Abstract
In recent years, breakthroughs have been made in tumor immunotherapy. However, tumor immunotherapy, particularly anti-PD-1/PD-L1 immune checkpoint inhibitors, is effective in only a small percentage of patients in solid cancer. How to improve the efficiency of cancer immunotherapy is an urgent problem to be solved. As we all know, the state of the tumor microenvironment (TME) is an essential factor affecting the effectiveness of tumor immunotherapy, and the cancer-associated fibroblasts (CAFs) in TME have attracted much attention in recent years. As one of the main components of TME, CAFs interact with cancer cells and immune cells by secreting cytokines and vesicles, participating in ECM remodeling, and finally affecting the immune response process. With the in-depth study of CAFs heterogeneity, new strategies are provided for finding targets of combination immunotherapy and predicting immune efficacy. In this review, we focus on the role of CAFs in the solid cancer immune microenvironment, and then further elaborate on the potential mechanisms and pathways of CAFs influencing anti-PD-1/PD-L1 immunotherapy. In addition, we summarize the potential clinical application value of CAFs-related targets and markers in solid cancers.
Keywords: Cancer-associated fibroblasts, PD-1/PD-L1 inhibitors, Immunotherapy
Background
For cancer treatment, the advent of immunotherapy has reinvigorated the field. Immunotherapy mainly includes immune checkpoint inhibitors (ICIs), tumor vaccine therapy, oncolytic virus therapy, and adoptive cell therapy (ACT) [1]. Among them, anti-cancer therapies using ICIs have shown great potential in tumor treatment. Although immunotherapy is more effective and more tolerated than conventional and targeted therapies, many patients demonstrate congenital or acquired resistance [2]. Especially for most advanced solid tumors, inhibitor therapy that blocks the binding of Programmed death receptor 1(PD-1) to Programmed death ligand 1 (PD-L1) has become central [3]. Meanwhile, unfortunately, clinical studies have shown that not all patients are sensitive to this approach, with some patients still showing congenital non-response to PD-1/PD-L1 blockade, and some others developing acquired resistance after treatment [4]. Therefore, in-depth research on drug resistance is necessary to improve the efficacy of PD-1/PD-L1 inhibitors. Current studies have shown that the main factors influencing immunotherapy are: the tumor immune microenvironment, the tumor immunogenicity, the dysfunction of MHCs, irreversible T cell failure, and mutations in tumor genes including the interferon-gamma (IFN-γ) signaling pathway [5–7]. Among them, the tumor microenvironment (TME) status has been shown to play a pivotal role in tumor development and will influence the clinical outcome of treatment [1]. The high heterogeneity and dynamics of TME affect the infiltration of immune cells in tumor tissues, thereby affecting the role of PD-1/PD-L1 inhibitors. For example, the infiltration degree of CD8+ T lymphocytes in tumor tissues, the expression status of PD-L1 in tumor cells, the number of CD4+ T lymphocytes and the composition ratio of other immune cells in the microenvironment [8–10].
In addition, in recent years, the CAFs in TME have received growing attention. CAFs are a core component of the TME and can not only interact with cancer cells but also affect other components of the TME (such as extracellular matrix and immune infiltration) [11]. As a widespread cell population in the matrix, CAFs also secrete a variety of cytokines and extracellular vesicles, and other substances, and then participate in the remodeling of the extracellular matrix (ECM) [12]. There is a wide range of cellular origins, phenotypic and functional heterogeneity for CAFs [13, 14]. In research analysis of various cancers, subgroup types with different functions have been isolated [15–19]. This also demonstrates the heterogeneity of the CAFs population, suggesting that this phenomenon may be present in a wide range of cancers [19, 20]. Different subsets of CAFs regulate immune cells through different signaling pathways, and reveal different mechanisms of these subsets to exert drug resistance in the process of immunotherapy. What’s more, ncRNAs contained in CAFs-derived exosomes in colon cancer, have enhanced tumor cell proliferation and stem cell properties [21]. CAFs have also been reported to promote tumor progression in studies of solid tumors such as breast, pancreatic, prostate, and colorectal cancer (CRC) [22].
In summary, based on the heterogeneity of CAFs and their interaction with TME, the influence mechanism and related pathways of CAFs on PD-1/PD-L1 immunotherapy are described in solid tumors. In addition, this review also describes the markers associated with CAFs and potential combination therapy targets in solid cancers.
The heterogeneity and subpopulation types of CAFs
The occurrence and development of cancer are dynamic processes. As cancer progress, cancer cells evolve, resulting in tumor heterogeneity. In addition to genetic differences in cancer cells themselves, different components of TME also lead to changes in the state or phenotype of cancer cells by altering signaling pathways in cancer cells. CAFs are a dynamic part of the TME and play a coordinating role between cancer cells and host matrix responses [23]. With the attention to CAFs and the development of single-cell RNA sequencing (scRNA-seq) technology, researchers have gradually revealed the heterogeneity of CAFs in different cancers. In addition to having basic myofibroblast properties, the activated fibroblast also characteristically expresses alpha-smooth muscle actin (A-SMA/α-SMA), fibroblast activation protein (FAP), fibroblast-specific protein 1 (FSP1), and other factors [15, 24]. Recent observations in genetically engineered mouse models and clinical studies have demonstrated the existence of at least two functionally distinct CAFs, cancer-promoting CAFs (PCAFs) and tumor-suppressing CAFs (RCAFs) [17, 25]. For example, in pancreatic ductal adenocarcinoma (PDAC) [25], researchers have identified the presence of fibroblast-activation protein (FAP)+ CAFs in the pro-oncogenic form and fibroblast-smooth muscle actin (α SMA)+ CAFs in the anti-oncogenic form. These two subpopulations have completely different roles in the progression and immune landscape of PDAC. One experiment in which primary CAFs were co-cultured with colon cancer cells showed that subpopulations of CAFs with different migratory abilities had different gene expression signatures [21]. The differences in function may originate from the diversity of differentiation pathways. Studies has shown that [13], CAFs are differentiated from different progenitor cells, such as resident fibroblasts, circulating bone marrow mesenchymal cells, epithelial-mesenchymal transition (EMT), and endothelial-mesenchymal transition (ENDMT). The differences in these differentiation pathways might largely determine the heterogeneity of CAFs subsets. Furthermore, in a mouse model of breast cancer, mechanoresponsive (MR) CAFs may exhibit a lower differentiation state relative to immunomodulatory (IM) CAFs. By assessing the epigenomic changes of CAFs in tumors, this study suggests that the level of chromatin opening in different clusters may partly determine heterogeneity [26].
In a single cell analysis of 10 common solid cancers, subpopulations of CAFs with common activation characteristics were displayed. These subpopulations mainly overexpress genes related to collagen activation and matrix metalloproteinases. Three major subgroups were defined as cancer-associated myofibroblasts (CAF muscles), inflammatory CAF (CAF infra), and lipogenic CAF (CAF Ardy Wiranata). In addition, minor subgroups include endothelial-to-mesenchymal transition CAF (CAF ends MT), peripheral nerve-like CAF (CAF PN), and antigen-presenting CAF (CAF ap) [27]. These subsets are mainly related to multiple inflammatory pathways, angiogenesis, endothelium-mesenchymal transition, antigen presentation, etc. This suggests that the potential mechanism of CAFs subsets in promoting tumor progression. However, the subpopulations of CAFs are not always similar in different cancers, and specific subpopulations may exist in certain tumors. For example, in pancreatic cancer [28], researchers have found that a unique Meflin positive subpopulation plays an important role in inhibiting cancer progression.
During the development of cancer, each cluster of CAFs is in dynamic change. The proportion of CAFs subpopulations is not constant. Studies have shown that the number of canonical myofibroblasts (myCAFs, Subset 2) and VEGF+ CAFs (vCAFs, Subset 3) cells in breast cancer tissues decreased significantly after transforming growth factor-β(TGF-β) antibody blockade, indicating that these two subsets are very sensitive to this treatment. In addition, the emergence of another new subpopulation, interferon-licensed CAFs (ilCAFs), is refreshing. The cluster is closely related to interferon response signaling pathway and antigen processing and presentation [14]. Similarly, five CAF clusters were identified by scRNA-seq of human skin cancer before and after immunotherapy, of which clusters 3 and 4 (SSL,steady state-like) were present only in pre-treatment CAFs. The proportion of mechanoresponsive (MR) CAFs and immunomodulatory (IM) CAFs increased significantly after treatment. Immunotherapy has been shown to stimulate differentiation of CAFs into other subsets [26]. This also confirmed the homeostasis and plasticity among CAFs subsets.
In addition, the interaction between CAFs subsets and other cells is also different. In spatial transcriptomics analysis of mouse breast cancer models, antigen-presenting CAFs in immune clusters in early tumors colocalized with lymphoid immune cells, while myofibroblast CAFs colocalized with epithelial cells. However, in the later stages of the tumor, immune-associated cell clusters are more closely related to myeloid cells (including macrophage phenotype) and the interactions are more prominent [26]. This also suggests that CAFs subsets are spatially dynamic as tumors progress. Therefore, there are different signal regulation pathways between CAFs and other cells. The ecm-myCAF subset in breast cancer not only increased the proportion of the transcription factor forkhead box protein p3 (Foxp3) + T cells in the CD4+CD25+population, but also upregulated the expression of PD-1 and cytotoxic T lymphocyte associate protein-4(CTLA-4) on their surface, which in turn increased the proportion of TGF-β-myCAF [19]. This may reveal the underlying mechanisms of immunosuppression and drug resistance.
CAFs and tumor immunity
As early as 1909, Paul Ehrlich put forward the original hypothesis of cancer immunity. Cancer immune system distinguishes between “self” and “non-self”, and eliminates “non-self” without damaging “self”, which also forms the original TME concept. Different types of tumors can also promote angiogenesis and stimulate peripheral immune tolerance to form specific microenvironments. With the occurrence and development of cancer, various cellular and molecular mechanisms in the TME are also in the process of dynamic change. Over time, tumor tissue becomes resistant to immunotherapy, which is also the reason why some patients have a poor response to immunotherapy [3]. Among them, immune cells infiltrated in tumor tissue are an important part and an important factor in predicting patient survival [29]. These immune cells include tumor-associated macrophages (TAMs) and neutrophils that promote tumor development, natural killer cells (NKs), and cytotoxic CD8+T cells that resist tumor development [30, 31]. In addition to immune cells, CAFs, as an important component of tumor tissue stromal components, also play an extremely important role in cancer progression. It also occupies an important part of tumor tissue. On the one hand, CAFs affect the function of immune cells by secreting various cytokines and products; on the other hand, as a component of tumor stroma, CAFs form a permeability barrier by remodeling the stroma, thereby reducing the effect of drug therapy [11].
