Abstract
Purpose:
To address the gap in the literature and clarify the expanding role of wearable sensor data in stroke rehabilitation, we summarized the methods for upper extremity (UE) sensor-based assessment and sensor-based treatment.
Materials and methods:
The guideline outlined by the preferred reporting items for systematic reviews and meta-analysis extension for scoping reviews was used to complete this scoping review. Information pertaining to participant demographics, sensory information, data collection, data processing, data analysis, and study results were extracted from the studies for analysis and synthesis.
Results:
We included 43 articles in the final review. We organized the results into assessment and treatment categories. The included articles used wearable sensors to identify UE functional motion, categorize motor impairment/activity limitation, and quantify real-world use. Wearable sensors were also used to augment UE training by triggering sensory cues or providing instructional feedback about the affected UE.
Conclusions:
Sensors have the potential to greatly expand assessment and treatment beyond traditional clinic-based approaches. This capability could support the quantification of rehabilitation dose, the nuanced assessment of impairment and activity limitation, the characterization of daily UE use patterns in real-world settings, and augment UE training adherence for home-based rehabilitation.
Keywords: Wearable sensors, accelerometers, IMU, upper extremity, stroke, sensor-based assessment, sensor-assisted treatment
Introduction
Wearable motion sensors are small, light, non-invasive electronic devices [1]. These sensors are worn on specific body segments, such as the wrist or chest, to capture motion data. Accelerometers and inertial measurement units (IMUs) are the two main sensors used to measure upper extremity (UE) motion [2]. Accelerometers capture linear acceleration data in 1–3 planes of motion [3]. IMUs capture three-dimensional (3D) linear accelerations from accelerometers, and angular velocities from gyroscopes [1]. The combination of linear and rotational data enables a more complete picture of UE motion, as it has many degrees of freedom that are insufficiently characterized by three dimensions of linear acceleration. Thus, IMU sensors are data-rich and are used when high classification performance is needed from the data [4]. Conversely, accelerometers are data-sparse and used when magnetic interference is a concern, i.e., in remote or uncontrolled environments [4]. Wearable sensor technology has widespread applications in sports, animation, and clinical settings [1,5]. Applications in clinical populations include quantifying tremor severity in individuals with Parkinson’s disease, monitoring safety and fall risk in the elderly, and monitoring gait and habitual activity in older adults [3,5].
Researchers have also increasingly incorporated UE sensors into stroke rehabilitation research. Stroke is the leading cause of serious long-term disability in the USA [6]. Functional use of the affected UE after stroke is key for increased independence in basic and instrumental activities of daily living (ADLs), returning to work, and overall quality of life [7]. For individuals six months after a first-time ischemic stroke with severe hemiplegia, only 38% of patients demonstrate some hand dexterity (e.g., gross grasp, finger extension), and only 11.6% experience full recovery [8]. The implementation of effective UE rehabilitation approaches to reduce disability is therefore of critical relevance.
To address this need, clinical researchers have begun to using wearable sensors to capture UE motion in and outside of the clinic. The use of wearable sensors has the potential to expand existing methods for UE assessment and treatment after stroke. In principle, improved knowledge about impairment or activity limitation could enable therapists to better target their training, potentially improving functional return. The first publications in PubMed using accelerometers or IMUs in stroke appeared in 1983, with an exponential increase in publications in the last decade [2]. This sharp increase in the use of wearable sensors signals the need to understand how these modalities can be used for assessing and treating the affected UE [9].
Earlier studies largely focused on establishing sensor data as valid and reliable instruments for measuring UE motion. UE accelerometer metrics (activity counts) demonstrate excellent test-retest reliability and construct validity for assessing UE motion in acute and chronic stroke [10]. Accelerometer and IMU metrics demonstrate convergent validity [10–15] and divergent validity [14,16]. Accelerometer metrics demonstrate discriminant ability for UE use in healthy individuals versus stroke survivors, upper versus lower extremity use in stroke, and severity of UE impairment in acute stroke [10,14]. These clinimetric studies underscore the reliability and validity of using sensors to capture UE motion after stroke.
Three recent reviews examined the clinical application of sensors in rehabilitation. The first broadly overviewed clinical applications of wearable sensors in neurological (stroke, spinal cord injury, cerebral palsy), musculoskeletal (arthritis, frozen shoulder trauma), pain management, and general rehabilitation populations [2]. This review found that accelerometers and IMUs were commonly used to provide extrinsic feedback (knowledge of results, knowledge of performance) for posture and UE movements in stroke [2], but did not examine the methods used for data capture and analyses. The second presented a broad overview of the past, present, and future directions for the clinical use of IMU, EMG, and e-textile based technologies in UE stroke rehabilitation [17]. This review identified limited clinical utility, bulky equipment, and difficulties with device set up with these technologies [17], but did not review the methods used for data capture and analyses. The third focused on the feasibility of using accelerometers for UE assessment in real-world home and community settings [18]. This didactic review provided detailed background information on the methods for data capture and analysis for sensor-based assessment in home and community settings [18], but did not present a comprehensive review for other types of sensor-based assessments or sensor-based treatment approaches.
Since these reviews, there have been continued advancements in sensor technology and data analysis, which have expanded UE assessment and treatment methods. In addition to the limitations of the reviews noted above, there currently exists no detailed review of emerging sensor-based assessment and treatment approaches in UE stroke rehabilitation. We thus completed a scoping review to systematically map the research in this area. The aims of this study were to summarize UE sensor-based assessment and UE sensor-based treatment approaches in individuals with stroke.
Materials and methods
We followed the guidelines outlined by the preferred reporting items for systematic reviews and meta-analysis extension for scoping reviews to conduct this scoping review [19]. We used the population, concept, context (PCC) structure to identify the key elements to conceptualize the scoping review: stroke (population), UE wearable sensors (concept), and rehabilitation (context) [19,20].
Databases and systematic search
We completed a systematically search of PubMed, Embase, Web of Science, and CINAHL databases on 17 December 2020. We used the following key words and their iterations: accelerometry OR IMU OR stroke OR upper extremity. See Supplement Appendix A for detailed search strategies used for each database. We also reviewed the references of included articles to search for additional relevant articles.
Selection criteria
We used the following criteria to determine eligibility.
Inclusion criteria:
adults with stroke;
use of wearable sensors;
measurement of UE motion;
sensor data used to develop methods for UE assessment;
sensor data used to augment UE training;
studies published in peer-reviewed journals from 2009 to 2020.
Exclusion criteria:
didactic papers, posters, book chapters, reviews;
validation or reliability studies of sensor-based metrics (clinimetric properties – validity and reliability);
studies examining the relationship between sensor data and clinical measures (clinimetric properties – concurrent validity);
sensor data captured by robotic devices/gaming consoles;
sensor data used to measure treatment outcomes (e.g., the effectiveness of robotics training or task specific practice).
Studies with stroke and healthy participants were included. Studies identified by the initial search were imported into Covidence, a review management software (https://www.covidence.org). Duplicates were removed and remaining abstracts were screened by ST and GK for eligibility. Abstracts without consensus were carried over into full text review. The remaining abstracts were advanced to full text review and examined independently by ST, GK, and AP. Any remaining uncertainty about eligibility was resolved by discussion between GK, AP, and HS.
Data extraction and analysis
The following data were extracted from each article: study aims, participant demographics, sensor characteristics, data collection methods, data processing methods, data analysis approaches, and study results. The extracted data were examined and studies were organized into assessment or treatment categories based on study aim. Studies in the assessment category were further analyzed and refined into three subcategories based on clinical application. Discrepancies with categorization were discussed with all co-authors until agreement was reached.
Results
We found 704 abstracts after searching the databases. The initial screen of titles and abstracts eliminated 575 abstracts, and 129 full text articles were reviewed. We excluded an additional 86 articles because they did not meet inclusion criteria, leaving 43 articles for full review. See Figure 1 for the PRISMA flowchart. We organized the results into assessment and treatment categories. In the assessment category, wearable sensor data were used to appraise three aspects of UE motion: functional motion, motor impairment/activity limitation, and real-world use. Functional motion is defined as any motion that is goal-directed and volitional in the context of ADLs [21]. Motor impairment is partial or total loss of body function or structures, while activity limitation is difficulty with executing or completing activities [22] Real-world use is motion captured under non-standardized conditions for sustained time periods. In the treatment category, wearable sensors were used to trigger sensory cues or provide instructional feedback for the affected UE during training. We present summarized results for each category below. See Tables 1–4 for detailed information about each study including participant information, study sample size, sensor type and placement, data collection conditions, data processing and analyses, and study results.
Figure 1.
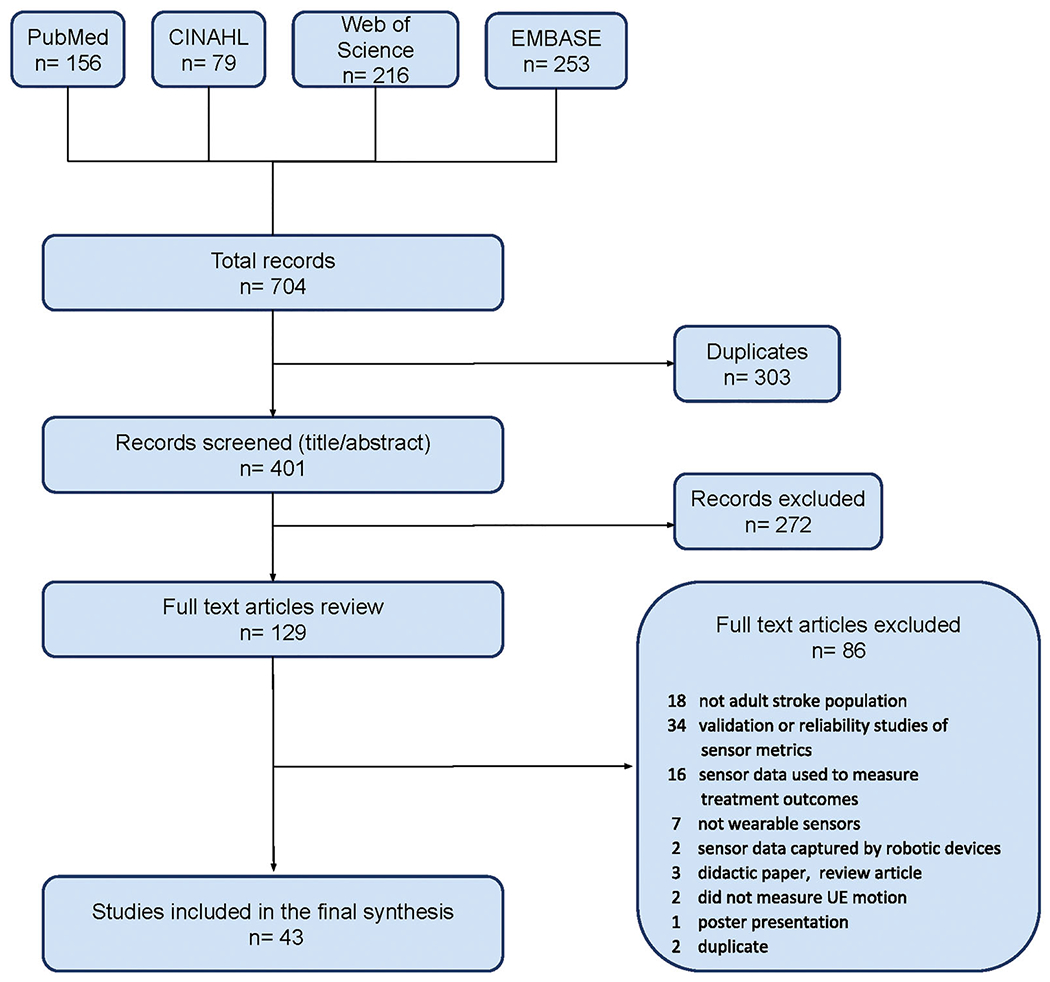
Flowchart of the screening and review process for included studies.
