Aims
This large cohort study aimed to assess the role of chronic statin use on COVID-19 disease severity.
Methods
An observational retrospective study from electronic medical records of hospitalized patients (n = 43 950) with COVID-19 between January and September 2020 in 185 hospitals in the United States. A total of 38 875 patients met inclusion criteria; 23 066 were included in the propensity-matched sampling with replacement cohort; 11 533 were prehospital statin users. The primary outcome was all-cause death; secondary outcomes were death from COVID-19 and serious complications. Mean, standard deviation, chi-square test, Student's t-test, linear regression, and binary and multinomial logistic regressions were used for statistical analysis.
Results
Among 38 875 patients, 30% were chronic statin users [mean age, 70.82 (±12.25); 47.1% women] and 70% were statin nonusers [mean age, 58.44 (±18.27); 48.5% women]. Key propensity-matched outcomes among 11 533 chronic statin users showed 20% lower risk of all-cause mortality (OR 0.80, 95% CI 0.74–0.86, P < 0.001), 23% lower risk of mortality from COVID-19 (OR 0.77, 95% CI 0.71–0.84, P < 0.001), 16% lower risk of ICU admission (OR 0.84, 95% CI 0.79–0.89, P < 0.001), 24% lower risk of critical acute respiratory distress syndrome with COVID-19 (OR 0.76, 95% CI 0.70–0.83, P < 0.001), 23% lower risk of mechanical ventilation (OR 0.77, 95% CI 0.71–0.82, P < 0.001), 20% lower risk of severe sepsis with septic shock (OR 0.80, 95% CI 0.67–0.93, P = 0.004), shorter hospital length of stay [9.87 (±8.94), P < 0.001] and brief duration of mechanical ventilation [8.90 (±8.94), P < 0.001].
Conclusion
Chronic use of statins is associated with reduced mortality and improved clinical outcomes in patients hospitalized for COVID-19.
Keywords: coronavirus, coronavirus disease 2019, epigenetic drugs, severe acute respiratory distress coronavirus 2, statins
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has claimed over six and a half million lives worldwide.1 In the United States, the Centers for Disease Control and Prevention Data Tracker reported a total of 96 581 755 cases and 1 057 975 deaths since 21 January 2020.2 Despite aggressive efforts to control the disease spread, the emergence of new variants poses an ongoing threat.
Severe acute respiratory distress coronavirus 2 (SARS-CoV-2) may cause a wide spectrum of respiratory manifestations, varying from mild upper respiratory infections to severe pneumonia. SARS-CoV-2 enters the host cell mainly through the angiotensin-converting enzyme 2 (ACE2) receptor, induces epigenetic modifications, such as DNA methylation, RNA modifications, histone, and nonhistone modifications, triggering viral replication and the release of proinflammatory cytokines, and activation of inflammatory cells, leading to oxidative stress, endothelial dysfunction, apoptosis, acute respiratory distress syndrome (ARDS) and, ultimately, death from multiorgan failure.3,4 Repurposed drugs (e.g. sartans, statins, metformin) that interfere with epigenetic pathways may provide novel therapeutic approaches.4–6
Statins may have beneficial effects on COVID-19 disease because of their cholesterol-independent pleiotropic effects, such as inhibition of viral entry, antiinflammation, antithrombosis, and immunomodulation.7 Statins showed some encouraging beneficial clinical results in viral infections such as seasonal flu, Middle East respiratory syndrome coronavirus (MERS-CoV), and Ebola virus.8–10 Therefore, we performed a retrospective study to evaluate the effects of the preexisting use of statins on mortality and morbidity outcomes in a large population of patients hospitalized with COVID-19. We hypothesized that chronic, routine use of statins might be protective against COVID-19 disease severity in hospitalized patients.11
Methods
Study design and population
A retrospective observational propensity-matched cohort study was conducted through the electronic medical records (EMR) of 185 primarily community hospitals of a wide-scale health system across the USA. All participating hospitals are part of a single healthcare system and use the same EMR software. The data were entered into a centralized data set in a secured server within the hospital system's clinical data warehouse and subsequently abstracted as de-identified data. This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board (IRB) Manager system. The requirement for written informed consent was waived as the obtained data were de-identified.
All patients hospitalized for COVID-19 (ICD10 U07.1) from 1 January 2020 to 30 September 2020 were included in this study. Patients were considered to have confirmed COVID-19 infection if the initial nasopharyngeal swab result was positive for SARS-CoV-2 by PCR testing. Patients were excluded if they were less than 18 years old, the length of stay was less than 2 days, patients were admitted to trauma, and on chronic renal dialysis.
Study cohort and variables
The cohort study included 43 950 laboratory-confirmed, hospitalized COVID-19-positive patients. Categorical, binary, and numerical data were retrieved from a structured relational database management system using diagnostic, procedural, and ICU admission codes constructed on a structured query language (SQL) programming interface (see Appendix 1 Table, Supplemental Digital Content).
Demographic information included age, BMI, gender, race, smoking status, payer type, and comorbidities. Comorbidities and secondary outcomes were captured using ICD-10 codes (see Appendix 1, Supplemental Digital Content). Statins included in the study were simvastatin, atorvastatin, pravastatin, rosuvastatin, fluvastatin, pitavastatin, and lovastatin, and data were collected from the active medication list for the patient encounter. Statin users (exposure group) were defined as those with prescribed oral statin prior to admission. Duration of statin outpatient use was not reported at the time of admission. Nonstatin users were the control group.
Outcomes
The primary outcome of the study was all-cause of death during hospitalization. Secondary outcomes were mortality from COVID-19, discharge to hospice, ICU admission, the severity of ARDS, need for invasive mechanical ventilation, extracorporeal membrane oxygenation, severe sepsis with and without shock, acute kidney injury, thrombosis, pulmonary embolism, disseminated intravascular coagulation, duration of mechanical ventilation, and length of stay.
Patient demographics, diagnosis, available laboratory data, and procedures were also reviewed.
Statistical analyses
Continuous data were expressed as mean with standard deviation (SD), and the difference between the groups was compared. Parametric data expressed as proportions were evaluated by chi-square test for categorical variables and Student's t-test for continuous variables. Primary and secondary outcomes were tested using the chi-square, Student's t-test, linear regression, and binary and multinomial logistic regression. The laboratory data were analyzed using mean and SD; differences were tested using an independent samples t-test.
Propensity score analyses (PSA) were performed on baseline characteristics to minimize confounding by selection using propensity score matching (PSM) with sampling without and with replacement. Chronic statin users were matched with nonusers on the following covariates – age, gender, BMI, race, smoking status, payer type, and comorbidities, including atrial fibrillation, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, malignancy, peripheral arterial disease, previous myocardial infarction, renal disease, and stroke. In conducting PSA, logistic regression was performed on the unmatched cohort, adjusting for covariates to build a predictive model. The resulting propensity variables were used to select controls for the cases. Case–control match tolerance was set at 0.1, and a fuzzy matching technique was utilized to identify approximately similar patients. We performed two separate PSM analyses: matchings without replacements for better precision and matchings with replacements for lesser selection bias.12 Standardized mean difference (SMD) was used to examine the balance of covariate distribution between exposed and unexposed groups. A 0.1 threshold was used to determine whether the balance had been achieved or not. A 10% SMD indicated substantial overlap in covariates between exposed and unexposed and better matching.
Missing values
Missing values were encountered for patients’ baseline characteristics and laboratory data during the preliminary analysis. Given the lack of control on data collection on retrospective data, the following baseline characteristics of COVID-19 patients were omitted from our study design because of a significant number of missing values: home medication list, statin dosages, types of statins, duration of statin intake, SOFA score on ICU admission, and in-hospital treatments. For laboratory variables, we performed a listwise deletion for missing values. No imputation was performed for missing values in our study.
All statistical analyses were performed using IBM SPSS version: 28.0.1.0 (142). Outcomes were determined to be statistically significant if the adjusted odds ratios did not overlap with 1.00. P-values less than 0.05 were considered statistically significant.
