Abstract
Bifurcation coronary artery disease is common as the development of atherosclerosis is facilitated by altered endothelial shear stress. Multiple anatomical and physiological factors need to be considered when treating bifurcation lesions. To achieve optimal results, various stenting techniques have been developed, each with benefits and limitations. In this state-of-the-art review we describe technically important characteristics of bifurcation lesions and summarise the evidence supporting contemporary bifurcation techniques.
Introduction
While atherosclerosis can develop throughout the coronary arterial tree, bifurcations are particularly vulnerable. Haemodynamic shear stress has been identified as an important risk factor for atherosclerosis in addition to traditional systemic comorbidities1,2.
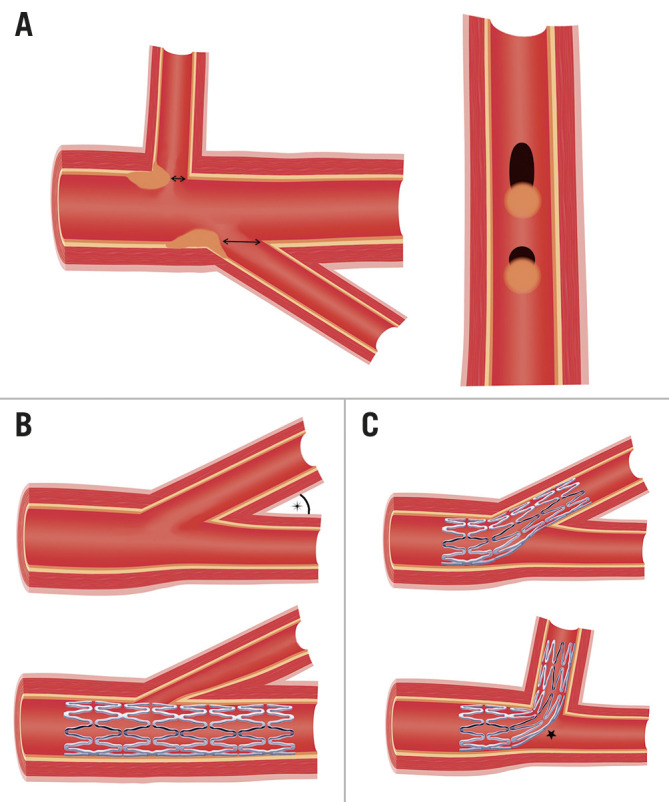
Bifurcations are composed of a proximal main vessel (PMV) that divides into 2 smaller branches, referred to as the distal main vessel (DMV) and side branch (SB). The carina is the flow-dividing septum between the 2 branches. Endothelia at the carina and adjacent inner walls are subjected to higher shear stress and are relatively protected from atherosclerosis. When plaque does develop in this region, it tends to be less voluminous3,4. In contrast, the endothelia of the opposing lateral walls experience low shear stress and oscillatory flow - conditions favouring plaque formation2. These cells demonstrate increased expression of proinflammatory proteins and reduced protective enzymes, resulting in vulnerability to injury5. There are also data suggesting that plaques which develop in regions of low shear stress are more likely to possess vulnerable characteristics such as a thin, fibrous cap and increased inflammation6,7.
These factors mean that bifurcation lesions are common, accounting for approximately 20% of percutaneous coronary interventions (PCIs)8. It is therefore important for the interventionalist to be familiar with the unique challenges of bifurcation disease and the available techniques for treatment. This is especially true as multiple studies show worse procedural and long-term outcomes in this cohort9,10,11.
Bifurcation variables
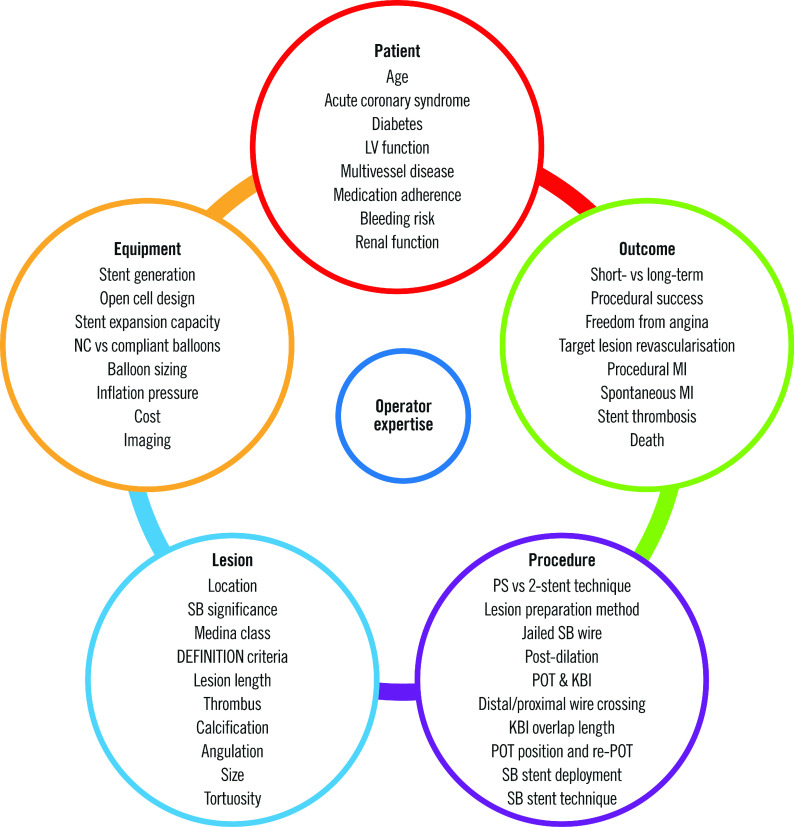
Bifurcation disease may occur at any branch point in the coronary circulation but is most often encountered in the left main coronary artery (LMCA) and left anterior descending coronary artery (LAD). When assessing a bifurcation lesion, numerous factors must be considered (Central illustration). If PCI is clinically indicated, the first decision is whether the SB requires preservation. A significant SB has been defined as one “that you do not want to lose in the global context of a particular patient (symptoms, location of ischaemia, branch responsible for symptoms or ischaemia, viability, collateral vessel, left ventricular function, and so forth)”12. As these factors vary between patients, it is up to the operator to assess the SB and arrive at an appropriate decision. Coronary computed tomography (CT) and invasive fractional flow reserve (FFR) have shown that SB length is a better predictor of myocardial mass supplied than SB diameter13. Only 20% of non-left main SBs supply ≥10% of the overall myocardial mass.
Central illustration. Variables involved in bifurcation PCI.
KBI: kissing balloon inflation; LV: left ventricular; MI: myocardial infarction; NC: non-compliant; POT: proximal optimisation technique; PS: provisional strategy; SB: side branch
Defining bifurcation anatomy by the Medina classification is useful to assess the distribution of disease14. A score of 1 is assigned to any of the PMV, DMV or SB with ≥50% diameter stenosis. Lesions involving both the main vessel (MV) and SB (Medina classification of 0,1,1, 1,0,1 or 1,1,1) have been associated with higher 36-month all-cause mortality and major adverse cardiovascular events (MACE) following treatment with both first- and second-generation drug-eluting stents15,16.
There are many additional variables not described in the Medina classification that also influence outcomes. Calcium remains the nemesis of the interventional cardiologist, and is overrepresented in bifurcation disease, with a recent registry reporting ≥moderate calcification in 33% of cases and increased rates of myocardial infarction (MI) and death within this group17. As there are no randomised trials comparing methods of lesion preparation in calcified bifurcation lesions, operators should use the techniques they are familiar with. Rotational atherectomy for plaque modification is an established therapy18, associated with reduced rates of SB compromise compared to the use of scoring or cutting balloons19. Early data for orbital atherectomy show 30-day MACE and target vessel revascularisation (TVR) comparable to non-bifurcation procedures20, and acceptable 1-year MACE in a high-risk left main cohort21. Intravascular lithotripsy (IVL) offers a novel solution that may be delivered over standard coronary wires and performed with a protective SB wire in place. Hybrid approaches combining rotational atherectomy and IVL have also been reported22.
While the bifurcation angle (defined as subtending the centrelines of the DMV and SB) has important technical implications, a consistent link with clinical outcomes has not been established23. The large COBIS II registry did not show a relationship between the bifurcation angle and rates of SB occlusion24. In contrast, an observational study by Zhang et al found that wider bifurcation angles (>52°) were predictive of occlusion of smaller calibre SBs25. COBIS I identified higher rates of SB stenting in patients with acute angles (<50°), but similar rates of MACE and TLR at 21 months26.
Angulation does appear to influence the suitability and results of different bifurcation techniques. A 90° take-off facilitates the use of T-stenting, whereas a more acute angle will result in a T and protrude (TAP) result. A wide angle has been found to predict poor outcomes after culotte stenting27, potentially due to the higher degree of stent deformation, and is a setting where outcomes may be better with the use of DK-crush28. However, bench studies have also shown the potential for incomplete SB stent apposition with the crush technique in bifurcations >80°29,30 (Figure 1). An earlier study demonstrated increased MACE with crush stenting in bifurcation angles >50°31, but this was not present in a larger study analysing outcomes from the DKCRUSH-I database32. While wider bifurcation angles have been associated with increased MACE and angina in patients treated with either crush or culotte techniques, the outcomes of patients treated with only a single stent appear unaffected33. Expert consensus is that both very narrow and very wide angles increase the likelihood of a poor outcome. The likely mechanisms for this are depicted in Figure 2. From a practical perspective, widely angulated SBs can be challenging for stent delivery and may influence the operator to consider upfront stenting into an SB at high risk of compromise.
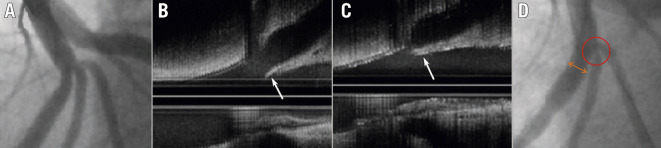
Figure 1. Incomplete SB stent apposition with the crush technique.
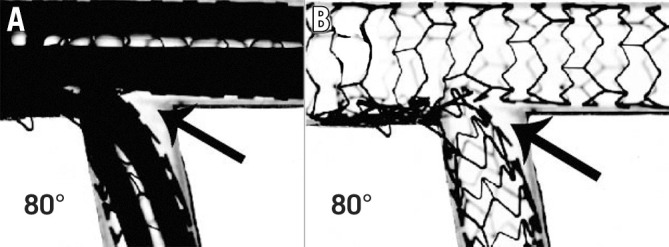
SB stent balloon deformation (A) leading to malapposition of the SB stent following crush in a wide-angle bifurcation (B, arrow). This may be minimised by sequential high-pressure inflations prior to KBI. Note the potential for wiring external to the stent if the SB is entered distally (Adapted from29). KBI: kissing balloon inflation; SB: side branch
Figure 2. Impact of the bifurcation angle.
A) SBs with the same diameter possess ostia of different shapes and areas depending on their angle of take-off. For the same volume of plaque prolapse, the round ostia of wide-angle SBs are more prone to obstruction than the elliptical ostia of acute angle SBs. B) Acute angle bifurcation SBs are more prone to ostial "pinching" from carinal shift if the DMV is over-expanded by stenting. However, due to the elliptical shape and larger areas of these SB ostia, this is less likely to be haemodynamically significant. C) Increased stent distortion is required to accommodate wide-angle bifurcations (star), which may lead to poor stent expansion and malapposition. DMV: distal main vessel; SB: side branch
The incidence of acute SB occlusion with MV stenting has been reported at approximately 8% and is associated with increased rates of cardiac death and MI24. SB ostial stenosis or occlusion occurs due to both carinal and plaque shift. Carinal shift occurs when the MV stent is oversized for the DMV, leading to displacement of the carina into the SB. This effect is more apparent in highly angulated and small-calibre SBs, leading to a thin carinal segment appearing as an "eyebrow" sign on intravascular imaging34 (Figure 3). An intravascular ultrasound (IVUS) study demonstrated that 85% of the loss of SB ostial lumen volume following MV stenting was due to carinal shift35. Importantly, FFR studies have shown that despite significant angiographic appearances, isolated carinal shift is rarely associated with physiological significance36. Plaque shift refers to the displacement of plaque into the SB following stent deployment in the MV. IVUS has shown that plaque shift is associated with PMV plaque volume decrease, suggesting that the shift tends to occur primarily from the PMV35. This is consistent with other reports that PMV disease severity is a strong risk factor for SB compromise24,37. Significant SB FFR decreases are almost always accompanied by plaque shift36.
Figure 3. IVUS imaging demonstrating carinal shift.
A) LAD trifurcation prior to PCI. B) IVUS demonstrating long carina “eyebrow sign” (arrow). C) Displacement of carina (arrow) into SB following MV PCI. D) Ostial diagonal “pinching” due to carinal shift (circle). Note mild oversizing of DMV (double arrow). (Adapted from34). DMV: distal main vessel; IVUS: intravascular ultrasound; LAD: left anterior descending artery; MV: main vessel; PCI: percutaneous coronary intervention; SB: side branch
While IVUS analysis has demonstrated that angiographic ostial SB disease can in fact be a false angiographic appearance generated by MV plaque, SB ostia with true circumferential plaque are much more likely to occlude38. The V-RESOLVE scoring system was developed to quantify the risk of SB occlusion following MV stenting39. The 6 independent predictors identified for occlusion were greater PMV stenosis, MV plaque distribution ipsilateral to SB, reduced MV Thrombolysis in Myocardial Infarction (TIMI) flow before stenting, a bifurcation angle >70°, a larger MV:SB ratio and greater SB stenosis. In a validation cohort, the SB occlusion rate was 26% in the high-risk group and 3.5% in the low-risk group40.
Important additional properties of the SB to consider are the calibre and length of the disease. Coronary arteries follow Murray’s law, where the PMV diameter3 = DMV diameter3 + SB diameter3. Therefore, a significant size mismatch may be present across the bifurcation branches. Accordingly, operators must be aware of the overexpansion limits of their stent platform, especially when deciding upon a 2-stent strategy.
The distal bifurcation is involved in 60-85% of left main disease41 and is a location that is associated with poorer outcomes following PCI42. There are several unique anatomical factors that contribute to this; the vessel calibre is large, and care needs to be taken when sizing as diffuse disease may be present along the entire shaft. Highlighting this fact, IVUS has revealed that ostial LAD lesions are frequently associated with significant distal LMCA disease43, not readily apparent on angiography. Accordingly, rates of TVR following stenting from the left main into the LAD are significantly lower than after ostial LAD stenting alone43,44. There is no true SB from the left main as both daughter vessels are important, and the consequences of occlusion or residual stenosis are clinically significant. Fortunately, the risk of acute vessel occlusion appears reduced compared to non-left main disease24. The angle of separation between daughter vessels is generally larger than in non-LM bifurcations45, and the proximal left circumflex (LCx) is often tortuous. Trifurcation lesions are encountered in approximately 10% of cases, and while technically more complex, long-term outcomes appear similar to those for bifurcation disease46. The optimal technique for trifurcations is unknown. As every additional procedural step and stent insertion increases the chance of complications, operators should deploy the simplest strategy that they are experienced in. Finally, the risk of intraprocedural longitudinal stent deformation needs to be considered due to the proximity of the guide to the proximal stent edge, especially with thin-strut modern stents.
