The synthesis and structures of dinuclear palladium complexes with 1,3-benzimidazolidine-2-thione and 1,3-imidazoline-2-thione are reported.
Keywords: crystal structure, dinuclear palladium complexes, benzimidazolidine- 2-thione complexes, imidazoline-2-thione complexes
Abstract
The synthesis and structures of dinuclear palladium complexes with 1,3-benzimidazolidine-2-thione (bzimtH) and 1,3-imidazoline-2-thione (imtH) are reported, namely, bis(μ-1H-benzimidazole-2-thiolato)-κ2 N 3:S;κ2 S:N 3-bis[cyanido(triphenylphosphine-κP)palladium(II)], [Pd2(C7H5N2S)2(CN)2(C18H15P)2] or [Pd2(μ-N,S-bzimtH)2(CN)2(PPh3)2] (1), and bis(μ-1H-imidazole-2-thiolato)-κ2 N 3:S;κ2 S:N 3-bis[cyanido(triphenylphosphine-κP)palladium(II)] acetonitrile 0.58-solvate, [Pd2(C3H3N2S)2(CN)2(C18H15P)2]·0.58C2H3N or [Pd2(μ-N,S-imtH)2(CN)2(PPh3)2]·0.58C2H3N (2). The compound [Pd2(μ-N,S-bzimtH)2(CN)2(PPh3)2] is located on a crystallographic twofold axis while [Pd2(μ-N,S-imtH)2(CN)2(PPh3)2]. 0.58(C2H3N) contains two partially occupied acetonitrile solvent molecules with occupancies of 0.25 and 0.33. In both of these compounds, the anionic bzimtH− and imtH− ligands coordinate through N,S-donor atoms in a bridging mode, covering four coordination sites of two metal centers and other two sites are occupied by two PPh3 ligand molecules. Finally, the remaining two sites of two metal centers are occupied by cyano groups, abstracted by the metals from the solvent during reaction. In the packing of the 1,3-benzimidazolidine- 2-thione and 1,3-imidazoline-2-thione complexes, there are intramolecular π–π interactions involving the thione moiety as well as an N—H⋯N hydrogen bond linking the thione and cyano ligands. In addition, in 2, as well as the π–π interaction involving the thione moieties, there is an additional π–π interaction involving one of the thione moieties and an adjacent phenyl ring from the triphenylphosphine ligand. There are also C—H⋯N interactions between the imidazoline rings and the acetonitrile N atoms.
1. Chemical context
The coordination chemistry of N,S-donor heterocyclic-2-thione ligands has been in focus for the past four decades, describing synthetic methods, bonding and structures of metal complexes (Raper, 1985 ▸, 1994 ▸, 1996 ▸, 1997 ▸; García-Vázquez et al., 1999 ▸; Akrivos, 2001 ▸), analytical chemistry (Koch, 2001 ▸), charge-transfer complexes (Serpe et al., 2008 ▸) and anion receptors (Bondy & Loeb, 2003 ▸). A recent survey revealed that the reactions of heterocyclic-2-thiones with group 10–12 metals (Ni–Pt, Cu–Au, Zn–Hg; Lobana, 2021 ▸) have led not only to the formation of a variety coordination compounds, but have also displayed other aspects of chemical reactivity. For instance, some reactions of heterocyclic thiones involved copper-mediated activation, and rupture of C—S (thione) bonds followed by their transformations to other forms of thio-ligands, bonded to the copper metal. Further, there has been an upsurge in explorations of the bio-activity and bio-safe potential of coordination compounds, as antimicrobial and anticancer agents (Lobana, 2021 ▸).
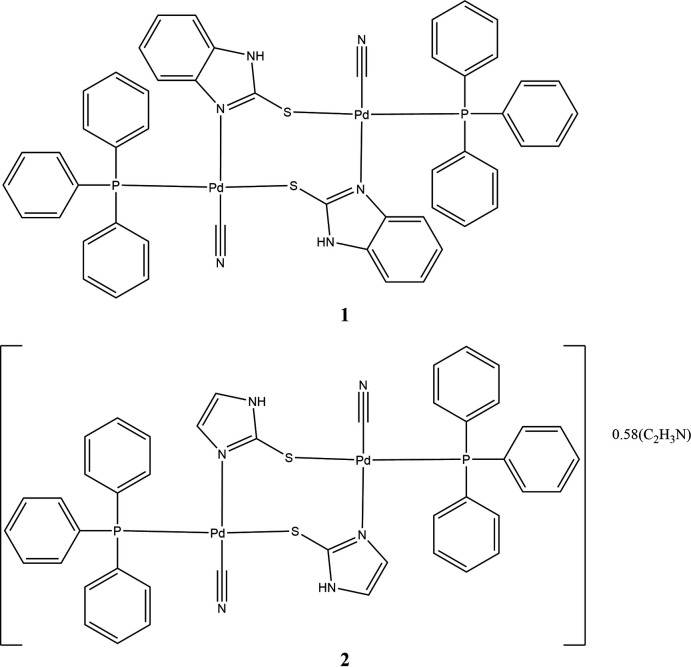
The chemistry of palladium is interesting because of the coordination flexibility and catalytic role of this metal in several reactions (Kostas & Steele, 2020 ▸; Lobana, 2021 ▸). In the literature, pyridine-2-thione (pytH) with palladium(II) has been reported to form dinuclear complexes, namely, [Pd2(μ-N,S-pyt)4] (Umakoshi et al., 1990 ▸), [Pd2(μ-N,S-pyt)(μ-S-pyt)(κ1:S-pyt)2(μ-P,P-dppm)] and [Pd2(μ-κ2:N,S-pyt)3(κ2:P,P-dppm)]Cl (Mendía et al., 2006 ▸), [Pd2Cl2(μ-N,S-pyt)2(PMe3)2] (Yamamoto et al., 1991 ▸), [Pd2Cl2(μ-N,S-pymt)2(PMe3)2] (Yap & Jensen, 1992 ▸). Benz-1,3-imidazoline-2-thioe (bzimtH2) has formed one dimer, [PdII 2(μ-κ2:N,S-bzimt)2(κ1-S-bzimt)(PPh3)3]Cl·2H2O (Lobana et al. 2017 ▸). In this manuscript, some reactions of this metal with a few heterocyclic-2-thione ligands (bzimtH2 and imtH2) are described.
2. Structural commentary
The reaction of PdCl2(PPh3)2 with bzimtH2 in a 1:2 molar ratio in the presence of Et3N base was designed to form [Pd(κ1 S-bzimtH)2(PPh3)2] after removal of both halogens as [Et3NH]+Cl−. However, the X-ray crystal structure of the product revealed the formation of the unexpected dinuclear compound [Pd2(μ-N,S-bzimtH)2(CN)2(PPh3)2] (1). Another thio-ligand, imtH2 yielded a similar dinuclear compound, [Pd2(μ-N,S-imtH)2-(CN)2(PPh3)2] (2). In both these compounds, the anionic bzimtH− and imtH− ligands coordinate through N,S donor atoms in a bridging mode, covering four coordination sites of two metal centers, and other two sites are occupied by two PPh3 ligand molecules. Finally, the remaining two sites of two metal centers are occupied by cyano groups, abstracted by the metals from the solvent during reaction.
Compound 1 crystallizes in the monoclinic space group C2/c, and compound 2 in the monoclinic space group, P21/c. Selected bond distances and bond angles are given in Tables 1 ▸ and 2 ▸, respectively. The molecular structure of compound 1 is shown in Fig. 1 ▸, while that of compound 2 is shown in Fig. 2 ▸ (leaving out the acetonitrile solvent molecules). Considering first the structure of compound 1, here only half of the molecule is unique as the molecule lies on a crystallographic twofold axis. In 1, the Pd metal atom is bonded to one P, one S, one N and one C atoms with the respective bond distances as follows: Pd—P = 2.2861 (6), Pd—S = 2.3547 (6), Pd—N = 2.0545 (17), and Pd—C = 1.959 (2) Å. The trans bond angles, P—Pd—S and N—Pd—C, of 172.26 (2) and 178.31 (8)°, as well as the cis bond angles in the range 84.93 (6)–94.24 (5)°, reveal the distorted square-planar geometry of each metal center. One of the major factors in the conformation adopted by the molecule is the strong π–π interaction between the thione moieties [Cg⋯Cg, 3.1905 (12) Å], as seen in Fig. 1 ▸. In addition, there is also a π–π interaction between the thione moieties and an adjacent phenyl ring from the triphenylphosphine ligand [Cg⋯Cg = 3.3560 (9) Å with a slippage of 1.408 Å].
Table 1. Selected geometric parameters (Å, °) for 1 .
| C1—S1 | 1.728 (2) | N1—Pd1 | 2.0545 (17) |
| N3—C8 | 1.127 (3) | P1—Pd1 | 2.2861 (6) |
| C8—Pd1 | 1.959 (2) | Pd1—S1i | 2.3547 (6) |
| C8—Pd1—N1 | 178.31 (8) | C8—Pd1—S1i | 84.93 (6) |
| C8—Pd1—P1 | 87.53 (6) | N1—Pd1—S1i | 94.24 (5) |
| N1—Pd1—P1 | 93.34 (5) | P1—Pd1—S1i | 172.26 (2) |
Symmetry code: (i)
 .
.
Table 2. Selected geometric parameters (Å, °) for 2 .
| Pd1—C1 | 1.957 (2) | Pd2—P2 | 2.2984 (5) |
| Pd1—N11 | 2.0346 (17) | Pd2—S1 | 2.3542 (5) |
| Pd1—P1 | 2.2914 (5) | S1—C11 | 1.734 (2) |
| Pd1—S2 | 2.3541 (5) | S2—C21 | 1.733 (2) |
| Pd2—C2 | 1.943 (2) | N1—C1 | 1.143 (3) |
| Pd2—N21 | 2.0345 (17) | N2—C2 | 1.148 (3) |
| C1—Pd1—N11 | 179.31 (8) | C2—Pd2—N21 | 178.38 (8) |
| C1—Pd1—P1 | 87.19 (6) | C2—Pd2—P2 | 89.18 (7) |
| N11—Pd1—P1 | 92.80 (4) | N21—Pd2—P2 | 92.44 (5) |
| C1—Pd1—S2 | 87.99 (6) | C2—Pd2—S1 | 86.54 (7) |
| N11—Pd1—S2 | 92.06 (5) | N21—Pd2—S1 | 91.84 (5) |
| P1—Pd1—S2 | 173.855 (19) | P2—Pd2—S1 | 174.489 (19) |
Figure 1.
Diagram of 1 showing the atom labeling for unique atoms (the molecule lies of a twofold axis; symmetry operation to generate the rest of the molecule is −x, y,
 − z) and the strong intramolecular π–π interactions involving both the thione rings and adjacent phenyl rings from the triphenylphosphine ligand. Atomic displacement parameters are at the 30% probability level.
− z) and the strong intramolecular π–π interactions involving both the thione rings and adjacent phenyl rings from the triphenylphosphine ligand. Atomic displacement parameters are at the 30% probability level.
Figure 2.
Diagram of 2 showing the atom labeling and the strong intramolecular π–π interactions involving both the thione rings and adjacent phenyl rings from the triphenylphosphine ligand. Atomic displacement parameters are at the 30% probability level.
The coordination pattern of compound 2 is similar to that of 1. Nevertheless, there are minor differences in the bond distances and angles pertaining to the two metal centers of compound 2 (Fig. 2 ▸). Thus, the respective Pd—P, Pd—S, Pd—N and Pd—C bond distances of 2 are 2.2914 (5), 2.3541 (5), 2.0345 (17) and 1.957 (2) Å (Pd1 metal center), and 2.2984 (5), 2.3542 (5), 2.0345 (17) and 1.943 (2) Å (Pd2 metal center). For both metal centers, the trans bond angles [P—Pd—S and N—Pd—C = 173.86 (2)–179.31 (8)°] and the adjacent bond angles [86.54 (7)– 92.80 (4)°] are similar to those of compound 1. These bond angles again reveal the distorted square-planar geometry of each metal center of compound 2. The various bond distances described above are normal and none unusual. Compound 1 has carbon–sulfur (C—S) bond distance of 1.728 (2), while in compound 2 it is 1.734 (2) Å. These distances are in between single (1.81 Å) and double-bond (1.68 Å) C—S distances (Huheey et al., 1993 ▸). It shows a weakening of the C—S bond as a result of S to Pd coordination. The C≡N distance of the coordinated cyano group is 1.127 (3) in compound 1 and 1.143 (3) /1.148 (3) Å in compound 2. These distances are less than the expected C=N double bond (1.28 Å) and are close to the C≡N triple bond distance (1.15 Å; Huheey et al., 1993 ▸). The structure of 2 contains partially occupied acetonitrile solvent molecules with occupancies of 0.33 and 0.25. As in the case of 1, in 2 one of the major factors in the conformation adopted by the molecule is the strong π–π interaction between the thione moieties [Cg⋯Cg = 3.3559 (12) Å], as seen in Fig. 2 ▸. In addition, there is also a π–π interaction between each of the thione moieties and an adjacent phenyl ring from the triphenylphosphine ligand [Cg⋯Cg distances of 3.3065 (8) Å and 3.3218 (8), respectively, with a slippage for the latter of 1.154 Å].
