Abstract
Background
Ileus commonly occurs after abdominal surgery, and is associated with complications and increased length of hospital stay (LOHS). Onset of ileus is considered to be multifactorial, and a variety of preventative methods have been investigated. Chewing gum (CG) is hypothesised to reduce postoperative ileus by stimulating early recovery of gastrointestinal (GI) function, through cephalo‐vagal stimulation. There is no comprehensive review of this intervention in abdominal surgery.
Objectives
To examine whether chewing gum after surgery hastens the return of gastrointestinal function.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (via Ovid), MEDLINE (via PubMed), EMBASE (via Ovid), CINAHL (via EBSCO) and ISI Web of Science (June 2014). We hand‐searched reference lists of identified studies and previous reviews and systematic reviews, and contacted CG companies to ask for information on any studies using their products. We identified proposed and ongoing studies from clinicaltrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform and metaRegister of Controlled Trials.
Selection criteria
We included completed randomised controlled trials (RCTs) that used postoperative CG as an intervention compared to a control group.
Data collection and analysis
Two authors independently collected data and assessed study quality using an adapted Cochrane risk of bias (ROB) tool, and resolved disagreements by discussion. We assessed overall quality of evidence for each outcome using Grades of Recommendation, Assessment, Development and Evaluation (GRADE). Studies were split into subgroups: colorectal surgery (CRS), caesarean section (CS) and other surgery (OS). We assessed the effect of CG on time to first flatus (TFF), time to bowel movement (TBM), LOHS and time to bowel sounds (TBS) through meta‐analyses using a random‐effects model. We investigated the influence of study quality, reviewers’ methodological estimations and use of Enhanced Recovery After Surgery (ERAS) programmes using sensitivity analyses. We used meta‐regression to explore if surgical site or ROB scores predicted the extent of the effect estimate of the intervention on continuous outcomes. We reported frequency of complications, and descriptions of tolerability of gum and cost.
Main results
We identified 81 studies that comprised 9072 participants for inclusion in our review. We categorised many studies at high or unclear risk of the bias' assessed. There was statistical evidence that use of CG reduced TFF [overall reduction of 10.4 hours (95% CI: ‐11.9, ‐8.9): 12.5 hours (95% CI: ‐17.2, ‐7.8) in CRS, 7.9 hours (95% CI: –10.0, ‐5.8) in CS, 10.6 hours (95% CI: ‐12.7, ‐8.5) in OS]. There was also statistical evidence that use of CG reduced TBM [overall reduction of 12.7 hours (95% CI: ‐14.5, ‐10.9): 18.1 hours (95% CI: ‐25.3, ‐10.9) in CRS, 9.1 hours (95% CI: ‐11.4, ‐6.7) in CS, 12.3 hours (95% CI: ‐14.9, ‐9.7) in OS]. There was statistical evidence that use of CG slightly reduced LOHS [overall reduction of 0.7 days (95% CI: ‐0.8, ‐0.5): 1.0 days in CRS (95% CI: ‐1.6, ‐0.4), 0.2 days (95% CI: ‐0.3, ‐0.1) in CS, 0.8 days (95% CI: ‐1.1, ‐0.5) in OS]. There was statistical evidence that use of CG slightly reduced TBS [overall reduction of 5.0 hours (95% CI: ‐6.4, ‐3.7): 3.21 hours (95% CI: ‐7.0, 0.6) in CRS, 4.4 hours (95% CI: ‐5.9, ‐2.8) in CS, 6.3 hours (95% CI: ‐8.7, ‐3.8) in OS]. Effect sizes were largest in CRS and smallest in CS. There was statistical evidence of heterogeneity in all analyses other than TBS in CRS.
There was little difference in mortality, infection risk and readmission rate between the groups. Some studies reported reduced nausea and vomiting and other complications in the intervention group. CG was generally well‐tolerated by participants. There was little difference in cost between the groups in the two studies reporting this outcome.
Sensitivity analyses by quality of studies and robustness of review estimates revealed no clinically important differences in effect estimates. Sensitivity analysis of ERAS studies showed a smaller effect size on TFF, larger effect size on TBM, and no difference between groups for LOHS.
Meta‐regression analyses indicated that surgical site is associated with the extent of the effect size on LOHS (all surgical subgroups), and TFF and TBM (CS and CRS subgroups only). There was no evidence that ROB score predicted the extent of the effect size on any outcome. Neither variable explained the identified heterogeneity between studies.
Authors' conclusions
This review identified some evidence for the benefit of postoperative CG in improving recovery of GI function. However, the research to date has primarily focussed on CS and CRS, and largely consisted of small, poor quality trials. Many components of the ERAS programme also target ileus, therefore the benefit of CG alongside ERAS may be reduced, as we observed in this review. Therefore larger, better quality RCTS in an ERAS setting in wider surgical disciplines would be needed to improve the evidence base for use of CG after surgery.
Keywords: Humans, Chewing Gum, Abdomen, Abdomen/surgery, Gastrointestinal Motility, Gastrointestinal Motility/physiology, Ileus, Ileus/therapy, Length of Stay, Postoperative Complications, Postoperative Complications/therapy, Postoperative Period, Randomized Controlled Trials as Topic, Recovery of Function, Recovery of Function/physiology, Time Factors
Plain language summary
Chewing gum after surgery to help recovery of the digestive system
Background
When people have surgery on their abdomen, the digestive system can stop working for a few days. This is called ileus, and can be painful and uncomfortable. There are different causes of ileus, and several ways of treating or preventing it. One possible way of preventing ileus is by chewing gum. The idea is that chewing gum tricks the body into thinking it is eating, causing the digestive system to start working again. It is important to do this review because ileus is common: it is estimated that up to a third of people having bowel surgery suffer from ileus.
Main Findings
This review found 81 relevant studies that recruited over 9000 participants in total. The studies mainly focussed on people having bowel surgery or caesarean section, but there were some studies of other surgery types. There were few studies of children. Most studies were of poor quality, which may mean their results are less reliable. We found some evidence that people who chewed gum after an operation were able to pass wind and have bowel movements sooner than people who did not chew gum. We also found some evidence that people who chewed gum after an operation had bowel sounds (gurgling sounds heard using a stethoscope held to the abdomen) slightly sooner than people who did not chew gum. There was a small difference in how long people stayed in hospital between people who did or did not chew gum. There were no differences in complications (such as infection or death) between people who did or did not chew gum. There was also no difference in the overall cost of treatment between people who did or did not chew gum.
Conclusions
There is some evidence that chewing gum after surgery may help the digestive system to recover. However, the studies included in this review are generally of poor quality, which meant that their results may not be reliable. We also know that there are many factors affecting ileus, and that modern treatment plans attempt to reduce risk of ileus. Therefore to further explore using chewing gum after surgery, more studies would be needed which are larger, of better quality, include different types of surgery, and consider recent changes in health care systems.
Summary of findings
Summary of findings for the main comparison. Summary of findings ‐ continuous outcomes.
| Chewing gum compared with control for improving postoperative recovery of gastrointestinal function in people undergoing abdominal surgery | |||||
|
Patient or population: individuals undergoing abdominal surgery Settings: hospital setting Intervention: chewing gum Comparison: standard care (no chewing gum) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Control group | Intervention group | ||||
|
Time to first flatus Hours |
The mean time to first flatus in the control group was 49.9 hours | The mean time to first flatus in the intervention group was 10.4 hours shorter (11.9 to 8.9 hours shorter) | 8293 (77) | ⊕⊕⊝⊝ low | High risk of bias in outcome reporting as participants cannot be blinded for this outcome Small to moderate confidence intervals |
|
Time to first bowel movement Hours |
The mean time to first bowel movement in the control group was 75.4 hours | The mean time to first bowel movement in the intervention group was 12.7 hours shorter (14.5 to 10.9 hours shorter) | 7283 (62) | ⊕⊕⊝⊝ low | High risk of bias in outcome reporting as participants cannot be blinded for this outcome Some suspicion of publication bias based on visual inspection of the funnel plot Small to moderate confidence intervals |
|
Length of hospital stay Days |
The mean length of hospital stay in the control groups was 6.8 days | The mean length of hospital stay in the intervention group was 0.7 days shorter (0.8 to 0.5 days shorter) | 5278 (50) | ⊕⊕⊕⊝ moderate | High risk of bias in outcome reporting as blinding methods poorly reported Some suspicion of publication bias based on visual inspection of the funnel plot Small to moderate confidence intervals |
|
Time to first bowel sounds Hours |
The mean time to first bowel sounds in the control group was 21.9 hours | The mean time to first bowel sounds in the intervention group was 5.0 (6.4 to 3.7 hours shorter) | 3981 (23) | ⊕⊕⊝⊝ low | High risk of bias in outcome reporting as blinding methods poorly reported Few studies reported accurately recording this outcome Moderate confidence intervals |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
For each continuous outcome, many studies' results were statistically manipulated or estimated to allow inclusion in our meta‐analyses (see Table 3)
For each continuous outcome, there were studies whose results could not be included in this meta‐analysis (see Table 4), therefore the evidence provided here does not include all evidence available
All evidence used is directly relevant to the research question
High heterogeneity between studies for each continuous outcome. Heterogeneity is not well explained by the pre‐specified subgroup analyses
Summary of findings 2. Summary of findings ‐ descriptive outcomes.
| Chewing gum compared with control for improving postoperative recovery in people undergoing abdominal surgery | |||
|
Patient or population: individuals undergoing abdominal surgery Settings: hospital setting Intervention: chewing gum Comparison: standard care (no chewing gum) | |||
| Outcomes | Relative effect | Quality of the evidence (GRADE) | Comments |
|
Complications Frequency |
Potential small reduction in frequency of nausea and vomiting Little difference reported in frequency of mortality Little difference reported in frequency of infection Little difference reported in frequency of readmission Potential small reduction in frequency of other complications Only one study reported complications which authors believed may have been related to the intervention (due to aerophagia whilst chewing gum) |
⊕⊕⊝⊝ low | Methods used for recording complications is poorly reported Low frequency provides little substantial evidence A diverse range of complications are reported; therefore it is difficult to group these together to draw meaningful comparisons High risk of bias in outcome reporting as blinding methods poorly reported |
|
Tolerability of gum Anecdotal evidence, interviews, questionnaires and surveys |
Gum was generally well‐tolerated by participants | ⊕⊕⊝⊝ low | The majority of evidence is anecdotal This outcome is generally measured and reported in an insufficient manner |
| Cost | One study found that cost of hospitalisation was lower in the intervention group, but did not reach significance (intervention group: 2379 ± 195 USD, control group: 2672 ± 265 USD) One study found that hospital charges did not differ significantly between the groups (intervention group: 2451 ± 806 YTL, 1493 to 4619 YTL; control group: 2102 ± 678 YTL, 1073 to 3497 YTL; P = 0.206) |
⊕⊝⊝⊝ very low | Only 2 studies reported cost analyses |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Background
Description of the condition
Although there is not currently one widely accepted definition of ileus (Vather 2013), this condition has previously been described as a transient impairment of bowel motility after abdominal surgery or other trauma (Holte 2000). Ileus is therefore considered to be an inevitable consequence of abdominal surgery (Tu 2014; Gervaz 2006), and commonly occurs following colorectal, gynaecological, thoracic and urological surgical procedures (Bashankaev 2009). Prevalence of ileus is difficult to estimate due to the lack of accurate reporting and lack of a standardised definition (Barletta 2014; Vather 2013). Evidence indicates that ileus is most prolonged following large bowel surgery, and reports in this surgical discipline range from 3 to 32% of patients (Kronberg 2011; Vasquez 2009). There is evidence however that the introduction of laparoscopic surgery may reduce incidence of ileus (Fujii 2014; Hosono 2006).
Resolution of ileus is an important factor in the speed of postoperative recovery. Ileus can lead to nausea, vomiting, abdominal discomfort (Johnson 2009), increased length of hospital stay (LOHS) (Schuster 2006) and therefore increased costs (Fitzgerald 2009). Additionally, it has been suggested that postoperative ileus can result in poorer wound healing, delays in time to mobilisation and resumption of oral intake, and reduced patient satisfaction (Behm 2003).
The pathogenesis of postoperative ileus is multifactorial (Bonventre 2014; Le Blanc‐Louvry 2002), as numerous factors influencing the surgical stress response contribute to the development and duration of ileus. These include degree of bowel manipulation, level of surgical trauma, anaesthesia and effects of postoperative modifiers such as pain management with opiates (Holte 2000; Lim 2013; Tu 2014). Additionally, suggested risk factors for postoperative ileus include increasing age, high body mass index and ethnic minority (Chang 2002; Svatek 2010).
Resolution of ileus usually occurs two to five days postoperatively (Livingston 1990; Warren 2011). Generally the small intestine is the first part of the digestive system to recover postoperatively within 24 hours, followed by the stomach within 24 to 48 hours, and the large bowel after 48 to 72 hours (Gervaz 2006; Nimarta 2013). Various approaches have been investigated to prevent onset and reduce duration of ileus, incorporating both reducing surgical stress and optimising postoperative care. These include providing nasogastric decompression, performing minimally invasive surgery, promoting early ambulation, avoiding preoperative bowel preparation, limiting intravenous fluid administration, using prokinetic agents, using epidural analgesia and reducing opiate use for pain management (Story 2009). Many of these practices have been incorporated into the Enhanced Recovery After Surgery (ERAS) programme endorsed across UK National Health Service (NHS) hospitals nationally. Early postoperative feeding is another component of ERAS that may stimulate gut motility, thereby reducing onset and duration of ileus (Fanning 2011). However, early postoperative feeding is not universally accepted, as it is not always well tolerated by patients. For example, vomiting and the risk of postoperative complications such as aspiration may be increased (Basaran 2009; Lewis 2001).
Additionally, a number of non‐clinical approaches to reduce postoperative ileus have been suggested. These include drinking coffee, herbal formulae, acupuncture, mechanical abdominal massage and rocking‐chair motion (Endo 2014; Garcia 2008; Le Blanc‐Louvry 2002; Massey 2010; Müller 2012).
Description of the intervention
It has been suggested that chewing gum (CG) postoperatively may help recovery of gastrointestinal (GI) function by stimulating earlier resumption of bowel activity (Asao 2002; Lim 2013). CG is a form of sham feeding that replicates the process of eating without ingestion of food. Thus, it may stimulate GI function without producing the complications associated with early feeding e.g. nausea, vomiting. CG is a cheap and widely available product which most people have previously experienced. Therefore it is an intervention which is likely to be well tolerated by individuals postoperatively.
How the intervention might work
In 2002, results from a small randomised controlled trial (RCT) suggested that use of CG may hasten postoperative recovery (Asao 2002). Since that time, a number of trials have examined the effect of CG on postoperative ileus, and several have demonstrated benefits (Abd‐El‐Maeboud 2009; Ledari 2012; Marwah 2012). It is thought that there are three main mechanisms by which CG may reduce duration and prevent onset of ileus (Tandeter 2009). First, stimulation of gut motility by cephalo‐vagal stimulation which in turn leads to release of GI hormones. Second, ‘sham feeding’ tricks parts of the digestive system and stimulates motility. Third, encouragement of release of pancreatic juices and saliva (Tandeter 2009). This intervention may provide a means to reduce the duration of postoperative ileus without the adverse effects of increased vomiting and nausea associated with early postoperative feeding. In addition, this may provide an intervention in patients where food cannot be tolerated.
Serious adverse events are unlikely to occur with this intervention; studies have reported no adverse events (Choi 2011; Husslein 2013). However incidents such as indigestion or bloating, potentially due to aerophagia whilst chewing, may occur (Zaghiyan 2013). Additionally CG may cause choking in individuals with dysphagia and in people who have difficulty chewing, such as individuals with dental problems, poor/loosely fitting dentures and young children.
Why it is important to do this review
Chewing gum may offer an innovative intervention for improving postoperative GI function recovery. Earlier resolution of ileus may result in reductions in patient discomfort, complications and LOHS. Considering the number of people who undergo abdominal operations each year globally, and the high prevalence of ileus within these, this could have implications for healthcare costs and recovery. It is therefore essential that benefits and costs are carefully evaluated. This systematic review (SR) summarises the available evidence on the use of CG in reducing the onset and duration of ileus by improving the rate of return of postoperative GI function.
Objectives
The objective of this review is to examine whether chewing gum (CG) after surgery hastens the return of gastrointestinal (GI) function. The review considers the impact of CG on indicators of bowel function [time to first flatus (TFF), bowel movement (TBM) and bowel sounds (TBS)] and on recovery [length of hospital stay (LOHS) and postoperative complications]. The review also considers tolerability of CG and the financial costs and benefits associated with using this intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs that used chewing gum as an intervention regardless of publication language. Quasi‐randomised trials were not included.