Table 1.
Identification of functional motion.
| Author | Participants | UE disability | Sensor information | Data collection | Data processing | Data analysis | Results |
|---|---|---|---|---|---|---|---|
| Biswas et al. [27] |
n=4 control n=4 stroke, chronicity not specified |
Not specified | Brand: Shimmer IMU Number: 1 Placement: affected wrist |
Outpatient: “make cup of tea” activity consisting of reach and retrieve object, drinking, and pouring | Labeling: observation notes Preprocessing: band-pass filtering of ACC data only |
Model type: non-ML, rule-based algorithm to determine sensor orientation, position transitions, and sequence detection Training: N/A Classification: functional movements (reach and retrieve object, drinking, pouring) Validation: N/A |
Accuracy: 0.91–0.99 control 0.70–0.85 stroke |
| Biswas et al. [28] |
n=4 control n=4 stroke, chronicity not specified |
Not specified | Brand: Shimmer IMU Number: 1 Placement: affected wrist |
Outpatient: reach and retrieve, drinking, pouring water | Labeling: observation notes Preprocessing: band-pass filtering |
Model type: ML, supervised K means clustering Training: supervised Classification: functional movements (reach and retrieve object, drinking, pouring) Validation: out-of-subject testing |
Accuracy: 0.88 control (ACC) 0.83 control (gyroscope) 0.70 stroke (ACC) 0.66 stroke (gyroscope) |
| Bochniewicz et al. [33] |
n=10 control n=10 stroke, chronic |
ARAT = 5–41 | Brand: Analog Devices IMU Number: 1 Placement: affected UE |
Outpatient: (mock apartment) laundry, shopping, kitchen activity, and making the bed | Labeling: video footage Preprocessing: band-pass filtering |
Model type: ML, random forest Training: supervised Classification: functional vs. non-functional movement Validation: in-subject testing (intrasubject approach), and out-of-subject testing (inter-subject approach) |
Accuracy: 0.91 control 0.70 stroke |
| Guerra et al. [32] |
n=10 control n=6 stroke, chronic |
FMA = 52.8 (mean) | Brand: XSens IMU Number: 11 Placement: head, sternum, pelvis, scapulae, BUEs |
Outpatient: tabletop and shelf reaching tasks, feeding task, drinking task | Labeling: video footage Preprocessing: band-pass filtering |
Model type: ML, sliding window approach, hidden Markov model, and logistical regression Training: supervised Classification: functional primitives (rest, reach, transport, retract) Validation: out-of-subject testing |
Sensitivity: 0.82 control 0.75 stroke |
| Lemmens et al. [26] |
n=30 control n=1 stroke, chronicity not specified |
Not specified | Brand: Shimmer IMU Number: 7 Placement: bilateral hand, wrist, upper arm, and chest |
Outpatient: drinking, eating, hair brushing Home: 30 min daily life recording (control, n=1) |
Labeling: time borders of summed gyroscope data Preprocessing: band-pass filtering |
Model type: non-ML, threshold-based approach, template matching algorithm using 2D convolution Training: N/A Classification: activity and functional movement (drinking, eating, hair brushing) Validation: out of subject testing |
Sensitivity: 1.0 control 1.0 stroke Sensitivity, drinking: 1.0 control (non-standardized) |
| Lum et al. [34] |
n=10 control n=10 stroke, chronic |
ARAT = 23.5 (mean) | Brand: custom built IMU Number: 2 Placement: bilateral wrist |
Outpatient: (mock apartment) laundry, shopping, kitchen activity, and making the bed | Labeling: video footage (functional, non-functional, unknown) Preprocessing: band-pass filtering |
Model type: ML, random forest, radial basis function support vector machine Training: supervised Classification: functional vs. non-functional movement Validation: in-subject testing (intrasubject approach), and out-of-subject testing (inter-subject approach) |
Accuracy, intra-subject approach 0.96 control 0.93 stroke Accuracy, inter-subject approach: 0.91 controls 0.74 stroke |
| Mazomenos et al. [29] |
n=18 control n=4 stroke, chronicity not specified |
Not specified | Brand: Shimmer IMU Number: 2 Placement: proximal affected wrist and elbow |
Outpatient: reach and retrieve, pouring water, drinking | Labeling: pre-defined start/end positions during data collection Preprocessing: band-pass filtering |
Model type: non-ML, rule-based detection algorithm to recognize kinematic patterns Training: N/A Classification: functional movement (reach and retrieve, drinking, pouring) Validation: N/A |
Accuracy: 0.89–0.96 control 0.84–0.93 stroke |
| Panwar et al. [31] | N=14 stroke, chronic | Not specified | Brand: Shimmer IMU and Physilog IMU Number: 1 (outpatient), 3 (home) Placement: affected wrist (outpatient), bilateral wrist and sternum (home) |
Outpatient: “make cup of tea” activity consisting of reach and retrieve, lift arm, rotate arm Home: 2 continuous hours during weekday, encouraged to use UE for daily activities (non-standardized) |
Labeling: observational notes Data augmentation Preprocessing: band-pass filtering, signal length standardized, signal transformation |
Model type: ML, convolutional neural network (deep learning), 10-fold cross validation Training: supervised Classification: functional movements (reach and retrieve object, drinking, pouring) Validation: Out-of-subject testing |
Accuracy, 0.98 0.89 non-standardized condition |
| Roy et al. [30] | N=10 stroke, chronic | Brunnstrom levels 3–5 | Brand: Analog Devices ACC Number: 8 (ACC), 8 (sEMG) Placement: thigh, trunk, bilateral shoulder, bilateral forearm |
Outpatient: 11 ADL activities based on the FIM in the areas of: feeding, grooming, dressing, functional mobility |
Labeling: not specified Preprocessing: band-pass filtering |
Model type: ML, artificial neural network, adaptive neuro-fuzzy adaptive system, scaled conjugate gradient algorithm Training: supervised Classification: functional movements (feeding, brushing teeth, hair combing, buttoning shirt, sit to stand, walking, toileting) Validation: out-of-subject testing |
Sensitivity: 0.95 ACC+sEMG 0.93 ACC Specificity: 0.99 ACC+sEMG 0.97 ACC |
| Zambrana et al. [35] |
n=15 control, n=6 stroke, chronicity not specified |
Not specified | Brand: BNO055 IMU Number: 2 Placement: bilateral wrist |
Outpatient: eating, pouring water, drinking, brushing teeth, folding a towel, walking | Labeling: video footage Preprocessing: band-pass filtering |
Model type: module 1 – non-ML, threshold-based analysis module 2 – ML, K-nearest neighbor, support vector machines, random forest Training: supervised Classification: module 1 – movement vs. non-movement module 2 – functional vs. non-functional movement Validation: in-subject testing (intrasubject approach), and out-of-subject testing (inter-subject approach) |
Accuracy, module 1: 0.89 Accuracy, module 2: 0.91 intra-subject control 0.90 inter-subject control 0.61 stroke |
ACC: accelerometer; IMU: inertial measurement unit; FIM: Functional Independence Measure; FMA: Fugl-Meyer Assessment; ML: machine learning; non-ML: non-machine learning; sEMG: surface electromyography. Participants: number of healthy and/or stroke participants, level of chronicity (acute: onset – 1 month, subacute 1–6 months, chronic >6 months); UE disability: motor impairment (FMA, Brunnstrom stages) or activity limitation (ARAT); sensor information: name and type of device, number and location of sensors placed on the UE; data collection: location of data collection, type of UE motion collected; data processing: methods for data labeling and preprocessing; data analysis: model type (machine learning or non-machine learning), training (supervised/unsupervised), classification of UE motion (activity, functional movement, functional primitive, functional vs. non-functional), and validation of the algorithm (out-of-subject, in-subject testing); results: performance of algorithm (sensitivity or accuracy), unless otherwise reported, results reflect out-of-subject testing and data collected under standardized conditions (controlled environment with pre-set tasks).
Table 4.