The IR spectrum of the bzimtH2 ligand showed a ν(N—H) band at 3113 (m), and in compound 1, this band appeared at a lower energy, 3055 (m) cm−1. The ligand showed a diagnostic ν(C=S) band at 1179 cm−1, which shifted to ν(C=S), 1033(s) cm−1, owing to the change of neutral bzimtH2 ligand to the bzimtH− anionic form, coordinating through N,S donor atoms. The PPh3 ligand showed its characteristic ν(P—CPh) band at 1097(s) cm−1 in compound 1. A band at 1734 cm−1 was assigned to the coordinated cyano group. The IR spectroscopic bands of compound 2 are similarly assigned: ν(N—H), 3050 (m), ν(C=S), 1020 (m), ν(P—CPh), 1105 (s) and ν(C≡N), 1740(s) cm−1.
In conclusion, the chemistry of heterocyclic-2-thiones remains enigmatic, probably due to the angular flexibility at sulfur, and also due to the short bite angle of the N,S-donor set in case it chelates with the formation of four-membered rings. This leads to a greater tendency of these thio-ligands in anionic forms to adopt bridging modes, noted as for example in dinuclear complexes (Raper, 1997 ▸; Lobana, 2021 ▸). Benz-1,3-imidazoline-2-thione (bzimtH2) has formed an N,S-bonded symmetrically bridged dinuclear compound, and so is the case with 1,3-imidazolidine-2-thione, and these are analogous to literature reports (Yamamoto et al., 1991 ▸; Yap & Jensen, 1992 ▸).
3. Supramolecular features
In the packing of 1 and 2 there are similar trends in both hydrogen-bond patterns and intramolecular interactions. In both structures, there are strong intramolecular π–π interactions involving the thione moiety and adjacent phenyl rings from the triphenylphosphine ligand as discussed above. Both 1 and 2 have a similar hydrogen-bonding pattern (numerical details in Tables 3 ▸ and 4 ▸), as shown in Figs. 3 ▸ and 4 ▸. In each, the N—H group of the thione moiety forms an intermolecular hydrogen bond with an adjacent N atom from the coordinated cyanide anion and these form
 (7) chains (Etter et al., 1990 ▸) in the [110] and [
(7) chains (Etter et al., 1990 ▸) in the [110] and [
 10] directions. In addition, in 2 there are also C—H⋯N interactions between the imidazoline rings and the partially occupied acetonitrile N atoms and this is shown in Fig. 5 ▸.
10] directions. In addition, in 2 there are also C—H⋯N interactions between the imidazoline rings and the partially occupied acetonitrile N atoms and this is shown in Fig. 5 ▸.
Table 3. Hydrogen-bond geometry (Å, °) for 1 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯N3ii | 0.80 (3) | 2.00 (3) | 2.796 (3) | 177 (3) |
Symmetry code: (ii)
 .
.
Table 4. Hydrogen-bond geometry (Å, °) for 2 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N12—H12A⋯N2i | 0.88 | 1.90 | 2.770 (3) | 169 |
| N22—H22A⋯N1ii | 0.88 | 1.92 | 2.760 (3) | 160 |
| C12—H13A⋯N1S | 0.95 | 2.35 | 3.261 (7) | 161 |
| C22—H23A⋯N1T | 0.95 | 2.29 | 3.081 (12) | 141 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 .
.
Figure 3.
Diagram showing the packing for 1 showing the two intermolecular
 (7) N—H⋯N hydrogen-bonded chains in the [110] and [
(7) N—H⋯N hydrogen-bonded chains in the [110] and [
 10] directions. Hydrogen atoms not involved in hydrogen bonding are omitted for clarity. Symmetry operation to generate the rest of the molecule is −x, y,
10] directions. Hydrogen atoms not involved in hydrogen bonding are omitted for clarity. Symmetry operation to generate the rest of the molecule is −x, y,
 − z.
− z.
Figure 4.
Diagram showing the packing for 2 showing the two intermolecular
 (7) N—H⋯N hydrogen bonding chains in the [110] and [
(7) N—H⋯N hydrogen bonding chains in the [110] and [
 10] directions. Hydrogen atoms not involved in hydrogen bonding are omitted for clarity.
10] directions. Hydrogen atoms not involved in hydrogen bonding are omitted for clarity.
Figure 5.
Diagram for 2 showing the packing viewed along the a-axis direction. N—H⋯N hydrogen bonds and C—H⋯N interactions involving the acetonitrile molecules are shown as dashed lines.
4. Database survey
A search of the Cambridge Structural Database for complexes of palladium with either 1, 3-benzimidazolidine- 2-thione or 1,3-imidazoline-2-thione returned nine hits for the former (BEYRUV and BEYWAG, Sandhu et al., 2018 ▸; PONKOT, PONKUZ, PONLAG, PONLEK and PONLIO, Talismanova et al., 2008 ▸; SANMOK, Talismanova et al., 2004 ▸; SAQPEI, Lobana et al., 2017 ▸) and three hits for the latter (APIYII, Ahmad et al., 2010 ▸; BEYVUZ, Sandhu et al., 2018 ▸; HAWYEJ, Kahn et al., 1993 ▸, SAQPIM, Lobana et al., 2017 ▸).
5. Synthesis and crystallization
The starting materials, namely palladium(II) chloride, triphenylphosphine (PPh3), 1,3-benzimidazoline-2-thione (bzimtH2), 1,3-imidazoline-2-thione (imtH2), and triethylamine were procured from Aldrich. The solvents (acetonitrile, ethanol, methanol and dichloromethane) were of HPLC grade and were stored over molecular sieves. The precursor, PdCl2(PPh3)2, was prepared by a literature procedure (Steffen & Palenik, 1976 ▸). The melting points were determined with a Gallenkamp electrically heated apparatus using the dried samples in capillary tubes. The analysis for carbon, hydrogen and nitrogen were performed by using CHNS-O analyzer Flash- EA-1112 series. The IR spectra of the compounds were recorded on FTIR–SHIMADZU 8400 Fourier transform spectrophotometer in the range of 4000–400 cm−1 using KBr pellets.
Preparation of the precursor, [PdCl2(PPh3)2]
Palladium(II) chloride (0.050 g, 0.282 mmol) was dissolved in hot acetonitrile (25 mL) in a 50 mL round-bottom flask, and to it was added triphenylphosphine (0.148 g, 0.564 mol). The contents were refluxed for 1 h and the yellow complex formed was filtered and dried in vacuo, m.p. 551-553 K
Preparation of 1
To a solution of PdCl2(PPh3)2 (0.030 g, 0.043 mmol) in 10 mL of CH3CN, was added solid bzimtH2 (0.013 g, 0.086 mmol) followed by the addition of Et3N base (0.5 mL). The solution became yellowish orange and was refluxed for 6 h. The orange compound was formed on refluxing. It was separated and dissolved in a solution of methanol (4 mL) and dichloromethane (1 mL) in a culture tube. A slow evaporation of the reaction mixture over a period of one month, resulted in the formation of orange crystals of compound 1. Yield: 0.015 g; 65%; m.p. 511–513 K. Analysis found: C, 57.71; H, 3.84; N, 7.50; C52H40N6P2Pd2S2 (1087.8) requires: C, 57.40; H, 3.70; N, 7.72%. IR Data (KBr, cm−1): ν(N—H), 3055 (m); ν(C–H), 2950 (m), 2920 (s), 2852 (m); ν(C≡N), 1734 (s), ν(C—C) + δ(N—H) + δ(C—H), 1635 (m), 1440 (s), 1380 (m); ν(P—CPh), 1097 (s); ν(C=S), 1033 (s). Ligand IR Data: ν(N—H), 3113 (m), ν(C—H), 3078 (m); 2981 (s); ν(C≡N) 1513 (s), δ(N—H), 1467 (s), 1381 (m); ν(C=S), 1179 (s). The compound is partially soluble in dichloromethane, but soluble in methanol and chloroform.
Preparation of 2
To the solution of PdCl2(PPh3)2 (0.040 g, 0.060 mmol) in 10 mL of CH3CN, was added solid imtH2 (0.012 g, 0.120 mmol) followed by the addition of Et3N base (0.5 mL). The solution became yellowish orange and was refluxed for 6 h. The orange compound was formed on refluxing and was separated. It was dissolved in a solution of methanol (4 mL) and dichloromethane (1 mL) in a culture tube. Slow evaporation of the reaction mixture over a period of one month formed yellowish-orange crystals of compound 2. Yield: 0.020 g; 69%; m.p. 485–488 K. Analysis found: C, 53.21; H, 3.92; N, 8.48; C44H36N6P2Pd2S2·0.58(CH3CN) (1011.5) requires: C, 53.58; H, 3.73; N, 8.36%. IR bands (KBr, cm-1): ν(N—H), 3050 (m); ν(C—H), 3081 (s), 3005 (m), 2968 (m), 2938 (m); ν(C≡N), 1740 (s), d(N—H) + ν(C≡N) + δ(C—H), 1581 (s), 1479 (s), 1401(s); ν(C=S), 1020 (m); ν(P—CPh), 1105 (s); Ligand IR data: ν(N—H), 3130 (s), ν(C—H), 2983 (m); 2876 (s); ν(C≡N) 1586 (s), δ(N—H), 1478 (s), 1266 (m); ν(C=S), 1120 (m). The compound is soluble in methanol, chloroform and partially in dichloromethane.
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. Hydrogen atoms were fixed geometrically (C—H = 0.93–0.98 Å) with their U iso(H) = 1.2U eq(C). The structure of 2 contains partially occupied acetonitrile solvent molecules with occupancies of 0.33 and 0.25.
