Abstract
Cross-sectional magnetic resonance enterography (MRE) and intestinal ultrasonography (IUS) provide valuable and noninvasive information to accurately assess disease activity, severity, and extent; detect complications; and monitor the response to treatment, as well as predict the postoperative recurrence of Crohn’s disease and a negative disease course. Therefore, both imaging modalities are emerging as pivotal diagnostic tools to achieve the emerging therapeutic target of transmural healing associated with better disease outcomes. Despite its numerous potential advantages over endoscopy and even MRE and its good availability, IUS is still widely underused to monitor and manage inflammatory bowel disease (IBD) patients and help in making clinical decisions in routine practice. This situation is clearly due to the absence of validated, reliable, and responsive indices, as well as the lack of trained gastroenterologists and radiologists, as IUS is a component of radiologist expertise in several countries but not yet integrated into the training program of gastroenterologists. However, there is an increasing body of evidence in the literature that IUS and MRE are both becoming essential imaging resources to help clinicians in making reliable decisions. Here, we discuss the up-to-date evidence about the usefulness and performance of cross-sectional imaging, focusing on the ability of bowel US and MRE to aid clinical decision-making for the optimal management and monitoring of IBD.
Keywords: clinical decision, Crohn’s disease, MRI, ulcerative colitis, ultrasound
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are life-long, relapsing, and destructive chronic inflammatory bowel disorders that can result in progressive bowel damage, impairment of the quality of life, and disability. The management of inflammatory bowel disease (IBD) has considerably evolved over the last several decades with a novel effective medical armamentarium, and marked changes in recommended therapeutic strategies, including tight control with targeted treatment accompanied by promising imaging modalities. Beyond disease-related symptoms, physicians need objective information on disease activity and severity and the extent of the disease, as well as on complications to adequately adapt the treatment of IBD patients (drug changes or optimization). Although ileocolonoscopy is still the reference standard and is widely used, both in clinical practice and in therapeutic trials, alternative less expensive, less invasive, and more acceptable tools, including cross-sectional imaging modalities [intestinal ultrasonography (IUS), magnetic resonance enterography (MRE), and computed tomography (CT)] represent alternatives to ileocolonoscopy for assessing disease activity and closely and accurately monitoring the response to treatment. In contrast to endoscopy, CT and magnetic resonance imaging (MRI) allow assessment of the entire length and integrity of the small bowel and colon at a time when transmural healing appears to be of great interest in improving disease outcomes, vascularity, and also the perienteric environment. Except for the detection of abdominal complications in emergency settings, in which abdominal CT is still useful, we focused on IUS and MRE for monitoring and decision-making to guide the management of IBD patients because of the cumulative radiation dose exposure potentially associated with CT and the potential associated risk of induced cancers, making its routine use impractical. Despite growing data available in the field of IBD and its strengths, IUS remains widely underused actually to guide therapeutic decision in clinical practice. Relative to ileocolonoscopy, MRE and IUS are minimally invasive or noninvasive and better tolerated by patients, who consider them to be acceptable for monitoring, as reported in a French patient self-questionnaire-based survey of 618 CD and 298 UC patients from both public and private centers using visual analog scales.1 In addition, IUS and MRE do not require prior bowel preparation or anesthesia. MRE has the key advantages of providing good discrimination between various soft tissue types, a wide field of view, multiplanar image acquisition, and no ionizing radiation. However, IUS, despite not being able to explore the entire bowel (especially proximal small bowel), provides additional advantages over MRE, including lower cost, better acceptability, minimal invasiveness, neither requiring oral administration of a hyperosmotic solution nor intravenous administration of gadolinium contrast agents (which can be associated with allergic-like reactions, nephrogenic systemic fibrosis, and gadolinium retention in the brain), and not requiring fasting. Moreover, IUS is easy to perform, readily available, and reproducible and therefore well adapted for disease monitoring on a regular basis. It can be also performed on a point-of-care (POC) basis, providing real-time information that can extend the physical examination to guide clinical decisions. Both IUS and MRE provide objective disease activity-related information that can help clinicians in their decision-making. These imaging modalities are thus of paramount interest for the optimal management of IBD patients and the subsequent adaptation of their treatment (need of drug changes or intensification) or referral for hospitalization or surgery. However, despite its cost-effectiveness, favorable benefit–risk balance, and high patient acceptability, the widespread use of IUS is still limited for IBD management in routine clinical practice due to concerns about its validity, reliability, and responsiveness. This review summarizes and discusses the up-to-date evidence concerning the accuracy and usefulness of cross-sectional imaging, focusing on the comparison of bowel US with MRE for clinical decision-making for optimal management and monitoring of patients with IBD.
Assessment of disease activity and severity
The objective control of intestinal inflammation is becoming the central driver for optimal medical management in IBD to reduce disease progression and intestinal damage. For this purpose, clinicians need to assess and monitor disease activity on a regular basis to assess disease control and make decisions concerning treatment intensification or modification. IUS and MRE are two available imaging modalities that are able to accurately detect signs of disease activity and severity. Several systematic reviews and meta-analyses have reported high accuracy and agreement between IUS and MRE in assessing disease activity in CD without any significant differences.2–7 Relative to ileocolonoscopy, used as the reference standard, high specificities were also reported for both IUS and MRE for predicting active colonic inflammation in a recent meta-analysis.8 Various parameters have been shown to be associated with inflammation as measured by IUS, including bowel wall thickness (BWT), mural and extramural changes, and the modification of bowel wall flow (Figures 1 and 2). Mural changes include ulceration(s) described as focal linear hypoechoic lesion crossing wall layers and edema shown as disruption of bowel wall stratification. Perienteric inflammation and creeping fat are also associated with inflammation as well as enlarged mesenteric lymph nodes, although nonspecific, may be supportive of disease activity. Detection of intramural and extramural abnormal blood flow by color Doppler on IUS is also representative of intestinal hyperemia in inflammatory bowel segments. Among these various parameters, BWT is the most consistent individual quantifiable and reliable measurement to assess intestinal inflammation and disease activity in both the small bowel and colon (except in the rectum) in both CD and UC. Based on expert consensus, given the optimal sensitivity and specificity of inflammation detected by IUS in correlation to endoscopy, the optimal threshold for detecting active disease has been defined as 3 mm.9,10 A recent systematic review and meta-analysis confirmed the pooled sensitivity and specificity to be high for detecting colorectal segments with inflammation at the threshold of BWT ⩾3 mm (86.4% and 88.3%, respectively).10 The addition of an increased Doppler signal and abnormal or loss of bowel wall stratification resulted in even higher diagnostic accuracy of intestinal inflammation (Figure 3(a) and (b)). In contrast with other colonic segments, the diagnostic accuracy of transabdominal IUS with color Doppler for detecting active disease was lower in the rectum due to its deep location in the pelvis even if it is feasible through the bladder. In addition, transperineal ultrasound (TUS) has been proposed to accurately assess rectal BWT in a cohort of 53 UC patients.11 With this technique, BWT ⩽4 mm was the only independent predictor of rectal endoscopic and histologic healing. Therefore, TUS performed with conventional probes, positioned directly above the anus may capture images of the anal canal and rectum and may be of great interest to monitor patients with proctitis. Similarly, ultrasound examination may also be sometimes substantially limited in obese patients with thick abdominal adipose tissue. Abnormal BWT and bowel wall flow could be combined with elevated levels of fecal calprotectin to compensate for the lower sensitivity and improve the specificity of IUS to detect active disease in these situations.12,13 In UC, combining BWT > 2 mm or fecal calprotectin levels >200 µg/g resulted in a sensitivity of 94.9% and a specificity of 66.7% for the detection of endoscopically active disease, defined by an endoscopic Mayo subscore of 1–3.14 The likelihood of identifying intestinal inflammation by combining BWT >3 mm and increased Doppler signal and loss of bowel wall stratification was also reported to be higher than that of using BWT >3 mm alone10 (Figures 2 and 3). Beyond abnormal BWT, other relevant imaging-based findings concerning mural changes have been reported for assessing inflammation (Figures 3–5). The presence of ulcerations featured on IUS by a focal loss of bowel wall stratification with a hypoechoic area crossing wall layers, as well as parietal edema featured on IUS as a disruption of bowel wall stratification and on MRE as an increased intramural T2 signal, reflects transmural inflammation. Other mural changes detected by MRE associated with intestinal inflammation include bowel wall hyperenhancement after the injection of contrast medium, as well as extramural perienteric changes, such as fat wrapping or the presence of enlarged abdominal lymph nodes. Other additional imaging features may reflect intestinal inflammation, such as a reduction in intestinal motility and restricted diffusion. Diffusion-weighted imaging (DWI), initially established as an indicator of early ischemic tissular changes, is an imaging procedure that relies on differences in the motion of water molecules between tissues. DWI is feasible both in CD and UC and has demonstrated its utility for assessing inflammatory activity relative to colonoscopy and MRE. Restricted diffusion in combination with the apparent diffusion coefficient (ADC) offers additional diagnostic values to conventional MRE, without the need for contrast administration, to accurately distinguish between active and inactive IBD. In UC, the loss or lack of haustration has been found to be highly related with endoscopically active disease.14 For MRE, BWT measurement ideally requires a distended bowel segment on either T2 or contrast-enhanced T1 sequences to minimize the risk of overestimation of inflammation. Physicians should be aware that adequate bowel luminal distension (requiring oral and/or rectal administration of negative contrast agent) not only influences the quality of MRE and image interpretation, but also impacts the acceptability of the patients. Overall, IUS or MRE associated with ileocolonoscopy for the detection and grading of the severity of mucosal and transmural inflammation is considered to be the most complete approach to assess disease activity.
