This case series reports the prevalence and clinical characteristics of osteoradionecrosis of the jaw in patients with oral and oropharyngeal cancer treated with proton radiation therapy.
Key Points
Question
What is the prevalence of osteoradionecrosis (ORN) of the jaw in patients with head and neck cancer following proton radiation therapy (PRT)?
Findings
In this case series of 122 radiation therapy–naive patients with oral and oropharyngeal cancer receiving PRT, the prevalence of ORN was 10.6%.
Meaning
The prevalence of ORN following PRT appears to be higher than that of intensity-modulated radiation therapy reported in the literature, indicating that ORN remains a clinical challenge even in the era of PRT.
Abstract
Importance
Proton radiation therapy (PRT) has reduced radiation-induced toxic effects, such as mucositis and xerostomia, over conventional photon radiation therapy, leading to significantly improved quality of life in patients with head and neck cancers. However, the prevalence of osteoradionecrosis (ORN) of the jaw following PRT in these patients is less clear.
Objective
To report the prevalence and clinical characteristics of ORN in patients with oral and oropharyngeal cancer (OOPC) treated with PRT.
Design, Setting, and Participants
This case series reports a single-institution experience (Memorial Sloan Kettering Cancer Center, New York, New York) between November 2013 and September 2019 and included 122 radiation therapy–naive patients with OOPC treated with PRT. Data were analyzed from 2013 to 2019.
Main Outcomes and Measures
Clinical parameters, including sex, age, comorbidities, tumor histology, concurrent chemotherapy, smoking, comorbidities, and preradiation dental evaluation, were obtained from the medical record. Patients with clinical or radiographic signs of ORN were identified and graded using the adopted modified Glanzmann and Grätz grading system. Characteristics of ORN, such as location, clinical presentation, initial stage at diagnosis, etiology, time to diagnosis, management, and clinical outcome at the last follow-up, were also collected.
Results
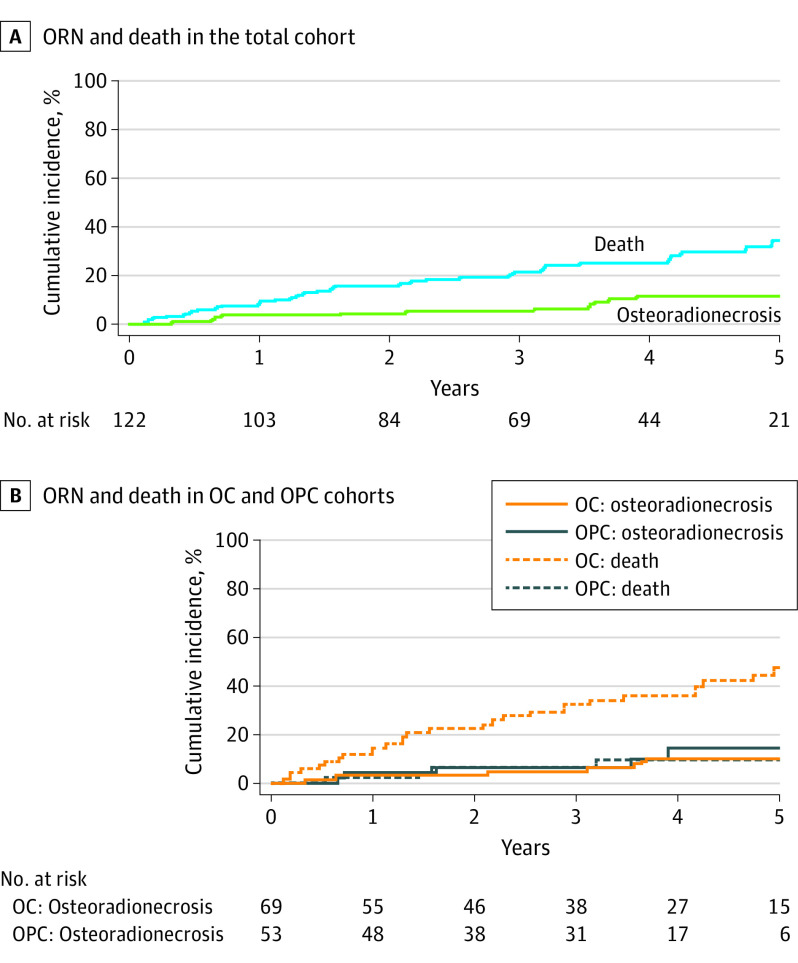
Of the 122 patients (mean [SD] age, 63 [13] years; 45 [36.9%] women and 77 [63.1%] men) included in this study, 13 (10.6%) developed ORN following PRT during a median (range) follow-up time of 40.6 (<1-101) months. All patients had spontaneous development of ORN. At the time of initial diagnosis, grade 0, grade 1, grade 2, and grade 3 ORN were seen in 2, 1, 9, and 1 patient, respectively. The posterior ipsilateral mandible within the radiation field that received the full planned PRT dose was the most involved ORN site. At a median (range) follow-up of 13.5 (0.2-58.0) months from the time of ORN diagnosis, complete resolution, stable condition, and progression of ORN were seen in 3, 6, and 4 patients, respectively. The 3-year rates of ORN and death in the total cohort were 5.2% and 21.5%, while the 5-year rates of ORN and death were 11.5% and 34.4%, respectively.
Conclusions and Relevance
In this case series, the prevalence of ORN following PRT was found to be 10.6%, indicating that ORN remains a clinical challenge even in the era of highly conformal PRT. Clinicians treating patients with OOPC with PRT should be mindful of this complication.