Sensor-assisted treatment.
| Author | Participants | UE disability | Sensor information | Data collection/treatment | Data processing | Data analysis | Results |
|---|---|---|---|---|---|---|---|
| Chae et al. [70] |
N=23 stroke, chronic n=6 control group (no feedback) n=17 treatment group (feedback) |
FMA = 29 (control group), 36.6 (treatment group) | Brand: LG smartwatch, IMU Number: 1 Placement: affected wrist |
Home: sensor data collected whenever smartphone app used by participants. Treatment: completed 4 home exercises daily over 12-weeks, treatment group received weekly feedback on type and amount of exercises they completed |
Labeling: Home exercise tasks, observation notes Preprocessing: not specified |
Model type: ML, convolutional neural network (deep learning), cross-validation testing Training: supervised Classification: home exercise tasks (shoulder flexion, wall pushups, scapular exercise, towel slides) Validation: out-of-subject testing |
Accuracy: 0.99 (personal data) 0.95 (total data) Training time for home exercises (minutes per day): 13.6 control group (self-report) 22.6 treatment group(sensor data) Change in WMFT scores, baseline to discharge: 3.4 control 2.8 treatment Change in shoulder flexion, baseline to discharge (degrees): 5.5 control 20.2 treatment Change in shoulder internal rotation, baseline to discharge (degrees): 6.5 control 13.1 treatment |
| Da-Silva et al. [68] | N=11 stroke, acute, and subacute | ARAT = 39 (mean) | Brand: CueS, ACC Number: 1 Placement: affected wrist |
Inpatient: 1 week to set prompt levels Home: 12 h/day ×4 weeks. Treatment: provided external vibration cue if UE activity did not reach customized threshold level |
Labeling: NA Preprocessing: not specified |
Metrics: Median signal vector magnitude (calculated every 3 days to set threshold for vibration cues) |
Adherence to wearing sensors = 89% 11–29% increase in mean activity one-hour post vibration cue Cueing frequency preference higher for severe group and lower for mild group |
| Da-Silva et al. [69] |
N=33 stroke, subacute n=19 control group(no vibration) n=14 treatment group (vibration) |
ARAT = 15 (control group), 37 (treatment group) | Brand: CueS, ACC Number: 1 Placement: affected wrist |
Inpatient and home: Sensors worn 12 h/day ×4 weeks. Treatment: provided external vibration cue if UE activity did not reach customized ACC threshold level |
Labeling: NA Preprocessing: not specified |
Metrics: Median signal vector magnitude (calculated every 3 days to set threshold for vibration cues) |
Adherence to wearing sensors = 79% Responses to receiving wrist vibration cue (self-report): 43% practiced activities from daily activities list 38% practiced their own self-chosen activity 17% ignored the cue Cueing frequency preference: 8 cues per day, hourly basis |
| Lee et al. [72] |
n=10 control n=20stroke, chronic |
FMA = 37 (mean) | Brand: Shimmer, IMU Number: 2 Placement: bilateral wrist |
Outpatient: ADLs targeting unilateral, bilateral and stabilization skills, strength and range of motion exercises Stakeholder survey of therapists (n=13) and stroke survivors (n=17) on user feasibility of proposed approach |
Labeling: video Preprocessing: band-pass filtering |
Model type: ML, logistic regression, nearest neighbor with dynamic time warping algorithm, random forest algorithm Training: supervised Classification: functional/non-functional movement, feedback/no feedback, types of feedback categories (compensatory or accuracy) Validation: out-of-subject testing |
Accuracy, functional/non-functional movement classification: 0.87 control and stroke unaffected UE Sensitivity, feedback/no-feedback classification: 0.83 stroke Sensitivity, type of feedback, compensatory classification: 0.64 stroke Sensitivity, type of feedback, accuracy classification: 0.76 stroke Survey, therapists: 91.2% willing to use in clinical practice Survey, stroke survivors: 76.5% willing to use the system 88.2% willing to use the system with therapist recommendation Survey, stroke survivors, preferred method of receiving UE feedback: 29.4% visual 23.5% vibration 35.3% sound and vibration |
| Whitford et al. [71] | N=8 stroke, chronic | FMA = 32 (mean) | Brand: Actigraph, ACC Number: 2 Placement: bilateral wrist |
Home: sensors worn for 3-week treatment period Treatment: 7 feedback sessions provided over 3 weeks on duration of use, acceleration magnitude, use ratio |
Labeling: NA Preprocessing: built-in software |
Metrics: Activity counts (proprietary software) Duration of use Use ratio Acceleration magnitude |
Adherence to wearing sensors: 100% Perceived UE use at home: 1.93 MAL AOU baseline 2.84 MAL AOU discharge |
ACC: accelerometer; ARAT: Action Research Arm Test; FMA: Fugl-Meyer Assessment; IMU: inertial measurement unit; MAL AOU: Motor Activity Log, Amount of Use Subscale; ML: machine learning; WMFT: Wolf Motor Function Test.
Participants: number of healthy and/or stroke participants, level of chronicity (acute: onset – 1 month, subacute 1–6 months, chronic >6 months); UE disability: motor impairment (FMA) or activity limitation (ARAT); sensor information: name and type of device, number and location of sensors placed on the UE; data collection/treatment: location and type of UE motion collected; summary of study intervention; data processing: methods for data labeling and preprocessing; data analysis: motion data metrics calculated OR model type (machine learning or non-machine learning), training type (supervised/unsupervised), classification of UE motion (performance feedback categories), and validation of algorithm (out-of-subject, in-subject testing); results: UE training augmentation, performance of algorithm (sensitivity or accuracy).
Category 1a: identification of functional motion
We identified 10 studies that identified functional motion of the affected UE while participants completed typical ADLs. A taxonomy of UE function [21] was used to define functional motion terminology in this section. Functional motion is any purposeful motion/minimal-motion that engages the environment in the context of basic or instrumental ADLs [21]. Functional motion can be deconstructed in a hierarchical manner based on decreasing time durations and goals. Activities are long-duration motions with many goals (e.g., UE dressing); functional movements are moderate-duration motions with a few goals (e.g., putting arm through sleeve, zipping up jacket) [23,24]; and functional primitives are short-duration motions or minimal-motions with one goal (e.g., reach, stabilize, transport) [25]. Within this framework, one study identified activity [26], five studies identified functional movements [27–31], and one study identified functional primitives [32]. Three studies identified the presence or absence of functional motion rather than recognizing a specific hierarchical level of functional motion [33–35].
Data capture
All studies in this category used IMU sensor data with the exception of one study, which used a combination of accelerometers and surface electromyography [30]. The number of sensors varied from a single sensor affixed to the affected wrist [27,31,33] to 11 sensors affixed to the head, bilateral UE, trunk, and pelvis [32]. All studies collected data in outpatient settings, and two studies also collected data in the home setting [26,31]. All study participants completed functional activities or functional movements for a specific set of repetitions in standardized conditions. Two studies also collected non-standardized data in a home setting from healthy [26] and stroke [31] participants. Stroke participants were in the chronic phase of recovery [30–34] or it was not specified [26–29,35].
Data processing and analysis
Sensor data were pre-processed using low- and high-band pass filtering to remove noise at the minimum and maximum end of the frequency spectrum, typically 0.2–15 Hz. Supervised machine learning (ML) was used in seven studies to classify UE motion as functional movements or functional primitives [27,30–35]. Supervised ML entails training an algorithm with a motion dataset that has been labeled by human observers, then testing the trained algorithm on another labeled motion dataset to assess its classification performance [4]. It is beyond the scope of this paper to detail the specific methods of ML classification, but practical reviews can be found elsewhere [4,9]. ML approaches used to classify motion data included K-means cluster analysis [28], random forest [33–35], hidden Markov model with logistical regression [32], multi-layered neural network and adaptive neurofuzzy system [30], convolutional neural network (deep learning) [31], radial basis function support vector machine [34], K-nearest neighbor, and support vector machines [35]. Once the algorithm was trained, out-of-subject testing was performed in all studies.
Three studies used non-ML approaches, including a threshold-based approach and a mathematical rule-based approach to classify UE motion as an activity or functional movement [26,27,29]. Rule-based or threshold-based approaches apply human-crafted rules and expert-determined thresholds to recognize functional motion, for instance identifying a reaching motion from a simultaneous increase in shoulder flexion and elbow extension. For non-ML approaches, algorithms were created to recognize kinematic patterns associated with functional movements or activities of interest [26,29], or to determine sensor orientation and movement sequences associated with specific functional movements [27].
Outcomes
The majority of studies assessed the performance of their algorithms by calculating sensitivity or accuracy. Sensitivity is calculated as the proportion of correct positive classifications relative to the total number of positive samples (true positive rate). Accuracy is calculated as the proportion of correctly classified samples (true positive and true negative) relative to the total sample [36]. The range for sensitivity or accuracy is 0–1, with 1 indicating perfect classification by the algorithms [4]. Unless noted, we report classification performance on out-of-subject data, where the algorithm assessed subject data that it had not previously seen.
The classification of three functional activities for controls and stroke had a high sensitivity of 1.0 [26]. The classification of functional movements had a sensitivity of 0.93 for stroke [30], and an accuracy of 0.83–0.99 for controls and 0.66–0.98 for stroke [27–29,31]. The classification of functional primitives had a sensitivity of 0.82 in controls and 0.75 in stroke [32]. The binary classification (functional versus non-functional motion) had an accuracy of 0.90–0.96 in controls and 0.61–0.93 in stroke [33–35].
Category 1b: identification of motor impairment and activity limitation
We identified 14 studies that classified UE motion at the motor impairment or activity limitation levels. Motor impairments are partial or total loss of body function or structures (e.g., strength, endurance, and motor control), while activity limitation is difficulty with executing or completing activities [22]. The purpose of the studies was to identify the presence of motor impairment [37–39] or to classify UE motion into distinct motor impairment/activity limitation levels [40–50].
Data capture
Various combinations of IMUs, accelerometers, and flex sensors were used to collect UE motion data. Flex sensors measure the amount of bend or deflection using thin strips of conductive materials and can be embedded into a glove or garment to capture movement of the wrist or fingers [51]. The number of sensors ranged from a single sensor at the affected wrist [42] to a 16-IMU hand glove [39,43]. Data were collected in the inpatient hospital [37,38,40,47–49], outpatient lab [43–45], outpatient clinic and home [46], or the location was not specified [42,50]. Data were collected during performance of standardized clinical assessments [40,42–46,50], study-specific shoulder/hand tasks [39,42,43,48,49], or non-standardized arm motion [37,38,41,47,50]. Stroke participants were in the acute [37,38,40,41,47–49], subacute [46,50], or chronic phase of recovery [46,50], or chronicity was not specified [39,42–45].
Data processing and analysis
Sensor data were pre-processed using low- and high-band pass filtering. All studies extracted accelerometry signals from motion data to derive kinematic data such as velocity, smoothness, and magnitude.
Eleven studies used supervised ML methodology described above to classify levels of motor impairment. UE motion data were labeled using scores from the Oxford Motor Strength Scale [47], Fugl-Meyer Assessment (FMA) [40,46], Brunnstrom levels [39,42,43], Wolf Motor Function Test (WMFT) [44,45], and the National Institute of Health Stroke Scale (NIHSS) [41,48,49]. The investigators used various ML approaches to classify arm impairment levels, including decision tree [40], bagging forest [40], dynamic time warping [42], K-means clustering [43], K-fold validation [43], extreme learning and RRelief [46], naïve Bayes classifier [44], random forest [45], logistic regression [39], support vector machines [47], hierarchical discriminant analysis [48,49], and non-linear mixed effects models with Gaussian process [50]. Once the algorithm was trained, out-of-subject testing was performed [40,42,43,46–50] or testing procedures were not specified [39,44,45].
Three studies used a non-ML threshold-based approach to classify motion data into presence or absence of motor impairment [37,38] and multiple class impairment categories [41]. In these studies, investigators used expert-knowledge to generate thresholds for sensor data (e.g., acceleration value) or statistical metrics derived from sensor data (e.g., cross-correlation, energy). A reading above this threshold indicates a “match” (stroke patient) and a value below indicates “not a match” (healthy control). Studies using threshold-based analysis did not split the motion data into testing and training sets. They generated the threshold using domain knowledge and used the entire dataset to test the threshold-based classification.