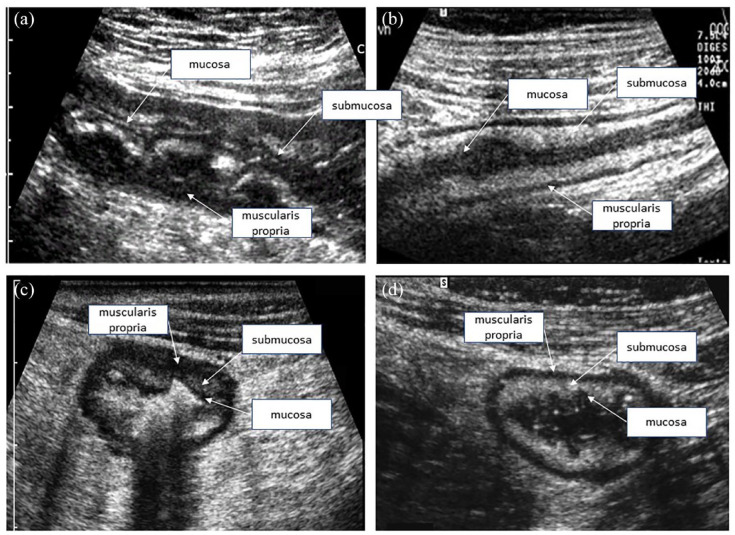
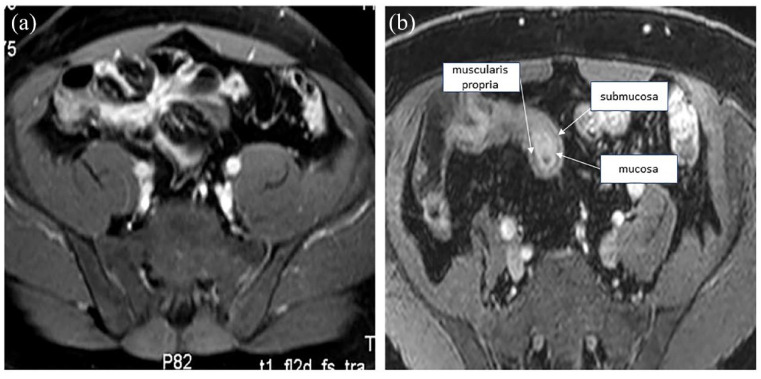
Figure 1.
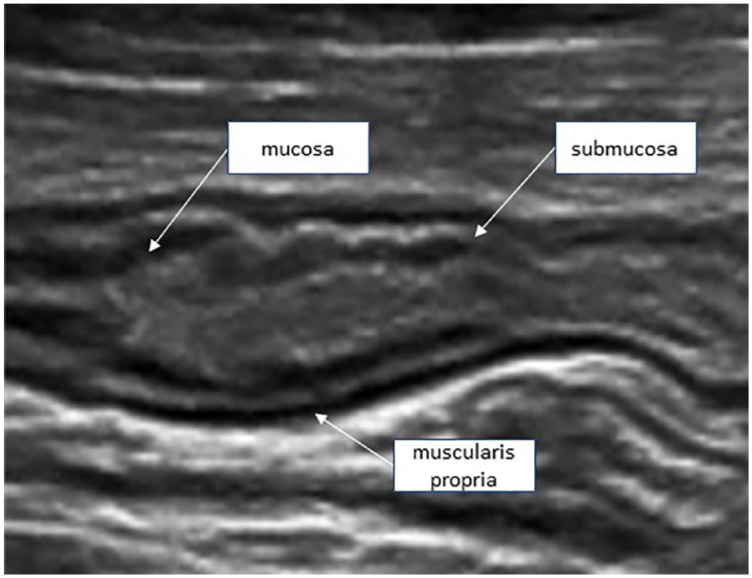
Normal ultrasound appearance of the small bowel, allowing the differentiation of the mucosa, submucosa, muscularis propria, and serosa (echostratification).
Figure 2.
Sagittal and axial views of the small bowel in CD (a, b) and UC (c, d). In CD, the bowel wall shows transmural thickening and involvement, with diffuse low echogenicity. In UC, the thickening is limited to the mucosa and submucosa, with no transmural changes.
CD, Crohn’s disease; UC, ulcerative colitis.
Figure 3.
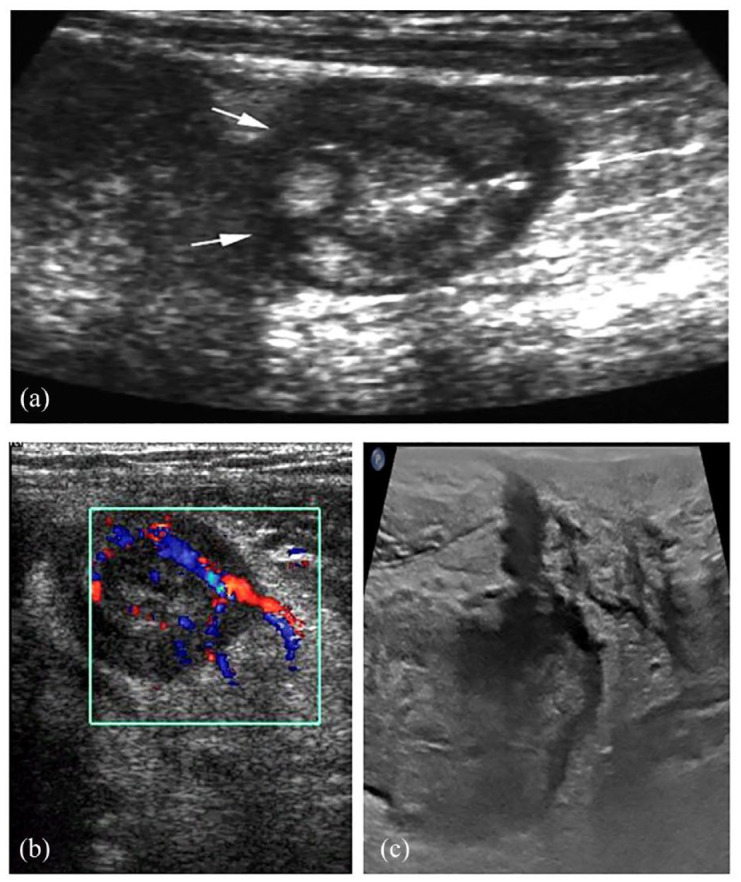
Representative images of small bowel lesions in CD. (a) Transverse images showing deep ulcerations (arrows). (b) Increased vascularity on color Doppler images. (c) A fistula is well identified as a low echogenicity tract in the adjacent fat.
CD, Crohn’s disease.
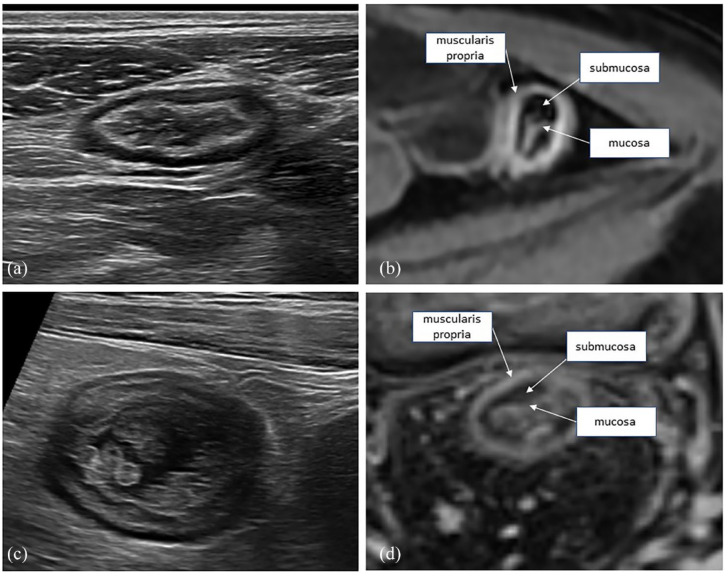
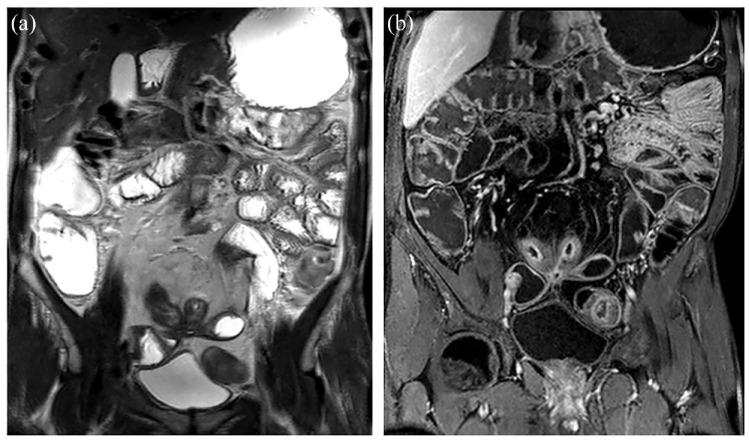
Figure 4.
Representative images of large bowel involvement in UC. IUS (a) and MRE (b) of the descending colon, showing slight colon wall thickening, with increased enhancement of the mucosal/submucosal complex on T1wMRE. IUS (c) and MRI (d) showing moderate thickening of the rectal wall, with the development of pseudo-polyps. Note the slight enhancement of the rectal wall, as well as the low signal of the submucosa, corresponding to fatty replacement, as seen in chronic presentations of the disease.
IUS, intestinal ultrasonography; MRI, magnetic resonance imaging; T1wMRE, T1-weighted magnetic resonance enterography; UC, ulcerative colitis.
Figure 5.
Axial MRE of CD patients before and after contrast injection. After the injection of contrast, marked enhancement of the wall of the terminal ileum (a) can be observed, indicating disease activity. Slight contrast enhancement, indicating quiescent disease (b).
CD, Crohn’s disease; MRE, magnetic resonance enterography.
