Abstract
Background:
Malignant hyperthermia (MH) is a potentially lethal disorder triggered by certain anesthetics. Mutations in the ryanodine receptor 1 (RYR1) gene account for about half of MH cases. Discordance between the low incidence of MH and a high prevalence of mutations has been attributed to incomplete penetrance, which has not been quantified yet. The authors aimed to examine penetrance of MH-diagnostic RYR1 mutations and the likelihood of mutation carriers to develop MH, and to identify factors affecting severity of MH clinical expression.
Methods:
In this multicenter case–control study, data from 125 MH pedigrees between 1994 and 2017 were collected from four European registries and one Canadian registry. Probands (survivors of MH reaction) and their relatives with at least one exposure to anesthetic triggers, carrying one diagnostic RYR1 mutation, were included. Penetrance (percentage of probands among all genotype-positive) and the probability of a mutation carrier to develop MH were obtained. MH onset time and Clinical Grading Scale score were used to assess MH reaction severity.
Results:
The overall penetrance of nine RYR1 diagnostic mutations was 40.6% (93 of 229), without statistical differences among mutations. Likelihood to develop MH on exposure to triggers was 0.25 among all RYR1 mutation carriers, and 0.76 in probands (95% CI of the difference 0.41 to 0.59). Penetrance in males was significantly higher than in females (50% [62 of 124] vs. 29.7% [30 of 101]; P = 0.002). Males had increased odds of developing MH (odds ratio, 2.37; 95% CI, 1.36 to 4.12) despite similar levels of exposure to trigger anesthetics. Proband’s median age was 12 yr (interquartile range 6 to 32.5).
Conclusions:
Nine MH-diagnostic RYR1 mutations have sex-dependent incomplete penetrance, whereas MH clinical expression is influenced by patient’s age and the type of anesthetic. Our quantitative evaluation of MH penetrance reinforces the notion that a previous uneventful anesthetic does not preclude the possibility of developing MH.
Malignant hyperthermia (MH) is a rare life-threatening disorder caused by dysregulation of intracellular calcium homeostasis in skeletal muscle and triggered by exposure to certain anesthetics in genetically predisposed individuals.1 A progressively better understanding of the pathomechanism of MH, advances in anesthesia monitoring, and the introduction of dantrolene have been crucial in reducing MH mortality, which remains around 10%.2
Variants in ryanodine receptor 1 (RYR1),3 calcium voltage-gated channel subunit alphal S (CACNA1S),4-6 and in SH3 and cysteine rich domain 3 (STAC3) genes7 are associated with MH. The RYR1 gene—encoding the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1)—is the major MH-associated locus, involved in more than half of MH cases, whereas variants in CACNA1S and STAC3 account for less than 1%. At present, 48 RYR1 and 2 CACNA1S variants are recognized as MH-causative mutations.8 Recent availability of data on thousands of human exomes9 has allowed to determine the true combined prevalence of all known MH-causative mutations as 1:2,750,10 which is close to earlier estimates.11-13 The prevalence of MH diagnostic mutations is considerably greater than the reported incidence of clinical MH episodes (1:35,000 to 1:68,000 surgical discharges).2 This striking discrepancy can be attributable to the fact that many mutation carriers may never be exposed to anesthetic triggers.14 The discrepancy may also reflect a reduced—or incomplete—penetrance of the MH trait.10,11,15 Indeed, not all subjects carrying a causative mutation develop MH on first exposure to anesthesia, and some may have several uneventful anesthetics before developing MH in the operating room.16,17 In addition, the onset, progression, and severity of MH reaction are variable. The time of onset of MH seems to be influenced by the type and dose of volatile anesthetic, whereas severity is also dependent on the duration of exposure.18-20
Although our knowledge of factors influencing MH penetrance is limited, it is known that MH penetrance may depend on the additive effect of more than one genetic factor,21 and allele-specific differences in RyR1 mRNA expression levels may explain the observed reduced penetrance and variations in MH phenotype among individuals.22 Several studies23-26 indicate higher incidence of MH in younger males, but the reason for that remains unclear.
Challenged by a paucity of clinically affected individuals with variable phenotype and known genotype, quantification of the MH trait penetrance remains an elusive subject, albeit being essential for an optimal risk assessment at the time of genetic counseling. In this study, we analyzed the available clinical and genetic data on 125 European and North American MH families collected at MH centers in Europe and Canada. We hypothesized that the penetrance of diagnostic RYR1 mutations is incomplete, and that reduced penetrance and MH clinical expression are genotype-specific. Therefore, our objectives were to examine the penetrance of RYR1 diagnostic mutations and the likelihood of RYR1 mutation carriers to develop MH, and to identify factors that may affect MH clinical expression.
Materials and Methods
After research ethics board approval and institutional authorization, for this multicenter case–control study, we collected clinical and genetic data on MH susceptible individuals from families with a positive history of MH reaction from the registries of three German, one Belgian, and one Canadian MH diagnostic centers between January 1, 1994 and December 31, 2017. Because of the retrospective nature of the study, consent was waived by the ethics board of all the participating centers.
Selection Process and Data Collection
Probands (survivors of MH reactions) and their relatives (family members who carried a familial RYR1 mutation and had one or more uneventful exposures to anesthetic triggers) were included in the study provided that: (1) they carried only a single MH diagnostic RYR1 mutation and had no additional potentially pathogenic RYR1 variants; (2) data on two or more probands sharing a RYR1 mutation were available; (3) three or more relatives (excluding the probands) shared a RYR1 mutation and each had a documented history of at least one uneventful exposure to MH triggers (fig. 1).
Fig. 1.
The five diamonds on the flowchart contain the criteria used in this research for screening, allocation and inclusion/exclusion according to: (1) variant pathogenicity as per the list of diagnostic malignant hyperthermia (MH) mutations from the European Malignant Hyperthermia Group; (2) history (Hχ) of uneventful exposure to trigger anesthetics in relatives bearing a given mutation; (3) available data from at least two probands sharing a ryanodine receptor 1 (RYR1) mutation. VUSs, variants of unknown significance.