At present, increasing studies have opened up new fields in exploring the role of immune resistance in cancer progression [32]. What’s more, the regulation of CAFs to relieve their inhibitory effect on immune cells and overcome their barrier effect, become a new means of tumor therapy.
The mechanism of CAFs regulating TME to affect PD-1/PD-L1 inhibitor immunotherapy
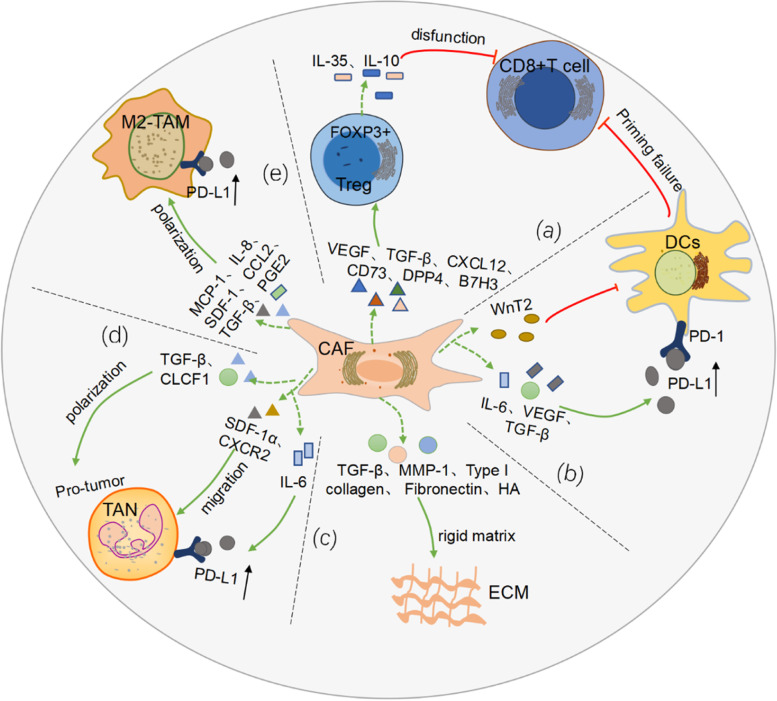
As key components of the TME, CAFs are not only involved in the remodeling process of the ECM, but also interact with other cells in the TME [11]. CAFs influence the recruitment and activity of immune cells through direct and indirect regulation of ECM remodeling [33]. For example, CAFs promote immune cell differentiation and enhance immune resistance by secreting a series of cytokines and other effector molecules, including TGF-β, interleukin 6(IL-6), c-x-c chemokine ligand 2(CXCL2), collagen, and laminin, etc. [11]. Moreover, different subsets of CAFs differentially regulate cancer-related pathways and the accumulation of regulatory T cells by interacting with tumor cells [25].Therefore, with an in-depth understanding of the immune microenvironment, people are increasingly aware of the importance of CAFs in affecting immune efficacy. CAFs were summarized the mechanism of regulating the TME and consequently affecting the PD-1/PD-L1 inhibitor immunotherapy (Fig. 1).
Fig. 1.
The mechanism of CAFs regulating TME to affect PD-1/PD-L1 inhibitor immunotherapy. a CAFs secrete VEGF, TGF-β, CXCL12, etc., which promote Treg recruitment, migration and FOXP3+Treg differentiation. The latter promotes CD8+ T cell dysfunction by secreting IL-35 and IL-10. b CAFs secrete WnT2, etc., thereby inhibiting the anti-tumor response of DC-cell-mediated CD8+ T cells. c CAFs secrete TGF-β, MMP-1, HA, etc. to remodel ECM, increase rigidity, and prevent immune cell infiltration. d TGF-β and CLCF1 differentiate TAN into tumor-promoting types; IL-6 promotes the expression of PD-L1 by TAN, leading to the formation of immune tolerance; SDF-1α and CXCR2 promote TAN migration to tumor tissue. e CAFs produce MCP-1, IL-8, SDF-1, etc. to promote monocyte recruitment, induce TAMs to M2 phenotypic differentiation, and impair effector T cell function, and increase the expression level of PD-L1 on the surface of the TAMs, impairing its phagocytosis
CAFs-Treg-CD8 + T
Regulatory T cells (Tregs) belong to the immunosuppressive subset of CD4+ T cells and are important in maintaining immune homeostasis. Tregs accelerate immune evasion of tumor cells by disrupting tumor immune surveillance and impairing anti-tumor immune responses [34]. The mechanism that a differentiated immunosuppressive Treg phenotype is induced in the TME, remains to be further explored. It is known that in terms of spatial distribution, Tregs are distributed in the tumor stroma in far greater numbers than cancer nests, and CAFs are also usually distributed in the stroma [35]. The spatial intersection leads to mutual crosstalk between the two. On the one hand, CAFs promote Treg recruitment and migration, resulting in increased infiltration at tumor sites. For example, in CRC [36], CD70+ CAFs stimulated Treg migration and significantly increased their frequency at tumor sites. In addition, Vascular endothelial growth factor-A (VEGF-A) released by CAFs directly or indirectly participates in the induction and maintenance of Treg cells [37].
On the other hand, CAFs also promote the transformation of Treg cells and are closely related to immunosuppression. In the stromal of lung adenocarcinoma [35], Tregs and CAFs were found to be spatially close, and in vitro experiments showed that CAFs in high-Treg adenocarcinoma had higher secretion capacity of immunoregulatory cytokines, leading to the induction of Treg; and further proved that TGF-β and VEGF were highly expressed in stroma-derived CAFs containing high concentrations of Tregs. CD8+ T cells are induced to express Foxp3 in the presence of TGF-β. These FOXp3-positive cells are widely present in the tumor immunosuppressive microenvironment and promote the differentiation of Treg cells [38, 39]. Furthermore, after co-culture of cancer cells and fibroblasts in vitro, the results showed that the expression of COX-2 was induced to increase in CAFs [40]. This enzyme and its product prostaglandin E2 (PGE2) play an important role in Treg function in tumors. PGE2 induces the expression of FOXP3 in Tregs [41]. Additionally, a study on breast cancer [42], indicated that FAP+PDGFRβ+ CAF in tissues not only released CXCL12 to promote the migration of CD4+CD25+ T cells, but also expressed CD73, dipeptidyl peptidase IV (DPP4) and B7H3 to promote the conversion of CD4+ T cells to FOXP3+ Treg cells. Subsequently, Tregs inhibit CD8+ T cells function and promote their depletion by producing IL-35 and IL-10 [43, 44]. In summary, CAFs can affect the killing ability of CD8+ T cells to tumors by regulating the differentiation of Treg (Fig. 1a).Moreover, Treg cells inhibit the secretion of IFN γ by CD8+ T cells, indirectly promoting M2-like TAM dominance, thereby enhancing cancer progression [45].
CAFs-DCs-CD8 + T
Dendritic cells (DCs) are the most efficient antigen-presenting cells (APCs), linking innate and adaptive immunity. DCs are phenotypically and functionally heterogeneous under physiological conditions. DC1s (type 1 dendritic cells) are capable of presenting antigens of MHC class I molecules to CD8 T cells, while DC2s (type 2 dendritic cells) are capable of presenting MHC class II molecules to CD4 T cells [46]. CAFs act an essential role in interfering with DC maturation, antigen presentation and immune response. Both TGF-β1 and TGF-β2 are upregulated in CAFs, and the both factors critical for maintaining an activated fibroblast phenotype [47]. TGF-β immobilizes DCs by blocking the migration of DCs, which in turn blocks the transport of antigens to the draining lymphatic system [48]. What’s more, under the stimulation of TGF-β, the expression of MHC-II, CD80, CD40, etc. on the surface of DCs are down-regulated, thereby changing the phenotype, which is beneficial to indirectly participating in the process of immunosuppression [49] (Fig. 1b). Likewise, in hepatocellular carcinoma [50], CAFs recruit normal DCs and induce their differentiation into regulatory DCs(RDCs) and dysfunctional DCs by activating the IL-6-mediated STAT3 pathway. These subpopulations are characterized by low levels of expression of costimulatory molecules and little antigens are present, while suppressive cytokines such as indoleamine 2,3-dioxygenated (IDO) are secreted. In addition, VEGF released by CAFs participates in the abnormal differentiation process of DCs and impairs their antigen-presenting function by inhibiting the activation of nuclear factor kappa-B(NF-κB) [51]. Strikingly, VEGF also promotes immune tolerance by upregulating the expression of PD-L1 on the DC surface [52]. In a related study of primary esophageal squamous cell carcinoma (ESCC), we found that WNT2+ CAFs were negatively correlated with CD8+ T cells. The further mechanistic analysis confirmed that WNT2 secreted by CAFs, inhibited DCs-mediated antitumor T cell responses through the SOCS3/p-JAK2/p-STAT3 signaling cascade [53] (Fig. 1b).
CAFs-ECM
The ECM is a complex network of extracellular proteins, proteoglycans, and glycoproteins [54]. As an important component of TME, ECM plays a significant role in tumor growth, migration and metastasis [55]. Studies showed that in the process of CAFs activation, the extracellular matrix continuously deduced the process of remodeling through matrix deposition and protein degradation [56]. This gives solid tumors rich in CAFs an “innate advantage” to form a rigid matrix. With the in-depth study of ECM remodeling, the researchers found that [57, 58], CAFs played an essential role in matrix remodeling by secreting a variety of matrix proteins (such as type I collagen, fibronectin, matrix metalloproteinase-1(MMP-1)) and releasing cytokines(Fig. 1c). For example, the growth factor TGF-β1 released by CAFs was found to be indispensable in the regulation of matrix remodeling [59]. In another animal experiment [14], a substantial reduction in the overall pro-fibrotic program in tumors of mice treated with anti-TGFβ. Furthermore, it was reconfirmed by analyzing the genetic signatures associated with collagen deposition and fibrosis. More in-depth research revealed that TGF-β signaling activation in CAFs was associated with transcription program dysregulation. Tumors activating the program carry characteristic gene profiles, such as BRAF, TP53 mutations, and MYC amplification, which are effective indicators of PD-1 blockade failure [59]. Hyaluronic acid is also often found to be excessively deposited in malignant tumors. The study reported that fibroblasts deficient in the hyaluronan synthase gene (HAS2) showed severe impairments in recruiting macrophages, and the HA-deficient stromal environment was also detrimental to tumor vascular and lymphangiogenesis [60].
