Abstract
Background
Sleep disorders are common in patients with multiple sclerosis and have a bidirectional interplay with fatigue and depression.
Objective
To evaluate the effect of treatment with oral dimethyl fumarate on the quality of sleep in relapsing-remitting multiple sclerosis.
Methods
This was a multicentre observational study with 223 relapsing-remitting multiple sclerosis subjects starting treatment with dimethyl fumarate (n=177) or beta interferon (n=46). All patients underwent subjective (Pittsburgh Sleep Quality Index) and objective (wearable tracker) measurements of quality of sleep. Fatigue, depression, and quality of life were also investigated and physical activity was monitored.
Results
Patients treated with dimethyl fumarate had significant improvement in the quality of sleep as measured with the Pittsburgh Sleep Quality Index (p<0.001). At all-time points, no significant changes in Pittsburgh Sleep Quality Index score were observed in the interferon group. Total and deep sleep measured by wearable tracker decreased at week 12 with both treatments, then remained stable for the total study duration. Depression significantly improved in patients treated with dimethyl fumarate. No significant changes were observed in mobility, fatigue and quality of life.
Conclusion
In patients with relapsing-remitting multiple sclerosis, the treatment with dimethyl fumarate was associated with improvements in patient-reported quality of sleep. Further randomised clinical trials are needed to confirm the benefits of long-term treatment with dimethyl fumarate.
Keywords: Dimethyl fumarate, sleep, wearable tracker, relapsing remitting multiple sclerosis
Introduction
Sleep disorders are linked to a heterogeneous spectrum of problems and are frequent in patients with multiple sclerosis (MS)1 contributing to worse daily functioning and quality of life.2–4 Epidemiological studies have shown that sleep disorders are undiagnosed in several MS patients5,6; however, they are more common in MS compared to healthy subjects,7 in particular in females, in patients with longer disease duration,8 with high psychological burden and treated with immunotherapy.7 Therefore, these disorders may be associated not only with the disease itself but also with psychological problems secondary to MS, side effects of therapies or other comorbidities.
Fatigue, the most common MS symptom,9 is associated with sleep disorders2,10,11 and this association could be partially explained by disruption of sleep microstructure, poor subjective sleep quality and depression.12 There is a limited number of studies on the impact of disease-modifying treatments on sleep and most of them with relevant methodological problems.13 Interferon (IFN)-β treatment may affect sleep and contribute to fatigue, depression, and poor quality of life14; conversely, treatment with natalizumab generally improves sleep quality.15
Dimethyl fumarate (DMF), a current MS therapy, may provide neuroprotection and immunomodulation by activating the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathways16 the primary cellular defence system to the potentially toxic oxidative and inflammatory stress that may be associated with sleep disruption in MS.17 DMF demonstrated robust efficacy and an acceptable safety profile in relapsing-remitting MS (RRMS) patients in the Phase 3 DEFINE18 and CONFIRM19 studies; it was shown to have a positive impact on health-related quality-of-life (HRQoL) outcomes as early as 6 months, which was maintained over the 2 years.20 Moreover, it was demonstrated that in obstructive sleep apnea (OSA), DMF mitigates OSA severity through suppression of systemic inflammation.21,22 The decrease in inflammatory activity, the good safety profile and the oral route of administration may have a positive impact on the quality of sleep. To date, there is no available data on the long-term effects of DMF on the quality of sleep.
The primary objective of this case-control observational study was to evaluate the effects of DMF on sleep in the treatment of naive MS patients. The impact on sleep was estimated by a combination of patient-reported evaluations and objective data recorded by a wearable device.
The primary objective was to assess the longitudinal change in sleep quality measured by the Pittsburgh Sleep Quality Index (PSQI) questionnaire. The secondary objectives were to evaluate the impact of fatigue, mood and quality of life and to assess the feasibility of periodic electronic remote monitoring. Digitalisation and remote monitoring may allow a constant objective evaluation of functioning in MS patients with a high time resolution. Conversely, the use of wearable devices might be limited by technical problems and suboptimal adherence.23 We have focused our attention on several sleep parameters measurable using a wearable device, which may have an important influence on MS patients’ quality of life.24 Moreover, a 1-year survey has been conducted on a relatively high sample of patients to assess whether DMF has a longitudinal effect, not only from a biological aspect but also on psychological perspective. Patients who started treatment with IFNs, the most frequently prescribed first-line DMT in Italy, were observed as a control group and evaluated as an exploratory objective.