Table 5. Experimental details.
| 1 | 2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | [Pd2(C7H5N2S)2(CN)2(C18H15P)2] | [Pd2(C3H3N2S)2(CN)2(C18H15P)2]·0.58C2H3N |
| M r | 1087.76 | 1011.46 |
| Crystal system, space group | Monoclinic, C2/c | Monoclinic, P21/c |
| Temperature (K) | 100 | 110 |
| a, b, c (Å) | 13.6026 (12), 13.9719 (12), 25.097 (2) | 12.7916 (2), 14.6718 (3), 25.3760 (4) |
| β (°) | 97.417 (1) | 101.3491 (15) |
| V (Å3) | 4729.8 (7) | 4669.34 (14) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.96 | 0.97 |
| Crystal size (mm) | 0.26 × 0.15 × 0.09 | 0.44 × 0.38 × 0.18 |
| Data collection | ||
| Diffractometer | Bruker SMART APEX CCD | Oxford Diffraction Gemini R (Mo) |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (CrysAlis PRO; Oxford Diffraction, 2009 ▸) |
| T min, T max | 0.811, 0.917 | 0.962, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 23706, 5857, 5308 | 15546, 15546, 10002 |
| R int | 0.024 | 0.031 |
| (sin θ/λ)max (Å−1) | 0.667 | 0.761 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.028, 0.065, 1.12 | 0.035, 0.078, 0.95 |
| No. of reflections | 5857 | 15546 |
| No. of parameters | 294 | 561 |
| No. of restraints | 0 | 39 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.57, −0.89 | 0.98, −1.20 |
Supplementary Material
Crystal structure: contains datablock(s) 1, 2. DOI: 10.1107/S2056989023000166/ex2063sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989023000166/ex20631sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989023000166/ex20632sup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
TSL thanks the Guru Nanak Dev University for an Honorary Professorship. General research facilities for students (BT and RA) at the university are gratefully acknowledged.
supplementary crystallographic information
Bis(µ-1H-benzimidazole-2-thiolato)-κ2N3:S;κ2S:N3-bis[cyanido(triphenylphosphine-κP)palladium(II)] (1) . Crystal data
| [Pd2(C7H5N2S)2(CN)2(C18H15P)2] | F(000) = 2192 |
| Mr = 1087.76 | Dx = 1.528 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 13.6026 (12) Å | Cell parameters from 9977 reflections |
| b = 13.9719 (12) Å | θ = 2.2–31.8° |
| c = 25.097 (2) Å | µ = 0.96 mm−1 |
| β = 97.417 (1)° | T = 100 K |
| V = 4729.8 (7) Å3 | Block, orange |
| Z = 4 | 0.26 × 0.15 × 0.09 mm |
Bis(µ-1H-benzimidazole-2-thiolato)-κ2N3:S;κ2S:N3-bis[cyanido(triphenylphosphine-κP)palladium(II)] (1) . Data collection
| Bruker SMART APEX CCD diffractometer | 5857 independent reflections |
| Radiation source: fine-focus sealed tube | 5308 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.024 |
| ω scans | θmax = 28.3°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | h = −18→18 |
| Tmin = 0.811, Tmax = 0.917 | k = −18→18 |
| 23706 measured reflections | l = −33→33 |
Bis(µ-1H-benzimidazole-2-thiolato)-κ2N3:S;κ2S:N3-bis[cyanido(triphenylphosphine-κP)palladium(II)] (1) . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.028 | Hydrogen site location: mixed |
| wR(F2) = 0.065 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.12 | w = 1/[σ2(Fo2) + (0.0241P)2 + 9.9394P] where P = (Fo2 + 2Fc2)/3 |
| 5857 reflections | (Δ/σ)max < 0.001 |
| 294 parameters | Δρmax = 0.57 e Å−3 |
| 0 restraints | Δρmin = −0.89 e Å−3 |
Bis(µ-1H-benzimidazole-2-thiolato)-κ2N3:S;κ2S:N3-bis[cyanido(triphenylphosphine-κP)palladium(II)] (1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Bis(µ-1H-benzimidazole-2-thiolato)-κ2N3:S;κ2S:N3-bis[cyanido(triphenylphosphine-κP)palladium(II)] (1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | −0.07279 (15) | 0.26650 (15) | 0.30035 (8) | 0.0167 (4) | |
| C2 | −0.02067 (16) | 0.41441 (16) | 0.31826 (8) | 0.0204 (4) | |
| N3 | 0.20989 (15) | −0.05673 (15) | 0.29010 (8) | 0.0296 (4) | |
| C3 | −0.00951 (19) | 0.51282 (17) | 0.32406 (10) | 0.0285 (5) | |
| H3 | −0.065219 | 0.554027 | 0.323359 | 0.034* | |
| C4 | 0.0864 (2) | 0.54824 (18) | 0.33093 (10) | 0.0333 (6) | |
| H4 | 0.096907 | 0.615208 | 0.334994 | 0.040* | |
| C5 | 0.16821 (19) | 0.48698 (18) | 0.33198 (9) | 0.0290 (5) | |
| H5 | 0.233000 | 0.513645 | 0.336220 | 0.035* | |
| C6 | 0.15735 (17) | 0.38872 (16) | 0.32701 (8) | 0.0223 (4) | |
| H6 | 0.213217 | 0.347564 | 0.328414 | 0.027* | |
| C7 | 0.06114 (16) | 0.35283 (15) | 0.31987 (8) | 0.0184 (4) | |
| C8 | 0.17115 (16) | 0.01314 (17) | 0.29575 (9) | 0.0224 (4) | |
| C9 | −0.02560 (16) | 0.14880 (15) | 0.41389 (8) | 0.0192 (4) | |
| C10 | −0.12021 (17) | 0.11322 (17) | 0.41677 (9) | 0.0245 (5) | |
| H10 | −0.135247 | 0.048473 | 0.407466 | 0.029* | |
| C11 | −0.19289 (18) | 0.1723 (2) | 0.43325 (10) | 0.0319 (5) | |
| H11 | −0.257352 | 0.147566 | 0.435347 | 0.038* | |
| C12 | −0.1718 (2) | 0.26638 (19) | 0.44654 (10) | 0.0323 (6) | |
| H12 | −0.222246 | 0.307008 | 0.456694 | 0.039* | |
| C13 | −0.0770 (2) | 0.30173 (18) | 0.44508 (10) | 0.0303 (5) | |
| H13 | −0.061902 | 0.366039 | 0.455393 | 0.036* | |
| C14 | −0.00422 (18) | 0.24371 (17) | 0.42866 (9) | 0.0244 (5) | |
| H14 | 0.060499 | 0.268435 | 0.427421 | 0.029* | |
| C15 | 0.17398 (16) | 0.07885 (16) | 0.43693 (9) | 0.0221 (4) | |
| C16 | 0.26896 (17) | 0.06387 (18) | 0.42373 (10) | 0.0289 (5) | |
| H16 | 0.279017 | 0.056685 | 0.387184 | 0.035* | |
| C17 | 0.34938 (19) | 0.05938 (19) | 0.46410 (12) | 0.0361 (6) | |
| H17 | 0.414124 | 0.048636 | 0.454978 | 0.043* | |
| C18 | 0.3355 (2) | 0.07046 (19) | 0.51719 (11) | 0.0373 (6) | |
| H18 | 0.390378 | 0.065693 | 0.544619 | 0.045* | |
| C19 | 0.2424 (2) | 0.0884 (2) | 0.53042 (10) | 0.0374 (6) | |
| H19 | 0.233304 | 0.098140 | 0.566924 | 0.045* | |
| C20 | 0.16164 (19) | 0.0923 (2) | 0.49069 (9) | 0.0318 (5) | |
| H20 | 0.097369 | 0.104236 | 0.500179 | 0.038* | |
| C21 | 0.02075 (16) | −0.04251 (15) | 0.38040 (9) | 0.0205 (4) | |
| C22 | 0.04218 (19) | −0.10869 (17) | 0.42181 (9) | 0.0274 (5) | |
| H22 | 0.083356 | −0.091135 | 0.453803 | 0.033* | |
| C23 | 0.0030 (2) | −0.20054 (18) | 0.41604 (11) | 0.0367 (6) | |
| H23 | 0.017564 | −0.245817 | 0.444194 | 0.044* | |
| C24 | −0.0571 (2) | −0.22642 (18) | 0.36955 (11) | 0.0351 (6) | |
| H24 | −0.084438 | −0.289039 | 0.366194 | 0.042* | |
| C25 | −0.07770 (18) | −0.16150 (18) | 0.32787 (10) | 0.0293 (5) | |
| H25 | −0.118226 | −0.179735 | 0.295793 | 0.035* | |
| C26 | −0.03879 (16) | −0.06974 (16) | 0.33325 (9) | 0.0239 (5) | |
| H26 | −0.052690 | −0.025117 | 0.304710 | 0.029* | |
| N1 | 0.02621 (12) | 0.26021 (12) | 0.30959 (7) | 0.0160 (3) | |
| N2 | −0.10313 (14) | 0.35712 (13) | 0.30663 (7) | 0.0200 (4) | |
| H2 | −0.157 (2) | 0.3796 (18) | 0.3021 (11) | 0.024* | |
| P1 | 0.06743 (4) | 0.07947 (4) | 0.38495 (2) | 0.01712 (11) | |
| Pd1 | 0.10174 (2) | 0.13416 (2) | 0.30350 (2) | 0.01536 (5) | |
| S1 | −0.15249 (4) | 0.17333 (4) | 0.28003 (2) | 0.01886 (11) |
Bis(µ-1H-benzimidazole-2-thiolato)-κ2N3:S;κ2S:N3-bis[cyanido(triphenylphosphine-κP)palladium(II)] (1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0176 (10) | 0.0189 (10) | 0.0133 (9) | 0.0014 (8) | 0.0016 (7) | −0.0006 (7) |
| C2 | 0.0239 (11) | 0.0209 (11) | 0.0158 (10) | −0.0007 (8) | 0.0009 (8) | −0.0011 (8) |
| N3 | 0.0284 (10) | 0.0308 (11) | 0.0294 (11) | 0.0092 (9) | 0.0022 (8) | 0.0032 (9) |
| C3 | 0.0346 (13) | 0.0214 (11) | 0.0289 (12) | 0.0029 (10) | 0.0026 (10) | −0.0027 (9) |
| C4 | 0.0457 (15) | 0.0225 (12) | 0.0308 (13) | −0.0079 (11) | 0.0011 (11) | −0.0064 (10) |
| C5 | 0.0305 (13) | 0.0318 (13) | 0.0236 (11) | −0.0118 (10) | −0.0005 (9) | −0.0013 (10) |
| C6 | 0.0215 (10) | 0.0272 (11) | 0.0175 (10) | −0.0029 (9) | −0.0006 (8) | −0.0007 (8) |
| C7 | 0.0213 (10) | 0.0207 (10) | 0.0128 (9) | −0.0013 (8) | 0.0011 (7) | −0.0001 (7) |
| C8 | 0.0187 (10) | 0.0307 (12) | 0.0179 (10) | 0.0036 (9) | 0.0026 (8) | 0.0011 (9) |
| C9 | 0.0214 (10) | 0.0225 (11) | 0.0140 (9) | 0.0036 (8) | 0.0032 (8) | 0.0004 (8) |
| C10 | 0.0246 (11) | 0.0285 (12) | 0.0208 (10) | −0.0005 (9) | 0.0053 (8) | −0.0025 (9) |
