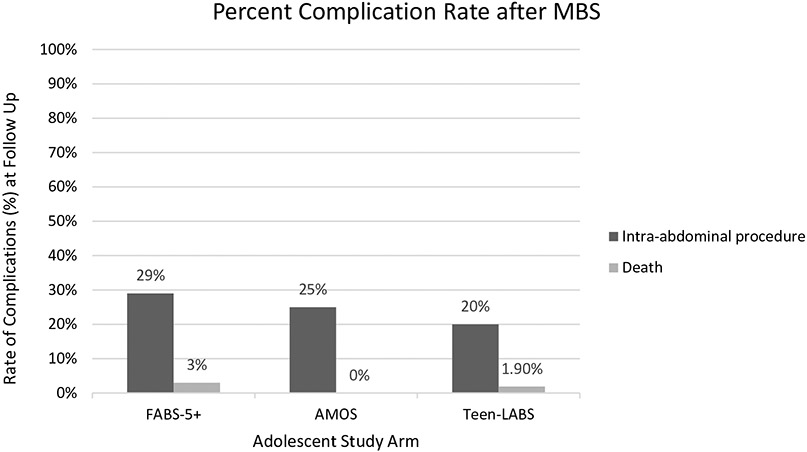
In the Teen-LABS and LABS cohorts, within the first 5-year post-operation, death occurred in three adolescent and seven adult patients [33]. Of the three adolescent patients, one death was attributed to a complication of Type 1 diabetes mellitus and suspected sepsis 3-year post-operation, and the other two deaths were attributed to acute drug overdose. Of the seven adult patients who died, three deaths occurred in the immediate postoperative period and were related to gastric bypass. Other adult deaths were either of indeterminate cause, suicide or secondary to colon cancer. No deaths occurred within the five-year study period in the AMOS trial [32]. Figure 1 shows a comparison of major complication rates among adolescents enrolled in each of the three studies at follow-up after MBS.
Figure 1.
Comparison of major complication rates among adolescents enrolled in each of the three studies at follow up after metabolic and bariatric surgery (MBS).
5.1. Effects on bones
While weight loss after bariatric surgery has been associated with improved functional mobility and reductions in musculoskeletal pains [43], MBS has also been noted to have deleterious effects on the bone health. In the FABS-5 + study, researchers found that nearly half of the cohort had elevated levels of parathyroid hormone (PTH) and low amounts of 25-OH vitamin D. In a more focused study, researchers found that whole-body bone mineral content declined by 7.4% at 2-year post-MBS with weight loss accounting for 14% of the decrease in bone mineral content in the first year after surgery [44]. Proposed mechanisms for postoperative bone loss include 1) weight loss resulting in loss of bone mass, 2) decreased food intake and altered calcium absorption (supporting the need for nutritional supplementation, which may be more challenging to enforce in adolescents), and 3) other hormonal changes, such as decreased levels of insulin-like growth factor 1, leptin and ghrelin, which may contribute to bone loss [44].
6. Special issues
As the rates of utilization of metabolic and bariatric surgery are increasing in the pediatric population [45-47], there remains a knowledge gap regarding its efficacy and safety among some providers and patients/families. A greater understanding of the long-term effects as they pertain to social and ethical ramifications is needed. Implicit and explicit biases are prevalent among physicians and care providers against metabolic and bariatric surgery; a survey study published in 2009 found that although physicians reported a greater than 80% rate of satisfaction with their patients’ bariatric procedure outcome, 88.5% of the practitioners would never or would be unlikely to refer a child for a bariatric procedure [48]. In another study published the following year, 48% of the physicians reported that they would never refer an adolescent for bariatric surgery [49]. A survey study published in 2013 revealed that far fewer practitioners, 15%, would not refer patients of any age for bariatric surgery [50].
Although this trend shows increased acceptance, it demonstrates a persistent physician bias against referring patients for surgical weight loss. Frequently cited reasons for reluctance to refer pediatric and adolescent patients to bariatric surgery included concern over the long-term effects and surgical risks/complications. Some physicians endorse a concern that weight loss surgery would not treat the main underlying psychological or behavioral problem leading to obesity [51,52], which demonstrates a flawed thinking and lack of understanding of the underlying pathophysiology of the disease of obesity. Other reported obstacles to referral for surgery included poor social supports [48], inability to adhere to lifestyle interventions [52], lack of access to a pediatric bariatric program [48], opinion that medical management was superior [48,52], and ethical concerns regarding the ability of this population to consent to a major and life-altering surgery [53]. Many clinicians reported feeling uncomfortable referring to pediatric patients under a certain age, with an identified appropriate age ranging from fourteen to 18 years old [49,50,54,55]. Physicians frequently recognized a need for minimum prerequisites prior to referral, which included activities such as a specific time-limited trial of medical management, a psychological evaluation, an expressed/demonstrated commitment to lifestyle change, and family support/parental counseling [49,50,55]. Minimal education for pediatricians who treat patients with obesity is a barrier to appropriate referral for MBS. A recent study of pediatricians in a large academic center reveals that only 20% would feel comfortable with pre and post-op care for bariatric surgery patients [56].