Results
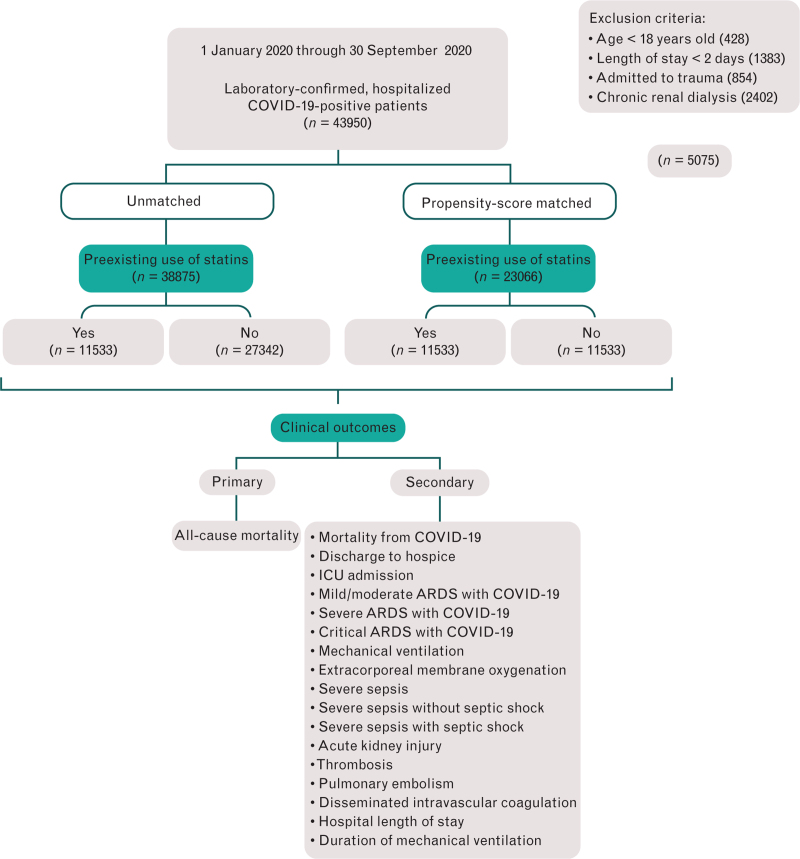
There was a total of 43 950 patients with confirmed cases of COVID-19 within the study period. A total of 38 875 patients met inclusion criteria (Fig. 1). Before PSM, the exposure group (statin users) included 11 533 (30%) COVID-19-positive patients; the control group (nonstatin users) included 27 342 (70%) COVID-19-positive patients. After PSM, we had 11 533 cases in the preexisting statin group and 11 533 controls in the nonstatin group. The output of the PSM revealed zero exact matches for sampling without replacement and 30 exact matches for sampling with replacement. The unmatched missing keys and valid keys were zero for both PSM models. Results of propensity score sampling with replacement showed a better covariate matching balance and exchangeability between the preexisting statin and nonstatin group than sampling without replacement, as shown with standardized mean differences in the tables. Therefore, we have included only PSM sampling with replacement analysis in our results and discussion. Results of PSM sampling without replacement are available in Appendix 2, Supplemental digital content 2.
Fig. 1.
A retrospective cohort flow diagram.
Table 1 provides baseline demographic characteristics between the unmatched and the propensity-matched cohorts sampling with replacement. In the unmatched cohorts, preexisting statin users were older (age: 65–74), the predominant race was white – not Hispanic, on Medicare, and with more comorbidities. The statin nonusers were younger (age: 51–64 years), the predominant race was Hispanics, payer type – others, and with fewer comorbidities. In the propensity-matched cohorts, the statin nonusers’ group had a mean age of 70.19 (±16.28) years, adjusted from 58.44 (±18.27) years. The two groups had a similar predominant age of between 65 and 74 years, BMI of 30+, gender: male, race: white – not Hispanic, on Medicare, nonsmoker status, and comorbidities, with diabetes being the most prevalent.
Table 1.
Comparison of baseline characteristics between preexisting statin users and nonusers before and after propensity matching
| Patient characteristics | Before propensity matching | After propensity matching, sampling with replacement, covariate-adjusted | ||||||||
| Preexisting use of statins | Preexisting use of statins | |||||||||
| Yes | No | Cohort | Std difa | P-value | Yes | No | Cohort | Std difa | Adjusted P-value | |
| (n = 11 533) | (n = 27 342) | (n = 38 875) | (n = 11 533) | (n = 11 533) | (n = 23 066) | |||||
| Demographics | ||||||||||
| Age (mean ± SD) | 70.82 ± 12.25 | 58.44 ± 18.27 | 62.11 ± 17.64 | −0.70 | <0.0001 | 70.82 ± 12.25 | 70.19 ± 13.26 | 70.50 ± 12.77 | −0.04 | <0.001 |
| Age range [no. (%)] | <0.0001 | <0.001 | ||||||||
| 18–30 | 19 (0.2) | 2152 (7.9) | 2171 (5.6) | −10.55 | 19 (0.2) | 51 (0.4) | 70 (0.3) | −1.03 | ||
| 31–50 | 688 (6.0) | 7201 (26.3) | 7889 (20.3) | −2.93 | 688 (6.0) | 697 (6.0) | 1385 (6.0) | −0.01 | ||
| 51–64 | 2662 (23.1) | 7428 (27.2) | 10 090 (26.0) | −1.07 | 2662 (23.1) | 2800 (24.3) | 5462 (23.7) | −0.05 | ||
| 65–74 | 3353 (29.1) | 4495 (16.4) | 7848 (20.2) | −0.29 | 3353 (29.1) | 3225 (28.0) | 6578 (28.5) | 0.04 | ||
| 75–84 | 3155 (27.4) | 3572 (13.1) | 6727 (17.3) | −0.12 | 3155 (27.4) | 2993 (26.0) | 6148 (26.7) | 0.05 | ||
| ≥85 | 1656 (14.4) | 2494 (9.1) | 4150 (10.7) | −0.41 | 1656 (14.4) | 1767 (15.3) | 3423 (14.8) | −0.06 | ||
| BMI (mean ± SD) | 30.30 ± 7.33 | 30.90 ± 7.93 | 30.7 ± 7.7 | 0.07 | <0.001 | 30.30 ± 7.33 | 30.07 ± 7.59 | 30.18 ± 7.46 | −0.03 | 0.53 |
| BMI range [no. (%)] | <0.001 | <0.01 | ||||||||
| <18.5 | 214 (1.9) | 625 (2.3) | 839 (2.2) | −1.12 | 214 (1.9) | 343 (2.1) | 457 (2.0) | −0.60 | ||
| 18.5–24.9 | 2540 (22.0) | 5558 (20.3) | 8098 (20.8) | −0.80 | 2540 (22.0) | 2760 (23.5) | 5246 (22.7) | −0.08 | ||
| 25–29.9 | 3603 (31.2) | 8152 (29.8) | 11 755 (30.2) | −0.84 | 3603 (31.2) | 3581 (31.1) | 7184 (31.1) | 0.01 | ||
| 30+ | 5176 (44.9) | 13 007 (47.6) | 18 183 (46.8) | −0.95 | 5176 (44.9) | 5003 (43.4) | 10 179 (44.1) | 0.03 | ||
| Gender [no. (%)] | 0.01 | 0.07 | ||||||||
| Female | 5431 (47.1) | 13 262 (48.5) | 18 693 (48.1) | −0.92 | 5431 (47.1) | 5565 (48.3) | 10 996 (47.7) | −0.02 | ||
| Male | 6102 (52.9) | 14 080 (51.5) | 20 182 (51.9) | −0.86 | 6102 (52.9) | 5968 (51.7) | 12 070 (52.3) | 0.02 | ||
| Race [no. (%)] | <0.001 | <0.67 | ||||||||
| Asian | 248 (2.2) | 457 (1.7) | 705 (1.8) | −0.62 | 248 (2.2) | 237 (2.1) | 485 (2.1) | 0.05 | ||
| Black not Hispanic | 2296 (19.9) | 5478 (20.0) | 7774 (20.0) | −0.90 | 2296 (19.9) | 2344 (20.3) | 4640 (20.1) | −0.02 | ||
| Other | 397 (3.4) | 1291 (4.7) | 1688 (34.0) | −1.25 | 397 (3.4) | 423 (3.7) | 820 (3.6) | −0.06 | ||