RYR1 mutation refers to nonsynonymous variants that have been functionally validated and included in the MH diagnostic mutations list of the European Malignant Hyperthermia Group.8 When referring to specific RYR1 mutations we describe the inferred amino acid change at the protein level using the Human Genome Variation Society recommendations for the description of sequence variants.27
Data collected on probands included RYR1 genotype, number of exposures (i.e., number of surgeries under general anesthesia with inhalational agents or succinylcholine, including the one during which the MH crisis occurred), trigger agent(s) used, sex, age at the time of the MH reaction, Clinical Grading Scale28 scores, and onset time defined as the period from the start of the trigger anesthetic to first sign of MH on record. Data were extracted from the anesthetic records, where available, or otherwise from the MH center records.
Available data on relatives of the probands included RYR1 genotype, sex, and number of exposures (i.e., uneventful anesthetics with trigger agents). The relatives’ ages at the time of triggered anesthetics were not extracted because they were not available for all.
Penetrance29 of an MH causative RYR1 mutation was defined as the percentage of probands among all carriers of the mutation who had been exposed to general anesthesia:
| (1) |
nprobands and nrelatives refer to the number of probands and of relatives, respectively. Because of the rarity of MH, penetrance in this study is a byproduct of pooled pedigrees with a shared RYR1 mutation and therefore it is subject to the influence of different familial genetic backgrounds.
MH susceptible individuals may have several uneventful exposures to triggering anesthetics before developing an MH reaction. We assessed the probability to develop MH on exposure to triggers in carriers of a RYR1 mutation both in probands (PMH probands) and in the entire cohort (PMH all), respectively, as follows:
| (2) |
Here, nprobands is the number of probands, which in this study is equal to the number of MH reactions, and Exp(x) is the number of exposures to triggers in probands or in relatives, as specified.30,31
Clinical Grading Scale scores and MH onset time were used as indices of MH phenotype severity for comparison of the different RYR1 genotypes.
Statistical Analysis
No statistical power calculation was conducted before the study, so the sample size was based on the available data. Hypothesis testing was two-tailed. Normality of the different variables was graphically assessed by histograms or by the Kolmogorov–Smirnov test for normality. Mean and SD were used to describe normally distributed data, whereas median and interquartile range were used for skewed variables. Chi-square test was used to look for associations between the phenotype groups (probands or relatives) and the RYR1 genotype, the number of exposures to anesthetic triggers, and sex. Pairwise comparisons of penetrance and PMH across the RYR1 mutations were performed using Z-test with Bonferroni’s correction to decrease the likelihood of committing type 1 error . Nonparametric between-subjects one-way ANOVA was used to assess differences in MH phenotype severity. Spearman’s coefficient was used to explore the correlation between proband’s age and each indicator of clinical MH severity (i.e., Clinical Grading Scale score and MH onset time). Only this latter analysis was data-driven (post hoc), whereas all the former were done in accordance to our original statistical plan. P < 0.05 was considered significant, unless otherwise specified. The software used for statistical analysis was SAS Studio (Enterprise Edition, version 3.7; SAS Institute Inc., USA).
Results
An initial screening of the four European and one Canadian MH registries revealed 125 unrelated MH pedigrees with 370 individuals carrying 37 different potentially pathogenic RYR1 variants. After applying the inclusion criteria (see Materials and Methods), 229 subjects from 93 MH pedigrees (93 probands and 136 relatives) carrying nine MH diagnostic mutations were included in this study (fig. 1). Among those excluded, there were eight families with more than one potentially pathogenic RYR1 variant. Data were extracted from anesthetic records in 76 probands and from the referring anesthesiologists’ reports in the rest of the study sample.
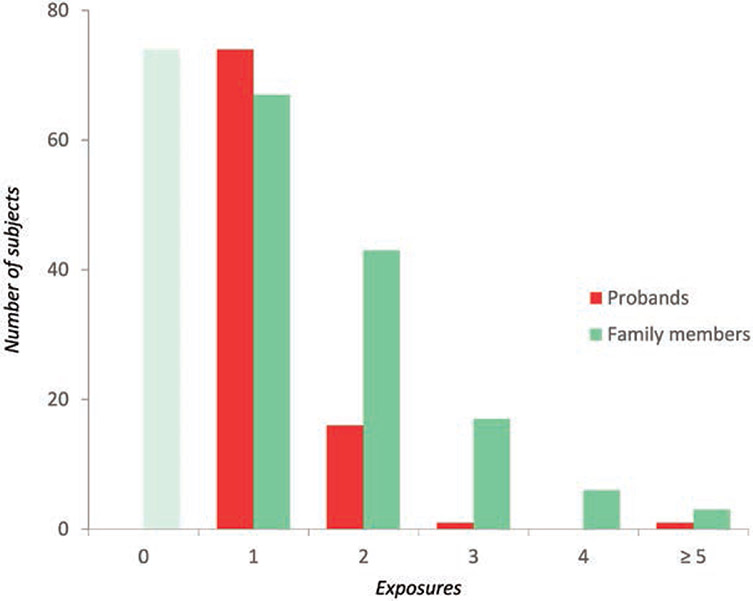
The selected RYR1 mutation carriers had in total 365 exposures to anesthesia with MH triggers, of which 93 resulted in MH reactions (table 1). The median exposure to MH triggers was higher in relatives than in probands (2 vs. 1 exposures per subject, P < 0.0001; table 1). Among probands, 79.6% (74 of 93) developed an MH reaction during the first anesthetic, 17.2% (16 of 93) during the second, and 3.2% (3 of 93) after more than two exposures to MH triggers (fig. 2).
Table 1.
