Abstract
Objective:
Older hospitalized adults with an existing diagnosis of cancer rarely receive cancer treatment after discharge to a skilled nursing facility (SNF). It is unclear to what degree these outcomes may be driven by cumulative effects of previous cancer treatment and their complications versus an absolute functional threshold from which it is not possible to return. We sought to understand post-acute care outcomes of adults newly diagnosed with cancer and explore functional improvement during their SNF stay.
Design:
Retrospective cohort study, 2011–2013
Setting and Participants:
Surveillance, Epidemiology, and End Results - Medicare database of patients with new stage II – IV colorectal, pancreatic, bladder, or lung cancer discharged to SNF.
Methods:
Primary outcome was time to death after hospital discharge. Covariates include cancer treatment receipt and hospice use. A Minimum Data Set (MDS)-Activities of Daily Living (ADL) score was calculated to measure changes in ADLs during SNF stay. Patient groups of interest were compared descriptively using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Logistic regression was used to compare patient groups.
Results:
A total of 6,791 cases were identified. Forty-six percent of patients did not receive treatment or hospice, 25.0% received no treatment but received hospice, 20.8% received treatment but no hospice, and 8.5% received both treatment and hospice. Only 43% of decedents received hospice. Patients who received treatment but not hospice had the best survival. There were limited improvements in MDS-ADL scores in the subset of patients for whom we have complete data. Those with greater functional improvement had improved survival.
Conclusions and Implications:
The majority of patients did not receive future cancer treatment or hospice care prior to death. There was limited improvement in MDS-ADL scores raising concern this population might not benefit from the rehabilitative intent of SNFs.
Keywords: Skilled nursing facility, cancer, palliative care, post-acute care
Brief summary:
This manuscript analyzes post-acute care outcomes of hospitalized older adults with new advanced cancer discharged to skilled nursing facilities and examines the association between functional improvement, cancer treatment, and hospice use on survivorship.
Introduction
Functional decline occurs frequently in older adults with cancer and is prognostic for overall survival. In a multicenter prospective study, inability to perform activities of daily living (ADLs) had the strongest prognostic value for overall survival in patients receiving chemotherapy who were 70 or older.1 Difficulty in performing ADLs is strongly associated with reduced independence and health related quality of life,2,3 increased hospital admissions, institutionalization, and health care costs. 4–6
Hospitalized patients with advanced cancer and functional impairment are older, have higher symptom burden, worse survival, and are more likely to discharge to a skilled nursing facility (SNF) compared to hospitalized patients with cancer and no functional impairment.7 Some patients may discharge to a SNF with the goal of becoming stronger to receive more cancer treatment. The intent of SNF care is to provide increased skilled nursing care and rehabilitative therapies for a limited time after a hospitalization. Medicare Part A or Medicare advantage plans cover these services and in 2015 accounted for > $59 billion dollars of Medicare spending for all patients, including those with a non-cancer diagnosis. 8,9 For other patients their decision to discharge to a SNF may be one of necessity as SNF care is the only means by which patients can get 24 hour supervised care paid for by insurance. To date, we are unaware of research that has evaluated patient and caregivers’ reasons and goals for discharging to SNF. Even without explicit rehabilitation goals, patients may opt for SNF discharge because they lack caregiver support or financial means to pay private caregivers to meet their care needs.
In our prior analysis of the Surveillance, Epidemiology and End Results (SEER)-Medicare dataset hospitalized patients with metastatic colorectal, pancreatic, bladder, or lung cancer who discharged to a SNF were significantly less likely to receive subsequent cancer treatment of any kind and had higher mortality compared with patients who discharged home. In the 2010–2013 sample of patients (21% discharged to a SNF, 23% to homecare, and 56% to home) 56% of SNF discharges died within 6 months compared with 36% of home discharges.10 It is unclear to what degree these outcomes may be driven by the cumulative effects of cancer treatment and their complications which might facilitate more rapid functional decline contributing to worse outcomes versus an absolute functional threshold from which it is not possible to return. Using the SEER-Medicare-Minimum Data Set (MDS), we sought to understand post-acute care outcomes of treatment naïve older adults newly diagnosed with advanced cancer during a hospitalization. We characterized the clinical outcomes of this population after discharge to a SNF and explored the association between functional improvement, cancer treatment, and hospice use with survivorship. We hypothesized that most patients would not demonstrate functional improvement and that improvement would be associated with future cancer treatment and increased survival.