The ECM modified by CAFs also modulates the activity of other immune cell populations. Complex crosstalk between cancerous collagen matrix and TAMs [61]. On the one hand, CAFs are closely related to the collagen-rich matrix, which not only promotes the activity of monocytes, but also induces the polarization process of M2 macrophages [62]. On the other hand, the induced formation of TAMs regulates collagen deposition, thereby increasing matrix rigidity [61]. Some studies showed [63, 64] that accumulation of collagen density or hardness activates the FAK pathway. This would not only drive CD8+ T cell depletion, but also promote the recruitment of other immune cells, such as Tregs and MDSCs. This process is closely related to the formation of immunosuppressive TME. It is undeniable that changes in matrix composition affect the migration and spatial differences of immune cells [65]. However, the specific crosstalk mechanism between ECM and immune cells still remains to be further explored.
CAFs-TANs
Similar to most immune cells in the TME, tumor-associated neutrophils (TANs) also exhibit diverse phenotypic and functional heterogeneity, influenced by other cells in the TME. According to different polarization states, neutrophils are divided into N1 type with anti-tumor function and N2 type with tumor-promoting effect [66]. CAFs affect TAN polarization by releasing a variety of cytokines. Among them, cardiotrophin-like cytokine 1 (CLCF1) released by CAFs is very important in inducing the polarization of tumor-promoting TANs, which is achieved by up-regulating the expression of CXCL6 and TGF-β in hepatoma cells [67] (Fig. 1d). What’ more, some studies confirmed that [68], in addition to TGF-β induction of a population of TANs that develop a tumor-promoting phenotype, blocking TGF-β results in the recruitment and activation of TANs with an anti-tumor phenotype. In addition to inducing polarization, CAFs also recruit surrounding neutrophils to aggregate into tumors, among which stromal-derived factor-1 alpha(SDF-1α),CXCR2 secreted by CAFs have been shown to promote the migration of TANs [69, 70] (Fig. 1d). CAFs may be involved in many stages of TANs progression to promote the formation of tumor immunosuppressive microenvironment through various mechanisms.
Remarkably, CAFs-derived IL-6 upregulates PD-L1 expression by activating the JAK-STAT3 signaling pathway of TANs, ultimately impairing T cell function and inducing immune tolerance in hepatocellular carcinoma(HCC) [69] (Fig. 1d). Similarly, in gastric cancer [71], tumor cell-derived MSCs (GC-MSCs) induce chemotaxis and activation of neutrophils by activating the IL-6-STAT3-ERK1/2 signaling pathway. Conversely, neutrophils activated by GC-MSCs induce normal MSCs to differentiate into CAFs. The interaction between GC-MSCs and TANs suggests a new mechanism for the remodeling of the cancer ecological environment. More importantly, by revealing the mechanism of action between CAFs and TANs, it provides a new idea for us to explore the mechanism of PD-1/PD-L1 immune resistance, and shows a new target site for the combined application of targeted drugs. However, due to the limited number of research reports, the specific mechanism of the interaction between the two remains to be further explored in the future.
CAFs-TAMs
The main sources of TAMs include peripheral blood monocytes and tissue-resident macrophages. The heterogeneity of TAMs is caused by the combined influence of the complexity of the growth environment and the plasticity of the cells themselves. Moreover, macrophage inflammation may promote tumor immune escape [72]. Notably, the high plasticity of TAMs is tightly regulated by specific chemokines and cytokines, which in turn polarize into a pro-inflammatory ‘M1 type’ or an immunosuppressive ‘M2 type’, upon application of differences in stimulated and induced transcription. Next, the M2 type is further divided into M2a, M2b, M2c, and M2d subtypes [73]. Most TAMs express markers of the M2 type, suggesting that infiltrating macrophages are reprogrammed to a ‘prominent’ phenotype by factors in the TME [74]. TAMs are one of the important components of TME and act an essential role in the regulation of tumor immune microenvironment, especially tumor immunosuppression. TAMs may exert both anti-tumor and tumor-promoting activities, while the molecular mechanisms known so far are very limited [75]. The study demonstrated [76], the proportion of macrophages in “fibrotic” matrices was significantly higher than in “inert” matrices, showing a potential interdependence between immunosuppressive TAMs and activating CAFs. In addition, studies showed that [74, 77, 78], CAFs promoted monocyte (macrophage precursor) recruitment and differentiation into cancer-promoting macrophage subsets (M2-type TAMs), thereby impairing effector T cell responses and inducing immunosuppression in the TME(Fig. 1e). In colon cancer, CAFs-induced M2-type TAMs had high expression of PD-1 on the cell surface, and the expression level was negatively correlated with the efficiency of phagocytosis of tumor cells [79] (Fig. 1e). Similarly, One study reported that the presence of TAMs aggravated the degree of immunosuppression in the pancreatic cancer microenvironment, leading to resistance to PD-1/PD-L1 blockade therapy [80]. CAFs might also inhibit certain aspects of TAM activity. In studies on targeted neuroblastoma models, blocking CAF-derived PGE2 reduced tumor proliferation and promoted the proportion of macrophages to differentiate into M2 type, thereby inhibiting tumor growth [81]. Clinical studies have demonstrated a correlation between accumulation of TAMs and adverse clinical outcomes [82]. In addition to the promotion of TAMs by CAFs, conversely, M2 macrophages also accelerate the progression of EMT by secreting soluble factors such as IL-6 and SDF-1, thereby stimulating the activation of CAFs. In other words, macrophages and CAFs have complex interaction mechanisms [83]. These mechanisms contribute to the formation of an immunosuppressive microenvironment and complicate immunotherapy. These facts lead us to explore the detailed mechanism of CAF-TAM in immune checkpoint therapy, which is also the current need for targeted therapy.
The related regulatory pathways of CAFs affecting PD-1/PD-L1 inhibitor immunotherapy
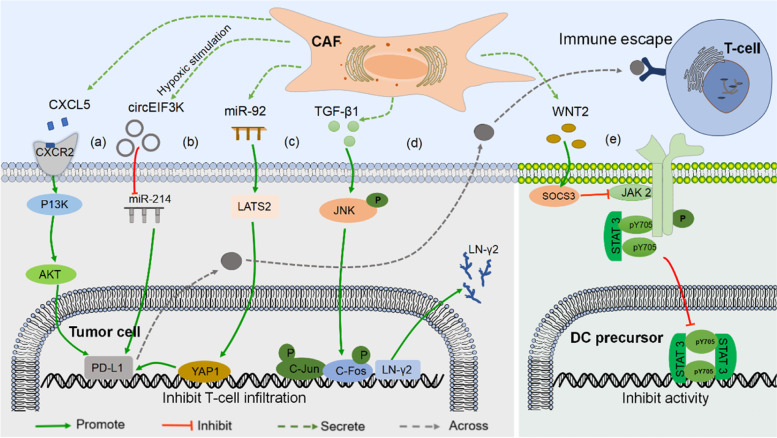
CAFs induce the expression of PD-L1 on cancer cells by deriving some cytokines and vesicles, thereby promoting tumor immune escape. Here, several related regulatory pathways of CAFs affecting PD-1/PD-L1 inhibitor immunotherapy are summarized (Fig. 2).
Fig. 2.
CAFs affect PD-1/PD-L1 inhibitor immunotherapy-related regulatory pathways. a WNT2 upregulates SOCS3 on DC precursors, thereby inhibiting the JAK2/STAT3 pathway and blocking DC differentiation and maturation. b TGF-β1 induces the expression of LN-γ2 in cancer cells through JNK/AP1 signal transduction, thereby hindering T cell invasion of cancer nests. c CAFs secrete CXCL5 to bind to CXCR2 on cancer cells, and then activate the PI3K/AKT pathway to promote PD-L1 expression on the cancer cell surface. d After miR-92 is taken up by cancer cells, it acts on LATS2/YAP1 signaling and increases PD-L1 transcriptional activity. e Hypoxia induces CAFS secretion of circeIF3K, which acts on the miR-214/PD-L1 axis, ultimately leading to immune evasion
In mouse melanoma and CRC [84], CAFs secrete CXCL5 to bind to c-x-c motif chemokine receptor 2(CXCR2) on cancer cells. The PI3K/AKT signaling pathway is then activated, promoting PD-L1 expression on the surface of cancer cells. Ultimately, immune evasion occurs (Fig. 2a). In addition, in vitro studies on CRC showed [85], hypoxia induced CAFS to secrete exosomal CirceIF3K, which induced PD-L1 expression in cancer cells, suggesting that this is achieved through the miR-214/PD-L1 axis (Fig. 2b). Moreover, another report is that [86], CAFs-derived exosomal miR-92 induced PD-L1 expression in breast cancer. After miR-92 is taken up by cancer cells, it acts on the target gene large tumor suppressor kinase 2 (LATS2). The latter interacts with transcriptional coactivator Yes-associated protein 1(YAP1) to promote nuclear translocation and binding to enhancers, thereby promoting PD-L1 transcriptional activity (Fig. 2c).
In addition to regulating the expression of PD-L1 on the surface of cancer cells, CAFs also affect immune efficacy by regulating immune cell differentiation and ECM remodeling. For example, in non–small cell lung cancer (NSCLC) and ESCC [16], TGF-β1 transduces the expression of laminin γ2 (LN-γ2) on tumor cells through JNK/AP1 signaling. Then LN-γ2 affects T cell receptor (TCR) gene transcription, which in turn inhibits T cell chemotaxis. This ultimately leads to ineffectiveness of anti-PD-1 therapy. Further analysis revealed that three AP1 (mainly c-jun and c-fos) binding sites were found upstream (− 92 to − 15 nucleotides) on the transcription factor binding site in the promoter region of the LN-γ2 gene. TGF-β1 stimulation significantly increased phosphorylated-c-jun (p–c-jun) and phosphorylated-c-fos (p–c-fos) binding to the LN-γ2 promoter (Fig. 2d). TGF-β1 inhibitors effectively inhibit this process. Therefore, the JNK/AP1 signaling pathway is involved in the upregulation of LN-γ2 mediated by TGF-β1. In addition, CAFs can also be indirectly regulated to weaken the killing effect of T cells. CAFs-derived WNT2 regulates the differentiation of DCs and attenuates the T cell killing effect in tumors. Tyrosine kinases (JAKs) and signal transducers and activators of transcription (STATs) are critical for inducing differentiation. WNT2 inhibits the p-JAK2/p-STAT3 (TYR705) pathway by up-regulating suppressor of cytokine signaling 3 (SOCS3) on DC precursors, thereby blocking DC differentiation and maturation [53] (Fig. 2e).
