Abstract
Background:
Streptococcus pneumoniae is a leading cause of severe infections among children. Despite vaccination, HIV-exposed, uninfected (HEU) children have a higher incidence of invasive pneumococcal disease than HIV-unexposed, uninfected (HUU) children. We sought to compare the immunogenicity of 13-valent pneumococcal conjugate vaccine (PCV-13) in HEU and HUU infants.
Methods:
We conducted a prospective cohort study of 134 mother-infant dyads in Botswana. Infants received PCV-13 doses at 2, 3, and 4 months through routine clinical care. We measured IgG antibodies specific to vaccine serotypes in sera collected from infants at 0, 5, and 12 months of age. We calculated the proportion of infants with protective IgG levels (≥0.35 μg/mL) to specific pneumococcal serotypes.
Results:
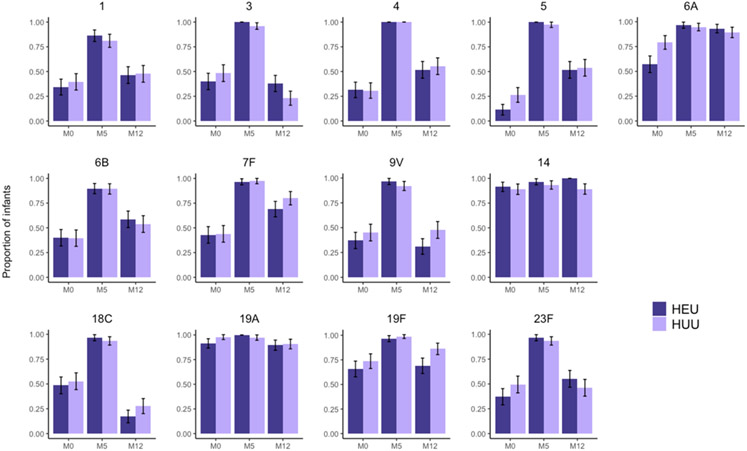
At birth, fewer than half of infants had protective IgG levels to serotypes 1 (38%), 3 (46%), 4 (33%), 5 (23%), 6B (40%), 7F (44%), 9V (44%), and 23F (46%). Compared to HUU infants (n=97), HEU infants (n=37) had lower antibody concentrations at birth to serotypes 5 (p=0.046) and 19A (p=0.008) after adjustment for maternal age and infant birth weight. More than 80% of HEU and HUU infants developed protective antibody levels to each of the 13 vaccine serotypes following PCV-13 vaccination. Median concentrations of antibodies to pneumococcal serotypes declined by 55–93% between 5 and 12 months of age, with fewer than half of infants having protective antibody levels to serotypes 1 (47%), 3 (28%), 9V (44%), 18C (24%), and 23F (49%) at 12 months of age.
Conclusions:
Both HEU and HUU infants developed protective antibody responses to PCV-13 administered in a 3+0 schedule. However, antibody concentrations to many pneumococcal serotypes waned substantially by 12 months of age, suggesting that a PCV-13 booster dose in the second year of life may be needed to maintain protective pneumococcal antibody levels in older infants and young children.
Keywords: HIV-exposed, uninfected, children, Streptococcus pneumoniae, 13-valent pneumococcal conjugate vaccine
INTRODUCTION
Globally, 1.4 million women with human immunodeficiency virus (HIV) give birth each year [1]. Fortunately, improved access to antiretroviral therapy during pregnancy has substantially reduced vertical transmission of HIV, resulting in a growing population of infants born to mothers with HIV who do not themselves acquire the virus. Despite the absence of HIV infection, these HIV-exposed, uninfected (HEU) infants have higher infectious morbidity and mortality than the children of mothers without HIV (HIV-unexposed, uninfected; HUU) [2]. HEU infants are recognized to have immune defects resulting from in utero exposure to HIV or antiretroviral medications including abnormalities of lymphocyte number and function, lower neutrophil counts, and lower levels of maternally derived antibodies to several common childhood pathogens [3, 4]. Moreover, although most studies suggest that HEU infants generate immune responses to common childhood vaccines that are comparable to those of HUU infants [4-7], several studies reported lower quantitative or qualitative antibody responses to vaccination among HEU infants [8-10].
Streptococcus pneumoniae is a leading cause of severe infections among children, resulting in more than 300,000 child deaths each year [11]. S. pneumoniae causes a broad range of infections ranging from mild respiratory illnesses to invasive pneumococcal disease (IPD), which includes life-threatening illnesses such as bloodstream infection and meningitis [12]. Pneumococcal conjugate vaccines effectively prevent IPD caused by vaccine serotypes [13, 14]. However, despite vaccination, HEU children have higher incidences of hospitalization and mortality from IPD than HUU children during the first year of life [15], suggesting that HEU infants may acquire lower levels of maternally-derived pneumococcal antibodies or have less robust immune responses to pneumococcal conjugate vaccination. The incidence of severe pneumococcal disease is particularly high in sub-Saharan Africa, where more than 4 million pneumococcal infections occur each year among children [16]. We previously demonstrated that introduction of 13-valent pneumococcal conjugate vaccine (PCV-13) was associated with substantial reductions in pneumonia hospitalizations and deaths among children in Botswana [17]. Notably, HIV exposure data were missing from most children hospitalized during the prevaccine period in this study, precluding evaluation of the association between PCV-13 introduction and pneumonia hospitalizations and deaths in HEU children [17].
In the current study, we evaluated the association between HIV exposure and pneumococcal antibody concentrations at birth and after PCV-13 vaccination in a cohort of 134 HEU and HUU infants in Botswana. As a secondary objective, we compared vaccine-elicited IgG subclass-specific pneumococcal antibodies in sera from 56 infants (28 HEU, 28 HUU) after PCV-13 vaccination.