Methods
Data Source
We extracted data from the SEER-Medicare database. The SEER program collects data from select cancer registries covering approximately 28% of the US population; 93% of persons aged >65 years in the SEER files are matched to the Medicare enrollment file. Our study evaluated outcomes of Medicare beneficiaries with fee-for-service coverage (FFS). During our study, 72% to 76% of the Medicare population enrolled in Medicare Fee-for-Service (FFS).11 All resident data came from MDS 3.0 assessments. The MDS is a standardized, comprehensive assessment instrument used for all individuals who receive care in Medicare or Medicaid certified nursing homes.12 The MDS captures information about patients’ comorbidities, physical, psychological, and psychosocial functioning in addition to any treatments (e.g., hospice care, dialysis) or therapies (physical, occupational, speech, restorative nursing) received.12 These assessments are conducted by trained nursing home clinicians and have been demonstrated to have strong reliability and validity.13–15 They are completed for patients receiving long term care in a nursing home and shorter stay patients who are receiving rehabilitation in a SNF for a limited period of time after a hospitalization. The Medicare-required assessment schedule includes 5-day, 14-day, 30-day, 60-day, and 90-day scheduled assessments. Assessments are also completed at admission, discharge, and when there is a significant change in clinical status. We considered 60 days as the maximum SNF stay as the average length of stay was 28 days for Medicare beneficiaries in 2013 nationally.16 We used residents’ first and last recorded assessments as admission and discharge assessments.17,18
Sample Selection
We selected patients with colorectal, pancreatic, bladder, or lung cancer diagnosed from 2011 to 2013. We excluded records from patients with a subsequent primary tumor or other prior cancer diagnoses other than Stage 0 or Stage 1 breast or cervical cancer, or non-metastatic prostate cancer diagnosed in the three years prior to the tumor of interest (total sample size, N = 225,900). Our analysis only included de novo cancers. We further restricted to patients with American Joint Committee on Cancer, 7th Edition (AJCC) Stage Group II-IV tumors at diagnosis to obtain the patient sample with regional or advanced disease (N = 142,506).19,20
We identified the patient sample for which we had complete claims data by restricting to patients age 66 years and older at diagnosis and with positive survival time (N =112,778) and excluding patients diagnosed at autopsy or with a missing diagnosis date. (n =95 and n=262 respectively). A positive survival time was defined as those who either died after admission to a SNF or were alive at the end of the observation time. We required that patients be continuously enrolled in Medicare FFS Parts A and B from 12 months prior to diagnosis through death or the end of the study follow-up, December 2014 or death (N = 68,122).
The study sample was further limited to patients with a paid claim for a short-term inpatient stay subsequent to diagnosis that did not end in death or discharge to hospice (N = 47,523). We assigned the first stay occurring in the month of diagnosis or later as the index inpatient stay. We required that the index stay occur by June 2014, with continuous enrollment in Medicare FFS Part A and Part B for at least six months after discharge, or until death if prior to six months, to ensure adequate follow-up for all outcome measures (N = 47,080). From the sample of patients with an inpatient stay, we selected patients discharging to a SNF (n = 8,962), excluding other discharge locations (such as home, home healthcare, long term care hospital, etc.). After excluding claims with prior oncology visits and prior chemotherapy, radiation, or targeted therapy treatments, our total sample size was 6,791. Study consort diagram detailed in Figure 1. Colorado Multiple Institutional Review Board and the Duke Institutional Review Board approved this study.
Figure 1.
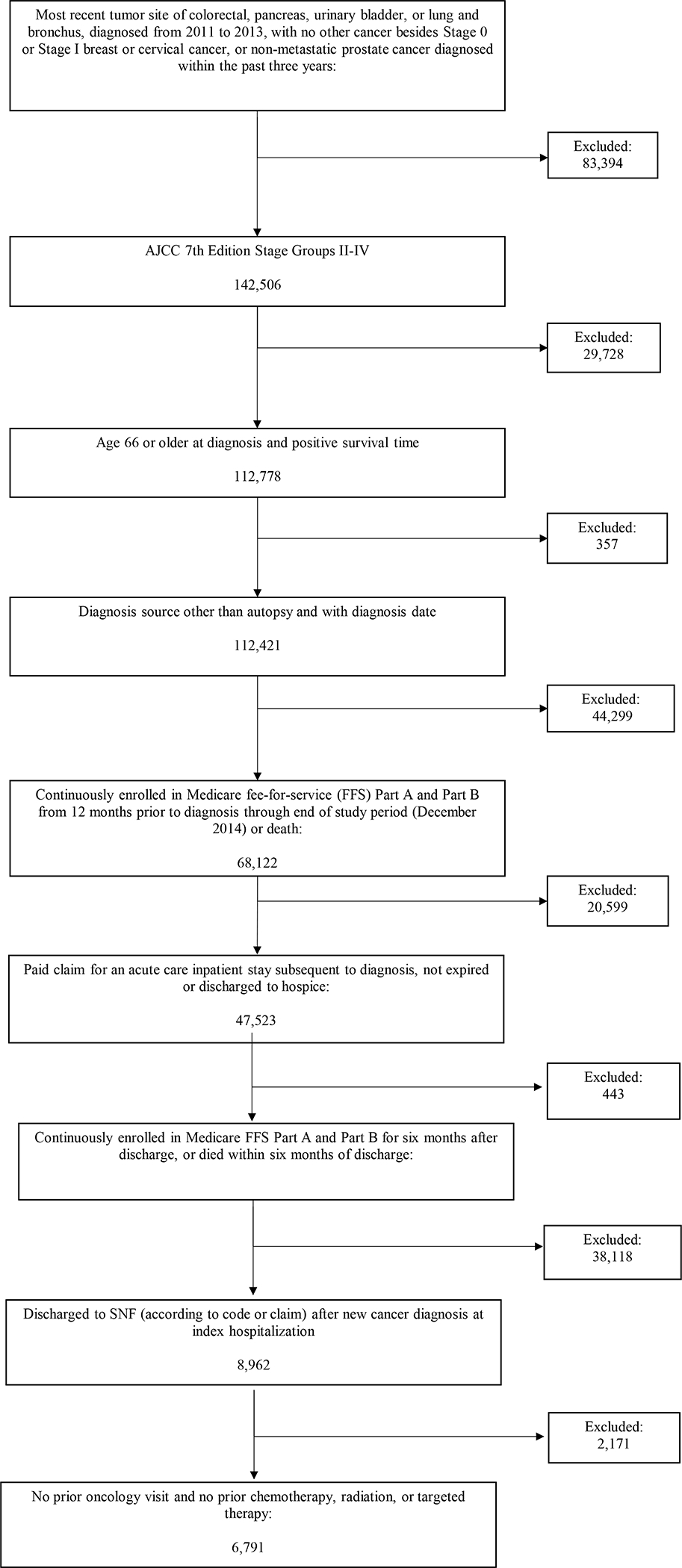
SEER-Medicare analysis sample derivation. Abbreviations: FFS, fee-for services; SNF, skilled nursing facility.