| C11 | 0.0218 (11) | 0.0448 (15) | 0.0302 (13) | 0.0023 (11) | 0.0076 (9) | −0.0033 (11) |
| C12 | 0.0343 (14) | 0.0378 (14) | 0.0264 (12) | 0.0136 (11) | 0.0103 (10) | −0.0011 (10) |
| C13 | 0.0422 (14) | 0.0262 (12) | 0.0246 (12) | 0.0047 (11) | 0.0117 (10) | −0.0031 (9) |
| C14 | 0.0277 (12) | 0.0257 (11) | 0.0208 (11) | −0.0007 (9) | 0.0074 (9) | −0.0006 (9) |
| C15 | 0.0217 (10) | 0.0221 (11) | 0.0213 (11) | −0.0006 (8) | −0.0016 (8) | 0.0029 (8) |
| C16 | 0.0231 (11) | 0.0321 (13) | 0.0304 (12) | 0.0034 (10) | −0.0012 (9) | −0.0043 (10) |
| C17 | 0.0240 (12) | 0.0332 (14) | 0.0480 (16) | 0.0037 (10) | −0.0075 (11) | −0.0064 (12) |
| C18 | 0.0366 (14) | 0.0310 (13) | 0.0385 (15) | −0.0026 (11) | −0.0174 (11) | 0.0020 (11) |
| C19 | 0.0425 (15) | 0.0458 (16) | 0.0214 (12) | −0.0073 (13) | −0.0058 (11) | 0.0062 (11) |
| C20 | 0.0277 (12) | 0.0449 (15) | 0.0217 (11) | −0.0019 (11) | −0.0003 (9) | 0.0048 (11) |
| C21 | 0.0207 (10) | 0.0186 (10) | 0.0231 (11) | 0.0032 (8) | 0.0062 (8) | 0.0013 (8) |
| C22 | 0.0370 (13) | 0.0237 (11) | 0.0220 (11) | 0.0045 (10) | 0.0062 (10) | 0.0021 (9) |
| C23 | 0.0587 (18) | 0.0229 (12) | 0.0297 (13) | 0.0036 (12) | 0.0106 (12) | 0.0055 (10) |
| C24 | 0.0473 (16) | 0.0206 (12) | 0.0399 (15) | −0.0035 (11) | 0.0154 (12) | −0.0047 (10) |
| C25 | 0.0275 (12) | 0.0268 (12) | 0.0338 (13) | −0.0018 (10) | 0.0049 (10) | −0.0062 (10) |
| C26 | 0.0230 (11) | 0.0229 (11) | 0.0252 (11) | 0.0015 (9) | 0.0009 (9) | 0.0001 (9) |
| N1 | 0.0154 (8) | 0.0176 (8) | 0.0150 (8) | −0.0001 (6) | 0.0018 (6) | −0.0001 (6) |
| N2 | 0.0181 (9) | 0.0213 (9) | 0.0204 (9) | 0.0049 (7) | 0.0020 (7) | −0.0011 (7) |
| P1 | 0.0167 (2) | 0.0192 (3) | 0.0156 (2) | 0.0018 (2) | 0.00211 (19) | 0.00103 (19) |
| Pd1 | 0.01371 (8) | 0.01746 (8) | 0.01506 (8) | 0.00304 (6) | 0.00240 (5) | 0.00079 (6) |
| S1 | 0.0167 (2) | 0.0223 (3) | 0.0181 (2) | −0.00317 (19) | 0.00424 (18) | −0.00229 (19) |
Bis(µ-1H-benzimidazole-2-thiolato)-κ2N3:S;κ2S:N3-bis[cyanido(triphenylphosphine-κP)palladium(II)] (1) . Geometric parameters (Å, º)
| C1—N1 | 1.339 (3) | C15—C16 | 1.390 (3) |
| C1—N2 | 1.347 (3) | C15—C20 | 1.394 (3) |
| C1—S1 | 1.728 (2) | C15—P1 | 1.820 (2) |
| C2—N2 | 1.378 (3) | C16—C17 | 1.393 (3) |
| C2—C3 | 1.389 (3) | C16—H16 | 0.9500 |
| C2—C7 | 1.403 (3) | C17—C18 | 1.378 (4) |
| N3—C8 | 1.127 (3) | C17—H17 | 0.9500 |
| C3—C4 | 1.385 (4) | C18—C19 | 1.374 (4) |
| C3—H3 | 0.9500 | C18—H18 | 0.9500 |
| C4—C5 | 1.401 (4) | C19—C20 | 1.386 (3) |
| C4—H4 | 0.9500 | C19—H19 | 0.9500 |
| C5—C6 | 1.385 (3) | C20—H20 | 0.9500 |
| C5—H5 | 0.9500 | C21—C22 | 1.394 (3) |
| C6—C7 | 1.391 (3) | C21—C26 | 1.398 (3) |
| C6—H6 | 0.9500 | C21—P1 | 1.817 (2) |
| C7—N1 | 1.391 (3) | C22—C23 | 1.390 (4) |
| C8—Pd1 | 1.959 (2) | C22—H22 | 0.9500 |
| C9—C10 | 1.390 (3) | C23—C24 | 1.384 (4) |
| C9—C14 | 1.397 (3) | C23—H23 | 0.9500 |
| C9—P1 | 1.818 (2) | C24—C25 | 1.386 (4) |
| C10—C11 | 1.391 (3) | C24—H24 | 0.9500 |
| C10—H10 | 0.9500 | C25—C26 | 1.387 (3) |
| C11—C12 | 1.378 (4) | C25—H25 | 0.9500 |
| C11—H11 | 0.9500 | C26—H26 | 0.9500 |
| C12—C13 | 1.387 (4) | N1—Pd1 | 2.0545 (17) |
| C12—H12 | 0.9500 | N2—H2 | 0.80 (3) |
| C13—C14 | 1.382 (3) | P1—Pd1 | 2.2861 (6) |
| C13—H13 | 0.9500 | Pd1—S1i | 2.3547 (6) |
| C14—H14 | 0.9500 | ||
| N1—C1—N2 | 110.96 (18) | C16—C17—H17 | 119.8 |
| N1—C1—S1 | 125.44 (16) | C19—C18—C17 | 120.0 (2) |
| N2—C1—S1 | 123.53 (16) | C19—C18—H18 | 120.0 |
| N2—C2—C3 | 132.2 (2) | C17—C18—H18 | 120.0 |
| N2—C2—C7 | 105.70 (19) | C18—C19—C20 | 120.2 (3) |
| C3—C2—C7 | 121.9 (2) | C18—C19—H19 | 119.9 |
| C4—C3—C2 | 117.1 (2) | C20—C19—H19 | 119.9 |
| C4—C3—H3 | 121.5 | C19—C20—C15 | 120.5 (2) |
| C2—C3—H3 | 121.5 | C19—C20—H20 | 119.7 |
| C3—C4—C5 | 121.1 (2) | C15—C20—H20 | 119.7 |
| C3—C4—H4 | 119.4 | C22—C21—C26 | 119.6 (2) |
| C5—C4—H4 | 119.4 | C22—C21—P1 | 122.52 (18) |
| C6—C5—C4 | 121.9 (2) | C26—C21—P1 | 117.92 (16) |
| C6—C5—H5 | 119.0 | C23—C22—C21 | 119.6 (2) |
| C4—C5—H5 | 119.0 | C23—C22—H22 | 120.2 |
| C5—C6—C7 | 117.2 (2) | C21—C22—H22 | 120.2 |
| C5—C6—H6 | 121.4 | C24—C23—C22 | 120.4 (2) |
| C7—C6—H6 | 121.4 | C24—C23—H23 | 119.8 |
| N1—C7—C6 | 130.7 (2) | C22—C23—H23 | 119.8 |
| N1—C7—C2 | 108.33 (18) | C23—C24—C25 | 120.4 (2) |
| C6—C7—C2 | 120.8 (2) | C23—C24—H24 | 119.8 |
| N3—C8—Pd1 | 178.4 (2) | C25—C24—H24 | 119.8 |
| C10—C9—C14 | 119.2 (2) | C24—C25—C26 | 119.6 (2) |
| C10—C9—P1 | 121.81 (17) | C24—C25—H25 | 120.2 |
| C14—C9—P1 | 118.68 (17) | C26—C25—H25 | 120.2 |
| C9—C10—C11 | 120.1 (2) | C25—C26—C21 | 120.4 (2) |
| C9—C10—H10 | 119.9 | C25—C26—H26 | 119.8 |
| C11—C10—H10 | 119.9 | C21—C26—H26 | 119.8 |
| C12—C11—C10 | 120.3 (2) | C1—N1—C7 | 106.42 (17) |
| C12—C11—H11 | 119.8 | C1—N1—Pd1 | 123.00 (14) |
| C10—C11—H11 | 119.8 | C7—N1—Pd1 | 130.47 (14) |
| C11—C12—C13 | 119.9 (2) | C1—N2—C2 | 108.50 (18) |
| C11—C12—H12 | 120.0 | C1—N2—H2 | 130.2 (19) |
| C13—C12—H12 | 120.0 | C2—N2—H2 | 121.1 (19) |
| C14—C13—C12 | 120.2 (2) | C21—P1—C9 | 105.62 (10) |
| C14—C13—H13 | 119.9 | C21—P1—C15 | 106.27 (10) |
| C12—C13—H13 | 119.9 | C9—P1—C15 | 104.31 (10) |
| C13—C14—C9 | 120.2 (2) | C21—P1—Pd1 | 111.54 (7) |
| C13—C14—H14 | 119.9 | C9—P1—Pd1 | 114.34 (7) |
| C9—C14—H14 | 119.9 | C15—P1—Pd1 | 114.00 (7) |
| C16—C15—C20 | 118.9 (2) | C8—Pd1—N1 | 178.31 (8) |
| C16—C15—P1 | 120.56 (17) | C8—Pd1—P1 | 87.53 (6) |
| C20—C15—P1 | 120.58 (18) | N1—Pd1—P1 | 93.34 (5) |
| C15—C16—C17 | 120.0 (2) | C8—Pd1—S1i | 84.93 (6) |
| C15—C16—H16 | 120.0 | N1—Pd1—S1i | 94.24 (5) |
| C17—C16—H16 | 120.0 | P1—Pd1—S1i | 172.26 (2) |
| C18—C17—C16 | 120.4 (3) | C1—S1—Pd1i | 101.12 (7) |
| C18—C17—H17 | 119.8 | ||
| N2—C2—C3—C4 | −174.0 (2) | P1—C21—C26—C25 | −179.03 (18) |
| C7—C2—C3—C4 | 0.8 (3) | N2—C1—N1—C7 | −2.9 (2) |
| C2—C3—C4—C5 | −0.1 (4) | S1—C1—N1—C7 | 174.31 (15) |
| C3—C4—C5—C6 | −0.9 (4) | N2—C1—N1—Pd1 | −179.43 (13) |
| C4—C5—C6—C7 | 1.2 (3) | S1—C1—N1—Pd1 | −2.2 (2) |
| C5—C6—C7—N1 | 174.3 (2) | C6—C7—N1—C1 | −173.3 (2) |
| C5—C6—C7—C2 | −0.5 (3) | C2—C7—N1—C1 | 2.0 (2) |
| N2—C2—C7—N1 | −0.4 (2) | C6—C7—N1—Pd1 | 2.9 (3) |
| C3—C2—C7—N1 | −176.4 (2) | C2—C7—N1—Pd1 | 178.18 (14) |
| N2—C2—C7—C6 | 175.44 (19) | N1—C1—N2—C2 | 2.7 (2) |
| C3—C2—C7—C6 | −0.5 (3) | S1—C1—N2—C2 | −174.57 (15) |
| C14—C9—C10—C11 | 1.2 (3) | C3—C2—N2—C1 | 174.0 (2) |
| P1—C9—C10—C11 | −172.37 (18) | C7—C2—N2—C1 | −1.3 (2) |
| C9—C10—C11—C12 | 0.3 (4) | C22—C21—P1—C9 | −90.0 (2) |
| C10—C11—C12—C13 | −2.0 (4) | C26—C21—P1—C9 | 90.01 (18) |
| C11—C12—C13—C14 | 2.1 (4) | C22—C21—P1—C15 | 20.4 (2) |
| C12—C13—C14—C9 | −0.6 (4) | C26—C21—P1—C15 | −159.57 (17) |
| C10—C9—C14—C13 | −1.0 (3) | C22—C21—P1—Pd1 | 145.20 (17) |
| P1—C9—C14—C13 | 172.69 (18) | C26—C21—P1—Pd1 | −34.77 (19) |
| C20—C15—C16—C17 | 2.1 (4) | C10—C9—P1—C21 | −14.5 (2) |
| P1—C15—C16—C17 | −177.2 (2) | C14—C9—P1—C21 | 171.94 (17) |
| C15—C16—C17—C18 | −0.5 (4) | C10—C9—P1—C15 | −126.31 (19) |
| C16—C17—C18—C19 | −1.6 (4) | C14—C9—P1—C15 | 60.13 (19) |
| C17—C18—C19—C20 | 2.1 (4) | C10—C9—P1—Pd1 | 108.52 (18) |
| C18—C19—C20—C15 | −0.5 (4) | C14—C9—P1—Pd1 | −65.04 (18) |
| C16—C15—C20—C19 | −1.6 (4) | C16—C15—P1—C21 | 92.0 (2) |
| P1—C15—C20—C19 | 177.7 (2) | C20—C15—P1—C21 | −87.2 (2) |
| C26—C21—C22—C23 | −0.9 (3) | C16—C15—P1—C9 | −156.63 (19) |
| P1—C21—C22—C23 | 179.13 (19) | C20—C15—P1—C9 | 24.1 (2) |
| C21—C22—C23—C24 | −0.1 (4) | C16—C15—P1—Pd1 | −31.2 (2) |
| C22—C23—C24—C25 | 1.0 (4) | C20—C15—P1—Pd1 | 149.50 (18) |
| C23—C24—C25—C26 | −0.9 (4) | N1—C1—S1—Pd1i | −61.89 (18) |
| C24—C25—C26—C21 | −0.1 (4) | N2—C1—S1—Pd1i | 114.98 (17) |
| C22—C21—C26—C25 | 1.0 (3) |
Symmetry code: (i) −x, y, −z+1/2.
Bis(µ-1H-benzimidazole-2-thiolato)-κ2N3:S;κ2S:N3-bis[cyanido(triphenylphosphine-κP)palladium(II)] (1) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···N3ii | 0.80 (3) | 2.00 (3) | 2.796 (3) | 177 (3) |
Symmetry code: (ii) x−1/2, y+1/2, z.