| White not Hispanic | 5422 (47.0) | 9201 (33.7) | 14 623 (37.6) | −0.54 | 5422 (47.0) | 5351 (46.4) | 10 773 (46.7) | 0.01 | ||
| Hispanic | 2980 (25.8) | 10 250 (37.5) | 13 230 (34.0) | −1.32 | 2980 (25.8) | 2967 (25.7) | 5947 (25.8) | 0.00 | ||
| Unknown | 190 (1.6) | 665 (2.4) | 855 (2.2) | −1.34 | 190 (1.6) | 211 (1.8) | 401 (1.7) | −0.10 | ||
| Smoking status [no. (%)] | <0.001 | <0.01 | ||||||||
| Nonsmoker | 8984 (77.9) | 20 832 (76.2) | 29 816 (76.7) | −0.87 | 8984 (77.9) | 8816 (76.4) | 17 800 (77.2) | 0.02 | ||
| Smoker | 522 (4.5) | 1919 (7.0) | 2441 (6.3) | −1.40 | 522 (4.5) | 587 (5.1) | 1109 (4.8) | −0.12 | ||
| Unknown | 2027 (17.6) | 4591 (16.8) | 6618 (17.0) | −0.84 | 2027 (17.6) | 2130 (18.5) | 4157 (18.0) | −0.05 | ||
| Payer type [no. (%)] | <0.0001 | <0.05 | ||||||||
| Blue Cross | 281 (2.4) | 1029 (3.8) | 1310 (3.4) | −1.39 | 281 (2.4) | 305 (2.6) | 586 (2.5) | −0.08 | ||
| Charity | 20 (0.2) | 277 (1.0) | 297 (0.8) | −3.45 | 20 (0.2) | 20 (0.2) | 40 (0.2) | 0.00 | ||
| Commercial | 33 (0.3) | 183 (0.7) | 216 (0.6) | −1.93 | 33 (0.3) | 27 (0.2) | 60 (0.3) | 0.20 | ||
| Medicaid | 583 (5.1) | 3426 (12.5) | 4009 (10.3) | −2.01 | 583 (5.1) | 639 (5.5) | 1222 (5.3) | −0.09 | ||
| Medicare | 8382 (72.7) | 10 797 (39.5) | 19 179 (49.3) | −0.25 | 8382 (72.7) | 8170 (70.8) | 16 552 (71.8) | 0.03 | ||
| Others | 2174 (18.9) | 11 177 (40.9) | 13 351 (34.3) | −1.83 | 2174 (18.9) | 2307 (20.0) | 4481 (19.4) | −0.06 | ||
| Self-pay | 28 (0.2) | 342 (1.3) | 370 (1.0) | −3.21 | 28 (0.2) | 40 (0.3) | 68 (0.3) | −0.36 | ||
| Worker's compensation | 32 (0.3) | 111 (0.4) | 143 (0.4) | −1.33 | 32 (0.3) | 25 (0.2) | 57 (0.2) | 0.25 | ||
| Comorbidities [no. (%)] | ||||||||||
| Atrial fibrillation | 2605 (22.6) | 3437 (12.6) | 6042 (15.5) | −0.27 | <0.001 | 2605 (22.6) | 2528 (21.9) | 5133 (22.3) | −0.01 | 0.22 |
| Chronic obstructive pulmonary disease | 2309 (20.0) | 2847 (10.4) | 5156 (13.3) | −0.01 | <0.001 | 2309 (20.0) | 2127 (18.4) | 4436 (19.2) | −0.04 | 0.002 |
| Congestive heart failure | 2802 (24.3) | 3452 (12.6) | 6254 (16.1) | −0.31 | <0.001 | 2802 (24.3) | 2564 (22.2) | 5366 (23.3) | −0.04 | <0.001 |
| Diabetes mellitus | 6934 (60.1) | 9949 (36.4) | 16 883 (43.4) | −0.28 | 0.0001 | 6934 (60.1) | 6551 (56.8) | 13 485 (58.5) | −0.06 | <0.001 |
| Malignancy | 465 (4.0) | 899 (3.3) | 1364 (3.5) | −0.47 | <0.001 | 465 (4.0) | 508 (4.4) | 973 (4.2) | 0.01 | 0.15 |
| Peripheral artery disease | 743 (6.4) | 901 (3.3) | 1644 (4.2) | −0.04 | <0.001 | 743 (6.4) | 654 (5.7) | 1397 (6.1) | −0.03 | 0.01 |
| Previous myocardial infarction | 1365 (11.8) | 1828 (6.7) | 3193 (8.2) | −0.18 | <0.001 | 1365 (11.8) | 1251 (10.8) | 2616 (10.1) | −0.03 | 0.01 |
| Renal disease | 3222 (27.9) | 3885 (14.2) | 7107 (18.3) | −0.15 | <0.001 | 3222 (27.9) | 2948 (25.6) | 6170 (26.7) | −0.05 | <0.001 |
| Stroke (cerebral infarction) | 170 (1.5) | 351 (1.3) | 521 (1.3) | −0.35 | 0.13 | 170 (1.5) | 180 (1.6) | 350 (1.5) | 0.00 | 0.59 |
Standardized difference adjusted covariates: age, BMI, gender, race, smoking status, payer type, and comorbidities.
Table 2 provides covariate unadjusted clinical outcomes data for preexisting statin users and nonusers in the unmatched and matched cohorts. Before matching, in the unmatched cohort, all-cause mortality was higher in the preexisting statin users compared with the statin nonusers (17.6 vs. 13.2%; P-value <0.001). For the secondary outcomes, preexisting statin users had higher in-hospital mortality from COVID-19 (12.4 vs. 10%; P-value <0.001), discharge to hospice (8 vs. 5.3%; P-value <0.001), ICU admission (35.9 vs. 34.8%; P-value 0.03), duration of mechanical ventilation (8.9 ± 8.94 vs. 10.46 ± 10.91 days; P-value <0.001), higher rates of acute kidney injury (3.9 vs. 3%; P-value <0.001) and longer hospital length of stay (9.87 ± 8.94 vs. 9.30 ± 9.76 days; P-value <0.001). After matching, in the propensity-matched cohort, the all-cause mortality was lower for preexisting statin users (17.6 vs. 20.1%; P-value <0.001). For the secondary outcomes, preexisting statin users had lower rates of mortality from COVID-19 (12.4 vs. 14.5%; P-value <0.001), ICU admission (35.9 vs. 39%; P-value <0.001), severe ARDS with COVID-19 (22.9 vs. 24%; P-value <0.001), critical ARDS with COVID-19 (12.9 vs. 15.1%; P-value <0.001), mechanical ventilation (15.1 vs. 17.8%; P-value <0.001), severe sepsis (3.6 vs. 4.4%; P-value <0.002), severe sepsis with septic shock (3.1 vs. 3.7%; P-value = 0.01); P-value = 0.05), AKI (3.9 vs. 4.6%; P = 0.01), thrombosis (0.3 vs. 0.4%; P-value = 0.03), hospital length of stay (9.87 ± 8.94 vs. 10.44 ± 9.53 days; P-value <0.001), duration of mechanical ventilation (8.90 ± 8.94 vs. 10.20 ± 10.17 days; P-value <0.001), and higher rates of mild/moderate ARDS with COVID-19 (64.1 vs. 61%; P-value < 0.001).
Table 2.