Types of participants
Participants of any age who underwent abdominal surgery for any indication.
Types of interventions
Interventions consisted of CG in the immediate postoperative recovery period and use of a control group for comparison. Studies in which the gum contained an active therapeutic agent were not considered unless the agent was also administered to the control group. Studies in which the intervention consisted of gum in combination with another intervention were not considered.
Types of outcome measures
Primary outcomes
Primary outcomes were time to first flatus (TFF) (hours) and time to first bowel movement (TBM) (hours).
Secondary outcomes
Secondary outcomes were length of hospital stay (LOHS) (days), time to first bowel sounds (TBS) (as an additional marker of return of GI function; hours), reports of postoperative complications (frequency), tolerability of gum and costs and benefits (descriptive outcomes).
Outcome measures were reported in units considered to be clinically meaningful.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 5, 2014), MEDLINE (via Ovid) from 1966 to present, MEDLINE (via PubMed) from 1966 to present, EMBASE (via Ovid) from 1980 to present, CINAHL (via EBSCO) from 1990 to present and ISI Web of Science from 1900 to present, using a combination of MeSH and key terms. The search terms included “gum”, “recovery” and “ileus” and any derivatives of those terms. Searching for RCTs was done by hand by screening abstracts and full‐texts where necessary.
No limitation based on language or date of publication was applied. One of the authors (RP) developed the search strategies, see Appendix 1 for CENTRAL; Appendix 2 for MEDLINE (via Ovid); Appendix 3 for MEDLINE (via PubMed); Appendix 4 for EMBASE (via Ovid); Appendix 5 for CINAHL (via EBSCO); and Appendix 6 for ISI Web of Science. The first search was run in June 2013, repeated in January 2014, and updated in June 2014.
Searching other resources
We hand‐searched reference lists of identified studies, previous reviews and SRs for additional relevant articles. We searched Google Scholar every two weeks up to page 20 with various combinations of key terms such as “gum, ileus”, “gum, bowel” and “gum, gastrointestinal”. We contacted authors for information on references from their reference lists if we could not access or identify them ourselves.
We searched the following registers for proposed and ongoing trials: clinicaltrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform and metaRegister of Controlled Trials using combinations of search terms including “gum chewing”, “gum AND ileus”, “gum AND bowel” and “sham feeding”. We did not impose any date or language restrictions. We approached principal investigators of identified ongoing trials that had not yet been published, to ask for relevant data. In addition, we contacted CG manufacturers (Wrigley Company, Cadbury Trebor Bassett, Lotte, Perfetti Van Melle and Hershey’s) to ask for information on published or unpublished material on their product.
Data collection and analysis
Selection of studies
Two review authors (VS and GH) independently examined the titles and abstracts of studies identified through the search strategy. Inconsistency between review authors regarding articles for full‐text reading was resolved by consultation with a third review author (RP or CP). We obtained full‐text papers for all studies that could not be excluded on the basis of title and abstract. The same review authors then independently refined their selection by examining the selected articles and excluding those not relevant to this review. Review authors recorded agreement on trial inclusion, and disagreement was resolved by predetermined co‐review authors (ST and SJL for clinical disputes, RP and CP for methodological disputes). We contacted original study authors where further clarity was needed in order to select a study for inclusion. We documented decisions on all studies and these are presented in the PRISMA flow chart (Figure 1).
1.
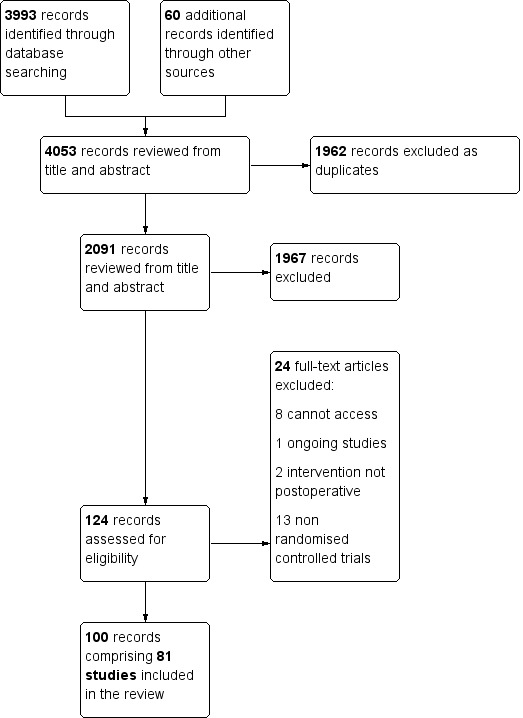
Study flow diagram.
Data extraction and management
Two review authors (VS and either GH or RP) independently extracted data from each study. Review authors were blinded to each other’s data. We developed a data extraction form adapted for this review from the original provided by Cochrane. Three authors (VS, GH and RP) examined this on several studies selected for inclusion, and revised it for ease of extraction and to include further useful data items. We extracted data regarding participant demographics, participant disease status, surgical procedures, control group postoperative care and the intervention (frequency and duration of CG) using these predesigned data extraction forms. In order to ensure accurate data extraction, three review authors (VS, GH and RP) independently extracted and compared data from 16 (20%) studies for consistency.
Many of the identified studies were published in other languages. Titles and abstracts were generally available in English, and where studies appeared to meet the inclusion criteria, they were either translated or directly extracted onto the data extraction form. Eighteen studies were directly extracted from Chinese (Mandarin), and 19 were translated from Chinese (Mandarin), Farsi, German, Korean and Spanish and then extracted by reviewers.
Assessment of risk of bias in included studies
Either two or three review authors (VS and either GH or RP) independently assessed risk of bias (ROB). We developed our own ROB tool based on the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), tailored to this review. We developed this as data extraction continued. We included specific examples and numerical cut‐off points in the adapted ROB tool (Appendix 7), to ensure consistency of ROB assessments. We then discussed ROB for all studies to ensure uniformity and agreement. Where possible, we sought protocols to aid assessment of selective outcome reporting bias. We reported use of sample size/power calculations and intention‐to‐treat analyses as measures of methodological quality. We labelled ROB as ‘high’, ‘low’ or ‘unclear’ for the following categories: random sequence generation, allocation concealment, blinding of personnel, blinding of outcome assessment (for TFF, TBM, LOHS, TBS and complications), incomplete outcome data, selective outcome reporting and ‘other’ risks (e.g. differences in baseline demographics, study sample size).
Measures of treatment effect
We considered continuous variables (TFF, TBM, LOHS and TBS) as weighted mean differences (WMDs), and included 95% confidence intervals. We reported complications as frequency of nausea and vomiting, mortality, infection, readmissions, other complications, and complications related to the intervention. We descriptively recorded any information on tolerability of gum or financial burden/benefit reported in the studies.
Unit of analysis issues
We used individual participants as the unit of analysis. No studies used cluster randomisation.
Dealing with missing data
We contacted authors when key information was missing. When no further information was provided or authors could not be contacted, we estimated results or used the available data where appropriate (see Data synthesis). Table 3 summarises these estimates and transformations.
1. Estimated results and assumptions.
| Study | Estimated results |
| Atkinson 2014 | Time to first flatus, time to first bowel movement, length of hospital stay and time to first bowel sounds reported as median, interquartile range and range (unpublished information). Mean and standard deviation calculated using the formulae described by Hozo 2005 |
| Bonventre 2014 | Time to first flatus, time to first bowel movement and length of hospital stay reported as median, interquartile range and range (unpublished information). Mean and standard deviation calculated using the formulae described by Hozo 2005 |
| Choi 2011 | Time to first flatus, time to first bowel movement and length of hospital stay reported as median and range (assumed to be range due to broad range of numbers and authors' later paper, Choi 2014). Mean and standard deviation calculated using the formulae described by Hozo 2005 |
| Choi 2014 | Time to first flatus, time to first bowel movement and length of hospital stay reported as median and range. Mean and standard deviation calculated using the formulae described by Hozo 2005 |
| Crainic 2009 | Time to first flatus and time to first bowel movement reported as mean and standard error of the mean. Standard deviation calculated from the standard error of the mean |
| Garshasbi 2011 | Time to first flatus and time to first bowel movement reported as a median (assumed to be means for analyses), time to first bowel sounds reported as a mean. Standard deviation estimations assumed from the most conservative reliable value within the caesarean section subgroup (time to first flatus, time to first bowel movement and time to first bowel sounds: Shang 2010 for both intervention and control groups). Complications reported as % of participants: 2% in gum chewing group and 10% in control group; these have been rounded to the nearest whole number (4.76 rounded to 5 in the gum chewing group, 26.2 rounded to 26 in the control group) |
| Husslein 2013 | Time to first flatus, time to first bowel movement and length of hospital stay reported as median and range. Mean and standard deviation calculated using the formulae described by Hozo 2005 |
| Jakkaew 2013 | Time to first flatus and length of hospital stay reported as median and range. Mean and standard deviation calculated using the formulae described by Hozo 2005 |
| Jin 2010 | Complications reported as % of participants: 8.7% in gum chewing group and 28.6% in control group; these have been rounded to the nearest whole number (4.002 rounded to 4 in the gum chewing group, 12.012 rounded to 12 in the control group) |
| Kafali 2010 | Postoperative antiemetic requirement assumed to indicate frequency of nausea and vomiting. Intestinal enema for discharge assumed to indicate an 'other' complication |
| Lee 2004 | Time to first flatus, time to first bowel movement and length of hospital stay reported as a mean. Assumed that a t‐test was conducted. P values reported as P < 0.03, P < 0.83 and P < 0.42. Conservative assumption of P = 0.03, P = 0.83 and P = 0.42 used to permit estimation of the t value. Standard deviation estimations assumed from the most conservative reliable value within the other surgery subgroup (time to first flatus: Park 2009 for the intervention group, Schweizer 2010 for the control group; time to first bowel movement: Webster 2007 for the intervention group, Chou 2006 for the control group; length of hospital stay: Schweizer 2010 for both intervention and control groups) |
| Lim 2013 | Time to first flatus and time to first bowel movement reported as mean and standard error of the mean. Study data from laparoscopic and open surgery subgroups combined to provide mean values for one intervention and one control group for length of hospital stay (unpublished data), standard deviation estimations assumed from the most conservative reliable value within the colorectal surgery subgroup (Bahena‐Aponte 2010 for both intervention and control groups) |
| Lu 2010a | Length of hospital stay reported as a mean. Standard deviation estimations assumed from the most conservative reliable value within the other surgery subgroup (Schweizer 2010 for both intervention and control groups) |
| Lu 2011 | Time to first flatus, length of hospital stay and time to first bowel sounds reported as a mean. P = 0.001 for time to first flatus, used to estimate the t value. P values presented as P < 0.001 for time to first bowel sounds, conservative assumption of P = 0.001 used to permit estimation of the t value. Standard deviation estimations assumed from the most conservative reliable value within the other surgery subgroup (time to first flatus: Park 2009 for the intervention group, Schweizer 2010 for the control group; length of hospital stay: Schweizer 2010 for both intervention and control groups; time to first bowel sounds: Marwah 2012 for both intervention and control groups) |
| Qiao 2011 | Time to first flatus and time to first bowel movement reported as a mean. Standard deviation estimations assumed from the most conservative reliable value within the other surgery subgroup (time to first flatus: Park 2009 for the intervention group, Schweizer 2010 for the control group; time to first bowel movement: Webster 2007 for the intervention group, Chou 2006 for the control group) |
| Ray 2008 | Time to first flatus and time to first bowel movement assumed to be reported as a mean. Length of hospital stay reported as median (assumed to be mean for analyses). Standard deviation estimations assumed from the most conservative reliable value within the other surgery subgroup (time to first flatus: Park 2009 for the intervention group, Schweizer 2010 for the control group; time to first bowel movement: Webster 2007 for the intervention group, Chou 2006 for the control group; length of hospital stay: Schweizer 2010 for both intervention and control groups). Number of participants per group not specifically stated; numbers used in analyses assumed from the text |
| Safdari‐Dehcheshmehi 2011 | Time to first defaecation and time to first bowel movement reported. Time to first defaecation results used in this review as reviewers anticipated that bowel movement was likely to occur after passage of flatus, and the results for time to first defaecation fitted this criterion whereas results for time to first bowel movement did not. Additionally there may have been a translation error in definition for 'time to first bowel movement' in the manuscript, as this study was translated from Farsi |
| Satij 2006 | Results reported as 'time to bowel function', defined as either passing flatus or a bowel movement; assumed to be time to flatus in this review |
| Schluender 2005 | Time to first flatus, time to first bowel movement and length of hospital stay reported as a mean. Study data from laparoscopic and open surgery subgroups combined to provide mean values for one intervention and one control group, standard deviation estimations assumed from the most conservative reliable value within the colorectal surgery subgroup (time to first flatus: Forrester 2014 for both intervention and control groups; time to first bowel movement: Forrester 2014 for the intervention group, Hirayama 2006 for the control group; length of hospital stay: Bahena‐Aponte 2010 for both intervention and control groups) |
| Watson 2008 | Time to first flatus, time to first bowel movement and length of hospital stay reported as median and interquartile range (unpublished information). Range estimated. Mean and standard deviation calculated using the formulae described by Hozo 2005 |
| Yi 2013 | Length of hospital stay reported as a mean. Standard deviation estimations assumed from the most conservative reliable value within the other surgery subgroup (Schweizer 2010 for both intervention and control groups) |
| Zhao 2008 | Time to first flatus reported as a mean. Standard deviation estimations assumed from the most conservative reliable value within the other surgery subgroup (Park 2009 for the intervention group, Schweizer 2010 for the control group) |
Assessment of heterogeneity
We assessed statistical heterogeneity across studies by visual inspection of the forest plot and using the Chi2 measurement. Heterogeneity is more difficult to detect when sample sizes and number of events are small, so we used a cut off of P < 0.01 for the Chi2 measurement to decide if there was statistical evidence of heterogeneity (Higgins 2011). As a measure of the variation in intervention effect due to statistical heterogeneity, we also assessed the I2 statistic; we considered values greater than 50% to be indicative of significant heterogeneity (Higgins 2011).
Assessment of reporting biases
We assessed reporting bias using funnel plots of included studies.
Data synthesis
We performed analyses in RevMan 5.3. Analyses comprised only within‐study comparisons rather than individual‐level data. Comparisons were based on an intention‐to‐treat analysis. We used a random‐effects model for the meta‐analysis of results, as there was a high level of heterogeneity among included studies. Three authors (VS, CP and RP) discussed results for each outcome measure within each study, to determine the inclusion of data in the meta‐analyses. Where data were not provided in the form of a mean and standard deviation, we derived these from the reported test statistics or estimated them from the reported data if suitable test statistics were not reported. We used the following methods to transform or estimate data:
· We estimated missing standard deviations using the most conservative reliable standard deviation from another study in the same surgical subgroup
· We considered medians as means if reported alone, and applied the most conservative reliable standard deviation from another study in the same surgical subgroup
· Where results were presented as median and range, we calculated mean and standard deviation using the formulae described by Hozo 2005
· Where complications were reported as % incidence, we converted this into the number of participants who experienced complications.
Co‐authors checked 100% of continuous outcome data entered into Revman for included studies. We assessed all of our outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) protocol and reported this in Table 1 and Table 2; we classed evidence as very low, low, moderate or high quality.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses to determine the sensitivity of overall conclusions to the surgical site. The key surgical disciplines reporting trials in this research area are colorectal surgery (CRS) and caesarean section (CS); we therefore created three subgroups: ‘CRS’, ‘CS’ and ‘other surgery’ (OS).
We used meta‐regression to assess whether the overall effect size was associated with the surgical site and whether this was a source of heterogeneity between studies using the 'metareg' package for the statistical software 'Stata 13' (StataCorp 2013). We also assigned each study a ROB score based on the combination of high and unclear risks for random sequence generation, allocation concealment, incomplete outcome data, selective outcome reporting or ‘other’ types of bias (a score of one was given for each unclear risk and a score of two for each high risk). Based on the spread of ROB scores, we categorised studies into subgroups by overall score: zero to three, four to five and six to ten. We used meta‐regression to assess the association between ROB score and overall effect size and whether this was a source of heterogeneity between studies.
Sensitivity analysis
We conducted sensitivity analyses based on the methodological and reporting qualities of the studies analysed. We considered the impact of methodological quality by excluding studies of lower quality, and we assessed how robust our overall results were to the use of estimates for missing data. We also explored the use of CG in an ERAS setting.