Introduction
Radiation therapy (RT), with or without chemotherapy, plays a crucial role in the management of head and neck cancers in the adjuvant and definitive clinical settings.1,2 Despite effective disease control and improved overall survival, radiation-induced toxic effects can significantly affect quality of life in long-term survivors.3,4 Over the past 3 decades, there have been technological advancements in radiation techniques, from conventional methods (using x-rays, gamma rays, or electron beams) to conformal 3-dimensional and more contemporary image-guided photon intensity-modulated RT (IMRT). These advances have led to a dramatic evolution in terms of appropriate targeting of tumor volumes, more precise dose distribution, and superior sparing of normal healthy tissues, all of which have resulted in reduced radiation-associated toxic effects.5 However, due to the limited physical properties of photons and the presence of exit dose, normal adjacent tissues may receive an excessive amount of moderate-dose radiation, resulting in radiation toxic effects even with IMRT.6
Currently, there is an increasing awareness of the dosimetric advantage of proton RT (PRT) in the management of head and neck cancers.7 Due to its high conformality and potential superior tissue-sparing capability, PRT has been shown to further decrease radiation-induced morbidity over photon IMRT.8,9 While photons continue to deposit the radiation dose in normal tissues beyond the tumor target, protons deposit an intermediate dose near the surface, sharply localize their maximum energy within the tumor target (Bragg peak), and have negligible exit dose. These unique physical properties have a great advantage given the complex anatomy and proximity of tumors to major organs at risk in the head and neck region. Hence, PRT is now being increasingly used in the management of head and neck cancers.10,11
Several studies have defined potential advantages of PRT over photon IMRT, demonstrating considerably lower rates of acute radiation toxic effects, such as mucositis and xerostomia, especially in the increasing proportion of young patients diagnosed with complex human papillomavirus–positive oropharyngeal cancers.12,13 Osteoradionecrosis (ORN) of the jaw is a major complication of RT that can significantly affect the quality of life of patients with head and neck cancer.14,15 Since head and neck cancers have large target volumes and are irregularly shaped, the Bragg peak needs to be spread out to encompass the entire tumor, and as a result, the entrance dose significantly increases.16 Superficially located organs, such as the skin, have been shown to experience significant toxic effects with PRT.10 In addition, the mandible is situated right beneath the skin and may be affected; however, the prevalence of ORN of the jaw following PRT is poorly characterized. The purpose of this case series is to determine the prevalence and clinical characteristics of ORN in patients with oral and oropharyngeal cancer (OOPC) treated with PRT at our institution.
Methods
Patient Characteristics
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC) (protocol No. 16-058). Informed consent was waived due to the study’s retrospective nature, minimal risk to the participants, and noninvasive procedures. This was an observational study of RT-naive patients with OOPC who were treated with PRT at our proton facility (Procure, Somerset, New Jersey) between November 2013 and September 2019. Patients with prior head and neck RT were excluded from the study. The electronic medical records for eligible patients were retrospectively reviewed for clinical information, including demographics, primary tumor site, tumor histology, social history, comorbidities (diabetes and hypertension), and concomitant chemotherapy. We also collected detailed clinical information for patients with ORN, including laterality and location, clinical presentation, etiology, stage at initial presentation, management, and clinical outcome at their last follow-up.
PRT Technique and Dose
Patients receiving PRT underwent computed tomography (CT) simulation in the supine position with 3-point or 5-point head/neck masks. Staging positron emission tomography (PET)/CT and/or magnetic resonance images were co-registered to the CT simulation scans to facilitate target volume delineation. The PRT was delivered using a Proteus 235 system (Ion Beam Applications). Uniform scanning beam using the match field technique was used for all patients treated before 2016. Starting in April 2016, patients were treated with either uniform scanning beam or pencil beam scanning (PBS), depending on target geometry and scheduling availability. The PBS was delivered in 2 ways: single-field uniform dose with each beam angle delivering a uniform dose covering the whole target volume, or multifield optimization using the sum of heterogeneous beam doses to cover each point in the target volume. Monte Carlo algorithm was used as a PBS calculation model. Setup variations of 3 to 5 mm and range uncertainties of plus or minus 3.5% were accounted for in the planning optimization. A relative biological effectiveness (RBE) value of 1.1 was applied in planning and plan evaluation. All areas of gross disease received 60 to 70 gray RBE (GyRBE), and regions of elective nodal radiation received 50 to 60 GyRBE. For patients receiving postoperative RT, the dose to the surgical bed was typically 60 GyRBE. For patients receiving quad shots for palliative treatment, the total doses were in the range of 13 to 44 GyRBE.
ORN Evaluation
For the purpose of this study, diagnostic criteria for ORN included “an area of exposed necrotic bone in a previously irradiated area that persisted over a period of 3 months, in the absence of new primary tumor, recurrence, or metastatic disease.”14 Radiographic ORN with clinically intact mucosa was also considered criteria for ORN diagnosis, as has been well documented in the literature.17 This was identified either as a lytic lesion on a panoramic radiograph or a contrast-enhanced lesion on postcontrast T1-weighted image and/or T2-weighted signal consistent on magnetic resonance scan. A dental oncologist (A.S., C.L.E.) graded ORN using the adopted modified Glanzmann and Grätz grading system18:
Grade 0—Radiographic ORN with intact mucosa
Grade 1—Exposed necrotic bone without signs of infection for at least 3 months
Grade 2—Exposed necrotic bone with signs of infection or sequestrum, but not grade 3 or 4
Grade 3—ORN resulting in pathologic fracture or ORN treated with surgical resection, with satisfactory result
Grade 4—ORN refractory to surgical resection
Follow-up and Outcomes
All patients were followed up at MSKCC at approximate intervals of 1 to 3 months after PRT completion. The follow-up time was calculated from the time of PRT completion to the time of the patients’ last clinical visit at MSKCC or the date of death in deceased patients. The time to ORN was calculated from PRT completion to the time of ORN clinical or radiographic diagnosis. Once diagnosed with ORN, patients were regularly monitored in the Dental Service. The clinical status of ORN at the last follow-up was assessed as either complete resolution (complete healing without any clinical symptoms), stable (no change from initial symptoms), or progression (increase in clinical symptoms, such as increase in area of exposed bone, purulent discharge, or a resulting pathologic fracture).
Statistical Analysis
Patient characteristics between those with and those without ORN were compared using appropriate effect size measures and 95% CIs. For continuous variables, mean difference was used. For categorical data, the odds ratio was used. The cumulative incidence of ORN was calculated using a competing risk analysis with death as the competing event. All statistical analyses were performed using R packages, version 4.2.1 (R Foundation for Statistical Computing).
Results
Between November 2013 and September 2019, 122 RT-naive patients (69 with oral cancer and 53 with oropharyngeal cancer) were treated with PRT in the definitive and adjuvant setting and followed up for a median of 40.6 months. There was no clinically meaningful difference in characteristics between the patients with and without ORN (Table 1).
