Abstract
Deep Brain Stimulation (DBS) is an established intervention for use in pediatric movement disorders, especially dystonia. While multiple publications have provided guidelines for DBS patient selection and programming in adults, there are no evidence-based or consensus statements published for pediatrics. The result is lack of standardized care and underutilization of this effective treatment. To this end, we assembled a focus group of 13 pediatric movement disorder specialists and one neurosurgeon experienced in pediatric DBS to review recent literature and current practices and propose a standardized approach to candidate selection, implantation target site selection, and programming algorithms. For pediatric dystonia, we provide algorithms for 1) Programming for initial session and follow-up sessions, and 2) Troubleshooting side effects encountered during programming. We discuss common side effects, how they present, and recommendations for management. This topical review serves as a resource for movement disorders specialists interested in utilizing DBS for pediatric dystonia.
Keywords: deep brain stimulation, programming, pediatrics, dystonia, movement
Introduction
Deep Brain stimulation (DBS) is an established and evolving tool for reversible and titratable neuromodulation in children, providing precisely targeted electrical stimulation. DBS is currently FDA-approved for treatment of Parkinson Disease1, essential tremor2, and epilepsy3,4, with humanitarian device exemptions in dystonia5 and obsessive-compulsive disorder6. It is also being studied in Tourette Syndrome7, autism8–10, self-injurious behavior8, and depression11,12. Pediatric DBS use has been increasing, especially for treatment of dystonia13–19 when pharmacologic treatment is ineffective or poorly tolerated20–22.
There is a need for clear recommendations for DBS programming in children with dystonia, similar to standardized protocols published for adults23–27. This review summarizes existing recommendations for DBS programming in children with dystonia, with additional input from a focus group of clinicians experienced in pediatric DBS. It provides guidance for the management of DBS for pediatric dystonia, including pre-implantation considerations, programming strategies, and troubleshooting recommendations.
Methods
A focus group consisting of 13 pediatric movement disorder specialists and 1 neurosurgeon experienced in deep brain stimulation from 9 academic institutions in the United States met monthly for 6 months to discuss literature and review institutional practices. Literature was reviewed from PubMed, including manuscripts published in English up to August 2021, with search terms including “pediatric”, “deep brain stimulation”, “dystonia”, and “programming”. Additional manuscripts were reviewed through manual searches of related reviews and reference lists of original articles. Programming recommendations were compiled based on recommendations from published literature, with information extracted from adult literature when not described in pediatric literature. Additional pediatric specific practices noted were based on majority group consensus.
Pre-Implantation Considerations
Candidate Evaluation and Selection
Specific guidelines for pediatric patient selection, an ongoing topic of discussion, are beyond the scope of this article. Generally accepted criteria for DBS implantation include: age 7 years or older, dystonia refractory to medical treatment, and significant disability28. Prior to decisions about implantation, neuroimaging should be obtained, with consideration for genetic testing in cases without clear acquired or structural etiology. DBS is considered most effective in childhood-onset monogenic dystonia, including early-onset isolated dystonia (DYT-TOR1A), myoclonus-dystonia syndrome (DYT-SGCE), DYT-THAP1, and more recently KMT2B-related dystonia (DYT-KMT2B)29–38. DBS can be therapeutic in acquired dystonia, although response may be more limited. Likely reasons include heterogenous patterns of brain injury, co-occurring DBS-resistant phenomenology such as spasticity, lack of objective measures for patient selection and identification of optimal target site,13,18,39–43 and longer proportion of life lived with dystonia42.
Pre-operative evaluation and post-operative management by an experienced, multidisciplinary team comprised of a pediatric movement disorder specialist, functional neurosurgeon, neuropsychologist, pediatric neuroradiologist, physiatrist, physical and occupational therapists, nurses, and social workers are recommended. Roles for individual members may vary based on institution, but it is important to involve a multidisciplinary team in pre-operative evaluation to ensure appropriate candidate selection and implantation site. Post-operative management is typically led by the DBS programming practitioner (most often the neurologist), with the neuropsychologist and therapists aiding in evaluating response to treatment, and nurses and social workers helping to address potential barriers to care.
Intracranial Target Tissue Selection
Successful DBS therapy and programming rely heavily on individualized site selection and accurate electrode implantation to facilitate maximum therapeutic response and avoid unwanted side effects. The bilateral globus pallidus internus (GPi) is the typical implantation site for both inherited and acquired dystonia in children19,37,44 and will be the focus of this paper. However, some children with destructive injury to the GPi may benefit from implantation in an alternate target such as the subthalamic nucleus45, thalamus46, or cerebellum47,48.
Understanding target tissue anatomy is critical to effective DBS programming (Figure 1a/b). The GPi is the smallest and most medial portion of the lentiform nucleus. It is bordered laterally by the globus pallidus externus (GPe) and the putamen and dorsomedially by the internal capsule. The optic tract is ventromedial to the GPi. The GPi is somatotopically arranged, with legs represented anterodorsally and the head posteroventrally (Figure 1c)49, providing potential guidance for targeted areas of stimulation to alleviate focal symptoms. Prior publications have reported that stimulation of the ventral portion of the GPi may be more beneficial for dystonia but can induce parkinsonian symptoms50,51. These location-specific responses may help optimize contact selection to achieve functional goals.
Figure 1: GPi Anatomy and Effective DBS Lead Placement.
a) View of effectively-placed bilateral globus pallidus internus (GPi) leads with four contacts. Note GPi in red, GPe (globus pallidus externus) in blue, and Putamen in brown. The GPi is bordered laterally by the GPe and the putamen and ventrally (inferiorly) by the optic radiations. b) The posterior limb of the internal capsule is located posterior and medial to the GPi c) Visual representation of the GPi homunculus with the head and face represented posterior and ventral/inferior (green) and the limbs represented in the anterior and dorsal/superior (orange) portions of the Gpi.
