ABSTRACT
Despite improved survival outcomes across many cancer types, the prognosis remains grim for certain solid organ cancers including glioblastoma and pancreatic cancer. Invariably in these cancers, the control achieved by time-limited interventions such as traditional surgical resection, radiation therapy, and chemotherapy is short-lived. A new form of anti-cancer therapy called therapeutic alternating electric fields (AEFs) or tumor treating fields (TTFields) has been shown, either by itself or in combination with chemotherapy, to have anti-cancer effects that translate to improved survival outcomes in patients. Although the pre-clinical and clinical data are promising, the mechanisms of TTFields are not fully elucidated. Many investigations are underway to better understand how and why TTFields is able to selectively kill cancer cells and impede their proliferation. The purpose of this review is to summarize and discuss the reported mechanisms of action of TTFields from pre-clinical studies (both in vitro and in vivo). An improved understanding of how TTFields works will guide strategies focused on the timing and combination of TTFields with other therapies, to further improve survival outcomes in patients with solid organ cancers.
Keywords: alternating electric fields (AEFs), cancer, mechanism of action, pre-clinical, tumor treating fields (TTFields)
Introduction
Glioblastoma (GBM) is the most common and aggressive type of primary brain cancer in adults. It has a high rate of recurrence, resulting in a poor prognosis. The median overall survival of GBM ranges from 12 to 16 months with a 5-year survival rate of 5.6% (Ostrom et al., 2019). The standard treatment for newly-diagnosed GBM (nGBM) involves surgery followed by chemoradiation, then adjuvant (i.e. maintenance) chemotherapy (Stupp et al., 2005; Gallego, 2015). The chemotherapy agent used to treat nGBM is temozolomide, an alkylating agent that works by sensitizing cells to radiation (Stupp et al., 2005; Dall'Oglio et al., 2008). Temozolomide was approved by the Food and Drug Administration (FDA) for the treatment of nGBM in March 2005, after Stupp et al. (2005) reported a 2.5-month median overall survival prolongation when temozolomide was added to radiation therapy (RT) and used as adjuvant chemotherapy, compared to the previous standard of care (RT alone).
The effects of an electric field on living cells were initially described in 1922 by Northrop (1922), who studied the migration of Bacillus typhosus in a field with an intensity of 4.5 V/cm. The work of Kirson et al. (2004) showed that therapeutic alternating electric fields (AEFs), or tumor treating fields (TTFields), interfered with cancer cell proliferation in vitro (100–300 kHz, 1–4 V/cm peak-to-peak) and in vivo (100–200 kHz, <2 V/cm peak-to-peak), by using mice implanted intradermally with syngeneic cancer cell lines (murine malignant melanoma B16F1 or murine colon carcinoma CT-26). They also examined various mechanisms of action (MOAs) through which TTFields impairs cancer cell growth (Kirson et al., 2004). They later showed that 200 kHz TTFields also arrested glioma growth in an orthotopic rat model and in patients with recurrent GBM (rGBM) (Kirson et al., 2007). Based on this seminal work, a novel therapy, now commonly referred to as TTFields, was approved by the FDA in April 2011 for use in rGBM (Stupp et al., 2012). In October 2015, the FDA approved 200 kHz TTFields for nGBM after a randomized phase III clinical trial by Stupp et al. (2015, 2017) showing that TTFields in combination with adjuvant chemotherapy prolonged median overall survival by 4.9 months compared to the use of adjuvant chemotherapy alone. The efficacy of TTFields recently led to the expansion of 150 kHz TTFields approval by the FDA for unresectable malignant pleural mesothelioma based on the data from a multicentered single-arm phase II trial in which TTFields was combined with pemetrexed and platinum-based chemotherapy (Ceresoli et al., 2019). In addition to improving overall survival compared to historical controls, TTFields resulted in a reduction in tumor size and slowed disease progression (Salzberg et al., 2008; Stupp et al., 2012; Pless et al., 2013; Vymazal and Wong, 2014; Wong et al., 2015b; Lu et al., 2019; Kim et al., 2020a). Furthermore, patients with GBM treated with TTFields and chemotherapy reported improvements in their quality of life, emotional well-being, physical function, and cognitive ability compared to patients on chemotherapy only (Onken et al., 2019). Due to the effectiveness of TTFields, clinical trials of TTFields in combination with standard chemotherapies are currently underway for many other cancer types, e.g. hepatocellular carcinoma with sorafenib (ClinicalTrials: NCT03606590, https://www.clinicaltrials.gov/ct2/show/NCT03606590; Gkika et al., 2022), non-small cell lung cancer (NSCLC) with immune checkpoint inhibitors or docetaxel (ClinicalTrials: NCT02973789, https://www.clinicaltrials.gov/ct2/show/NCT02973789). The scope of this review focuses on the published mechanisms of TTFields, as derived from the cell-based and pre-clinical in vivo literature, to unravel the underlying mechanisms of TTFields and its anti-cancer activities.
Since the initial work, there have been many improvements in the methods of delivering TTFields to the body and understanding its MOAs that impede cancer cell growth (Kinzel et al., 2019). TTFields is a non-invasive technique in which AEFs are delivered locally to the tumor through transducer electrode arrays placed on the overlying skin. The electric field's magnitude is proportional to the voltage differences between the two electrodes, inversely proportional to the distance between the two electrodes, and reported in units of V/cm (Wang et al., 2019). Cells contain both positively and negatively charged proteins that have local dipole moments (e.g. tubulin). When such molecules are placed in electric fields, they rotate and align to the oppositely charged electrode in a process called dipole alignment (Wang et al., 2019). Additionally, AEFs allow molecules to move towards the higher field intensity under the principle of dielectrophoresis (Wang et al., 2019). These two principles play important roles in how TTFields interferes with cellular processes (Wang et al., 2019). TTFields has been shown to disrupt mitotic spindle assembly via interference with the large dipole moment of microtubules, resulting in metaphase arrest, prolonged mitosis, dysregulated daughter cell production, and eventual cell death (Gera et al., 2015; Giladi et al., 2016). Additionally, the concentrated (i.e. non-uniform) electric fields at the cleavage furrow in rapidly dividing cells result in cell fragmentation and tumor cell apoptosis (Gera et al., 2015; Giladi et al., 2016).
Many studies to date have focused on the effect of TTFields on disrupting cancer cell division. More recently, investigations have demonstrated that the effects of TTFields are not confined only to the hindrance of mitotic cell division in rapidly growing tumor cells. For instance, the work of Chang et al. (2018) showed that TTFields permeabilizes the GBM cell membrane. Additionally, recent work from Kessler et al. (2018) and Keßler et al. (2019) demonstrated that TTFields increases the permeability of the blood–brain barrier (BBB) through reduced expression of tight junction proteins between neurovascular endothelial cells. Taken together, these two recent findings may help to explain the anti-GBM efficacy of TTFields in combination with chemotherapy, whereby it allows such drugs to transit more easily through the BBB and into cancer cells. There are likely more unexplained mechanisms by which TTFields prevents tumor growth and invasion. The purpose of this review is to summarize the known MOAs of TTFields that have been reported by in vitro and in vivo studies of cancer.
Anti-cancer MOAs of TTFields
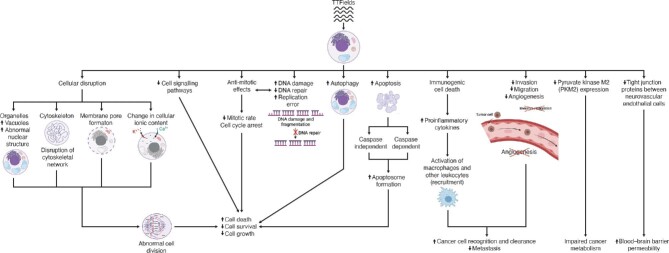
Most data on the MOAs of TTFields were obtained through experimentation in different cancer cell types and in vivo in animal models. Figure 1 summarizes the currently known anti-cancer mechanisms of TTFields. Various publications demonstrate that the effect of TTFields on tumor cell growth depends on the frequency, intensity, duration, and field direction for various cell types, with the optimal therapeutic frequency window of 100–300 kHz (Kirson et al., 2007, 2009b; Giladi et al., 2014a; Voloshin et al., 2016; Porat et al., 2017; Silginer et al., 2017; Jo et al., 2018a, 2019, 2020; Berkelmann et al., 2019; Lee et al., 2019; Gencturk et al., 2020; Bobkova et al., 2021; Jeong et al., 2021; Linder et al., 2021; Mumblat et al., 2021; Pfeifer et al., 2021; Sabri and Brosseau, 2021; Wu et al., 2021; Branter et al., 2022). Higher frequency and durations of exposure >72 h did not more significantly reduce cell proliferation and did not more significantly improve treatment outcomes (Kirson et al., 2007, 2009b; Giladi et al., 2014a; Voloshin et al., 2016; Porat et al., 2017; Silginer et al., 2017; Jo et al., 2018a, 2019; Berkelmann et al., 2019; Lee et al., 2019; Gencturk et al., 2020). The optimal TTFields frequencies for various cancer cell lines are summarized in Supplementary Table S1. It is important to note that the frequency of TTFields is not likely high enough to cause heat generation and ionize the cells; therefore, heat production is less likely one of the potential mechanisms through which TTFields disrupts tumor cell growth (Castellví et al., 2015). The spatial relation of TTFields transducer arrays to one another was found to be an important factor in impeding cancer cell migration; the placement of arrays perpendicular to the leading edge of cell movement was found to be most effective in reducing cancer cell migration velocity (Voloshin et al., 2020b).
Figure 1.
An overview of the MOAs of TTFields, a form of AEFs therapy in cancer.
Additionally, the effects of TTFields combined with other therapies (e.g. drugs and radiation), are summarized for both cell culture studies (Supplementary Table S2) and animal studies of cancer (Supplementary Table S3) (Kirson et al., 2009b; Schneiderman et al., 2010; Giladi et al., 2014a; Castellví et al., 2015; Voloshin et al., 2016; Chang et al., 2017; Clark et al., 2017; Silginer et al., 2017; Kessler et al., 2018; Lei et al., 2018; Lee et al., 2019, 2021; Jo et al., 2020, 2022; Karanam et al., 2020; Vargas-Toscano et al., 2020; Yoon et al., 2020; Voloshin et al., 2020a; Bai et al., 2021; Kim et al., 2021; Linder et al., 2021; Mumblat et al., 2021; Shi et al., 2022). Kirson et al. (2009b) showed that chemotherapeutic agents had a synergistic effect with TTFields in reducing cancer cell growth. Thus, a lower dose of chemotherapeutic agents and radiation might be able to be used when adding TTFields to these traditional cancer treatments (Kirson et al., 2009b). Of note, the synergistic effect of TTFields and chemotherapy lingered following treatment cessation, in that the number of cells continued to decline compared to control (Kirson et al., 2009b). Additionally, the study of GBM cells (MZ-54 and U251) in vitro (250 and 200 kHz, respectively, for 24–72 h of exposure) by Linder et al. (2021) showed that concomitant dexamethasone administration during TTFields exposure does not alter TTFields efficacy both in vitro and in patients with GBM, while it limits the therapeutic effect of RT. Therefore, along with TTFields, dexamethasone could continue to be used as the standard treatment for edema to improve neurological deficits (Linder et al., 2021). Certainly, the untoward effects of dexamethasone's interference with the immune system in GBM is a separate, but related, issue that warrants further investigation in the setting of TTFields usage (Wong et al., 2015a).