Materials and methods
The study included adult outpatients of either sex with RRMS within 5 years diagnosed as per 2010 revised McDonald criteria,25 relapse-free and in stable neurological condition in the last 30 days prior to screening, with EDSS 0.0–5.5 and started DMF or an IFN within 1–4 weeks before enrolment according to local clinical practice. Exclusion criteria were: other MS forms according to standard definitions26; onset of MS relapse in the previous 30 days and/or no partial recover from a previous relapse; any other previous MS treatment within 1–4 weeks before enrolment; steroids administration in the last 30 days (IV methylprednisolone was allowed for relapse treatment after enrolment); concomitant treatment with hypnotics; history of malignancy (except for cured basal cell carcinoma), severe allergic or anaphylactic reactions or known drug hypersensitivity, clinically significant concomitant diseases or laboratory abnormalities, drug or alcohol abuse in the past 2 years; pregnant or breastfeeding women or women not using appropriate contraception. Patients with major sleep disorders were also excluded.
Following a one-week screening phase, visits at the clinic took place at baseline and after 12, 24 and 48 weeks of treatment. The primary endpoint was the change from baseline to Week 24 in quality of sleep, as measured by means of the PSQI questionnaire. The PSQI27 includes 19 individual items that generate seven different areas of sleep. Scoring of answers is based on a 0–3 scale, whereby a score of 3 reflects the negative extreme on the Likert Scale. A global sum of ≥5 indicates poor sleep quality.
Secondary end points based on patient-reported outcome measures included: changes from baseline in quality of sleep and daily activity as measured by a wearable tracker (Withings Pulse™); changes from baseline in quality of life, measured with the Multiple Sclerosis International Quality of Life (MusiQoL),28 changes in fatigue measured with the Fatigue Severity Scale (FSS),29 and in depression measured with the Asberg Depression Rating Scale (MADRS)30; treatment satisfaction was assessed with the Treatment Satisfaction Questionnaire for Medication-9 (TSQM-9).31 The comparison between DMF and IFN groups for all the variables was an exploratory objective.
Patients wore the tracker during the week before the planned visit. The technology used by Withings Pulse™ is based on Actigraphy, a method for monitoring human rest/activity cycles through a sensor called an accelerometer; using Micro-Electro-Mechanical Systems technology (MEMS) the micro-movements are converted into electrical signals and subsequently translated into data through a specific pre-made algorithm. The following parameters of sleep were evaluated on a weekly basis: quality of sleep (duration deep sleep/duration total sleep), total sleep, deep sleep, light sleep duration, number of awakenings. The daily mobility was estimated by the number of steps and the total distance covered. The weekly based assessment of remote monitoring was based on the availability of at least 4 days of recording. Adverse events were recorded throughout the study.
The study protocol was approved by the Ethics Committee of each study site and all participants signed the informed consent.
Analysis of efficacy was performed in the full analysis set (FAS), which included all patients that started treatment with DMF or IFN who had the baseline and at least one post-baseline assessment of PSQI. The analysis of the primary endpoint was repeated in the per-protocol (PP) set, which included all the FAS patients that completed the study with no major protocol violations. All statistical tests were two-sided and were carried out at the 5% level of significance.
Changes in PSQI and sleep parameters over time were analysed using a mixed-effects regression model, with the patient as a random factor and visit and treatment as a fixed factor, taking into account repeated value for each subject over time. Models with visit variables as continuous were estimated to evaluate linear trends over time. Missing data were interpolated from the model. Subgroup analyses by gender and age range (< or > 35 years) were also performed. The relationship between changes from baseline in PSQI score and quality of sleep (wearable tracker), fatigue, quality of life, mood and treatment satisfaction was evaluated using Pearson's or Spearman's correlation coefficient, respectively, when variables were assessed on an ordinal level of measurement or when one variable was an ordinal-level variable and the other was an interval- or ratio-level variable. A Wilcoxon Signed Rank test was used to analyze the changes from baseline in quality of life, fatigue and depression in patients treated with DMF.
Results
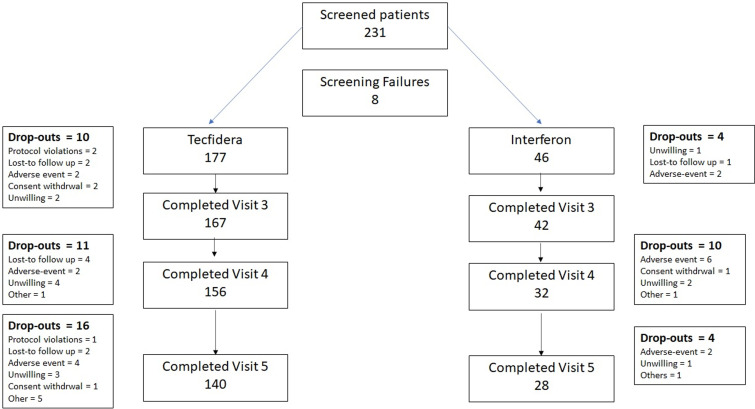
A total of 231 patients were screened in 25 Italian sites and 223 patients accepted to participate, signed the informed consent and started treatment (177 with DMF and 46 with IFN). Figure 1 summarises the disposition of patients. Overall, 168 patients (75.3%, 140 treated with DMF and 28 with IFN) completed the 48-week study period. Adverse events (8 and 10 patients, respectively in the DMF and IFN group) were the most common reasons for study discontinuation. Seventeen patients (12 and 5, respectively in the two groups) did not have post-baseline efficacy data and were excluded from the FAS (165 and 41 patients, respectively). The PP population comprised 136 patients treated with DMF and 27 treated with IFN. Table 1 shows the demographic and other baseline characteristics of patients without significant differences.