Outcomes
Algorithm performance was evaluated using sensitivity, accuracy, coefficient of determination (R2), and error estimate calculations (root mean square (RMS) values). The coefficient of determination is calculated as the proportion of variance of the predicted data that is explained by the observed data [42]. Values range from 0 to 1, with scores approaching 1 representing a better fit. Error estimates represent misclassifications that are both false positives (“false alarms”) and false negatives (“accidental misses”), and are considered the complement of accuracy [36]. Error estimates do not have a set score range and have the same units as their target value, but scores approaching zero reflect better fit [52].
The classification of functional dependence versus ability to move against gravity had a sensitivity of 0.87 and an accuracy of 0.82 [47]. The classification of the presence versus absence of impairment had a sensitivity of 0.87–0.98 [37–39,48,49], mild versus moderate/severe impairment had a sensitivity of 0.80 and an accuracy of 0.80 [49], and moderate versus severe impairment had a sensitivity of 0.82–0.88 and an accuracy of 0.87–0.96 [48,49]. FMA scores were predicted with an error rate of 0.025 ± 0.025 [40] and an R2 of 0.92 [46]. Brunnstrom levels 3–6 were predicted with an accuracy of 0.82 [42], and Brunnstrom levels 4 and 5 were predicted with an accuracy of 0.70 [43]. WFMT scores were predicted with RMS values of 0.45 [44] and 2.25 [45]. NIHSS motor scores were predicted with an accuracy of 0.81 [41]. Chedoke Arm and Hand Activity Inventory (CAHAI) scores were predicted with RMS values of 6.9 for subacute and 3.4 for chronic participants [50].
Category 1c: quantification of real-world use
We identified 14 studies that quantified real-world use. This approach is considered “real-world” because data were captured under non-standardized conditions for sustained time periods in the home or hospital. This approach quantifies general arm motion rather than specific functional motion. These studies compared use of the affected UE to the unaffected UE of stroke participants or to the UEs of healthy participants.
Data capture
All studies in this group used accelerometer data to capture motion. Nine studies used a sensor on each wrist [53–61], whereas five other studies used bilateral wrist sensors plus 1 −3 sensors at the bilateral lower extremities and/or hip [62–66]. Sensor data were collected in community, outpatient, and inpatient settings. Participants did not complete any predetermined UE tasks. Observational UE motion data were captured continuously for durations ranging from daytime waking hours [56,66] to 30 contiguous days [59]. Four longitudinal studies collected UE data at multiple time points post-stroke, including at hospital admission and 3 weeks [62], at admission and 3 months [55], at hospital discharge and 12 months [63], and from start of outpatient therapy to discharge from therapy [54]. Stroke participants were in the acute [54,55,57,60–63,66], subacute [55], and chronic [53,54,56,58,59,63,64] phases of recovery.
Data processing and analysis
Studies with commercial accelerometers used built-in proprietary software for initial pre-processing of raw acceleration data [53,54,57,60–63]. Some studies reported little information on pre-processing and calculation of metrics [55,56,58,59,65]. Mathematical calculations determined common metrics including duration of use, activity counts, and acceleration magnitude. Activity counts are defined as the sum of acceleration signals over a pre-determined epoch or period of time [67]. Activity counts are specific for each study, and are often used as the basic unit representing UE use. Activity counts were reported [55,56,62,63,65] or further processed to characterize UE duration of use [53,54,57–61,64]. Acceleration magnitude, calculated as the sum of the vector magnitude for every second of activity, was used to characterize UE motion intensity [53,54,58,60,61,64,66]. Several studies calculated laterality indices – the ratio of affected to unaffected UE motion – examining duration of use (bilateral use ratio) or acceleration magnitude (bilateral magnitude ratio) [53,54,57,58,60–63,66].
Outcome
Individuals in the acute, subacute, and chronic phase of recovery used their affected UE significantly less than their unaffected UE or the UE of healthy controls, as measured by activity counts [55,62,63], duration of use [53,57,58,60,61,64], and acceleration magnitude [53,54,58,60,61,64,66]. In acute stroke, activity counts in the affected UE were 1.3 times lower than the unaffected UE and 2.4 times lower than in individuals with transient ischemic attack [65]. Activity counts in the affected UE were 4.0 times lower at 3 weeks and 3.0 times lower at 12 months compared to the unaffected UE [62,63]. Another study found activity counts in the affected UE to be 2.5 times lower at 2 weeks and 1.8 times lower at 3 months compared to the unaffected UE [55]. Mean activity counts for moderately impaired individuals were 3.5 times lower than mildly impaired individuals [56]. Duration of use ranged from 1.5 to 6.7 h on the affected side and 5.3–9.1 h on the unaffected side [57,58,60,61,64]. The use ratio was 0.22–0.86 in stroke and 0.89–0.95 in controls [54,57,60,61]. The bilateral magnitude ratio ranged from −2.2 to −7 in stroke participants and −0.1 to −0.26 in controls [53,54,60,61], and was significantly lower on weekends than weekdays in the acute rehabilitation setting [66]. A bilateral magnitude ratio score approaching zero indicates equal contribution of both UEs, while a negative ratio indicates more motion in the unaffected UE relative to the affected UE [53].
Category 2: sensor-assisted treatment
We identified five studies that used sensor-derived data to help with an aspect of treatment. Two studies used motion data to set the timing of vibration cues delivered to the affected UE during home-based arm training [68,69]. Two studies provided intermittent performance feedback to participants during home-based interventions, using UE motion data to calculate general UE use or to classify the type and quality of motion completed by participants [70,71]. One study assessed the initial feasibility of multiple types of performance feedback and stakeholder acceptance of the sensor-based training approach in preparation for a clinical trial [72].
Data capture
Three studies used accelerometers [68,69,71], while two studies used IMUs [70,72]. The studies used 1–2 sensors affixed to the affected or bilateral UEs. Data were collected during inpatient rehabilitation [68,69], in the home [68–71], or an outpatient lab [72]. Participants completed pre-determined functional tasks and UE exercises determined by research staff [70,72] or were instructed to complete their usual activities under non-standardized conditions [68,69,71]. Studies included participants with subacute [68,69] and chronic stroke [70–72].
Data processing and analysis
Sensor data were pre-processed using low- and high-band pass filtering [72] or procedures were not specified [68–71]. Two studies calculated acceleration magnitude every three days to set a threshold for vibration cues during an arm training protocol [68,69]. One study calculated general use metrics (duration of use, use ratio, acceleration magnitude) twice a week during a 3-week treatment period [71]. Two studies used a supervised ML approach to identify several aspects of UE motion [70,72]. The first of these studies classified whether assigned home exercises were performed (convolutional neural network) [70]. The second study determined performance feedback in multiple steps and classified UE motion as functional/non-functional (logistic regression), feedback or no feedback (nearest neighbor with dynamic time warping algorithm), and accuracy or compensatory (random forest) [72]. Once algorithms were trained, out-of-subject testing was performed for both studies [70,72].
Outcomes
Two studies delivered vibration cues based on acceleration magnitude thresholds to prompt UE activity, finding that they increased arm activity by 11–29% [68] or increased the practice of assigned or self-chosen activities [69]. Three studies provided intermittent performance feedback based on sensor data [70–72]. The first study gave feedback based on general UE use metrics, improving scores on perceived actual arm use after a 3-week home intervention [71]. The second study gave feedback based on the classification of study-specific UE exercises (accuracy of 0.95–0.9) [70], significantly improving shoulder range of motion and WMFT scores for the treatment group compared to the control group. The third study demonstrated the feasibility of their multi-step approach by initially identifying functional/non-functional motion (accuracy of 0.87) [72]. Functional motion was then classified by whether it needed feedback or no feedback (sensitivity of 0.76), compensatory feedback (sensitivity of 0.67), or accuracy feedback (sensitivity of 0.76) [72]. Stakeholder willingness to use this proposed approach for home rehabilitation was 91.7% for therapists and 88.2% for stroke survivors [72].
Discussion
Publication trends reflect the growing interest in using wearable sensors to study stroke recovery and rehabilitation. We reviewed and discussed the current literature to showcase the potential applications of this technology for UE assessment and treatment. We summarized how sensors have been used to assess UE functional motion, motor impairment and activity limitation, and real-world use. We also summarized the use of sensors to deliver sensory cues to prompt UE training or provide intermittent performance feedback during the UE treatment.
The number and placement of sensors were variable in the assessment of functional motion, impairment, and activity limitation. While the optimal number and placement of sensors did not emerge, sensor data were collected at the affected wrist in all studies, indicating that this could be a sensible placement location. The complexity of data analysis methodologies used for assessment and treatment were dependent on the level of specificity needed. Studies that identified specific functional ADLs or level of motor impairment required more complex ML and non-ML classification approaches. Studies that quantified general UE use used straightforward mathematical calculation of acceleration kinematics. Studies that used sensor data to assist in UE training had the greatest variability, using all aforementioned approaches.
Sensor-based assessment
The first group of studies used advanced analyses (ML and non-ML) to identify UE motion data at the three levels of functional motion: activity, functional movement, and functional primitives. Classification performance was generally higher for healthy control data and decreased with stroke data. While overall performance was high in these studies, comparison is challenged by their use of variable sensor systems, data types, functional tasks, classification approaches (ML versus non-ML), and ML algorithms.
The classification of UE functional motion has the potential to objectively monitor training content. The calculation of training dose for remote UE training is currently based on self-report. This sensor-based assessment could be used to quantify the dose of rehabilitation training in terms of time spent in functional motion [33] or repetitions of functional motion [26,32]. The classification of UE functional motion in non-standardized settings is more challenging than in standardized settings, but it is an important area for future work if measuring home-based rehabilitation is to be undertaken.
A limitation of the studies was the inconsistent terminology used to define the UE motion that was being classified. We suggest the adoption of a uniform language based on a functional motion hierarchy, which would facilitate the consistent identification and reporting of functional motion in future studies [21]. Future studies are also needed to systematically compare classification performance with different data capture, processing, and analysis methodologies and in standardized versus non-standardized (real-world) rehabilitation conditions.
The second group of studies used sensors to classify UE impairment and activity limitation by predicting scores of traditional standardized assessments such as the FMA [40,46], Brunnstrom stages [42,43], WMFT [44,45], and the CAHAI [50]. Studies used advanced analyses (ML and non-ML approaches) to classify data into impairment or activity limitation categories.