Additionally, parents and pediatric/adolescent patients also share similar beliefs. Qualitative studies indicate that significant psychosocial negative attitudes toward bariatric surgery exist. Many parents viewed their child’s obesity as a psychological or behavioral problem and that weight loss surgery was an ‘easy’ solution [51]. Adolescents felt similarly, reporting a belief that non-surgical intervention could be just as effective, and that undergoing weight loss surgery was a sign of weakness of moral character [51]. This viewpoint is shared by the greater public; studies have shown that people who lose weight via bariatric surgery are seen as lazy and less competent than their counterparts who lose weight through diet and exercise alone [57,58]. In other studies, parents who viewed their child’s weight as a future health problem were more likely to consider bariatric surgery, indicating that a barrier to acceptance of surgery is a lack of understanding or awareness of the long-term health consequences of obesity [54]. Physician attitudes are also an important factor in influencing parents’ willingness to consider bariatric surgery; a study by Singh and colleagues found that counseling by a pediatric provider was more likely to influence parents to consider surgery than participating in a structured weight loss program or exposure to bariatric surgery [54].
Given these findings, it is possible that the 2019 American Academy of Pediatrics (AAP) policy statement in support of bariatric surgery for pediatric and adolescent patients with severe obesity may lead to increased acceptance of the practice [29]. In this policy statement and associated technical report [28], the AAP makes several practice-level and system-level recommendations to ensure that appropriate patients are able to access multidisciplinary, pediatric-focused metabolic and bariatric surgery programs, regardless of demographics. Unfortunately, access continues to be a major barrier. The general expert consensus published by the ASMBS and supported by the AAP highlight the importance of the establishment of specialized weight management centers for the pediatric population [28,29]. These centers should include multidisciplinary services such as access to dieticians, social workers, behavioral health specialists, and subspecialists such as cardiologist, endocrinologist, and gastroenterologists as needed to meet individual patient health needs. While these recommendations will help ensure the highest quality of care for patients, this approach limits pediatric surgical centers to primarily urban academic centers that can provide the requisite highly specialized providers. This may lead to decreased care access, particularly for the pediatric and adolescent population in rural areas which have higher rates of obesity compared to urban counterparts [59,60].
Though the AAP encourages that public and private insurers cover evaluations for and treatment with bariatric surgery, research has shown that having a low income and having public insurance are significant barriers to accessing surgical management for obesity [60]. Socioeconomic status has been shown in one study to be the underlying catalyst of race-based disparities in bariatric surgery access [61], and an independent negative predictor of undergoing bariatric surgery [62]. In addition, some studies have revealed that race is an independent negative predictor of bariatric surgery rates. Despite the overall improvement in access to surgery with the implementation of the Affordable Care Act, race and ethnicity-based differences persist [63]. This is highlighted by the evidence that minority race status is independently associated with lower odds for receiving bariatric surgery [60,64] or being considered for it [65], even though ethnic/racial minorities have higher rates of obesity [66,67]. Insurance coverage seems to impact access to surgical care differentially based on the patient’s race and ethnicity. Perez and colleagues found that when comparing Medicaid coverage and private insurance coverage, there was a higher rate of bariatric surgery for white adolescents with Medicaid and a lower rate of bariatric surgery for minority adolescents with Medicaid [64]. These findings indicate significant disparities to access, based on location (rural versus urban), race (minority versus Caucasian), and socioeconomic status, including coverage by public versus private insurance.
7. Pregnancy after metabolic and bariatric surgery
Pregnancy and neonatal outcomes after MBS are vital and important concerns. Encouragingly, patients who underwent MBS showed several beneficial trends in maternal health [68], especially remarkably lower incidence of gestational diabetes mellitus and hypertension, reduced likelihood for postpartum hemorrhage and lower rates of c-section delivery [68]. However, it is also an important factor in neonatal outcomes. A meta-analysis investigating findings from 33 studies noted that post-MBS pregnancies have risks higher by 57% for preterm birth, 29% for congenital anomalies, 41% NICU admissions, and 38% perinatal mortality compared to children born to mothers who never underwent MBS [69]. It is helpful to note that risk factors like small for gestational age (SGA) and intrauterine growth restriction (IUGR) are reduced in procedures like sleeve gastrectomy and gastric banding compared to gastric bypass procedures (GBP) [70]. There is currently, however, no clear consensus on the best choice of surgical procedure among women of reproductive age [71]. Identifying the risk factors specific to each procedure type will enable women to make an informed decision. Further, these risks can be overcome with targeted nutritional support during preconception and antenatal periods in the future for post-MBS adolescents [69].