Penetrance and Likelihood to Develop MH, by RYR1 Genotype
| RYR1 Mutation | Relatives |
Probands |
Penetrance (%) |
MH Probability (PMH) |
|||
|---|---|---|---|---|---|---|---|
| n | #Exp | n | #Exp | P MH all | P MH probands | ||
| p.Gly341Arg | 18 | 25 | 11 | 17 | 37.9 | 0.26 | 0.65 |
| p.Arg614Cys | 33 | 64 | 30 | 39 | 47.6 | 0.29 | 0.77 |
| p.Arg614Leu | 3 | 5 | 5 | 6 | 62.5 | 0.45 | 0.83 |
| p.Thr2206Met | 36 | 57 | 18 | 19 | 33.3 | 0.24 | 0.95 |
| p.Arg2336His | 5 | 12 | 3 | 5 | 37.5 | 0.18 | 0.60 |
| p.Ala2350Thr | 4 | 9 | 3 | 4 | 42.9 | 0.23 | 0.75 |
| p.Gly2375Ala | 7 | 11 | 4 | 5 | 36.4 | 0.25 | 0.80 |
| p.Gly2434Arg | 9 | 17 | 12 | 18 | 57.1 | 0.34 | 0.67 |
| p.Arg2454His | 21 | 43 | 7 | 9 | 25 | 0.13 | 0.78 |
| Overall | 136 | 243 | 93 | 122 | 40.6 ± 0.12 | 0.25 ± 0.09* | 0.76 ± 0.11 |
| Exposures | 2 [1–2]* | 1 [1-1] | |||||
Overall penetrance and PMH expressed as mean ± SD; exposures expressed as median [interquartile range]. Penetrance = nprobands / (nrelatives + nprobands) × 100; PMH probands = nprobands / #Expprobands; PMH all = nprobands / (#Expprobands + #Exprelatives). #Exp, number of exposures (i.e., general anesthetics received with trigger agents); MH, malignant hyperthermia; RYR1, ryanodine receptor 1. Range (highest and lowest) values are showed in boldface, P = 0.047, 0.054 and 0.038 for penetrance, PMH all and PMH probands range values, respectively.
P < 0.001.
Fig. 2.
Exposures to general anesthetics with trigger agents in carriers of ryanodine receptor 1 mutations. On the vertical and horizontal axes are represented the number of subjects exposed to trigger anesthetics and the total number of exposures, respectively. Exposures in probands (red) include the general anesthetic during which the actual malignant hyperthermia crisis occurred, whereas in family members (green) they comprise the total number of uneventful anesthetics with trigger agents; family members with no anesthetic history (shaded green bar) were excluded from analysis.
Penetrance
RYR1 Genotype.
The MH-diagnostic RYR1 mutations harbored by the study participants included three amino-terminal mutations (p.Gly341Arg, p.Arg614Cys, and p.Arg614Leu) and six mutations within the central RyR1 region (p.Thr2206Met, p.Arg2336His, p.Ala2350Thr, p.Gly2375Ala, p.Gly2434Arg, and p.Arg2454His).
There were no missing data for genotype, neither in probands nor in relatives. There were 93 MH cases among 229 genotype-positive subjects with previous recorded exposure to trigger anesthetics; that yields an overall penetrance for the analyzed RYR1 mutations of 40.6% (95% CI, 34.3 to 47.3%). Notably, levels of penetrance were not significantly different (P = 0.303) among the analyzed RYR1 mutations (table 1). Even the difference between mutations with the highest and lowest penetrance did not reach statistical significance (62.5% [5 of 8] for p.Arg614Leu, and 25% [7 of 28] for p.Arg2454His, respectively; P = 0.047, whereas P < 0.0014 was required after Bonferroni’s correction for 36 pairwise comparisons).
The overall probability that a carrier of any of the nine RYR1 diagnostic mutations will develop MH on exposure to triggers, PMH all, was 0.25 (95% CI, 0.21 to 0.30). PMH all ranged from 0.45 to 0.13 for the same aforementioned pair of mutations (P = 0.054). However, if only probands were considered, the probability of developing MH (P) increased to 0.76 (95% CI, 0.67 to 0.83; table 1).
Demographic Factors and MH Triggers.
There was a significant association between sex and phenotype: 67.4% (62 of 92) probands were males, whereas among relatives males comprised 46.6% (62 of 133; P = 0.002; table 2). One proband and three relatives did not have data for sex and were excluded from the analysis.
Table 2.
Sex Distribution by Phenotype Group and RYR1 Genotype (Upper Section) and Penetrance, Likelihood to Develop MH, and Exposure Rate by Sex (Lower Section)
| Males |
Females |
|||
|---|---|---|---|---|
| RYR1 mutation | Proband | Relative | Proband | Relative |
| p.Gly341Arg | 8 | 6 | 3 | 11 |
| p.Arg614Cys | 18 | 15 | 12 | 18 |
| p.Arg614Leu | 3 | 2 | 2 | 1 |
| p.Thr2206Met | 11 | 19 | 6 | 17 |
| p.Arg2336His | 2 | 2 | 1 | 3 |
| p.Ala2350Thr | 3 | 2 | 0 | 2 |
| p.Gly2375Ala | 4 | 3 | 0 | 4 |
| p.Gly2434Arg | 9 | 4 | 3 | 5 |
| p.Arg2454His | 4 | 9 | 3 | 10 |
| N | 62 | 62 | 30 | 71 |
| # Exposures | 87 | 110 | 34 | 126 |
| Penetrance | 50 ± 0.11* | 29.7 ± 0.18 | ||
| Exposures | 1 [1-1] | 1 [1-1] | ||
| P MH-all | 0.31 ± 0.09 | 0.19 ± 0.12 | ||
| P MH-probands | 0.71 ± 0.16 | 0.88 ± 0.14 | ||
Sex data were missing in one proband and three relatives. Penetrance and PMH are expressed as mean ± SD; Exposures as median [interquartile range]. MH, malignant hyperthermia; RYR1, ryanodine receptor 1.
P < 0.01.
The overall penetrance of the MH trait was significantly higher in males compared with females (50% [62 of 124] vs. 29.7% [30 of 101]; 95% CI of the difference 7 to 32%, P = 0.002]. Moreover, males had increased odds of developing MH compared with females (odds ratio, 2.37; 95% CI, 1.36 to 4.12) despite similar levels of exposure to trigger anesthetics for both sexes, with an overall rate of 1.6 exposures per subject (table 2).
