Key Points
Question
Can necrotizing conjunctivitis be seen in human mpox (monkeypox) infection?
Findings
In this case report, a patient with mpox presented with ophthalmic manifestations before the onset of skin lesions with manifestations of necrotizing blepharoconjunctivitis.
Meaning
Human mpox could initially present with ophthalmic manifestations and can also be sight threatening.
This case report describes a patient with mpox (monkeypox) who presented with sight-threatening necrotizing blepharoconjunctivitis.
Abstract
Importance
Ophthalmic manifestations occur in less than 5% of patients with human mpox (monkeypox), most commonly presenting with self-limiting conjunctivitis and keratitis. Cases with severe ophthalmic complication are uncommon.
Objective
To present a case of human mpox with sight-threatening necrotizing blepharoconjunctivitis.
Design, Setting, and Participants
This is a report of a patient who developed necrotizing conjunctivitis due to the monkepox virus at a large university hospital. Data were collected from July to October 2022.
Main Outcomes and Measures
Description of the progression and clinical evaluation of the ocular condition and the management.
Results
A 63-year-old HIV-positive man presented initially with conjunctivitis and eyelid swelling and developed skin lesions from monkeypox virus 2 days later. Despite remaining stable systemically, after 4 days, his ophthalmic condition evolved to necrotizing blepharoconjunctivitis for which systemic antiviral treatment with tecovirimat was given along with topical trifluoridine, 1%, eye drops. In addition, he required repeated tissue debridement with amniotic membrane grafting to preserve the eye integrity.
Conclusions and Relevance
The severity of this observation was associated with a coexisting immunocompromised state and appeared similar to findings associated with other orthopoxviruses. Ophthalmic manifestations could be the initial presentation of human mpox and could also be severe. Early recognition and intervention may limit the likelihood of substantial ocular morbidity.
Introduction
Mpox (monkeypox) is a zoonotic disease caused by a double-stranded DNA virus classified in the orthopoxvirus genus of the Poxviridae family.1,2 It is endemic in sub-Saharan Africa, with nearly all cases occurring in Africa prior to 2022 and only a few sporadic cases elsewhere; however, its human epidemiology appears to be changing, with more recent outbreaks and atypical clinical features in nonendemic countries.3,4,5 With sporadic reports of human infections with other orthopox viruses, it has been speculated that emergent or reemergent human mpox might fill the epidemiological void left by smallpox.3,4
Typically, after an incubation period of 1 to 2 weeks, patients develop fever, malaise, and lymphadenopathy, followed by a rash that evolves through macular, papular, vesicular, and pustular morphologies before scabbing over and resolving. Multiorgan involvement may occur in more severe forms, but the mortality rate in Africa remains low.1 Since the beginning of the 2022 outbreak in the US, from a total of 26 384 cases, only 6 deaths have been reported.6
Ophthalmic involvement in mpox infections has been described in less than 5% of cases, resulting in mainly mild and self-limited conjunctivitis and keratitis.2,3,7,8 We report a patient with mpox infection presenting with severe and sight-threatening necrotizing blepharoconjunctivitis for which we followed the reporting guidelines described by Kempen et al.9
Report of a Case
A 63-year-old man presented with a 5-day history of worsening left eye redness, itching, discharge, and painful swelling of the upper and lower eyelid as well as fever and malaise 2 days after the onset of his eye symptoms. He had received vaccination for SARS-CoV-2 (3 doses) and was known to have HIV well controlled with dolutegravir and lamivudine. On examination, his visual acuity was hand motion OS and 20/20 OD. He had left upper eyelid swelling causing a complete mechanical ptosis, conjunctival hyperemia with purulent discharge and corneal edema, and a 4-millimeter central epithelial defect. Intraocular pressures were measured with rebound tonometry (iCare IC100; iCare) and were within normal limits. B-scan ultrasonography showed clear vitreous cavity with no structural globe abnormality. Computed tomography imaging of the orbits and brain showed diffuse preseptal soft tissue thickening with no postseptal or intracranial involvement. He was admitted and presumptively treated for infective preseptal cellulitis and keratoconjunctivitis with intensive topical guttae moxifloxacin, 0.5%, intravenous ceftriaxone, 2 g, once a day, and oral metronidazole, 500 mg, 3 times a day as well as oral doxycycline, 100 mg, once a day after obtaining conjunctival swabs and corneal scrapes for Gram staining and cultures.
