This post hoc analysis of the XIRIUS and XOLARIS studies investigates how changes in visual function associated with cotoretigene toliparvovec gene therapy in participants with RPGR-variant X-linked retinitis pigmentosa compare with those of untreated individuals.
Key Points
Question
How do visual function changes associated with cotoretigene toliparvovec (BIIB112/AAV8-RPGR) subretinal gene therapy in participants with X-linked retinitis pigmentosa with RPGR gene variations compare with those of untreated individuals?
Findings
In this post hoc analysis of 18 participants in the Clinical Trial of Retinal Gene Therapy for X-linked Retinitis Pigmentosa Using BIIB112 (XIRIUS) study and 103 participants in the Natural History of the Progression of X-Linked Retinitis Pigmentosa (XOLARIS) trial, early and sustained improvements in visual function were associated with this gene therapy through 12 months in some of the 12 participants who received the 4 highest doses of therapy in the XIRIUS trial compared with untreated individuals in the XOLARIS trial. There were no dose-limiting toxicities; reported serious adverse events included reduced visual acuity (2 participants) and noninfective retinitis (1 participant).
Meaning
Study results support pursuit of additional clinical trials with cotoretigene toliparvovec.
Abstract
Importance
X-linked retinitis pigmentosa (XLRP) is a severe cause of early-onset RP in male individuals, characterized by degeneration of photoreceptors, an extinguished electroretinogram, and vision loss.
Objective
To assess the duration of improvements in retinal sensitivity associated with a single, subretinal injection of cotoretigene toliparvovec (BIIB112/AAV8-RPGR) gene therapy after vitrectomy surgery in the dosed eye over 12 months in part 1 of the Clinical Trial of Retinal Gene Therapy for X-linked Retinitis Pigmentosa Using BIIB112 (XIRIUS) study, compared with untreated fellow eyes and eyes from the untreated subgroup from the Natural History of the Progression of X-Linked Retinitis Pigmentosa (XOLARIS) study.
Design, Setting, and Participants
This was a post hoc analysis of the XIRIUS and XOLARIS studies. Part 1 of the XIRIUS study was a phase 1, dose-escalation study of 18 male participants 18 years or older enrolled between March 8, 2017, and October 16, 2018, with genetically confirmed RPGR-variant XLRP with active disease and best-corrected visual acuity better than or equal to light perception (cohort 1), 34 to 73 letters (20/40 to 20/200 Snellen equivalent; cohorts 2-3), or greater than or equal to 34 letters (better than or equal to 20/200 Snellen equivalent; cohorts 4-6). Participants from the noninterventional, multicenter, global, prospective XOLARIS clinical study who met the inclusion and exclusion criteria of part 1 of XIRIUS were included as a comparator group (n = 103). Safety assessments included all XIRIUS participants; post hoc associations of retinal sensitivity assessments in XIRIUS only included the 12 participants receiving the 4 highest doses of cotoretigene toliparvovec. Data were analyzed on June 30, 2021.
Main Outcomes and Measures
Incidence of dose-limiting toxicities (DLTs), treatment-emergent adverse events, changes from baseline in retinal sensitivity (as assessed by macular integrity assessment microperimetry), retinal sensitivity response (achievement of ≥7-dB improvement from baseline at ≥5 of 16 central loci), and low-luminance visual acuity were assessed over 24 months.
Results
A total of 18 participants (mean [SD] age, 31.9 [9.4] years; male, 100%) were enrolled and completed the XIRIUS study. A subgroup of 103 participants (mean [SD] age, 30.8 [11.4] years; male, 100%) from the XOLARIS study was included. Administration of the 4 highest doses of cotoretigene toliparvovec (n = 12) among the 18 XIRIUS participants was associated with early improvements in retinal sensitivity. One of 103 untreated participants (1%) in the XOLARIS subgroup achieved improved retinal sensitivity at month 12. No DLTs were noted at any dose, and serious adverse events of reduced visual acuity (n = 2) and noninfective retinitis (n = 1) occurred.
Conclusions and Relevance
Results suggest that early and sustained improvements in retinal sensitivity and low-luminance visual acuity in some participants through 12 months support consideration of additional clinical trials.
Trial Registration
ClinicalTrials.gov Identifier: XIRIUS: NCT03116113; XOLARIS: NCT04926129
Introduction
X-linked retinitis pigmentosa (XLRP) is a severe and not uncommon cause of early-onset retinitis pigmentosa (RP) in male individuals, characterized by degeneration of photoreceptors, an extinguished electroretinogram response, and vision loss.1,2,3 Individuals with XLRP may experience onset of night blindness in childhood and early adolescence, followed by a reduction of visual field and visual acuity (VA) and progressively severe visual impairment.4,5 In developed countries, the prevalence of XLRP caused by variations in the RPGR gene is estimated to be 3.4 to 4.4 cases per 100 000 male individuals (eAppendix 1 in Supplement 1).6 There are no currently established or approved therapies available for modifying the progression of RPGR-variant XLRP; however, gene therapy using an adenoassociated virus (AAV) vector shows considerable promise.7,8,9
Cotoretigene toliparvovec (BIIB112/AAV8-RPGR)—a codon-optimized RPGR gene therapy delivered via the AAV8 vector, which contains recombinant complementary DNA encoding the RPGR ORF15 protein—is currently being investigated to potentially halt disease progression and improve vision in individuals with RPGR-variant XLRP by providing a full-length RPGR protein. Six-month results from the phase 1/2 Clinical Trial of Retinal Gene Therapy for X-linked Retinitis Pigmentosa Using BIIB112 (XIRIUS) study demonstrated that 7 of 18 participants experienced gains in visual function in eyes dosed with cotoretigene toliparvovec beginning at month 1 that were sustained at month 6 of follow-up.9 We present 12-month results assessing improvements in retinal sensitivity associated with cotoretigene toliparvovec subretinal gene therapy by comparing dosed eyes with untreated fellow eyes in the XIRIUS study and eyes from a subgroup of untreated individuals with similar baseline characteristics from the Natural History of the Progression of X-Linked Retinitis Pigmentosa (XOLARIS) RPGR-variant XLRP trial.
