Abstract
Panax notoginseng is an ancient Chinese medicinal plant that has great clinical value in regulating cardiovascular disease in China. As a single component of panax notoginosides, notoginsenoside R1 (NGR1) belongs to the panaxatriol group. Many reports have demonstrated that NGR1 exerts multiple pharmacological effects in ischemic stroke, myocardial infarction, acute renal injury, and intestinal injury. Here, we outline the available reports on the pharmacological effects of NGR1 in ischemia-reperfusion (I/R) injury. We also discuss the chemistry, composition and molecular mechanism underlying the anti-I/R injury effects of NGR1. NGR1 had significant effects on reducing cerebral infarct size and neurological deficits in cerebral I/R injury, ameliorating the impaired mitochondrial morphology in myocardial I/R injury, decreasing kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin in renal I/R injury and attenuating jejunal mucosal epithelium injury in intestinal I/R injury. The various organ anti-I/R injury effects of NGR1 are mainly through the suppression of oxidative stress, apoptosis, inflammation, endoplasmic reticulum stress and promotion of angiogenesis and neurogenesis. These findings provide a reference basis for future research of NGR1 on I/R injury.
Keywords: Notoginsenoside R1, Ischemia/reperfusion injury, Chemistry, Pharmacological properties, Molecular mechanism
Abbreviations: NGR1, notoginsenoside R1; I/R, ischemia/reperfusion; MIRI, myocardial ischemic/reperfusion injury; ER, endoplasmic reticulum; ROS/RNS, reactive oxygen/nitrogen species; GSH, glutathione; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, nicotinamide adenosine dinucleotide phosphate; Cyt-C, cytochrome-C; MDA, malondialdehyde; OGD/R, oxygen-glucose deprivation/reoxygenation; Δψm, mitochondrial membrane potential; Bax, Bcl-2-associated X protein; HBMECs, human brain microvascular endothelial cells; PERK, protein kinase R-like endoplasmic reticulum kinase; ATP, adenosine triphosphate; GRP78, proteins glucose regulatory protein 78
Introduction
The restoration of blood supply to tissues or organs after ischemia will aggravate the damage to the ischemic tissue. This phenomenon is called ischemia/reperfusion (I/R) injury. I/R injury mainly includes ischemic stroke, myocardial infarction, acute kidney injury and intestinal injury. Many factors can cause or affect I/R injury, such as coronary artery bypass, thrombolytic therapy and other vascular recanalization procedures, as well as heart/lung/brain resuscitation after cardiac arrest and organ transplantation.1 At present, antiplatelet drugs have shown good effect on the prevention of platelet adhesion and aggregation, but the effect on energy metabolism disorders and oxidative stress is poor.2 Currently, tissue plasminogen activator is the only drug approved by the US Food and Drug Administration for treating acute ischemic stroke. Unfortunately, because of the narrow treatment window, contraindications, and complications, only 3%–5% of patients currently receive tissue plasminogen activator treatment. In addition, reocclusion after thrombolysis leads to impaired neurological function and higher in-hospital mortality.3 Therefore, the search for new drugs to improve I/R injury remains an urgent issue in both basic research and clinical studies.
Ischemic stroke is a highly complex pathophysiological process that involves cerebrovasculature and parenchymal tissues.4, 5, 6 Most therapeutic targets/drugs have focused on multiple mechanisms underlying cerebral I/R injury, including neuronal excitotoxicity, excessive glutamate release, inflammation, oxidative injury, apoptosis, free radical release, mitochondrial responses, angiogenesis and neurogenesis.7 Accumulating evidence obtained from laboratory investigations shows that traditional Chinese medicine has made remarkable achievements in decreasing the risk of ischemic stroke.8, 9, 10, 11 There is evidence that also suggests the potent pro-neuroprotective and pro-neurorestorative benefits of isolated compounds, which provides a promising therapeutic strategy to protect against ischemic stroke.8,10,11
Panax notoginseng (Burk.) F.H. Chen (PN) is known as “aspirin” in Chinese medicine. This plant has been widely used for over 400 years and still holds an iconic position in traditional Chinese medicine. PN roots have been used as herbal medicines for several thousand years to promote hemostasis and blood circulation. Inspired by this history, a large number of modern studies have made substantial efforts to explore the diverse bioactive components and multifarious pharmacological properties to regulate circulatory disorders. The primary effects of PN are mainly attributed to panax notoginosides, which exert versatile protective and therapeutic effects against complex cardiovascular12,13 and cerebrovascular diseases.14, 15, 16 Individual PNSs have been used to explore pharmacological activities and underlying mechanisms due to their different chemical structures. With the development of advanced technology, more than 80 PNSs have been isolated and identified from the roots, leaves and/or stems and flower heads of PN.17
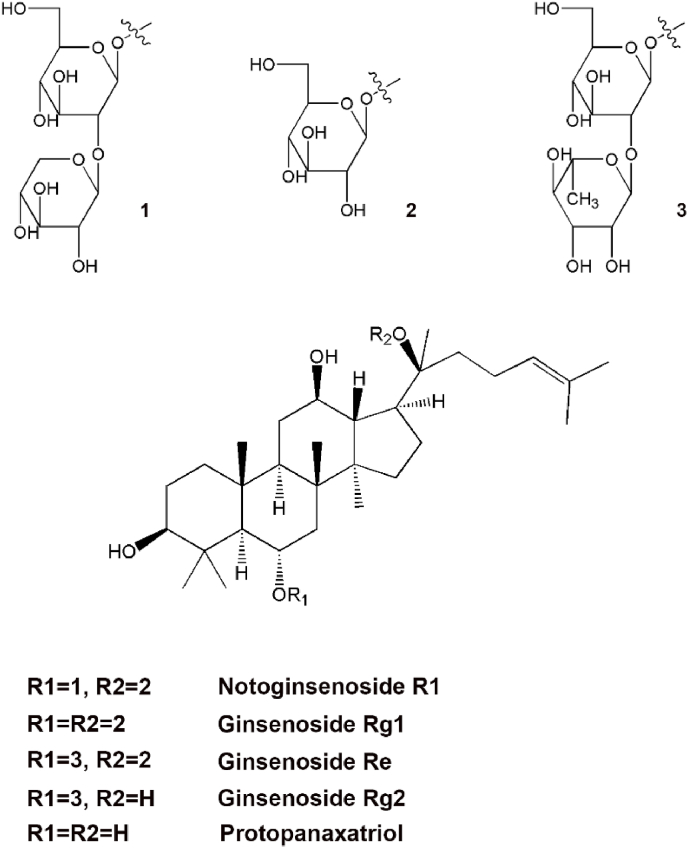
Notoginsenoside R1 (NGR1, chemical structure and related ginsenosides are shown in Fig. 1) is a major PNS and unique constituent of PN roots. The literature has shown that NGR1 exhibits anti-I/R injury effects via multiple complex mechanisms both in vivo and in vitro.18,19 However, the molecular mechanisms underlying this effect have not yet been systematically outlined in the literature. The purpose of this paper is to review the chemistry, therapeutic effects, and molecular mechanisms of NGR1 on I/R injury.
