To the Editor: Earlier in the COVID-19 pandemic, we studied the effects of tumor necrosis factor inhibitors (TNFis) and methotrexate (MTX) on disease severity following COVID-19. There were fewer patients on these therapies who required hospitalization or died compared to controls; however, this association was insignificant.1 A study conducted during this period by Veenstra and coworkers also examined the relationship between TNFi and COVID-19, and found these medications to be associated with less severe disease.2 Another study found MTX was associated with reduced mortality.3 Since a much greater number of COVID-19 cases are now available for analysis, we re-examined the TriNetX database to clarify the relationship between COVID-19 severity and MTX or TNFi exposure.
TriNetX is a global federated research network that provides access to statistics on electronic medical records across 66 health care organizations, as described previously.1 Since our prior study, the TriNetX COVID-19 research network has almost doubled in size from over 53 million to over 95 million patients. Those with a COVID-19-related diagnosis since January 20, 2020 also increased from 32,076 to over 1.7 million. Among adults with documented COVID-19, 24,068 were exposed to a TNFi or MTX within 1 year before diagnosis compared with our previous cohort of 214 patients.
We queried the TriNetX COVID-19 research network on June 14, 2022 for adult patients with documented exposure to a TNFi (adalimumab, infliximab, etanercept, certolizumab, or golimumab) or MTX within 1 year of COVID-19 diagnosis. Outcomes examined were hospitalization or death within 45 days of COVID-19 diagnosis. Cohorts included TNFi and MTX as well as patients on both medications. 1:1 propensity score matching was performed for comorbidities associated with poor COVID-19-related outcomes (Supplementary Tables I and II, available via Mendeley at https://doi.org/10.17632/tvvwmbry4h.2).4 Analyses were assembled using the International Classification of Diseases, 10 th Revision, Clinical Modification diagnoses and terminology recommended by the World Health Organization and Centers for Disease Control and Prevention (Supplementary Appendix, available via Mendeley at https://doi.org/10.17632/tvvwmbry4h.2).
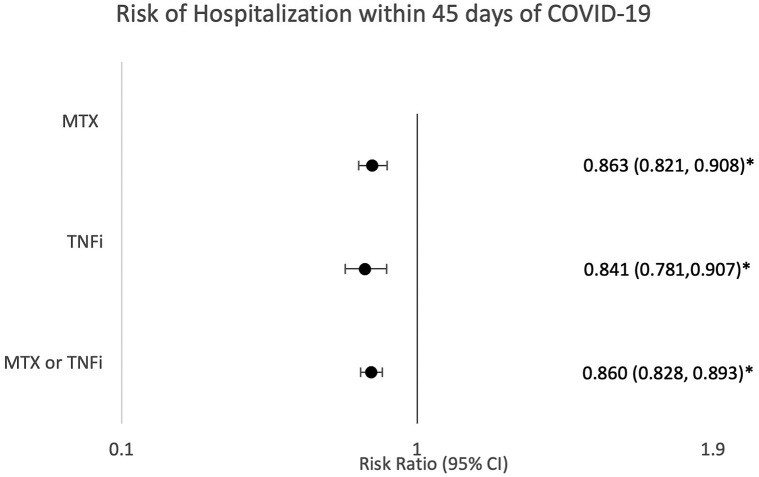
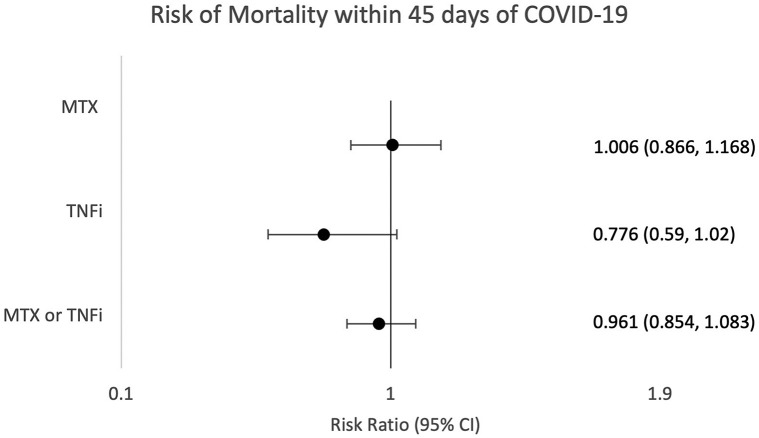
For each matched group, the likelihood of hospitalization was significantly decreased. Risk differences were 2.786% in the combined TNFi/MTX group (risk ratio [RR] = 0.860 [95% CI 0.828, 0.893]), 2.933% in the TNFi group (RR = 0.841 [95% CI 0.781, 0.907]), and 2.952% in the MTX group (RR = 0.863 [95% CI 0.821, 0.908]) for hospitalization (Fig 1 ). Mortality for matched groups did not reach significance with risk differences of 0.087% for TNFi/MTX, 0.385% for TNFi, and 0.016% for MTX (RR = 0.961 [95% CI 0.854, 1.083], RR = 0.776 [95% CI 0.59, 1.02], RR = 1.006 [95% CI 0.866, 1.168], respectively) (Fig 2 ).
Fig 1.
Risk of hospitalization within 45 days of COVID-19. Risk ratios and 95% CIs were calculated to assess the likelihood of hospitalization within 45 days of receiving a diagnosis associated with COVID-19 in patients on methotrexate or tumor necrosis factor inhibitor after 1:1 propensity matching. MTX, Methotrexate; TNFi, tumor necrosis factor inhibitor. ∗P < .05 was considered significant.
Fig 2.
Risk of mortality within 45 days of COVID-19. Risk ratios and 95% CIs were calculated to assess the likelihood of mortality within 45 days of receiving a diagnosis associated with COVID-19 in patients on methotrexate or tumor necrosis factor inhibitor after 1:1 propensity matching. MTX, Methotrexate; TNFi, tumor necrosis factor inhibitor. ∗P < .05 was considered significant.
Consistent with the study by Veenstra and coworkers, our results suggest that patients on MTX and TNFi are not at increased risk of more severe COVID-19-related sequelae and that TNFi may be associated with less severe disease.2 Ours are among the first to suggest MTX may also be associated with milder COVID-19. These findings continue to support ongoing use of TNFi and MTX without interruption due to fear of worse COVID-19 outcomes and also support the rationale for ongoing randomized trials testing TNFi and MTX as therapy for COVID-19.5
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Yousaf A., Gayam S., Feldman S., Zinn Z., Kolodney M. Clinical outcomes of COVID-19 in patients taking tumor necrosis factor inhibitors or methotrexate: a multicenter research network study. J Am Acad Dermatol. 2021;84(1):70–75. doi: 10.1016/j.jaad.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veenstra J., Buechler C.R., Robinson G., et al. Antecedent immunosuppressive therapy for immune-mediated inflammatory diseases in the setting of a COVID-19 outbreak. J Am Acad Dermatol. 2020;83(6):1696–1703. doi: 10.1016/j.jaad.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FAI2R /SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2021;80(4):527–538. doi: 10.1136/annrheumdis-2020-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 5.Hachem H., Godara A., Schroeder C., et al. Rapid and sustained decline in CXCL-10 (IP-10) annotates clinical outcomes following TNFα-antagonist therapy in hospitalized patients with severe and critical COVID-19 respiratory failure. J Clin Transl Sci. 2021;5(1):e146. doi: 10.1017/cts.2021.805. [DOI] [PMC free article] [PubMed] [Google Scholar]