Methods
Study Design
This study, conducted from February 24 to April 9, 2021, was a post hoc analysis of the XIRIUS and XOLARIS studies and was approved by the following institutional review boards (IRBs)/research ethics committees (RECs): South Central–Oxford A REC of Bristol REC Centre, Bristol, UK; University of Pennsylvania IRB, Philadelphia, Pennsylvania, US; and Western IRB, Puyallup, Washington, US. Although part 1 of the XIRIUS trial was not a randomized study, Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines were followed where applicable for the reporting of results from the XIRIUS trial in this subanalysis and in its primary report.
Both the XIRIUS and XOLARIS studies were conducted in accordance with all applicable laws and regulations, including the International Conference on Harmonisation Guidelines for Good Clinical Practice, Good Epidemiological Practice guidelines, and the relevant articles of the Declaration of Helsinki, where permissible. Written and verbal versions of participant information and informed consent were presented to participants before evaluations for eligibility were performed. The protocol, informed consent/assent forms, and other relevant study documentation were submitted to appropriate ethics committees, regulatory authorities, and host institutions for written approval in accordance with local requirements. Participants were reimbursed for travel expenses but did not receive a stipend.
The XIRIUS Trial
The XIRIUS study was a 2-part, first-in-human, multicenter trial of a single subretinal injection of cotoretigene toliparvovec in male individuals with a genetically confirmed diagnosis of XLRP with RPGR gene variations (eMethods and eAppendix 3 in Supplement 1). Part 1 of the XIRIUS trial was a phase 1, dose-escalation study designed to identify the maximum-tolerated dose of cotoretigene toliparvovec by assessing safety and visual evaluations over a 24-month period (eFigure 1A in Supplement 1). The 3 + 3 dose-escalation scheme10 includes 6 dose cohorts of up to 100 μL of vector suspension containing: 5 × 109 vector genomes (vg; cohort 1), 1 × 1010 vg (cohort 2), 5 × 1010 vg (cohort 3), 1 × 1011 vg (cohort 4), 2.5 × 1011 vg (cohort 5), and 5 × 1011 vg (cohort 6). All participants underwent vitrectomy and received a single subretinal injection of cotoretigene toliparvovec in their dosed eye, as described previously.9,11 Selection of the dosed eye was made in conjunction with a surgical consultant and study sponsor and was generally the worse-affected eye. Participants were assessed for safety and visual evaluations throughout the study, and an independent data monitoring committee reviewed safety data before confirming whether escalation to a higher dose level should occur.
Eligible participants were male individuals 18 years or older with genetically confirmed XLRP caused by a variation in RPGR and active disease clinically visible within the macular region in both eyes (ellipsoid zone as measured by spectral domain optical coherence tomography at screening must have been within the nasal and temporal border of any B scan and not visible on the most inferior and superior B scan).9 Minimum best-corrected VA (BCVA) required for inclusion was better than or equal to light perception in cohort 1, 34 to 73 Early Treatment Diabetic Retinopathy Study (ETDRS) letters in cohorts 2 and 3 (approximate Snellen equivalent, 20/40 to 20/200), and greater than or equal to 34 ETDRS letters in cohorts 4 to 6 (approximate Snellen equivalent, better than or equal to 20/200).
The XOLARIS Study Subanalysis
The XOLARIS study is an ongoing, noninterventional, global, prospective, observational clinical study evaluating the natural progression of XLRP caused by RPGR gene variations in untreated male and female participants (planned enrollment, N = 300) (eMethods and eAppendix 3 in Supplement 1) followed up over a 24-month period (eFigure 1B in Supplement 1). Included as a comparator was a subgroup of male participants from the XOLARIS study 18 years and older who met XIRIUS part 1 inclusion criteria (both eyes were required to have BCVA ≥34 ETDRS letters at baseline) and had completed 12 months of follow-up. The study eye was defined as the worse eye as assessed by BCVA (or the right eye if both eyes were assessed as equal).
In both the XIRIUS and XOLARIS trials, participant race was categorized as American Indian or Alaskan Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, White, and other (with a field to specify). Ethnicity was categorized in both trials as Hispanic or Latino, not Hispanic or Latino, not reported, or unknown.
Clinical Assessments
Primary safety end points included incidence of dose-limiting toxicities (DLTs) and treatment-emergent adverse events (TEAEs) over 24 months (eMethods in Supplement 1). Changes from baseline in retinal sensitivity (as assessed by macular integrity assessment [MAIA] microperimetry under dark/mesopic conditions) and change from baseline in low-luminance VA (LLVA) at 12 months were secondary end points (eAppendix 2 in Supplement 1). Comparisons of retinal sensitivity with the control XOLARIS subgroup were post hoc analyses. Mesopic microperimetry (MAIA [CenterVue SpA]) was conducted for both eyes at various study points using a standard 10-2 grid. The retinal sensitivity responder criterion was defined as achievement of a 7-dB or greater improvement from baseline at 5 or more of 16 central loci using the 10-2 grid; this definition was expected to capture improved sensitivity in the central fovea that was likely to be clinically meaningful. LLVA was assessed over 12 months using a standard ETDRS chart by placing a 2.0–log unit neutral density filter over the front of each eye during examination.