Fig. 1.
Chemical structure of NGR1 and related ginsenosides.
Chemistry and composition of NGR1
Most of the various saponins isolated from PN are dammarane-type tetracyclic triterpenes, which have strong biological activities. Based on the presence or absence of aglycone, dammarane-type saponins can be divided into protopanaxadiol (e.g., ginsenoside Rb1, Rb3, Rg3 and Rd) and protopanaxatriol groups (e.g., NGR1, ginsenoside Rg1 and Re) according to whether the C-6 position of the molecular structure is replaced by a hydroxyl group.20 Among them, NGR1, ginsenoside Rb1, Rg1, Rd and Re account for 90% of the total PNSs used in pharmacological experiments,21 among which ginsenoside Rb1 (30%–36%)22,23 and Rg1 (20% – 40%)22,24 are the most studied.
Different types of PNSs are enriched in different parts of the PN plant. Rb1 is the major ginsenoside in the PN root, rhizome and flower bud. Rg1 is abundant in the PN root and rhizome.25,26 Rb3 is particularly enriched in flower buds.21,27,28 NGR1 belongs to the protopanaxatriol group and was reported to have neuroprotective effects. Xia et al.29 showed that the quantity of NGR1 in PN roots and rhizomes is much higher than that in other parts of the plant. These findings indicate that PN roots and rhizomes may be a valuable source from which to extract NGR1.
The PNS content in PN is affected by multiple biological and environmental factors, including species, plant parts, age, place of origin, skill of cultivation, and drying methods. By studying the effect of different drying methods on the saponin content in PN slices, Ma et al.30 found that sunlight exposure and drying at 50 °C are more suitable for PN slice processing than other methods. Gao et al.31 used microwave to dry PN and found that different intensities of microwave radiation resulted in a decrease in the levels of the main saponins in PN. These results indicate that the traditional drying method can better maintain the levels of effective components in the roots of PN, but is time-consuming. Researchers urgently need to explore new and rapid PN drying methods for large-scale applications. A report also showed that the saponin content in PN roots is also affected by root diseases. In Sun et al.'s study,32 it has been proved that the content of PNS decreased with increasing root rot. However, root rot had no significant effect on the ratios of NGR1, ginsenoside Rg1 and Rb1.20 Therefore, to ensure the production of high-quality PN, the prevention and control of pests and diseases is particularly important in the actual production process.
Pharmacological properties and mechanism of NGR1
Several reports have indicated that NGR1 possesses anti-I/R injury effects, including balancing ion metabolism disorder,33, 34, 35 alleviating free radical damage,18 inhibiting inflammation and apoptosis,36, 37, 38 as well as promoting angiogenesis39 and neurogenesis.40
Anti-cerebral I/R injury activity of NGR1
Antioxidant effect
The brain is more sensitive to oxidative stress than any other organs because of the inherently higher oxidative metabolism, fewer antioxidant enzymes and higher membranous content in the brain.41,42 Oxidative stress refers to a condition in which the imbalance favors reactive oxygen/nitrogen species (ROS/RNS) levels over antioxidative ability, which causes cellular injury and pathological changes.7 An increase in the secondary messenger system associated with enzymatic production of free radicals (such as cyclooxygenase or nitric oxide synthase) leads to ROS/RNS stress as a partial downstream consequence of excitotoxicity.42, 43, 44 ROS/RNS are mainly generated in the ischemic penumbra, particularly after reperfusion and are continuously removed through powerful free radical scavenging systems (e.g., superoxide dismutase, glutathione (GSH), catalase, and nicotinamide adenine dinucleotide phosphate (NADP)/nicotinamide adenosine dinucleotide phosphate (NADPH)) to maintain a dynamic balance.45 Under cerebral I/R conditions, increased generation of active free radicals can induce lipid peroxidation, protein denaturation, intracellular Ca2+ overload, enzyme inactivation and DNA and mitochondrial damage, which promotes the release of cytochrome-C (Cyt-C) and apoptosis inducing factor from mitochondria, cellular signaling pathway disorders and the generation of downstream apoptotic cascade reactions.46 During this process, the increase in malondialdehyde (MDA) caused by biofilm damage is an important index that reflects the severity of nerve cell damage. Thus, pharmaceutical interventions targeting the scavenging of free radicals to prevent neuronal oxidative stress are one of the major neuroprotective strategies for stroke treatment.47,48
Excessive ROS-induced damage causes a marked increase in the level of the lipid peroxidation product MDA and the protein carbonyl and DNA damage indicator 8-hydroxyguanine during cerebral I/R injury. However, NGR1 effectively inhibits the oxidative stress pathway to protect against oxidative stress-induced neuronal injury.49 In addition, increasing attention has been focused on treatment strategies for ischemic stroke to attempt to inhibit the source of ROS rather than scavenge these ROS after formation.50 Accumulating evidence indicates that NADPH oxidase is an important site of ROS formation induced by cerebral I/R injury. As we previously reported, NGR1 administration decreased the levels of NADPH oxidase activity in middle cerebral artery occlusion/reperfusion-induced rats and oxygen-glucose deprivation/reoxygenation (OGD/R)-induced primary cortical neurons. Mitochondria are another major site of superoxide in the pathological process of cerebral I/R injury. In vivo and in vitro, NGR1 exhibited neuroprotective and antioxidant effects by suppressing ROS levels and mitochondrial superoxide concentrations. Moreover, NGR1 exhibited robust antioxidant effects by inhibiting the loss of mitochondrial membrane potential (Δψm) via estrogen receptor-dependent activation of Akt/Nrf2/oxygenase-1 pathways.18
Targeting intracellular antioxidant and endogenous antioxidant enzyme generation to prevent oxidative stress after cerebral I/R injury is another promising therapeutic strategy. Preconditioning by NGR1 treatment confers neuroprotection by enhancing the expression of phase II antioxidant enzymes, including oxygenase-1, γ-glutamylcysteine synthetase heavy subunit and NAD(P)H quinone oxidoreductase-1, as well as reducing the intracellular GSH concentration through activating Nrf2/ARE signaling in neurons.49
Antiapoptotic effects
