Abstract
Introduction:
Medications for opioid use disorder (MOUD) reduce illicit opioid use and overdose mortality, but effectiveness remains limited by poor treatment retention. Understanding multilevel barriers and facilitators to retention from the patient perspective can guide intervention strategies to improve retention.
Methods:
We conducted semi-structured telephone interviews to elicit perspectives of individuals with opioid use disorder (OUD) currently (n = 19) and formerly (n = 16) receiving treatment from a multi-clinic outpatient MOUD program in Pennsylvania in July 2020 to January 2021. The Capability, Opportunity, Motivation, Behavior model provided a theoretical framework for analysis.
Results:
Based on interview themes, physical, rather than psychological, capability was more salient to MOUD engagement, and pertained to individual-level factors such as side effects, withdrawal, and the degree to which MOUD addressed participants’ need for pain management. Co-existing mental health conditions also challenged participants’ physical ability to attend appointments. The opportunity domain contained both physical and social aspects. Physical opportunity for MOUD engagement centered on community-level factors related to MOUD access (e.g., distance, transportation) and clinical-level factors including program policies. Themes related to social opportunity included interpersonal influences—such as therapeutic and social support—and stigma associated with OUD and MOUD. Motivation emerged as the dominant domain for patients. Reflective motivation factors included individual-level factors such as participants’ recognition of their addiction and “readiness” to quit illicit opioid use, attitudes toward MOUD, future treatment expectations, motivation to engage in MOUD, and perceived consequences of disengagement. Automatic motivation factors included the degree to which MOUD created a sense of normalcy for participants and the use of illicit drugs to numb emotions.
Conclusions:
Factors at the individual, interpersonal, clinical, community, and societal levels influenced patients’ capability, opportunity, and motivation to engage in MOUD. Understanding such factors can inform implementation strategies to improve retention.
Keywords: Addiction, Buprenorphine, Opioid-related disorders, Qualitative research, Rural health
1. Introduction
The U.S. drug overdose epidemic reached a tragic new record in 2021 of 108,000 overdose deaths, the majority of which involved opioids (CDC, 2022). Overdose mortality provides a glimpse of a much larger population of individuals suffering from opioid use disorder (OUD). In 2020, 2.7 million Americans aged 12 years and older had an OUD and 9.5 million had misused opioids in the past year (SAMHSA, 2021a). Rural regions have been disproportionately affected, particularly in Appalachia (Heffernan et al., 2021).
Medications for the treatment of OUD (MOUD)—including methadone, buprenorphine, and naltrexone—safely and effectively reduce illicit opioid use and overdose mortality (SAMHSA, 2021b). Maintenance treatment is often most effective at preventing relapse to illicit opioid use (SAMHSA, 2021b). MOUD retention of over one year (versus less than one year) is associated with lower mortality (Ma et al., 2019), and buprenorphine maintenance for one year (compared to three to five months) is associated with lower risk of all-cause hospitalizations and emergency department visits (Lo-Ciganic et al., 2016). Maintenance treatment also allows individuals the opportunity to make life changes and develop skills needed for recovery (SAMHSA, 2021b).
Despite the existence of effective treatment, a substantial addiction care gap remains (Socias et al., 2016). MOUD access presents an enormous hurdle, but even among those who initiate treatment, retention remains a challenge, leading to few individuals achieving long-term remission (Williams et al., 2017). A review of observational studies found wide variation in MOUD retention, with median retention of 57% at 12 months (O’Connor et al., 2020). Individuals face elevated risk of illicit opioid use (Bentzley et al., 2015) and overdose mortality (Sordo et al., 2017) in the first month following MOUD dropout. Determining the underlying reasons for persistently low MOUD retention is a research priority (Chan et al., 2021a).
Research on addiction treatment discontinuation has often focused on individual-level factors, reflecting a traditional medical model that views dropout as an abnormality of the patient (Brorson et al., 2013). Yet increasing MOUD retention requires identifying engagement factors at multiple levels. Qualitative research is crucial for accessing patient perspectives to understand multilevel factors that influence individuals to maintain or discontinue treatment. Prior qualitative studies have highlighted a range of factors related to MOUD engagement—such as individual attitudes toward MOUD, internal motivation, stigma, support from clinical staff, and transportation barriers (Beharie et al., 2022; Filteau et al., 2022; Granerud and Toft, 2015; Hewell et al., 2017; Lai et al., 2021; Notley et al., 2015; Scorsone et al., 2020)—but with few exceptions (e.g., Kahn et al., 2022), studies have not taken an explicitly multilevel approach. In this qualitative study, we explored multilevel barriers and facilitators to MOUD engagement from the perspective of two groups of individuals who had received buprenorphine treatment through an outpatient MOUD program—those currently engaged in treatment and those who had left the outpatient program. The Capability, Opportunity, and Motivation model of behavior (COM-B), often used to explore implementation problems, provided a theoretical framework for this study that facilitated a multilevel perspective (Michie et al., 2011).
2. Methods
2.1. Study design
We applied a collective case study design to gain an in-depth, multi-faceted understanding of patient engagement in MOUD (Crowe et al., 2011). We examined two cases, eliciting perspectives of individuals currently engaged in buprenorphine treatment through an outpatient MOUD program (in treatment) and those who had left the program (left treatment). We examined factors within and across cases influencing patient engagement and summarized common themes and differences. The study was approved by Geisinger’s Institutional Review Board.
2.2. Conceptual framework
The COM-B model provided a theoretical framework from which to understand multilevel factors related to MOUD engagement. COM-B is a comprehensive model of behavioral determinants that posits that behavior is determined by an interaction between three domains: (1) an individual’s psychological and physical capability to engage in a behavior; (2) physical and social opportunities that facilitate behavior; and (3) reflective (evaluative) and automatic (emotional) motivation that direct behavior (Michie et al., 2011).
2.3. Study setting
Pennsylvania is among the Appalachian states with the highest prevalence of drug overdose (Beatty et al., 2019). Geisinger, an integrated health system serving central and northeast Pennsylvania, has prioritized addressing the region’s opioid epidemic in part through an outpatient MOUD program. The program, selected as a Pennsylvania Center of Excellence for OUD (Pennsylvania Department of Human Services, 2022), uses a hub-and-spoke model (Barbour et al., 2020). The hubs comprise four geographically dispersed outpatient addiction treatment clinics—two located in small towns and two in an urban area—and serve roughly 2000 patients with OUD annually through provision and management of MOUD. This geographic diversity allowed for examination of cross-cutting and unique factors influencing MOUD engagement among urban and rural populations.
The outpatient addiction treatment clinics are staffed with physicians with fellowship training in addiction medicine, advanced practice providers, and embedded case managers. Available medications include buprenorphine and naltrexone; most patients with OUD receive a buprenorphine/naloxone sublingual preparation, with a sizable minority receiving a depot formulation of buprenorphine that involves a monthly injection. The program’s care model differentiates patients by one of two treatment objectives depending on patient goals: 1) functional improvement, with a goal of gradual cessation of substance use; and 2) harm reduction, with a goal of keeping patients engaged and alive and reducing the spread of infectious diseases (Barbour et al., 2020).
2.4. Sampling and recruitment
Using Geisinger electronic health record (EHR) data, we identified 1491 individuals who met inclusion criteria: 18 years of age or older; had a clinical encounter at one of Geisinger’s addiction medicine clinics between June 2018 and June 2020; had an International Classification of Diseases 10th Revision diagnostic code for OUD (F11.X); and had received at least two medication orders for buprenorphine. We recruited two groups of participants, based on clinic encounter dates: 1) individuals currently receiving buprenorphine (in treatment); and 2) individuals with no record of treatment in at least the past two months (left treatment). We used maximum variation sampling to recruit a balance of males and females of varying ages (<40 vs. ≥ 40 years of age) from each of the four addiction medicine clinics.