Clinical outcomes before and after propensity matching with replacement between statin users and nonusers
| Outcomes | Before propensity matching, covariate unadjusted analysis (n = 38 875) | After propensity matching with replacement, covariate unadjusted analysis (n = 23 066) | ||||||||
| Preexisting use of statins | Preexisting use of statins | |||||||||
| Yes | No | Cohort | Std difa | P-value | Yes | No | Cohort | Std difa | P-value | |
| (n = 11 533) | (n = 27 342) | (n = 38 875) | (n = 11 533) | (n = 11 533) | (n = 23 066) | |||||
| Primary [no. (%)] | ||||||||||
| All-cause mortality | 2032 (17.6) | 3611 (13.2) | 5643 (14.5) | -0.12 | <0.001 | 2032 (17.6) | 2316 (20.1) | 4348 (18.9) | 0.06 | <0.001 |
| Secondary [no. (%)] | ||||||||||
| In-hospital mortality from COVID-19 | 1430 (12.4) | 2727 (10.0) | 4157 (10.7) | −0.65 | <0.001 | 1430 (12.4) | 1672 (14.5) | 3102 (13.4) | −0.15 | <0.001 |
| Discharge to hospice | 927 (8.0) | 1454 (5.3) | 2381 (6.1) | −0.45 | <0.001 | 927 (8.0) | 992 (8.6) | 1919 (8.3) | −0.06 | <0.001 |
| ICU admission | 4139 (35.9) | 9510 (34.8) | 136 469 (35.1) | −0.02 | 0.03 | 4139 (35.9) | 4500 (39.0) | 8639 (37.5) | 0.06 | <0.001 |
| Mild/moderate ARDS with COVID-19 | 7394 (64.1) | 17 832 (65.2) | 25 226 (64.9) | −0.90 | 0.11 | 7394 (64.1) | 7033 (61.0) | 14 427 (62.5) | 0.05 | <0.001 |
| Severe ARDS with COVID-19 | 2646 (22.9) | 6059 (22.2) | 8705 (22.4) | −0.85 | 0.11 | 2646 (22.9) | 2764 (24.0) | 5410 (23.5) | −0.04 | <0.001 |
| Critical ARDS with COVID-19 | 1493 (12.9) | 3451 (12.6) | 4944 (12.7) | −0.86 | 0.11 | 1493 (12.9) | 1736 (15.1) | 3229 (14.0) | −0.15 | <0.001 |
| Mechanical ventilation | 1737 (15.1) | 3986 (14.6) | 5723 (14.7) | −0.01 | 0.22 | 1737 (15.1) | 2049 (17.8) | 3786 (16.4) | 0.07 | <0.001 |
| Extracorporeal membrane oxygenation | 3 (0.0) | 26 (0.1) | 29 (0.1%) | 0.02 | 0.02 | 3 (0.0) | 8 (0.1) | 11 (0.0) | 0.01 | 0.13 |
| Severe sepsis | 416 (3.6) | 911 (3.3) | 1327 (3.4) | −0.01 | 0.17 | 416 (3.6) | 507 (4.4) | 923 (4.0) | 0.04 | 0.002 |
| Severe sepsis without septic shock | 58 (0.5) | 140 (0.5) | 198 (0.5) | 0.00 | 0.90 | 58 (0.5) | 81 (0.7) | 139 (0.6) | 0.02 | 0.05 |
| Severe sepsis with septic shock | 358 (3.1) | 772 (2.8) | 1130 (2.9) | −0.01 | 0.13 | 358 (3.1) | 426 (3.7) | 784 (3.4) | 0.03 | 0.01 |
| Acute kidney injury | 454 (3.9) | 812 (3.0) | 1266 (3.3) | −0.05 | <0.001 | 454 (3.9) | 531 (4.6) | 985 (4.3) | 0.03 | 0.01 |
| Thrombosis | 32 (0.3) | 99 (0.4) | 131 (0.3) | 0.01 | 0.18 | 32 (0.3) | 51 (0.4) | 83 (0.4) | 0.02 | 0.03 |
| Pulmonary embolism | 43 (0.4) | 102 (0.4) | 145 (0.4) | 0.00 | 0.99 | 43 (0.4) | 53 (0.5) | 96 (0.4) | 0.01 | 0.30 |
| Disseminated intravascular coagulation | 24 (0.2) | 52 (0.2) | 76 (0.2) | −0.00 | 0.71 | 24 (0.2) | 23 (0.2) | 47 (0.2) | −0.00 | 0.88 |
| Hospital length of stay | 9.87 ± 8.94 | 9.30 ± 9.76 | 9.47 ± 9.53 | −0.06 | <0.001 | 9.87 ± 8.94 | 10.44 ± 9.53 | 10.15 ± 9.2 | 0.06 | <0.001 |
| Duration of mechanical ventilation | 8.90 ± 8.94 | 10.46 ± 10.91 | 9.99 ± 10.3 | 0.15 | <0.001 | 8.90 ± 8.94 | 10.20 ± 10.17 | 9.60 ± 9.65 | 0.13 | <0.001 |
ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019.
Standardized difference.
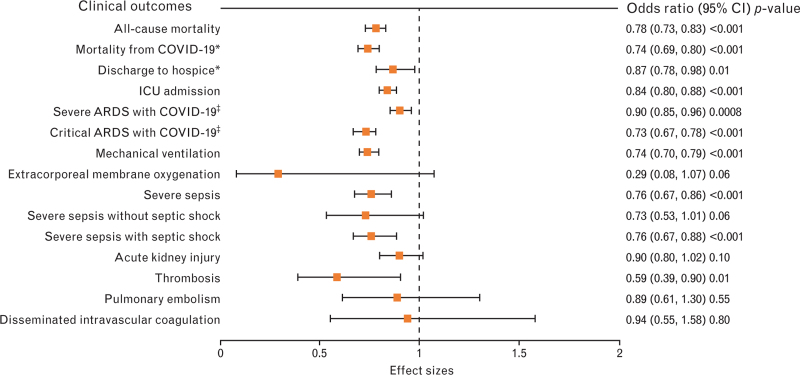
Table 3 provides the covariate-adjusted odd ratios before and after matching in preexisting statin users and nonusers. Logistic regression analysis in the unmatched preexisting statin users showed significantly lower rates of all-cause mortality (OR 0.78, 95% CI 0.73–0.83; P-value <0.001), mortality from COVID-19 (OR 0.74, 95% CI 0.69–0.80; P-value <0.001), discharge to hospice (OR 0.87, 95% CI 0.78–0.98; P-value 0.01), ICU admission (OR 0.84, 95% CI 0.80–0.88; P-value <0.001), severe ARDS with COVID-19 (OR 0.90, 95% CI 0.85–0.96; P-value <0.001), critical ARDS with COVID-19 (OR 0.73, 95% CI 0.67–0.78; P-value <0.001), mechanical ventilation (OR 0.74, 95% CI 0.70–0.79; P-value <0.001), severe sepsis (OR 0.76, 95% CI 0.67–0.88; P-value <0.001), severe sepsis with septic shock (OR 0.76, 95% CI 0.67–0.88; P-value <0.001) and thrombosis (OR 0.89, 95% CI 0.39–0.90; P-value = 0.01). The same results of logistic regression in an unmatched cohort are illustrated in Fig. 2.
Table 3.