Table 1. Patient Characteristics Including Estimates and Confidence Intervals.
| Characteristic | No. (%) | Categorical variables, OR (95% CI) | Continuous variables, difference (95% CI)a | |
|---|---|---|---|---|
| Patients with no ORN (n = 109) | Patients with ORN (n = 13) | |||
| Age, y | NA | 3.8 (−4.3 to 12) | ||
| Median (range) | 65 (21 to 96) | 62 (37 to 87) | ||
| Mean (SD) | 63 (13) | 59 (13) | ||
| Sex | ||||
| Female | 41 (91) | 4 (9) | 1 [Reference] | NA |
| Male | 68 (88) | 9 (12) | 1.36 (0.41 to 5.26) | NA |
| Primary sites | ||||
| Oral cavity | 61 (88) | 8 (12) | 1 [Reference] | NA |
| Oropharynx | 48 (91) | 5 (9) | 0.79 (0.23 to 2.54) | NA |
| Histology | ||||
| ACC | 13 (87) | 2 (13) | 1 [Reference] | NA |
| Others | 6 (75) | 2 (25) | 2.17 (0.22 to 22.0) | NA |
| SCC | 90 (91) | 9 (9) | 0.65 (0.15 to 4.57) | NA |
| T category | ||||
| T0-Tx/T1-T2 | 72 (91) | 7 (9) | 1 [Reference] | NA |
| T3-T4 | 30 (83) | 6 (17) | 2.06 (0.62 to 6.70) | NA |
| Unknown | 7 | 0 | NA | NA |
| N category | ||||
| N0-N1/Nx | 63 (85) | 11 (15) | 1 [Reference] | NA |
| N2-N3 | 39 (95) | 2 (5) | 0.29 (0.04 to 1.17) | NA |
| Unknown | 7 | 0 | NA | NA |
| Hypertension | ||||
| No | 68 (92) | 6 (8) | 1 [Reference] | NA |
| Yes | 41 (85) | 7 (15) | 1.93 (0.60 to 6.39) | NA |
| Diabetes | ||||
| No | 97 (90) | 11 (10) | 1 [Reference] | NA |
| Yes | 12 (86) | 2 (14) | 1.47 (0.21 to 6.38) | NA |
| Smoking history | ||||
| Never | 47 (89) | 6 (11) | 1 [Reference] | NA |
| Former | 62 (90) | 7 (10) | 0.88 (0.28 to 2.91) | NA |
| Concurrent chemotherapy | ||||
| No | 63 (90) | 7 (10) | 1 [Reference] | NA |
| Yes | 46 (88) | 6 (12) | 1.17 (0.36 to 3.76) | NA |
| Type of radiation | ||||
| IMRT + proton boost | 8 (80) | 2 (20) | 1 [Reference] | NA |
| Proton only | 95 (90) | 11 (10) | 0.46 (0.10 to 3.32) | NA |
| Quad shot | 6 (100) | 0 | 0.00 | NA |
| Radiation dose, GyRBE | NA | −1.9 (−13 to 8.8) | ||
| Median (range) | 66 (6 to 76) | 66 (20 to 76) | ||
| Mean (SD) | 58 (18) | 60 (17) | ||
| Dose by radiation type | ||||
| Proton only | 66 (20 to 76) | 66 (60 to 76) | NA | NA |
| IMRT + proton boost | 12 (6 to 40) | 23 (20 to 26) | NA | NA |
| Quad shot | 14 (13 to 44) | NA | NA | NA |
| Pre PRT-dental evaluation | ||||
| No | 68 (91) | 7 (9) | 1 [Reference] | NA |
| Yes | 41 (38) | 6 (46) | 1.42 (0.43 to 4.57) | NA |
| HPV 16+ | ||||
| No | 62 (89) | 8 (11) | 1 [Reference] | NA |
| Yes | 43 (90) | 5 (10) | 0.90 (0.26 to 2.89) | NA |
| Unknown | 4 | 0 | NA | NA |
Abbreviations: ACC, adenoid cystic carcinoma; GyRBE, gray relative biological effectiveness; HPV 16+, human papillomavirus 16–positive status; IMRT, intensity-modulated radiation therapy; N, node; NA, not applicable; OR, odds ratio; ORN, osteoradionecrosis; PRT, proton radiation therapy; SCC, squamous cell carcinoma; T, tumor.
Difference in means.
A total of 13 patients (9 men and 4 women) were identified with clinical/radiographic ORN following PRT (13 of 122; 10.6%) (eTable in the Supplement). Nearly half of these were T1 to T2 tumors (7 of 13; 53.8%). Most patients had squamous cell carcinoma histology (9 of 13; 69.2%). The primary tumor sites included base of tongue (n = 3) followed by buccal mucosa (n = 2), hard palate (n = 2), retromolar trigone (n = 2), tonsil (n = 2), floor of mouth (n = 1), and maxillary alveolus (n = 1). Out of 13, 5 (38.5%) patients with oropharynx primary tumors were human papillomavirus positive, and 7 of 13 (53.8%) patients were former smokers. Eight (61.5%) patients had hypertension and 2 of 13 (15.4%) had diabetes, while 5 of 13 (38.5%) patients did not have any comorbidities. Seven (53.8%) patients received concurrent chemotherapy. A pre-PRT dental evaluation was performed in 6 of 13 (46.2%) patients. Patient characteristics with ORN are summarized in the eTable in the Supplement.