Statistical Analysis
Unless otherwise specified, the methods for part 1 of the XIRIUS trial were part of a preplanned statistical analysis including all enrolled study participants who received cotoretigene toliparvovec. System organ class and preferred term were used to summarize AEs. Serious AEs (SAEs), AE severity, and the association of AE with study drug/procedure were also summarized. Visual function analyses included a comparison of dosed eyes vs untreated fellow eyes in the XIRIUS trial. The comparison of changes in retinal sensitivity associated with cotoretigene toliparvovec and that of the XOLARIS subgroup was a post hoc analysis.
Continuous variables and their change from baseline are summarized using descriptive statistics (number of participants, mean, and SD or SE). For categorical variables, the number and proportion of participants pertaining to each category are presented. This analysis presents visual function results through month 12 for the therapeutic doses assessed in XIRIUS trial dose cohorts 3 to 6. The correlation analysis of LLVA and microperimetry (ad hoc) included individual data for each visit up to 12 months for each participant in cohorts 3 to 6 for the dosed eyes and the untreated fellow eyes. Safety results are presented separately for cohorts 3 to 6 and for all participants in the XIRIUS trial. Data were analyzed on June 30, 2021, using SAS software.
Results
Participant Disposition and Baseline Characteristics
In part 1 of the XIRIUS trial, 18 male participants (mean [SD] age, 31.9 [9.4] years; Hispanic or Latino, 1 [5.6%]; White, 18 [100%]) were enrolled and completed the study. A subgroup of 103 male participants (mean [SD] age, 30.8 [11.4] years; Asian, 4 [3.9%]; Black or African American, 1 [1.0%]; Hispanic or Latino, 4 [3.9%]; Native Hawaiian or other Pacific Islander, 1 [1.0%]; White, 96 [93.2%]) from the XOLARIS study was included (data cut: electronic data capture, March 1, 2021; Doheny Image Reading Center, February 24, 2021). Demographics and baseline disease characteristics were generally comparable between participants in dose cohorts 3 to 6 (therapeutic doses, n = 12) of the XIRIUS trial and the untreated subgroup from the XOLARIS study (Table 1).12
Table 1. Participant Demographics and Baseline Disease Characteristics in the Clinical Trial of Retinal Gene Therapy for X-Linked Retinitis Pigmentosa Using BIIB112 (XIRIUS) and Natural History of the Progression of X-Linked Retinitis Pigmentosa (XOLARIS) Studies.
| Parameter | XIRIUS (cohorts 3-6) (n = 12) | XOLARIS subgroup meeting criteria for XIRIUS (n = 103) |
|---|---|---|
| Age, mean (range), y | 30.6 (20.7-50.7) | 30.8 (18.0-84.0) |
| Race, No. (%) | ||
| Asian | 0 | 4 (3.9) |
| Black or African American | 0 | 1 (1.0) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (1.0) |
| White | 12 (100) | 96 (93.2) |
| Ethnicity, No. (%) | ||
| Hispanic or Latino | 1 (8.3) | 4 (3.9) |
| Not reported | 0 | 1 (1.0) |
| Retinal sensitivity, mean (SD), dB (16 central loci) | ||
| Study eyea | 7.8 (5.9) | 8.8 (6.7) |
| Untreated fellow eye | 9.1 (6.2) | 9.1 (6.5) |
| Retinal sensitivity, mean (SD), dB (68 central loci) | ||
| Study eyea | 3.9 (6.4) | 4.4 (4.5) |
| Untreated fellow eye | 4.6 (7.2) | 4.7 (4.6) |
| BCVA, ETDRS letters, mean (SD) [approximate Snellen equivalent] b | ||
| Study eyea | 66.5 (11.8) [20/40 to 20/50] | 63.2 (10.9) [20/50 to 20/63] |
| Untreated fellow eye | 69.3 (11.2) [20/40 to 20/50] | 67.5 (10.1) [20/40 to 20/50] |
| Ellipsoid zone width, mean (SD), μm | ||
| Study eyea | 966.8 (1623.0) | 543.3 (680.8) |
| Untreated fellow eye | 1379.1 (2462.7) | 611.1 (701.5) |
| Ellipsoid zone area, mean (SD), mm2 | ||
| Study eyea | 2.9 (7.2) | 0.5 (0.8) |
| Untreated fellow eye | 5.4 (13.0) | 0.6 (0.9) |
Abbreviations: BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study.
In the XIRIUS trial part 1, the study eye was the dosed eye.
Snellen equivalent approximate is given as ranges on the basis of previously published conversion tables.12
Visual Function Changes
Microperimetry
Cotoretigene toliparvovec in dose cohorts 3 to 6 was associated with an early and sustained therapeutic benefit, as defined by the retinal sensitivity responder criterion (≥7-dB improvement from baseline at ≥5 of 16 central loci). At month 1, 6 of 12 participants (50%) in cohorts 3 to 6 met the responder definition (Figure 1A; example microperimetry images in a responder participant in cohort 4 are shown in eFigure 2 in Supplement 1). Through 12 months of follow-up, these improvements in retinal sensitivity were maintained in 4 of 12 participants (33%). One untreated contralateral eye (8%), which would have met the entry criteria as a study eye (ie, dosed eye), in XIRIUS cohorts 3 to 6 met the responder definition at month 1, and another untreated contralateral eye (8%) met the responder definition at month 12 (Figure 1A). Untreated contralateral eyes from 2 participants (17%) met the responder definition at month 9. At 12 months, 1 of 102 untreated participants (1%) in the XOLARIS study subgroup met the responder definition in the study eye (Figure 1B). The correlation between change from baseline in mean retinal sensitivity in 16 central loci assessed by MAIA microperimetry and mean LLVA was observed only in the dosed eyes through 12 months of follow-up (Pearson r = 0.6185; untreated eyes: r = −0.1574; Figure 1C and D).