Different mechanisms of tumor cells driving CAFs to affect the immune efficacy of PD-1/PD-L1 inhibitors
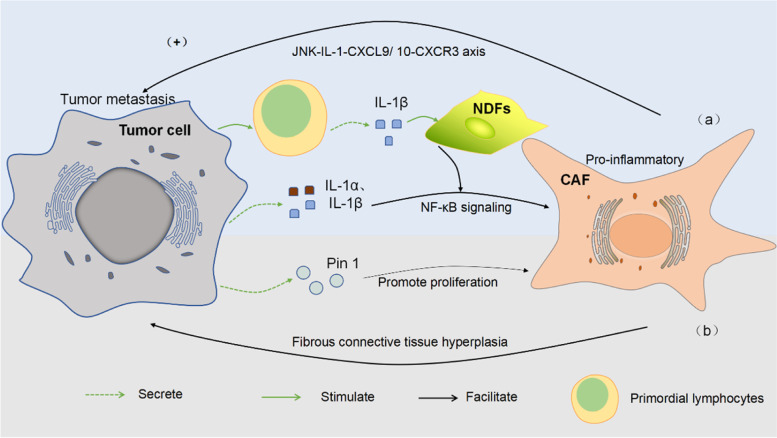
Tumor cells educate surrounding cells to enhance tumor immunity, thereby supporting their own proliferation, migration, and invasion [87]. In other words, cancer cells exploit the plasticity of stromal cells to enhance a microenvironment conducive to cancer cell growth. This is also one of the reasons for the poor immune efficacy of PD-1/PD-L1 inhibitors. This article summarizes several mechanisms by which tumor cells drive CAFs to affect immune efficacy (Fig. 3).
Fig. 3.
Tumor cells drive CAFs through different mechanisms to affect immune efficacy. a By directly secreting cytokines or indirectly stimulating primitive lymphocytes, tumor cells act on the NF-κB signaling pathway, activate fibroblasts, differentiate them into pro-inflammatory phenotypes, and then CAF acts on the JNK-IL-1-CXCL9/ 10-CXCR3 axis to promote tumor metastasis. b Pin1 promotes CAF proliferation, promotes fibrous connective tissue proliferation and immunosuppressive TME formation, which in turn is conducive to cancer progression
Related studies have shown that normal skin fibroblasts (NDFs) are “educated” by tumor cells to express pro-inflammatory genes, mainly by activating the NF-κB pathway in activated fibroblasts. This pro-inflammatory process occurs in early tumors. The adaptive immune cells induce the expression of IL-1β by primary immune cells, thus promoting the activation of CAFs [88] (Fig. 3a). Likewise, in CRC [89] and its liver metastases in research [90], NF-κB target genes COX-2 and IL-8 were also found to be highly expressed in human myofibroblasts, suggesting that NF-κB signaling may also play a role in the activation of CAFs in CRC. Another niche study of fibroblasts in metastases found that IL-1α and IL-1β secreted by breast cancer cells induced CXCL9 and CXCL10 production in lung fibroblasts, which was also achieved through NF-κB signaling and ultimately promoted the progression of lung metastasis. Surprisingly, the CXCL9/10-binding chemokine receptor CXCR3 was specifically expressed in a small subset of breast cancer cells, exhibited tumor-initiating capacity when co-transplanted with fibroblasts, and had high JNK signaling Drives IL-1α/β expression. Importantly, disruption of the intercellular JNK-IL-1-CXCL9/10-CXCR3 axis reduced metastatic colonization in xenograft and syngeneic mouse models [91]. In the study of PDAC [92], the researchers analyzed tumor samples and found a unique proline isomerase, Pin1, overexpressed in both CAFs and cancer cells. This study revealed that Pin1 drives both desmoplastic and immunosuppressive TME by acting on CAFs (Fig. 3b). Furthermore, Pin1 binds to the pSer929-Pro site in HIP1R, which in turn promotes actin binding and ultimately drives PD-L1 on cancer cells into lysosomal degradation. In addition, in vivo animal experiments have also shown amazing results. Pin1 inhibitor combined with PD-1 antibody and gemcitabine (GEM) synergistically destroys tumor fibrosis, improves the inhibition of TME, and achieves the level of tumor elimination [92]. Undoubtedly, it is necessary to study the interaction mechanism between tumor cells and CAFs, which provides a new idea for us to study immunotherapy resistance.
The value of TGF-β in CAFs regulating the immune efficacy of PD-1/PD-L1 inhibitors
The production, storage, and release of TGF-β (transforming growth factor beta) are strictly controlled processes. Active TGF-β stored in the ECM complex is released into the TME through cleavage mediated by various serine proteins or cathepsins, especially the matrix metalloproteinases present in the TME [93]. In contrast, high levels of TGF-β in the stroma are mainly contributed by CAFs [94]. Meanwhile, there is substantial evidence that [95], CAFs contribute to the active release process of TGF-β. However, this point is still not confirmed by in vivo experiments.
In recent years, the study on TGF-β has become growing in-depth, especially its dual roles in the TME. In normal tissues and precancerous cells, TGF-β inhibits cell stagnation, differentiation and apoptosis processes. It also reduces inflammation and interstitial-derived mitocin, thereby maintaining homeostasis and inhibiting tumor progression [96]. Conversely, in tumors, the advantages of TGF-β is utilized by cancer cells, which stimulates fibrosis, promotes EMT and drives tumor metastasis [97]. Dysregulation of the C-ECM (cancer extracellular matrix) transcriptional program is associated with activation of TGF-β signaling in CAFs and with immunosuppression in other immunocompetent tumors [59].
Most tumor cells can secrete TGF-β in the late stage. Once the level of TGF-β increases, it hinders the differentiation of naive T cells to Th1 subsets, promotes their transformation into Treg cells, and inhibits the antigen presentation function of dendritic cells, leading to immune escape of tumor cells [98]. In experimental research [99], they found that TGF-β negatively regulated innate CD4+ T cell subsets through the production of the pro-inflammatory cytokine IL-6 and signals to promote colonic epithelial cell survival and proliferation in an inflammatory environment, ultimately enhancing risk of dysplasia. Similarly, in castration-resistant prostate cancer, prostate cancer [100], skin melanoma [101] verified that the inhibition of immune response by TGF-β was achieved by antagonizing Th1 differentiation. In a gene expression profiling study of CRC [102], the predictive power of subpopulations with poor prognosis was not derived from the gene expression of epithelial cancer cells but from stromal cells. Meanwhile, all subtypes with poor prognosis had a gene program induced by TGF-β. In addition, in CRC studies, in genetically engineered mice [94], the results also demonstrated that TGF-β blockade significantly enhanced the effect of antitumor immunity by modulating the CAFs-mediated T cell rejection phenotype. In a mouse model of metastatic urothelial carcinoma (mUC) that replicated this immune rejection phenotype, therapeutic administration of TGF-β blocking antibodies and anti-PD-L1 did reduce TGF-β signaling in stromal cells. That increased the penetration of T cells into the tumor center, ultimately leading to immune escape and strong tumor regression [103].
On the one hand, blocking TGF-β synergistically with anti-PD-L1 reprogrammed peritumoral stromal fibroblasts, increased the abundance of infiltrating T cells in tumors, and in particular significantly enhanced CD8+ T effector(TEFF) cell signaling [103]. Therefore, increased immune cell infiltration with TGF-β inhibitors might promote sensitivity to anti-PD-1/PD-L1 immune checkpoint therapy and be particularly useful for treating TGF-β-enriched cancers in the stroma [94]. On the other hand, normalization of aberrant transcriptomes in fibroblasts using blockers of TGF-β signaling, or depletion of CAFs may serve as a potential strategy to enhance checkpoint blockade [59]. Therefore, blocking the TGF-β signaling pathway is conducive to regulate the immune efficacy of PD-1/PD-L1 inhibitors.
The role of exosomes in CAFs affecting tumor immune efficacy
Exosomes are the main components of extracellular vesicles, with a diameter of 30–150 nm, which contain complex RNA components and proteins [104]. Exosomes are used as delivery vehicles to change the behavior of target cells by loading genes, proteins, drugs and small molecules, etc. [105]. Tumor cell-derived exosomes not only act on immune cells to produce immunosuppressive functions, but also participate in processes such as angiogenesis, matrix remodeling, and tumor cell invasion [106]. Furthermore, a large number of stromal cells (eg, CAFs) in the TME also derives exosomes, which plays an important role in tumorigenesis and development [107]. CAFs-derived exosomes affect the behavior of immune cells and cancer cells by activating different signaling pathways, thereby affecting the effect of immunotherapy. This study summarizes the signaling pathways by which CAFs-derived exosomes affect immunotherapy in several cancers (Table 1).
Table 1.
CAFs-derived exosomes affect signaling pathways in immunotherapy
| Cancer type | Exosomes | Functions | Molecular mechanisms | Reference |
|---|---|---|---|---|
| Breast cancer | miR-181D-5P | Promote cancer cell proliferation, invasion and accelerate EMT | Target CDX2 and downregulate CDX2 and HOXA5 | [108] |
| miR-92 | Increased apoptosis and impaired proliferation of T cells | miR-92/YAP1-LATS2/PD-L1 | [86] | |
| SNHG3 (lncRNA) | Increase glycolysis metabolism and inhibit immune cell activity | Target miR-330-5p and increase the PKM expression | [109] | |
| Lung cancer | OIP5-AS1 | Inhibition of PBMCs-induced killing of lung cancer cells | OIP5-AS1/miR-142-5p/PD-L1 axis | [110] |
| Colorectal cancer | CircEIF3K | Inhibits colorectal cancer cell proliferation, invasion and tube formation | circEIF3K/miR-214/PD-L1 | [85] |
In addition to affecting immune efficacy, exosomes also intervene in tumor cell behavior through other pathways, thereby promoting cancer development. Exosomal miR-500a-5p molecule affects the expression level of ubiquitin-specific peptidase 28 (USP28) through targeting transport, and ultimately promote cancer proliferation and metastasis [111]. Studies have shown [112], hypoxia up-regulated CAFs-derived exosomal circHIF1A (circ_0032138) higher than normal CAFs. circHIF1A, acting as a miR-580-5P sponge, enhances stem cell properties of breast cancer cells by regulating miR-580-5p targeting CD44 expression. CAFs containing miR-181d-5p accelerate EMT and promote cancer progression by inhibiting the CDX2/HOXA5 axis [108]. SNHG3 (lncRNA) secreted by CAFs promotes breast cancer cell proliferation by regulating PKM (Pyruvate Kinase M1/M2) at the transcriptional level of cancer cells [109]. In PDAC, CAFs induce tumor cell drug resistance via exosomes [20]. In CRC, CAFs secrete long noncoding RNAs (lncRNAs) LINC00659, which interact directly with miR-342-3P in cancer cells. Though upregulating the expression of annexin A2, it promotes EMT and cancer progression [113]. In addition, miR-92a-3p is also taken up by CRC cells to increase its internal expression level, thereby activating the Wnt/β-catenin pathway and directly inhibiting the downstream targets FBXW7 and MOAP1, ultimately attenuating mitochondrial apoptosis and increasing tumor aggressiveness and chemotherapy resistance [114]. Through in vitro culture and animal experiments, CAFs-derived exosomal opa-interacting protein 5 antisense RNA 1 (OIP5-AS1) inhibited the effect of PBMCs on inducing and killing lung cancer cells through the miR-142-5p/PD-L1 axis, thereby promoting lung cancer progress [110]. Meanwhile exosomes offer potential for cancer diagnosis and treatment.