ORN Characteristics
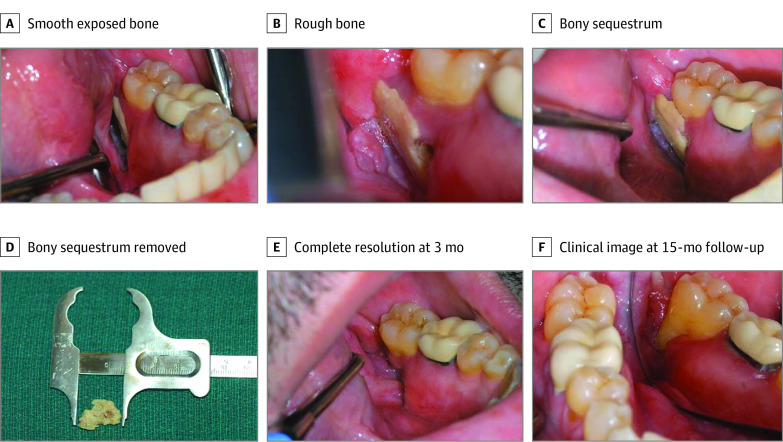
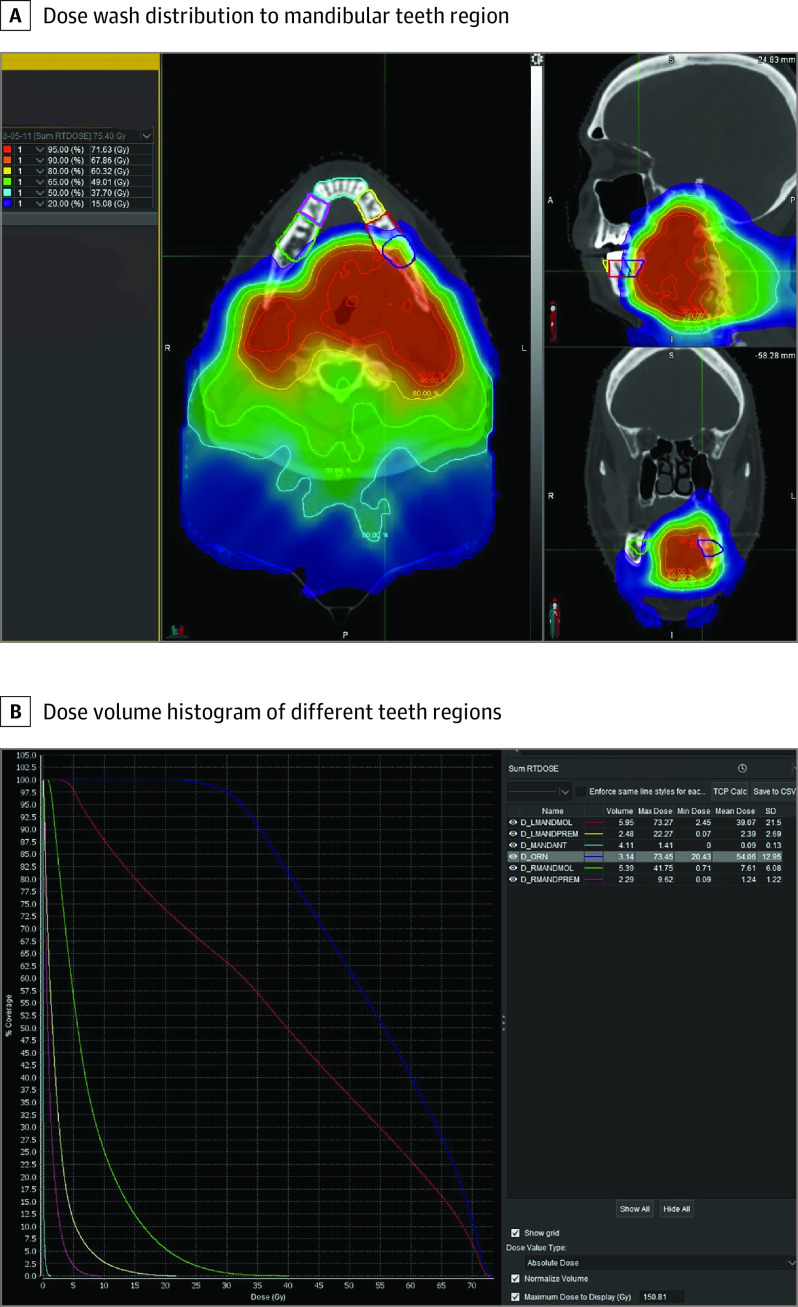
A summary of ORN characteristics is presented in Table 2. All 13 patients developed spontaneous ORN with a median (range) time to ORN diagnosis of 50.0 (2.0-83.0) months from PRT completion. Clinical presentation included avulsed tooth (n = 1), tooth mobility (n = 2), pain (n = 7), exposed bone area (n = 6) (Figure 1A, B, and C), purulent discharge (n = 3), and draining fistulae (n = 1). Two patients were asymptomatic at the time of ORN diagnosis (patients 1 and 4). Radiographic findings in patient 1 showed a lytic lesion in the region of avulsed maxillary anterior tooth on a panoramic radiograph. Magnetic resonance imaging scan at the time of disease evaluation in patient 4 showed a focal marrow infiltration and cortical destruction of left mandibular ramus and angle. This correlated with an increased fluorodeoxyglucose uptake (standardized uptake value, 9.5) on PET/CT, consistent with ORN. Overall, the posterior mandible was the most affected ORN site (10 of 13 [77%]). In 12 (92.3%) patients, ORN developed in the ipsilateral primary tumor site within the highest-dose fields. In contrast, patient 6 developed ORN on the contralateral side, possibly due to the large size of the primary tumor that received higher radiation dose. Based on adopted modified Glanzmann and Grätz grading system, 2 patients had grade 0, 1 patient had grade 1, 9 patients had grade 2, and 1 patient had grade 3 ORN at the time of initial diagnosis. To confirm the dosimetry, we also contoured the specific areas of ORN using the radiation treatment planning software (Figure 2). The ORN-affected site revealed an average Dmean of 71.5 GyRBE (range, 45.1-76.5 GyRBE) and average Dmax of 75.1 GyRBE (range, 61.8-81.2 GyRBE ); the doses are provided in the eTable in the Supplement.