Comparator Groups
Analyses were conducted among patients discharging to a SNF with a new diagnosis of cancer in four comparison groups based on subsequent clinical trajectory after an acute care hospitalization: 1) no treatment and no hospice (N=3,103) 2) no treatment and hospice (N=1,696) 3) treatment and no hospice (N=1,413) and 4) treatment and hospice (N=579). We defined a confirmed discharge to a SNF as the presence of a SNF claim with an admission date equal to the index discharge date. Treatment was defined as the receipt of anti-cancer therapy, and hospice was measured using the number of covered days; both were identified using information reported on the patient claims in the six months following acute care hospitalization discharge. Current Procedural Terminology (CPT) codes, Healthcare Common Procedure Coding System (HCPCS) codes, International Classification of Diseases, 9th Revision (ICD-9) procedure and diagnosis codes, and National Drug Codes (NDC) were used to identify treatment received, including radiation, chemotherapy, and targeted therapy. We included targeted therapy drugs approved by the Food and Drug Administration (FDA) for the tumor sites of interest that had specific HCPCS codes initiated prior to the December 2014. The selected drugs included bevacizumab, cetuximab, everolimus, panitumumab, ramucirumab, and ziv-aflibercept.
Outcomes
The primary outcome was time to death after discharge from acute care hospitalization. The maximum observed or censored time was 48 months.
Functional Measures
The ADL self-performance items include bed mobility, transfer, walking in room, walking in corridor, locomotion on unit, locomotion off unit, dressing, eating, toilet use, and personal hygiene. Each activity must occur 3 or more times within the past 7 days to be coded on a scale of 0 (independent) to 4 (total dependence). If the activity occurred 2 or fewer times within the past 7 days, the item is coded 7 (occurred only once or twice) or 8 (activity did not occur).
We examined each ADL self-performance item to determine completeness on both admission and discharge assessments for our sample. We calculated change in ADL self-performance between admission and discharge by using the long-form MDS-ADL scale. The long-form MDS-ADL scale includes measures for bed mobility, transfer, locomotion on unit, dressing, eating, toilet use, and personal hygiene. The scale ranges from 0 to 28, with higher scores indicating greater impairment.21 We recoded any items with scores of 7 or 8 (activity occurred only once or twice or activity did not occur) as totally dependent, code 4. This is consistent with the calculation of the long-form ADL scale from the MDS 2.0, in which items with scores of 8 were recoded to a score of 4.21 ADL change was calculated as the admission score minus the discharge score, so positive scores indicate improvement, whereas negative scores indicate decline. Further, negative scores were recoded as zero (indicating no improvement) to define ADL improvement.17
Control Variables
We used SEER variables to obtain patient demographics and tumor characteristics at diagnosis. We used claims to identify characteristics of the index inpatient stay, prior health conditions, health care services received, and to generate the Charlson Comorbidity Index and identify specific conditions of interest using Chronic Conditions Data Warehouse algorithms, as potential covariates in the analysis.22
Statistical Analysis
A total of 6,791 cases were identified and the four comparison groups of interest were compared descriptively using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Kaplan-Meier estimates were used to compare survival curves by patient group. Cox proportional hazards regression was used to estimate the Hazard Ratio (HR) of death (ratio of conditional probabilities of death given alive) by clinical trajectory (4 patient groups), adjusting for ADL score and other covariates. Statistically significant predictors for the outcomes were identified using a stepwise algorithm. The regression models included variables in the unreported developed/validated model for patients with unestablished cancer in a previously published article using 2010–2013 data and were calibrated by adding clinical trajectory and ADL score.10 Statistical significance was defined as p<0.05. SAS v9.4 (SAS Institute, Cary, NC) was used for all analyses.
Results
Patient Characteristics
The study population consisted of 6, 791 people who discharged to a SNF after a hospitalization with a new diagnosis of stage II – IV colorectal, lung, bladder, or pancreatic cancer (Table 1). The majority of the patients were female (58%) with an average age of 81.2 (± Standard Deviation [SD] 7.4). Of these, 45.7% of patients did not receive future treatment or hospice (group 1), 25.0% received no treatment but did receive hospice (group 2), 20.8% received treatment but no hospice (group 3), and 8.5% received both treatment and hospice care (group 4). Patients who did not receive future treatment or hospice (group 1) were more likely to have colorectal cancer and stage II cancer; and patients who received both treatment and hospice care (group 4) were less likely to have colorectal cancer and stage III cancer, compared to other groups. Among 5,295 decedents, 43% received hospice before death. The mean SNF length of stay for all patient groups ranged from 17.0 – 18.7 days for 6,317 patients with non-missing and plausible values. The mean number of days between admission and discharge ADL assessments for all patient groups was 13.1 – 14.4.
Table 1.