We emailed eligible participants informing them of the opportunity to participate in a research interview without divulging the study topic to avoid breaches in privacy. We then called them to describe the study and invite them to participate. The email also invited individuals to directly contact the study team via email or telephone to learn about the study and schedule an interview. We continued recruitment until we reached our sampling goal of at least 16 participants from each group; at this point, both interviewers were satisfied that data saturation had been reached. Telephone interviews were scheduled at a time convenient to the participant.
2.5. Data collection
We developed an interview guide with open-ended questions regarding participants’ addiction and treatment experience (Supplement). Although some questions drew from prior literature on MOUD retention factors, most questions allowed participants’ to share their unique treatment journey. The interview guide was developed with input from clinicians with expertise in addiction and qualitative researchers on the study team. Early in data collection, the interviewers met after each interview and discussed the guide’s effectiveness (e.g., clarity and usefulness of questions), but changes were not deemed necessary. Interviews were semi-structured and so covered questions in the interview guide, but with the interviewer maintaining discretion to follow leads and reframe questions (H. Russell Bernard, 2006).
During the telephone interview, we first obtained verbal consent and explained to participants the study’s purpose of learning from their experience with treatment to improve future care and how their responses would be kept confidential. Two study authors conducted the interviews—MP, a PhD researcher with expertise in qualitative research and interest in conducting research to improve treatment for OUD; and PA, an MD research assistant with training in qualitative research and interest in addiction medicine. Interviews were conducted between July 2020 and January 2021 and ranged from 21 to 84 min. Participants received a $50 e-gift card for participating. Immediately following each interview, interviewers wrote memos summarizing their observations. Demographic data were collected from the EHR.
2.6. Data analysis
Interviews were audio-recorded and transcribed verbatim, with post-interview memos incorporated into transcripts. We used a general inductive approach for data analysis (Thomas, 2006). Analysis began with immersion in the data by reading all transcripts. This was followed by a phase of open coding, with MP and PB coding a sample of transcripts line-by-line to capture emergent concepts. Using these concepts, a set of initial codes was developed and applied to new transcripts, with new codes added as new concepts emerged. The next step involved focused coding, with the most salient and frequent open codes used to develop a coding scheme. A single coder applied the coding scheme to all transcripts using HyperRESEARCH 4.5.1 (Researchware, Inc.), with a second coder checking codes. Few codes were inconsistent or changed upon review by the second coder. Based on the codes that emerged during analysis, the COM-B model was selected inductively given its conceptual fit with the data, and the codes were mapped onto COM-B model components. Coded data were summarized and combined into the themes presented herein, with similarities and differences across the two cases (in treatment versus left treatment) highlighted.
We took several steps to enhance the trustworthiness of the data analysis (Lincoln and Guba, 1986). Regarding credibility, data triangulation was achieved by including participants who had sought treatment from four different clinics and the use of two interviewees. A process of member-checking was also undertaken, with preliminary results presented and feedback solicited during a meeting with staff, providers, and administrators from the MOUD program. Reflexivity was addressed through the post-interview memo-writing process. Reporting of this research followed the COREQ checklist (Tong et al., 2007).
3. Results
3.1. Participant characteristics
We attempted to contact 296 individuals by telephone; 214 (72%) could not be reached, 14 (5%) declined participation (too busy, not interested), one (<1%) was incarcerated, and 32 (11%) agreed to participate but could not be reached at the time of the scheduled interview. We conducted interviews with 35 individuals (in treatment n = 19; left treatment n = 16) (Table 1). Most participants were non-Hispanic White, reflecting the region’s demographics. Participants were split as to whether they had initiated opioid use via prescription or illicit use. Most had previous treatment attempts. Among participants who had left treatment at Geisinger, eight reported receiving MOUD through a non-Geisinger program and eight no longer received MOUD. Hereafter, we categorize study participants as G-MOUD (receiving MOUD at Geisinger), O-MOUD (receiving MOUD through a non-Geisinger program), and former patient (not currently receiving MOUD).
Table 1.
Characteristics of participants by treatment status.
| Characteristic | Geisinger treatment status at time of interview | |
|---|---|---|
| In treatment | Left treatment | |
| Total number | 19 | 16 |
| Age in years, mean (min, max) | 46 (22, 70) | 38 (28, 50) |
| Female, n (%) | 11 (58) | 8 (50) |
| Race, n (%) | ||
| Black | 3 (16) | 0 (0) |
| White | 16 (84) | 16 (100) |
| Non-Hispanic ethnicity, n (%) | 18 (95) | 16 (100) |
| Opioid initiation (self-reported), n (%) | ||
| Prescription from provider | 9 (47) | 6 (38) |
| Illicit use | 9 (47) | 7 (44) |
| Unclear | 1 (5) | 2 (13) |
| Previously attempted treatment, n (%) | 12 (63) | 15 (94) |
| Medication type at time of interview, n (%) | ||
| Suboxone | 15 (79) | 5 (31) |
| Sublocade | 4 (21) | 0 (0) |
| Subutex | 0 (0) | 1 (6) |
| Methadone | 0 (0) | 2 (13) |
| Marijuana (medical or illicit) | 0 (0) | 3 (19) |
| None | 0 (0) | 5 (31) |
O-MOUD participants reported switching programs out of convenience (based on location or schedule); to obtain methadone, which was not offered at Geisinger; due to dissatisfaction with Geisinger program rules or providers; or due to discharge from the Geisinger program. Former patient participants reported discontinuing MOUD due to a belief that they no longer needed treatment, a desire to be free from a perceived addiction to MOUD, intolerable medication side-effects, incarceration, or due to discharge from the Geisinger program.
3.2. Interview themes
Fig. 1 maps themes from the interview findings onto the COM-B model. As theorized (Michie et al., 2011), capability and opportunity factors can influence motivation to engage in the behavior of interest—engagement in MOUD. We conceptualized MOUD engagement as remaining in a MOUD program for the duration recommended by a provider (i.e., not dropping out) and attending scheduled appointments. Arrows depict relationships between factors within each domain and with MOUD engagement. For example, as described in the following sections, understanding how MOUD works (psychological capability) may increase motivation to engage, whereas experiencing stigma of MOUD (social opportunity) can decrease motivation for some individuals to engage while motivating others. In turn, motivation factors, such as attitudes toward MOUD, can lead individuals to take further actions that may improve their capability or opportunity for MOUD engagement.
Fig. 1.