Table 2. Summary of Clinical Data and ORN Characteristics Following PRT.
| Patient No. | ORN | Calculated radiation dose to ORN site, GyRBE | Etiology | Time to ORN, mo | Management of ORN | Follow-up, mo | Outcome at last follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laterality | Location | Clinical presentation | Grade at initial diagnosis | Mean | Maximum | ||||||
| 1 | I/L | Maxillary anterior | Avulsed tooth | 1 | 76.5 | 81.2 | Spontaneous | 41 | CHX | 36 | Complete resolution |
| 2 | I/L | Mandibular posterior | Pain, EBA (3 × 1 cm) | 2 | 60.9 | 66.2 | Spontaneous | 2 | CHX, ABX | 25 | Progression |
| 3 | I/L | Mandibular posterior | Pain, purulent discharge EBA (1 × 1 cm) | 2 | 57.1 | 69.3 | Spontaneous | 42 | CHX, ABX, PENT-E | 32 | Progression |
| 4 | I/L | Mandibular ramus | Asymptomatic | 0 | 68.1 | 69.4 | Spontaneous | 24 | CHX | 31 | Stable |
| 5 | I/L | Mandibular posterior | Pain, purulent discharge | 2 | 57.6 | 66.2 | Spontaneous | 7 | CHX, ABX | 42 | Progression |
| 6 | C/L | Maxillary posterior | Draining fistula | 3 | 73.9 | 79.0 | Spontaneous | 35 | CHX, ABX, surgical resection + graft | 35 | Complete resolution |
| 7 | I/L | Mandibular posterior | Pain, tooth mobility, EBA (1.5 × 1 cm) | 2 | 52.5 | 72.9 | Spontaneous | 6 | CHX, ABX, debridement, PENT-E | 35 | Complete resolution |
| 8 | I/L | Mandibular posterior | Pain, EBA (2 × 2 cm) | 2 | 45.1 | 73.6 | Spontaneous | 6 | CHX, ABX, PENT-E, HBOT | 58 | Progression |
| 9 | I/L | Mandibular posterior | EBA (0.8 × 1 cm) | 1 | 57.1 | 71.3 | Spontaneous | 40 | CHX, PENT-E | 36 | Stable |
| 10 | I/L | Mandibular posterior | Pain, swelling, purulent discharge | 2 | 62.7 | 69.8 | Spontaneous | 45 | CHX, ABX, PENT-E | 0 | Stable |
| 11 | I/L | Mandibular posterior | Swelling | 2 | 64.8 | 68.5 | Spontaneous | 17 | CHX, ABX, PENT-E | 16 | Stable |
| 12 | I/L | Maxillary posterior | 3 EBAs (0.3 × 0.3 cm, 0.2 × 0.3 cm, 0.1 × 0.5 cm) | 1 | 45.5 | 61.8 | Spontaneous | 83 | CHX, PENT-E | 11 | Stable |
| 13 | I/L | Mandibular posterior | Pain, tooth mobility | 2 | 66.6 | 69.1 | Spontaneous | 74 | CHX, ABX | 7 | Stable |
Abbreviations: ABX, antibiotics; CHX, chlorhexidine; C/L, contralateral; EBA, exposed bone area; GyRBE, gray relative biological effectiveness; HBOT, hyperbaric oxygen therapy; I/L, ipsilateral; ORN, osteoradionecrosis; PENT-E, pentoxifylline–vitamin E.
Figure 1. Clinical Presentation of Adopted Modified Glanzmann and Grätz Grade 2 Osteoradionecrosis (ORN).
A, Smooth exposed bone measuring 1.5 cm × 1 cm seen on the lingual surface of the mandibular alveolar ridge distal to the left mandibular second molar. B, Rough bone noted 6 months after diagnosis of ORN causing traumatic ulcer on left side of tongue. C, Bony sequestrum appeared to be mobile 8 months after ORN diagnosis. D, The mobile bony sequestrum was removed gently with a pair of forceps. E, Clinical view showing complete resolution at 3 months. F, Clinical view at 15-month follow-up.
Figure 2. Dosimetric Analysis .
A, Dose wash distribution to mandibular teeth region. The teeth-bearing regions are outlined in different colors. Note that the osteoradionecrosis (ORN) area (outlined in indigo) lies within the highest dose distribution area in the left mandible. B, Dose volume histogram of different teeth regions: right mandibular molars (green), right mandibular premolars (pink), mandibular anterior (blue), left mandibular premolars (yellow), and left mandibular molars (red). The ORN area (indigo) shows a maximum and mean dose of 73.45 gray relative biological effectiveness (GyRBE) and 54.06 GyRBE, respectively.
Only 39.3% (48 of 122) patients underwent a pre-PRT dental evaluation at our Dental Service, of whom 6 of 48 (12.5%) developed ORN. In addition, 5 of 48 patients underwent pre-PRT dental extraction; 1 of 5 who underwent extraction in the mandibular left first molar, first and second premolar due to extensive decay and infection, developed ORN in the region of primary tumor that received the maximum PRT dose (patient 1). The remaining 74 of 122 patients did not undergo a pre-PRT dental evaluation, of whom 7 (9.4%) developed ORN.
With an overall median follow-up time of 40.6 months, the 3-year rates of ORN and death in the total cohort were 5.2% and 21.5%, while the 5-year rates of ORN and death were 11.5% and 34.4%, respectively (Figure 3A). In this study, ORN developed within a median (range) of 50.0 (2.0-83.0) months after completion of PRT. We also identified that patients with oropharyngeal cancer developed ORN earlier than patients with oral cancer (median [range], 11.5 [6-45] months). The 3-year rates of ORN and death in this cohort was calculated as 6.1% and 6.2%, while the 5-year rates of ORN and death were 14.4% and 9.1%, respectively (Figure 3B). Patients with oral cancer developed ORN with a median (range) time of 57.5 (2-83) months from PRT completion and displayed a poor survival. The 3-year rates of ORN and death in this cohort were calculated as 4.6% and 32.3%, while the 5-year rates of ORN and death were 9.9% and 47.3%, respectively (Figure 3B).
Figure 3. Cumulative Incidence Curve of Osteoradionecrosis (ORN) With Death Serving as the Competing Event.
A, The 3-year rates of ORN and death in the total cohort were 5.2% and 21.5%, while the 5-year rates of ORN and death were 11.5% and 34.4%, respectively. B, The 3-year rates of ORN and death in patients with oropharyngeal cancer (OPC) was calculated as 6.1% and 6.2%, while the 5-year rates of ORN and death were 14.4% and 9.1%, respectively. The 3-year rates of ORN and death in patients with oral cancer (OC) was calculated as 4.6% and 32.3%, while the 5-year rates of ORN and death were 9.9% and 47.3%, respectively.