Bowel wall vascularization
Intestinal hyperemia, as well as neoangiogenesis, has been reported to be associated with inflammation and disease activity. Abnormal intramural and extramural intestinal blood flow can be observed by IUS coupled with color Doppler in inflammatory bowel segments and represent additional parameters of inflammation in active IBD (Figure 3(b)). Increased contrast hyperenhancement allows the qualitative assessment of the presence of inflammation by MRE (Figures 4–6). In addition, when present, a comb sign, defined as regional dilatation of the vasa recta, is also suggestive of disease activity.15 The added value of abdominal color Doppler or contrast enhancement US (CEUS) over conventional US to detect inflammation is still a subject of debate. The accuracy of these ultrasonographic modalities is almost the same, with a sensitivity of 90%, 90%, and 94% and a specificity of 94%, 94%, and 94% for conventional US, color Doppler US, and CEUS to detect activity, respectively.16 The performance of intestinal CEUS was also found to be quite similar to that of conventional US using histopathological analysis as the standard reference.17 In this prospective study comparing IUS coupled with CEUS and MRE for the stratification of CD patients based on the presence or absence of severe active CD assessed by histopathological analysis, the accuracy of IUS based on BWT did not differ from that of MRE to predict highly active disease. When associated with injection of a contrast agent allowing a quantitative assessment of bowel wall flow, CEUS provides several quantitative parameters from the time–intensity curve analysis. However, data on the patient’s acceptability of CEUS relative to IUS are lacking and despite numerous studies that have investigated the value of such time–intensity curve-based parameters in the assessment of CD activity, none were able to identify reliable and clinically pertinent parameters responsive to change.17–20 Altogether, compared with histopathology, CEUS cannot distinguish between inflammatory activity and fibrosis.21 In addition, one of the most common concerns about CEUS is the lack of reproducibility in most of the steps leading to contrast agent injection and the need to manually select regions of interest, as well as the limitation that the entire procedure is time-consuming.
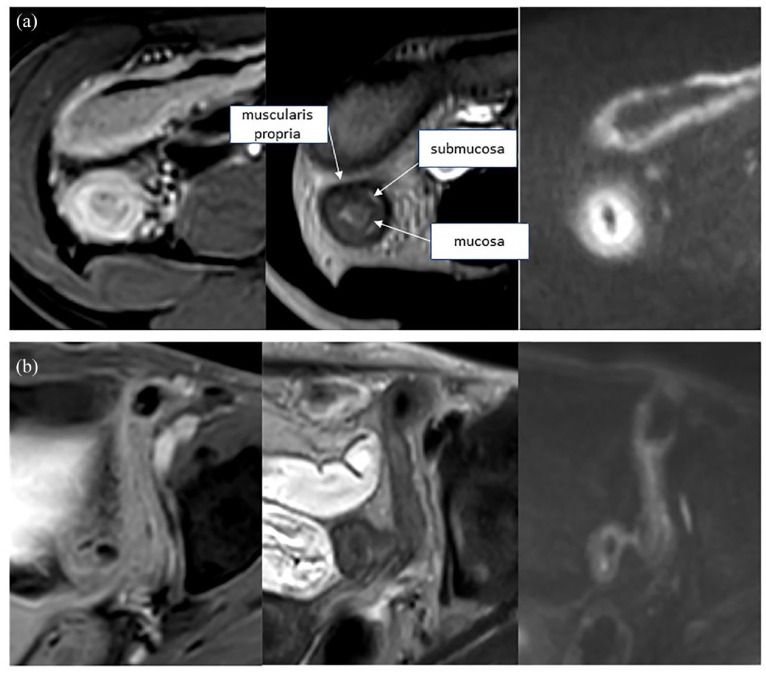
Figure 6.
Axial MRE of CD patients. Marked enhancement, wall edema on T2, and restricted diffusion, indicating active disease (a). Slight enhancement, low T2 signal, and the absence of restricted diffusion, indicating quiescent/chronic disease (b).
MRE, magnetic resonance enterography.
Diagnostic accuracy for determining the degree of intestinal fibrosis
Small bowel strictures are often characterized by different degrees of inflammation and fibrosis. It is important to evaluate the predominant inflammatory or fibrotic status of gut CD-related strictures; such information has a significant influence on the therapeutic decision since inflammatory strictures usually tend to respond to anti-inflammatory medications, whereas predominant fibrotic stenoses are more likely to require balloon dilation or surgery.22 Except for histopathological analysis, there is no validated tool for the assessment of inflammatory or fibrotic stricture. A systematic review, including only studies with histopathological results taken as the reference standard, investigated the accuracy of various cross-sectional imaging for assessment of CD-associated small bowel strictures and fibrosis.23 IUS estimates of sensitivity for stricture diagnosis were 80–100% with specificity rates of 63–75%. The sensitivity and specificity of small intestinal contrast ultrasonography (SICUS) were increased and ranged from 88% to 98% and 88% and 100%, respectively. The diagnostic accuracy of MRE was quite similar with sensitivity between 75% and 100% and specificity rates of 91–96%. However, contrast-enhanced US may assist in determining the degree of fibrosis and inflammation within a stricture. Indeed, by applying a dichotomized histopathology inflammatory versus fibrotic score, the vast majority of CD-associated strictures (82%) were correctly classified in a small cohort of 25 patients.24 Zappa et al. have studied the performance of MRE findings to accurately discriminate inflammation from fibrosis in small bowel CD-associated strictures, taking surgical pathologic analysis as reference.25 They found that BWT, mural hyperintensity, comb sign, and the presence of fistula correlated with fibrosis as well as inflammation. In a cohort of 41 CD patients who performed MRI within 4 months before undergoing surgery, using the analysis of surgical specimens as reference, the degree of inflammation was associated with various MRE parameters, including mucosal enhancement, ulcerations, and blurred margins, whereas fibrosis was significantly correlated with the percentage of enhancement gain, the pattern of enhancement at 7 min, and the presence of stenosis.26 New MRE sequences, including the perfusion fraction (intravoxel incoherent motion used to estimate blood flow in tissues), T1 mapping sequence, and reduction of relaxation time, have been recently associated with fibrosis in a prospective, single-center cohort study including 33 CD patients.27 In clinical practice, the association of IUS examination and Doppler with SICUS or CEUS or MRE with such novel sequences may increase the accuracy in detecting and better quantifying transmural intestinal fibrosis.
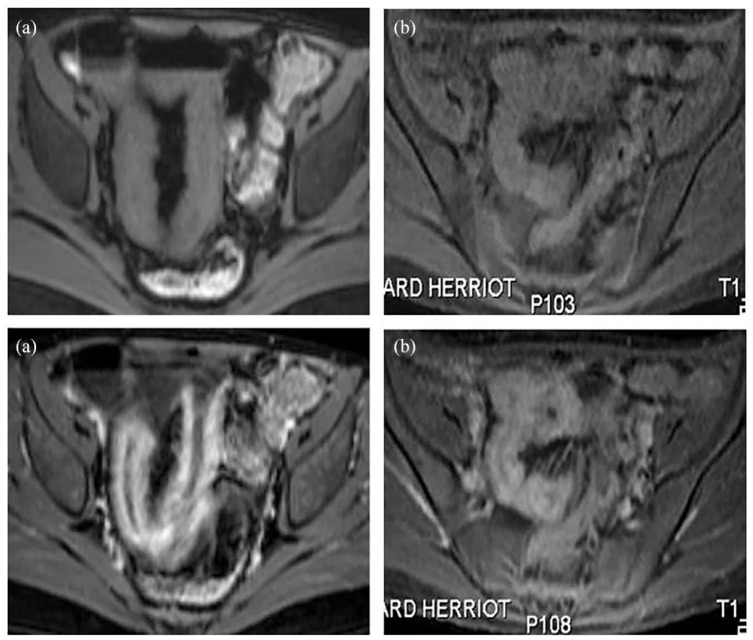
Specificity in perianal CD
In perianal CD, MRE appears to be superior to other imaging modalities, including CT, in detecting perianal complex fistulas and sinus tracts, with pooled sensitivities of more than 80%, and remains the preferred technique for assessing the location, number, and inflammatory status of perianal fistula tracts and associated complications.28,29 According to the European Crohn’s and Colitis Organisation (ECCO) guidelines, MRE is the reference standard for the accurate morphological assessment of perianal CD9 and to follow disease outcomes under treatment. Changes in the MRI-based score were shown to correlate with positive clinical outcomes, in particular, T2 hyperintensity in responding patients.30 Various MRI-based parameters, including a description of the number and features of each fistula tract, a high intensity of the T2 signal in the active fistulas, Park’s classification for each fistula, the presence and location of abscesses, and the assessment of sphincter integrity should be reported. In addition, an MRE-based Van Aasche score that combines various criteria to feature the anatomy (extension) and complexity of the fistula tracts (signs of activity, abscess) has been proposed.31 In addition, a simpler and more convenient simplified MRI-parameter-based score that is more feasible in routine practice has been proposed to provide an accurate cartography of the perianal fistula tracts and may be a useful indicator to assess the clinical status of patients before surgery, to follow them, and to evaluate treatment responses.6 A novel and robust index for assessment of perianal fistula at MRE (MAGNIFI-CD) has been developed and validated to determine perianal fistulizing CD activity.32 It was recently reported that the MAGNIFI-CD index was capable of accurately predicting long-term treatment outcome in perianal fistulizing CD patients receiving only antitumor necrosis factor (TNF) therapy or surgical closure after anti-TNF induction therapy.33 Interestingly, all the patients with a completely fibrotic fistula tract at MRE experienced long-term fistula closure, suggesting that this parameter should be considered as critical in radiological fistula healing. TUS has also been reported in small studies to be an accurate, well accepted, and cost-effective tool for documenting perianal fluid collections and fistulas. A systematic review and meta-analysis pooling 12 studies (enrolling 565 patients) investigating the accuracy of TUS in the assessment of perianal fistula and abscesses reported its good overall performance with a sensitivity of 98% on a per-lesion basis in detecting perianal fistula with a positive predictive value of 95%.34 TUS is therefore an accurate examination in trained hands for diagnosing, classifying, and managing perianal fistulas and abscesses and can overcome several limitations of endo-anal ultrasound (in case of anal strictures) and of MRE (in patients with metallic clips or claustrophobia). The recent ECCO/European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus recognized TUS as a possible modality for the imaging of perianal fistulas and abscesses in CD according to local availability and expertise.12 However, TUS still has significant limitations in terms of imaging complex and high fistula tracts, including the exclusion of deep anorectal abscesses.