Changes in cellular and subcellular morphology
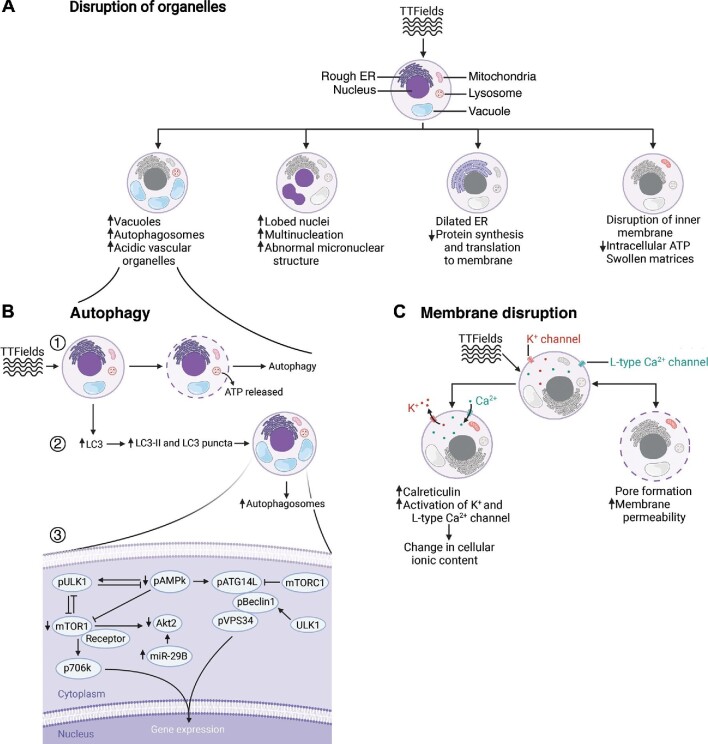
One of the ways that TTFields interferes with tumor cell growth is by altering the structure and function of cellular organelles. As shown in Figure 2, TTFields affects many subcellular structures in a variety of ways. For instance, TTFields increases autophagosome and vacuole formation, which is represented as an increase in granularity on microscopy at various orders of magnification (Silginer et al., 2017; Chang et al., 2018; Shteingauz et al., 2018; Kim et al., 2019). Shteingauz et al. (2018) exposed human GBM U373 and U87 cell lines to TTFields (200 kHz for 24–72 h) and quantified LC3 protein as a marker for autophagosome formation using western blotting. They detected a 4-fold increase in LC3 compared to the unexposed cells (Shteingauz et al., 2018). To further verify the results, treating cells with 20 μM chloroquine (CQ), a known lysosomal degradation drug, for 4 h after TTFields exposure resulted in a 2.5-fold increase in LC3 concentration (Shteingauz et al., 2018). Transmission electron microscopy further showed that the number of autophagic vesicles increased in samples exposed with TTFields and CQ in comparison with control samples that were treated only with CQ (Shteingauz et al., 2018). The addition of an autophagic inhibiting agent, such as 2 mM of 3-methyladenine, showed a decrease in the number of visible vesicles in U373 and U87 cells by immunofluorescence microscopy (Kim et al., 2019). Additionally, quantification of acidic vesicular organelles (AVOs) using flow cytometry showed a time-dependent increase in AVOs in both U373 (+16%) and U87 (+23%) cells that were exposed to 150 kHz TTFields for 48 h (Kim et al., 2019). Similar experimentation in Lewis lung carcinoma (LLC-1) cells yielded comparable results (Voloshin et al., 2020a). Following 48 h of 150 kHz TTFields exposure, LLC-1 cells were analyzed using scanning transmission electron microscopy. Voloshin et al. (2020a) found that the number of autophagosomes and autolysosomes was increased. Similar results were obtained when studying TTFields in an animal model (Kim et al., 2019; Lee et al., 2019). Western blotting analysis of human U87 GBM cells xenografted into mouse models followed by 7 days of TTFields caused an increase in LC3 expression compared to unexposed controls (Kim et al., 2019). Similarly, human colorectal cancer (HCT116) cells xenografted in nude mice that were exposed to TTFields, either with or without 5-fluorouracil (5-FU), for 7 days resulted in a significant increase in LC3 in comparison to mice exposed to 5-FU alone or control, based on immunohistochemistry (Lee et al., 2019). This further supports a role for TTFields in increasing phagolysosome formation and resultant cancer cell death.
Figure 2.
TTFields disrupts subcellular organelles, promotes autophagy, and perturbs cell membranes of cancer cells. (A) TTFields disrupts the structure of vacuoles, nuclei, ER, and inner membrane of cancer cells. (B) Increased autophagosome formation through upregulation of the depicted genes. (C) The disrupted cancer cell membrane in the presence of TTFields leads to changes in cellular ionic content and increased membrane permeability to molecules at least 20 kDa in size. These TTFields-induced alterations impair normal function, responses to external stimuli, and the induction of stress response in cancer cells. These effects, when combined with the increased rate of autophagosome formation, cause more cancer cell entry into cell death pathways.
As previously discussed and as shown in Figure 2B, upregulation of autophagy was detected by an increase in leakage of adenosine triphosphate (ATP) to the extracellular matrix; autophagosome formation was monitored vis-à-vis increases in LC3II and LC3 puncta formation in TTFields-exposed cancer cells, which resulted in increased cell death (Giladi et al., 2016; Kim et al., 2016, 2019; Silginer et al., 2017; Shteingauz et al., 2018; Voloshin et al., 2020a; Bai et al., 2021). Autophagy is considered a survival or cytoprotective response during stressful conditions such as radiation and chemotherapy. However, in other settings, such as TTFields exposure, autophagy is cytotoxic and serves as a cellular death mechanism (Sharma et al., 2014). Shteingauz et al. (2018) determined that autophagy activation in TTFields exposed cells is dependent on the adenosine monophosphate-activated protein kinase (AMPK) pathway. Depletion of AMPK or autophagy-related protein 7 (ATG7) in GBM cell lines via cell transfection with shRNA inhibited the upregulation of autophagy in TTFields-exposed cells compared to control (Shteingauz et al., 2018). Kim et al. (2019) additionally detected the upregulation of autophagy-related genes in human GBM cells following 48 h of 150 kHz TTFields exposure. They utilized various techniques, such as immunoblotting assay and fluorescence assisted cell sorting (FACS), to detect alterations in the specific proteins involved in the AMPK pathway responsible for the upregulation of TTFields-mediated autophagy (Kim et al., 2019). As shown in Figure 2B, upon TTFields exposure to human GBM cells, Beclin1 and Atg5 are upregulated in a time-dependent manner. Meanwhile, mTOR/p70D6K and Akt2, which are the critical negative modulators of autophagy, are downregulated. The downregulation of Akt2 further stimulates LC3 puncta accumulation and induces autophagy. Additionally, in human GBM cells transfected with miR-29b-3p, TTFields upregulated miR-29 that binds to its complementary sequence motifs, 3′-UTR of Akt2, and further downregulated Akt2 expression (Kim et al., 2019). The genes that upregulate autophagosome formation were overexpressed, while the genes that suppress autophagy were downregulated, upon TTFields exposure. Considering autophagosome formation as a cellular death mechanism, this could be considered as one of the mechanisms through which TTFields induces cancer cell death.
The electric field disturbance triggered by TTFields leads to alteration in nuclear structure and abnormality in chromosomal number and distribution (Gera et al., 2015; Giladi et al., 2016; Silginer et al., 2017; Kessler et al., 2018; Jo et al., 2018b). As shown in Figure 2A, TTFields induces cellular stress, which results in nuclear dysmorphologies (Gera et al., 2015; Giladi et al., 2016; Silginer et al., 2017; Kessler et al., 2018; Jo et al., 2018b). TTFields exposure increased the number of GBM and mesothelioma cells with multiple nuclei and micronuclear structures by 6% and 16%, respectively (Giladi et al., 2016). Additionally, studying human breast adenocarcinoma (MCF-7) cells via fluorescence microscopy following 8 h of 150 kHz TTFields exposure showed a 10% increase in the number of enlarged cells (Gera et al., 2015). The enlargement was a consequence of cellular stress induced by TTFields that results in an increase in the number of nuclei present in each cell (Gera et al., 2015). The aberrant effect of TTFields on normal nuclear formation in cancer cells can be intensified by combining TTFields with chemotherapies. Analyzing human GBM with fluorescence microscopy following exposure with TTFields and an inhibitor of monopolar spindle 1 (Mps1-IN-3) resulted in a significant increase in the number of cells with aberrant nuclei and abnormal shape and morphologies (73% compared to 9% in the control group). Each treatment alone also increased the number of cells with abnormal nuclei (38% for TTFields exposure and 65% for IN-3 exposure), but the increase was more significant under combination treatment (Kessler et al., 2018). Time-lapse fluorescence microscopy of human cervical adenocarcinoma (HeLa), breast adenocarcinoma (MDA-MB-231 and MCF-7), and colorectal cancer (HCT116) cells demonstrated a 25% increase on average in the number of cells with a higher number of micronuclear structures (Gera et al., 2015). This indicates that TTFields can induce stress in cancer cells, which results in an increase in genotoxic events, chromosomal instability, and cell death.
Further morphological changes in the endoplasmic reticulum (ER) were observed in cancer cells exposed to TTFields. The ER is required for protein production and ensuring the function and stability of cells. Thus, ER dilation as a result of TTFields exposure disrupts protein synthesis and their translocation to the cell membrane, which could further contribute to cancer cell death (Silginer et al., 2017; Voloshin et al., 2020a). ER dilation was observed under electron microscopy (EM) in human long-term glioma (LN-18) and gastrointestinal cancer (ZH-161) cells (Silginer et al., 2017). Additionally, immunoblotting analysis of LLC-1 and CT-26 cells exposed to TTFields for 72 h showed a respective 3-fold and 6-fold increase in phosphorylation of elF2a over time (Voloshin et al., 2020a). Such observations further demonstrate that TTFields induces stress in the ER that could disrupt various cellular functions and interactions with the intracellular environment. Induction of ER stress could also be the underlying reason for the increase in localization of calreticulin (CRT) in the cell membrane following TTFields exposure (Voloshin et al., 2020a).
Mitochondria are another important organelle that could be affected by TTFields. Analyzing LN-18 and ZH-161 cells with EM showed that the mitochondria were swollen and enlarged following 100 kHz TTFields exposure for 48 h (Silginer et al., 2017). Analysis of the mitochondria with the tetramethylrhodamine ethyl ester perchlorate (TMRE) mitochondria assay kit and measurement of the mitochondrial inner membrane potential (DYm) in human glioblastoma cells (T98G and U251) showed low TMRE staining and dissipation of DYm after TTFields exposure (Neuhaus et al., 2019); this effect could be augmented with benidipine in T98G cells by 2-fold, while no such synergistic effect was observed in U251 cells. Additionally, assessing LLC-1, CT-26, spontaneously transformed murine ovarian surface epithelial (MOSE-L), human hepatocellular carcinoma (HEPG2), and human lung squamous cell carcinoma (H520) cells with flow cytometry and enzymatic assay demonstrated that the quinacrine signal, an indicator of ATP levels, decreased over time, while the signal increased in extracellular compartments (Voloshin et al., 2020a). Thus, 24–72 h of TTFields exposure, at the indicated frequencies for LLC-1 [150 kHz], CT-26 [200 kHz], MOSE-L [200 kHz], and H520 and HEPG2 [150 kHz], was found to disrupt the mitochondrial inner membrane, which allows ATP to leak out of the cell into the extracellular environment. Cancer cells are rapidly growing and dividing cells, which makes them more vulnerable to ATP depletion and disruptions in protein production. As a result, these morphological changes observed upon TTFields exposure are related to apoptosis and autophagy in cancer cells in a cell type-dependent manner.