Introduction to the Deep Brain Stimulator
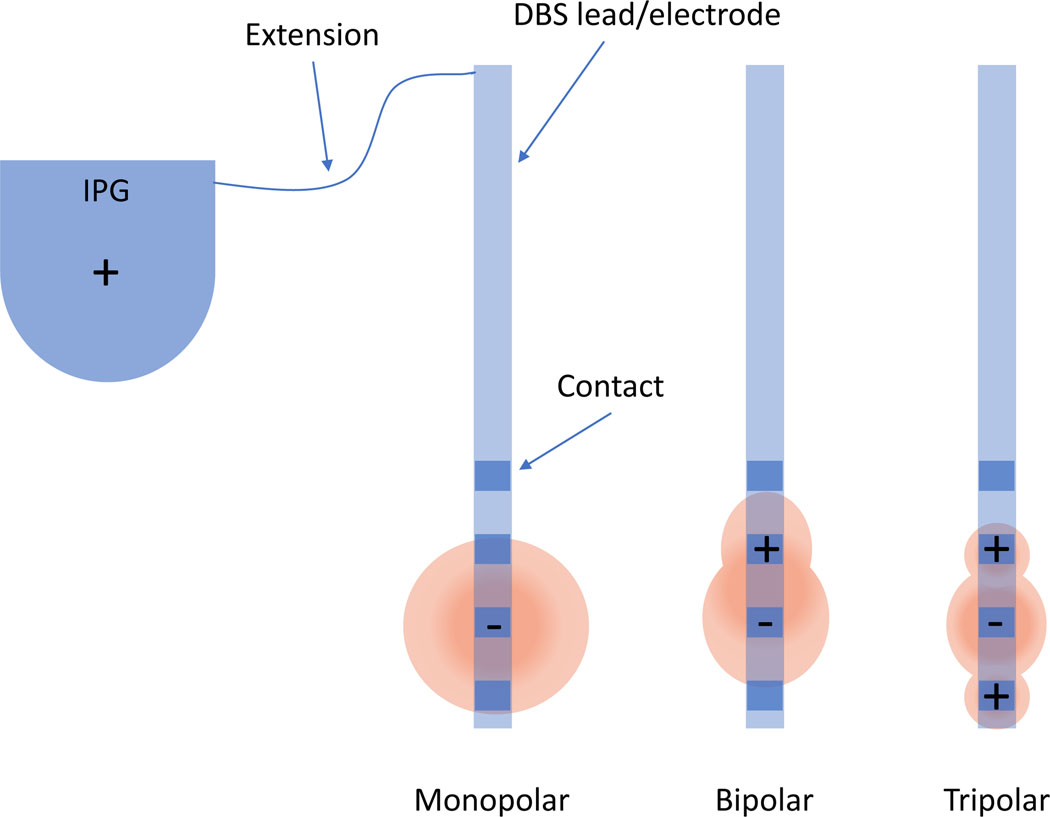
While Deep Brain Stimulators are currently produced by several manufacturers, they share similar general components (Figure 2). An implantable pulse generator (IPG), surgically placed under the skin of the chest or abdomen, contains the battery and computer to program the device. IPGs may have a rechargeable battery, which allows the patients to recharge the battery using a handheld device placed over the skin at the location of the IPG, or it may have a non-rechargeable battery. The IPG is connected via an insulated wire (the extension) under the skin to the DBS leads (also called electrodes) which are stereotactically implanted into the desired region of the brain.
Figure 2: Deep Brain Stimulator Components and Stimulation configurations.
A DBS system is made up of an implantable pulse generator (IPG) which is surgically placed under the skin and connected via a thin insulated wire (the extension) to the DBS leads (or electrodes) that are stereotactically implanted into the desired region of the brain. Common electrode stimulation configurations including monopolar, bipolar, and tripolar configurations. In the monopolar configuration, the implantable pulse generator (IPG) acts as the anode (+) while the electrode contact is the cathode (−). In bipolar configuration, one electrode contact serves as the anode and another as the cathode, creating a narrower field of stimulation. In tripolar configuration, the electrode contact serving as the cathode is bordered by two anodic contacts, focusing the field of stimulation on the cathodic contact to decrease spread of stimulation into the superior and inferior tissues.
Each DBS lead contains multiple programmable contacts. This paper will focus on the most common configuration with four cylindrical contacts on each lead. Other systems, with differing configurations and options for directional stimulation, have, to date, more limited use in children.
Deep Brain Stimulation: Basic Principles
Effective DBS programming requires understanding of current, voltage, resistance, and impedance and their relationships. Impedance is a measure of resistance to the propagation of current in an alternating current system52. According to Ohm’s law (current = voltage/resistance), the propagation of current through a conductor with any given voltage can vary based on the resistance, i.e. impedance, across a circuit. The DBS device provides values for both lead integrity impedance, which is dependent on the structure of the DBS system and surrounding parenchyma, and therapeutic impedance, which is affected by the stimulation settings. A rising lead integrity impedance could indicate an open circuit (break in the circuit), while a low impedance could indicate a short circuit53. An open circuit could result in immediate loss of efficacy. A short circuit may develop due to gradual decrease in impedances, shortening battery life and reducing symptom control54.
For any given voltage stimulation, a change in impedance will change the current through the circuit. This becomes especially relevant as different DBS systems use constant voltage stimulation (CVS) versus constant current stimulation (CCS). CVS systems maintain a constant voltage stimulation despite fluctuations in impedance. In CCS systems, the clinician sets the desired current, and the DBS device automatically adjusts the voltage depending on the measured impedances to maintain that constant current. Some argue that the CCS system is more stable, especially in the immediate post-operative period when levels of parenchymal edema can significantly affect impedance and therefore the supplied current in a CVS system26,55.
DBS Programming in Pediatric Dystonia
Prior to beginning DBS programming, it is important to understand key programming configurations (Figure 2). The most common configuration for initial programming is unipolar/monopolar, in which the IPG case serves as positive (anode) and the electrode contact is negative (cathode). Other configurations include bipolar, in which one electrode contact serves as the anode and another contact as the cathode. Multipolar configurations, such as tripolar configuration, include multiple contacts serving as the anode and another contact as the cathode. Various configurations can be used to maximize benefit to side effect ratio.
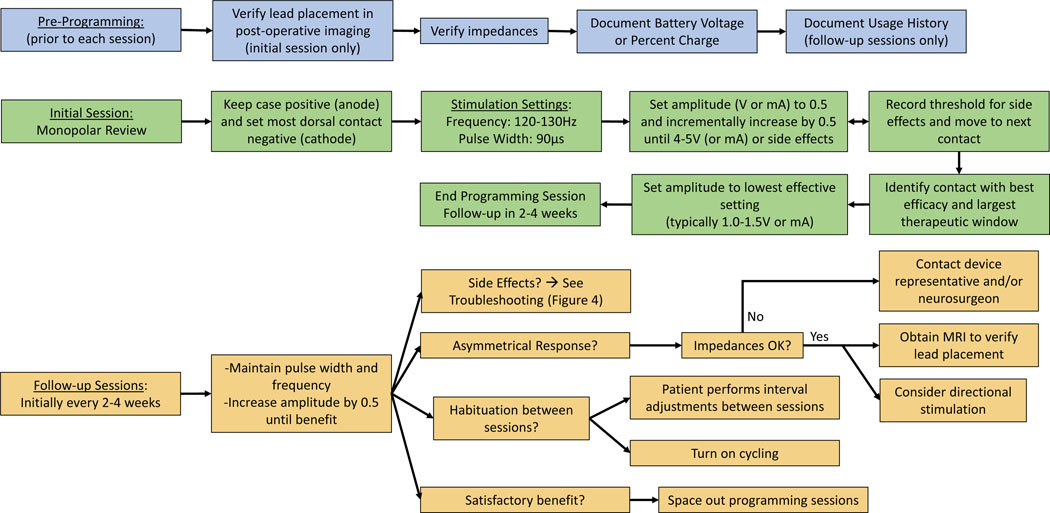
Many children with dystonia have limited or no verbal communication. Assessing communication skills before the programming sessions, involving a Child Life team, and treating underlying anxiety (including use of music and/or videos during programming) may enhance or clarify expectations for post-operative reporting of symptomatic relief or side effects. A suggested algorithm for GPi DBS programming in pediatric dystonia is presented in Figure 3, including pre-programming, initial, and follow up sessions. The algorithm in Figure 4 provides tips for troubleshooting side effects.