A minimum of 12–18 months of interval has been advised prior to conception in women who have undergone MBS; therefore, contraception initiation is recommended ahead of the surgery [71,72]. Oral contraceptive pills (OCP), given their increased risk of thromboembolic events, must be discontinued from, at least, 4–6 weeks prior to surgery up to 6 weeks postoperatively [73]. Further, due to concerns of lowered efficacy with oral agents especially post-MBS, contraceptives that are long-acting and reversible like levonorgestrel implants and intrauterine devices are the most recommended options [74]. Nutritional surveillance and management should be initiated ideally before conception in women of reproductive age who have undergone MBS, by physicians with expertise in the management of bariatric-surgery patients [75]. Deficiencies in certain nutrients such as maternal folate, vitamin A, and also total energy during pregnancy can have detrimental effects on normal fetal development, specifically, on fetal renal structure and function [76]. This makes future nutritional monitoring vital throughout pregnancy and postpartum for post-MBS adolescents [77]. Evidence is insufficient at present, in terms of suggesting differing weight-gain recommendations in post-MBS women compared to the general population [71,78].
Most importantly, it needs to be highlighted to patients that future pregnancy will not have any significant negative impact on the weight loss facilitated by MBS. Evidence shows that the long-term effects of pregnancy on the weight management outcomes in MBS patients are clinically insignificant, thereby rendering MBS as an efficient treatment option for successful long-term weight management [79].
7.1. Fetal programming of adult disease
Literature supports obesity management prior to conception. In addition to the maternal diet and its impact on the fetus, in utero exposure to obesity has been associated with gene expression alteration and metabolic abnormalities in offspring [80]. The term ‘programming’ was coined nearly three decades ago [81] to explain the effects of environment the developing fetus was developing in on its phenotype. Maternal nutritional and metabolic status were shown to exert a permanent impact on fetal developing tissue predisposing to elevated risk for chronic disorders like metabolic syndrome and coronary artery disease later in the fetus’ adult life [82]. It is hypothesized that such permanent structural changes to developing organs are mediated through epigenetic alterations such as DNA methylation, histone modification, and microRNA [82]. This is a relevant concern even in the setting of paternal obesity. Neonates of men with obesity have been noted with altered DNA methylation patterns at imprinted genes [83]. It has been suggested that developing sperms may be susceptible to environmental insults, and the resulting imprint instability in those with severe obesity, may be passed on to future generations [83]. This makes it fundamental to recognize the need to treat severe obesity prior to conception.
Weighing in on the benefits of MBS in treating reproductive age groups of women with severe obesity, evidence demonstrates markedly lower health risks in children born to mothers who achieved weight reduction prior to conception [84]. Compared to children born to mothers before MBS (pre-MBS offspring), those born post-MBS were noted to have lower weight, waist-to-hip ratios, and body fat percentage. Post-MBS offspring also showed improved fasting insulin levels, homeostatic model of insulin resistance index (HOMA-IR) and lower systolic and diastolic blood pressures [85]. Studies have demonstrated results as promising as threefold lowered prevalence of severe obesity in post-MBS offspring in addition to improved lipid profiles and improved cardiometabolic markers sustaining well into adolescence [85].
8. Conclusion
We are witnessing a major health crisis of severe obesity and its myriad of comorbidities among adolescents. Metabolic and bariatric surgery is an effective, evidence-based, and safe treatment strategy for the treatment of severe obesity in adolescents. However, major stigma against the disease of obesity, lack of knowledge among physicians, and inadequate access to an already limited number of multidisciplinary pediatric centers is some of the notable limitations for timely and effective treatment of severe obesity in the adolescents. Enhanced education efforts of the medical community and improved access of patients to timely and appropriate care are critical, barring them from being at serious risk of worsening disease severity and an overall impaired quality of life.
9. Expert opinion
According to the Centers for Disease Control and Prevention, obesity has more than quadrupled in adolescents in the past three decades. Current adolescent obesity treatment guidelines focus primarily on intensive lifestyle intervention with changes in diet and physical activity. To date, there are very few pharmacotherapeutic agents approved by the U.S. Food and Drug Administration such as orlistat, phentermine and liraglutide, for treatment of adolescent obesity. Recent studies have demonstrated that the most effective strategy for severe obesity is MBS; however, its utilization is much lower than expected in adolescents.