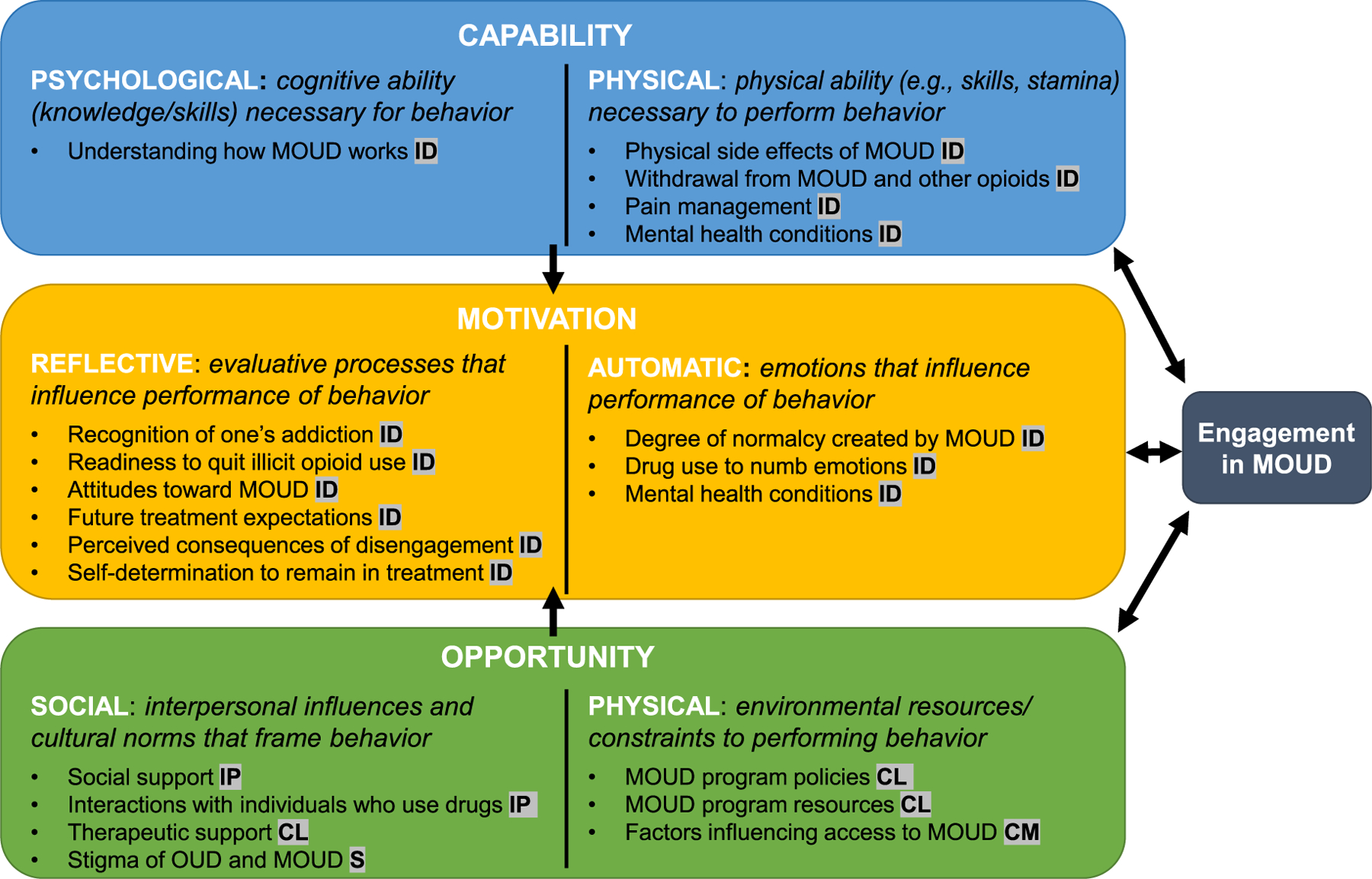
Application of COM-B model components to factors related to patient engagement in MOUD, based on themes from interview findings. The level(s) at which each factor operates is depicted in highlighted abbreviations following the description of each factor: ID, individual; IP, interpersonal; CL, clinical; CM, community; S, societal.
Fig. 1 also displays the level (individual, interpersonal, clinical, community, societal) of each factor. These levels were adapted from the social-ecological framework of the opioid crisis (Jalali et al., 2020). Due to our study’s focus on the clinical setting, we differentiate the “clinical” level from the “community” level.
3.2.1. Psychological capability
When queried, participants displayed general understanding of how buprenorphine works to block the action of other opioids, a factor that appeared to enhance their psychological capability to avoid illicit opioid use. One participant noted, “There were times I was in an environment [where] everybody is shooting up, and I know I can’t get high because I’m on [Sublocade], so … I am not even fiending for it.” Participants had received information from treatment providers regarding buprenorphine’s action, but with varying quality. For example, one participant attributed his success in Geisinger’s program to a “better set of teachers” who took time to explain how treatment works, “that it wasn’t an overnight wonder drug,” comparing this to more “regimented” explanations he had received from other programs.
3.2.2. Physical capability
Most factors related to physical capability pertained to MOUD’s physical effects. While most participants tolerated side effects, there were exceptions. Some participants had discontinued short-acting buprenorphine formulations because of the highs and lows they experienced. Participants who had discontinued methadone complained of the intolerable lethargy it caused. Uneasiness with having become physically dependent on, or “addicted to,” buprenorphine had led two former patient participants to stop treatment altogether.
Most participants described past treatment efforts that were punctuated by relapse to illicit opioid use. For several participants, previous experience with “excruciating” withdrawal symptoms after discontinuing short-acting buprenorphine—perceived to be more severe than heroin withdrawal—was a physical deterrent to restarting the medication. This was particularly problematic for participants who cycled in and out of jail.
Suboxone withdrawal … is the most horrible feeling … diarrhea, anxiety, you’re clamming up, gonna have a heart attack, you’re sweating but freezing, you get chills, your blood pressure drops. … it lasts almost a month. And I’ve gone through it about 40 times between detoxes and prisons and getting kicked off Suboxone programs … it’s a horrible cycle. I’ll never go back …
–Former patient
One former patient participant noted that Sublocade was helpful for avoiding withdrawal symptoms that occurred during incarceration.
Pain management presented an issue for several participants whose OUD had begun with pain medication prescriptions, making them wary of taking opioids for pain, despite being in severe pain. Participants had mixed views as to whether buprenorphine helped alleviate pain. Some thought it helped “somewhat,” whereas others reported that it did not help, including one participant who had switched to a methadone clinic based on her need for pain management, explaining that buprenorphine “did not touch my pain.” Several interviewees said they were willing to tolerate pain to remain in MOUD.
Mental health conditions created a physical constraint to MOUD engagement for participants who suffered from severe social anxiety or agoraphobia, which made it difficult to attend in-person MOUD appointments.
I hate going outside my house because I have such bad anxiety … I get to points where I want to push the appointment off because I can’t get myself to come out of my house.
–G-MOUD patient
Telemedicine appointments—which became an option with the onset of the COVID-19 pandemic—appeared to overcome these difficulties for some participants, although a few felt that in-person visits, despite being challenging, were an important part of their recovery.
3.2.3. Social opportunity
Almost all participants described having social support, including family, partners, or friends, who supported their treatment by ensuring they got to appointments; providing material support such as transportation, childcare, or housing; and through moral support. Several participants found people familiar with recovery most supportive, such as one participant who worked at a rehab facility:
[M]y job is huge for me. [It] brought certain people in my life that have encouraged me along the way, who I felt comfortable going to. … I’ve probably avoided relapses because I talked to them. … Not being embarrassed as much when I mess up …
–G-MOUD participant
Most participants described the supportive people in their lives as beneficial—if not crucial—to their recovery. However, a few had a more ambivalent perspective, describing personal determination as more important.
I guess [treatment] is easier, [with] family, everyone being supportive and the clinic being supportive … But I know what I want to do and whether someone is supporting me or not, it’s what my goal is.
–O-MOUD participant
Many participants spoke about the importance of reducing temptations to use drugs by avoiding individuals who continue to use illicit drugs. They described past attempts at treatment or abstinence that were unsuccessful due to a relationship with someone using illicit opioids or being in environments in which drug use was occurring. “Start[ing] fresh” with new relationships was seen as difficult, particularly for participants living in small towns where avoiding old relationships is impossible.
This isn’t the biggest town in the world … I grew up around most of these people, bought drugs off them, or have gotten high with them, and I just don’t want to be around them.
–Former patient
Participants also complained about treatment clinics in which other patients discussed drug use in the waiting room.
I’ve seen [other patients] in [the clinic] that make me not want to physically go back when they’re there, especially if I have to bring my child with me. … They’ll sit in the waiting room and literally talk about drug deals … I’m trying to stay clean, and you’re putting all these ideas in my head. … this is the one place that is supposed to be like a safe zone, and I’m not safe from those thoughts here because these people are telling war stories and then setting up a drug deal for after.
–G-MOUD participant
One Geisinger clinic reportedly overcame this problem by putting up a sign reading ‘no war stories,’ which “made it better because now nobody … talks at all.”