The classification of UE impairment and activity limitation has the potential for objectively monitoring recovery. This sensor-based assessment could quantify UE impairment and activity limitation with fewer items than the FMA and WMFT, reducing the need for time-consuming clinical testing. An additional advantage of this approach is that sensor data can be transmitted wirelessly to external work stations, enabling the monitoring of a patient’s motor status in real-time [73]. One important consideration for implementation in the home would be having caretaker help to don the sensors. We are not suggesting that this approach should replace clinical outcomes; however, this approach may surmount the challenge of administering clinical tests in specific circumstances including the first few hours after stroke or in community settings where resources (e.g., trained assessors, testing kits) may be limited.
A limitation of these studies was their focus on recapitulating the scores of existing clinical tests. The FMA, WMFT, and Brunnstrom stages use ordinal scoring systems, which precludes the detailed appraisal of impairment or activity limitation. Sensors capture continuous kinematic data, and could provide a more nuanced assessment of impairment and activity limitations. This would require the establishment of new metrics that break from traditional bedside tests. Work on this type of measurement is its infancy [74]. An additional limitation of the studies was the lack of participants with severe arm impairment, who may require additional sensors (e.g., EMG) to detect subtle muscle activation. Future work could include severely impaired participants to expand the classification capabilities of the approaches, making their application more universal.
All studies in the third group used sensors to quantify UE use over a sustained period of time [53,54,62–65]. Results from this group demonstrated that healthy individuals use both UEs fairly equally during everyday activities regardless of hand dominance, while stroke survivors used their affected UE less than the unaffected UE in acute, subacute, and chronic stages of recovery.
The quantification of real-world use has provided a unique understanding of the patterns of bilateral and unilateral arm motion over extended periods in natural settings, but it does not identify UE functional motion. Measurements based on activity counts (duration of use and use ratios) are commonly reported as proxies for amount of functional UE motion; however, these measurements have been shown to significantly overestimate functional UE motion, even when presented as a use ratio [34]. Metrics based on acceleration magnitude may be more promising and less prone to overestimation [34,75].
Unlike earlier studies, recent work in the acute stroke setting has stratified real-world use by a patient’s impairment level, which clarifies the full spectrum of general use patterns outside of therapy [60,61]. Studies that stratify real-world use by motor impairment are needed in subacute and chronic stroke. In addition, the reporting of data processing methods and the calculation of metrics varied widely in this category, which limits reproducibility. To increase methodological consistency, we suggest the standardized sensor set-up, motion data preprocessing, and selection of metrics discussed elsewhere [18].
Sensor-assisted treatment
The use of sensor data to facilitate UE training has recently emerged in the literature. The five studies in this category used motion data to increase training or to provide feedback to improve UE performance. The methodologies used in this category were based on an aspect of the intervention that the researchers wanted to facilitate using sensor data. For example, the straightforward mathematical calculation of acceleration magnitude was used to determine UE motion threshold levels to remind participants to move their arm [69] while complex ML approaches (deep learning algorithms) were used to identify the completion of assigned home exercises during a home-based protocol [70].
The use of sensors has the potential for increasing adherence to home-based interventions. Compared to traditional methods of adherence (activity diaries or checklists), sensor data have the potential to increase the frequency of arm training in real-time by triggering sensory reminders to move. They may also promote engagement and adherence by providing objective feedback about the type and amount of UE exercises completed in a home-based program. The same is likely true for in-clinic rehabilitation settings, where objective feedback to therapists and patients will keep training aligned with training and recovery goals. Future work with larger sample sizes is needed to validate the feasibility and clinical efficacy of these approaches for home-based rehabilitation. As the adoption of telerehabilitation and remote health care delivery increases in stroke rehabilitation [76], sensors are a promising tool to facilitate adherence and improve UE performance in home settings. The cost-effectiveness of sensor-based approaches will need to be systematically examined, as this area of research develops further.
Limitations
The aim of the review was to address the current gap in the literature for a detailed review of emerging assessment and treatment approaches in UE stroke rehabilitation. To map out emerging applications, we included a variety of research designs, including proof-of-concept and feasibility studies. We did not weigh the strength of the evidence or appraise efficacy of the approaches, which is beyond the scope of this review. We included studies that examined function, impairment, and use, but not other contributors to UE disability such as spasticity or impaired energy expenditure.
Conclusions
Wearable sensors have the potential to greatly expand assessment and treatment, both early after stroke and later in home and community settings. As demonstrated by the studies discussed here, sensor data can be used to assess UE functional motion, motor impairment, activity limitation, and real-world use. Sensor data can also be used to facilitate UE training by delivering sensory cues, and providing objective performance feedback during home-based UE training. This capability supports several emerging areas of research relevant to UE recovery: the quantification of rehabilitation dose, the nuanced assessment of impairment and activity limitation, the characterization of daily UE use patterns in real-world settings, and the adherence to home-based rehabilitation. Wearable sensors could enable us to respond to changing needs in rehabilitation delivery, opening new horizons in the automated assessment and treatment of stroke disability.
Supplementary Material
Table 2.
Identification of motor impairment and activity limitation.
| Author | Participants | UE disability | Sensor information | Data collection | Data processing | Data analysis | Results |
|---|---|---|---|---|---|---|---|
| Chaeibakhsh et al. [40] | N=8 stroke, acute | FMA = 4–53 | Brand: ADPM Opal IMU Number: 5 Placement: sternum, bilateral wrist, bilateral arm |
Inpatient: FMA items | Labeling: video Preprocessing: band-pass filtering |
Model type: ML, decision tree algorithm and bagging forest algorithm Training: supervised Classification: movement categories – synergy, out of synergy, wrist/hand function, fine motor coordination (based on FMA scores) Validation: out-of-subject testing |
Error rate: 0.03 ± 0.03 bagging forest 0.18 ± 0.01 decision tree |
| Datta et al. [48] |
n=15 control n=32 stroke, acute |
NIHSS 1–4 | Brand: Eoxys, ACC Number: 2 Placement: bilateral wrist |
Acute: finger tapping and swiping, hand opening/closing, wrist torsion, elbow flexion and extension, 3 min of spontaneous movement | Labeling: NIHSS scores (control, moderate, severe) Processing: band-pass filtering, marker-based segmentation for structured and spontaneous motion |
Model type: ML, hierarchical discriminant analysis, AUC and ROC (classification performance) Training: supervised Classification: healthy or stroke, moderate or severe Validation: out-of-subject testing |
Sensitivity: 0.87 overall 0.91–0.93 healthy or stroke 0.82–0.86 moderate or severe Accuracy: 0.91 overall 0.92 healthy or stroke 0.87–0.96 moderate or severe |
| Datta et al. [49] |
n=15 control n=40 stroke, acute |
NIHSS 1–4 | Brand: Eoxys, ACC Number: 2 Placement: bilateral wrist |
Acute: finger tapping and swiping, hand opening/closing, wrist torsion, elbow flexion and extension, 3 min of spontaneous movement | Labeling: NIHSS scores (control, mild, moderate, severe) Processing: band-pass filtering, segmentation for short-term activity |
Model type: ML, hierarchical discriminant analysis, the Kruskal–Wallis test (feature extraction), AUC and ROC (classification performance) Training: supervised Classification: healthy or stroke, mild or moderate-to-severe, moderate or severe, mild-to-moderate or severe Validation: out-of-subject testing |
Sensitivity: 0.78 overall 0.87 healthy or stroke 0.80 mild or moderate-to-severe 0.88 moderate or severe 0.92 mild-to-moderate or severe Accuracy: 0.78 overall 0.94 healthy or stroke 0.80 mild or moderate-to-severe 0.95 moderate or severe 0.87 mild-to-moderate or severe |
| Gubbi et al. [38] |
n=7 control n=15 stroke, acute |
Not specified | Brand: Crossbow iMote2 ACC Number: 2 Placement: bilateral wrist |
Inpatient: non-standardized motion – 4h immediately post stroke, 1 h after day 1 | Labeling: not specified Preprocessing: band-pass filtering |
Model type: non-ML, threshold-based algorithm using cross correlation of ACC magnitude and difference in cross correlation of ACC magnitude of 3 axes Training: N/A Classification: impaired or not impaired Validation: N/A |
Sensitivity: 0.87 cross correlation of ACC magnitude 0.95 correlation of 3 axes |
| Gubbi et al. [41] |
n=10 control n=15 stroke, acute |
NIHSS >0 | Brand: Crossbow iMote2 ACC Number: 2 Placement: bilateral wrist |
Inpatient: non-standardized motion – 4h immediately post stroke, 1 h after day 1 | Labeling: not specified Preprocessing: band-pass filtering |
Model type: non-ML, threshold-based algorithm correlation of activity indices to NIHSS motor score Training: N/A Classification: NIHSS motor score Validation: N/A |
Accuracy: 0.72 norm-based 0.73 SMA-based 0.81 energy-based |
| Heron et al. [37] |
n=10 control n=30 stroke, acute |
NIHSS, motor UE = 2 (median) | Brand: Crossbow iMote2 ACC Number: 2 Placement: bilateral wrist |
Inpatient: non-standardized motion – 5 h over 25 h period | Labeling: not specified Preprocessing: band-pass filtering |
Model type: non-ML, threshold-based algorithm, ICC, ROC curve analysis (diagnostic threshold) Training: N/A Classification: impaired or not impaired Validation: N/A |