Some down-regulated miRNAs in CAFs-derived exosomes were also associated with cancer progression. A study on head and neck cancer [115], showed that reduced levels of miR-3188 in CAF-derived exosomes promoted the proliferation of HNC cells and inhibited their apoptosis by inhibiting the expression of B-cell lymphoma 2 in recipient cells in vitro and in vivo. In sequencing analysis of oral squamous cell carcinoma [116], the researchers also found that MiR-34a-5p with low exosome expression was transferred to cancer cells by CAFs. miR-34a-5p binds to its direct downstream target AXL, induces EMT and promotes cancer progression through the AKT/GSK-3β/β-catenin/snail signaling cascade.
In melanoma [117], cancer-derived exosomes carry PD-L1 and can be upregulated by IFN-γ. Upregulated PD-L1 impairs the killing function of CD8+ T cells, which in turn mediates immunosuppression. In addition, because exosomes can also be detected in the blood circulation, researchers have long used them as biomarkers in many clinical trial studies to explore the value of exosomes in early cancer diagnosis and prognosis. However, there are still uncertainties about the source of exosomes as biomarkers and exosomes are promising to have broad prospects in cancer liquid biopsy [118].
The mechanisms of CAFs affecting PD-1/PDL-1 inhibitor immunotherapy in different solid cancers
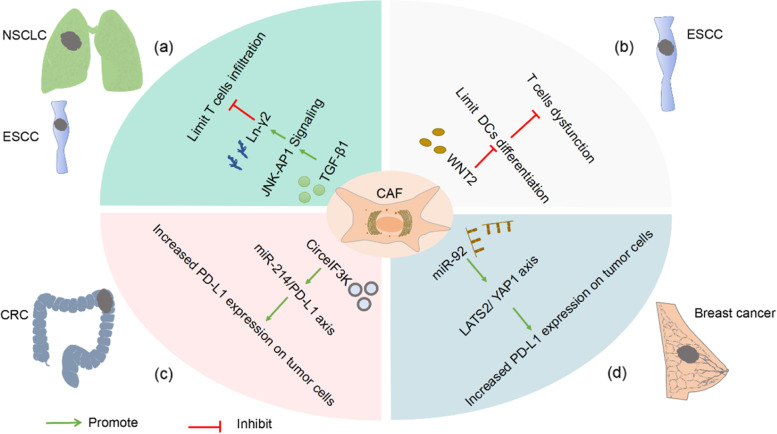
Immunotherapy that eliminates tumor cells by inhibiting immune checkpoints to promote anti-tumor immunity has received increasing attention. Antibodies to PD-1 or PD-L1 have proven to change the treatment landscape for many advanced cancers, including melanoma, lung, breast, kidney, etc. A growing number of studies report good long-term results with these treatments compared to traditional treatments. However, in most cases, only 20%-40% of patients respond, and fewer patients achieve long-term disease remission [119]. Different cancer types and stromal heterogeneity are factors that cannot be ignored for the differences in immune efficacy. Since the functions of CAFs in different subgroups are also heterogeneous, the mechanisms of CAFs in different tumor immunotherapy are also different (Fig. 4).
Fig. 4.
CAFs affect PD-1/PD-L1 inhibitor immunotherapy in different solid cancers. a In non–small cell lung cancer (NSCLC) or esophageal squamous cell carcinoma (ESCC), CAFs prevent T cells from penetrating into the cancer nest by promoting the secretion of Ln-γ2 by cancer cells. b CAFs-derived WNT2 inhibits the differentiation of DC cells and ultimately impairs the killing of T cells in ESCCs. c Hypoxia induces CAFS secretion of CirceIF3K, induces PD-L1 expression in colorectal cancer (CRC) cells through the miR-214/PD-L1 axis, leading to immune escape. d Exosome miR-92 acts on the LATS2-YAP1 axis to increase the transcription level of PD-L1 in breast cancer cells. Ultimately, T cell apoptosis and proliferation disorders are induced
In studies of advanced NSCLC and ESCC [16], they found that TGF-β1 secreted by CAFs utilized the c-Jun N-terminal kinase/activator protein 1(JNK/AP1) signaling pathway to promote the expression of Ln-γ2 in cancer cells, thereby building a protective barrier and limiting the penetration of T cells into the cancer nest (Fig. 4a). In the study of mUC, it was also found that TGF-β could shape the TME by limiting T cell infiltration, thereby suppressing anti-tumor immunity [103]. Besides, heat shock protein 90 inhibitor (XL888) has demonstrated sensitization advantages in immunotherapy for pancreatic cancer. For one thing, it limits PSC/CAF growth and for another, it inhibits JAK/STAT phosphorylation, and reshapes TME in combination with anti-PD-1 therapy [120].
In addition, CAFs also act on immune cells and cancer cells by secreting different cytokines, thereby affecting the immune efficacy. It has been confirmed in animal experiments that WNT2 secreted by CAFs reduces the generation of effector T cells by inhibiting the differentiation of DC cells, and ultimately attenuates the killing effect of T cells in the tumor [53] (Fig. 4b). Additionally, the role of CAFs-derived extracellular vesicles in information transmission should not be underestimated. According to research analysis, CAF-secreted exosomal CirceIF3K inhibits CRC cell proliferation, invasion, and tube formation in vitro, suggesting that the miR-214/PD-L1 axis increases the expression level of PD-L1 in cancer cells [85] (Fig. 4c). In breast cancer [86], exosomal miR-92 acts on the LATS2-YAP1 axis, thereby increasing the transcription level of PD-L1 in cancer cells, ultimately inducing T cell apoptosis and proliferation disorders, and blocking the killing function of NK cells (Fig. 4d).
The application of CAFs in clinical solid cancer immunity
CAFs are predominantly ‘oncogenic’ in cancer progression. However, in some cases, CAFs also have a tumor suppressor phenotype [17, 25]. Based on the functional heterogeneity of CAFs, prognostic-related markers for different cancers have gradually become the object of attention (Table 2). α-SMA is the most common marker of CAFs and marks activated fibroblasts [121]. Recent studies have reported that it has a dual role in cancer progression. For example, αSMA+CAFs promote tumor progression by changing the TME, which is associated with poor prognosis of liver cancer [122]. However, in a study on PDAC, deletion of α-SMA+CAFs promotes tumor progression by increasing the number of CD4+ FOXP3+ Treg cells in tumors [123], suggesting that this subset has an important anticancer role in PDAC. As another well-known biomarker of CAFs, the subset of CAFs with high FAP expression promote cancer cell proliferation, invasion and treatment resistance. Therefore, FAP often indicates poor prognosis of patients [124]. Previous clinical trials have shown that antibodies specific for FAP have shown safe and effective results in phase I trials in advanced cancer [125]. However, in a phase II trial of metastatic colorectal cancer, FAP inhibitors (FAPI) alone did not have a sensitizing advantage over chemotherapy [126]. At present, clinical trials of FAPI are mostly used to improve the diagnostic efficiency of malignant tumors in combination with radioactive tracers and to explore the efficacy in solid cancers in combination with antibodies (Table 3).
Table 2.
Application of CAFs-related markers in clinical solid cancer immunity
| Type of effect | Marker | Immune resistance mechanisms | Clinical application |
|---|---|---|---|
| Double effect | α-SMA | Pro-tumor: promote immunosuppressive microenvironment; Anti-cancer: deletion inhibits immune surveillance and reduces survival | Prognostic indicator |
| Tumor-promoting | FAP | Promote cancer cell proliferation, invasion, treatment resistance |
Preclinical experiments have mixed results, prognostic indicator |
| TGF-β | Limit T cell infiltration and suppress antitumor immunity | TGF-β blockade sensitizes anti-PD-L1 immunotherapy and LRRC15 may serve as a prognostic marker | |
| FGF, PDGF, VEGF | Recruit immunosuppressive cells, promote immune evasion | Nintedanib (BIBF1120) has been used in the clinical trial phase in lung adenocarcinoma | |
| WNT 2 | Disrupting cancer immune surveillance, tumor immune evasion and immunotherapy resistance | Antibodies, inhibitors, activators have been developed and are in clinical trials | |
| Tumor-suppressing | Meflin | Negatively correlated with tumor progression | Prognostic potential markers |
| VCAN | Inhibits fibroblast proliferation and promotes tumor growth | Potential markers |
Table 3.