Logistic regression of clinical outcomes before and after propensity matching with replacement in daily statin users
| Outcomes | Before propensity matching and covariate-adjusted effect size in prehospital statin users (n = 11533) | After propensity matching with replacement and covariate-adjusted effect size in prehospital statin users (n = 11533) (same as Fig. 2) | ||||||||||
| OR | (95% CI) | Adjusted P-value | OR | (95% CI) | Adjusted P-value | |||||||
| Primary [no. (%)] | ||||||||||||
| All-cause mortality | 0.78 | 0.73–0.83 | <0.001 | 0.80 | 0.74–0.86 | <0.001 | ||||||
| Secondary [no. (%)] | ||||||||||||
| In-hospital mortality from COVID-19 vs. normal or other discharge | 0.74 | 0.69–0.80 | <0.001 | 0.77 | 0.71–0.84 | <0.001 | ||||||
| Discharge to hospice vs. normal or other discharge | 0.87 | 0.78–0.98 | 0.01 | 0.88 | 0.80–0.97 | 0.01 | ||||||
| ICU admission | 0.84 | 0.80–0.88 | <0.001 | 0.84 | 0.79–0.89 | <0.001 | ||||||
| Severe ARDS with COVID-19 vs. mild/moderate | 0.90 | 0.85–0.96 | 0.0008 | 0.89 | 0.83–0.94 | <0.001 | ||||||
| Critical ARDS with COVID-19 vs. mild/moderate | 0.73 | 0.67–0.78 | <.0001 | 0.76 | 0.70–0.83 | <0.001 | ||||||
| Mechanical ventilation | 0.74 | 0.70–0.79 | <0.001 | 0.77 | 0.71–0.82 | <0.001 | ||||||
| Extracorporeal membrane oxygenation | 0.29 | 0.08–1.07 | 0.06 | 0.30 | 0.05–1.57 | 0.15 | ||||||
| Severe sepsis | 0.76 | 0.67–0.86 | <0.001 | 0.78 | 0.68–0.89 | <0.001 | ||||||
| Severe sepsis without septic shock | 0.73 | 0.53–1.01 | 0.06 | 0.68 | 0.48–0.96 | 0.03 | ||||||
| Severe sepsis with septic shock | 0.76 | 0.67–0.88 | <0.001 | 0.80 | 0.67–0.93 | 0.004 | ||||||
| Acute kidney injury | 0.90 | 0.80–1.02 | 0.10 | 0.81 | 0.71–0.93 | 0.003 | ||||||
| Thrombosis | 0.59 | 0.39–0.90 | 0.01 | 0.60 | 0.38–0.93 | 0.02 | ||||||
| Pulmonary embolism | 0.89 | 0.61–1.30 | 0.55 | 0.82 | 0.54–1.23 | 0.34 | ||||||
| Disseminated intravascular coagulation | 0.94 | 0.55–1.58 | 0.80 | 0.80 | 0.74–0.86 | <0.001 | ||||||
| Estimate, standard error | (95% CI) | Adjusted P-value | Estimate, standard error | (95% CI) | Adjusted P-value | |||||||
| Hospital length of stay | −0.66, 0.11 | (−0.88 to −0.44) | <0.001 | −0.66, 0.12 | (−0.89 to −0.42) | <0.001 | ||||||
| Duration of mechanical ventilation | −1.19, 0.32 | (−1.83 to −0.56) | <0.001 | −1.27, 0.32 | (−1.91 to −0.63) | <0.001 | ||||||
ARDS, acute respiratory distress syndrome; CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
Fig. 2.
Before matching, covariate-adjusted odds ratio in preexisting statin users (n = 11 533) (same as right column above). ∗Normal or other discharge is the reference category; ‡mild/moderate acute respiratory distress syndrome (ARDS) with COVID-19 is the reference category. CI, confidence interval.
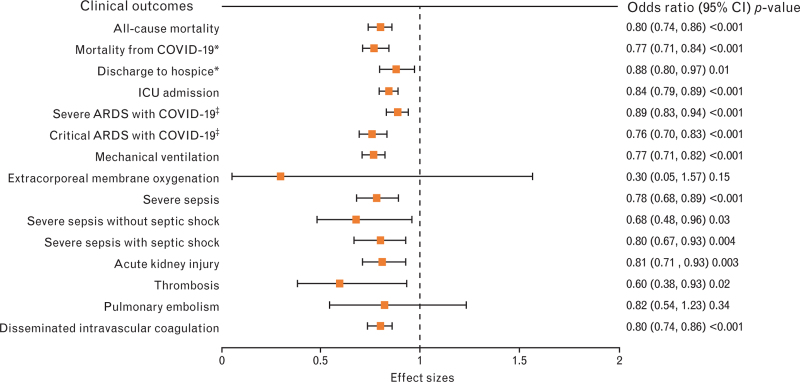
Logistic regression in the matched preexisting statin users showed lower rates of all-cause mortality (OR 0.80, 95% CI 0.74–0.86; P-value <0.001), mortality from COVID-19 (OR 0.77, 95% CI 0.71–0.84; P-value <0.001), discharge to hospice (OR 0.88, 95% CI 0.80–0.97; P-value 0.01), ICU admission (OR 0.84, 95% CI 0.79–0.89; P-value <0.001), severe ARDS with COVID-19 (OR 0.89, 95% CI 0.83–0.94; P-value <0.001), critical ARDS with COVID-19 (OR 0.76, 95% CI 0.70–0.83; P-value <0.001), mechanical ventilation (OR 0.77, 95% CI 0.71–0.82; P-value <0.001), severe sepsis (OR 0.78, 95% CI 0.68–0.89; P-value <0.001), severe sepsis without septic shock (OR 0.68, 95% CI 0.48–0.96; P-value = 0.03), severe sepsis with septic shock (OR 0.80, 95% CI 0.67–0.93; P-value = 0.004), AKI (OR 0.81, 95% CI 0.71–0.93; P-value = 0.003), thrombosis (OR 0.60, 95% CI 0.38–0.93; P-value = 0.02), and DIC (OR 0.80, 95% CI 0.74–0.86; P-value < 0.001). The rates were lower for ECMO and pulmonary embolism, but the P-values were not statistically significant. The same results of logistic regression in PSM with replacement are illustrated in Fig. 3. Linear regression of adjusted continuous variables showed a shorter hospital length of stay among home statin users before and after matching.
Fig. 3.
After matching, propensity score-matched, sampling with replacement, and covariate-adjusted odds ratio in preexisting statin users (n = 11 533). ∗Normal or other discharge is the reference category; ‡mild/moderate ARDS with COVID-19 is the reference category. ARDS, acute respiratory distress syndrome; CI, confidence interval.
Table 4 describes the available laboratory data in the unmatched cohort of COVID-19 patients. The preexisting statin users had significantly lower levels of CRP (96.87 ± 98.37 vs. 100.39 ± 111.29; P-value = 0.01), hemoglobin (12.45 ± 2.10 vs. 12.83 ± 2.19; P-value <0.001), platelet count (280.75 ± 125.11 vs. 295.23 ± 133.10; P-value <0.001), PaO2 (110.33 ± 73.16 vs. 114.49 ± 80.29; P-value <0.001), PaCO2 (47.01 ± 20.46 vs. 48.06 ± 21.04, P-value = 0.05), low-density lipoprotein (LDL) (64.09 ± 30.76 vs. 80.52 ± 35.85, P-value <0.001), VLDL (24.12 ± 10.62 vs. 26.69 ± 14.08; P-value = 0.01), and total bilirubin (0.75 ± 0.89 vs. 0.82 ± 1.46; P-value <0.001). The preexisting statin users had significantly higher levels of IL-6 (266.58 ± 623.78 vs. 197.16 ± 484.44; P-value <0.001), leukocyte (11.59 ± 9.44 vs. 11.31 ± 7.38; P-value = 0.01), aPTT (56.10 ± 45.72 vs. 52.97 ± 42.48; P-value <0.001), PT (16.53 ± 13.38 vs. 15.05 ± 10.02; P-value <0.001), INR (1.51 ± 1.10 vs. 1.37 ± 0.85; P-value <0.001), BUN (37.86 ± 28.43 vs. 31.09 ± 27.55; P-value <0.001), and creatinine (1.49 ± 1.25 vs. 1.28 ± 1.23; P-value <0.001).
Table 4.