Figure 1.
Disposition of patients.
Table 1.
Demographic and other baseline characteristics of patients.
| DMF (N=177) |
Interferon (N=46) |
|
|---|---|---|
| Sex, N (%) Males Females |
n=176 57 (32.4%) 119 (67.6%) |
n=45 16 (35.6%) 29 (64.4%) |
| Age, years Mean + SD Median (range) |
n=176 35.7 + 10.3 35.0 (18–61) |
n=45 37.4 + 12.7 34.0 (19–68) |
| BMI, kg/m2 Mean + SD Median (range) |
n=153 24.3 + 4.2 23.4 (16–36) |
n=41 24.2 + 5.1 23.0 (18–41) |
| Time from first RRMS diagnosis, days Mean + SD Median (range) |
n=176 236.2 + 411.1 65.5 (0–2537) |
n=46 216.6 + 427.3 52.0 (19–1927) |
| EDSS score Mean + SD Median (range) |
n=176 1.6 + 1.1 1.5 (0–5) |
n=45 1.6 + 0.9 1.5 (0–4) |
| PSQI score Mean + SD Median (range) |
n=165 6.0 + 3.7 5.0 (0–18) |
n=41 5.8 + 3.3 6.0 (1–13) |
N: number of patients; n: number of observations; DMF: dimethyl fumarate; RRMS: relapsing-remitting multiple sclerosis; PSQI: Pittsburgh Sleep Quality Index; BMI: body mass index.
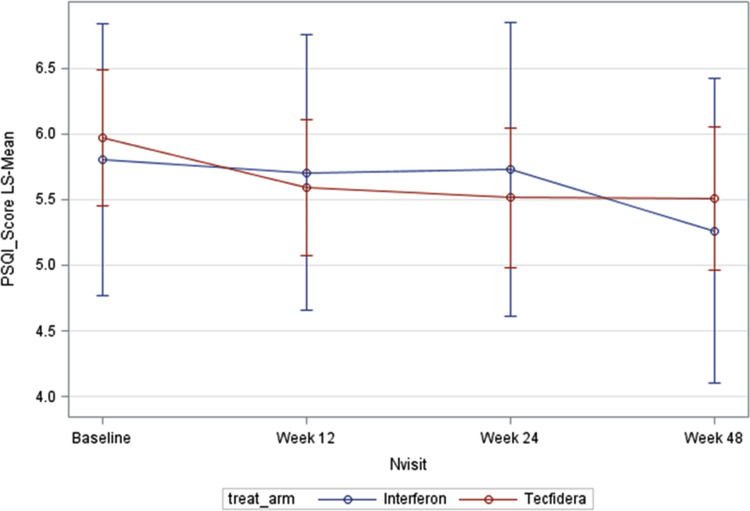
Patients treated with DMF had significant improvement over time in quality of sleep as measured with the PSQI (p<0.001); the treatment-by-interaction effect was not statistically significant. However, the PSQI score decreased from baseline to Week 24 in patients treated with DMF (mean change: −0.46; 9% CI: −0.94 to 0.02, p=0.062) and then remained stable at week 48. No significant changes at any time points were observed in the IFN group (Figure 2).
Figure 2.
Results of PSQI (FAS). Values are adjusted means. PSQI: Pittsburgh sleep quality index; FAS: full analysis set.
Similar findings of the PSQI score from baseline to Week 24 were observed for the DMF group in the baseline-adjusted analysis (p=0.06) and in the PP population (p=0.048). Moreover, a significant effect over time and significant changes from baseline to any post-baseline time point (p<0.001), were observed in the subgroup of 101 patients treated with DMF who had poor sleep at baseline (PSQI score > 5. In the DMF group, depression significantly improved from baseline to Week 24 (p=0.041): the mean (SD) change in MADRS score was −0.99 (5.46). No significant changes in depression were observed in IFN group. Conversely, there were no statistically significant changes from baseline to Week 24 in quality of life (mean [SD] change in MusiQoL score: −1.07 [12.09], p=0.366) and fatigue (mean [SD] change in FSS score: −0.95 [11.73], p=0.273) in both groups. Statistically, significant direct correlations were observed between PSQI score and FSS score at week 48 in the overall population (r=0.17, p=0.048) and in patients with poor sleep at baseline (r=0.30, p=0.013), and between PSQI score and MADRS score at week 12 (r=0.19, p=0.018) and week 24 (r=0.24, p=0.005).
