Abstract
Introduction
Ceftazidime–avibactam has proven activity against multidrug-resistant (MDR) bacteria in clinical trials and real-world studies. This study was conducted to describe the patterns of use of ceftazidime–avibactam (including indications and associated antibiotics), and the effectiveness and safety of ceftazidime–avibactam in real-world clinical practice.
Methods
This non-interventional medical chart review study was conducted in 11 countries across the European and Latin American (LATAM) regions. Consecutive patients treated in clinical practice with at least one dose of ceftazidime–avibactam for an approved indication per country label since 01 January 2018 (or launch date in the country if posterior) were enrolled. Effectiveness analyses were conducted in patients treated with ceftazidime–avibactam for at least 72 h.
Results
Of the 569 eligible patients enrolled, 516 (90.7%) were treated for at least 72 h (354 patients from Europe and 162 patients from LATAM); 390 patients (75.7%) had switched from another antibiotic line for Gram-negative coverage. Infection sources were intra-abdominal, urinary, respiratory, bloodstream infections, and other infections (approximately 20% each). K. pneumoniae was the most common microorganism identified in the latest microbiological evaluation before starting ceftazidime–avibactam (59.3%). Two-thirds of microorganisms tested for susceptibility were MDR, of which 89.3% were carbapenem-resistant. The common MDR mechanisms for K. pneumoniae were carbapenemase (33.9%), oxacillinase 48 (25.2%), extended-spectrum beta-lactamase (21.5%), or metallo-beta-lactamase (14.2%) production. Without prior patient exposure, 17 isolates (mostly K. pneumoniae) were resistant to ceftazidime–avibactam. Treatment success was achieved in 77.3% of patients overall (88.3% among patients with urinary infection), regardless of first or second treatment line. In-hospital mortality rate was 23.1%. Adverse events were reported for six of the 569 patients enrolled.
Conclusion
This study provides important real-world evidence on treatment patterns, effectiveness, and safety of ceftazidime–avibactam in clinical practice through its recruitment in the European and LATAM regions. Ceftazidime–avibactam is one of the antibiotics to consider for treatment of MDR bacteria.
Trial Registration
ClinicalTrials.gov identifier, NCT03923426.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00762-9.
Keywords: Bloodstream infection, Ceftazidime–avibactam, Europe, Intra-abdominal infection, K. pneumoniae, LATAM, Multidrug-resistant, Respiratory infection, Urinary infection
Key Summary Points
| Why carry out this study? |
| Comprehensive real-world data examining the treatment characteristics, resource use, effectiveness, and safety of ceftazidime–avibactam against multidrug-resistant (MDR) bacteria are needed. |
| This study was conducted to describe the patterns of use of ceftazidime–avibactam and to determine the effectiveness and safety of ceftazidime–avibactam in real-world clinical practice. |
| What was learned from the study? |
| Among patients who received ceftazidime–avibactam for at least 72 h in real-world clinical practice across the European and LATAM regions: |
| Infection sources were mainly intra-abdominal, urinary, respiratory, bloodstream infection, and other infections (approximately 20% each). |
| K. pneumoniae was the most common microorganism identified in the latest microbiological evaluation before the start of ceftazidime–avibactam (59.3%). |
| Treatment success was achieved in 77.3% of patients overall (88.3% among patients with urinary infection). |
| The in-hospital mortality rate was 23.1%. |
Introduction
Antimicrobial resistance and healthcare-associated infections are global patient safety concerns that can result in prolonged hospital stay, long-term disability, financial burden, and mortality [1–3]. According to a World Health Organization report in 2008, healthcare-associated infections resulted in approximately 16 million days of hospitalization and 37,000 deaths per year in Europe and contributed to an additional 110,000 deaths [4].
According to a point prevalence survey of healthcare-associated infections and antimicrobial use in acute care hospitals in Europe, conducted in 2011–2012, of a total of 15,000 reported healthcare-associated infections, the major infections were ventilator-associated respiratory tract infections (pneumonia and lower respiratory tract; 19.4% and 4.1% respectively), surgical site infections (SSI; 19.6%), catheter-associated urinary tract infection (UTI; 19.0%), central-line associated bloodstream infections (CLABSI; 10.7%), and gastrointestinal infections (7.7%) [5]. The most frequent hospital-acquired infections (HAI) in Europe and Latin America (LATAM) include lower respiratory tract infections, UTI, and SSI [6, 7]. One of the most common mechanisms behind antibiotic resistance in Gram-negative bacteria is the production of extended-spectrum beta-lactamases (ESBLs), which can hydrolyze a large number of beta-lactam antibiotics, including third-generation cephalosporins and aztreonam [8]. A systematic review was conducted among 42 national and regional surveillance systems for antimicrobial resistance in Europe; 90.4%, 85.7%, 83.3%, and 80.9% of those 42 national and regional surveillance systems reported that Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, respectively, were frequently identified multidrug-resistant (MDR) Gram-negative bacteria [9].
MDR Gram-negative bacteria are associated with mortality rates of up to 45% among hospitalized patients [10, 11]. Carbapenems alone or in combination with other antibacterial agents have been the mainstay for the treatment of infections due to MDR Gram-negative bacteria for decades [12, 13]. However, the emergence and spread of carbapenemase-producing bacteria has highlighted the urgent need for new antimicrobial agents [14, 15]. Avibactam, a non-beta-lactam beta-lactamase inhibitor, can inhibit Ambler class A and class C beta-lactamases and a few class D enzymes, including ESBLs, K. pneumoniae carbapenemases (KPC) and oxacillinase 48 (OXA-48) carbapenemases, and ampicillinase C (AmpC) enzymes [16, 17]. Ceftazidime–avibactam has been approved in Europe in 2016 and subsequently in Russia (2017) and Latin American countries (Argentina and Brazil in 2018, and Colombia in 2019) for the treatment of adults with complicated UTI (cUTI) [including pyelonephritis], complicated intra-abdominal infection (cIAI), and hospital-acquired pneumonia/ventilator-associated pneumonia (HAP/VAP), including cases of bacteremia associated with these infections (bacteremia can arise as a primary bloodstream infection [BSI] [primary bacteremia] or secondary to acute systemic infections [secondary bacteremia]) [18], and for the treatment of infections due to aerobic Gram-negative organisms with limited treatment options. The latter indication was not approved in Brazil and Colombia [17, 19].
In recent years, real-world studies have reported the effectiveness of ceftazidime–avibactam against MDR Gram-negative bacterial infections on carbapenem-resistant Enterobacteriaceae (CRE) across the United States (USA) and Europe, but these studies were often limited to certain types of infection, or specific countries [20, 21].
This non-interventional medical chart review study was conducted to collect comprehensive real-world data examining the span of indications, treatment characteristics, and efficacy and safety of ceftazidime–avibactam in real-world clinical settings in Europe and LATAM. The primary objective of the EZTEAM study was to describe the patterns of use of ceftazidime–avibactam in real-world practice, with particular focus on its indications and use for the treatment of infections due to aerobic Gram-negative organisms with limited treatment options, available microbiological evidence, and use in combination regimens. Secondary objectives included evaluation of clinical effectiveness, resource utilization, mortality, and adverse events. Data was collected after the end of the treatment, without interfering with medical care.
Methods
Study Population
Hospitalized adult patients (at least 18 years of age) who had undergone treatment with at least one dose of ceftazidime–avibactam for an approved indication (as per the country label) in routine clinical practice since 01 January 2018 (or the date of launch in the country if posterior) were included in the study. By inclusion criteria, patients should have undergone microbiological sampling within 5 days before the initiation of ceftazidime–avibactam treatment (irrespective of actual microbiology results). Patients previously exposed to ceftazidime–avibactam including participants of compassionate care programs were not eligible to participate in the study.
Study Design
This non-interventional medical chart review study was conducted at 30 European sites (Austria, France, Germany, Greece, Italy, Spain, the United Kingdom [UK], and Russia) and 15 hospital sites in LATAM (Argentina, Brazil, and Colombia). No drugs were supplied for this study.
All data were collected through the abstraction of hospital medical records. Indication, treatments, hospital length of stay (LOS), readmissions, and clinical outcomes (refer to Supplementary Table S1 for definitions of clinical outcomes) were collected in patients who were exposed to ceftazidime–avibactam for at least 72 h. Data collected for patients who were exposed to ceftazidime–avibactam for less than 72 h were limited to indication, use of ceftazidime–avibactam, adverse events, and mortality.
This study was conducted in 45 enrolling hospital sites after receiving approval from the independent ethics committees where applicable by local regulation (a list of participating sites is provided in Supplementary Table S6), as well as in accordance with the Helsinki Declaration of 1964 and its later amendments.
Written informed consent was obtained from each patient or the patient’s legally acceptable representative before any study-specific activity was performed. In France, patients could only be recruited retrospectively. There was no requirement for obtaining informed consent by local regulation; however, a patient information sheet was sent to the enrolled patient. In Austria, Germany, Greece, Italy, and Spain, next of kin’s consent was not applicable for deceased patients. In the UK, an informed consent waiver was approved for all patients, however late in the recruitment period. In all LATAM countries an informed consent waiver was approved for patients who were deceased or who could not be located. Details are provided in Supplementary Table S6.
Data Collection
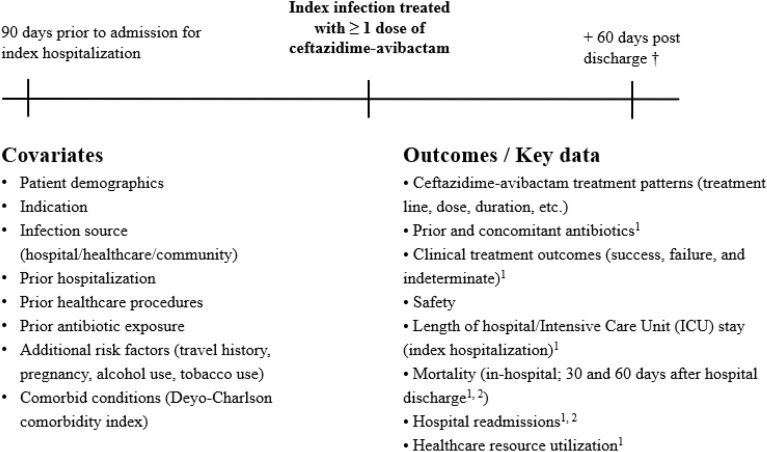
Collected data included patient characteristics, clinical and microbiological characteristics of the infection (by local laboratory and any standards used by the site), treatment patterns (dose, frequency, duration of infusion, start and stop dates, associated antibiotics if any), effectiveness, and safety of ceftazidime–avibactam, medical resource utilization during hospital stay, mortality and hospital readmissions up to 60 days post-discharge or a censoring event (death, withdrawal, or lost-to-follow-up, whichever occurred first). Post-discharge information on hospital readmissions or survival status not available via medical chart abstraction was obtained by contacting the patient or their legal representative by phone. The study data collection and assessment schedule are described in Fig. 1.