METHODS
Setting
Botswana is a landlocked country in southern Africa with a semi-arid climate and a short rainy season that typically occurs from November to March. The country’s under-five child mortality rate was estimated to be 41.6 per 1,000 live births in 2019 [18]. Gaborone, the capital and largest city in Botswana, is located in the country’s South-East district and was estimated to have a population of 231,626 in 2011 [19]. In July 2012, 13-valent pneumococcal conjugate vaccine (Prevnar 13®, Pfizer; PCV-13) was included in the national immunization program as a 3-dose primary series without a booster (3+0 schedule) with doses administered at 2, 3, and 4 months of age. There was no national program for vaccination of adults or pregnant women against pneumococcus during the study period. The HIV prevalence among individuals 15 to 49 years of age in Botswana was 20.7% in 2019 [20]. More than 95% of pregnant women with HIV in Botswana receive antiretroviral therapy, and the vertical HIV transmission rate is estimated to be less than 2% [20].
Data and sample collection
Mother-infant dyads were recruited within 72 hours of delivery at an academic hospital and two public clinics near Gaborone, Botswana between February 2016 and January 2020, as previously described [21]. Exclusion criteria included maternal age less than 18 years, infant birth weight less than 2000 grams, multiple gestation pregnancy, and Caesarian delivery. Participants were seen for monthly study visits until the infant was six months of age and every other month thereafter until the infant was 12 months of age. Mid-upper arm circumference (MUAC) was measured at all visits starting at 6 months of age, and current World Health Organization (WHO) guidelines were used to classify infants as having moderate (MUAC between 11.5 cm and 12.5 cm) or severe (MUAC less than 11.5 cm) malnutrition [22]. Serum samples were collected from infants at 0, 5, and 12 months of age. A dried blood spot was collected from HIV-exposed infants by heel prick at two months of age and, for infants who were breastfed for any duration, again at 12 months of age. These samples were tested for HIV-1 DNA using the Cobas AmpliPrep/Cobas TaqMan HIV-1 Qualitative Assay, version 2.0 (Roche) [23]. The analyses presented herein were limited to HEU or HUU infants who received 3 doses of PCV-13 prior to 150 days of age with the 3rd dose being administered 14 days or more prior to collection of the 5-month serum sample.
Measurement of pneumococcal immunoglobulin G antibodies
Enzyme-linked immunosorbent assays (ELISA) were developed based on the WHO protocol and used to measure immunoglobulin G (IgG) binding to the 13 pneumococcal serotypes included in PCV-13 [24]. High-binding 384-well plates were coated with optimized concentrations of 5 μg/ml or 10 μg/ml of pneumococcal serotype-specific carbohydrates (ATCC, Rockville, MD, USA) and incubated for 5 hours at 37°C and 5% CO2 and subsequently stored overnight at 4°C. Sera from HEU and HUU infants (1:50, 1:250, 1:1000 dilutions) were prepared in assay diluent (1xPBS, 0.05% Tween, 5 μg/ml of pneumococcal cell wall polysaccharide and serotype 22F pneumococcal capsular polysaccharide) and incubated for 30 minutes. Diluted sera were then transferred to the plate and incubated for 2 hours at 20°C. IgG was detected by a horse radish peroxidase-conjugated goat anti-human IgG polyclonal antibody (Jackson ImmunoResearch, West Grove, PA, USA). WHO international standard 007SP, a sample of pooled sera from humans immunized with Pneumovax II (National Institute for Biological Standards and Control, Hertfordshire, England), was used as a positive control [25]. ELISA plates were developed with SureBlue Reserve TMB substrate for 5 minutes (KPL Inc., Gaithersburg, MD, USA). Plates were read immediately after addition of stop solution at 450 nm on a SpectraMax Plus 384 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Softmax Pro 6.3 software was used to interpolate concentrations from standard curves. Antibody concentrations that were above (or below) levels that could be quantitated were set to the upper (or lower) limit of quantification (Supplemental Table 1).
Measurement of pneumococcal immunoglobulin G antibody subclasses
Measurement of IgG subclass-specific pneumococcal antibodies was performed on sera collected from infants at 5 months of age using a previously described binding antibody multiplex assay [26]. Briefly, the 13 pneumococcal serotypes tested above were coupled to carboxylated fluorescent beads (Bio-Rad Laboratories, Inc., Hercules, CA, USA) following the DMTMM-coupling protocol [27]. The coupled beads were incubated with diluted plasma (at 1:100 dilution) for 30 minutes at 20°C. Antigen-specific subclass response was detected using biotin-conjugated mouse anti-human IgG1 (BD Pharmingen, San Diego, CA, USA; 4 μg/L), IgG2 (Southern Biotech, Birmingham, AL, USA; 5 μg/mL), IgG3 (Calbiochem, San Diego, CA, USA; 2 μg/mL), or IgG4 (BD Pharmingen; 2 μg/mL) and tertiary detection agent streptavidin-phycoerythrin (BD Biosciences, Franklin Lakes, New Jersey, USA) at 5 μg/mL. Beads were washed and read on a Bio-Plex 200 instrument (Bio-Rad Laboratories, Inc.). IgG levels were expressed as mean fluorescence intensity (MFI) and all MFI values were corrected by subtracting the intensity of a blank control. IgG levels below 100 MFI were considered to be below the lower limit of quantitation for the assay. Consistency between assays was measured by tracking the 50% effective concentration, MFI, and area under the curve of the positive control (007SP) by Levey-Jennings charts.