The most common types of MBS in adolescents include VSG and RYGB with VSG becoming more popular and common in the recent years due to superior outcomes related to weight loss and resolution of comorbidities. The AAP published a policy statement in December 2019 regarding the expanded use of MBS in adolescent patients with severe obesity. The AAP recommends a multidisciplinary team approach with a shared decision-making process for managing adolescents with obesity. The 2019 ASMBS pediatric metabolic and bariatric surgery guidelines recommend that adolescents with class II obesity (120% BMI percentile for age and sex) along with an obesity-related comorbidity or with class III obesity (140% BMI percentile for age and sex) should be considered for MBS.
Much of the information regarding outcomes of bariatric surgery in adolescents come from three landmark studies – Follow-up of Adolescent Bariatric Surgery (FABS-5+), Adolescent Morbid Obesity Surgery (AMOS), and Teen-Longitudinal Assessment of Bariatric Surgery (Teens-LABS). These studies demonstrated durable and sustainable weight loss, improvements in cardiometabolic health, resolution of obesity-related comorbidities such as diabetes, hypertension, sleep apnea, and improvement in weight-related quality of life. The benefits must also be viewed in the context of risks associated with adolescent bariatric surgery such as increased risk of substance use, self-harm, micronutrient deficiencies, and the possibility of future abdominal procedures in some patients.
Due to the dynamic physiological and psychological changes, the period of adolescence presents unique opportunities and challenges for management of obesity in this population. Adolescent decision-making skills are still developing and not yet fully evolved. Thus, the decision of whether to per the issues related to adolescents’ informed consent and assent as well as the long-lasting consequences of bariatric surgery. In addition, psychopathology either due to or because of obesity can complicate the process of provider’s identification of appropriate adolescents for bariatric surgery. Other factors that prevent the adoption of MBS in clinical practice may include failure to recognize adolescent obesity as a disease, stigma and bias around the disease of obesity, misconceptions about bariatric surgery (i.e. thinking of MBS as an easy way out) in addition to access and insurance-related barriers.
Given the rapidly expanding data on surgical options in the past decade with results on its profound metabolic benefits, we speculate that adolescent MBS will continue to evolve as the best treatment option for a group of adolescents with severe obesity in whom lifestyle and pharmacotherapeutic interventions are ineffective. Futurelongitudinal studies may help uncover adolescents’ characteristics, genetic and biomarkers that can provide us the ability to predict more accurately which patients will achieve distinct benefits from specific types of interventions such as use of anti-obesity medications and/or MBS that can improve health outcomes in these patients.
Article Highlights.
The American Society for Metabolic and Bariatric Surgery (ASMBS) recommends metabolic and bariatric surgery (MBS) as a safe and effective treatment option for adolescents with BMI ≥ 120% of the 95th percentile with comorbidities including hyperlipidemia, hypertension (HTN), Type 2 Diabetes Mellitus (T2DM), Non- alcoholic fatty liver disease (NAFLD) gastroesophageal reflux disease or those with BMI ≥ 140% of the 95th percentile.
It is imperative to understand the criticalness for timely treatment of obesity and recognizing it as a disease. This includes referral of patients who meet criteria for surgery to an MBS center with advanced treatments and support.
The most common types of MBS in adolescents include Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG), with VSG now considered the gold standard for bariatric surgery.
Available literature demonstrating the 5-year outcomes after MBS has shown promising results.
Barriers to adolescent bariatric surgery include lack of knowledge and negative psychosocial attitudes from providers, patients and their families, and inability to access specialized pediatric weight centers. Disparities in access due to socioeconomic status, public health insurance coverage, and minority race are evident.
Post-MBS women show better maternal health during pregnancy and nutritional monitoring should be implemented well ahead of conception to tackle neonatal health risks in post-MBS women.
Funding
This project was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, NIH P30 DK040561 (FCS), L30 DK118710 (FCS).