Fig. 1.
Study design. 1Indicates outcome that was examined for patients exposed to ceftazidime–avibactam for ≥ 72 h. 2Information about hospital readmissions or death after hospital discharge not available via medical chart abstraction was ascertained by contacting the patient or their legal representative by phone > 60 days after hospital discharge. † Patients followed from ceftazidime–avibactam initiation until 60 days after hospital discharge or another censoring event (in-hospital death, withdrawal from the study, or loss-to-follow-up, whichever occurs first). Data had been abstracted from patient medical charts. Note: Data collection began 01 Jan 2018 or since the date of the launch if after 01 Jan 2018
Sample Size
Overall, the study planned to enroll 525 hospitalized patients with at least 72 h of exposure to ceftazidime–avibactam across the European and LATAM regions.
Statistical Analyses
The full analysis set (FAS) contained all enrolled patients meeting the study eligibility criteria. Descriptive and effectiveness analyses were performed for patients exposed for at least 72 h (FAS72+). Safety data was analyzed for all patients enrolled.
The results were presented overall and stratified by indication (cIAI, cUTI, HAP/VAP, and others, including patients with BSI/sepsis).
Continuous/quantitative variables were summarized using the number of observations, mean, standard deviation (SD), median, and range as appropriate. Categorical/qualitative data were presented using frequency counts and percentages with exact 95% confidence intervals (CIs).
Univariate logistic regression was modeled for clinical success versus clinical failure excluding clinical indeterminate outcome to investigate the association between potential factors and clinical success at initial hospitalization (refer to Supplementary Table S1 for definitions of clinical outcomes). The results of logistic regression models were reported as odds ratio (OR) with 95% CI.
Results
A total of 569 patients met the eligibility criteria, of whom 516 (90.7%) and 53 patients (9.3%) were assigned to the FAS72+ and FAS72− (patients exposed for less than 72 h) data sets, respectively. Out of 516 patients in the FAS72+ group, 354 patients were enrolled from the European region and 162 patients from the LATAM region. Unless specified otherwise, all the results presented herein are for patients included in the FAS72+ data set. Baseline characteristics of patients in the FAS72− data set are available in Supplementary Table S2.
For the 516 patients treated by ceftazidime–avibactam for at least 72 h, the main indications were HAP/VAP (22.1%), cUTI (20.0%), BSI (18.8%), and cIAI (17.4%) (Table 1). About 40% of patients were enrolled in the study before the coronavirus disease 2019 (COVID-19) pandemic; overall, 63 patients had a diagnosis of COVID-19 at admission or during hospital stay.
Table 1.
Indication for ceftazidime–avibactam
| Total (N = 516) | |
|---|---|
| Indication, n (%) | |
| HAP/VAP | 114 (22.1) |
| cUTI | 103 (20.0) |
| BSI | 97 (18.8) |
| cIAI | 90 (17.4) |
| Other | 112 (21.7) |
| Febrile neutropenia | 24 (4.7) |
| SSI | 15 (2.9) |
| CLABSI | 15 (2.9) |
| Osteomyelitis | 7 (1.4) |
| Others | 51 (9.9) |
| Diagnosis of COVID-19, n (%) | |
| Yes | 63 (12.2) |
| No | 247 (47.9) |
| Patients enrolled before COVID-19 pandemic | 206 (39.9) |
BSI bloodstream infection, cIAI complicated intra-abdominal infections, CLABSI central-line associated bloodstream infections, COVID-19 coronavirus disease 2019, cUTI complicated urinary tract infections, HAP hospital-acquired pneumonia, SSI surgical site infections, VAP ventilator-acquired pneumonia
Demographic and baseline characteristics are summarized in Table 2. Age ranged from 18 to 98 years, with a median of 62.0 years; most patients belonged to the 60–79 years age group (n = 231, 44.8%). The majority of patients (n = 352, 68.2%) were male.
Table 2.
Demographic and baseline characteristics by indication
| Characteristic | cIAI (n = 90) |
cUTI (n = 103) |
HAP/VAP (n = 114) |
Other (n = 209)a |
Total (n = 516) |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 61.0 (15.9) | 60.7 (18.5) | 59.2 (18.7) | 55.5 (18.8) | 58.3 (18.4) |
| Median | 63.0 | 64.0 | 63.0 | 58.0 | 62.0 |
| Q1, Q3 | 53.0, 70.0 | 46.0, 74.0 | 49.0, 73.0 | 41.0, 71.0 | 45.0, 72.0 |
| Min, max | 19, 90 | 19, 95 | 18, 93 | 18, 98 | 18, 98 |
| Age group, n (%) | |||||
| < 40 | 12 (13.3) | 14 (13.6) | 18 (15.8) | 44 (21.1) | 88 (17.1) |
| 40–49 | 4 (4.4) | 14 (13.6) | 11 (9.6) | 34 (16.3) | 63 (12.2) |
| 50–59 | 17 (18.9) | 15 (14.6) | 19 (16.7) | 32 (15.3) | 83 (16.1) |
| 60–69 | 33 (36.7) | 22 (21.4) | 32 (28.1) | 40 (19.1) | 127 (24.6) |
| 70–79 | 15 (16.7) | 25 (24.3) | 20 (17.5) | 44 (21.1) | 104 (20.2) |
| 80–89 | 8 (8.9) | 8 (7.8) | 11 (9.6) | 12 (5.7) | 39 (7.6) |
| ≥ 90 | 1 (1.1) | 5 (4.9) | 3 (2.6) | 3 (1.4) | 12 (2.3) |
| Gender, n (%) | |||||
| Male | 54 (60.0) | 73 (70.9) | 86 (75.4) | 139 (66.5) | 352 (68.2) |
| Female | 36 (40.0) | 30 (29.1) | 28 (24.6) | 70 (33.5) | 164 (31.8) |
| DCCI score, n (%) | |||||
| 0 | 8 (8.9) | 6 (5.8) | 25 (21.9) | 20 (9.6) | 59 (11.4) |
| 1 | 9 (10.0) | 5 (4.9) | 17 (14.9) | 22 (10.5) | 53 (10.3) |
| 2 | 21 (23.3) | 7 (6.8) | 18 (15.8) | 44 (21.1) | 90 (17.4) |
| 3 | 10 (11.1) | 5 (4.9) | 12 (10.5) | 23 (11.0) | 50 (9.7) |
| ≥ 4 | 42 (46.7) | 80 (77.7) | 42 (36.8) | 100 (47.8) | 264 (51.2) |
| DCCI score | |||||
| Mean (SD) | 4.5 (3.7) | 6.5 (3.8) | 3.4 (3.31) | 4.3 (3.2) | 4.6 (3.6) |
| Median | 3.0 | 6.0 | 2.0 | 3.0 | 4.0 |
| Q1, Q3 | 2.0, 6.0 | 4.0, 9.0 | 1.0, 6.0 | 2.0, 7.0 | 2.0, 7.0 |
| Min, max | 0, 16 | 0, 16 | 0, 14 | 0, 18 | 0, 18 |
| Immunocompromised patients, n (%) | 47 (52.2) | 60 (58.3) | 29 (25.4) | 107 (51.2) | 243 (47.1) |
| Patients with bacteremia, n (%) | |||||
| Primary | – | – | – | 97 (46.4) | 97 (18.8) |
| Secondary | 36 (40.0) | 34 (33.0) | 29 (25.4) | 35 (16.7) | 134 (26.0) |
| No or unknown | 54 (60.0) | 69 (67.0) | 85 (74.6) | 77 (36.8) | 285 (55.2) |
| Infection origin, n (%) | |||||
| Healthcare-associatedb | 30 (33.3) | 41 (39.8) | 15 (13.2) | 30 (14.4) | 116 (22.5) |
| Hospital-acquired | 52 (57.8) | 49 (47.6) | 90 (78.9) | 163 (78.0) | 354 (68.6) |
| Community-acquired | 8 (8.9) | 13 (12.6) | 9 (7.9) | 16 (7.7) | 46 (8.9) |
BSI bloodstream infection, cIAI complicated intra-abdominal infection, cUTI complicated urinary tract infection, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia, DCCI Deyo-Charlson Comorbidity Index, Max maximum, Min minimum, Q1 quartile one, Q3 quartile three, SD standard deviation
aIncludes patients with BSI/sepsis
bExcluding hospital-acquired infections
Half of the patients (n = 264, 51.2%) had a Deyo-Charlson Comorbidity Index (DCCI) score of at least 4. The mean DCCI score was 4.6 (SD 3.6, median 4.0). A total of 243 patients (47.1%) were considered immunocompromised as a result of underlying cancer (metastatic/non-metastatic), hematological malignancy/leukemia or lymphoma, bone marrow or solid organ transplantation, or acquired immunodeficiency syndrome (AIDS), with the highest proportion of immunocompromised patients among those with cUTI (n = 60/103 [58.3%]). Additionally, 113 (21.9%) had moderate/severe renal disease.
The majority of patients had HAIs (n = 354, 68.6%), followed by healthcare-associated infections (n = 116, 22.5%) and community-acquired infections (n = 46, 8.9%). Healthcare-associated infections were most frequently observed among patients with cUTI (n = 41, 39.8%). A total of 97 (18.8%) and 134 patients (26.0%) had primary (BSI/sepsis) and secondary bacteremia, respectively; the latter mainly among patients with cIAI (n = 36/90, 40.0%) and or cUTI (n = 34/103, 33.0%) (Table 2).
As presented in Table 3 the majority of patients had received other antibiotics for the same infection, before the initiation of ceftazidime–avibactam, wherein 390 patients (75.6%) had received antibiotics for Gram-negative coverage, 147 (28.5%) for Gram-positive coverage, and 20 (3.9%) had received metronidazole for anaerobic coverage. Specifically, the proportion of patients with at least one prior antibiotic line was 87.7% among patients with HAP/VAP, 82.2% of patients with cIAI, 72.8% of patients with cUTI, and 70.8% of patients treated for “other indications” (including BSI/sepsis).
Table 3.
