Abstract
Introduction: Obesity is associated with a worse prognosis in COVID-19 patients with acute respiratory distress syndrome (ARDS). Veno-venous (V-V) Extracorporeal Membrane Oxygenation (ECMO) can be a rescue option, however, the direct impact of morbid obesity in this select group of patients remains unclear.
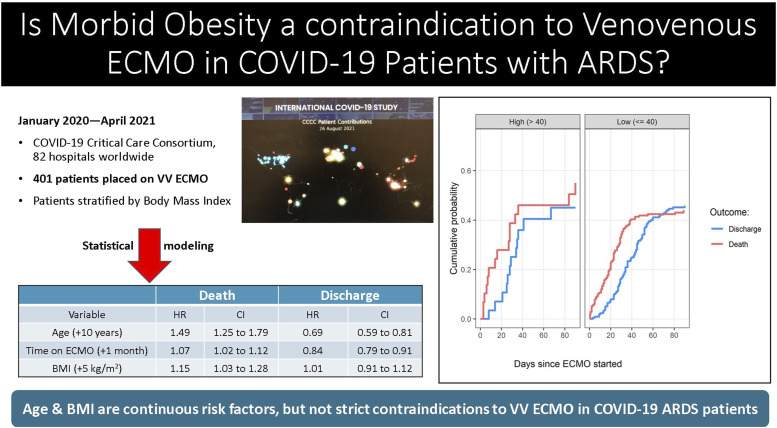
Methods: This is an observational study of critically ill adults with COVID-19 and ARDS supported by V-V ECMO. Data are from 82 institutions participating in the COVID-19 Critical Care Consortium international registry. Patients were admitted between 12 January 2020 to 27 April 2021. They were stratified based on Body Mass Index (BMI) at 40 kg/m2. The endpoint was survival to hospital discharge.
Results: Complete data available on 354 of 401 patients supported on V-V ECMO. The characteristics of the high BMI (>40 kg/m2) and lower BMI (≤40 kg/m2) groups were statistically similar. However, the ‘high BMI’ group were comparatively younger and had a lower APACHE II score. Using survival analysis, older age (Hazard Ratio, HR 1.49 per-10-years, CI 1.25–1.79) and higher BMI (HR 1.15 per-5 kg/m2 increase, CI 1.03–1.28) were associated with a decreased patient survival. A safe BMI threshold above which V-V ECMO would be prohibitive was not apparent and instead, the risk of an adverse outcome increased linearly with BMI.
Conclusion: In COVID-19 patients with severe ARDS who require V-V ECMO, there is an increased risk of death associated with age and BMI. The risk is linear and there is no BMI threshold beyond which the risk for death greatly increases.
Keywords: extracorporeal membrane oxygenation, morbid obesity, COVID-19, acute respiratory distress syndrome
Graphical Abstract.
Introduction
The potential effectiveness of veno-venous (V-V) Extracorporeal Membrane Oxygenation (ECMO) for severe acute respiratory distress syndrome (ARDS) has been demonstrated in two randomized-controlled trials.1,2 During the Influenza A H1N1 pandemic, V-V ECMO was associated with a 71% survival to intensive care unit (ICU) discharge.3 More broadly in patients with severe respiratory failure, EOLIA and CESAR showed improved outcomes for patients who received their care at a high volume ECMO center.1,2 A portion of patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) go on to develop severe respiratory failure refractory to mechanical ventilation and require extracorporeal support. Similar to its use in the treatment of other respiratory viruses, ECMO provided a viable rescue option. In an early analysis of Extracorporeal Life Support Organization (ELSO) registry data, the estimated in-hospital 90-day mortality was 37% for COVID-19 infected patients on V-V ECMO.4
Obesity has been associated with worse outcomes in COVID-19 infected patients and is prevalent in the United States.5 Twenty percent of the population have Class 2 Obesity with a Body Mass Index (BMI) 35–39.9 kg/m2 and 10% have Class 3 obesity (BMI >40 kg/m2).6 Patients with Class 3 Obesity often have metabolic syndrome and carry the associated comorbidities of diabetes and hypertension.7 Additional risk factors for poor outcomes in COVID-19 include advanced age, and chronic pulmonary disease.8 Obese patients with COVID-19 infection have experienced higher rates of hospitalization and ICU admission for severe respiratory difficulties,9,10 This is likely attributed to the impaired respiratory mechanics and exercise intolerance caused by morbid obesity. As such, effective respiratory support in obese patients requiring mechanical ventilation or V-V ECMO can be a challenge.11
The impact of Class 2 or 3 Obesity on patients with ARDS secondary to COVID-19 who then require ECMO remains under explored. In the initial COVID-19 ELSO guidelines, Class 3 Obesity or a BMI >40 kg/m2 was a relative contraindication for ECMO.12 However, this was based on the limited available evidence at the time and through gained experience, reconsideration of patient selection criteria may be needed.
The COVID-19 Critical Care Consortium (CCCC) is an international registry currently of 240 hospitals in 54 nations enrolling critically ill patients with COVID-19. It was formed to create a platform to prospectively collect data in ICU patients with the SARS-CoV-2 virus and allow for real time analysis of a litany of variables and factors impacting outcomes such as patient survival. This included collecting data in collaboration with ELSO on patients requiring ECMO. The aim of our study is to use the CCCC database to examine the effect of BMI on the outcome of obese patients with COVID-19 that went on to develop ARDS requiring veno-venous ECMO. We will assess for an association between obesity and poor outcomes so that it may better inform selection criteria for V-V ECMO.