Figure 3: Programming Algorithm.
Programming algorithm for GPi DBS in pediatric dystonia. The blue section (pre-programming) details steps that should be performed prior to each programming session. Of note, lead placement only needs to be verified in the initial session unless concern for lead migration. Usage and group history only needs to be noted in follow-up programming sessions. The green section details the programming algorithm for the initial programming session. The orange section details the programming algorithm for the follow-up programming sessions.
Figure 4:
Algorithm for troubleshooting side effects during programming sessions
Pre-programming steps
Verify DBS lead location
It is good practice to visualize the location of the DBS lead implants on post-operative high-resolution volumetric MRI or CT images prior to the first programming session, making note of any slight rotation of the patient in the scanner that could contribute to the appearance of asymmetric lead placement. Independently created toolboxes such as Lead-DBS (https://www.lead-dbs.org 56) create 3D reconstructions of lead placement based on the patient’s postoperative imaging. They can also include stimulation simulation maps and other programming tools. More recently, toolboxes and commercial DBS software are incorporating personalized or normative diffusion MRI-based tractography to estimate locations of white-matter motor pathways in relation to the simulation maps. These connectomic approaches to DBS programming have been fueled by literature revealing abnormal structural and functional network connectivity in dystonia57–60 and implicating specific neural pathways as predictors of outcome in DBS for movement disorders61,62. While these toolboxes are still research-based and have some limitations in pediatric clinical care, it is likely such programs will soon be used to optimize DBS programming in children.
Verify Impedances
The integrity of the circuit can be interrogated by measuring lead impedances using the clinician programmer. The programmer will typically alert if any impedances are out of range. Normal lead impedances should be 250–2000 Ω for monopolar and 250–4000 Ω for bipolar configurations. Impedance > 40000 Ω suggests an open circuit (i.e. negligible current flow, which may indicate a connector break). Although electrode encapsulation in extracellular matrix components may occur over time and affect tissue conductivity53, a pattern of steadily increasing Ωs could indicate a developing problem. If the impedance is trending below 500 Ω, this is also worth noting: < 250 Ω suggests a possible short circuit (unimpeded current); < 50 Ω indicates a true short. If an open circuit is suspected, the next step is to recheck impedances at higher voltage (or current, as applicable) to ensure accuracy. Many programming systems have an automated process to check impedances and will automatically test at increasing voltages/currents if lead impedances are elevated beyond the normal range. If there is a short circuit, there is no need to recheck at higher voltages, but it is recommended to avoid the involved contacts.
If multiple lead impedances are abnormal, it is worthwhile to check therapeutic impedances. Therapeutic impedance, assessed based on the programmer-specified parameters, can differ from lead impedance, especially at lower voltages52. In addition to the benefit of checking impedances to ensure system integrity, therapeutic impedance values can help with programming. DBS benefits and side effects are related to the volume of brain tissue activated by stimulation, which is proportional to current density. Relatively high therapeutic impedances can increase the current density and lead to more focused tissue activation, theoretically facilitating maximal benefit while reducing side effects52. Consistent abnormalities in either lead impedances or therapeutic impedances may necessitate a lead or IPG replacement.
Document Battery Voltage or Percent Charge
After verifying electrode impedances, the next step is documentation of battery voltage or percent charge. The programmer will typically indicate whether and when battery replacement is needed. Rechargeable devices may not require surgical replacement for up to 15 years, while non-rechargeable devices usually require replacement in approximately 5 years. Children with dystonia may require higher stimulation settings, resulting in faster battery consumption63. The University of Florida has developed a DBS Battery Life Estimator that can be used to pre-emptively plan for battery replacement (https://movementdisorders.ufhealth.org/surgery/dbs-battery-estimator/)64. If a non-rechargeable battery voltage is decreasing rapidly, or a rechargeable battery is not holding charge for at least 24 hours, verify that there is not a short circuit.
Stimulation Parameters: initial session
The first session of DBS programming often requires two hours or more and is critical for successful subsequent programming sessions. Standardized dystonia rating scales (such as the Barry-Albright Dystonia Rating Scale65, Burke Fahn Marsden Dystonia Rating Scale66) and video documentation should be completed prior to programming at the first session and periodically thereafter to document response. These may be most effective when combined with additional metrics assessing patient-specific goals, function, and quality of life67.
Key parameters in DBS programming include frequency, pulse width, amplitude (voltage or current), and contact selection. The frequency is the number of electrical pulses delivered per second (Hz) while the pulse width is the duration of each electrical pulse (μs). The amplitude is the magnitude of the electrical pulse, measured either as voltage (V) or current (mA).
The goal in the first DBS programming session should be to complete monopolar review, a systematic exploration of each available contact to determine the therapeutic window, or range of stimulation settings which produce benefit without intolerable side effects. In monopolar review, the user first selects and sets the frequency and pulse width which are kept constant throughout the programming session while current/voltage settings are adjusted. Specific stimulation parameters listed below are largely based on adult literature with specific pediatric recommendations by majority author consensus based on institutional practices.
Frequency
Initial frequency for Gpi DBS in children and adults is typically 120–130Hz.23–25
Pulse width
Most adult literature recommends an initial pulse width from 60–120μs,24,25 with typical initial pulse width of 90μs in pediatric patients based on author consensus.
Amplitude + Contact optimization
After selecting settings for frequency and pulse width, the next step is to systematically test each contact point on the lead, typically beginning with the most ventral contact, starting with 0.5V or mA and increasing in stepwise increments of 0.5, until reaching 4–5V (or mA) or side effects observed, whichever is sooner. If excessive stimulation extends posterior or medial into the internal capsule, the patient may develop spasticity, painful cramping of the contralateral limb(s), dyskinesias, or dysarthria. Extension ventromedially to the optic tract, often by stimulation of the most ventral/deepest contact, may induce phosphenes or other visual disturbances. Stimulation of the amygdala lateral to the optic tract may induce sudden sadness, mania, or emotional lability (See Table 1). In children with cognitive impairment or limited expressive language abilities, stimulation-induced discomfort may present as sudden irritability, somnolence, or behavior changes, and visual changes may manifest as squinting or rapid blinking, with no visible changes in motor control. Thus, it is important to perform a thorough baseline exam and periodic targeted examinations to assess tone, vision, behavior, and mood, and query the patient and/or caregiver about side effects throughout the programming session. Side effects typically begin immediately with changes in stimulation settings and can be alleviated by decreasing the settings or turning off the programmer. However, the possibility of delayed stimulation-induced side effects should be considered for any new symptom in a patient with DBS. Anxious patients or caregivers may also over-report side effects and others may experience a placebo response, in which case it can be helpful to blind them to changes made during the session.