The mean TSQM-9 score remained quite stable during DMF treatment, with a trend of small non-significant improvement from Week 12 (mean [SD], 42.79 [7.36]) to Week 24 (mean [SD], 43.73 [8.54]), which was maintained at Week 48 (mean [SD], 43.47 [11.54]).
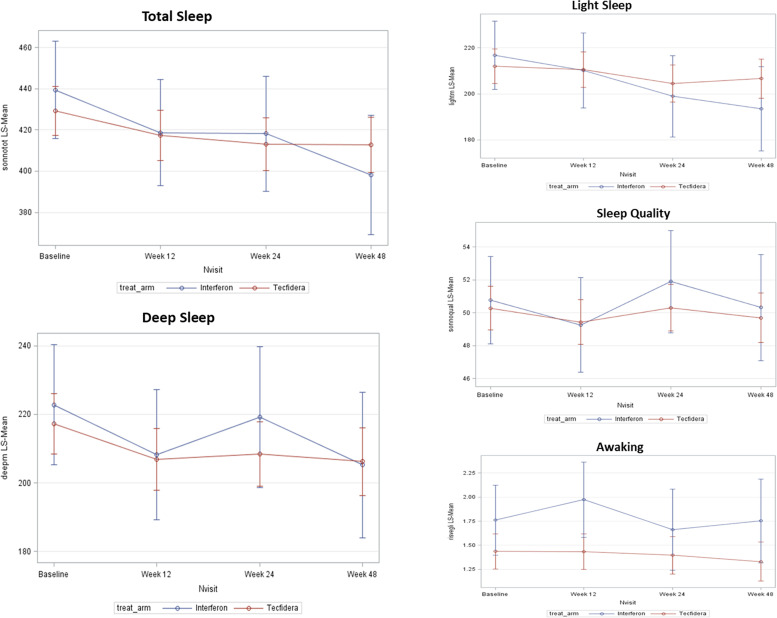
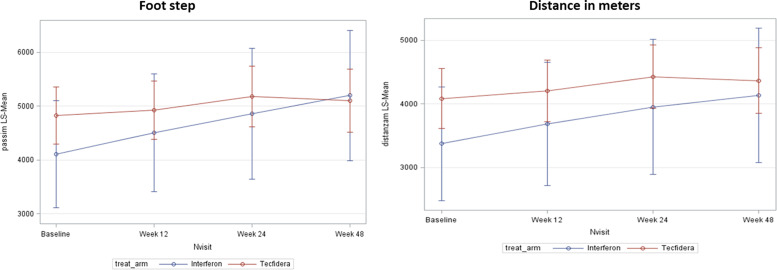
Overall, the tracker records were downloaded for all 210 patients at baseline, for 188 at Week 12, for 170 at Week 24 and for 151 at Week 48. Quality of sleep measured by wearable tracker was not significantly modified, however, both total and deep sleep were significantly modified by the onset of treatment. Treatment with DMF was associated with statistically significant decreases from baseline in the mean duration of deep sleep at Week 12 (p=0.020) and Week 48 (p=0.024), and in mean duration of total sleep at Week 24 (p=0.014) and Week 48 (p=0.017) (Figure 3). Similar longitudinal changes of totals sleep duration and deep sleep duration were observed in the IFN group, however, the intragroup longitudinal changes were statistically significant for total sleep duration only at 48 Weeks (p=0.005). The profile of recorded sleep parameters was confirmed by the results observed in the PP population and in the population with PSQI score >5. There were no significant changes in number of awakenings in both treatment groups. The results of mobility are shown in Figure 4. An increase from baseline in number of footsteps and in covered distance to any post-baseline time point was observed in both groups. The increase in covered distance from baseline to Week 24 was statistically significant in patients treated with DMF (p=0.037) and the increase in number of footsteps from baseline to Week 48 was statistically significant in IFN group (p=0.037).
Figure 3.
Results of quality of sleep measured with the wearable tracker (full analysis set (FAS)). Values are adjusted means.
Figure 4.
Results of mobility measured with the wearable tracker (full analysis set (FAS)).Values are adjusted means.
In the DMF group, the analysis of the correlation of changes from baseline showed significant inverse correlations between PSQI and deep sleep at week 48 in the overall population (r=−0.36, p=0.001) and in patients with poor sleep at baseline (r=−0.48, p=0.002), and between PSQI and quality sleep at week 48 in the overall population (r=−0.286, p=0.016) and in patients with poor sleep at baseline (r=−0.35, p=0.028). There were no statistically significant correlations between PSQI score and light sleep, and between MADRS score and deep sleep. In the overall sample, statistically significant inverse correlations between the EDSS score and the number of steps were observed at both the baseline (r=−0.22, p=0.004) and all post-baseline visits (Week 12: r=−0.20, p=0.012; Week 24: r=−0.17, p=0.046; Week 48: r=−0.23, p=0.010).