Antibiotic treatment prior to ceftazidime–avibactam therapy by indication
| Characteristic | cIAI (n = 90) |
cUTI (n = 103) |
HAP/VAP (n = 114) |
Other (n = 209)a |
Total (n = 516) |
|---|---|---|---|---|---|
| Any antibiotic(s) used for current infection before ceftazidime–avibactam, n (%) | |||||
| No | 16 (17.8) | 28 (27.2) | 14 (12.3) | 61 (29.2) | 119 (23.1) |
| Yes | 74 (82.2) | 75 (72.8) | 100 (87.7) | 148 (70.8) | 397 (76.9) |
| Reason for discontinuation per antibiotic, n (%) | |||||
| n | 73 | 75 | 100 | 141 | 389 |
| Perceived clinical failure/disease progression | 27 (37.0) | 14 (18.7) | 44 (44.0) | 49 (34.8) | 134 (34.4) |
| Isolation of a resistant bacteria | 44 (60.3) | 54 (72.0) | 50 (50.0) | 88 (62.4) | 236 (60.7) |
| Preference for empiric coverage | 5 (6.8) | 5 (6.7) | 11 (11.0) | 11 (7.8) | 32 (8.2) |
| Secondary infection requiring regimen change | 9 (12.3) | 1 (1.3) | 13 (13.0) | 12 (8.5) | 35 (9.0) |
| Any antibiotic for Gram-negative coverage used for current infection before ceftazidime–avibactam, n (%) | |||||
| No (ceftazidime–avibactam as first line) | 18 (20.0) | 31 (30.1) | 15 (13.2) | 62 (29.7) | 126 (24.4) |
| Yes (ceftazidime–avibactam as second line) | 72 (80.0) | 72 (69.9) | 99 (86.8) | 147 (70.3) | 390 (75.6) |
One reason per antibiotic—patients could receive several antibiotics
BSI bloodstream infection, cIAI complicated intra-abdominal infection, cUTI complicated urinary tract infection, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia
aIncludes patients with BSI/sepsis
Meropenem (n = 195, 49.1% of patients with prior treatment), piperacillin-tazobactam (n = 113, 28.5%), and vancomycin (n = 70, 17.6%) were the most frequently used antibiotics. The reason for discontinuation was collected for each antibiotic (a patient could have several antibiotics); 60.7% of these patients (n = 236/389; data missing for one patient) had at least one antibiotic discontinued as a result of the isolation of resistant bacteria and 34.4% (n = 134/389; data missing for one patient) because of perceived clinical failure/disease progression. Considering only Gram-negative coverage, 126 patients (24.4%) received ceftazidime–avibactam therapy for the current infection as first-line therapy and 390 patients (75.6%) as second-line therapy, respectively.
Table 4 shows the patterns of treatment. A total of 158 patients (30.6%) received ceftazidime–avibactam as monotherapy (mostly [66.0%] among patients with cUTI) and 358 patients (69.4%) in combination with other antibiotics, most commonly with aztreonam (n = 104, 20.2%), vancomycin (n = 65, 12.6%), colistin (n = 60, 11.6%), metronidazole (n = 60, 11.6%), and amikacin (n = 58, 11.2%).
Table 4.
Ceftazidime–avibactam usage by indication
| Characteristic | cIAI (n = 90) |
cUTI (n = 103) |
HAP/VAP (n = 114) |
Other (n = 209)a |
Total (n = 516) |
|---|---|---|---|---|---|
| Use of ceftazidime–avibactam overall, n (%) | |||||
| Monotherapy | 26 (28.9) | 68 (66.0) | 25 (21.9) | 39 (18.7) | 158 (30.6) |
| Combination therapy | 64 (71.1) | 35 (34.0) | 89 (78.1) | 170 (81.3) | 358 (69.4) |
| Gram-negative coverage | 22 (24.4) | 17 (16.5) | 43 (37.7) | 94 (45.0) | 176 (34.1) |
| Other coverageb | 17 (18.9) | 8 (7.8) | 19 (16.7) | 20 (9.6) | 64 (12.4) |
| Gram-negative and other coverage | 25 (27.8) | 10 (9.7) | 27 (23.7) | 56 (26.8) | 118 (22.9) |
| Total duration of administration of ceftazidime–avibactam (days), n (%) | |||||
| Mean (SD) | 13.6 (12.5) | 9.3 (5.7) | 10.3 (6.6) | 13.3 (14.3) | 11.9 (11.4) |
| Median | 9.5 | 7.0 | 9.0 | 10.0 | 9.0 |
| Q1, Q3 | 6.0, 16.0 | 6.0, 12.0 | 6.0, 12.0 | 7.0, 15.0 | 7.0, 14.0 |
| Total dose of ceftazidime–avibactam (g) | |||||
| Mean (SD) | 76.0 (76.5) | 42.6 (35.0) | 55.8 (41.3) | 69.3 (57.9) | 62.2 (55.8) |
| Median | 54.0 | 36.0 | 42.0 | 51.0 | 48.0 |
| Q1, Q3 | 30.0, 90.0 | 16.5, 60.0 | 30.0, 69.0 | 38.5, 84.0 | 30.0, 78.0 |
| Missing, nc | 1 | 1 | 0 | 0 | 2 |
| Daily dose of ceftazidime–avibactam (g) | |||||
| Mean (SD) | 5.5 (1.3) | 4.5 (1.9) | 5.4 (1.4) | 5.4 (1.4) | 5.2 (1.5) |
| Median | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Q1, Q3 | 6.0, 6.0 | 3.0, 6.0 | 6.0, 6.0 | 6.0, 6.0 | 5.6, 6.0 |
| Missing, nc | 1 | 1 | 0 | 0 | 2 |
| Patients with average daily dose of ceftazidime–avibactam < 4 g, n (%)d | 9 (10.0) | 39 (37.9) | 18 (15.8) | 28 (13.4) | 94 (18.2) |
| Outcome/reason for discontinuation of ceftazidime–avibactam, n (%) | |||||
| n | 89 | 103 | 113 | 201 | 506 |
| Adverse event | 0 (0.0) | 2 (1.9) | 1 (0.9) | 0 (0.0) | 3 (0.6) |
| Perceived clinical failure/disease progression | 3 (3.4) | 1 (1.0) | 2 (1.8) | 4 (2.0) | 10 (2.0) |
| Isolation of a resistant bacteria | 4 (4.5) | 1 (1.0) | 3 (2.7) | 1 (0.5) | 9 (1.8) |
| Preference for empiric coverage | 1 (1.1) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 2 (0.4) |
| Secondary infection with regimen change | 1 (1.1) | 1 (1.0) | 5 (4.4) | 4 (2.0) | 11 (2.2) |
| Switch to oral therapy | 0 (0.0) | 1 (1.0) | 1 (0.9) | 3 (1.5) | 5 (1.0) |
| De-escalation | 11 (12.4) | 11 (10.7) | 12 (10.6) | 25 (12.4) | 59 (11.7) |
| Cure | 54 (60.7) | 82 (79.6) | 58 (51.3) | 135 (67.2) | 329 (65.0) |
| Death | 11 (12.4) | 2 (1.9) | 22 (19.5) | 15 (7.5) | 50 (9.9) |
| Other | 4 (4.5) | 2 (1.9) | 9 (8.0) | 13 (6.5) | 28 (5.5) |
| Main antibiotics used in combination with ceftazidime–avibactam (≥ 10% of patients) | |||||
| Amikacin | 10 (11.1) | 8 (7.8) | 12 (10.5) | 28 (13.4) | 58 (11.2) |
| Vancomycin | 14 (15.6) | 8 (7.8) | 11 (9.6) | 32 (15.3) | 65 (12.6) |
| Aztreonam | 9 (10.0) | 11 (10.7) | 18 (15.8) | 66 (31.6) | 104 (20.2) |
| Metronidazole | 25 (27.8) | 5 (4.9) | 7 (6.1) | 23 (11.0) | 60 (11.6) |
| Colistin | 3 (3.3) | 2 (1.9) | 19 (16.7) | 36 (17.2) | 60 (11.6) |
BSI bloodstream infection, cIAI complicated intra-abdominal infection, cUTI complicated urinary tract infection, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia, Q1 quartile one, Q3 quartile three, SD standard deviation
aIncludes patients with BSI/sepsis
bOther coverage included coverage for anaerobes, antiviral, antimycotic, and antiparasitic drugs
cTotal dose and average daily dose could not be computed for 2 patients treated after dialysis
dPatients with dose adjustments due to renal impairment
The median (interquartile range [IQR]) duration of ceftazidime–avibactam administration was 9.0 (7.0–14.0) days. Four hundred patients (77.5%) received a 2.0 g/0.5 g dose three times daily (TID) and for most of these patients (n = 361, 90.3%) ceftazidime–avibactam was administered as a 2-h infusion. The median (IQR) daily dose of ceftazidime–avibactam was 6.0 g (5.6–6.0). Ninety-four patients (18.2%) had average daily doses of less than 4 g, due to renal impairment.
The main reasons for discontinuation of ceftazidime–avibactam were cure (n = 329/506, 65.0%) and de-escalation (n = 59, 11.7%); however, 50 (9.9%) patients died while on treatment, of whom 22 were affected by HAP/VAP.
Table 5 shows the microbiological evaluations conducted before the start of ceftazidime–avibactam therapy. K. pneumoniae (n = 306, 59.5% of patients) was the most common pathogen identified, followed by P. aeruginosa (n = 69, 13.4%), Klebsiella spp. (n = 50, 9.7%), E. coli (n = 45, 8.5%), and Enterobacter cloacae (n = 34, 6.6%). Forty-four patients (8.5%) had no bacterial pathogen identified.
Table 5.