Patients and methods
Study design
This is an observational study of critically ill, adult patients with COVID-19 supported by V-V ECMO. At the time of analysis the data were drawn from 82 institutions participating in the CCCC international registry. Each participating institution obtained Institutional Review Board approval and waivers of informed consent were granted at each site. Site-specific investigators received detailed instructions by the Consortium’s data team. A data dictionary was used to gather and record the patient data. (Supplemental Data Dictionary) Specialized case report forms were completed for patients on ECMO. The data were then de-identified and using Research Electronic Data Capture (REDCap) submitted to an encrypted central repository, hosted by the Oxford University, United Kingdom. The full protocol for the wider study has been published.13–15
This study examined patients admitted between 12 January 2020 to 27 April 2021. We included adults (18 years or older) with confirmed COVID-19 who required V-V ECMO in addition to invasive mechanical ventilation. The primary outcomes were survival to discharge and death at 90 days following ECMO cannulation.
Data collection
The collected data included demographics, comorbid conditions, date of admission and discharge from the ICU, the Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II) Score, and the Sequential Organ Failure Assessment (SOFA) score, relevant laboratory tests, mechanical ventilation settings and the time of initiation and discontinuation of invasive mechanical ventilation, and adjunctive therapies including prone positioning before and during ECMO support. For patients supported on ECMO, the time of cannulation and decannulation, mode of ECMO support, and ECMO complications were recorded. Center-specific data included the country and location, and the number of patients on ECMO support.16
Statistical analysis
For descriptive statistics we dichotomized the patients into those with a BMI greater than 40 kg/m2 vs. less than or equal 40 kg/m2. We used median values with interquartile ranges (IQR) to summarize continuous variables. Multi-state survival analysis was used to explore the effect of Class 3 Obesity on patient survival to hospital discharge and death. To implement the model, we followed patients from the date of ECMO initiation until death or date of discharge, which are competing risks. To model the impact of patients with Class 2 Obesity we revised the BMI threshold to 35 kg/m2. This then shifted Class 2 and 3 Obesity into the ‘High BMI’ cohort, to assess for a clinical difference in V-V ECMO patients.
The data were examined to gain insights on the effects of BMI on the final outcome of death or discharge. Potential confounders of the association between BMI and death/discharge were identified based on previously published studies, the clinically relevant factors for decision making, and differences in baseline characteristics.17,18 The confounders included age, sex, date and APACHE II score. Date was added to adjust for trends over time in death and discharge. Continuous variables were scaled so that the hazard ratios (HR) were more clinically relevant, using a per 10-year increase for age and per 5 kg/m2 increase for BMI.