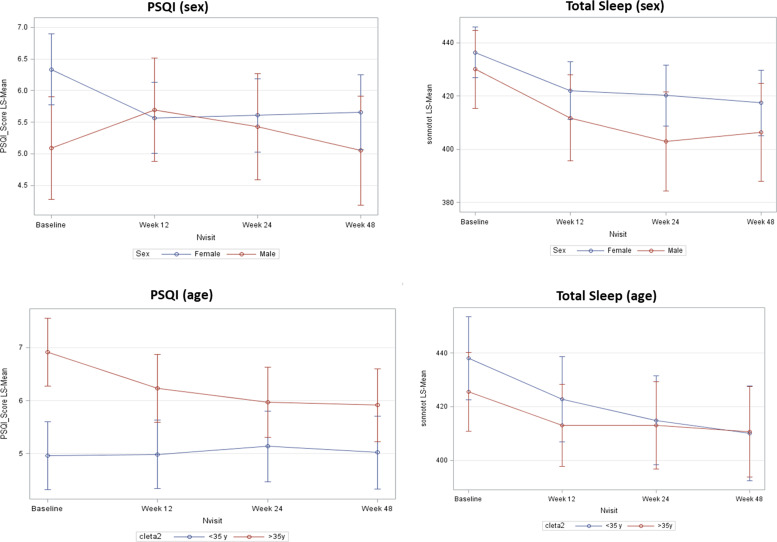
The subgroup analyses (overall population) showed that the mean PSQI score significantly decreased from baseline to any post-baseline time point in females, without important changes in males, as well as statistically significant decreases in mean PSQI from baseline to any post-baseline time point were observed in patients aged ≥ 35 years, but not in younger patients (Figure 5). Mean duration of total sleep decreased from baseline to any post-baseline time point both in males and females, and decreased significantly at Week 24 and Week 48 in patients aged <35 years, with less evident and non-significant decreases in patients aged ≥35 years.
Figure 5.
Results of PSQI and total sleep by age and gender subgroups (FAS). Values are adjusted means. PSQI: Pittsburgh sleep quality index; FAS: full analysis set.
Discussion
The main findings of the study have shown that treatment with DMF was associated with a patient-perceived improvement of quality of sleep as measured with the PSQI: improvements in patients treated with DMF were observed as early as after 12 weeks of treatment and were sustained over 48 weeks. Even if a treatment per time interaction was not evident, a significant improvement of the PSQI score was observed only in DMF group. The absence of improvement in IFN group is probably explained by the negative effects of IFN on the quality of sleep, mostly because of its flu-like symptoms. This subjective improvement of the sleep quality after the onset of treatment with DMF was confirmed in PP and in the baseline adjusted analysis; an even larger improvement was observed in patients with sleep problems at entry.
The subgroup analyses revealed that females and patients older than 35 years of age had a larger improvement in perceived sleep quality, which is not surprising because these groups were those with a poorer sleep quality at entry. In fact, patients with some impairment of sleep (PSQI >5) at entry were those with a larger improvement after the onset of DMF. No significant benefits were reported by patients who started IFN treatment.
The benefit of sleep of starting DMF was not confirmed by remote monitoring: the sleep quality was not significantly influenced by the onset of both treatments and the total sleep and deep sleep decreased, mostly in the first 12 weeks, with both treatments, then remained stable for the total study duration. With both treatments, there were no significant changes from baseline in the number of awakenings. It is important to underline the superior sensitivity of patients reported outcome compared to an objective assessment of the sleep profile in revealing an improvement of the quality of sleep. The correlation tests showed significant correlations between subjective (PSQI) and objective (wearable tracker) improvement of quality of sleep at Week 48 in DMF group, which were more evident in patients with poor sleep at baseline. Treatment with DMF was associated with significant and sustained improvements in depression, with no significant changes in fatigue. Both depression and fatigue improved with a subjective improvement of sleep quality, whereas there was no correlation between MADRS score and FSS and sleep parameters collected by wearable tracker. Adherence to the wearable tracker declined over time but records at Week 48 were available from approximately 90% of patients who completed the overall observational period. As none of the patients had previous experience with the wearable tracker used in the study and no registration was performed before enrolment, it can be hypothesised that adherence to the device may be further enhanced after a period of familiarisation.
Quality of life and satisfaction with treatment did not significantly improve over time. Notably, other observational studies with DMF in RRMS,32,33 reporting significant improvements in quality of life, included higher sample size and used different scales for the assessment of the quality of life.
There were no gender differences in the duration of total sleep, whereas significant improvements in PSQI score were observed only in female patients, which suggests that, in line with data reported in the literature ,34 female patients may have a worse perception of quality of sleep than males, but this perception is not reflected on measurements done by wearable tracker. Furthermore, as gender differences were more evident at Week 12 than thereinafter, it is likely that gender may influence the initial adaptation to sleep perception.