All participant groups spoke positively about therapeutic support they received at Geisinger addiction medicine clinics. Participants described ways in which clinic staff provided important emotional support that made them feel known, cared for, and encouraged, such as being available “just to talk,” and getting to know patients and their individual struggles.
[T]hey went a little deeper with helping me find out why I needed to stay on [the medication] and get my life in order … The staff … come around, they see you, they talk to you, and it showed a lot more concern to me … That kind of hit close to make me do and get where I’m at right now.
–G-MOUD participant
Participants also highlighted how clinic staff celebrated their successes and cared for them as people rather than as “addicts” or a source of money.
[T]hey mark your milestones. Like you might go in this appointment and [they] say ‘wow, 6 months you’ve been without an incident.’ … So, everybody knows—the doctors, the secretaries, everybody. … they actually kept track … Then you feel good all day …
–G-MOUD participant
It felt like I was going home. … They’d legitimately be happy to see me. … The other [treatment clinics] it was more, here’s my money, get going.
–O-MOUD participant
The patient-provider relationship was central to the therapeutic support experienced by participants. A few O-MOUD participants had negative interactions with providers, which led them to change treatment programs. For the most part, however, participants described positive relationships with providers, emphasizing providers’ dedication to patients’ recovery.
All the other places were like just ‘here, take [the medication], come see me in 30 days. … if you don’t comply with that, we’re going to … throw you out … ‘ Instead of having something where people talk to you and say ‘okay, this isn’t working so let’s talk about it and find out what the real underlying problem is.’
–G-MOUD participant
[W]hen I met [the doctor], he was like, ‘look, I will fight for you, for your sobriety. … This is my passion. I care about this. I care about you.’ … And that hit me.
–O-MOUD participant
Empathy was viewed as a critical trait for providers that influenced engagement. For example, one participant contrasted her experience at two different Geisinger clinics, noting her increased willingness to engage when providers were perceived as nonjudgmental:
In [one clinic], you would get written up for relapsing or not passing your urine test, and that would make me get embarrassed. … They made you feel like … you’re being punished more, which made me want to avoid it. Versus at the [other] clinic … They are just like, ‘we understand, it happens, please don’t be embarrassed by it.’ It’s easier to go in there and be honest about it and know I’m not going to get written up or kicked out of the clinic. They might make you come in more [often], but that’s okay because they are nonjudgmental.
–G-MOUD participant
Providers’ distrust of patients was seen as a hindrance to the patient-provider relationship, particularly when patients had demonstrated their trustworthiness through treatment successes. Although participants readily acknowledged that individuals with addiction may be difficult to trust, approaching them with an attitude of “care” rather than “suspicion” was important to building a cooperative relationship conducive to treatment. Building trust was acknowledged to take time and consistency in providers. Some participants expressed frustration at not seeing the same provider each appointment, requiring them to explain their drug use history repeatedly.
Most participants reported experiencing stigma related to OUD, feeling held “hostage for your past,” and for their participation in MOUD.
I don’t like that there’s a stigma behind it. ‘Oh, you’re not clean’ because you’re on Sublocade or Suboxone or Vivitrol … Like the idea that people can’t be clean and still be on some type of maintenance program. … there’s always people saying it’s a choice, you can stop whenever you want … you’re just substituting, you’re still getting high.
–G-MOUD participant
Participants also commented on the judgement they felt by health care providers outside of addiction medicine and the difficulty in being honest about their participation in MOUD. One participant noted that receiving MOUD in Geisinger’s integrated health system had made it much easier to be honest about her treatment and to manage her overall health because there was communication between specialists.
Responses to stigma varied. Many participants had kept their treatment hidden, including from friends and family, and some had avoided treatment.
[M]y issue I guess with the whole starting treatment thing is my wife … does not like the Suboxone or anything else. … that’s why I always tried to hide it and bought it off the street and never went to treatment because I knew that she would be against it.
–O-MOUD participant
Family counseling at the addiction medicine clinics had facilitated some participants’ family members’ understanding and acceptance of MOUD.
In contrast, several participants expressed ambivalence to others’ opinions, and some even found the judgement to be a “driving force” to “prove everybody wrong.”
It’s made it embarrassing [to get treatment], but it’s not hard at all, because I know what the alternative is, and that is way worse. So I’ll take a little bit of judgement for being on the shot instead of destroying my life for a bunch of people that really don’t matter.
–G-MOUD participant
Stigma of OUD was even apparent among participants. For example, some participants contrasted Geisinger’s clinics with other treatment programs that they viewed as being “junky centers” filled with patients “trying to sell you drugs in the waiting room.” Methadone clinics were disdained by some, who saw “no difference between standing out in front of the methadone clinic and standing on the corner and copping dope.” One participant associated attendance at a specialty clinic for MOUD with being “a junkie” and noted she would prefer receiving treatment from her primary care provider.
Notably, a few participants felt that attitudes were shifting as awareness of treatment—and the prevalence of addiction—has grown.
There are so many people that are on the [MOUD] program anymore, I think that stigma is kind of dying a little bit.
–O-MOUD participant
3.2.4. Physical opportunity
Physical opportunity for MOUD engagement pertained to factors both internal and external to MOUD programs. In terms of MOUD program policies, some participants believed Geisinger’s harm reduction orientation had helped them stay in treatment.
[B]y rights, any other clinic … would have kicked you out, but they were dedicated. … They had seen me coming in there struggling. … I was always making the effort, so [the provider] made sure I stayed in the program. He said, ‘One day you’re going to get it. I just want to make sure this is available for you … when that day comes.’
–Former patient
Providers’ willingness to give patients the benefit of the doubt at critical points in their treatment also appeared to facilitate engagement. For example, participants shared stories of providers taking extra steps to confirm their explanations for suspicious drug screening results rather than discharging them from the program or withholding medications. In contrast, one participant described the lack of repercussions for illicit drug use as detrimental to his treatment:
I started getting into [meth], but they were like letting it go. They were just like telling me, ‘okay, it’s not dope, it’s not opiates,’ … it was almost like they didn’t care. … It definitely did not help my treatment. … It kind of made me think like, ‘okay I can just get high, like they’re gonna still give me my Suboxone … ‘ It was like I was going to see my drug dealer.
–Former patient
Participants across groups described Geisinger’s program rules as straightforward and easy to follow and appreciated transparency in what was expected of them. Even when rules such as appointment frequency were seen as burdensome, some participants understood the need for accountability in their recovery. In contrast, one participant who had been on MOUD for many years expressed ambivalence regarding such accountability—on one hand, understanding the need for it, while on the other hand, feeling a lack of agency in his recovery.
I got to come … and I got to give you urines and all this shit. I’m fed up with that part. … Just give me my damn medication management and let me live my life please. … But then again, it helps me out because sometimes I got to be accountable to somebody more than myself. … my history says, yeah, I did need a little looking after sometimes. … my biggest trouble is the dependency on someone else.
–G-MOUD participant
Participants’ main critique of program rules centered on inflexibility in appointment frequency. Frequent appointments were viewed by some as a hardship, interfering with work schedules and other time demands, and also in terms of co-pay costs.
Every time I go to the clinic, it costs me $75 out of pocket. Once a month is fine. But when they want you to go every week … And then I pay $25 for my prescription. … If I have to go every week, then it’s $100 a week, and it’s not economically feasible.
–G-MOUD participant
Some participants expressed frustration regarding providers’ inflexibility or perceived lack of empathy for patients’ hardships and being penalized by having to attend more frequent appointments. A similar complaint related to obtaining bridge prescriptions. One participant described a series of unexpected events, including a car breakdown and a car accident, through which the provider would not provide a bridge prescription.
[E]ven when I was in unimaginable circumstances … there was no way to extend my prescription a couple days … The provider said, ‘come in or go into withdrawal and reschedule your appointment, it’s that simple.’ … There should be protocols in place for that to make it easier for the patient and not stress then out to the point where they want to quit the program.