Another mechanism by which TTFields interferes with cellular function is disruption of cellular membrane permeability. Chang et al. (2018) evaluated ethidium D and FITC-dextran uptake in 200 kHz TTFields-exposed human GBM cells, which resulted in a 1.5-fold and 3-fold increase compared to control, respectively, an indication of pore formation in the cellular membrane. Utilizing various sized FITC-dextran probes, they demonstrated that the fenestrae in the membrane are between 20 kDa and 50 kDa in size due to TTFields-exposed cells permitting a significant increase in uptake of the 20 kDa, but not 50 kDa, probe compared to unexposed controls (Chang et al., 2018). The visualization of these pores was confirmed with EM. Additionally, they showed that at 24 h after TTFields cessation, the pores were reversibly closed such that there was no longer any increase in ingress of the 4 kDa and 20 kDa probes (Chang et al., 2018). They also observed that following TTFields exposure, uptake of 5-aminolevulinic acid (an agent used to demarcate the GBM-brain border intraoperatively) increased over time in human GBM cells. This effect was not observed in human non-cancer fibroblast cells, suggesting a cancer-specific effect of TTFields (Chang et al., 2018). Yoon et al. (2020) made similar observations when they introduced barium titanate nanoparticles (BTNPs) of 100–200 nm size in combination with 72 h of 150 kHz TTFields exposure. They found that the combination increased the cell permeability and resulted in the accumulation of 100 nm and 200 nm BTNPs inside MCF7 and BT-549 cancer cells (Yoon et al., 2020). As shown in Figure 2C, TTFields has also been shown to upregulate the membrane ion channels and alter cytoplasmic ionic content (Neuhaus et al., 2019). This effect is different from the cell membrane permeabilization effect identified by Chang et al. (2018). The fenestrae studied by Chang et al. (2018) were greater than 51.8 nm2 in size, which is significantly larger than the ion channels in the cellular membrane (∼1 nm) (Berg et al., 2002). Neuhaus et al. (2019) utilized voltage-clamp measurements and showed that potassium channels were selectively activated by 200 kHz TTFields (various durations of exposure) in GBM cells. Additionally, increasing extracellular calcium resulted in an increase in free calcium in the cells, which suggested that calcium channels were also being activated by TTFields (Neuhaus et al., 2019). Using different calcium channel blockers, they could specifically pinpoint that nifedipine-sensitive L-type Cav channels were responsible for the entry of calcium into the cells upon TTFields exposure (Neuhaus et al., 2019). The cellular membrane is an important structure that helps with cellular maintenance and regulation, and any perturbation of it could result in cellular electrolyte imbalances. These findings, coupled with the alterations in subcellular structures, lead to disruption of normal cellular function and responses to external stimuli and induction of stress responses in cancer cells exposed to TTFields. Maladaptation in combination with upregulation of autophagosome formation leads to increased cancer cell death.
Alteration in cell division and cell growth
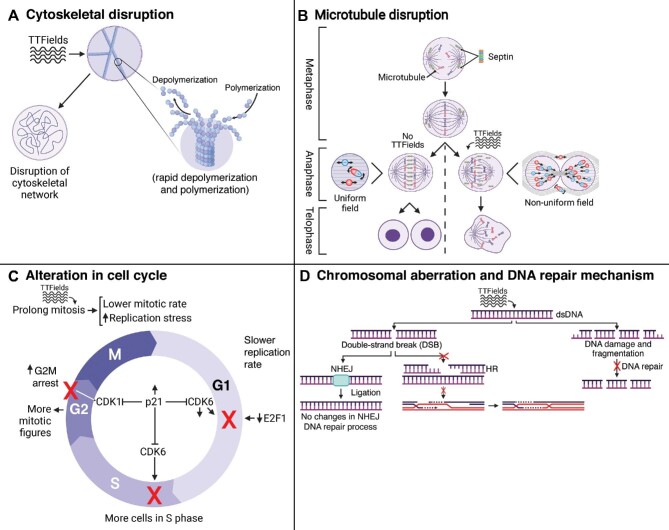
TTFields induces dielectrophoretic forces that interfere with normal microtubule, cilia, and mitotic spindle formation and severely impair mitotic progression and cell growth, predisposing factors for increased cell death (Wang et al., 2019; Shi et al., 2022). Interference with normal microtubule formation and cytoskeletal network that is provoked by electrophoresis was one of the earliest hypothesized mechanisms by which TTFields interferes with cell division and cell growth (Kirson et al., 2004). As shown in Figure 3A, these changes include altered dynamics by reducing polymerization and rapid polymerizations and depolymerizations (Giladi et al., 2014a, 2016; Gera et al., 2015; Voloshin et al., 2016; Chang et al., 2017; Kessler et al., 2018; Riley et al., 2019). Visualizing lung adenocarcinoma (A549) and breast adenocarcinoma (MDA-MB-231) cells by confocal fluorescence microscopy following 24 h of 150 kHz TTFields exposure demonstrated a halted spindle assembly and disrupted normal microtubule networks (Giladi et al., 2016). Western blotting analysis of cells following 24 h of TTFields exposure showed a 12.7% decrease in levels of polymerized microtubules compared to the unexposed cells as well as cells treated with only paclitaxel or vinorelbine. Western blotting analysis also showed an increase in tubulin depolymerization in TTFields-exposed cells (Giladi et al., 2016). Performing experiments under conditions of rapid vs. slower microtubule turnover rates, it was found that TTFields reduced the ratio of polymerized tubulin to total tubulin (Giladi et al., 2016). Visualizing cells under light microscopy further showed that the cytoskeletal network within TTFields-exposed cells was disrupted, and it was more evident when TTFields was combined with chemotherapies such as paclitaxel and withaferin A that are known to disrupt microtubule assembly (Giladi et al., 2014b; Chang et al., 2017). Not only does TTFields alter the connective network of microtubules, but it also disrupts mitotic spindle assembly, which results in unsuccessful chromosomal division, deranged chromosomal segregation, cytokinetic furrow function disruption, cell division impairment, and aberrant mitotic exit and cell death (Giladi et al., 2014a; Gera et al., 2015; Voloshin et al., 2016; Silginer et al., 2017; Kessler et al., 2018; Riley et al., 2019). Furthermore, TTFields has been shown to dampen DNA replication through downregulation of mini-chromosome maintenance (MCM) family and suppressed formation of filamentous structures including tunneling nanotubes (TNTs) (Gera et al., 2015; Giladi et al., 2016; Karanam et al., 2020; Farmani et al., 2022). TNTs play a critical role in mediating the transfer of cellular contents from networked cancer cells, and TTFields-induced disruption of these structures may serve as another anti-cancer mechanism (Lou et al., 2012).
Figure 3.
TTFields disrupts the cell cycle, growth, and division and induces stress in cancer cells. (A–C) TTFields exposure disrupts cytoskeletal network (A), impairs mitotic cell division (B), and further slows cell cycle progression with alterations in checkpoint regulators (C). Thus, more mitotic figures and cells in S phase are visible under TTFields exposure. (D) Additionally, TTFields causes DSBs and disrupts DNA repair mechanism (HR), resulting in DNA fragmentation and DNA damage. NHEJ is another DNA repair mechanism that is not affected by TTFields.
In addition to the effects of TTFields on microtubule assembly and turnover rates, perturbing septin localization and septin–microtubule complex formation contributes to abnormal chromosomal separation and asymmetrical cellular division in mitosis (Gera et al., 2015; Giladi et al., 2016; Riley et al., 2019). As shown in Figure 3B, TTFields has been shown to prevent accumulation and localization of septin-7 protein at the anaphase spindle midline and proper interaction with microtubule and complex formation (Gera et al., 2015). Upon TTFields exposure, the septin filaments align with the direction of the TTFields and would not be able to properly interact with the microtubules. Septin is a filamentous guanosine-5′-triphosphate-binding protein that plays an important role in membrane trafficking, chromosomal separation, and cell division (Nakos et al., 2019). Thus, as shown in Figure 3B, any disruption in the interaction between septin and microtubules results in disruption of the cleavage furrow, abnormal cell division, increase in aneuploid or polyploid nuclei, and increased hyper-G subpopulation, with a resultant increase in cancer cell death (Gera et al., 2015; Neuhaus et al., 2019).
Alterations in the cell cycle due to TTFields result in abnormal chromosomal segregation, micronucleation, alterations in the duration of various mitotic phases, and decrease in cellular proliferation (Kirson et al., 2007; Gera et al., 2015; Giladi et al., 2016; Kim et al., 2016, 2019; Voloshin et al., 2016; Chang et al., 2017; Porat et al., 2017; Kessler et al., 2018; Lei et al., 2018; Jo et al., 2018b; Yoon et al., 2020; Branter et al., 2022). Prolonged mitotic phase and decreased mitotic rate were also evident in animal models of cancer under TTFields exposure (Giladi et al., 2014a). This effect is more evident in rapidly dividing cancer cells, as more cells are in the mitotic phase at a given time point (Giladi et al., 2016). TTFields application additionally could alter the duration of other phases of the cell cycle (Figure 3C). There is a collective agreement across different studies that the number of cancer cells in the S and M phases increases following TTFields exposure (Giladi et al., 2014a, 2016; Neuhaus et al., 2019), while the cancer cell population in the G1 and G2 phases decreases or is not significantly altered by the presence of TTFields (Giladi et al., 2014a; Neuhaus et al., 2019). The cell population in each phase might change depending on the duration of TTFields exposure. Silginer et al. (2017) analyzed human long-term glioma cells (LTCs) LN-18 and LN-229 by flow cytometry after 24 and 48 h of 100 kHz TTFields exposure. They found that the number of cells in the G2/M phase increased after 24 h, while there was a significant increase in the sub-G1 phase after 48 h of exposure (Silginer et al., 2017). A similar phenomenon was found by Lee et al. (2021); when they exposed rat GBM (F98) cells to 150 kHz TTFields for 48 h in combination with proton beam radiation, they found an increase in the sub-G1 cell population. Although the M phase of the cell cycle was prolonged, using time-lapse fluorescence microscopy, Gera et al. (2015) found that in human cervical adenocarcinoma (HeLa) cells, the intervals between chromatid condensation and metaphase plate formation were similar in the control and TTFields-exposed cells (150 kHz for 8 h). TTFields-exposed cells showed disruption and blebbing of the membrane during the same time as control cells undergoing metaphase exit (Gera et al., 2015). The alteration in the timing of various phases might be due to alterations in the regulation of cell cycle checkpoints. Utilizing immunoblotting and flow cytometry, an increase in p21 expression was detected (Yoon et al., 2020). The p21 protein downregulates various cyclin-dependent kinases (CDKs) including CDK1 and CDK6, which play an important role in various cell cycle checkpoints including G1/S, S, and G2/M phases. In TTFields-exposed cells, the increase in cyclin B and p-CDC2 prevented G2/M progression and resulted in G2/M arrest and an increase in the number of visible mitotic figures (Jo et al., 2018b, 2019; Kim et al., 2019; Mumblat et al., 2021). Additionally, similar to paclitaxel-treated human breast adenocarcinoma (MDA-MB-231), the number of cyclin B and pH3 double-positive cells increased, which further demonstrated the effects of TTFields on G2/M arrest (Gera et al., 2015). Utilizing western blotting, Yoon et al. (2020) demonstrated that TTFields combined with nanoparticles (BTNPs) resulted in a decrease in expression of CDK6 and E2F1, which are key regulators of G1 progression. Another factor that could play a role in cell cycle alteration is the expression of chromosomal maintenance genes (Karanam et al., 2020). Karanam et al. (2020) showed that the expression of MCM10 and MCM6 genes decreased in human NSCLC cell lines following 12 h of TTFields exposure (H157 [100 kHz], H4006 [150 kHz], A549 [200 kHz], and H1299 [100 kHz]). MCM6 and MCM10 are essential in the replication initiation and DNA elongation process. Thus, a TTFields-induced reduction in MCM6 and MCM10 expression could interfere with normal cell cycle progression (Karanam et al., 2020). On the similar note, TTFields can reduce viral genomic replication through dampening the expression of various DNA replication complexes from MCMs and impede, for example, COVID-19 viral amplification (Farmani et al., 2022).