Management and Outcomes
Management of ORN following PRT varied depending on the stage of initial ORN diagnosis. All patients received a prescription of chlorhexidine gluconate, 0.12%, for oral hygiene optimization. Conservative management with a course of antibiotics when indicated (typically amoxicillin clavulanic acid, 875 mg twice daily) and pain medication was prescribed in 9 patients. A prescription of pentoxifylline, 400 mg, and vitamin E, 600 IU twice daily, was recommended in 7 patients. A gentle debridement of the mobile necrotic bone segment was performed in patient 7 followed by pentoxifylline, 400 mg, and vitamin E, 600 IU twice daily, for 2 months resulting in a complete resolution (Figure 1D, E, and F). In addition to medical management, patient 8 received 30 dives of hyperbaric oxygen (HBO) at an outside institution due to progressive necrotic lesions. For patient 6, with an advanced grade of ORN, surgical resection of necrotic palatal bone followed by graft placement and fabrication of obturator prosthesis were performed where conservative management failed. The median (range) follow-up time after ORN diagnosis was 13.5 (0.2-58.0) months. At the last follow-up at MKSCC, 3 patients had complete resolution, 6 patients remained stable, and 4 patients progressed. Progressive lesions of ORN were seen in the form of increased purulent drainage (patients 5 and 8) or pathologic fracture (patients 2 and 3).
Discussion
In this single-institution case series, we found a 10.6% rate of ORN following PRT in patients with OOPC. Osteoradionecrosis is a well-documented radiation-induced toxic effect with a varying prevalence in the literature.15,19,20 With the evolution of RT modalities, there has been a reduction in the prevalence of ORN from 13% with 3-dimensional conformal RT to 6% with IMRT.19,21 In a retrospective study of 1023 patients with OOPC treated with IMRT at our institution, the prevalence of ORN was reported to be as low as 4.3%.22 However, the prevalence of ORN following PRT has not been well established. In a retrospective study, Zhang et al23 showed the minimized radiation dose to the mandible with intensity-modulated proton therapy resulted in a 2% risk of ORN with PRT as compared to IMRT (7.7%) in patients with oropharyngeal cancer. Fossum et al24 reported 1 of 11 (9.1%) patients with Common Terminology Criteria for Adverse Events grade 3 ORN in the right mandible following PRT with 60 GyRBE for right mandibular gingival cancer. Takayama et al25 reported 1 of 15 (6.6%) patients with grade 2 CTCAE ORN after PRT and intra-arterial infusion chemotherapy for adenoid cystic carcinoma of the base of the tongue. Thus, the ORN rate in our study (10.6%) is higher than the previously reported prevalence of 4% to 8% in the modern era.21,22,26
Osteoradionecrosis can occur at any time, even beyond 10 years following RT.27,28 However, it is most frequently diagnosed (70%-94%) in the first 3 years after completion of RT.18,29 A study from our institution reported a median (range) time of 19.1 (0-120.2) months to the diagnosis of ORN following IMRT, and patients with oropharyngeal cancer were prone to develop ORN earlier compared to patients with oral cancer.22
Several risk factors have been associated with ORN.30,31 Patients with oral cancer with prior ablative surgery, including bone resections or osteotomies, are known to be at increased risk for ORN.19,32 In our study, 11 of 13 (85%) patients underwent surgical resection followed by postoperative PRT. Since all patients developed spontaneous ORN without a preceding dentoalveolar procedure, we believe it is unlikely that a pre-RT dental evaluation would have prevented ORN.
Spontaneous ORN is known to be dependent on higher radiation dose.22,31 Most of the ORN sites in our study developed spontaneously in the posterior mandible located within the proton radiation field. This finding aligns with a prior report9 that areas of the mandible within the planning target volume receive full radiation dose and are at an increased risk for ORN. The mean radiation dose to the contralateral side of the mandible is negligible, and therefore ORN in that region is less likely.9
Despite the dosimetric benefits of PRT, there is concerning uncertainty regarding the RBE (ie, the ratio of the photon dose to the proton dose to cause the same level of biological effect) of protons.33,34 Currently, PRT is planned and delivered assuming a proton RBE scaling factor of 1.1 (1 Gy of proton beam = 1.1 Gy of photon beam); thus, protons result in approximately 10% more biological damage per unit of physical dose than photons.35 Charged proton particles have greater biological effectiveness than photon beams because of their higher rate of energy deposition to tissues and a higher linear energy transfer (LET), thus greater relative ability to damage cellular DNA.36,37 Yet there is ongoing debate regarding the biological effects in tissues and increased LET at the end and distal to the proton Bragg peak.38,39 A recent study40 suggested that RBEs were larger than 1.1 at moderate doses (between 40 and 60 GyRBE) with high LET for mandible ORN. The authors also suggest that reducing LET at moderate doses may minimize the incidence of ORN following PRT. Studies have shown that IMRT doses greater than 60 Gy to the mandible are associated with high risk of developing ORN.20,22 Considering the RBE of protons may be larger than 1.1, the areas of the mandible within the planned target volume that receive greater than 60 Gy equivalent (a higher equivalent to a photon beam) may be at a high risk of developing ORN. In our study, 95.1% (116 of 122) patients received 60 GyRBE or greater to their primary tumor site (Table 1).
Management of ORN following PRT is multimodal based on contemporary management, as was seen in our study cohort.41 A conservative approach for the management of early-stage ORN consists of close monitoring, strict oral hygiene maintenance, and regular use of chlorhexidine mouth rinses.42,43 Medical management for mildly symptomatic patients or patients with active ORN infection includes systemic antibiotic therapy, anti-inflammatory agents, and analgesics. A simple surgical intervention involves gentle debridement of mobile bony sequestrum to promote healing. Studies have shown that early intervention with minor surgical procedures combined with pharmacological methods may improve the prognosis of ORN.44 Surgical management is generally used when conservative management has failed and there is progressive ORN resulting in pathological fractures and draining fistulae. Those with more advanced ORN may require extensive surgical resections.45 The use of hyperbaric oxygen (HBO) therapy for prevention or management of ORN is controversial. A recent study showed a lack of evidence to support the routine use of HBO for the prevention or management of ORN. However, adjunctive HBO may be considered for use on an individual basis.46 In our study, patient 8 received HBO at an outside institution, which showed no clinical benefit. Based on the pathophysiology of ORN as a radiation-induced fibro-atrophic process, the current literature supports the combined use of pentoxifylline (antifibrotic agent) and tocopherol (antioxidant) in the management of ORN, which has shown promising results.47
Limitations
This study is limited by its retrospective nature, small sample size, and relatively short duration of follow-up.