Figure 1. Responder Analysis of Dosed Eyes in the Clinical Trial of Retinal Gene Therapy for X-Linked Retinitis Pigmentosa Using BIIB112 (XIRIUS) and Natural History of the Progression of X-Linked Retinitis Pigmentosa (XOLARIS) Studies.
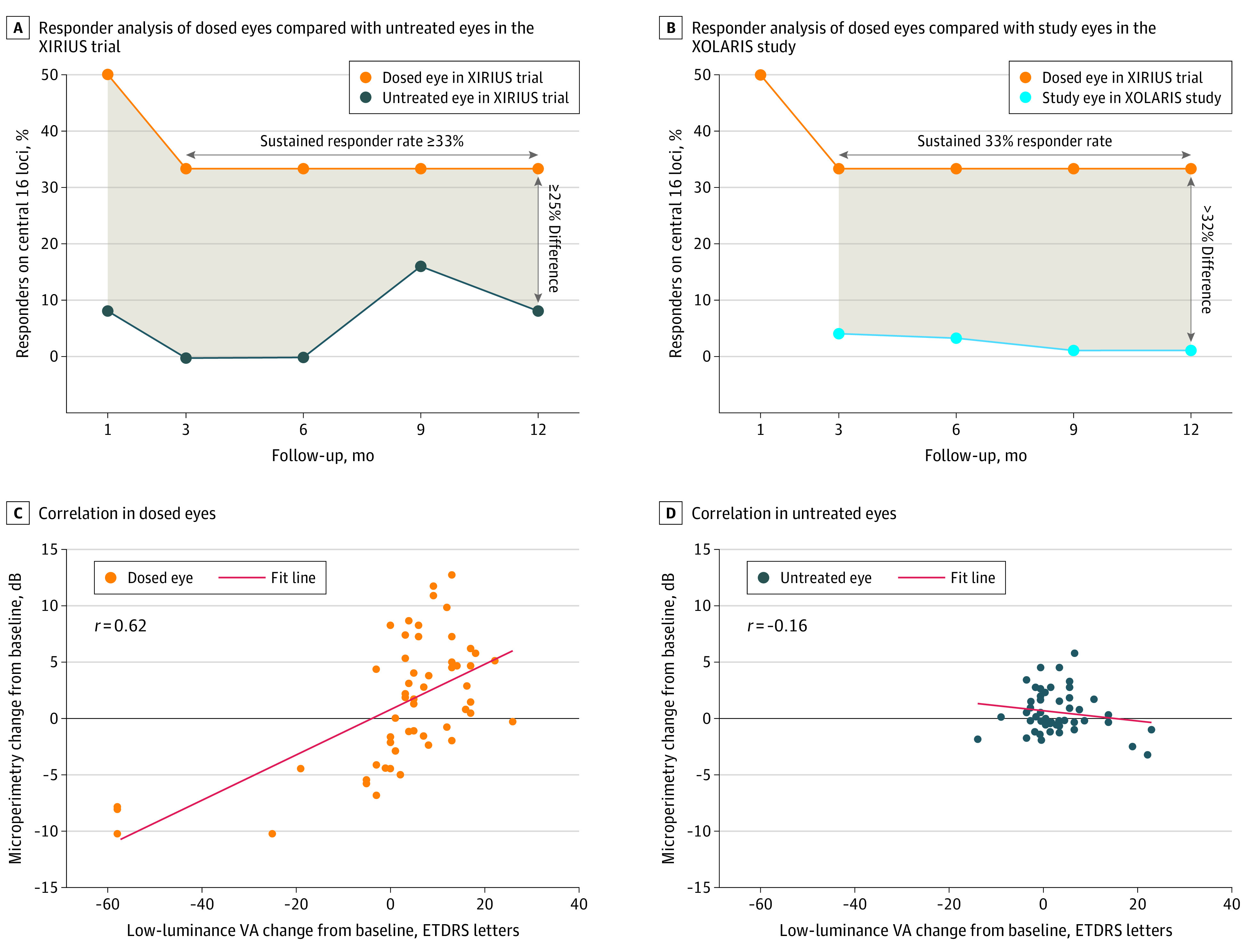
Responder analysis (achievement of ≥7-dB improvement from baseline at ≥5 of 16 central loci) in the XIRIUS trial part 1 therapeutic dose cohorts 3 to 6 using the dosed eyes compared with untreated eyes in the XIRIUS trial (A) and study eyes from participants in the XOLARIS study (B). The shading in panels A and B indicates the difference between the dosed and untreated eye in the XIRIUS trial (A) and the dosed eye in the XIRIUS trial and the study eye in the XOLARIS study (B). Mean retinal sensitivity in 16 central loci vs low-luminance visual acuity (VA) change from baseline for all visits up to month 12 in dosed (C) and untreated (D) fellow eyes among participants in XIRIUS part 1 cohorts 3 to 6 (n = 11; 1 participant had no baseline low-luminance VA). The correlation between change from baseline in mean retinal sensitivity in the 16 central loci assessed by macular integrity assessment microperimetry and low-luminance VA is shown. ETDRS indicates Early Treatment Diabetic Retinopathy Study.