Statistical analysis
Sociodemographic characteristics of HEU and HUU infants were compared using Wilcoxon rank-sum tests for continuous variables and Chi-squared tests or Fisher’s exact tests for categorical variables. Antibody concentrations for each serotype and at each time point were compared between HEU and HUU infants using Wilcoxon rank-sum tests. Proportions of infants with protective antibody concentrations to pneumococcal serotypes were determined based on the protective threshold of ≥0.35 μg/mL proposed by the WHO [28]. Chi-squared tests were used to evaluate associations between HIV exposure status and the presence of protective antibody concentrations to pneumococcal serotypes at each time point. Linear regression models were fit to evaluate associations between HIV exposure status and the log-transformed serum concentrations of antibodies to each serotype at birth, adjusting for infant birth weight and maternal age. Because concentrations of antibodies to different pneumococcal serotypes were anticipated to be correlated in a given infant, analyses were performed without adjustment for multiple comparisons. Analyses were conducted using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
Of 134 infants, 37 (28%) were HEU and 97 (72%) were HUU (Table 1); no infants tested positive for HIV by DNA PCR. Median [interquartile range (IQR)] birth weight was 3225 (2940–3440) grams and 70 (52%) infants were female. One-third (34%) of infants resided in a rural area and 9 (7%) infants met criteria for moderate malnutrition at one or more study visits. Of 37 mothers with HIV, 36 (97%) received antiretroviral therapy during pregnancy for a median (IQR) duration of 9 (5, 9) months; the most common maternal antiretroviral regimens were tenofovir disoproxil fumarate, emtricitabine, and efavirenz (n=17, 46%) and tenofovir disoproxil fumarate, emtricitabine, and dolutegravir (n=10, 27%). Median (IQR) CD4 count was 455 (300, 646) cells/μL, and 29 of 30 (97%) mothers with available data had undetectable viral loads (<400 copies/mL) during pregnancy. Compared to HUU infants, HEU infants had lower birth weight (p=0.01), were less likely to be ever breastfed (p<0.0001), and had older mothers (p=0.0003) who were less likely to have completed secondary or tertiary education (p=0.03). No episodes of invasive pneumococcal disease were identified among infants in the study population.
Table 1.
Sociodemographic characteristics of the study population by HIV exposure status
| Overall (n=134) |
HEU Infants (n=37) |
HUU Infants (n=97) |
p | ||
|---|---|---|---|---|---|
| Infant sex, n (%) | 0.40 | ||||
| Female | 70 (52%) | 22 (59%) | 48 (49%) | ||
| Male | 64 (48%) | 15 (41%) | 49 (51%) | ||
| Infant birth weight (g), median (IQR) | 3225 (2940–3440) | 3040 (2745–3285) | 3275 (2970–3560) | 0.01 | |
| Infant breastfeeding, n (%) | 109 (81%) | 13 (35%) | 96 (99%) | <0.0001 | |
| Nutritional statusa | >0.99 | ||||
| No malnutrition | 120 (93%) | 34 (94%) | 86 (92%) | ||
| Moderate malnutrition | 9 (7%) | 2 (6%) | 7 (8%) | ||
| Maternal age, median (IQR) | 26.8 (22.8–31.8) | 31.1 (25.4–36.1) | 25.9 (22.6–30.1) | 0.0003 | |
| Maternal education, n (%) | 0.03 | ||||
| None or primary | 9 (7%) | 6 (16%) | 3 (3%) | ||
| Secondary | 109 (81%) | 26 (70%) | 83 (86%) | ||
| Tertiary | 16 (12%) | 5 (14%) | 11 (11%) | ||
| Location of residence, n (%) | 0.62 | ||||
| Rural | 46 (34%) | 11 (30%) | 35 (36%) | ||
| Urban | 88 (66%) | 26 (70%) | 62 (64%) | ||
| Household size, median (IQR) | 5.5 (4.0–9.0) | 6.0 (4.0–8.0) | 5.0 (4.0–9.0) | 0.71 | |
HEU, HIV-exposed uninfected; HUU, HIV-unexposed uninfected; IQR, interquartile range
Data on nutritional status were missing for 5 infants
Serum antibody concentrations to pneumococcal serotypes among infants at birth
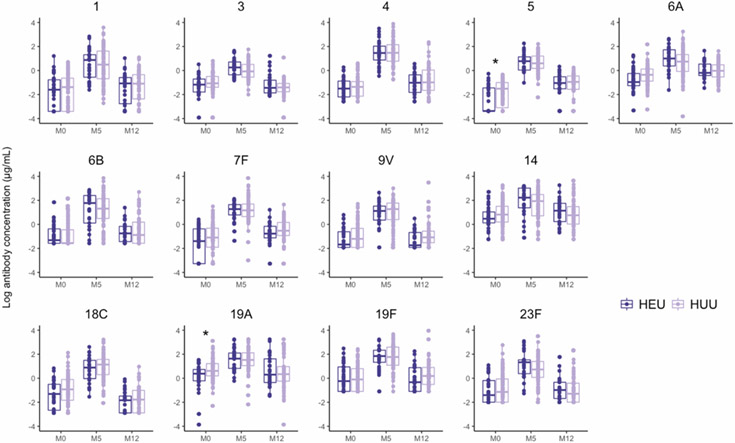
Serum concentrations of IgG antibodies specific to pneumococcal serotypes among infants are shown in Figure 1. In multivariable linear regression models adjusting for maternal age and infant birth weight, infant HIV exposure was independently associated with lower concentrations of antibodies to pneumococcal serotypes 5 (p=0.046) and 19A (p=0.008) at birth (Table 2). No significant associations were observed between maternal age or infant birth weight and antibody levels to pneumococcal serotypes at birth (data not shown).