Footnotes
Declaration of interest
S Malhotra has served as a speaker for Rhythm Pharmaceutical. Apart from those disclosed, the authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Reviewer disclosures
Peer reviewers in this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Skinner AC, Perrin EM, Skelton JA. Prevalence of obesity and severe obesity in US children, 1999-2014. Obesity. 2016. May;24(5):1116–1123. Silver Spring: [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015; (219):1–8. Nov. [PubMed] [Google Scholar]
- 3.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;8(314):1–27. Jun [PubMed] [Google Scholar]
- 4.Ryder JR, Jacobs DR Jr., Sinaiko AR, et al. Longitudinal changes in weight status from childhood and adolescence to adulthood. J Pediatr. 2019;214(187–192):e2. Nov. [DOI] [PubMed] [Google Scholar]
- 5.Llewellyn A, Simmonds M, Owen CG, et al. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev. 2016;17(1):56–67. Jan. [DOI] [PubMed] [Google Scholar]
- 6.He Y, Tian J, Oddy WH, et al. Association of childhood obesity with female infertility in adulthood: a 25-year follow-up study. Fertil Steril. 2018;110(4):596–604 e1. Sep. [DOI] [PubMed] [Google Scholar]
- 7.Zohar L, Rottenberg Y, Twig G, et al. Adolescent overweight and obesity and the risk for pancreatic cancer among men and women: a nationwide study of 1.79 million Israeli adolescents. Cancer. 2019;125(1):118–126. Jan 1. [DOI] [PubMed] [Google Scholar]
- 8.Landberg A, Falt A, Montgomery S, et al. Overweight and obesity during adolescence increases the risk of renal cell carcinoma. Int J Cancer. 2019;145(5):1232–1237. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shreberk-Hassidim R, Galili E, Hassidim A, et al. Epidemiology and comorbidities of psoriasis among Israeli adolescents: a large cross-sectional study. Dermatology. 2019;235(6):488–494. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen AR, Aarestrup J, Baker JL, et al. Association of birth weight, childhood body mass index, and height with risk of hidradenitis suppurativa. JAMA Dermatol. 2020;156(7):746–753. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinicato NA, Postal M, Peres FA, et al. Obesity and cytokines in childhood-onset systemic lupus erythematosus. J Immunol Res. 2014;2014:162047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng X, Ma J, Yuan Y, et al. Association between overweight or obesity and the risk for childhood asthma and wheeze: an updated meta-analysis on 18 articles and 73 252 children. Pediatr Obes. 2019;14(9):e12532. Sep. [DOI] [PubMed] [Google Scholar]
- 13.Quek YH, Tam WWS, Zhang MWB, et al. Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obes Rev. 2017;18(7):742–754. Jul. [DOI] [PubMed] [Google Scholar]
- 14.Danielsson P, Kowalski J, Ekblom O, et al. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166(12):1103–1108. Dec. [DOI] [PubMed] [Google Scholar]
- 15.Levine MD, Ringham RM, Kalarchian MA, et al. Is family-based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30(3):318–328. Nov. [DOI] [PubMed] [Google Scholar]
- 16. Griggs CL, Perez NP Jr., Goldstone RN, et al. National trends in the use of metabolic and bariatric surgery among pediatric patients with severe obesity. JAMA Pediatr. 2018;172(12):1191–1192. Dec 1. • Offers factual commentary on the recent trends of MBS among pediatric patients with severe obesity. For the years 2005 to 2014, less than 0.04% of children and adolescents with severe obesity were treated with MBS each year.
- 17. Pratt JS, Browne A, Browne NT, et al. , ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obesity Related Dis. 2018;14(7): 882–901. • Guidelines compiled by the American Society for Metabolic and Bariatric Surgery Pediatric Committee is included to provide background on the evolution of and rapid shift in expert opinions (compared to now outdated practice guidelines published in 2012). With more available data on weight loss and co-morbidity outcomes in pediatric MBS patients, the guidelines have shifted to support MBS as the standard of care for severe obesity.