A sub-distribution hazard to examine the cumulative risks of BMI on death and discharge was used. Ninety-five percent confidence intervals (CI) for the hazard ratios instead of p-values were used as this permitted focus on the size of the estimated effects and their uncertainty, which are more clinically relevant than tests of statistical significance.19 Missing data for BMI by was handled by conducting multiple imputation. Patients with an unknown outcome or still alive at 90 days were censored and so still contributed to the estimates of risk for death and discharge. Leave one out sensitivity analysis were used to check if the survival results were strongly influenced by one site. The deviance information criteria was used to select the best model and to determine the BMI threshold for death or discharge by testing thresholds from 25 to 40 kg/m2 in steps of 5 kg/m2.
We used R Version 4.0.2 (R Foundation for Statistical Computing, Vienna Austria) for all analyses with the corresponding reference.20,21 The cumulative frequency models were fitted using the “cmprsk” package.22 All the R code used in the analysis is available online.23
Results
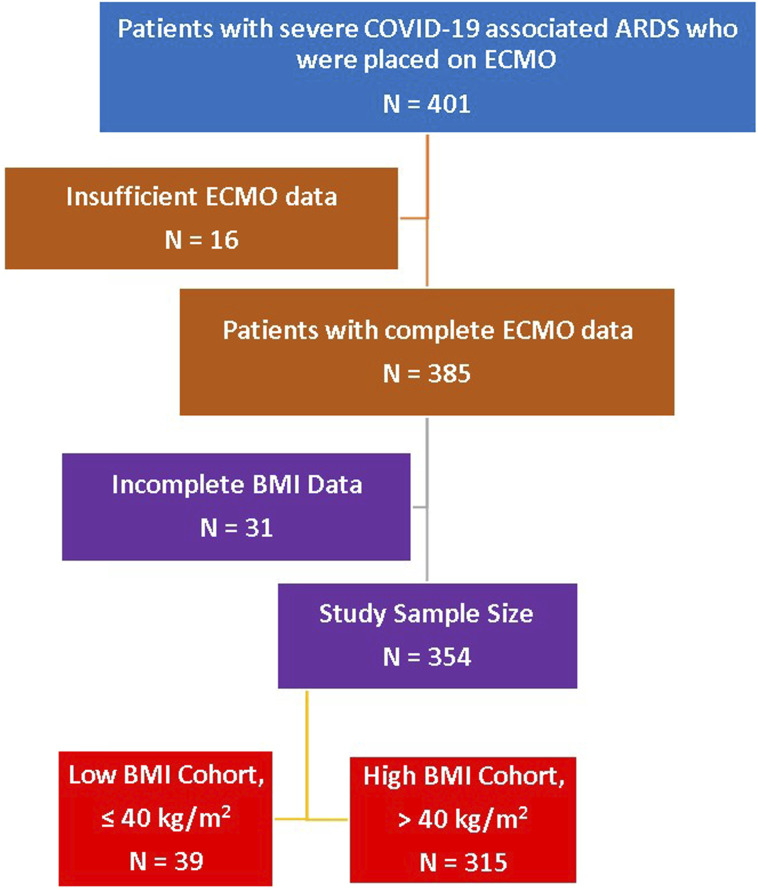
A total of 401 patients with severe COVID-19 associated ARDS required V-V ECMO for respiratory support at 82 global collaborating sites. Sixteen patients were excluded as they had insufficient information on the dates of ECMO, discharge and death, leaving a total of 385 patients available for analysis. A further 31 patients were marked as missing due to incomplete BMI data. The resulting study sample size was 354 patients. (Figure 1) The admission dates were between 12 January 2020 and 27 April 2021. Most cases were in the United States, Western Europe (Italy, Belgium, and Germany) and Colombia. (Supplemental Table 5) Patients were predominantly male (71%) and white (44%). The median age (IQR) was 52 years (IQR 44–60) and the median BMI was 30 kg/m2 (26–35). The cannulations for V-V ECMO were exclusively percutaneous and no reported cannula site vascular injuries. There was a 2% incidence of significant cannulation site bleeding among the 354 patients. There was no statistical difference when the data was stratified by BMI for subgroup analysis.
Figure 1.
Patient selection algorithm. BMI at 40 kg/m2 threshold. ARDS: acute respiratory distress syndrome; BMI: body mass index.
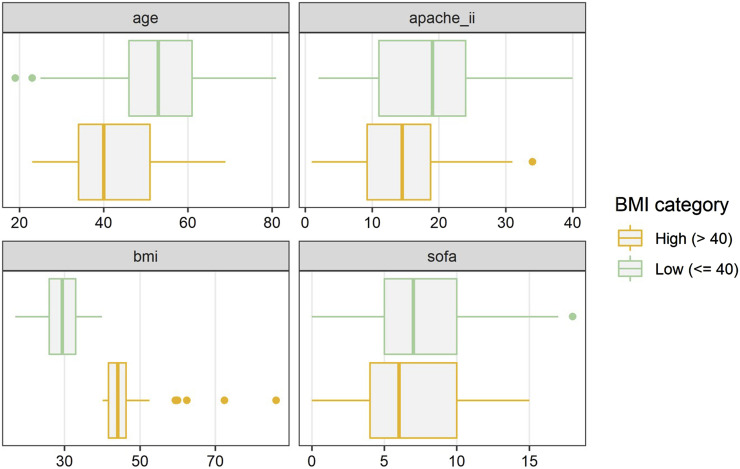
Patients were stratified into a ‘Lower BMI’ group (BMI ≤40 kg/m2), which included normal BMI, as well as Class 1 & 2 Obesity (N = 315), and ‘High BMI’ group (Class 3 Obesity, BMI >40 kg/m2, N = 39). The severity of their critical illness was comparable, however, there was a trend towards the high BMI group being younger [median age (IQR) 40 years (34–51) versus 53 years (46–61)] and with a lower median APACHE II score [high BMI group 14 (9–19) versus lower BMI group 19 (11–24)]. (Figure 2) Otherwise the co-morbidities were clinically similar in the two groups with diabetes mellitus, hypertension, and smoking being the most common in COVID-19 patients requiring ECMO. The time of onset of symptoms to hospital admission was slightly shorter (median 2 days less) in the high BMI group. This was also true for time from intubation to ECMO cannulation. (Table 1)
Figure 2.
Study group characteristics, BMI at 40 kg/m2 threshold: There was a trend towards the high BMI group being younger and with a lower APACHE II score. BMI: body mass index.
Table 1.
Patients characteristics comparing lower body mass index patients to high bmi patients at time of veno-venous ECMO initiation.