Table 1:
Stimulation-induced side effects of GPi Deep Brain Stimulation
| Spread of Stimulation Relative to GPi | Affected anatomy | Side Effect |
|---|---|---|
| Lateral or Anterior or Superior/Dorsal |
Globus pallidus externus, putamen | None |
| Medial or Posterior |
Posterior limb of internal capsule | Stiffness, muscle spasms, speech impairment, dyskinetic movements *child may become irritable or cry in pain |
| Inferior/Ventral | Optic tract | Phosphenes (flashing lights) in contralateral visual hemifield *child may blink eyes rapidly, squint, become irritable |
| Amygdala | Emotional lability, sadness, mania *child may have sudden change of mood, crying |
Once a maximum setting is identified that does not cause any side effects, the programmer should document these settings and proceed to the next contact. Commercial software also allows for documentation of side effects when encountered. The range from minimal beneficial voltage/current to maximal tolerated voltage/current is the therapeutic window. However, it may be difficult to fully determine the therapeutic window in the first programming session. Phasic dystonia (characterized by irregular, non-sustained, or rhythmic movements) may respond more quickly than tonic dystonia (sustained movements), which will continue to improve over several weeks or months25,68. A post-surgical microlesion effect may necessitate smaller stimulation settings initially, with larger increases in amplitude required 10–12 weeks after surgery. Children specifically may lack the endurance to tolerate full determination of the therapeutic window for each contact in a single programming session. Further, children may initially be sensitive to small increases in stimulation settings, but over time their tolerance and therapeutic window may gradually increase.
After testing all contacts on each lead, it is recommended to use the contact with fewest side effects and a large therapeutic window, as this will provide more options for future adjustments. Voltage/current selection is typically set at 1.0–2.0V (or mA) at the end of the first programming session.
Stimulation parameters: follow-up sessions
Follow-up, 1-hour programming sessions are usually scheduled every 2–4 weeks for the first 6–12 months, or until reaching stable stimulation settings. Initial steps at each follow-up session include 1) checking usage history to quantify any on and off periods as well as usage of multiple programming groups (discussed further below); 2) checking electrode impedances; and 3) documenting battery voltage or percent charge.
The goal of programming is optimization of symptom control within the therapeutic window and avoidance of side effects. Stimulation parameters for follow-up sessions follow some general guidelines; however, individual patient responses are also considered. Generally, voltage/current amplitudes should be increased in increments of 0.1 every 2–3 minutes while observing for side effects, keeping frequency and pulse width parameters unchanged. Total amplitude change within a session is often 0.5–1, varying based on individual patient tolerance. When amplitude is increased gradually, most patients will tolerate 4.5–5.5V or mA before exhibiting side effects (often triggering worsening tone). If a patient experiences a side effect while increasing their programming settings, return to the last, best-tolerated setting. Alternative setting parameters and/or configurations, i.e. different programming groups, can be created and the DBS provider can train caregivers to switch between groups depending on delayed appreciation of side effects or efficacy. Device representatives can be an additional resource in training patients/caregivers to use the patient programmer in this manner.
The concept of average current can be used to guide programming strategy69. Average current is the current delivery per second (typically < 120 μA) and is calculated as the product of frequency (Hz), pulse width (μs), and pulse current (mA) divided by 1000 (μA=Hz x μs x mA/1000). If increasing the pulse current (amplitude) leads to side effects, decreasing pulse width and/or frequency may allow the use of a higher pulse current while maintaining the average current (and associated benefit) and reducing side effects.
Once amplitude has been optimized to maximize clinical benefit while avoiding side effects, programming may shift to focus on pulse width. Typically, pulse width is increased in increments of 10–20μs per session. As with amplitude, gradual changes may result in tolerance of higher simulation settings with fewer side effects. While literature for DBS in dystonia has documented pulse widths up to 450μs23, in the authors’ experience, optimal pulse width in pediatric patients typically ranges from 60–200μs.
Generally, frequency is the last parameter to adjust, in increments of 10Hz. An initial frequency of 120Hz may be used, with subsequent increases to 140Hz or as high as 190Hz for cranio-cervical dystonia23. Higher frequencies may also help patients with predominantly phasic dystonia70. Low frequency stimulation should be considered for patients with dystonia with poor efficacy or tolerability at traditional settings or with some component of chorea, where low frequency stimulation <40Hz may be effective71–73.
If at any point the programming device generates a warning that the chosen settings may lead to tissue damage (indicating the risk of excessively high current density), the amplitude and/or pulse width should be immediately lowered to a safe level.
Additional Troubleshooting
(See Figure 3, follow-up sessions and supplemental case vignettes).
Asymmetrical stimulation response
In patients with significantly asymmetrical hemi-body response to stimulation, it is important to verify DBS lead location, as slight lead misplacement or migration from original coordinates could result in poor stimulation response or a smaller therapeutic window. Post-operative lead migration is reported to occur in 0.5–10% of adult DBS patients74–76. While comparable literature in children is lacking, small changes in head circumference could affect lead placement over time and follow-up neuroimaging can be obtained for comparison. Recent studies suggest that directional DBS,77 with post-operative confirmation of direction, 78 may provide a larger therapeutic window for some patients. In cases of sudden asymmetrical response, it is critical to verify circuit integrity by checking impedances, as an open circuit could lead to insufficient current.
Limitations of Therapeutic Window
Each individual’s therapeutic window is dynamic, and may narrow or widen over time, related to both external and internal factors (medication use, concurrent illness, stress, fatigue, duration of stimulation, puberty, ongoing neurodevelopment, menstrual cycle, etc). Late emergence of side effects may indicate a change in the therapeutic window, in which case several interventions should be considered (Figure 4):
Adjusting stimulation settings Small changes at each programming session typically allow patients to tolerate higher settings over time. Another option is to decrease pulse width while increasing amplitude, preserving the average current69.
Adjusting contact configuration Contact reconfiguration could include a switch to bipolar or double monopolar configuration to modify the shape of the stimulation field. Another similar technique is commonly referred to as “guarding”, in which the stimulation field is constrained by “sandwiching” a negative contact (cathode) with a positive contact (anode) on either side (tripolar configuration). This focuses the volume of tissue activated centrally at the cathodic contact to diminish overflow of stimulation into surrounding tissue79 (See Figure 2 and supplemental case vignette #1).