Latest microbiological evaluation before start of ceftazidime–avibactam therapy by indication
| cIAI (n = 90) |
cUTI (n = 103) |
HAP/VAP (n = 114) |
Other (n = 209)a |
Total (n = 516) |
|
|---|---|---|---|---|---|
| Patient level | |||||
| Samples taken, n (%) | |||||
| n | 90 | 103 | 114 | 209 | 516 |
| Yes | 90 (100) | 103 (100) | 114 (100) | 209 (100) | 516 (100) |
| Number of bacterial pathogens, n (%) | |||||
| N | 90 | 103 | 114 | 209 | 516 |
| 0 | 11 (12.2) | 6 (5.8) | 16 (14.0) | 11 (5.3) | 44 (8.5) |
| 1 | 52 (57.8) | 76 (73.8) | 71 (62.3) | 146 (69.9) | 345 (66.9) |
| 2 | 15 (16.7) | 16 (15.5) | 19 (16.7) | 41 (19.6) | 91 (17.6) |
| > 2 | 8 (8.9) | 4 (3.9) | 6 (5.3) | 9 (4.3) | 27 (5.2) |
| Same pathogen in > 1 sampleb | 8 (8.9) | 19 (18.4) | 10 (8.8) | 22 (10.5) | 59 (11.4) |
| Not known | 4 (4.4) | 1 (1.0) | 2 (1.8) | 2 (1.0) | 9 (1.7) |
| Fungal pathogen identified, n (%) | |||||
| n | 90 | 103 | 114 | 208 | 515 |
| Yes | 9 (10.0) | 1 (1.0) | 19 (16.7) | 18 (8.7) | 47 (9.1) |
| Type of specimen, n (%) | |||||
| n | 90 | 103 | 114 | 208 | 515 |
| Blood | 41 (45.6) | 31 (30.1) | 42 (36.8) | 133 (63.9) | 247 (48.0) |
| Catheter tip | 2 (2.2) | 4 (3.9) | 3 (2.6) | 9 (4.3) | 18 (3.5) |
| Urine | 7 (7.8) | 85 (82.5) | 9 (7.9) | 17 (8.2) | 118 (22.9) |
| Bronchoalveolar lavage | 4 (4.4) | 1 (1.0) | 49 (43.0) | 13 (6.3) | 67 (13.0) |
| Sputum | 1 (1.1) | 0 (0.0) | 20 (17.5) | 18 (8.7) | 39 (7.6) |
| Abscess drainage | 22 (24.4) | 1 (1.0) | 0 (0.0) | 5 (2.4) | 28 (5.4) |
| Swab | 5 (5.6) | 0 (0.0) | 11 (9.6) | 26 (12.5) | 42 (8.2) |
| Surgical/biopsy specimen | 9 (10.0) | 1 (1.0) | 2 (1.8) | 15 (7.2) | 27 (5.2) |
| Other | 20 (22.2) | 6 (5.8) | 20 (17.5) | 23 (11.1) | 69 (13.4) |
| Pathogens identified | |||||
| n | 90 | 103 | 114 | 209 | 516 |
| Gram-negative, n (%) | |||||
| Escherichia coli | 18 (20.0) | 8 (7.8) | 2 (1.8) | 17 (8.1) | 45 (8.7) |
| Klebsiella pneumoniae | 54 (60.0) | 70 (68.0) | 60 (52.6) | 122 (58.4) | 306 (59.3) |
| Klebsiella spp. | 5 (5.6) | 9 (8.7) | 3 (2.6) | 33 (15.8) | 50 (9.7) |
| Proteus mirabilis | 2 (2.2) | 2 (1.9) | 4 (3.5) | 2 (1.0) | 10 (1.9) |
| Proteus spp. | 1 (1.1) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 3 (0.6) |
| Acinetobacter baumannii | 3 (3.3) | 1 (1.0) | 10 (8.8) | 4 (1.9) | 18 (3.5) |
| Acinetobacter spp. | 0 (0.0) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (0.2) |
| Enterobacter cloacae | 6 (6.7) | 6 (5.8) | 8 (7.0) | 14 (6.7) | 34 (6.6) |
| Enterobacter spp. | 3 (3.3) | 0 (0.0) | 2 (1.8) | 2 (1.0) | 7 (1.4) |
| Citrobacter spp. | 3 (3.3) | 1 (1.0) | 2 (1.8) | 1 (0.5) | 7 (1.4) |
| Serratia spp. | 2 (2.2) | 1 (1.0) | 3 (2.6) | 4 (1.9) | 10 (1.9) |
| Morganella morganii | 2 (2.2) | 0 (0.0) | 1 (0.9) | 2 (1.0) | 5 (1.0) |
| Pseudomonas aeruginosa | 6 (6.7) | 13 (12.6) | 22 (19.3) | 28 (13.4) | 69 (13.4) |
| Pseudomonas spp. | 1 (1.1) | 1 (1.0) | 0 (0.0) | 5 (2.4) | 7 (1.4) |
| Haemophilus influenzae | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Haemophilus spp. | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Other | 2 (2.2) | 2 (1.9) | 6 (5.3) | 3 (1.4) | 13 (2.5) |
| Gram-positive, n (%)c | |||||
| Staphylococcus aureus | 2 (2.2) | 2 (1.9) | 3 (2.6) | 6 (2.9) | 13 (2.5) |
| Staphylococcus spp. | 1 (1.1) | 0 (0.0) | 5 (4.4) | 11 (5.3) | 17 (3.3) |
| Enterococcus spp. | 12 (13.3) | 6 (5.8) | 4 (3.5) | 15 (7.2) | 37 (7.2) |
| Streptococcus spp. | 3 (3.3) | 1 (1.0) | 2 (1.8) | 5 (2.4) | 11 (2.1) |
| Other | 3 (3.3) | 1 (1.0) | 4 (3.5) | 5 (2.4) | 13 (2.5) |
| Anaerobes, n (%) | 1 (1.1) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 2 (0.4) |
| Other bacterial agent, n (%) | 0 (0.0) | 2 (1.9) | 1 (0.9) | 2 (1.0) | 5 (1.0) |
BSI bloodstream infection, cIAI complicated intra-abdominal infection, cUTI complicated urinary tract infection, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia
aIncludes patients with BSI/sepsis
bAmong patients with more than one pathogen identified
cIdentified mainly in patients with “other” indication or cIAI, along with Gram-negative bacteria
Table 6 displays the susceptibility of MDR pathogens to antibiotics. From the latest sampling prior to starting ceftazidime–avibactam therapy, 68.5% (364/531) of Gram-negative bacteria identified were MDR, with the highest proportion noted among patients with cUTI (n = 94/106, 88.7%). Gram-negative MDR isolates were mainly resistant to penicillin/beta-lactamase inhibitor (98.0%), cephalosporins (97.4%), fluoroquinolones (90.6%), carbapenems (89.3%), and aminoglycosides (65.1%). With respect to K. pneumoniae and Klebsiella spp. altogether, 88.5% of all isolates and 92.4% of MDR isolates were resistant to carbapenems.
Table 6.
Susceptibility of multidrug-resistant pathogens to antibiotics
| All pathogen isolates | Klebsiella spp. isolates | Pseudomonas spp. isolates | Enterobacter spp. isolates | |
|---|---|---|---|---|
| Pathogen level (isolates) | ||||
| Gram-negative bacterial pathogens with susceptibility data | 531 | 332 | 65 | 37 |
| Antibiotic resistancea | ||||
| Carbapenems | 438 (76.9) | 269 (88.5) | 56 (80.4) | 33 (66.7) |
| Gram-negative multi-resistant pathogens, n (%) | 364 (68.5) | 252 (75.9) | 45 (69.2) | 28 (75.6) |
| Antibiotic resistancea | ||||
| n (pathogens) | 364 | 252 | 45 | 28 |
| Aminoglycosides | 352 (65.1) | 249 (68.7) | 38 (57.9) | 28 (53.6) |
| Amphenicol | 16 (75.0) | 13 (84.6) | ||
| Carbapenems | 355 (89.3) | 250 (92.4) | 42 (95.2) | 27 (81.5) |
| Doripenem | 63 (96.8) | 53 (96.2) | 4 (100) | 1 (100) |
| Ertapenem | 208 (86.1) | 163 (91.4) | – | 22 (77.3) |
| Imipenem | 254 (71.7) | 188 (71.3) | 24 (100) | 19 (57.9) |
| Meropenem | 323 (80.8) | 228 (82.9) | 42 (92.9) | 22 (68.2) |
| Cephalosporins | 343 (97.4) | 240 (98.8) | 44 (88.6) | 26 (100) |
| Cephalosporin/beta-lactamase inhibitor | 222 (18.5) | 155 (18.1) | 26 (23.1) | 20 (10.0) |
| Ceftazidime/avibactam | 219 (7.8) | 154 (9.1) | 25 (4.0) | 20 (5.0) |
| Cefoperazone/sulbactam | 14 (85.7) | 9 (100) | 3 (33.3) | |
| Ceftolozane/tazobactam | 39 (61.5) | 20 (80.0) | 16 (31.3) | 1 (100) |
| Glycylcycline | 139 (39.6) | 117 (42.7) | 4 (100) | 9 (0.0) |
| Monobactam | 99 (76.8) | 72 (84.7) | 16 (56.3) | 6 (33.3) |
| Penicillins | 147 (98.0) | 112 (100) | 4 (50.0) | 18 (100) |
| Penicillins and beta-lactamase inhibitors | 325 (97.8) | 232 (98.3) | 37 (97.3) | 25 (100) |
| Ampicillin–sulbactam | 80 (98.8) | 61 (98.4) | 3 (100) | 8 (100) |
| Amoxicillin–clavulanate | 146 (98.6) | 118 (99.2) | – | 12 (100) |
| Piperacillin–tazobactam | 232 (90.9) | 153 (93.5) | 37 (91.9) | 21 (90.5) |
| Ticarcillin–clavulanate | 8 (62.5) | 4 (100) | 1 (0.0) | 1 (0.0) |
| Fluoroquinolones | 329 (90.6) | 228 (96.1) | 40 (80.0) | 24 (70.8) |
| Tetracycline | 19 (52.6) | 17 (52.9) | 1 (0.0) | |
| Colistin | 175 (25.7) | 126 (31.7) | 24 (8.3) | 16 (12.5) |
| Trimethoprim-sulfamethoxazole | 152 (79.6) | 113 (83.2) | 3 (100) | 19 (68.4) |
| Fosfomycin | 140 (45.0) | 111 (44.1) | 5 (100) | 16 (31.3) |
| Nitrofurantoin | 21 (42.9) | 17 (41.2) | – | 3 (33.3) |
Susceptibility data for pathogens identified at the latest sampling time before the start of ceftazidime–avibactam, by genus. Each table cell gives the number of isolates tested against the particular antibiotic (or class) followed in brackets by the percentage of isolates which were found resistant to the particular antibiotic (or class)
Beta-lactamases were identified in 401/555 (72.3%) isolates tested; 154 isolates (21.9%) did not carry any beta-lactamase, and 72 isolates (13.0%) carried more than one beta-lactamase. Table 7 displays the mechanisms of resistance for the main pathogens. The most common type was KPC (n = 135/390, 34.6%), followed by OXA-48 (n = 78/390, 20.0%), ESBL (n = 78/390, 20.0%), and metallo-beta-lactamases (MBL, including data entries of MBL, New Delhi metallo-beta-lactamase [NDM], Verona integron-encoded metallo-beta-lactamase [VIM], and imipenem-hydrolyzing beta-lactamase [IMP]; n = 70/390, 17.9%). Ninety percent of the patients in whom bacteria carrying MBL were identified were treated by the combination of ceftazidime–avibactam and aztreonam.
Table 7.