Mitochondria are one of the most important organs in brain neurons and play important roles in regulating Ca2+ homeostasis in the cytoplasm, maintaining intracellular pH, and generating adenosine triphosphate through the tricarboxylic acid cycle and electron transport chains. Mitochondria are not only a major source of ROS but also an important target organelle for ROS damage.51 Disorders of ROS and ion metabolism can damage the integrity of the mitochondrial inner membrane, depolarize and decrease the Δψm, and open the proteinic pores (mitochondrial permeability transition pore) in the membrane. Irreversible, excessive opening of the permeability transition pore can release Cyt-C from the mitochondria into the cytoplasm, to form a complex with the apoptotic protease activator apoptotic protease activating factor-1, and then activate caspase-9, an aspartic acid-specific proteolytic enzyme containing cysteine. Caspase-9 activates the downstream effector protein caspase-3 to decompose most of the functional proteins in cells and induce apoptosis.52, 53, 54 This apoptotic process induced by the release of Cyt-C from mitochondria and activation of downstream caspase-9 proteins is called apoptosis through the mitochondrial pathway. The classic apoptosis signaling pathway also includes apoptosis induced by the death receptor pathway.55 During death receptor pathway-induced apoptosis, proapoptotic ligands such as FasL bind to death receptors on the membrane surface and recruit Fas-associated with death domain protein to form a death-inducing signaling complex, which induces the cleavage of caspase-8 precursors into active caspase-8, further activates downstream caspase-3 and induces the apoptotic cascade.56 Excessive ROS damage results in mitochondrial dysfunction, caspase-3 activation and cell apoptosis. Mitochondrial membrane potential assays with JC-1 and Annexin-V/propidium iodide, followed by flow cytometry analysis, were used to detect Δψm and apoptosis. NGR1 preconditioning effectively reduced the loss of Δψm and resulted in an obvious decrease in caspase-3 activation.18 Terminal deoxyribonucleotidyl transferse-mediated biotin-16-dUTP nick-end labeling staining also verified its antiapoptotic effects in the OGD/R model.34 Ischemia also increases the expression of proapoptotic proteins, such as Bcl-2-associated death promoter, Bcl-2-associated X protein (Bax) and BH3-interacting domain death agonist, and decreases the expression of antiapoptotic proteins, such as Bcl-2 and Bcl-xL, in the brain. The mitochondrial-mediated apoptotic effect caused by ACR-induced neurotoxicity was mitigated by incubation with NGR1, as evidenced by the downregulation of Bax, caspase-9 and caspase-3 expression and the upregulation of Bcl-2 and Trx-1 protein levels.36 In addition, NGR1 treatment in rats in the cerebral I/R model group significantly increased brain derived neurotrophic factor gene expression in the hippocampus by increasing the expression of the antiapoptotic factor Bcl-2 and synergistically reducing expression of the Bax.57 A recent study showed that NGR1 inhibits neuronal apoptosis and promotes cell survival to protect against neonatal rat brain injury caused by hypoxia-ischemia through the PI3K-Akt-mTOR/JNK signaling pathways by targeting estrogen receptors.19 Another study showed that after hypoxic-ischemic encephalopathy, the expression of proapoptotic proteins (C/EBP homologous protein, protein kinase R-like endoplasmic reticulum (ER) kinase (PERK), ER oxidoreductin-1α, and inositol-requiring enzyme-1a) and caspase-12 was increased. However, neuronal apoptosis was attenuated by NGR1 treatment.33
The effect on suppressing ER calcium release
The loss of Ca2+ homeostasis plays a crucial role in ischemia-induced neuronal damage after stroke. Excessive intracellular Ca2+ accumulation, mainly reflected in Ca2+ influx, inhibits the mitochondrial electron transport chain, accelerates the generation of ROS/RNS and superoxide anion and regulates Ca2+ homeostasis in mitochondria.7 In addition, the activation of Ca2+ transporters in the ER caused by the increase in Ca2+ concentration in the cytoplasm leads to Ca2+ release from the ER into the cytoplasm, which in turn activates the downstream cascade.58 Targeting Ca2+ overload has been proposed as a potential therapeutic strategy to prevent neuronal damage after ischemic stroke.
In the OGD/R model, Ca2+ released from the ER causes an increase in the Ca2+ concentration in the cytoplasm.59 The severe depletion of Ca2+ in the ER induces glucose regulatory protein 78 (GRP78) to dissociate from PERK and inositol-requiring enzyme-1a, resulting in subsequent activation of ER stress and apoptosis.60,61 In a neonatal cerebral hypoxic-ischemic injury model induced by unilateral ligation of the common carotid artery in rats, the ER chaperone GRP78 was activated, and expression of ERS-associated proapoptotic proteins were increased. However, the ERS response and neuronal apoptosis were attenuated by NGR1 treatment.33 In addition, NGR1 was found to inhibit activation of the PLC/IP3R pathway, subsequently suppressing ERS, ER Ca2+ release and CaMKII levels and resulting in suppression of cell apoptosis.34
The effect on promoting neurovascular production
In recent years, angiogenesis and neurogenesis have become a topic of widespread concern. Endogenous angiogenesis plays an important role in improving brain tissue recovery and functional prognosis after ischemic stroke. Angiogenesis also promotes the sprouting and neurogenesis of axons and dendrites.7 The literature reports that at least two areas of the adult rodent brain contain neural stem cells, namely the subventricular zone in the lateral ventricle and the subgranular zone in the hippocampal dentate gyrus.62,63 Removal of stroke-induced neuroblasts in transgenic mice will exacerbate ischemic damage and worsen the neurological prognosis during stroke recovery,64 indicating that newly generated neuroblasts are involved in the brain repair process. Therefore, the exploration and development of new natural active compounds based on therapeutic strategies that promote the formation of new blood vessels and regeneration of new neurons is of great significance for repairing neurological function after ischemic stroke.
NGR1 exhibits potent proangiogenic activity, as reflected mainly by its ability to increase the numbers of CD31+/EdU+ cells, and repair the damage to the structure of cerebral microvascular endothelial cells. Human brain microvascular endothelial cells (HBMECs) pretreated with different concentrations of NGR1 for 12 h significantly promoted the migration, proliferation, and tube formation of HBMECs. Furthermore, the proangiogenic effects of NGR1 were related to increased ATP, adenosine diphosphate, adenosine monophosphate and guanosine monophosphate expressions, attenuated tricarboxylic acid cycle dysfunction.35,39 OGD-induced PC12 cells models are used to mimic cerebral I/R injury in animals and are extensively used in neurogenesis studies. NGR1 (20 mg/kg) was proven to increase the number of DCX+/EdU+, Nestin+/EdU+ or NeuN+/EdU+ cells significantly, decrease the microglia and reactive astrocyte marker vimentin, and increase neurotrophic factor expression and promote synaptic formation.40 These results highlight the effects of NGR1 on angiogenesis and neurogenesis and reveal the therapeutic potential of this compound in cerebral I/R injury.