Patient and Index Hospitalization/SNF Discharged to Characteristics by Treatment Decision (n (%) or mean ± SD unless otherwise specified) – SEER-Medicare 2011–2013
| Group 1 | Group 2 | Group 3 | Group 4 | ||
|---|---|---|---|---|---|
| Characteristic | All | No Treatment No Hospice | No Treatment Hospice | Treatment No Hospice | Treatment Hospice |
| Total patients - N (% of the whole study sample 6791) | 6791 (100%) | 3103 (45.7%) | 1696 (25.0%) | 1413 (20.8%) | 579 (8.5%) |
| Male (vs. Female) | 2853 (42.0%) | 1283 (41.4%) | 696 (41.0%) | 618 (43.7%) | 256 (44.2%) |
| Age at Index Admission (years) | 81.2 ± 7.4 | 81.6 ± 7.2 | 81.5 ± 7.2 | 77.8 ± 6.8 | 78.3 ± 6.8 |
| Months between Diagnosis and Index Admission | 1.3 ± 1.4 | 1.4 ± 1.6 | 1.2 ± 1.4 | 1.2 ± 0.7 | 1.1 ± 0.7 |
| Race | |||||
| White NH | 5603 (82.6%) | 2565 (82.8%) | 1432 (84.5%) | 1113 (78.8%) | 493 (85.2%) |
| Black NH | 576 (8.5%) | 250 (8.1%) | 133 (7.9%) | 146 (10.3%) | 47 (8.1%) |
| Hispanic | 319 (4.7%) | 132 (4.3%) | 76 (4.5%) | 89 (6.3%) | 22 (3.8%) |
| Asian or Pacific Islander/ American Indian/Alaska Native NH | 284 (4.2%) | 150 (4.8%) | 53 (3.1%) | 64 (4.5%) | 17 (2.9%) |
| Married or Partnered (vs. Non-married) | 2181 (32.1%) | 955 (30.8%) | 507 (29.9%) | 501 (35.5%) | 218 (37.7%) |
| Registry Region at Diagnosis | |||||
| East | 2245 (33.1%) | 987 (31.8%) | 508 (30.0%) | 540 (38.2%) | 210 (36.3%) |
| Midwest | 768 (11.3%) | 324 (10.4%) | 257 (15.2%) | 121 (8.6%) | 66 (11.4%) |
| South | 1256 (18.5%) | 580 (18.7%) | 327 (19.3%) | 238 (16.8%) | 111 (19.2%) |
| West | 2522 (37.1%) | 1212 (39.1%) | 604 (35.6%) | 514 (36.4%) | 192 (33.2%) |
| Census Tract Socioeconomic Status (SES) | |||||
| Median Income ($) | 62894.6 ± 25447.0 | 62599.6 ± 25414.1 | 62284.6 ± 24906.7 | 63889.9 ± 26295.4 | 63817.4 ± 25057.3 |
| % of Residents below Poverty | 13.5 ± 9.3 | 13.6 ± 9.2 | 13.6 ± 9.7 | 13.4 ± 9.1 | 13.1 ± 9.2 |
| % of Non-High School Grads | 13.9 ± 9.6 | 14.0 ± 9.6 | 13.6 ± 9.3 | 14.2 ± 9.8 | 13.6 ± 9.2 |
| Charlson Comorbidity Index | |||||
| 0 | 1937 (28.5%) | 877 (28.3%) | 409 (24.1%) | 486 (34.4%) | 165 (28.5%) |
| 1 | 1657 (24.4%) | 722 (23.3%) | 427 (25.2%) | 356 (25.2%) | 152 (26.3%) |
| 2 | 1233 (18.2%) | 572 (18.4%) | 313 (18.5%) | 232 (16.4%) | 116 (20.0%) |
| 3 or more | 1964 (28.9%) | 932 (30.0%) | 547 (32.3%) | 339 (24.0%) | 146 (25.2%) |
| Year of Diagnosis | |||||
| 2011 | 2280 (33.6%) | 1075 (34.6%) | 596 (35.1%) | 427 (30.2%) | 182 (31.4%) |
| 2012 | 2304 (33.9%) | 1039 (33.5%) | 577 (34.0%) | 494 (35.0%) | 194 (33.5%) |
| 2013 | 2207 (32.5%) | 989 (31.9%) | 523 (30.8%) | 492 (34.8%) | 203 (35.1%) |
| Cancer Type | |||||
| Colorectal | 2865 (42.2%) | 1731 (55.8%) | 495 (29.2%) | 563 (39.8%) | 76 (13.1%) |
| Lung | 2993 (44.1%) | 1017 (32.8%) | 864 (50.9%) | 680 (48.1%) | 432 (74.6%) |
| Pancreas | 559 (8.2%) | 209 (6.7%) | 214 (12.6%) | 105 (7.4%) | 31 (5.4%) |
| Bladder | 374 (5.5%) | 146 (4.7%) | 123 (7.3%) | 65 (4.6%) | 40 (6.9%) |
| Cancer Stage | |||||
| Stage II | 1925 (28.3%) | 1255 (40.4%) | 298 (17.6%) | 320 (22.7%) | 52 (9.0%) |
| Stage III | 1646 (24.2%) | 760 (24.5%) | 336 (19.8%) | 456 (32.3%) | 94 (16.2%) |
| Stage IV | 3220 (47.4%) | 1088 (35.1%) | 1062 (62.6%) | 637 (45.1%) | 433 (74.8%) |
| One or More SNF Stays prior to Index Admission | 1861 (27.4%) | 835 (26.9%) | 595 (35.1%) | 279 (19.8%) | 152 (26.3%) |
| Conditions prior to Index Admission | |||||
| Hypertension | 5055 (74.4%) | 2344 (75.5%) | 1240 (73.1%) | 1039 (73.5%) | 432 (74.6%) |
| Diabetes | 2205 (32.5%) | 1031 (33.2%) | 538 (31.7%) | 456 (32.3%) | 180 (31.1%) |
| COPD | 1936 (28.5%) | 878 (28.3%) | 496 (29.3%) | 364 (25.8%) | 198 (34.2%) |