Laboratory data with preexisting statin use and nonuse in the unmatched cohort of coronavirus disease 2019 patients
| Laboratory data | Prehospital use of statins | ||||
| Yes | No | P-value | |||
| n | Mean (SD) | n | Mean (SD) | ||
| Inflammatory response | |||||
| ESR (mm/h) | 893 | 62.9 (±35.45) | 2056 | 58.17 (±35.68) | 0.41 |
| CRP (mg/l) | 4032 | 96.87 (±98.37) | 9324 | 100.39 (±111.29) | 0.01 |
| High-sensitivity CRP (mg/l) | 299 | 96.76 (±82.07) | 694 | 104.39 (±82.34) | 0.03 |
| Ferritin (ng/ml) | 3645 | 930.20 (±2330.15) | 8463 | 993.63 (±2735.89) | 0.09 |
| IL-6 (pg/ml) | 297 | 266.58 (±623.78) | 739 | 197.16 (±484.44) | <0.001 |
| Hematologic profile | |||||
| Hemoglobin concentration (g/dl) | 8735 | 12.45 (±2.10) | 19 839 | 12.83 (±2.19) | <0.001 |
| Leukocyte count (103 cells/μl) | 8663 | 11.59 (±9.44) | 19 889 | 11.31 (±7.38) | 0.01 |
| Neutrophil count (103 cells/μl) | 6438 | 8.82 (±5.69) | 14 531 | 8.78 (±5.78) | 0.93 |
| Lymphocyte count (103 cells/μl) | 6699 | 1.44 (±4.57) | 15 367 | 1.47 (±2.32) | 0.18 |
| Platelet count (103 cells/μl) | 8611 | 280.75 (± 25.11) | 19 524 | 295.23 (±133.10) | <0.001 |
| Coagulation profile | |||||
| aPTT (s) | 2177 | 56.10 (±45.72) | 4842 | 52.97 (±42.48) | <0.001 |
| PT (s) | 2446 | 16.53 (±13.38) | 5353 | 15.05 (±10.02) | <0.001 |
| INR (s) | 370 | 1.51 (±1.10) | 941 | 1.37 (±0.85) | <0.001 |
| Fibrinogen (mg/dl) | 540 | 509.39 (±198.46) | 1427 | 516.80 (±208.03) | 0.27 |
| D-dimer (μg/ml) | 4435 | 3.99 (±15.63) | 10 177 | 3.67 (±10.11) | 0.26 |
| Metabolic profile | |||||
| pH | 2566 | 7.43 (±0.09) | 5843 | 7.44 (±0.09) | 0.06 |
| paO2 (mmHg) | 2691 | 110.33 (±73.16) | 5914 | 114.49 (±80.29) | <0.001 |
| paCO2 (mmHg) | 2761 | 47.01 (±20.46) | 6038 | 48.06 (±21.04) | 0.05 |
| Lactic acid (mmol/l) | 3197 | 2.29 (±2.36) | 7355 | 2.31 (±2.49) | 0.07 |
| LDH (U/l) | 3554 | 467.18 (±685.56) | 8221 | 473.12 (±1139.30) | 0.70 |
| Renal profile | |||||
| BUN (mg/dl) | 8803 | 37.86 [±28.43) | 19 679 | 31.09 (±27.55) | <0.001 |
| Creatinine (mg/dl) | 8970 | 1.49 (±1.25) | 19 924 | 1.28 (±1.23) | <0.001 |
| Lipid profile | |||||
| LDL (mg/dl) | 493 | 64.09 (±30.76) | 1098 | 80.52 (±35.85) | <0.001 |
| VLDL (mg/dl) | 99 | 24.12 (±10.62) | 195 | 26.69 (±14.08) | 0.01 |
| Liver profile | |||||
| AST (U/l) | 6815 | 121.05 (±721.08) | 15 462 | 129.44 (±824.58) | 0.28 |
| ALT (U/l) | 6816 | 91.66 (±410.19) | 15 366 | 101.18 (±375.97) | 0.43 |
| Total bilirubin (mg/dl) | 6769 | 0.75 (±0.89) | 15 440 | 0.82 (±1.46) | <0.001 |
ALT, alanine aminotransaminase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransaminase; BUN, blood urea nitrogen; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IL-6, interleukin 6; INR, international normalized ratio; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; paCO2, partial arterial carbon dioxide; paO2, partial arterial oxygen; PT, prothrombin time; VLDL, very low-density lipoprotein.
Discussion
This retrospective propensity scores matched study in a large cohort of US patients hospitalized with COVID-19 showed that daily statin users had a lower mortality risk from COVID-19 – 26% lower risk in an unmatched, covariate-adjusted cohort, 37% lower risk in propensity-matched, sampling without replacement, covariate-adjusted cohort, and 23% lower risk in propensity-matched, sampling with replacement, covariate-adjusted cohort. Preexisting statin users had lower odds of all-cause mortality, discharge to hospice, ICU admissions, need for mechanical ventilation, severe and critical ARDS, severe sepsis, severe sepsis with and without septic shock, AKI, thrombosis, DIC, and hospital length of stay.
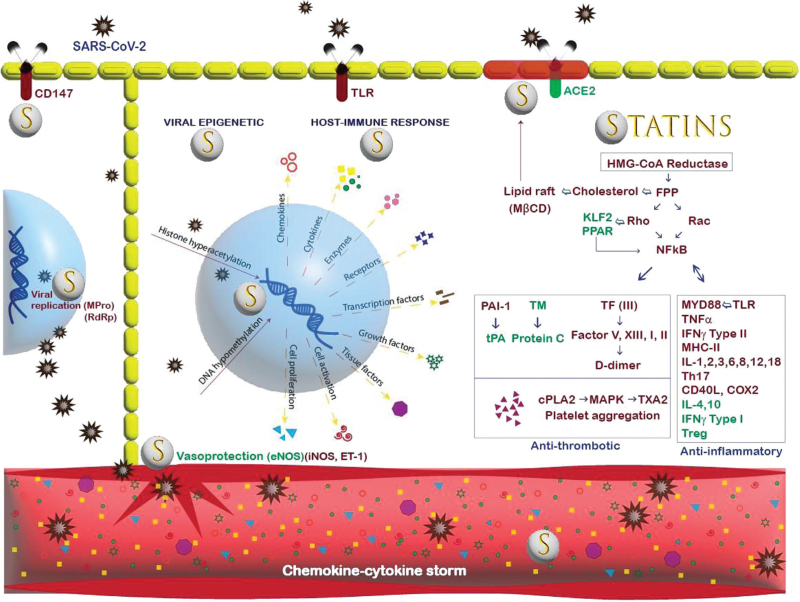
Cholesterol-independent, immunomodulatory effects of statins might interfere with SARS-CoV-2 pathogenic pathways and host hyperinflammatory response, attenuating the severity and mortality from COVID-19 (Fig. 4). SARS-CoV-2 gains cell entry through lipid rafts, Toll-like-receptor (TLR), and various host cell receptors.7,13–15 Statins can prevent viral entry by inhibiting the synthesis of cholesterol, isoprenoid, and small GTPases necessary for lipid raft composition and TLR activation.13 After gaining cell entry, SARS-CoV-2 induces epigenetic changes by hyperacetylating histones and hypomethylating DNA for viral replication.4 Statins, through their histone deacetylase inhibitory properties, reverse epigenetic histone modification.14 Pulmonary viral invasion and epigenetic changes activate the host's immune and inflammatory response with a cytokine storm through the TLR-MYD88-NF-κB axis. Statins downregulate the MYD88 gene leading to statin-mediated TLR-MYD88-NF-κB axis suppression and decreased release of cytokines.15 High blood concentrations of IL-6 are observed early in the course of COVID-19 infection and are associated with higher mortality.16 Interestingly, our study showed higher levels of IL-6 among home statin users. One possibility for higher IL-6 levels among statin users could be the development of drug resistance from chronic statin use. A study on the epigenetic benefits of statins in colorectal cancer showed that the pleiotropic effects of statins wane after approximately 2 weeks of treatment; the findings were correlated with an increase in Phosphatase and Tensin homolog (PTEN) levels.12 Other possibilities for higher IL-6 levels could be a normal elevation early in the course of infection and/or delayed onset of statin-induced TLR-MYD88-NF-κB axis suppression because of the intracellular and nuclear mechanisms involved in pleiotropic anti-inflammatory effects that may fail to keep pace with the rapidly progressive devastating COVID-19 disease.