Sensitivity: 0.95 NIHSS and ICC correlation: −0.53, p = 0.02 Spearman’s rho |
| Lin et al. [43] |
n=15 control n=15 stroke, chronicity not specified |
Brunnstrom levels 4–6 | Brand: custom built IMU glove Number: 16 Placement: affected hand |
Outpatient: grasp and release, thumb task, card turning | Labeling: Brunnstrom levels Preprocessing: band-pass filtering |
Model type: ML, K-means clustering, K-fold cross validation algorithm, twofold, 10-fold, and leave-one-out Training: supervised Classification: Brunnstrom categories 4, 5, 6 Validation: out of subject testing |
Accuracy: 0.73 B6 (healthy), 0.70 B5, 0.75 B4 |
| Lin et al. [39] |
n=15 control n=15 stroke, chronicity not specified |
Brunnstrom levels >3 | Brand: custom built IMU glove Number: 16 Placement: affected hand |
Outpatient: thumb task, grip task, card-turning | Labeling: Brunnstrom levels Preprocessing: band-pass filtering |
Model type: ML, logistic regression, principle component analysis (feature extraction), AUC and ROC (classification performance) Non-ML, the Kruskal–Wallis test Training: supervised Classification: impaired or not impaired, Brunnstrom levels 4, 5 and healthy subject (non-ML, the Kruskal–Wallis test) Validation: not specified |
Sensitivity, impaired or not impaired: 0.98 |
| Lucas et al. [47] | N=4 stroke, acute | Oxford Motor Scale, 0–5 | Brand: Axivity AX3, ACC Number: 4 Placement: bilateral wrists and ankles |
Inpatient: non-standardized motion for duration of ICU stay (up to 14 days) | Labeling: Oxford Motor Scale scores 0–2 = dependent 3–5 = antigravity Preprocessing: band-pass filtering |
Model type: ML, support vector machines Training: Supervised Classification: dependent or antigravity UE Validation: out-of-subject testing |
Sensitivity: 0.87 Accuracy: 0.82 |
| Parnandi et al. [44] | N=1 stroke, chronicity not specified | Not specified | Brand: IMU custom built Number: 1 Placement: affected wrist |
Outpatient: 15 WMFT tasks | Labeling: video Preprocessing: band-pass filtering |
Model type: ML, naïve Bayesian classifier Training: supervised Classification: WMFT-FAS score Validation: not specified |
RMS value (error estimate): 0.45 |
| Patel et al. [45] | N = 24 stroke, chronicity not specified | WMFT-FAS = 47.2 (mean) | Brand: not specified, ACC Number: 6 Placement: Index finger, thumb, trunk, hand, forearm, upper arm |
Outpatient: 8 WMFT tasks – 4 reaching, 4 manipulation | Labeling: digital markers, performance videotaped Preprocessing: band-pass filtering |
Model type: ML, random forest, RRelief algorithm and Davies Bouldin index (feature selection) Training: supervised Classification: WMFT-FAS score Validation: not specified |
RMS value (error estimates): 0.056 for 8 tasks 0.091 for 4 tasks |
| Tang et al. [50] |
N=59 stroke, n=26 subacute, n = 33 chronic |
Not specified | Brand: Axivity AX3, ACC Number: 2 Placement: bilateral wrist |
Setting not specified: 9 CAHAI items, non-standardized motion of 3-day period for 8 weeks | Labeling: not specified Processing: band-pass filtering, day time activity only (9 am–9 pm) |
Model type: ML, Gaussian Mixture Model and principle component analysis (feature clustering and extraction), linear mixed effects model, non-linear mixed effects model with Gaussian process (NMM-GP) Training: supervised Classification: CAHAI scores Validation: out-of-subject testing |
RMS value (error estimates) for NMM-GP: 6.9 (subacute stroke) 3.4 (chronic stroke) |
| Yu et al. [46] | N=24 stroke, subacute and chronic | FMA = 18 (mean) | Brand: Analog Devices ACC, not specified, flex sensors Number: 2 (ACC), 7 (flex sensors) Placement: forearm, upper arm (ACC); wrist and hand (flex sensors) |
Outpatient: 7 FMA items – 4 proximal and 3 distal 10x Home: 7 FMA items every week for 3 months (n=5) |
Labeling: FMA scores Preprocessing: band-pass filtering |
Model type: ML, Extreme Learning Machine algorithm (feature extraction and selection), RRelief algorithm (feature selection refinement) Training: supervised Classification: FMA scores for each item Validation: out-of-subject testing. |
Coefficient of determination, overall: 0.92 (R2) |
| Zhang et al. [42] |
n=9 control n=21 stroke, chronicity not specified |
Brunnstrom levels ≥3 | Brand: InvenSense IMU Number: 1 Placement: wrist |
Setting not specified: single shoulder touching task completed 5x | Labeling: Brunnstrom levels Preprocessing: band-pass filtering |
Model type: ML, constrained dynamic time warping algorithm, Naïve Bayes, quadratic discriminant analysis, K-nearest neighbor (KNN) Training: supervised Classification: Brunnstrom levels 3, 4, 5, 6 Validation: out-of-subject testing |
Sensitivity (most accurate using KNN): 0.93 B3, 0.88 B4, 0.80 B5, 0.93 B6, 0.82 overall |
ACC: accelerometer; B4–6: Brunnstrom stage 4–6; AUC: area under the curve; CAHAI: Chedoke Arm and Hand Activity Inventory; FMA: Fugl-Meyer Assessment; ICC: intra-class correlation; IMU: inertial measurement unit; ML: machine learning; N/A: not applicable; NIHSS: National Institute of Health Stroke Scale; non-ML: non-machine learning; ROC: receiver operating characteristic; RMS: root mean square; WMFT-FAS: Wolf Motor Function Test, Functional Ability Scale.
Participants: number of healthy and/or stroke participants, level of chronicity (acute: onset – 1 month, subacute 1–6 months, chronic >6 months); UE disability: motor impairment (Brunnstrom stages, FMA, NIHSS, Oxford Scale) or activity limitation (WMFT-FAS); sensor information: name and type of device, number and location of sensors placed on the UE; data collection: location of data collection, type of UE motion collected; data processing: methods for data labeling and preprocessing, and wireless data transfer for real time processing (if applicable); data analysis: model type (machine learning or non-machine learning), training type (supervised/unsupervised for machine learning models), classification of UE motion (presence/absence of impairment, motor impairment, or activity limitation levels), and validation of algorithm (out-of-subject, in-subject testing for machine learning models); results: reported outcomes vary based on machine learning or non-machine learning approach (sensitivity, accuracy, error estimates).
Table 3.
Quantification of real-world use.
| Author | Participants | UE disability | Sensors information | Data collection | Data processing | Data analysis | Results |
|---|---|---|---|---|---|---|---|
| Bailey et al. [53] | n=74 control, n=48 stroke, chronic | ARAT = 10–48 | Brand: Actigraph, ACC Number: 2 Placement: bilateral wrist |
Home: 24 h | Preprocessing: built-in ActiLife Software | Metrics: Activity count (proprietary software) Bilateral acceleration magnitude (sum of accelerations for all 3 axes) Magnitude ratio |
Duration of use (hours): 7.2 control 4.1 stroke Unilateral duration of use (hours): 1.5 non-dominant UE control 1.9 dominant UE control 0.8 affected UE stroke 3.4 unaffected UE stroke Bilateral acceleration magnitude: 132.6 control 82.4 stroke Bilateral magnitude ratio (score range: +7, unilateral affected UE use to −7 unilateral unaffected UE use): −0.1 control −2.2 stroke |
| Barth et al. [57] | n=12 control, n=24 stroke, acute | FMA = 35.4 (mean) | Brand: Actigraph GT9X Link, ACC Number: 2 Placement: bilateral wrist |
Inpatient: 24 h | Preprocessing: built-in ActiLife Software | Metrics: Activity count (proprietary software) Duration of use Use ratio |
Duration of use (hours): 6.2 non-dominant UE (control) 7.0 dominant UE (control) 3.7 affected UE (stroke) 6.9 unaffected UE (stroke) Unilateral duration of use (hours): 1.1 non-dominant UE (control) 2.2 dominant UE (control) 0.93 affected UE (stroke) 3.9 unaffected UE (stroke) Bilateral movement (hours): 5.0 control 2.8 stroke Duration of use ratio: 0.89 control 0.56 stroke |
| Bhatnagar et al. [58] | N=20 stroke, chronic | FMA = 36.1 (mean) | Brand: Actigraph GT3X+, ACC Number: 2 Placement: bilateral wrist |
Home: 3 contiguous days | Preprocessing: not specified | Metrics: Activity counts Duration of use Use ratio Acceleration magnitude Magnitude ratio |
Duration of use (hours): 5.3 affected UE 9.1 unaffected UE Unilateral duration of use (hours): 0.6 affected UE 4.3 unaffected UE Bilateral movement (hours): 4.8 Magnitude ratio (negative values indicate greater nonparetic activity, 0 score indicates equal arm contribution) −1.1 |
| Chin et al. [60] | N=12 stroke, acute | Mild: FMA = 65 (mean) Moderate: FMA = 32 (mean) Severe: FMA = 5 (mean) |
Brand: Actigraph GT3X+, ACC Number: 2 Placement: bilateral wrist |
Inpatient: 24 h | Preprocessing: built-in ActiLife Software | Metrics: Activity count (proprietary software) Duration of use Use ratio Bilateral acceleration magnitude Magnitude ratio Variation ratio |
Duration of use, affected UE (hours): 5.4 mild 4.1 moderate 1.5 severe Duration of use unaffected UE (hours): 8.7 mild 8.5 moderate 7.1 severe Use ratio (duration of use): 0.59 mild 0.45 moderate 0.22 severe Magnitude ratio (score range: +7 unilateral affected UE use to −7 unilateral unaffected UE use): −2.79 mild −5.25 moderate −7 severe |
| Chin et al. [61] | n=30 control, n=60 stroke, acute | Mild: FMA = 61 (mean) Moderate: FMA = 36 (mean) Severe: FMA = 8 (mean) |
Brand: Actigraph GT3X+, ACC Number: 2 Placement: bilateral wrist |
Inpatient: 24 h | Preprocessing: built-in ActiLife Software | Metrics: Activity count (proprietary software) Duration of use Use ratio Bilateral acceleration magnitude Magnitude ratio Variation ratio |
Duration of use, non-dominant, affected UE (hours): 8.72 control 6.71 mild 4.49 moderate 1.67 severe Duration of use dominant, unaffected UE (hours): 9.43 control 7.92 mild 7.68 moderate 6.97 severe Use ratio (duration of use): 0.93 control 0.86 mild 0.50 moderate 0.25 severe Magnitude ratio (score range: +7 unilateral affected/non-dominant UE use to −7 unilateral unaffected/dominant UE use): −0.26 control −2.79 mild −5.25 moderate −7 severe |
| Doman et al. [54] | N=15 stroke, acute and chronic | Not specified | Brand: Actigraph, ACC Number: 2 Placement: bilateral wrist |
Home: 24 h at baseline, every 10th outpatient therapy visit, discharge |
Preprocessing: built-in ActiLife Software | Metrics: Activity counts (proprietary software) Bilateral use ratio Bilateral acceleration magnitude Bilateral magnitude ratio |
Bilateral use ratio: 0.95 control 0.57 stroke Bilateral magnitude ratio (score range: +7 unilateral affected UE use to −7 unilateral unaffected UE use): −0.1 control −4.04 stroke |
| Lee et al. [55] | N=16 stroke, acute and subacute | Not specified | Brand: Actiwatch ACC Number: 2 Placement: bilateral wrist |
Inpatient: 24 h × 4 timepoints (onset to 3 months) Not specified if any of these timepoints were collected at home |