Conclusions
In this case series, we report a 10.6% prevalence of ORN in RT-naive patients with OOPC treated with PRT. The 3-year rates of ORN and death in the total cohort were 5.2% and 21.5%, respectively, while the 5-year rates of ORN and death were 11.5% and 34.4%, respectively. Although the present study is limited by its retrospective design, small sample size, and relatively short duration of follow-up, it highlights that ORN remains a clinical challenge even in the era of highly conformal PRT. Future studies with larger sample sizes, prospective clinical data, and longer follow-up will be important to confirm these findings. Clinicians treating patients with head and neck cancer with PRT should be mindful of this complication, and patients should be monitored closely for ORN and appropriately managed to ensure optimal outcomes and quality of life.
eTable. Summary of Patient Demographics, Tumor Characteristics, and Radiation Therapy Details
References
- 1.Gutiontov SI, Shin EJ, Lok B, Lee NY, Cabanillas R. Intensity-modulated radiotherapy for head and neck surgeons. Head Neck. 2016;38(suppl 1):E2368-E2373. doi: 10.1002/hed.24338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson G, Ebadi M, Vo K, Novak J, Govindarajan A, Amini A. An updated review on head and neck cancer treatment with radiation therapy. Cancers (Basel). 2021;13(19):4912. doi: 10.3390/cancers13194912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi: 10.1200/JCO.2007.14.6647 [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Ridge JA, Li T, et al. Long-term toxicities in 10-year survivors of radiation treatment for head and neck cancer. Oral Oncol. 2017;71:122-128. doi: 10.1016/j.oraloncology.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grégoire V, Langendijk JA, Nuyts S. Advances in Radiotherapy for Head and Neck Cancer. J Clin Oncol. 2015;33(29):3277-3284. doi: 10.1200/JCO.2015.61.2994 [DOI] [PubMed] [Google Scholar]
- 6.Gujral DM, Nutting CM. Patterns of failure, treatment outcomes and late toxicities of head and neck cancer in the current era of IMRT. Oral Oncol. 2018;86:225-233. doi: 10.1016/j.oraloncology.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 7.Moreno AC, Frank SJ, Garden AS, et al. Intensity modulated proton therapy (IMPT)—the future of IMRT for head and neck cancer. Oral Oncol. 2019;88:66-74. doi: 10.1016/j.oraloncology.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118(2):286-292. doi: 10.1016/j.radonc.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owosho AA, Yom SK, Han Z, et al. Comparison of mean radiation dose and dosimetric distribution to tooth-bearing regions of the mandible associated with proton beam radiation therapy and intensity-modulated radiation therapy for ipsilateral head and neck tumor. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(5):566-571. doi: 10.1016/j.oooo.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leeman JE, Romesser PB, Zhou Y, et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol. 2017;18(5):e254-e265. doi: 10.1016/S1470-2045(17)30179-1 [DOI] [PubMed] [Google Scholar]
- 11.Blanchard P, Gunn GB, Lin A, Foote RL, Lee NY, Frank SJ. Proton therapy for head and neck cancers. Semin Radiat Oncol. 2018;28(1):53-63. doi: 10.1016/j.semradonc.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 12.Sio TT, Lin HK, Shi Q, et al. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys. 2016;95(4):1107-1114. doi: 10.1016/j.ijrobp.2016.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchard P, Garden AS, Gunn GB, et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—a case matched analysis. Radiother Oncol. 2016;120(1):48-55. doi: 10.1016/j.radonc.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(5):283-288. doi: 10.1016/0278-2391(83)90294-X [DOI] [PubMed] [Google Scholar]
- 15.Frankart AJ, Frankart MJ, Cervenka B, Tang AL, Krishnan DG, Takiar V. Osteoradionecrosis: exposing the evidence not the bone. Int J Radiat Oncol Biol Phys. 2021;109(5):1206-1218. doi: 10.1016/j.ijrobp.2020.12.043 [DOI] [PubMed] [Google Scholar]
- 16.Ahn PH, Lukens JN, Teo BK, Kirk M, Lin A. The use of proton therapy in the treatment of head and neck cancers. Cancer J. 2014;20(6):421-426. doi: 10.1097/PPO.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 17.Owosho AA, Kadempour A, Yom SK, et al. Radiographic osteoradionecrosis of the jaw with intact mucosa: proposal of clinical guidelines for early identification of this condition. Oral Oncol. 2015;51(12):e93-e96. doi: 10.1016/j.oraloncology.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glanzmann C, Grätz KW. Radionecrosis of the mandibula: a retrospective analysis of the incidence and risk factors. Radiother Oncol. 1995;36(2):94-100. doi: 10.1016/0167-8140(95)01583-3 [DOI] [PubMed] [Google Scholar]
- 19.Moon DH, Moon SH, Wang K, et al. Incidence of, and risk factors for, mandibular osteoradionecrosis in patients with oral cavity and oropharynx cancers. Oral Oncol. 2017;72:98-103. doi: 10.1016/j.oraloncology.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 20.Chronopoulos A, Zarra T, Ehrenfeld M, Otto S. Osteoradionecrosis of the jaws: definition, epidemiology, staging and clinical and radiological findings: a concise review. Int Dent J. 2018;68(1):22-30. doi: 10.1111/idj.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai CJ, Hofstede TM, Sturgis EM, et al. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85(2):415-420. doi: 10.1016/j.ijrobp.2012.05.032 [DOI] [PubMed] [Google Scholar]