-O-MOUD participant
Another participant attributed such inflexibility to providers’ lack of trust of patients with addiction.
I have seen other people get screwed because things happen outside of their control because [the providers] don’t trust anybody. I don’t blame them, I get it. I was a junkie … I lived that life, but at some point, you have to give an inch.
–G-MOUD participant
Participants also critiqued the clinic policy of observed urine sample collection. Most understood the need for drug testing, but some noted that other clinics did not require physical observation. Others found it difficult, or even anxiety-provoking, to urinate within a fixed amount of time. This had made one O-MOUD participant consistently late for work, contributing to her decision to switch programs.
Program resources mentioned by participants included referrals to services such as counseling, transportation, and food banks. A few had received direct assistance such as getting help with admittance into a detox program, accessing Medical Assistance, and coordinating care for other health issues. Services noted as lacking included on-site counseling, transportation to appointments, and peer recovery support.
Participants described several factors that influenced MOUD access, the most common of which related to clinic distance and lack of transportation. Numerous participants lacked a driver’s license or vehicle access, often relying on a family member for transportation. Some participants found public transportation or transportation services helpful but noted the lack of flexibility in timing often turned a quick appointment into a multi-hour venture. One participant described how challenging this was early in treatment:
I was hauling my purse, a diaper bag, a baby in a car seat and trying to coordinate [transit] to and from my appointment … that is not an easy task for anyone, especially when you are first trying to straighten out. Your brain isn’t right … your emotions are all out of whack. … you have to really want it or … you’re just going to give up.
–G-MOUD participant
Several participants reported selecting a clinic due to its nearby location, but even those who could walk or bike to the clinic found transportation difficult during the winter. Participants also noted the difficulty of balancing demands in their life with the frequency of MOUD appointments. This was particularly true for individuals with little flexibility, for example, those working two jobs, in the service industry, who could not be honest with employers about MOUD appointment attendance, or who required childcare.
Most participants found Geisinger clinics’ schedules sufficiently accommodating, with efficient appointments. Several particularly appreciated evening appointments that had made it easier to balance work. One former patient described how a Geisinger clinic made time to reinitiate treatment when he showed up “5 minutes” after release from jail. In contrast, some O-MOUD participants described the lack of scheduling flexibility as one reason they sought treatment elsewhere. Participants who had attended telemedicine appointments appreciated the convenience and described how telemedicine helped overcome barriers to attending appointments such as distance, lack of transportation, and competing demands.
All interviewees were on Medical Assistance or had commercial insurance that covered most treatment costs; however, for those with large co-pays, frequent appointments were difficult to afford. Participants acknowledged that their insurance status determined their future MOUD access and some reported that lapses in insurance had forced them to leave treatment in the past.
3.2.5. Reflective motivation
Regarding reflective motivation, participants described recognition of one’s addiction as a necessary precursor to treatment initiation and engagement—“admit[ting] to yourself that you actually have a problem and need help.” This was difficult when participants did not view themselves as a stereotypical “addict.” Participants recalled mental models they invoked to distinguish themselves from “drug addicts,” such as their ability to maintain a functioning life, only using prescribed pain medications, or not using or injecting heroin. Not fitting the stereotype of a “drug addict” also influenced where participants were willing to seek MOUD.
I never did a needle. I don’t consider myself better than the other drug addicts, but I had a good job. I have a good family. I couldn’t bring myself to go to [name of clinic]. They were like people from the alleys.
–G-MOUD participant
Participants dependent on prescribed opioids often recognized their OUD once their prescription was cut off and they began experiencing withdrawal symptoms or seeking illicit opioids. Others described recognition arising when using opioids was no longer fun or began to take over their lives or paychecks, or after being incarcerated.
Participants predominantly attributed their current success in treatment to intrinsic motivation, particularly their ‘readiness’ to quit illicit opioid use. They described past treatment efforts as failed due to a lack of such readiness, driven instead by a desire to “please other people” or to obtain buprenorphine to sell or use to get high.
I wasn’t ready last time. I said ‘I’m gonna do this for everybody else. I’m gonna do this for my mom. I’m gonna do this to make this person happy, to make that person happy, that way I could see my kid.’ It just sort of looks good, you know ‘I’m on Suboxone.’
–G-MOUD participant
Treatment success came when they were ready to put their “heart into it”; otherwise, the logistical, emotional, and cognitive challenges were too great. Several participants explained that once they were ready to quit, initiating and maintaining MOUD was easy. The importance of self-determination to “get sober” was also cited by many former patient participants.
When queried about what had made them ready to quit, participants described coming to a point in their addiction that they felt was untenable—“hitting rock bottom.”
You have to … live your worst nightmare to let go of heroin … there’s nothing that nobody can say to you for you to stop … your children aren’t enough, your family’s not enough, you aren’t enough. … Death isn’t enough. … the homeless part did it for me. I was eating out of garbage cans … nobody let me in their house this time. … nobody helped me. I lost my kid, I lost everything. And that’s what it took for me to realize that I didn’t want to live like that anymore …
–G-MOUD patient
What constituted “rock bottom” differed by person, though many described a sense of exhaustion—from the chaos of their lives, constant drug-seeking to avoid withdrawal, and of never feeling good. There were exceptions—a few participants recognized their dependence on prescribed opioids and sought help before their lives unraveled.
Participants primarily expressed positive attitudes toward MOUD. Treatment was not only viewed as allowing participants to regain a “normal” life but also as giving them energy and motivation and providing the “mental space” needed for recovery.
[Suboxone] is a helping aid … it leaves you with a clear mind and ability to think for yourself … It lets you take what you need to not crave the usage of the opioids and then at the same time, lets you work through some of the problems … that you have, and get a better grip on who you are and what you need to do to maintain this type of lifestyle.
–G-MOUD participant
Several participants attributed MOUD as saving their lives.
I liked the fact that I finally had something that didn’t make me want to do heroin again. It was much safer, and I didn’t have to go into the projects, risk getting murdered or robbed or thrown in jail, overdosing, none of that.
–Former patient
However, a few participants—primarily former patients—felt that MOUD was simply trading one addiction for another or had stopped MOUD because they believed they had become addicted to buprenorphine.
In my opinion, it’s the next high, because I got addicted to [Suboxone]. … enough’s enough, and I went to rehab. … I just wanted to get off of them and be clean and not need a pill to stay clean.
–Former patient
When questioned about their future treatment expectations, some participants expressed a desire to be free of medication, as one participant described, to not wake up “relying on having to take something just to be able to make it through the day.” For at least one participant, this had been a barrier to re-starting treatment. However, most participants recognized that they were not yet at a point where they could safely stop treatment without relapsing. Many had no plans to stop, commenting that they would stay in treatment for as long as they needed it.
I don’t want to be on [Sublocade] forever, but if I have to be to maintain my life, I will be. … I’m not emotionally in a position where I think I would remain sober if I were to go off the shot … I rely on the shot to keep me straight, so I can be a good mom to my kid, so I can be a good employee at my job, and until I can do that without the shot I don’t want to go off of it.
–G-MOUD participant
Across all groups, participants’ motivation to remain on MOUD centered on avoiding a return to a “life of drugs,” and the perceived consequences of disengagement, with participants recognizing that MOUD was necessary for them to avoid relapse. Having experienced the destructiveness of addiction—overdose, loss of relationships, financial devastation—most participants were highly aware of all they had to lose.
[I]t has not [been] hard for me to stay in treatment because I know what the alternative is and that’s just not an option for me. … if I leave treatment, I won’t stay clean. If I don’t stay clean, I lose my life, my kid, my job, my everything …
–G-MOUD participant
Many participants described a desire to be healthy to take care of or spend time with children, grandchildren, and other family members. Overall, however, participants tended to cite their own self-determination as the main facilitator to remaining in treatment.