Mechanisms of resistance for the main pathogens identified (latest sampling before the start of ceftazidime–avibactam)
| Total | |
|---|---|
| Pathogen level (Isolates) | |
| All isolates | 710 |
| Number of beta-lactamases identified, n (%) | |
| n | 704 |
| 0 | 154 (21.9) |
| 1 | 329 (46.7) |
| 2 | 67 (9.5) |
| ≥ 3 | 5 (0.7) |
| Unknown/not tested | 149 (21.2) |
| Ambler's type, n (%) | |
| n | 390 |
| KPC | 135 (34.6) |
| OXA-48 | 78 (20.0) |
| MBLa | 70 (17.9) |
| ESBL | 78 (20.0) |
| Otherb | 42 (10.8) |
| Principal pathogens identified | |
| Escherichia coli | 58 |
| Beta-lactamases identified, n (%) | |
| N | 58 |
| 0 | 29 (50.0) |
| 1 | 14 (24.1) |
| 2 | 1 (1.7) |
| 3 | 1 (1.7) |
| Unknown/not tested | 13 (22.4) |
| Ambler's type, n (%) | |
| n | 14 |
| KPC | 1 (7.1) |
| OXA-48 | 4 (28.6) |
| MBLa | 1 (7.1) |
| ESBL | 6 (42.9) |
| Otherb | 2 (14.3) |
| Klebsiella pneumoniae, n (%) | 383 |
| Beta-lactamases identified, n (%) | |
| n | 383 |
| 0 | 44 (11.5) |
| 1 | 215 (56.1) |
| 2 | 60 (15.7) |
| 3 | 2 (0.5) |
| Unknown/not tested | 62 (16.2) |
| Ambler's type, n (%) | |
| n | 274 |
| KPC | 93 (33.9) |
| OXA-48 | 69 (25.2) |
| MBLa | 39 (14.2) |
| ESBL | 59 (21.5) |
| Otherb | 26 (9.5) |
| Klebsiella spp., n (%) | 50 |
| Beta-lactamases identified, n (%) | |
| n | 50 |
| 0 | 2 (4.0) |
| 1 | 44 (88.0) |
| 2 | 3 (6.0) |
| Unknown/not tested | 1 (2.0) |
| Ambler's type, n (%) | |
| n | 45 |
| KPC | 16 (35.6) |
| OXA-48 | 2 (4.4) |
| MBLa | 20 (44.4) |
| ESBL | 4 (8.9) |
| Otherb | 3 (6.6) |
| Enterobacter cloacae, n (%) | 38 |
| Beta-lactamases identified, n (%) | |
| n | 38 |
| 0 | 8 (21.1) |
| 1 | 22 (57.9) |
| 2 | 1 (2.6) |
| Unknown/not tested | 7 (18.4) |
| Ambler's type, n (%) | |
| n | 22 |
| KPC | 15 (68.2) |
| OXA-48 | 2 (9.1) |
| MBLa | 5 (22.7) |
| ESBL | 1 (4.5) |
| Pseudomonas aeruginosa, n (%) | 83 |
| Beta-lactamases identified, n (%) | |
| n | 83 |
| 0 | 41 (49.4) |
| 1 | 11 (13.3) |
| 2 | 1 (1.2) |
| Unknown/not tested | 30 (36.1) |
| Ambler's type, n (%) | |
| n | 12 |
| KPC | 4 (33.3) |
| MBLa | 5 (41.7) |
| ESBL | 2 (16.7) |
| Otherb | 1 (8.3) |
ESBL extended-spectrum beta-lactamase, KPC Klebsiella pneumoniae carbapenemase, MBL metallo-beta-lactamase, OXA-48 oxacillinase 48
aMBL includes date entries of metallo-beta-lactamase (MBL), New Delhi metallo-beta-lactamase (NDM), Verona integron-encoded metallo-beta-lactamase (VIM), and imipenem-hydrolyzing beta-lactamase (IMP)
bOther includes ampicillinase C, CTX-M (Cefotaxime-M), cephalosporinases, penicillinases, serine beta-lactamase, CARBA-R (carbapenem resistance)
Without prior patient exposure to ceftazidime–avibactam (per inclusion criteria), 17 of 219 (7.8%) tested isolates were found to be resistant to ceftazidime–avibactam (Table 6). These 17 resistant isolates were identified in 15 patients (two patients had the same resistant isolates identified at two different timepoints). Thirteen isolates (5.4%) were K. pneumoniae, 2 (0.9%) belonged to Klebsiella spp., and 1 (0.4%) each was E. cloacae and P. aeruginosa, respectively. Data for beta-lactamases were available for nine out of 13 K. pneumoniae isolates, one isolate from Klebsiella spp., and one E. cloacae isolate. The most common Ambler’s types were MBL (n = 11) followed by OXA-48 (n = 3), ESBL (n = 2), and KPC (n = 1).
A total of 19 patients had isolation of A. baumannii or Acinetobacter spp. before the start of ceftazidime–avibactam (Table 5); however, ceftazidime–avibactam was not initiated for this pathogen (other Gram-negative bacteria were identified). All patients had received one or several prior lines of antibiotics, including mostly carbapenems and polymyxins. The duration of treatment with ceftazidime–avibactam varied from 3 days to 1 month; and associated antibiotics included colistin or polymyxin B, tigecycline, aztreonam, metronidazole, gentamicin, and daptomycin.
Table 8 shows the mode of initial hospitalization and LOS. The majority of patients (n = 384, 74.4%) had undergone emergency hospitalization; most patients (n = 364, 70.8%) were outpatients prior to hospitalization, although 73 patients (14.2%) were transferred from another acute care facility.
Table 8.
Initial hospitalization by indication
| cIAI (n = 90) |
cUTI (n = 103) |
HAP/VAP (n = 114) |
Other (n = 209)a |
Total (n = 516) |
|
|---|---|---|---|---|---|
| Mode of admission, n (%)b | |||||
| Emergency | 60 (66.7) | 88 (85.4) | 99 (86.8) | 137 (65.6) | 384 (74.4) |
| Scheduled | 30 (33.3) | 15 (14.6) | 15 (13.2) | 72 (34.4) | 132 (25.6) |
| Source of admission, n (%)b | |||||
| N | 90 | 103 | 114 | 207 | 514 |
| Outpatient | 64 (71.1) | 79 (76.7) | 72 (63.2) | 149 (72.0) | 364 (70.8) |
| Long-term care facility | 1 (1.1) | 7 (6.8) | 2 (1.8) | 11 (5.3) | 21 (4.1) |
| Transfer from acute care hospital | 16 (17.8) | 11 (10.7) | 21 (18.4) | 25 (12.1) | 73 (14.2) |
| Other | 9 (10.0) | 6 (5.8) | 19 (16.7) | 22 (10.6) | 56 (10.9) |
| Admission or transfer to ICU during hospitalization, n (%)b | |||||
| n | 90 | 103 | 112 | 209 | 514 |
| ICU | 51 (56.7) | 35 (34.0) | 94 (83.9) | 96 (45.9) | 276 (53.7) |
| LOS from hospital admissionc | |||||
| n | 90 | 103 | 113 | 208 | 514 |
| Mean (SD) | 68.6 (72.0) | 31.3 (42.7) | 60.9 (66.3) | 61.7 (60.7) | 56.6 (62.2) |
| Median | 46.0 | 17.0 | 38.0 | 43.5 | 36.0 |
| Q1, Q3 | 25.0, 84.0 | 10.0, 34.0 | 25.0, 78.0 | 27.0, 73.0 | 20.0, 72.0 |
| Min, max | 3, 418 | 3, 292 | 7, 408 | 3, 369 | 3, 418 |
| LOS from start of ceftazidime–avibactamd | |||||
| n | 90 | 103 | 114 | 208 | 515 |
| Mean (SD) | 38.4 (55.1) | 17.2 (20.4) | 30.3 (35.4) | 34.8 (47.4) | 30.9 (42.9) |
| Median | 17.0 | 11.0 | 18.0 | 21.0 | 17.0 |
| Q1, Q3 | 9.0, 41.0 | 7.0, 19.0 | 10.0, 37.0 | 12.0, 39.5 | 10.0, 37.0 |
| Min, max | 2, 404 | 1, 161 | 2, 246 | 2, 364 | 1, 404 |
| Cumulative ICU LOS from hospital admissione | |||||
| n | 51 | 35 | 93 | 96 | 275 |
| Mean (SD) | 34.0 (57.8) | 25.5 (33.3) | 47.5 (62.4) | 37.9 (43.5) | 38.8 (52.6) |
| Median | 21.0 | 15.0 | 29.0 | 24.0 | 24.0 |
| Q1, Q3 | 8.0, 38.0 | 4.0, 34.0 | 17.0, 58.0 | 9.0, 50.0 | 10.0, 48.0 |
| Min, max | 1, 399 | 1, 173 | 3, 405 | 1, 213 | 1, 405 |
BSI bloodstream infection, ICU intensive care unit, LOS length of stay, Min minimum, Max maximum, Q1 quartile one, Q3 quartile three, SD standard deviation
aIncludes patients with BSI/sepsis
bThe ICU was the ward of admission for 116 patients (22.5%)
cConsecutive hospital LOS: (date of hospital discharge − date of hospital admission)
dHospital LOS from treatment initiation: (date of hospital discharge − date of ceftazidime–avibactam initiation)
eConsecutive or non-consecutive ICU LOS: (date of ICU discharge − date of ICU admission)
During hospitalization, 276 patients (53.7%) stayed in the intensive care unit (ICU), which was the ward of admission for 116 patients (22.5%). Admission or transfer to the ICU was mostly observed in patients with HAP/VAP (n = 94/114, 83.9%), and was the least observed among patients with cUTI (n = 35/103, 34.0%). The median LOS calculated from hospital admission was 36.0 days (IQR 20.0–72.0 days), whereas the median LOS from the start of ceftazidime–avibactam was 17.0 days (IQR 10.0–37.0 days). For patients admitted or transferred to the ICU, the median cumulative ICU LOS was 24.0 days (IQR 10.0–48.0 days).
Clinical outcomes (treatment success, treatment failure, or indeterminate) evaluated during the hospitalization are summarized in Table 9. Treatment success was achieved in 399 patients (77.3%), treatment failure in 60 patients (11.6%), and indeterminate outcome in 57 patients (11.0%). Treatment success was most frequent in patients with cUTI (n = 91/103, 88.3%) and “other indications”, including BSI/sepsis (n = 172/209, 82.3%) compared to patients with HAP/VAP (n = 78/114, 68.4%) or cIAI (n = 58/90, 64.4%). Subgroup analyses showed that the rate of treatment success did not vary by first or second treatment line, for patients with immunocompromised status or those with lower dose of ceftazidime–avibactam due to renal impairment.
Table 9.