Fig. 4.
Illustration of SARS-CoV-2–host–statin interactions. The center (‘nuclear’ blue area) shows the relationships among virus and epigenetic modifications (left) and aspects of the host immune response in response to the epigenetic changes (right). The upper right is a lipid raft (maroon) with contents regulated by statins and pleiotropic effects linked to isoprenoid group deficiency. ACE2 receptor gains function. Suppression of inflammatory and thrombotic mediators by statins, directly and indirectly, modifies the level of inflammation and other COVID-19-induced responses, that is, the ‘cytokine storm’. Following left underneath under the nuclear area, the vasoprotective actions of statins drugs include normalization of nitric oxide production and endothelial function. ACE2, angiotensin-converting enzyme 2; COX2, cyclooxygenase2; cPLA2, c-phospholipase; ET, endothelin; FPP, Farnesyl pyrophosphate; IL, interleukin; MHC, major histocompatibility; MPro, main protease; MYD88, myeloid differentiation factor; NOS, nitric oxide synthase; PAI-1, plasminogen activator inhibitor; RdRp, RNA-dependent RNA polymerase; TF, tissue factor; TM, thrombomodulin; TNF, tumor necrosis factor; TXA2, thromboxane.
Statins can inhibit both the SARS-CoV-2 entry into the cells by translocating the ACE-2 receptors, normally located in lipid rafts, into nonlipid rafts domain so reducing the virus binding to these receptors and SARS-CoV-2 intracellular replication by binding with the main protease and inhibiting the phospholipase A2 alpha.17 Statins can also mitigate the inflammatory storm by inhibiting the TLR-MYD88-NF-κB pathway and increasing intracellular ACE-2 expression, normally downregulated by SARS-CoV-2, via epigenetic mechanisms and improving endothelial function, decreasing oxidative stress and the risk of thrombosis.15
In addition, viral inflammation increases endothelial permeability, promoting infiltration of cytotoxic T-cell lymphocytes, macrophages, neutrophils, and fibrocytes into the alveoli, resulting in the destruction of capillary–alveolar beds and hypoxic lung injury.15 Hypoxia induces pulmonary vasoconstriction and releases endothelin-1(ET-1) and inducible nitric oxide synthase (iNOS). Statins are mostly vasoprotective through their upregulation of vasodilatory endothelial nitric oxide synthase (eNOS) and downregulation of vasoconstrictive ET-1 and iNOS.15 Statins can help to restore endothelial dysfunction, which is frequently observed in COVID-19 disease.18 Unsurprisingly, our study showed that preexisting statin users had higher odds of less severe ARDS with COVID-19, lower rates of AKI, lesser need for mechanical ventilation, lower ICU admission, and shorter hospital length of stay than those not taking home statins.
Endothelial damage and massive inflammation caused by COVID-19 infection can result in platelet aggregation, NF-κB-mediated activation of prothrombotic plasminogen activator inhibitor-1, tissue factor promoting a hypercoagulable state that can lead to thrombosis, thrombocytopenia, and disseminated intravascular coagulation.19 Statins may have antithrombotic effects. In a meta-analysis of 16 randomized clinical trial (RCT) studies, statins, mainly atorvastatin, lowered plasminogen activator inhibitor-1 concentrations and reduced the risk of thrombosis.20 A recent prospective cohort study found reduced recurrence of venous thromboembolism in the elderly, suggesting the use of statins when anti-coagulation is not indicated or feasible.21 Our study supports these findings by showing lower odds of thrombosis and DIC in preexisting statin users.
Aging can exacerbate the clinical course of the SARS-CoV-2 infection through immunosenescence and a dysfunctional immune response.22 Diabetes is another important risk factor associated with mortality in patients with COVID-19.23 Increased expression of glycated ACE2 receptors in diabetes could open the door for SARS-CoV-2 entry into the cardiomyocyte, contributing to the development of arrhythmias and myocarditis, which raises mortality.24,25 Most elderly and diabetic patients with coronary heart disease are being treated with ACE inhibitors, sartans, aspirin, statins, and oral anticoagulants that may reduce the disease severity.26 Continued statin use decreases chronic LDL and VLDL-mediated low-grade inflammation seen in obesity, metabolic syndrome, diabetes, heart disease, renal disease, and stroke.27 Mendelian randomization studies have shown a causal relationship between higher triglyceride levels and increased susceptibility to and severity of COVID-19, suggesting statins could be protective in COVID-19 patients through their lipid-lowering effects.28 Interestingly, our study revealed lower levels of LDL, VLDL, CRP and hs-CRP, and lower mortality and morbidity outcomes in preexisting statin users compared with nonusers.
Our study included a large cohort of hospitalized COVID-19 patients across the United States. We adopted a combined analysis using PSM to minimize the likelihood of confounders because of selection biases and model-based regression adjustments for prognostic covariates predictive of the outcome variables. Interestingly, in the unmatched unadjusted cohort, the preexisting statin users showed higher all-cause mortality (17.6 vs. 13.2%, P-value <0.001), acute kidney injury (3.9 vs. 3.0; P-value <0.001) and longer hospital length of stay (9.87 ± 8.94 vs. 9.30 ± 9.76 days; P-value < 0.001) compared with the nonusers. In the PSM unadjusted analysis, all-cause mortality (17.6 vs. 20.1%; P-value <0.001) and acute kidney injury (3.9 vs. 4.6%; P-value = 0.03) were lower between the statin user and nonuser groups; the statin user group had shorter hospital length of stay (9.87 ± 8.94 vs. 10.44 ± 9.53 days; P-value <0.001) compared with the statin nonuser group. In the PSM and covariate-adjusted analysis, the preexisting statin users showed significantly lower rates of all-cause mortality (OR 0.80, 95% CI 0.74–0.86; P-value <0.001) and acute kidney injury (OR 0.81, 95% CI 0.71–0.93; P-value = 0.003). These results show the importance of a rigorous statistical analysis to obtain correct data and make accurate conclusions. Our study has a uniquely large sample size of preexisting statin users (n = 11 533) compared with most of the above-mentioned studies.
To note, a few studies do not support the protective role of statins and have shown an association between chronic use of statins with higher mortality and/or morbidity;29–31 in particular, patients with type 2 diabetes showed the worst outcomes.29,30 Our study does not support these findings despite the preexisting statin users in the propensity matching score analysis having a higher incidence of diabetes than the statin nonusers (60.1 vs. 56.8%; P-value <0.001).
Limitations
Our study has several limitations that have been acknowledged. This is a retrospective cohort design, and no statement can be made about causality. Also, we included different statins, and each statin has different properties and pharmacokinetics, which are further compounded by individual responses. This study did not stratify patients based on different statin agents, statin intensity, dosage, dose–response effect, the role of time since statin initiation on outcomes, or the potential additional impact of nonstatin cholesterol-lowering medications such as ezetimibe or PCSK9 inhibitors. Moreover, the use of PSM has its own limitations. PSA only controls for measured covariates leaving the risks of unmeasured confounding. Risks of residual confounding not investigated in our study include concomitant use of various home medications such as aspirin, metformin, ACE inhibitors, sartans, oral anticoagulants, and in-hospital treatments, including the continuation of home medications during hospitalization, in-hospital use of antiviral treatments, in-hospital early initiation of prophylactic or intermediate low-molecular-weight heparin. Additionally, routine use of oral anticoagulants and antiplatelet agents such as aspirin and clopidogrel in patients with diabetes, heart disease, and stroke for primary or secondary prevention of atherosclerotic cardiovascular disease and stroke could also contribute to thrombosis protectiveness in COVID-19 disease that may not be solely attributed to statins.32,33 It might also be possible that patients with multiple comorbidities, especially those conditions requiring statins, may receive more aggressive care, such as early ventilatory support for COVID-19, leading to better morbidity and mortality outcomes in COVID-19. This selective vigilance in care may contribute to the risk of residual confounding that was not measured or statistically controlled in our study.34 Other studies have demonstrated that the presence of statin resistance after 2 weeks of statin therapy in colorectal cancer correlated with an increase in PTEN levels questioning the long-lasting epigenetic pleiotropic effects of statins.12 Furthermore, the data gathered in the EMR of hospitalized patients during the dramatic first wave of COVID-19 may lack detailed medical history because of the threats of disease exposure, the priority of emergency treatment versus data collection, and patient acuity and cooperation. Such challenges in data collection may contribute to the risk of residual confounding and the accuracy of our retrospective study results. Lastly, this study was conducted during the early phase of the COVID-19 pandemic and did not consider the effects of the occurrence of new SARS-CoV-2 variants and the application of new therapeutical practices and vaccination.