We therefore conducted the following sensitivity analyses:
We removed studies judged at ‘high risk’ of bias for at least two of the following components: random sequence generation, allocation concealment, incomplete outcome data, selective outcome reporting or ‘other’ types of bias
We removed studies which did not report on complications (deemed by co‐authors to be an indicator of low quality)
We excluded studies with any estimated results
We applied less conservative methods for dealing with missing data (e.g. instead of using the most conservative standard deviations, the mean standard deviation across all reliable values in the relevant subgroup was used)
We only included studies conducted within the context of an ERAS programme.
As we observed publication bias across studies reporting TBM and LOHS, we decided to also conduct post‐hoc meta‐analyses using a fixed‐effect model.
Results
Description of studies
See tables of Characteristics of included studies, Characteristics of excluded studies.
Results of the search
The electronic search identified 3993 hits. We identified 60 further records through hand‐searching: 54 from Google and Google Scholar and six through scanning reference lists of included studies and relevant SRs. After screening titles and abstracts, we excluded 1962 duplicates and 1967 irrelevant records. We sought full‐texts for the remaining 124 records; upon screening we excluded a further 24 records (see Characteristics of excluded studies). One hundred publications met the full inclusion criteria, of which 19 were subsequently found to be duplicate publications. We therefore identified 81 unique studies for inclusion comprising 9072 participants, as shown in Figure 1.
Included studies
We included 81 studies (see Characteristics of included studies). For 10 studies reported in multiple publications, we used the reference that provided the most comprehensive information (Abdollahi 2013; Asao 2002; Forrester 2014; Huang 2012a; Ledari 2012; Lim 2013; Matros 2006; McCormick 2005; Ren 2010; Schuster 2006).
Twelve studies were published as abstracts (Atkinson 2014; Garshasbi 2011; Lee 2004; Lu 2011; McCormick 2005; Ray 2008; Satij 2006; Schluender 2005; Schweizer 2010; Watson 2008; Webster 2007; Zamora 2012). We could obtain one publication only in part (Jin 2010). We sought extra information for 22 studies; unpublished data were provided for 11 (Atkinson 2014; Bonventre 2014; Ertas 2013; Jernigan 2014; Lim 2013; Matros 2006; McCormick 2005; Satij 2006; Schweizer 2010; Watson 2008; Zamora 2012) (see Characteristics of included studies).
Studies were conducted in 20 countries. Multiple trials were identified from the following countries: 35 in China (Cao 2008; Chen 2010; Chen 2011; Chen 2012; Fan 2009; Gong 2011; Guangqing 2011; Han 2011; Huang 2012a; Huang 2012b; Jin 2010; Li 2007a; Li 2012a; Li 2012b; Liang 2007; Lu 2010a; Lu 2010b; Lu 2011; Luo 2010; Qiao 2011; Qiu 2006; Ren 2010; Shang 2010; Sun 2005; Tan 2011; Tian 2013; Wang 2008; Wang 2009a; Wang 2011a; Wang 2011b; Yang 2011; Yi 2013; Zhang 2008; Zhao 2008; Zhong 2009), 12 in the USA (Crainic 2009; Forrester 2014; Jernigan 2014; Lee 2004; Matros 2006; McCormick 2005; Ray 2008; Satij 2006; Schluender 2005; Schuster 2006; Webster 2007; Zaghiyan 2013), eight in Iran (Abdollahi 2013; Akhlaghi 2008; Askarpour 2009; Garshasbi 2011; Ghafouri 2008; Ledari 2012; Pilehvarzadeh 2014; Safdari‐Dehcheshmehi 2011), four in Turkey (Çavuşoğlu 2009; Ertas 2013; Kafali 2010; Terzioglu 2013), three in Korea (Choi 2011; Choi 2014; Park 2009), three in the UK (Atkinson 2014; Quah 2006; Watson 2008), two in Japan (Asao 2002; Hirayama 2006) and two in Thailand (Chuamor 2014; Jakkaew 2013).
We identified only four paediatric studies (Çavuşoğlu 2009; Yang 2011; Zhang 2008; Zhao 2008). Studies applied various exclusion criteria, commonly postoperative complications, previous abdominal/bowel surgery, inability to chew gum and co‐morbidities (including chronic constipation, diabetes, pre‐eclampsia/eclampsia, hypothyroidism and pancreatitis).
One study used sugared gum for the intervention (Zaghiyan 2013); all other studies did not specify or used sugar‐free/sugar‐less gum. Ten studies included placebo or alternative treatment groups alongside a control group. Placebo interventions were sucking hard candy (Crainic 2009) and wearing a silicone‐adhesive patch (Forrester 2014) or an acupressure wrist bracelet (Matros 2006). Alternative treatments were early ambulation and sphincter exercises (Huang 2012a), stomach massage (Lu 2010a), chewing green tea leaves (Zhong 2009), early oral feeding (Safdari‐Dehcheshmehi 2011), laxatives or early feeding (Askarpour 2009), combinations of early oral hydration and early mobilisation (Terzioglu 2013) or combinations of olive oil and water (Bonventre 2014).
Controls received either standard care or a similar care regimen to the intervention group in 52 studies. Four studies were conducted in the context of an ERAS programme (Atkinson 2014; Lim 2013; Watson 2008; Zaghiyan 2013). Fourteen either did not specify or stated that the control group did not chew gum or receive GI stimulants or special treatment (Abdollahi 2013; Cabrera 2012; Choi 2014; Chou 2006; Crainic 2009; Garshasbi 2011; Lee 2004; Liang 2007; Lu 2011; Park 2009; Qiu 2006; Satij 2006; Schluender 2005; Zhang 2008). The control group underwent mobilisation protocols in four studies (Chen 2011; Huang 2012b; Wang 2008; Yi 2013). The control group had sips of clear liquid in one study (McCormick 2005), two studies created their own control group protocol (Akhlaghi 2008; Terzioglu 2013), and controls were nil‐by‐mouth in four studies (Abd‐El‐Maeboud 2009; Askarpour 2009; Marwah 2012; Shang 2010).
Eight studies reported results in subgroups by surgical site (Abdollahi 2013; Bonventre 2014; Schweizer 2010) or surgical approach: open and robot‐assisted (Choi 2011) or open and laparoscopic (Crainic 2009; Lim 2013; McCormick 2005; Schluender 2005). Zaghiyan 2013 conducted age and operative time subgroup analyses following identification of baseline differences.
Of our outcomes, TFF was most commonly reported, followed by TBM, LOHS, tolerability of gum, TBS, complications and cost. Other than these, the most frequently reported outcome was time to first food consumption. Additional reported outcomes included blood catecholamines (Zhang 2008; Zhao 2008), blood motilin (Guangqing 2011; Wang 2011b), blood/serum gastrin (Chen 2010; Zhang 2008; Zhao 2008), blenching (Chuamor 2014), analgesic use (Ertas 2013; Husslein 2013; Kafali 2010), antiemetic use (Ertas 2013; Kafali 2010), time to tolerance or first oral fluids (Crainic 2009; Watson 2008), tolerance of first meal (Jakkaew 2013), time to first hunger (Fan 2009; Forrester 2014; Jakkaew 2013; Ledari 2012; Marwah 2012; McCormick 2005; Schuster 2006), discomfort (Huang 2012a), pain (Lim 2013; Lu 2011), time until ready for discharge (Matros 2006) and time to feeling first intestinal movement (Rashad 2013).
Excluded studies
Upon reading the full texts where possible, we excluded 24 records (see Characteristics of excluded studies). Thirteen were not RCTs (Anon 2006b; Anon 2006c; Anon 2008; Chathongyot 2010; Darvall 2011; Harma 2009; Hwang 2013; Keenahan 2014; Kim 2010; Nimarta 2013; Slim 2014; Takagi 2012; Utli 2013), we could not source eight (Alcántara 2010; Alper 2006; Anon 2006a; Duluklu 2012; Li 2007b; Starly 2009; Wang 2003; Wang 2009b), two described a non‐postoperative intervention (Apostolopoulos 2008; Svarta 2012) and one was incomplete (reported in the Ongoing studies section) (van Leersum 2012).
We identified a further 15 ongoing trials that could not be included in this review (see Ongoing studies). Seven were complete but not yet published (Abd‐El‐Maeboud 2010; Andersson 2011; Clark 2008; Fakari 2011; Lopez 2012; Lv 2011; Sabo 2012).
Risk of bias in included studies
ROB for each study is described in detail in the Characteristics of included studies section. Details of ROB judgements for each study are presented in Figure 2, with an overall summary graph in Figure 3. The largest ROB was reporting bias due to Selective reporting (reporting bias). The smallest ROB was attrition bias due to Incomplete outcome data (attrition bias). Allocation concealment methods were most poorly reported, resulting in the greatest number of 'unclear' ROB assessments [see Allocation (selection bias)].
2.
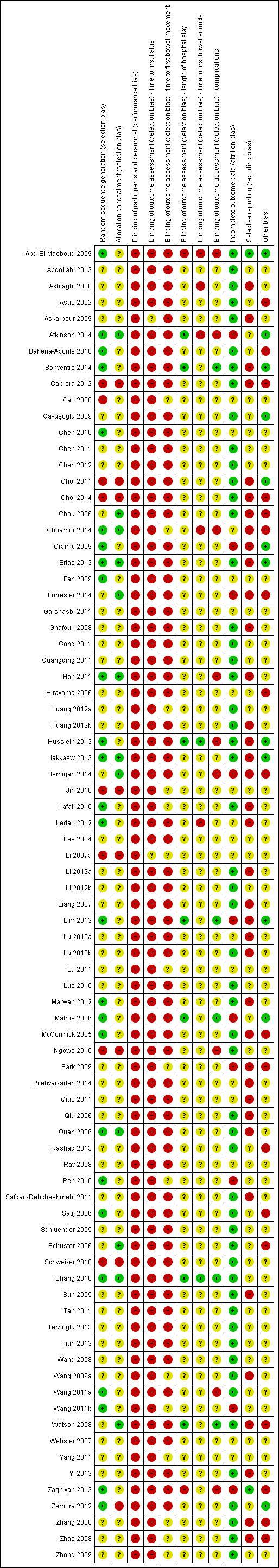
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
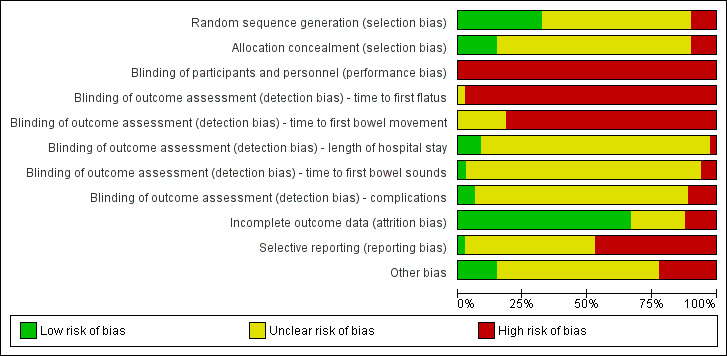
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We categorised 26 studies at low ROB due to acceptable randomisation sequence generation through use of computer‐generated randomisation, a random number table, a draw or an online program (Abd‐El‐Maeboud 2009; Atkinson 2014; Bahena‐Aponte 2010; Bonventre 2014; Chen 2010; Chuamor 2014; Crainic 2009; Ertas 2013; Fan 2009; Han 2011; Husslein 2013; Jakkaew 2013; Kafali 2010; Ledari 2012; Lim 2013; Marwah 2012; Matros 2006; McCormick 2005; Quah 2006; Ren 2010; Satij 2006; Shang 2010; Wang 2011a; Wang 2011b; Zaghiyan 2013; Zamora 2012).
We classed eight studies at high ROB through inadequate random sequence generation. Methods used included randomisation by order of hospital admission (Cabrera 2012; Cao 2008), hospital bed number (Li 2007a), operating time (Jin 2010), alternate randomisation (Choi 2011; Ngowe 2010), allocation by an investigator (Choi 2014) or allocation by participant preference (Schweizer 2010). We categorised all other studies at unclear ROB.
Allocation concealment
We considered 12 studies to be at low ROB due to adequate allocation concealment methods. Methods included sequentially numbered, opaque, sealed envelopes, a sequential card‐pull design, an Access database or central telephone assignment (Atkinson 2014; Chou 2006; Chuamor 2014; Ertas 2013; Forrester 2014; Han 2011; Jakkaew 2013; Jernigan 2014; Quah 2006; Schuster 2006; Shang 2010; Watson 2008). We classed eight studies at high ROB due to inadequate methods for allocation concealment (Cabrera 2012; Choi 2011; Choi 2014; Jin 2010; Li 2007a; Ngowe 2010; Schweizer 2010; Zamora 2012). We classed all remaining studies at unclear ROB.
Blinding
Participants
Participants cannot be adequately blinded with this intervention, therefore we judged all studies to be at high ROB.
Personnel
Personnel were not blinded in four studies (Abd‐El‐Maeboud 2009; Ertas 2013; Jernigan 2014; Zaghiyan 2013). Eight studies described methods used to blind some personnel (Atkinson 2014; Bonventre 2014; Choi 2011; Choi 2014; Lim 2013; Matros 2006; Shang 2010; Watson 2008) and three studies reported personnel blinding but did not describe methods used (Çavuşoğlu 2009; Han 2011; Schluender 2005). No other studies discussed personnel blinding.
Outcome assessment
We considered TFF and TBM as participant‐reported outcomes, therefore we judged all studies reporting these outcomes at high ROB. One study described TFF and TBM with a stoma, which could have been reported by staff (Quah 2006). However, as 45% of participants in this study did not have a stoma placed, we also categorised this study at high ROB.
We assumed that staff reported LOHS (as it is likely to have been taken from medical notes or administration records). We judged two studies at high ROB: authors stated that blinding of outcome assessment was not possible (Abd‐El‐Maeboud 2009; Zaghiyan 2013). We classed seven studies at low ROB, where participants or ward staff were taught not to reveal group allocation to outcome assessors (Atkinson 2014; Bonventre 2014; Husslein 2013; Lim 2013; Matros 2006; Shang 2010; Watson 2008), participants hid gum (Husslein 2013; Matros 2006; Shang 2010), containers for gum disposal were provided (Lim 2013), concealed charts identifying intervention participants (for nurses) were kept in patient records (Lim 2013), or clinical rounds and CG periods were separated (Bonventre 2014; Husslein 2013; Matros 2006). We classed all other studies at unclear ROB, as methods for blinding of outcome assessment were not discussed.
We assumed that staff reported TBS (unless otherwise stated). We classed five studies as at high ROB where authors reported that blinding of staff was not possible (Abd‐El‐Maeboud 2009; Atkinson 2014), TBS was participant‐reported (Akhlaghi 2008; Ledari 2012) or investigators providing the gum assessed TBS (Chuamor 2014). We classed two studies at low ROB (same methods used for LOHS assessment) (Husslein 2013; Shang 2010). We classed all other studies at unclear ROB.
Complications were reported by participants or staff. We classed nine studies at high ROB where complications were participant‐reported or staff were not blinded or inadequately blinded (Abd‐El‐Maeboud 2009; Atkinson 2014; Chuamor 2014; Han 2011; Husslein 2013; Jernigan 2014; Ngowe 2010; Wang 2011a; Zaghiyan 2013). We categorised five studies at low ROB (same methods used for LOHS assessment) (Bonventre 2014; Lim 2013; Matros 2006; Shang 2010; Watson 2008). We classed all other studies at unclear ROB.
ROB through blinding of assessment of tolerability of gum was not reported as this was not possible nor relevant to both groups. Additionally, ROB for assessment of cost was not reported as we considered blinding to have had little effect.
Incomplete outcome data
We judged ROB as high in 10 studies. One had a greater than 10% difference in missing data between groups (Zaghiyan 2013). One stated use of intention‐to‐treat analyses, but only 157 of 168 participants were included in analyses (Lim 2013). Eight reported more than 10% missing data for an outcome of interest (Atkinson 2014; Crainic 2009; Forrester 2014; Jernigan 2014; Matros 2006; Park 2009; Ren 2010; Wang 2011b).
Sixteen studies did not state the number of participants included in analyses (Cao 2008; Chen 2010; Chuamor 2014; Fan 2009; Garshasbi 2011; Hirayama 2006; Jin 2010; Lee 2004; Li 2007a; Lu 2010a; Lu 2011; Pilehvarzadeh 2014; Qiao 2011; Ray 2008; Webster 2007; Yang 2011) and one study reported a 9% attrition rate of randomised participants, but did not state to which group(s) they had been allocated (Ledari 2012). We considered these to be at unclear ROB. We classed all remaining studies at low ROB.
Selective reporting
We judged two studies to be at low ROB, where all outcomes pre‐specified in the available protocol were reported in the publication (Abd‐El‐Maeboud 2009; Zaghiyan 2013).