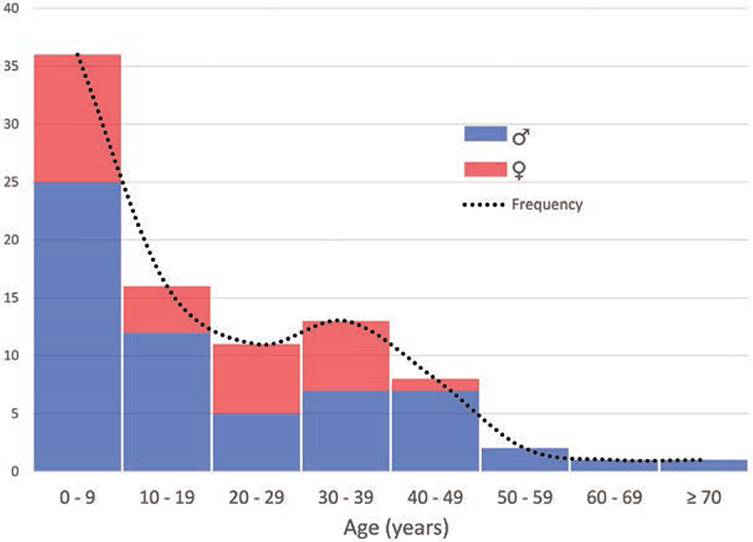
Age distribution in probands was positively skewed (fig. 3), with a median age of 12 yr and an overwhelming majority being younger than 33 yr old (interquartile range 6 to 32.5; table 3). Age was missing for five probands, who were excluded from the MH phenotype severity analysis.
Fig. 3.
Age distribution at the time of malignant hyperthermia (MH) crisis by sex, in MH probands carrying ryanodine receptor 1 mutations.
Table 3.
Proband’s Age at MH Crisis and Clinical MH Indices as per RYR1 Mutation
| RYR1 Mutation | Age (yr) | Onset t (min) | CGS |
|---|---|---|---|
| p.Gly341Arg | 23 | 25 | 55 ± 9 |
| [14–34] | [8–45] | ||
| p.Arg614Cys | 8 | 5 | 47 ± 21 |
| [6–32] | [2–5] | ||
| p.Arg614Leu | 12 | 2 | 46 ± 10 |
| [6–23] | [2–11] | ||
| p.Thr2206Met | 11 | 10 | 48 ± 16 |
| [5–25] | [5–79] | ||
| p.Arg2336His | 9.5 | 10 | 46 ± 11 |
| [7.75–11.25] | — | ||
| p.Ala2350Thr | 31.5 | 60 | 33 |
| [23.75–39.25] | — | ||
| p.Gly2375Ala | 41.5 | 33 | 50 ± 15 |
| [28.5–49.5] | [19–46] | ||
| p.Gly2434Arg | 6 | 12 | 46 ± 20 |
| [5.5–28.5] | [10–80] | ||
| p.Arg2454His | 13 | 98 | 45 ± 20 |
| [8.5–26] | [56–161] | ||
| Overall | 12 | 10 | 50 ± 17 |
| [6–32.5] | [5–60] |
Age and MH onset time are shown as median [interquartile range]; Clinical Grading Scale (CGS) score is expressed as mean ± SD. MH, malignant hyperthermia; RYR1, ryanodine receptor 1.
There were no missing data regarding the anesthetic triggers used in probands. Succinylcholine was used in 76.3% (71 of 93) MH cases, either in combination with volatiles (71%, 66 of 93) or alone (5.4%, 5 of 93), whereas volatile agents were administered without succinylcholine in 23.7% (22 of 93) cases. Succinylcholine use did not differ significantly in male (77.4%, 48 of 62) versus female probands (73.3%, 22 of 30; P = 0.667).
MH Phenotype Severity
We used Clinical Grading Scale scores and MH onset time as quantitative indicators of MH phenotype severity. The median time to MH onset was 10 min (interquartile range 5 to 60) after exposure to trigger anesthetics, whereas the mean Clinical Grading Scale score was 47.9 (SD: 17.1). There was no significant association between the RYR1 genotypes and either indicator of MH phenotype severity (table 3).
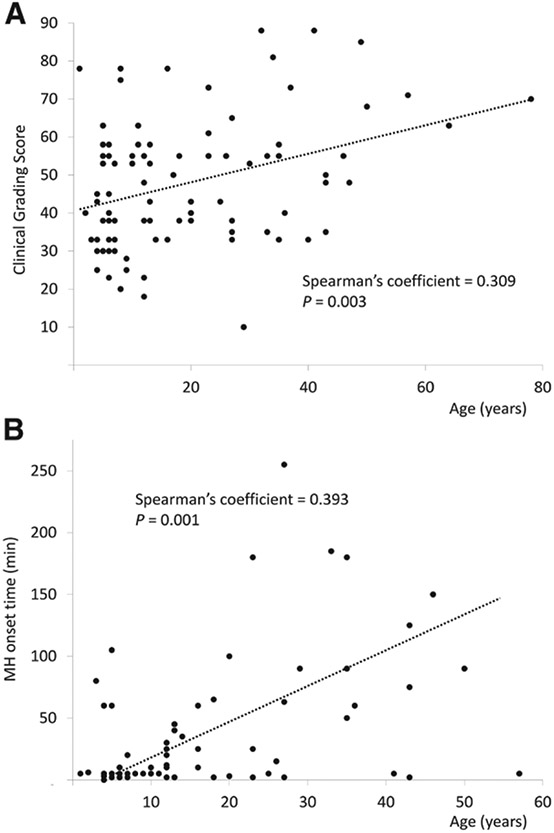
Both Clinical Grading Scale score and MH onset time positively correlated with age (Spearman’s correlation coefficient 0.31, P = 0.003; and 0.39, P = 0.001, respectively), which implies that MH reactions in older patients were identified later and were more severe as per Clinical Grading Scale score (fig. 4).
Fig. 4.