Pulse Interleaving Pulse interleaving is a technique in which the IPG rapidly alternates between two beneficial programs involving separate contacts. Pulse width and amplitude are independently chosen for each program, but frequency must be equal, with a maximum of 125Hz set by most manufacturers. Pulse interleaving can be especially beneficial for patients with complex movement phenomenology to allow targeting of multiple symptoms and can also be used to reduce side effects or maximize efficacy in patients with a small therapeutic window25,80,81.
Directional programming Some DBS leads allow for directional programming, with radially segmented contacts that allow for current steering using individualized configurations of anodes and cathodes based on a patient’s particular anatomy and response82. This novel configuration can widen a patient’s therapeutic window by directing current away from the neural pathways inducing side effects.
Pharmacologic treatment Adjustments to pharmacologic treatments for dystonia (including botulinum toxin) may continue even after DBS implantation and on the day of programming. However, it is best to avoid medication changes within the first 6 months of DBS programming to minimize confusion.
Habituation
Patients may appear to achieve significant reduction in symptoms during a programming session, but the effect wears off within the first 1–2 weeks. Some programming strategies can help to mitigate this.
Cycling with on/off periods of stimulation Cycling the DBS on and off has been beneficial to prevent tremor habituation in some patients with Parkinson’s disease83, and similar benefits are noted in some pediatric patients with dystonia. The IPG can be programmed to automatically cycle (for example 30 seconds on and 5 seconds off). Other patients may respond better to manual cycling in which the patient or caregiver turns the stimulator off at night and on again during the day. In addition to prolonging battery life, this can help some patients retain benefit from programmed settings for a longer period.
Interval patient-initiated adjustments Virtual DBS programming sessions are infrequently performed in children, due to difficulty communicating side effects and evaluating stimulation response. However, with certain patients/families, it can be appropriate to identify a range of tolerated voltage/current amplitude during an in-person programming session, and then allow the family to use their patient programmer to increase amplitude by small increments each week within this pre-established range. The gradual increase in amplitude between programming sessions can retain the beneficial effects of stimulation.
Overstimulation
The lack of an objective measure of stimulation effects plus the latency between the programming change and improvement in dystonia may lead to overstimulation. Overstimulation can be subtle and easily missed. It is important to consider it in any patient who seems more dyskinetic or dystonic, especially when stimulation is increased. In these cases, it may be best to perform a programming overhaul, in which settings are returned to previously tolerated values or amplitude and pulse width are reduced and a monopolar review is repeated, as in the initial phases of programming. Patients may not need to return to their initial settings, but a reduction of stimulation may lead to improvement in tone and movement (see supplemental case vignette #2).
Conclusion
DBS is an established and effective tool that can be used in conjunction with pharmacologic therapy to treat dystonia in children. While not appropriate for all children with dystonia, DBS remains notably underutilized in pediatrics, likely due to a lack of familiarity and education regarding utility, feasibility, and availability of this therapy. Standardized recommendations for patient selection and DBS programming are imperative to ensure appropriate access for children who could benefit from DBS. This review summarizes current recommendations for DBS programming in dystonia and applies this evidence to inform pediatric DBS programming strategies based on institutional experiences of 9 pediatric centers. Troubleshooting tips can address common hurdles, with the goal of maximizing each child’s response to DBS. While pediatric patients may struggle with communication barriers and may ultimately require higher stimulation settings, the initial stimulation parameters for DBS programming in pediatric dystonia are similar to adults. The suggested programming algorithms allow for a systematic approach to DBS programming in pediatric dystonia to maximize functional benefit while minimizing adverse effects. As pediatric practice evolves, best-practice recommendations and evidence-based guidelines should evolve to include directional stimulation, management of multiple targets, and virtual DBS programming in children.
Supplementary Material
Acknowledgments
Rose Gelineau-Morel receives funding from the National Institute of Child Health and Human Development (2T32HD069038-11).
Dr. Gelineau-Morel receives support from the NIH (NICHD) through a T32 Pediatric Clinical and Developmental Pharmacology Training Program (2T32HD069038-11). Dr. Kruer receives grant support from the NIH, Medtronic, the Cerebral Palsy Alliance Research Foundation, Doris Duke Charitable Foundation, PTC Therapeutics, and BridgeBio, all outside of the submitted work. Dr. Coffman has received honoraria and/or travel support from the Tourette Association of America/Centers for Disease Control and Prevention, The American Board of Psychiatry and Neurology, and the FamilieSCN2A foundation outside of the submitted work. He has served as a consultant for Lundbeck Pharmaceuticals, TEVA Pharmaceuticals, PTC Therapeutics, and EuMentis Therapeutics. He has received research support from the NIH (NICHD) outside the submitted work. Dr. Zea Vera has received travel support from the Child Neurology Society and has received research support from the Tourette Association of America, American Brain Foundation, and American Academy of Neurology, all outside the submitted work. Dr. Wu receives support from NIH, outside the submitted work. Dr. Wu also consulted for Medtronic from 2018–2020. Dr. Gilbert has received payment for medical expert opinions through Advanced Medical/Teladoc. He has served as a consultant for Applied Therapeutics and Eumentics Therapeutics. He has received research support from the NIH (NIMH) and the DOD outside the submitted work. He has received book/publication royalties from Elsevier, Wolters Kluwer, and the Massachusetts Medical Society. Dr. O’Malley has attended educational dinners hosted by Boston Scientific and Medtronic.
Abbreviations:
- DBS
Deep Brain Stimulation
- GPi
Globus pallidus internus
- GPe
Globus pallidus externus
- IPG
Implantable pulse generator
- CVS
Constant voltage stimulation
- CCS
Constant current stimulation
Footnotes
Conflict of Interest Statement:
All other authors have no conflicts of interest to disclose.