DMF exhibited a satisfactory safety profile, which was in line with the safety profile highlighted from randomised clinical trials.18,19
Despite the evidence of the benefits of DMF in improving sleep quality in RRMS patients, this study has some limitations, particularly for the comparison between DMF and IFN groups, which was an exploratory endpoint. First, the study was conducted according to an open-label design, and thus the awareness of the treatment received might have influenced the subjective measurements of patients. Second, caution should be used in the interpretation of results in patients treated with IFN and of comparisons between DMF and IFN treatment, due to the small sample of patients treated with IFN (approximately a quarter of DMF recipients), which might have limited the probability of detecting statistically significant changes. Also, the difference by gender in subjective sleep perception might have been biased by the larger sample of females than males enrolled. Finally, the potential pre-selection of patients and assigned therapy that could have excluded potentially treating difficult might have led to potential biases in treatment selection and hence in the interpretation of the results. Great caution should also be used to interpret the tracker findings, both for technical and methodological reasons.23 The polysomnography would be of help in validating and interpreting the tracker findings, on the other side it would enhance the study burden impacting adherence. Because there were 1–4 weeks between the onset of treatment and the enrolment, we may have missed the initial phase of adaptation to the treatment. This choice was intentional because the scope of the study was to examine if the anti-inflammatory effects of DMF could improve the quality of sleep.
Despite these limitations, the main findings of this study suggest that treatment with DMF may improve the quality of sleep in RRMS patients naïve to treatment, in which sleep disruption and fatigue are important determinants in impaired quality of life that often remain under-diagnosed and under-treated in routine evaluation. Further randomised controlled clinical trials would be helpful to confirm the benefits of long-term treatment with DMF in the management of sleep disorders in patients with RRMS.
List of the investigators of the hi-tec Italian multicentre study group
Pasquale Annunziata – Azienda Ospedaliera Universitaria Senese – Siena
Mauro Zaffaroni – Centro Sclerosi Multipla Ospedale di Gallarate ASST Valle Olona – Varese
Maria Rosa Rottoli – UO Neurologia, Ospedale Papa Giovanni XXIII – Bergamo
Marta Zaffira Conti – UO Neurologia, Ospedale Papa Giovanni XXIII – Bergamo
Marco Ronzoni – Centro Sclerosi Multipla, Ospedale G. Salvini – Garbagnate – (MI)
Patrizia Perrone – UO di Neurologia, Ospedale Civile di Legnano – Legnano – (MI)
Marco Peresson – UO Neurologia Ospedale San Pietro FateBenefratelli – Roma
Daniela De Pascalis – Centro regionale per la Diagnosi e Cura della Sclerosi Multipla e delle Malattie Demielinizzanti Ospedale S. Eugenio – Roma
Girolama Alessandra Marfia – Centro Sclerosi Multipla Dipartimento Neuroscienze Policlinico Tor vergata – Roma
Angela Marsili – Centro Regionale per la Sclerosi Multipla, Università di Napoli Federico II – Napoli
Enrico Millefiorini – Istituto Neurotraumatologico Italiano – Roma
Vincenzo Di Lazzaro – Università Campus Bio-Medico – Roma
Carlo Pozzilli – UOC Neurologia Centro Sclerosi Multipla A.O. Sant’Andrea – Roma
Maurizio Assetta – UO Neurologia Ospedale Civile di Teramo – Teramo
Alessia Bianchi – UOC Neurologia e Neurofisiopatologia Policlinico ‘Paolo Giaccone’ – Palermo
Francesca Ruscica – Centro Sclerosi Multipla Fondazione Istituto San Raffaele ‘G. Giglio’ – Cefalù – (PA)
Graziella Callari – Centro Sclerosi Multipla Fondazione Istituto San Raffaele ‘G. Giglio’ – Cefalù – (PA)
Marinella Clerico - Dipartimento di Scienze Cliniche e Biologiche Azienda Ospedaliera Universitaria San Luigi Gonzaga – Orbassano – (TO)
Cristoforo Comi – Neurologia Università del Piemonte Orientale – Novara
Maria Grazia Piscaglia – U.O. Neurologia Ospedale S. Maria delle Croci – Ravenna
Antonio Palmieri – Ospedale Civile di Portogruaro – (VE)
Antonio Gallo – I Clinica Neurologica AOU, Università degli Studi della Campania ‘L. Vanvitelli’ – Napoli
Maria Trojano – UO Neurofisiopatologia AOU Policlinico Bari – Bari
Umberto Aguglia – Cattedra di Neurologia Ospedale Riuniti Bianchi-Melacrino-Morelli – Reggio Calabria
Elisabetta Signoriello – Centro Sclerosi Multipla, II Divisione di Neurologia, Università degli studi della Campania ‘L. Vanvitelli’ – Napoli
Barbara Nieddu – UO Neurologia Ospedale Antonio Segni di Ozieri, Ozieri – Sassari, Italy
Acknowledgments
The authors are thankful for the writing and editorial support for the preparation of this abstract by Excel Scientific Solutions (Southport, CT, USA) and Fullcro S.r.l.