Clinical evaluation outcome (success, failure, indeterminate) by indication
| Characteristic | cIAI (n = 90) |
cUTI (n = 103) |
HAP/VAP (n = 114) |
Other (n = 209)a |
Total (n = 516) |
|---|---|---|---|---|---|
| Overall outcome of ceftazidime–avibactam (any therapy), n (%) | |||||
| Treatment success | 58 (64.4) | 91 (88.3) | 78 (68.4) | 172 (82.3) | 399 (77.3) |
| Treatment failure | 18 (20.0) | 6 (5.8) | 14 (12.3) | 22 (10.5) | 60 (11.6) |
| Indeterminate | 14 (15.6) | 6 (5.8) | 22 (19.3) | 15 (7.2) | 57 (11.0) |
| Overall outcome by subgroups | |||||
| Ceftazidime–avibactam in monotherapy regimen, n (%) | |||||
| n | 26 | 68 | 25 | 39 | 158 |
| Treatment success | 17 (65.4) | 60 (88.2) | 17 (68.0) | 34 (87.2) | 128 (81.0) |
| Treatment failure | 7 (26.9) | 2 (2.9) | 4 (16.0) | 1 (2.6) | 14 (8.9) |
| Indeterminate | 2 (7.7) | 6 (8.8) | 4 (16.0) | 4 (10.3) | 16 (10.1) |
| Ceftazidime–avibactam in combination therapy regimens, n (%) | |||||
| n | 64 | 35 | 89 | 170 | 358 |
| Treatment success | 41 (64.1) | 31 (88.6) | 61 (68.5) | 138 (81.2) | 271 (75.7) |
| Treatment failure | 11 (17.2) | 4 (11.4) | 10 (11.2) | 21 (12.4) | 46 (12.8) |
| Indeterminate | 12 (18.8) | 0 (0.0) | 18 (20.2) | 11 (6.5) | 41 (11.5) |
| Patients treated in first line, n (%)b | |||||
| n | 18 | 31 | 15 | 62 | 126 |
| Treatment success | 12 (66.7) | 26 (83.9) | 11 (73.3) | 48 (77.4) | 97 (77.0) |
| Treatment failure | 3 (16.7) | 3 (9.7) | 2 (13.3) | 9 (14.5) | 17 (13.5) |
| Indeterminate | 3 (16.7) | 2 (6.5) | 2 (13.3) | 5 (8.1) | 12 (9.5) |
| Patients treated in second line, n (%)c | |||||
| n | 72 | 72 | 99 | 147 | 390 |
| Treatment success | 46 (63.9) | 65 (90.3) | 67 (67.7) | 124 (84.4) | 302 (77.4) |
| Treatment failure | 15 (20.8) | 3 (4.2) | 12 (12.1) | 13 (8.8) | 43 (11.0) |
| Indeterminate | 11 (15.3) | 4 (5.6) | 20 (20.2) | 10 (6.8) | 45 (11.5) |
| Immunocompromised patients, n (%)d | |||||
| n | 47 | 60 | 29 | 107 | 243 |
| Treatment success | 30 (63.8) | 57 (95.0) | 17 (58.6) | 87 (81.3) | 191 (78.6) |
| Treatment failure | 11 (23.4) | 3 (5.0) | 6 (20.7) | 14 (13.1) | 34 (14.0) |
| Indeterminate | 6 (12.8) | 0 (0.0) | 6 (20.7) | 6 (5.6) | 18 (7.4) |
| Non-immunocompromised patients, n (%) | |||||
| n | 43 | 43 | 85 | 102 | 273 |
| Treatment success | 28 (65.1) | 34 (79.1) | 61 (71.8) | 85 (83.3) | 208 (76.2) |
| Treatment failure | 7 (16.3) | 3 (7.0) | 8 (9.4) | 8 (7.8) | 26 (9.5) |
| Indeterminate | 8 (18.6) | 6 (14.0) | 16 (18.8) | 9 (8.8) | 39 (14.3) |
| Patients with daily dose < 4 ge | |||||
| n | 9 | 39 | 18 | 28 | 94 |
| Treatment success | 4 (44.4) | 35 (89.7) | 9 (50.0) | 21 (75.0) | 69 (73.4) |
| Treatment failure | 4 (44.4) | 3 (7.7) | 2 (11.1) | 3 (10.7) | 12 (12.8) |
| Indeterminate | 1 (11.1) | 1 (2.6) | 7 (38.9) | 4 (14.3) | 13 (13.8) |
| Patients with daily dose ≥ 4 g | |||||
| n | 81 | 64 | 96 | 181 | 422 |
| Treatment success | 54 (66.7) | 56 (87.5) | 69 (71.9) | 151 (83.4) | 330 (78.2) |
| Treatment failure | 14 (17.3) | 3 (4.7) | 12 (12.5) | 19 (10.5) | 48 (11.4) |
| Indeterminate | 13 (16.0) | 5 (7.8) | 15 (15.6) | 11 (6.1) | 44 (10.4) |
BSI bloodstream infection, cIAI complicated intra-abdominal infection, cUTI complicated urinary tract infection, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia
aIncludes patients with BSI/sepsis
bPatients with no antibiotics for Gram-negative coverage before the start of ceftazidime–avibactam
cPatients with at least one antibiotic for Gram-negative coverage used for the same infection before the start of ceftazidime–avibactam
dPatients with underlying cancer (metastatic/non-metastatic), hematological malignancy/leukemia or lymphoma, bone marrow or solid organ transplantation, or acquired immunodeficiency syndrome (AIDS)
eDose adjustment due to renal impairment
Overall, across the etiology of infections, treatment success with ceftazidime–avibactam was reported for 40/47 patients (85.1%) infected by E. coli, 34/40 patients (85.0%) infected by Enterobacter spp., 280/352 patients (79.5%) infected by Klebsiella spp. (including K. pneumoniae), 55/74 patients (74.3%) infected by Pseudomonas spp. (including P. aeruginosa), and 39/59 patients (66.1%) infected by other Gram-negative bacteria (Supplementary Table S3).
The latest available records report the deaths of 145 patients (28.2%, one patient with missing date of death), of whom 119 (82.6%) died during index hospitalization (Table 10). The total in-hospital mortality rate was 23.1% (95% CI 19.5–26.9), the cumulative mortality rate was 24.6% (n = 127; 95% CI 21.0–28.6) at 30 days post-discharge and 27.9% (n = 144; 95% CI 24.1–32.0) at 60 days post-discharge. The lowest mortality rates were observed for patients with cUTI (60 days post-discharge: n = 15, 14.6%), in contrast to cIAI (n = 29, 32.2%), HAP/VAP (n = 39, 34.2%), and “other indications” including BSI/sepsis (n = 62, 29.8%).
Table 10.
Vital status and mortality rates by indication
| Characteristic | cIAI (n = 90) |
cUTI (n = 103) |
HAP/VAP (n = 114) |
Other (n = 209)a |
Total (n = 516) |
|---|---|---|---|---|---|
| Patient status at date of last available record, n (%) | |||||
| n | 90 | 103 | 114 | 208 | 515 |
| Alive | 61 (67.8) | 88 (85.4) | 75 (65.8) | 146 (70.2) | 370 (71.8) |
| Patient died | 29 (32.2) | 15 (14.6) | 39 (34.2) | 62 (29.8) | 145 (28.2) |
| Timing of death (if patient died), n (%) | |||||
| n | 29 | 15 | 39 | 61 | 144 |
| During Index hospitalization | 26 (89.7) | 10 (66.7) | 37 (94.9) | 46 (75.4) | 119 (82.6) |
| Within 30 days of discharge | 0 (0.0) | 1 (6.7) | 0 (0.0) | 7 (11.5) | 8 (5.6) |
| Within 31–60 days of discharge | 3 (10.3) | 4 (26.7) | 2 (5.1) | 8 (13.1) | 17 (11.8) |
| Total in-hospital mortality rate | 26 (28.9) | 10 (9.7) | 37 (32.5) | 46 (22.0) | 119 (23.1) |
| 95% CI | 19.8, 39.4 | 4.8, 17.1 | 24.0, 41.9 | 16.6, 28.2 | 19.5, 26.9 |
| Cumulative mortality rate at 30 days post-discharge | 26 (28.9) | 11 (10.7) | 37 (32.5) | 53 (25.4) | 127 (24.6) |
| 95% CI | 19.8, 39.4 | 5.5, 18.3 | 24.0, 41.9 | 19.6, 31.8 | 21.0, 28.6 |
| Cumulative mortality rate at 60 days post-discharge | 29 (32.2) | 15 (14.6) | 39 (34.2) | 61 (29.2) | 144 (27.9) |
| 95% CI | 22.8, 42.9 | 8.4, 22.9 | 25.6, 43.7 | 23.1, 35.9 | 24.1, 32.0 |
BSI bloodstream infection, CI confidence interval, cIAI complicated intra-abdominal infection, cUTI complicated urinary tract infection, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia
aIncludes patients with BSI/sepsis
Lowest 60-day mortality rates were also observed for patients infected by E. coli (n = 8/47, 17.0%) and Enterobacter spp. (n = 9/40, 22.5%), versus patients infected by Klebsiella spp. (n = 103/351, 29.3%), Pseudomonas spp. (n = 22/74, 29.7%), and other Gram-negative bacteria (n = 16/59, 27.1%). For all these subsets, death occurred mostly in-hospital (Supplementary Table S4A–E).
The association between potential factors at initial hospitalization with clinical success was explored by logistic regression, and was modeled for clinical success versus clinical failure, excluding clinically indeterminate outcomes. A summary of the univariate model is available in Table 11 (the model is presented in Supplementary Table S5). Important variables decreasing the rate of clinical success included age (> 80 years vs. < 60 years), identification of “other” Gram-negative bacteria (other than E. coli, Klebsiella spp. (including K. pneumoniae), Enterobacter spp. (including E. cloacae), P. aeruginosa), indications of cIAI and HAP/VAP (in reference to cUTI), and concomitant colistin use. The chance of clinical success decreased as the age increased and was the lowest among the patients over 80 (odds ratio [OR] 0.37, 95% confidence interval [CI] 0.16–0.85, p = 0.0186). By contrast, hospitalization within 90 days prior to the index date was associated with a higher chance of clinical success. Sex, bacteremia, immunocompromised status, reduced dose due to renal impairment, and treatment line of ceftazidime–avibactam were not significant covariates (Supplementary Table S5).
Table 11.