Conclusion
Our study showed a statistically significant relationship between preexisting statin intake and decreased mortality and severe disease in hospitalized COVID-19 patients. Statins’ cholesterol-independent effects (pleiotropy) can be protective against inflammation, thrombosis, organ failure, and death observed in COVID-19 patients. Statins are among the most studied drugs over the past decades, available in inexpensive generic forms with well known pharmacology and adverse events. Statins do not represent a financial burden upon the disadvantaged and in countries with moderate incomes. Given the emergence of new variants that pose an ongoing threat from COVID-19, our study emphasized that long-term persistent use of statins and their extended benefits on COVID-19 severity beyond the cholesterol-lowering effects could be a cost-effective, repurposed drug solution against COVID-19 disease severity. Ongoing RCTs will better define the role of initiating statins in healthy subjects to prevent COVID-19.35 Further RCT studies are required to elucidate their use as a repurposing drug against COVID-19, their dosage, different types of statins, duration of administration, and dose–response effect.
Acknowledgements
The authors would like to thank Sara Wilson, MS, for providing research guidance and reviewing statistical methods and analyses.
Funding: This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
E.C. and U.R. contributed equally.
Supplemental digital content is available for this article.
References
- 1. WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int. [Accessed 24 April 2022] [Google Scholar]
- 2. CDC. COVID Data Tracker. Centers for Disease Control and Prevention. Published 28 March 2020. Available at: https://covid.cdc.gov/covid-data-tracker. [Accessed 14 July 2022] [Google Scholar]
- 3.Gustine JN, Jones D. Immunopathology of Hyperinflammation in COVID-19. Am J Pathol 2021; 191:4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crimi E, Benincasa G, Figueroa-Marrero N, Galdiero M, Napoli C. Epigenetic susceptibility to severe respiratory viral infections and its therapeutic implications: a narrative review. Br J Anaesth 2020; 125:1002–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napoli C, Benincasa G, Criscuolo C, Faenza M, Liberato C, Rusciano M. Immune reactivity during COVID-19: implications for treatment. Immunol Lett 2021; 231:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaziano L, Giambartolomei C, Pereira AC, et al. VA Million Veteran Program COVID-19 Science Initiative. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med 2021; 27:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlos A, Niedzielski M, Gorzelak-Pabiś P, Broncel M, Woźniak E. COVID-19: direct and indirect mechanisms of statins. Int J Mol Sci 2021; 22:4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandermeer ML, Thomas AR, Kamimoto L, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis 2012; 205:13–19. [DOI] [PubMed] [Google Scholar]
- 9.Yuan S. Statins may decrease the fatality rate of Middle East respiratory syndrome infection. mBio 2015; 6:e01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedson DS, Rordam OM. Treating Ebola patients: a ’bottom up’ approach using generic statins and angiotensin receptor blockers. Int J Infect Dis 2015; 36:80–84. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 12.Ouahoud S, Jacobs RJ, Peppelenbosch MP, et al. Kinome-wide analysis of the effect of statins in colorectal cancer. Br J Cancer 2021; 124:1978–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proto MC, Fiore D, Piscopo C, et al. Lipid homeostasis and mevalonate pathway in COVID-19: basic concepts and potential therapeutic targets. Prog Lipid Res 2021 101099; 82:101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dje N’Guessan P, Riediger F, Vardarova K, et al. Statins control oxidized LDL-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler Thromb Vasc Biol 2009; 29:380–386. [DOI] [PubMed] [Google Scholar]
- 15.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005; 4:977–987. [DOI] [PubMed] [Google Scholar]
- 16.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 2021; 326:499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashour T, Halwani R, Arabi YM, et al. Statins as an adjunctive therapy for COVID-19: the biological and clinical plausibility. Immunopharmacol Immunotoxicol 2021; 43:37–50. [DOI] [PubMed] [Google Scholar]
- 18.Margaritis M, Channon KM, Antoniades C. Statins as regulators of redox state in the vascular endothelium: beyond lipid lowering. Antioxid Redox Signal 2014; 20:1198–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahebkar A, Catena C, Ray KK, et al. Impact of statin therapy on plasma levels of plasminogen activator inhibitor-1: a systematic review and meta-analysis of randomised controlled trials. Thromb Haemost 2016; 116:162–171. [DOI] [PubMed] [Google Scholar]
- 21.Kronenberg RM, Beglinger S, Stalder O, et al. Statin therapy and recurrent venous thromboembolism in the elderly: a prospective cohort study. Sci Rep 2019; 9:14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napoli C, Tritto I, Mansueto G, Coscioni E, Ambrosio G. Immunosenescence exacerbates the COVID-19. Arch Gerontol Geriatr 2020; 90:104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grasselli G, Greco M, Zanella A, et al. COVID-19 Lombardy ICU Network. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Onofrio N, Scisciola L, Sardu C, et al. Glycated ACE2 receptor in diabetes: open door for SARS-COV-2 entry in cardiomyocyte. Cardiovasc Diabetol 2021; 20:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020; 5:831–840. [DOI] [PubMed] [Google Scholar]
- 26.Napoli C, Tritto I, Benincasa G, Mansueto G, Ambrosio G. Cardiovascular involvement during COVID-19 and clinical implications in elderly patients: a review. Ann Med Surg (Lond) 2020; 57:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milajerdi A, Larijani B, Esmaillzadeh A. Statins influence biomarkers of low grade inflammation in apparently healthy people or patients with chronic diseases: a systematic review and meta-analysis of randomized clinical trials. Cytokine 2019; 123:154752. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa M, Asaba K, Nakayama T. Estimating causal effects of atherogenic lipid-related traits on COVID-19 susceptibility and severity using a two-sample Mendelian randomization approach. BMC Med Genomics 2021; 14:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cariou B, Goronflot T, Rimbert A, et al. CORONADO investigators. Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab 2021; 47:101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitacchione G, Schiavone M, Curnis A, et al. Impact of prior statin use on clinical outcomes in COVID-19 patients: data from tertiary referral hospitals during COVID-19 pandemic in Italy. J Clin Lipidol 2021; 15:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuin M, Rigatelli G, Bilato C, Rigatelli A, Roncon L, Ribichini F. Preexisting coronary artery disease among coronavirus disease 2019 patients: a systematic review and meta-analysis. J Cardiovasc Med 2022; 23:535–545. [DOI] [PubMed] [Google Scholar]
- 32.Rizk JG, Lippi G, Rizk Y. Thromboprophylaxis in outpatients with COVID-19: a safe bet or tilting at windmills? Minerva Cardiol Angiol 2022; 70:1–4. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava R, Kumar A. Use of aspirin in reduction of mortality of COVID-19 patients: a meta-analysis. Int J Clin Pract 2021; 75:e14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabrini L, Ghislanzoni L, Severgnini P, et al. Early versus late tracheal intubation in COVID-19 patients: a ‘pros/cons’ debate also considering heart-lung interactions. Minerva Cardiol Angiol 2021; 69:596–605. [DOI] [PubMed] [Google Scholar]
- 35.Talasaz AH, Sadeghipour P, Aghakouchakzadeh M, et al. Investigating lipid-modulating agents for prevention or treatment of COVID-19: JACC state-of-the-art review. J Am Coll Cardiol 2021; 78:1635–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.