Figure 1. Serum concentrations of antibodies to pneumococcal serotypes among 134 infants immunized with PCV-13 in Botswana by infant HIV exposure status.
Box plots depict natural log-transformed serum antibody concentrations (μg/mL) to each of the 13 vaccine serotypes in HIV-exposed, uninfected (HEU) and HIV-unexposed, uninfected (HUU) infants receiving PCV-13 in a 3+0 schedule with doses at 2, 3, and 4 months of age. Each point represents the concentration in a single sample; boxes represent the 25th to 75th percentile with median values shown as a line. The dark purple points and boxes correspond to antibody concentrations of HEU infants and the light purple points and boxes correspond to antibody concentrations of HUU infants. Statistical comparisons of serum antibody concentrations by HIV exposure status were performed using linear regression adjusting for infant birth weight and maternal age with significant differences indicated by asterisks. M0, birth (0 months); M5, 5 months of age; M12, 12 months of age
Table 2.
Serum concentrations of immunoglobulin G antibodies to pneumococcal serotypes among 126 infants with sera at birth by HIV exposure status
| Serotype | Median (IQR) Serum Antibody Concentration (μg/mL) |
padja | |
|---|---|---|---|
| HEU Infants (n= 35) |
HUU Infants (n= 91) |
||
| 1 | 0.20 (0.03–0.46) | 0.25 (0.03–0.53) | 0.59 |
| 3 | 0.31 (0.17–0.49) | 0.34 (0.25–0.61) 0.26 (0.12, 0.40) |
0.50 |
| 4 | 0.22 (0.11–0.42) 0.03 (0.03, 0.23) |
0.26 (0.12–0.40) | 0.64 |
| 5 | 0.03 (0.03–0.24) 0.27 (0.21, 0.69) |
0.22 (0.05–0.37) | 0.046 |
| 6A | 0.38 (0.28–0.78) | 0.70 (0.41–1.13) | 0.09 |
| 6B | 0.27 (0.21–0.69) 0.19 (0.15, 0.54) |
0.21 (0.21–0.64) | 0.84 |
| 7F | 0.25 (0.04–0.69) | 0.33 (0.15–0.73) | 0.06 |
| 9V | 0.19 (0.15–0.54) | 0.30 (0.15–0.72) 1.60 (1.13, 2.82) |
0.20 |
| 14 | 1.60 (1.14–2.82) 0.27 (0.07, 0.60) |
2.26 (1.22–4.51) | 0.39 |
| 18C | 0.27 (0.07–0.60) 0.79 (0.33, 2.57) |
0.40 (0.16–0.91) | 0.13 |
| 19A | 1.46 (0.81–2.08) 0.24 (0.14, 0.83) |
1.85 (1.22–3.38) 0.32 (0.14, 0.94) |
0.008 |
| 19F | 0.79 (0.33–2.57) | 0.90 (0.33–2.23) | 0.66 |
| 23F | 0.25 (0.14–0.83) | 0.32 (0.14–0.94) | 0.27 |
IQR, interquartile range; μg, microgram; mL, milliliter; HEU, HIV-exposed uninfected; HUU, HIV-unexposed uninfected
Adjusted p values were estimated from linear regression models evaluating associations between HIV exposure status and log-transformed serum antibody concentrations and adjusted for infant birth weight and maternal age
Pneumococcal antibody responses following receipt of PCV-13
Both HEU and HUU infants mounted robust antibody responses to 3 doses of PCV-13. At 5 months of age, corresponding to 1 month after completion of the 3-dose primary vaccination series, the proportion of infants with protective antibody concentrations to specific pneumococcal serotypes was 83%–100% (Figure 2), and 59% of infants developed protective antibody concentrations to all 13 vaccine serotypes. No significant differences in antibody concentrations to any of the 13 vaccine serotypes were found between HEU and HUU infants at 5 months of age (Figure 1). Between 5 and 12 months of age, median serum concentrations of antibodies specific to pneumococcal serotypes declined by 55–93%, with marked differences in the degree of antibody waning by pneumococcal serotype (Table 3). For instance, the concentration of antibodies specific to pneumococcal serotype 18C declined by a median (IQR) of 93% (86%–97%) between 5 and 12 months of age, while the concentration of antibodies specific to serotype 6A declined by a median (IQR) of 55% (8%–75%) over this same time period. No consistent trend was observed in the degree of antibody waning by infant HIV exposure status, although serum concentrations of antibodies to pneumococcal serotype 19F did decline to a greater extent among HEU infants than among HUU infants [median (IQR): 90% (74-95%) vs. 77% (50%-90%); p=0.04]. At 12 months of age, fewer than half of infants had protective antibody levels to serotypes 1 (47%), 3 (28%), 9V (44%), 18C (24%), and 23F (49%) (Figure 2).
Figure 2. Proportion of infants with protective concentrations of serum antibodies to specific pneumococcal serotypes.
Bar charts depict the proportion of infants with serum antibody concentrations at or above a protective threshold of 0.35 μg/mL. The dark purple bars correspond to HIV-exposed, uninfected (HEU) infants and the light purple bars correspond to HIV-unexposed uninfected (HUU) infants. Error bars are shown representing the 95% confidence interval for each proportion.
Table 3.