Univariate logistic regression models for clinical success at initial hospitalization (summary)
| n (%) | Odds ratio | 95% CI | p value | |
|---|---|---|---|---|
| Age class | 459 (100) | |||
| < 60 | 216 (47.1) | Ref | ||
| 60–69 | 106 (23.1) | 0.65 | 0.32, 1.33 | 0.2385 |
| 70–79 | 93 (20.3) | 0.61 | 0.29, 1.25 | 0.1781 |
| Above 80 | 44 (9.6) | 0.37 | 0.16, 0.85 | 0.0186 |
| Recent hospitalizationa | 457 (99.6) | |||
| Yes | 225 (49.0) | 2.01 | 1.13, 3.57 | 0.0177 |
| No | 232 (50.5) | Ref | ||
| Indication | 459 (100) | |||
| cIAI | 76 (16.6) | 0.21 | 0.08, 0.57 | 0.0020 |
| cUTI | 97 (21.1) | Ref | ||
| HAP/VAP | 92 (20.0) | 0.37 | 0.13, 1.00 | 0.0504 |
| Otherb | 194 (42.3) | 0.52 | 0.20, 1.32 | 0.1661 |
| Bacteria identified | 459 (100) | |||
| Escherichia coli | ||||
| Yes | 47 (10.2) | 1.29 | 0.49, 3.41 | 0.6023 |
| No | 412 (89.8) | Ref | ||
| Klebsiella pneumoniae | ||||
| Yes | 281 (61.2) | 0.90 | 0.51, 1.58 | 0.7187 |
| No | 178 (38.8) | Ref | ||
| Klebsiella spp. | ||||
| Yes | 51 (11.1) | 1.87 | 0.65, 5.39 | 0.2470 |
| No | 408 (88.9) | Ref | ||
| Enterobacter cloacae | ||||
| Yes | 34 (7.4) | 2.53 | 0.59, 10.84 | 0.2115 |
| No | 425 (92.6) | Ref | ||
| Enterobacter spp. | ||||
| Yes | 7 (1.5) | 0.37 | 0.07, 1.94 | 0.2387 |
| No | 452 (98.5) | Ref | ||
| Pseudomonas aeruginosa | ||||
| Yes | 61 (13.3) | 0.73 | 0.35, 1.54 | 0.4101 |
| No | 398 (86.7) | Ref | ||
| Pseudomonas spp. | ||||
| Yes | 9 (2.0) | 1.21 | 0.15, 9.83 | 0.8603 |
| No | 450 (98.0) | Ref | ||
| Other Gram-negative bacteria | ||||
| Yes | 59 (12.9) | 0.42 | 0.21, 0.82 | 0.0111 |
| No | 400 (87.1) | Ref | ||
| Colistin use | 459 (100) | |||
| Concomitant to ceftazidime–avibactam | 47 (10.2) | 0.38 | 0.19, 0.79 | 0.0092 |
| Subsequent to ceftazidime–avibactam | 12 (2.6) | 0.66 | 0.14, 3.08 | 0.5927 |
| Both concomitant and subsequent use | 3 (0.7) | – | – | 0.9906 |
| No usage | 397 (86.5) | Ref | ||
The complete logistic regression analysis is presented in the supplementary materials
This analysis was conducted on patients with clinical success or failure at the initial hospitalization (excluding patients with indeterminate clinical outcome). It examined the association of several variables with clinical success
BMI body mass index, BSI bloodstream infection, CI confidence interval, cIAI complicated intra-abdominal infection, cUTI complicated urinary tract infection, DCCI Deyo-Charlson Comorbidity Index, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia
aWithin 90 days prior to date of admission for index hospitalization
bIncludes patients with BSI/sepsis
Adverse events were reported for six patients out of the 569 enrolled. Five patients (0.9%) experienced seven serious adverse events, including altered state of consciousness, neurotoxicity, cholestasis, hepatocellular injury, multiple organ dysfunction syndrome, Clostridium difficile colitis, and Stevens–Johnson syndrome. Adverse events leading to the discontinuation of ceftazidime–avibactam included altered state of consciousness, cholestasis, and hepatocellular injury. One patient who had received ceftazidime–avibactam in combination with linezolid died from multiorgan failure, secondary to Stevens–Johnson syndrome.
Discussion
This real-world non-interventional medical chart review study describes treatment patterns (indications and use), the type of infection treated (indication and microbiology), effectiveness (clinical outcomes), and the safety of ceftazidime–avibactam in 516 patients enrolled from Europe and LATAM who received ceftazidime–avibactam for at least 72 h in routine clinical practice. Patients had heavy comorbidity burden, with a mean DCCI score of 4.6. A substantial proportion of patients had conditions associated with immune deficiency, such as hematological malignancies or solid organ transplant.
Infection sources identified were mainly intra-abdominal (17.4%), urinary (20.0%), respiratory (22.1%), and other indications (40.5%). The most common of “other indications” was BSI (18.8% out of 40.5%). Besides patients with BSI, 26.0% of patients had secondary bacteremia across the four indications. Around two-thirds of the patients had HAIs, and K. pneumoniae was the most common bacteria identified (n = 306, 59.3%), followed by P. aeruginosa (n = 69, 13.4%). The common MDR mechanisms for K. pneumoniae were KPC (33.9%), OXA-48 (25.2%), ESBL (21.5%), and MBLs (14.2%). Although the patients did not have prior exposure to ceftazidime–avibactam per the inclusion criteria, 7.8% of isolates tested were found resistant to that antibiotic before its initiation (mostly K. pneumoniae).
Ceftazidime–avibactam was mainly used as second-line treatment for Gram-negative coverage, and in combination with other antibiotics (notably aztreonam); the daily dose, frequency, and duration of infusion observed were according to the approved prescribing information, with the recommended dose adjustments in patients with renal impairment. Ceftazidime–avibactam therapy achieved treatment success in 77.3% of patients, particularly among patients with urinary infection (88.3%). The in-hospital mortality rate was 23.1%. Adverse experience collected from hospital records was uncommon.
In complement to other real-world evidence studies on ceftazidime–avibactam, this study provides important information about treatment patterns for ceftazidime–avibactam in routine clinical practice, by recruitment of patients across 11 countries from the European and LATAM regions, the size of the subset treated for “other indications” (including BSI/sepsis, febrile neutropenia, SSI, CLABSI, osteomyelitis, and other diagnoses), and the high proportion of patients with primary (BSI/sepsis) or secondary bacteremia. The primary objective of this study was to inform on the patterns of use of ceftazidime–avibactam in real-world practice; as such inclusion criteria were broad to fulfill this purpose, and data were collected after the end of the treatment, without interfering with medical care. In this regard, the study provides an overview of the use of this antibiotic in daily hospital practice.
A retrospective cohort study was conducted by Jorgensen et al. [20] among 203 patients treated with ceftazidime–avibactam (for at least 72 h) at six centers in the USA. The authors reported that the major indications were respiratory (37.4%), urinary (19.7%), intra-abdominal (18.7%), skin and soft tissue (8.9%), and osteoarticular (6.9%) infections, whereas the major indications of the current study were HAP/VAP (22.1%), cUTI (20.0%), BSI (18.8%), and cIAI (17.4%). In the study by Jorgensen et al., the most identified Gram-negative bacteria were CRE (57.6%) and Pseudomonas spp. (31.0%), whereas K. pneumoniae (59.3%) and P. aeruginosa (13.4%) were the most identified Gram-negative bacteria in the current study. The authors reported clinical failure (29.1%) and 30-day mortality (17.2%) rates, which were comparable to the results of the current study (treatment success 77.3%; in-hospital mortality rate 23.1%). The population of the study by Jorgensen et al. had a high burden of medical comorbidities (median DCCI score 4; IQR 2–6), which was similar to the current study findings (median DCCI score 4; IQR 2–7).
Another study conducted by Calvo-García et al. [21] in Spain among 63 patients aged over 18 years who received ceftazidime–avibactam for more than 24 h reported infection sources [respiratory (n = 13, 20.6%), urinary (n = 13, 20.6%), and intra-abdominal (n = 20, 31.7%)], main Gram-negative bacteria identified [K. pneumoniae (n = 43, 68.3%) and P. aeruginosa (n = 6, 9.5%)], and clinical cure rate (n = 47, 74.6%), which were comparable to the current study findings. Some of the parameters, like infection recurrence at 90 days (n = 23, 36.5%), and cumulative mortality rate 30 days after initiating ceftazidime–avibactam therapy (n = 10, 15.9%), were different from the current study findings [infection recurrence leading to hospital readmission n = 25; cumulative mortality rate at 30 days post-discharge n = 127, 24.6%]. Noteworthily, 79% of patients required dose adjustment due to renal impairment, versus 18% of patients in the current study. In addition, renal adjustment was not associated with poor outcome in the present study in comparison to previous reports [20, 22], probably because of the higher awareness among prescribers about the need to adapt the ceftazidime–avibactam dose as soon as the renal function changes.
A systematic literature review of 72 publications (62 peer-reviewed articles and 10 abstracts) on the real-world use of ceftazidime–avibactam monotherapy or combination therapy revealed the main indications were pneumonia, UTIs, abdominal infections, bacteremia, or skin/soft tissue infections caused by CRE or carbapenemase-producing Enterobacteriaceae and carbapenem-resistant, MDR, and extensively drug resistant P. aeruginosa. In a few of the included studies, treatment by ceftazidime–avibactam resulted in statistically superior clinical success rates (45–100%) and reported 30-day mortality (0–63%) compared to comparators/controls. Emergence of ceftazidime–avibactam resistance was observed mostly among KPC-producing strains, which is reflected in our study [23]. In another retrospective review, 577 adults (including 391 patients with BSI), infected by KPC-producing strains of K. pneumoniae, were treated with ceftazidime–avibactam monotherapy (n = 165, 28.6%) or combination therapy (n = 412, 71.4%); in-hospital mortality rate was 25.0%. These findings are similar to those in the current study [24]. In our study, the combination with colistin was associated with a worse clinical outcome. A potential explanation is that these patients had a higher nephrotoxicity rate leading to higher failure and mortality rates.
In randomized controlled trials, the clinical cure rate of ceftazidime–avibactam therapy against Gram-negative bacterial infections was reported as 90.3% in patients with UTIs caused by Gram-negative bacteria (including Enterobacteriaceae and P. aeruginosa) [25], 68.8% of patients with nosocomial pneumonia (including VAP; modified intention to treat analysis) due to Gram-negative bacterial infections (including K. pneumoniae and P. aeruginosa) [26], and 91.0% of patients with cUTIs or cIAIs caused by ceftazidime-resistant Enterobacteriaceae or P. aeruginosa (clinical cure of 92.0% in cUTI and 80.0% in cIAI) [27]. These clinical trials results are consistent with the findings from the current study, observed in the real-world setting.
Gales et al. conducted a review of 28 real-world studies to understand effectiveness and safety of ceftazidime–avibactam in more than 1050 adult patients (across the 28 studies) with infections due to aerobic Gram-negative bacteria with limited treatment options, including CRE and MDR Pseudomonas spp. Ceftazidime–avibactam was an effective alternative to standard of care antibiotics for treating infection caused by MDR Gram-negative bacteria including CRE and MDR Pseudomonas spp. Ceftazidime–avibactam was also well tolerated in patients who had MDR Gram-negative bacteria infections including CRE and MDR Pseudomonas spp., and also in patients with multiple comorbidities, severely or critically ill patients, or those with bacteremia [28].