- 18.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–123. Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulus GF, De Vaan LE, Verdam FJ, et al. Bariatric surgery in morbidly obese adolescents: a systematic review and meta-analysis. Obes Surg. 2015. May;25(5):860–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alqahtani AR, Elahmedi MO, Al Qahtani A. Co-morbidity resolution in morbidly obese children and adolescents undergoing sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(5):842–850. Sep-Oct [DOI] [PubMed] [Google Scholar]
- 21.Algorithm for the assessment and management of childhood obesity in patients 2 years and older c2015 ed: AAP institute for healthy childhood weight; 2015. [Google Scholar]
- 22.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007. Dec;120 (Suppl 4):S164–92. [DOI] [PubMed] [Google Scholar]
- 23.Stanley T, Misra M. Endocrine conditions in pediatrics: a practical guide. Cham, Switzerland: Springer; 2021. p. 119–122. [Google Scholar]
- 24.Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017. Mar 1;102(3):709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Pediatric Endosurgery Group S, Safety C. IPEG guidelines for surgical treatment of extremely obese adolescents. J Laparoendosc Adv Surg Tech A. 2008. Dec;18(6):xiv–xvi. [DOI] [PubMed] [Google Scholar]
- 26.Kolotkin RL, Zeller M, Modi AC, et al. Assessing weight-related quality of life in adolescents. Obesity. Silver Spring 2006. Mar;14 (3):448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sogg S, Lauretti J, West-Smith L. Recommendations for the pre-surgical psychosocial evaluation of bariatric surgery patients. Surg Obes Relat Dis. 2016. May;12(4):731–749. [DOI] [PubMed] [Google Scholar]
- 28.Bolling CF, Armstrong SC, Reichard KW, et al. Metabolic and bariatric surgery for pediatric patients with severe obesity. Pediatrics. 2019. Dec;144(6):e20193224. [DOI] [PubMed] [Google Scholar]
- 29. Armstrong SC, Bolling CF, Michalsky MP, et al. Pediatric metabolic and bariatric surgery: evidence, barriers, and best practices. Pediatrics. 2019. Oct 27;144(6):e20193223. • Citations 27 and 28 are the most recent Position Statement and accompanying Technical Report on the subject of Metabolic and Bariatric Surgery (MBS) released by the American Academy of Pediatrics in December 2019. These statements published in the journal Pediatrics provide updated, practice-level, and system-level guidance on the use of MBS in pediatric patients with severe obesity. Recommendations included in the Position Statement support the use of multi-disciplinary care teams pre- and post-operatively and emphasize the importance of expanding access to MBS for all “pediatric patients of all racial, ethnic, and socioeconomic backgrounds.” Citing evidence from three robust clinical trials: AMOS, FABS-5 + and Teen-LABS, the Technical Report outlines the most common approaches for MBS and stresses that these are proven safe and effective forms of surgical weight loss in children and adolescents. These publications are potentially groundbreaking in shifting opinions in pediatric practices towards MBS for treating severe obesity
- 30.Kallies K, Rogers AM; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. American society for metabolic and bariatric surgery updated statement on single-anastomosis duodenal switch. Surg Obes Relat Dis. 2020. Jul;16(7):825–830. [DOI] [PubMed] [Google Scholar]
- 31.Inge TH, Jenkins TM, Xanthakos SA, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 2017;5(3):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olbers T, Beamish AJ, Gronowitz E, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017;5(3):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inge TH, Courcoulas AP, Jenkins TM, et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med. 2019. May 30;380(22):2136–2145. • Provides support for sustained weight loss and remission from comorbidities in adolescents five years post-operatively following gastric bypass. The authors offer an important comparison of outcomes between adolescents and adults from two prospective, multicenter, observational studies (1) Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study and the (2) LABS study. These findings strengthen the manuscript’s sections on outcomes and complications. Lead author, Dr. Thomas Inge, is considered a thought leader in the application of bariatric surgery for children and adolescents. He is a professor of pediatrics and surgery at the Children’s Hospital of Colorado and is well-regarded for his expertise in the field. Some of his other research on the topic is included and cited throughout the manuscript
- 34.Ryder JR, Xu P, Inge TH, et al. Thirty-year risk of cardiovascular disease events in adolescents with severe obesity. Obesity (Silver Spring). 2020. Mar;28(3):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalsky MP, Inge TH, Jenkins TM, et al. Cardiovascular risk factors after adolescent bariatric surgery. Pediatrics. 2018. Feb;141(2):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price PH, Kaizer AM, Daniels SR, et al. Physical activity improves lipid and weight-loss outcomes after metabolic bariatric surgery in adolescents with severe obesity. Obesity (Silver Spring). 2019. Jun;27(6):989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007. Aug 23;357(8):741–752. [DOI] [PubMed] [Google Scholar]