We classed 38 studies at high ROB. Six studies deviated in outcome reporting from pre‐specifications in the protocol (Bonventre 2014; Ertas 2013; Husslein 2013; Jernigan 2014; Lim 2013; Safdari‐Dehcheshmehi 2011). Five studies did not pre‐specify any outcomes in the publication (Askarpour 2009; Li 2012a; Lu 2010b; Sun 2005; Wang 2009a). In 27 studies data pre‐specified as an outcome measure or collected as part of the research methodology were not presented fully, or outcome reporting deviated from pre‐specifications in the publication (Akhlaghi 2008; Cabrera 2012; Choi 2011; Choi 2014; Chou 2006; Chuamor 2014; Crainic 2009; Forrester 2014; Ghafouri 2008; Han 2011; Huang 2012b; Jakkaew 2013; Kafali 2010; Ledari 2012; Liang 2007; Lu 2010a; Marwah 2012; McCormick 2005; Park 2009; Pilehvarzadeh 2014; Qiao 2011; Qiu 2006; Quah 2006; Watson 2008; Yi 2013; Zhang 2008; Zhao 2008).
We classed all other studies at unclear ROB. We categorised studies that were reported only as abstracts as unclear so as not to penalise for exclusion of information within the confines of an abstract.
Visual inspection of the funnel plots for each continuous outcome indicated that reporting bias may be present for TBM and LOHS.
Other potential sources of bias
We detected three additional potential biases:
Baseline differences between groups. Onset and duration of ileus are considered to be multifactorial, hence some baseline differences between groups could introduce bias. We classed six studies at high ROB due to significant baseline differences in age (Park 2009), operative time (Rashad 2013), age and operative time (Zaghiyan 2013), operative blood loss (Chuamor 2014), BMI, ethnicity and use of epidural (Jernigan 2014) and BMI, stoma creation and pain relief (Watson 2008). Zaghiyan 2013 conducted further subgroup analyses to explore the implications of the identified baseline differences in age and operative time.
We considered sample sizes that were more than 10% below the sample size calculations, or which were likely to be too small to adequately test the research question, to be at high ROB. We considered 20 participants per arm as an arbitrary value for acceptable sample sizes; we classed 11 studies at high ROB with sample sizes less than 20 per arm (Asao 2002; Bahena‐Aponte 2010; Cabrera 2012; Choi 2014; Chou 2006; Hirayama 2006; Park 2009; Satij 2006; Schuster 2006; Zhang 2008; Zhao 2008). We classed 12 studies at low ROB where sample sizes were within 10% of the calculated sample size requirement (Abd‐El‐Maeboud 2009; Atkinson 2014; Bonventre 2014; Çavuşoğlu 2009; Choi 2011; Crainic 2009; Ertas 2013; Husslein 2013; Jakkaew 2013; Lim 2013; Matros 2006; Zamora 2012); three futher studies met the calculated sample size requirement (within 10%) but were still judged at high risk due to baseline differences between groups (Chuamor 2014; Watson 2008; Zaghiyan 2013). We classed two studies at high ROB where sample size requirements more than 10% below the sample size calculations (Forrester 2014; Jernigan 2014).
Non‐specified differences in randomisation to treatment groups. We decided that a greater than 10% difference in randomisation to each arm, which was not pre‐specified, constituted a ROB. Two studies were classed at high ROB due to a 17% and 34% difference in randomisation between groups (Hirayama 2006; McCormick 2005).
We judged all other studies at unclear ROB for these additional potential biases.
Effects of interventions
Evidence for effects of interventions are summarised in the Table 1 and Table 2.
Time to first flatus
A reduction in TFF with postoperative CG was observed across subgroups. The overall combined analysis of 8239 participants from 77 studies showed a reduction of 10.4 hours (95% CI ‐11.9, ‐8.9) (see Analysis 1.1, Figure 4). In the CRS subgroup, analysis of 1668 participants from 22 studies showed a reduction of 12.5 hours (95% CI ‐17.2, ‐7.8). In the CS subgroup, analysis of 2401 participants from 14 studies showed a reduction of 7.9 hours (95% CI –10.0, ‐5.8). In the OS subgroup, analysis of 4224 participants from 43 studies showed a reduction of 10.6 hours (95% CI ‐12.7, ‐8.5). There was evidence of statistical heterogeneity between studies in all analyses (overall: I2 = 96%, P < 0.001, CRS: I2 = 89%, P < 0.001, CS: I2 = 93%, P < 0.001, OS: I2 = 97%, P < 0.001). Visual inspection of the funnel plot did not indicate the presence of publication bias (see Figure 5). Post‐hoc meta‐analyses using a fixed‐effect model showed a reduced effect estimate, but no difference in direction of effect [overall reduction of 9.1 hours (95% CI ‐9.3, ‐8.8), CRS: reduction of 12.5 hours (95% CI ‐13.9, ‐11.2), CS: overall reduction of 7.2 hours (95% CI ‐7.7, ‐6.7), OS: overall reduction of 9.5 hours (95% CI ‐9.8, ‐9.2)] (see Appendix 8).
1.1. Analysis.
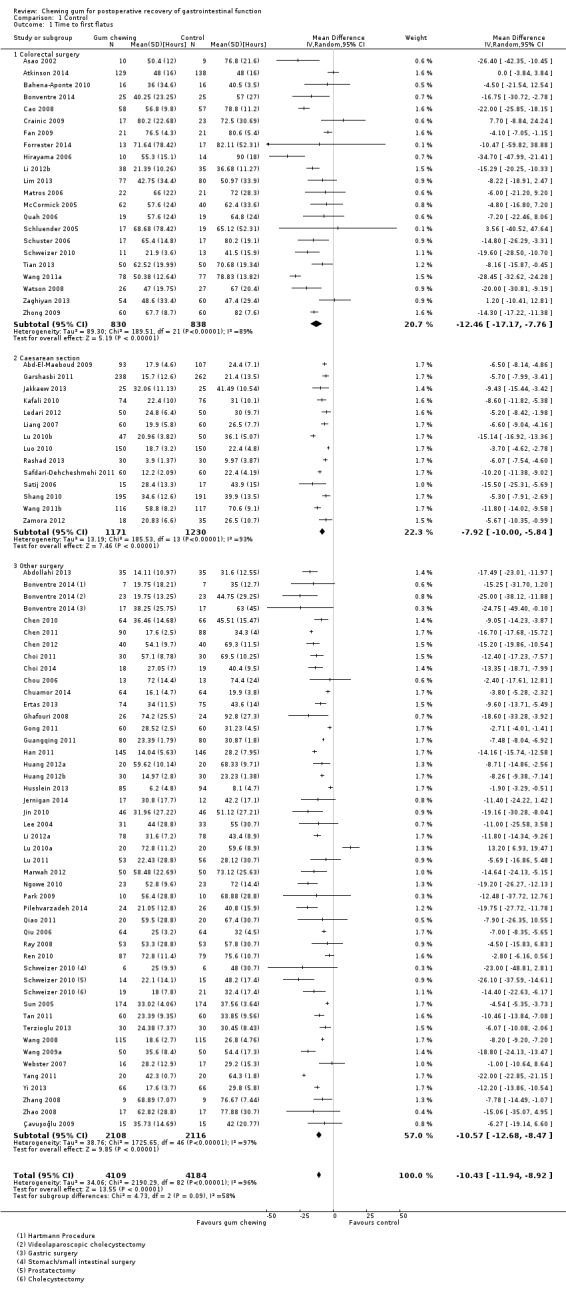
Comparison 1 Control, Outcome 1 Time to first flatus.
4.
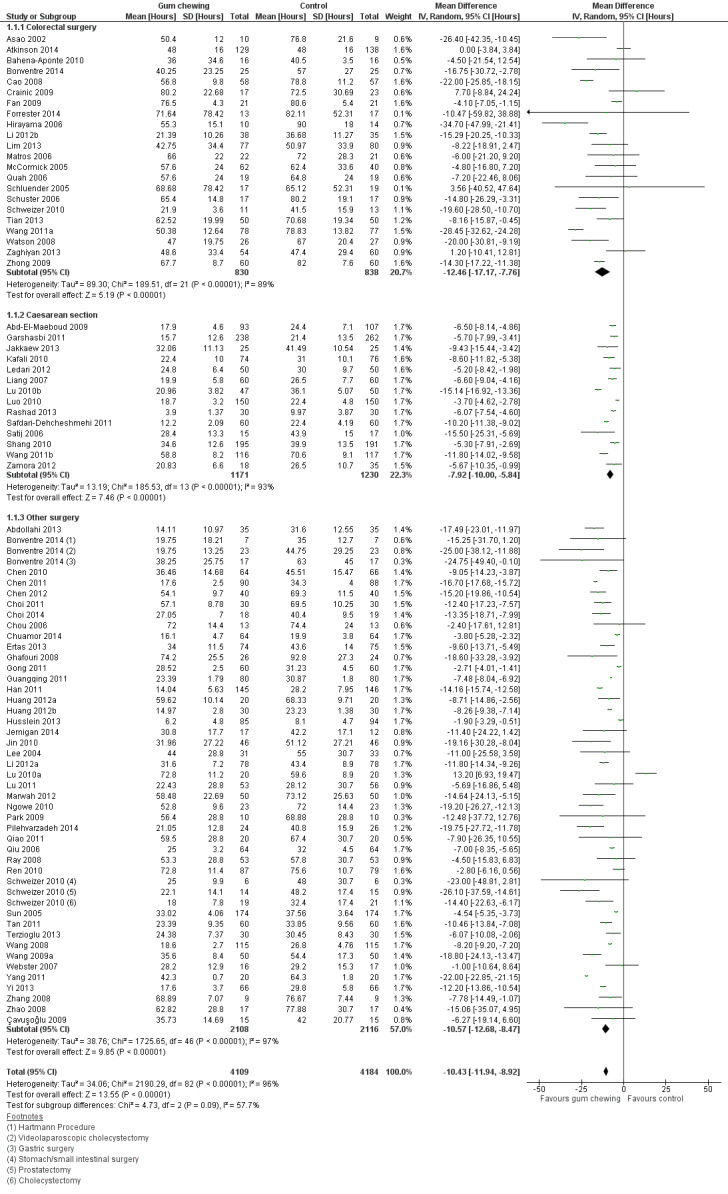
Forest plot of comparison: 1 Control, outcome: 1.1 Time to first flatus [Hours].
5.
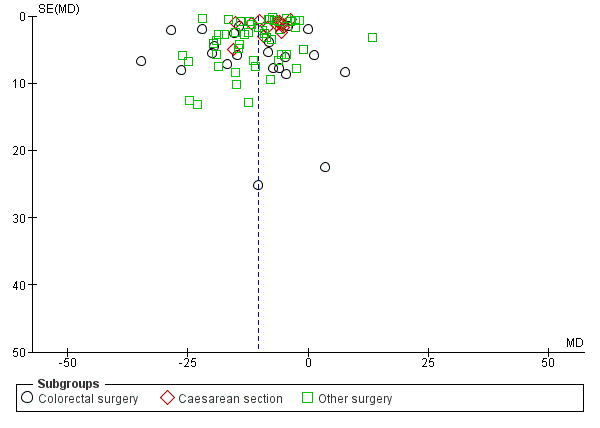
Funnel plot of comparison: 1 Control, outcome: 1.1 Time to first flatus [Hours].
Time to first bowel movement
A reduction in TBM with postoperative CG was observed across subgroups. The overall combined analysis of 7282 participants from 62 studies showed a reduction of 12.7 hours (95% CI ‐14.5, ‐10.9) (see Analysis 1.2, Figure 6). In the CRS subgroup, analysis of 1470 participants from 20 studies showed a reduction of 18.1 hours (95% CI ‐25.3, ‐10.9). In the CS subgroup, analysis of 2336 participants from 11 studies showed a reduction of 9.1 hours (95% CI ‐11.4, ‐6.7). In the OS subgroup, analysis of 3477 participants from 33 studies showed a reduction of 12.3 hours (95% CI ‐14.9, ‐9.7). There was evidence of statistical heterogeneity between studies in all analyses (overall: I2 = 96%, P < 0.001, CRS: I2 = 91%, P < 0.001, CS: I2 = 93%, P < 0.001, OS: I2 = 97%, P < 0.001). Visual inspection of the funnel plot indicated that publication bias may be present (see Figure 7). Post‐hoc meta‐analyses using a fixed‐effect model showed a reduced effect estimate, but no difference in direction of effect [overall reduction of 9.2 hours (95% CI ‐9.4, ‐8.9), CRS: reduction of 17.6 (95% CI ‐19.4, ‐15.9), CS: overall reduction of 8.4 hours (95% CI ‐9.0, ‐7.9), OS: overall reduction of 9.2 hours (95% CI ‐9.4, ‐8.9)] (see Appendix 8).
1.2. Analysis.
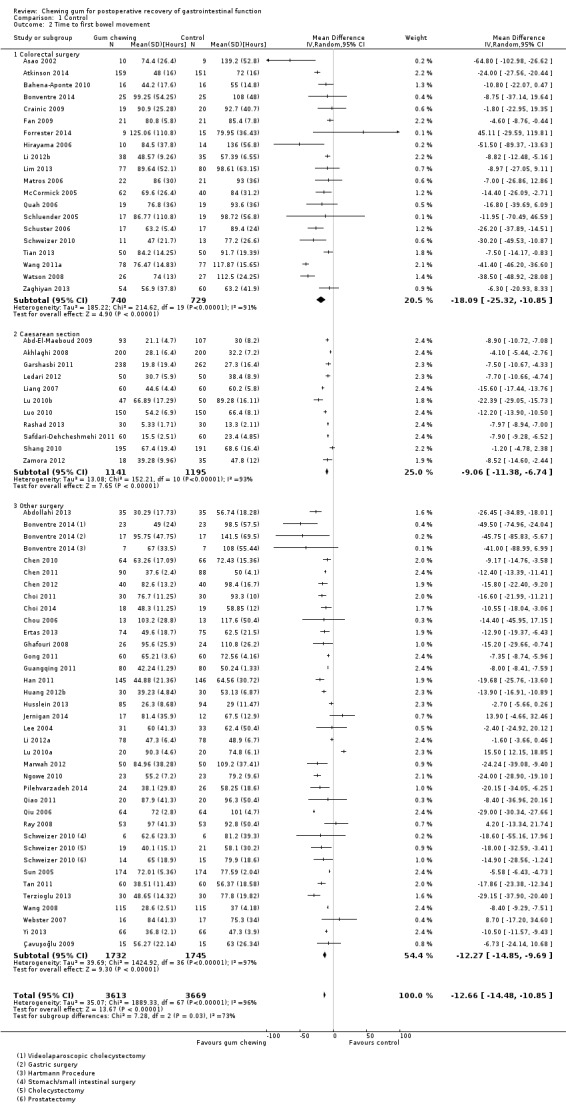
Comparison 1 Control, Outcome 2 Time to first bowel movement.
6.
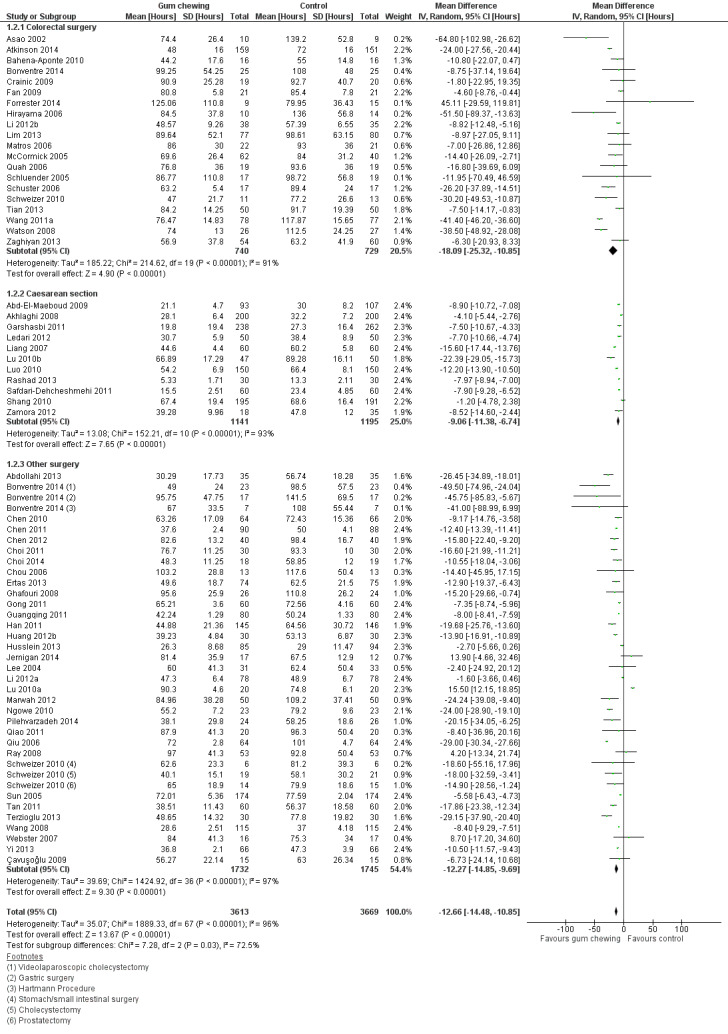
Forest plot of comparison: 1 Control, outcome: 1.2 Time to first bowel movement [Hours].