\ Bis(µ-1H-imidazole-2-thiolato)-κ2N3:S;\ κ2S:N3-bis[cyanido(triphenylphosphine-κP)\ palladium(II)] acetonitrile 0.58-solvate (2) . Crystal data
| [Pd2(C3H3N2S)2(CN)2(C18H15P)2]·0.58C2H3N | F(000) = 2035 |
| Mr = 1011.46 | Dx = 1.439 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.7916 (2) Å | Cell parameters from 15979 reflections |
| b = 14.6718 (3) Å | θ = 4.7–32.7° |
| c = 25.3760 (4) Å | µ = 0.97 mm−1 |
| β = 101.3491 (15)° | T = 110 K |
| V = 4669.34 (14) Å3 | Plate, yellow |
| Z = 4 | 0.44 × 0.38 × 0.18 mm |
\ Bis(µ-1H-imidazole-2-thiolato)-κ2N3:S;\ κ2S:N3-bis[cyanido(triphenylphosphine-κP)\ palladium(II)] acetonitrile 0.58-solvate (2) . Data collection
| Oxford Diffraction Gemini R (Mo) diffractometer | 15546 independent reflections |
| Graphite monochromator | 10002 reflections with I > 2σ(I) |
| Detector resolution: 10.5081 pixels mm-1 | Rint = 0.031 |
| ω scans | θmax = 32.8°, θmin = 4.7° |
| Absorption correction: multi-scan (CrysAlisPro; Oxford Diffraction, 2009) | h = −18→18 |
| Tmin = 0.962, Tmax = 1.000 | k = −17→22 |
| 15546 measured reflections | l = −34→36 |
\ Bis(µ-1H-imidazole-2-thiolato)-κ2N3:S;\ κ2S:N3-bis[cyanido(triphenylphosphine-κP)\ palladium(II)] acetonitrile 0.58-solvate (2) . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.035 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.078 | H-atom parameters constrained |
| S = 0.95 | w = 1/[σ2(Fo2) + (0.0359P)2] where P = (Fo2 + 2Fc2)/3 |
| 15546 reflections | (Δ/σ)max = 0.002 |
| 561 parameters | Δρmax = 0.98 e Å−3 |
| 39 restraints | Δρmin = −1.20 e Å−3 |
\ Bis(µ-1H-imidazole-2-thiolato)-κ2N3:S;\ κ2S:N3-bis[cyanido(triphenylphosphine-κP)\ palladium(II)] acetonitrile 0.58-solvate (2) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
\ Bis(µ-1H-imidazole-2-thiolato)-κ2N3:S;\ κ2S:N3-bis[cyanido(triphenylphosphine-κP)\ palladium(II)] acetonitrile 0.58-solvate (2) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Pd1 | 0.35095 (2) | 0.87099 (2) | 0.30663 (2) | 0.02356 (4) | |
| Pd2 | 0.14320 (2) | 0.88485 (2) | 0.19323 (2) | 0.02559 (4) | |
| S1 | 0.08912 (4) | 0.94151 (4) | 0.27044 (2) | 0.03170 (12) | |
| S2 | 0.41813 (4) | 0.90816 (4) | 0.22938 (2) | 0.03362 (12) | |
| P1 | 0.30170 (4) | 0.82559 (4) | 0.38464 (2) | 0.02500 (11) | |
| P2 | 0.17961 (4) | 0.82426 (4) | 0.11528 (2) | 0.03002 (12) | |
| N1 | 0.43502 (18) | 0.67452 (15) | 0.29819 (7) | 0.0548 (6) | |
| N2 | 0.0059 (2) | 0.71602 (18) | 0.20621 (8) | 0.0727 (8) | |
| N11 | 0.29215 (13) | 0.99892 (11) | 0.31143 (6) | 0.0272 (4) | |
| N12 | 0.17481 (16) | 1.10817 (13) | 0.30467 (7) | 0.0410 (5) | |
| H12A | 0.113398 | 1.137168 | 0.298069 | 0.049* | |
| N21 | 0.23236 (13) | 0.99792 (12) | 0.18750 (6) | 0.0284 (4) | |
| N22 | 0.37399 (16) | 1.08292 (14) | 0.19348 (6) | 0.0416 (5) | |
| H22A | 0.441192 | 1.100160 | 0.199723 | 0.050* | |
| C1 | 0.40581 (17) | 0.74749 (16) | 0.30153 (7) | 0.0363 (5) | |
| C2 | 0.05709 (19) | 0.77821 (17) | 0.20056 (7) | 0.0412 (6) | |
| C11 | 0.18880 (16) | 1.01847 (15) | 0.29650 (7) | 0.0291 (5) | |
| C12 | 0.2718 (2) | 1.14654 (17) | 0.32489 (8) | 0.0480 (7) | |
| H13A | 0.285290 | 1.208633 | 0.334479 | 0.058* | |
| C13 | 0.3447 (2) | 1.07915 (16) | 0.32859 (8) | 0.0385 (5) | |
| H14A | 0.419358 | 1.085725 | 0.340892 | 0.046* | |
| C21 | 0.33859 (16) | 0.99883 (15) | 0.20226 (7) | 0.0311 (5) | |
| C22 | 0.2886 (2) | 1.13701 (17) | 0.17333 (9) | 0.0484 (7) | |
| H23A | 0.290465 | 1.199438 | 0.163621 | 0.058* | |
| C23 | 0.20113 (19) | 1.08439 (16) | 0.16993 (8) | 0.0381 (5) | |
| H24A | 0.129679 | 1.103756 | 0.157463 | 0.046* | |
| C1A | 0.24085 (17) | 0.71348 (15) | 0.37962 (7) | 0.0324 (5) | |
| C2A | 0.16469 (19) | 0.69425 (17) | 0.33331 (8) | 0.0424 (6) | |
| H2AA | 0.148149 | 0.738927 | 0.305829 | 0.051* | |
| C3A | 0.1134 (2) | 0.61103 (19) | 0.32715 (9) | 0.0575 (8) | |
| H3AA | 0.060788 | 0.599083 | 0.295887 | 0.069* | |
| C4A | 0.1386 (3) | 0.54504 (19) | 0.36657 (10) | 0.0654 (9) | |
| H4AA | 0.102770 | 0.487976 | 0.362623 | 0.079* | |
| C5A | 0.2166 (2) | 0.56257 (18) | 0.41211 (9) | 0.0563 (8) | |
| H5AA | 0.235385 | 0.516640 | 0.438678 | 0.068* | |
| C6A | 0.26698 (19) | 0.64645 (16) | 0.41884 (8) | 0.0383 (5) | |
| H6AA | 0.319336 | 0.658288 | 0.450210 | 0.046* | |
| C1B | 0.41603 (16) | 0.82116 (14) | 0.44038 (7) | 0.0295 (4) | |
| C2B | 0.51745 (18) | 0.80590 (18) | 0.43012 (8) | 0.0442 (6) | |
| H2BA | 0.527274 | 0.798236 | 0.394240 | 0.053* | |
| C3B | 0.6042 (2) | 0.8019 (2) | 0.47257 (9) | 0.0557 (8) | |
| H3BA | 0.673349 | 0.790636 | 0.465621 | 0.067* | |
| C4B | 0.5911 (2) | 0.81419 (18) | 0.52455 (9) | 0.0457 (6) | |
| H4BA | 0.651230 | 0.811916 | 0.553268 | 0.055* | |
| C5B | 0.4920 (2) | 0.82962 (18) | 0.53501 (8) | 0.0454 (6) | |
| H5BA | 0.483094 | 0.838279 | 0.570956 | 0.054* | |
| C6B | 0.40439 (18) | 0.83263 (17) | 0.49303 (7) | 0.0398 (6) | |
| H6BA | 0.335423 | 0.842714 | 0.500531 | 0.048* | |
| C1C | 0.20732 (16) | 0.89953 (15) | 0.40931 (7) | 0.0286 (4) | |
| C2C | 0.23993 (18) | 0.98632 (16) | 0.42808 (8) | 0.0363 (5) | |
| H2CA | 0.311953 | 1.004513 | 0.430333 | 0.044* | |
| C3C | 0.1672 (2) | 1.04648 (18) | 0.44355 (8) | 0.0457 (6) | |
| H3CA | 0.189705 | 1.105585 | 0.456258 | 0.055* | |
| C4C | 0.0636 (2) | 1.0207 (2) | 0.44049 (9) | 0.0533 (7) | |
| H4CA | 0.013952 | 1.062039 | 0.450704 | 0.064* | |
| C5C | 0.0311 (2) | 0.9349 (2) | 0.42266 (11) | 0.0607 (8) | |
| H5CA | −0.040582 | 0.916511 | 0.421319 | 0.073* | |
| C6C | 0.10260 (19) | 0.87539 (18) | 0.40664 (9) | 0.0446 (6) | |
| H6CA | 0.078935 | 0.816741 | 0.393571 | 0.054* | |
| C1D | 0.28204 (18) | 0.88581 (15) | 0.08915 (7) | 0.0335 (5) | |
| C2D | 0.25910 (19) | 0.97236 (17) | 0.06660 (8) | 0.0403 (6) | |
| H2DA | 0.188431 | 0.995509 | 0.061180 | 0.048* | |
| C3D | 0.3395 (2) | 1.02420 (19) | 0.05223 (8) | 0.0494 (7) | |
| H3DA | 0.323656 | 1.083075 | 0.037180 | 0.059* | |
| C4D | 0.4415 (2) | 0.9915 (2) | 0.05946 (9) | 0.0533 (7) | |
| H4DA | 0.496407 | 1.027784 | 0.049892 | 0.064* | |
| C5D | 0.4644 (2) | 0.9055 (2) | 0.08075 (11) | 0.0566 (7) | |
| H5DA | 0.534715 | 0.881930 | 0.084976 | 0.068* | |
| C6D | 0.3844 (2) | 0.85306 (18) | 0.09605 (9) | 0.0453 (6) | |
| H6DA | 0.400780 | 0.794418 | 0.111328 | 0.054* | |
| C1E | 0.06406 (17) | 0.82453 (15) | 0.05978 (7) | 0.0334 (5) | |
| C2E | 0.07759 (19) | 0.82814 (18) | 0.00681 (8) | 0.0446 (6) | |
| H2EA | 0.147239 | 0.832430 | −0.000792 | 0.054* | |
| C3E | −0.0108 (2) | 0.82547 (19) | −0.03519 (8) | 0.0497 (7) | |
| H3EA | −0.001292 | 0.828234 | −0.071351 | 0.060* | |
| C4E | −0.1110 (2) | 0.81890 (18) | −0.02460 (8) | 0.0459 (6) | |
| H4EA | −0.170923 | 0.816583 | −0.053380 | 0.055* | |
| C5E | −0.12546 (19) | 0.81559 (18) | 0.02794 (8) | 0.0446 (6) | |
| H5EA | −0.195388 | 0.811418 | 0.035189 | 0.054* | |
| C6E | −0.03805 (18) | 0.81836 (16) | 0.07012 (8) | 0.0375 (5) | |
| H6EA | −0.048286 | 0.816009 | 0.106170 | 0.045* | |
| C1F | 0.22604 (18) | 0.70707 (15) | 0.12111 (7) | 0.0351 (5) | |
| C2F | 0.20602 (18) | 0.64505 (16) | 0.07863 (8) | 0.0379 (5) | |
| H2FA | 0.162463 | 0.662691 | 0.045431 | 0.045* | |