Scatter plot showing a positive correlation between age and (A) the malignant hyperthermia (MH) Clinical Grading Score, or (B) the MH crisis onset time.
Discussion
This multicenter case–control study aimed to assess the penetrance of MH diagnostic RYR1 mutations and examine factors that influence their expression. MH is a rare genetic syndrome with an incidence of 1:35,000 to 1:68,000 MH crises per all surgical discharges,2,32 which makes it challenging for a single MH research center to amass sufficient data for a comprehensive penetrance study. Therefore, we combined clinical and genetic data on 125 European and Canadian MH families.
We quantified the penetrance of nine MH diagnostic mutations to be around 40%. We found that probands’ likelihood to develop MH on exposure to triggers is higher compared with other RYR1 mutation carriers. In fact, most probands in our sample developed MH during their first exposure to general anesthesia. We also found higher penetrance in males, despite similar exposures to triggers in both sexes.
Reduced penetrance is a phenomenon that blurs the distinction between genetically complex disorders and monogenic conditions with Mendelian inheritance, resulting from the interaction of multiple genetic and nongenetic factors that hamper establishing straightforward causation from known genotypes to specific phenotypes.33 Factors influencing the penetrance of a genetic trait include the degree of dysfunction caused by a specific mutation(s), the modulating influence of additional variants on allelic expression, interindividual variations in gene expression, allele dosage causing homozygotes or compound-heterozygotes to have more severe phenotype, age- and sex-specific epigenetic changes such as genomic imprinting leading to the mutually exclusive expression of either the maternal or the paternal allele, and environmental influences such as the diet, alcohol intake, drugs, body habitus, and comorbid disease states. Any of these factors may either ameliorate or exacerbate the impact of the underlying genetic predisposition.29
Concerning MH, although a possible role of allele silencing in relation to other MH loci has not been explored yet, monoallelic silencing does not seem to affect the penetrance of MH-associated RYR1 mutations,34 and the role of allele-specific differences in expression levels of RyR1 transcript remains to be elucidated.22
Our study may lack the necessary power to achieve statistical significance regarding the variation in penetrance among the analyzed RYR1 mutations. However this should not be interpreted as lack of differences in severity of phenotypes. Comparison of in vivo and in vitro in knock-in mouse models of MH demonstrate differences among the p.G2435R,35 p.R163C,36 and p.T4826I variants.37
In a large retrospective study by Carpenter et al.,38 different RYR1 genotypes in a group of MH probands were associated with magnitude of contracture in the in vitro contracture test and serum creatine kinase concentration, and both parameters were also associated with the clinical phenotype severity defined as MH onset time. The functional data from animal models alongside the human data from Carpenter et al. may suggest that the observed differences in severity of phenotype and penetrance among different variants may actually be real.
The Clinical Grading Scale scoring system was conceived to estimate the likelihood that an observed adverse anesthetic outcome was attributable to MH.28 However, because of its post hoc nature, Clinical Grading Scale reliability depends on the availability of clinical data. It may underestimate the likelihood of MH in cases where the crisis is promptly recognized and treated. Despite these, we deemed it reasonable to use Clinical Grading Scale as quantitative indicator of MH phenotype severity because it rates the importance of clinical variables that appear during an MH event by assigning them a score. The positive correlation of both Clinical Grading Scale scores and time of MH onset with the patient’s age observed in this study may suggest that diagnosis tends to be more delayed with increasing age.
We also explored the influence of nongenetic factors, such as sex and age, on MH penetrance. In our pool of probands, there were at least two males for every female, but there was a slightly greater proportion of women among relatives (71 of 133, 53.3%). A bias from sex imbalance would not arise if our study groups were matched according to sex, but then no effect estimate for sex could be derived.
Sex differences pervade the literature on MH, from the earliest epidemiologic reports23,24 to the most recent survey demonstrating that the prevalence of MH in male patients doubles that of females.2 It was also shown that more males than females test positive on the diagnostic contracture test for MH.26 Sex-dependent susceptibility to MH is also present in mouse models.37 Recently, male sex and body build subjectively assessed as muscular have been reported as independent predictors of MH susceptibility.39 However, whether a larger muscle mass is associated with MH sex discrepancy warrants objective assessment. On the whole, the pathomechanism leading to sex differences in the penetrance of MH is still unknown, but epigenetic RYR1 allele silencing has been ruled out as a cause of reduced penetrance of MH susceptibility in females.34
Age distribution in MH probands is known to be positively-skewed with younger people being most affected.1,40 Several studies found that children aged 15 or younger comprised more than 50% of all reactions.1,41,42 Although the majority of cases described here involved also children younger than 15 yr old, our probands’ median age of 12 yr is lower than previously reported.40 This possibly reflects the difference in composition of the investigated cohorts, because the former study included mostly adult patients whose MH status were confirmed by muscle biopsy and contracture testing.
The contrast between the low penetrance of some RYR1 mutations and the high likelihood of probands to develop MH (PMH probands) strengthens the notion of a multifactorial origin of MH, where genetic predisposition is necessary but not sufficient to unleash the MH syndrome. Although co-inheritance of other genetic factors should be taken into account, nongenetic influences, such as the anesthetic technique, the type of surgery, and patient’s comorbidities, may play a pivotal role. In fact, we observed that in our series succinylcholine was used in 76% of MH cases (71% along with volatile anesthetics), which is far above the 10% average use in a typical North American hospital nowadays.43 Because a number of our cases date back to the 1990s, this may reflect the bygone routine use of succinylcholine. Despite lacking relevant data about the use of succinylcholine in the relatives of our probands, the observed bias in the drug use in probands could be explained by the higher occurrence of MH during emergency surgery where succinylcholine is commonly used. Although ear, nose, and throat procedures seem to be the most common reason for surgery in the majority of MH cases in children,2,44 orthopedic surgery and other emergent procedures are preponderant among adults.29,41,45 Anecdotal reports of MH occurring during or shortly after emergent surgery for acute appendicitis are not rare in the medical literature.46-54 It seems thus reasonable to suggest that a 10- to 20-fold increased risk of MH secondary to succinylcholine use42,43 might not be solely attributable to the effects of the drug on the intracellular calcium dynamics of genetically predisposed patients, but also to a threshold shift induced by fever or a concomitant inflammatory process like sepsis or trauma, priming the onset of MH. We deem our observation worthy of further investigation.