7.
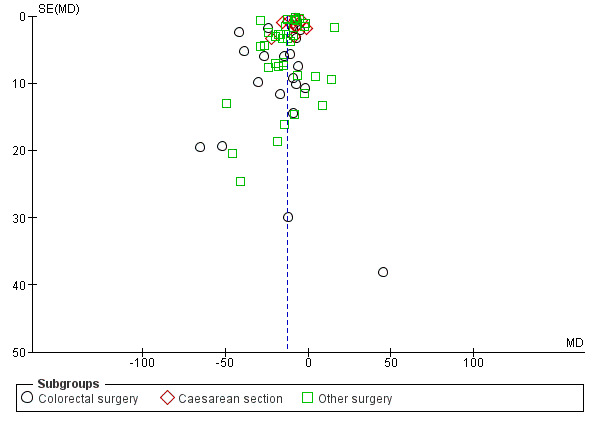
Funnel plot of comparison: 1 Control, outcome: 1.2 Time to first bowel movement [Hours].
Length of hospital stay
A reduction in LOHS with postoperative CG was observed across subgroups. The overall combined analysis of 5278 participants from 50 studies showed a reduction of 0.7 days (95% CI ‐0.8, ‐0.5) (see Analysis 1.3, Figure 8). In the CRS subgroup, analysis of 1523 participants from 18 studies showed a reduction of 1.0 days (95% CI ‐1.6, ‐0.4). In the CS subgroup, analysis of 1239 participants from 6 studies showed a reduction of 0.2 days (95% CI ‐0.3, ‐0.1). In the OS subgroup, analysis of 2516 participants from 28 studies showed a reduction of 0.8 days (95% CI ‐1.1, ‐0.5). There was evidence of statistical heterogeneity between studies in all analyses (overall: I2 = 86%, P < 0.001, CRS: I2 = 70%, P < 0.001, CS: I2 = 86%, P < 0.001, OS: I2 = 81%, P < 0.001). Visual inspection of the funnel plot indicated that publication bias may be present (see Figure 9). Post‐hoc meta‐analyses using a fixed‐effect model showed a reduced effect estimate, but no difference in direction of effect [overall reduction of 0.2 days (95% CI ‐0.3, ‐0.2), CRS: reduction of 0.9 days (95% CI ‐1.2, ‐0.6), CS: overall reduction of 0.2 days (95% CI ‐0.2, ‐0.1), OS: overall reduction of 0.7 (95% CI ‐0.8, ‐0.6)] (see Appendix 8).
1.3. Analysis.
Comparison 1 Control, Outcome 3 Length of hospital stay.
8.
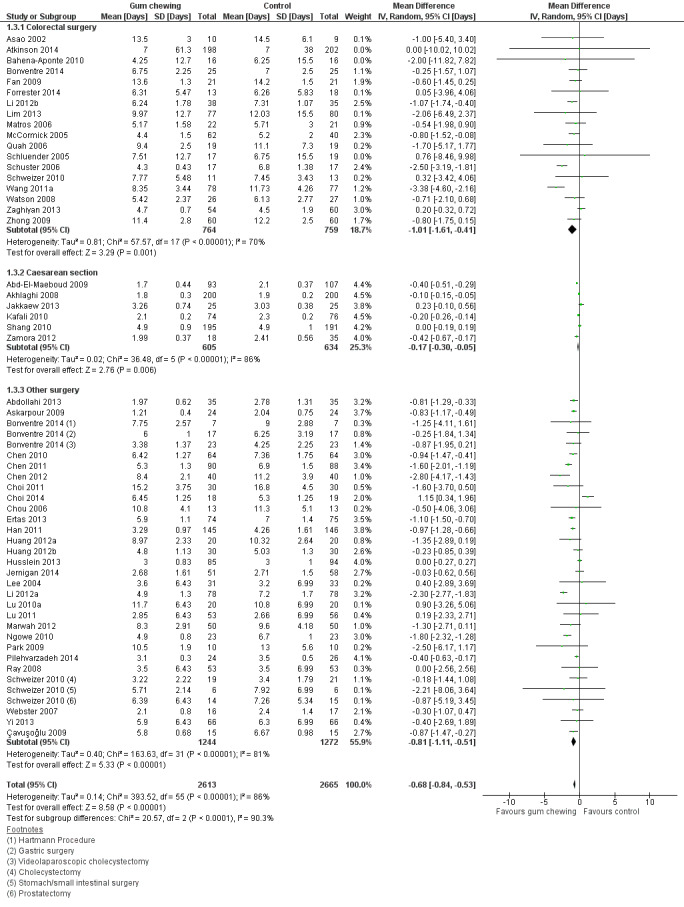
Forest plot of comparison: 1 Control, outcome: 1.3 Length of hospital stay [Days].
9.
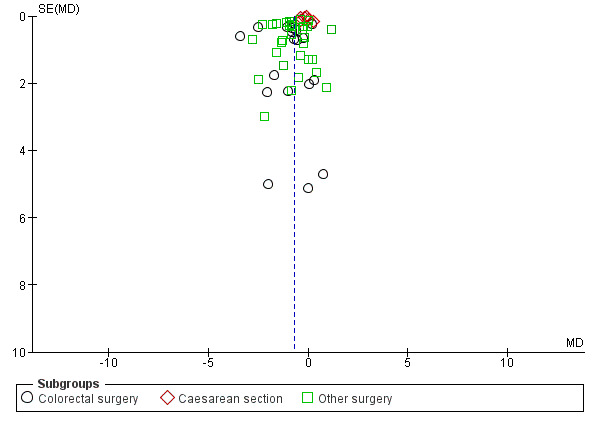
Funnel plot of comparison: 1 Control, outcome: 1.3 Length of hospital stay [Days].
Time to first bowel sounds
A reduction in TBS with postoperative CG was observed across subgroups. The overall combined analysis of 3981 participants from 23 studies showed a reduction of 5.0 hours (95% CI ‐6.4, ‐3.7) (see Analysis 1.4, Figure 10). In the CRS subgroup, analysis of 291 participants from 2 studies showed a reduction of 3.2 hours (95% CI ‐7.0, 0.6). In the CS subgroup, analysis of 2449 participants from 10 studies showed a reduction of 4.4 hours (95% CI ‐5.9, ‐2.8). In the OS subgroup, analysis of 1241 participants from 11 studies showed a reduction of 6.3 hours (95% CI ‐8.7, ‐3.8). There was evidence of statistical heterogeneity between studies in all analyses other than CRS (as only two studies were included) (overall: I2 = 97%, P < 0.001, CRS: I2 = 9%, P = 0.29, CS: I2 = 95%, P < 0.001, OS: I2 = 98%, P < 0.001). Visual inspection of the funnel plot did not indicate the presence of publication bias (see Figure 11). Post‐hoc meta‐analyses using a fixed‐effect model showed a reduced effect estimate, but no difference in direction of effect [overall reduction of 4.3 hours (95% CI ‐4.5, ‐4.1), CRS: reduction of 3.3 hours (95% CI ‐6.9, 0.2), CS: overall reduction of 5.0 hours (95% CI ‐5.3, ‐4.7), OS: overall reduction of 3.4 hours (95% CI ‐3.7, ‐3.1)] (see Appendix 8).
1.4. Analysis.
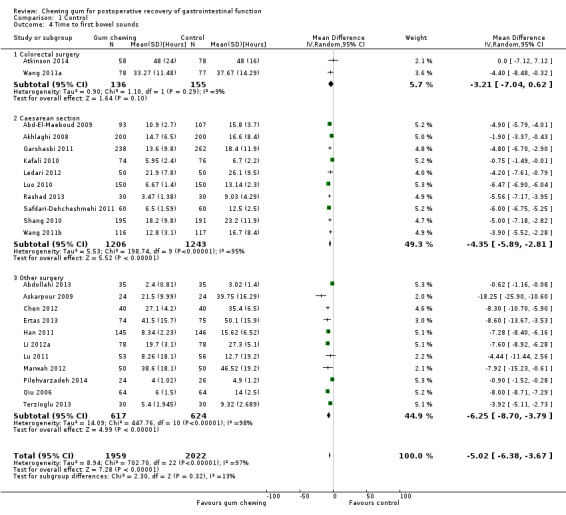
Comparison 1 Control, Outcome 4 Time to first bowel sounds.
10.
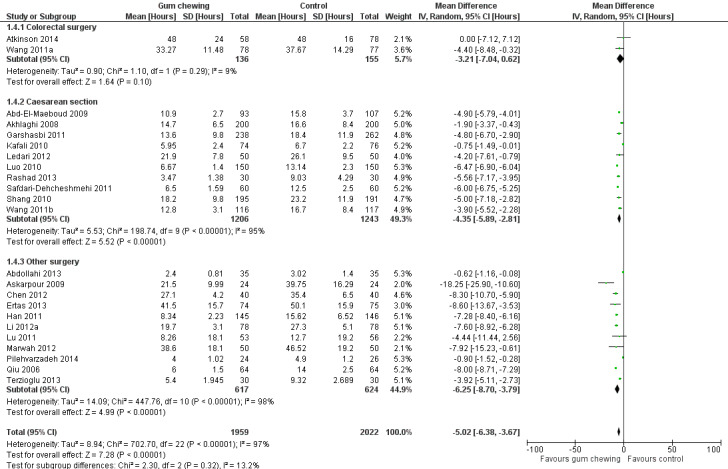
Forest plot of comparison: 1 Control, outcome: 1.4 Time to first bowel sounds [Hours].
11.
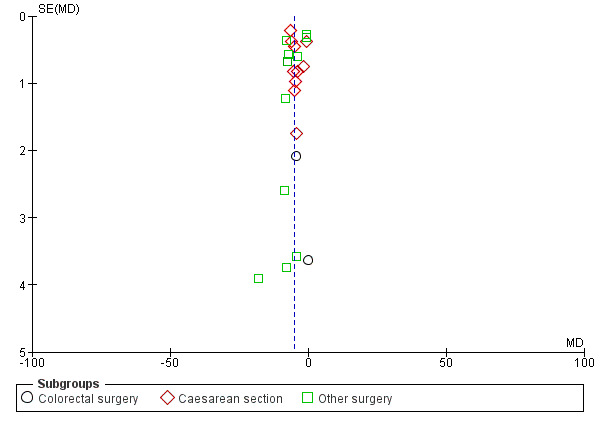
Funnel plot of comparison: 1 Control, outcome: 1.4 Time to first bowel sounds [Hours].
Complications
We reported nausea and vomiting, mortality, infection, readmissions, other complications, and complications related to the intervention.
Fifteen studies reported nausea and vomiting (six CRS, four CS, five OS) (see Analysis 1.5). Similar prevalence of nausea and vomiting were observed between groups in five CRS and three CS studies (Abd‐El‐Maeboud 2009; Atkinson 2014; Hirayama 2006; Jakkaew 2013; Lim 2013; Zaghiyan 2013; Zamora 2012; Zhong 2009). Nausea and vomiting reports were lower in the intervention group in one CRS, one CS and all five OS studies (Askarpour 2009; Han 2011; Jernigan 2014; Kafali 2010; Li 2012a; Marwah 2012; Wang 2011a).
1.5. Analysis.
Comparison 1 Control, Outcome 5 Complications ‐ Nausea and Vomiting [Frequency].
| Complications ‐ Nausea and Vomiting [Frequency] | ||
|---|---|---|
| Study | Intervention group | Control group |
| Colorectal surgery | ||
| Atkinson 2014 | 36 (vomiting on postoperative day 2, recorded for only 196 of 198 participants in the intervention group) | 34 (vomiting on postoperative day 2, recorded for all 202 participants in the control group) |
| Hirayama 2006 | 0 | 2 |
| Lim 2013 | 100 | 97 |
| Wang 2011a | 24 | 36 |
| Zaghiyan 2013 | 3 | 6 |
| Zhong 2009 | 22 | 21 |
| Caesarean section | ||
| Abd‐El‐Maeboud 2009 | 1 | 3 |
| Jakkaew 2013 | 2 | 3 |
| Kafali 2010 | 2 | 10 |
| Zamora 2012 | 0 ‐ no recorded postoperative ileus symptoms (such as nausea, vomiting, abdominal distension and diarrhoea) | 0 ‐ no recorded postoperative ileus symptoms (such as nausea, vomiting, abdominal distension and diarrhoea) |
| Other surgery | ||
| Askarpour 2009 | 0 | 4 |
| Han 2011 | 5 | 17 |
| Jernigan 2014 | 22 | 39 |
| Li 2012a | 20 | 33 |
| Marwah 2012 | 14 | 25 |
Seven studies reported mortality (five CRS, two OS) (details presented in Analysis 1.6). Four CRS and both OS studies reported either no or one death, with no differences between groups (Bahena‐Aponte 2010; Çavuşoğlu 2009; Lim 2013; Marwah 2012; Quah 2006; Watson 2008). One CRS study reported 11 deaths in the intervention group and none in the control group (Atkinson 2014); authors have however confirmed that mortality was not judged to be related to the intervention in these cases.
1.6. Analysis.
Comparison 1 Control, Outcome 6 Complications ‐ Mortality [Frequency].
| Complications ‐ Mortality [Frequency] | ||
|---|---|---|
| Study | Intervention group | Control group |
| Colorectal surgery | ||
| Atkinson 2014 | 11 ‐ 9 prior to 12‐week follow‐up, 2 after 12‐week follow‐up | 0 |
| Bahena‐Aponte 2010 | 0 | 0 |
| Lim 2013 | 0 | 1 ‐ 30 day mortality |
| Quah 2006 | 1 | 0 |
| Watson 2008 | 0 | 1 |
| Other surgery | ||
| Marwah 2012 | 0 | 0 |
| Çavuşoğlu 2009 | 0 | 0 |
Thirteen studies reported on infections (six CRS, one CS, six OS) (details presented in Analysis 1.7). No studies found any clinically important differences between groups in reports of infections (Abd‐El‐Maeboud 2009; Asao 2002; Çavuşoğlu 2009; Chou 2006; Hirayama 2006; Marwah 2012; Matros 2006; Ngowe 2010; Park 2009; Quah 2006; Watson 2008; Zaghiyan 2013; Zhang 2008).
1.7. Analysis.
Comparison 1 Control, Outcome 7 Complications ‐ Infection [Frequency].
| Complications ‐ Infection [Frequency] | ||
|---|---|---|
| Study | Intervention group | Control group |
| Colorectal surgery | ||
| Asao 2002 | 0 | 0 |
| Hirayama 2006 | 2 ‐ wound infection | 4 ‐ wound infection |
| Matros 2006 | 0 ‐ in hospital, 2 ‐ within 30 days wound infection and intra‐abdominal abscess | 2 ‐ in hospital wound infection and pneumonia, 4 ‐ within 30 days wound infection, pneumonia and intra‐abdominal abscess |
| Quah 2006 | 2 ‐ wound infection | 2 ‐ chest infection and urinary tract infection |
| Watson 2008 | 1 ‐ wound infection | 4 ‐ wound infection and MRSA |
| Zaghiyan 2013 | 1 ‐ wound infection/pelvic abscess | 1 ‐ abdominal abscess |
| Caesarean section | ||
| Abd‐El‐Maeboud 2009 | 7 ‐ febrile morbidity | 10 ‐ febrile morbidity |
| Other surgery | ||
| Chou 2006 | 1 ‐ pneumonia | 0 |
| Marwah 2012 | 3 ‐ purulent wound discharge | 3 ‐ purulent wound discharge and pneumonitis |
| Ngowe 2010 | 3 ‐ parietal sepsis | 2 ‐ parietal sepsis |
| Park 2009 | 0 ‐ no postoperative complications such as postoperative infection or haemorrhage | 0 ‐ no postoperative complications such as postoperative infection or haemorrhage |
| Zhang 2008 | 0 | 0 |
| Çavuşoğlu 2009 | 0 | 2 ‐ intra‐abdominal abscess and superficial surgical site infection |
Twelve studies reported readmissions (seven CRS, five OS) (details presented in Analysis 1.8). One CRS and four OS studies reported no readmissions in either study arm (Choi 2014; Ertas 2013; Husslein 2013; Schuster 2006; Zhang 2008). Six CRS and one OS study reported no difference in readmissions between groups (Asao 2002; Jernigan 2014; Lim 2013; Matros 2006; Quah 2006; Watson 2008; Zaghiyan 2013).