Another mechanism by which TTFields halts cell cycle progression is through DNA damage and disruption of repair mechanisms, based on cancer cell culture and animal studies (Figure 3D; Giladi et al., 2017; Kim et al., 2019; Karanam et al., 2020; Jeong et al., 2021; Mumblat et al., 2021). Immunofluorescence microscopy of TTFields combined with radiation-treated human NSCLC, human GBM, and malignant pleural mesothelioma cell lines showed an increase in the amount of residual γH2AX that was significantly higher than that in the RT-alone group (Giladi et al., 2017; Karanam et al., 2017; Mumblat et al., 2021). This increase in γH2AX indicated that TTFields caused DNA damage. Additionally, western blotting analysis of TTFields-exposed GBM cells showed a decrease in ATM expression in GBM cell lines (Giladi et al., 2017). ATM is an early activation factor in response to DNA double-strand breaks (DSBs) and a decrease in ATM expression suggested that TTFields can impede the initiation and progression of DNA repair mechanisms in cancer cells. Homologous recombination (HR) and non-homologous end-joining (NHEJ) are the two main DNA repair mechanisms. Giladi et al. (2017) analyzed human GBM (U-118) cells with immunoblotting assay and plasmid end-joining assay after 72 h of 200 kHz TTFields exposure. Immunoblotting showed a similar increase in phosphorylation of DNA-PK at 1 and 4 h post-RT and post-(TTFields + RT) exposure (Giladi et al., 2017). Localization of DNA-PKcs phosphorylated at S2056 (pS2056) foci with γ-H2AX foci initiates NHEJ mechanisms. The absence of a difference in ligation of the vectors in the plasmid end-joining assay in TTFields-exposed and unexposed cells demonstrated that NHEJ was not disrupted by TTFields (Giladi et al., 2017). Giladi et al. (2017) assessed the effect of TTFields on the HR pathway by monitoring Rad51 foci formation under TTFields exposure alone and in combination with RT. They found that exposing human GBM cells to 24 h of 200 kHz TTFields following RT increased Rad51 foci formation, which indicates that TTFields impairs HR cellular pathways (Giladi et al., 2017). Additionally, Lee et al. (2020) and Karanam et al. (2017) used RNA sequencing and microarray analysis on TTFields-exposed GBM and NSCLC cell lines, respectively, to analyze the alteration in expression of various genes. Lee et al. (2020) showed that TTFields alters many genes that play roles in various cellular responses such as cell cycle, death, immune response, neurogenesis, secretion, and cellular migration. Karanam et al. (2017) and Mumblat et al. (2021) found that many altered genes following TTFields exposure are involved in the BRCA pathway including BRCA1, FANC(A, B, C, D2, E, J), and RFC3. Since the BRCA pathway is activated in response to DNA damage, downregulation of gene expression and subsequent protein expression results in a decrease in DNA damage repair and cell cycle progression, and subsequently upregulation of cell death (Karanam et al., 2017; Mumblat et al., 2021).
A recent study by Patel et al. (2021) demonstrated that 200 kHz TTFields impairs GBM aberrant glycolytic metabolism by decreasing the expression of pyruvate kinase M2 (PKM2) (Patel et al., 2021). They utilized [18F]DASA-23, a radiotracer that targets PKM2 and has higher tumor-to-background ratio in the brain compared to the frequently used [18F]FDG radiotracer, and validated the findings using western blotting and immunofluorescent microscopy (Patel et al., 2021; Beinat et al., 2021). They found that TTFields exposure reduced PKM2 expression by 49% after 3 days and by 80% after 6 days of exposure compared to control (Patel et al., 2021). Cancer cells grow rapidly and invade the surrounding normal tissue. In doing so, they rely heavily on glycolysis as an energy source, despite the presence of oxygen. Impeding deranged cancer metabolism with TTFields, and possibly inducing a shift from aberrant glycolysis to oxidative phosphorylation, could be another mechanism through which TTFields stymies cancer cell growth and results in higher cell death without affecting surrounding non-cancer cells (Figure 1).
Impaired cellular proliferation
TTFields also impedes cancer cell growth and proliferation through interference with normal signaling pathways within cancer cells (Kim et al., 2016, 2019; Lee et al., 2019, 2021). As shown in Supplementary Figure S1, some of these pathways include NF-κB, MAPK, and PI3K/Akt signaling pathways that regulate various cellular responses to environmental factors, including but not limited to inflammatory responses, cellular growth, and apoptosis. Western blotting analysis following 24 h of TTFields exposure to human GBM U373 and U87 cells showed a decrease in NF-κB translation and IκBα, p38, ERK, JNK, and Akt phosphorylation (Kim et al., 2016). Meanwhile, there was no significant reduction in the level of MAPK expression after TTFields exposure (Kim et al., 2016). As shown in Supplementary Figure S1, TTFields exposure downregulates these pathways at each step within the cytoplasm, which is exacerbated by the reduction in NF-κB translation in the ER due to the induction of cellular stress. Additionally, immunofluorescence assay confirmed that TTFields blocked the translocation of NF-κB p65 from the cytoplasm to the nucleus of cancer cells, which, in combination with its effects on DNA damage and fragmentation and inhibition of HR, resulted in a greater decrease in cancer cell survival, growth, and proliferation compared to cells unexposed to TTFields (Kim et al., 2016).
Although TTFields exposure downregulates the MAPK and NF-κB pathways in cancer cells, western blotting analysis of mouse macrophages (RAW 264.7) following 72 h of TTFields exposure showed an increase in phosphorylation of IκBα and NF-κB p65 (Park et al., 2019). The phosphorylated p65 subunit of NF-κB was released from IκBα and ready for translocation into the nucleus to upregulate necessary genes for macrophage activation (Park et al., 2019). The alternative effect of TTFields exposure on macrophages allowed for immune activation and eradication of cancer cells through phagocytosis. The concept of TTFields-induced immunogenic cell death and increased activation and recruitment of macrophages and other leukocytes to enhance cancer recognition and clearance is shown in Figure 1.
TTFields induces apoptosis in cancer cells
As previously described (Figures 1–3), mitotic catastrophe as a result of TTFields, which includes irregular alignment of chromosomes during anaphase, asymmetrical cell division, and genomic instability, is associated with an increase in cell death. As the duration of TTFields exposure increases, more cells were arrested at various points of the cell cycle, which resulted in a higher rate of cell death (Jo et al., 2019). There are various proposed cell death mechanisms induced by TTFields.
As shown in Supplementary Figure S2, TTFields-induced cancer cell apoptosis is either caspase-mediated or caspase-independent (Giladi et al., 2016; Li et al., 2016; Kessler et al., 2018; Jo et al., 2018a; Lee et al., 2019, 2021; Yoon et al., 2020; Voloshin et al., 2020a). Both early apoptosis and late apoptosis were upregulated by TTFields (Giladi et al., 2016; Kessler et al., 2018; Yoon et al., 2020; Voloshin et al., 2020a). Cell death analysis of TTFields-exposed cells using annexin V and 7-amino-actinomycin D (7AAD) demonstrated an elevation in the percentage of early apoptotic cells (Annexin V+/7AAD−) and late apoptotic cells (Annexin V+/7AAD+) after various durations of exposure (Voloshin et al., 2020a). As shown in Supplementary Figure S2, in caspase-dependent cell apoptosis, various factors are upregulated and result in apoptosome formation and eventual cell death. Giladi et al. (2016) showed that there is a 2-fold increase in caspase activity in TTFields-exposed ovarian carcinoma (A2780) cells. Cell death was also halted upon caspase system inhibition (Giladi et al., 2016). Within the nucleus, TTFields induced DSBs (Figure 3B), increased poly(ADP-ribose) polymerase (PARP) cleavage, and enhanced reactive oxygen species (ROS) formation, which downregulated cellular repair and activated cellular apoptosis (Jo et al., 2018b, 2019; Lee et al., 2021). Caspase-dependent apoptosis is p53-dependant (Gera et al., 2015; Yoon et al., 2020). Gera et al. (2015) engineered a p53 knockout (KO) model of human colorectal cancer (HCT116) and analyzed the percentage of cell death following 8 h of TTFields exposure. They discovered that the KO cell line did not respond to TTFields and the percentage of cell death was similar to the unexposed group, whereas the wild-type cells experienced greater cell death upon TTFields exposure (Gera et al., 2015). As depicted in Supplementary Figure S2, p53 can bind to death receptors, upregulates the expression of BAX, and downregulates Bcl2 (Kim et al., 2019). This results in the activation of CytC and modulates downstream steps of apoptosome formation. Interestingly, TTFields exposure resulted in caspase-3 activation and PARP cleavage in non-cancer cells as well as in cancer cells (Jo et al., 2019). Furthermore, when TTFields was combined with hyperthermia (40°C for 30 min per day) in GBM U373 and A172 cells, Jo et al. (2022) reported that the levels of cleaved PPAR and caspase-3 were higher compared to TTFields alone or hyperthermia alone. Wu et al. (2020) studied the effects of TTFields in a rat model of GBM. Following an average of three weeks of 200 kHz TTFields exposure, they not only confirmed that the tumor shrank using magnetic resonance imaging (MRI) measurements, but also showed that TTFields increased caspase-3 expression (treated vs. non-treated: 68.57 ± 8.09 vs. 38.06 ± 10.04 cells/field, P < 0.001) in the TTFields-exposed cells, while no effect was observed in the liver, kidney, and various blood components (Wu et al., 2020). The caspase-dependent pathway activation became more evident in both non-cancer and cancer cells as the duration of TTFields increased (Jo et al., 2019). Thus, finding the optimal duration of TTFields that maximizes harm to cancer cells and minimizes harm to non-cancer cells will be crucial. Furthermore, Silginer et al. (2017) found that cell death induction in the presence of TTFields was independent of O6-methylguanine-DNA-methyltransferase (MGMT) status. MGMT is a protein that participates in stereochemical DNA repair independent from enzymatic pathways (Sharma et al., 2009). MGMT also has the potential to inactivate itself when necessary (Sharma et al., 2009). TTFields exposure to engineered LTCs revealed that cell death was not further enhanced based on the absence or presence of MGMT (Silginer et al., 2017).
The caspase-dependent pathway is not the only apoptotic pathway through which TTFields induces cell death. Silginer et al. (2017) showed that necroptosis, a form of caspase-independent cell death, might also play a role in TTFields-induced cell death in cancer cells. They treated LTC cell lines with necrostatin-1 (NEC-1), which is a potent necroptosis inhibitor (Silginer et al., 2017). After 72 h of TTFields exposure, flow cytometry analysis revealed a 10%–20% reduction in cell death in the TTFields + NEC-1 group compared to the TTFields-only and untreated groups (Silginer et al., 2017).