There are limitations inherent to our study design. Data collection was retrospective and based on prevalent cases, carrying the risk of recall bias. Although the sample of patients was selected based on all available data, its composition may differ from that of the whole MH population because of participation and ascertainment bias (e.g., identification of a causal mutation in a family preventing further exposures to MH triggers on its members, probands with weaker or abortive reactions remaining unrecognized, lower likelihood of asymptomatic relatives to be tested). We excluded patients with missing data and those with RYR1 variants of unknown significance, which may have affected the overall power of the study. Because data from first- and second-degree relatives only were available for this study, penetrance in higher-order relatives remains an important investigation for the future.
In conclusion, the penetrance of nine MH-diagnostic RYR1 mutations is incomplete and sex-dependent. Among genotype-positive subjects, probands have the highest risk of developing MH on exposure to trigger anesthetics. Young age, male sex, and the use of succinylcholine seem to be major nongenetic risk factors influencing expression of the RYR1 genotypes conferring MH susceptibility in our cohort. Our quantitative evaluation of MH penetrance reinforces the notion that a previous uneventful anesthetic does not preclude the possibility of developing MH.
EDITOR’S PERSPECTIVE.
What We Already Know about This Topic
Malignant hyperthermia is a rare life-threatening disorder triggered in genetically predisposed individuals by exposure to certain anesthetics
The ryanodine receptor 1 (RYR1) gene, which encodes the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum, is the major malignant hyperthermia-associated locus
Malignant hyperthermia diagnostic mutations are more prevalent than the reported incidence of clinical malignant hyperthermia episodes because many mutation carriers are never exposed to anesthetic triggers and some may have several uneventful anesthetics before developing malignant hyperthermia reaction
What This Article Tells Us That Is New.
In a multicenter case-control study of 229 genotype-positive subjects with previous recorded exposure to trigger anesthetics, there were 93 malignant hyperthermia cases, for an overall penetrance for the analyzed RYR1 mutations of 40.6%
The probability of developing malignant hyperthermia on exposure to triggers was 0.25 among all RYR1 mutation carriers and 0.76 in survivors of malignant hyperthermia reactions (95% CI of the difference 0.41 to 0.59)
Young age, male sex, and the use of succinylcholine were major nongenetic risk factors influencing expression of the RYR1 mutations conferring malignant hyperthermia susceptibility
Acknowledgments
The authors offer sincere thanks to Elena Zvaritch, Ph.D., from the Department of Anesthesia, University of Toronto, Toronto, Canada, for critical reading of the manuscript.
Research Support
This research was supported by a merit award from the Department of Anesthesia, University of Toronto, Toronto, Canada (to Dr. Riazi).
Footnotes
Competing Interests
Dr. Riazi received a one-time honorarium from Norgine B.V. (Amsterdam, The Netherlands). The other authors declare no competing interests.
Part of the work presented in this article has been presented at the International Anesthesia Research Society Meeting (IARS) on April 28, 2018, in Chicago, Illinois.
Contributor Information
Carlos A. Ibarra Moreno, Department of Anesthesia, University of Toronto and Malignant Hyperthermia Investigation Unit, Toronto General Hospital, Toronto, Ontario, Canada
Sally Hu, Department of Anesthesia, University of Toronto and Malignant Hyperthermia Investigation Unit, Toronto General Hospital, Toronto, Ontario, Canada
Natalia Kraeva, Department of Anesthesia, University of Toronto and Malignant Hyperthermia Investigation Unit, Toronto General Hospital, Toronto, Ontario, Canada
Frank Schuster, Department of Anesthesia and Critical Care, University of Würzburg, Würzburg, Germany
Stephan Johannsen, Department of Anesthesia and Critical Care, University of Würzburg, Würzburg, Germany
Henrik Rueffert, Department of Anaesthesiology and Intensive Care, Leipzig University Hospital, Leipzig, Germany; Helios Klinik Schkeuditz, Schkeuditz, Germany
Werner Klingler, Academic Hospital Sigmaringen, Sigmaringen, Germany; Experimental Anaesthesiology, Ulm University, Ulm, Germany
Luc Heytens, Department of Anaesthesiology, Antwerp University Hospital, Edegem, Belgium; MH Research Unit, University of Antwerp, Wilrijk, Belgium
Sheila Riazi, Department of Anesthesia, University of Toronto and Malignant Hyperthermia Investigation Unit, Toronto General Hospital, Toronto, Ontario, Canada; Department of Anesthesia and Critical Care, University of Würzburg, Würzburg, Germany
References
- 1.Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K: Malignant hyperthermia: A review. Orphanet J Rare Dis 2015; 10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu Z, Rosenberg H, Li G: Prevalence of malignant hyperthermia diagnosis in hospital discharge records in California, Florida, New York, and Wisconsin. J Clin Anesth 2017; 39:10–4 [DOI] [PubMed] [Google Scholar]
- 3.Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P: Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat 2006; 27:977–89 [DOI] [PubMed] [Google Scholar]
- 4.Stewart SL, Hogan K, Rosenberg H, Fletcher JE: Identification of the Arg1086His mutation in the alpha subunit of the voltage-dependent calcium channel (CACNA1S) in a North American family with malignant hyperthermia. Clin Genet 2001; 59:178–84 [DOI] [PubMed] [Google Scholar]
- 5.Carpenter D, Ringrose C, Leo V, Morris A, Robinson RL, Halsall PJ, Hopkins PM, Shaw MA: The role of CACNA1S in predisposition to malignant hyperthermia. BMC Med Genet 2009; 10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toppin PJ, Chandy TT, Ghanekar A, Kraeva N, Beattie WS, Riazi S: A report of fulminant malignant hyperthermia in a patient with a novel mutation of the CACNA1S gene. Can J Anaesth 2010; 57:689–93 [DOI] [PubMed] [Google Scholar]
- 7.Horstick EJ, Linsley JW, Dowling JJ, Hauser MA, McDonald KK, Ashley-Koch A, Saint-Amant L, Satish A, Cui WW, Zhou W, Sprague SM, Stamm DS, Powell CM, Speer MC, Franzini-Armstrong C, Hirata H, Kuwada JY: Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun 2013; 4:1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed January 3, 2019]. https://www.emhg.org/diagnostic-mutations.