Application of CAFs-related markers in clinical solid cancer immunity (Data source: ClinicalTrials.gov: https://beta.clinicaltrials.gov/ provided by the U.S. National Library of Medicine.)
| Target | Cancer type | Application phase | Purpose of application | Clinical Trial No |
|---|---|---|---|---|
| FAP | Breast cancer | Not Applicable | 68 Ga conjugated FAPI for targeted FAP and tumor-matrix imaging | NCT05574907 |
| Diagnostic value of AL18F-fluorodeoxyglucose(18F-FDG)- NOTA-FAPI PET/CT in cancer | NCT05574920 | |||
| Gastrointestinal cancers | Phase II | Assess efficacy of 18F-FAPI-74 to detect FAP expressing cells in patients | NCT05641896 | |
| Malignant neoplasms | Not Applicable | The diagnostic value of 68 Ga-FAPI PET/CT in malignant tumors, especially those with low FDG uptake | NCT05034146 | |
| The diagnostic value of 18F-FAPI PET / CT in various cancers and to compare it with 18F-FDG PET / CT | NCT05485792 | |||
| Advanced solid tumors | Phase I | Dose escalation study of OMTX7 as a single agent in combination with Pembrolizumab (PD-1 mAb) in patients with advanced solid tumors to assess safety and tolerability | NCT05547321 | |
| Meflin | Pancreatic cancer | Phase I/II | To evaluate the safety and tolerability of Am80 in combination with gemcitabine (GEM) and nattaxol in patients with unresectable pancreatic cancer and to determine the recommended dose | NCT05064618 |
| FGFR, PDGFR, VEGFR | Non-small cell lung cancer | Phase I/II | Efficacy and tolerability of Nintedanib in combination with PD-1 antibody and CTLA-4 antibody | NCT03377023 |
| Advanced solid tumors | Phase Ib | Anti-angiogenic combined with anti-PD-1 therapy to explore the efficacy | NCT02856425 | |
| MCT-4, caveolin-1 | Breast cancer | Phase I(completed) | To assess the feasibility of the effect of n-acetylcysteine on tumor cell metabolism | NCT01878695 |
| / | Pancreatic cancer | Not Applicable | Establishment of mixed organoid model to predict drug response | NCT05571956, NCT05196334 |
In addition to traditional biomarkers, other CAFs markers in different cancer types are also being gradually discovered. In a study of primary colon cancer, genes such as IGBP3, OAS2, MX1, and Robo2 were found as prognostic markers in an independent series of colon cancer patients. Meanwhile, studies have shown that the mRNA expression levels of some gene sets with a “CAF signature” appear to be useful for investigating additive effects on patient survival [21]. A subset of TGFβ-driven cells is the most prevalent CAFs in advanced PDAC, and this subset highly expresses a leucine-rich repeat containing 15 protein(LRRC15). While the LRRC15+ CAFs signature is associated with adverse responses to immune checkpoint blockade in several different human tumor types [20]. In contrast, studies have shown that Meflin is a marker of a subset of antitumor CAFs in PDAC. Its overexpression in CAFS is inversely correlated with tumor progression. Therefore, the infiltration of Meflin-positive subpopulations often suggests a favorable prognosis in patients [17]. Currently, Am80(common name:Tamibarotene) converts a Meflin negative subpopulation to positive, thereby increasing sensitivity to treatment (clinicaltrials.gov NCT05064618). In addition, versican (VCAN) is also a potential marker of anti-tumor CAFs. VCAN depletion reduces collagen stiffness by inhibiting collagen synthesis and fibroblast proliferation, which in turn promotes tumor growth [127]. These anti-cancer CAFs are beneficial to the remodeling of TME and search for new therapeutic targets.
Some cytokines secreted by CAFs, such as CXCL5, TGF-β, hepatocyte growth factor(HGF), and pro-angiogenic factors, play an important role in promoting malignant transformation and the proliferation and invasion of cancer cells [128–131].
Small molecule inhibitors of tyrosine kinases target fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and VEGF receptor (VEGFR) [132]. Meanwhile, small-molecule tyrosine kinase inhibitors in combination with immune checkpoint inhibitors have been used in phase I/II clinical trials in non-small cell lung cancer (NCT03377023) and phase I studies in advanced solid tumors (NCT02856425). Accumulating findings suggest that aberrant WNT signaling favors disruption of cancer immune surveillance, leading to tumor immune evasion and resistance to immune checkpoint inhibitors [133]. Molecular modulators targeting the WNT pathway have shown promising clinical application potential. It is gratifying that the combined effect of anti-WNT 2 and anti-PD-1 significantly enhanced the anti-tumor response of intratumoral T cells and enhanced the efficacy of anti-PD-1 [53]. This promises us to solve the problem of PD-1/PD-L1 immune resistance. Furthermore, in animal experiments with melanoma and CRC, the up-regulated expression of tumor PD-L1 induced by CAFs was reversed after silencing CXCL5’s receptor CXCR2.This suggests that the CXCL5-CXCR2 axis may be a promising therapeutic target [84].
Based on the above studies, there are differences in the subtypes and functions of CAFs in different cancer types, suggesting that the effectiveness of non-targeted therapy may remain to be tested [12]. It is important to note that biomarkers of opposite function that suggest prognostic cancer progression might be less targeted by immunotherapy.
Conclusion
In TME, stromal cells crosstalk with immune cells and ECM to promote the formation and stability of an immunosuppressive microenvironment. As an important part of the TME, CAFs stimulate the differentiation and function of immune cells, and ECM in the microenvironment by secreting a variety of cytokines and conducting different signaling pathways. Based on the immunosuppressive effects and related mechanisms of CAFs in resisting PD-1/PD-L1 immunotherapy, researchers have discovered several key molecules, such as TGF-β, Ln-γ2, Wnt2, and exosome molecules. At present, therapeutic strategies of targeting CAFs biomarkers, “star” molecules in the mechanism and limiting ECM remodeling have been developed in clinical experiments, thereby enhancing tumor immune efficacy. Strikingly, the combined targeted blockade approach has seen promising results, namely improved patient survival and enhanced immune efficacy against PD-1/PD-L1. However, the adaptive resistance of solid cancers remains an unsolved problem. The existence of subpopulations and functional heterogeneity of CAFs poses a hurdle for us to elucidate the mechanism. Meanwhile, it also implies that there may be more complex and surprising mechanisms in different tumors. Therefore, in order to improve the precision of combined targeted therapy, finding more reliable and specific anti-tumor CAFs markers in different solid cancers, the rich of fibrous stroma in cancers also has important reference value for the prognosis of solid tumors. In the near future, the deeper mechanisms to promote or activate anti-tumor immune responses remain to be explored in solid cancers.
Acknowledgements
Not applicable.
Abbreviations
- PD-1
Programmed death receptor 1
- PD-L1
Programmed death ligand 1
- IFNγ
Interferon-gamma
- TME
The tumor microenvironment
- CAFs
Cancer-associated fibroblasts
- ICIs
Immune checkpoint inhibitors (ICIs)
- ACT
Adoptive cell therapy
- ECM
Extracellular matrix
- CRC
Colorectal cancer
- scRNA-seq
Single-cell RNA sequencing
- A-SMA
Alpha-smooth muscle actin
- FAP
Fibroblast activation protein
- FSP1
Fibroblast-specific protein 1
- PDAC
Pancreatic ductal adenocarcinoma
- EMT
Epithelial-mesenchymal transition
- ENDMT
Endothelial-mesenchymal transition
- VEGF
Vascular endothelial growth factor
- TGF-β
Transforming growth factor-β
- Foxp3
Forkhead box protein p3
- CTLA-4
Cytotoxic T lymphocyte associate protein-4
- TAMs
Tumor-associated macrophage
- NKs
Natural killer cells
- IL-6
Interleukin 6
- CXCL2
C-x-c chemokine ligand 2
- Tregs
Regulatory T cells
- PGE2
Product prostaglandin E2
- DPP4
Dipeptidyl peptidase IV
- DCs
Dendritic cells
- APCs
Antigen presenting cells
- IDO
Indoleamine 2,3-dioxygenated
- NF-κB
Nuclear factor kappa-B
- MMP-1
Matrix metalloproteinase-1
- HAS2
Hyaluronan synthase gene
- TANs
Tumor-associated neutrophil
- CLCF1
Cardiotrophin-like cytokine 1
- SDF-1α
Stromal-derived factor-1 alpha
- HCC
Hepatocellular carcinoma
- CXCR2
C-x-c motif chemokine receptor 2
- LATS2
Large tumor suppressor kinase 2
- YAP1
Yes-associated protein 1
- SOCS3
Suppressor of cytokine signaling 3
- NSCLC
Non–small cell lung cancer
- ESCC
Esophageal squamous cell carcinoma
- LN-γ2
Laminin γ2
- p–c-jun
Phosphorylated-c-jun
- NDFs
Normal skin fibroblasts
- GEM
Gemcitabine
- TEFF
T effector
- mUC
Metastatic urothelial carcinoma
- USP28
Ubiquitin-specific peptidase 28
- lncRNAs
Long noncoding RNAs
- OIP5-AS1
Opa-interacting protein 5 antisense RNA 1
- JNK/AP1
C-Jun N-terminal kinase/activator protein 1
- LRRC15
Leucine-rich repeat containing 15 protein
- VCAN
Versican
Authors’ contributions
ZQS, YZ, CZW, and LL provided direction and guidance throughout the preparation of this manuscript. LPP, YL, and LL wrote and edited the manuscript. SCG and XYG reviewed and made significant revisions to the manuscript. YDF collected and prepared the related papers. All authors read and approved the final manuscript.
Funding
This study was supported by The National Natural Science Foundation of China (82173055, 81972663), The Science Project of Henan Natural Science Foundation (212300410074, 202300410446), The Youth Talent Innovation Team Support Program of Zhengzhou University (32320290), The Provincial and Ministry co-constructed key projects of Henan medical science and technology (SBGJ202102134), Key scientific and technological research projects of Henan Provincial Department of Science and Technology (212102310117), Henan Provincial Health Commission and Ministry of Health Co-construction Project, and Henan Provincial Health and Health Commission Joint Construction Project (LHGJ20200158), Henan Province young and middle-aged health science and technology innovation leading talent project (YXKC2022016), Henan Province Medical Affairs Technology Promotion Project (SYJS2022109).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved to publish this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liping Pei, Yang Liu and Lin Liu contributed equally to this work.
Contributor Information
Liping Pei, Email: metheuslp@163.com.
Yang Liu, Email: zlyyliuyang1440@zzu.edu.cn.
Lin Liu, Email: liulin@zzu.edu.cn.
Shuochen Gao, Email: fccgaosc@zzu.edu.cn.
Xueyan Gao, Email: zzugxy2022@163.com.
Yudi Feng, Email: fengyudi00@163.com.
Zhenqiang Sun, Email: fccsunzq@zzu.edu.cn.
Yan Zhang, Email: fcczhangy@zzu.edu.cn.
Chengzeng Wang, Email: czw202112@zzu.edu.cn.