Waning of serum concentrations of antibodies to specific pneumococcal serotypes following PCV-13 vaccination
| Serotype | Median (IQR) Serum Antibody Concentration (μg/mL) |
Median (IQR) Percent Decline |
|
|---|---|---|---|
| Early Post-Vaccine (Age 5 Months) |
Late Post-Vaccine (Age 12 Months) |
||
| 1 | 1.66 (0.51–4.97) | 0.34 (0.06–0.66) | 83% (67%–90%) |
| 3 | 1.04 (0.60–1.71) | 0.24 (0.17–0.37) | 75% (58%–86%) |
| 4 | 4.34 (2.20–8.57) | 0.37 (0.18–0.80) | 91% (82%–96%) |
| 5 | 1.96 (1.14–3.29) | 0.37 (0.21–0.63) | 83% (69%–90%) |
| 6A | 2.29 (0.97–4.37) | 0.86 (0.58–1.61) | 55% (8%–75%) |
| 6B | 3.82 (1.51–10.10) | 0.43 (0.21–1.10) | 86% (62%–93%) |
| 7F | 3.32 (1.91–5.26) | 0.58 (0.38–0.96) | 81% (68%–89%) |
| 9V | 3.38 (1.42–5.75) | 0.30 (0.15–0.56) | 90% (82%–94%) |
| 14 | 8.10 (2.40–13.35) | 2.42 (1.19–5.12) | 59% (18%–81%) |
| 18C | 2.99 (1.29–4.71) | 0.17 (0.06–0.33) | 93% (86%–97%) |
| 19A | 4.71 (2.41–6.88) | 1.39 (0.76–4.78) | 62% (0%–85%) |
| 19F | 6.24 (2.96–11.55) | 1.08 (0.52–2.47) | 84% (61%–93%) |
| 23F | 2.32 (1.21–4.21) | 0.34 (0.14–0.70) | 85% (61%–94%) |
IQR, interquartile range; μg, microgram; mL, milliliter
Pneumococcal antibody subclasses
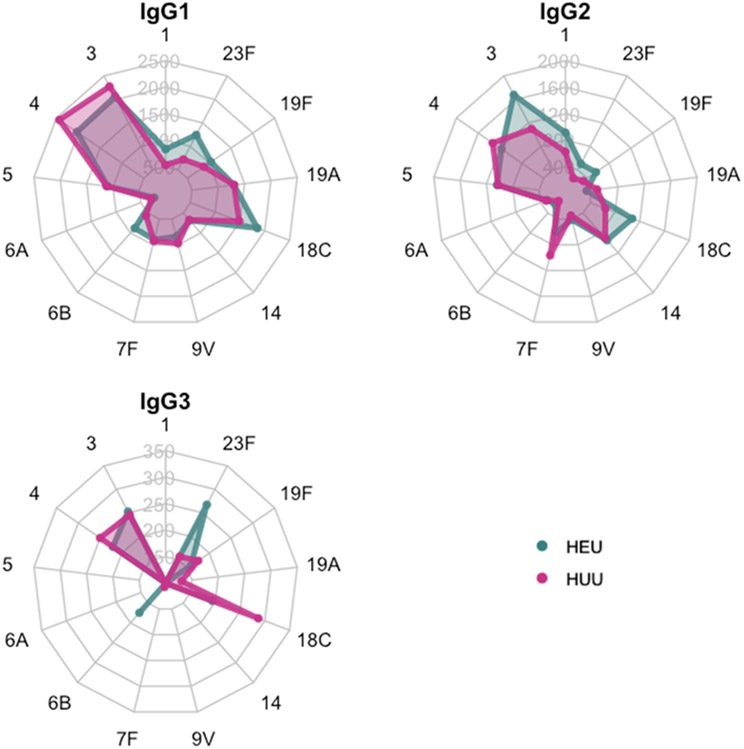
To further evaluate the association between HIV exposure and immune responses to PCV-13, we measured levels of IgG subclass antibodies to each pneumococcal serotype contained within PCV-13 in sera from 56 infants (28 HEU, 28 HUU) at 5 months of age (Figure 3, Supplemental Table 2). We observed no significant differences in the total IgG subclass-specific antibody to pneumococcal serotypes contained in PCV-13. Notably, for most pneumococcal serotypes, a majority of infants had levels of IgG3 and IgG4 antibodies below the level of quantitation for the assay following PCV-13 vaccination. Levels of IgG1 and IgG2 antibodies specific to pneumococcal serotypes were broadly similar in HEU and HUU infants, although HEU infants developed statistically higher levels of IgG2 subclass antibodies specific to serotype 23F (p=0.02).
Figure 3. Serum immunoglobulin G subclass antibodies among 56 infants following PCV-13 vaccination.
Radar plots depict median fluorescence intensities of IgG subclass antibodies to each of the 13 serotypes contained within 13-valent pneumococcal conjugate vaccine. The green points and corresponding shaded areas represent the median antibody levels for HIV-exposed, uninfected (HEU) infants. The pink points and corresponding shaded areas represent the median antibody levels for HIV-unexposed, uninfected (HUU) infants. IgG4 subclass antibodies are not shown because of the high proportion of infants with values below the level of quantitation for the assay.
DISCUSSION
In this longitudinal study of 134 infants in Botswana, both HEU and HUU infants developed protective humoral immune responses after three doses of PCV-13 administered at 2, 3, and 4 months of age (3+0 schedule). However, serum concentrations of antibodies to specific pneumococcal serotypes declined by 55–93% between 5 and 12 months of age, with fewer than half of infants having protective antibody levels to many vaccine serotypes at 12 months of age.