- 9. [Accessed January 3, 2019]. http://exac.broadinstitute.org/gene/ENSG00000196218.
- 10.Riazi S, Kraeva N, Hopkins PM: Malignant hyperthermia in the post-genomics era: New perspectives on an old concept. Anesthesiology 2018; 128:168–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monnier N, Krivosic-Horber R, Payen JF, Kozak-Ribbens G, Nivoche Y, Adnet P, Reyford H, Lunardi J: Presence of two different genetic traits in malignant hyperthermia families: Implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology 2002; 97:1067–74 [DOI] [PubMed] [Google Scholar]
- 12.Ibarra M CA, Wu S, Murayama K, Minami N, Ichihara Y, Kikuchi H, Noguchi S, Hayashi YK, Ochiai R, Nishino I: Malignant hyperthermia in Japan: Mutation screening of the entire ryanodine receptor type 1 gene coding region by direct sequencing. Anesthesiology 2006; 104:1146–54 [DOI] [PubMed] [Google Scholar]
- 13.Wolak S, Rücker B, Kohlschmidt N, Doetsch S, Bartsch O, Zechner U, Tzanova I: Homozygous and compound heterozygous RYR1 mutations: New findings on prevalence and penetrance of malignant hyperthermia [in German]. Anaesthesist 2014; 63:643–50 [DOI] [PubMed] [Google Scholar]
- 14.Hirshey Dirksen SJ, Larach MG, Rosenberg H, Brandom BW, Parness J, Lang RS, Gangadharan M, Pezalski T: Special article: Future directions in maligant hyperthermia research and patient care. Anesth Analg 2011; 113:1108–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britt BA: Malignant Hyperthermia. Boston, MA, Springer, 1987 [Google Scholar]
- 16.Larach MG, Gronert GA, Allen GC, Brandom BW, Lehman EB: Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg 2010; 110:498–507 [DOI] [PubMed] [Google Scholar]
- 17.Brandom BW, Muldoon SM: Unexpected MH deaths without exposure to inhalation anesthetics in pediatric patients. Paediatr Anaesth 2013; 23:851–4 [DOI] [PubMed] [Google Scholar]
- 18.Hopkins PM: Malignant hyperthermia: Pharmacology of triggering. Br J Anaesth 2011; 107:48–56 [DOI] [PubMed] [Google Scholar]
- 19.Visoiu M, Young MC, Wieland K, Brandom BW: Anesthetic drugs and onset of malignant hyperthermia. Anesth Analg 2014; 118:388–96 [DOI] [PubMed] [Google Scholar]
- 20.Migita T, Mukaida K, Kobayashi M, Hamada H, Kawamoto M: The severity of sevoflurane-induced malignant hyperthermia. Acta Anaesthesiol Scand 2012; 56:351–6 [DOI] [PubMed] [Google Scholar]
- 21.Kraeva N, Heytens L, Jungbluth H, Treves S, Voermans N, Kamsteeg E, Ceuterick-de Groote C, Baets J, Riazi S: Compound RYR1 heterozygosity resulting in a complex phenotype of malignant hyperthermia susceptibility and a core myopathy. Neuromuscul Disord 2015; 25:567–76 [DOI] [PubMed] [Google Scholar]
- 22.Grievink H, Stowell KM: Allele-specific differences in ryanodine receptor 1 mRNA expression levels may contribute to phenotypic variability in malignant hyperthermia. Orphanet J Rare Dis 2010; 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Britt BA, Kalow W: Malignant hyperthermia: A statistical review. Can Anaesth Soc J 1970; 17:293–315 [DOI] [PubMed] [Google Scholar]
- 24.Ording H: Incidence of malignant hyperthermia in Denmark. Anesth Analg 1985; 64:700–4 [PubMed] [Google Scholar]
- 25.Mauritz W, Sporn P, Steinbereithner K: Malignant hyperthermia in Austria. I. Epidemiology and clinical aspects [in German]. Anaesthesist 1986; 35:639–50 [PubMed] [Google Scholar]
- 26.Islander G, Rydenfelt K, Ranklev E, Bodelsson M: Male preponderance of patients testing positive for malignant hyperthermia susceptibility. Acta Anaesthesiol Scand 2007; 51:614–20 [DOI] [PubMed] [Google Scholar]
- 27. [Accessed December 23, 2018]. http://varnomen.hgvs.org/recommendations/protein/.