1.8. Analysis.
Comparison 1 Control, Outcome 8 Complications ‐ Readmissions [Frequency].
| Complications ‐ Readmissions [Frequency] | ||
|---|---|---|
| Study | Intervention group | Control group |
| Colorectal surgery | ||
| Asao 2002 | 0 | 1 ‐ due to ileus, 2 days post‐discharge |
| Lim 2013 | 6 | 6 |
| Matros 2006 | 1 ‐ due to ileus, within 30 days | 2 ‐ due to ileus, within 30 days |
| Quah 2006 | 0 | 1 ‐ within 30 days |
| Schuster 2006 | 0 | 0 |
| Watson 2008 | 1 ‐ due to abdominal abscess, within 30 days | 0 |
| Zaghiyan 2013 | 0 | 2 ‐ due to ileus, within 30 days |
| Other surgery | ||
| Choi 2014 | 0 | 0 |
| Ertas 2013 | 0 | 0 |
| Husslein 2013 | 0 | 0 |
| Jernigan 2014 | 2 ‐ within 30 days | 3 ‐ within 30 days |
| Zhang 2008 | 0 | 0 |
Fifty‐four studies reported on other types of complications (including halitosis, dry mouth, bloating, oral ulcers, intestinal obstruction and anastomotic leak) (see Analysis 1.9). Eight studies reported none in either group (Abdollahi 2013; Asao 2002; Bonventre 2014; Li 2012b; Ngowe 2010; Park 2009; Zamora 2012; Zhang 2008). Three reported none in the intervention group but no information for the control group (Gong 2011; Huang 2012b; Qiu 2006). Markedly higher numbers of other complications were reported in the control group in four CRS, six CS and 11 OS studies (Abd‐El‐Maeboud 2009; Chen 2012; Ertas 2013; Garshasbi 2011; Guangqing 2011; Han 2011; Huang 2012a; Husslein 2013; Jin 2010; Kafali 2010; Liang 2007; Li 2012a; Luo 2010; Qiao 2011; Shang 2010; Sun 2005; Tan 2011; Tian 2013; Wang 2008; Wang 2011a; Zhong 2009). The remaining 22 studies did not report clinically important differences in other complications.
1.9. Analysis.
Comparison 1 Control, Outcome 9 Complications ‐ Other [Frequency].
| Complications ‐ Other [Frequency] | ||
|---|---|---|
| Study | Intervention group | Control group |
| Colorectal surgery | ||
| Asao 2002 | 0 | 0 |
| Bahena‐Aponte 2010 | 0 | 2 |
| Bonventre 2014 | 0 ‐ no postoperative complications were observed | 0 ‐ no postoperative complications were observed |
| Cao 2008 | 16 | 12 |
| Forrester 2014 | 0 | 1 |
| Hirayama 2006 | 2 | 1 |
| Li 2012b | 0 ‐ no participants experienced adverse effects | 0 ‐ no participants experienced adverse effects |
| Lim 2013 | 57 | 63 |
| Matros 2006 | 1 | 2 |
| Quah 2006 | 2 | 3 |
| Schluender 2005 | 2 | 2 |
| Schuster 2006 | 1 | 2 |
| Tian 2013 | 4 | 13 |
| Wang 2011a | 28 | 58 |
| Watson 2008 | 6 | 7 |
| Zaghiyan 2013 | 6 | 8 |
| Zhong 2009 | 11 | 20 |
| Caesarean section | ||
| Abd‐El‐Maeboud 2009 | 11 | 31 |
| Garshasbi 2011 | 5 | 26 |
| Jakkaew 2013 | 5 | 2 |
| Kafali 2010 | 5 ‐ inestinal enema for gas passage (after 48 hr without passage of flatus). May have confounded results for time to first flatus | 16 ‐ inestinal enema for gas passage (after 48 hr without passage of flatus). May have confounded results for time to first flatus |
| Liang 2007 | 12 | 23 |
| Luo 2010 | 24 | 60 |
| Satij 2006 | 1 | 4 |
| Shang 2010 | 23 | 41 |
| Zamora 2012 | 0 ‐ no recorded complications (such as fever or temperature >38°C or wound dehiscence) | 0 ‐ no recorded complications (such as fever or temperature >38°C or wound dehiscence) |
| Other surgery | ||
| Abdollahi 2013 | 0 ‐ there were no surgicical complications in the 2 groups | 0 ‐ there were no surgicical complications in the 2 groups |
| Bonventre 2014 | 0 ‐ no postoperative complications were observed | 0 ‐ no postoperative complications were observed |
| Chen 2012 | 9 | 47 |
| Choi 2011 | 9 | 8 |
| Choi 2014 | 2 | 3 |
| Ertas 2013 | 12 | 37 |
| Gong 2011 | 0 ‐ no complications in the intervention group | No information |
| Guangqing 2011 | 5 | 80 |
| Han 2011 | 4 | 37 |
| Huang 2012a | 13 | 31 |
| Huang 2012b | 0 ‐ no bloating, pain nor complications in the intervention group | No information |
| Husslein 2013 | 9 | 63 |
| Jernigan 2014 | 2 | 5 |
| Jin 2010 | 4 | 12 |
| Li 2012a | 19 | 39 |
| Lu 2010a | 10 | 11 |
| Lu 2011 | 2 | 4 |
| Marwah 2012 | 14 | 16 |
| Ngowe 2010 | 0 | 0 |
| Park 2009 | 0 ‐ no postoperative complications such as postoperative infection or haemorrhage | 0 ‐ no postoperative complications such as postoperative infection or haemorrhage |
| Qiao 2011 | 7 | 28 |
| Qiu 2006 | 0 ‐ no complications in the intervention group | No information |
| Ray 2008 | 8 | 11 |
| Sun 2005 | 57 | 142 |
| Tan 2011 | 3 | 22 |
| Wang 2008 | 22 | 190 |
| Yi 2013 | 8 | 10 |
| Zhang 2008 | 0 | 0 |
| Çavuşoğlu 2009 | 1 | 4 |
Ten studies considered complications associated with CG (see Analysis 1.10). Nine reported no complications caused by the intervention (Bonventre 2014; Choi 2014; Ertas 2013; Hirayama 2006; Lee 2004; Li 2007a; Lu 2010a; Schluender 2005; Schweizer 2010). Cabrera 2012 reported abdominal distension, lack of gas and stool passage, and increased postoperative pain in two participants in the intervention group; authors believed this to be due to aerophagia whilst chewing gum.
1.10. Analysis.
Comparison 1 Control, Outcome 10 Complications related to the intervention [Frequency].
| Complications related to the intervention [Frequency] | |
|---|---|
| Study | |
| Colorectal surgery | |
| Bonventre 2014 | No treatment‐related complications were observed |
| Hirayama 2006 | No side effects or clinical problems were caused by gum‐chewing in any participants during this study |
| Schluender 2005 | There were no adverse events related to chewing. One participant was excluded due to nausea from chewing |
| Schweizer 2010 | In the entire participant population, no complications caused by the chewing gum were observed |
| Other surgery | |
| Bonventre 2014 | No treatment‐related complications were observed |
| Cabrera 2012 | 2 participants (12%) experienced abdominal distension, lack of gas and stool passage, and increased postoperative pain. Authors believed that this was due to aerophagia from chewing gum, therefore the intervention was stopped and nasogastric tubes put in place |
| Choi 2014 | There were no cases of side effects from gum chewing |
| Ertas 2013 | There were no reports of complications associated with gum chewing |
| Lee 2004 | No major complications with gum chewing were noted |
| Li 2007a | There were no adverse effects (e.g. abdominal pain/distension) in the gum chewing group |
| Lu 2010a | Not even one case of chewing gum was found to give an adverse effect on participants |
| Schweizer 2010 | In the entire participant population, no complications caused by the chewing gum were observed |
Tolerability of gum
Twenty‐nine studies reported on participants’ tolerability of gum (eight CRS, nine CS, 10 OS and two including both CRS and OS subgroups) (see Analysis 1.11). Eight CRS, seven CS and seven OS studies reported that gum was tolerated by all participants or that none of the participants were dissatisfied with it (Abd‐El‐Maeboud 2009; Abdollahi 2013; Akhlaghi 2008; Asao 2002; Bonventre 2014; Ertas 2013; Garshasbi 2011; Ghafouri 2008; Kafali 2010; Ledari 2012; Lee 2004; Lim 2013; Marwah 2012; McCormick 2005; Ngowe 2010; Quah 2006; Satij 2006; Schuster 2006; Wang 2011a; Watson 2008; Zamora 2012).
1.11. Analysis.
Comparison 1 Control, Outcome 11 Tolerability of gum.
| Tolerability of gum | ||
|---|---|---|
| Study | Description | Method of reporting |
| Colorectal surgery | ||
| Asao 2002 | All the patients tolerated gum chewing from the first AM after the operation | Patient tolerance of postoperative gum chewing was queried and recorded on the chart with information concerning flatus and defecation |
| Bonventre 2014 | All of the patients tolerated treatment from the first postoperative morning | Not discussed |
| Crainic 2009 | Anecdotal findings indicated that 20% of subjects (13 of 66: gum chewing and placebo group) said chewing gum or sucking on hard candy increased nausea | Not discussed |
| Lim 2013 | All patients in the intervention arm were compliant with and tolerated the chewing gum | Data were collected by an independent investigator, not involved with clinical management, during the postoperative period on a daily basis, via a patient questionnaire |
| McCormick 2005 | Gum chewing was well tolerated in both the open and laparoscopic participants | Not discussed |
| Quah 2006 | Gum chewing was well tolerated by all of the intervention group. 14 of 16 participants from the intervention group reported that chewing gum helped to keep their mouth moist and gave them a sense of well‐being and 13 of 16 participants from the intervention group reported that they were very satisfied with being able to chew gum postoperatively. One participant had difficulty in chewing gum due to ill‐fitting dentures | Documented |
| Schuster 2006 | All gum chewing patients tolerated the gum | Not discussed |
| Schweizer 2010 | Of the 50 participants in the intervention group (across all surgical subgroup), 30 participants (60%) considered the chewing gum to be positive by improving salivation and the coverage of negative gustatory sensations. 18 participants (36%) reported an indifferent attitude to the chewing gum. 2 participants (4%) had a negative opinion of the chewing gum: 1 participant had not experienced chewing gum previously and found it unusual, 1 participant felt that it induced nausea. Authors do not report which surgical subgroups these participants were in | Participants were questioned on positive or negative feelings about the chewing gum. Participants completed a survery after finishing the intervention |
| Wang 2011a | All subjects were capable of tolerating postoperative gum chewing and no chewing gums were accidentally swallowed | An in‐hospital medical staff member recorded the tolerance of patients in postoperative gum chewing |
| Watson 2008 | Chewing gum appeared to be tolerated well by all study participants. Two patients in the gum group that developed an ileus declined the gum when their symptoms were at their worst (abdominal pain, distension, nausea and vomiting) and one who developed the anastomotic leak ceased chewing gum on the day of the leak and did not re‐start it when recovered | Data on patients' tolerance of the gum was collected by ward staff and not disclosed to the research team until the time of data analysis |
| Caesarean section | ||
| Abd‐El‐Maeboud 2009 | All gum chewing patients tolerated and completed their course of gum chewing until bowel function | Not discussed |
| Akhlaghi 2008 | All patients in the intervention group tolerated gum chewing immediately after surgery, three times a day. All women in the intervention group indicated that chewing gum helped reduce or eradicate dryness and bitter taste in the mouth | Not discussed |
| Garshasbi 2011 | Chewing gum was well tolerated by all patients immediately after caesarean section | Not discussed |
| Kafali 2010 | All gum chewing patients completed their course of gum chewing and tolerated it very well | Not discussed |
| Ledari 2012 | None of the participants felt dissatisfied with chewing gum | Positive tolerance of gum chewing was documented |
| Safdari‐Dehcheshmehi 2011 | There were significant differences between groups in terms of patients’ satisfaction of interventions (p = 0.001). The gum chewing group reported the highest satisfaction, with 83.3% being highly satisfied with the intervention | Participants’ satisfaction with the intervention was investigated using a questionnaire with five 5‐point Likert type questions on: satisfaction with the time of intervention; satisfaction with the type of intervention; willingness to use the intervention in future; willingness to recommend the intervention to others; and satisfaction with the time to recovery and leaving the bed. The responses to each question ranged from “entirely unsatisfied” to “entirely satisfied” |
| Satij 2006 | Gum chewing was easily tolerated without any complications | Not discussed |
| Shang 2010 | 3 gum chewing participants were dissatisfied with the gum (1.6%), but all completed the course until passage of stool | Documented |
| Zamora 2012 | Gum chewing was considered by the authors as safe and well‐tolerated | Upon discharge patients were interviewed regarding their hospital progress and satisfaction with the study protocol |
| Other surgery | ||
| Abdollahi 2013 | All gum‐chewing patients tolerated the gum | Not discussed |
| Bonventre 2014 | All of the patients tolerated treatment from the first postoperative morning | Not discussed |
| Chuamor 2014 | Satisfaction with treatment was measured (score 0 to 10). Intervention group: 8.09 ± 1.30, control group: 6.45 ± 0.94 (P < 0.001) | The patient satisfaction for postoperative ileus management was evaluated using numeric rating scale (0‐10) i.e. 0 = very unsatisfied, and 10 = very satisfied. The overall satisfaction was evaluated using a 5‐point Likert scale, i.e., very satisfied, satisfied, uncertain, dissatisfied, and very dissatisfied |
| Ertas 2013 | All gum chewing patients tolerated and completed their course of gum chewing until bowel function | Not discussed |
| Ghafouri 2008 | All gum‐chewing patients tolerated gum | Patients were monitored daily by a general surgery specialist trainee, and assessed for sugarfree chewing gum intolerance |
| Han 2011 | One patient withdrew from the trial due to intolerance of gum. All the participants tolerated the chewing gum well | A nurse asked patients about tolerability of gum every hour |
| Husslein 2013 | A total of 81 women (95%) out of 85 would repeat gum chewing after the next surgery, which was highly significant (P = 0.001; binomial test) | At the day of discharge, patient satisfaction concerning postoperative gum chewing was assessed using a visual analog scale. Patients in the intervention roup were asked to rate treatment satisfaction and if they would like to repeat postoperative gum chewing in case of another surgery. The lowest score was one reflecting maximal dissatisfaction and the highest score was 10 reflecting maximal satisfaction |
| Lee 2004 | Well tolerated | Not discussed |
| Marwah 2012 | All patients in the study group tolerated gum chewing quite well. 12 participants continued to chew gum even after being asked to stop since they had passed flatus as they found it refreshing and appetising | Not discussed |
| Ngowe 2010 | All patients selected for chewing gum tolerated it well | Not discussed |
| Park 2009 | All the patients in the ‘gum‐chewing’ group commented positively regarding chewing gum as it enhanced saliva flow therefore kept their oral cavity from being too dry | Not discussed |
| Schweizer 2010 | Of the 50 participants in the intervention group (across all surgical subgroup), 30 participants (60%) considered the chewing gum to be positive by improving salivation and the coverage of negative gustatory sensations. 18 participants (36%) reported an indifferent attitude to the chewing gum. 2 participants (4%) had a negative opinion of the chewing gum: 1 participant had not experienced chewing gum previously and found it unusual, 1 participant felt that it induced nausea. Authors do not report which surgical subgroups these participants were in | Participants were questioned on positive or negative feelings about the chewing gum. Participants completed a survery after finishing the intervention |
Additional positive reports were presented in six studies where the CG group recorded the highest level of intervention satisfaction at 83.3% (Safdari‐Dehcheshmehi 2011), all intervention participants said that CG helped reduce or prevent dryness and a bitter taste in the mouth (Akhlaghi 2008), 12 participants continued CG after reaching the intervention endpoint as they found it refreshing and appetising (Marwah 2012), a higher satisfaction rating was observed in the intervention group (Chuamor 2014), positive comments about the intervention were received as it increased saliva flow and prevented mouth dryness (Park 2009), and 81 participants (95%) would repeat CG after the next surgery (Husslein 2013).