Recruitment of immunomodulatory mechanisms and alteration in tumor invasion and angiogenesis
As previously discussed, an increase in macrophage activation suggests that TTFields exposure could have immunomodulatory effects and help with tumor cell clearance in the body (Figure 1; Kirson et al., 2009a; Park et al., 2019; Weinberg et al., 2019; Diamant et al., 2021). In lung cancer and colon cancer mouse models, flow cytometry on peritoneal exudate cells exposed for 24–48 h to 150 kHz TTFields showed a 1.5-fold increase in CD45 signal, which is an indicator of leukocyte recruitment and upregulation of immune-mediated cell apoptosis (Voloshin et al., 2020a). However, the recruited immune cells differed depending on cancer type. In the lung cancer mouse model, flow cytometry analysis following combined anti-PD-1 therapy and TTFields showed an increase in activation of innate immunity by 25% in macrophages and 4% in dendritic cells compared to control groups (Voloshin et al., 2020a). The parallel analysis in the syngeneic colon carcinoma (CT-26) mouse model depicted that combination of anti-PD-1 therapy and TTFields increased the population of CD8+ and CD4+ T cells by 2% and 5%, respectively, compared to the control; this may indicate activation of adaptive immunity in the presence of TTFields (Voloshin et al., 2020a). In addition to upregulation of immune response, recent work by Diamant et al. (2021) demonstrated that TTFields had minimal effects on the antitumor activity of activated T cells. Using flow cytometry to examine the effects of 3.5 days of 200 kHz TTFields exposure to peripheral blood mononuclear cells, they found that the PD-1 upregulation, cytotoxic degranulation, and IFN-γ secretion were comparable to that of controls, with similar polyfunctional patterns (Diamant et al., 2021). They also evaluated multiple myeloma cell lines ectopically expressing HER2 CAG in the presence of chimeric antigen receptor (CAR) T-cells and found similar functionality and viability between CAR T-cells exposed or unexposed to TTFields (Diamant et al., 2021).
An important factor that allows for the recruitment of immune cells is the upregulation of proinflammatory cytokines (Park et al., 2019; Chen et al., 2022). Quantitative polymerase chain reaction analysis of mouse macrophages (RAW 264.7) following 24 h of TTFields exposure showed a 5-fold increase in IL-1β and a 4-fold increase in TNFα compared to controls (Park et al., 2019). Both factors are proinflammatory cytokines and play a crucial role in the activation and recruitment of macrophages (Park et al., 2019). Further gene expression analysis on dissected patient GBM samples following TTFields exposure demonstrated that a cytotoxic molecule (granulysin), a natural killer (NK) cell activator receptor associated with TTFields-induced NK killing of glioma cells (NKG2D), and a hallmark Th1 transcription factor (t-bet) were the upregulated antitumor genes; protumor genes including a chemokine driving suppressive tumor microenvironments (CXCL14), a marker of T cell exhaustion (PDL2), a proinflammatory cytokine (L18), and cytokines that polarize T helper cells to ineffective or protumor functions (IL-4 and IL-17RB) were downregulated (Diamant et al., 2021). Additionally, as shown in Supplementary Figure S3, TTFields upregulated CRT on the surface of lung cancer and colon cancer cells exposed to TTFields for 24–72 h (Voloshin et al., 2020a). Similarly, the concentration of the released high mobility group box 1 protein (HMGB1) increased over time in the same cell lines that were exposed to TTFields (Voloshin et al., 2020a). HMGB1 is required for the processing and cross-presentation of antigens and serves as a reliable marker of immunogenic cell death (Wang, 1999). Combined with the upregulation of CRT on the cell surface, Voloshin et al. (2020a) found that TTFields could induce immunogenic-dependent cell death.
A hallmark of metastatic cancers is their ability to co-opt vasculature (resulting in vasculogenesis and angiogenesis), migrate, and invade nearby tissues to obtain nutrients (Jahroudi and Greenberger, 1995). TTFields has been shown to alter and downregulate metastasis mechanisms in various cancers (Kim et al., 2016; Silginer et al., 2017; Jo et al., 2018b, 2022; Lee et al., 2019; Oh et al., 2020). Following 48 h of 150 kHz TTFields exposure to human colorectal cancer cell lines (HCT116, SW480, HT29, and SW620), transwell and Matrigel invasion assays demonstrated a decrease in cellular migration and invasion of treated cancer cells (Lee et al., 2019). This inhibitory effect of TTFields was further enhanced when combined with 5-FU, a commonly used chemotherapy (Lee et al., 2019). Western blotting analysis of human osteosarcoma cells exposed to TTFields showed a decrease in expression of vimentin, N-cadherin, TWIST, and SNAIL as epithelial–mesenchymal transition (EMT) markers in TTFields-exposed cells compared to controls (Oh et al., 2020). This observation demonstrated that TTFields inhibited invasion and migration of cancer cells through EMT-related markers (Oh et al., 2020; Lee et al., 2021). Furthermore, TTFields interfered with cancer-related angiogenesis, a finding that could help reduce the metastatic potential of cancer (Kim et al., 2016; Oh et al., 2020). TTFields suppressed angiogenesis by downregulating matrix metalloproteinase 2 (MMP2), MMP9, vascular endothelial growth factor, and hypoxia-inducible factor 1 genes that are known to promote tumor growth, angiogenesis, and metastasis (Kim et al., 2016; Oh et al., 2020). Additionally, photomicrographs of in vitro tube formation assays showed that TTFields exposure decreased vascular tubule development in endothelial (2H11) and human osteosarcoma (U2OS) cells, which provides additional evidence that TTFields suppressed angiogenesis (Oh et al., 2020). Combining 28 h of 150 kHz TTFields with an anti-angiogenic drug (e.g. sorafenib) was shown to increase the inhibition of tube formation in endothelial (2H11) cells compared to unexposed controls (Jo et al., 2018b). Recently published work from Voloshin et al. (2020b) also demonstrated that TTFields activated the RhoA/ROCK signaling pathway, which could be one explanatory mechanism by which TTFields interfered with cancer cell migration. Rho plays a central role in regulating the assembly of stress fibers and the formation of focal adhesions. Voloshin et al. (2020b) found that RhoA is upregulated in GBM cell lines. They also found that the phosphorylation of GEF-H1 and active ROCK increased in TTFields-exposed cells, which further implicates this signaling pathway in the disruption of normal microtubule formation and GBM cell migration (Voloshin et al., 2020b). A recent investigation by Kim et al. (2021) studied the effects of combining TTFields with trastuzumab (TRZ) in treating TRZ-resistant breast cancers. In addition to an increase in cell death in vivo and in vitro upon TTFields exposure, they found that when the mouse model (xenografted with BT-474 cells and exposed to 150 kHz TTFields for 5 days) was injected with 150 μg of Alexa 488-TRZ, the penetration via the tumor vessels into the tumor increased by 33% (P = 0.021), which supports a role for TTFields causing morphological changes in the tumor that could potentiate combination approaches with antibody-based therapy (Kim et al., 2021).
Perspectives
Despite the therapeutic effects of TTFields demonstrated in numerous cell culture, animal, and human studies of cancer, its use as standard-of-care varies by geographic region, with 84% of German nGBM patients using TTFields during the period from August 2017 to May 2019 (Bähr et al., 2019) and ∼45% of US nGBM patients using TTFields during the period from October 2011 to February 2019 (Shi et al., 2020; Lin et al., 2021). A fast-growing body of research, both in vitro and in vivo, is converging to demonstrate the multiple inhibitory effects of TTFields on cancer growth. There is an increased effort to ascertain the mechanisms through which TTFields adversely affects rapidly dividing cancer cells. The electrophysiological alterations caused by TTFields interferes with normal cellular functions and disrupts cellular stability and organelle formation, which in turn disrupts the cancer cell's capacity to respond to external stressors. Another compendium of studies demonstrated that TTFields promotes cancer cell death by causing DNA damage, interfering with cell division and DNA repair, and upregulating cell death mechanisms and immunogenic antitumor responses. Despite the disparate nature of the anti-cancer mechanisms of TTFields, they converge on (i) the impairment of cancer cell proliferation, survival, migration, and metabolism and (ii) the promotion of cancer cell death and recognition and clearance by the immune system; effects on blood vessels have also been observed in regard to reduced angiogenesis and increased BBB permeability (Figure 1).
Although there was agreement across different studies regarding the ability of TTFields to dampen cancer cell replication rate and tumor growth, there were also a few controversies regarding the MOAs of TTFields, which could be addressed by analyzing the differences in experimental conditions, cell lines, TTFields frequency, TTFields duration of exposure, and readout assays used. One of the controversies was whether TTFields upregulates cell apoptosis in a caspase-mediated manner. Giladi et al. (2016) studied this effect in human ovarian carcinoma (A2780) and GBM (U-87MG) cells following 24–48 h of 200 kHz TTFields exposure. They concluded that TTFields-induced cell death was caspase-dependent after finding that apoptosis was not different between unexposed cells vs. cells exposed to a combination of TTFields and a broad-spectrum caspase inhibitor (Giladi et al., 2016). Silginer et al. (2017) performed similar experiments in LTCs (LN-18 and LN-229) and glioma initiating cells (ZH-161) but, instead, used 100 kHz TTFields with a reduced exposure duration of 6 h. They did not detect caspase-3 processing upon TTFields exposure, indicating that TTFields-induced cell death occurred in a caspase-independent manner (Silginer et al., 2017). The effect of TTFields on cancer cells has been shown to depend on the cell type as well as the frequency, intensity, duration, number of cycles, and field direction of the TTFields (Kirson et al., 2007, 2009b; Giladi et al., 2014a; Voloshin et al., 2016; Porat et al., 2017; Silginer et al., 2017; Jo et al., 2018a, 2019; Berkelmann et al., 2019; Lee et al., 2019; Gencturk et al., 2020; Pfeifer et al., 2021; Wu et al., 2021; Ye et al., 2022). For instance, Wu et al. (2021) studied human glioma (U251) cells exposed to 200 kHz TTFields (field strength 2.2 V/cm) for 24–72 h in two different electric field output sequence modes: random and fixed. They found that the random sequence mode resulted in a more effective cancer cell death in vitro and reduced tumor growth in vivo, compared to the fixed sequence mode (Wu et al., 2021). The same logic could be applied to the discrepancy between the observed effects of TTFields on mitotic spindle formation. Utilizing time-lapse EM following 8 h of TTFields exposure in human cervical adenocarcinoma (HeLa) cells, Gera et al. (2015) observed that the mitotic spindle and metaphase plate were intact whereas microtubule–septin complex formation was disrupted. Giladi et al. (2016) performed a similar experiment in lung adenocarcinoma (A549) and breast adenocarcinoma (MDA-MB-231) cells. They analyzed the cells following 24 h of TTFields exposure with confocal fluorescence microscopy and detected abnormal microtubule polymerization, which resulted in abnormal spindle assembly (Giladi et al., 2016). Additionally, when they analyzed cells 8 h following TTFields exposure with time-lapse fluorescence microscopy, they observed a disrupted spindle structure that resulted in the formation of binucleate or polynucleate daughter cells (Giladi et al., 2016).
Whereas an agreement was observed in regard to the interference by TTFields on microtubule production, variations were observed based on the different phases of the cell cycle. For instance, many studies showed an increase in the G2/M subpopulation following TTFields exposure (Voloshin et al., 2016; Karanam et al., 2017; Silginer et al., 2017; Jo et al., 2018a, b; Kim et al., 2019). FACS analysis in other studies demonstrated a decrease in the G2/M cell population (Giladi et al., 2014a; Kessler et al., 2018; Neuhaus et al., 2019). The work of Kessler et al. (2018) may help to reconcile such apparent discrepancies. FACS analysis following 72 h of TTFields exposure to two human GBM cell lines resulted in differential effects on the G2/M population based on the cell line (a 10% increase in U87 cells and a 20% decrease in GaMG cells) (Kessler et al., 2018). Therefore, the proper interpretation of the effects of TTFields and the degree of such effects must take into account not only the TTFields properties (frequency, field strength, and exposure duration) but also the cell line(s) being studied and possible differences in culture conditions.