The current study displayed several strengths. This was a large-scale study conducted in 11 countries pertaining to two regions: Europe (including Russia) and LATAM, enrolling 569 patients. Collected data represented patients’ medical records from routine clinical practice in their respective countries. Compared to restrictive eligibility criteria in randomized controlled trials, this real-world evidence study allowed selection of heterogeneous patient populations with various comorbidities and concomitant treatments. Ceftazidime–avibactam was used per the approved prescribing information (dose, frequency, duration of infusion), with the recommended dose adjustments in patients with renal impairment. This allows generalizability of study results to patients eligible for receiving ceftazidime–avibactam in routine clinical practice across Europe and the LATAM region.
However, the outcomes of the study should be interpreted considering its limitations. First, the present investigation being a non-comparative study, the results could not be compared against another treatment. Second, there might have been a selection bias toward the patients who had already died, as those cases were eligible for waived informed consent in European countries (except in France and the Russian Federation) and LATAM countries. Third, the study centers were mostly teaching and tertiary care hospitals; thus, the patients may not be representative of the general patient population treated in other types of hospitals in terms of indications, severity of the infection, and underlying conditions. Finally, the patients were followed up for 60 days after discharge for any indication of the same infection leading to readmission. Since no visit was mandated, the patients’ readmission at a different hospital during that period might remain uncaptured (outcome misclassification).
Conclusion
The findings of the current study provide important real-world evidence on treatment patterns, resource use, clinical outcomes, and the safety of ceftazidime–avibactam in routine clinical practice across the European and LATAM regions among patients treated for the array of approved indications for ceftazidime–avibactam, with a high proportion of patients treated for BSI. Ceftazidime–avibactam is one of the antibiotics to consider for treatment of MDR bacteria. These findings also complement the results of randomized controlled trials and offer insight to assess and improve clinical practice worldwide.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
All authors would like to thank Nathalie Baillon-Plot (Pfizer P.I.O., Paris, France) for her support and contribution in this study.
Funding
The EZTEAM study was funded by Pfizer, however, no payments or honoraria were made to the authors in respect to manuscript preparation. Pfizer also funded the journal’s Rapid Service fees.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) authorship guidelines for this manuscript.
Author Contributions
All named authors contributed to interpretation of the study results, drafting and revision of the manuscript, and approved the final version of the manuscript.
Disclosures
Alex Soriano has received honoraria for lectures and advisory boards from Pfizer, MSD, Menarini, Shionogi, and Angelini. Philippe Montravers has received research funds for lectures and advisory boards for Menarini, MSD and Pfizer. Galina Klyasova has received honoraria for lectures from Pfizer, MSD, Gilead. Paurus Irani is an employee of Pfizer Ltd, UK. Gregory Stone is an employee of Pfizer, Groton, USA. Richard Chambers is an employee of Pfizer Inc, New York, USA. Pascale Peeters and Claire Hulin are employees of IQVIA, France. Mitesh Shah is an employee of IQVIA, India. Francesco Menichetti served as local PI for an AstraZeneca–sponsored trial and a Toscana Life Science-sponsored trial evaluating monoclonal antibodies for SARS-CoV-2 (for which no personal fees were received), and received in the last 3 years (outside the submitted work) speaker honoraria or advisory board or support for meetings from Angelini, Menarini, Correvio, MSD, Pfizer, Astellas, Gilead, BMS, Janssen, ViiV, BioMerieux, Biotest, Becton–Dickinson, Pfizer, Shionogi, Roche, GSK, Advanz Pharma, and ThermoFisher. Mathias W. Pletz has received research funds and speaker fees from Pfizer and has participated in related advisory boards. Marisa Sanchez received speaker fees from Pfizer and MSD. Anita Verma was the chief investigator for UK and principal investigator at King’s College Hospital for this study and received research fund from Pfizer. Maria Lavinea N. de Figueiredo is an employee of Pfizer, Sao Paulo, Brazil. Claudie Charbonneau is an employee of Pfizer P.I.O., Paris, France. The remaining authors declared no conflict of interest.
Compliance with Ethics Guidelines
This observational study was conducted after receiving approval from the ethics committees. Where applicable by local regulation, written informed consent was obtained from each patient or the patient’s legally acceptable representative before any study-specific activity was performed. In France, patients could only be recruited retrospectively, and although there was no requirement for obtaining informed consent (by local regulation), a patient information sheet was sent to the enrolled patient. In the UK, an informed consent waiver was approved for all patients, however late in the recruitment period.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available due to Pfizer’s internal policies. Pfizer will provide access to individual de-identified subject data and related study documents (such as protocol, statistical analysis plan, clinical study report) upon request from qualified researchers, and subject to certain criteria, conditions, and exceptions. Pfizer's data sharing criteria and process for requesting access are available at https://www.pfizer.com/science/clinical_trials/trial_data_and_results/data_requests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 2.Bates DW, Larizgoitia I, Prasopa-Plaizier N, Jha AK. Global priorities for patient safety research. BMJ. 2009;338:b1775. doi: 10.1136/bmj.b1775. [DOI] [PubMed] [Google Scholar]
- 3.Zarb P, Coignard B, Griskeviciene J, et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 2012. 10.2807/ese.17.46.20316-en. [DOI] [PubMed]
- 4.World Health Organizations. Annual epidemiological report on communicable disease in Europe. 2008. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0812_SUR_Annual_Epidemiological_Report_2008.pdf. Accessed 22 Feb 2018.
- 5.Annual epidemiological report Reporting on 2011 surveillance data and 2012 epidemic intelligence data. 2013. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/annual-epidemiological-report-2013.pdf. Accessed 21 Oct 2021.
- 6.Healthcare associated infections (HAI): point prevalence survey, England. 2016. https://www.gov.uk/government/publications/healthcare-associated-infections-hcai-point-prevalence-survey-england. Accessed 21 Oct 2021.
- 7.Huerta-Gutiérrez R, Braga L, Camacho-Ortiz A, et al. One-day point prevalence of healthcare-associated infections and antimicrobial use in four countries in Latin America. Int J Infect Dis. 2019;86:157–166. doi: 10.1016/j.ijid.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Rawat D, Nair D. Extended-spectrum beta-lactamases in Gram negative bacteria. J Glob Infect Dis. 2010;2(3):263–274. doi: 10.4103/0974-777X.68531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Núñez-Núñez M, Navarro MD, Palomo V, et al. The methodology of surveillance for antimicrobial resistance and healthcare-associated infections in Europe (SUSPIRE): a systematic review of publicly available information. Clin Microbiol Infect. 2018;24(2):105–109. doi: 10.1016/j.cmi.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Patolia S, Abate G, Patel N, Patolia S, Frey S. Risk factors and outcomes for multidrug-resistant Gram-negative bacilli bacteremia. Ther Adv Infect Dis. 2018;5(1):11–18. doi: 10.1177/2049936117727497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin TL, Chang PH, Chen IL, et al. Risk factors and mortality associated with multi-drug-resistant Gram-negative bacterial infection in adult patients following abdominal surgery. J Hosp Infect. 2022;119:22–32. doi: 10.1016/j.jhin.2021.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Zowawi HM, Harris PN, Roberts MJ, et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 2015;12(10):570–584. doi: 10.1038/nrurol.2015.199. [DOI] [PubMed] [Google Scholar]
- 13.Pitout JD. Infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70(3):313–333. doi: 10.2165/11533040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Cantón R, Akóva M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18(5):413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 15.Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. http://remed.org/wp-content/uploads/2017/03/lobal-priority-list-of-antibiotic-resistant-bacteria-2017.pdf. Accessed 26 Oct 2021.
- 16.Wong D, van Duin D. Novel beta-lactamase inhibitors: unlocking their potential in therapy. Drugs. 2017;77(6):615–628. doi: 10.1007/s40265-017-0725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medical Association. Zavicefta® Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/zavicefta-epar-product-information_en.pdf. Accessed 28 Apr 2022.
- 18.Mazuski JE, Wagenlehner F, Torres A, et al. Clinical and microbiological outcomes of ceftazidime–avibactam treatment in adults with gram-negative bacteremia: a subset analysis from the phase 3 clinical trial program. Infect Dis Ther. 2021;10(4):2399–2414. doi: 10.1007/s40121-021-00506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply "precision medicine" to antimicrobial chemotherapy? Expert Opin Pharmacother. 2016;17(6):761–781. doi: 10.1517/14656566.2016.1145658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen SCJ, Trinh TD, Zasowski EJ, et al. Real-world experience with ceftazidime–avibactam for multidrug-resistant Gram-negative bacterial infections. Open Forum Infect Dis. 2019;6(12):ofz 522. [DOI] [PMC free article] [PubMed]
- 21.Calvo-García A, Ibanez Zurriaga MD, Ramírez Herráiz E, Pérez Abánades M, Sáez Béjar C, Morell Baladrón A. Ceftazidime–avibactam: effectiveness and safety in the clinical practice. A third hospital level experience. Rev OFIL·ILAPHAR 2020;32(1):52–62.
- 22.Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidimeavibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano A, Carmeli Y, Omrani AS, Moore LSP, Tawadrous M, Irani P. Ceftazidime–avibactam for the treatment of serious gram-negative infections with limited treatment options: a systematic literature review. Infect Dis Ther. 2021;10(4):1989–2034. doi: 10.1007/s40121-021-00507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumbarello M, Raffaelli F, Giannella M, et al. Ceftazidime–avibactam use for Klebsiella pneumoniae carbapenemase-producing K. pneumoniae infections: a retrospective observational multicenter study. Clin Infect Dis. 2021;73(9):1664–1676. [DOI] [PubMed]
- 25.Mendes RE, Castanheira M, Woosley LN, Stone GG, Bradford PA, Flamm RK. Molecular beta-lactamase characterization of Gram-negative pathogens recovered from patients enrolled in the ceftazidime–avibactam phase 3 trials (RECAPTURE 1 and 2) for complicated urinary tract infections: efficacies analysed against susceptible and resistant subsets. Int J Antimicrob Agents. 2018;52(2):287–292. doi: 10.1016/j.ijantimicag.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Torres A, Zhong N, Pachl J, et al. Ceftazidime–avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285–295. doi: 10.1016/S1473-3099(17)30747-8. [DOI] [PubMed] [Google Scholar]
- 27.Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime–avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis. 2016;16(6):661–673. doi: 10.1016/S1473-3099(16)30004-4. [DOI] [PubMed] [Google Scholar]
- 28.Gales AC, Carmargo LFA, Cuba GT, et al. A review of real-world use of ceftazidime–avibactam for multidrug-resistant gram-negative bacterial infections. J Infect Dis Ther. 2022;10:483. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available due to Pfizer’s internal policies. Pfizer will provide access to individual de-identified subject data and related study documents (such as protocol, statistical analysis plan, clinical study report) upon request from qualified researchers, and subject to certain criteria, conditions, and exceptions. Pfizer's data sharing criteria and process for requesting access are available at https://www.pfizer.com/science/clinical_trials/trial_data_and_results/data_requests.