Preprocessing: not specified | Metrics: Raw activity count |
Mean activity counts (104 counts/day): Onset: 8 affected, 20.5 unaffected 1-month: 27.1 unaffected, 10.8 affected 2-month: 28.3 unaffected, 14.1 affected 3-month: 34.5 unaffected,19.4 affected |
| Lee et al. [59] |
N=29 stroke, chronic, right hemiparesis n=14 non-dominant UE affected, n=15 dominant UE affected |
FMA = 45.3 (mean) | Brand: Fitness Tracker Mi Band-3, ACC Number: 2 Placement: bilateral wrist |
Home: 30 contiguous days | Preprocessing: not specified | Metrics: Activity counts Differential activity (non-dominant activity counts minus dominant activity counts) Asymmetry index |
Activity counts, dominant UE (counts/day): 4126 non-dominant affected group 3053 dominant affected group Activity counts, non-dominant UE (counts/day): 3365 non-dominant affected group 4505 dominant affected group Differential activity (counts/day): 761 non-dominant affected group −1450 dominant affected group Asymmetry index (positive or negative index represents more contributions from dominant or non-dominant hand, respectively): 4.2 non-dominant affected group −30.5 dominant affected group |
| Michielsen et al. [64] |
n=18 control n=38 stroke, chronic |
Brunnstrom levels 3–5 | Brand: ULAM, ACC Number: 5 Placement: sternum, bilateral thigh, bilateral wrist |
Home: 24 h | Preprocessing: band-pass filtering | Metrics: Duration of use Movement intensity (root mean square values of acceleration) |
Duration of use (hours): 5.1 non-dominant UE (control) 5.3 dominant UE (control) 2.4 affected UE (stroke) 5.3 unaffected UE (stroke) Movement intensity (g/min): 0.04 dominant and nondominant UE (control) 0.02 affected UE (stroke) 0.04 unaffected UE (stroke) |
| Alt Murphy et al. [66] |
n=10 controls n=28 stroke, acute |
FMA = 35 (median) | Brand: Shimmer 3, ACC Number: 5 Placement: bilateral wrist and ankles, trunk |
Inpatient: 8 am–8 pm for 2 days during weekdays and weekend | Preprocessing: visual inspection, band-pass filtering | Metrics: Signal magnitude area (SMA) Signal magnitude area ratio |
Signal magnitude area, weekday, (m/s2): 2.51 non-dominant UE (control) 1.20 affected UE (stroke) 2.51 dominant UE (control) 2.34 unaffected UE (stroke) Signal magnitude area, weekend, (m/s2): 2.45 non-dominant UE (control) 0.91 affected UE (stroke) 2.74 dominant UE (control) 2.10 unaffected UE (stroke) Signal magnitude area ratio (0 value indicated perfect symmetry between UEs, negative value indicates lower magnitude of affected UE or non-dominant UE): −0.07 weekday control −0.33 weekday stroke −0.04 weekend control −0.36 weekend stroke |
| Rand and Eng [62] | n=40 control, n=60 stroke, acute | Not specified | Brand: Actical ACC Number: 3 Placement: bilateral wrist, hip |
Inpatient: 3 contiguous days at admission and at 3 weeks | Preprocessing: built-in software | Metrics: Activity counts (proprietary software) UE motion during ambulation removed |
Change in activity counts (admission to 3 weeks): no difference affected and unaffected UE Change in use ratio (admission to 3 weeks): no change Activity count (at 3 weeks): unaffected UE 4x more than affected UE |
| Rand and Eng [63] | N=32 stroke, acute, and chronic | FMA = 51 (median) | Brand: Actical ACC Number: 3 Placement: bilateral wrist, hip |
Inpatient: 3 contiguous weekdays at discharge Home: 3 contiguous days at 12 months |
Preprocessing: built-in software | Metrics: Activity counts-proprietary software UE motion during ambulation removed |
Change in activity counts (discharge to 12 months): no difference in affected and unaffected UE Activity count (at 12 months): Unaffected UE 3x more than affected UE |
| Shim et al. [56] | N=40 stroke, chronic | Mild: FMA >45 Moderate: FMA = 19–44 |
Brand: Fittmeter ACC Number: 2 Placement: bilateral wrist |
Inpatient: 8 am–11 pm × 5 days | Preprocessing: not specified | Metrics: Raw activity counts Use ratios |
Mean activity count (m/s2): Moderate impairment group- 552–734 (therapy time) 540 (free time) Mild impairment group – 1831–2191 (therapy time) 1868 (free time) |
| Strommen et al. [65] | N=100 stroke, acute | Not specified | Brand: Actical, ACC Number: 5 Placement: bilateral wrist, bilateral ankle, hip |
Inpatient: contiguously until discharge or up to 7 days (0–7 days) | Preprocessing: Not specified |
Metrics: Activity counts (proprietary software) |
Activity count: 71% lower for stroke vs. TIA 80% less in affected UE vs. unaffected UE |
ACC: accelerometer; ARAT: Action Research Arm Test; FMA: Fugl-Meyer Assessment; TIA: temporary ischemic attack.
Participants: number of healthy and/or stroke participants, level of chronicity (acute: onset – 1 month, subacute 1–6 months, chronic >6 months); UE disability: UE motor impairment (FMA, Brunnstrom levels) or activity limitation (ARAT); sensor information: name and type of device, number and location of sensors placed on the UE; data collection: location and duration of time of data collection; data processing: methods for motion data labeling and preprocessing; data analysis: motion data metrics calculated; results: duration of use, activity counts, acceleration magnitude, laterality indices (ratio of affected to unaffected UE motion).
IMPLICATIONS FOR REHABILITATION.
Sensor data have been used to assess UE functional motion, motor impairment/activity limitation, and real-world use.
Sensor-assisted treatment approaches are emerging, and may be a promising tool to augment UE adherence in home-based rehabilitation.
Wearable sensors may extend our ability to objectively assess UE motion beyond supervised clinical settings, and into home and community settings.
Funding
This work was supported by an AHA AWS Postdoctoral Training Grant 19AMTG35210398 (AP) and NIH R01 NS110696, R01 LM013316, K02 NS104207 (HMS).
Footnotes
Disclosure statement
The authors declare that there is no conflict of interest.
References
- [1].Iosa M, Picerno P, Paolucci S, et al. Wearable inertial sensors for human movement analysis. Expert Rev Med Devices. 2016;13(7):641–659. [DOI] [PubMed] [Google Scholar]
- [2].Wang Q, Markopoulos P, Yu B, et al. Interactive wearable systems for upper body rehabilitation: a systematic review. J Neuroeng Rehab. 2017;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Green LB. Assessment of habitual physical activity and paretic arm mobility among stroke survivors by accelerometry. Top Stroke Rehabil. 2007;14(6):9–21. [DOI] [PubMed] [Google Scholar]
- [4].Parnandi A, Uddin J, Nilsen DM, et al. The pragmatic classification of upper extremity motion in neurological patients: a primer. Front Neurol. 2019;10:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patel S, Park H, Bonato P, et al. A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil. 2012;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Houwink A, Nijland RH, Geurts AC, et al. Functional recovery of the paretic upper limb after stroke: who regains hand capacity? Arch Phys Med Rehabil. 2012;94(5):839–844. [DOI] [PubMed] [Google Scholar]
- [8].Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. [DOI] [PubMed] [Google Scholar]
- [9].Lara OD, Labrador MA. A survey on human activity recognition using wearable sensors. IEEE Commun Surv Tutor. 2013;15(3):1192–1209. [Google Scholar]
- [10].Gebruers N, Vanroy C, Truijen S, et al. Monitoring of physical activity after stroke: a systematic review of accelerometry-based measures. Arch Phys Med Rehabil. 2010;91(2):288–297. [DOI] [PubMed] [Google Scholar]
- [11].Repnik E, Puh U, Goljar N, et al. Using inertial measurement units and electromyography to quantify movement during Action Research Arm Test execution. Sensors. 2018;18(9):2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van Meulen FB, Reenalda J, Buurke JH, et al. Assessment of daily-life reaching performance after stroke. Ann Biomed Eng. 2015;43(2):478–486. [DOI] [PubMed] [Google Scholar]
- [13].Noorkoiv M, Rodgers H, Price CI. Accelerometer measurement of upper extremity movement after stroke: a systematic review of clinical studies. J Neuroeng Rehab. 2014;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lang CE, Bland MD, Bailey RR, et al. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther. 2013;26(2):104–114; quiz 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen HL, Lin KC, Hsieh YW, et al. A study of predictive validity, responsiveness, and minimal clinically important difference of arm accelerometer in real-world activity of patients with chronic stroke. Clin Rehabil. 2018;32(1):75–83. [DOI] [PubMed] [Google Scholar]
- [16].Lang CE, Wagner JM, Edwards DF, et al. Upper extremity use in people with hemiparesis in the first few weeks after stroke. J Neurol Phys Ther. 2007;31(2):56–63. [DOI] [PubMed] [Google Scholar]
- [17].Sethi A, Ting J, Allen M, et al. Advances in motion and electromyography based wearable technology for upper extremity function rehabilitation: a review. J Hand Ther. 2020;33(2):180–187. [DOI] [PubMed] [Google Scholar]
- [18].Hayward KS, Eng JJ, Boyd LA, et al. Exploring the role of accelerometers in the measurement of real world upper-limb use after stroke. Brain Impair. 2016;17(1):16–33. [Google Scholar]
- [19].Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. [DOI] [PubMed] [Google Scholar]
- [20].Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–2126. [DOI] [PubMed] [Google Scholar]
- [21].Schambra HM, Parnandi A, Pandit NG, et al. A taxonomy of functional upper extremity motion. Front Neurol. 2019;10:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].International Classification of Functioning, Disability, and Health: ICF. Version 1.0. Geneva: World Health Organization; 2001. [Google Scholar]
- [23].Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther. 2007;31(1):3–10. [DOI] [PubMed] [Google Scholar]
- [24].Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in one hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair. 2010;24(7):620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Del Vecchio D, Murray RM, Perona P. Decomposition of human motion into dynamics-based primitives with application to drawing tasks. Automatica. 2003;39(12):2085–2098. [Google Scholar]
- [26].Lemmens RJM, Janssen-Potten YJM, Timmermans AAA, et al. Recognizing complex upper extremity activities using body worn sensors. PLOS One. 2015;10(3):e0118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Biswas D, Corda D, Baldus G, et al. Recognition of elementary arm movements using orientation of a tri-axial accelerometer located near the wrist. Physiol Meas. 2014;35(9):1751–1768. [DOI] [PubMed] [Google Scholar]
- [28].Biswas D, Cranny A, Gupta N, et al. Recognizing upper limb movements with wrist worn inertial sensors using k-means clustering classification. Hum Mov Sci. 2015;40:59–76. [DOI] [PubMed] [Google Scholar]