Footnotes
Conflict of interest: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Giancarlo Comi has received personal compensation for consulting and speaking activities from Biogen, Novartis, Teva, Sanofi Genzyme, Merck, Roche, Almirall, Celgene, Forward Pharma, Medday and Excemed. Letizia Leocani has received honoraria for consulting services from Merck, Roche, Novartis and for speaking activities from Teva; research support from Merck, Biogen, Novartis; travel support from Merck, Roche over the last 3 years. Marta Radaelli received compensation for speaking activities and travelling support to congresses by Biogen, Novartis, Merck Serono, Genzyme and Teva. Roberta Lanzillo received personal compensation for speaking or consultancy from Biogen, Teva, Genzyme, Merck, Mylan, Novartis and Roche. Giacomo Lus received research grants and honoraria as a speaker and member of advisory boards by Bayer, Biogen, Merck Serono, Novartis, Sanofi Genzyme, Teva, Almirall, Allergan, Merz, Ipsen, Roche. Fioravante Capone received travel grants from Biogen, Merck, and Sanofi-Genzyme. Luigi Grimaldi received funding for travel to attend scientific events or speaker honoraria from Merck Serono, Biogen Idec, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd, Roche, Novartis and Bayer Schering Pharma; and received institutional research support from Biogen Idec and Novartis. Giuseppe Salemi: received research grants from Biogen, Merck Serono, Novartis, Sanofi Genzyme, Teva, Almirall, and Roche. Valentina Zipoli and Alessandra Cardillo are employees of and hold stock/stock options in Biogen. Luigi Ferini-Strambi, Gloria Dalla Costa, Valentina Bianchi and Sebastiano Traccis have nothing to disclose.
Data availability statements: The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was sponsored by Biogen Italia S.R.L, Milan, Italy
ORCID iDs: Giancarlo Comi https://orcid.org/0000-0002-6989-1054
Letizia Leocani https://orcid.org/0000-0001-9326-6753
Gloria D Costa https://orcid.org/0000-0002-8761-9337
Fioravante Capone https://orcid.org/0000-0002-7639-5104
Contributor Information
Giancarlo Comi, Università Vita Salute San Raffaele, Centro Sclerosi Multipla Ospedale Gallarate, Milan, Italy.
Letizia Leocani, Experimental Neurophysiology Unit, INSPE IRCCS Ospedale San Raffaele, Università Vita Salute San Raffaele, Milan, Italy.
Luigi Ferini-Strambi, Department of Clinical Neurosciences, San Raffaele Scientific Institute, Sleep Disorders Center, Università Vita Salute San Raffaele, Milan, Italy.
Marta Radaelli, Experimental Neurophysiology Unit, INSPE IRCCS Ospedale San Raffaele, Università Vita Salute San Raffaele, Milan, Italy.
Gloria D Costa, Experimental Neurophysiology Unit, INSPE IRCCS Ospedale San Raffaele, Università Vita Salute San Raffaele, Milan, Italy.
Roberta Lanzillo, Neurosciences, Reproductive and Odontostomatological Sciences Department, Federico II, University of Naples, Italy.
Giacomo Lus, Multiple Sclerosis Center, II Division of Neurology, University of Campania ‘L. Vanvitelli’ Napoli, Italy.
Valentina Bianchi, UOC Neurologia, Azienda Ospedaliera Sant’Andrea, Roma, Italy.
Sebastiano Traccis, UO Neurologia Ospedale Antonio Segni di Ozieri, Ozieri, Italy.
Fioravante Capone, Università Campus Bio-Medico, Roma, Italy.
Luigi ME Grimaldi, Centro Sclerosi Multipla Fondazione Istituto ‘G. Giglio’, Cefalù (PA), Italy.
Giuseppe Salemi, UOC Neurologia e Neurofisiopatologia Policlinico ‘Paolo Giaccone’, Palermo, Italy.