| Study population(N = 354) | Lower BMI (≤40 kg/m2)(N = 315) | High BMI (>40 kg/m2)(N = 39) | |
|---|---|---|---|
| Age, years | 52 (44–60) | 53 (46–61) | 40 (34–51) |
| Sex | |||
| Male | 250 (71%) | 229 (73%) | 21 (54%) |
| Female | 104 (29%) | 86 (27%) | 18 (46%) |
| Race/ethnicity | |||
| White | 154 (44%) | 144 (46%) | 10 (26%) |
| Hispanic | 74 (21%) | 69 (22%) | 5 (13%) |
| Asian | 44 (12%) | 40 (13%) | 4 (10%) |
| Black | 35 (10%) | 22 (6%) | 13 (33%) |
| Others | 47 (13%) | 40 (13%) | 7 (18%) |
| BMI (kg/m2) | 30 (26–35) | 29 (26–33) | 44 (42–46) |
| Acute physiology score II (APACHE II) | 18 (11–24) | 19 (11–24) | 14 (9–19) |
| Sequential organ-function assessment (SOFA) score | 7 (5–10) | 7 (5–10) | 6 (4–10) |
| Pregnancy | 6 (2%) | 3 (1%) | 3 (8%) |
| Co-morbidities | |||
| Immunocompromised or transplant patient | 3 (1%) | 3 (1%) | 0 |
| Hypertension | 149 (42%) | 136 (43%) | 13 (33%) |
| Diabetes | 88 (25%) | 77 (24%) | 11 (28%) |
| Active smoker | 65 (18%) | 59 (19%) | 6 (15%) |
| Chronic pulmonary disease | 10 (3%) | 8 (3%) | 2 (5%) |
| Asthma | 8 (2%) | 8 (3%) | 0 |
| Chronic cardiac disease | 21 (6%) | 20 (6%) | 1 (3%) |
| Chronic kidney disease | 12 (3%) | 12 (4%) | 0 |
| Alcohol abuse | 8 (2%) | 8 (3%) | 0 |
| Malignancy | 9 (3%) | 8 (3%) | 1 (3%) |
| Time from first symptoms to hospital admission, d | 10 (6–16) | 10 (6–16) | 8 (4–12) |
| Time from intubation to ECMO, d | 6 (2–10) | 6 (2–10) | 4 (0–6) |
Summary Statistics are median (inter-quartile range) or n (%). ECMO: Extracorporeal membrane oxygenation; BMI: body mass index; d: days.
Both groups met the clinical definition of ARDS at time of ECMO cannulation, but the P:F ratio was worse in high BMI group. The median PaO2/FiO2 (IQR) was 71 (57–101) vs. 88 (65–129) in the lower BMI group. The pre-ECMO median PaCO2 (IQR) was 51 mmHg (45–60 mmHg) in the high BMI group vs. 49 mmHg (40–60 mmHg) in the lower BMI group. The static compliance was slightly lower in the high BMI group with median (IQR) 24 mL/cm H2O (19–30 mL/cm H2O) in comparison to those in the lower BMI group 25 mL/cm H2O (19–33 mL/cm H2O). The median duration on ECMO, mechanical ventilation, and ICU length of stay were comparatively lower in the high BMI group. (Table 2)
Table 2.
Patients pre-ECMO respiratory support characteristics and post-ECMO outcomes, grouped by Body Mass Index (BMI 40 kg/m2 threshold).
| Study population (N = 354) | Lower BMI (≤40 kg/m2) (N = 315) | High BMI (>40 kg/m2) (N = 39) | |
|---|---|---|---|
| Ventilatory parameters | |||
| FiO2 | 100% | 100% | 100% |
| PEEP, cm H2O | 12 (10–14) | 12 (10–14) | 12 (11–16) |
| Static compliance, mL/cm H2O | 25 (19–33) | 25 (19–33) | 24 (19–30) |
| PaO2/FiO2 | 86 (63–127) | 88 (65–129) | 71 (57–101) |
| PaCO2 | 50 (40–60) | 49 (40–60) | 51 (45–60) |
| Pre-ECMO prone positioning | 211 (60%) | 191 (61%) | 20 (51%) |
| Duration of mechanical ventilation, d | 24 (14–38) | 24 (14–40) | 16 (9–32) |
| Duration on ECMO, d | 16 (8–28) | 16 (8–28) | 14 (8–25) |
| Hospital length of stay, d | 32 (18–52) | 32 (20–52) | 28 (14–36) |
| Intensive care unit length of stay, d | 28 (18–46) | 30 (18–46) | 24 (13–36) |
Data are median (inter-quartile range) or n (%). ECMO: extracorporeal membrane oxygenation; PEEP: positive end-expiratory pressure; BMI: body mass index; d: days.
To further assess the impact of Class 2 and 3 Obesity on V-V ECMO patients with COVID-19 related ARDS we lowered the BMI threshold to 35 kg/m2. The demographics were similar when patients were stratified at the lower BMI. (Supplemental Table 1) This was also the case for the pre-ECMO respiratory support characteristics and post-ECMO outcomes. (Supplemental Table 2) The outcomes of patients on V-V ECMO at both thresholds were comparable in the high and lower BMI groups. (Table 3 and Supplemental Table 3)
Table 3.
Outcomes on V-V ECMO (threshold, 40 kg/m2).
| Outcome | Study population (N = 354) | Lower BMI (≤40 kg/m2) (N = 315) | High BMI (>40 kg/m2) (N = 39) |
|---|---|---|---|
| Discharged to other facilities | 136 (39%) | 117 (37%) | 19 (49%) |
| Remain in the hospital | 75 (21%) | 72 (23%) | 3 (8%) |
| In-hospital death | 143 (40%) | 126 (40%) | 17 (43%) |
Data are n (%), BMI: body mass index; d: days.