References
- 1.Kogan M, McGuire M, Riley J. Deep Brain Stimulation for Parkinson Disease. Neurosurg Clin N Am. Apr 2019;30(2):137–146. doi: 10.1016/j.nec.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 2.Lake W, Hedera P, Konrad P. Deep Brain Stimulation for Treatment of Tremor. Neurosurg Clin N Am. Apr 2019;30(2):147–159. doi: 10.1016/j.nec.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 3.Hachem LD, Yan H, Ibrahim GM. Invasive Neuromodulation for the Treatment of Pediatric Epilepsy. Neurotherapeutics. Jan 2019;16(1):128–133. doi: 10.1007/s13311-018-00685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salanova V. Deep brain stimulation for epilepsy. Epilepsy Behav. Nov 2018;88s:21–24. doi: 10.1016/j.yebeh.2018.06.041 [DOI] [PubMed] [Google Scholar]
- 5.Dietz N, Neimat J. Neuromodulation: Deep Brain Stimulation for Treatment of Dystonia. Neurosurg Clin N Am. Apr 2019;30(2):161–168. doi: 10.1016/j.nec.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 6.Arya S, Filkowski MM, Nanda P, Sheth SA. Deep brain stimulation for obsessive-compulsive disorder. Bull Menninger Clin. Winter 2019;83(1):84–96. doi: 10.1521/bumc.2019.83.1.84 [DOI] [PubMed] [Google Scholar]
- 7.Coulombe MA, Elkaim LM, Alotaibi NM, et al. Deep brain stimulation for Gilles de la Tourette syndrome in children and youth: a meta-analysis with individual participant data. J Neurosurg Pediatr. Oct 26 2018;23(2):236–246. doi: 10.3171/2018.7.peds18300 [DOI] [PubMed] [Google Scholar]
- 8.Sturm V, Fricke O, Bührle CP, et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2012;6:341. doi: 10.3389/fnhum.2012.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stocco A, Baizabal-Carvallo JF. Deep brain stimulation for severe secondary stereotypies. Parkinsonism Relat Disord. Sep 2014;20(9):1035–6. doi: 10.1016/j.parkreldis.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 10.Segar DJ, Chodakiewitz YG, Torabi R, Cosgrove GR. Deep brain stimulation for the obsessive-compulsive and Tourette-like symptoms of Kleefstra syndrome. Neurosurg Focus. Jun 2015;38(6):E12. doi: 10.3171/2015.3.FOCUS1528 [DOI] [PubMed] [Google Scholar]
- 11.Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. Aug 2012;37(9):1975–85. doi: 10.1038/npp.2012.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubu CS, Brelje T, Butters MA, et al. Cognitive outcome after ventral capsule/ventral striatum stimulation for treatment-resistant major depression. J Neurol Neurosurg Psychiatry. Mar 2017;88(3):262–265. doi: 10.1136/jnnp-2016-313803 [DOI] [PubMed] [Google Scholar]
- 13.Cif L, Coubes P. Historical developments in children’s deep brain stimulation. Eur J Paediatr Neurol. Jan 2017;21(1):109–117. doi: 10.1016/j.ejpn.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 14.Elkaim LM, De Vloo P, Kalia SK, Lozano AM, Ibrahim GM. Deep brain stimulation for childhood dystonia: current evidence and emerging practice. Expert Rev Neurother. Oct 2018;18(10):773–784. doi: 10.1080/14737175.2018.1523721 [DOI] [PubMed] [Google Scholar]
- 15.Lin JP, Kaminska M, Perides S, et al. Bilateral globus pallidus internus deep brain stimulation for dyskinetic cerebral palsy supports success of cochlear implantation in a 5-year old ex-24 week preterm twin with absent cerebellar hemispheres. Eur J Paediatr Neurol. Jan 2017;21(1):202–213. doi: 10.1016/j.ejpn.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 16.Perides S, Lin JP, Lee G, et al. Deep brain stimulation reduces pain in children with dystonia, including in dyskinetic cerebral palsy. Dev Med Child Neurol. 08 2020;62(8):917–925. doi: 10.1111/dmcn.14555 [DOI] [PubMed] [Google Scholar]
- 17.Marks WA, Honeycutt J, Acosta F, et al. Dystonia due to cerebral palsy responds to deep brain stimulation of the globus pallidus internus. Mov Disord. Aug 2011;26(9):1748–51. doi: 10.1002/mds.23723 [DOI] [PubMed] [Google Scholar]
- 18.Koy A, Hellmich M, Pauls KAM, et al. Effects of deep brain stimulation in dyskinetic cerebral palsy: A meta-analysis. Movement Disorders. 2013;28(5):647–654. [DOI] [PubMed] [Google Scholar]
- 19.Larsh T, Wu SW, Vadivelu S, Grant GA, O’Malley JA. Deep Brain Stimulation for Pediatric Dystonia. Semin Pediatr Neurol. Jul 2021;38:100896. doi: 10.1016/j.spen.2021.100896 [DOI] [PubMed] [Google Scholar]
- 20.Jankovic J. Treatment of dystonia. Lancet Neurol. Oct 2006;5(10):864–72. doi: 10.1016/s1474-4422(06)70574-9 [DOI] [PubMed] [Google Scholar]
- 21.Fehlings D, Brown L, Harvey A, et al. Pharmacological and neurosurgical interventions for managing dystonia in cerebral palsy: a systematic review. Dev Med Child Neurol. Apr 2018;60(4):356–366. doi: 10.1111/dmcn.13652 [DOI] [PubMed] [Google Scholar]
- 22.Lumsden DE, Kaminska M, Tomlin S, Lin JP. Medication use in childhood dystonia. Eur J Paediatr Neurol. Jul 2016;20(4):625–9. doi: 10.1016/j.ejpn.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 23.Magown P, Andrade RA, Soroceanu A, Kiss ZHT. Deep brain stimulation parameters for dystonia: A systematic review. Parkinsonism Relat Disord. 09 2018;54:9–16. doi: 10.1016/j.parkreldis.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 24.Picillo M, Lozano AM, Kou N, Munhoz RP, Fasano A. Programming Deep Brain Stimulation for Tremor and Dystonia: The Toronto Western Hospital Algorithms. Brain Stimul. 2016. May-Jun 2016;9(3):438–452. doi: 10.1016/j.brs.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 25.Koeglsperger T, Palleis C, Hell F, Mehrkens JH, Bötzel K. Deep Brain Stimulation Programming for Movement Disorders: Current Concepts and Evidence-Based Strategies. Front Neurol. 2019;10:410. doi: 10.3389/fneur.2019.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagle Shukla A, Zeilman P, Fernandez H, Bajwa JA, Mehanna R. DBS Programming: An Evolving Approach for Patients with Parkinson’s Disease. Parkinsons Dis. 2017;2017:8492619. doi: 10.1155/2017/8492619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steigerwald F, Kirsch AD, Kühn AA, et al. Evaluation of a programming algorithm for deep brain stimulation in dystonia used in a double-blind, sham-controlled multicenter study. Neurol Res Pract. 2019;1:25. doi: 10.1186/s42466-019-0032-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills KA, Starr PA, Ostrem JL. Neuromodulation for dystonia: target and patient selection. Neurosurg Clin N Am. Jan 2014;25(1):59–75. doi: 10.1016/j.nec.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 29.Aravamuthan BR, Waugh JL, Stone SS. Deep brain stimulation for monogenic dystonia. Curr Opin Pediatr. Dec 2017;29(6):691–696. doi: 10.1097/mop.0000000000000548 [DOI] [PubMed] [Google Scholar]