Preparation for bifurcation lesion treatment is important, noting that longer procedural times and greater contrast use are to be expected. While 6 Fr catheters are adequate in most scenarios, the use of 2 simultaneous stents or ≥3 balloons will require a 7 Fr system. Adequate guide support is required as advancing equipment into angulated vessels and through stent cells can be challenging. Low-profile single marker compliant balloons can aid when crossing is difficult. Examples include the Ryurei (Terumo) and Sapphire II Pro (OrbusNeich) families.
Imaging
Due to the importance of adequate lesion preparation and accurate sizing in all 3 limbs of a bifurcation, intravascular imaging is likely to offer even greater benefits than when used in standard lesions. Recent propensity-matched analysis of the unprotected left main cohort within the British Cardiovascular Intervention Society National PCI Audit dataset showed a lower rate of in-hospital MACE (odds ratio [OR] 0.47, 95% confidence interval [CI]: 0.37-0.59) and improved 30-day (OR 0.54, 95% CI: 0.43-0.68) and 12-month (OR 0.66, 95% CI: 0.57-0.77) mortality with imaging use47. Even greater mortality benefit was observed in the bifurcation subgroup who underwent PCI from the left main into the LAD (p for interaction=0.048).
While no specific bifurcation imaging randomised control trials (RCTs) have been published, a 3-year follow-up of the ULTIMATE trial showed a significant reduction of target vessel failure (TVF) with IVUS in the bifurcation subgroup (hazard ratio [HR] 0.48, 95% CI: 0.27-0.87), in concordance with the finding in the overall trial population48. The 5-year results from DKCRUSH-II identified a reduced rate of MI in patients who underwent IVUS assessment (1.8% vs 5.4%; p=0.043)49. A meta-analysis of left main disease has also shown reduced MACE with IVUS guidance50. Dedicated IVUS (DKCRUSH-VIII51) and optical coherence tomography (OCT) (OCTOBER52 and ILUMIEN IV53) bifurcation studies are currently underway. Another more unique application of OCT imaging has been the development of online 3D bifurcation models to confirm distal cell wire crossing. The clinical impact of this will be determined in the OPTIMUM trial54.
As both IVUS and OCT provide information regarding plaque composition and vessel sizing, it is recommended that operators use the modality they are most comfortable with. While OCT offers superior resolution, the limitations of reduced imaging depth, increased contrast use, and difficulty assessing the left main ostium must be kept in mind. It should also be noted that any benefit from imaging is implicit on the operator interpreting and responding to the information to ensure optimal lesion preparation, stent coverage and stent expansion.
Bifurcation strategies
A range of strategies have been developed to tackle bifurcation disease. Many have undergone bench testing in models designed to simulate the elasticity of atherosclerotic arterial walls, or through computer simulations with computational flow dynamics. However, the limitations of these models are important to remember. Coronary disease is seldom distributed in an even circumferential pattern, and calcification, especially, leads to heterogenous arterial compliance and stent expansion that is neither circular nor complete. Stent expansion in vivo into coronary atheroma also provides grip to resist stent migration, which is often seen in bench models. Finally, bench testing is usually performed under direct visualisation, allowing fine control of crossing location and balloon positioning. It is, therefore, not currently possible to recreate true representations of bifurcation strategy performance in vitro.
Provisional strategy
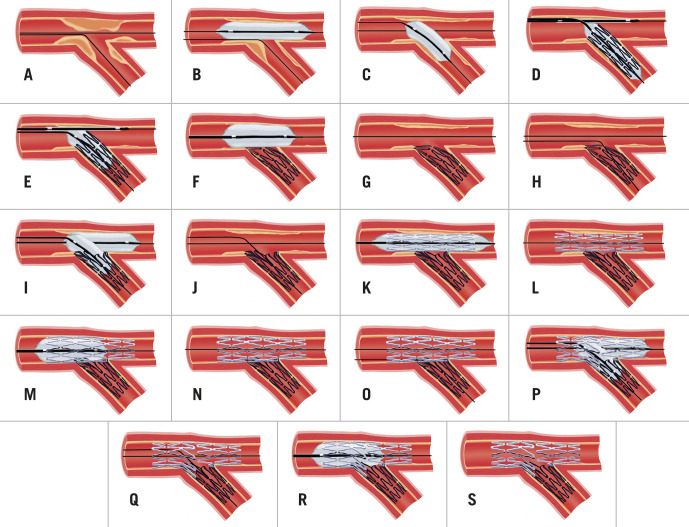
The provisional strategy (PS) is the most versatile technique for addressing bifurcation lesions (Figure 4A-G). Following wire placement into both the DMV and SB, predilation is performed in the MV to ensure adequate lesion preparation. Routine SB predilation is not recommended by the European Bifurcation Club (EBC) due to potential SB dissection and occlusion55. In observational studies, SB predilation has not been demonstrated to influence the incidence of SB occlusion at the end of the case24, but it has been associated with higher crossover to SB stenting with an increased risk of TVR56. An RCT comparing routine SB predilation to none found that SB TIMI flow <3 following MV stent placement was less common in the predilation group (4% vs 18%; p<0.001)57. However, 68% of predilation patients still required SB post-dilation (for SB stenosis >50% or TIMI flow <3), and the time taken to rewire was similar to those patients who had not undergone predilation. Rates of SB stenting were low in both groups, and final SB residual stenosis and TIMI flow were almost identical. A single-centre, observational study has also been performed comparing the strategy of kissing balloon predilation to sequential predilation, identifying a lower rate of TIMI flow <3 in the kissing balloon group and reduced MACE at 6-8 months58. More studies are required prior to recommending this approach.
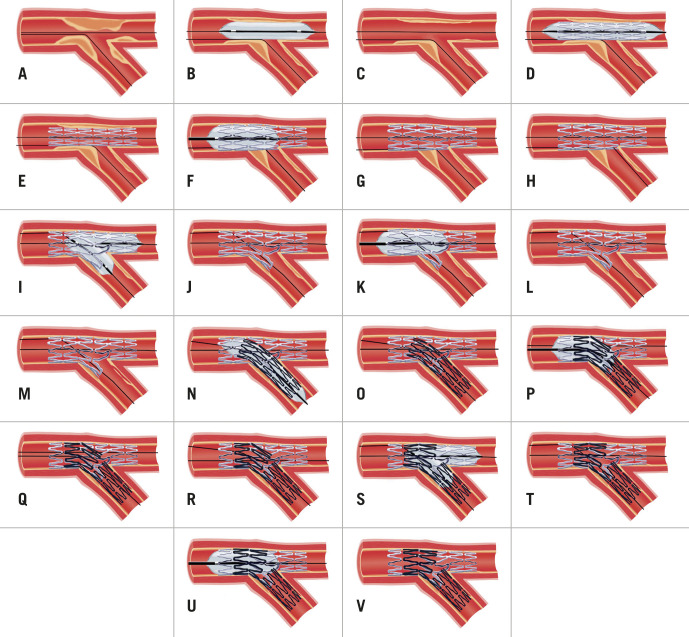
Figure 4. Provisional strategy (A-G), provisional strategy with KBI (A-L) and provisional strategy to DK mini-culotte (A-V).
A) Wiring of bifurcation lesion. B&C) MV predilation with PMV plaque-shift into SB. D&E) MV stent deployment (sized to DMV to avoid excessive carinal shift) with jailed wire in SB. F&G) POT. H) Distal cell rewiring of SB. I&J) NC KBI following initial sequential inflation. K&L) Repeat POT to correct mild deformation of PMV stent. M) SB dissection requiring stent. N&O) SB stent deployment with minimal protrusion into MV. P&Q) Repeat POT. R) Distal cell rewiring of DMV. S&T) NC KBI following initial sequential inflation. U&V) Repeat POT to correct mild deformation of PMV stent. DK: double kiss; DMV: distal main vessel; KBI: kissing balloon inflation; LAD: left anterior descending artery; MV: main vessel; NC: non-compliant; PMV: proximal main vessel; POT: proximal optimisation technique; PS: provisional strategy; SB: side branch
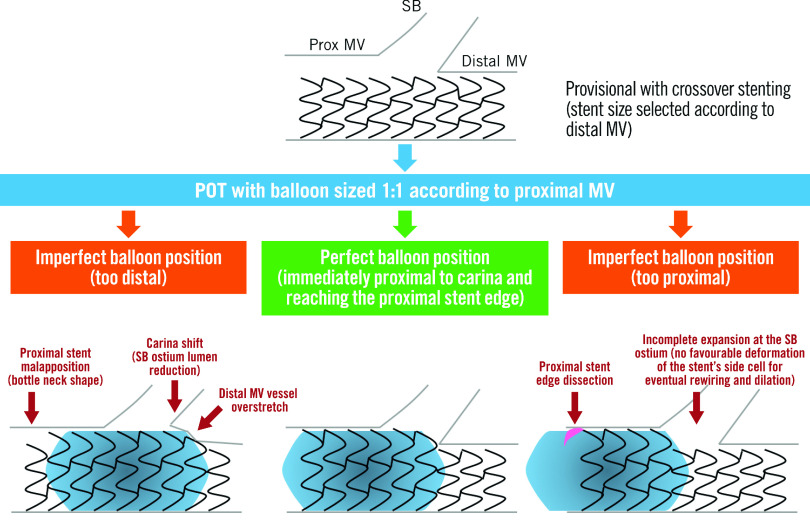
Following lesion preparation, stent sizing for the MV should match the diameter of the DMV landing zone to avoid excessive carinal shift. Due to bifurcation architecture (Murray’s law), this will result in a degree of stent malapposition in the PMV, especially in the setting of a large calibre SB. Accordingly, it is important to subsequently expand the proximal section of stent. This proximal optimisation technique (POT) is undertaken with a short non-compliant (NC) balloon. This will appose the stent and prevent wire passage under the struts during SB rewiring (if required). Appropriate POT positioning places the distal balloon shoulder immediately proximal to the carina and results in a favourable “bay-window” expansion of the stent cells into the SB ostium to facilitate re-entry59 (Figure 5). Unfortunately, the relationship between the balloon shoulder and marker varies between manufacturers, and balloon positioning within the 3-dimensional carina guided by 2-dimensional angiography is challenging. Regardless, the use of POT has been linked to significant reductions in 12-month TLF and stent thrombosis60.
Figure 5. Importance of appropriate POT balloon placement (reproduced from55).
MV: main vessel; POT: proximal optimisation technique; SB: side branch
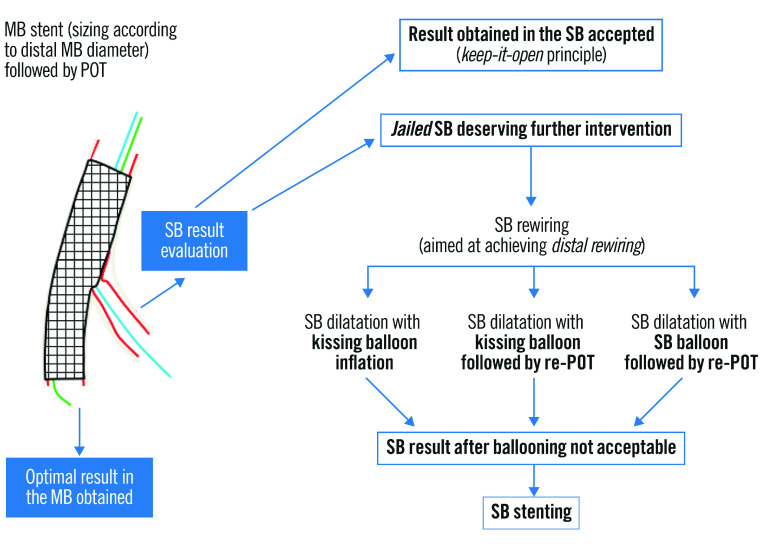
Following POT, a decision must be made regarding further intervention on the SB (Figure 6). FFR analysis has revealed that most jailed SBs do not experience physiologically important flow disturbance and that angiographic severity of ostial lesions correlates poorly to significant FFR values61. In DKCRUSH-VI, using an FFR <0.80 to guide SB intervention rather than an ostial stenosis of >70% resulted in a significant reduction of SB stent implantation without any worsening of MACE62. In the absence of reduced TIMI flow, routine SB balloon dilation has not shown any clinical benefit. The NORDIC-Baltic Bifurcation Study III found no improvement in 6-month MACE in patients assigned to routine final kissing balloon inflation (KBI), but also no penalty63. A reduced rate of SB stenosis >50% at 8 months was seen in patients with true bifurcation disease who underwent KBI (7.6% vs 20%; p=0.02), but this did not impact clinical outcomes. The THUEBIS study randomised patients to mandatory SB intervention, or intervention only in the event of TIMI flow <264. Final SB TIMI 3 flow was similar between both groups, with no difference in 6-month MACE or need for SB PCI during follow-up. The SMART-STRATEGY RCT compared SB intervention for TIMI flow <3 vs >75% stenosis for non-LMCA bifurcations, and >75% stenosis vs >50% stenosis for LMCA bifurcations65. The 3-year follow-up found reduced rates of TVF (11.7% vs 20.8%; p=0.049) and cardiac death or MI (0.8% vs 6.2%; p=0.036) with the more conservative strategy. The need for a second stent was higher with more aggressive SB intervention (30% vs 7%) and associated with an HR of 5.42 (95% CI: 2.03-14.5) for TVF66. Contrastingly, the PROTECT-SB RCT of 113 patients with non-left main bifurcation disease treated with second-generation stents and low rates of 2-stent implantation found no difference in MACE with routine KBI at 3 years67.
Figure 6. Possible outcomes of the provisional strategy following MV stent placement and POT (adapted from59).
MB: main branch; MV: main vessel; POT: proximal optimisation technique; SB: side branch.
p>It should be noted that data beyond 12 months are still limited, so it remains unclear whether routine SB dilation through KBI carries any benefit or harm in the longer term. An observational OCT study at 6-12 months after stent implantation showed increased rates of uncovered struts with subclinical thrombus attachment in patients who did not receive KBI68. Based on available studies, our recommendation is for non-LMCA SB KBI in the settings of TIMI flow <3 and/or acute symptoms or signs of ischaemia. For the LMCA bifurcation, >50-75% residual ostial LAD or LCx stenosis should undergo KBI, especially if PCI may be required in the future.