- 38.Stanford FC, Mushannen T, Cortez P, et al. Comparison of short and long-term outcomes of metabolic and bariatric surgery in adolescents and adults [Original research]. Front Endocrinol (Lausanne). 2020. [2020 Mar 24];11(157). DOI: 10.3389/fendo.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manco M, Mosca A, De Peppo F, et al. The benefit of sleeve gastrectomy in obese adolescents on nonalcoholic steatohepatitis and hepatic fibrosis. J Pediatr. 2017. Jan;180:31–37 e2. [DOI] [PubMed] [Google Scholar]
- 40.Inge TH, Xanthakos SA. Reversal of nonalcoholic steatohepatitis in adolescents after metabolic surgery. J Pediatr. 2017. Jan;180:6–7. [DOI] [PubMed] [Google Scholar]
- 41.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: cosponsored by American association of clinical endocrinologists, the obesity society, and American society for metabolic & bariatric surgery. Surg Obes Relat Dis. 2013. Mar-Apr;9(2):159–191. [DOI] [PubMed] [Google Scholar]
- 42.Anveden A, Peltonen M, Naslund I, et al. Long-term incidence of gallstone disease after bariatric surgery: results from the nonrandomized controlled Swedish obese subjects study. Surg Obes Relat Dis. 2020. Oct;16(10):1474–1482. [DOI] [PubMed] [Google Scholar]
- 43.Ryder JR, Edwards NM, Gupta R, et al. Changes in functional mobility and musculoskeletal pain after bariatric surgery in teens with severe obesity: teen-Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Pediatr. 2016. Sep 1;170(9):871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaulfers AM, Bean JA, Inge TH, et al. Bone loss in adolescents after bariatric surgery. Pediatrics. 2011. Apr;127(4):e956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ke K, Jl B, H M, et al. Trends in volume and utilization outcomes in adolescent metabolic and bariatric surgery at children’s hospitals. J Adolesc Health. 2019;65:3:331–336. [DOI] [PubMed] [Google Scholar]
- 46.Pl S, Mm D, Ct A, et al. National trends in adolescent bariatric surgical procedures and implications for surgical centers of excellence. J Am Coll Surg. 2008;206:1:1–12.doi: 10.1016/j.jamcollsurg.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 47.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161(3):217–221. [DOI] [PubMed] [Google Scholar]
- 48.Cw I, K S, Ad I, et al. Perspectives on pediatric bariatric surgery: identifying barriers to referral. Surg Obes Relat Dis. 2009;5(1):88–93. doi: 10.1016/j.soard.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 49.Woolford SJ, Clark SJ, Gebremariam A, et al. To cut or not to cut: physicians' perspectives on referring adolescents for bariatric surgery. Obes Surg. 2010. Jul;20(7):937–42.doi: 10.1007/s11695-010-0152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanguri P, Lanning D, Wickham EP, et al. Pediatric health care provider perceptions of weight loss surgery in adolescents: [research-article]. Clin Pediatr (Phila). 2014. Jan;53(1):60–5. [DOI] [PubMed] [Google Scholar]
- 51.vG SM, Il B, vdB-S OH, et al. The controversy over pediatric bariatric surgery: an explorative study on attitudes and normative beliefs of specialists, parents, and adolescents with obesity. J Bioeth Inq. 2013;10:2. 2013 Jun [DOI] [PubMed] [Google Scholar]
- 52.Aj B, R T. Should bariatric surgery be performed in adolescents? Eur J Endocrinol. 2017;176(4):D1–D15. DOI: 10.1530/EJE-16-0906. [DOI] [PubMed] [Google Scholar]
- 53.C A, Wj C. Bariatric surgery needs a seat at the children’s table: bridging the perception and reality of the role of bariatric surgery in the treatment of obesity in adolescents. Clin Ther. 2018;40 (10):1648–1654. doi: 10.1016/j.clinthera.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Ud S, C. A. Parental attitudes toward bariatric surgery in adolescents with obesity. Surg Obes Relat Dis. 2020;16(3)):406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penna M, Markar S, Hewes J, et al. Adolescent Bariatric Surgery — thoughts and perspectives from the UK [Article]. Int J Environ Res Public Health. 2013;11(1):573–582. 2013-December-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campoverde Reyes KJ, Perez NP, Czepiel KS, et al. Exploring pediatric obesity training, perspectives, and management patterns among pediatric primary care physicians. Obesity. Silver Spring 2021;29(1):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lr V, F. J. The stigma of obesity surgery: negative evaluations based on weight loss history. Obes Surg. 2013;23(10):154–550. doi: 10.1007/s11695-013-0918-y. [DOI] [PubMed] [Google Scholar]
- 58.F J, Lr V. Changes in weight bias following weight loss: the impact of weight-loss method. Int J Obesity. 2005. 2012 2012 Feb;36 (2):314–9. DOI: 10.1038/ijo.2011.26. [DOI] [PubMed] [Google Scholar]
- 59.Ja J, Am J. Urban-rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis. Childhood Obesity. 2015;11:3. Print. 2015 Jun. [DOI] [PubMed] [Google Scholar]
- 60.Wallace AE, Young-Xu Y, Hartley D, et al. Racial, socioeconomic, and rural–urban disparities in obesity-related Bariatric Surgery [Original Paper]. Obes Surg. 2010;20(10):1354–1360. 2010-January-06. [DOI] [PubMed] [Google Scholar]