References
- 1.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 3.Kim TK, Vandsemb EN, Herbst RS, Chen L. Adaptive immune resistance at the tumour site: mechanisms and therapeutic opportunities. Nat Rev Drug Discov. 2022;21:529–40. doi: 10.1038/s41573-022-00493-5. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 5.Xia H, Wang W, Crespo J, Kryczek I, Li W, Wei S, et al. Suppression of FIP200 and autophagy by tumor-derived lactate promotes naïve T cell apoptosis and affects tumor immunity. Sci Immunol. 2017;2:4631. doi: 10.1126/sciimmunol.aan4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–41. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squadrito ML, Cianciaruso C, Hansen SK, De Palma M. EVIR: chimeric receptors that enhance dendritic cell cross-dressing with tumor antigens. Nat Methods. 2018;15:183–186. doi: 10.1038/nmeth.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavazoie MF, Pollack I, Tanqueco R, Ostendorf BN, Reis BS, Gonsalves FC, et al. LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer. Cell. 2018;172:825–840.e18. doi: 10.1016/j.cell.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16:282–95. doi: 10.1038/s41575-019-0115-0. [DOI] [PubMed] [Google Scholar]
- 14.Grauel AL, Nguyen B, Ruddy D, Laszewski T, Schwartz S, Chang J, et al. TGFβ-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat Commun. 2020;11:6315. doi: 10.1038/s41467-020-19920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y, Cai Q, Chen Y, Shi T, Liu W, Mao L, et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology. 2022;75:28–42. doi: 10.1002/hep.32099. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Wei J-R, Dong J, Lin Q-G, Tang H, Jia Y-X, et al. Laminin γ2-mediating T cell exclusion attenuates response to anti-PD-1 therapy. Sci Adv. 2021;7:eabc8346. doi: 10.1126/sciadv.abc8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, et al. Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer Res. 2019;79:5367–81. doi: 10.1158/0008-5472.CAN-19-0454. [DOI] [PubMed] [Google Scholar]
- 18.Pm G, X Z, D Z. Molecular Features of Cancer-associated Fibroblast Subtypes and their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clinical cancer research : an official journal of the American Association for Cancer Research. Clin Cancer Res; 2021;27. Available from: https://pubmed.ncbi.nlm.nih.gov/33622705/. [cited 2022 May 11]. [DOI] [PMC free article] [PubMed]
- 19.Kieffer Y, Hocine HR, Gentric G, Pelon F, Bernard C, Bourachot B, et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 2020;10:1330–1351. doi: 10.1158/2159-8290.CD-19-1384. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez CX, Müller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15+ Myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discovery. 2020;10:232–53. doi: 10.1158/2159-8290.CD-19-0644. [DOI] [PubMed] [Google Scholar]
- 21.Herrera M, Islam ABMMK, Herrera A, Martín P, García V, Silva J, et al. Functional Heterogeneity of Cancer-Associated Fibroblasts from Human Colon Tumors Shows Specific Prognostic Gene Expression Signature. Clin Cancer Res. 2013;19:5914–26. doi: 10.1158/1078-0432.CCR-13-0694. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi: 10.1016/j.pharmthera.2020.107753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018;11:dmm029447. doi: 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804. doi: 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAndrews KM, Chen Y, Darpolor JK, Zheng X, Yang S, Carstens JL, et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL-6 mediated therapy resistance in pancreatic cancer. Cancer Discov. 2022;12:1580–97. doi: 10.1158/2159-8290.CD-20-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster DS, Januszyk M, Delitto D, Yost KE, Griffin M, Guo J, et al. Multiomic analysis reveals conservation of cancer-associated fibroblast phenotypes across species and tissue of origin. Cancer Cell. 2022;40:1392–1406.e7. doi: 10.1016/j.ccell.2022.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo H, Xia X, Huang L-B, An H, Cao M, Kim GD, et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat Commun. Nature Publishing Group. 2022;13:6619. doi: 10.1038/s41467-022-34395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, et al. Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer Res. 2019;79:5367–5381. doi: 10.1158/0008-5472.CAN-19-0454. [DOI] [PubMed] [Google Scholar]
- 29.Pandey PR, Young KH, Kumar D, Jain N. RNA-mediated immunotherapy regulating tumor immune microenvironment: next wave of cancer therapeutics. Mol Cancer. 2022;21:58. doi: 10.1186/s12943-022-01528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albrengues J, Shields M, Ng D, Park CG, Ambrico A, Poindexter M, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woan KV, Miller JS. Harnessing Natural Killer Cell Antitumor Immunity: From the Bench to Bedside. Cancer Immunol Res. 2019;7:1742–1747. doi: 10.1158/2326-6066.CIR-19-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarchoan M, Johnson BA, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209–22. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harper J, Sainson RCA. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin Cancer Biology. 2014;25:69–77. doi: 10.1016/j.semcancer.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Koyama S, Nishikawa H. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. BMJ Specialist Journals. 2021;9:e002591. doi: 10.1136/jitc-2021-002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinoshita T, Ishii G, Hiraoka N, Hirayama S, Yamauchi C, Aokage K, et al. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Science. 2013;104:409–15. doi: 10.1111/cas.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs J, Deschoolmeester V, Zwaenepoel K, Flieswasser T, Deben C, Van den Bossche J, et al. Unveiling a CD70-positive subset of cancer-associated fibroblasts marked by pro-migratory activity and thriving regulatory T cell accumulation. Oncoimmunology. 2018;7:e1440167. doi: 10.1080/2162402X.2018.1440167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourhis M, Palle J, Galy-Fauroux I, Terme M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Front Immunol. 2021;12:616837. doi: 10.3389/fimmu.2021.616837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA. Cutting edge: tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol. 2009;183:4848–52. doi: 10.4049/jimmunol.0900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, et al. Conversion of Peripheral CD4+CD25− Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson IC, Mari SE, Broderick RJ, Mari BP, Shipp MA. The angiogenic factor interleukin 8 is induced in non-small cell lung cancer/pulmonary fibroblast cocultures. Cancer Res. 2000;60:269–272. [PubMed] [Google Scholar]
- 41.Baratelli F, Lin Y, Zhu L, Yang S-C, Heuzé-Vourc’h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–90. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 42.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell. 2018;33:463–479.e10. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, et al. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20:724–35. doi: 10.1038/s41590-019-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turnis ME, Sawant DV, Szymczak-Workman AL, Andrews LP, Delgoffe GM, Yano H, et al. Interleukin-35 Limits Anti-Tumor Immunity. Immunity. 2016;44:316–329. doi: 10.1016/j.immuni.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Chikina M, Deshpande R, Menk AV, Wang T, Tabib T, et al. Treg Cells Promote the SREBP1-Dependent Metabolic Fitness of Tumor-Promoting Macrophages via Repression of CD8+ T Cell-Derived Interferon-γ. Immunity. 2019;51:381–397.e6. doi: 10.1016/j.immuni.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferris ST, Durai V, Wu R, Theisen DJ, Ward JP, Bern MD, et al. cDC1 prime and are licensed by CD4 T cells to induce anti-tumour immunity. Nature. 2020;584:624–9. doi: 10.1038/s41586-020-2611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber F, Byrne SN, Le S, Brown DA, Breit SN, Scolyer RA, et al. Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer Immunol Immunother. 2005;54:898–906. doi: 10.1007/s00262-004-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bekeredjian-Ding I, Schäfer M, Hartmann E, Pries R, Parcina M, Schneider P, et al. Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology. 2009;128:439–450. doi: 10.1111/j.1365-2567.2009.03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng J, Deng Y, Yi H, Wang G, Fu B, Chen W, et al. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. doi: 10.1038/oncsis.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clinical Cancer Research. 2019;25:5449–57. doi: 10.1158/1078-0432.CCR-18-1543. [DOI] [PubMed] [Google Scholar]
- 52.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7–H1 improves myeloid dendritic cell–mediated antitumor immunity. Nat Med. Nature Publishing Group. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 53.Huang T-X, Tan X-Y, Huang H-S, Li Y-T, Liu B-L, Liu K-S, et al. Targeting cancer-associated fibroblast-secreted WNT2 restores dendritic cell-mediated antitumour immunity. Gut. 2022;71:333–44. doi: 10.1136/gutjnl-2020-322924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019;36:171–198. doi: 10.1007/s10585-019-09966-1. [DOI] [PubMed] [Google Scholar]
- 55.Asimakopoulos F, Hope C, Johnson MG, Pagenkopf A, Gromek K, Nagel B. Extracellular matrix and the myeloid-in-myeloma compartment: balancing tolerogenic and immunogenic inflammation in the myeloma niche. J Leukoc Biol. 2017;102:265–275. doi: 10.1189/jlb.3MR1116-468R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 57.Lopez JI, Kang I, You W-K, McDonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integr Biol (Camb) 2011;3:910–921. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato T, Ota T, Watanabe M, Imada K, Nomizu M, Ito A. Identification of an active site of EMMPRIN for the augmentation of matrix metalloproteinase-1 and -3 expression in a co-culture of human uterine cervical carcinoma cells and fibroblasts. Gynecol Oncol. 2009;114:337–342. doi: 10.1016/j.ygyno.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun. 2018;9:4692. doi: 10.1038/s41467-018-06654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, et al. Hyaluronan Deficiency in Tumor Stroma Impairs Macrophage Trafficking and Tumor Neovascularization. Cancer Res. 2010;70:7073–83. doi: 10.1158/0008-5472.CAN-09-4687. [DOI] [PubMed] [Google Scholar]
- 61.Varol C. Tumorigenic Interplay Between Macrophages and Collagenous Matrix in the Tumor Microenvironment. Methods Mol Biol. 2019;1944:203–220. doi: 10.1007/978-1-4939-9095-5_15. [DOI] [PubMed] [Google Scholar]
- 62.Stahl M, Schupp J, Jäger B, Schmid M, Zissel G, Müller-Quernheim J, et al. Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS ONE. 2013;8:e81382. doi: 10.1371/journal.pone.0081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/26406376/. [cited 2022 Sep 10]. [DOI] [PMC free article] [PubMed]
- 64.Bae YH, Mui KL, Hsu BY, Liu S-L, Cretu A, Razinia Z, et al. A FAK-Cas-Rac-Lamellipodin Signaling Module Transduces Extracellular Matrix Stiffness into Mechanosensitive Cell Cycling. Sci Signal. 2014;7:ra57. doi: 10.1126/scisignal.2004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mushtaq MU, Papadas A, Pagenkopf A, Flietner E, Morrow Z, Chaudhary SG, et al. Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers. J Immunother Cancer. 2018;6:65. doi: 10.1186/s40425-018-0376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. Nature Publishing Group. 2016;16:431–46. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 67.Song M, He J, Pan Q-Z, Yang J, Zhao J, Zhang Y-J, et al. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology. 2021;73:1717–1735. doi: 10.1002/hep.31792. [DOI] [PubMed] [Google Scholar]