- 30.Russ JB, Nallappan AM, Robichaux-Viehoever A. Management of Pediatric Movement Disorders: Present and Future. Semin Pediatr Neurol. Apr 2018;25:136–151. doi: 10.1016/j.spen.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 31.Tisch S, Kumar KR. Pallidal Deep Brain Stimulation for Monogenic Dystonia: The Effect of Gene on Outcome. Front Neurol. 2020;11:630391. doi: 10.3389/fneur.2020.630391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cif L, Demailly D, Lin JP, et al. KMT2B-related disorders: expansion of the phenotypic spectrum and long-term efficacy of deep brain stimulation. Brain. 12 2020;143(11):3242–3261. doi: 10.1093/brain/awaa304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moro E, LeReun C, Krauss JK, et al. Efficacy of pallidal stimulation in isolated dystonia: a systematic review and meta-analysis. Eur J Neurol. Apr 2017;24(4):552–560. doi: 10.1111/ene.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruber D, Kühn AA, Schoenecker T, et al. Pallidal and thalamic deep brain stimulation in myoclonus-dystonia. Mov Disord. Aug 2010;25(11):1733–43. doi: 10.1002/mds.23312 [DOI] [PubMed] [Google Scholar]
- 35.Kosutzka Z, Tisch S, Bonnet C, et al. Long-term GPi-DBS improves motor features in myoclonus-dystonia and enhances social adjustment. Mov Disord. 01 2019;34(1):87–94. doi: 10.1002/mds.27474 [DOI] [PubMed] [Google Scholar]
- 36.Danielsson A, Carecchio M, Cif L, et al. Pallidal Deep Brain Stimulation in DYT6 Dystonia: Clinical Outcome and Predictive Factors for Motor Improvement. J Clin Med. Dec 06 2019;8(12)doi: 10.3390/jcm8122163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brüggemann N, Kühn A, Schneider SA, et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology. Mar 2015;84(9):895–903. doi: 10.1212/WNL.0000000000001312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jinnah HA, Alterman R, Klein C, et al. Deep brain stimulation for dystonia: a novel perspective on the value of genetic testing. J Neural Transm (Vienna). 04 2017;124(4):417–430. doi: 10.1007/s00702-016-1656-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koy A, Timmermann L. Deep brain stimulation in cerebral palsy: Challenges and opportunities. Eur J Paediatr Neurol. Jan 2017;21(1):118–121. doi: 10.1016/j.ejpn.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 40.Elia AE, Bagella CF, Ferré F, Zorzi G, Calandrella D, Romito LM. Deep brain stimulation for dystonia due to cerebral palsy: A review. Eur J Paediatr Neurol. Mar 2018;22(2):308–315. doi: 10.1016/j.ejpn.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 41.Lumsden DE, Kaminska M, Ashkan K, Selway R, Lin JP. Deep brain stimulation for childhood dystonia: Is ‘where’ as important as in ‘whom’? Eur J Paediatr Neurol. Jan 2017;21(1):176–184. doi: 10.1016/j.ejpn.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 42.Lumsden DE, Kaminska M, Gimeno H, et al. Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Developmental Medicine & Child Neurology. 2013;55(6):567–574. [DOI] [PubMed] [Google Scholar]
- 43.Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. Dec 2010;81(12):1383–9. doi: 10.1136/jnnp.2010.207993 [DOI] [PubMed] [Google Scholar]
- 44.Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. Dec 2012;11(12):1029–38. doi: 10.1016/S1474-4422(12)70257-0 [DOI] [PubMed] [Google Scholar]
- 45.Tambirajoo K, Furlanetti L, Samuel M, Ashkan K. Subthalamic Nucleus Deep Brain Stimulation in Post-Infarct Dystonia. Stereotact Funct Neurosurg. 2020;98(6):386–398. doi: 10.1159/000509317 [DOI] [PubMed] [Google Scholar]
- 46.San Luciano M, Robichaux-Viehoever A, Dodenhoff KA, et al. Thalamic deep brain stimulation for acquired dystonia in children and young adults: a phase 1 clinical trial. J Neurosurg Pediatr. Nov 27 2020:1–10. doi: 10.3171/2020.7.PEDS20348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horisawa S, Kohara K, Nonaka T, Mochizuki T, Kawamata T, Taira T. Case Report: Deep Cerebellar Stimulation for Tremor and Dystonia. Front Neurol. 2021;12:642904. doi: 10.3389/fneur.2021.642904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown EG, Bledsoe IO, Luthra NS, Miocinovic S, Starr PA, Ostrem JL. Cerebellar Deep Brain Stimulation for Acquired Hemidystonia. Mov Disord Clin Pract. Feb 2020;7(2):188–193. doi: 10.1002/mdc3.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeharia N, Hertz U, Flash T, Amedi A. New whole-body sensory-motor gradients revealed using phase-locked analysis and verified using multivoxel pattern analysis and functional connectivity. J Neurosci. Feb 2015;35(7):2845–59. doi: 10.1523/JNEUROSCI.4246-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berman BD, Starr PA, Marks WJ, Ostrem JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotact Funct Neurosurg. 2009;87(1):37–44. doi: 10.1159/000195718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schrader C, Capelle HH, Kinfe TM, et al. GPi-DBS may induce a hypokinetic gait disorder with freezing of gait in patients with dystonia. Neurology. Aug 2011;77(5):483–8. doi: 10.1212/WNL.0b013e318227b19e [DOI] [PubMed] [Google Scholar]
- 52.Deeb W, Patel A, Okun MS, Gunduz A. Management of Elevated Therapeutic Impedances on Deep Brain Stimulation Leads. Tremor Other Hyperkinet Mov (N Y). 2017;7:493. doi: 10.7916/D8BR94MV [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol. Feb 2006;117(2):447–54. doi: 10.1016/j.clinph.2005.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samura K, Miyagi Y, Okamoto T, et al. Short circuit in deep brain stimulation. J Neurosurg. Nov 2012;117(5):955–61. doi: 10.3171/2012.8.JNS112073 [DOI] [PubMed] [Google Scholar]
- 55.Bronstein JM, Tagliati M, McIntyre C, et al. The rationale driving the evolution of deep brain stimulation to constant-current devices. Neuromodulation. Feb 2015;18(2):85–8; discussion 88–9. doi: 10.1111/ner.12227 [DOI] [PubMed] [Google Scholar]