- 61.Fc S, Db J, Be S, et al. Patient race and the likelihood of undergoing bariatric surgery among patients seeking surgery. Surg Endosc. 2015;29(9):2794–9. DOI: 10.1007/s00464-014-4014-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O A, K J, B Q, et al. Socioecological factors associated with ethnic disparities in metabolic and bariatric surgery utilization: a qualitative study. Surg Obes Relat Dis. 2020;16(6):786–795. DOI: 10.1016/j.soard.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Km G, Z A, Ks C, et al. Bariatric surgery among vulnerable populations: the effect of the affordable care act’s Medicaid expansion. Surgery. 2019;166(5):820–828.doi: 10.1016/j.surg.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Np P, Ml W, Sm S, et al. Beyond insurance: race-based disparities in the use of metabolic and bariatric surgery for the management of severe pediatric obesity. Surg Obes Relat Dis. 2020;16:3. 2020 Mar [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cc W, Kw H, B-j D, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med. 2014;29(3):414–419. doi: 10.1016/j.soard.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogden CL, Carroll MD, Lawman HG. National center for health statistics UCfDCaP, Hyattsville, Maryland, Carroll MD, et al. trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA 2016;315 (21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pj C, A-w P. The obesity epidemic: are minority individuals equally affected? Prim Care. 2009;36(2):307–17. doi: 10.1016/j.pop.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Kwong W, Tomlinson G, Feig DS. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am J Obstet Gynecol. 2018. Jun;218(6):573–580. [DOI] [PubMed] [Google Scholar]
- 69.Akhter Z, Rankin J, Ceulemans D, et al. Pregnancy after bariatric surgery and adverse perinatal outcomes: a systematic review and meta-analysis. PLoS Med. 2019. Aug;16(8):e1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chevrot A, Kayem G, Coupaye M, et al. Impact of bariatric surgery on fetal growth restriction: experience of a perinatal and bariatric surgery center. Am J Obstet Gynecol. 2016. May;214(5):655 e1–7. [DOI] [PubMed] [Google Scholar]
- 71.Ciangura C, Coupaye M, Deruelle P, et al. Clinical practice guidelines for childbearing female candidates for Bariatric Surgery, pregnancy, and post-partum management after Bariatric Surgery. Obes Surg. 2019. Nov;29(11):3722–3734. [DOI] [PubMed] [Google Scholar]
- 72.Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing Bariatric procedures - 2019 update: cosponsored by American association of clinical endocrinologists/American college of endocrinology, the obesity society, American society for metabolic and Bariatric Surgery, obesity medicine association, and American society of anesthesiologists. Obesity. 2020. Apr;28(4):O1–O58. Silver Spring. [DOI] [PubMed] [Google Scholar]
- 73.Robinson GE, Burren T, Mackie IJ, et al. Changes in haemostasis after stopping the combined contraceptive pill: implications for major surgery. BMJ. 1991. Feb 2;302(6771):269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hillman JB, Miller RJ, Inge TH. Menstrual concerns and intrauterine contraception among adolescent bariatric surgery patients. J Womens Health (Larchmt). 2011. Apr;20(4):533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rottenstreich A, Elazary R, Goldenshluger A, et al. Maternal nutritional status and related pregnancy outcomes following bariatric surgery: a systematic review. Surg Obes Relat Dis. 2019. Feb;15 (2):324–332. [DOI] [PubMed] [Google Scholar]
- 76.Lee YQ, Collins CE, Gordon A, et al. The relationship between maternal nutrition during pregnancy and offspring kidney structure and function in humans: a systematic review. Nutrients. Feb 21. 2018;10(2). DOI: 10.3390/nu10020241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Slater C, Morris L, Ellison J, et al. Nutrition in pregnancy following Bariatric Surgery. Nutrients. 2017;9(12):1338. Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grandfils S, Demondion D, Kyheng M, et al. Impact of gestational weight gain on perinatal outcomes after a bariatric surgery. J Gynecol Obstet Hum Reprod. 2019. Jun;48(6):401–405. [DOI] [PubMed] [Google Scholar]
- 79.Rottenstreich A, Shufanieh J, Kleinstern G, et al. The long-term effect of pregnancy on weight loss after sleeve gastrectomy. Surg Obes Relat Dis. 2018. Oct;14(10):1594–1599. [DOI] [PubMed] [Google Scholar]
- 80.Ogunwole SM, Zera CA, Stanford FC. Obesity management in women of reproductive age. JAMA. 2021. Feb 2;325(5):433–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156: 38–50. discussion 50-5. [PubMed] [Google Scholar]
- 82.Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients. 2014. Jun 2;6(6):2165–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soubry A, Murphy SK, Wang F, et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int J Obes (Lond). 2015. Apr;39(4):650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guenard F, Deshaies Y, Cianflone K, et al. Differential methylation in glucoregulatory genes of offspring born before vs after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci USA. 2013. Jul 9;110(28):11439–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009. Nov;94(11):4275–4283. [DOI] [PubMed] [Google Scholar]