Prior studies indicate that HEU infants generally mount robust humoral immune responses to other childhood vaccines, including vaccines for pertussis, tetanus, hepatitis B, and Haemophilus influenzae type b [4-6]. However, few prior studies compared the immune responses of HEU and HUU infants to pneumococcal conjugate vaccines. In a study of 109 mother-infant dyads in South Africa, HEU infants had higher concentrations of antibodies to pneumococcus than HUU infants two weeks after receipt of a second dose of 7-valent pneumococcal conjugate vaccine (PCV-7) when all infants were approximately four months of age [4]. Subsequent studies reported similar concentrations of antibodies to specific pneumococcal serotypes in HEU and HUU infants receiving PCV-7 at 4 to 6 months of age [7, 8]; however, these studies evaluated antibody responses after a three-dose primary series of PCV-7. Notably, evaluation of antibody-mediated opsonophagocytic activity in HEU and HUU infants suggested that pneumococcal conjugate vaccines may induce antibodies with different functional profiles in HEU and HUU infants [8]. Our study provides the first comparative data on the quantitative antibody responses of HEU and HUU infants to PCV-13. We demonstrate that infant HIV exposure does not influence total serum IgG or IgG subclass responses to serotypes contained in PCV-13.
We observed lower pre-vaccination levels of antibodies against pneumococcal serotypes 5 and 19A among HEU infants compared with HUU infants. In the aforementioned study of mother-infant dyads in South Africa, HEU infants were found to have lower levels of IgG antibodies to pneumococcal polysaccharide at birth [4]. Similarly, Madhi and colleagues observed lower pre-vaccination antibody concentrations to most vaccine serotypes among HEU infants compared with HUU infants immunized with PCV-7; antibodies to pneumococcal serotypes 5 and 19A were not measured in this study because PCV-7 does not include capsular polysaccharide for these serotypes [7]. These subtle differences in findings across studies may reflect variability in the age of infants at the time of sera collection, differences in the immune statuses of mothers with HIV, variable pneumococcal immunization statuses of mothers, or differences in the timing of these studies relative to introduction of pneumococcal conjugate vaccine in these settings, which could influence the risk of maternal colonization or infection by circulating vaccine serotypes. HEU infants were also previously reported to have lower pre-vaccination levels of antibodies to several other pathogens, including Haemophilus influenzae type B, pertussis, and tetanus [4-7]. This is believed to result from reduced acquisition of maternal antibodies because of lower levels of pathogen-specific antibodies among mothers with HIV or impaired transplacental antibody transfer [29, 30]. Importantly, because infants do not begin most childhood vaccine series until the first or second month of life, these differences in acquisition of maternal antibodies suggest that HEU infants may be particularly vulnerable to vaccine-preventable infections in early infancy.
We observed that both HEU and HUU infants exhibited marked declines in pneumococcal antibody levels between 5 and 12 months of age. Interestingly, the degree of decline in pneumococcal antibodies varied markedly by serotype. Differences in serotype-specific waning have previously been reported in a post-licensure observational cohort study [31], and these findings have important implications for serotype-specific susceptibility to both pneumococcal colonization and infections. Importantly, children are at greatest risk of IPD during the first year of life, particularly from pneumococcal serotypes for which antibodies may wane rapidly after vaccination [32]. The World Health Organization’s Strategic Advisory Group of Experts on Immunization recommends administration of 10-valent or 13-valent pneumococcal conjugate vaccines as either a 2-dose primary series with a booster dose (2+1 schedule) or as a 3-dose primary series without a booster (3+0 schedule), while acknowledging that 3+1 schedules are also used in some countries [33]. However, recent data from several countries indicate that a 3+0 schedule may be associated with a higher incidence of breakthrough IPD in older children and continued circulation of vaccine serotypes. In Australia, reductions in vaccine-type IPD following the sequential introductions of PCV-7 and PCV-13 in a 3+0 schedule were inferior to those seen in other high-income settings using schedules that included a booster dose [34]. Moreover, high vaccine efficacies were seen for PCV-7 and PCV-13 among Australian infants, but the odds of vaccine-type IPD increased more than 5-fold starting 12 months after completion of the primary vaccine series [35]. High levels of residual circulation of vaccine serotypes have persisted in Malawi despite >90% coverage of infants with PCV-13 administered in a 3+0 schedule [36], and we recently reported similar findings among infants and young children in Botswana [37]. In response to these observations, many countries have shifted or are considering shifting to a 2+1 schedule, despite a lack of direct comparative data on 2+1 and 3+0 schedules for pneumococcal conjugate vaccines. Although our study did not evaluate for IPD in the study population, the marked decrease in protective levels of antibodies against pneumococcal serotypes among both HEU and HUU infants suggests that a booster dose of PCV-13 in the second year of life may be needed to maintain protective pneumococcal antibody levels in older infants and young children [24, 35].
We observed only slight differences in the levels of pneumococcal antibody subclasses between HEU and HUU infants following PCV-13. Though levels of IgG1 and IgG2 antibodies to specific pneumococcal serotypes were broadly similar in HEU and HUU infants, we did observe higher levels of serotype 23F-specific IgG2 in HEU infants compared to HUU infants. Serotype 23F polysaccharide has previously been shown to induce poor antibody responses in young children [38], and this serotype is highly prevalent among children with pneumococcal carriage [39]. A previous study that measured antibody levels at 6 weeks of age among HEU and HUU infants in Malawi found lower levels of IgG2 in HEU infants, indicating that maternal HIV infection may be associated with decreased placental transfer of the IgG2 subclass or could alternatively reflect lower breastfeeding rates among mothers living with HIV [40]. It is possible that lower levels of maternally derived IgG2 at birth may result in a more robust antibody response to vaccination due to decreased maternal antibody interference [41].