References
- 1.Fleming WE, Pollak CP. Sleep disorders in multiple sclerosis. Semin Neurol 2005; 25: 64–68. [DOI] [PubMed] [Google Scholar]
- 2.Kaminska M, Kimoff RJ, Schwartzman K, et al. Sleep disorders and fatigue in multiple sclerosis: evidence for association and interaction. J Neurol Sci 2011; 302: 7–13. [DOI] [PubMed] [Google Scholar]
- 3.Bamer AM, Johnson KL, Amtmann D, et al. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler 2008; 14: 1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caminero A, Bartolomé M. Sleep disturbances in multiple sclerosis. J Neurol Sci 2011; 309: 86–91. [DOI] [PubMed] [Google Scholar]
- 5.Brass SD, Li CS, Auerbach S. The underdiagnosis of sleep disorders in patients with multiple sclerosis. J Clin Sleep Med 2014; 10: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braley TJ, Boudreau EA. Sleep disorders in multiple sclerosis. Curr Neurol Neurosci Rep 2016; 16: 50. [DOI] [PubMed] [Google Scholar]
- 7.Bøe Lunde HM, Aae TF, Indrevåg W, et al. Poor sleep in patients with multiple sclerosis. PLoS One 2012; 7: e49996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitkova M, Gdovinova Z, Rosenberger J, et al. Factors associated with poor sleep quality in patients with multiple sclerosis differ by disease duration. Disabil Health J 2014; 7: 466–471. [DOI] [PubMed] [Google Scholar]
- 9.Ayache SS, Chalah MA. Fatigue in multiple sclerosis – insights into evaluation and management. Neurophysiol Clin 2017; 47: 139–171. [DOI] [PubMed] [Google Scholar]
- 10.Leocani L, Colombo B, Comi G. Physiopathology of fatigue in multiple sclerosis. Neurol Sci 2008; 29: S241–S243. [DOI] [PubMed] [Google Scholar]
- 11.Nociti V, Losavio FA, Gnoni V, et al. Sleep and fatigue in multiple sclerosis: a questionnaire-based, cross-sectional, cohort study. J Neurol Sci 2017; 372: 387–392. [DOI] [PubMed] [Google Scholar]
- 12.Kaynak H, Altintaş A, Kaynak D, et al. Fatigue and sleep disturbance in multiple sclerosis. Eur J Neurol 2006; 13: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 13.Lanza G, Ferri R, Bella R, et al. The impact of drugs for multiple sclerosis on sleep. Mult Scler 2017; 23: 5–13. [DOI] [PubMed] [Google Scholar]
- 14.Mendozzi L, Tronci F, Garegnani M, et al. Sleep disturbance and fatigue in mild relapsing remitting multiple sclerosis patients on chronic immunomodulant therapy: an actigraphic study. Mult Scler 2010; 16: 238–247. [DOI] [PubMed] [Google Scholar]
- 15.Svenningsson A, Falk E, Celius EG, et al. Natalizumab treatment reduces fatigue in multiple sclerosis. Results from the TYNERGY trial; a study in the real life setting. PLoS One 2013; 8: e58643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 2012; 341: 274–284. [DOI] [PubMed] [Google Scholar]
- 17.Morris G, Stubbs B, Köhler CA, et al. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med Rev 2018; 41: 255–265. [DOI] [PubMed] [Google Scholar]
- 18.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 19.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 20.Kita M, Fox RJ, Gold R, et al. Effects of delayed-release dimethyl fumarate (DMF) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: an integrated analysis of the phase 3 DEFINE and CONFIRM studies. Clin Ther 2014; 36: 1958–1971. [DOI] [PubMed] [Google Scholar]
- 21.Braley TJ, Segal BM, Chervin RD. Sleep-disordered breathing in multiple sclerosis. Neurology 2012; 79: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braley TJ, Huber AK, Segal BM, et al. A randomized, subject and rater-blinded, placebo-controlled trial of dimethyl fumarate for obstructive sleep apnea. Sleep 2018; 41: 1–10. [DOI] [PubMed] [Google Scholar]
- 23.Sparaco M, Lavorgna L, Conforti R, et al. The role of wearable devices in multiple sclerosis. Mult Scler Int 2018; 2018: 7627643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes AJ, Parmenter BA, Haselkorn JK, et al. Sleep and its associations with perceived and objective cognitive impairment in individuals with multiple sclerosis. J Sleep Res 2017; 26: 428–435. [DOI] [PubMed] [Google Scholar]
- 25.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 28.Simeoni M, Auquier P, Fernandez O, et al. Validation of the multiple sclerosis international quality of life questionnaire. Mult Scler 2008; 14: 219–230. [DOI] [PubMed] [Google Scholar]
- 29.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- 31.Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated treatment satisfaction questionnaire for medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes 2009; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger T, Brochet B, Brambilla L, et al. Effectiveness of delayed-release dimethyl fumarate on patient-reported outcomes and clinical measures in patients with relapsing-remitting multiple sclerosis in a real-world clinical setting: PROTEC. Mult Scler J Exp Transl Clin 2019; 5: 2055217319887191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kresa-Reahl K, Repovic P, Robertson D, et al. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther 2018; 40: 2077–2087. [DOI] [PubMed] [Google Scholar]
- 34.Leonavicius R, Adomaitiene V. Features of sleep disturbances in multiple sclerosis patients. Psychiatr Danub 2014; 26: 249–255. [PubMed] [Google Scholar]