Models of death and discharge
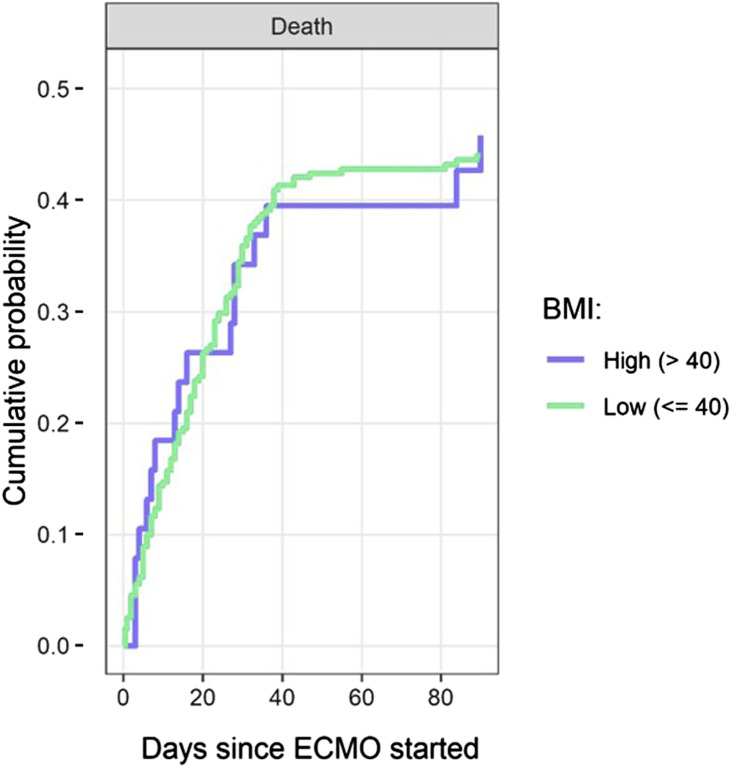
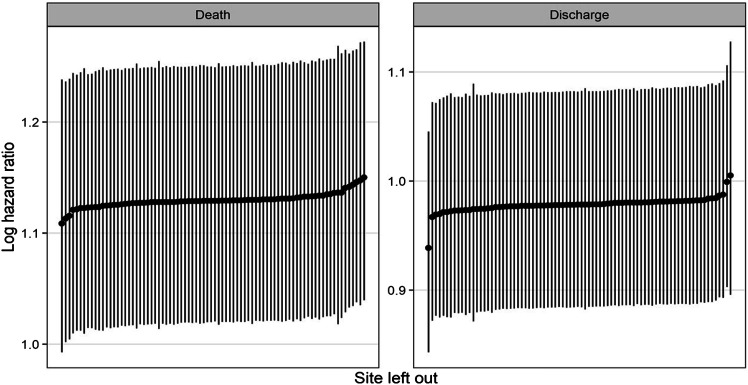
A multi-state survival model to estimate the effect of continuous BMI on the patients’ outcomes was used. There was a steady accumulation of death and discharge in both groups up to 90 days from initiation of ECMO. (Supplemental Figure 1) Older age (per-every-10 years) was associated with a decrease in the hazard of discharge (HR 0.69, CI 0.59–0.81) and increase the hazard of death (HR 1.49, CI 1.25–1.79). Higher BMI (per-every-5 kg/m2) was associated with decreased survival or increased hazard of death (HR = 1.15 per 5 kg/m2, CI 1.03–1.28). (Table 4) The cumulative survival curves show no significant difference at the BMI threshold of 40 kg/m2 which is consistent with this continuous variable carrying an additive risk. (Figure 3) Sensitivity analyses showed that variabilities between sites were small and no one site overly influenced outcomes. (Figure 4)
Table 4.
Estimated risk of death and discharge, (threshold, 40 kg/m2).
| Death | Discharge | |||
|---|---|---|---|---|
| Variable | HR | 95% CI | HR | 95% CI |
| Age (+10 years) | 1.49 | 1.25–1.79 | 0.69 | 0.59–0.81 |
| Time on ECMO (+1 month) | 1.07 | 1.02–1.12 | 0.84 | 0.79–0.91 |
| Male sex | 1.31 | 0.90–1.91 | 0.90 | 0.62–1.31 |
| BMI (+5 kg/m2) | 1.15 | 1.03–1.28 | 1.01 | 0.91–1.12 |
| APACHE II score <18 | 1.11 | 0.68–1.79 | 1.20 | 0.74–1.95 |
ECMO: extracorporeal membrane oxygenation; BMI: body mass index; APACHE II Score: acute physiologic assessment and chronic health evaluation ii score; HR: hazard ratio; CI = confidence interval. *The median APACHE II score was 18.
Figure 3.
Cumulative probabilities over time of survival, stratified by a BMI threshold of 40 kg/m2: The cumulative survival curves show no significant difference at the BMI threshold of 40 kg/m2, however, the risk of death is a continuous variable (HR 1.15 per-5 kg/m2 increase, CI 1.03–1.28). BMI: body mass index.
Figure 4.
Sensitivity analyses show that variability between sites was small and no one site overly influenced outcomes.
Of note, two patients from different centers had extremely high BMIs (>70 kg/m2). A sensitivity analysis was repeated with these two patients excluded. There was no difference in the end results of age (+10 years, HR 1.37 CI 1.13–1.68) and BMI (every 5 kg/m2, HR 1.20 CI 1.06–1.35) carrying a negative additive impact on patient survival.