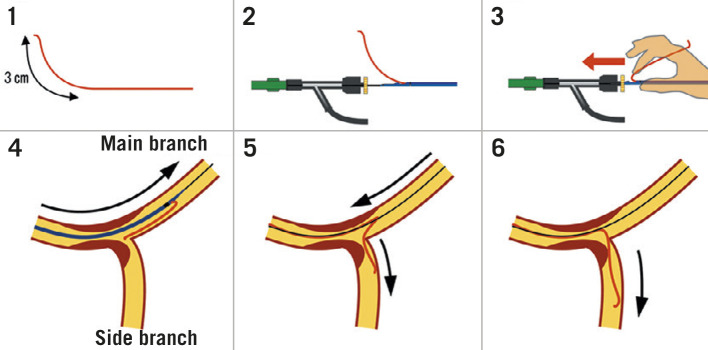
If SB intervention is required (Figure 4H-L), the combination of POT and a large bend on a new coronary wire should allow knuckled entry into the MV stent with minimal chance of wire misadventure outside the stent lumen. Rewiring of the SB should then be via the most distal cell; this is best achieved by withdrawing the angulated wire slowly from the DMV and allowing it to drop into the SB at the carina. Once the wire tip engages the SB, torque in the opposite direction is often required with gentle wire advancement to prevent wire prolapse back into the DMV. Balloon trackability tends to be optimised when crossing distally, and dilation through this cell minimises the metallic neo-carina formed and allows for optimal scaffolding of the SB ostium. This, in turn, improves the chances of T-stenting with minimal protrusion, if required. In the event of difficult SB rewiring, low tip-load polymer-jacketed wires such as the Whisper MS or Pilot 50 (both Abbott) are recommended. Occasionally stiffer hydrophilic wires or more directional wires may be needed to regain access to a calcified jailed ostium. Examples include the Pilot 200 (Abbott), Ultimate 3 or Gaia Second (both Asahi Intecc). Dual lumen microcatheters can also improve wire control and support69. An additional technique involves making a smooth reverse bend in a hydrophilic wire and inserting it ahead of the dual lumen microcatheter side-port (Figure 7). The system can be advanced beyond a highly angulated SB and then withdrawn into it.
Figure 7. Dual lumen microcatheter facilitated reverse wire technique.
1) In addition to standard tip shaping, a smooth bend is created approximately 3 cm from the tip of a polymer-jacketed wire. 2&3) The MV wire is introduced into the distal microcatheter port. The SB wire is advanced out of the side port and inserted into the Y-connector ahead of the microcatheter. 4) The microcatheter is advanced beyond the bifurcation. 5) Careful simultaneous retraction of the microcatheter and SB wire will lead to intubation of a highly angled SB. 6) The wire may then be advanced and the microcatheter removed. (Adapted from69). MV: main vessel; SB: side branch
The traditional method of SB dilation has been with KBI. Without KBI, isolated SB dilation can displace stent struts opposite to the carina and compromise MV stent performance70. Despite this concern, 1 randomised trial comparing KBI to single balloon SB dilation alone in 244 patients could not identify any benefit in clinical endpoints at 12 months4. To perform KBI a 2-stage process is preferred, starting with sequential higher-pressure dilation of the DMV and SB, with NC balloons sized according to the distal reference diameters to ensure full expansion. A lower-pressure simultaneous inflation is then performed to centralise the neo-carina while maintaining stent apposition. The balloons should be deflated simultaneously to preserve this modified architecture. KBI has been found to be effective at restoring SB FFR ≥0.75 in 92% of patients with an SB FFR <0.75 following MV stenting61. NC balloons are preferred as they are resistant to deformation and overexpansion. The COBIS II bifurcation registry reported reduced rates of SB dissection (0.1% vs 1.1%; p=0.046) and a lower risk of MACE (HR 0.64, 95% CI: 0.46-0.91; p=0.01) when NC balloons were used for KBI71.
Despite favourable carinal modification, KBI causes elliptical distortion of the PMV stent and can induce stent malapposition72. The COBIS registry identified a higher incidence of MACE and TLR in patients undergoing KBI, with most TLR occurring in the MV (HR 3.39, 95% CI: 1.86-6.19 for KBI group)73. A meta-analysis of 5 randomised studies showed that KBI significantly reduced SB restenosis (OR 0.44, 95% CI: 0.30-0.64) at the cost of increased MV restenosis (OR 2.96, 95% CI: 1.74-5.01 )74. Finally, 5-year follow-up of DKCRUSH-II revealed significantly greater TLR in PS patients who underwent KBI (19.4% vs 5.2%; p=0.036)49. One method of minimising KBI-induced stent distortion is to use short balloons to reduce the length of balloon overlap into the PMV75. A final POT should also be performed to remould the proximal segment of the stent, although a degree of residual elliptical distortion may persist76. This repeat POT (re-POT) should be positioned proximal to the carina to avoid displacement.
Due to the limitations of KBI, an alternative strategy of POT, SB-only dilation and re-POT has been proposed. Bench tests have showed encouraging mechanical results77. However, this technique is highly dependent on achieving both optimal POT placement and distal cell crossing, which may be difficult in practice. The final POT should cover the SB ostium to address opposing wall stent malapposition induced by SB dilation. In the event of a proximal cell crossing into the SB, this can lead to adverse neo-carinal shift78. Nevertheless, OCT of 106 patients treated with this technique showed excellent stent apposition and a low percentage of SB ostial obstruction, with only 1 TLR episode at 6 months79. The development of ultra-short SB-dedicated balloons may assist in minimising MV stent deformation with SB ballooning80 and further improve outcomes without necessitating KBI.
It is recommended that the SB wire is jailed until POT and rewiring has been successfully completed81. While deploying the MV stent with a jailed SB wire has not been shown to reduce rates of SB occlusion, it has been associated with increased rates of flow recovery24 , favourable optimisation of bifurcation geometry for SB reaccess and acts as a marker for the SB origin. Extra support is also provided for SB balloon delivery. Finally, jailed wires allow a bailout in case of inability to rewire the SB by providing a track for a low-profile balloon to be passed and dilated for flow restoration. It is important to note that cases of jailed wire entrapment and fracture have been described. The degree of jailed wire damage on removal has been linked to the length the of entrapped wire, rather than vascular calcification or stent deployment pressure82. To avoid an excessive length of jailed wire looping around the MV stent, a final small retraction of the SB wire has been suggested prior to stent deployment83. If resistance is encountered during jailed wire removal, care should be taken to avoid deep guide engagement and excessive force on the wire. The advancement of a balloon or microcatheter over the wire can isolate traction onto the trapped segment. Polymer-jacketed wires may be an attractive option as they are more efficient in crossing SB ostia and sustain less overt structural trauma when jailed82. They have also been reported to cause less damage to the stent coating on wire withdrawal84. However, electron microscopy has revealed a loss of polymer coating from these wires85, and there have been contradictory reports regarding the clinical significance of this82,86. Alternative techniques with jailed balloons87 and microcatheters88 to protect SB patency have also been described in small studies.
The development of drug-coated balloons (DCBs) has introduced another variable that could improve outcomes with provisional bifurcation stenting. A 2018 meta-analysis of 3 RCTs and one observational study found that DCB use in SBs was associated with reduced late lumen loss, when compared to standard balloons, at 9 months89. These results were consistent with the BEYOND RCT, published in 2020, that randomised 222 patients with true bifurcation disease to DCB or standard balloon SB angioplasty following KBI90. SB diameter stenosis was less at 9 months in the DCB cohort (29% vs 40%; p<0.0001), but clinical outcomes were unchanged.
In the event of persistent SB TIMI flow <3, FFR-significant disease or extensive dissection of the SB following KBI, SB stenting should be undertaken. Reported rates of SB stenting vary widely, likely due to different thresholds for KBI and balloon sizing. In the 2 EBC randomised trials of provisional versus dual-stenting strategies, SB stenting rates were low at 1 in 691 and 1 in 592, respectively. If SB stent placement is required, multiple strategies are available, with common options including T-stenting, TAP and culotte. These were investigated in the BBK II RCT, which assigned 300 patients who required SB stenting following a planned PS (for extensive dissection, TIMI<3 or ≥75% stenosis) to culotte or TAP techniques93. Nine-month angiographic follow-up revealed an increased binary restenosis rate in the TAP group (17% vs 6.5%; p=0.006), predominantly due to differences in the SB result, whilst MACE was unchanged. The culotte technique may, therefore, be the preferred option when anatomically suitable.
Two-stent strategies
Multiple systematic 2-stent strategies have been described, with DK-crush and culotte the most widely used. The selection of strategy is partly determined by the pattern of bifurcation disease. If there is minimal concern regarding SB access, the MV may be treated first, followed by T/TAP or culotte stenting. In the event of expected difficulties with SB access, the SB should be secured first, followed by DK-crush, reverse-culotte or reverse-T.
DK-crush
The elegant double kiss modification to the original crush technique (Colombo et al94) was described by Chen et al in 200595. It had been recognised that final KBI was essential to achieve optimal SB stent expansion, but due to the presence of 2 layers of struts across the SB ostium, rewiring and balloon delivery was often not possible. Even once delivered, balloon expansion was frequently suboptimal96. To address this limitation, a sequential process of SB optimisation by a double kiss was described. In the initial report, the success rate of final KBI was significantly greater with double kissing (100% vs 80%; p<0.01)95. The multicentre DKCRUSH-I RCT compared clinical outcomes between DK-crush and classic crush in 311 patients97. The importance of final KBI was highlighted by the fact that the 76% of classic crush patients who achieved final KBI had significantly lower rates of stent thrombosis than those who did not (1.7% vs 5.1%; p<0.001). All DK-crush patients achieved final KBI and had a reduced rate of MACE at 8 months (11.4% vs 24.4%; p=0.02).
To perform a DK-crush, both the MV and SB are accessed, and predilation is performed to ensure lesion preparation (Figure 8). A stent is then advanced into the SB and a balloon sized to at least the DMV is positioned in the MV across the SB ostium. The SB stent is then delivered with approximately 2 mm of protrusion into the MV. To optimise ostial stent expansion, the stent delivery balloon can then be withdrawn slightly into the PMV and reinflated to a high pressure, and/or further NC balloon dilation can be performed98. After complete withdrawal of the stent delivery balloon and wire, the MV balloon is inflated to compress the protruding stented segment. It is also recommended to perform a further crush with a short NC balloon sized to the PMV. This is followed by rewiring the SB. Unlike in the provisional or culotte strategies, rewiring should be through a proximal strut. This is because the process of crushing the SB stent can cause malapposition at the distal SB wall, and inadvertent wiring of this space will result in incomplete SB coverage (Figure 1). Once wiring is accomplished, the first KBI is performed. This opens the SB ostium whilst simultaneously deflecting the neo-carina away from the MV lumen. A stent is then positioned in the MV (sized to the DMV), and the SB wire is removed prior to deployment. After stent deployment, POT is performed. The SB is rewired and a final KBI and re-POT completed.
Figure 8. DK-crush technique.
A) Wiring of bifurcation lesion. B&C) MV and SB predilation. D&E) SB stent deployment with crush balloon positioned in MV. Stent balloon reinflated to higher pressure more proximally. F&G) Crush balloon inflation with deformation of SB stent. H) Proximal cell rewiring of SB stent, away from region of malapposition. I&J) KBI following initial sequential inflation. K&L) MV stent deployment, sized to DMV to avoid carinal shift. M&N) POT. O) Proximal cell rewiring of SB stent. P&Q) KBI following initial sequential inflation. R&S) Re-POT to correct mild deformation of PMV stent. DK: double kiss; DMV: distal main vessel; KBI: kissing balloon inflation; MV: main vessel; PMV: proximal main vessel; POT: proximal optimisation technique; SB: side branch
It is recommended that balloon dilations are done with short NC devices at high pressure. Bench testing has demonstrated that at wider bifurcation angles, KBI alone may not achieve full expansion of the SB29. Therefore, sequential high-pressure dilations into the DMV and SB prior to a lower pressure KBI may offer benefit, as with culotte.
Technical advantages of DK-crush over other 2-stent strategies include maintenance of the MV wire positioning throughout the entire procedure and the ability to accommodate a significant size mismatch between the PMV and SB without the need for stent overexpansion.
Culotte
Culotte was first described by Chevalier et al in 199899 and developed to ensure complete coverage of the bifurcation. The technique involves the wiring of both branches, followed by the predilation and stenting of the MV. Enough PMV coverage for POT is required, which is performed prior to the rewiring of the SB through a distal cell. Following balloon dilation into the SB, a stent is delivered with some protrusion into the MV. POT of the protruding segment is then performed. The DMV is then rewired, once again via a distal cell. KBI is used, followed by a final POT to optimise the result.
Several modifications have since been described to improve outcomes (Figure 4M-V), although clinical data are lacking. The ‘mini-culotte’ refers to reduced protrusion of the second stent into the PMV to minimise the length of double-stent layering100. The ‘DK culotte’ introduces a KBI after the first rewiring101, in recognition of the beneficial effects of double kissing in DK-crush. In bench testing this has been found to minimise neo-carinal formation and reduce the degree of stent malapposition and SB ostial stenosis102.
The culotte avoids formation of a triple-layer stent segment in the PMV, although an area of double-stent layers is present. When performed optimally, the metal neo-carina is minimised. It is not well suited to bifurcations with very wide angles or when there are large calibre differences between the PMV and daughter branches.
KBI with 2-stent strategies
While the role of routine KBI has not been established for the PS, it is a mandatory part of all 2-stent strategies and has consistently been shown to improve clinical outcomes75,97,99. Difficulty in crossing a stent cell with a balloon may be solved by using low-profile single-marker balloons. Re-POT and wiring of a different cell to change the balloon approach angle can also assist. If resistance to passing the balloon occurs prior to reaching the carina, either wire wrap or wire passage under the proximal stent edge should be considered. As a practical tip, it is often easier to advance NC kissing balloons simultaneously through the guide (holding the devices together), rather than sequentially.
Comparisons between bifurcation strategies
Trials comparing bifurcation strategies are difficult to perform for multiple reasons. Each technique has undergone many subtle iterations to optimise results, and heterogeneity in implementation exists between operators. Thresholds for KBI and rates of SB stent placement following provisional MV stenting vary widely and may influence the results. Drug-eluting stents have also undergone generational improvements between studies. Medical therapy has evolved with higher potency antiplatelet agents and statins now available, and the duration of dual antiplatelet therapy (DAPT) differs between trials. As MACE rates have subsequently declined, studies are often underpowered for clinical outcomes. Observational trials are prone to bias with 2-stent strategies generally preferred for more complex disease. Trials also vary in their inclusion criteria, and caution must be taken when generalising results. The significance of endpoints such as TLR can be difficult to interpret as the threshold for revascularisation varies between centres and can be influenced by the use of routine angiographic follow-up. Follow-up has also, generally, been limited to <5 years, so truly long-term outcomes are unknown. Finally, it is unlikely that any 1 technique offers the best results for all bifurcations. Nevertheless, here we discuss the evidence from the major RCTs investigating this area (Table 1, Table 2).