| C3F | 0.2487 (2) | 0.55839 (18) | 0.08427 (9) | 0.0477 (6) | |
| H3FA | 0.233104 | 0.516470 | 0.055249 | 0.057* | |
| C4F | 0.3142 (2) | 0.53223 (18) | 0.13203 (10) | 0.0546 (7) | |
| H4FA | 0.345070 | 0.473104 | 0.135597 | 0.066* | |
| C5F | 0.3342 (2) | 0.59283 (19) | 0.17436 (10) | 0.0614 (8) | |
| H5FA | 0.378526 | 0.575078 | 0.207321 | 0.074* | |
| C6F | 0.2903 (2) | 0.67900 (18) | 0.16923 (9) | 0.0513 (7) | |
| H6FA | 0.303984 | 0.719733 | 0.198891 | 0.062* | |
| N1S | 0.2657 (6) | 1.3556 (5) | 0.3677 (3) | 0.0700 (15) | 0.33 |
| C1S | 0.2748 (8) | 1.4030 (6) | 0.3321 (4) | 0.0744 (14) | 0.33 |
| C2S | 0.2930 (8) | 1.4590 (7) | 0.2900 (4) | 0.0818 (15) | 0.33 |
| H2S1 | 0.334222 | 1.512578 | 0.304829 | 0.123* | 0.33 |
| H2S2 | 0.224550 | 1.478602 | 0.268390 | 0.123* | 0.33 |
| H2S3 | 0.332939 | 1.424861 | 0.267223 | 0.123* | 0.33 |
| N1T | 0.1809 (10) | 1.3230 (8) | 0.1440 (5) | 0.0995 (19) | 0.25 |
| C1T | 0.1767 (12) | 1.3567 (10) | 0.1851 (5) | 0.0967 (18) | 0.25 |
| C2T | 0.1924 (12) | 1.4067 (10) | 0.2333 (5) | 0.0912 (17) | 0.25 |
| H2T1 | 0.268941 | 1.410818 | 0.248353 | 0.137* | 0.25 |
| H2T2 | 0.156359 | 1.375999 | 0.259002 | 0.137* | 0.25 |
| H2T3 | 0.163014 | 1.468107 | 0.226043 | 0.137* | 0.25 |
\ Bis(µ-1H-imidazole-2-thiolato)-κ2N3:S;\ κ2S:N3-bis[cyanido(triphenylphosphine-κP)\ palladium(II)] acetonitrile 0.58-solvate (2) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Pd1 | 0.02286 (7) | 0.03057 (9) | 0.01702 (7) | 0.00603 (6) | 0.00335 (5) | 0.00011 (6) |
| Pd2 | 0.02643 (8) | 0.03380 (9) | 0.01603 (7) | −0.00787 (7) | 0.00293 (5) | 0.00017 (6) |
| S1 | 0.0255 (3) | 0.0476 (4) | 0.0223 (2) | 0.0063 (2) | 0.00533 (18) | 0.0017 (2) |
| S2 | 0.0291 (3) | 0.0485 (4) | 0.0253 (2) | −0.0039 (3) | 0.01024 (19) | −0.0011 (2) |
| P1 | 0.0293 (3) | 0.0286 (3) | 0.0164 (2) | 0.0046 (2) | 0.00299 (18) | 0.00043 (19) |
| P2 | 0.0365 (3) | 0.0359 (3) | 0.0171 (2) | −0.0075 (3) | 0.00384 (19) | −0.0021 (2) |
| N1 | 0.0692 (15) | 0.0542 (14) | 0.0369 (11) | 0.0373 (12) | 0.0003 (9) | −0.0038 (10) |
| N2 | 0.0887 (18) | 0.0875 (19) | 0.0348 (11) | −0.0607 (16) | −0.0051 (11) | 0.0123 (11) |
| N11 | 0.0345 (9) | 0.0289 (9) | 0.0172 (7) | 0.0039 (8) | 0.0028 (6) | −0.0003 (6) |
| N12 | 0.0564 (13) | 0.0378 (11) | 0.0256 (9) | 0.0245 (10) | 0.0002 (8) | −0.0022 (8) |
| N21 | 0.0337 (9) | 0.0324 (10) | 0.0183 (7) | −0.0077 (8) | 0.0034 (6) | 0.0014 (7) |
| N22 | 0.0480 (12) | 0.0498 (13) | 0.0247 (9) | −0.0249 (10) | 0.0017 (8) | 0.0027 (8) |
| C1 | 0.0385 (12) | 0.0475 (14) | 0.0213 (10) | 0.0216 (11) | 0.0018 (8) | −0.0009 (9) |
| C2 | 0.0484 (14) | 0.0545 (16) | 0.0182 (9) | −0.0221 (12) | 0.0001 (9) | 0.0018 (9) |
| C11 | 0.0373 (11) | 0.0330 (12) | 0.0166 (8) | 0.0129 (10) | 0.0039 (7) | 0.0013 (8) |
| C12 | 0.0769 (19) | 0.0327 (13) | 0.0291 (11) | 0.0036 (13) | −0.0023 (11) | −0.0074 (10) |
| C13 | 0.0486 (14) | 0.0383 (13) | 0.0247 (10) | −0.0040 (11) | −0.0020 (9) | −0.0030 (9) |
| C21 | 0.0383 (12) | 0.0382 (12) | 0.0171 (9) | −0.0130 (10) | 0.0058 (8) | −0.0007 (8) |
| C22 | 0.0741 (19) | 0.0377 (14) | 0.0299 (12) | −0.0152 (14) | 0.0015 (11) | 0.0070 (10) |
| C23 | 0.0488 (14) | 0.0380 (13) | 0.0252 (10) | −0.0022 (11) | 0.0021 (9) | 0.0061 (9) |
| C1A | 0.0416 (12) | 0.0319 (12) | 0.0231 (9) | 0.0003 (10) | 0.0055 (8) | 0.0001 (8) |
| C2A | 0.0558 (15) | 0.0428 (14) | 0.0249 (10) | −0.0114 (12) | −0.0013 (9) | 0.0040 (9) |
| C3A | 0.077 (2) | 0.0572 (18) | 0.0314 (12) | −0.0266 (16) | −0.0046 (12) | 0.0011 (11) |
| C4A | 0.104 (2) | 0.0472 (17) | 0.0410 (14) | −0.0374 (17) | 0.0040 (14) | −0.0005 (12) |
| C5A | 0.092 (2) | 0.0407 (15) | 0.0342 (13) | −0.0140 (15) | 0.0065 (13) | 0.0072 (11) |
| C6A | 0.0557 (15) | 0.0338 (13) | 0.0240 (10) | −0.0039 (11) | 0.0043 (9) | 0.0022 (9) |
| C1B | 0.0363 (11) | 0.0294 (11) | 0.0202 (9) | 0.0032 (9) | −0.0011 (8) | 0.0016 (8) |
| C2B | 0.0401 (13) | 0.0606 (17) | 0.0287 (11) | 0.0152 (12) | −0.0010 (9) | −0.0082 (11) |
| C3B | 0.0399 (14) | 0.081 (2) | 0.0400 (13) | 0.0209 (14) | −0.0074 (10) | −0.0121 (13) |
| C4B | 0.0489 (15) | 0.0501 (16) | 0.0305 (12) | 0.0024 (13) | −0.0105 (10) | −0.0006 (10) |
| C5B | 0.0552 (15) | 0.0591 (17) | 0.0191 (10) | −0.0084 (13) | 0.0007 (9) | 0.0023 (10) |
| C6B | 0.0401 (13) | 0.0564 (16) | 0.0220 (10) | 0.0003 (12) | 0.0042 (8) | 0.0013 (10) |
| C1C | 0.0318 (10) | 0.0367 (12) | 0.0180 (9) | 0.0049 (9) | 0.0066 (7) | 0.0008 (8) |
| C2C | 0.0412 (12) | 0.0409 (14) | 0.0296 (10) | 0.0073 (11) | 0.0138 (9) | −0.0022 (9) |
| C3C | 0.0614 (17) | 0.0448 (15) | 0.0342 (12) | 0.0128 (13) | 0.0175 (11) | −0.0029 (10) |
| C4C | 0.0507 (16) | 0.071 (2) | 0.0425 (14) | 0.0266 (15) | 0.0195 (11) | −0.0012 (13) |
| C5C | 0.0335 (14) | 0.090 (3) | 0.0632 (17) | 0.0040 (15) | 0.0216 (12) | −0.0104 (16) |
| C6C | 0.0374 (13) | 0.0560 (17) | 0.0429 (13) | −0.0036 (12) | 0.0139 (10) | −0.0054 (11) |
| C1D | 0.0412 (12) | 0.0432 (14) | 0.0174 (9) | −0.0092 (10) | 0.0088 (8) | −0.0040 (8) |
| C2D | 0.0445 (13) | 0.0522 (15) | 0.0243 (10) | −0.0115 (12) | 0.0070 (9) | 0.0041 (10) |
| C3D | 0.0653 (17) | 0.0568 (17) | 0.0289 (11) | −0.0141 (15) | 0.0158 (11) | 0.0059 (11) |
| C4D | 0.0581 (17) | 0.070 (2) | 0.0386 (13) | −0.0245 (15) | 0.0250 (11) | −0.0068 (12) |
| C5D | 0.0507 (16) | 0.066 (2) | 0.0603 (17) | −0.0054 (15) | 0.0290 (13) | −0.0093 (15) |
| C6D | 0.0494 (15) | 0.0484 (16) | 0.0423 (13) | −0.0043 (13) | 0.0193 (11) | −0.0062 (11) |
| C1E | 0.0427 (12) | 0.0358 (13) | 0.0198 (9) | −0.0067 (10) | 0.0015 (8) | −0.0032 (8) |
| C2E | 0.0484 (14) | 0.0616 (17) | 0.0230 (10) | −0.0045 (13) | 0.0049 (9) | −0.0039 (10) |
| C3E | 0.0586 (16) | 0.0666 (19) | 0.0203 (10) | −0.0040 (15) | −0.0013 (10) | −0.0051 (10) |
| C4E | 0.0530 (15) | 0.0497 (16) | 0.0278 (11) | −0.0079 (13) | −0.0098 (10) | −0.0034 (10) |
| C5E | 0.0435 (13) | 0.0538 (16) | 0.0327 (12) | −0.0080 (12) | −0.0021 (10) | 0.0043 (11) |
| C6E | 0.0448 (13) | 0.0431 (14) | 0.0227 (10) | −0.0098 (11) | 0.0018 (9) | 0.0014 (9) |
| C1F | 0.0431 (13) | 0.0376 (13) | 0.0242 (10) | −0.0076 (10) | 0.0059 (8) | −0.0015 (9) |
| C2F | 0.0463 (13) | 0.0431 (14) | 0.0248 (10) | −0.0099 (11) | 0.0084 (9) | −0.0027 (9) |
| C3F | 0.0661 (17) | 0.0425 (15) | 0.0375 (13) | −0.0076 (13) | 0.0171 (11) | −0.0096 (11) |
| C4F | 0.078 (2) | 0.0376 (15) | 0.0483 (15) | 0.0073 (14) | 0.0135 (13) | 0.0001 (12) |
| C5F | 0.088 (2) | 0.0457 (17) | 0.0427 (15) | 0.0098 (16) | −0.0065 (14) | 0.0002 (12) |
| C6F | 0.0735 (18) | 0.0430 (16) | 0.0313 (12) | 0.0033 (14) | −0.0045 (11) | −0.0044 (10) |
| N1S | 0.084 (3) | 0.040 (3) | 0.098 (4) | 0.014 (3) | 0.049 (3) | −0.006 (2) |
| C1S | 0.089 (3) | 0.046 (3) | 0.101 (4) | 0.015 (3) | 0.051 (3) | −0.002 (2) |
| C2S | 0.098 (3) | 0.055 (3) | 0.105 (4) | 0.016 (3) | 0.051 (3) | 0.004 (2) |
| N1T | 0.113 (4) | 0.071 (4) | 0.119 (5) | 0.021 (4) | 0.033 (4) | −0.004 (3) |
| C1T | 0.109 (4) | 0.069 (4) | 0.118 (4) | 0.020 (3) | 0.037 (4) | 0.000 (3) |
| C2T | 0.104 (4) | 0.065 (3) | 0.114 (4) | 0.017 (3) | 0.044 (3) | 0.004 (3) |