Anti-myocardial I/R injury activity of NGR1
The incidence of ischemic heart disease ranks first in primary heart disease. Percutaneous coronary intervention and coronary artery bypass grafting are common methods for surgical treatment of ischemic heart disease. However, ischemic myocardial reperfusion will aggravate heart damage and cause myocardial ischemic/reperfusion injury (MIRI). Drug intervention is currently a common method of clinical treatment of MIRI. In recent years, more and more natural medicines have achieved remarkable curative effects in the treatment of MIRI.
NGR1 ameliorates myocardial infarction, histological injury, and cardiac function, and the protective mechanisms may be associated with inhibiting Rho associated coiled-coil forming protein kinase 1 expression and enhancing mitochondrial ATP synthase δ-subunits.65 In the H9c2 cells oxygen and glucose deprivation/reoxygenation model, NGR1 was found to improve H9c2 cell viability, maintain actin skeleton and mitochondria morphology, and attenuate apoptosis.65 Also, NGR1 pretreatment prevents cell apoptosis and delays the onset of ERS by decreasing the protein expression levels of ERS-responsive proteins GRP78, phosphorylated-PERK, activating transcription factor 6, IRE, and inhibiting the expression of pro-apoptosis proteins C/EBP homologous protein, caspase-12, and P-JNK. Besides, NGR1 scavenges free radical, and increases the activity of antioxidase.66 NGR1 is also capable of inhibiting inflammation in the rat model of MIRI. The results of a study by Xia et al.67 show that after MIRI, NGR1 treatment can ameliorate the impaired mitochondrial morphology and oxidation system, inhibit inflammatory factor expression through inhibiting p-IκBα, NF-κBP65, p-NF-κBP65 protein levels and increasing VDUP1 protein level.
Anti-I/R injury activity of NGR1 in other organs
Renal I/R injury is the most common cause of acute renal injury in clinical practice. Its mechanism is very complicated, which is an important cause of renal failure and affects the prognosis. However, there is still a lack of effective treatment strategies. Fan et al.37 confirmed that NGR1 can suppress the production of I/R-induced inflammatory cytokines, by down-regulating oxidative stress markers of MDA, protein carbonyl, and 8-hydroxyguanine levels, and up-regulating antioxidant enzymes such as superoxide dismutase, catalase, and GSH levels. This suggests that NGR1 is a promising drug candidate for treatment of renal dysfunction.
Intestinal I/R injury can lead to local tissue necrosis of the intestine, trigger intestinal endotoxemia and systemic inflammation, and is an important pathological basis for acute severe pancreatitis. Therefore, the prevention and treatment of intestinal I/R injury is one of the key points in the field of critical illness research. Li et al.68 found that treatment with 10 mg/kg NGR1 attenuated intestinal I/R-induced microvascular hyperpermeability, inflammatory cytokine production, NF-κB activation, and loss of tight junction proteins, as well as improved energy metabolism during I/R. The results of the present study suggest NGR1 as an option in protecting against intestinal I/R injury.
Conclusion and future directions
NGR1 belongs to the panaxatriol group and is a major notoginsenoside and unique constituent of the root of Panax notoginseng. The available data suggest that the antioxidant, anti-inflammatory, anti-apoptotic, pro-angiogenic and pro-neurogenic activities of NGR1, as well as its ability to suppress ER calcium release, may play important roles in the anti-I/R effects (Table 1). These findings demonstrate the potential of NGR1 for the treatment of ischemia-induced organs disorders. However, due to the lack of therapeutic effects of NGR1 in patients, it is difficult to make a clear decision. We recommend that promising research on NGR1 should focus on the following areas: (1) reliable therapeutic targets and novel treatment strategies underlying NGR1-mediated functional recovery after I/R injury; (2) development of a delivery system with pharmacokinetic studies to find effective methods to increase the bioavailability of NGR1; and (3) precise clinical positioning and rational drug use to explore the best effective doses of NGR1. If the anti-I/R injury effect of NGR1 is fully explored in animal models and patients, NGR1 treatment could be a strategy to reduce the high mortality of clinical patients with I/R injury.
Table 1.
Summarized effects and mechanisms of NGR1 on different organs related to ischemia/reperfusion injury in vivo and in vitro studies.