Our study has several strengths and limitations. Strengths of this study include the evaluation of a large cohort with longitudinal data from birth through 12 months of age. Though the overall cohort is large, it is limited by a relatively small number of HEU infants, which potentially reduced our ability to detect differences in vaccine responses to individual pneumococcal serotypes by infant HIV exposure status. Moreover, sera was not available from mothers and we were thus unable to determine if differences in the serum antibody concentrations to pneumococcal serotypes at birth were related to lower maternal antibody concentrations or reduced placental antibody transfer. The assays that we used to measure antibody concentrations are subject to both lower and upper limits of detection, limiting our ability to fully observe true concentration levels for some infants and potentially lessening our ability to detect biological differences that exist by HIV exposure status. In addition, given the number of pairwise comparisons performed, there is a reasonably high probability of false-positive findings; however, this may have been mitigated to some extent by the fact that the comparisons were not independent. We did not assess for cross-reactivity between serotypes in the multiplex assays that were used to measure pneumococcal serotype-specific IgG subclasses. However, prior studies that have used this technology for measurement of serum pneumococcal antibodies have generally only observed cross-reactivity between structurally related serotypes such as 6A and 6B [42, 43]. Finally, we did not assess neutralization or opsonization activity, and thus were unable to determine if functional differences in the antibody responses to PCV-13 may exist by HIV exposure status. Despite these limitations, this study provides valuable insights into the immune responses of HEU infants to PCV-13.
In conclusion, we demonstrated that both HEU and HUU infants in Botswana developed robust antibody responses to a 3-dose primary series of PCV-13. Given the rapid decline in antibodies to multiple pneumococcal serotypes by 12 months of age, it may be necessary to provide a booster dose to optimize protection of children after the first year of life.
Supplementary Material
HIGHLIGHTS.
HEU and HUU infants develop protective antibodies to PCV-13 given in a 3+0 schedule
Levels of pneumococcal IgG subclass antibodies were similar in HEU and HUU infants
Pneumococcal antibodies following PCV-13 waned substantially by 12 months of age
Funding
This research was supported by the Children’s Hospital of Philadelphia, the Pincus Family Foundation, and through core services from the Penn Center for AIDS Research, a National Institutes of Health (NIH)-funded program (P30-AI045008). MSK was supported by a NIH Career Development Award (K23-AI135090) and a research grant from the Society for Pediatric Research. APS received financial support from the NIH through the Penn Center for AIDS Research (P30-AI045008).
Acknowledgements
We offer sincere gratitude to the children and families who participated in this research.
ABBREVIATIONS
- cm
centimeter
- ELISA
enzyme-linked immunosorbent
- HEU
HIV-exposed uninfected
- HIV
human immunodeficiency virus
- HUU
HIV-unexposed uninfected
- IgG
immunoglobulin G
- IQR
interquartile range
- IPD
invasive pneumococcal disease
- MFI
mean fluorescence intensity
- mL
milliliter
- PCV-7
7-valent pneumococcal conjugate vaccine
- PCV-13
13-valent pneumococcal conjugate vaccine
- WHO
World Health Organization
- μg
microgram
Footnotes
Ethics approval and consent to participate
This study was approved by the Botswana Ministry of Health, the Princess Marina Hospital ethics committee, and institutional review boards at the University of Pennsylvania, Duke University, and McMaster University. Written informed consent was obtained from all participants or their legal guardians.
Competing Interests
The authors have no competing interests to declare.
REFERENCES
- [1].WHO, Eliminating mother-to-child HIV transmission. 2018, World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mofenson LM, Editorial commentary: New challenges in the elimination of pediatric HIV infection: the expanding population of HIV-exposed but uninfected children. Clin Infect Dis, 2015. 60(9): p. 1357–60. [DOI] [PubMed] [Google Scholar]
- [3].Farquhar C, et al. , High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr, 2005. 40(4): p. 494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jones CE, et al. , Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. Jama, 2011. 305(6): p. 576–84. [DOI] [PubMed] [Google Scholar]
- [5].Reikie BA, et al. , Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. Clin Vaccine Immunol, 2013. 20(1): p. 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Simani OE, et al. , Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. Aids, 2014. 28(4): p. 531–41. [DOI] [PubMed] [Google Scholar]
- [7].Madhi SA, et al. , Immunogenicity following the first and second doses of 7-valent pneumococcal conjugate vaccine in HIV-infected and -uninfected infants. Vaccine, 2013. 31(5): p. 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Madhi SA, et al. , Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. Journal of Infectious Diseases, 2010. 202(3): p. 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abramczuk BM, et al. , Impaired humoral response to vaccines among HIV-exposed uninfected infants. Clin Vaccine Immunol, 2011. 18(9): p. 1406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hesseling AC, et al. , Delayed BCG immunization does not alter antibody responses to EPI vaccines in HIV-exposed and -unexposed South African infants. Vaccine, 2016. 34(32): p. 3702–9. [DOI] [PubMed] [Google Scholar]
- [11].Wahl B, et al. , Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. The Lancet Global Health, 2018. 6(7): p. e744–e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bogaert D, de Groot R, and Hermans P, Streptococcus pneumoniae colonisation: the key to pneumococcal disease. The Lancet infectious diseases, 2004. 4(3): p. 144–154. [DOI] [PubMed] [Google Scholar]