Imaging scores
IUS scoring indices
A recent systematic review reported that, to date, more than 21 ultrasound scoring indices have been proposed to assess IBD activity.35 Among them, the three most commonly used parameters were BWT, color Doppler imaging, and bowel wall stratification. According to the reference standard (ileocolonoscopy being the most common), the accuracy, sensitivity, and specificity of ultrasound indices range from 73% to 100%, 39% to 100%, and 63% to 100%, respectively. A quantitative bowel US-based score was recently devised from a large prospective cohort of ileocolonic CD patients using BWT, bowel wall pattern, bowel wall flow, and the presence of complications, mesenteric hypertrophy, and enlarged mesenteric lymph nodes. These explanatory parameters were all compared with the findings of endoscopic activity, used as the reference standard. By multivariable analysis, BWT and bowel wall flow were identified to be two strong independent predictors of endoscopic activity and a bowel US score (BUSS) was defined as follows: BUSS = 0.75 × BWT + 1.65 × BWF, where BWF = 1 if present or BWF = 0 if absent36 (Table 1). In UC, a novel ultrasonographic activity index, called the UC-IUS index, was proposed by Bots et al. by comparing IUS parameters and endoscopy findings on a colonic segment from 60 consecutive UC patients.14 The four most predictive IUS parameters and their related best cutoffs that were able to distinguish between endoscopically inactive (endoscopic Mayo subscore 0) and active (Mayo subscores 1–3) UC were identified and the UC-IUS index generated including BWT, color Doppler signal, abnormal haustrations, and fat wrapping. Among the 207 colonic segments analyzed, the UC-IUS strongly correlated with the endoscopic Mayo score (r = 0.83) and, to a lesser degree, the UCEIS index (r = 0.75), with a good interobserver agreement. However, both the BUSS and UC-IUS indices need to be prospectively validated in independent cohorts of CD and UC patients, respectively. Full validation against endoscopy, MRE, or existing or novel IUS scoring activity indices with adequate operating property data (i.e. validity, reliability, responsiveness, sensitivity, specificity, accuracy, positive predictive value, and negative predictive value) is needed before any specific score can be recommended.
Table 1.
Main intestinal ultrasound and MRE scoring indices for assessing disease activity in CD and UC.
| Scoring indices | Disease | Parameters and formula | Advantages and limitations |
|---|---|---|---|
| BUSS36 | CD | 0.75 × BWT + 1.65 × BWF | Validated |
| Good indicator of endoscopic activity | |||
| BUSS > 3.52 as a threshold to discriminate patients with endoscopically active versus inactive CD (AUROC = 0.864; Sen = 83%; Spe = 85% | |||
| UC-ultrasound index14 | UC | 4 parameters (graded on an arbitrary 7-point scale): BWT; Doppler signal; abnormal haustration; fat wrapping | Point-based index for grading disease activity |
| Strong correlation with the endoscopic Mayo score and the UCEIS index | |||
| Not yet validated and lack of data for its sensitivity to change | |||
| MaRIA37 | CD | 1.5 × BWT + 0.02 × RCE + 5 × edema + 10 × ulcer | Validated |
| Well correlated with the endoscopic disease severity CDEIS | |||
| RCE = [(WSI postgadolinium − WSI pregadolinium)/(WSI pregadolinium)] × 100 × (SD noise pregadolinium/SD noise postgadolinium) | Contrast agent required | ||
| Segmental index from 6 defined intestinal regions or combined into a global index | |||
| Complex measurements for measurement of RCE | |||
| Simplified MaRIA30 | CD | (1 × BWT > 3 mm) + (1 × wall edema) + (1 × fat stranding) + (2 × ulcers) | Easier to obtain compared with the segmental MaRIA |
| No contrast agents required | |||
| Lack of data on its reproducibility and specificity | |||
| Clermont score31 | CD | 1.646 × BWT − 1.321 × ADC + 5.613 × edema + 8.306 × ulceration + 5.039 | Requires diffusion-weighted sequences |
ADC, apparent diffusion coefficient; AUROC, area under the receiver operating characteristic; BUSS, bowel US score; BWF, bowel wall flow; BWT, bowel wall thickness; CD, Crohn’s disease; CDEIS, Crohn’s Disease Endoscopic Index of Severity; MaRIA, magnetic resonance index of activity; MRE, magnetic resonance enterography; RCE, relative contrast enhancement; UC, ulcerative colitis; WSI, wall signal intensity.
MRE scoring indices
Various MRE-based activity scores have been proposed, of which the first and best validated is the MaRIA score, which has shown a good correlation with indices of activity in capsule endoscopy and ileocolonoscopy and high sensitivity and specificity.37–41 Because one of the major drawbacks of the MaRIA score is the need of contrast injection, the validated simplified MaRIA (which excludes contrast enhancement) was proposed as a reliable and less time-consuming alternative that is as accurate as the MRE-based activity score, which correlates well with endoscopic CD severity. The Clermont score, derived from the MaRIA score, was also developed to assess CD activity without the need for contrast enhancement and uses DWI sequences with the ADC42 (Table 1). These activity scores, which can all be applied to the whole small bowel and colon or by individual intestinal segment, accurately distinguish active from inactive CD and show good inter-reader and intra-reader reproducibility (Figures 5 and 6). In addition, their satisfactory responsiveness allows their use to follow the response to therapy. All these scores, which provide quantitative and objective assessment of disease activity, have all been widely used in clinical trials to select and stratify patients and as therapeutic endpoints. Imaging scores which provide quantitative values of disease activity should be reported systematically in daily practice to minimize the interobserver variability, especially in case of different operators over time. However, given their relative complexity and the length of time required for their calculation (requiring manual selection of the regions of interest to assess bowel wall contrast enhancement before and after contrast injection), the use of these scores is still relatively limited in routine daily practice.
Assessment of the extent of disease and detection of complications
Several studies have investigated the respective performance of IUS and MRE to assess the extent of CD and reported high concordance between IUS and MRE in assessing the length of diseased intestine in IBD (Figures 3(c) and 7).17,43 Relative to MRE or surgery, the pooled sensitivity and specificity of IUS for detecting the extent of CD has been shown to be 86% [95% confidence interval (CI): 83–88%] and 94% (95% CI: 93–95%), respectively.3,4,44,45 Notably, most of the studies reported that MRE outperformed IUS in assessing disease location and extent, with significantly higher sensitivity and specificity of MRE for the extension of small bowel disease than IUS.2,3,17 Except for the rectum, IUS is accurate for determining the location of colonic disease and its extent in UC patients, with a pooled sensitivity and specificity of 74% and 93% per segment.6,45,46 Capsule endoscopy is a highly sensitive tool to detect small bowel mucosal lesions in CD and may be of interest to guide therapeutic decisions. In patients with suspected CD and with negative ileocolonoscopy, a prospective cohort study comparing capsule endoscopy and IUS found similar diagnostic yield for both imaging modalities.47 This was confirmed in a systematic review of the literature and meta-analysis identifying 13 studies comparing the diagnostic yield of capsule endoscopy, MRE, and SICUS in the evaluation of small bowel CD.48 The diagnostic yields of capsule endoscopy, MRE, and SICUS were found to be similar for detection of small bowel CD in both suspected and established CD. Interestingly, capsule endoscopy was superior to MRE [pooled odds ratio (OR) 2.79; 95% CI: 1.2–6.48 from 7 studies, 251 patients] and to IUS (OR: 2.76; 95% CI: 0.84–9.02 from 3 studies, 95 patients) for detecting proximal small bowel lesions. The current ECCO/ESGAR guidelines support the use of capsule endoscopy, MRE, and IUS for detection and follow-up of small bowel CD but clinicians should keep in mind the risk of capsule retention and the usefulness of patency capsule test in established CD. In addition, complications, such as strictures, fistulas, and abscesses, can be accurately detected by both MRE and IUS (Figures 7 and 8). Relative to surgery, high and similar sensitivity (74% and 76%) and specificity (95% and 96%) of IUS and MRE were reported for the detection of enteric fistula, with a linear hypoechoic area in direct contact with the affected bowel segment.4 The overall performance of IUS and MRE to detect intra-abdominal abscesses is quite similar, with sensitivities of 84% and 86%, respectively, and a specificity of 93% for both relative to surgery.4 CEUS shows high diagnostic accuracy (98%) relative to surgery, percutaneous drainage, and MRE in differentiating inflammatory phlegmons from intra-abdominal collected abscesses, with high interobserver agreement (kappa = 0.95).49 Beyond the usefulness of IUS in detecting and monitoring intra-abdominal abscesses over time, IUS-guided percutaneous drainage of accessible abscesses is a component of the optimal management of such complicated penetrating CD. Of note, deep fistula tracts, complicated or not with abscesses, particularly those located in the pelvis or proximal small bowel, represent the main limitation of IUS, and cross-sectional imaging should be favored in the presence of strong clinical suspicion. IUS and MRE can also detect enteric and colonic strictures characterized by an area of thickened bowel wall with a narrowed lumen and subsequent proximal gut dilatation. Relative to surgery specimens as the reference, the pooled sensitivity of IUS and MRE was 79% and 89% and the pooled specificity was 92% and 94%, respectively, to detect small and large bowel strictures in CD patients.4 Up to now, there is no internationally recognized consensus regarding the definition of imaging-based stricture. IUS allows exploring thickened bowel wall but not proximal bowel dilation. The possibility to explore gut motility in a real-time setting represents one of the major advantages of IUS relative to other cross-sectional imaging techniques and makes it possible to distinguish between strictures caused by either spasms, on one hand, or active inflammation or fibrosis, on the other, which often coexist in CD-related strictures. Quantification of MRE-based terminal ileal motility has been reported to be of interest for assessing CD endoscopic and histopathologic activity.50 In addition, MRE-based motility was also sensitive to change with a close relationship between improvement in gut motility and improvement in disease activity.