| Organs | Model | Objects | Dose/concentration | Tissue sites | Effects | Mechanisms | RF |
|---|---|---|---|---|---|---|---|
| Brain | Rats MCAO/R model | In vivo | 20 mg/kg, i.p. | Ischemic stroke | ↓ Infarct size and neurological deficits ↓ Neuronal apoptosis and astrocyte activation ↓ Abnormal accumulation of glucose and citric acid ↑ Glutamate and malate-aspartate shuttle content ↑ ATP metabolism and antioxidant content |
Regulate brain small molecule metabolism | 35 |
| Rats MCAO/R model PC12 cells OGD/R model |
In vivo In vitro |
20 mg/kg, i.p. 25 μM |
Ischemic stroke | ↓ Infarct volume and neuronal loss ↑ DCX+/EdU+, Nestin+/EdU+, NeuN+/EdU+ and APC+/EdU+ numbers ↑ BDNF, NGF and NT-4 levels ↑ SYN, PSD95, MAP-2 and Tau-1 levels |
↑ Neurogenesis via the BDNF/Akt/CREB pathway | 40 | |
| Rats BCCAO model | In vivo | 100 mg/kg, i.g. | Cerebral I/R injury | ↓ Cerebral infarction and neuronal apoptosis | ↑ BDNF and Bcl-2 expression ↓ Bax expression |
57 | |
| Rats MCAO model PC12 cells OGD/R model |
In vivo In vitro |
100 mg/kg, p.o. 0.1, 1 and 10 μM |
Ischemic stroke | ↓ BBB permeability, cerebral infarction volume and neurological impairments ↓ MMPs activity ↑ Zo1 and caludin-5 expressions |
Intervene degradation and redistribution of zo1, caludin5 and occludin by cav-1/MMP2/9 pathway | 69 | |
| Rats MCAO model Primary cortical neurons OGD/R model |
In vivo In vitro |
20 mg/kg, i.p. 25 μM |
Cerebral I/R injury | ↓ MDA, PC and 8-OHdG ↑ ERα, ERβ, phospho-Akt, phospho-GSK3β, nuclear Nrf2 and HO-1 expression |
↓ NADPH oxidase activity and mitochondrial dysfunction via ER-dependent activation of Akt/Nrf2 pathways | 18 | |
| Rats MCAO model HBMEC cells OGD/R model |
In vivo In vitro |
20 mg/kg, i.p. 25 μM |
Ischemic stroke | ↑ Cerebral blood flow, mitochondrial energy metabolism and angiogenesis ↑ Migration, proliferation, and tube formation |
↑ NAMPT-NAD+-SIRT1 cascade ↓ Notch signaling ↑ VEGFR-2 expression |
39 | |
| Rats HIE model Primary cortical neurons OGD/R model |
In vivo In vitro |
5, 10 and 15 mg/kg, i.p. 0.5, 1, 2, 5, 10, 20 μmol/L |
Neonatal cerebral hypoxic-ischemic injury | ↑ GRP78, CHOP, PERK, ERO1-α and IRE1α ↑ Caspase-12 level ↓ BCL-2 level |
Estrogen receptor-dependent activation of endoplasmic reticulum stress pathways | 33 | |
| Heart | IR-induced myocardial injury rat model | In vivo | 20, 40 and 60 mg/kg, i.g. | Myocardial I/R injury | ↓ LDH, CK, MPO, T-SOD and MDA ↓ IL-1β, IL-8 and TNF-α ↓ p-IκBα, NF-κBP65 and p-NF-κBP65 levels ↑ VDUP1 level |
Regulate VDUP1/NF-κB pathway | 67 |
| IR-induced myocardial injury rat model | In vivo | 1 mg/kg, femoral vein | Myocardial I/R injury | ↓ F-actin and myocardial fiber rupture ↓ Myocardial infarction size ↑ Velocities of RBC in venules ↑ MBF and heart function |
↓ Sirt-1/NDUFA10/Complex I ↓ RhoA/ROCK-1 activation ↓ ATP/ATP 5D/Complex V expression |
70 | |
| H9c2 cells H/R model | In vitro | 20 μM | Myocardial I/R injury | ↓ GRP78, P-PERK, ATF6 and IRE ↓ CHOP, caspase-12 and P-JNK ↓ Free radical ↑ Antioxidase activity |
Regulate oxidative stress- and endoplasmic reticulum stress-related signaling pathways | 66 | |
| IR-induced myocardial injury rabbit model | In vivo | 25 mg/kg, i.v. | Acute myocardial infarction | ↓ SOD ↑ MDA ↓ Caspase-3, -8 and -9 |
↑ TGF-β1/TAK1 signaling pathway | 38 | |
| H9c2 cells H/R model | In vitro | 20 μM | Myocardial I/R injury | ↑ Cell viability ↓ Cell apoptosis ↓ LDH and MDA |
↑ miR-132 expression ↓ HBEGF expression |
71 | |
| IR-induced myocardial injury rat model H9c2 cells H/R model |
In vivo In vitro |
5 mg/kg, femoral vein 0.1 mM |
Myocardial I/R injury | ↓ Myocardial infarction, histological injury, and cardiac function ↑ H9c2 cell viability Maintain actin skeleton and mitochondria morphology ↓ Apoptosis and energy abnormity |
↓ ROCK expression ↑ Mitochondrial ATP synthase δ-subunits |
65 | |
| Renal | IR-induced renal injury rat model | In vivo | 4.2 mg/kg, i.p. | Transient cerebral ischemia | ↓ MDA, PCO and 8-OHdG ↑ SOD, catalase and GSH ↓ TNF-α, TGF-β1, INF-γ and IL-6 ↑ IL-10 |
↓ Inflammatory cytokines production by suppressing oxidative stress | 37 |
| Intestinal | IR-induced intestinal injury rat model | In vivo | 10 mg/kg, i.p. | Intestinal I/R injury | ↓ Neurological score, infarct volume and cerebral water content ↓ IL-6, IL-1β and TNF-α ↓ ROS, MDA and PARP-1 ↑ SOD, catalase and GSH |
↓ Apoptosis signal, NF-κB signaling and microglia | 68 |
RF: reference; I/R: ischemia/reperfusion; MCAO/R: middle cerebral artery occlusion/reperfusion; OGD/R: oxygen-glucose deprivation/reoxygenation; ATP: adenosine triphosphate; BDNF: brain derived neurotrophic factor; CREB: cAMP-responsive element-binding protein; BCCAO: bilateral common carotid artery occlusion; NGF: nerve growth factor; NT-4: neurotrophin-3; SYN: synaptophysin; PSD95: post-synaptic density protein 95; Bax: Bcl-2-associated X protein; BBB: blood brain barrier; MMPs: matrix metalloproteinases; MDA: malondialdehyde; 8-OHdG: 8-hydroxyguanine; ERα: estrogen receptors alpha; ERβ: estrogen receptors beta; GSK3β: glycogen synthase kinase 3β; HO-1: oxygenase-1; NADPH: nicotinamide adenosine dinucleotide phosphate; ER: endoplasmic reticulum; NAMPT: nicotinamide phosphoribosyltransferase; NAD: nicotinamide adenine dinucleotide; SIRT1: Sirtuin 1; HBMEC: human brain microvascular endothelial cells; VEGFR-2: vascular endothelial growth factor receptor-2; HIE: hypoxic-ischemic encephalopathy; GRP78: proteins glucose regulatory protein 78; CHOP: C/EBP homologous protein; PERK: protein kinase R-like endoplasmic reticulum kinase; ERO-1α: ER oxidoreductin-1α; IRE1a: inositol-requiring enzyme-1a; LDH: lactate dehydrogenase; CK: creatine kinase; MPO: myeloperoxidase; MDA: malondialdehyde; IL-1β: Interleukin-1 beta; IL-8: Interleukin-8; TNF-α: tumor necrosis factor-α; p-IκBα: phosphorylation inhibitor of nuclear factor kappa B alpha; NF-κBP65: nuclear factors-κB p65; VDUP1: vitamin D(3) up-regulating protein 1; ROCK-1: Rho associated coiled-coil forming protein kinase 1; PCO: protein carbonyl; SOD: superoxide dismutase; GSH: glutathione; TGF-β1: transforming growth factor-β1; INF-γ: interferon-γ; IL-6: interleukin-6; IL-10: interleukin-10; ROS: reactive oxygen species; PARP-1: poly (ADP-ribose) polymerase 1.
↓: downregulation or inhibition; ↑: upregulation or activation.