One study observed that 30 participants (60%) reported positive feelings towards the CG, 18 (36%) felt indifferent towards it, and 2 (4%) had a negative opinion (Schweizer 2010). Negative reports were presented in four other studies. In one study, 20% of participants (in either the CG or hard candy placebo group) stated that the intervention increased nausea (Crainic 2009). In another study, one participant had difficulty chewing the gum due to ill‐fitting dentures (Quah 2006). One participant withdrew from a study due to intolerance of gum (although authors also stated that all the participants tolerated the CG well) (Han 2011), and in another study three gum chewing participants were dissatisfied with the gum (1.6%), but all completed the course until passage of stool (Shang 2010).
Economic effect
Only two studies (both OS) investigated the economic effect of postoperative CG (see Analysis 1.12). Both studies observed reduced hospital charges for the intervention group, but there was no statistical evidence to support these findings in either trial (Chou 2006; Çavuşoğlu 2009).
1.12. Analysis.
Comparison 1 Control, Outcome 12 Cost.
| Cost | |
|---|---|
| Study | |
| Chou 2006 | Cost of hospitalisation was lower in the intervention group, but did not reach significance (intervention group: 2379 ± 195 USD, control group: 2672 ± 265 USD) |
| Çavuşoğlu 2009 | Hospital charges were evaluated from the invoice sent to the social security institution. These did not differ significantly between the groups (intervention group: 2451 ± 806 YTL, 1493 to 4619 YTL; control group: 2102 ± 678 YTL, 1073 to 3497 YTL; p = 0.206) |
Sensitivity analyses
We conducted the following sensitivity analyses for the continuous outcomes included in this review:
Sensitivity Analysis 1: removing studies with at least two high risks of bias
We considered 19 studies to be of poor methodological quality as we judged them to be at high ROB for at least two elements from: random sequence generation, allocation concealment, incomplete outcome data, selective outcome reporting or ‘other’ types of bias (Cabrera 2012; Choi 2011; Choi 2014; Chou 2006; Chuamor 2014; Crainic 2009; Forrester 2014; Jernigan 2014; Jin 2010; Li 2007a; Lim 2013; McCormick 2005; Ngowe 2010; Park 2009; Schweizer 2010; Watson 2008; Zaghiyan 2013; Zhang 2008; Zhao 2008). All results were similar between the sensitivity analyses and original estimates. A summary table is presented in Appendix 9.
Sensitivity analysis 2: removing studies which do not report complications
We considered 17 studies to be of poor methodological quality as they did not report complications (Chen 2010; Chen 2011; Crainic 2009; Fan 2009; Ghafouri 2008; Ledari 2012; Lu 2010b; Pilehvarzadeh 2014; Rashad 2013; Ren 2010; Safdari‐Dehcheshmehi 2011; Terzioglu 2013; Wang 2009a; Wang 2011b; Webster 2007; Yang 2011; Zhao 2008). All results were similar between the sensitivity analyses and original estimates. A summary table is presented in Appendix 10).
Sensitivity analysis 3: removing studies with any estimated results
Co‐authors estimated results for 22 studies. The calculations conducted and assumptions made are presented in Table 3. We conducted sensitivity analyses to assess if these imputed results affected our summary effect size estimates, by excluding these results from the meta‐analyses. All results were similar between the sensitivity analyses and original estimates. A summary table is presented in Appendix 11.
Sensitivity analysis 4: use of less conservative estimated results
Co‐authors estimated standard deviations or ranges for 11 studies (Garshasbi 2011; Lee 2004; Lim 2013; Lu 2010a; Lu 2011; Qiao 2011; Ray 2008; Schluender 2005; Watson 2008; Yi 2013; Zhao 2008) (see Table 3). In this sensitivity analysis, we applied less conservative estimations for these values. All results were similar between the sensitivity analyses and original estimates. A summary table is presented in Appendix 12.
Sensitivity analysis 5: ERAS studies
Four studies (all CRS) reported using an ERAS programme (Atkinson 2014; Lim 2013; Watson 2008; Zaghiyan 2013). Effect estimates were reduced for TFF and slightly increased for TBM [TFF: analysis of 591 participants from 4 studies showed a reduction of 6.2 hours (95% CI ‐15.4, 3.0), TBM: 634 participants from 4 studies showed a reduction of 21.1 hours (95% CI ‐33.0, ‐9.1)]. There was no difference between the intervention and control groups in LOHS: analysis of 724 participants from 4 studies showed an increase of 0.1 days (95% CI ‐0.4, 0.5). Atkinson 2014 was the only study conducted in an ERAS context that reported TBS. A summary table is presented in Appendix 13.
Meta‐regression
Meta‐regression was considered more appropriate than the standard Chi2 test available in the RevMan software due to the differences in our subgroups (Higgins 2011) (discussed in Overall completeness and applicability of evidence). As observed in the overall analyses, meta‐regression models indicated an association between surgical site and effectiveness of the intervention (see Appendix 14). Effects were greatest in CRS, followed by OS, with the smallest effect sizes in the CS subgroup. There was weak evidence that the extent of effect of CG on LOHS was greater in both the CRS and OS subgroups than the CS subgroup (CRS compared to CS: regression coefficient = ‐0.9 days, P = 0.026; OS compared to CS: regression coefficient = ‐0.7 days, P = 0.045). There was also weak evidence that the extent of effect of CG on TFF and TBM was greater in the CRS subgroup than the CS subgroup (TFF: regression coefficient = ‐4.7 hours, P = 0.067; TBM: regression coefficient = ‐8.7 hours, P = 0.047). There was no evidence of an influence of surgical site on the extent of effect of CG on TBS. ROB score was not associated with the extent of effect of CG on TFF, TBM, TBS or LOHS. In a mutually adjusted model, adjusting for both surgical site and ROB score, the association between surgical site and extent of effect on LOHS and TBM persisted (LOHS CRS subgroup: regression coefficient = ‐0.9 days, P = 0.035; LOHS OS subgroup: regression coefficient = ‐0.8 days, P = 0.026; TBM CRS subgroup: regression coefficient = ‐8.9 hours, P = 0.044), and there was evidence of a weak association between ROB score and TBS (TBS ROB score 6 to 10 subgroup: regression coefficient = 6.5 hours, P = 0.047). There was no longer evidence for an influence of surgical site on extent of effect on TFF. I2 values did not support surgical site or ROB score as a source of heterogeneity between studies.
Discussion
Summary of main results
Our review shows that there is some evidence for a reduction in TFF and TBM with use of postoperative CG (reductions of 10.4 and 12.7 hours respectively), with a modest clinical difference in LOHS and TBS. There was also no clear difference in mortality, infection and readmissions between groups. Although we were unable to formally meta‐analyse complications, some studies reported reduced nausea and vomiting and other complications in the intervention group. CG was generally well‐tolerated by participants. There was little difference in cost between groups, but only two studies reported this outcome. Findings are summarised in the Table 1 and Table 2.
Sensitivity analyses for study quality and use of estimated data showed no clinically important changes to the findings. The effect of CG on outcomes was generally reduced in the analysis of studies conducted within an ERAS context. Meta‐regression analyses indicated that surgical site is associated with the effectiveness of chewing gum on LOHS (for all surgical subgroups), and TFF and TBM (for CS and CRS). ROB score was not associated with the extent of effect of the intervention.
Overall completeness and applicability of evidence
1. Completeness
We attempted to identify and synthesise all existing research to provide a comprehensive estimate of the effect of CG on postoperative recovery of GI function. We included 81 studies that comprised 9072 participants; the largest SR to date prior to ours included only 17 RCTs that recruited 1374 participants (Li 2013). However, our search strategies may not have identified all of the existing literature. Additionally, eight identified publications could not be located through our library resources (see Characteristics of excluded studies), potentially biasing our results. Several studies also reported results in a format that could not be used in the review (see Table 4). However, where possible we estimated and made assumptions about the data, which allowed us to use the majority of identified information.
2. Results not included in this review.
| Study | Excluded results |
| Akhlaghi 2008 | Reported duration of abdominal distension and postoperative ileus |
| Askarpour 2009 | Time to first bowel movement reported as number of participants within 24 hours |
| Atkinson 2014 | Abdominal pain and nausea reported as visual analogue scales on postoperative day 2 |
| Bahena‐Aponte 2010 | Reported change in abdominal distension in cm from preoperatively to the first 24 hours postoperatively |
| Cabrera 2012 | Time to first flatus reported as number of participants within 12 hours, time to first bowel movement reported as number of participants within 48 hours, length of hospital stay reported as number of participants within 5 days |
| Chuamor 2014 | Time to first bowel sounds reported categorically as number per minute within 12 hours, on day 1, day 2 and day 3. Reported severity of ileus (mild/moderate/severe) and abdominal distension scores (0 to 100) within 12 hours, on day 1, day 2 and day 3 |
| Garshasbi 2011 | No numerical data provided for length of hospital stay; authors state that there was virtually no difference between the groups |
| Gong 2011 | Time to ease of bloating reported |
| Husslein 2013 | Bowel sounds reported as number of participants at 3, 5 and 7 hours |
| Jakkaew 2013 | Reported visual analogue scale scores for nausea, abdominal cramping and abdominal distension |
| Kafali 2010 | Postoperative mefenamic acid requirement reported in mg |
| Li 2007a | Complication (fungal infections, dry mouth, bad breath and mouth ulcers) frequency was statistically significant between the groups, but no numerical data provided |
| Luo 2010 | Time taken to alleviate abdominal distension reported |
| McCormick 2005 | Nausea and vomiting and time to first bowel sounds presented in graph format indicating % of participants experiencing these incidents on postoperative days 1 to 8 and > 8 |
| Qiu 2006 | Time to bloating relief reported |
| Terzioglu 2013 | Length of hospital stay reported categorically as % of participants with 3 to 4 days, 5 to 6 days and ≥ 7 days |
We looked at similar outcomes to other SRs, but further outcomes reported in studies such as time to first solid food consumption could have been assessed as another marker of recovery. Inclusion of reports of subjective markers of recovery, such as self‐report measures of pain, hunger and fatigue may also have been helpful to incorporate into this review. Nonetheless, the outcomes that we have presented are useful measures of postoperative recovery of GI function.
2. Applicability
Most studies applied exclusion criteria to individuals for study participation. These frequently included previous abdominal surgery, thereby limiting the applicability of findings for people with recurrent surgical problems. Many studies also had upper or lower age restrictions, and children have been particularly neglected in this research area (only four studies in this review were conducted in children). Furthermore, studies often excluded individuals with intraoperative or postoperative complications, and common co‐morbidities such as diabetes. This restricts the applicability of findings for groups other than ‘healthy’ people.
Studies included in this review were conducted in various countries, incorporating a range of cultures and health care systems which may have an effect on outcomes. For example, Shang 2010 state that in Chinese culture it is not acceptable for women to take anti‐emetics during lactation. Therefore CG following CS may be more effective in minimising nausea and vomiting among women living in China. Future analyses focusing on country or health care system may be useful to determine the applicability of results to different parts of the world. In addition, standard health care practice is likely to vary across countries. For example, ERAS is employed to different degrees internationally, which may impact on the effectiveness of CG.
A priori, we grouped studies into CRS, CS and OS subgroups. However, we had not anticipated the volume of studies or the broad range of surgical disciplines encompassed by the OS subgroup. Therefore, the overall meta‐analysed result may not be applicable to the individual surgical specialties. Future reviews could further sub‐divide this category by specific surgery type, such as gynaecological procedures or cholecystectomy. We conducted an exploratory analysis investigating gynaecological studies from the OS subgroup, which demonstrated a smaller effect of CG on TFF, but little difference in other outcomes (data not shown).
There are likely to be a number of important differences between our chosen subgroups. For example, the CS participants (of childbearing age) are generally much younger than the CRS subgroup. In addition, CS participants are likely to be a healthier population than the other subgroups, as surgery is for pregnancy rather than disease. Therefore these underlying assumptions about the overall surgical population should be considered when interpreting our results and for any comparisons between subgroups that may be made.
Quality of the evidence
Assessements of quality of evidence for each outcome are presented in the Table 1 and Table 2.
1. Methodology
Methodological quality and ROB were difficult to assess in many studies due to poor reporting. Those with available information were of variable methodological rigour. Several studies applied inadequate methods for randomisation sequence generation and allocation concealment. Allocation concealment methods were the most poorly reported of all evaluated ROB elements. There were also few reports of attempts to to blind outcome assessors and other personnel. The majority of ROB assessments were therefore classified as ‘unclear’ or ‘high’ risk. However, the sensitivity analyses refining by study quality did not change the direction or extent of effect estimates. Meta‐regression also did not identify an association between ROB score and effectiveness of the intervention for any outcome.
Few studies reported use of sample size or power calculations; of those that did, several did not meet the target sample size. Many other studies included small sample sizes, reducing the power of the trial to observe clinically important differences in outcomes.
2. Outcome Assessment
Ileus is the clinical outcome of interest in this research area. However, there is not currently a widely accepted definition of ileus (Vather 2013). Instead, TFF, TBM and TBS are used as proxy markers of ileus resolution. A more consistent outcome using a combination of individual markers as described in Tan 2006 would allow for a more precise measure of ileus resolution, which could improve the quality of the evidence base.
TFF and TBM rely on self‐report. Postoperative participants may feel unwell or disorientated; hence these self‐reported outcomes may be open to misreporting or bias. Additionally blinding of participants with this intervention is not possible, and an awareness of treatment allocation may result in participants misreporting these outcomes. However, this risk of bias is applicable to all studies reporting these outcomes, as TFF and TBM cannot be more accurately recorded by any another means. Reporting of TBS may also be inaccurate as it is generally dependent on clinicians’ availability to listen as opposed to actual time to event. Furthermore, protocols for listening for bowel sounds may differ across studies. Finally, LOHS is likely to be influenced by variation in discharge criteria, which may result in differences between studies. This lack of uniformity across centres may introduce variability among some outcomes.
3. Heterogeneity
Considerable heterogeneity was observed in all of our results. Despite this, we are confident that this heterogeneity indicates variation in size of effect as opposed to direction, given that most studies’ findings suggested a beneficial effect of CG on postoperative recovery outcomes. Our meta‐regression analyses did not identify surgical site or ROB score as key sources of heterogeneity between studies. Additionally, visual inspection of the forest plots and associated data did not indicate that size of study affected effect size. However, visual inspection of the funnel plots for each continuous outcome indicated that publication bias may be present for TBM and LOHS (see Figure 5, Figure 7, Figure 9, Figure 11).
Potential biases in the review process
1. Search strategy
Although we believe that our electronic and hand‐searching strategies identified the majority of relevant trials, it is possible that we may have missed some available literature or unpublished material. We stopped hand‐searching at the end of August 2014, and in the time period until publication other trials may have been published or made available. These will be incorporated into future updates to this review.
2. Assumptions about the mechanism of effect
CG is assumed to work through cephalo‐vagal stimulation for GI hormone production, ‘sham feeding’ to cause GI motility, and release of pancreatic juices and saliva (Tandeter 2009). The chewing action may not be the only mechanism by which CG might improve postoperative GI recovery. It has been suggested that the ingredients in some types of gum (particularly sugar‐free gum) such as hexitols, may have a laxative effect (Tandeter 2009). These may produce the GI stimulatory effect which is generally associated with the chewing action of gum. Our focus on the action of the CG intervention, rather than the ingredients, may have limited our approach and analyses. However, only one study used sugared gum, therefore stratification by intervention was not feasible in this review. Additionally, the small dosages of hexitols within a CG protocol [estimated to be approximately 3.75g of sorbitol per day, plus maxitols (Tandeter 2009)] may not be great enough to produce considerable GI stimulation effects.
3. Assumptions about the meta‐analyses and results
We may have introduced bias in the review process through the degree of data manipulation required to conduct meta‐analyses. A diverse range of outcome metrics were reported across studies, requiring conversion to common units for use in this review. In addition, a large number of studies did not report data completely or conventionally. We therefore had to make estimations and assumptions using the available data (see Table 3). We conducted a sensitivity analysis removing all results that had been estimated, and the direction of effect for each subgroup within each outcome remained the same. Effect sizes remained similarly unchanged when less conservative estimates were applied to results, indicating that the quality of our statistical manipulations did not substantially affect our findings. Given the extent of heterogeneity present between studies a random‐effects model was deemed appropriate (Higgins 2011). This may have resulted in smaller studies being granted a larger weighting than necessary, potentially biasing the overall meta‐analysed results. As publication bias was identified from visual inspection of the funnel plots (see Figure 7, Figure 9), which may explain some of the heterogeneity between studies, we also ran the main meta‐analyses using a fixed‐effect model. This diminished effect estimates (by 1.3 hours, 3.5 hours, 0.5 days and 0.7 hours for TFF, TBM, LOHS and TBS respectively), but the direction of effect remained the same (see Appendix 8).