Table 1. General bifurcation RCTs (PS vs 2-stent).
| Variable | Nordic | BBK | CACTUS | BBC ONE | EBC TWO | Nordic-Baltic IV | Lin et al | DK CRUSH II | |
|---|---|---|---|---|---|---|---|---|---|
| n | 413 | 202 | 350 | 500 | 200 | 450 | 108 | 370 | |
| Stable/ACS | Stable/unstable angina or silent ischaemia | Stable angina or positive stress test | Stable/unstable angina or silent ischaemia | Stable/unstable angina or ACS (excluding STEMI) | Stable/unstable angina or ACS (excluding STEMI) | Stable/unstable angina or silent ischaemia | Stable/unstable angina or silent ischaemia | Stable/unstable angina or ACS. Includes CTO | |
| LMCA | 2.0% | 0.0% | 0.0% | 0.0% | 0.0% | 2.0% | 0.0% | 16.8% | |
| Stent generation | 1st | 1st | 1st | 1st | 2nd | 1st and 2nd | 1st | 1st | |
| Anatomy | Any bifurcation | Any bifurcation |
94% Medina 1,1,1/1,0, 1/0,1,1 |
82% Medina 1,1,1/1,0, 1/0,1,1 |
100% Medina 1,1,1/1,0, 1/0,1,1 |
100% Medina 1,1,1/1,0, 1/0,1,1 |
100% Medina 1,1,1/1,0, 1/0,1,1 |
100% Medina 1,1,1/0,1,1 | |
| Average dimensions | |||||||||
| PMV (SD) | 3.3 mm (0.4) | 3.1 mm (0.4) | 2.8 mm (0.3) | ≥2.5 mm* | 3.0 mm (0.3)** | 3.2 mm (0.5) | 4.0 mm (0.4) | 2.8 mm (0.3) | |
| SB (SD) | 2.6 mm (0.5) | 2.4 mm (0.3) | 2.2 mm (0.3) | ≥2.25 mm* | 2.7 mm (0.3)** | 2.4 mm (0.5) | 2.8 mm (0.2) | 2.3 mm (0.3) | |
| SB disease length (SD) | 6.2 mm (4.8) | 10.2 mm (4.2) | 5.8 mm (4.5) | 16 mm (5)*** | 10.3 mm (7.2) | 7.1 mm (4.5) | 12.8 mm (2.9) | 15.2 mm (11.9) | |
| PS | SB predilation | NS | NS | 90.8% | NS | NS | 64.2% | NS | 36.8% |
| KBI | 32.0% | 100.0% | 90.2% | 26.0% | 94.0% | 36.1% | 94.4% | 79.5% | |
| SB stented | 4.3% | 19.0% | 31.0% | 3.0% | 16.0% | 3.7% | 16.7% | 28.6% | |
| 2-stent | Crush var | (Crush) 50.0% | – | (Crush) 100.0% | (Crush) 68.0% | – | (Mini) 21.0% | (DK) 65.0% | (DK) 100.0% |
| Culotte | 21.0% | – | – | 30.0% | 100.0% | 66.0% | 25.0% | – | |
| Other | 29.0% | T (100.0%) | – | – | – | 13.0% | 10.0% | – | |
| SB stented | 95.1% | 98.2% | 100.0% | 98.0% | 97.0% | 96.5% | 94.4% | 100.0% | |
| FKBI | 74.0% | 100.0% | 92.1% |
72.0% crush/ 89.0% culotte |
96.0% | 91.2% | 90.7% | 100.0% | |
| MACE definitions | Cardiac death, non-procedural MI, or TVR | Cardiac death, non-procedural MI, or TLR | Cardiac death, MI (including periprocedural), or TVR | Cardiac death, MI (including periprocedural), or TVR | Cardiac death, MI (including periprocedural), or TVR | Cardiac death, non-procedural MI, and TLR | Cardiac death, MI (including periprocedural), or TVR | Cardiac death, MI (including periprocedural), or TVR | |
| Last reported outcomes (PS vs 2-stent) |
5 years: 15.8% vs 21.8%; p=0.15 |
5 years: 22.8% vs 22.9%; p=0.91 |
6 months: 15.0% vs 15.8%; p=0.95 |
9 months: 8.0% vs 15.2%; p=0.009 Driven by excess procedural MI |
12 months: 7.7% vs 10.3%; p=0.53 |
2 years: 12.9% vs 8.4% (HR 0.63, 95% CI: 0.35 to 1.13; p=0.12) |
8 months: 38.9% vs 11.1%; p<0.01 Driven by TLR |
5 years: 23.8% vs 15.7%; p=0.051 Trend driven by TLR |
|
| *as per inclusion criteria. **ave DES diameter. ***ave DES length. ACS: acute coronary syndrome; CI: confidence interval; CTO: chronic total occlusion; DES: drug-eluting stent; DK: double kiss; EBC: European Bifurcation Club; HR: hazard ratio; (F)KBI: (final) kissing balloon inflation; LMCA: left main coronary artery; MACE: major adverse cardiovascular events; MI: myocardial infarction; NS: non-significant; PMV: proximal main vessel; PS: provisional strategy; RCT: randomised control trial; SB: side branch; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction; TLR: target lesion revascularisation; TVR: target vessel revascularisation | |||||||||
Table 2. LMCA bifurcation RCTs (PS vs 2-stent).
| Variable | DK-CRUSH V | EBC MAIN |
|---|---|---|
| n | 482 | 467 |
| Stable/ACS | Stable/unstable angina or silent ischaemia or MI >24 hr before treatment | Stable angina or silent ischaemia or ACS (excluding STEMI <72 hr before treatment) |
| Trifurcations | 17.8% | 5.0% |
| Stent generation | 2nd | 2nd |
| Anatomy | Medina 1,1,1/0,1,1 | Medina 1,1,1/0,1,1 |
| Mean SYNTAX (SD) | 31 (8) | 23 (6) |
| Average dimensions | ||
| PMV (SD) | 3.1 mm (0.5) | 3.8 mm (0.7) |
| SB (SD) | 2.7 mm (0.4) | 2.7 mm (0.6) |
| SB disease length (SD) | 16.4 mm (13) | 6.9 mm (4.9) |
| PS | ||
| SB predilation | NS | 49.0% |
| KBI | 78.9% | 89.0% |
| SB stented | 47.1% | 22.0% |
| 2-stent | ||
| SB stented | 100.0% | 94.0% |
| DK-crush | 100.0% | 5.0% |
| Culotte | – | 53.0% |
| T/TAP | – | 33.0% |
| FKBI | 99.6% | 93.0% |
| MACE definitions | Cardiac death, target vessel MI or clinically driven TLR | All-cause death, MI, or TLR |
| Last reported outcomes (PS vs 2-stent) |
12 months: 10.7% vs 5.0%; p=0.02 Driven by TVMI (ST) 3 years: 16.9% vs 8.3%, p=0.005 Driven by TVMI (ST) and TLR |
12 months: 14.7% vs 17.7%; p=0.34 |
| ACS: acute coronary syndrome; DK: double kiss; EBC: European Bifurcation Club; (F)KBI: (final) kissing balloon inflation; LMCA: left main coronary artery; MACE: major adverse cardiovascular events; MI: myocardial infarction; PMV: proximal main vessel; PS: provisional strategy; RCT: randomised control trial; SB: side branch; SD: standard deviation; ST: stent thrombosis; STEMI: ST-segment elevation myocardial infarction; TAP: T and protrude; TLR: target lesion revascularisation; TVMI: target vessel myocardial infarction | ||
Studies addressing predominantly non-LMCA bifurcations
The Nordic I bifurcation study compared an upfront 2-stent strategy to the PS in 413 patients with all bifurcation types103. A range of 2-stent techniques were used with first-generation stents. Only 4% of the PS group proceeded to SB stenting due to the very high threshold of TIMI 0 flow for this step. The 2-stent strategy showed significantly longer procedural times and higher procedural-related biomarker increases. MACE at 6 months was similar (3.4% vs 2.9%) and the 8-month quantitative angiography showed no difference in the angiographic endpoint of >50% MV stenosis and/or SB occlusion (5.1% vs 5.3%) between the 2-stent or PS groups. Five-year outcomes were also consistent, showing no difference in mortality, MI or TVR104.
The BBK trial assessed routine T-stenting compared to the PS plus routine KBI in 202 patients105. Only 68% of patients had a Medina classification of 1,1,1/1,0,1/0,1,1. KBI was performed in all patients and 19% of PS patients proceeded to T-stenting. Binary restenosis rates in either the MV or SB were not different between the 2 groups at 9 months. Increased initial luminal gain with T-stenting was countered by increased in-stent luminal loss at follow-up. MACE at 1 year was also not different (12.9% vs 11.9%; p=0.83), a finding that was similar at 5 years (22.8% vs 22.9%; p=0.91)106.
The CACTUS trial compared the classic crush strategy to the PS in 350 patients107. In total, 94% of patients had true bifurcation disease. The rates of KBI were >90% in both groups, and 31% of PS patients required SB stent placement. MACE at 6 months was not significantly different (15.8% vs 15%; p=0.95). While diameter stenosis of the SB was less in the crush group immediately following the procedure, this was no longer significant at 6 months.
The BBC ONE trial randomised 500 patients to either the PS with optional KBI or upfront crush/culotte with mandatory final KBI108. A total of 26% of PS patients underwent KBI, and only 3% progressed to T-stenting. Despite the focus on final KBI, only 72% of crush and 89% of culotte cases completed successful KBI. At 9 months, an increased incidence of MACE was present in the 2-stent group (15.2% vs 8.0%; p=0.009), driven by excess MI (predominantly periprocedural; 11.2% vs 3.6%; p=0.001). Improvement in angina and quality of life was significant in both groups109. Five-year pooled patient-level data combining outcomes from the NORDIC I and BBC ONE trials show lower mortality with the PS compared to systematic dual-stenting110.
The above studies collectively identified clinical neutrality or potential harm when using routine 2-stent strategies in smaller calibre bifurcations, where SB disease was less likely to be functionally significant. The mean SB disease length was generally ≤10 mm and first-generation stents were used.
The EBC TWO study addressed some of these limitations by comparing the PS to the culotte technique in 200 patients with true bifurcation lesions, with branch diameters ≥2.5 mm and SB lesion lengths ≥5 mm91. Rates of KBI were ≥95% in both groups, with 16% of the PS proceeding to a second stent. MACE at 12 months was similar between the PS and culotte (7.7% vs 10.3%; p=0.53), as was relief from angina, although the trial was underpowered due to a lower-than-expected event rate.
The Nordic-Baltic Bifurcation Study IV included 450 patients with true bifurcation disease, visual SB diameters ≥2.75 mm and SB lesion lengths up to 15 mm111. Both visual and quantitative comparative analysis (QCA) measurements were reported, and notably the average SB diameter by QCA was approximately 2.4 mm, suggesting that SB diameters may be routinely overestimated by operators. SB disease severity was also incongruous, with an average visual estimate of 75% and a QCA estimate of 46%. The PS was performed with 36% receiving KBI and only 3.7% requiring a second stent. Over 90% of the 2-stent technique cases achieved final KBI. No difference in periprocedural biomarker release was found. The 8-month angiography showed increased binary restenosis of the SB in the PS group (20.3% vs 5.2%; p<0.001). However, MACE at 2 years was 12.9% vs 8.4% (HR 0.63; p=0.12) for PS vs routine 2-stent techniques, and no difference in the improvement of angina was identified.
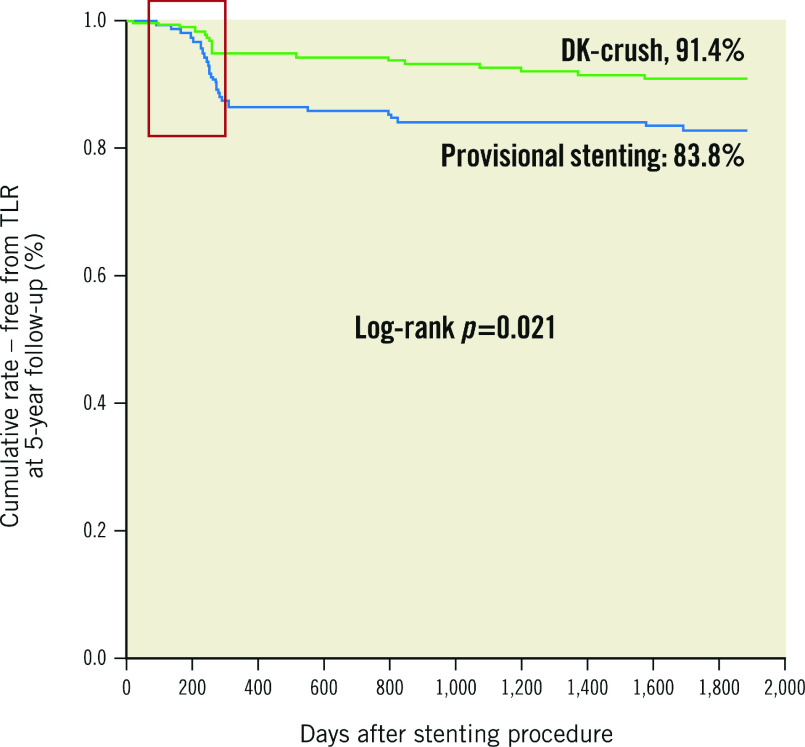
DKCRUSH-II compared the performance of the PS to DK-crush in 370 patients112. All patients had a Medina classification of 1,1,1 or 0,1,1, with a mean SB disease length of approximately 15 mm. In all, 29% of PS patients received an SB stent. Routine 8-month angiography showed increased restenosis in both the MV and SB with the PS. The incidence of MACE at 12 months was not significantly different between the groups, although the rate of TVR was significantly reduced with DK-crush (6.5% vs 14.6%; p=0.017). Findings were similar at 5-year follow-up, with reduced TLR with DK-crush (8.6% vs 16.2%; p=0.027) and a low rate of stent thrombosis in both groups (2.7%)49. Subgroup analysis showed that a reduction in TLR was seen if SB lesion length was >5 mm or stenosis was >70%. It is important to note that the majority of TLR was heavily focused around the time of study-mandated angiographic follow-up, suggesting treatment of angiographic, rather than symptomatic, lesions (Figure 9).
Figure 9. DKCRUSH-II 5-year Kaplan-Meier analysis.
DKCRUSH-II 5-year Kaplan-Meier analysis showing TLR events heavily clustered around the period of mandatory 8-month angiographic follow-up (red box). Event rates in both groups appear to stabilise after this period. (Adapted from49). DK: double kiss; TLR: target lesion revascularisation
These trials predominantly involved LAD/diagonal bifurcation lesions. The majority indicated that a provisional approach, undertaken with a high threshold for SB stenting, offers excellent clinical outcomes with no difference in MACE when compared to the routine use of 2 stents. The use of first-generation stents in 2-stent techniques was, in fact, associated with increased periprocedural MI and increased long-term mortality at 5 years. This effect was not present in the more recent EBC TWO and Nordic-Baltic IV studies, in which second-generation stents were used with higher rates of final KBI. It should be noted that the clinical outcomes were similar despite increased acute SB luminal gain in the 2-stent groups post-procedure. This reflects the fact that many ostial SB stenoses are not haemodynamically significant. In contrast, when performed by experienced operators, the optimised DK-crush technique has shown specific benefits over the PS (when undertaken with a low threshold for SB stenting) for TVR in patients with longer SB lesion lengths (>10 mm)113.
An objective method of assessing bifurcation complexity has been developed to better identify patients who may benefit from stents to both vessels. The DEFINITION criteria (Figure 10) are based on a bifurcation cohort of 1,550 patients114. When applied to a validation cohort, the PS resulted in lower rates of in-hospital stent thrombosis (ST) and 12-month TLR in simple bifurcations, while 2-stent techniques achieved a reduction in 12-month cardiac death in lesions fulfilling the complex criteria. DEFINITION II was a randomised trial of the PS vs 2 stents on patients with true bifurcation (Medina classification of 1,1,1 or 0,1,1) lesions with SB diameters ≥2.5 mm and meeting DEFINITION criteria for complexity115. Most patients had multivessel disease, and the mean SB lesion length was approximately 20 mm. SB stenting was performed in 23% of the PS group compared to 92% of the 2-stent group. DK-crush was used in 77.8% of the 2-stent group, and culotte in 17.9%. TLF at 12 months was lower in the 2-stent group (6.1% vs 11.4%; p=0.019), driven by lower rates of early target vessel myocardial infarction (TVMI) and TLR. This study provided additional support for tailoring the bifurcation strategy towards each patient.