- [13].Kaplan SL, et al. , Decrease of invasive pneumococcal infections in children among 8 children’s hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics, 2004. 113(3): p. 443–449. [DOI] [PubMed] [Google Scholar]
- [14].Hammitt LL, et al. , Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: a longitudinal surveillance study. The Lancet, 2019. 393(10186): p. 2146–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].von Mollendorf C, et al. , Increased Risk for and Mortality From Invasive Pneumococcal Disease in HIV-Exposed but Uninfected Infants Aged <1 Year in South Africa, 2009–2013. Clinical Infectious Diseases, 2015. 60(9): p. 1346–1356. [DOI] [PubMed] [Google Scholar]
- [16].O'Brien KL, et al. , Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet, 2009. 374(9693): p. 893–902. [DOI] [PubMed] [Google Scholar]
- [17].Congdon M, et al. , Effect of Haemophilus influenzae type b and 13-valent pneumococcal conjugate vaccines on childhood pneumonia hospitalizations and deaths in Botswana. Clin Infect Dis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].UN Inter-agency Group for Child Mortality Estimation. Levels & trends in child mortality, report 2020. Available at: https://childmortality.org/wp-content/uploads/2020/09/UNICEF-2020-Child-Mortality-Report.pdf. Accessed Nov. 3, 2020. . [Google Scholar]
- [19].Statistics Botswana. 2011. Population and Housing Census Analytical Report. Available at: http://www.cso.gov.bw/images/analytical_report.pdf. Accessed 24 July 2016. [Google Scholar]
- [20].Joint United Nations Programme on HIV/AIDS. UNAIDS estimates 2019: Botswana. Available at: http://www.unaids.org/en/regionscountries/countries/botswana. Accessed February 3, 2021. . [Google Scholar]
- [21].Kelly MS, et al. , Non-diphtheriae Corynebacterium species are associated with decreased risk of pneumococcal colonization during infancy. The ISME journal, 2021: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Organization WH, Guideline: updates on the management of severe acute malnutrition in infants and children. 2013: World Health Organization. [PubMed] [Google Scholar]
- [23].Gueye SB, et al. , Performance of Roche CAP/CTM HIV-1 qualitative test version 2.0 using dried blood spots for early infant diagnosis. Journal of virological methods, 2016. 229: p. 12–15. [DOI] [PubMed] [Google Scholar]
- [24].Wernette CM, et al. , Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clinical and diagnostic laboratory immunology, 2003. 10(4): p. 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goldblatt D, et al. , Establishment of a new human pneumococcal standard reference serum, 007sp. Clinical and vaccine immunology : CVI, 2011. 18(10): p. 1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tomaras GD, et al. , Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. Journal of virology, 2008. 82(24): p. 12449–12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schlottmann SA, et al. , A novel chemistry for conjugating pneumococcal polysaccharides to Luminex microspheres. J Immunol Methods, 2006. 309(1-2): p. 75–85. [DOI] [PubMed] [Google Scholar]
- [28].Organization WH, Recommendations for the production and control of pneumococcal conjugate vaccines. WHO Tech Rep Ser, 2005. 927(2): p. 64–98. [Google Scholar]
- [29].Fouda GG, et al. , The Impact of IgG transplacental transfer on early life immunity. Immunohorizons, 2018. 2(1): p. 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patel SM, et al. , Placental transfer of respiratory syncytial virus antibody among HIV-exposed, uninfected infants. J Pediatric Infect Dis Soc, 2020. 9(3): p. 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Andrews NJ, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis, 2014;14(9):839–46. [DOI] [PubMed] [Google Scholar]
- [32].van Westen E, et al. Serotype-specific IgG antibody waning after pneumococcal conjugate primary series vaccinations with either the 10-valent or the 13-valent vaccine. Vaccines, 2018;6(4):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].World Health Organization. Summary of WHO Position Paper on Pneumococcal conjugate vaccines in infants and children under 5 years of age, February 2019. Available at: https://www.who.int/immunization/policy/position_papers/who_pp_pcv_2019_summary.pdf. Accessed October 7, 2021. [Google Scholar]
- [34].Jayasinghe S, et al. , Long-term Impact of a “3 + 0” Schedule for 7- and 13-Valent Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Australia, 2002–2014. Clinical Infectious Diseases, 2016. 64(2): p. 175–183. [DOI] [PubMed] [Google Scholar]
- [35].Jayasinghe S, et al. , Effectiveness of 7- and 13-Valent Pneumococcal Conjugate Vaccines in a Schedule Without a Booster Dose: A 10-Year Observational Study. Clin Infect Dis, 2018. 67(3): p. 367–374. [DOI] [PubMed] [Google Scholar]
- [36].Swarthout TD, et al. , High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat Commun, 2020. 11(1): p. 2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Patel SM, et al. , Evolution of pneumococcal serotype epidemiology in Botswana following introduction of 13-valent pneumococcal conjugate vaccine. PloS one, 2022. 17(1): p. e0262225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Douglas RM, et al. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis, 1983. 148(1):131–7. [DOI] [PubMed] [Google Scholar]
- [39].Austrian R Some aspects of the pneumococcal carrier state. J Antimicrob Chemother, 1986. 18(Supplement A):35–45. [DOI] [PubMed] [Google Scholar]
- [40].Baroncelli S, et al. Immunoglobulin G passive transfer from mothers to infants: total IgG, IgG subclasses and specific antipneumococcal IgG in 6-week Malawian infants exposed or unexposed to HIV. BMC Infect Dis, 2022. 22(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Niewiesk S Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol, 2014. 16;5:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Elberse KE, et al. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol, 2010. 17(4):674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pavliakova D, et al. Development and validation of 13-plex Luminex-based assay for measuring human serum antibodies to Streptococcus pneumoniae capsular polysaccharides. mSphere, 2018. 3(4):e00128–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.