- 56.Horn A, Kühn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. Feb 15 2015;107:127–135. doi: 10.1016/j.neuroimage.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Prudente CN, Stilla R, Sathian K, Jinnah HA, Hu X. Alterations of resting-state fMRI measurements in individuals with cervical dystonia. Hum Brain Mapp. Aug 2017;38(8):4098–4108. doi: 10.1002/hbm.23651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Premi E, Diano M, Gazzina S, et al. Functional Connectivity Networks in Asymptomatic and Symptomatic DYT1 Carriers. Mov Disord. 11 2016;31(11):1739–1743. doi: 10.1002/mds.26725 [DOI] [PubMed] [Google Scholar]
- 59.Fuertinger S, Simonyan K. Task-specificity in focal dystonia is shaped by aberrant diversity of a functional network kernel. Mov Disord. 12 2018;33(12):1918–1927. doi: 10.1002/mds.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanekamp S, Simonyan K. The large-scale structural connectome of task-specific focal dystonia. Hum Brain Mapp. Apr 2020;doi: 10.1002/hbm.25012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horn A, Reich M, Vorwerk J, et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. Jul 2017;82(1):67–78. doi: 10.1002/ana.24974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Fatly B, Ewert S, Kübler D, Kroneberg D, Horn A, Kühn AA. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain. 10 01 2019;142(10):3086–3098. doi: 10.1093/brain/awz236 [DOI] [PubMed] [Google Scholar]
- 63.Lumsden DE, Kaminska M, Tustin K, et al. Battery life following pallidal deep brain stimulation (DBS) in children and young people with severe primary and secondary dystonia. Childs Nerv Syst. Jul 2012;28(7):1091–7. doi: 10.1007/s00381-012-1728-6 [DOI] [PubMed] [Google Scholar]
- 64.Montuno M, Kohner A, Okun M. DBS Battery Estimator. Accessed August 12, 2021. https://movementdisorders.ufhealth.org/surgery/dbs-battery-estimator/
- 65.Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. Jun 1999;41(6):404–11. doi: 10.1017/s0012162299000870 [DOI] [PubMed] [Google Scholar]
- 66.Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. Jan 1985;35(1):73–7. doi: 10.1212/wnl.35.1.73 [DOI] [PubMed] [Google Scholar]
- 67.Gimeno H, Tustin K, Selway R, Lin JP. Beyond the Burke-Fahn-Marsden Dystonia Rating Scale: deep brain stimulation in childhood secondary dystonia. Eur J Paediatr Neurol. Sep 2012;16(5):501–8. doi: 10.1016/j.ejpn.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 68.Yokochi F, Kato K, Iwamuro H, et al. Resting-State Pallidal-Cortical Oscillatory Couplings in Patients With Predominant Phasic and Tonic Dystonia. Front Neurol. 2018;9:375. doi: 10.3389/fneur.2018.00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanger TD. Deep brain stimulation for cerebral palsy: where are we now? Dev Med Child Neurol. Jan 2020;62(1):28–33. doi: 10.1111/dmcn.14295 [DOI] [PubMed] [Google Scholar]
- 70.Barow E, Neumann WJ, Brücke C, et al. Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain. Nov 2014;137(Pt 11):3012–3024. doi: 10.1093/brain/awu258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dy ME, Chang FC, Jesus SD, et al. Treatment of ADCY5-Associated Dystonia, Chorea, and Hyperkinetic Disorders With Deep Brain Stimulation: A Multicenter Case Series. J Child Neurol. 07 2016;31(8):1027–35. doi: 10.1177/0883073816635749 [DOI] [PubMed] [Google Scholar]
- 72.Edwards TC, Zrinzo L, Limousin P, Foltynie T. Deep brain stimulation in the treatment of chorea. Mov Disord. Mar 2012;27(3):357–63. doi: 10.1002/mds.23967 [DOI] [PubMed] [Google Scholar]
- 73.Shin H, Ki CS, Cho AR, et al. Globus pallidus interna deep brain stimulation improves chorea and functional status in a patient with chorea-acanthocytosis. Stereotact Funct Neurosurg. 2012;90(4):273–7. doi: 10.1159/000338216 [DOI] [PubMed] [Google Scholar]
- 74.Boviatsis EJ, Stavrinou LC, Themistocleous M, Kouyialis AT, Sakas DE. Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta neurochirurgica. 2010;152(12):2053–2062. [DOI] [PubMed] [Google Scholar]
- 75.Morishita T, Hilliard JD, Okun MS, et al. Postoperative lead migration in deep brain stimulation surgery: Incidence, risk factors, and clinical impact. PLOS ONE. 2017–09-13 2017;12(9):e0183711. doi: 10.1371/journal.pone.0183711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bouwens van der Vlis TA, van de Veerdonk MG, Ackermans L, et al. Surgical and Hardware-Related Adverse Events of Deep Brain Stimulation: A Ten-Year Single-Center Experience. Neuromodulation : journal of the International Neuromodulation Society. 2022. Feb 2022;25(2)doi: 10.1016/j.neurom.2021.12.011 [DOI] [PubMed] [Google Scholar]
- 77.Dembek TA, Reker P, Visser-Vandewalle V, et al. Directional DBS increases side-effect thresholds—A prospective, double-blind trial. Movement Disorders. 2017;32(10):1380–1388. doi: 10.1002/mds.27093 [DOI] [PubMed] [Google Scholar]
- 78.Dembek TA, Hoevels M, Hellerbach A, et al. Directional DBS leads show large deviations from their intended implantation orientation. Parkinsonism & Related Disorders. 2019/10/01/ 2019;67:117–121. doi: 10.1016/j.parkreldis.2019.08.017 [DOI] [PubMed] [Google Scholar]
- 79.Valente V, Demosthenous A, Bayford R. A tripolar current-steering stimulator ASIC for field shaping in deep brain stimulation. IEEE Trans Biomed Circuits Syst. Jun 2012;6(3):197–207. doi: 10.1109/TBCAS.2011.2171036 [DOI] [PubMed] [Google Scholar]
- 80.Miocinovic S, Khemani P, Whiddon R, et al. Outcomes, management, and potential mechanisms of interleaving deep brain stimulation settings. Parkinsonism Relat Disord. Dec 2014;20(12):1434–7. doi: 10.1016/j.parkreldis.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 81.Kovács N, Janszky J, Nagy F, Balás I. Changing to interleaving stimulation might improve dystonia in cases not responding to pallidal stimulation. Movement Disorders. 2012–01-01 2012;27(1):163–165. doi: 10.1002/mds.23962 [DOI] [PubMed] [Google Scholar]
- 82.Schüpbach WMM, Chabardes S, Matthies C, et al. Directional leads for deep brain stimulation: Opportunities and challenges. Mov Disord. 10 2017;32(10):1371–1375. doi: 10.1002/mds.27096 [DOI] [PubMed] [Google Scholar]
- 83.Enatsu R, Kitagawa M, Morishita T, et al. Effect of Cycling Thalamosubthalamic Stimulation on Tremor Habituation and Rebound in Parkinson Disease. World Neurosurg. 12 2020;144:64–67. doi: 10.1016/j.wneu.2020.08.141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.