| Heart Disease | 3075 (45.3%) | 1502 (48.4%) | 757 (44.6%) | 580 (41.1%) | 236 (40.8%) |
| Stroke | 535 (7.9%) | 272 (8.8%) | 148 (8.7%) | 71 (5.0%) | 44 (7.6%) |
| Cognitive Disorder | 1193 (17.6%) | 604 (19.5%) | 424 (25.0%) | 117 (8.3%) | 48 (8.3%) |
| Depressive Disorder | 1259 (18.5%) | 567 (18.3%) | 352 (20.8%) | 231 (16.4%) | 109 (18.8%) |
| Alcohol Use Disorder | 166 (2.4%) | 75 (2.4%) | 31 (1.8%) | 46 (3.3%) | 14 (2.4%) |
| Index LOS (days) | 11.0 ± 7.7 | 11.3 ± 8.1 | 10.2 ± 7.5 | 11.5 ± 7.4 | 10.8 ± 6.3 |
| Index Hospital Characteristics | |||||
| Location | |||||
| Urban (vs. Rural) | 6146 (91.2%) | 2783 (90.7%) | 1525 (90.7%) | 1301 (92.9%) | 537 (93.1%) |
| Type | |||||
| Teaching (vs. Non-teaching) | 3609 (53.7%) | 1601 (52.2%) | 901 (53.6%) | 794 (56.7%) | 313 (54.3%) |
Data were rarely missing (<1%, except SES variables ~4%).
P-values are not shown because all comparisons were highly significant due to the large sample size.
MDS-ADL Scores
Changes in long form MDS-ADL scores are presented in Table 2 for 2,758 patients in our cohort who had both an admission and discharge assessment available. The mean (SD) ADL scores at admission and discharge were 17.1 (4.6) and 15.1 (5.9), respectively. Patients had a mean ADL improvement of 1.5 – 3.1 between admission and discharge long-form ADL scale assessments. Patients who only went on to receive hospice care (group 2) had the smallest improvement with a mean ADL change score of 1.5 between admission and discharge assessments. The 2,758 patients with ADL scores available both at admission and discharge had longer mean length of stay ranging from 20.7 – 22.1.
Table 2.
Minimum Data Set (MDS)-Activities of Daily Living (ADL) Score in First Assessment and Improvement at SNF Discharged to by Treatment Decision (mean ± SD unless otherwise specified, range) – SEER-Medicare 2011–2013
| All Available Patients | Group 1 | Group 2 | Group 3 | Group 4 | |
| Characteristic | All | No Treatment No Hospice | No Treatment Hospice | Treatment No Hospice | Treatment Hospice |
| Total patients - N (% of the whole study sample 6791) | 6791 (100%) | 3103 (45.7%) | 1696 (25.0%) | 1413 (20.8%) | 579 (8.5%) |
| SNF LOS (days) | 17.7 ± 13.3 [1, 60] N=6317 |
17.7 ± 13.6 [1, 60] N=2870 |
17.0 ± 13.0 [1, 60] N=1591 |
18.3 ± 13.0 [1, 60] N=1307 |
18.7 ± 13.2 [1, 60] N=549 |
| Days to First ADL Assessment | 6.3 ± 3.1 [1, 59] N=3984 |
6.3 ± 3.1 [1, 59] N=1823 |
6.2 ± 3.5 [1, 59] N=998 |
6.6 ± 3.1 [1, 52] N=821 |
6.3 ± 2.2 [1, 26] N=342 |
| Total ADL Score: First Assessment | 17.4 ± 4.9 [0,28] N=3984 |
17.9 ± 4.8 [0, 28] N=1823 |
18.2 ± 4.6 [0, 28] N=998 |
15.8 ± 5.1 [0, 28] N=821 |
16.7 ± 4.5 [0, 26] N=342 |
| Patients with First and Last ADL Assessments | Group 1 | Group 2 | Group 3 | Group 4 | |
| Characteristic | All | No Treatment No Hospice | No Treatment Hospice | Treatment No Hospice | Treatment Hospice |
| Total patients - N (% of 2758 patients with first and last ADL assessments) | 2758 (100%) | 1216 (44.1%) | 644 (23.4%) | 637 (23.1%) | 261 (9.5%) |
| SNF LOS (days) | 21.6 ± 12.4 [1, 60] |
22.1 ± 12.8 [1, 60] |
21.5 ± 11.9 [1, 60] |
20.7 ± 12.0 [1, 58] |
22.0 ± 12.7 [1, 60] |
| Days to First ADL Assessment | 6.7 ± 1.6 [1, 28] |
6.8 ± 1.8 [1, 28] |
6.7 ± 1.4 [1, 26] |
6.8 ± 1.5 [1, 28] |
6.7 ± 1.7 [1, 26] |
| Total ADL Score: First Assessment | 17.1 ± 4.6 [0,28] |
17.4 ± 4.5 [0, 28] |
17.8 ± 4.3 [0, 28] |
15.9 ± 4.8 [0, 27] |
16.6 ± 4.3 [1, 26] |
| Days between First and Last ADL Assessments | 13.5 ± 11.4 [0, 54] |
13.7 ± 11.8 [0, 53] |
13.1 ± 10.9 [0, 49] |
13.1 ± 11.0 [0, 51] |
14.4 ± 12.2 [0, 54] |
| Total ADL Score: Improvement | 2.3 ± 3.5 [0,21] |
2.4 ± 3.6 [0, 21] |
1.5 ± 2.6 [0, 17] |