3.2.6. Automatic motivation
The degree to which MOUD created a sense of normalcy emerged as a prominent factor related to automatic motivation for participants across groups, influencing their satisfaction with, and preferences for, treatment. Participants recognized the benefit buprenorphine provided in alleviating opioid craving, which contributed to them feeling “normal,” an immense improvement to the “hell” of addiction. This was most evident among participants who had experience using the monthly injectable, Sublocade, particularly in comparison with other MOUD types. For example, Sublocade eliminated the daily cycle of highs and lows some participants had experienced with short-acting MOUD formulations. Several participants preferred Sublocade because taking a daily medication—particularly in pill form—felt overly reminiscent of their addiction.
[Sublocade] takes away some of the addict behavior … When I would be doing Suboxone strips, for me, it’s addict behavior because I still woke up to knowing that I had something to put in my body.
–G-MOUD participant
Additionally, the convenience of a once-monthly injection was noted to be a benefit, eliminating frequent medical visits. In contrast, among participants who had used methadone, a sense of normalcy appeared constrained by the lethargy or high experienced by some, its potential for misuse, and the hassle of going to the methadone clinic each day. That said, one participant preferred methadone as it allowed her to get “back on track quickly” when she relapsed.
Exceptions to this preference for normalcy appeared to reflect a few participants’ tenuous state of recovery. For example, one participant welcomed the discomfort of sublingual buprenorphine wearing off at the end of the day because it reminded her of the pain of addiction, keeping her “grounded.” One participant preferred taking methadone because of the accountability required to obtain her dose in person every day, stating “you cannot give [addicts] all that responsibility and think that we are going to not mess it up.”
Some participants found it difficult to deal with emotions that re-emerged during treatment that had previously been “camouflaged” by illicit drug use. Participants described using illicit drugs to numb emotions—for example, to “numb the pain” of a loss of a loved one, to fill a “void” in their life, or to escape the “nightmare” of an abusive relationship. As one former patient participant explained, “it was like putting a Band-Aid over all the drama and all the BS of dealing with the real world.” The reemergence of these emotions sometimes led to relapse.
When you spend 10 years of your life turning off your emotions, it’s not easy to all of a sudden have them, and so life is not easy. When you add all of those things together it’s just like overwhelming, and that’s when people relapse.
–G-MOUD participant
Some participants were dismayed to discover that stopping drug use did not “ease” their mental health conditions.
[E]verybody made it seem like the drug itself was the issue and then once I got off the drugs, the depression, anxiety, bipolar all of that would go away … The depression and all that was there, which is what led me to start using drugs. It wasn’t vice versa. … I thought … the lack of narcotics would ease the situation and it really didn’t.
–Former patient
Almost every participant reported having at least one, if not many, mental health conditions such as depression or anxiety. For some, these conditions made it difficult to avoid relapse.
I have a lot of trauma, PTSD, anxiety, depression, bipolar, so I have a lot of other things that factor into me having difficulty trying to stay sober, and saying screw the clinic, and go get high. I have an emotional weakness that I’m fighting with every day, just to maintain going to the clinic and doing this the right way.
–O-MOUD participant
Participants acknowledged that addressing “the root of the problem” was necessary for recovery. One participant described MOUD as providing the clarity needed to work through the underlying issues that had led to drug use.
I think [the] program gave me enough leeway to where I have been able to get a hold of things with myself that I have never had before, and some things that I have known and just pushed off to the side forever and ever and refused to deal with. But now I have dealt with them and I don’t see myself falling back.
–G-MOUD participant
4. Discussion
With analysis facilitated by the COM-B model, this qualitative exploration identified multilevel factors that facilitate and constrain MOUD retention from the perspective of a geographically diverse patient population. Multilevel conceptualizations of MOUD engagement broaden understanding of the complex factors and circumstances that distinguish individuals’ treatment experiences (Kahn et al., 2022) and are essential for informing efforts to increase retention. Elicitation of perspectives from current and former patients in Geisinger’s MOUD program revealed important insights regarding factors influencing engagement and allowed for data triangulation, with consistency across groups enhancing the validity of our findings. Specifically, examination of participants’ reasons for leaving the program, in combination with reflections from participants across groups regarding their treatment experience, sheds light on key determinants of MOUD retention, with implications for implementation strategies to enhance engagement. Importantly, the finding that half of participants who had left Geisinger’s treatment program reported seeking MOUD elsewhere (and that several current patients had switched to Geisinger from another program), demonstrates the need for research to inform optimal treatment, not only in terms of MOUD formulations or types, but also in terms of treatment environment and policies.
4.1. Individual-level factors
Encompassing individuals’ cognitive and physical abilities and internal motivation, factors identified as relating to capability and motivation comprised a range of individual-level barriers and facilitators. We identified few factors related to psychological capability, aside from participants’ understanding of how MOUD works to block the action of other opioids and relieve craving. However, physical effects of medications—which influenced some participants’ decision to leave Gei-singer’s MOUD program—may warrant retention strategies to increase patients’ psychological capability. For example, uneasiness with feeling “addicted” to MOUD and intolerance to side effects could be alleviated through education about the effectiveness of maintenance therapy and the variety of MOUD options. Other aspects of physical capability, such as need for pain management or physical constraints caused by mental health conditions, are best addressed with improved access to comprehensive healthcare services.
Reflective motivation emerged as a predominant domain influencing MOUD engagement. Consistent with prior studies (Hewell et al., 2017; Kahn et al., 2022; Notley et al., 2015), participants often ascribed their treatment success to internal motivation—particularly their ‘readiness’ to stop opioid use and self-determination to change—whereas initiating treatment due to external pressure led to failed efforts. MOUD itself can be a tool for developing internal motivation to change (Hewell et al., 2017), as reflected by current participants’ primarily positive attitudes toward MOUD. In contrast, negative attitudes toward MOUD, particularly the idea that MOUD is “trading one drug for another,” was most prevalent among former patient participants, reinforcing evidence that attitudes may be associated with reduced retention (O’Connor et al., 2020), and indicating an opportunity for intervention.
Overall, participants across groups expressed appreciation for MOUD, as it allowed them to escape the chaos of constant opioid-seeking and feel “normal,” facilitating recovery. This finding aligns with past research, which has identified a sense of freedom from opioid dependence and improved quality of life as contributing to patients’ positive perceptions of MOUD (Granerud and Toft, 2015; Notley et al., 2015; Scorsone et al., 2020). Notably, our findings suggest that in comparison with short-acting MOUD, the monthly injectable form of buprenorphine excels at bringing about this sense of normalcy. A qualitative study of MOUD preferences similarly found that long-acting MOUD formulations were perceived as helping individuals achieve ‘normalcy’ by eliminating the need for daily medication dosing, though many individuals with OUD remained unwilling to use injectable formulations due to a desire to maintain agency over their treatment or a fear of injections (Saunders et al., 2020). Given the potential advantages to patients, additional research is warranted to inform potential strategies to increase the acceptability and use of long-acting MOUD. While randomized control trials have not shown differences in retention in comparing short-versus long-acting MOUD (Chan et al., 2021a), the availability of and access to a variety of MOUD types and formulations may prove essential to MOUD engagement.
A cross-cutting theme at the individual-level related to co-occurring mental health conditions. Studies assessing mental health diagnoses or symptoms and MOUD retention have shown mixed results (O’Connor et al., 2020). Our findings indicate that such conditions impeded behavioral regulation to avoid illicit opioid use and contributed to illicit drug use to numb emotions (automatic motivation), and—particularly for patients with agoraphobia or social anxiety—presented a physical barrier to attending in-person patients (physical capability). With almost every participant reporting depression or anxiety, aligning with the prevalence of these conditions within the OUD population (Jones and McCance-Katz, 2019), the need for mental healthcare for patients with OUD cannot be understated.