Funding
Nil.
Ethical statement
Not applicably.
Declaration of competing interest
The authors declared no competing interest.
Author contributions
Ting Zhu: Conceptualization, writing - review & editing; Qi Wan: Supervision, project administration.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Zhang W., Song J.K., Du G.H., et al. Apoptosis signal transduction pathways in ischemia-reperfusion injury. Chin Pharmaceut J. 2015;50:565–569. https://10.11669/cpj.2015.07.001 [Google Scholar]
- 2.Han J.Y., Li Q., Ma Z.Z., et al. Effects and mechanisms of compound Chinese medicine and major ingredients on microcirculatory dysfunction and organ injury induced by ischemia/reperfusion. Pharmacol Ther. 2017;177:146–173. doi: 10.1016/j.pharmthera.2017.03.005. https://10.1016/j.pharmthera.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 3.Lee K.Y., Heo J.H., Lee S.I., et al. Rescue treatment with abciximab in acute ischemic stroke. Neurology. 2001;56:1585–1587. doi: 10.1212/wnl.56.11.1585. https://10.1212/wnl.56.11.1585 [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz M.A., Lo E.H., Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. https://10.1016/j.neuron.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez J.C., Hurko O., Barone F.C., et al. Pharmacologic interventions for stroke: looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke. 2009;40:e558–563. doi: 10.1161/STROKEAHA.109.559914. https://10.1161/strokeaha.109.559914 [DOI] [PubMed] [Google Scholar]
- 6.Lo E.H., Dalkara T., Moskowitz M.A. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. https://10.1038/nrn1106 [DOI] [PubMed] [Google Scholar]
- 7.Zhou Z., Lu J., Liu W.W., et al. Advances in stroke pharmacology. Pharmacol Ther. 2018;191:23–42. doi: 10.1016/j.pharmthera.2018.05.012. https://10.1016/j.pharmthera.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 8.Wang F.J., Wang S.X., Chai L.J., et al. Xueshuantong injection (lyophilized) combined with salvianolate lyophilized injection protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress. Acta Pharmacol Sin. 2018;39:998–1011. doi: 10.1038/aps.2017.128. https://10.1038/aps.2017.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M., Zou W., Chen M., et al. Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur J Pharmacol. 2018;833:221–229. doi: 10.1016/j.ejphar.2018.06.012. https://10.1016/j.ejphar.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 10.Yao Y., Chen L., Xiao J., et al. Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. Int J Mol Sci. 2014;15:20913–20926. doi: 10.3390/ijms151120913. https://10.3390/ijms151120913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Wang M., Ju J., et al. Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am J Transl Res. 2019;11:199–209. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X., Zhang F., Wang Y. Proteomic analysis reveals Xuesaitong injection attenuates myocardial ischemia/reperfusion injury by elevating pyruvate dehydrogenase-mediated aerobic metabolism. Mol Biosyst. 2017;13:1504–1511. doi: 10.1039/c7mb00140a. https://10.1039/c7mb00140a [DOI] [PubMed] [Google Scholar]
- 13.Hua Y., Shao M., Wang Y., et al. Xuesaitong injection treating acute myocardial infarction: a systematic review and meta-analysis. Medicine (Baltim) 2021;100 doi: 10.1097/MD.0000000000027027. https://10.1097/md.0000000000027027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu T., Meng X.B., Dong D.X., et al. Xuesaitong injection (lyophilized) combined with aspirin and clopidogrel protect against focal cerebral ischemic/reperfusion injury in rats by suppressing oxidative stress and inflammation and regulating the NOX2/IL-6/STAT3 pathway. Ann Palliat Med. 2021;10:1650–1667. doi: 10.21037/apm-20-1681. https://10.21037/apm-20-1681 [DOI] [PubMed] [Google Scholar]
- 15.Li F., Zhao H., Han Z., et al. Xuesaitong may protect against ischemic stroke by modulating microglial phenotypes and inhibiting neuronal cell apoptosis via the STAT3 signaling pathway. CNS Neurol Disord: Drug Targets. 2019;18:115–123. doi: 10.2174/1871527317666181114140340. https://10.2174/1871527317666181114140340 [DOI] [PubMed] [Google Scholar]
- 16.Wang L., Zhu T., Xu H.B., et al. Effects of notoginseng leaf triterpenes on small molecule metabolism after cerebral ischemia/reperfusion injury assessed using MALDI-MS imaging. Ann Transl Med. 2021;9:246. doi: 10.21037/atm-20-4898. https://10.21037/atm-20-4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Chu Y., Li W., et al. Advances in study on saponins in Panax notoginseng and their pharmacological. Chin Tradit Herb Drugs. 2015;46:1381–1392. https://10.7501/j.issn.0253-2670.2015.09.023 [Google Scholar]
- 18.Meng X., Wang M., Wang X., et al. Suppression of NADPH oxidase- and mitochondrion-derived superoxide by Notoginsenoside R1 protects against cerebral ischemia-reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways. Free Radic Res. 2014;48:823–838. doi: 10.3109/10715762.2014.911853. https://10.3109/10715762.2014.911853 [DOI] [PubMed] [Google Scholar]
- 19.Tu L., Wang Y., Chen D., et al. Protective effects of notoginsenoside R1 via regulation of the PI3K-Akt-mTOR/JNK pathway in neonatal cerebral hypoxic-ischemic brain injury. Neurochem Res. 2018;43:1210–1226. doi: 10.1007/s11064-018-2538-3. https://10.1007/s11064-018-2538-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia P.G., Zhang S.C., Liang Z.S., et al. Research history and overview of chemical constituents of Panax notoginseng. Chin Tradit Herb Drugs. 2014;45:2564–2570. https://10.7501/j.issn.0253-2670.2014.17.026 [Google Scholar]
- 21.Liu J., Wang Y., Qiu L., et al. Saponins of Panax notoginseng: chemistry, cellular targets and therapeutic opportunities in cardiovascular diseases. Expet Opin Invest Drugs. 2014;23:523–539. doi: 10.1517/13543784.2014.892582. https://10.1517/13543784.2014.892582 [DOI] [PubMed] [Google Scholar]