Threshold for body mass index effect
To account for the definitions of Class 2 and Class 3 Obesity, BMI thresholds of 40 kg/m2 (Tables 1, 2, 3, and 4) and 35 kg/m2 (Supplemental Tables 1–4) were modeled. They showed two similarly characterized cohorts and there were no clinically significant differences at the two thresholds. The deviance information criteria results showed that the best model was that without a clear threshold. This indicates that there may not be a “safe” BMI, or a BMI beyond which risk greatly increases. The models for death similarly showed that the risk was linear and there was no clear risk threshold.
Discussion
V-V ECMO is an increasingly utilized management modality for refractory respiratory failure secondary to ARDS in patients with COVID-19. As with any treatment or surgical procedure, patient selection directly impacts outcomes. In the case of ECMO where there often is a limit of physical ICU space, ECMO circuits or specialized staff to care of patients on ECMO, the pandemic makes responsible resource stewardship all the more important. Simply put, there are not enough ECMO circuits for all the adults with severe ARDS. As clinicians, we circle back to patient selection to determine who would truly benefit from this limited resource.
Traditionally, Class 3 Obesity has not been associated with worse outcomes in patients on V-V ECMO.24–26 A study by Swol et al. in the pre-COVID era had a median BMI of 30 kg/m2 (19–88.5 kg/m2), which was similar to our study, and showed no association between BMI and mortality.27 Indeed, the presence of obesity was observed to be “protective” within in the PRESERVE Score analysis and thus it was not been considered a contraindication to ECMO.28 This is, however, with the caveat that at the extreme upper limits of BMI it was often center specific or ECMO utilization is determined on a case-by-case basis. In patients with COVID-19, conversely, obesity has been reported as a leading risk factor for worse outcomes, including mortality. As such, this dichotomy served as the basis for this investigation within the context of the current pandemic when selecting patients for V-V ECMO cannulation.
To investigate the question: “Is there a threshold BMI above which it is unsafe or likely futile to place a patient with COVID-19 related severe ARDS on ECMO?”, we modeled the data at two clinically relevant BMI thresholds. They were 35 kg/m2 to include Class 2 and 3 Obese patients in the High BMI group, and 40 kg/m2 to include only the Class 3 Obese patients in the High BMI group. Importantly, the results of this multinational analysis, demonstrate that there is no specific inflection point in the survival curve, but rather continuously increased risk with increasing patient BMI. (Figure 3) It appears that—like age—BMI is a continuous variable and carries a linear risk. (Table 4 and Supplemental Table 4)
This observation is supported by the rationale that obesity is not a binary medical condition, but rather exists on a spectrum. When examining BMI as a continuous variable in the statistical modeling we observed a decrease in survival for every 5 kg/m2 increase in the BMI. (Table 4) The significance of this observation indicates that the initial guidelines where a specific BMI alone was a contraindication may have been perhaps too narrow in scope. However, in this unique patient population the negative impact on patient survival can accumulate with every 5-point increase in the BMI. This represents a departure from pre-COVID-19 era where BMI was not a predictor of survival.24,28
The authors do acknowledge that V-V ECMO in an extremely obese patient (BMIs in the 60–70 kg/m2) is not routine and perhaps should be limited to centers that are experienced in the care of such patients. The cannulation can be technically challenging and the day-to-day management is nuanced.11,29 In this study 2 of the 3 patients with a BMI >60 kg/m2—all of whom were in their 30 years—survived to discharge. However, the vast majority of the patients in this study were not in the very high end of the BMI range (60–70 kg/m2), and so no meaningful conclusion can be drawn about the safety and efficacy in the extremely obese patient based on this small sample size.
The median BMI in the “high BMI” cohort was 44 kg/m2 (IQR 42–46 kg/m2), and so it is not surprising that there was a low incidence of cannulation-related adverse events—namely a 2% cannula site bleeding rate. This was likely further helped by the fact that percutaneous Veno-venous ECMO cannulation in the morbidly obese is technically much easier than percutaneous or open Veno-arterial ECMO cannulation—where limb ischemia and cannula migration are unique hurdles. It should be further noted that this study was not powered to show a difference among the low incidence of adverse event when the subgroups were stratified by BMI threshold.
In this study the lower BMI cohort was older. (Figure 2) Not surprisingly, older age—a known risk factor for adverse outcomes in boarder COVID-19 patients—was also associated with adverse outcomes in COVID-19 patients on ECMO. We can therefore deduce that older patients with Class 3 Obesity who develop COVID-19 associated ARDS requiring ECMO would be at even higher risk for worse outcomes.
When stratified by BMI, the patient groups were relatively evenly matched in terms of demographics and comorbidities. It should be acknowledged that the patients in the high BMI group had a slightly worse P:F ratio and static compliance, meaning their ARDS was functionally more severe at time of ECMO cannulation. However, the difference of a P:F of 16 points or a static compliance of 1 mL/cm H2O may not be clinically significant.
Study limitations
This observational study is limited by its retrospective nature and incomplete data, which resulted in 47 patients being eliminated from the final analysis. However, when we modeled to account for the missing data, we did not see clinically significant changes in the results. It should also be noted that much of the data was entered prospectively. In addition, the leave-one-out sensitivity analysis showed that no one study site had a disproportionate impact on the results. (Figure 4) Similarly, the two patients with BMIs >70 kg/m2 were from different centers. When excluded in a repeat sensitivity analysis, the additive risk of death associated with advancing age (ever 10 + years) and BMI (every 5 kg/m2) remained unchanged. This counters any concern that one aggressive site or a patient with an extreme BMI could bias the study’s findings.