- 28.Larach MG, Localio AR, Allen GC, Denborough MA, Ellis FR, Gronert GA, Kaplan RF, Muldoon SM, Nelson TE, Ording H: A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 1994; 80:771–9 [DOI] [PubMed] [Google Scholar]
- 29.Cooper DN, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H: Where genotype is not predictive of phenotype: Towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet 2013; 132:1077–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendixen D, Skovgaard LT, Ording H: Analysis of anaesthesia in patients suspected to be susceptible to malignant hyperthermia before diagnostic in vitro contracture test. Acta Anaesthesiol Scand 1997; 41:480–4 [DOI] [PubMed] [Google Scholar]
- 31.Møller P, Clark N, Mæhle L: A Simplified method for Segregation Analysis (SISA) to determine penetrance and expression of a genetic variant in a family. Hum Mutat 2011; 32:568–71 [DOI] [PubMed] [Google Scholar]
- 32.Riazi S, Kraeva N, Muldoon SM, Dowling J, Ho C, Petre MA, Parness J, Dirksen RT, Rosenberg H: Malignant hyperthermia and the clinical significance of type-1 ryanodine receptor gene (RYR1) variants: Proceedings of the 2013 MHAUS scientific conference. Can J Anaesth 2014; 6:1040–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dipple KM, McCabe ER: Modifier genes convert “simple” Mendelian disorders to complex traits. Mol Genet Metab 2000; 71:43–50 [DOI] [PubMed] [Google Scholar]
- 34.Robinson RL, Carpenter D, Halsall PJ, Iles DE, Booms P, Steele D, Hopkins PM, Shaw MA: Epigenetic allele silencing and variable penetrance of malignant hyperthermia susceptibility. Br J Anaesth 2009; 103:220–5 [DOI] [PubMed] [Google Scholar]
- 35.Lopez JR, Kaura V, Diggle CP, Hopkins PM, Allen PD: Malignant hyperthermia, environmental heat stress, and intracellular calcium dysregulation in a mouse model expressing the p.G2435R variant of RYR1. Br J Anaesth 2018; 121:953–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang T, Riehl J, Esteve E, Matthaei KI, Goth S, Allen PD, Pessah IN, Lopez JR: Pharmacologic and functional characterization of malignant hyperthermia in the R163C RyR1 knock-in mouse. Anesthesiology 2006; 105:1164–75 [DOI] [PubMed] [Google Scholar]
- 37.Yuen B, Boncompagni S, Feng W, Yang T, Lopez JR, Matthaei KI, Goth SR, Protasi F, Franzini-Armstrong C, Allen PD, Pessah IN: Mice expressing T4826I-RYR1 are viable but exhibit sex- and genotype-dependent susceptibility to malignant hyperthermia and muscle damage. FASEB J 2012; 26:1311–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpenter D, Robinson RL, Quinnell RJ, Ringrose C, Hogg M, Casson F, Booms P, Iles DE, Halsall PJ, Steele DS, Shaw MA, Hopkins PM: Genetic variation in RYR1 and malignant hyperthermia phenotypes. Br J Anaesth 2009; 103:538–48 [DOI] [PubMed] [Google Scholar]
- 39.Butala B, Brandom B: Muscular body build and male sex are independently associated with malignant hyperthermia susceptibility. Can J Anaesth 2017; 64:396–401 [DOI] [PubMed] [Google Scholar]
- 40.Riazi S, Larach MG, Hu C, Wijeysundera D, Massey C, Kraeva N: Malignant hyperthermia in Canada: Characteristics of index anesthetics in 129 malignant hyperthermia susceptible probands. Anesth Analg 2014; 118:381–7 [DOI] [PubMed] [Google Scholar]
- 41.Strazis KP, Fox AW: Malignant hyperthermia: A review of published cases. Anesth Analg 1993; 77:297–304 [DOI] [PubMed] [Google Scholar]
- 42.Klingler W, Heiderich S, Girard T, Gravino E, Heffron JJ, Johannsen S, Jurkat-Rott K, Rüffert H, Schuster F, Snoeck M, Sorrentino V, Tegazzin V, Lehmann-Horn F: Functional and genetic characterization of clinical malignant hyperthermia crises: A multi-centre study. Orphanet J Rare Dis 2014; 9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dexter F, Epstein RH, Wachtel RE, Rosenberg H: Estimate of the relative risk of succinylcholine for triggering malignant hyperthermia. Anesth Analg 2013; 116:118–22 [DOI] [PubMed] [Google Scholar]
- 44.Nelson P, Litman RS: Malignant hyperthermia in children: An analysis of the North American malignant hyperthermia registry. Anesth Analg 2014; 118:369–74 [DOI] [PubMed] [Google Scholar]
- 45.Pinyavat T, Rosenberg H, Lang BH, Wong CA, Riazi S, Brady JE, Sun LS, Li G: Accuracy of malignant hyperthermia diagnoses in hospital discharge records. Anesthesiology 2015; 122:55–63 [DOI] [PubMed] [Google Scholar]
- 46.Liebenschutz F, Mai C, Pickerodt VW: Increased carbon dioxide production in two patients with malignant hyperpyrexia and its control by dantolene. Br J Anaesth 1979; 51:899–903 [DOI] [PubMed] [Google Scholar]
- 47.Stovner J, Innes KR, Holen A: Ten cases of malignant hyperthermia in Norway. Can Anaesth Soc J 1976; 23:518–26 [DOI] [PubMed] [Google Scholar]
- 48.Kemp DR, Choong LS: Malignant hyperthermia and the conscious patient. Aust N Z J Surg 1988; 58:423–7 [DOI] [PubMed] [Google Scholar]
- 49.Denborough MA, Galloway GJ, Hopkinson KC: Malignant hyperpyrexia and sudden infant death. Lancet 1982; 2:1068–9 [DOI] [PubMed] [Google Scholar]
- 50.Ellis FR, Halsall PJ, Harriman DG: Malignant hyperpyrexia and sudden infant death syndrome. Br J Anaesth 1988; 60:28–30 [DOI] [PubMed] [Google Scholar]
- 51.Strecker G, Adnet P, Forget AP, Krivosic-Horber R: Hyperthermie maligne et état infectieux d’origine appendiculaire: Peut-on les differencier en tours d’intervention? [Malignant hyperthermia and appendicular sepsis: Can they be differentiated during surgical procedure?]. Ann Fr Anesth Reanim 1997; 16:234–8 [DOI] [PubMed] [Google Scholar]
- 52.Baek SH, Son GM, Park BS, Park JH, Paik HJ: Malignant hyperthermia followed by rhabdomyolysis during laparoscopic surgery with sevoflurane: 1AP6-3. Eur J Anaesthesiol Supp 2014; 52:20–1 [Google Scholar]
- 53.Kakihara T, Sasaki S, Nakayama H, Watanabe T, Oto H, Ichikawa K, Nakase H: Postoperative malignant hyperthermia following appendectomy. Surgery Curr Res 2014; 4:181 [Google Scholar]
- 54.Toh H, Shahani JM, Ali AJ: Malignant hyperthermia in a young adolescent: A case report. Natl Med J India 2014; 27:259–60 [PubMed] [Google Scholar]