3.1 ± 4.0 [0, 21] |
1.7 ± 2.9 [0, 16] |
Survivorship
Figure 2 depicts Kaplan-Meier survival curves for all patient groups. Patients who only went on to receive hospice (group 2) had the worst survival and patients who received treatment but not hospice (group 3) had the best survival. Patients who received treatment then hospice (group 4) had worse survival than patients who did not receive treatment or hospice (group 1). Cox regression proportional hazards models are shown in Table 3. Variables affecting survival were examined in Model 0, with pronounced effects for cancer type and stage, and grade. Model 1 estimated unadjusted HRs for clinical trajectory, confirming the findings using Kaplan-Meier curves. Model 2 estimated HRs for clinical trajectory, adjusting for the variables in Model 0, and the effects of treatment decision did not change much. ADL score was included in Model 3, although, as noted above, more than half of patients could not be included due to the severe missing data in ADL reporting. Covariates largely maintained magnitude and significance, suggesting no substantial bias in the reduced sample. Patients with a higher admission MDS-ADL score in the SNF had worse survival. Patients who had greater improvement in the MDS-ADL score between their admission and discharge assessment had improvement in survival.
Figure 2.

Kaplan Meier Survival Curve: Group 1 (No Treatment/No Hospice), Group 2 (No Treatment/Hospice), Group 3 (Treatment/No Hospice), and Group 4 (Treatment/Hospice). Censoring marks omitted to mask individual results.
Table 3.
Cox Models for Months to Death
| Model 0 | Model 1 | Model 2 | Model 3* | |
|---|---|---|---|---|
| N | 6523 | 6791 | 6523 | 2758 |
| n (%) Censored | 1447 (22.2%) | 1496 (22.0%) | 1447 (22.2%) | 705 (25.6%) |
| Variable | HR (95% CI)** | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Treatment Decision (vs. Treatment/No Hospice [Group 3]): | ||||
| No Treatment/No Hospice [Group 1] | 1.08 (1.002–1.17) | 1.61 (1.48–1.75) | 1.40 (1.23–1.58) | |
| Treatment/Hospice [Group 4] | 2.98 (2.68–3.31) | 2.09 (1.88–2.33) | 1.99 (1.69–2.35) | |
| No Treatment/Hospice [Group 2] | 3.40 (3.13–3.69) | 2.90 (2.66–3.18) | 2.84 (2.48–3.25) | |
| Total ADL score: | ||||
| First Assessment | 1.04 (1.03–1.05) | |||
| Improvement | 0.93 (0.92–0.95) | |||
| Male (vs. Female) | 1.16 (1.10–1.23) | 1.17 (1.11–1.24) | 1.20 (1.10–1.32) | |
| Age at Index Admission (10 years) | 1.19 (1.14–1.23) | 1.06 (1.02–1.11) | ||
| Months between Diagnosis and Index Admission | 0.93 (0.90–0.96) | 0.93 (0.91–0.96) | 0.94 (0.90–0.98) | |
| SES | ||||
| Median Income ($10,000) | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) | ||
| Charlson Comorbidity Index Categories | 1.07 (1.05–1.10) | 1.05 (1.03–1.08) | ||
| Cancer Type (vs. Colorectal) | ||||
| Lung | 1.87 (1.72–2.03) | 1.88 (1.73–2.04) | 2.06 (1.81–2.35) | |
| Pancreas | 2.29 (2.05–2.57) | 2.20 (1.96–2.46) | 2.36 (1.97–2.84) | |
| Bladder | 1.89 (1.64–2.18) | 1.80 (1.56–2.07) | 1.86 (1.51–2.30) | |
| Cancer Stage (vs. Stage IV) | ||||
| Stage II | 0.34 (0.31–0.37) | 0.35 (0.32–0.38) | 0.38 (0.33–0.43) | |
| Stage III | 0.52 (0.48–0.56) | 0.56 (0.52–0.60) | 0.58 (0.52–0.66) | |
| Grade (vs. 9) | ||||
| 1 | 0.43 (0.36–0.52) | 0.48 (0.40–0.58) | 0.48 (0.36–0.64) | |
| 2 | 0.61 (0.56–0.67) | 0.66 (0.60–0.73) | 0.70 (0.61–0.82) | |
| 3 | 0.87 (0.80–0.94) | 0.93 (0.86–1.005) | 0.97 (0.86–1.10) | |
| 4 | 0.90 (0.78–1.03) | 0.98 (0.85–1.12) | 1.16 (0.94–1.43) | |
| Index LOS (10 days) | 1.07 (1.03–1.10) | 1.09 (1.05–1.13) |
Many cases were not used in the model since improvement in total ADL score was available for only 2758 patients.