- [29].Mazomenos EB, Biswas D, Cranny A, et al. Detecting elementary arm movements by tracking upper limb joint angles with MARG sensors. IEEE J Biomed Health Inform. 2016;20(4):1088–1099. [DOI] [PubMed] [Google Scholar]
- [30].Roy SH, Cheng MS, Chang SS, et al. A combined sEMG and accelerometer system for monitoring functional activity in stroke. IEEE Trans Neural Syst Rehabil Eng. 2009;17(6):585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Panwar M, Biswas D, Bajaj H, et al. Rehab-Net: deep learning framework for arm movement classification using wearable sensors for stroke rehabilitation. IEEE Trans Biomed Eng. 2019;66(11):3026–3037. [DOI] [PubMed] [Google Scholar]
- [32].Guerra J, Uddin J, Nilsen D, et al. Capture, learning, and classification of upper extremity movement primitives in healthy controls and stroke patients. IEEE Int Conf Rehabil Robot. 2017;2017:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bochniewicz EM, Emmer G, McLeod A, et al. Measuring functional arm movement after stroke using a single wrist-worn sensor and machine learning. J Stroke Cerebrovasc Dis. 2017;26(12):2880–2887. [DOI] [PubMed] [Google Scholar]
- [34].Lum PS, Shu L, Bochniewicz EM, et al. Improving accelerometry-based measurement of functional use of the upper extremity after stroke: machine learning versus counts threshold method. Neurorehabil Neural Repair. 2020;34(12):1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zambrana C, Idelsohn-Zielonka S, Claramunt-Molet M, et al. Monitoring of upper-limb movements through inertial sensors – preliminary results. Smart Health. 2019;13:100059. [Google Scholar]
- [36].Tharwat A. Classification assessment methods. Appl Comput Inform. 2018;17(1):168–192. [Google Scholar]
- [37].Heron CL, Fang K, Gubbi J, et al. Wireless accelerometry is feasible in acute monitoring of upper limb motor recovery after ischemic stroke. Cerebrovasc Dis. 2014;37(5):336–341. [DOI] [PubMed] [Google Scholar]
- [38].Gubbi J, Kumar D, Rao AS, et al. A pilot study on the use of accelerometer sensors for monitoring post acute stroke patients. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:957–960. [DOI] [PubMed] [Google Scholar]
- [39].Lin BS, Lee IJ, Hsiao PC, et al. An assessment system for post-stroke manual dexterity using principal component analysis and logistic regression. IEEE Trans Neural Syst Rehabil Eng. 2019;27(8):1626–1634. [DOI] [PubMed] [Google Scholar]
- [40].Chaeibakhsh S, Phillips E, Buchanan A, et al. , editors. Upper extremity post-stroke motion quality estimation with decision trees and bagging forests. Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2016. [DOI] [PubMed] [Google Scholar]
- [41].Gubbi J, Rao AS, Fang K, et al. Motor recovery monitoring using acceleration measurements in post acute stroke patients. Biomed Eng Online. 2013;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang Z, Fang Q, Gu XD. Objective assessment of upper-limb mobility for poststroke rehabilitation. IEEE Trans Biomed Eng. 2016;63(4):859–868. [DOI] [PubMed] [Google Scholar]
- [43].Lin B-S, Hsiao P-C, Yang S-Y, et al. Data glove system embedded with inertial measurement units for hand function evaluation in stroke patients. IEEE Trans Neural Syst Rehabil Eng. 2017;25(11):2204–2213. [DOI] [PubMed] [Google Scholar]
- [44].Parnandi A, Wade E, Mataric M. Motor function assessment using wearable inertial sensors. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:86–89. [DOI] [PubMed] [Google Scholar]
- [45].Patel S, Hughes R, Hester T, et al. Tracking motor recovery in stroke survivors undergoing rehabilitation using wearable technology. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:6858–6861. [DOI] [PubMed] [Google Scholar]
- [46].Yu L, Xiong DX, Guo LQ, et al. A remote quantitative Fugl-Meyer assessment framework for stroke patients based on wearable sensor networks. Comput Methods Prog Biomed. 2016;128:100–110. [DOI] [PubMed] [Google Scholar]
- [47].Lucas A, Hermiz J, Labuzetta J, et al. Use of accelerometry for long term monitoring of stroke patients. IEEE J Transl Eng Health Med. 2019;7:2100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Datta S, Karmakar CK, Rao AS, et al. Automated scoring of hemiparesis in acute stroke from measures of upper limb co-ordination using wearable accelerometry. IEEE Trans Neural Syst Rehabil Eng. 2020;28(4):805–816. [DOI] [PubMed] [Google Scholar]
- [49].Datta S, Karmakar C, Yan B, et al. Novel measures of similarity and asymmetry in upper limb activities for identifying hemiparetic severity in stroke survivors. IEEE J Biomed Health Inform. 2020;25(6):1964–1974. [DOI] [PubMed] [Google Scholar]
- [50].Tang L, Halloran S, Shi JQ, et al. Evaluating upper limb function after stroke using the free-living accelerometer data. Stat Methods Med Res. 2020;29(11):3249–3264. [DOI] [PubMed] [Google Scholar]
- [51].Saggio G, Riillo F, Sbernini L, et al. Resistive flex sensors: a survey. Smart Mater Struct. 2016;25(1):13001. [Google Scholar]
- [52].Roy K, Das RN, Ambure P, et al. Be aware of error measures. Further studies on validation of predictive QSAR models. Chemometr Intell Lab Syst. 2016;152:18–33. [Google Scholar]
- [53].Bailey RR, Klaesner JW, Lang CE. Quantifying real-world upper-limb activity in nondisabled adults and adults with chronic stroke. Neurorehabil Neural Repair. 2015;29(10):969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Doman CA, Waddell KJ, Bailey RR, et al. Changes in upper-extremity functional capacity and daily performance during outpatient occupational therapy for people with stroke. Am J Occup Ther. 2016;70(3):7003290040p1–7003290040p11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee B, Kurihara J, Tokuda K, et al. Evaluation of the motor recovery process in stroke patients using a laterality index based on the paretic and non-paretic upper limbs’ actigraphic activity. J Phys Ther Sci. 2011;23(3):361–363. [Google Scholar]
- [56].Shim S, Kim H, Jung J. Comparison of upper extremity motor recovery of stroke patients with actual physical activity in their daily lives measured with accelerometers. J Phys Ther Sci. 2014;26(7):1009–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Barth J, Geed S, Mitchell A, et al. Characterizing upper extremity motor behavior in the first week after stroke. PLOS One. 2020;15(8):e0221668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bhatnagar K, Bever CT, Tian J, et al. Comparing home upper extremity activity with clinical evaluations of arm function in chronic stroke. Arch Rehabil Res Clin Transl. 2020;2(2):100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lee JP, Chen S, Tsai CT, et al. Characteristics associated with the differential activity of nondominant and dominant affected hands in patients with poststroke right hemiparesis. Occup Ther Int. 2020;2020:2387378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chin LF, Hayward KS, Soh AJA, et al. An accelerometry and observational study to quantify upper limb use after stroke during inpatient rehabilitation. Physiother Res Int. 2019;24(4):e1784. [DOI] [PubMed] [Google Scholar]
- [61].Chin LF, Hayward KS, Brauer S. Upper limb use differs among people with varied upper limb impairment levels early post-stroke: a single-site, cross-sectional, observational study. Top Stroke Rehabil. 2020;27(3):224–235. [DOI] [PubMed] [Google Scholar]
- [62].Rand D, Eng JJ. Disparity between functional recovery and daily use of the upper and lower extremities during subacute stroke rehabilitation. Neurorehabil Neural Repair. 2012;26(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rand D, Eng JJ. Predicting daily use of the affected upper extremity 1 year after stroke. J Stroke Cerebrovasc Dis. 2015;24(2):274–283. [DOI] [PubMed] [Google Scholar]
- [64].Michielsen ME, Selles RW, Stam HJ, et al. Quantifying nonuse in chronic stroke patients: a study into paretic, nonparetic, and bimanual upper-limb use in daily life. Arch Phys Med Rehabil. 2012;93(11):1975–1981. [DOI] [PubMed] [Google Scholar]
- [65].Strommen AM, Christensen T, Jensen K. Quantitative measurement of physical activity in acute ischemic stroke and transient ischemic attack. Stroke. 2014;45(12):3649–3655. [DOI] [PubMed] [Google Scholar]
- [66].Alt Murphy M, Andersson S, Danielsson A, et al. Comparison of accelerometer-based arm, leg and trunk activity at weekdays and weekends during subacute inpatient rehabilitation after stroke. J Rehabil Med. 2019;51(6):426–433. [DOI] [PubMed] [Google Scholar]
- [67].Uswatte G, Miltner WH, Foo B, et al. Objective measurement of functional upper-extremity movement using accelerometer recordings transformed with a threshold filter. Stroke. 2000;31(3):662–667. [DOI] [PubMed] [Google Scholar]
- [68].Da-Silva RH, van Wijck F, Shaw L, et al. Prompting arm activity after stroke: a clinical proof of concept study of wrist-worn accelerometers with a vibrating alert function. J Rehabil Assist Technol Eng. 2018;5:2055668318761524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Da-Silva RH, Moore SA, Rodgers H, et al. Wristband Accelerometers to motiVate arm Exercises after Stroke (WAVES): a pilot randomized controlled trial. Clin Rehabil. 2019;33(8):1391–1403. [DOI] [PubMed] [Google Scholar]
- [70].Chae SH, Kim Y, Lee KS, et al. Development and clinical evaluation of a web-based upper limb home rehabilitation system using a smartwatch and machine learning model for chronic stroke survivors: prospective comparative study. JMIR Mhealth Uhealth. 2020;8(7):e17216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Whitford M, Schearer E, Rowlett M. Effects of in home high dose accelerometer-based feedback on perceived and actual use in participants chronic post-stroke. Physiother Theory Pract. 2020;36(7):799–809. [DOI] [PubMed] [Google Scholar]
- [72].Lee SI, Adans-Dester CP, Grimaldi M, et al. Enabling stroke rehabilitation in home and community settings: a wearable sensor-based approach for upper-limb motor training. IEEE J Transl Eng Health Med. 2018;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yuce MR. Implementation of wireless body area networks for healthcare systems. Sens Actuators A Phys. 2010;162(1):116–129. [Google Scholar]
- [74].Arac A. Machine learning for 3D kinematic analysis of movements in neurorehabilitation. Curr Neurol Neurosci Rep. 2020;20(8):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bailey RR, Klaesner JW, Lang CE. An accelerometry-based methodology for assessment of real-world bilateral upper extremity activity. PLOS One. 2014;9(7):e103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cramer SC, Dodakian L, Le V, et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: a randomized clinical trial. JAMA Neurol. 2019;76(9):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