Although, the percentage of Class 3 Obesity in our study population mirrored that in the US population (∼11%), the smaller sample size of the Class 3 Obese patient group may not be adequately powered to show statistical differences at a set threshold. Even though most patients came from two continents, the breath and scope of this international consortium of 82 participating institutions supports the generalizability of the data. This diversity of the catchment pool of patients allows this data to support clinical decision making for the obese COVID-19 patients with severe ARDS.
The “high BMI” cohort was a median of 13 years younger than the “lower BMI” group. As evidenced by the data in this study there is potential a survival advantage associated with >10 years age difference. This age difference could have muted some of the overall mortality seen in the high BMI group. This study was not powered to do a subset analysis on older high BMI patients compared to younger high BMI patients—especially given the lack of a threshold age or BMI.
In a study of patients treated during an ongoing pandemic, a potential limitation can be selection bias, as clinicians were building the proverbial plane while flying it. The COVID-19 ELSO criteria still carry a relative contraindication for BMI >40 kg/m2. However, standard of care is still being determined by the work of consortiums such as this. For example, prior to the COVID-19 pandemic, obesity was shown to be protective or not to have a negative mortality effect on patients requiring ECMO. Initially, in the early phase of the pandemic this may have impacted a physician’s decisions to use ECMO in obese COVID-19 patients. However, any concerns for selection bias can be countered by the notion that during this study period, the surge in COVID-19 patients with ARDS coupled with the limitations in ECMO resources likely ensured that nearly all patients placed on ECMO were optimally selected independent of BMI. It would stand to reason that the low-BMI-cohort survival was not skewed by centers placing lower BMI patients with multi-organ failure and long ventilator times on ECMO.
Another limitation of this study is the time period during which patient data was collected. The study time spanned the initial 1.5 years of the pandemic. During that time much knowledge has been rapidly gained, and management strategies were likely updated in real time. As healthcare teams refined their management processes around COVID-19 patients with severe ARDS, it is reasonable to assume that ECMO selection criteria were adjusted. Unfortunately, this study was not designed and is not powered to determine the effect of changes in practice pattern over time when groups are stratified by BMI.
With these limitations in mind, the data supports a measured and nuanced approach in this group of patients. BMI is a continuous variable and therefore no strict cutoff should be used. If their BMIs are 40, 50 or 60 and above, patients with COVID-19 and ARDS can be still considered for V-V ECMO. In these instances, it is prudent that a center’s comfort and experience guide its patient selection.11
Conclusion
In patients with COVID-19 and severe ARDS who require V-V ECMO, there is a decreased survival and additive risk associated with higher BMI and advanced age. There was no specific BMI cutoff beyond which the risk for death greatly increases. A nuanced approach in COVID-19 patients with BMIs of 40 or above should be considered when evaluating them for V-V ECMO. Our results are hypothesis-generating and any attempt to better define the suitability of V-V ECMO in morbidly obese patients with COVID-19 ARDS, might be best answered using a prospective randomized study.
Supplemental Material
Supplemental Material for Morbid obesity’s impact on COVID-19 patients requiring venovenous extracorporeal membrane oxygenation: The covid-19 critical care consortium database review by Jeffrey Javidfar, Akram M Zaaqoq, Ahmed Labib, Adrian G Barnett, JW Awori Hayanga, Greg Eschun, Michael H Yamashita, Jeffrey P Jacobs, Silver Heinsar, Jacky Y Suen, John F Fraser, Gianluigi Li Bassi, Rakesh C Arora and Giles J Peek; on behalf of the Covid-19 Critical Care Consortium (COVID Critical) in Perfusion
Acknowledgements
We acknowledge all members of the COVID-19 Critical Care Consortium for their hard work and tireless efforts to better understand and manage this disease. Please see the online supplemental for a detailed list of the various collaborators.
Appendix.
Abbreviations
| Acute Respiratory Distress Syndrome | ARDS |
| Veno-venous | V-V |
| Extracorporeal membrane oxygenation | ECMO |
| Body Mass index | BMI |
| Severe acute respiratory syndrome coronavirus 2 | SARS-CoV-2 |
| Extracorporeal life support organization | ELSO |
| ECMO to rescue lung injury in severe ARDS trial | EOLIA trial |
| Conventional ventilatory support versus ECMO for severe adult respiratory failure trial | CESAR trial |
| COVID-19 critical care consortium | CCCC |
| Acute physiologic assessment and chronic health evaluation II score | APACHE II score |
| Sequential organ failure assessment score | SOFA score |
| Interquartile range | IQR |
| PaO2/FiO2 ratio | P:F ratio |
| Hazard ratio | HR |
| Confidence interval | CI |
| Predicting death for severe ARDS on V-V ECMO score | PRESERVE score |
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Dr Gianluigi Li Bassi acknowledges receipt of a “BITRECS” fellowship; the “BITRECS” project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 754550 and from the “La Caixa” Foundation (ID 100010434), under the agreement LCF/PR/GN18/50310006.