Figure 10. DEFINITION criteria to guide bifurcation stenting strategy.
. DK: double kiss; LMCA: left main coronary artery; MV: main vessel; RVD: reference vessel diameter; SB: side branch
LMCA-dedicated trials
Due to the important differences between left main and non-left main bifurcations, several studies have specifically investigated this lesion subset.
DKCRUSH-III tackled the question of whether DK-crush or culotte offers a better result in the distal LMCA bifurcation28. Patients with heavy calcification, LMCA >5 mm diameter or a difference in diameter between the LAD and LCx of >1 mm were excluded. In the study, 419 patients were randomised, with IVUS used in >70%. The culotte group experienced significantly increased MACE at 1 year (16.3% vs 6.2%; p=0.001), driven predominantly by increased TVR (11% vs 4.3%; p=0.016). Three-year follow-up revealed increased MACE in the culotte arm (23.7% vs 8.2%; p<0.001), driven by increased MI (8.2% vs 3.4%; p=0.037) in addition to TVR (18.8% vs 5.8%; p<0.001)116. Definite ST was significantly elevated with culotte (3.4% vs 0%; p=0.007), with all patients still taking DAPT. Prespecified culotte subgroups with poorer outcomes included those with a bifurcation angle of >70° and complex lesions (per DEFINITION criteria).
Following those results, DK-CRUSH-V compared the PS to DK-crush in complex LMCA bifurcation disease in 482 patients117. The SB lesion length was ≥10 mm in nearly 50% of cases. The rate of SB stenting in the PS group was high at 47%, reflecting the severity of the disease. Twelve-month TLF (composite of cardiac death, target vessel MI or TLR) was increased with the PS compared to DK-crush (10.7% vs 5.0%; p=0.02). Significantly higher rates of TVMI were driven by definite or probable ST in the PS cohort (3.3% vs 0.4%; p=0.02). Three-year TLF remained worse with the PS (16.9% vs 8.3%; p=0.005), with significantly more TVMI, TLR and stent thrombosis118. POT and KBI were performed less frequently in the PS cohort. Thirty-day stent thrombosis with the PS (2.5%) was associated with both longer SB lesion lengths (31.9±13.3 mm vs 12.4±5.6 mm; p=0.004) and wider bifurcation angles (110°±23° vs 66.7°±2.5°; p=0.01).
EBC MAIN compared the PS to systematic 2-stent strategies in 467 European patients with LMCA bifurcation disease92. SB lesion length averaged only 7 mm, with a requirement for SB stenting in 22% of the PS group. Two-stent techniques were predominantly culotte or T/TAP, with a 93% rate of final KBI. The incidence of MACE at 12 months was similar between both the PS and 2-stent groups (14.7% vs 17.7%; p=0.34), with no difference in individual endpoints. Improvement in angina and reduction of medication use were highly significant regardless of randomisation. Stent thrombosis rates were similar at 1.7% for the PS and 1.3% for 2 stents. Procedural times and consumables used were less with the PS.
Despite seemingly discordant results, these trials are in fact complementary, covering a range of both LMCA bifurcation complexity and international operator expertise. When compared to DK-CRUSH-V, the population studied in EBC MAIN was older (71 years vs 64 years), with higher rates of recent myocardial infarction (37% vs 12%). All-cause mortality and MI were used as MACE endpoints (rather than cardiac death and TVMI), which also likely contributed to the higher reported event rates. Patients in DK-CRUSH-V had higher mean SYNTAX scores (31 vs 23) and longer mean SB lesion lengths (16 mm vs 7 mm), which may explain the 2-fold increase in SB stenting with the PS. In DK-CRUSH-V, PS patients who did not require an SB stent (52.9%) had numerically lower TLF at 1 year (8.6% vs 13.2%; p=0.30) and significantly lower ST (0.8% vs 6.1%; p=0.03) than those who did117. PS for complex disease (as per DEFINITION criteria) was more likely to progress to SB stenting (64.9% vs 38.8%; p=0.001), and it was these complex patients who obtained the greatest absolute benefit with DK-crush (HR for TLF 0.27, 95% CI: 0.05-0.54)118.
The low rate of DK-crush (5%) used in the EBC MAIN trial demonstrates that European operators are not as familiar with this technique. Indeed, each trial result favours the strengths of the teams who designed and implemented them, and it is likely that each stenting strategy was performed better by its champion group. Two-stent techniques remain challenging procedures, and in the DK-CRUSH-V trial, the DK-crush patients required significantly greater procedural time (82 minutes vs 66 minutes; p<0.001) and contrast volume (227 ml vs 191 ml; p<0.001) than the PS patients. This is despite only exceptionally high-volume operators participating (≥300 PCIs/year for 5 years, including at least 20 LMCA PCIs per year), and a requirement for interventionalists to have steering committee reviews of 3-5 DK-crush cases prior to commencement. This depth of operator experience was achievable due to the preference for PCI over surgery in Asian countries. The EBC MAIN trial had a more liberal expectation that participating operators would perform >150 PCIs/year. It is important to remember that any bifurcation technique can lead to multiple challenges, and even with the seemingly simpler PS, successful stenting of an acutely occluded SB can be challenging. Individual physicians must therefore be cognisant of their own experience and limitations when embarking on these cases.
Conclusions
For most bifurcation lesions, stepwise provisional stenting should be the treatment of choice, with a conservative approach to any side branch intervention. As bifurcation lesion complexity increases, the outcomes with 2-stent strategies appear more favourable. Operators should familiarise and upskill themselves with the 2-stent technique they prefer. The difference between techniques may be less important than their fastidious application, although the existing data support the application of DK-crush for complex left main bifurcation lesions in particular.
Acknowledgments
Conflict of interest statement
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations
- CT
computed tomography
- DK
double kiss
- DMV
distal main vessel
- EBC
European Bifurcation Club
- FFR
fractional flow reserve
- IVL
intravascular lithotripsy
- IVUS
intravascular ultrasound
- KBI
kissing balloon inflation
- LAD
left anterior descending
- LCx
left circumflex
- LMCA
left main coronary artery
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- MV
main vessel
- NC
non-compliant
- OCT
optical coherence tomography
- PCI
percutaneous coronary intervention
- PMV
proximal main vessel
- POT
proximal optimisation technique
- PS
provisional strategy
- RCT
randomised control trial
- TAP
T and protrude
- TIMI
Thrombolysis in Myocardial Infarction
- TLF
target lesion failure
- TLR
target lesion revascularisation
- TVF
target vessel failure
- TVMI
target vessel myocardial infarction
Contributor Information
David Hildick-Smith, Sussex Cardiac Centre, University Hospitals Sussex NHS Trust, Brighton, United Kingdom.
Sandeep Arunothayaraj, Sussex Cardiac Centre, University Hospitals Sussex NHS Trust, Brighton, United Kingdom.
Goran Stankovic, Department of Cardiology, University Clinical Center of Serbia, and Faculty of Medicine, University of Belgrade, Belgrade, Serbia.
Shao-Liang Chen, Department of Cardiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China..
References
- Morbiducci U, Kok AM, Kwak BR, Stone PH, Steinman DA, Wentzel JJ. Atherosclerosis at arterial bifurcations: evidence for the role of haemodynamics and geometry. Thromb Haemost. 2016;115:484–92. doi: 10.1160/TH15-07-0597. [DOI] [PubMed] [Google Scholar]
- Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- Nakazawa G, Yazdani SK, Finn AV, Vorpahl M, Kolodgie FD, Virmani R. Pathological findings at bifurcation lesions: the impact of flow distribution on atherosclerosis and arterial healing after stent implantation. J Am Coll Cardiol. 2010;55:1679–87. doi: 10.1016/j.jacc.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Pan M, Medina A, Suárez de, Romero M, Segura J, Martín P, Suárez de, Hernández E, Mazuelos F, Moreno A, Pavlovic D, Ojeda S, Toledano F, Leon C. Coronary bifurcation lesions treated with simple approach (from the Cordoba & Las Palmas [CORPAL] Kiss Trial). Am J Cardiol. 2011;107:1460–5. doi: 10.1016/j.amjcard.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99:315–27. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Tempel D, van Haperen, van der, Grosveld F, Daemen MJ, Krams R, de Crom. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–53. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- Yamamoto E, Siasos G, Zaromytidou M, Coskun AU, Xing L, Bryniarski K, Zanchin T, Sugiyama T, Lee H, Stone PH, Jang IK. Low Endothelial Shear Stress Predicts Evolution to High-Risk Coronary Plaque Phenotype in the Future: A Serial Optical Coherence Tomography and Computational Fluid Dynamics Study. Circ Cardiovasc Interv. 2017;10:e005455. doi: 10.1161/CIRCINTERVENTIONS.117.005455. [DOI] [PubMed] [Google Scholar]
- Lassen JF, Holm NR, Banning A, Burzotta F, Lefèvre T, Chieffo A, Hildick-Smith D, Louvard Y, Stankovic G. Percutaneous coronary intervention for coronary bifurcation disease: 11th consensus document from the European Bifurcation Club. EuroIntervention. 2016;12:38–46. doi: 10.4244/EIJV12I1A7. [DOI] [PubMed] [Google Scholar]
- Kogame N, Chichareon P, De Wilder, Takahashi K, Modolo R, Chang CC, Tomaniak M, Komiyama H, Chieffo A, Colombo A, Garg S, Louvard Y, Jüni P, G Steg, Hamm C, Vranckx P, Valgimigli M, Windecker S, Stoll HP, Onuma Y, Janssens L, Serruys PW. Clinical relevance of ticagrelor monotherapy following 1-month dual antiplatelet therapy after bifurcation percutaneous coronary intervention: Insight from GLOBAL LEADERS trial. Catheter Cardiovasc Interv. 2020;96:100–11. doi: 10.1002/ccd.28428. [DOI] [PubMed] [Google Scholar]
- Grundeken MJ, Wykrzykowska JJ, Ishibashi Y, Garg S, de Vries, Garcia-Garcia HM, Onuma Y, de Winter, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice MC, di Mario, Meier B, Jüni P, Yazdani A, Copt S, Windecker S, Serruys PW. First generation versus second generation drug-eluting stents for the treatment of bifurcations: 5-year follow-up of the LEADERS all-comers randomized trial. Catheter Cardiovasc Interv. 2016;87:e248–60. doi: 10.1002/ccd.26344. [DOI] [PubMed] [Google Scholar]
- Al Suwaidi, Yeh W, Cohen HA, Detre KM, Williams DO, Holmes DR. Immediate and one-year outcome in patients with coronary bifurcation lesions in the modern era (NHLBI dynamic registry). Am J Cardiol. 2001;87:1139–44. doi: 10.1016/s0002-9149(01)01482-5. [DOI] [PubMed] [Google Scholar]
- Louvard Y, Thomas M, Dzavik V, Hildick-Smith D, Galassi AR, Pan M, Burzotta F, Zelizko M, Dudek D, Ludman P, Sheiban I, Lassen JF, Darremont O, Kastrati A, Ludwig J, Iakovou I, Brunel P, Lansky A, Meerkin D, Legrand V, Medina A, Lefèvre T. Classification of coronary artery bifurcation lesions and treatments: time for a consensus! Catheter Cardiovasc Interv. 2008;71:175–83. doi: 10.1002/ccd.21314. [DOI] [PubMed] [Google Scholar]
- Kim HY, Doh JH, Lim HS, Nam CW, Shin ES, Koo BK, Lee JM, Park TK, Yang JH, Song YB, Hahn JY, Choi SH, Gwon HC, Lee SH, Kim SM, Choe Y, Choi JH. Identification of Coronary Artery Side Branch Supplying Myocardial Mass That May Benefit From Revascularization. JACC Cardiovasc Interv. 2017;10:571–81. doi: 10.1016/j.jcin.2016.11.033. [DOI] [PubMed] [Google Scholar]
- Medina A, Suárez de, Pan M. [A New Classification of Coronary Bifurcation Lesions]. Rev Esp Cardiol. 2006;59:183. [Article in Spanish]. [PubMed] [Google Scholar]
- Perl L, Witberg G, Greenberg G, Vaknin-Assa H, Kornowski R, Assali A. Prognostic significance of the Medina classification in bifurcation lesion percutaneous coronary intervention with second-generation drug-eluting stents. Heart Vessels. 2020;35:331–9. doi: 10.1007/s00380-019-01504-z. [DOI] [PubMed] [Google Scholar]
- Park TK, Park YH, Song YB, Oh JH, Chun WJ, Kang GH, Jang WJ, Hahn JY, Yang JH, Choi SH, Choi JH, Lee SH, Jeong MH, Kim HS, Lee JH, Yu CW, Rha SW, Jang Y, Yoon JH, Tahk SJ, Seung KB, Park JS, Gwon HC. Long-term Clinical Outcomes of True and Non-True Bifurcation Lesions According to Medina Classification - Results from the COBIS (COronary BIfurcation Stent) II Registry. Circ J. 2015;79:1954–62. doi: 10.1253/circj.CJ-15-0264. [DOI] [PubMed] [Google Scholar]
- Murphy JL, Patel N, Vengrenyuk Y, Okamoto N, Barman N, Sweeny J, Kapur V, Hasan C, Krishnan P, Vijay P, Jhaveri V, Dangas G, Mehran R, Aquino M, Baber U, Sharma SK, Kini AS. Cardiovascular outcomes after percutaneous coronary intervention on bifurcation lesions with moderate to severe coronary calcium: A single-center registry study. Catheter Cardiovasc Interv. 2021;98:35–42. doi: 10.1002/ccd.29069. [DOI] [PubMed] [Google Scholar]