On the second day of admission, periorbital swelling deteriorated, and visual acuity was reduced to light perception OS, with repeated computed tomography imaging showing increased swelling of preseptal tissues (Figure 1A). Systemic examination revealed left-sided cervical lymphadenopathy, with pustules and vesicles on his face, arms, back, and groin region (Figure 1B), raising the suspicion of mpox. Further inquiry revealed that the patient’s male partner had recently had similar skin lesions, for which he self-isolated and did not seek medical advice. Polymerase chain reaction results from skin lesions and conjunctiva were both positive for mpox DNA, and blood tests showed an HIV viral load of less than 50 copies/mL and CD4 count of 495 cells/μL.
Figure 1. Clinical Features at the Moment of Diagnosis of Mpox and After Initial Debridement.
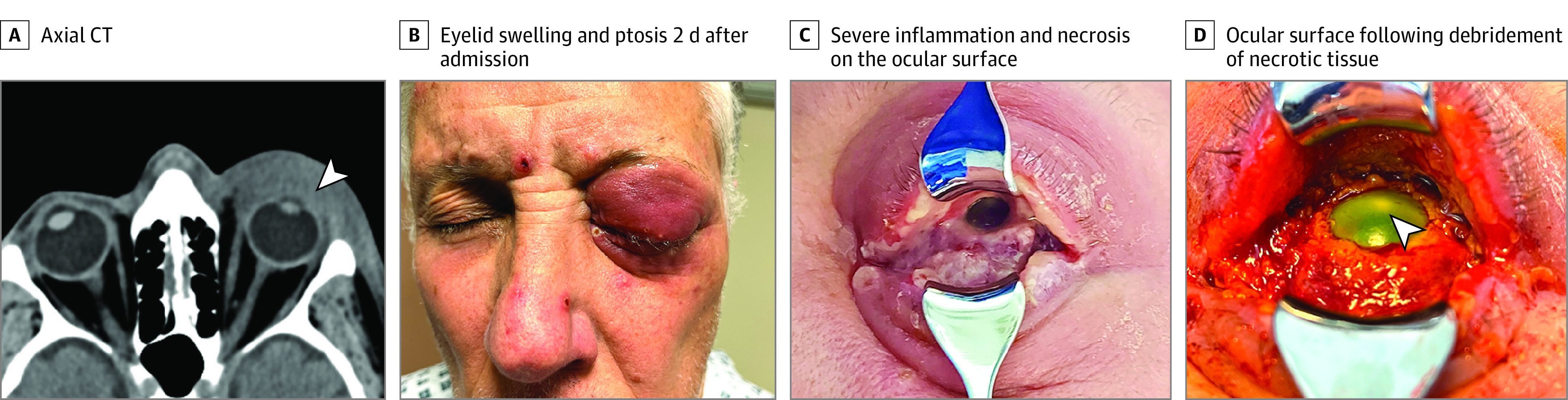
A, Axial computed tomography section showing extensive eyelid and epibulbar soft tissue swelling with no postseptal extension (arrowhead). B, Eyelid swelling and ptosis 2 days after admission when the patient developed skin lesions. C, Severe inflammation and necrosis on the ocular surface. D, Ocular surface following debridement of necrotic tissue, the cornea shows complete epithelial defect (staining after fluorescein drops instillation) and an infiltrate (2 mm).
Gram staining and cultures from ocular samples found no microorganisms, and systemic antibiotics were discontinued. Further tests for Chlamydia trachomatis, Neisseria gonorrhea, and syphilis were negative, and his general condition otherwise remained stable.
On day 4 of admission, a more detailed examination revealed white discharge, fibrotic membranes, and significant necrosis on the bulbar and tarsal conjunctiva (Figure 1C). Examination also revealed corneal epithelial defect, edema, stromal keratitis, and a small 2-mm central nonsupurative infiltrate without corneal melting or perforation (Figure 1D). Extensive debridement of membranes and necrotic tissue was performed at the bedside, and samples were sent for histopathology study. Moxifloxacin eye drops were replaced by unpreserved chloramphenicol, 0.5%, 4 times a day. Because of the severity of his ophthalmic condition, it was decided to start antiviral treatment with oral tecovirimat, 600 mg, twice a day and topical trifluorodine, 1%, eye drops 5 times per day. Necrotic tissue debridement was repeated every 2 days, and on the sixth day after his hospitalization, hourly dexamethasone, 0.1%, eye drops were initiated. Trifluoridine eye drops were discontinued on day 9 to prevent toxic effects of the affected ocular surface. Two weeks after his admission, the ocular surface showed signs of improvement, dexamethasone, 0.1%, eye drops were reduced to 4 times per day, and a dry amniotic membrane (OmniGen) was stuck to the ocular surface and fornices using fibrin glue (Tisseel) to attempt reepithelialization of the cornea. Histopathology of the conjunctival biopsy revealed degenerate multinucleate squamous epithelial cells consistent with a viropathic origin. The ocular surface improved following application of amniotic membrane, showing conjunctival growth and corneal epithelium healing (Figure 2B). Four weeks after his admission, visual acuity improved only to counting fingers OS because of remaining dense central corneal scarring; the upper eyelid swelling decreased but blepharoptosis persisted. Despite corneal scarring, fundus examination after dilation did not reveal gross retinal damage, and B-scan confirmed his retina remained fully attached.