4.2. Interpersonal-level factors
At the interpersonal-level, social support appeared to facilitate treatment engagement, with most participants reporting having family, partners, or friends who supported their treatment through emotional or material support. In contrast, interactions with individuals who continued to use illicit drugs challenged treatment engagement. Engaging social networks in treatment and supporting separation of patients from substance using peers both show promise for improving treatment outcomes (Cooper and Nielsen, 2017). As noted by study participants, such strategies may be more challenging for rural populations, where small towns provide little opportunity to avoid old relationships and build new ones (Notley et al., 2015; Scorsone et al., 2020).
4.3. Clinical-level factors
Clinical-level factors pertained to both social and physical opportunity. Therapeutic support emerged as a salient aspect of social opportunity. Negative views of such support influenced some O-MOUD participants to switch treatment programs. Most study participants, however, viewed the support they received in Geisinger’s MOUD program as central to their recovery. The importance of therapeutic support for MOUD engagement is well supported by prior research, which has shown that nonjudgmental, personal, and dedicated care motivates patients to remain in treatment (Beharie et al., 2022; Filteau et al., 2022; Scorsone et al., 2020).
MOUD program policies related to physical opportunity, with a central tension emerging around the MOUD program’s harm reduction orientation. Geisinger’s addiction medicine program differentiates patients’ acuity levels into harm reduction or functional improvement categories based on patients’ recovery goals, an approach that has been attributed to improving patient engagement (Barbour et al., 2020). In line with this effort, study participants tended to describe a compassionate harm reduction approach as facilitating their engagement in MOUD, similar to prior research (Kahn et al., 2022; Lai et al., 2021). Participants’ perspectives diverged regarding flexibility in appointment frequency. Some agreed with a need for accountability. Others felt a lack of agency regarding their recovery, a theme identified in past research (Granerud and Toft, 2015), or associated inflexibility in appointment frequency and provision of bridge prescriptions with a lack of empathy for patients’ hardships. The harm reduction orientation includes low-barrier approaches to MOUD, such as reducing visit burden through telemedicine and requiring drug screening only when results will change care management (Taylor et al., 2021). With increasing calls for low-threshold buprenorphine, research is needed on best practices for low-threshold MOUD to maximize population health without compromising treatment effectiveness.
4.4. Community-level factors
At the community-level, aspects of physical opportunity appeared to play a key role in participants’ decisions regarding where to seek treatment and whether to continue. Constraints to MOUD access such as transportation, distance, and competing demands like childcare have been previously cited as barriers to initiation and engagement (Hall et al., 2021; Mackey et al., 2020), with travel burden consistently identified as limiting MOUD access among rural populations (Lister et al., 2020). In our study, distance to clinics and lack of transportation was the most salient constraint to access, likely because the program serves many rural patients. Yet even urban participants described the coordination and scheduling of public transportation as burdensome. Travel barriers could be reduced with greater access to telemedicine or mobile treatment and expansion of MOUD services to primary care (Chan et al., 2021b; Lister et al., 2020). Flexible appointment scheduling, particularly evening and weekend hours, could alleviate constraints related to competing demands. Participants also identified incarceration as an access barrier, a factor consistently associated with reduced MOUD retention (O’Connor et al., 2020), highlighting the need for universal MOUD access in correctional facilities (Wakeman and Rich, 2015).
4.5. Societal-level factors
Shaped by the larger social context, harmful misconceptions regarding OUD and MOUD lead to stigmatizing interpersonal interactions in a variety of settings. To enhance social opportunity for MOUD engagement, stigma must be addressed. As among our participants, experiences of stigma appear ubiquitous among individuals with OUD, leading to marginalization and loneliness and hindering MOUD initiation and engagement (Hewell et al., 2017; Notley et al., 2015; Richard et al., 2020; Scorsone et al., 2020). Stigma towards MOUD is in part due to an abstinence-oriented belief that MOUD is equivalent to “trading one addiction for another,” particularly in regions with a conservative culture such as Appalachia (Richard et al., 2020). Participants’ reports of increased acceptance of MOUD (their own and family members’) when providers dedicated time to explaining how treatment works underscores the important role providers can play in shifting attitudes. In contrast, participants described stigma from other health care providers for engaging in MOUD, making it difficult to be honest about their treatment. Prior research on perceived stigma in healthcare settings has found that individuals may conceal MOUD participation or abstain from seeking healthcare to avoid stigma, with potential negative effects on the quality of their healthcare (Garpenhag and Dahlman, 2021). Our findings also illustrate how OUD-related stigma pervades the OUD population itself, with the persistence of stereotypes of “drug addicts” constraining study participants’ recognition of their OUD and their willingness to attend treatment programs they view as “below” themselves. To reduce stigma and promote treatment, efforts are needed at multiple levels that humanize people who use drugs and counter stereotypes, including individual-level contact-based interventions, provider education and training, and population-level stigma reduction campaigns (Cheetham et al., 2022).
4.6. Study limitations
To understand barriers to MOUD engagement, it is critical to seek out the perspectives of individuals who have left treatment. Although our study elicited perspectives from current and former MOUD patients, we may not have reached former patients experiencing severe OUD and who likely faced the greatest barriers to maintaining MOUD. That said, study participants reflected upon the worst periods of their opioid use disorder and offered perspectives regarding their capacity, motivation, and opportunity to engage in MOUD at the time. Additionally, although the study population was geographically diverse, most participants were non-Hispanic White, reflecting the demographics of the region but limiting the transferability of our findings to other populations. Finally, interviews were conducted by phone, which may have limited rapport-building with participants who prefer in-person contact. Additionally, in some instances it was clear that participants were in the presence of others, which may have influenced their responses. However, this approach facilitated recruitment by increasing the convenience of participation and allowing the study to proceed during the emergence of the COVID-19 pandemic.
5. Conclusion
Poor MOUD retention presents a challenge to effectively addressing the opioid epidemic, with few individuals achieving long-term remission (O’Connor et al., 2020; Williams et al., 2017). We identified multilevel factors influencing MOUD engagement from the patient perspective. Our findings are consistent with prior qualitative literature but present a comprehensive view of the multiple levels at which barriers and facilitators to MOUD engagement exist. Such an approach views treatment discontinuation not as an abnormality of the patient, but rather as the result of a complex set of factors and circumstances that constrain individuals’ capability, opportunity, and motivation to continue treatment. Framing our analysis using the COM-B model of behavior facilitated identification of explanatory factors underpinning MOUD engagement that can inform implementation strategies to improve MOUD retention. To address the burgeoning overdose crisis and achieve maximum individual- and population-level impact, greater understanding is needed as to how to optimize all aspects of treatment to minimize multilevel barriers to MOUD engagement.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr. Margaret Jarvis for her support of this study and for reviewing a draft of the manuscript.
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number K01DA049903. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- COM-B
Capability, Opportunity, and Motivation model of behavior
- MOUD
medications for opioid use disorder
- OUD
opioid use disorder
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Melissa N. Poulsen: Conceptualization, Formal analysis, Funding acquisition, Investigation, Writing – original draft. Patrick B. Asdell: Investigation, Writing – review & editing. Wade Berrettini: Conceptualization, Supervision, Writing – review & editing. Kortney McBryan: Project administration, Writing – review & editing. Alanna K. Rahm: Conceptualization, Writing – review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmmh.2022.100151.
References
- Barbour JS, Jarvis MA, & Withers DJ (2020). How Geisinger dramatically reduced deaths from opioid use disorder. NEJM Catal., 1. [Google Scholar]
- Beatty Kate, Hale Nathan, Meit Michael, Heffernan Megan, Dougherty Michelle, Rocha Luciana, et al. (2019). Issue brief: Health disparities related to opioid misuse in Appalachia - practical strategies and recommendations for communities. https://www.arc.gov/report/health-disparities-opioid-misuse/. (Accessed 26 August 2022).