\ Bis(µ-1H-imidazole-2-thiolato)-κ2N3:S;\ κ2S:N3-bis[cyanido(triphenylphosphine-κP)\ palladium(II)] acetonitrile 0.58-solvate (2) . Geometric parameters (Å, º)
| Pd1—C1 | 1.957 (2) | C6B—H6BA | 0.9500 |
| Pd1—N11 | 2.0346 (17) | C1C—C6C | 1.374 (3) |
| Pd1—P1 | 2.2914 (5) | C1C—C2C | 1.394 (3) |
| Pd1—S2 | 2.3541 (5) | C2C—C3C | 1.394 (3) |
| Pd2—C2 | 1.943 (2) | C2C—H2CA | 0.9500 |
| Pd2—N21 | 2.0345 (17) | C3C—C4C | 1.366 (3) |
| Pd2—P2 | 2.2984 (5) | C3C—H3CA | 0.9500 |
| Pd2—S1 | 2.3542 (5) | C4C—C5C | 1.373 (4) |
| S1—C11 | 1.734 (2) | C4C—H4CA | 0.9500 |
| S2—C21 | 1.733 (2) | C5C—C6C | 1.383 (3) |
| P1—C1A | 1.813 (2) | C5C—H5CA | 0.9500 |
| P1—C1C | 1.823 (2) | C6C—H6CA | 0.9500 |
| P1—C1B | 1.8247 (18) | C1D—C6D | 1.373 (3) |
| P2—C1F | 1.815 (2) | C1D—C2D | 1.400 (3) |
| P2—C1D | 1.820 (2) | C2D—C3D | 1.384 (3) |
| P2—C1E | 1.830 (2) | C2D—H2DA | 0.9500 |
| N1—C1 | 1.143 (3) | C3D—C4D | 1.369 (4) |
| N2—C2 | 1.148 (3) | C3D—H3DA | 0.9500 |
| N11—C11 | 1.333 (2) | C4D—C5D | 1.381 (4) |
| N11—C13 | 1.382 (3) | C4D—H4DA | 0.9500 |
| N12—C11 | 1.350 (3) | C5D—C6D | 1.395 (3) |
| N12—C12 | 1.367 (3) | C5D—H5DA | 0.9500 |
| N12—H12A | 0.8800 | C6D—H6DA | 0.9500 |
| N21—C21 | 1.337 (3) | C1E—C6E | 1.385 (3) |
| N21—C23 | 1.377 (3) | C1E—C2E | 1.390 (3) |
| N22—C21 | 1.348 (3) | C2E—C3E | 1.394 (3) |
| N22—C22 | 1.365 (3) | C2E—H2EA | 0.9500 |
| N22—H22A | 0.8800 | C3E—C4E | 1.363 (3) |
| C12—C13 | 1.349 (3) | C3E—H3EA | 0.9500 |
| C12—H13A | 0.9500 | C4E—C5E | 1.383 (3) |
| C13—H14A | 0.9500 | C4E—H4EA | 0.9500 |
| C22—C23 | 1.348 (3) | C5E—C6E | 1.388 (3) |
| C22—H23A | 0.9500 | C5E—H5EA | 0.9500 |
| C23—H24A | 0.9500 | C6E—H6EA | 0.9500 |
| C1A—C6A | 1.392 (3) | C1F—C6F | 1.393 (3) |
| C1A—C2A | 1.400 (3) | C1F—C2F | 1.395 (3) |
| C2A—C3A | 1.381 (3) | C2F—C3F | 1.380 (3) |
| C2A—H2AA | 0.9500 | C2F—H2FA | 0.9500 |
| C3A—C4A | 1.384 (3) | C3F—C4F | 1.385 (3) |
| C3A—H3AA | 0.9500 | C3F—H3FA | 0.9500 |
| C4A—C5A | 1.395 (3) | C4F—C5F | 1.379 (3) |
| C4A—H4AA | 0.9500 | C4F—H4FA | 0.9500 |
| C5A—C6A | 1.384 (3) | C5F—C6F | 1.379 (4) |
| C5A—H5AA | 0.9500 | C5F—H5FA | 0.9500 |
| C6A—H6AA | 0.9500 | C6F—H6FA | 0.9500 |
| C1B—C6B | 1.383 (3) | N1S—C1S | 1.163 (10) |
| C1B—C2B | 1.391 (3) | C1S—C2S | 1.405 (11) |
| C2B—C3B | 1.387 (3) | C2S—H2S1 | 0.9800 |
| C2B—H2BA | 0.9500 | C2S—H2S2 | 0.9800 |
| C3B—C4B | 1.374 (3) | C2S—H2S3 | 0.9800 |
| C3B—H3BA | 0.9500 | N1T—C1T | 1.167 (10) |
| C4B—C5B | 1.364 (3) | C1T—C2T | 1.405 (11) |
| C4B—H4BA | 0.9500 | C2T—H2T1 | 0.9800 |
| C5B—C6B | 1.387 (3) | C2T—H2T2 | 0.9800 |
| C5B—H5BA | 0.9500 | C2T—H2T3 | 0.9800 |
| C1—Pd1—N11 | 179.31 (8) | C6B—C5B—H5BA | 120.1 |
| C1—Pd1—P1 | 87.19 (6) | C1B—C6B—C5B | 120.9 (2) |
| N11—Pd1—P1 | 92.80 (4) | C1B—C6B—H6BA | 119.6 |
| C1—Pd1—S2 | 87.99 (6) | C5B—C6B—H6BA | 119.6 |
| N11—Pd1—S2 | 92.06 (5) | C6C—C1C—C2C | 118.4 (2) |
| P1—Pd1—S2 | 173.855 (19) | C6C—C1C—P1 | 122.32 (18) |
| C2—Pd2—N21 | 178.38 (8) | C2C—C1C—P1 | 119.14 (16) |
| C2—Pd2—P2 | 89.18 (7) | C3C—C2C—C1C | 120.2 (2) |
| N21—Pd2—P2 | 92.44 (5) | C3C—C2C—H2CA | 119.9 |
| C2—Pd2—S1 | 86.54 (7) | C1C—C2C—H2CA | 119.9 |
| N21—Pd2—S1 | 91.84 (5) | C4C—C3C—C2C | 120.2 (2) |
| P2—Pd2—S1 | 174.489 (19) | C4C—C3C—H3CA | 119.9 |
| C11—S1—Pd2 | 103.47 (7) | C2C—C3C—H3CA | 119.9 |
| C21—S2—Pd1 | 103.09 (7) | C3C—C4C—C5C | 120.0 (2) |
| C1A—P1—C1C | 105.05 (10) | C3C—C4C—H4CA | 120.0 |
| C1A—P1—C1B | 106.83 (9) | C5C—C4C—H4CA | 120.0 |
| C1C—P1—C1B | 103.83 (9) | C4C—C5C—C6C | 120.1 (3) |
| C1A—P1—Pd1 | 112.97 (6) | C4C—C5C—H5CA | 120.0 |
| C1C—P1—Pd1 | 115.90 (6) | C6C—C5C—H5CA | 120.0 |
| C1B—P1—Pd1 | 111.44 (7) | C1C—C6C—C5C | 121.2 (2) |
| C1F—P2—C1D | 104.60 (11) | C1C—C6C—H6CA | 119.4 |
| C1F—P2—C1E | 105.46 (10) | C5C—C6C—H6CA | 119.4 |
| C1D—P2—C1E | 104.41 (9) | C6D—C1D—C2D | 119.2 (2) |
| C1F—P2—Pd2 | 114.49 (7) | C6D—C1D—P2 | 121.09 (18) |
| C1D—P2—Pd2 | 113.82 (7) | C2D—C1D—P2 | 119.39 (18) |
| C1E—P2—Pd2 | 113.06 (7) | C3D—C2D—C1D | 119.9 (2) |
| C11—N11—C13 | 107.43 (18) | C3D—C2D—H2DA | 120.0 |
| C11—N11—Pd1 | 122.63 (14) | C1D—C2D—H2DA | 120.0 |
| C13—N11—Pd1 | 129.94 (15) | C4D—C3D—C2D | 120.7 (3) |
| C11—N12—C12 | 108.74 (19) | C4D—C3D—H3DA | 119.7 |
| C11—N12—H12A | 125.6 | C2D—C3D—H3DA | 119.7 |
| C12—N12—H12A | 125.6 | C3D—C4D—C5D | 119.7 (3) |
| C21—N21—C23 | 107.19 (18) | C3D—C4D—H4DA | 120.1 |
| C21—N21—Pd2 | 122.77 (15) | C5D—C4D—H4DA | 120.1 |
| C23—N21—Pd2 | 130.03 (15) | C4D—C5D—C6D | 120.2 (3) |
| C21—N22—C22 | 108.90 (19) | C4D—C5D—H5DA | 119.9 |
| C21—N22—H22A | 125.6 | C6D—C5D—H5DA | 119.9 |
| C22—N22—H22A | 125.6 | C1D—C6D—C5D | 120.3 (3) |
| N1—C1—Pd1 | 178.1 (2) | C1D—C6D—H6DA | 119.8 |
| N2—C2—Pd2 | 178.2 (2) | C5D—C6D—H6DA | 119.8 |
| N11—C11—N12 | 108.68 (19) | C6E—C1E—C2E | 119.17 (18) |
| N11—C11—S1 | 125.60 (16) | C6E—C1E—P2 | 120.21 (14) |
| N12—C11—S1 | 125.72 (16) | C2E—C1E—P2 | 120.60 (17) |
| C13—C12—N12 | 106.7 (2) | C1E—C2E—C3E | 120.1 (2) |
| C13—C12—H13A | 126.6 | C1E—C2E—H2EA | 119.9 |
| N12—C12—H13A | 126.6 | C3E—C2E—H2EA | 119.9 |
| C12—C13—N11 | 108.4 (2) | C4E—C3E—C2E | 120.3 (2) |
| C12—C13—H14A | 125.8 | C4E—C3E—H3EA | 119.9 |
| N11—C13—H14A | 125.8 | C2E—C3E—H3EA | 119.9 |
| N21—C21—N22 | 108.60 (19) | C3E—C4E—C5E | 120.2 (2) |
| N21—C21—S2 | 126.07 (16) | C3E—C4E—H4EA | 119.9 |
| N22—C21—S2 | 125.32 (17) | C5E—C4E—H4EA | 119.9 |
| C23—C22—N22 | 106.5 (2) | C4E—C5E—C6E | 120.1 (2) |
| C23—C22—H23A | 126.8 | C4E—C5E—H5EA | 119.9 |
| N22—C22—H23A | 126.8 | C6E—C5E—H5EA | 119.9 |
| C22—C23—N21 | 108.8 (2) | C1E—C6E—C5E | 120.17 (19) |
| C22—C23—H24A | 125.6 | C1E—C6E—H6EA | 119.9 |
| N21—C23—H24A | 125.6 | C5E—C6E—H6EA | 119.9 |
| C6A—C1A—C2A | 119.2 (2) | C6F—C1F—C2F | 118.1 (2) |
| C6A—C1A—P1 | 123.45 (16) | C6F—C1F—P2 | 118.58 (17) |
| C2A—C1A—P1 | 117.38 (16) | C2F—C1F—P2 | 123.18 (16) |
| C3A—C2A—C1A | 120.7 (2) | C3F—C2F—C1F | 120.8 (2) |
| C3A—C2A—H2AA | 119.7 | C3F—C2F—H2FA | 119.6 |
| C1A—C2A—H2AA | 119.7 | C1F—C2F—H2FA | 119.6 |
| C2A—C3A—C4A | 119.9 (2) | C2F—C3F—C4F | 120.3 (2) |
| C2A—C3A—H3AA | 120.0 | C2F—C3F—H3FA | 119.8 |
| C4A—C3A—H3AA | 120.0 | C4F—C3F—H3FA | 119.8 |
| C3A—C4A—C5A | 119.8 (2) | C5F—C4F—C3F | 119.4 (3) |
| C3A—C4A—H4AA | 120.1 | C5F—C4F—H4FA | 120.3 |
| C5A—C4A—H4AA | 120.1 | C3F—C4F—H4FA | 120.3 |
| C6A—C5A—C4A | 120.4 (2) | C4F—C5F—C6F | 120.5 (2) |
| C6A—C5A—H5AA | 119.8 | C4F—C5F—H5FA | 119.7 |
| C4A—C5A—H5AA | 119.8 | C6F—C5F—H5FA | 119.7 |
| C5A—C6A—C1A | 120.0 (2) | C5F—C6F—C1F | 120.8 (2) |
| C5A—C6A—H6AA | 120.0 | C5F—C6F—H6FA | 119.6 |
| C1A—C6A—H6AA | 120.0 | C1F—C6F—H6FA | 119.6 |
| C6B—C1B—C2B | 118.82 (17) | N1S—C1S—C2S | 176.2 (11) |
| C6B—C1B—P1 | 121.48 (16) | C1S—C2S—H2S1 | 109.5 |
| C2B—C1B—P1 | 119.70 (14) | C1S—C2S—H2S2 | 109.5 |
| C3B—C2B—C1B | 119.6 (2) | H2S1—C2S—H2S2 | 109.5 |
| C3B—C2B—H2BA | 120.2 | C1S—C2S—H2S3 | 109.5 |
| C1B—C2B—H2BA | 120.2 | H2S1—C2S—H2S3 | 109.5 |
| C4B—C3B—C2B | 120.7 (2) | H2S2—C2S—H2S3 | 109.5 |
| C4B—C3B—H3BA | 119.7 | N1T—C1T—C2T | 167.3 (16) |
| C2B—C3B—H3BA | 119.7 | C1T—C2T—H2T1 | 109.5 |
| C5B—C4B—C3B | 120.1 (2) | C1T—C2T—H2T2 | 109.5 |
| C5B—C4B—H4BA | 119.9 | H2T1—C2T—H2T2 | 109.5 |