- 68.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of Tumor-Associated Neutrophil (TAN) Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Y, Li H, Deng Y, Tai Y, Zeng K, Zhang Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9:422. doi: 10.1038/s41419-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan X, et al. The IL-6–STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014;5:e1295. doi: 10.1038/cddis.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23:1148–1156. doi: 10.1038/s41590-022-01267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 74.Tan B, Shi X, Zhang J, Qin J, Zhang N, Ren H, et al. Inhibition of Rspo-Lgr4 Facilitates Checkpoint Blockade Therapy by Switching Macrophage Polarization. Cancer Res. 2018;78:4929–42. doi: 10.1158/0008-5472.CAN-18-0152. [DOI] [PubMed] [Google Scholar]
- 75.Hu B, Wang Z, Zeng H, Qi Y, Chen Y, Wang T, et al. Blockade of DC-SIGN+ tumor-associated macrophages reactivates antitumor immunity and improves immunotherapy in muscle-invasive bladder cancer. Cancer Res. 2020;80:1707–1719. doi: 10.1158/0008-5472.CAN-19-2254. [DOI] [PubMed] [Google Scholar]
- 76.Sadozai H, Acharjee A, Eppenberger-Castori S, Gloor B, Gruber T, Schenk M, et al. Distinct stromal and immune features collectively contribute to long-term survival in pancreatic cancer. Front Immunol. 2021;12:643529. doi: 10.3389/fimmu.2021.643529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–72. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, et al. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019;10:273. doi: 10.1038/s41419-019-1435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumor-associated macrophages inhibits phagocytosis and tumor immunity. Nature. NIH Public Access. 2017;545:495. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–69. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kock A, Larsson K, Bergqvist F, Eissler N, Elfman LHM, Raouf J, et al. Inhibition of microsomal prostaglandin e synthase-1 in cancer-associated fibroblasts suppresses neuroblastoma tumor growth. EBioMedicine. 2018;32:84–92. doi: 10.1016/j.ebiom.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiu Y, Chen T, Hu R, Zhu R, Li C, Ruan Y, et al. Next frontier in tumor immunotherapy: macrophage-mediated immune evasion. Biomark Res. 2021;9:72. doi: 10.1186/s40364-021-00327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Z, Zhou J, Zhang J, Li S, Wang H, Du J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. International Journal of Cancer. 2019;145:1946–57. doi: 10.1002/ijc.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang K, Zhang J, Bao C. Exosomal circEIF3K from cancer-associated fibroblast promotes colorectal cancer (CRC) progression via miR-214/PD-L1 axis. BMC Cancer. 2021;21:933. doi: 10.1186/s12885-021-08669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dou D, Ren X, Han M, Xu X, Ge X, Gu Y, et al. Cancer-associated fibroblasts-derived exosomes suppress immune cell function in breast cancer via the miR-92/PD-L1 pathway. Front Immunol. 2020;11:2026. doi: 10.3389/fimmu.2020.02026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Erez N, Truitt M, Olson P, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-κB-Dependent Manner. Cancer Cell. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 89.Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res. 2004;10:5870–5879. doi: 10.1158/1078-0432.CCR-0431-03. [DOI] [PubMed] [Google Scholar]
- 90.Mueller L, Goumas FA, Affeldt M, Sandtner S, Gehling UM, Brilloff S, et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol. 2007;171:1608–1618. doi: 10.2353/ajpath.2007.060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pein M, Insua-Rodríguez J, Hongu T, Riedel A, Meier J, Wiedmann L, et al. Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs. Nat Commun. Nature Publishing Group. 2020;11:1494. doi: 10.1038/s41467-020-15188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koikawa K, Kibe S, Suizu F, Sekino N, Kim N, Manz TD, et al. Targeting Pin1 renders pancreatic cancer eradicable by synergizing with immunochemotherapy. Cell. NIH Public Access. 2021;184:4753. doi: 10.1016/j.cell.2021.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tauriello DVF, Sancho E, Batlle E. Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22:25–44. doi: 10.1038/s41568-021-00413-6. [DOI] [PubMed] [Google Scholar]
- 94.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. Nature Publishing Group. 2018;554:538–43. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 95.Brown NF, Marshall JF. Integrin-Mediated TGFβ Activation Modulates the Tumour Microenvironment. Cancers (Basel) 2019;11:E1221. doi: 10.3390/cancers11091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Massagué J. TGFβ in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, et al. TGF-β and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci. 2018;19:E1294. doi: 10.3390/ijms19051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tauriello DVF, Sancho E, Batlle E. Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22:25–44. doi: 10.1038/s41568-021-00413-6. [DOI] [PubMed] [Google Scholar]
- 99.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, et al. TGF-β Suppresses Tumor Progression in Colon Cancer by Inhibition of IL-6 trans-Signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan B, Miura Y, et al. Differences in tumor microenvironment dictate t helper lineage polarization and response to immune checkpoint therapy. Cell. 2019;179:1177–1190.e13. doi: 10.1016/j.cell.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 101.Ravi R, Noonan KA, Pham V, Bedi R, Zhavoronkov A, Ozerov IV, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nature Communications. Nature Publishing Group; 2018;9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5821872/. [cited 2022 Aug 3]. [DOI] [PMC free article] [PubMed]
- 102.Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47:320–9. doi: 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 103.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kunou S, Shimada K, Takai M, Sakamoto A, Aoki T, Hikita T, et al. Exosomes secreted from cancer-associated fibroblasts elicit anti-pyrimidine drug resistance through modulation of its transporter in malignant lymphoma. Oncogene. Nature Publishing Group. 2021;40:3989–4003. doi: 10.1038/s41388-021-01829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luga V, Wrana JL. Tumor-stroma interaction: revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res. 2013;73:6843–7. doi: 10.1158/0008-5472.CAN-13-1791. [DOI] [PubMed] [Google Scholar]
- 108.Wang H, Wei H, Wang J, Li L, Chen A, Li Z. MicroRNA-181d-5p-Containing Exosomes Derived from CAFs Promote EMT by Regulating CDX2/HOXA5 in Breast Cancer. Mole Ther Nucleic Acids. 2020;19:654–67. doi: 10.1016/j.omtn.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Y, Zhao Z, Liu W, Li X. SNHG3 Functions as miRNA sponge to promote breast cancer cells growth through the metabolic reprogramming. Appl Biochem Biotechnol. 2020;191:1084–99. doi: 10.1007/s12010-020-03244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang Y, Wang K, Lu X, Wang Y, Chen J. Cancer-associated fibroblasts-derived exosomes promote lung cancer progression by OIP5-AS1/ miR-142-5p/ PD-L1 axis. Mol Immunol. 2021;140:47–58. doi: 10.1016/j.molimm.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 111.Chen B, Sang Y, Song X, Zhang D, Wang L, Zhao W, et al. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11:3932–3947. doi: 10.7150/thno.53412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhan Y, Du J, Min Z, Ma L, Zhang W, Zhu W, et al. Carcinoma-associated fibroblasts derived exosomes modulate breast cancer cell stemness through exonic circHIF1A by miR-580-5p in hypoxic stress. Cell Death Discov. 2021;7:141. doi: 10.1038/s41420-021-00506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou L, Li J, Tang Y, Yang M. Exosomal LncRNA LINC00659 transferred from cancer-associated fibroblasts promotes colorectal cancer cell progression via miR-342-3p/ANXA2 axis. J Transl Med. 2021;19:8. doi: 10.1186/s12967-020-02648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18:91. doi: 10.1186/s12943-019-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X, Qin X, Yan M, Shi J, Xu Q, Li Z, et al. Loss of exosomal miR-3188 in cancer-associated fibroblasts contributes to HNC progression. J Exp Clin Cancer Res : CR. BioMed Central; 2019;38. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6454737/. [cited 2022 Jul 29]. [DOI] [PMC free article] [PubMed]
- 116.Li Y-Y, Tao Y-W, Gao S, Li P, Zheng J-M, Zhang S-E, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209–220. doi: 10.1016/j.ebiom.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 Response. Nature. 2018;560:382–6. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.LeBleu VS, Kalluri R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer. 2020;6:767–774. doi: 10.1016/j.trecan.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 119.Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–62. doi: 10.1038/s41571-021-00473-5. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Ware MB, Zaidi MY, Ruggieri AN, Olson BM, Komar H, et al. Heat shock protein-90 inhibition alters activation of pancreatic stellate cells and enhances the efficacy of pd-1 blockade in pancreatic cancer. Mol Cancer Ther. 2021;20:150–160. doi: 10.1158/1535-7163.MCT-19-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han C, Liu T, Yin R. Biomarkers for cancer-associated fibroblasts. Biomark Res. 2020;8:64. doi: 10.1186/s40364-020-00245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lau EYT, Lo J, Cheng BYL, Ma MKF, Lee JMF, Ng JKY, et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016;15:1175–1189. doi: 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 123.Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu C-C, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2015;28:831–833. doi: 10.1016/j.ccell.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 124.Hussain A, Voisin V, Poon S, Karamboulas C, Bui NHB, Meens J, et al. Distinct fibroblast functional states drive clinical outcomes in ovarian cancer and are regulated by TCF21. J Exp Med. 2020;217:e20191094. doi: 10.1084/jem.20191094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scott AM, Wiseman G, Welt S, Adjei A, Lee F-T, Hopkins W, et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res. 2003;9:1639–1647. [PubMed] [Google Scholar]
- 126.Narra K, Mullins SR, Lee H-O, Strzemkowski-Brun B, Magalong K, Christiansen VJ, et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting fibroblast activation protein in patients with metastatic colorectal cancer. Cancer Biol Ther. 2007;6:1691–1699. doi: 10.4161/cbt.6.11.4874. [DOI] [PubMed] [Google Scholar]
- 127.Fanhchaksai K, Okada F, Nagai N, Pothacharoen P, Kongtawelert P, Hatano S, et al. Host stromal versican is essential for cancer-associated fibroblast function to inhibit cancer growth. Int J Cancer. 2016;138:630–641. doi: 10.1002/ijc.29804. [DOI] [PubMed] [Google Scholar]
- 128.Fahey S, Dempsey E, Long A. The role of chemokines in acute and chronic hepatitis C infection. Cell Mol Immunol. 2014;11:25–40. doi: 10.1038/cmi.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Butera D, Marukian S, Iwamaye AE, Hembrador E, Chambers TJ, Di Bisceglie AM, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175–1182. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Unterleuthner D, Neuhold P, Schwarz K, Janker L, Neuditschko B, Nivarthi H, et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159–77. doi: 10.1007/s10456-019-09688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kugeratski FG, Atkinson SJ, Neilson LJ, Lilla S, Knight JRP, Serneels J, et al. Hypoxic cancer-associated fibroblasts increase NCBP2-AS2/HIAR to promote endothelial sprouting through enhanced VEGF signaling. Sci Signal. 2019;12:eaan8247. doi: 10.1126/scisignal.aan8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kato R, Haratani K, Hayashi H, Sakai K, Sakai H, Kawakami H, et al. Nintedanib promotes antitumour immunity and shows antitumour activity in combination with PD-1 blockade in mice: potential role of cancer-associated fibroblasts. Br J Cancer. 2021;124:914–924. doi: 10.1038/s41416-020-01201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galluzzi L, Spranger S, Fuchs E, López-Soto A. WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol. 2019;29:44–65. doi: 10.1016/j.tcb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.