- Ito H, Piel S, Das P, Chhokar V, Khadim G, Nierzwicki R, Williams A, Dieter RS, Leya F. Long-term outcomes of plaque debulking with rotational atherectomy in side-branch ostial lesions to treat bifurcation coronary disease. J Invasive Cardiol. 2009;21:598–601. [PubMed] [Google Scholar]
- Allali A, Abdel-Wahab M, Traboulsi H, Hemetsberger R, Mankerious N, Byrne R, Geist V, El-Mawardy M, Sulimov D, Toelg R, Richardt G. Impact of Lesion Preparation Technique on Side Branch Compromise in Calcified Coronary Bifurcations: A Subgroup Analysis of the PREPARE-CALC Trial. J Interv Cardiol. 2020;2020:9740938. doi: 10.1155/2020/9740938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, Armstrong EJ, Benhuri B, Okamoto N, Vengrenyuk Y, Shlofmitz E, Revtyak GE, Martinsen BJ, Igyarto Z, Valle JA, Waldo SW, Aksut B, Bell S, Gardner R, Lee M, Zakir R, Shroff A, Don C, Shlofmitz R, Chambers JW, Kini A, Sharma S. Orbital Atherectomy for Treatment of Severely Calcified Coronary Artery Bifurcation Lesions: A Multicenter Analysis. Cardiovasc Revasc Med. 2021;26:34–8. doi: 10.1016/j.carrev.2020.10.023. [DOI] [PubMed] [Google Scholar]
- Lee MS, Shlofmitz E, Park KW, Goldberg A, Jeremias A, Schlofmitz R. Orbital Atherectomy of Severely Calcified Unprotected Left Main Coronary Artery Disease: One-Year Outcomes. J Invasive Cardiol. 2018;30:270–4. [PubMed] [Google Scholar]
- Pawłowski T, Legutko J, Modzelewski P, Gil RJ. Synergistic application of high-speed rotational atherectomy and intravascular lithotripsy for a severely calcified undilatable proximal left anterior descending coronary artery bifurcation lesion: Case of rotalithoplasty-facilitated DK-CRUSH. Cardiol J. 2021;28:181–2. doi: 10.5603/CJ.2021.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Dou K. Coronary Bifurcation Intervention: What Role Do Bifurcation Angles Play? J Interv Cardiol. 2015;28:236–48. doi: 10.1111/joic.12203. [DOI] [PubMed] [Google Scholar]
- Hahn JY, Chun WJ, Kim JH, Song YB, Oh JH, Koo BK, Rha SW, Yu CW, Park JS, Jeong JO, Choi SH, Choi JH, Jeong MH, Yoon JH, Jang Y, Tahk SJ, Kim HS, Gwon HC. Predictors and outcomes of side branch occlusion after main vessel stenting in coronary bifurcation lesions: results from the COBIS II Registry (COronary BIfurcation Stenting). J Am Coll Cardiol. 2013;62:1654–59. doi: 10.1016/j.jacc.2013.07.041. [DOI] [PubMed] [Google Scholar]
- Zhang D, Xu B, Yin D, Li Y, He Y, You S, Qiao S, Wu Y, Yan H, Yang Y, Gao R, Dou K. How bifurcation angle impacts the fate of side branch after main vessel stenting: a retrospective analysis of 1,200 consecutive bifurcation lesions in a single center. Catheter Cardiovasc Interv. 2015;85:706–15. doi: 10.1002/ccd.25858. [DOI] [PubMed] [Google Scholar]
- Yang JH, Song YB, Song PS, Hahn JY, Choi SH, Choi JH, Lee SH, Kim HS, Jang Y, Tahk SJ, Seung KB, Park SJ, Gwon HC. Impact of coronary bifurcation angle on clinical outcomes after percutaneous coronary intervention in real-world practice: results from the COBIS registry. Cardiol. 2012;122:216–24. doi: 10.1159/000338817. [DOI] [PubMed] [Google Scholar]
- Adriaenssens T, Byrne RA, Dibra A, Iijima R, Mehilli J, Bruskina O, Schömig A, Kastrati A. Culotte stenting technique in coronary bifurcation disease: angiographic follow-up using dedicated quantitative coronary angiographic analysis and 12-month clinical outcomes. Eur Heart J. 2008;29:2868–76. doi: 10.1093/eurheartj/ehn512. [DOI] [PubMed] [Google Scholar]
- Chen SL, Xu B, Han YL, Sheiban I, Zhang JJ, Ye F, Kwan TW, Paiboon C, Zhou YJ, Lv SZ, Dangas GD, Xu YW, Wen SY, Hong L, Zhang RY, Wang HC, Jiang TM, Wang Y, Chen F, Yuan ZY, Li WM, Leon MB. Comparison of double kissing crush versus Culotte stenting for unprotected distal left main bifurcation lesions: results from a multicenter, randomized, prospective DKCRUSH-III study. J Am Coll Cardiol. 2013;61:1482–8. doi: 10.1016/j.jacc.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Ormiston JA, Currie E, Webster MWI, Kay P, Ruygrok PN, Stewart JT, Padgett RC, Panther MJ. Drug-eluting stents for coronary bifurcations: insights into the crush technique. Catheter Cardiovasc Interv. 2004;63:332–6. doi: 10.1002/ccd.20120. [DOI] [PubMed] [Google Scholar]
- Ormiston JA, Webster MW, Webber B, Stewart JT, Ruygrok PN, Hatrick RI. The ‘crush’ technique for coronary artery bifurcation stenting: insights from micro-computed tomographic imaging of bench deployments. JACC Cardiovasc Interv. 2008;1:351–7. doi: 10.1016/j.jcin.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Dzavik V, Kharbanda R, Ivanov J, Ing DJ, Bui S, Mackie K, Ramsamujh R, Barolet A, Schwartz L, Seidelin PH. Predictors of long-term outcome after crush stenting of coronary bifurcation lesions: importance of the bifurcation angle. Am Heart J. 2006;152:762–9. doi: 10.1016/j.ahj.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Chen SL, Zhang JJ, Ye F, Chen YD, Fang WY, Wei M, He B, Sun XW, Yang S, Chen JG, Shan SJ, Tian NL, Li XB, Liu ZZ, Kan J, Michael L, W KT. Effect of coronary bifurcation angle on clinical outcomes in Chinese patients treated with crush stenting: a subgroup analysis from DKCRUSH-1 bifurcation study. Chin Med J (Engl) 2009;122:396–402. [PubMed] [Google Scholar]
- Collins N, Seidelin PH, Daly P, Ivanov J, Barolet A, Mackie K, Bui S, Schwartz L, Dzavík V. Long-term outcomes after percutaneous coronary intervention of bifurcation narrowings. Am J Cardiol. 2008;102:404–10. doi: 10.1016/j.amjcard.2008.03.075. [DOI] [PubMed] [Google Scholar]
- Suárez de, Medina A, Martín P, Novoa J, Suárez de, Pan M, Caballero E, Melián F, Mazuelos F, Quevedo V. Predictors of ostial side branch damage during provisional stenting of coronary bifurcation lesions not involving the side branch origin: an ultrasonographic study. EuroIntervention. 2012;7:1147–54. doi: 10.4244/EIJV7I10A185. [DOI] [PubMed] [Google Scholar]
- Xu J, Hahn JY, Song YB, Choi SH, Choi JH, Lu C, Lee SH, Hong KP, Park JE, Gwon HC. Carina shift versus plaque shift for aggravation of side branch ostial stenosis in bifurcation lesions: volumetric intravascular ultrasound analysis of both branches. Circ Cardiovasc Interv. 2012;5:657–62. doi: 10.1161/CIRCINTERVENTIONS.112.969089. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Kim WJ, Lee JY, Park DW, Lee SW, Kim YH, Lee CW, Mintz GS, Park SW, Park SJ. Hemodynamic impact of changes in bifurcation geometry after single-stent cross-over technique assessed by intravascular ultrasound and fractional flow reserve. Catheter Cardiovasc Interv. 2013;82:1075–82. doi: 10.1002/ccd.24956. [DOI] [PubMed] [Google Scholar]
- Park JJ, Chun EJ, Cho YS, Oh IY, Yoon CH, Suh JW, Choi S, Youn TJ, Koo BK, Chae IH, Choi DJ. Potential predictors of side-branch occlusion in bifurcation lesions after percutaneous coronary intervention: a coronary CT angiography study. Radiology. 2014;271:711–20. doi: 10.1148/radiol.14131959. [DOI] [PubMed] [Google Scholar]
- Furukawa E, Hibi K, Kosuge M, Nakatogawa T, Toda N, Takamura T, Tsukahara K, Okuda J, Ootsuka F, Tahara Y, Sugano T, Endo T, Kimura K, Umemura S. Intravascular ultrasound predictors of side branch occlusion in bifurcation lesions after percutaneous coronary intervention. Circ J. 2005;69:325–30. doi: 10.1253/circj.69.325. [DOI] [PubMed] [Google Scholar]
- Dou K, Zhang D, Xu B, Yang Y, Yin D, Qiao S, Wu Y, Yan H, You S, Wang Y, Wu Z, Gao R, Kirtane AJ. An angiographic tool for risk prediction of side branch occlusion in coronary bifurcation intervention: the RESOLVE score system (Risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion). JACC Cardiovasc Interv. 2015;8:39–46. doi: 10.1016/j.jcin.2014.08.011. [DOI] [PubMed] [Google Scholar]
- He Y, Zhang D, Yin D, Zhu C, Feng L, Song C, Chen C, Xu B, Dou K. Validation of the V-RESOLVE (Visual Estimation for Risk prEdiction of Side Branch OccLusion in Coronary Bifurcation interVEntion) score system. Catheter Cardiovasc Interv. 2018;91:591–8. doi: 10.1002/ccd.27499. [DOI] [PubMed] [Google Scholar]
- Lefèvre T, Girasis C, Lassen JF. Differences between the left main and other bifurcations. EuroIntervention. 2015;11:V106–10. doi: 10.4244/EIJV11SVA24. [DOI] [PubMed] [Google Scholar]
- Song PS, Song YB, Lee JM, Hahn JY, Choi SH, Choi JH, Lee SH, Park KW, Kim HS, Jang Y, Seung KB, Oh JH, Gwon HC. Major Predictors of Long-Term Clinical Outcomes After Percutaneous Coronary Intervention for Coronary Bifurcation Lesions With 2-Stent Strategy: Patient-Level Analysis of the Korean Bifurcation Pooled Cohorts. JACC Cardiovasc Interv. 2016;9:1879–86. doi: 10.1016/j.jcin.2016.06.049. [DOI] [PubMed] [Google Scholar]
- Rigatelli G, Zuin M, Baracca E, Galasso P, Carraro M, Mazza A, Lanza D, Roncon L, Daggubati R. Long-Term Clinical Outcomes of Isolated Ostial Left Anterior Descending Disease Treatment: Ostial Stenting Versus Left Main Cross-Over Stenting. Cardiovasc Revasc Med. 2019;20:1058–62. doi: 10.1016/j.carrev.2019.01.030. [DOI] [PubMed] [Google Scholar]
- Yang ZK, Hu J, Ding FH, Ni JW, Zhang RY, Shen WF. One-year outcome of single-stent crossover versus accurate ostial stenting for isolated left anterior descending ostial stenosis. Coron Artery Dis. 2022;31:e67–72. doi: 10.1097/MCA.0000000000001071. [DOI] [PubMed] [Google Scholar]
- Medrano-Gracia P, Ormiston J, Webster M, Beier S, Ellis C, Wang C, Smedby Ö, Young A, Cowan B. A Study of Coronary Bifurcation Shape in a Normal Population. J Cardiovasc Transl Res. 2017;10:82–90. doi: 10.1007/s12265-016-9720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandzari DE, Gershlick AH, Serruys PW, Leon MB, Morice MC, Simonton CA, Lembo NJ, Mansour S, Sabaté M, Sabik JF, Kappetein AP, Dressler O, Stone GW. Procedural characteristics and clinical outcomes in patients undergoing percutaneous coronary intervention for left main trifurcation disease: the EXCEL trial. EuroIntervention. 2020;16:e982–8. doi: 10.4244/EIJ-D-19-00686. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Johnson T, Anderson R, Gallagher S, Sirker A, Ludman P, de Belder, Copt S, Oldroyd K, Banning A, Mamas M, Curzen N. Intravascular Imaging and 12-Month Mortality After Unprotected Left Main Stem PCI: An Analysis From the British Cardiovascular Intervention Society Database. JACC Cardiovasc Interv. 2020;13:346–57. doi: 10.1016/j.jcin.2019.10.007. [DOI] [PubMed] [Google Scholar]
- Gao XF, Ge Z, Kong XQ, Kan J, Han L, Lu S, Tian NL, Lin S, Lu QH, Wang XY, Li QH, Liu ZZ, Chen Y, Qian XS, Wang J, Chai DY, Chen CH, Pan T, Ye F, Zhang JJ, Chen SL. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc Interv. 2021;14:247–57. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- Chen SL, Santoso T, Zhang JJ, Ye F, Xu YW, Fu Q, Kan J, Zhang FF, Zhou Y, Xie DJ, Kwan TW. Clinical Outcome of Double Kissing Crush Versus Provisional Stenting of Coronary Artery Bifurcation Lesions: The 5-Year Follow-Up Results From a Randomized and Multicenter DKCRUSH-II Study (Randomized Study on Double Kissing Crush Technique Versus Provisional Stenting Technique for Coronary Artery Bifurcation Lesions). Circ Cardiovasc Interv. 2017;10:e004497. doi: 10.1161/CIRCINTERVENTIONS.116.004497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZG, Gao XF, Li XB, Shao MX, Gao YL, Chen SL, Tian NL. The outcomes of intravascular ultrasound-guided drug-eluting stent implantation among patients with complex coronary lesions: a comprehensive meta-analysis of 15 clinical trials and 8,084 patients. Anatol J Cardiol. 2017;17:258–68. doi: 10.14744/AnatolJCardiol.2016.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Kan J, Gao XF, Kong XQ, Zuo GF, Ye F, Tian NL, Lin S, Liu ZZ, Sun ZQ, He PC, Wei L, Yang W, He YQ, Xue YZ, Wang LM, Miao LF, Pu J, Sun YW, Nie SP, Tao JH, Wen SY, Yang Q, Su X, Yao QC, Huang YJ, Xia Y, Shen FR, Qiu CG, Mao YL, Liu Q, Hu XQ, Du ZM, Nie RQ, Han YL, Zhang JJ, Chen SL. Comparison of intravascular ultrasound-guided with angiography-guided double kissing crush stenting for patients with complex coronary bifurcation lesions: Rationale and design of a prospective, randomized, and multicenter DKCRUSH VIII trial. Am Heart J. 2021;234:101–10. doi: 10.1016/j.ahj.2021.01.011. [DOI] [PubMed] [Google Scholar]