The in vivo studies of TTFields continued in both animal models of cancer as well as patients with GBM, NSCLC, malignant melanoma, and breast cancer metastasis to the skin (Kirson et al., 2004, 2009a; Salzberg et al., 2008; Stupp et al., 2012; Pless et al., 2013; Wong et al., 2015b; Lu et al., 2019). To date, TTFields has been approved by the FDA for the treatment of rGBM as monotherapy, nGBM in combination with adjuvant chemotherapy, and malignant pleural mesothelioma in combination with chemotherapy (Stupp et al., 2012, 2015, 2017; Ceresoli et al., 2019). Additionally, TTFields is safe, and the most common side effect that was observed in vivo and in various clinical trials was mild to moderate dermatitis underlying the electrode transducer arrays, which can be managed with slight repositioning of the arrays or topical corticosteroids (Salzberg et al., 2008; Stupp et al., 2012, 2015, 2017; Pless et al., 2013; Mrugala et al., 2014; Kesari et al., 2017; Zhu et al., 2017; Taphoorn et al., 2018; Vergote et al., 2018; Ceresoli et al., 2019; Lu et al., 2019; Rivera et al., 2019; Bokstein et al., 2020; Lacouture et al., 2020; Shi et al., 2020; Song et al., 2020; Wu et al., 2020, 2021; Kim et al., 2020a; Nour et al., 2021). Due to the survival benefits and minimal side effects of TTFields observed in clinical trials thus far (Supplementary Table S4), additional pre-clinical investigations and clinical trials are currently underway with an aim to understand the efficacy of this novel therapeutic modality in various cancer types including nGBM and NSCLC brain metastases in pediatric and adult patients (Johnson et al., 2017; Lau et al., 2017), hepatocellular carcinoma, pancreatic cancer, ovarian cancer, malignant pleural mesothelioma, and gastric adenocarcinoma (https://www.novocure.com). Understanding the cancer-specific MOAs of TTFields will be crucial in the rational design of combination strategies for various cancer types.
Future directions
TTFields has been shown in humans to be safe and effective when used as a monotherapy or in combination with chemotherapy (Stupp et al., 2012, 2015, 2017; Ceresoli et al., 2019). However, human tissue-based validation of the numerous MOAs identified by pre-clinical in vitro and in vivo studies is limited, warranting further investigation to ensure translatability of the pre-clinical findings. As summarized in Supplementary Tables S2 and S3, many cell culture and animal studies have been performed to understand the effectiveness of combining TTFields with standard-of-care chemotherapy regimens (Kirson et al., 2009b; Schneiderman et al., 2010; Giladi et al., 2014a; Castellví,et al., 2015; Voloshin et al., 2016, 2020a; Chang et al., 2017; Clark et al., 2017; Silginer et al., 2017; Kessler et al., 2018; Lei et al., 2018; Lee et al., 2019; Karanam et al., 2020; Yoon et al., 2020; Kim et al., 2020b; Branter et al., 2022). Collectively, they demonstrated that combining TTFields could have an additive or synergistic anti-cancer effect, as reflected in clinical studies (Kirson et al., 2009b; Pless et al., 2013; Vymazal and Wong, 2014; Wong et al., 2014, 2015a, b; Stupp et al., 2015, 2017; Kesari et al., 2017; Guzauskas et al., 2018, 2019; Taphoorn et al., 2018; Vergote et al., 2018; Ceresoli et al., 2019; Lu et al., 2019; Rivera et al., 2019; Toms et al., 2019; Kim et al., 2020a; Lazaridis et al., 2020; Song et al., 2020). TTFields alone has also been shown to improve overall survival, reduce tumor size, and/or enhance quality of life in patients with cancer (Salzberg et al., 2008; Stupp et al., 2012; Pless et al., 2013; Vymazal and Wong, 2014; Wong et al., 2015b; Lu et al., 2019; Onken et al., 2019; Kim et al., 2020a; Palmer et al., 2021). With technological improvements that reduce patient burden (e.g. the second-generation TTFields system for patients with nGBM, which has less heavy batteries), the necessary duration of TTFields exposure for anti-cancer efficacy can be achieved by more patients (Kinzel et al., 2019). There are some contraindications to the use of TTFields in patients with cancer. For example, patients with GBM who wish to use TTFields must be ≥ 22 years of age [it is currently being studied in the pediatric population (Johnson et al., 2017; Lau et al., 2017)] and not have implanted medical devices, skull defects, or bullet fragments.
Understanding the MOAs of TTFields would aid in the selection of chemotherapies that are most likely to synergize via complementary treatment effects. Further investigation is necessary into the processes through which TTFields induces cell death. Supplementary Figure S2 depicts data from the literature that were based on various mechanisms of caspase-dependent cell death. Meanwhile, there are minimal data on caspase-independent pathways that could be activated upon TTFields exposure. Silginer et al. (2017) studied the possible activation of the caspase-independent pathway by TTFields exposure, and additional studies would help to further elucidate the caspase-independent apoptotic pathways induced by TTFields (e.g. necroptosis and ferroptosis). A recent work by Slangen et al. (2022), which provides a protocol for live cell imaging during TTFields exposure, could provide a means to understand the temporal dynamics of TTFields initiation and its downstream effects.
Another area that could be further investigated to understand the safety of TTFields is its effect on non-cancer cells. There have been only a few studies that investigated the effect of TTFields on non-cancer cells. Chang et al. (2018) showed that the membrane permeability of non-cancer cells (human fibroblast PCS-201) remained unchanged under 200 kHz TTFields exposure, whereas human GBM cell membrane permeability was significantly increased under the same settings. Many studies have demonstrated that the effects of TTFields on cells are associated with the rate of cell division. This is one manner in which TTFields affects cancer cells disproportionally compared to non-cancer cells (Giladi et al., 2014a). This raises a concern about the effect of TTFields exposure to rapidly dividing cells such as non-cancer epithelial cells of the gut. Furthermore, prolonging the duration of TTFields exposure was shown to have a similar effect on both normal and cancer cell types. Jo et al. (2019) found that increasing the duration of TTFields exposure delayed DNA repair, increased cell cycle arrest, and increased the rate of apoptosis in normal intestinal epithelial (IEC-6) as well as human glioblastoma (U373) cells. A recent study by Jeong et al. (2021) investigated the effect of TTFields exposure on non-cancer keratinocytes and thymidine-treated GBM cells that rendered the GBM cells arrested in the G1/S phase of the cell cycle. Similar to previous studies, they found that TTFields had minimal effects on non-cancer cells but they also found that TTFields did not affect thymidine-treated cancer cells (Jeong et al., 2021). When TTFields exposure preceded thymidine treatment, not only was there a significant reduction in the number of colonies compared to unexposed control, but also the genes involved in cell cycle arrest and DNA damage (BRCA1, PCNA, CDC25C, and MAD2) had comparable expression to that in the control group (Jeong et al., 2021). Additionally, an animal study and various human trials reported that the most frequent side effect observed in TTFields-exposed patients was mild to moderate dermatitis at the site of electrode transducer array placement (Salzberg et al., 2008; Stupp et al., 2012, 2015, 2017; Pless et al., 2013; Mrugala et al., 2014; Kesari et al., 2017; Zhu et al., 2017; Taphoorn et al., 2018; Vergote et al., 2018; Ceresoli et al., 2019; Lu et al., 2019; Rivera et al., 2019; Bokstein et al., 2020; Kim et al., 2020a; Shi et al., 2020; Song et al., 2020; Wu et al., 2020). Increased knowledge of the effects of TTFields on non-cancer cells could be used, as necessary, to develop strategies to protect non-cancer cells against apoptosis and DNA damage when utilizing TTFields in a clinical setting.
In addition to being a completely different therapeutic modality compared to traditional surgery, radiation, and chemotherapy that are taught in medical school curricula and graduate medical education, there are other factors that may hinder the understanding of this biophysical therapeutic modality by the oncologists responsible for discussing treatment options with their patients. First, the rate of identification of the MOAs of TTFields based on pre-clinical studies outpaces the ability of clinical trials to validate them. Thus, it would be important to design into TTFields clinical trials the necessary correlative studies to reliably corroborate or reject the leading MOAs that have been identified by multiple independent groups and have the highest potential of being validated in patient tissue samples. Second, the improvements in median overall survival achieved through the use of TTFields are not yet accompanied by biomarkers of response or failure to this form of therapy, beyond traditional oncology response assessment criteria. Thus, there is a need for further research into biospecimen-based (e.g. cancer tissue, blood, cerebrospinal fluid, immunological profiling, etc.) and non-invasive imaging-based (e.g. positron emission tomography, advanced MRI, etc.) indicators of response to TTFields, which could then be monitored over time. Third, pre-clinical studies of TTFields could be designed to maximize the probability of the mechanistic findings being confirmed in human patients enrolled in TTFields clinical trials. For example, in vitro studies might consider using biomimetic 3D models of cancer that take into account the tumor microenvironmental factors, perfusion, immune system, etc. Similarly, in vivo studies could evaluate TTFields with particular attention to the use of syngeneic models of cancer (i.e. in immunocompetent animals), orthotopic implantation of cancer cells, initiation of TTFields only when a threshold tumor size is achieved (this would require non-invasive imaging to confirm tumor size in experimental vs. control groups, as opposed to starting TTFields at a pre-defined time point after cancer cell inoculation), and customized hardware systems to focus the TTFields on the anatomic location of interest. Finally, the vast majority of TTFields studies have focused on its effects on cancer cells. To understand any potential untoward effects of TTFields on cells adjacent to the tumor, the non-cancer cells could be exposed to TTFields to understand whether the mechanisms identified in cancer cells (Figure 1) are also identified in non-cancer cells. If there is little overlap in the effects of TTFields on cancer cells compared to non-cancer cells, then additional studies could be performed to improve our understanding of the intrinsic differences between the cell types that render the cancer cells more vulnerable to TTFields. Through coordinated efforts among biomedical scientists, engineers, biophysicists, and clinicians, the key mechanisms of TTFields will likely be identified and clinically validated, which will enable improved efficacy of this novel form of anti-cancer therapy.
Resources and methods
Search term strategy
A literature search in PubMed was performed to find relevant peer-reviewed articles. Search terms included ‘TTFields’ OR ‘Optune’ OR ‘Novo-TTF’ OR ‘NovoTAL’ OR ‘Novocure’ OR ‘inovitro’ OR ‘tumor treating fields’ OR ‘NovoTTF’ OR ‘alternating electric fields’. The search was last performed on April 22, 2022, to ensure the most up-to-date content.
Exclusion and inclusion criteria
The initial search resulted in 466 publications. The studies that did not have ‘TTFields’ or ‘alternating electric fields’ in their abstract were excluded from further review. Additionally, case reports, reviews, commentaries, theoretical and computational modeling studies, and conference abstracts were excluded. This resulted in the inclusion of peer-reviewed manuscripts focused on the MOAs of TTFields.
Data extraction and data analysis
After determining the final set of studies to be reviewed, relevant data were systematically extracted from each manuscript.
Supplementary Material
Acknowledgements
We thank the American Academy of Neurology (AAN) Medical Student Research Scholarship (S.S.). We also thank Jim Strommer for creating the illustrations (created with BioRender.com).