Author’s note: The Covid-19 Critical Care Consortium is supported by Wesley Medical Research, Fisher and Paykel, and Queensland Health.
Ethical approval: Institutional Review Board: The IRB at each consortium member site gave their approval to participate in the study.
Data availability: The underlying data can be accessed via the Covid-19 Critical Care Consortium Web site: https://www.covid-critical.com.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Jeffrey Javidfar https://orcid.org/0000-0002-2070-0528
Akram M Zaaqoq https://orcid.org/0000-0003-3147-5044
Ahmed Labib https://orcid.org/0000-0003-1863-0484
JW Awori Hayanga https://orcid.org/0000-0001-5091-7069
References
- 1.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018; 378: 1965–1975. [DOI] [PubMed] [Google Scholar]
- 2.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374: 1351–1363. [DOI] [PubMed] [Google Scholar]
- 3.Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 Influenza A (H1N1) acute respiratory distress syndrome. JAMA 2009; 302: 1888–1895. [DOI] [PubMed] [Google Scholar]
- 4.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the extracorporeal life support organization registry. Lancet 2020; 396: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammad S, Aziz R, Al Mahri S, et al. Obesity and Covid-19: what makes obese host so vulnerable? Immun Ageing. 2021; 18: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kass DA. COVID-19 and severe obesity: a big problem? Ann Intern Med 2020; 173: 840–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third national health and nutrition examination survey. JAMA 2002; 287(3): 356–359. [DOI] [PubMed] [Google Scholar]
- 8.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180(10): 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis 2020; 71: 896–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lockhart SM, O’Rahilly S. When Two Pandemics Meet: Why Is Obesity Associated with Increased COVID-19 Mortality? Med (N Y) 2020; 1(1): 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javidfar J, Zaaqoq AM, Yamashita MH, et al. Venovenous extracorporeal membrane oxygenation in obese patients. JTCVS Tech 2021; 10: 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett RH, Ogino MT, Brodie D, et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J 2020; 66: 472–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 critical care consortium. Available at:https://www.covid-critical.com/ (2021, accessed 26 September 2021).
- 14.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform 2009; 42(2): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners, J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaaqoq AM, Barnett AG, Griffee MJ, et al. Beneficial effect of prone positioning during venovenous extracorporeal membrane oxygenation for coronavirus disease 2019. Crit Care Med 2022; 50(2): 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazard D, Kaier K, von Cube M, et al. Joint analysis of duration of ventilation, length of intensive care, and mortality of COVID19 patients: a multistate approach. BMC Med Res Methodol 2020; 20: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306: 1659–1668. [DOI] [PubMed] [Google Scholar]
- 19.Lunn D, Jackson C, Best N, et al. The bugs book: a practical introduction to bayesian analysis. Chapman & Hall/Crc Texts in Statistical Science. New York: Taylor & Francis, 2012. [Google Scholar]
- 20.Wolkewitz M, Cooper BS, Bonten MJM, et al. Interpreting and comparing risks in the presence of competing events. BMJ 2014; 349: g5060. [DOI] [PubMed] [Google Scholar]
- 21.https://www.r-project.org/, accessed November 1, 2022.
- 22.Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat 2016; 70: 129–133. [Google Scholar]
- 23.Gray B. Cmprsk: subdistribution analysis of competing risks. Available at: https://CRAN.R-project.org/package=cmprsk (2020, accessed 11 November 2020).
- 24.Al-Soufi S, Buscher H, Nguyen ND, et al. Lack of association between body weight and mortality in patients on veno-venous extracorporeal membrane oxygenation. Intensive Care Med 2013; 39(11): 1995–2002. [DOI] [PubMed] [Google Scholar]
- 25.Kon ZN, Dahi S, Evans CF, et al. Class III obesity is not a contraindication to venovenous extracorporeal membrane oxygenation support. Ann Thorac Surg 2015; 100(5): 1855–1860. [DOI] [PubMed] [Google Scholar]
- 26.Swol J, Buchwald D, Dudda M, et al. Veno-venous extracorporeal membrane oxygenation in obese surgical patients with hypercapinic lung failure. Acta Anaesthesiol Scand 2014; 58(5): 534–538. [DOI] [PubMed] [Google Scholar]
- 27.Swol J, Buchwald D, Strauch JT, et al. Effect of body mass index on outcome of surgical patients receiving extracorporeal devices (VV ECMO, pECLA) for respiratory failure. Int J Artif Organs 2017; 40: 102–108. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013; 39(10): 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ull C, Buchwald D, Strauch J, et al. Extremely obese patients treated with venovenous ECMO—an intensivist’s challenge. Am J Emerg Med 2015; 33(11): 1720.e3–1720.e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Morbid obesity’s impact on COVID-19 patients requiring venovenous extracorporeal membrane oxygenation: The covid-19 critical care consortium database review by Jeffrey Javidfar, Akram M Zaaqoq, Ahmed Labib, Adrian G Barnett, JW Awori Hayanga, Greg Eschun, Michael H Yamashita, Jeffrey P Jacobs, Silver Heinsar, Jacky Y Suen, John F Fraser, Gianluigi Li Bassi, Rakesh C Arora and Giles J Peek; on behalf of the Covid-19 Critical Care Consortium (COVID Critical) in Perfusion