| C3B—C4B—H4BA | 119.9 | C1T—C2T—H2T3 | 109.5 |
| C4B—C5B—C6B | 119.8 (2) | H2T1—C2T—H2T3 | 109.5 |
| C4B—C5B—H5BA | 120.1 | H2T2—C2T—H2T3 | 109.5 |
| C13—N11—C11—N12 | −1.0 (2) | Pd1—P1—C1C—C6C | 107.61 (17) |
| Pd1—N11—C11—N12 | 179.69 (12) | C1A—P1—C1C—C2C | 166.82 (15) |
| C13—N11—C11—S1 | 178.50 (14) | C1B—P1—C1C—C2C | 54.80 (17) |
| Pd1—N11—C11—S1 | −0.8 (2) | Pd1—P1—C1C—C2C | −67.74 (16) |
| C12—N12—C11—N11 | 0.5 (2) | C6C—C1C—C2C—C3C | −0.1 (3) |
| C12—N12—C11—S1 | −179.08 (15) | P1—C1C—C2C—C3C | 175.43 (15) |
| Pd2—S1—C11—N11 | −58.55 (17) | C1C—C2C—C3C—C4C | 0.1 (3) |
| Pd2—S1—C11—N12 | 120.92 (16) | C2C—C3C—C4C—C5C | 0.7 (4) |
| C11—N12—C12—C13 | 0.3 (2) | C3C—C4C—C5C—C6C | −1.5 (4) |
| N12—C12—C13—N11 | −1.0 (2) | C2C—C1C—C6C—C5C | −0.7 (3) |
| C11—N11—C13—C12 | 1.3 (2) | P1—C1C—C6C—C5C | −176.07 (19) |
| Pd1—N11—C13—C12 | −179.55 (14) | C4C—C5C—C6C—C1C | 1.5 (4) |
| C23—N21—C21—N22 | −1.0 (2) | C1F—P2—C1D—C6D | −23.41 (19) |
| Pd2—N21—C21—N22 | 179.80 (12) | C1E—P2—C1D—C6D | −133.98 (18) |
| C23—N21—C21—S2 | 177.87 (14) | Pd2—P2—C1D—C6D | 102.27 (17) |
| Pd2—N21—C21—S2 | −1.3 (2) | C1F—P2—C1D—C2D | 163.28 (16) |
| C22—N22—C21—N21 | 0.7 (2) | C1E—P2—C1D—C2D | 52.72 (18) |
| C22—N22—C21—S2 | −178.16 (16) | Pd2—P2—C1D—C2D | −71.03 (17) |
| Pd1—S2—C21—N21 | −57.77 (17) | C6D—C1D—C2D—C3D | −0.8 (3) |
| Pd1—S2—C21—N22 | 120.92 (16) | P2—C1D—C2D—C3D | 172.62 (16) |
| C21—N22—C22—C23 | −0.1 (2) | C1D—C2D—C3D—C4D | 0.4 (3) |
| N22—C22—C23—N21 | −0.5 (2) | C2D—C3D—C4D—C5D | 0.8 (4) |
| C21—N21—C23—C22 | 0.9 (2) | C3D—C4D—C5D—C6D | −1.6 (4) |
| Pd2—N21—C23—C22 | −179.96 (14) | C2D—C1D—C6D—C5D | 0.0 (3) |
| C1C—P1—C1A—C6A | −98.1 (2) | P2—C1D—C6D—C5D | −173.35 (18) |
| C1B—P1—C1A—C6A | 11.7 (2) | C4D—C5D—C6D—C1D | 1.2 (4) |
| Pd1—P1—C1A—C6A | 134.63 (18) | C1F—P2—C1E—C6E | 96.5 (2) |
| C1C—P1—C1A—C2A | 82.64 (19) | C1D—P2—C1E—C6E | −153.57 (19) |
| C1B—P1—C1A—C2A | −167.49 (17) | Pd2—P2—C1E—C6E | −29.3 (2) |
| Pd1—P1—C1A—C2A | −44.6 (2) | C1F—P2—C1E—C2E | −81.6 (2) |
| C6A—C1A—C2A—C3A | 2.1 (4) | C1D—P2—C1E—C2E | 28.4 (2) |
| P1—C1A—C2A—C3A | −178.7 (2) | Pd2—P2—C1E—C2E | 152.62 (17) |
| C1A—C2A—C3A—C4A | −1.2 (4) | C6E—C1E—C2E—C3E | −0.1 (4) |
| C2A—C3A—C4A—C5A | −0.7 (5) | P2—C1E—C2E—C3E | 178.0 (2) |
| C3A—C4A—C5A—C6A | 1.8 (5) | C1E—C2E—C3E—C4E | −0.3 (4) |
| C4A—C5A—C6A—C1A | −0.9 (4) | C2E—C3E—C4E—C5E | 0.5 (4) |
| C2A—C1A—C6A—C5A | −1.0 (4) | C3E—C4E—C5E—C6E | −0.4 (4) |
| P1—C1A—C6A—C5A | 179.8 (2) | C2E—C1E—C6E—C5E | 0.2 (3) |
| C1A—P1—C1B—C6B | −83.0 (2) | P2—C1E—C6E—C5E | −177.91 (18) |
| C1C—P1—C1B—C6B | 27.7 (2) | C4E—C5E—C6E—C1E | 0.1 (4) |
| Pd1—P1—C1B—C6B | 153.15 (17) | C1D—P2—C1F—C6F | 90.2 (2) |
| C1A—P1—C1B—C2B | 97.0 (2) | C1E—P2—C1F—C6F | −160.00 (19) |
| C1C—P1—C1B—C2B | −152.31 (19) | Pd2—P2—C1F—C6F | −35.1 (2) |
| Pd1—P1—C1B—C2B | −26.9 (2) | C1D—P2—C1F—C2F | −85.8 (2) |
| C6B—C1B—C2B—C3B | 0.4 (4) | C1E—P2—C1F—C2F | 24.1 (2) |
| P1—C1B—C2B—C3B | −179.6 (2) | Pd2—P2—C1F—C2F | 148.98 (17) |
| C1B—C2B—C3B—C4B | −0.9 (4) | C6F—C1F—C2F—C3F | 0.0 (4) |
| C2B—C3B—C4B—C5B | 0.6 (4) | P2—C1F—C2F—C3F | 175.98 (19) |
| C3B—C4B—C5B—C6B | 0.2 (4) | C1F—C2F—C3F—C4F | −1.4 (4) |
| C2B—C1B—C6B—C5B | 0.4 (4) | C2F—C3F—C4F—C5F | 1.6 (4) |
| P1—C1B—C6B—C5B | −179.63 (19) | C3F—C4F—C5F—C6F | −0.5 (5) |
| C4B—C5B—C6B—C1B | −0.7 (4) | C4F—C5F—C6F—C1F | −0.8 (5) |
| C1A—P1—C1C—C6C | −17.83 (19) | C2F—C1F—C6F—C5F | 1.1 (4) |
| C1B—P1—C1C—C6C | −129.86 (18) | P2—C1F—C6F—C5F | −175.1 (2) |
\ Bis(µ-1H-imidazole-2-thiolato)-κ2N3:S;\ κ2S:N3-bis[cyanido(triphenylphosphine-κP)\ palladium(II)] acetonitrile 0.58-solvate (2) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N12—H12A···N2i | 0.88 | 1.90 | 2.770 (3) | 169 |
| N22—H22A···N1ii | 0.88 | 1.92 | 2.760 (3) | 160 |
| C12—H13A···N1S | 0.95 | 2.35 | 3.261 (7) | 161 |
| C22—H23A···N1T | 0.95 | 2.29 | 3.081 (12) | 141 |
Symmetry codes: (i) −x, y+1/2, −z+1/2; (ii) −x+1, y+1/2, −z+1/2.
Funding Statement
JPJ acknowledges the NSF–MRI program (Grant No. CHE-1039027) for funds to purchase an X-ray diffractometer. MZ acknowledges the NSF Grant CHE 0087210, Ohio Board of Regents Grant CAP-491, and Youngstown State University for funds to purchase an X-ray diffractometer.
References
- Ahmad, S., Rüffer, T., Lang, H., Nadeem, S., Tirmizi, S. A., Saleem, M. & Anwar, A. (2010). Russ. J. Coord. Chem. 36, 520–524.
- Akrivos, P. D. (2001). Coord. Chem. Rev. 213, 181–210.
- Bondy, C. R. & Loeb, S. J. (2003). Coord. Chem. Rev. 240, 77–99.
- Bruker (2007). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- García-Vázquez, J. A., Romero, J. & Sousa, A. (1999). Coord. Chem. Rev. 193–195, 691–745.
- Hübschle, C. B., Sheldrick, G. M. & Dittrich, B. (2011). J. Appl. Cryst. 44, 1281–1284. [DOI] [PMC free article] [PubMed]
- Huheey, J. E., Keiter, E. A. & Keiter, R. L. (1993). Inorganic Chemistry: Principles of Structure and Reactivity, 4th ed. New York: Harper Collins College Publishers.
- Kahn, E. S., Rheingold, A. L. & Shupack, S. I. (1993). J. Crystallogr. Spectrosc. Res. 23, 697–710.
- Koch, K. R. (2001). Coord. Chem. Rev. 216–217, 473–488.
- Kostas, I. D. & Steele, B. R. (2020). Catalysts 10, 1107; doi: 10.3390/catal10101107.
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Lobana, T. S. (2021). Coord. Chem. Rev. 441, 213884.
- Lobana, T. S., Sandhu, A. K., Mahajan, R. K., Hundal, G., Gupta, S. K., Butcher, R. J. & Castineiras, A. (2017). Polyhedron, 127, 25–35.
- Mendía, A., Cerrada, E., Arnáiz, F. J. & Laguna, M. (2006). Dalton Trans. pp. 609–616. [DOI] [PubMed]
- Oxford Diffraction (2009). CrysAlis PRO, CrysAlis CCD , and CrysAlis RED. Oxford Diffraction Ltd, Abingdon, England.
- Raper, E. S. (1985). Coord. Chem. Rev. 61, 115–184.
- Raper, E. S. (1994). Coord. Chem. Rev. 129, 91–156.
- Raper, E. S. (1996). Coord. Chem. Rev. 153, 199–255.
- Raper, E. S. (1997). Coord. Chem. Rev. 165, 475–567.
- Sandhu, A. K., Lobana, T. S., Sran, B. S., Hundal, G. & Jasinski, J. P. (2018). J. Organomet. Chem. 861, 112–124.
- Serpe, A., Artizzu, F., Mercuri, M. L., Pilia, L. & Deplano, P. (2008). Coord. Chem. Rev. 252, 1200–1212.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Steffen, W. L. & Palenik, G. J. (1976). Inorg. Chem. 15, 2432–2439.
- Talismanova, M. O., Sidorov, A. A., Aleksandrov, G. G., Charushin, V. N., Kotovskaya, S. K., Ananikov, V. P., Eremenko, I. L. & Moiseeva, I. I. (2008). Russ. Chem. Bull. 57, 47–55.
- Talismanova, M. O., Sidorov, A. A., Aleksandrov, G. G., Oprunenko, Y. F., Eremenko, I. L. & Moiseev, I. I. (2004). Russ. Chem. Bull. 53, 1507–1510.
- Umakoshi, K., Ichimura, A., Kinoshita, I. & Ooi, S. (1990). Inorg. Chem. 29, 4005–4010.
- Yamamoto, J. H., Yoshida, W. & Jensen, C. M. (1991). Inorg. Chem. 30, 1353–1357.
- Yap, G. P. A. & Jensen, C. M. (1992). Inorg. Chem. 31, 4823–4828.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2. DOI: 10.1107/S2056989023000166/ex2063sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989023000166/ex20631sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989023000166/ex20632sup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report