Contributor Information
Shadi Shams, Rowan University School of Osteopathic Medicine, Stratford, NJ 08028, USA.
Chirag B Patel, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; Neuroscience Graduate Program, The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, Houston, TX 77030, USA; Cancer Biology Program, The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, Houston, TX 77030, USA.
Funding
C.B.P. is a McNair Scholar supported by the McNair Medical Institute at The Robert and Janice McNair Foundation.
Conflict of interest: C.B.P. is a co-inventor on a patent (US11103698B2) titled ‘Using alternating electric fields to increase cell membrane permeability’ and a co-inventor on a patent application (US17/133,853) titled ‘Methods of normalizing aberrant glycolytic metabolism in cancer cells’, received consulting honoraria from Novocure, Ltd, and is a recipient of an AACR–Novocure Career Development Award for tumor treating fields (TTFields) research.
References
- Bähr O., Tabatabai G., Fietkau R.et al. (2019). ACTR-31. The use of TTFields for newly diagnosed GBM patients in Germany in routine clinical care (TIGER: TTFields in Germany in routine clinical care). Neuro Oncol. 21, vi20. 10.1093/neuonc/noz175.074 [DOI] [Google Scholar]
- Bai L., Pfeifer T., Gross W.et al. (2021). Establishment of Tumor Treating Fields combined with mild hyperthermia as novel supporting therapy for pancreatic cancer. Front. Oncol. 11, 738801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinat C., Patel C.B., Haywood Tet al. (2021). A Clinical PET imaging tracer ([18F]DASA-23) to monitor pyruvate kinase M2-induced glycolytic reprogramming in glioblastoma. Clin. Cancer Res. 27, 6467–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J.M., Tymoczko J.L., Stryer L. (2002). Biochemistry (5th edn). New York: W.H. Freeman Publishing. [Google Scholar]
- Berkelmann L., Bader A., Meshksar S.et al. (2019). Tumour-treating fields (TTFields): investigations on the mechanism of action by electromagnetic exposure of cells in telophase/cytokinesis. Sci. Rep. 9, 7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobkova I.V., Bobkov A.M., Silaev M.A. (2021). Dynamic spin-triplet order induced by alternating electric fields in superconductor–ferromagnet–superconductor josephson junctions. Phys. Rev. Lett. 127, 147701. [DOI] [PubMed] [Google Scholar]
- Bokstein F., Blumenthal D., Limon D.et al. (2020). Concurrent Tumor Treating Fields (TTFields) and radiation therapy for newly diagnosed glioblastoma: a prospective safety and feasibility study. Front. Oncol. 10, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branter J., Estevez-Cebrero M., Diksin M.et al. (2022). Genome-wide expression and anti-proliferative effects of electric field therapy on pediatric and adult brain tumors. Int. J. Mol. Sci. 23, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellví Q., Ginestà M.M., Capellà G.et al. (2015). Tumor growth delay by adjuvant alternating electric fields which appears non-thermally mediated. Bioelectrochemistry 105, 16–24. [DOI] [PubMed] [Google Scholar]
- Ceresoli G.L., Aerts J.G., Dziadziuszko R.et al. (2019). Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 20, 1702–1709. [DOI] [PubMed] [Google Scholar]
- Chang E., Patel C.B., Pohling C.et al. (2018). Tumor Treating Fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 4, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E., Pohling C., Beygui N.et al. (2017). Synergistic inhibition of glioma cell proliferation by Withaferin A and tumor treating fields. J. Neurooncol. 134, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Le S.B., Hutchinson T.E.et al. (2022). Tumor Treating Fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J. Clin. Invest. 132, e149258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P.A., Gaal J.T., Strebe J.K.et al. (2017). The effects of tumor treating fields and temozolomide in MGMT expressing and non-expressing patient-derived glioblastoma cells. J. Clin. Neurosci. 36, 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Oglio S., D'Amico A., Pioli F.et al. (2008). Dose-intensity temozolomide after concurrent chemoradiotherapy in operated high-grade gliomas. J. Neurooncol. 90, 315–319. [DOI] [PubMed] [Google Scholar]
- Diamant G., Simchony Goldman H., Gasri Plotnitsky L.et al. (2021). T cells retain pivotal antitumoral functions under tumor-treating electric fields. J. Immunol. 207, 709–719. [DOI] [PubMed] [Google Scholar]
- Farmani A.R., Mahdavinezhad F., Scagnolari C.et al. (2022). An overview on tumor treating fields (TTFields) technology as a new potential subsidiary biophysical treatment for COVID-19. Drug Deliv. Transl. Res. 12, 1605–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego O. (2015). Nonsurgical treatment of recurrent glioblastoma. Curr. Oncol. 22, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gencturk E., Ulgen K.O., Mutlu S. (2020). Thermoplastic microfluidic bioreactors with integrated electrodes to study tumor treating fields on yeast cells. Biomicrofluidics 14, 034104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gera N., Yang A., Holtzman T.S.et al. (2015). Tumor Treating Fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One 10, e0125269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi M., Munster M., Schneiderman R.S.et al. (2017). Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat. Oncol. 12, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi M., Schneiderman R.S., Porat Y.et al. (2014a). Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 14, 54–63. [DOI] [PubMed] [Google Scholar]
- Giladi M., Schneiderman R.S., Voloshin T.et al. (2016). Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci. Rep. 5, 18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi M., Weinberg U., Schneiderman R.S.et al. (2014b). Alternating electric fields (Tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin. Oncol. 41, S35–S41. [DOI] [PubMed] [Google Scholar]
- Gkika E., Grosu A.-L., Macarulla Mercade T.et al. (2022). Tumor Treating Fields concomitant with sorafenib in advanced hepatocellular cancer: results of the HEPANOVA phase II study. Cancers 14, 1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzauskas G.F., Pollom E.L., Stieber V.W.et al. (2019). Tumor treating fields and maintenance temozolomide for newly-diagnosed glioblastoma: a cost-effectiveness study. J. Med. Econ. 22, 1006–1013. [DOI] [PubMed] [Google Scholar]
- Guzauskas G.F., Salzberg M., Wang B.C. (2018). Estimated lifetime survival benefit of tumor treating fields and temozolomide for newly diagnosed glioblastoma patients. CNS Oncol. 7, CNS23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahroudi N., Greenberger J.S. (1995). The role of endothelial cells in tumor invasion and metastasis. J. Neurooncol. 23, 99–108. [DOI] [PubMed] [Google Scholar]
- Jeong H., Jo Yunhui, Yoon Myonggeunet al. (2021). Thymidine decreases the DNA damage and apoptosis caused by tumor-treating fields in cancer cell lines. Genes Genomics 43, 995–1001. [DOI] [PubMed] [Google Scholar]
- Jo Y., Han Y.I., Lee E.et al. (2022). The combination of tumor treating fields and hyperthermia has synergistic therapeutic effects in glioblastoma cells by downregulating STAT3. Am. J. Cancer Res. 12, 1423–1432. [PMC free article] [PubMed] [Google Scholar]
- Jo Y., Hwang S.-G., Jin Y.B.et al. (2018a). Selective toxicity of tumor treating fields to melanoma: an in vitro and in vivo study. Cell Death Discov. 4, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y., Kim E., Sai S.et al. (2018b). Functional biological activity of sorafenib as a tumor-treating field sensitizer for glioblastoma therapy. Int. J. Mol. Sci. 19, 3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y., Oh G., Gi Y.et al. (2020). Tumor Treating Fields (TTF) treatment enhances radiation-induced apoptosis in pancreatic cancer cells. Int. J. Radiat. Biol. 96, 1528–1533. [DOI] [PubMed] [Google Scholar]
- Jo Y., Sung J., Jeong H.et al. (2019). Effectiveness of a fractionated therapy scheme in tumor treating fields therapy. Technol. Cancer Res. Treat. 18, 1533033819845008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T., Aguilera D., Albasheer A.et al. (2017). PDCT-06. Radio-immunotherapy using the IDO-inhibitor indoximod in combination with re-irradiation for children with progressive brain tumors in the phase 1 setting: an updated report of safety and tolerability (NCT02502708). Neuro Oncol. 19, vi185. 10.1093/neuonc/nox168.750 [DOI] [Google Scholar]
- Karanam N.K., Ding L., Aroumougame A.et al. (2020). Tumor treating fields cause replication stress and interfere with DNA replication fork maintenance: implications for cancer therapy. Transl. Res. 217, 33–46. [DOI] [PubMed] [Google Scholar]
- Karanam N.K., Srinivasan K., Ding L.et al. (2017). Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 8, e2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesari, S., Ram, Z.; EF-14 Trial Investigators . (2017). Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: a post hoc analysis of the EF-14 trial. CNS Oncol. 6, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A.F., Frömbling G.E., Gross F.et al. (2018). Effects of tumor treating fields (TTFields) on glioblastoma cells are augmented by mitotic checkpoint inhibition. Cell Death Discov. 4, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keßler A.F., Salvador E., Domröse D.et al. (2019). P11.28 Alteration of blood brain barrier (BBB) permeability by Tumor Treating Fields (TTFields) in vivo. Neuro Oncol. 21, iii49. 10.1093/neuonc/noz126.174 [DOI] [Google Scholar]
- Kim C.-Y., Paek S.H., Nam D.et al. (2020a). Tumor treating fields plus temozolomide for newly diagnosed glioblastoma: a sub-group analysis of Korean patients in the EF-14 phase 3 trial. J. Neurooncol. 146, 399–406. [DOI] [PubMed] [Google Scholar]
- Kim E.H., Jo Y., Sai S.et al. (2019). Tumor-treating fields induce autophagy by blocking the Akt2/miR29b axis in glioblastoma cells. Oncogene 38, 6630–6646. [DOI] [PubMed] [Google Scholar]
- Kim E.H., Song H.S., Yoo S.H.et al. (2016). Tumor treating fields inhibit glioblastoma cell migration, invasion, and angiogenesis. Oncotarget 7, 65125–65136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Cho J.M., Kim H.et al. (2021). Tumor treating fields can effectively overcome trastuzumab resistant breast cancer multiplication. Am. J. Cancer Res. 11, 3935–3945. [PMC free article] [PubMed] [Google Scholar]
- Kim J.-Y., Jo Y., Oh H.-K.et al. (2020b). Sorafenib increases tumor treating fields-induced cell death in glioblastoma by inhibiting STAT3. Am. J. Cancer Res. 10, 3475–3486. [PMC free article] [PubMed] [Google Scholar]
- Kinzel A., Ambrogi M., Varshaver M.et al. (2019). Tumor treating fields for glioblastoma treatment: patient satisfaction and compliance with the second-generation optune® system. Clin. Med. Insights Oncol. 13, 1179554918825449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson E.D., Dbaly V., Tovarys F.et al. (2007). Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl Acad. Sci. USA 104, 10152–10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson E.D., Giladi M., Gurvich Z.et al. (2009a). Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin. Exp. Metastasis 26, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson E.D., Gurvich Z., Schneiderman R.et al. (2004). Disruption of cancer cell replication by alternating electric fields. Cancer Res. 64, 3288–3295. [DOI] [PubMed] [Google Scholar]
- Kirson E.D., Schneiderman R.S., Dbalý V.et al. (2009b). Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med. Phys. 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture M.E., Anadkat M.J., Ballo M.T.et al. (2020). Prevention and management of dermatologic adverse events associated with tumor treating fields in patients with glioblastoma. Front. Oncol. 10, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L.S., Stampar M., Rivero-Hinojosa S.et al. (2017). PDTM-24. The proteogenomics of medulloblastoma. Neuro Oncol. 19, vi195. 10.1093/neuonc/nox168.788 [DOI] [Google Scholar]