Figure 7.
Axial MRE of complicated CD patients. Multiple enhancing tracts correspond to bowel-to-bowel fistulae (a). Focal dilation of small bowel loops can be seen upstream of the focal stenoses. Note the marked associated mural thickening (b).
CD, Crohn’s disease; MRE, magnetic resonance enterography.
Figure 8.
Coronal T2w- (a) and T1w-enhanced (b) images of CD patients. Note the detection of the bowel-to-bowel fistulae, as well as the marked mural enhancement consistent with active disease.
CD, Crohn’s disease.
Assessment of the response to treatment
From the perspective of the ultimate treat-to-target approach, imaging modalities are shifting toward becoming a monitoring tool for predicting the response to therapy. Hence, the usefulness of both IUS and MRE is based on their responsiveness to changes and the definition of remission after treatment. A systematic review and expert consensus recently proposed recommendations for defining the IUS response, transmural healing, and optimal timing of ultrasonographic assessment during treatment of IBD.51 Treatment response has been recently defined as a reduction in BWT > 25% or >2.0 mm or >1.0 mm and one color Doppler signal reduction.51 Although there is still no expert consensus on the definition of transmural healing of the small and large bowel in CD, it is commonly defined by BWT ⩽ 3 mm with normal color Doppler signal. Despite the fact that transmural healing in CD (defined as the complete normalization of BWT of all inflamed segments) is not yet considered to be a formal therapeutic target,52 it has been increasingly recognized to reflect deep remission. Consistent findings have shown that transmural healing is an independent predictor of more favorable long-term outcomes than mucosal healing.53
Response to treatment in CD
Transmural remission of the small and large bowel is defined by BWT ⩽3 mm with a normal color Doppler signal. The true benefits of attaining transmural remission in long-term CD outcomes have been reported in a number of studies, resulting in a lower risk of hospitalization, surgery, and need for steroids.54,55 In addition, an early MRE-based transmural response, as soon as 12 weeks after initiation with an anti-TNF agent, was found to be predictive of corticosteroid-free remission assessed at 1 year.56 In an observational study that included 80 CD patients treated with anti-TNF and undergoing serial SICUS over time, more than half were considered to be complete responders. The ultrasound-based response to treatment was associated with a better long-term disease course, less need of steroids, and less hospitalization and surgery.55 A recent prospective multicenter study that included 188 adult CD patients (the vast majority treated with adalimumab, and to a lesser degree with infliximab, vedolizumab, or ustekinumab) investigated changes in IUS parameters, such as BWT, echo pattern, blood flow modifications, extent of the disease, and transmural healing, over a 1-year period under different biological therapies.57 In this cohort, transmural healing was achieved in more than a quarter of patients at 12 months and IUS-based parameters improved or normalized in responding patients, demonstrating the usefulness of close IUS-based monitoring of remission or the response to biologics over time. The largest study, a 12-month observational German study that included 234 adult CD patients receiving an anti-TNF agent who experienced a flare requiring drug intensification and followed up by serial IUS over time, confirmed the clinical value of ultrasound parameters in assessing the response to therapy. After 12 months of drug modification, BWT, as well as the echo pattern, was improved, fibrofatty proliferation was reduced, and the color Doppler signal was attenuated.58 Moreover, these sonographic components of disease activity were modified as soon as 3 months after drug intensification. The early prediction of the response to therapy is a challenge of paramount interest in IBD. A recent cohort of 40 consecutive active CD patients starting anti-TNF were followed by IUS with and without CEUS over time and the endoscopic response/remission was assessed 12–34 weeks later. A reduction in BWT was able to predict a further endoscopic response early, with strong performance [area under the receiver operating characteristic (AUROC) = 0.71, OR: 10.8, p = 0.012]. The quantitative parameter washout rate from CEUS and the absence of a color Doppler signal were both associated with improved accuracy of the prediction model.59 In addition, the therapeutic response guided by early IUS has also been recently evaluated for patients under treatment with ustekinumab in an ancillary ultrasonography substudy from the treat-to-target versus standard-of-care treatment strategy STARDUST study.60 Among the 77 CD patients under ustekinumab therapy who underwent serial IUS over time, the segmental IUS response, prospectively defined as a reduction in BWT ⩾ 25% in the target segments, was detectable as early as 4 weeks after treatment initiation. The agreement between IUS and endoscopy was satisfactory, especially for identifying the most diseased bowel segments, whereas its reliability with endoscopic findings and biomarkers to assess the response to therapy was considered to be fair to moderate, regardless of the time point. In addition, a progressive IUS response and transmural healing were observed in 46% and 24% of ustekinumab-treated patients at Week 48, with significantly better results in terms of BWT and bowel blood flow normalization in patients with colonic disease location and in biologic-naïve patients. Interestingly, the discrepancy between the rate of and time to response to treatment between the disease in the colon and ileum, with generally earlier and better resolution of colonic lesions than those in the small bowel, is common. In this setting, IUS appears to be a valuable and accurate imaging tool to follow-up and monitor the differences in the timing of transmural improvement and healing between the colon and ileum. Overall, components visible by IUS, in particular BWT, are responsive to changes in therapy and therefore, IUS, in synergistic combination with the clinical disease activity score and inflammatory biomarkers, such as fecal calprotectin, is potentially of great interest for long-term close monitoring of patients under treatment, as well as for guiding the optimization of therapy. BWT measured just before initiating treatment is the best ultrasonographic component to accurately predict further response to therapy (the greater the BWT before treatment, the higher the likelihood of treatment failure and absent or incomplete normalization of the bowel wall after treatment). The agreement between IUS and MRE in assessing the rate of transmural remission was investigated in a 2-year observational longitudinal study of 80 CD patients treated with anti-TNF.61 Transmural healing was achieved in approximately 25%, and this rate was similar, regardless of the imaging modality used. In this study, in accordance with others, good agreement was reported between transmural healing and endoscopic mucosal remission.3,61
Response to treatment in UC
There are currently only a few studies examining in UC the relationship between IUS treatment response over time with endoscopy as the reference standard. Significant improvement in increased baseline BWT, consistent with that observed with endoscopic-based monitoring in the sigmoid over time during therapy, was reported in UC patients in a large prospective and observational study by Maaser et al.62 In addition, IUS showed the potential to distinguish between responders and non-responders early, as soon as 2 weeks after starting treatment, allowing the identification of candidates for early drug intensification. In addition, a small pilot study has reported the ability of IUS to predict treatment outcome in patients hospitalized for a flare of severe acute UC and treated with steroids.63 Transmural healing of the large bowel in UC is defined by both BWT ⩽3 mm and no color Doppler signal. Although based on limited evidence, the current recommendations based on our clinical experience and expert opinion suggest using the same definitions of treatment response and transmural healing in UC as for CD. However, more dedicated studies are needed both in adult and pediatric population.
Special clinical situations
The feasibility and performance of IUS to objectify IBD activity throughout pregnancy was evaluated in a recent pilot study that included 38 patients with CD or UC. The sensitivity and specificity to detect active disease during pregnancy were high (84% and 98%, respectively). However, reduced feasibility of IUS to explore the distal ileum and sigmoid was reported during the third trimester.64 It is still not clear whether fecal calprotectin and IUS should be combined throughout pregnancy to optimize the diagnostic accuracy to detect inflammation and subsequently to further improve the disease course by guiding clinical decisions. Although the risk of nephrogenic systemic fibrosis or nephrotoxicity following administration of a standard dose of gadolinium is considered extremely low by a joint expert consensus from the American College of Radiology and the National Kidney Foundation, monitoring of such IBD patients with severe renal dysfunction by IUS appears to be a more appropriate option. Of note, some IBD patients who achieved endoscopic mucosal healing under treatment retained evident signs of residual active mural or extramural intestinal inflammation. Although the clinical relevance of such persistent active imaging-based lesions and the subsequent need for treatment optimization warrant further clarification, such clinical situations highlight not only the synergistic and complementary impact of using imaging modalities for close monitoring of IBD, but also its impact on making therapeutic decisions.