- Beharie N, Kaplan-Dobbs M, Urmanche A, Paone D, & Harocopos A (2022). I didn’t feel like a number”: the impact of nurse care managers on the provision of buprenorphine treatment in primary care settings. J. Subst. Abuse Treat, 132, Article 108633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Barth KS, Back SE, & Book SW (2015). Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J. Subst. Abuse Treat, 52, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson HH, Ajo Arnevik E, Rand-Hendriksen K, & Duckert F (2013). Drop-out from addiction treatment: a systematic review of risk factors. Clin. Psychol. Rev, 33, 1010–1024. [DOI] [PubMed] [Google Scholar]
- CDC. (2022). U.S. Overdose Deaths in 2021 Increased Half as Much as in 2020 - but Are Still up 15%. Centers for Disease Control and Prevention National Center for Health Statistics. [Google Scholar]
- Chan B, Gean E, Arkhipova-Jenkins I, Gilbert J, Hilgart J, Fiordalisi C, et al. (2021a). Retention strategies for medications for opioid use disorder in adults: a rapid evidence review. J. Addiction Med, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B, Hoffman KA, Bougatsos C, Grusing S, Chou R, & McCarty D (2021b). Mobile methadone medication units: a brief history, scoping review and research opportunity. J. Subst. Abuse Treat, 129, Article 108483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Picco L, Barnett A, Lubman D, & Nielsen S (2022). The impact of stigma on people with opioid use disorder, opioid treatment, and policy. Subst. Abuse Rehabil, 13, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, & Nielsen S (2017). Stigma and social support in pharmaceutical opioid treatment populations: a scoping review. Int. J. Ment. Health Addiction, 15, 452–469. [Google Scholar]
- Crowe S, Cresswell K, Robertson A, Huby G, Avery A, & Sheikh A (2011). The case study approach. BMC Med. Res. Methodol, 11, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filteau MR, Kim FL, & Green B (2022). “It’s more than just a job to them”: a qualitative examination of patient and provider perspectives on medication-assisted treatment for opioid use disorder. Community Ment. Health J, 58(2), 321–327. 10.1007/s10597-021-00824-7 [DOI] [PubMed] [Google Scholar]
- Garpenhag L, & Dahlman D (2021). Perceived healthcare stigma among patients in opioid substitution treatment: a qualitative study. Subst. Abuse Treat. Prev. Pol, 16, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granerud A, & Toft H (2015). Opioid dependency rehabilitation with the opioid maintenance treatment programme - a qualitative study from the clients’ perspective. Subst. Abuse Treat. Prev. Pol, 10, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NY, Le L, Majmudar I, & Mihalopoulos C (2021). Barriers to accessing opioid substitution treatment for opioid use disorder: a systematic review from the client perspective. Drug Alcohol Depend., 221, Article 108651. [DOI] [PubMed] [Google Scholar]
- Heffernan M, Meit M, Cherney M, & Hallman V (2021). Tracking the impact of diseases of despair in Appalachia—2015 to 2018. J. Appalachian Health, 3, 56–67. [Google Scholar]
- Hewell VM, Vasquez AR, & Rivkin ID (2017). Systemic and individual factors in the buprenorphine treatment-seeking process: a qualitative study. Subst. Abuse Treat. Prev. Pol, 12, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali MS, Botticelli M, Hwang RC, Koh HK, & McHugh RK (2020). The opioid crisis: a contextual, social-ecological framework. Health Res. Pol. Syst, 18, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, & McCance-Katz EF (2019). Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend, 197, 78–82. [DOI] [PubMed] [Google Scholar]
- Kahn LS, Wozniak ML, Doscher T, Moore C, & Vest BM (2022). Treatment experiences among people who use opioids: a social ecological approach. Qual. Health Res, 32, 1386–1398. [DOI] [PubMed] [Google Scholar]
- Lai S, Li E, Silverio A, DeBates R, Kelly EL, & Weinstein LC (2021). “It’s a place that gives me hope”: a qualitative evaluation of a buprenorphine-naloxone group visit program in an urban federally qualified health center. Subst. Abuse, 42, 858–864. [DOI] [PubMed] [Google Scholar]
- Lincoln YS, & Guba EG (1986). But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. N. Dir. Progr. Eval, 1986, 73–84. [Google Scholar]
- Lister JJ, Weaver A, Ellis JD, Himle JA, & Ledgerwood DM (2020). A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am. J. Drug Alcohol Abuse, 46, 273–288. [DOI] [PubMed] [Google Scholar]
- Lo-Ciganic WH, Gellad WF, Gordon AJ, Cochran G, Zemaitis MA, Cathers T, et al. (2016). Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction, 111, 892–902. [DOI] [PubMed] [Google Scholar]
- Ma J, Bao Y-P, Wang R-J, Su M-F, Liu M-X, Li J-Q, et al. (2019). Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol. Psychiatr, 24, 1868–1883. [DOI] [PubMed] [Google Scholar]
- Mackey K, Veazie S, Anderson J, Bourne D, & Peterson K (2020). Barriers and facilitators to the use of medications for opioid use disorder: a rapid review. J. Gen. Intern. Med, 35, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, van Stralen MM, & West R (2011). The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement. Sci, 6, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notley C, Blyth A, Maskrey V, Pinto H, & Holland R (2015). Exploring the concepts of abstinence and recovery through the experiences of long-term opiate substitution clients. Subst. Abuse, 36, 232–239. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Cousins G, Durand L, Barry J, & Boland F (2020). Retention of patients in opioid substitution treatment: a systematic review. PLoS One, 15, Article e0232086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennsylvania Department of Human Services. (2022). Centers of Excellence. Commonwealth of Pennsylvania. [Google Scholar]
- Richard EL, Schalkoff CA, Piscalko HM, Brook DL, Sibley AL, Lancaster KE, et al. (2020). “You are not clean until you’re not on anything”: perceptions of medication-assisted treatment in rural Appalachia. Int. J. Drug Pol, 85, Article 102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell Bernard H (2006). Interviewing: unstructured and semistructured. In Bernard H. Russell (Ed.), Research Methods in Anthropology: Qualitative and Quantitative Approaches. United States of America: AltaMira Press. [Google Scholar]
- SAMHSA. (2021a). Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- SAMHSA. (2021b). Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63 Publication No. PEP21–02-01–002. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Saunders EC, Moore SK, Walsh O, Metcalf SA, Budney AJ, Scherer E, et al. (2020). Perceptions and preferences for long-acting injectable and implantable medications in comparison to short-acting medications for opioid use disorders. J. Subst. Abuse Treat, 111, 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorsone KL, Haozous EA, Hayes L, & Cox KJ (2020). Overcoming barriers: individual experiences obtaining medication-assisted treatment for opioid use disorder. Qual. Health Res, 30, 2103–2117. [DOI] [PubMed] [Google Scholar]
- Socias ME, Volkow N, & Wood E (2016). Adopting the ‘cascade of care’ framework: an opportunity to close the implementation gap in addiction care? Addiction, 111, 2079–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. (2017). Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ, 357, j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Johnson S, Cruz R, Gray JR, Schiff D, & Bagley SM (2021). Integrating harm reduction into outpatient opioid use disorder treatment settings. J. Gen. Intern. Med, 36, 3810–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR (2006). A general inductive approach for analyzing qualitative evaluation data. Am. J. Eval, 27, 237–246. [Google Scholar]
- Tong A, Sainsbury P, & Craig J (2007). Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care, 19, 349–357. [DOI] [PubMed] [Google Scholar]
- Wakeman SE, & Rich JD (2015). Addiction treatment within U.S. Correctional facilities: bridging the gap between current practice and evidence-based care. J. Addict. Dis, 34, 220–225. [DOI] [PubMed] [Google Scholar]
- Williams AR, Nunes E, & Olfson M (2017). To battle the opioid overdose epidemic, deploy the ‘Cascade of Care’ model. Health Affairs Blog. 10.1377/forefront.20170313.059163 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