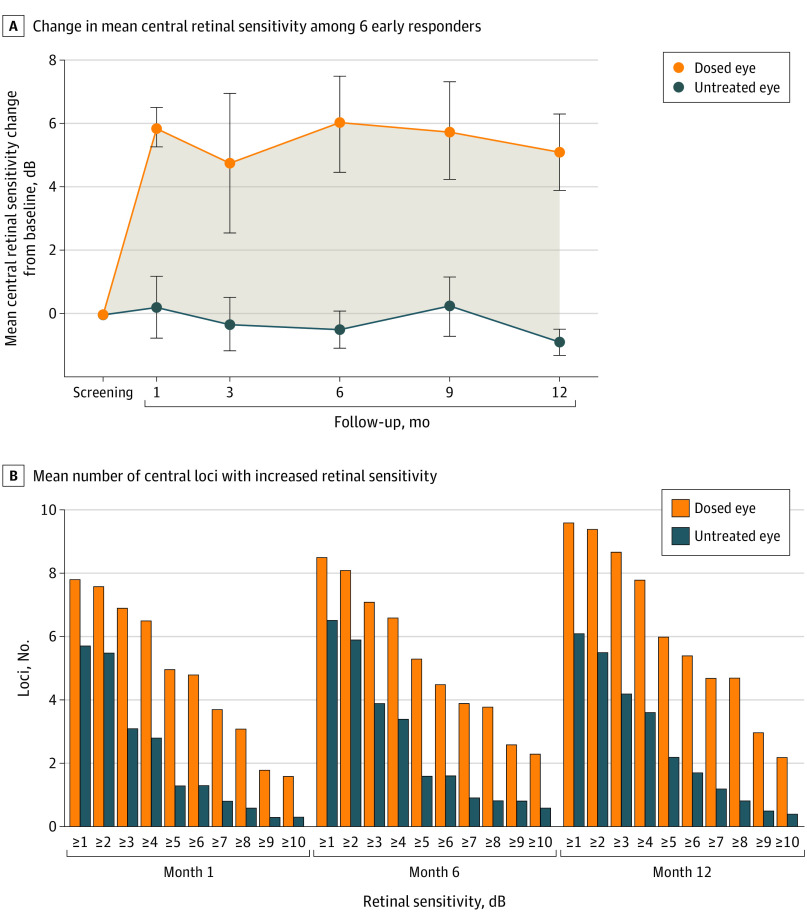
Among the 6 responders in cohorts 3 to 6 at month 1 (the therapeutic range), the mean change from baseline in mean central retinal sensitivity among dosed eyes at month 12 was 5.1 dB, compared with −0.9 dB in untreated fellow eyes (Figure 2A). Improvements in retinal sensitivity (measured by number of 16 central loci with increased retinal sensitivity from baseline) in the dosed eye were observed across a wide range of decibel intervals (Figure 2B). On average, 4.7 loci improved by 7 dB or more in the dosed eyes at month 12, compared with 1.2 loci in the fellow eyes.
Figure 2. Retinal Sensitivity in the Clinical Trial of Retinal Gene Therapy for X-Linked Retinitis Pigmentosa Using BIIB112 (XIRIUS) Study Part 1 Therapeutic Dose Cohorts 3 to 6.
A, Change from baseline in mean central retinal sensitivity for 6 early (month 1) responders. The shading in panel A indicates the difference between the dosed and untreated eye. Error bars represent the SE. B, Mean number of 16 central loci with increased retinal sensitivity from baseline.
LLVA
Improvements in LLVA response in cohorts 3 to 6 (ie, gains of ≥10 and ≥15 ETDRS letters) were observed in dosed eyes in the XIRIUS trial as early as month 1 and were sustained through 12 months of follow-up (Figure 3A and B). Among 11 participants with available baseline LLVA assessment score, improvements in LLVA responses (gain of ≥15 ETDRS letters at month 12) were observed in 3 dosed eyes (27%) compared with 1 untreated fellow eye (9%).
Figure 3. Low-Luminance Visual Acuity (VA) in Dosed and Untreated Eyes in the Clinical Trial of Retinal Gene Therapy for X-Linked Retinitis Pigmentosa Using BIIB112 (XIRIUS) Study Part 1.
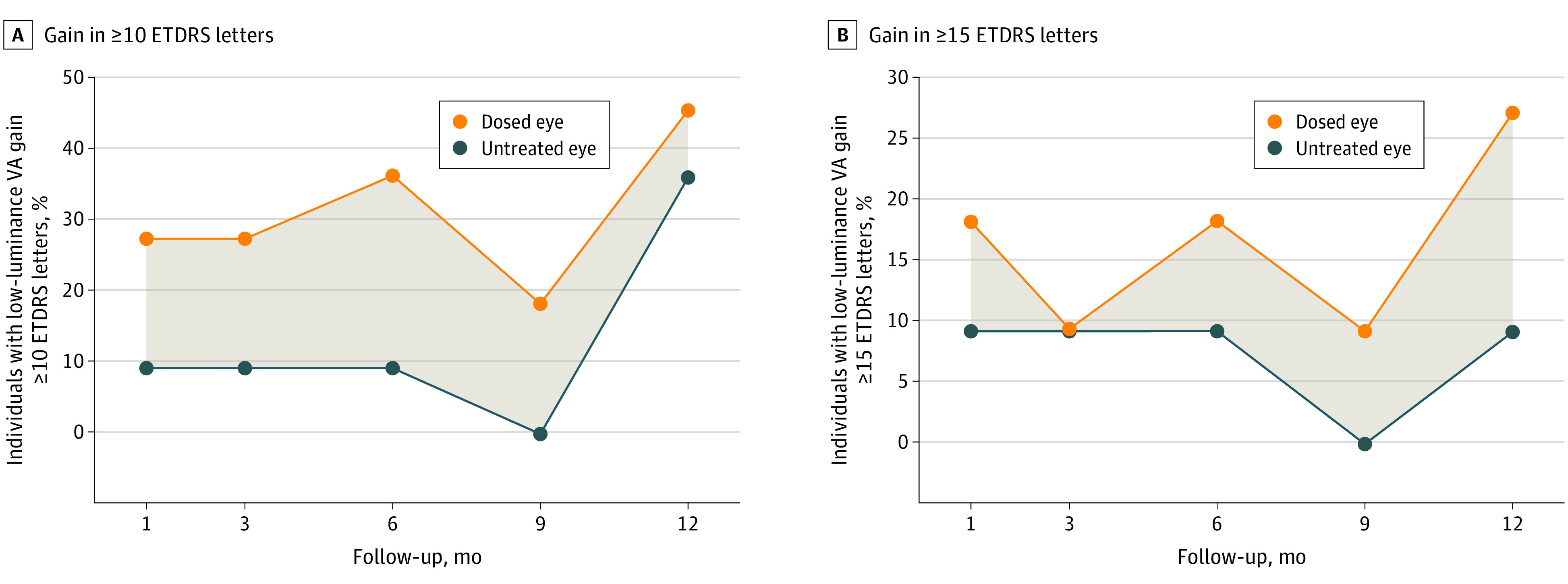
A, Gain in low-luminance VA Early Treatment Diabetic Retinopathy Study (ETDRS) score greater than or equal to 10 letters. B, Gain in low-luminance VA ETDRS score greater than or equal to 15 letters. The shading in panels A and B indicates the difference between the dosed and untreated eye.