Prediction of disease course
The discovery of imaging-based quantitative parameters that contribute to predicting a negative disease course requiring treatment changes or the need of hospitalization or surgery and to stratifying the risk of occurrence of complications in IBD patients is crucial to guide appropriate therapeutic decisions. However, data on the accuracy of IUS and MRE in predicting the disease course are still scarce. In a recent large prospective cohort of 225 ileal and/or colonic CD patients, a baseline BUSS, including BWT and vascularization changes, was reported to be an independent predictor of a worse disease outcome (defined as the need for steroids, change of therapy, hospitalization, or surgery) throughout the 12-month follow-up.36 In addition, baseline detection of at least one complication by IUS, such as stricture, fistula, or abscess, predicted the need for further surgery. Furthermore, greater BWT assessed by IUS at baseline was identified to be an independent predictor of a lower likelihood of transmural healing at 3 and 12 months in a recent prospective cohort of CD patients under biological therapies.57 Using a threshold BWT > 7 mm, IUS was capable of identifying CD patients with a high risk of surgery suggesting that repeated monitoring by IUS during the follow-up of patients could help to stratify disease severity and guide therapeutic decisions.65 The occurrence of intestinal complications such as strictures, fistula, or abscesses is common in CD and characterized bowel damage. MRE has the major advantage of being capable of qualifying both bowel damage and inflammation simultaneously. In a dual-center prospective study including 142 consecutive CD patients, whereas the disease activity assessed by the MaRIA score failed to predict the disease course, they found that bowel damage on imaging was associated with the risk of CD-related hospitalization and surgery.66 A recent study analyzed the relationship between MRE-based parameters and the risk of surgery within 5 years and identified the presence of restricted diffusion, complex fistula, increased upstream dilatation from a stricture, perienteric inflammation, fibrofatty proliferation, and increased length of disease involvement as risk factors of progression to surgery.67 In newly diagnosed adult CD patients, the ability of MRE features at diagnosis to improve prediction of disabling CD within 5 years of follow-up is actually addressed in a prospective multicenter study (METRIC-EF) that is actually in progress using various scoring systems including the MaRIA global, the simplified MaRIA Scores, and the Lémann Index (ISRCTN76899103).68
Detection of postoperative recurrence in CD
Although ileocolonoscopy is still the gold standard for identifying patients at high risk of postoperative recurrence, IUS has shown high accuracy to detect postoperative recurrence, with a sensitivity of 77% and a specificity of 94%, and the use of small intestine contrast enhancement substantially improved its sensitivity to 82%, with similar specificity.69 A strong relationship between BWT > 3 mm and the Rutgeerts endoscopic severity score was reported in a cohort of 72 CD patients, and IUS showed high accuracy (more than 87%) in the detection of postoperative recurrence in a more convenient condition than endoscopy. In a prospective cohort of 45 CD patients who had undergone bowel resection, IUS was found to be useful in grading the severity of postoperative recurrence and identifying patients at high risk of recurrence. Hence, abnormal BWT (>3 mm) was associated with postoperative recurrence in CD. Using the best cutoff of BWT ⩾ 3 mm, the sensitivity, specificity, and positive and negative predictive values of IUS to assess endoscopic recurrence (according to the Rutgeerts score ⩾ i2) were 79%, 95%, 95%, and 80%, respectively, and the performance of IUS was reported to be higher for the assessment of severe endoscopic recurrence.70 A systematic review and meta-analysis established that the best cutoff for BWT capable of predicting the presence of severe postoperative recurrence (defined by the Rutgeerts score ⩾ i3) was ⩾5.5 mm with a sensitivity of 84% and a specificity of 97%.71 Overall, increasing evidence supports the usefulness of IUS with an optimal cutoff point for BWT > 5 mm for monitoring CD patients who have undergone ileocolonic surgery in daily practice. The pooled diagnostic sensitivity, specificity, and accuracy assessed by the area under the curve (ROC) of MRE to detect postoperative endoscopic recurrence in CD patients were 97%, 84%, and 0.98, respectively.72 In addition, a recent study found that diffusion-weighted MRE also had similar diagnostic ability compared with contrast-enhanced MRE to detect postoperative recurrence in CD patients.73 The first MRE index MONITOR (Magnetic Resonance Imaging in CD to Predict Postoperative Recurrence) designed specifically for the prediction of postoperative recurrence by considering the Rutgeerts score as the gold standard and including the seven items, wall thickening, T2 signal increase, ulcers, contrast enhancement, diffusion-weighted signal increase, edema, and length of the diseased segment,74 has been recently proposed. Using the best cutoff of ⩾1 point, the AUROC of the MONITOR index was 0.80 with a sensitivity of 79%, a specificity of 55% and predictive positive and negative values of 68% to predict endoscopic postoperative recurrence (Rutgeerts score > i1). However, the MONITOR index has to be formally validated and its negative predictive value is not high enough to accurately discriminate CD patients at low risk of postoperative endoscopic recurrence, to avoid endoscopy. Because the measurement of fecal calprotectin has been reported to be useful within the first year after surgery, mainly to exclude endoscopic postoperative recurrence, thus avoiding approximately one-third of colonoscopies,75 the usefulness of the MRE index can be improved by combining it with monitoring of fecal calprotectin. The added value of an integrated approach that includes serial IUS or MRE and fecal calprotectin monitoring warrants further investigation in a dedicated study.
Impact of cross-sectional imaging on clinical decision-making
Few studies have investigated the true performance of imaging-driven management in IBD. A prospective study that included 60 consecutive ileal and/or colonic CD patients who underwent IUS, MRE, and colonoscopy within 1 week reported good overall accuracy (96%, 95% CI: 89–99%) of IUS alone relative to MRE in association with colonoscopy in assessing active disease.7 Moreover, in this study, the respective contribution of IUS or MRE to the medical decision process for continuing/changing/optimizing therapy was addressed. The concordance of the management of patients between IUS and MRE, as well as among IUS or MRE alone compared to the medical decision based on the combination of clinical, imaging, and endoscopic findings and biological markers, was high (kappa ranging from 0.76 to 0.80). Imaging modalities are therefore valid and represent clinically useful tools that can positively influence decision-making in CD and help in potentially avoiding colonoscopy procedures when not necessary and subsequently increasing patient acceptability and adhesion to monitoring. A comparison of the preferences and points of view of CD patients concerning IUS and MRE showed that the vast majority prefer IUS, as they find it to be more tolerable and less time-consuming, and they appreciate the fact that it does not require unpleasant bowel preparation.7 A recent retrospective study that evaluated 345 POC IUS examinations performed on a large cohort of IBD outpatients (including 280 CD and 65 UC patients) in a real-world setting showed that disease management after such imaging was modified for almost two-thirds of patients, including substantial changes in medication for 48%.59 The medical records of 54 patients with IBD who underwent MRE and IUS within 3 months were reviewed to investigate the impact of each imaging modality to guide the therapeutic decisions of clinicians. High sensitivities (90.5% and 88.1%) and moderate specificities (≈50% for both) for detecting intestinal inflammation were reported using MRE and IUS, respectively. In all, 19 patients were identified with complicated disease requiring clinical decisions, such as the need for steroids or surgical or percutaneous drainage. In this study, IUS and MRE contributed similarly to trigger and guide such clinical decisions.76 Beyond the influence of IUS to contribute to disease management, POC IUS may also help to accelerate the clinical decision-making process. Beyond its good patient acceptability and satisfactory feasibility for trained gastroenterologists and radiologists, the ability of POC IUS to accurately extend the scope of the patient’s physical examination and to help physicians make correct and relevant real-time clinical decisions makes IUS of paramount interest to drive the monitoring and management of IBD patients (Figures 9 and 10). Common issues to keep in mind when using IUS are its inability to evaluate the entire bowel, and potential interobserver and inter-equipment variability (even if the inter-reader variability of IUS was found high in practice), suggesting that at least theoretically, IUS monitoring should be ideally performed by the same sonographer using the same US device and probe to minimize confounding factors and ensure that any IUS-related changes observed are attributable to true inflammation-induced tissue modifications rather than machine/acquisition settings. The choice between IUS and MRE to monitor IBD patients could depend on local expertise, imaging availability, costs, and the wait required to perform these procedures. Beyond its ability to diagnose IBD and to distinguish active from inactive disease, we strongly believe that the use of both imaging modalities and fecal calprotectin will represent a well-accepted, valid, and reliable instrument for closely monitoring patients with IBD, predicting disease course and also response to treatment. Therefore, these noninvasive tools will definitely play a key role for adjusting therapeutic approach and making clinical decisions in the near future.
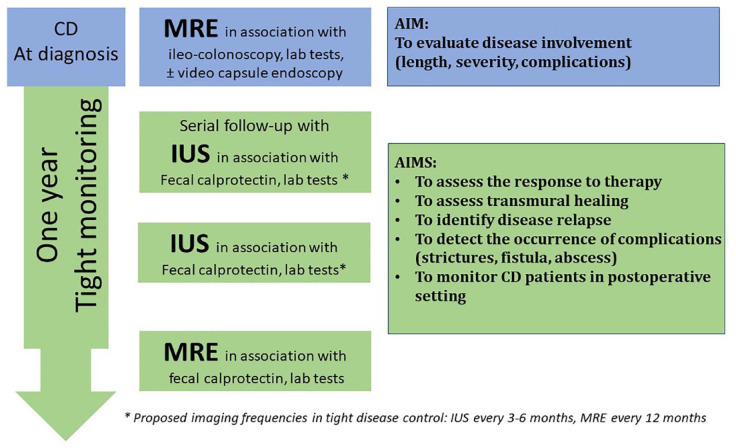
Figure 9.
Tight and noninvasive disease monitoring using imaging modalities (baseline MRE and serial follow-up with IUS every 3–6 months in alternance with yearly MRE) in patients with CD.
CD, Crohn’s disease; IUS, intestinal ultrasonography; MRE, magnetic resonance enterography.
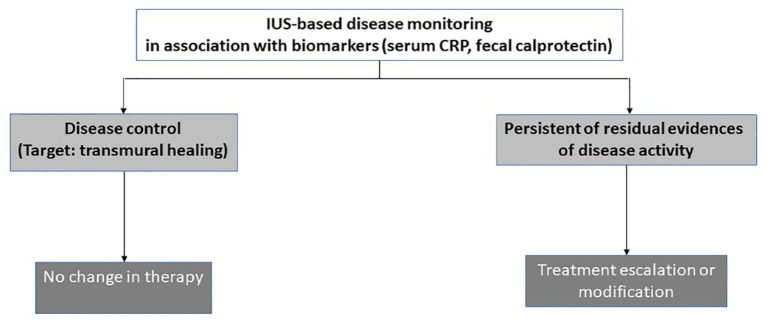
Figure 10.
Practical therapeutic algorithm according to the findings of noninvasive monitoring of disease activity in IBD using IUS, MRE in association with biomarkers including fecal calprotectin.
IBD, inflammatory bowel disease; IUS, intestinal ultrasonography; MRE, magnetic resonance enterography.