Hazard Ratio (95% Confidence Interval)
Discussion
This study describes the post-acute care outcomes of patients who discharged to a SNF with a new diagnosis of stage II – IV pancreatic, colorectal, lung, or bladder cancer after an index hospitalization. Patients who received subsequent cancer treatment had increased survival however 71% of patients did not go on to receive future cancer therapy. Only 43% of patients received hospice care before death. Approximately 40% of patients had complete admission and discharge ADL self-performances scores limiting our ability to assess changes in MDS-ADL scores of our cohort. Patients whose MDS-ADL score improved more between their first and last assessment, however, had a decreased risk of death.
The MDS-ADL long form scale provides an opportunity to measure changes in ADLs and gives an indication of the extent to which SNF rehabilitative care affects an individual’s health status and ability for independent mobility and self-care.17 More than half the patients in our study did not have ADL self-performance scores completed at both admission and discharge. This missing data indicates that nursing facilities are not fulfilling requirements of assessing physical function, limiting our ability to assess patient conditions most likely to benefit from rehabilitative post-acute care. As post-acute care use continues to rise, it will be imperative to capture accurate ADL measures to track clinical progress and improve patient selection for different types of post-acute care.9 Patients discharged to SNFs with limited rehabilitation potential and poor prognosis are at risk for being “rehabbed to death,” transitioning between hospital and post-acute care facilities near the end of life in a burdensome and costly pattern of care.23
There were limited improvements in MDS-ADL scores across all four patient groups in the subset of patients for whom we have complete admission and discharge MDS-ADL data. Most studies documenting functional change in nursing homes have been restricted to long stay patients who permanently reside in the facility.12,24 Patients who were admitted to SNFs after sustaining a hip fracture had a mean ADL improvement of 14%.17 In a sample of 59, 383 older adults receiving SNF care after an intensive care hospitalization, about 60% improved function. Among those with improved ADL scores the average first MDS-ADL score was 18.0 and the last was 13.4 (out of a total score of 28), demonstrating an improvement of 16% of the total scale range. 25 The 2,758 patients with complete data in our study only demonstrated an average ADL improvement (defined as non-negative) of 8% of the total scale range with an average admission MDS-ADL score of 17.1 and discharge of 15.1. Less improvement was associated with worse survival.
Post-acute care health utilization outcomes differ for patients with new advanced cancer compared to patients with an established diagnosis of cancer and receipt of prior cancer treatment (chemotherapy, radiation, targeted therapy). In our earlier analysis of SEER-Medicare data, 42% of patients with a pre-existing diagnosis of advanced cancer received future cancer treatment after discharge to SNF compared to 30% in this study. Hospice use in our study was substantially lower, only 34% of all patients received hospice care after SNF discharge compared to 55% of patients with a known cancer diagnosis.10 The low rates of hospice use in our study suggest that there is a critical need to improve palliative care delivery during care transitions from acute to post-acute care. 26–28 Palliative care has been shown to improve quality of life, decrease burdensome end of life treatment, improve hospice utilization and is recommended for patients with advanced cancer by the American Society of Clinical Oncology.29–31
Providers need to recognize that for some patients the disability they are experiencing after a hospitalization may be part of a patient’s end-of-life trajectory. Recognizing and acknowledging the uncertainty of the situation and providing patients and caregivers language to communicate what is important is imperative. Framing a SNF stay as a trial of treatment, developing a back-up plan before hospital discharge, and completing portable medical orders such as POLST and MOST forms are some options to enhance the receipt of goal-concordant care between care setting transitions.
Our study has several strengths but also some limitations. Our analysis was limited to patients diagnosed with new advanced cancer between 2011 – 2013 limiting the generalizability of these results. The analysis does not include the current impact of newer targeted therapies and immunotherapy. Admission and discharge ADL scores were recorded for fewer than one half of the patients. We grouped patients based on treatment and hospice receipt rather than cancer type and stage. This issue might be less relevant given the global lack of improvement in MDS-ADL score for all groups suggesting that patients with different cancer types might be more alike than different in the SNF setting. We did not assess facility level characteristics such as size, staffing, and payment sources and identifying modifiable facility level factors should be investigated.32 Secondary analysis of data may not allow for other outcomes of importance to patients such as quality of life and receipt of goal-concordant care.
Conclusion and Implications
This study characterizes the post-acute care trajectory of hospitalized patients with newly diagnosed cancer discharged to a SNF and reveals that the majority do not experience significant rehabilitative gains, receive cancer treatment or hospice care. Further development of novel palliative care delivery models in post-acute care is needed to provide high quality care for this high-risk population.