Safety
Cotoretigene toliparvovec was not associated with any DLTs. Overall, 73 AEs occurred in the dosed eye in the XIRIUS trial, with most events reported in cohorts 3 to 6 (58 of 73 events) (Table 2). The majority of TEAEs in the dosed eye were mild (n = 10, 55 of 73 events; cohorts 3-6, 42 of 58 events) and recovered/resolved completely (all participants, 60 of 73 events [for remainder, 8 were unresolved, 4 were resolved with sequalae, and 1 was of unknown status]; cohorts 3-6, 47 of 58 events [for remainder, 6 were unresolved, 4 were resolved with sequalae, and 1 was of unknown status]).
Table 2. Summary of Treatment-Emergent Adverse Events (TEAEs) in Part 1 of the Clinical Trial of Retinal Gene Therapy for X-Linked Retinitis Pigmentosa Using BIIB112 (XIRIUS) Study.
| Parameter | All participants (n = 18) | Cohorts 3-6 (n = 12) | ||
|---|---|---|---|---|
| Participants, No. (%) | Events, No. | Participants, No. (%) | Events, No. | |
| All TEAEs | 18 (100) | 109 | 12 (100) | 84 |
| Non-ocular TEAEs | 9 (50) | 30 | 5 (42) | 23 |
| Ocular TEAEs | 18 (100) | 79 | 12 (100) | 61 |
| Dosed eye | 18 (100) | 73 | 12 (100) | 58 |
| Untreated fellow eye | 5 (28) | 6 | 3 (25) | 3 |
| Most common ocular TEAEs in the dosed eyea | ||||
| Increased intraocular pressure | 6 (33) | 10 | 5 (42) | 9 |
| Noninfective retinitis | 5 (28) | 8 | 5 (42) | 8 |
| Eye inflammation | 4 (22) | 5 | 4 (33) | 5 |
| Anterior chamber inflammation | 3 (17) | 3 | 1 (8) | 1 |
| Conjunctival hemorrhage | 3 (17) | 3 | 0 | 0 |
| Vision blurred | 3 (17) | 3 | 3 (25) | 3 |
| Severity of TEAEs in the dosed eye | ||||
| Mild | 10 (56) | 55 | 6 (50) | 42 |
| Moderate | 4 (22) | 14 | 4 (33) | 14 |
| Severe | 3 (17) | 4 | 1 (8) | 2 |
| All withdrawals related to TEAEs | 0 | 0 | 0 | 0 |
| All TEAEs related to treatment | 18 (100) | 64 | 12 (100) | 52 |
| TEAEs related to study drug in the dosed eye | 6 (33) | 16 | 6 (50) | 16 |
| TEAEs related to procedure in the dosed eye | 17 (94) | 35 | 11 (92) | 25 |
| Most common TEAEs related to treatment in the dosed eyeb | ||||
| Noninfective retinitis | 3 (17) | 5 | 3 (25) | 5 |
| Corneal deposits | 2 (11) | 2 | 2 (17) | 2 |
| All serious TEAEs | 5 (28) | 6 | 3 (25) | 4 |
| Serious TEAEs in the dosed eye | 5 (28) | 5 | 3 (25) | 3 |
| Visual acuity reduced | 2 (11) | 2 | 2 (17) | 2 |
| Noninfective retinitis | 1 (6) | 1 | 1 (8) | 1 |
| Retinal detachment | 1 (6) | 1 | 0 | 0 |
| Visual impairment | 1 (6) | 1 | 0 | 0 |
Occurring in more than 15% of all participants.
Occurring in more than 10% of all participants.
Among all participants, 17 participants (94%) experienced 35 TEAEs associated with study procedure, and 6 participants (33%) experienced 16 TEAEs associated with the study drug. Ocular inflammation–associated TEAEs occurred in 12 participants (67%; 20 events, 19 of which were in the dosed eye). Ocular inflammation–associated TEAEs were treated with corticosteroids, and all except 1 (described in detail in the following paragraph) were considered recovered by month 24. Fifteen ocular inflammation–associated TEAEs (75%) occurred 30 days or more after study drug administration. A total of 10 TEAEs associated with reduction in VA were experienced by 8 participants (44.4%). Eight of the events were considered recovered by month 24, and the other 2 were reported as SAEs.