- Holm NR, Andreasen LN, Walsh S, Kajander OA, Witt N, Eek C, Knaapen P, Koltowski L, Gutiérrez-Chico JL, Burzotta F, Kockman J, Ormiston J, Santos-Pardo I, Laanmets P, Mylotte D, Madsen M, Hjort J, Kumsars I, Råmunddal T, Christiansen EH. Rational and design of the European randomized Optical Coherence Tomography Optimized Bifurcation Event Reduction Trial (OCTOBER). Am Heart J. 2018;205:97–109. doi: 10.1016/j.ahj.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Ali Z, Landmesser U, Karimi Galougahi, Maehara A, Matsumura M, Shlofmitz RA, Guagliumi G, Price MJ, Hill JM, Akasaka T, Prati F, Bezerra HG, Wijns W, Mintz GS, Ben-Yehuda O, McGreevy RJ, Zhang Z, Rapoza RR, West NEJ, Stone GW. Optical coherence tomography-guided coronary stent implantation compared to angiography: a multicentre randomised trial in PCI - design and rationale of ILUMIEN IV: OPTIMAL PCI. EuroIntervention. 2021;16:1092–9. doi: 10.4244/EIJ-D-20-00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Muramatsu T, Asano T, Katagiri Y, Sotomi Y, Nakatani S, Takahashi K, Kogame N, Higuchi Y, Ishikawa M, Kyono H, Yano M, Ozaki Y, Serruys PW, Okamura T, Onuma Y. Online three-dimensional OFDI-guided versus angiographyguided PCI in bifurcation lesions: design and rationale of the randomised OPTIMUM trial. EuroIntervention. 2021;16:1333–41. doi: 10.4244/EIJ-D-18-00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzotta F, Lassen JF, Lefèvre T, Banning AP, Chatzizisis YS, Johnson TW, Ferenc M, Rathore S, Albiero R, Pan M, Darremont O, Hildick-Smith D, Chieffo A, Zimarino M, Louvard Y, Stankovic G. Percutaneous coronary intervention for bifurcation coronary lesions: the 15th consensus document from the European Bifurcation Club. EuroIntervention. 2021;16:1307–17. doi: 10.4244/EIJ-D-20-00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song PS, Song Y, Yang JH, Hahn JY, Choi SH, Choi JH, Koo BK, Seung KB, Park SJ, Gwon HC. The impact of side branch predilatation on procedural and long-term clinical outcomes in coronary bifurcation lesions treated by the provisional approach. Rev Española Cardiol (English Ed) 2014;67:804–12. doi: 10.1016/j.rec.2014.01.032. [DOI] [PubMed] [Google Scholar]
- Pan M, Medina A, Romero M, Ojeda S, Martin P, Suarez de, Segura J, Mazuelos F, Novoa J, Suarez de. Assessment of side branch predilation before a provisional T-stent strategy for bifurcation lesions. A randomized trial. Am Heart J. 2014;168:374–80. doi: 10.1016/j.ahj.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Ohya H, Kyo E, Tsuji T, Watanabe S, Katoh O. Impact on clinical outcomes of predilatation using the kissing-balloon technique for crossover stenting in true coronary bifurcation lesions. J Invasive Cardiol. 2013;25:512–8. [PubMed] [Google Scholar]
- Lassen JF, Burzotta F, Banning A, Lefèvre T, Darremont O, Hildick-Smith D, Chieffo A, Pan M, Holm N, Louvard Y, Stankovic G. Percutaneous coronary intervention for the left main stem and other bifurcation lesions: 12th consensus document from the European Bifurcation Club. EuroIntervention. 2018;13:1540–53. doi: 10.4244/EIJ-D-17-00622. [DOI] [PubMed] [Google Scholar]
- Chevalier B, Mamas MA, Hovasse T, Rashid M, Gómez-Hospital JA, Pan M, Witkowski A, Crowley J, Aminian A, McDonald J, Beygui F, Fernandez Portales, Roguin A, Stankovic G. Clinical outcomes of proximal optimization technique (POT) in bifurcation stenting. EuroIntervention. 2021;17:e910–8. doi: 10.4244/EIJ-D-20-01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BK, Park KW, Kang HJ, Cho YS, Chung WY, Youn TJ, Chae IH, Choi DJ, Tahk SJ, Oh BH, Park YB, Kim HS. Physiological evaluation of the provisional side-branch intervention strategy for bifurcation lesions using fractional flow reserve. Eur Heart J. 2008;29:726–32. doi: 10.1093/eurheartj/ehn045. [DOI] [PubMed] [Google Scholar]
- Chen SL, Ye F, Zhang JJ, Xu T, Tian NL, Liu ZZ, Lin S, Shan SJ, Ge Z, You W, Liu YQ, Qian XS, Li F, Yang S, Kwan TW, Xu B, Stone GW. Randomized Comparison Of FFR-Guided and Angiography-Guided Provisional Stenting of True Coronary Bifurcation Lesions: The DKCRUSH-VI Trial (Double Kissing Crush Versus Provisional Stenting Technique For Treatment Of Coronary Bifurcation Lesions VI). JACC Cardiovasc Interv. 2015;8:536–46. doi: 10.1016/j.jcin.2014.12.221. [DOI] [PubMed] [Google Scholar]
- Niemelä M, Kervinen K, Erglis A, Holm NR, Maeng M, Christiansen EH, Kumsars I, Jegere S, Dombrovskis A, Gunnes P, Stavnes S, Steigen TK, Trovik T, Eskola M, Vikman S, Romppanen H, Mäkikallio T, Hansen KN, Thayssen P, Aberge L, Jensen LO, Hervold A, Airaksinen J, Pietilä M, Frobert O, Kellerth T, Ravkilde J, Aarøe J, Jensen JS, Helqvist S, Sjögren I, James S, Miettinen H, Lassen JF, Thuesen L Nordic- Baltic PCI Study Group. Randomized comparison of final kissing balloon dilatation versus no final kissing balloon dilatation in patients with coronary bifurcation lesions treated with main vessel stenting: the Nordic-Baltic Bifurcation Study III. Circulation. 2011;123:79–86. doi: 10.1161/CIRCULATIONAHA.110.966879. [DOI] [PubMed] [Google Scholar]
- Korn HV, Yu J, Ohlow MA, Huegl B, Schulte W, Wagner A, Wassmer G, Gruene S, Petek O, Lauer B. Interventional therapy of bifurcation lesions: a TIMI flow-guided concept to treat side branches in bifurcation lesions -- a prospective randomized clinical study (Thueringer bifurcation study, THUEBIS study as pilot trial). Circ Cardiovasc Interv. 2009;2:535–42. doi: 10.1161/CIRCINTERVENTIONS.108.833046. [DOI] [PubMed] [Google Scholar]
- Song Y, Hahn JY, Song PS, Yang JH, Choi JH, Choi SH, Lee SH, Gwon HC. Randomized comparison of conservative versus aggressive strategy for provisional side branch intervention in coronary bifurcation lesions: results from the SMART-STRATEGY (Smart Angioplasty Research Team-Optimal STRATEGY for Side Branch Intervention in CBifurcation Lesions) randomized trial. JACC Cardiovasc Interv. 2012;5:1133–40. doi: 10.1016/j.jcin.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Song Y, Park TK, Hahn JY, Yang JH, Choi JH, Choi SH, Lee SH, Gwon HC. Optimal Strategy for Provisional Side Branch Intervention in Coronary Bifurcation Lesions: 3-Year Outcomes of the SMART-STRATEGY Randomized Trial. JACC Cardiovasc Interv. 2016;9:517–26. doi: 10.1016/j.jcin.2015.11.037. [DOI] [PubMed] [Google Scholar]
- Yamawaki M, Fujita M, Sasaki S, Tsurugida M, Nanasato M, Araki M, Hirano K, Ito Y, Tsukahara R, Muramatsu T. Randomized comparison between provisional and routine kissing-balloon technique after main vessel crossover stenting for coronary bifurcation lesions. Heart Vessels. 2017;32:1067–76. doi: 10.1007/s00380-017-0977-4. [DOI] [PubMed] [Google Scholar]
- Hariki H, Shinke T, Otake H, Shite J, Nakagawa M, Inoue T, Osue T, Iwasaki M, Taniguchi Y, Nishio R, Hiranuma N, Kinutani H, Konishi A, Hirata KI. Potential benefit of final kissing balloon inflation after single stenting for the treatment of bifurcation lesions-insights from optical coherence tomography observations. Circ J. 2013;77:1193–201. doi: 10.1253/circj.cj-12-0848. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Saito N, Bao B, Tokushige A, Watanabe H, Yamamoto E, Kawase Y, Kimura T. Microcatheter-facilitated reverse wire technique for side branch wiring in bifurcated vessels: an in vitro evaluation. EuroIntervention. 2013;9:870–7. doi: 10.4244/EIJV9I7A141. [DOI] [PubMed] [Google Scholar]
- Ormiston JA, Webster MW, El Jack, Ruygrok PN, Stewart JT, Scott D, Currie E, Panther MJ, Shaw B, O’Shaughnessy B. Drug-eluting stents for coronary bifurcations: bench testing of provisional side-branch strategies. Catheter Cardiovasc Interv. 2006;67:49–55. doi: 10.1002/ccd.20453. [DOI] [PubMed] [Google Scholar]
- Park TK, Lee JH, Song Y, Jeong JO, Hahn JY, Yang JH, Choi SH, Choi JH, Lee SH, Jeong MH, Kim HS, Oh JH, Yu CW, Rha SW, Jang Y, Yoon JH, Tahk SJ, Seung KB, Park JS, Gwon HC. Impact of non-compliant balloons on long-term clinical outcomes in coronary bifurcation lesions: esults from the COBIS (COronary BIfurcation Stent) II registry. EuroIntervention. 2016;12:456–64. doi: 10.4244/EIJV12I4A79. [DOI] [PubMed] [Google Scholar]
- Murasato Y, Iwasaki K, Yamamoto T, Yagi T, Hikichi Y, Suematsu Y, Yamamoto T. Optimal kissing balloon inflation after single-stent deployment in a coronary bifurcation model. EuroIntervention. 2014;10:934–41. doi: 10.4244/EIJV10I8A160. [DOI] [PubMed] [Google Scholar]
- Gwon HC, Hahn JY, Koo BK, Song Y, Choi SH, Choi JH, Lee SH, Jeong MH, Kim HS, Seong IW, Yang JY, Rha SW, Jang Y, Yoon JH, Tahk SJ, Seung KB, Park SJ. Final kissing ballooning and long-term clinical outcomes in coronary bifurcation lesions treated with 1-stent technique: results from the COBIS registry. Heart. 2012;98:225–31. doi: 10.1136/heartjnl-2011-300322. [DOI] [PubMed] [Google Scholar]
- Zhong M, Tang B, Zhao Q, Cheng J, Jin Q, Fu S. Should kissing balloon inflation after main vessel stenting be routine in the one-stent approach? A systematic review and meta-analysis of randomized trials. PLoS One. 2018;13:e0197580. doi: 10.1371/journal.pone.0197580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaido L, D’Ascenzo F, Imori Y, Wojakowski W, Saglietto A, Figini F, Mattesini A, Trabattoni D, Rognoni A, Tomassini F, Bernardi A, Ryan N, Muscoli S, Helft G, De Filippo, Parma R, De Luca, Ugo F, Cerrato E, Montefusco A, Pennacchi M, Wańha W, Smolka G, de Lio, Bruno F, Huczek Z, Boccuzzi G, Cortese B, Capodanno D, Omedè P, Mancone M, Nuñez-Gil I, Romeo F, Varbella F, Rinaldi M, Escaned J, Conrotto F, Burzotta F, Chieffo A, Perl L, D’Amico M, di Mario, Sheiban I, Gagnor A, Giammaria M, De Ferrari. Impact of Kissing Balloon in Patients Treated with Ultrathin Stents for Left Main Lesions and Bifurcations: An Analysis from the RAIN-CARDIOGROUP VII Study. Circ Cardiovasc Interv. 2020;13:e008325. doi: 10.1161/CIRCINTERVENTIONS.119.008325. [DOI] [PubMed] [Google Scholar]
- Dérimay F, Rioufol G, Cellier G, Souteyrand G, Finet G. Benefits of final proximal optimization technique (POT) in provisional stenting. Int J Cardiol. 2019;274:71–3. doi: 10.1016/j.ijcard.2018.09.041. [DOI] [PubMed] [Google Scholar]
- Finet G, Derimay F, Motreff P, Guerin P, Pilet P, Ohayon J, Darremont O, Rioufol G. Comparative Analysis of Sequential Proximal Optimizing Technique Versus Kissing Balloon Inflation Technique in Provisional Bifurcation Stenting: Fractal Coronary Bifurcation Bench Test. JACC Cardiovasc Interv. 2015;8:1308–17. doi: 10.1016/j.jcin.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Andreasen LN, Holm NR, Webber B, Ormiston JA. Critical aspects of balloon position during final proximal optimization technique (POT) in coronary bifurcation stenting. Catheter Cardiovasc Interv. 2020;96:31–9. doi: 10.1002/ccd.28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dérimay F, Finet G, Souteyrand G, Maillard L, Aminian A, Lattuca B, Cayla G, Cellier G, Motreff P, Rioufol G. Benefit of a new provisional stenting strategy, the re-proximal optimisation technique: the rePOT clinical study. EuroIntervention. 2018;14:e325–32. doi: 10.4244/EIJ-D-17-00941. [DOI] [PubMed] [Google Scholar]
- Murasato Y, Nishihara M, Mori T, Meno K, Shibao K, Takenaka K, Iwasaki K. Feasibility and efficacy of an ultra-short side branch-dedicated balloon in coronary bifurcation stenting. EuroIntervention. 2021;17:e425–32. doi: 10.4244/EIJ-D-20-00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banning AP, Lassen JF, Burzotta F, Lefèvre T, Darremont O, Hildick-Smith D, Louvard Y, Stankovic G. Percutaneous coronary intervention for obstructive bifurcation lesions: the 14th consensus document from the European Bifurcation Club. EuroIntervention. 2019;15:90–8. doi: 10.4244/EIJ-D-19-00144. [DOI] [PubMed] [Google Scholar]
- Pan M, Ojeda S, Villanueva E, Chavarria J, Romero M, Suarez de, Mazuelos F, Segura J, Carrasco F, Hidalgo F, Lopez Aguilera, Rodriguez S, Puente M, Suarez de. Structural Damage of Jailed Guidewire During the Treatment of Coronary Bifurcation Lesions: A Microscopic Randomized Trial. JACC Cardiovasc Interv. 2016;9:1917–24. doi: 10.1016/j.jcin.2016.06.030. [DOI] [PubMed] [Google Scholar]
- Burns AT, Gutman J, Whitbourn R. Side-branch wire entrapment during bifurcation PCI: Avoidance and management. Catheter Cardiovasc Interv. 2010;75:351–3. doi: 10.1002/ccd.22269. [DOI] [PubMed] [Google Scholar]
- Gao L, Zhang C, Wang H, Zhang Y, Gao Z, Xu B, Chen J, Yuan J, Qiao S, Chen J. Scanning Electron Microscopic Assessment of Stent Coating Integrity in Jailed Wire Technique for Bifurcation Treatment. J Interv Cardiol. 2021;2021:262393. doi: 10.1155/2021/2629393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Brott BC, Foley R, Alli O, Sasse M, Ahmed M, Al Solaiman, Reddy G, Ather S, Leesar MA. Safety of hydrophilic guidewires used for side-branch protection during stenting and proximal optimization technique in coronary bifurcation lesions. Cardiovasc Revasc Med. 2016;17:456–62. doi: 10.1016/j.carrev.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, White JS, Hashim T, Leesar MA. Jailing polymer jacketed guide-wires during bifurcation coronary interventions is associated with procedural myocardial infarction. World J Cardiol. 2017;9:442–447. doi: 10.4330/wjc.v9.i5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou K, Zhang D, Pan H, Guo N, Li L, Li Y, Zhang Q, Liu B, Shen Z, Zhang B, Liu J, Han W, Wang Y, Zhao Y, Yang Y, Chen S, Xie L, Guan C, Kirtane AJ, Xu B CIT-RESOLVE Investigators. Active SB-P Versus Conventional Approach to the Protection of High-Risk Side Branches: The CIT-RESOLVE Trial. JACC Cardiovasc Interv. 2020;13:1112–22. doi: 10.1016/j.jcin.2020.01.233. [DOI] [PubMed] [Google Scholar]