Supplementary Material
Funding Sources:
This research was supported by the National Palliative Care Research Center Kornfeld Scholar Award and by the National Cancer Institute grant number P30CA046934, University of Colorado Cancer Center Core Support Grant. Analyses were completed by the Population Health Shared Resource (P30CA046934).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Kenis C, Decoster L, Bastin J, et al. Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J Geriatr Oncol. 2017;8(3):196–205. [DOI] [PubMed] [Google Scholar]
- 2.Giebel CM, Sutcliffe C, Stolt M, et al. Deterioration of basic activities of daily living and their impact on quality of life across different cognitive stages of dementia: a European study. Int Psychogeriatr. 2014;26(8):1283–1293. [DOI] [PubMed] [Google Scholar]
- 3.Han L, Allore H, Murphy T, Gill T, Peduzzi P, Lin H. Dynamics of functional aging based on latent-class trajectories of activities of daily living. Ann Epidemiol. 2013;23(2):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meddings J, Reichert H, Smith SN, et al. The Impact of Disability and Social Determinants of Health on Condition-Specific Readmissions beyond Medicare Risk Adjustments: A Cohort Study. J Gen Intern Med. 2017;32(1):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millan-Calenti JC, Tubio J, Pita-Fernandez S, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50(3):306–310. [DOI] [PubMed] [Google Scholar]
- 7.Lage DE, El-Jawahri A, Fuh CX, et al. Functional Impairment, Symptom Burden, and Clinical Outcomes Among Hospitalized Patients With Advanced Cancer. J Natl Compr Canc Netw. 2020;18(6):747–754. [DOI] [PubMed] [Google Scholar]
- 8.Osakwe ZT, Larson E, Agrawal M, Shang J. Assessment of Activity of Daily Living Among Older Adult Patients in Home Healthcare and Skilled Nursing Facilities: An Integrative Review. Home Healthc Now. 2017;35(5):258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner RM, Konetzka RT. Trends in Post-Acute Care Use Among Medicare Beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S, Eguchi M, Min SJ, Fischer S. Outcomes of Patients With Cancer Discharged to a Skilled Nursing Facility After Acute Care Hospitalization. J Natl Compr Canc Netw. 2020;18(7):856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program; National Cancer Institute. Overview of the SEER Program. https://seer.cancer.gov/about/overview.html. Accessed. [Google Scholar]
- 12.Thomas KS, Boyd E, Mariotto AB, Penn DC, Barrett MJ, Warren JL. New Opportunities for Cancer Health Services Research: Linking the SEER-Medicare Data to the Nursing Home Minimum Data Set. Med Care. 2018;56(12):e90–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman M, Tyler D, Acquah JK, Lima J, Mor V. Sensitivity and specificity of the Minimum Data Set 3.0 discharge data relative to Medicare claims. J Am Med Dir Assoc. 2014;15(11):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Rio RA, Goldman M, Kapella BK, Sulit L, Murray PK. The accuracy of Minimum Data Set diagnoses in describing recent hospitalization at acute care facilities. J Am Med Dir Assoc. 2006;7(4):212–218. [DOI] [PubMed] [Google Scholar]
- 15.Gruber-Baldini AL, Zimmerman SI, Mortimore E, Magaziner J. The validity of the minimum data set in measuring the cognitive impairment of persons admitted to nursing homes. J Am Geriatr Soc. 2000;48(12):1601–1606. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services (CMS). Legacy Medicare Provider Utilization and Payment Data: Skilled Nursing Facilities
- 17.Wysocki A, Thomas KS, Mor V. Functional Improvement Among Short-Stay Nursing Home Residents in the MDS 3.0. J Am Med Dir Assoc. 2015;16(6):470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595–601. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute, Division of Cancer Control and Population Sciences. SEER-Medicare: Medicare Enrollment & Claims Data. https://healthcaredelivery.cancer.gov/seermedicare/medicare/. Accessed.
- 20.National Cancer Institute, Division of Cancer Control and Population Sciences. SEER-Medicare: How the SEER & Medicare Data are Linked. . https://healthcaredelivery.cancer.gov/seermedicare/overview/linked.html. Accessed.
- 21.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546–553. [DOI] [PubMed] [Google Scholar]
- 22.Center for Medicare and Medicaid Services. Chronic Conditions Data Warehouse. . https://www.ccwdata.org/web/guest/condition-categories. Accessed.
- 23.Flint LA, David DJ, Smith AK. Rehabbed to Death. N Engl J Med. 2019;380(5):408–409. [DOI] [PubMed] [Google Scholar]
- 24.Mor V, Gruneir A, Feng Z, Grabowski DC, Intrator O, Zinn J. The effect of state policies on nursing home resident outcomes. J Am Geriatr Soc. 2011;59(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downer B, Pritchard K, Thomas KS, Ottenbacher K. Improvement in Activities of Daily Living during a Nursing Home Stay and One-Year Mortality among Older Adults with Sepsis. J Am Geriatr Soc. 2021;69(4):938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boorsma M, Frijters DH, Knol DL, Ribbe ME, Nijpels G, van Hout HP. Effects of multidisciplinary integrated care on quality of care in residential care facilities for elderly people: a cluster randomized trial. CMAJ. 2011;183(11):E724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Retrum JH, Gozansky WS, Lahoff DG, et al. A Need for More Palliative Focused Care: A Survey of Colorado Skilled Care Facilities. J Am Med Dir Assoc. 2015;16(8):712–713. [DOI] [PubMed] [Google Scholar]
- 28.Kelley AS, Morrison RS. Palliative Care for the Seriously Ill. N Engl J Med. 2015;373(8):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahlin CM, Kelley JM, Jackson VA, Temel JS. Early palliative care for lung cancer: improving quality of life and increasing survival. Int J Palliat Nurs. 2010;16(9):420–423. [DOI] [PubMed] [Google Scholar]
- 30.Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973–979. [DOI] [PubMed] [Google Scholar]
- 31.Ferrell BR, Temel JS, Temin S, et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(1):96–112. [DOI] [PubMed] [Google Scholar]
- 32.Hakkarainen TW, Arbabi S, Willis MM, Davidson GH, Flum DR. Outcomes of Patients Discharged to Skilled Nursing Facilities After Acute Care Hospitalizations. Ann Surg. 2016;263(2):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