Conclusion
IUS and MRE have become an integral part of the clinical monitoring and management of IBD patients due to their reliability and high performance to accurately assess disease activity and extent and detect complications and offer better patient acceptability relative to endoscopy (Figure 9). Although MRE has been extensively explored, novel technological developments have renewed interest in IUS, which appears to be a highly promising and useful noninvasive, accurate, cheaper, better accepted, and suitable tool to provide objective parameters of inflammation and guide and speed up clinical decisions (Figure 10). In addition, it could be used in a POC setting, providing reliable information to facilitate the clinical decision-making process to change or optimize treatment and avoid endoscopies, reducing the need and cost of additional imaging, even if interobserver variability in image acquisition is still a major limitation. Because superficial mucosal lesions may not be detected by imaging modalities, the strategy of combining IUS with fecal calprotectin levels to provide synergistic information on mucosal and transmural inflammation and increase the diagnostic accuracy of disease activity may have added value and could be helpful for clinicians in guiding therapeutic decisions; however, data on the true performance of this combined approach are lacking, as well as studies investigating the cost-effectiveness of such integrated strategies. Beyond the key role of IUS and MRE in aiding the clinical decision-making process, cross-sectional image-guided interventions, such as the drainage of abscesses, represent a less-invasive alternative to surgical procedures.
In future, IUS should be better integrated into the training program of gastroenterologists. MRE and IUS now play a pivotal role in the close monitoring and tight control of the therapy of IBD patients, which are both crucial in the management of IBD in daily practice. They are effective, useful, easy-to-use, reproducible, and noninvasive tools that are complementary or an alternative to endoscopy for determining the extent of the disease and the severity of intestinal inflammation, evaluating disease activity, and monitoring the course of the disease during therapy to guide decisions.
Acknowledgments
None.
Footnotes
ORCID iDs: Stéphane Nancey  https://orcid.org/0000-0003-2088-1442
https://orcid.org/0000-0003-2088-1442
Xavier Roblin  https://orcid.org/0000-0002-2840-0108
https://orcid.org/0000-0002-2840-0108
Contributor Information
Stéphane Nancey, Department of Gastroenterology, Lyon Sud Hospital, Hospices Civils de Lyon, University Claude Bernard Lyon 1, 165 Chemin du Grand Revoyet, Pierre-Bénite, France; INSERM U1111, CIRI, Lyon, France.
Mathurin Fumery, Department of Gastroenterology, University Hospital of Amiens, Amiens, France.
Mathias Faure, INSERM U1111, CIRI, Lyon, France.
Gilles Boschetti, Department of Gastroenterology, Lyon Sud Hospital, Hospices Civils de Lyon, University Claude Bernard Lyon 1, Pierre-Bénite, France; INSERM U1111, CIRI, Lyon, France.
Claire Gay, Department of Gastroenterology, Lyon Sud Hospital, Hospices Civils de Lyon, University Claude Bernard Lyon 1, Pierre-Bénite, France.
Laurent Milot, Department of Radiology, Hospices Civils de Lyon, University Claude Bernard Lyon 1, Lyon, France.
Xavier Roblin, Department of Gastroenterology, Immunology, University Hospital of Saint-Etienne, Saint-Etienne, France.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Stéphane Nancey: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Mathurin Fumery: Data curation; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Mathias Faure: Data curation; Methodology; Writing – review & editing.
Gilles Boschetti: Data curation; Writing – review & editing.
Claire Gay: Data curation; Writing – review & editing.
Laurent Milot: Data curation; Investigation; Writing – original draft; Writing – review & editing.
Xavier Roblin: Data curation; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Buisson A, Gonzalez F, Poullenot F, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis 2017; 23: 1425–1433. [DOI] [PubMed] [Google Scholar]
- 2. Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol 2018; 3: 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castiglione F, Testa A, Rea M, et al. Transmural healing evaluated by bowel sonography in patients with Crohn’s disease on maintenance treatment with biologics. Inflamm Bowel Dis 2013; 19: 1928–1934. [DOI] [PubMed] [Google Scholar]
- 4. Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011; 34: 125–145. [DOI] [PubMed] [Google Scholar]
- 5. Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn’s disease. A review with recommendations of an international panel of experts. Inflamm Bowel Dis 2016; 22: 1168–1183. [DOI] [PubMed] [Google Scholar]
- 6. Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008; 247: 64–79. [DOI] [PubMed] [Google Scholar]
- 7. Allocca M, Fiorino G, Bonifacio C, et al. Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn’s disease and guiding clinical decision-making. J Crohns Colitis 2018; 12: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 8. Alshammari MT, Stevenson R, Abdul-Aema B, et al. Diagnostic accuracy of non-invasive imaging for detection of colonic inflammation in patients with inflammatory bowel disease: a systematic review and meta-analysis. Diagn Basel Switz 2021; 11: 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kucharzik T, Tielbeek J, Carter D, et al. ECCO-ESGAR topical review on optimizing reporting for cross-sectional imaging in inflammatory bowel disease. J Crohns Colitis 2022; 16: 523–543. [DOI] [PubMed] [Google Scholar]
- 10. Sagami S, Kobayashi T, Miyatani Y, et al. Accuracy of ultrasound for evaluation of colorectal segments in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021; 19: 908–921. [DOI] [PubMed] [Google Scholar]
- 11. Sagami S, Kobayashi T, Aihara K, et al. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment Pharmacol Ther 2020; 51: 1373–1383. [DOI] [PubMed] [Google Scholar]
- 12. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019; 13: 144–164. [DOI] [PubMed] [Google Scholar]
- 13. Sathananthan D, Rajagopalan A, Van De Ven L, et al. Point-of-care gastrointestinal ultrasound in inflammatory bowel disease: an accurate alternative for disease monitoring. JGH Open 2019; 4: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bots S, Nylund K, Löwenberg M, et al. Intestinal ultrasound to assess disease activity in ulcerative colitis: development of a novel UC-ultrasound index. J Crohns Colitis 2021; 15: 1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyers MA, McGuire PV. Spiral CT demonstration of hypervascularity in Crohn disease: “vascular jejunization of the ileum” or the “comb sign”. Abdom Imaging 1995; 20: 327–332. [DOI] [PubMed] [Google Scholar]
- 16. Migaleddu V, Scanu AM, Quaia E, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology 2009; 137: 43–52. [DOI] [PubMed] [Google Scholar]
- 17. Servais L, Boschetti G, Meunier C, et al. Intestinal conventional ultrasonography, contrast-enhanced ultrasonography and magnetic resonance enterography in assessment of Crohn’s disease activity: a comparison with surgical histopathology analysis. Dig Dis Sci 2022; 67: 2492–2502. [DOI] [PubMed] [Google Scholar]
- 18. Ripollés T, Martínez MJ, Paredes JM, et al. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 2009; 253: 241–248. [DOI] [PubMed] [Google Scholar]
- 19. Ripollés T, Martínez-Pérez MJ, Paredes JM, et al. The role of intravenous contrast agent in the sonographic assessment of Crohn’s disease activity: is contrast agent injection necessary? J Crohns Colitis 2019; 13: 585–592. [DOI] [PubMed] [Google Scholar]
- 20. Romanini L, Passamonti M, Navarria M, et al. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in inflammatory bowel disease. Eur J Radiol 2014; 83: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 21. Wilkens R, Hagemann-Madsen RH, Peters DA, et al. Validity of contrast-enhanced ultrasonography and dynamic contrast-enhanced MR enterography in the assessment of transmural activity and fibrosis in Crohn’s disease. J Crohns Colitis 2018; 12: 48–56. [DOI] [PubMed] [Google Scholar]
- 22. Van Assche G, Vermeire S, Rutgeerts P. The potential for disease modification in Crohn’s disease. Nat Rev Gastroenterol Hepatol 2010; 7: 79–85. [DOI] [PubMed] [Google Scholar]
- 23. Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut 2019; 68: 1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ripollés T, Rausell N, Paredes JM, et al. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohns Colitis 2013; 7: 120–128. [DOI] [PubMed] [Google Scholar]
- 25. Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 2011; 17: 984–993. [DOI] [PubMed] [Google Scholar]
- 26. Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015; 110: 432–440. [DOI] [PubMed] [Google Scholar]
- 27. Caron B, Laurent V, Odille F, et al. New magnetic resonance imaging sequences for fibrosis assessment in Crohn’s disease: a pilot study. Scand J Gastroenterol 2022; 57: 1450–1453. [DOI] [PubMed] [Google Scholar]
- 28. Haggett PJ, Moore NR, Shearman JD, et al. Pelvic and perineal complications of Crohn’s disease: assessment using magnetic resonance imaging. Gut 1995; 36: 407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziech M, Felt-Bersma R, Stoker J. Imaging of perianal fistulas. Clin Gastroenterol Hepatol 2009; 7: 1037–1045. [DOI] [PubMed] [Google Scholar]
- 30. Savoye-Collet C, Savoye G, Koning E, et al. Fistulizing perianal Crohn’s disease: contrast-enhanced magnetic resonance imaging assessment at 1 year on maintenance anti-TNF-alpha therapy. Inflamm Bowel Dis 2011; 17: 1751–1758. [DOI] [PubMed] [Google Scholar]
- 31. Van Assche G, Vanbeckevoort D, Bielen D, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol 2003; 98: 332–339. [DOI] [PubMed] [Google Scholar]