Six SAEs were reported (4 in cohorts 3-6), 5 of which were in the dosed eye. Two participants, 1 event each from cohort 5 and 6, experienced SAEs of reduced VA. In the participant from cohort 5, a reduction in BCVA by 16 ETDRS letters was reported at the month 9 visit, which was considered associated with study treatment and procedure. Ocular inflammation was observed in the dosed eye and was treated with topical corticosteroids. By the month 24 visit, the SAE was not resolved, and return to baseline VA was not expected because of loss of central photoreceptors secondary to ocular inflammation. The participant from cohort 6 experienced reduced VA from day 133 to day 154, which was considered associated with study drug and procedure (but with unknown attribution) and resolved after treatment with corticosteroids. One participant in cohort 1 experienced 1 SAE of visual impairment that occurred on day 734 (BCVA reduced from 22 ETDRS letters at baseline to 2 ETDRS letters). The event was not considered associated with study drug or study procedure but rather from natural disease progression because of presence of advanced retinal degeneration. One participant from cohort 6 experienced 1 SAE of noninfective retinitis that occurred on day 795 and was considered associated with study procedure (as indicated by patches of atrophy at the injection site) and study drug. Although VA recovered with sequelae, retinal sensitivity as assessed by microperimetry did not recover. In 1 participant from cohort 2, an SAE of retinal detachment was observed on day 823 and was considered associated with study procedure. The event was resolved on day 864 after a vitrectomy with gas tamponade.
Discussion
In this post hoc analysis of the XIRIUS and XOLARIS studies, in participants in dose cohorts 3 to 6 in the XIRIUS trial, cotoretigene toliparvovec was associated with early and sustained improvements up to 12 months in some participants, whereas improvement in retinal sensitivity was uncommon in similar untreated participants from the XOLARIS natural history study. No DLTs were observed at any dose. Most TEAEs were reported as associated with the surgical procedure. Of the TEAEs considered associated with study drug in the dosed eye, eye inflammation was the most common (6 events) and was reported only at higher doses (cohorts 4-6). One event of eye inflammation and 2 events of reduced VA were among the SAEs; therefore, risks of the study drug and procedure should be considered when evaluating the sustained improvements in retinal sensitivity.
One month after administration of the highest 4 doses of cotoretigene toliparvovec (cohorts 3-6), 6 of 12 participants (50%) met the retinal sensitivity responder definition as assessed by microperimetry at month 1. Four of 12 participants (33%) still maintained this level of improvement through 6 and 12 months (individual changes from baseline in microperimetry at 6 months were previously published).9 Analysis of the mean central retinal sensitivity change from baseline in early responders (n = 6) in cohorts 3 to 6 at 12 months showed a 6-dB improvement in dosed vs untreated eyes, providing additional evidence that improvement in visual function was sustained in dosed eyes.
The sustained nature of the improvement in cone function may be relevant to the design of the vector that expressed the full-length and fully glutamylated RPGR protein without any deletions of the ORF15 region.8 In-frame ORF15 deletions lead to reduced glutamylation of the RPGR protein, which is associated with cone dystrophy,13 and most likely result in progressive cone loss over time. At high doses, shortened RPGR proteins with ORF15 deletions may have a toxic effect on photoreceptors.14,15 Hence, the long-term clinical data presented here support the use of codon-optimization within the vector to prevent the cloning errors that are frequently seen in the wild-type RPGR sequence that may lead to in-frame ORF15 deletions.16
When comparing responder rates of dosed eyes in the XIRIUS study with the responder rates of untreated eyes in the subgroup of XOLARIS participants, cotoretigene toliparvovec administration was associated with a greater than 32% difference in retinal sensitivity response between the 2 groups after 12 months of treatment. Comparing dosed eyes of XIRIUS participants with untreated eyes of XOLARIS participants may better indicate potential improvements in visual function than comparing dosed eyes with contralateral eyes of XIRIUS participants. The 2020 US Food and Drug Administration guidelines for gene therapy in retinal disorders recommend use of untreated participants as a parallel comparator in clinical trials rather than the untreated fellow eye of treated participants.17 Among reasons cited are that the dosed eye and contralateral eye may be at different stages of disease progression at the time of trial entry and that ophthalmic assessments in contralateral eyes may be influenced by changes in the dosed eye.17,18
LLVA is a measure of mesopic visual function at low light levels.19 Loss of VA under low-luminance conditions is reported to precede loss of BCVA in RP.20 This may be due to LLVA being dependent on the preserved function of a larger central macular area than standard BCVA.20 Because of the relatively slow rate of decline in BCVA as XLRP progresses, a treatment benefit in LLVA might be observed earlier than a benefit in BCVA. Improvements in LLVA were observed in dosed eyes of participants in the XIRIUS trial as early as month 1 and were sustained through 12 months of follow-up. The difference in LLVA improvements between dosed and untreated eyes was most prominent in the analysis using a gain of 15 or more ETDRS letters over baseline. The high response rates in untreated fellow eyes, which were highest at month 12 using a gain of 10 or more ETDRS letters as a cutoff, may in part be due to test-retest variability observed with this assessment and also the natural history of variable visual function throughout the day. These changes in LLVA appeared to correlate with changes in retinal sensitivity, which is expected because both functions are considered cone-derived under low-light conditions with no preassessment dark adaptation. This correlation lends further support to the potential clinical benefit of cotoretigene toliparvovec administration.
Limitations
A limitation of this analysis is the small number of participants in each cohort (n = 3) in part 1 of the XIRIUS trial due to the dose-escalation study design. Therefore, no formal sample size computation was performed, and the study was not powered for efficacy comparisons. Also, there were no formal statistics performed to quantify differences in baseline disease characteristics between the XIRIUS study participants and the XOLARIS subgroup. The study design only included adults because pediatric participants may not have been able to comply, adequately perform study assessments, and have sufficiently advanced disease that was encroaching on the macula. Further, the open-label study design and the untreated concurrent parallel controls introduce the risk of response bias.