Figure 2. Progression of the Ocular Surface Following Treatment.
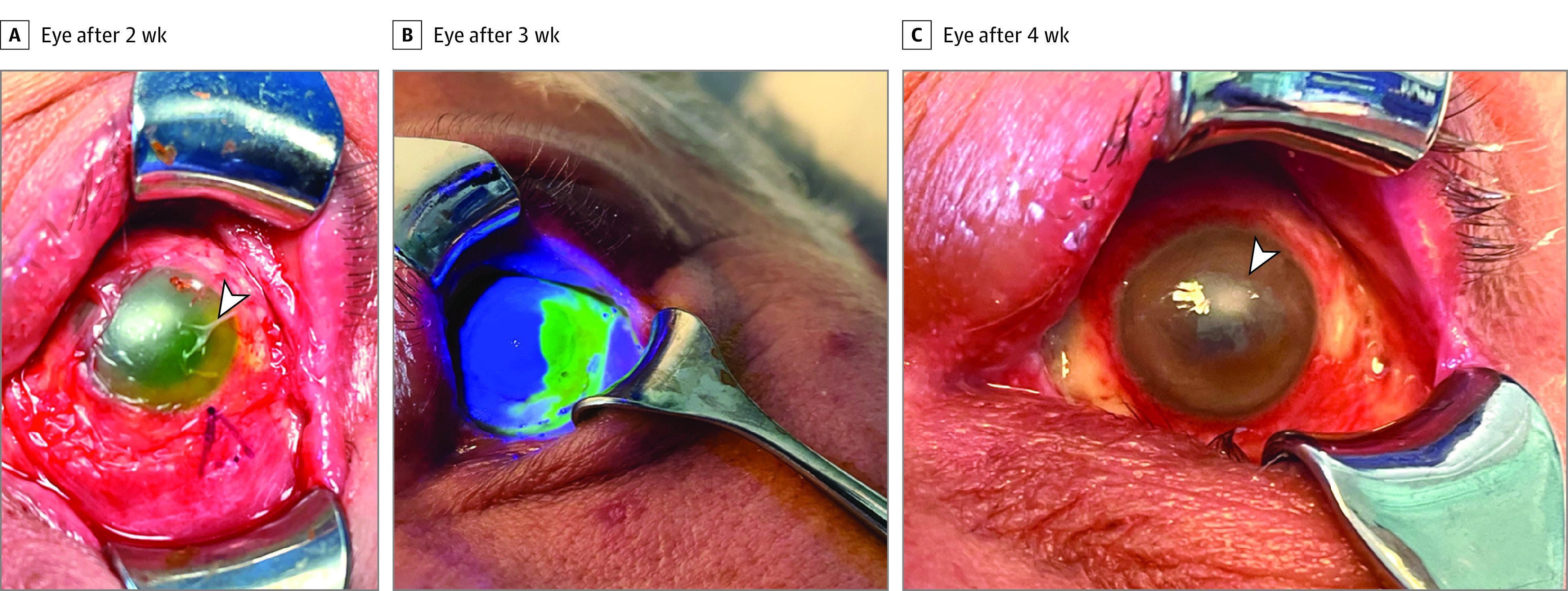
A, Eye after 2 weeks of regular debridement when a dry amniotic membrane was used to cover the ocular surface (arrowhead). B, Eye after 3 weeks showing superior corneal epithelialization on staining with fluorescein. C, Eye after 4 weeks with remaining areas of bare sclera and central corneal scarring (arrowhead).