- 22.Owosho AA, Tsai CJ, Lee RS, et al. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): the Memorial Sloan Kettering Cancer Center experience. Oral Oncol. 2017;64:44-51. doi: 10.1016/j.oraloncology.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Zhang X, Yang P, et al. Intensity-modulated proton therapy and osteoradionecrosis in oropharyngeal cancer. Radiother Oncol. 2017;123(3):401-405. doi: 10.1016/j.radonc.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fossum CC, Beltran CJ, Whitaker TJ, Ma DJ, Foote RL. Biological model for predicting toxicity in head and neck cancer patients receiving proton therapy. Int J Part Ther. 2017;4(2):18-25. doi: 10.14338/IJPT-17-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayama K, Kato T, Nakamura T, et al. Proton beam therapy combined with intra-arterial infusion chemotherapy for stage IV adenoid cystic carcinoma of the base of the tongue. Cancers (Basel). 2019;11(10):1413. doi: 10.3390/cancers11101413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studer G, Bredell M, Studer S, Huber G, Glanzmann C. Risk profile for osteoradionecrosis of the mandible in the IMRT era. Strahlenther Onkol. 2016;192(1):32-39. doi: 10.1007/s00066-015-0875-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000;58(10):1088-1093. doi: 10.1053/joms.2000.9562 [DOI] [PubMed] [Google Scholar]
- 28.Epstein J, van der Meij E, McKenzie M, Wong F, Lepawsky M, Stevenson-Moore P. Postradiation osteonecrosis of the mandible: a long-term follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(6):657-662. doi: 10.1016/S1079-2104(97)90314-0 [DOI] [PubMed] [Google Scholar]
- 29.Murray CG, Herson J, Daly TE, Zimmerman S. Radiation necrosis of the mandible: a 10 year study. part II. dental factors; onset, duration and management of necrosis. Int J Radiat Oncol Biol Phys. 1980;6(5):549-553. doi: 10.1016/0360-3016(80)90381-8 [DOI] [PubMed] [Google Scholar]
- 30.Mendenhall WM, Suárez C, Genden EM, et al. Parameters associated with mandibular osteoradionecrosis. Am J Clin Oncol. 2018;41(12):1276-1280. doi: 10.1097/COC.0000000000000424 [DOI] [PubMed] [Google Scholar]
- 31.Nabil S, Samman N. Risk factors for osteoradionecrosis after head and neck radiation: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(1):54-69. doi: 10.1016/j.tripleo.2011.07.042 [DOI] [PubMed] [Google Scholar]
- 32.Lee IJ, Koom WS, Lee CG, et al. Risk factors and dose-effect relationship for mandibular osteoradionecrosis in oral and oropharyngeal cancer patients. Int J Radiat Oncol Biol Phys. 2009;75(4):1084-1091. doi: 10.1016/j.ijrobp.2008.12.052 [DOI] [PubMed] [Google Scholar]
- 33.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy: variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59(22):R419-R472. doi: 10.1088/0031-9155/59/22/R419 [DOI] [PubMed] [Google Scholar]
- 34.Underwood T, Paganetti H. Variable proton relative biological effectiveness: how do we move forward? Int J Radiat Oncol Biol Phys. 2016;95(1):56-58. doi: 10.1016/j.ijrobp.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 35.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25(8):953-964. doi: 10.1200/JCO.2006.09.7816 [DOI] [PubMed] [Google Scholar]
- 36.Gerweck L, Paganetti H. Radiobiology of charged particles. In: DeLaney TF, Kooy HM, eds. Proton and Charged Particle Radiotherapy. Lippincott Williams & Wilkins; 2007:8-18. [Google Scholar]
- 37.Halperin EC. Particle therapy and treatment of cancer. Lancet Oncol. 2006;7(8):676-685. doi: 10.1016/S1470-2045(06)70795-1 [DOI] [PubMed] [Google Scholar]
- 38.Grassberger C, Trofimov A, Lomax A, Paganetti H. Variations in linear energy transfer within clinical proton therapy fields and the potential for biological treatment planning. Int J Radiat Oncol Biol Phys. 2011;80(5):1559-1566. doi: 10.1016/j.ijrobp.2010.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuaron JJ, Chang C, Lovelock M, et al. Exponential increase in relative biological effectiveness along distal edge of a proton Bragg peak as measured by deoxyribonucleic acid double-strand breaks. Int J Radiat Oncol Biol Phys. 2016;95(1):62-69. doi: 10.1016/j.ijrobp.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, Muller OM, Shiraishi S, et al. Empirical relative biological effectiveness (RBE) for mandible osteoradionecrosis (ORN) in head and neck cancer patients treated with pencil-beam-scanning proton therapy (PBSPT): a retrospective, case-matched cohort study. Front Oncol. 2022;12:843175. doi: 10.3389/fonc.2022.843175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobson AS, Buchbinder D, Hu K, Urken ML. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. 2010;46(11):795-801. doi: 10.1016/j.oraloncology.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 42.O’Dell K, Sinha U. Osteoradionecrosis. Oral Maxillofac Surg Clin North Am. 2011;23(3):455-464. doi: 10.1016/j.coms.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 43.Chrcanovic BR, Reher P, Sousa AA, Harris M. Osteoradionecrosis of the jaws—a current overview—part 2: dental management and therapeutic options for treatment. Oral Maxillofac Surg. 2010;14(2):81-95. doi: 10.1007/s10006-010-0205-1 [DOI] [PubMed] [Google Scholar]
- 44.Camolesi GC, Ortega KL, Medina JB, et al. Therapeutic alternatives in the management of osteoradionecrosis of the jaws: systematic review. Med Oral Patol Oral Cir Bucal. 2021;26(2):e195-e207. doi: 10.4317/medoral.24132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee M, Chin RY, Eslick GD, Sritharan N, Paramaesvaran S. Outcomes of microvascular free flap reconstruction for mandibular osteoradionecrosis: a systematic review. J Craniomaxillofac Surg. 2015;43(10):2026-2033. doi: 10.1016/j.jcms.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 46.Sultan A, Hanna GJ, Margalit DN, et al. The use of hyperbaric oxygen for the prevention and management of osteoradionecrosis of the jaw: a Dana-Farber/Brigham and Women’s Cancer Center multidisciplinary guideline. Oncologist. 2017;22(3):343-350. doi: 10.1634/theoncologist.2016-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolokythas A, Rasmussen JT, Reardon J, Feng C. Management of osteoradionecrosis of the jaws with pentoxifylline-tocopherol: a systematic review of the literature and meta-analysis. Int J Oral Maxillofac Surg. 2019;48(2):173-180. doi: 10.1016/j.ijom.2018.08.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Summary of Patient Demographics, Tumor Characteristics, and Radiation Therapy Details