- 32. Hindryckx P, Jairath V, Zou G, et al. Development and validation of a magnetic resonance index for assessing fistulas in patients with Crohn’s disease. Gastroenterology 2019; 157: 1233–1244.e5. [DOI] [PubMed] [Google Scholar]
- 33. van Rijn KL, Meima-van Praag EM, Bossuyt PM, et al. Fibrosis and MAGNIFI-CD activity index at magnetic resonance imaging to predict treatment outcome in perianal fistulizing Crohn’s disease patients. J Crohns Colitis 2022; 16: 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maconi G, Greco MT, Asthana AK. Transperineal ultrasound for perianal fistulas and abscesses - a systematic review and meta-analysis. Ultraschall Med 2017; 38: 265–272. [DOI] [PubMed] [Google Scholar]
- 35. Goodsall TM, Nguyen TM, Parker CE, et al. Systematic review: gastrointestinal ultrasound scoring indices for inflammatory bowel disease. J Crohns Colitis 2021; 15: 125–142. [DOI] [PubMed] [Google Scholar]
- 36. Allocca M, Craviotto V, Bonovas S, et al. Predictive value of bowel ultrasound in Crohn’s disease: a 12-month prospective study. Clin Gastroenterol Hepatol 2022; 20: 723–740. [DOI] [PubMed] [Google Scholar]
- 37. Rimola J, Alvarez-Cofiño A, Pérez-Jeldres T, et al. Comparison of three magnetic resonance enterography indices for grading activity in Crohn’s disease. J Gastroenterol 2017; 52: 585–593. [DOI] [PubMed] [Google Scholar]
- 38. Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastroenterol 2015; 110: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 39. Panes J, Jairath V, Levesque BG. Advances in use of endoscopy, radiology, and biomarkers to monitor inflammatory bowel diseases. Gastroenterology 2017; 152: 362–373. [DOI] [PubMed] [Google Scholar]
- 40. Ordás I, Rimola J, Rodríguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology 2014; 146: 374–382. [DOI] [PubMed] [Google Scholar]
- 41. Kim JS, Jang HY, Park SH, et al. MR enterography assessment of bowel inflammation severity in Crohn disease using the MR index of activity score: modifying roles of DWI and effects of contrast phases. Am J Roentgenol 2017; 208: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 42. Buisson A, Hordonneau C, Goutte M, et al. Diffusion-weighted magnetic resonance imaging is effective to detect ileocolonic ulcerations in Crohn’s disease. Aliment Pharmacol Ther 2015; 42: 452–460. [DOI] [PubMed] [Google Scholar]
- 43. Horjus Talabur Horje CS, Bruijnen R, Roovers L, et al. Contrast enhanced abdominal ultrasound in the assessment of ileal inflammation in Crohn’s disease: a comparison with MR enterography. PLoS One 2015; 10: e0136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis 2013; 7: 556–585. [DOI] [PubMed] [Google Scholar]
- 45. Parente F, Greco S, Molteni M, et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther 2003; 18: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 46. Ordas I, Rimola J, Rodriguez S, et al. Imaging of the colon in inflammatory bowel disease: ready for prime time? Curr Drug Targets 2012; 13: 1252–1260. [DOI] [PubMed] [Google Scholar]
- 47. Carter D, Katz LH, Bardan E, et al. The accuracy of intestinal ultrasound compared with small bowel capsule endoscopy in assessment of suspected Crohn’s disease in patients with negative ileocolonoscopy. Ther Adv Gastroenterol 2018; 11: 1756284818765908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kopylov U, Yung DE, Engel T, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn’s disease: systematic review and meta-analysis. Dig Liver Dis 2017; 49: 854–863. [DOI] [PubMed] [Google Scholar]
- 49. Ripollés T, Martínez-Pérez MJ, Paredes JM, et al. Contrast-enhanced ultrasound in the differentiation between phlegmon and abscess in Crohn’s disease and other abdominal conditions. Eur J Radiol 2013; 82: 525–531. [DOI] [PubMed] [Google Scholar]
- 50. Menys A, Puylaert C, Tutein Nolthenius CE, et al. Quantified terminal ileal motility during MR enterography as a biomarker of Crohn disease activity: prospective multi-institution study. Radiology 2018; 289: 428–435. [DOI] [PubMed] [Google Scholar]
- 51. Ilvemark JFKF, Hansen T, Goodsall TM, et al. Defining transabdominal intestinal ultrasound treatment response and remission in inflammatory bowel disease: systematic review and expert consensus statement. J Crohns Colitis 2022; 16: 554–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160: 1570–1583. [DOI] [PubMed] [Google Scholar]
- 53. Ma L, Li W, Zhuang N, et al. Comparison of transmural healing and mucosal healing as predictors of positive long-term outcomes in Crohn’s disease. Ther Adv Gastroenterol 2021; 14: 17562848211016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Serban ED. Treat-to-target in Crohn’s disease: will transmural healing become a therapeutic endpoint? World J Clin Cases 2018; 6: 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zorzi F, Ghosh S, Chiaramonte C, et al. Response assessed by ultrasonography as target of biological treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2020; 18: 2030–2037. [DOI] [PubMed] [Google Scholar]
- 56. Messadeg L, Hordonneau C, Bouguen G, et al. Early transmural response assessed using magnetic resonance imaging could predict sustained clinical remission and prevent bowel damage in patients with Crohn’s disease treated with anti-tumour necrosis factor therapy. J Crohns Colitis 2020; 14: 1524–1534. [DOI] [PubMed] [Google Scholar]
- 57. Calabrese E, Rispo A, Zorzi F, et al. Ultrasonography tight control and monitoring in Crohn’s disease during different biological therapies: a multicenter study. Clin Gastroenterol Hepatol 2022; 20: 711–722. [DOI] [PubMed] [Google Scholar]
- 58. Kucharzik T, Wittig BM, Helwig U, et al. Use of intestinal ultrasound to monitor Crohn’s disease activity. Clin Gastroenterol Hepatol 2017; 15: 535–542. [DOI] [PubMed] [Google Scholar]
- 59. de Voogd F, Bots S, Gecse K, et al. Intestinal ultrasound early on in treatment follow-up predicts endoscopic response to anti-TNFα treatment in Crohn’s disease. J Crohns Colitis 2022; 16: 1598–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kucharzik T, Wilkens R, D’Agostino MA, et al. Early ultrasound response and progressive transmural remission after treatment with ustekinumab in Crohn’s disease. Clin Gastroenterol Hepatol 2022; 14: 1542–3565. [DOI] [PubMed] [Google Scholar]
- 61. Castiglione F, Mainenti P, Testa A, et al. Cross-sectional evaluation of transmural healing in patients with Crohn’s disease on maintenance treatment with anti-TNF alpha agents. Dig Liver Dis 2017; 49: 484–489. [DOI] [PubMed] [Google Scholar]
- 62. Maaser C, Petersen F, Helwig U, et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut 2020; 69: 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smith RL, Taylor KM, Friedman AB, et al. Early assessment with gastrointestinal ultrasound in patients hospitalised for a flare of ulcerative colitis and predicting the need for salvage therapy: a pilot study. Ultrasound Med Biol 2021; 47: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 64. De Voogd F, Joshi H, Van Wassenaer E, et al. Intestinal ultrasound to evaluate treatment response during pregnancy in patients with inflammatory bowel disease. Inflamm Bowel Dis 2021; 28: 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Castiglione F, de Sio I, Cozzolino A, et al. Bowel wall thickness at abdominal ultrasound and the one-year-risk of surgery in patients with Crohn’s disease. Am J Gastroenterol 2004; 99: 1977–1983. [DOI] [PubMed] [Google Scholar]
- 66. Fiorino G, Morin M, Bonovas S, et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J Crohns Colitis 2017; 11: 274–280. [DOI] [PubMed] [Google Scholar]
- 67. Dane B, Qian K, Krieger R, et al. Correlation between imaging findings on outpatient MR enterography (MRE) in adult patients with Crohn disease and progression to surgery within 5 years. Abdom Radiol (NY) 2022; 47: 3424–3435. [DOI] [PubMed] [Google Scholar]
- 68. Kumar S, Plumb A, Mallett S, et al. METRIC-EF: magnetic resonance enterography to predict disabling disease in newly diagnosed Crohn’s disease-protocol for a multicentre, non-randomised, single-arm, prospective study. BMJ Open 2022; 12: 067265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Castiglione F, Bucci L, Pesce G, et al. Oral contrast-enhanced sonography for the diagnosis and grading of postsurgical recurrence of Crohn’s disease. Inflamm Bowel Dis 2008; 14: 1240–1245. [DOI] [PubMed] [Google Scholar]
- 70. Rispo A, Bucci L, Pesce G, et al. Bowel sonography for the diagnosis and grading of postsurgical recurrence of Crohn’s disease. Inflamm Bowel Dis 2006; 12: 486–490. [DOI] [PubMed] [Google Scholar]
- 71. Rispo A, Imperatore N, Testa A, et al. Diagnostic accuracy of ultrasonography in the detection of postsurgical recurrence in Crohn’s disease: a systematic review with meta-analysis. Inflamm Bowel Dis 2018; 24: 977–988. [DOI] [PubMed] [Google Scholar]
- 72. Yung DE, Har-Noy O, Tham YS, et al. Capsule endoscopy, magnetic resonance enterography, and small bowel ultrasound for evaluation of postoperative recurrence in Crohn’s disease: systematic review and meta-analysis. Inflamm Bowel Dis 2017; 24: 93–100. [DOI] [PubMed] [Google Scholar]
- 73. Djelouah M, Marical V, Kanagaratnam L, et al. Diagnosis of postoperative recurrence of Crohn disease with MR-enterography: value of diffusion-weighted imaging. Diagn Interv Imaging 2021; 102: 743–751. [DOI] [PubMed] [Google Scholar]
- 74. Schaefer M, Laurent V, Grandmougin A, et al. A magnetic resonance imaging index to predict Crohn’s disease postoperative recurrence: the MONITOR index. Clin Gastroenterol Hepatol 2022; 20: 1040–1049. [DOI] [PubMed] [Google Scholar]
- 75. Boschetti G, Laidet M, Moussata D, et al. Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn’s disease. Am J Gastroenterol 2015; 110: 865–872. [DOI] [PubMed] [Google Scholar]
- 76. Calavrezos L, Bannas P, Warncke M, et al. Transabdominal ultrasound and magnetic resonance enterography in inflammatory bowel disease: results of an observational retrospective single-center study. Ultrasound Int Open 2022; 8: E22–E28. [DOI] [PMC free article] [PubMed] [Google Scholar]