- Lazaridis L., Schäfer N., Teuber-Hanselmann S.et al. (2020). Tumour Treating Fields (TTFields) in combination with lomustine and temozolomide in patients with newly diagnosed glioblastoma. J. Cancer Res. Clin. Oncol. 146, 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.S., Seo S.-J., Chung H.K.et al. (2021). Tumor-treating fields as a proton beam-sensitizer for glioblastoma therapy. Am. J. Cancer Res. 11, 4582–4594. [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-J., Cho J.-M., Sai S.et al. (2019). 5-Fluorouracil as a tumor-treating field-sensitizer in colon cancer therapy. Cancers. 11, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-J., Seo H.W., Baek J.-H.et al. (2020). Gene expression profiling of glioblastoma cell lines depending on TP53 status after tumor-treating fields (TTFields) treatment. Sci. Rep. 10, 12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K.F., Hsieh S.-C., Goh A.et al. (2018). Proliferation arrest, selectivity, and chemosensitivity enhancement of cancer cells treated by a low-intensity alternating electric field. Biomed. Microdevices 20, 90. [DOI] [PubMed] [Google Scholar]
- Li J., Guo C., Wang Z.et al. (2016). Electrical stimulation towards melanoma therapy via liquid metal printed electronics on skin. Clin. Transl. Med. 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Wang M., Chen Y. (2021). Trends in intracranial glioma incidence and mortality in the United States, 1975–2018. Front. Oncol. 11, 748061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou E., Fujisawa S., Morozov A.et al. (2012). Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One 7, e33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B., Schiesl A., Voss M.et al. (2021). Dexamethasone treatment limits efficacy of radiation, but does not interfere with glioma cell death induced by Tumor Treating Fields. Front. Oncol. 11, 715031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Rao M., Zhu P.et al. (2019). Triple-drug therapy with bevacizumab, irinotecan, and temozolomide plus Tumor Treating Fields for recurrent glioblastoma: a retrospective study. Front. Neurol. 10, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrugala M.M., Engelhard H.H., Dinh Tran D.et al. (2014). Clinical practice experience with NovoTTF-100ATM system for glioblastoma: the patient registry dataset (PRiDe). Semin. Oncol. 41, S4–S13. [DOI] [PubMed] [Google Scholar]
- Mumblat H., Martinez-Conde A., Braten O.et al. (2021). Tumor Treating Fields (TTFields) downregulate the fanconi anemia-BRCA pathway and increase the efficacy of chemotherapy in malignant pleural mesothelioma preclinical models. Lung Cancer 160, 99–110. [DOI] [PubMed] [Google Scholar]
- Nakos K., Radler M.R., Spiliotis E.T. (2019). Septin 2/6/7 complexes tune microtubule plus-end growth and EB1 binding in a concentration- and filament-dependent manner. Mol. Biol. Cell 30, 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus E., Zirjacks L., Ganser K.et al. (2019). Alternating electric fields (TTFields) activate Cav1.2 channels in human glioblastoma cells. Cancers 11, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J.H. (1922). The stability of bacterial suspensions: I. A convenient cell for microscopic cataphoresis experiments. J. Gen. Physiol. 4, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour Y., Pöttgen C., Kebir S.et al. (2021). Dosimetric impact of the positioning variation of tumor treating field electrodes in the PriCoTTF-phase I/II trial. J. Appl. Clin. Med. Phys. 22, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.Y., Lee Y.-J., Kim E.H. (2020). Tumor-treating fields inhibit the metastatic potential of osteosarcoma cells. Technol. Cancer Res. Treat. 19, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken J., Goerling U., Heinrich M.et al. (2019). Patient reported outcome (PRO) among high-grade glioma patients receiving TTFields treatment: a two center observational study. Front. Neurol. 10, 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom Q.T., Cioffi G., Gittleman H.et al. (2019). CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 21(Suppl 5), v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J.D., Chavez G., Furnback W.et al. (2021). Health-related quality of life for patients receiving tumor treating fields for glioblastoma. Front. Oncol. 11, 772261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-I., Song K.-H., Jung S.-Y.et al. (2019). Tumor-treating fields induce RAW264.7 macrophage activation via NK-κB/MAPK signaling pathways. Technol. Cancer Res. Treat. 18, 1533033819868225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel C.B., Beinat C., Xie Y.et al. (2021). Tumor treating fields (TTFields) impairs aberrant glycolysis in glioblastoma as evaluated by [18F]DASA-23, a non-invasive probe of pyruvate kinase M2 (PKM2) expression. Neoplasia 23, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer T., Bai L., Gladkich J.et al. (2021). Therapy of pancreatic cancer with alternating electric fields: limitations of the method. Bioelectrochemistry 141, 107881. [DOI] [PubMed] [Google Scholar]
- Pless M., Droege C., von Moos R.et al. (2013). A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer 81, 445–450. [DOI] [PubMed] [Google Scholar]
- Porat Y., Giladi M., Schneiderman R.S.et al. (2017). Determining the optimal inhibitory frequency for cancerous cells using Tumor Treating Fields (TTFields). J. Vis. Exp. 123, e55820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M.M., San P., Lok E.et al. (2019). The clinical application of Tumor Treating Fields therapy in glioblastoma. J. Vis. Exp. 146, e58937. [DOI] [PubMed] [Google Scholar]
- Rivera F., Benavides M., Gallego J.et al. (2019). Tumor treating fields in combination with gemcitabine or gemcitabine plus nab-paclitaxel in pancreatic cancer: results of the PANOVA phase 2 study. Pancreatology 19, 64–72. [DOI] [PubMed] [Google Scholar]
- Sabri E., Brosseau C. (2021). Modeling cell membrane electrodeformation by alternating electric fields. Phys. Rev. E 104, 034413. [DOI] [PubMed] [Google Scholar]
- Salzberg M., Kirson E., Palti Y.et al. (2008). A pilot study with very low-intensity, intermediate-frequency electric fields in patients with locally advanced and/or metastatic solid tumors. Onkologie 31, 362–365. [DOI] [PubMed] [Google Scholar]
- Schneiderman R.S., Shmueli E., Kirson E.D.et al. (2010). TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer 10, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Le N., Alotaibi M.et al. (2014). Cytotoxic autophagy in cancer therapy. Int. J. Mol. Sci. 15, 10034–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Salehi F., Scheithauer B.W.et al. (2009). Role of MGMT in tumor development, progression, diagnosis, treatment, and prognosis. Anticancer Res. 29, 3759–3768. [PubMed] [Google Scholar]
- Shi P., Tian J., Ulm B.S.et al. (2022). Tumor Treating Fields suppression of ciliogenesis enhances temozolomide toxicity. Front. Oncol. 12, 837589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Blumenthal D.T., Oberheim Bush N.A.et al. (2020). Global post-marketing safety surveillance of tumor treating fields (TTFields) in patients with high-grade glioma in clinical practice. J. Neurooncol. 148, 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteingauz A., Porat Y., Voloshin T.et al. (2018). AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields). Cell Death. Dis. 9, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silginer M., Weller M., Stupp R.et al. (2017). Biological activity of tumor-treating fields in preclinical glioma models. Cell Death Dis. 8, e2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slangen P.L.G., Porat Y., Mertz M.et al. (2022). Protocol for live-cell imaging during Tumor Treating Fields treatment with inovitro live. STAR Protoc. 3, 101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A., Bar-Ad V., Martinez N.et al. (2020). Initial experience with scalp sparing radiation with concurrent temozolomide and tumor treatment fields (SPARE) for patients with newly diagnosed glioblastoma. J. Neurooncol. 147, 653–661. [DOI] [PubMed] [Google Scholar]
- Stupp R., Mason W.P., van den Bent M.J.et al. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996. [DOI] [PubMed] [Google Scholar]
- Stupp R., Taillibert S., Kanner A.et al. (2017). Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318, 2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R., Taillibert S., Kanner A.A.et al. (2015). Maintenance therapy with tumor-treating fields plus temozolomide vs. temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 314, 2535–2543. [DOI] [PubMed] [Google Scholar]
- Stupp R., Wong E.T., Kanner A.A.et al. (2012). NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur. J. Cancer 48, 2192–2202. [DOI] [PubMed] [Google Scholar]
- Taphoorn M.J.B., Dirven L., Kanner A.A.et al. (2018). Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncol. 4, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms S.A., Kim C.Y., Nicholas G.et al. (2019). Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J. Neurooncol. 141, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Toscano A., Nickel A.-C., Li G.et al. (2020). Rapalink-1 targets glioblastoma stem cells and acts synergistically with tumor treating fields to reduce resistance against temozolomide. Cancers 12, 3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergote I., von Moos R., Manso L.et al. (2018). Tumor Treating Fields in combination with paclitaxel in recurrent ovarian carcinoma: results of the INNOVATE pilot study. Gynecol. Oncol. 150, 471–477. [DOI] [PubMed] [Google Scholar]
- Voloshin T., Kaynan N., Davidi S.et al. (2020a). Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol. Immunother. 69, 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshin T., Munster M., Blatt R.et al. (2016). Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo: TTFields in combination with paclitaxel are therapeutically effective against ovarian cancer. Int. J. Cancer 139, 2850–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshin T., Schneiderman R.S., Volodin A.et al. (2020b). Tumor Treating Fields (TTFields) hinder cancer cell motility through regulation of microtubule and actin dynamics. Cancers 12, 3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vymazal J., Wong E.T. (2014). Response patterns of recurrent glioblastomas treated with tumor-treating fields. Semin. Oncol. 41, S14–S24. [DOI] [PubMed] [Google Scholar]
- Wang H. (1999). HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251. [DOI] [PubMed] [Google Scholar]
- Wang Y., Pandey M., Ballo M.T. (2019). Integration of tumor-treating fields into the multidisciplinary management of patients with solid malignancies. Oncologist 24, e1426–e1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg U., Kaynan N., Sela T.V.et al. (2019). Immunomodulatory effects of Tumor Treating Fields (TTFields) on lung cancer models. Ann. Oncol. 30, ii2–ii3. 10.1093/annonc/mdz072.006 [DOI] [Google Scholar]
- Wong E.T., Lok E., Gautam S.et al. (2015a). Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br. J. Cancer 113, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.T., Lok E., Swanson K.D.et al. (2014). Response assessment of NovoTTF-100A versus best physician's choice chemotherapy in recurrent glioblastoma. Cancer Med. 3, 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.T., Lok E., Swanson K.D. (2015b). Clinical benefit in recurrent glioblastoma from adjuvant NovoTTF-100A and TCCC after temozolomide and bevacizumab failure: a preliminary observation. Cancer Med. 4, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wang C., Liu J.et al. (2020). Evaluation of a tumor electric field treatment system in a rat model of glioma. CNS Neurosci. Ther. 26, 1168–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Yang L., Liu H.et al. (2021). Exploring the efficacy of tumor electric field therapy against glioblastoma: an in vivo and in vitro study. CNS Neurosci. Ther. 27, 1587–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye E., Lee J.E., Lim Y.-S.et al. (2022). Effect of duty cycles of tumor-treating fields on glioblastoma cells and normal brain organoids. Int. J. Oncol. 60, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.N., Lee D.-S., Park H.J.et al. (2020). Barium titanate nanoparticles sensitise treatment-resistant breast cancer cells to the antitumor action of tumor-treating fields. Sci. Rep. 10, 2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.-J., Demireva P., Kanner A.A.et al. (2017). Health-related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF-14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma. J. Neurooncol. 135, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.