- 22.Pan C., Huo Y., An X., et al. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vasc Pharmacol. 2012;56:150–158. doi: 10.1016/j.vph.2011.12.006. https://10.1016/j.vph.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 23.Chen W., Dang Y., Zhu C. Simultaneous determination of three major bioactive saponins of Panax notoginseng using liquid chromatography-tandem mass spectrometry and a pharmacokinetic study. Chin Med. 2010;5:12. doi: 10.1186/1749-8546-5-12. https://10.1186/1749-8546-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G., Wang B., Zhang J., et al. Total panax notoginsenosides prevent atherosclerosis in apolipoprotein E-knockout mice: role of downregulation of CD40 and MMP-9 expression. J Ethnopharmacol. 2009;126:350–354. doi: 10.1016/j.jep.2009.08.014. https://10.1016/j.jep.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 25.Zhu T., Wang L., Feng Y., et al. Classical active ingredients and extracts of Chinese herbal medicines: pharmacokinetics, pharmacodynamics, and molecular mechanisms for ischemic stroke. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/8868941. https://10.1155/2021/8868941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu T., Wang L., Wang L-p, et al. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: applications for natural compounds from medicinal herbs. Biomed Pharmacother. 2022;148 doi: 10.1016/j.biopha.2022.112719. https://10.1016/j.biopha.2022.112719 [DOI] [PubMed] [Google Scholar]
- 27.Wan J.B., Yang F.Q., Li S.P., et al. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J Pharm Biomed Anal. 2006;41:1596–1601. doi: 10.1016/j.jpba.2006.01.058. https://10.1016/j.jpba.2006.01.058 [DOI] [PubMed] [Google Scholar]
- 28.Dan M., Su M., Gao X., et al. Metabolite profiling of Panax notoginseng using UPLC-ESI-MS. Phytochemistry. 2008;69:2237–2244. doi: 10.1016/j.phytochem.2008.04.015. https://10.1016/j.phytochem.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 29.Xia P.G., Zhang S.C., Liang Z.S., et al. Research history and overview of chemical constituents of Panax notoginseng. Chin Tradit Herb Drugs. 2014;45:2564–2570. https://10.7501/j.issn.0253-2670.2014.17.026 [Google Scholar]
- 30.Ma N., Gao M.J., Zhou J.M., et al. Influence of different dehydrations on cotents of saponin in Sanqi slice. Special Wild Econ Anim Plant. 2010;32:40–42. https://10.16720/j.cnki.tcyj.2010.03.018 [Google Scholar]
- 31.Gao M., Feng G., Zeng H., et al. Effect of dehydrations on the effective components of Panax notoginseng saponins. J Chin Med Mater. 2010;33:198–200. https://10.13863/j.issn1001-4454.2010.02.017 [Google Scholar]
- 32.Sun Y., Ke J., Ma N., et al. Effects of root rot on saponin content in Panax notoginseng. J Chin Med Mater. 2004;27:79–80. https://10.13863/j.issn1001-4454.2004.02.001 [PubMed] [Google Scholar]
- 33.Wang Y., Tu L., Li Y., et al. Notoginsenoside R1 protects against neonatal cerebral hypoxic-ischemic injury through estrogen receptor-dependent activation of endoplasmic reticulum stress pathways. J Pharmacol Exp Therapeut. 2016;357:591–605. doi: 10.1124/jpet.115.230359. https://10.1124/jpet.115.230359 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Tu L., Li Y., et al. Notoginsenoside R1 alleviates oxygen-glucose deprivation/reoxygenation injury by suppressing endoplasmic reticulum calcium release via PLC. Sci Rep. 2017;7 doi: 10.1038/s41598-017-16373-7. https://10.1038/s41598-017-16373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu T., Wang L., Tian F., et al. Anti-ischemia/reperfusion injury effects of notoginsenoside R1 on small molecule metabolism in rat brain after ischemic stroke as visualized by MALDI-MS imaging. Biomed Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110470. https://10.1016/j.biopha.2020.110470 [DOI] [PubMed] [Google Scholar]
- 36.Wang W., Huang L., Hu Y., et al. Neuroprotective effects of notoginsenoside R1 by upregulating Trx-1 on acrylamide-induced neurotoxicity in PC12. Hum Exp Toxicol. 2020;39:797–807. doi: 10.1177/0960327120901586. https://10.1177/0960327120901586 [DOI] [PubMed] [Google Scholar]
- 37.Fan C., Chen Q., Ren J., et al. Notoginsenoside R1 suppresses inflammatory signaling and rescues renal ischemia-reperfusion injury in experimental rats. Med Sci Mon Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.920442. https://10.12659/msm.920442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge Z.R., Xu M.C., Huang Y.U., et al. Cardioprotective effect of notoginsenoside R1 in a rabbit lung remote ischemic postconditioning model via activation of the TGF-β1/TAK1 signaling pathway. Exp Ther Med. 2016;11:2341–2348. doi: 10.3892/etm.2016.3222. https://10.3892/etm.2016.3222 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Zhu T., Xie W.J., Wang L., et al. Notoginsenoside R1 activates the NAMPT-NAD(+)-SIRT1 cascade to promote postischemic angiogenesis by modulating Notch signaling. Biomed Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111693. https://10.1016/j.biopha.2021.111693 [DOI] [PubMed] [Google Scholar]
- 40.Zhu T., Wang L., Xie W., et al. Notoginsenoside R1 improves cerebral ischemia/reperfusion injury by promoting neurogenesis via the BDNF/Akt/CREB pathway. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.615998. https://10.3389/fphar.2021.615998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saeed S.A., Shad K.F., Saleem T., et al. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp Brain Res. 2007;182:1–10. doi: 10.1007/s00221-007-1050-9. https://10.1007/s00221-007-1050-9 [DOI] [PubMed] [Google Scholar]
- 42.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. https://10.1152/physrev.00029.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuyama N., Takizawa S., Ishida H., et al. Peroxynitrite formation in focal cerebral ischemia-reperfusion in rats occurs predominantly in the peri-infarct region. J Cerebr Blood Flow Metabol. 1998;18:123–129. doi: 10.1097/00004647-199802000-00001. https://10.1097/00004647-199802000-00001 [DOI] [PubMed] [Google Scholar]
- 44.Fabian R.H., DeWitt D.S., Kent T.A. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cerebr Blood Flow Metabol. 1995;15:242–247. doi: 10.1038/jcbfm.1995.30. https://10.1038/jcbfm.1995.30 [DOI] [PubMed] [Google Scholar]
- 45.Zhou P., Xie W., Sun Y., et al. Ginsenoside Rb1 and mitochondria: a short review of the literature. Mol Cell Probes. 2019;43:1–5. doi: 10.1016/j.mcp.2018.12.001. https://10.1016/j.mcp.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 46.Wu J.S., Tsai H.D., Cheung W.M., et al. PPAR-Γ ameliorates neuronal apoptosis and ischemic brain injury via suppressing NF-κB-Driven p22phox transcription. Mol Neurobiol. 2016;53:3626–3645. doi: 10.1007/s12035-015-9294-z. https://10.1007/s12035-015-9294-z [DOI] [PubMed] [Google Scholar]
- 47.Shirley R., Ord E.N., Work L.M. Oxidative stress and the use of antioxidants in stroke. Antioxidants. 2014;3:472–501. doi: 10.3390/antiox3030472. https://10.3390/antiox3030472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang T., Sun Y., Zhang F. Anti-oxidative aspect of inhaled anesthetic gases against acute brain injury. Med Gas Res. 2016;6:223–226. doi: 10.4103/2045-9912.196905. https://10.4103/2045-9912.196905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng X., Sun G., Ye J., et al. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: a novel mechanism of Nrf2/ARE signaling activation. Free Radic Res. 2014;48:445–460. doi: 10.3109/10715762.2014.885117. https://10.3109/10715762.2014.885117 [DOI] [PubMed] [Google Scholar]
- 50.Wingler K., Hermans J.J., Schiffers P., et al. NOX1, 2, 4, 5: counting out oxidative stress. Br J Pharmacol. 2011;164:866–883. doi: 10.1111/j.1476-5381.2011.01249.x. https://10.1111/j.1476-5381.2011.01249.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faraci F.M. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol. 2011;300:H1566–H1582. doi: 10.1152/ajpheart.01310.2010. https://10.1152/ajpheart.01310.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamarche F., Carcenac C., Gonthier B., et al. Mitochondrial permeability transition pore inhibitors prevent ethanol-induced neuronal death in mice. Chem Res Toxicol. 2013;26:78–88. doi: 10.1021/tx300395w. https://10.1021/tx300395w [DOI] [PubMed] [Google Scholar]
- 53.Franklin J.L. Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxidants Redox Signal. 2011;14:1437–1448. doi: 10.1089/ars.2010.3596. https://10.1089/ars.2010.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. https://10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 55.Muñoz-Pinedo C. Signaling pathways that regulate life and cell death: evolution of apoptosis in the context of self-defense. Adv Exp Med Biol. 2012;738:124–143. doi: 10.1007/978-1-4614-1680-7_8. https://10.1007/978-1-4614-1680-7_8 [DOI] [PubMed] [Google Scholar]
- 56.Kantari C., Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. https://10.1016/j.bbamcr.2011.01.026 [DOI] [PubMed] [Google Scholar]
- 57.Zou S., Zhang M., Feng L., et al. Protective effects of notoginsenoside R1 on cerebral ischemia-reperfusion injury in rats. Exp Ther Med. 2017;14:6012–6016. doi: 10.3892/etm.2017.5268. https://10.3892/etm.2017.5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar V.S., Gopalakrishnan A., Naziroglu M., et al. Calcium ion--the key player in cerebral ischemia. Curr Med Chem. 2014;21:2065–2075. doi: 10.2174/0929867321666131228204246. https://10.2174/0929867321666131228204246 [DOI] [PubMed] [Google Scholar]
- 59.Wei H., Inan S. Dual effects of neuroprotection and neurotoxicity by general anesthetics: role of intracellular calcium homeostasis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;47:156–161. doi: 10.1016/j.pnpbp.2013.05.009. https://10.1016/j.pnpbp.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alberdi E., Wyssenbach A., Alberdi M., et al. Ca(2+) -dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of Alzheimer disease. Aging Cell. 2013;12:292–302. doi: 10.1111/acel.12054. https://10.1111/acel.12054 [DOI] [PubMed] [Google Scholar]
- 61.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. https://10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- 62.Alvarez-Buylla A., Herrera D.G., Wichterle H. The subventricular zone: source of neuronal precursors for brain repair. Prog Brain Res. 2000;127:1–11. doi: 10.1016/s0079-6123(00)27002-7. https://10.1016/s0079-6123(00)27002-7 [DOI] [PubMed] [Google Scholar]
- 63.Gage F.H., Ray J., Fisher L.J. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. https://10.1146/annurev.ne.18.030195.001111 [DOI] [PubMed] [Google Scholar]
- 64.Wang X., Mao X., Xie L., et al. Conditional depletion of neurogenesis inhibits long-term recovery after experimental stroke in mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038932. https://10.1371/journal.pone.0038932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He K., Yan L., Pan C.S., et al. ROCK-dependent ATP5D modulation contributes to the protection of notoginsenoside NR1 against ischemia-reperfusion-induced myocardial injury. Am J Physiol Heart Circ Physiol. 2014;307:H1764–H1776. doi: 10.1152/ajpheart.00259.2014. https://10.1152/ajpheart.00259.2014 [DOI] [PubMed] [Google Scholar]
- 66.Yu Y., Sun G., Luo Y., et al. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep. 2016;6 doi: 10.1038/srep21730. https://10.1038/srep21730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia K.P., Ca H.M., Shao C.Z. Protective effect of notoginsenoside R1 in a rat model of myocardial ischemia reperfusion injury by regulation of Vitamin D3 upregulated protein 1/NF-κB pathway. Pharmazie. 2015;70:740–744. https://info:doi/10.1691/ph.2015.5694 [PubMed] [Google Scholar]
- 68.Li C., Li Q., Liu Y.Y., et al. Protective effects of Notoginsenoside R1 on intestinal ischemia-reperfusion injury in rats. Am J Physiol Gastrointest Liver Physiol. 2014;306:G111–G122. doi: 10.1152/ajpgi.00123.2013. https://10.1152/ajpgi.00123.2013 [DOI] [PubMed] [Google Scholar]
- 69.Liu B., Li Y., Han Y., et al. Notoginsenoside R1 intervenes degradation and redistribution of tight junctions to ameliorate blood-brain barrier permeability by Caveolin-1/MMP2/9 pathway after acute ischemic stroke. Phytomedicine. 2021;90 doi: 10.1016/j.phymed.2021.153660. https://10.1016/j.phymed.2021.153660 [DOI] [PubMed] [Google Scholar]
- 70.Yan L., Pan C.S., Liu Y.Y., et al. The composite of 3, 4-dihydroxyl-phenyl lactic acid and notoginsenoside R1 attenuates myocardial ischemia and reperfusion injury through regulating mitochondrial respiratory chain. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.538962. https://10.3389/fphys.2021.538962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin Z., Gan C., Luo G., et al. Notoginsenoside R1 protects hypoxia-reoxygenation deprivation-induced injury by upregulation of miR-132 in H9c2 cells. Hum Exp Toxicol. 2021;40:S29–S38. doi: 10.1177/09603271211025589. https://10.1177/09603271211025589 [DOI] [PubMed] [Google Scholar]