- Kuno T, Sugiyama T, Imaeda S, Hashimoto K, Ryuzaki T, Yokokura S, Saito T, Yamazaki H, Tabei R, Kodaira M, Numasawa Y. Novel Insights of Jailed Balloon and Jailed Corsair Technique for Percutaneous Coronary Intervention of Bifurcation Lesions. Cardiovasc Revasc Med. 2019;20:1065–72. doi: 10.1016/j.carrev.2019.01.033. [DOI] [PubMed] [Google Scholar]
- Megaly M, Rofael M, Saad M, Shishehbor M, Brilakis ES. Outcomes with Drug-Coated Balloons for Treating the Side Branch of Coronary Bifurcation Lesions. J Invasive Cardiol. 2018;30:393–9. [PubMed] [Google Scholar]
- Jing QM, Zhao X, Han YL, Gao LL, Zheng Y, Li ZQ, Yang P, Cong HL, Gao CY, Jiang TM, Li H, Li JX, Wang DM, Wang G, Cong ZC, Zhang Z. A drug-eluting Balloon for the trEatment of coronarY bifurcatiON lesions in the side branch: A prospective multicenter ranDomized (BEYOND) clinical trial in China. Chin Med J (Engl) 2020;133:899–908. doi: 10.1097/CM9.0000000000000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildick-Smith D, Behan MW, Lassen JF, Chieffo A, Lefèvre T, Stankovic G, Burzotta F, Pan M, Ferenc M, Bennett L, Hovasse T, Spence MS, Oldroyd K, Brunel P, Carrie D, Baumbach A, Maeng M, Skipper N, Louvard Y. The EBC TWO Study (European Bifurcation Coronary TWO): A Randomized Comparison of Provisional T-Stenting Versus a Systematic 2 Stent Culotte Strategy in Large Caliber True Bifurcations. Circ Cardiovasc Interv. 2016;9:e003643. doi: 10.1161/CIRCINTERVENTIONS.115.003643. [DOI] [PubMed] [Google Scholar]
- Hildick-Smith D, Egred M, Banning A, Brunel P, Ferenc M, Hovasse T, Wlodarczak A, Pan M, Schmitz T, Silvestri M, Erglis A, Kretov E, Lassen JF, Chieffo A, Lefèvre T, Burzotta F, Cockburn J, Darremont O, Stankovic G, Morice MC, Louvard Y. The European bifurcation club Left Main Coronary Stent study: a randomized comparison of stepwise provisional vs. systematic dual stenting strategies (EBC MAIN). Eur Heart J. 2021;42:3829–39. doi: 10.1093/eurheartj/ehab283. [DOI] [PubMed] [Google Scholar]
- Ferenc M, Gick M, Comberg T, Rothe J, Valina C, Toma A, Löffelhardt N, Hochholzer W, Riede F, Kienzle RP, Achtari A, Neumann FJ. Culotte stenting vs. TAP stenting for treatment of de-novo coronary bifurcation lesions with the need for side-branch stenting: the Bifurcations Bad Krozingen (BBK) II angiographic trial. Eur Heart J. 2016;37:3399–405. doi: 10.1093/eurheartj/ehw345. [DOI] [PubMed] [Google Scholar]
- Colombo A, Stankovic G, Orlic D, Corvaja N, Liistro F, Airoldi F, Chieffo A, Spanos V, Montorfano M, Di Mario. Modified T-stenting technique with crushing for bifurcation lesions: immediate results and 30-day outcome. Catheter Cardiovasc Interv. 2003;60:145–51. doi: 10.1002/ccd.10622. [DOI] [PubMed] [Google Scholar]
- Chen SL, Ye F, Zhang JJ, Zhu ZS, Lin S, Shan SJ, Liu ZZ, Liu Y, Duan BX, Ge JB. DK crush technique: Modified treatment of bifurcation lesions in coronary artery. Chin Med J (Engl) 2005;118:1746–50. [PubMed] [Google Scholar]
- Chen S, Zhang J, Ye F, Chen Y, Fang W, Wei M, He B, Sun X, Yang S, Kwan TW. Final kissing balloon inflation by classic crush stenting did not improve the clinical outcomes for the treatment of unprotected left main bifurcation lesions: The importance of double-kissing crush technique. Catheter Cardiovasc Interv. 2008;71:166–72. doi: 10.1002/ccd.21317. [DOI] [PubMed] [Google Scholar]
- Chen SL, Zhang JJ, Ye F, Chen YD, Patel T, Kawajiri K, Lee M, Kwan TW, Mintz G, Tan HC. Study comparing the double kissing (DK) crush with classical crush for the treatment of coronary bifurcation lesions: the DKCRUSH-1 Bifurcation Study with drug-eluting stents. Eur J Clin Invest. 2008;38:361–71. doi: 10.1111/j.1365-2362.2008.01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavarra F. Proximal side optimization: A modification of the double kissing crush technique. US Cardiology Review. 2020;14:e02. [Google Scholar]
- Chevalier B, Glatt B, Royer T, Guyon P. Placement of coronary stents in bifurcation lesions by the “culotte” technique. Am J Cardiol. 1998;82:943–9. doi: 10.1016/s0002-9149(98)00510-4. [DOI] [PubMed] [Google Scholar]
- Chen LL, Fan L, Chen ZY, Zhen XC, Luo YK, Lin CG, Peng YF. Modified culotte stenting for treatment of complex coronary bifurcation lesions: Immediate and 9-month outcomes in apilot study. Chin Med J (Engl) 2011;124:1943–50. [PubMed] [Google Scholar]
- Fan L, Chen L, Luo Y, Zhang L, Zhong W, Lin C, Chen Z, Peng Y, Zhen X, Dong X. DK mini-culotte stenting in the treatment of true coronary bifurcation lesions: a propensity score matching comparison with T-provisional stenting. Heart Vessels. 2016;31:308–21. doi: 10.1007/s00380-014-0611-7. [DOI] [PubMed] [Google Scholar]
- Hu F, Tu S, Cai W, Jiang Z, Zheng H, Xiao L, Qiu C, Xiong C, Yao Y, Chen L. Double kissing mini-culotte versus mini-culotte stenting: Insights from micro-computed tomographic imaging of bench testing. EuroIntervention. 2019;15:465–72. doi: 10.4244/EIJ-D-18-00688. [DOI] [PubMed] [Google Scholar]
- Steigen TK, Maeng M, Wiseth R, Erglis A, Kumsars I, Narbute I, Gunnes P, Mannsverk J, Meyerdierks O, Rotevatn S, Niemelä M, Kervinen K, Jensen JS, Galløe A, Nikus K, Vikman S, Ravkilde J, James S, Aarøe J, Ylitalo A, Helqvist S, Sjögren I, Thayssen P, Virtanen K, Puhakka M, Airaksinen J, Lassen JF, Thuesen L Nordic PCI Study Group. Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation. 2006;114:1955–61. doi: 10.1161/CIRCULATIONAHA.106.664920. [DOI] [PubMed] [Google Scholar]
- Maeng M, Holm NR, Erglis A, Kumsars I, Niemelä M, Kervinen K, Jensen JS, Galløe A, Steigen TK, Wiseth R, Narbute I, Gunnes P, Mannsverk J, Meyerdierks O, Rotevatn S, Nikus K, Vikman S, Ravkilde J, James S, Aarøe J, Ylitalo A, Helqvist S, Sjögren I, Thayssen P, Virtanen K, Puhakka M, Airaksinen J, Christiansen EH, Lassen JF, Thuesen L Nordic-Baltic Percutaneous Coronary Intervention Study Group. Long-term results after simple versus complex stenting of coronary artery bifurcation lesions: Nordic Bifurcation Study 5-year follow-up results. J Am Coll Cardiol. 2013;62:30–4. doi: 10.1016/j.jacc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Ferenc M, Gick M, Kienzle RP, Bestehorn HP, Werner KD, Comberg T, Kuebler P, Büttner HJ, Neumann FJ. Randomized trial on routine vs. provisional T-stenting in the treatment of de novo coronary bifurcation lesions. Eur Heart J. 2008;29:2859–67. doi: 10.1093/eurheartj/ehn455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenc M, Ayoub M, Büttner HJ, Gick M, Comberg T, Rothe J, Valina CM, Hochholzer W, Neumann FJ. Long-term outcomes of routine versus provisional T-stenting for de novo coronary bifurcation lesions: five-year results of the Bifurcations Bad Krozingen I study. EuroIntervention. 2015;11:856–9. doi: 10.4244/EIJV11I8A175. [DOI] [PubMed] [Google Scholar]
- Colombo A, Bramucci E, Saccà S, Violini R, Lettieri C, Zanini R, Sheiban I, Paloscia L, Grube E, Schofer J, Bolognese L, Orlandi M, Niccoli G, Latib A, Airoldi F. Randomized study of the crush technique versus provisional side-branch stenting in true coronary bifurcations: the CACTUS (Coronary Bifurcations: Application of the Crushing Technique Using Sirolimus-Eluting Stents) Study. Circulation. 2009;119:71–8. doi: 10.1161/CIRCULATIONAHA.108.808402. [DOI] [PubMed] [Google Scholar]
- Hildick-Smith D, de Belder, Cooter N, Curzen NP, Clayton TC, Oldroyd KG, Bennett L, Holmberg S, Cotton JM, Glennon PE, Thomas MR, Maccarthy PA, Baumbach A, Mulvihill NT, Henderson RA, Redwood SR, Starkey IR, Stables RH. Randomized trial of simple versus complex drug-eluting stenting for bifurcation lesions: the British Bifurcation Coronary Study: old, new, and evolving strategies. Circulation. 2010;121:1235–43. doi: 10.1161/CIRCULATIONAHA.109.888297. [DOI] [PubMed] [Google Scholar]
- Sirker A, Sohal M, Oldroyd K, Curzen N, Stables R, de Belder, Hildick-Smith D. The impact of coronary bifurcation stenting strategy on health-related functional status: a quality-of-life analysis from the BBC One (British Bifurcation Coronary; Old, New, and Evolving Strategies) study. JACC Cardiovasc Interv. 2013;6:139–45. doi: 10.1016/j.jcin.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Behan MW, Holm NR, de Belder, Cockburn J, Erglis A, Curzen NP, Niemelä M, Oldroyd KG, Kervinen K, Kumsars I, Gunnes P, Stables RH, Maeng M, Ravkilde J, Jensen JS, Christiansen EH, Cooter N, Steigen TK, Vikman S, Thuesen L, Lassen JF, Hildick-Smith D. Coronary bifurcation lesions treated with simple or complex stenting: 5-year survival from patient-level pooled analysis of the Nordic Bifurcation Study and the British Bifurcation Coronary Study. Eur Heart J. 2016;37:1923–8. doi: 10.1093/eurheartj/ehw170. [DOI] [PubMed] [Google Scholar]
- Kumsars I, Holm NR, Niemelä M, Erglis A, Kervinen K, Christiansen EH, Maeng M, Dombrovskis A, Abraitis V, Kibarskis A, Trovik T, Latkovskis G, Sondore D, Narbute I, Terkelsen CJ, Eskola M, Romppanen H, Laine M, Jensen LO, Pietila M, Gunnes P, Hebsgaard L, Frobert O, Calais F, Hartikainen J, Aarøe J, Ravkilde J, Engstrøm T, Steigen TK, Thuesen L, Lassen JF Nordic baltic bifurcation study group. Randomised comparison of provisional side branch stenting versus a two-stent strategy for treatment of true coronary bifurcation lesions involving a large side branch: The Nordic-Baltic Bifurcation Study IV. Open Heart. 2020;7:e000947. doi: 10.1136/openhrt-2018-000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Santoso T, Zhang JJ, Ye F, Xu YW, Fu Q, Kan J, Paiboon C, Zhou Y, Ding SQ, Kwan TW. A randomized clinical study comparing double kissing crush with provisional stenting for treatment of coronary bifurcation lesions: results from the DKCRUSH-II (Double Kissing Crush versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions) trial. J Am Coll Cardiol. 2011;57:914–20. doi: 10.1016/j.jacc.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Di Gioia, Sonck J, Ferenc M, Chen SL, Colaiori I, Gallinoro E, Mizukami T, Kodeboina M, Nagumo S, Franco D, Bartunek J, Vanderheyden M, Wyffels E, De Bruyne, Lassen JF, Bennett J, Vassilev D, Serruys PW, Stankovic G, Louvard Y, Barbato E, Collet C. Clinical Outcomes Following Coronary Bifurcation PCI Techniques: A Systematic Review and Network Meta-Analysis Comprising 5,711 Patients. JACC Cardiovasc Interv. 2020;13:1432–44. doi: 10.1016/j.jcin.2020.03.054. [DOI] [PubMed] [Google Scholar]
- Chen SL, Sheiban I, Xu B, Jepson N, Paiboon C, Zhang JJ, Ye F, Sansoto T, Kwan TW, Lee M, Han YL, Lv SZ, Wen SY, Zhang Q, Wang HC, Jiang TM, Wang Y, Chen LL, Tian NL, Cao F, Qiu CG, Zhang YJ, Leon MB. Impact of the complexity of bifurcation lesions treated with drug-eluting stents: the DEFINITION study (Definitions and impact of complEx biFurcation lesIons on clinical outcomes after percutaNeous coronary IntervenTIOn using drug-eluting steNts). JACC Cardiovasc Interv. 2014;7:1266–76. doi: 10.1016/j.jcin.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Ye F, Xu K, Kan J, Tao L, Santoso T, Munawar M, Tresukosol D, Li L, Sheiban I, Li F, Tian NL, Rodríguez AE, Paiboon C, Lavarra F, Lu S, Vichairuangthum K, Zeng H, Chen L, Zhang R, Ding S, Gao F, Jin Z, Hong L, Ma L, Wen S, Wu X, Yang S, Yin WH, Zhang J, Wang Y, Zheng Y, Zhou L, Zhou L, Zhu Y, Xu T, Wang X, Qu H, Tian Y, Lin S, Liu L, Lu Q, Li Q, Li B, Jiang Q, Han L, Gan G, Yu M, Pan D, Shang Z, Zhao Y, Liu Z, Yuan Y, Chen C, Stone GW, Han Y, Chen SL. Multicentre, randomized comparison of two-stent and provisional stenting techniques in patients with complex coronary bifurcation lesions: the DEFINITION II trial. Eur Heart J. 2020;41:2523–36. doi: 10.1093/eurheartj/ehaa543. [DOI] [PubMed] [Google Scholar]
- Chen SL, Xu B, Han YL, Sheiban I, Zhang JJ, Ye F, Kwan TW, Paiboon C, Zhou YJ, Lv SZ, Dangas GD, Xu YW, Wen SY, Hong L, Zhang RY, Wang HC, Jiang TM, Wang Y, Sansoto T, Chen F, Yuan ZY, Li WM, Leon MB. Clinical Outcome After DK Crush Versus Culotte Stenting of Distal Left Main Bifurcation Lesions: The 3-Year Follow-Up Results of the DKCRUSH-III Study. JACC Cardiovasc Interv. 2015;8:1335–42. doi: 10.1016/j.jcin.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Chen SL, Zhang JJ, Han Y, Kan J, Chen L, Qiu C, Jiang T, Tao L, Zeng H, Li L, Xia Y, Gao C, Santoso T, Paiboon C, Wang Y, Kwan TW, Ye F, Tian N, Liu Z, Lin S, Lu C, Wen S, Hong L, Zhang Q, Sheiban I, Xu Y, Wang L, Rab TS, Li Z, Cheng G, Cui L, Leon MB, Stone GW. Double Kissing Crush Versus Provisional Stenting for Left Main Distal Bifurcation Lesions: DKCRUSH-V Randomized Trial. J Am Coll Cardiol. 2017;70:2605–17. doi: 10.1016/j.jacc.2017.09.1066. [DOI] [PubMed] [Google Scholar]
- Chen X, Li X, Zhang JJ, Han Y, Kan J, Chen L, Qiu C, Santoso T, Paiboon C, Kwan TW, Sheiban I, Leon MB, Stone GW, Chen SL DKCRUSH-V Investigators. 3-Year Outcomes of the DKCRUSH-V Trial Comparing DK Crush With Provisional Stenting for Left Main Bifurcation Lesions. JACC Cardiovasc Interv. 2019;12:1927–37. doi: 10.1016/j.jcin.2019.04.056. [DOI] [PubMed] [Google Scholar]