Conclusions
In conclusion, as reported through 12 months, results of this post hoc analysis of the XIRIUS and XOLARIS trials suggest that participants receiving cotoretigene toliparvovec administration experienced no DLTs, and some participants showed early and sustained improvements in important measures of visual function. Considering that SAEs of reduced VA and eye inflammation were observed, additional data from these studies will further assess the safety and visual evaluations of a single subretinal injection of cotoretigene toliparvovec in individuals with RPGR-variant XLRP and natural progression of the disease.
eAppendix 1. Background
eMethods.
eAppendix 2. Discussion
eFigure 1. Study Design
eFigure 2. Microperimetry Image of a Participant From XIRIUS Part 1 Cohort 4 With Sustained Improvement in Retinal Sensitivity at 1 Month Sustained Through 12 Months
eAppendix 3. Study Groups
eReferences
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Martinez-Fernandez De La Camara C, Nanda A, Salvetti AP, Fischer MD, MacLaren RE. Gene therapy for the treatment of X-linked retinitis pigmentosa. Expert Opin Orphan Drugs. 2018;6(3):167-177. doi: 10.1080/21678707.2018.1444476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang WC, Wright AF, Roman AJ, et al. RPGR-associated retinal degeneration in human X-linked RP and a murine model. Invest Ophthalmol Vis Sci. 2012;53(9):5594-5608. doi: 10.1167/iovs.12-10070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong DH, Pawlyk BS, Shang J, Sandberg MA, Berson EL, Li T. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc Natl Acad Sci U S A. 2000;97(7):3649-3654. doi: 10.1073/pnas.97.7.3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40. doi: 10.1186/1750-1172-1-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kousal B, Skalicka P, Valesova L, et al. Severe retinal degeneration in women with a c.2543del mutation in ORF15 of the RPGR gene. Mol Vis. 2014;20:1307-1317. [PMC free article] [PubMed] [Google Scholar]
- 6.Vinikoor-Imler LC, Simpson C, Narayanan D, Abbasi S, Lally C. Prevalence of RPGR-mutated X-linked retinitis pigmentosa among males. Ophthalmic Genet. 2022;43(5):581-588. doi: 10.1080/13816810.2022.2109686 [DOI] [PubMed] [Google Scholar]
- 7.Megaw RD, Soares DC, Wright AF. RPGR: its role in photoreceptor physiology, human disease, and future therapies. Exp Eye Res. 2015;138:32-41. doi: 10.1016/j.exer.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer MD, McClements ME, Martinez-Fernandez de la Camara C, et al. Codon-optimized RPGR improves stability and efficacy of AAV8 gene therapy in 2 mouse models of X-linked retinitis pigmentosa. Mol Ther. 2017;25(8):1854-1865. doi: 10.1016/j.ymthe.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cehajic-Kapetanovic J, Xue K, Martinez-Fernandez de la Camara C, et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med. 2020;26(3):354-359. doi: 10.1038/s41591-020-0763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storer BE. Design and analysis of phase 1 clinical trials. Biometrics. 1989;45(3):925-937. doi: 10.2307/2531693 [DOI] [PubMed] [Google Scholar]
- 11.Xue K, Groppe M, Salvetti AP, MacLaren RE. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond). 2017;31(9):1308-1316. doi: 10.1038/eye.2017.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194-205. doi: 10.1016/S0002-9394(02)01825-1 [DOI] [PubMed] [Google Scholar]
- 13.Sun X, Park JH, Gumerson J, et al. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc Natl Acad Sci U S A. 2016;113(21):E2925-E2934. doi: 10.1073/pnas.1523201113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong D-H, Pawlyk BS, Adamian M, Li T. Dominant, gain-of-function mutant produced by truncation of RPGR. Invest Ophthalmol Vis Sci. 2004;45(1):36-41. doi: 10.1167/iovs.03-0787 [DOI] [PubMed] [Google Scholar]
- 15.Wu Z, Hiriyanna S, Qian H, et al. A long-term efficacy study of gene replacement therapy for RPGR-associated retinal degeneration. Hum Mol Genet. 2015;24(14):3956-3970. doi: 10.1093/hmg/ddv134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Fernandez de la Camara C, Cehajic-Kapetanovic J, MacLaren RE. RPGR gene therapy presents challenges in cloning the coding sequence. Expert Opin Biol Ther. 2020;20(1):63-71. doi: 10.1080/14712598.2020.1680635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration . Human gene therapy for retinal disorders: guidance for industry. Accessed May 16, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-gene-therapy-retinal-disorders
- 18.Zweifel SA, Saroj N, Shapiro H, Freund KB. The effect of fellow eye visual acuity on visual acuity of study eyes receiving ranibizumab for age-related macular degeneration. Retina. 2012;32(7):1243-1249. doi: 10.1097/IAE.0b013e3182469064 [DOI] [PubMed] [Google Scholar]
- 19.Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(9):1480-1488, 1488.e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood LJ, Jolly JK, Josan AS, Buckley TMW, MacLaren RE. Low luminance visual acuity and low luminance deficit in choroideremia and RPGR-associated retinitis pigmentosa. Transl Vis Sci Technol. 2021;10(2):28. doi: 10.1167/tvst.10.2.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Background
eMethods.
eAppendix 2. Discussion
eFigure 1. Study Design
eFigure 2. Microperimetry Image of a Participant From XIRIUS Part 1 Cohort 4 With Sustained Improvement in Retinal Sensitivity at 1 Month Sustained Through 12 Months
eAppendix 3. Study Groups
eReferences
Nonauthor Collaborators
Data Sharing Statement