Discussion
We describe a patient with a severe ophthalmic complication of human mpox, which developed 4 days after presentation and prior to systemic and topical antiviral treatment. Previous reports have described self-limiting conjunctivitis and keratitis associated with this infection3,7,8; however, severe necrotizing conjunctivitis has been described in prairie dogs.10 Conjunctival necrosis has been also observed in a patient with human cowpox, which is another species of orthopoxvirus genus, suggesting a common pathogenesis.11 Mpox seems to have a predilection for conjunctival epithelial cells, and as in this case, conjunctivitis can be the initial presenting feature in animals.10,11 The mechanism for the severe ocular surface damage in this patient is unclear; however, it was associated with mpox and HIV coinfection. Although this patient had low viral load and almost normal CD4 cell count, it has been suggested that coinfection with HIV can alter the natural history and course of infection of mpox because of immunosuppression.1 However, a multicenter study found that patients with and without HIV with mpox c-infection has similar clinical presentations and severity, suggesting that the pathogenesis and host immune response to mpox are still poorly understood.3 Smallpox vaccination has also been thought to confer some protection against severe mpox infection; however, this patient had been previously vaccinated for smallpox, albeit more than 6 decades ago, and vaccine-induced immunity may be therefore insufficient.2
Four days after presentation, the patient developed necrotizing blepharoconjunctivitis, and we started treatment with topical trifluoridine and systemic tecovirimat. Although no US Food and Drug Administration–approved product for treatment of mpox exist, tecovirimat has been considered in the US Centers for Disease Control and Prevention management recommendation for cases with or at risk for severe monkeypox.6 As described in a case of necrotizing conjunctivitis due to cowpox virus, recovery of the ocular surface can be achieved by regular debridement as well as topical anti-inflammatory and antibiotic treatment with the hope of reducing the chance of secondary bacterial infection. In this case, we used an amniotic membrane, which has been shown to promote ocular surface healing and allow faster recovery of the corneal epithelium; however, in association with the severity of the keratitis, this patient developed corneal scarring with a negative effect on his vision.
Limitations
This study has limitations. This was a single case report and had a short follow-up period.
Conclusions
In summary, mpox infection can initially present with ophthalmic manifestations that may be self-limiting and managed with simple ocular surface therapies; however, it can be associated with severe and sight-threatening complications, such as necrotizing conjunctivitis. In patients presenting with severe ocular disease, systemic tecovirimat should be considered. Early recognition and intervention may limit the likelihood of substantial ocular morbidity.
Data Sharing Statement
References
- 1.Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210-e214. doi: 10.1093/cid/ciaa143 [DOI] [PubMed] [Google Scholar]
- 2.Damon IK. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29(suppl 4):D54-D59. doi: 10.1016/j.vaccine.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 3.Thornhill JP, Barkati S, Walmsley S, et al. ; SHARE-net Clinical Group . Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387(8):679-691. doi: 10.1056/NEJMoa2207323 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Monkeypox: public health advice for gay, bisexual and other men who have sex with men. Accessed August 11, 2022. https://www.who.int/news/item/25-05-2022-monkeypox--public-health-advice-for-gay--bisexual-and-other-men-who-have-sex-with-men
- 5.Kaufman AR, Chodosh J, Pineda R II. Monkeypox virus and ophthalmology—a primer on the 2022 monkeypox outbreak and monkeypox-related ophthalmic disease. JAMA Ophthalmol. Published online November 3, 2022. doi: 10.1001/jamaophthalmol.2022.4567 [DOI] [PubMed] [Google Scholar]
- 6.Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17-October 6, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(45):1449-1456. doi: 10.15585/mmwr.mm7145a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Learned LA, Reynolds MG, Wassa DW, et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73(2):428-434. doi: 10.4269/ajtmh.2005.73.428 [DOI] [PubMed] [Google Scholar]
- 8.Huhn GD, Bauer AM, Yorita K, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742-1751. doi: 10.1086/498115 [DOI] [PubMed] [Google Scholar]
- 9.Kempen JH. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol. 2011;151(1):7-10.e1. doi: 10.1016/j.ajo.2010.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langohr IM, Stevenson GW, Thacker HL, Regnery RL. Extensive lesions of monkeypox in a prairie dog (Cynomys sp). Vet Pathol. 2004;41(6):702-707. doi: 10.1354/vp.41-6-702 [DOI] [PubMed] [Google Scholar]
- 11.Kiernan M, Koutroumanos N. Orbital cowpox. N Engl J Med. 2021;384(23):2241. doi: 10.1056/NEJMicm2033620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement


