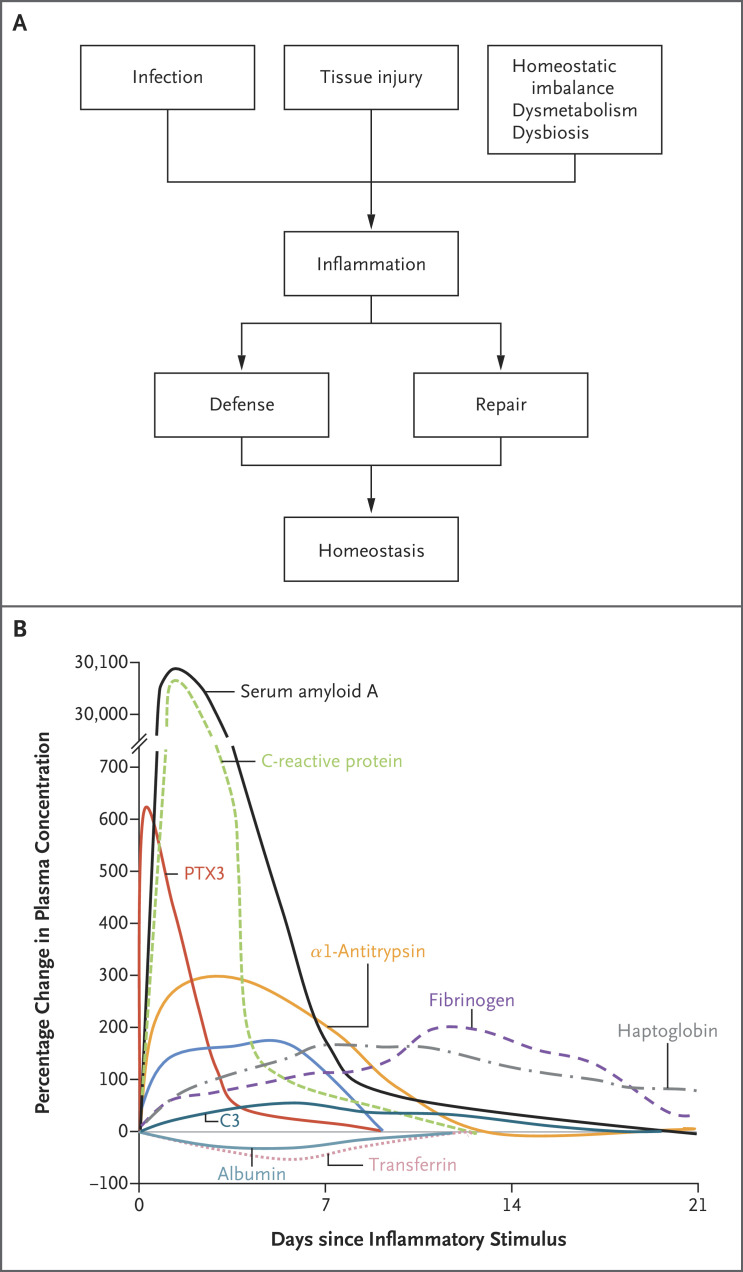
The broad term “inflammation” encompasses a diverse set of tissue reactions classically triggered by microbial recognition and by tissue damage.1,2 More recently, it has been recognized that dysmetabolic conditions, ranging from diabetes to obesity, elicit overt or subclinical inflammatory reactions. The general role of inflammatory reactions is in the amplification of innate resistance and tissue repair, leading to a return to homeostasis (Figure 1A).
Figure 1. Role of Inflammation and Changes in the Circulation of Acute-Phase Proteins.
Panel B is adapted from Gitlin and Colten3 with permission from the publisher. PTX3 denotes pentraxin 3.
Systemic manifestations of inflammation include fever, alterations in leukocyte counts, cardiovascular reactions, endocrine responses, and reorientation of metabolism in association with increased production of a diverse set of molecules referred to as acute-phase proteins.4,5 The prototypic acute-phase protein, C-reactive protein, was originally described as a molecule that was present in the circulation of patients with infections and that was capable of recognizing the C-type polysaccharides of Streptococcus pneumoniae.6,7 The appearance of increased levels of acute-phase proteins in blood and other body fluids (Figure 1B) is part of a more complex response to local inflammation or to systemic inflammation (e.g., sepsis) that has been referred to as the acute-phase response,5 which is characterized by decreased production of albumin by hepatocytes, reorientation of iron metabolism, and hormonal changes.4,5 These alterations are also observed in the context of chronic inflammatory conditions and subclinical inflammation.
Almost a century after the discovery of C-reactive protein, acute-phase proteins continue to serve as fundamental diagnostic tools that have applications in patients with a range of conditions, including infection, cardiovascular illness, cancer, neurodegeneration, and dysmetabolism.8-10 During the coronavirus disease 2019 (Covid-19) pandemic, acute-phase proteins such as C-reactive protein, fibrinogen and its degradation product d-dimer, and ferritin have served as invaluable tools in the day-to-day management of illnesses and as prognostic indicators (Table 1). Progress has been made in dissecting the production, structure, and function of many of these molecules, and the findings have indicated that a fundamental function of the acute-phase response is to amplify antimicrobial resistance and tissue repair, with many of the acute-phase proteins serving as key components of humoral innate immunity (“ante-antibodies”).25 From this general perspective, we review key aspects of the production, structure, and function of selected acute-phase proteins, which continue to represent pillar diagnostic tools that could be integrated more systematically into the molecular signatures that have recently emerged from transcriptomic and proteomic profiles.
Table 1. Main Acute-Phase Proteins and Their Role in Covid-19.*.
| Function and Protein or Proteins | Degree and Type of Change in Inflammatory Conditions† | Role or Roles in Covid-19 and Associated Conditions‡ |
|---|---|---|
| Humoral innate immunity | ||
| C-reactive protein | ↑↑↑↑ | Association with death, ICU admission, need for interleukin-6 inhibition, and PASC11-14 |
| Serum amyloid P | ↑ or → | ND |
| Serum amyloid A | ↑↑↑↑ | Association with severity15 |
| PTX3 | ↑↑↑ | Association with death, lung lesions on CT, response to interleukin-6 inhibition, intubation, thrombotic events, and PASC16-19 |
| C1q, C3, and C4 | ↑ | Association with pathogenesis20,21 |
| C4-binding protein | ↑ | ND |
| Mannose-binding lectin | ↑↑ or → | Viral inhibition, association with thromboembolism22 |
| Interleukin-1Ra | ↑↑ | Association between anti–interleukin-1Ra autoantibodies and severity, MIS-C, or myocarditis after SARS-CoV-2 vaccination23 |
| Coagulation or tissue repair and remodeling | ||
| Fibrinogen | ↑↑ | Association of d-dimer with thromboembolism14 |
| Prothrombin | → | ND |
| Fibronectin | ↑ or → | ND |
| α2-Macroglobulin | ↑ | ND |
| Antithrombin III | ↓ | ND |
| α1-Antitrypsin | ↑↑ | ND |
| α1-Antichymotrypsin | ↑↑ | ND |
| Urokinase-type plasminogen activator | ↑ | ND |
| Thrombopoietin | ↑↑ | ND |
| Iron metabolism | ||
| Transferrin | ↓ | ND |
| Ferritin | ↑↑ | Association with ICU admission and mechanical ventilation14,24 |
| Haptoglobin | ↑↑ | ND |
| Hemopexin | ↑ | ND |
| Hepcidin | ↑↑ | ND |
| Other carrier proteins | ||
| Albumin | ↓ | ND |
| Ceruloplasmin | ↑ or → | ND |
| Apolipoproteins | ↓ | ND |
| α1-Acid glycoprotein | ↑↑ | ND |
Covid-19 denotes coronavirus disease 2019, CT computed tomography, ICU intensive care unit, MIS-C multisystem inflammatory syndrome in children, ND not determined, PASC postacute sequelae of Covid-19, PTX3 pentraxin 3, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Upward-pointing arrows (↑) and downward-pointing arrows (↓) indicate increases and decreases in concentration, respectively; a right-pointing arrow (→) indicates no change. Greater numbers of upward-pointing arrows indicate greater increases in concentration.
The references shown are selected references related to Covid-19.
The Context: Cellular and Humoral Innate Immunity
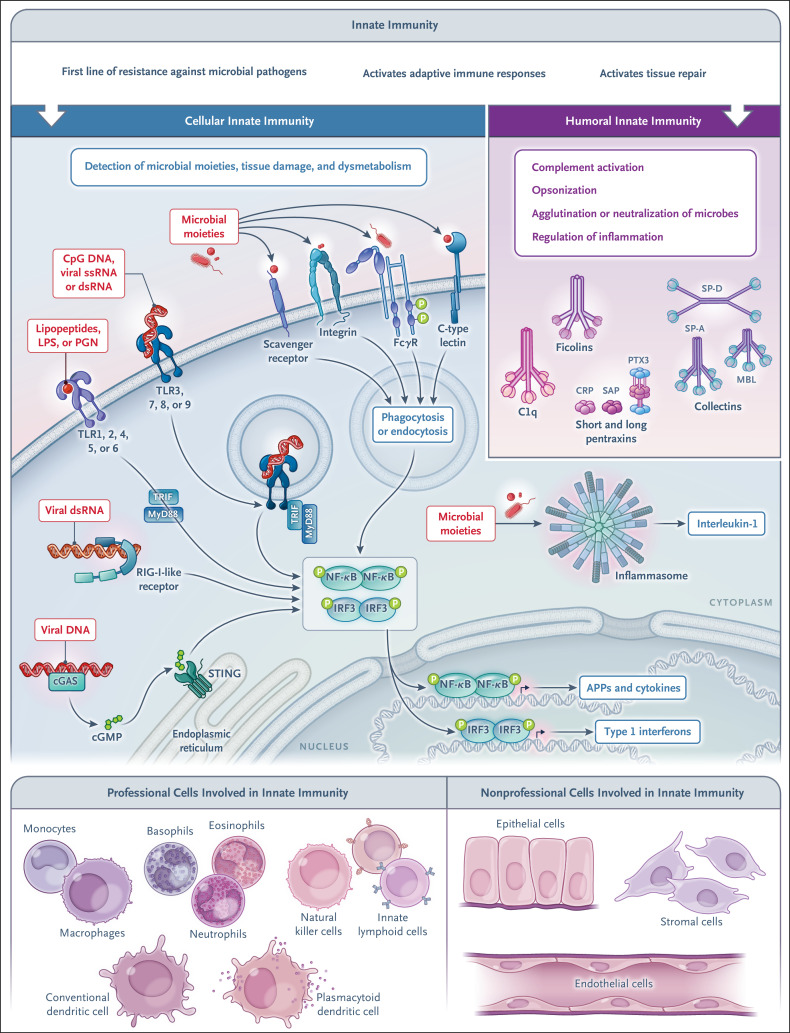
Innate immunity is a first line of resistance against microbial pathogens and is involved in the activation of adaptive immune responses, as well as in tissue repair. Innate immunity is made up of a cellular arm and a humoral arm. The molecular strategies used by the cellular arm to sense microbial moieties and tissue damage involve cell-associated pattern-recognition molecules located in different cellular compartments (plasma membrane, endosomes, and cytoplasm) and belonging to different molecular families, including toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)–like and retinoic acid–inducible gene I (RIG-I)–like receptors, inflammasomes, stimulator of interferon genes (STING), C-type lectins, and scavenger receptors (Figure 2). The activation of these receptors leads to the expression of cytokines (including interferons and chemokines), adhesion molecules, and antimicrobial effectors or to the scavenging of microbes through phagocytosis.1,2,25
Figure 2. Innate Immunity — a Cellular Arm and a Humoral Arm.
Cellular sensors of tissue damage, infection, and dysmetabolism are strategically localized on the cell surface, in the endosomal compartment, and in the cytoplasm, in both professional innate immune cells (i.e., those with innate immunity as their principal function) and nonprofessional innate immune cells (i.e., those with other principal functions). The latter include hepatocytes, a major source of acute-phase proteins. Under homeostatic conditions and in response to inflammation, components of the humoral arm of innate immunity are produced. These molecules serve complex functions, including immune resistance, by activating complement and having opsonic activity (ante-antibodies). APP denotes acute-phase protein, cGAS cyclic GMP–AMP synthase, cGMP cyclic guanosine monophosphate, CRP C-reactive protein, dsRNA double-stranded RNA, IRF interferon regulatory factor, LPS lipopolysaccharide, MBL mannose-binding lectin, MyD88 myeloid differentiation primary response 88, NF-κB nuclear factor kappa B, PGN peptidoglycan, RIG-I retinoid acid-inducible gene I, SAP serum amyloid P, SP-A surfactant protein A, SP-D surfactant protein D, ssRNA single-stranded RNA, STING stimulator of interferon genes, TLR toll-like receptor, TRIF toll/interleukin-1 receptor–domain–containing adapter-inducing interferon-β.
The humoral arm of the innate immune system is made up of different classes of molecules, such as pentraxins, collectins, and ficolins, which functionally act as ancestors of antibodies (ante-antibodies) by initiating complement activation, opsonizing microbes and damaged cells, agglutinating or neutralizing microbes, and regulating inflammation.25 As discussed below, some of these molecules are key components of the acute-phase response (Table 1) that are rapidly induced in liver cells or other cell types by primary inflammatory cytokines or microbial moieties.
Upstream of the Acute-Phase Response: the Cytokine Cascade
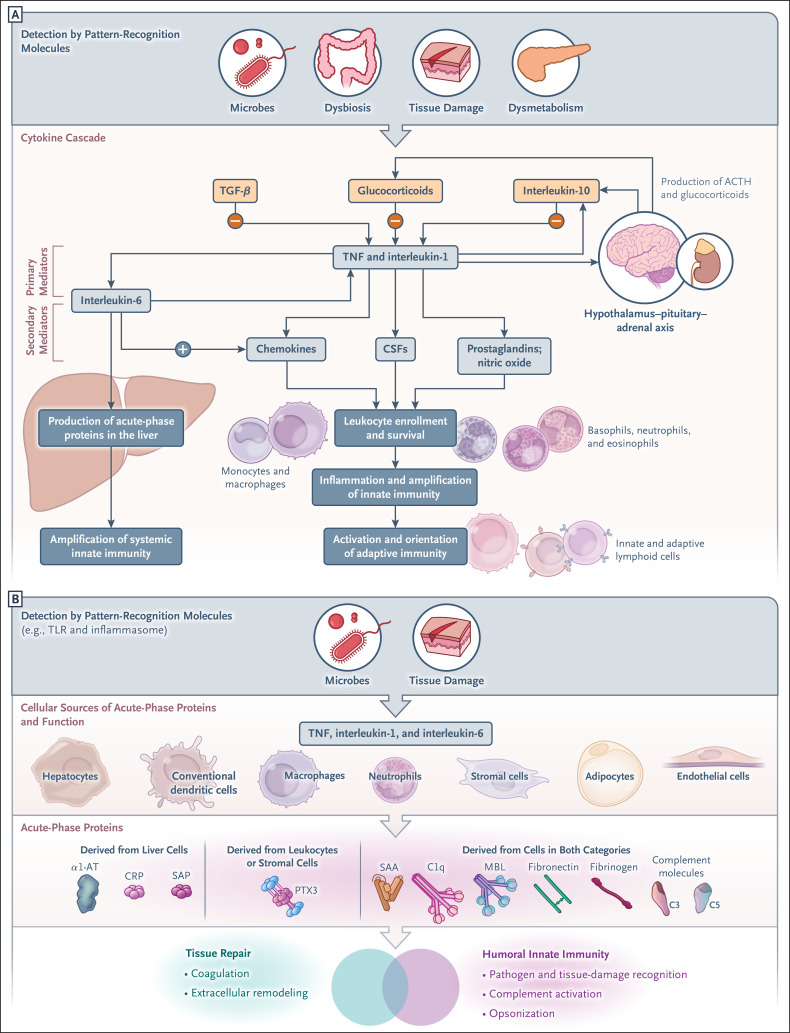
Sensing of microbial moieties, tissue damage, or dysmetabolism by cellular pattern-recognition molecules sets in motion a cytokine cascade that induces amplification and regulation of innate immunity and production of acute-phase proteins, as shown in Figure 3A. Primary inflammatory cytokines, typically interleukin-1, interleukin-6, and tumor necrosis factor (TNF), induce production of secondary mediators in tissues (e.g., interleukin-6 itself, chemokines, colony-stimulating factors, endothelial adhesion molecules, prostaglandins, and nitric oxide); these mediators amplify leukocyte recruitment, effector functions, and local innate immunity. Amplification of local innate immunity sets the stage for the activation and orientation of adaptive (antigen-specific) immune responses.
Figure 3. The Cytokine Cascade and Cellular Sources and Functions of Acute-Phase Proteins.
Panel A shows the cytokine cascade set in motion by pattern-recognition molecules; this process involves the production of primary and secondary mediators, activation of the acute-phase response, and promotion of leukocyte recruitment and leads to amplification of local and systemic innate immunity, as well as to the activation and orientation of adaptive immune responses. Inflammatory cytokines also promote the expression of negative regulators of inflammation (interleukin-10, transforming growth factor beta [TGF-β], and interleukin-1Ra) and the activation of the hypothalamus–pituitary–adrenal axis, which results in production of adrenocorticotropic hormone (ACTH) and glucocorticoid hormones. As shown in Panel B, in addition to hepatocytes, other cell types contribute to the synthesis of acute-phase proteins, which contribute to humoral innate immunity and tissue repair. α1-AT denotes α1-antitrypsin, CSF colony-stimulating factor, SAA serum amyloid A, and TNF tumor necrosis factor.
In addition to stimulating chemokine production and favoring the transition from acute to chronic inflammation, interleukin-6 is a potent inducer of the production of acute-phase proteins in the liver through reprogramming and reorientation of metabolic functions (e.g., decrease in albumin production and increased production of acute-phase proteins). Inflammatory cytokines also act on the central nervous system through their activation of the hypothalamus–pituitary–adrenal axis, resulting in production of adrenocorticotropic hormone and glucocorticoid hormones. Glucocorticoid hormones, among their many functions, act as negative regulators of inflammation by suppressing, for instance, interleukin-1 and inducing the interleukin-1 decoy receptor interleukin-1R2.26 Antiinflammatory cytokines (interleukin-10, transforming growth factor β, and interleukin-1Ra) are also part of pathways of negative regulation (Figure 3A). Among these antiinflammatory cytokines, interleukin-1Ra, which acts as an interleukin-1R antagonist, has classically been considered a liver-derived acute-phase protein27 and is a product of macrophages and other cell types in tissues. Interleukin-1 and interleukin-6 are the key regulators of acute-phase protein synthesis in the liver through their activation of a network of transcription factors (signal transducer and activator of transcription 3 [STAT3], nuclear factor κB, and CCAAT/enhancer-binding proteins) and methylation of CpG motifs in the binding sites of these transcription factors.28
Hepatic and Nonhepatic Sources of Acute-Phase Proteins
The liver has classically been considered the source of the elevated blood levels of acute-phase proteins4 (Figure 3A). Approximately 200 acute-phase proteins are produced — mainly by hepatocytes, but other cell types also contribute to the acute-phase reaction. These cell types include organ-infiltrating monocytes and tissue-resident macrophages such as Kupffer cells, hepatic stellate cells, and endothelial cells, all of which are sources of proinflammatory cytokines that activate acute-phase protein synthesis by hepatocytes.28
Evidence suggests that in addition to hepatocytes, cells in peripheral tissues can produce some acute-phase proteins (Figure 3B). For instance, macrophages and endothelial cells can produce complement components,29 serum amyloid A (SAA),30 iron transporters, α1-antitrypsin,31 and interleukin-1Ra. The pentraxin relative of C-reactive protein, pentraxin 3 (PTX3), is released mainly in peripheral tissues by diverse cell types, most notably phagocytes and endothelial cells, on induction by microbial moieties or inflammatory cytokines. At a local tissue level, local production complements the function of circulating acute-phase proteins produced by hepatocytes (Figure 3B). For instance, adipose tissue is an important source of the overall systemic concentration of acute-phase proteins in response to proinflammatory stimuli (interleukin-1 and interleukin-6). Adipocytes express large amounts of complement factors (C3, D, and B), αl-acid glycoprotein, and lipocalin-2, as well as plasminogen activator inhibitor 1 (PAI-1) and serum amyloid A3 (SAA3).32 In myopathy, skeletal muscle wasting, and atrophy associated with critical illness, locally produced interleukin-6 and TNF contribute to the induction of acute-phase proteins in muscle cells. In this condition, primary inflammatory cytokines and serum amyloid A1 (SAA1) contribute to muscle atrophy.33
Molecules and Functions
Pentraxins
Pentraxins are a family of evolutionarily conserved proteins characterized by a cyclic multimeric structure and by the presence of a conserved 200-amino-acid pentraxin domain. C-reactive protein (also called PTX1) and serum amyloid P component (SAP, or PTX2) are pentameric short pentraxins.34 PTX3 is an octameric molecule, and each protomer has a pentraxin-like domain associated with a long N-terminal domain unrelated to those of other known proteins.35
C-reactive protein is a prototypic liver-derived acute-phase protein in humans, whereas SAP is the main acute-phase protein in mice. In humans, C-reactive protein plasma levels increase by as much as 1000 times in response to an acute-phase stimulus, in particular to interleukin-6, whereas SAP is constitutively present in plasma. In contrast, PTX3 is rapidly induced in response to interleukin-1 and TNF or microbial components in various cell types — in particular, myelomonocytic cells (monocytes, macrophages, dendritic cells), vascular and lymphatic endothelial cells, and stromal cells.35 Neutrophils synthesize PTX3 during myelopoiesis, store it in lactoferrin-positive granules, and rapidly release it after microbial recognition.36 Thus, PTX3 differs from short pentraxins in terms of structure, cell source, and regulation.
C-reactive protein, SAP, and PTX3 bind various bacteria, fungi, and viruses, promoting innate immune responses to these pathogens.25,34,37,38 Pentraxins also bind to phospholipids and small nuclear ribonucleoproteins in apoptotic cells, promoting the disposal of these cells in a noninflammatory mode.39
C-reactive protein, SAP, and PTX3 interact with different complement molecules (e.g., C1q, ficolins, and mannose-binding lectin [MBL]), which leads to enhanced and broader recognition potential. In addition to promoting complement-dependent opsonization, short pentraxins and PTX3 promote phagocytosis of microbes and apoptotic cells by interacting with FcγR, particularly with FcγRIII (also called CD16) and FcγRII (CD32)40 (Figure 2). They also interact with complement regulators, such as factor H and C4BP, thereby promoting regulation of complement-dependent inflammation.
Genetic polymorphisms are associated with increased C-reactive protein levels, and C-reactive protein levels in blood are associated with the risk of coronary heart disease.8,9 This association suggested a pathogenetic role for C-reactive protein in atherosclerosis. Mendelian randomization analysis in a large cohort of patients with coronary disease showed that genetically increased concentrations of C-reactive protein are unrelated to conventional risk factors and the risk of cardiovascular events.41
SAP binds and stabilizes all forms of amyloid fibrils, contributing to amyloidosis.34,42 SAP also binds extracellular matrix components, such as laminin, type IV collagen, fibronectin, and proteoglycans, thereby regulating extracellular matrix deposition and inhibiting fibrosis. In idiopathic pulmonary fibrosis, human SAP (PRM-151) improves lung function43 by inhibiting alternative activation of macrophages and fibrocyte differentiation. In addition, PTX3 interacts with extracellular matrix proteins (TNF-α–induced protein 6 and inter–α-trypsin inhibitor), fibrinogen or fibrin, and plasminogen, which explains its involvement in matrix remodeling in tissue damage and repair.44
PTX3 plasma concentrations increase rapidly during a number of infections and are positively associated with disease severity and the risk of death,45-48 as discussed below for Covid-19. PTX3 plasma levels also reflect the severity of inflammatory vascular diseases ranging from atherosclerosis to vasculitis.49 In inflammatory conditions, PTX3 levels increase earlier than C-reactive protein levels, as shown in Figure 1B. The different kinetics of the appearance of PTX3 and C-reactive protein may well reflect their different cellular sources. PTX3 is stored in neutrophil granules, ready-made for release, and PTX3 serves as an immediate early gene in tissues, with its transcription induced by TLR agonists and inflammatory cytokines. In contrast, C-reactive protein production in the liver is downstream of the cytokine cascade, which results in a later appearance.
PTX3 and SAP genetic polymorphisms have been associated with susceptibility to fungal and bacterial infections38,48,50,51 and to lung granuloma formation in sarcoidosis through regulation of complement.52 Thus, results of mechanistic analyses, evidence from gene-targeted mice, and findings from analyses of human genetic polymorphisms are consistent with the view that the pentraxin trio of C-reactive protein–SAP–PTX3 plays a role in the amplification of innate resistance to selected pathogens and in the regulation of tissue remodeling.
Serum Amyloid A
Members of the SAA family are major acute-phase proteins in humans. In humans, four genes encode different members of the family; SAA1 and SAA2 are typical liver-derived acute-phase proteins and are collectively termed A-SAA. Extrahepatic synthesis of A-SAA in joints accounts for high SAA levels in the synovial fluid, in addition to high systemic plasma levels.53 In the small intestine, SAA is induced in epithelial cells by interleukin-22 and promotes local T helper 17 cell differentiation and effector function, favoring barrier integrity.54
Cytokine-like functional activities have been reported for SAA family members, including chemotaxis caused by direct interaction with the G protein–coupled formyl peptide receptors. In addition, the scavenger receptor B-I (CD36) acts as an endocytic SAA receptor and is involved in SAA-mediated immune and inflammatory functions. A-SAA also reportedly induces M2-like (antiinflammatory) skewing of macrophages and opsonizes gram-negative pathogenic bacteria, promoting their clearance and innate resistance to infections.55
Because of the massive increase in its plasma concentrations during inflammation, A-SAA has been used as a marker in several inflammatory conditions, such as rheumatoid arthritis, cardiovascular diseases, cancer, and infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.30,53,56 Long-term or recurrent high plasma SAA concentrations (e.g., due to tuberculosis or rheumatoid arthritis) in association with SAA1 allelic variants or other, unknown factors can lead to amyloid A (AA) amyloidosis, a condition caused by the accumulation of AA fibrils in several organs, including the kidneys, spleen, and liver, impairing their function. AA fibrils form as a consequence of SAA-derived C-terminal truncated AA protein folding into extremely hydrophobic β sheets that aggregate in oligomers, which generate insoluble, proteolysis-resistant fibrils.57
The Complement System
The complement system is an evolutionarily conserved central player in humoral innate immunity. It consists of approximately 50 soluble molecules, mostly produced by the liver and normally found in the circulation, and cell-associated receptors, which are expressed by several cell types.29,58
The liver is the major site for the synthesis of most complement molecules. Among them, both activating molecules (C3, C4, C9, and factor B) and negative regulators (C1 inhibitor and C4BP) are up-regulated during an acute-phase reaction, which underlines the relevance of activating balanced complement-mediated responses. However, their synthesis increases modestly as compared with that of major acute-phase reaction molecules and peaks at late time points4 (Figure 1B). In addition to hepatocytes, other cell types, including monocytes, macrophages, endothelial cells, fibroblasts, and adipocytes, can be local sources of complement proteins such as C1q, C3, and C529 (Figure 3B).
Mannose-Binding Lectin
MBL is a liver-derived C-type plasma lectin, a class of pattern-recognition molecules composed of a Ca2+-type lectin domain (also called the carbohydrate-recognition domain) and a collagen-like domain,59 acting as a humoral pattern-recognition molecule with high affinity for mannose and N-acetyl glucosamine exposed on microbes. MBL interacts with these carbohydrate moieties through the lectin domain, opsonizing pathogens for phagocytosis and leading to activation of MBL-associated serine proteases, thus initiating the complement cascade through the lectin pathway. Within MBL2, the gene encoding human MBL, three point mutations have been found in exon 1, which encodes the MBL collagenous region; in addition, several polymorphisms have been found in the promoter region. These mutations and polymorphisms affect the function and plasma concentration of the molecule. The combination of the promoter haplotypes and structural mutations results in MBL deficiency, which occurs in approximately 25% of persons and is associated with increased susceptibility to selected infections, particularly in children with primary or secondary immunodeficiency.60 MBL was originally defined as an acute-phase protein on the basis of the up-regulation of MBL2 in liver-biopsy specimens from patients with inflammatory conditions. However, subsequent studies have shown that in most persons with coding mutations, MBL is not up-regulated in the acute phase of infectious conditions. For instance, in studies involving patients with sepsis or pneumonia, MBL behaved as a positive or negative acute-phase protein or did not change during hospitalization, with its behavior depending mainly on the genotype of exon 1 and the promoter61,62 and possibly on other single-nucleotide polymorphisms in regulatory regions.22
Acute-Phase Proteins and Iron Homeostasis
Several acute-phase proteins are involved in the metabolism of iron, a nutrient required for a number of host-cell functions as well as for the growth of microbial pathogens. The general functions of acute-phase proteins in iron metabolism include binding to the nutrient, thus preventing utilization of circulating free iron by pathogens, and retention of iron inside cells. Therefore, the complex regulation of iron metabolism results in metabolic resistance to selected pathogens. The acute-phase proteins involved in free iron control include the circulating peptide hormone hepcidin, ferritin, haptoglobin, and hemopexin, which are up-regulated in the acute-phase reaction, whereas transferrin is a negative acute-phase protein that is down-regulated during the acute phase (Table 1). Hepcidin binds the transmembrane protein ferroportin, regulating the release of iron from cells to plasma. Ferritin normally directly reflects iron levels in blood; however, in contrast to iron-deficiency anemia, anemia associated with chronic inflammatory diseases is characterized by higher levels of ferritin than normal because of its induction by the acute-phase reaction. Indeed, a high plasma concentration of ferritin is observed in patients with severe pathologic inflammatory conditions, including macrophage-activation syndrome, septic shock, and Covid-19, in which ferritin is used as a marker of severity and prognosis (see below).
Haptoglobin and hemopexin are acute-phase proteins that act as soluble scavengers of free hemoglobin and heme, respectively. Free heme is highly toxic because it is a source of redox-active iron and has the ability to intercalate into lipid membranes, promoting lipid peroxidation. Up-regulation of haptoglobin and hemopexin in acute-phase reactions favors protection against heme-mediated oxidative stress, iron loss in inflammatory conditions associated with hemolysis, and infections by preventing the utilization of iron by pathogens.
Acute-Phase Proteins, Coagulation, and Tissue Repair
A number of acute-phase proteins are related to the coagulation cascade. Fibrinogen and its downstream degradation products (d-dimer and other fibrin degradation products) are widely used as diagnostic markers in inflammatory conditions, including Covid-19. Coagulation and tissue repair are strictly connected processes. The fibrin mesh formed downstream of the coagulation cascade serves as a provisional matrix essential for tissue repair. Its timely removal by means of fibrinolysis is a prerequisite for subsequent steps in matrix maturation. The acute-phase reaction fuels components involved in coagulation (e.g., fibrinogen) and extracellular matrix formation (fibronectin) (Table 1). PTX3, unlike its relative C-reactive protein, engages in a tripartite interaction with fibrinogen and plasminogen, promoting the timely degradation of the provisional fibrin mesh and subsequent tissue repair.44 In addition to extracellular matrix components, inhibitors of proteolytic enzymes (e.g., α1-antitrypsin, α2-macroglobulin, and α1-acid glycoprotein) are produced during the acute-phase reaction, and these inhibitors may limit tissue damage. For instance, α1-antitrypsin has host protective functions in autoimmunity and infection.31,63
Extracellular matrix proteins, including fibrinogen and fibronectin, bind microbes and facilitate their clearance by phagocytes. The ancestral function of fibrinogen domain–containing molecules was defense.25 Therefore, the production of some extracellular matrix proteins during systemic inflammation is at the intersection of tissue repair and innate immunity.
Covid-19
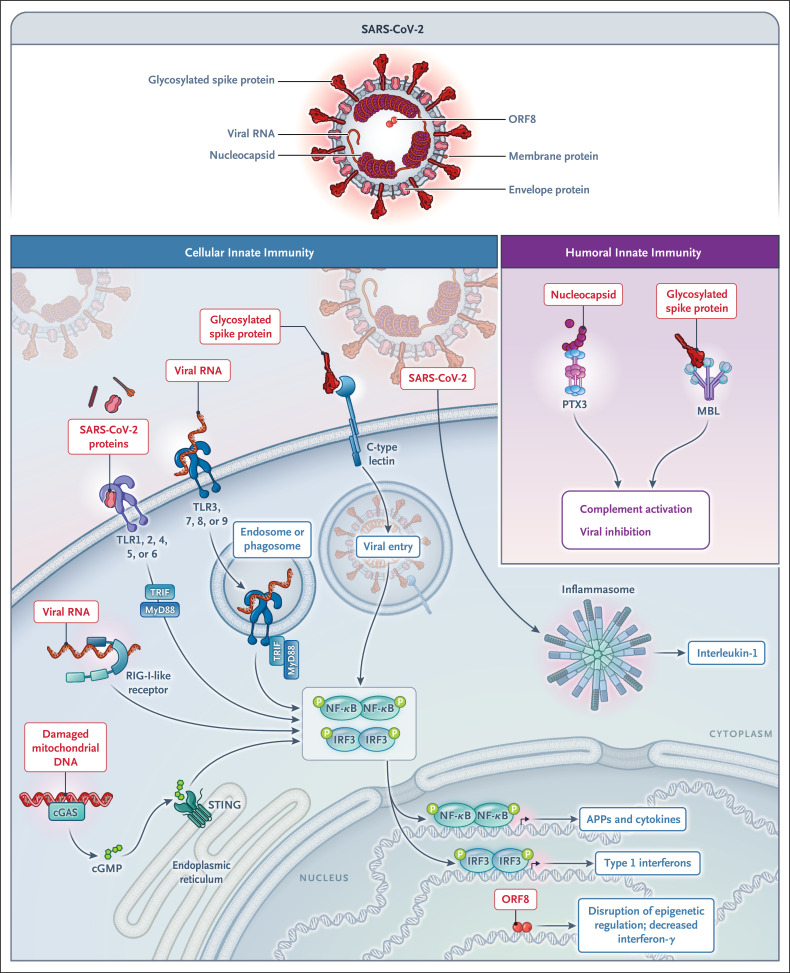
Innate immune recognition of SARS-CoV-2 acts as a fundamental first line of resistance, triggers adaptive immunity, and drives the immunopathologic effects of infection.64 The cellular sensors involved in the innate response to SARS-CoV-2 include membrane C-type lectins that recognize spike protein65; endosomal TLR3, TLR7, and TLR8, which recognize viral nucleic acids; the cytoplasmic cyclic GMP–AMP synthase (cGAS)–STING pathway; and the inflammasome (Figure 4). The SARS-CoV-2–encoded open reading frame 8 (ORF8) has recently been shown to inhibit interferon production through epigenetic mechanisms.66 SARS-CoV-2 components are recognized by selected acute-phase proteins. MBL binds the spike protein of all variants tested and has antiviral activity.22 Moreover, it activates the complement lectin pathway, thus possibly contributing to immunopathologic effects in advanced disease. PTX3, unlike C-reactive protein and SAP, binds the viral nucleoprotein,22 but the actual in vivo function of this interaction has not been defined.
Figure 4. Recognition of SARS-CoV-2 by Cellular and Humoral Pattern-Recognition Molecules, Including Acute-Phase Proteins.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins and nucleic acids are recognized by cellular receptors involved in innate immunity, including C-type lectins, TLRs, cGAS–STING, and the inflammasome. Open reading frame 8 (ORF8) inhibits interferon production through epigenetic mechanisms. The humoral pattern-recognition molecules MBL and PTX3 bind glycosylated spike and the viral nucleocapsid, respectively.
Although the clinical significance of virus recognition by selected acute-phase proteins remains to be fully elucidated, these molecules have served as invaluable diagnostic tools throughout the pandemic, in contexts ranging from outpatient clinics to intensive care units (ICUs) (Table 1). C-reactive protein, procalcitonin, and ferritin have been used extensively, and in most studies, high plasma concentrations at hospital admission have been associated with severe disease and poor survival.11-14 The concentration of d-dimer downstream of fibrinogen has been positively correlated with areas of hypoperfusion in patients with acute respiratory distress syndrome, a finding consistent with thromboembolic disease, and has been associated with higher mortality.67 Dysregulated iron homeostasis, which is common in hospitalized patients with Covid-19, as reflected by the presence of anemia and an increased ferritin:transferrin ratio, has been found to predict ICU admission and receipt of mechanical ventilation.24 The concentration of complement components is increased in patients with Covid-19, a finding consistent with a role of this pathway in immunopathologic effects.20 The complement pathway has emerged as a therapeutic target in Covid-19, with encouraging preliminary results in small cohort studies showing a reduced acute-phase reaction, reduced thrombin activity, and reduced neutrophil extracellular trap generation in association with complement inhibition.21 In a series of independent studies based on conventional immunoassays16,17,68-70 or proteomic approaches,71 PTX3 has emerged as a strong prognostic marker and independent predictor of death within 28 days in hospitalized patients. PTX3 was found to be expressed by myeloid cells in peripheral blood and lungs and by lung endothelial cells in patients with Covid-19.16 The strong independent prognostic significance of PTX3, which is better than that of C-reactive protein, interleukin-6, ferritin, or d-dimer, may reflect an integration of myeloid and microvascular endothelial cell activation.
The selection of patients for different therapies at different stages of the disease and their follow-up remain formidable challenges. Elevations in C-reactive protein levels combined with the determination of a need for supplemental oxygen have been used to select patients who may benefit from anti–interleukin-6 therapy.72 In a small study (involving 30 patients), the response to treatment with an anti–interleukin-6 monoclonal antibody (siltuximab) was measured with the use of a range of biomarkers. Levels of C-reactive protein were decreased irrespective of clinical benefit, reflecting the inhibition of production of this acute-phase protein by the liver, unrelated to tissue inflammation. In contrast, PTX3 and interleukin-8, which are produced in tissues, were better correlates of clinical response.68
Prediction of disease progression represents a holy grail for timely intervention. A low-cost signature that included C-reactive protein as an indicator of systemic inflammation, PTX3 as a correlate of tissue reaction, and lactate dehydrogenase as an indicator of cell and tissue damage was found to correlate with the severity of lesions on computed tomography and subsequent disease progression in patients with paucisymptomatic Covid-19.18 Integration of low-tech, low-cost measurement of selected acute-phase proteins with molecular signatures may pave the way to the development of tools allowing more patient-tailored early approaches.
Postacute sequelae of Covid-19 (PASC), also known as “long Covid,” is a challenge for patients and for health care systems. The pathogenesis of PASC is complex and depends on several driving factors, including the persistence of SARS-CoV-2 in different organs; reactivation and response to unrelated viruses, such as Epstein–Barr virus; autoimmunity; sustained inflammation; and microvascular thrombosis.73-75 Fatigue, muscle weakness, and exercise intolerance are among the most frequent symptoms of long Covid. This clinical picture is reminiscent of a condition known as chronic fatigue syndrome or myalgic encephalomyelitis, which occurs after viral infections, in which acute-phase proteins have emerged as correlates of disease activity.73,76 In a recent proteomic study aimed at defining a biomarker associated with subsequent development of PASC, a set of acute-phase proteins, including C-reactive protein and molecules involved in iron metabolism, emerged as part of an inflammation and stress-response signature predictive of long Covid.77 Profound perturbations of myeloid-cell function were observed 8 months after mild-to-moderate Covid-19. A set of biomarkers (interferon-β, interferon-γ, interferon-λ, interleukin-6, and PTX3) was associated with PASC.19 The borders of the long-Covid universe and the diversity of organ involvement remain ill-defined. Changes in acute-phase protein levels together with emerging signatures74,78 may help to define the actual borders of this universe, its diversity, and the prognosis of organ involvement.
Conclusions
Since the discovery of C-reactive protein, acute-phase proteins have been invaluable tools at the bedside in a wide range of diseases, including Covid-19 and long Covid,11-14,16 which points to inflammation as a metanarrative of medicine at present and in the foreseeable future.10,26 Acute-phase proteins have emerged as more than innocent bystanders of acute and chronic inflammation. Many of these molecules recognize microbial moieties and damaged cells or tissues. These ante-antibodies promote disposal of microbes and dead cells by activating and regulating the complement cascade and by mediating opsonic activity. Increased production of matrix molecules (fibrinogen and fibronectin) and protease inhibitors during the acute-phase response may be viewed as a general mechanism to promote tissue repair. Moreover, changes in iron metabolism have broad implications at a systemic (metabolic resistance) and cellular level.79 Thus, acute-phase proteins, and by and large the acute-phase response, are an essential component of humoral innate immunity, promoting antimicrobial resistance and tissue repair.
The recognition that some acute-phase proteins are more than biomarkers raises the possibility that they may represent therapeutic tools or targets. SAP binds to all forms of amyloid fibrils and is a therapeutic target in amyloidosis and neurodegeneration.34,42,80 However, on the basis of its inhibitory effect on fibrocyte differentiation, SAP has entered clinical assessment for the treatment of idiopathic pulmonary fibrosis43 and is being evaluated in randomized phase 3 trials (ClinicalTrials.gov numbers, NCT04594707 and NCT04552899). Genetic polymorphisms at the SAP and PTX3 loci and evidence from preclinical studies point to the therapeutic potential of these molecules for aspergillus infections, which pose a major clinical challenge.38,48 MBL has been administered to patients with genetic deficiencies81 and has been recently shown to recognize the spike protein of known SARS-CoV-2 variants and to mediate resistance against SARS-CoV-2.22 Thus, human genetics, safety, and preclinical findings call for efforts to explore the potential of acute-phase proteins for future therapeutic applications.
Comprehensive approaches that take advantage of state-of-the-art technology have identified candidate signatures associated with the risk and clinical course of Covid-19,19,74,77,78 and some of the molecules discussed here are part of these signatures. Integration of classic validated biomarkers in emerging signatures and their rigorous assessment in large population studies with sustainable technology hold promise for a “back to the future” for acute-phase proteins 100 years on from their initial discovery.6,7
Acknowledgments
We thank Dr. Antonio Voza (head of the emergency department, Humanitas Research Hospital) and Prof. Maurizio Cecconi (head of the intensive care unit, Humanitas Research Hospital) for discussion and suggestions.
Disclosure Forms
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Medzhitov R. The spectrum of inflammatory responses. Science 2021;374:1070-1075. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Nonresolving inflammation redux. Immunity 2022;55:592-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gitlin JD, Colten HR. Molecular biology of the acute phase plasma proteins. In: Pick E, Landy M, eds. Lymphokines. Vol. 14. San Diego: Academic Press, 1987:123-153. [Google Scholar]
- 4.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448-454. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med 1984;311:1413-1418. [DOI] [PubMed] [Google Scholar]
- 6.Tillett WS, Francis T. Serological reactions in pneumonia with a nonprotein somatic fraction of pneumococcus. J Exp Med 1930;52:561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abernethy TJ, Avery OT. The occurrence during acute infections of a protein not normally present in the blood: I. Distribution of the reactive protein in patients’ sera and the effect of calcium on the flocculation reaction with C polysaccharide of pneumococcus. J Exp Med 1941;73:173-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836-843. [DOI] [PubMed] [Google Scholar]
- 9.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 2006;6:508-519. [DOI] [PubMed] [Google Scholar]
- 10.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danwang C, Endomba FT, Nkeck JR, Wouna DLA, Robert A, Noubiap JJ. A meta-analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID-19). Biomark Res 2020;8:37-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol 2020;92:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji P, Zhu J, Zhong Z, et al. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine (Baltimore) 2020;99(10)e23315-e23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cecconi M, Piovani D, Brunetta E, et al. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for Covid-19 infection in Lombardy, Italy. J Clin Med 2020;9:1548-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zinellu A, Paliogiannis P, Carru C, Mangoni AA. Serum amyloid A concentrations, COVID-19 severity and mortality: an updated systematic review and meta-analysis. Int J Infect Dis 2021;105:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunetta E, Folci M, Bottazzi B, et al. Macrophage expression and prognostic significance of the long pentraxin PTX3 in COVID-19. Nat Immunol 2021;22:19-24. [DOI] [PubMed] [Google Scholar]
- 17.Lapadula G, Leone R, Bernasconi DP, et al. Long pentraxin 3 (PTX3) levels predict death, intubation and thrombotic events among hospitalized patients with COVID-19. Front Immunol 2022;13:933960-933960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folci M, Brunetta E, Lanza E, et al. A PTX3/LDH/CRP signature correlates with lung injury CTs scan severity and disease progression in paucisymptomatic COVID-19. September 29, 2021. (https://www.medrxiv.org/content/10.1101/2021.09.29.21264061v1). preprint.
- 19.Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022;23:210-216. [DOI] [PubMed] [Google Scholar]
- 20.Risitano AM, Mastellos DC, Huber-Lang M, et al. Complement as a target in COVID-19? Nat Rev Immunol 2020;20:343-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skendros P, Germanidis G, Mastellos DC, et al. Complement C3 inhibition in severe COVID-19 using compstatin AMY-101. Sci Adv 2022;8(33):eabo2341-eabo2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stravalaci M, Pagani I, Paraboschi EM, et al. Recognition and inhibition of SARS-CoV-2 by humoral innate immunity pattern recognition molecules. Nat Immunol 2022;23:275-286. [DOI] [PubMed] [Google Scholar]
- 23.Thurner L, Kessel C, Fadle N, et al. IL-1RA antibodies in myocarditis after SARS-CoV-2 vaccination. N Engl J Med 2022;387:1524-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellmann-Weiler R, Lanser L, Barket R, et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med 2020;9:2429-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol 2010;28:157-183. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 2019;50:778-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest 1997;99:2930-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehlting C, Wolf SD, Bode JG. Acute-phase protein synthesis: a key feature of innate immune functions of the liver. Biol Chem 2021;402:1129-1145. [DOI] [PubMed] [Google Scholar]
- 29.Reichhardt MP, Meri S. Intracellular complement activation — an alarm raising mechanism? Semin Immunol 2018;38:54-62. [DOI] [PubMed] [Google Scholar]
- 30.De Buck M, Gouwy M, Wang JM, et al. Structure and expression of different serum amyloid A (SAA) variants and their concentration-dependent functions during host insults. Curr Med Chem 2016;23:1725-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai X, Bai A, Tomasicchio M, et al. α1-Antitrypsin binds to the glucocorticoid receptor with anti-inflammatory and antimycobacterial significance in macrophages. J Immunol 2022;209:1746-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem 2001;276:42077-42083. [DOI] [PubMed] [Google Scholar]
- 33.Langhans C, Weber-Carstens S, Schmidt F, et al. Inflammation-induced acute phase response in skeletal muscle and critical illness myopathy. PLoS One 2014;9(3):e92048-e92048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepys MB. The pentraxins 1975–2018: serendipity, diagnostics and drugs. Front Immunol 2018;9:2382-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garlanda C, Bottazzi B, Magrini E, Inforzato A, Mantovani A. PTX3, a humoral pattern recognition molecule, in innate immunity, tissue repair, and cancer. Physiol Rev 2018;98:623-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaillon S, Peri G, Delneste Y, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 2007;204:793-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 2002;420:182-186. [DOI] [PubMed] [Google Scholar]
- 38.Doni A, Parente R, Laface I, et al. Serum amyloid P component is an essential element of resistance against Aspergillus fumigatus. Nat Commun 2021;12:3739-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bickerstaff MC, Botto M, Hutchinson WL, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med 1999;5:694-697. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature 2008;456:989-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wensley F, Gao P, Burgess S, et al. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ 2011;342:d548-d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodin K, Ellmerich S, Kahan MC, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature 2010;468:93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghu G, van den Blink B, Hamblin MJ, et al. Long-term treatment with recombinant human pentraxin 2 protein in patients with idiopathic pulmonary fibrosis: an open-label extension study. Lancet Respir Med 2019;7:657-664. [DOI] [PubMed] [Google Scholar]
- 44.Doni A, Musso T, Morone D, et al. An acidic microenvironment sets the humoral pattern recognition molecule PTX3 in a tissue repair mode. J Exp Med 2015;212:905-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caironi P, Masson S, Mauri T, et al. Pentraxin 3 in patients with severe sepsis or shock: the ALBIOS trial. Eur J Clin Invest 2017;47:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller B, Peri G, Doni A, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med 2001;29:1404-1407. [DOI] [PubMed] [Google Scholar]
- 47.Lee YT, Gong M, Chau A, et al. Pentraxin-3 as a marker of sepsis severity and predictor of mortality outcomes: a systematic review and meta-analysis. J Infect 2018;76:1-10. [DOI] [PubMed] [Google Scholar]
- 48.Cunha C, Aversa F, Lacerda JF, et al. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med 2014;370:421-432. [DOI] [PubMed] [Google Scholar]
- 49.Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 2009;29:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wójtowicz A, Lecompte TD, Bibert S, et al. PTX3 polymorphisms and invasive mold infections after solid organ transplant. Clin Infect Dis 2015;61:619-622. [DOI] [PubMed] [Google Scholar]
- 51.Jaillon S, Moalli F, Ragnarsdottir B, et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 2014;40:621-632. [DOI] [PubMed] [Google Scholar]
- 52.Gonçales RA, Bastos HN, Duarte-Oliveira C, et al. Pentraxin 3 inhibits complement-driven macrophage activation to restrain granuloma formation in sarcoidosis. Am J Respir Crit Care Med 2022;206:1140-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connolly M, Marrelli A, Blades M, et al. Acute serum amyloid A induces migration, angiogenesis, and inflammation in synovial cells in vitro and in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol 2010;184:6427-6437. [DOI] [PubMed] [Google Scholar]
- 54.Sano T, Huang W, Hall JA, et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 2015;163:381-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah C, Hari-Dass R, Raynes JG. Serum amyloid A is an innate immune opsonin for Gram-negative bacteria. Blood 2006;108:1751-1757. [DOI] [PubMed] [Google Scholar]
- 56.Fyfe AI, Rothenberg LS, DeBeer FC, Cantor RM, Rotter JI, Lusis AJ. Association between serum amyloid A proteins and coronary artery disease: evidence from two distinct arteriosclerotic processes. Circulation 1997;96:2914-2919. [DOI] [PubMed] [Google Scholar]
- 57.Westermark GT, Fändrich M, Westermark P. AA amyloidosis: pathogenesis and targeted therapy. Annu Rev Pathol 2015;10:321-344. [DOI] [PubMed] [Google Scholar]
- 58.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol 2017;18:1288-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol 2003;21:547-578. [DOI] [PubMed] [Google Scholar]
- 60.Koch A, Melbye M, Sørensen P, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA 2001;285:1316-1321. [DOI] [PubMed] [Google Scholar]
- 61.Dean MM, Minchinton RM, Heatley S, Eisen DP. Mannose binding lectin acute phase activity in patients with severe infection. J Clin Immunol 2005;25:346-352. [DOI] [PubMed] [Google Scholar]
- 62.Herpers BL, Endeman H, de Jong BAW, et al. Acute-phase responsiveness of mannose-binding lectin in community-acquired pneumonia is highly dependent upon MBL2 genotypes. Clin Exp Immunol 2009;156:488-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ebert M, Jerke U, Eulenberg-Gustavus C, et al. Protective α1-antitrypsin effects in autoimmune vasculitis are compromised by methionine oxidation. J Clin Invest 2022;132(23):e160089-e160089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science 2022;375:1122-1127. [DOI] [PubMed] [Google Scholar]
- 65.Lempp FA, Soriaga LB, Montiel-Ruiz M, et al. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature 2021;598:342-347. [DOI] [PubMed] [Google Scholar]
- 66.Kee J, Thudium S, Renner DM, et al. SARS-CoV-2 disrupts host epigenetic regulation via histone mimicry. Nature 2022;610:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 2020;8:1201-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gritti G, Raimondi F, Bottazzi B, et al. Siltuximab downregulates interleukin-8 and pentraxin 3 to improve ventilatory status and survival in severe COVID-19. Leukemia 2021;35:2710-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen CB, Sandholdt H, Møller MEE, et al. Prediction of respiratory failure and mortality in COVID-19 patients using long pentraxin PTX3. J Innate Immun 2022;14:493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schirinzi A, Pesce F, Laterza R, et al. Pentraxin 3: potential prognostic role in SARS-CoV-2 patients admitted to the emergency department. J Infect 2021;82:84-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gutmann C, Takov K, Burnap SA, et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat Commun 2021;12:3406-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. A guide to immunotherapy for COVID-19. Nat Med 2022;28:39-50. [DOI] [PubMed] [Google Scholar]
- 73.Mantovani A, Morrone MC, Patrono C, et al. Long Covid: where we stand and challenges ahead. Cell Death Differ 2022;29:1891-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022;185(5):881-895.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pretorius E, Vlok M, Venter C, et al. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 2021;20:172-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med 2022;28:911-923. [DOI] [PubMed] [Google Scholar]
- 77.Captur G, Moon JC, Topriceanu CC, et al. Plasma proteomic signature predicts who will get persistent symptoms following SARS-CoV-2 infection. EBioMedicine 2022;85:104293-104293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klein J, Wood J, Jaycox J, et al. Distinguishing features of long COVID identified through immune profiling. August 10, 2022. (https://www.medrxiv.org/content/10.1101/2022.08.09.22278592v1). preprint. [DOI] [PMC free article] [PubMed]
- 79.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol 2011;32:241-247. [DOI] [PubMed] [Google Scholar]
- 80.Pepys MB, Dyck RF, de Beer FC, Skinner M, Cohen AS. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol 1979;38:284-293. [PMC free article] [PubMed] [Google Scholar]
- 81.Garred P, Pressler T, Lanng S, et al. Mannose-binding lectin (MBL) therapy in an MBL-deficient patient with severe cystic fibrosis lung disease. Pediatr Pulmonol 2002;33:201-207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.