Studies reported a diverse range of complications which did not fall into natural categories. The categories we developed may therefore not completely represent the data, especially given that ‘other complications’ ranged in severity from dry mouth to myocardial infarction. Nonetheless, we feel that the groups presented encompassed the complications of interest and relevance to this review.
4. Assumptions about study methodology
We made a number of assumptions about the comparability of study methodology, including the control and intervention protocols, and compliance to the intervention.
There were a variety of care pathways for controls across studies including standard care, ERAS, early ambulation/exercise and nil‐by‐mouth. We combined all of these to form one control group, which may have given a different effect size compared to analyses stratified by control group care. In an exploratory analysis we expanded the ERAS sensitivity analysis by including a further ten studies employing early mobilisation (one of the components of ERAS) (Bahena‐Aponte 2010; Chen 2011; Gong 2011; Guangqing 2011; Huang 2012b; Li 2012a; Matros 2006; Tan 2011; Wang 2008; Yi 2013). Results did not differ greatly from those of the original analyses (data not shown).
Similarly, our analyses did not adjust for differences in CG protocols. Timing of intervention commencement, duration and frequency differed greatly across studies. It is possible that results may have varied due to a dose‐response effect or threshold effect, but we feel that this was unlikely to have greatly affected our findings.
We recorded reports of compliance in the included studies (see Appendix 15). Sixteen studies described methods to monitor or improve compliance; of these, only six studies reported compliance levels, stated as high in five trials. Based on this, we assumed good compliance levels generally, despite poor reporting. As we were unable to test compliance, our results may underestimate the effect of CG through our assumption of high compliance across studies. It is also possible that control participants may have independently decided to chew gum, and if this was the case then the overall effect of CG on recovery of postoperative GI function may have been attenuated.
Agreements and disagreements with other studies or reviews
Several SRs have been published on this topic, with similarly positive results. Compared to a recent meta‐analysis of 17 abdominal surgery studies (Li 2013), we observed a slightly greater reduction in TFF, and similar reductions in TBM and LOHS. Authors conducted the same subgroup analyses as in our review: CRS (eight studies), CS (four studies) and OS (five studies). Our review showed greater reductions in TFF in the CRS subgroup (12.5 hours compared to 7.2 hours), greater reductions in TBM and LOHS in the CS subgroup (9.1 hours compared to 6.24 hours and 0.8 days compared to 0.21 days), and a smaller reduction in TBM in the OS subgroup (12.3 hours compared to 21.36 hours). All other results were comparable between reviews. Li 2013 also showed statistical evidence of heterogeneity, but found no evidence of publication bias.
Several SRs have demonstrated reductions in TFF and TBM, with less consistent effects on LOHS (Chan 2007; Noble 2009; Parnaby 2009; Vasquez 2009). Variation in results may be due to differences in exclusion criteria; for example, one review excluded trials with unclear methodology and unclear statistical analyses (Parnaby 2009). Our review included all of the studies meta‐analysed in previous SRs, with the exception of one non‐RCT (Kouba 2007) that was included in Noble 2009. In agreement with our review, all previous SRs reported heterogeneity between included studies.
The most recent CRS SR that we are aware of identified 10 RCTs (Ho 2014). However, compared to both our review and previous SRs, they reported markedly reduced effect sizes (0.517 hours, 0.502 hours and 0.5 days for TFF, TBM and LOHS respectively). For their analyses they used standard mean difference rather than weighted mean difference (as in our review and previous reviews), and the validity of their reported effect sizes has recently been questioned (Zhuang 2014). Ho 2014 reported similarly reduced effect sizes compared to our review for an ERAS specific sensitivity analysis of two trials. Ho 2014 suggested that CG does not provide any additional benefit to ERAS, whereas our results indicate that there may be some small further benefit for GI recovery outcomes. However, it is difficult to draw valid conclusions with only four studies. A temporal decrease in effect size can be seen across SRs, with newer SRs showing smaller effect estimates compared to older SRs (Chan 2007; Purkayastha 2008; Li 2013; Yin 2013). It is possible that this may reflect general improvements in care over time and factors such as the implementation of ERAS programmes, which may have diminished the effect of CG seen in older trials. One study in this review pre‐empted the possible reduced effect of CG in an ERAS context, and chose not to use a fast‐track programme so as not to mask the extent of effect observed with the intervention alone (Bonventre 2014).
Although many trials and SRs have investigated CRS, there have been several SRs of CG use following CS. Zhu 2014 reported similar results to our review, with reductions of 6.42 hours, 6.58 hours, 3.62 hours and 5.94 hours in TFF, TBM, TBS and LOHS, based on results from six CS RCTs. TBS has not been frequently meta‐analysed in surgical disciplines outside of CS.
Unlike previous SRs (Chan 2007; Parnaby 2009), we did not meta‐analyse complications due to the diverse range reported in studies. Our results indicated that there may be reductions in nausea and vomiting and other general complications, but there was little difference in infections, mortality or readmissions between groups. Several previous reviews have suggested little or no effect of CG on frequency of complications (Belghazi 2012; Chan 2007; Noble 2009; Parnaby 2009). However, some SRs have reported a lower risk of complications with use of CG (Ho 2014; Li 2013), as well as suggestions that CG may be associated specifically with a reduction in risk of ileus (Craciunas 2014; Yuan 2011).
One prior review considered complications due the chewing gum itself (Parnaby 2009); no complications were found, which is in agreement with our overall findings. Previous SRs have not reported on tolerability of gum in detail; our review suggests that CG is generally well‐tolerated. Cost has not been commonly reported in SRs, but one previous review found that there was no difference between groups (Belghazi 2012). Our results support these findings, although they are based on only two studies.
Authors' conclusions
Implications for practice.
Ileus is a common problem following abdominal surgery. We found low quality evidence suggesting a clinically relevant decrease in TFF and TBM with CG. These results are based on many small, poor quality trials, with evidence of heterogeneity and publication bias for some outcomes. Our sensitivity analysis suggests that there is a reduced benefit of CG in the ERAS era. This is unsurprising, given that ERAS incorporates a range of components targeting ileus. This questions the benefit of adding CG to postoperative care within an established ERAS programme, although it must be noted that our findings are based on only four studies that explicitly stated that they were conducted within the context of an ERAS programme. However, there is also little chance of CG causing any adverse events. CG may be most clinically beneficial in centres where ERAS or fast track programmes are not in place, or where the application of some ERAS components is not practical, for example in people who cannot tolerate food or who have severe nausea and vomiting (Smith 2014).
The effect of CG on postoperative recovery outside CRS is also unclear. Our results suggest that the greatest benefits from CG may occur in CRS, and the least benefit in CS. This is not surprising given the differences in surgical trauma/duration of surgery, both of which can affect the extent of ileus. Given the overall poor quality of the evidence, it is not possible to draw firm conclusions regarding the inclusion of CG as part of routine practice.
Implications for research.
Now that ERAS is becoming more widespread, the usefulness of CG in the context of an ERAS programme is the relevant question to be answered. The majority of studies included in this review do not state use of ERAS or ‘fast‐track’ protocols, but these are becoming increasingly popular. The limited number of ERAS trials included in this review demonstrate a need for RCTs within an ERAS context: RCTs comparing ERAS with or without the use of gum would be required. Additionally, the increasing application of ERAS internationally may reduce heterogeneity across future trials. Furthermore, future work could also focus on groups that are at high risk of developing ileus (due to longer or more complicated surgery), where the potential benefits of CG may be more apparent. Future studies could also include exploration of other less commonly investigated outcomes which may be clinically relevant, such as time to first food consumption, use of nasogastric tube insertion, pain and discomfort. Additionally, preoperative informed consent with postoperative randomisation may reduce attrition rates due to intraoperative and postoperative complications. The poor quality and small size of the trials to date also emphasise the necessity for large, better quality, well‐designed trials.
The available literature largely focusses on CRS or CS. Our results show greater effects in CRS than CS, but further work would be required to establish the potential role of CG in other surgical disciplines. Similarly, the literature is also mainly limited to adults; further trials in children may be warranted.
Future trials would need to be higher quality and large, as differences between groups are likely to be smaller in ERAS populations. Given the modest effect size in ERAS trials in this review, the chance that such trials will show a clinically important difference is debatable. Therefore better quality, larger‐scale trials would be required to provide further evidence and greater confidence in findings.
What's new
| Date | Event | Description |
|---|---|---|
| 20 May 2015 | Amended | Minor correction to Time to Bowel Movement (TBM) data, sensitivity analyses 2 and 5, and meta‐regression incorporated in this version. Conclusions remain the same. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 2, 2015
| Date | Event | Description |
|---|---|---|
| 12 February 2015 | Amended | Feedback from Cochrane edit team incorporated into the Background, Methods, Discussion and Authors' Conclusions. Adapted risk of bias tool adjusted, and relevant associated changes made to sensitivity analysis 1, the meta‐regression and risk of bias scoring |
| 8 December 2014 | New search has been performed | Text and results added |
| 12 May 2014 | Amended | CEU proposed changes to the protocol accepted where appropriate. Clarification provided for 'Measures of treatment effect', 'Data synthesis' and 'Sensitivity analysis' sections. |
| 27 February 2014 | New citation required and major changes | This is a substantial update of the protocol published in 2007 by Griffiths and Watson. Title has been modified. Editing group proposed changes accepted. Additional descriptions of potential adverse events, search for ongoing trials, search of reference lists of previous trials, use of a PRISMA flow chart and units of analysis. |
Notes
May 20 2015: Minor correction to Time to Bowel Movement (TBM) data, sensitivity analyses 2 and 5, and meta‐regression incorporated in this version. Conclusions remain the same
Acknowledgements
The authors would like to acknowledge the support of Rachel Churchill in the overall review process, and Leala Watson for providing advice for the electronic search strategy. The authors would also like to acknowledge Vanessa Er, Peony Ng and Joon Seong for their help in providing translation and direct extraction of information for use in this review.
The authors would also like to acknowledge the CCCG editorial office for this RevMan version of the review.
Appendices
Appendix 1. CENTRAL
#1. MeSH descriptor: [Ileus] explode all trees
#2. MeSH descriptor: [Gastrointestinal Motility] explode all trees
#3. MeSH descriptor: [Intestinal Obstruction] explode all trees
#4. MeSH descriptor: [Peristalsis] explode all trees
#5. MeSH descriptor: [Gastrointestinal Transit] explode all trees
#6. MeSH descriptor: [Intestinal Pseudo‐Obstruction] explode all trees
#7. bowel* near/5 function:ti,ab
#8. intestin* near/5 pseudo‐obstruct*:ti,ab
#9. (postoperative or post‐operative) near/5 recover*:ti,ab
#10. postoperative near/5 ileus:ti,ab
#11. post‐operative near ileus:ti,ab
#12. post‐operative near/5 recover*:ti,ab
#13. postoperative near/5 recover*:ti,ab
#14. gastrointestin* near/5 function*:ti,ab
#15. gastro‐intestin* near/5 function*:ti,ab
#16. gastrointestin* near/5 dysmotilit*:ti,ab
#17. gastro‐intestin* near/5 dysmotilit*:ti,ab
#18. gastrointestin* near/5 motilit*:ti,ab
#19. gastro‐intestin* near/5 motilit*:ti,ab
#20. gastrointestin* near/5 transit*:ti,ab
#21. gastro‐intestin* near/5 transit*:ti,ab
#22. (duoden* or intestin*) near/5 obstruct*:ti,ab
#23. gut near/5 (motilit* or dysmotilit* or transit*):ti,ab
#24. bowel near/5 (motilit* or dysmotilit* or transit*):ti,ab
#25. colon near/5 (motilit* or dysmotilit* or transit*):ti,ab
#26. (colorect* or intestin* or colectom* or ileostom* or colonic or gynecolog* or gynaecolog* or cesarean or caesarean or C‐section or cystectom* or cholecystectom* or appendectom*):ti,ab
#27. (auscultation or ileus or peristalsis or colon resection or bowel resection or surger*):ti,ab
#28. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29. MeSH descriptor: [Chewing Gum] explode all trees
#30. sham* near/5 chew*:ti,ab
#31. gum* near/5 chew*:ti,ab
#32. chew* near/5 gum*:ti,ab
#33. sham* near/5 feed*:ti,ab
#34. vagal* near/5 cholinergic:ti,ab
#35. cephalic‐vagal:ti,ab
#36. gum*:ti,ab
#37. #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36
#38. #28 and #37
Appendix 2. MEDLINE (via Ovid)
1. (sham$ adj5 chew$).tw.
2. (gum$ adj5 chew$).tw.
3. (chew$ adj5 gum$).tw.
4. exp Chewing Gum/
5. (vagal$ adj5 cholinerg$ adj5 stimulation$).tw.
6. (cephalic‐vagal$ adj5 stimulation$).tw.
7. gum$.tw.
8. (sham$ adj5 feed$).tw.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. exp Gastrointestinal Motility/
11. exp Intestinal Obstruction/ or exp Intestinal Pseudo‐Obstruction/ or exp Ileus/ or exp Duodenal Obstruction/
12. exp Gastrointestinal Transit/
13. exp Peristalsis/
14. exp Auscultation/
15. ileus$.tw.
16. bowel$ function$.tw.
17. (intestin$ adj5 pseudo‐obstruct$).tw.
18. exp ileus/
19. ((postoperative or post‐operative) adj5 recover$).tw.
20. ((gastro‐intestinal or gastrointestinal) adj5 function$).tw.
21. ((gastrointestinal or gastro‐intestinal) adj5 dysmotilit$).tw.
22. ((gastrointestinal or gastro‐intestinal) adj5 motilit$).tw.
23. ((gastrointestinal or gastro‐intestinal) adj5 transit$).tw.
24. ((duoden$ or intestin$) adj5 obstruct$).tw.
25. (gut adj5 (motilit$ or dysmotilit$ or transit$)).tw.
26. (colorect$ or intestin$ or colectom$ or ileostom$ or colonic$ or gynecologic$ or gynaecolog$ or auscultation$ or peristalsis).tw.
27. ((postoperative or post‐operative) adj5 ileus).tw.
28. (bowel adj5 (motlit$ or dysmotilit$ or transit$)).ti,ab.
29. (colon adj5 (motilit$ or dysmotilit$ or transit$)).ti,ab.
30. (caesarean or cesarean or C‐section or cystectom$ or cholecystectom$ or appendectom$ or colon resection or bowel resection or surger$).tw.
31. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
32. 9 and 31
Appendix 3. MEDLINE (via PubMed)
(((chew*[All Fields] AND gum*[Title/Abstract]) OR (sham*[All Fields] AND chew*[Title/Abstract]) OR (gum*[All Fields] AND chew*[Title/Abstract]) OR (sham*[All Fields] AND feed*[Title/Abstract]) OR (chewing gum[MeSH Terms]) OR (vagal*[All Fields] AND cholinerg*[All Fields] AND stimulat*[Title/Abstract]) OR (cephalic‐vagal*[All Fields] AND stimulat*[Title/Abstract]) OR (gum*[Title/Abstract]))) AND ((gastrointestinal motility[MeSH Terms]) OR (intestinal obstruction[MeSH Terms]) OR (intestinal pseudo‐obstruction[MeSH Terms]) OR (ileus[MeSH Terms]) OR (duodenal obstruction[MeSH Terms]) OR (gastrointestinal transit[MeSH Terms]) OR (peristalsis[MeSH Terms]) OR (auscultation[MeSH Terms]) OR (ileus[Title/Abstract]) OR (gut motilit*[Title/Abstract]) OR ((caesarean[All Fields] OR cesarean[All Fields] OR c‐section[All Fields] OR cystectom*[All Fields] OR cholecystectom*[All Fields] OR appendectom*[All Fields] OR colon resection[All Fields] OR bowel resection[All Fields] OR surger*[All Fields] OR hospital stay[All Fields] OR anal exhaust*[All Fields]) AND title/abstract[All Fields]) OR (intestin*[All Fields] AND pseudo‐obstruct*[Title/Abstract]) OR ((postoperative[All Fields] OR post‐operative[All Fields]) AND recover*[Title/Abstract]) OR ((gastrointestin*[All Fields] OR gastro‐intestin*[All Fields]) AND function*[Title/Abstract]) OR ((gastrointestin*[All Fields] OR gastro‐intestin*[All Fields]) AND dysmotilit*[Title/Abstract]) OR ((gastrointestin*[All Fields] OR gastro‐intestin*[All Fields]) AND motilit*[Title/Abstract]) OR ((gastrointestin*[All Fields] OR gastro‐intestin*[All Fields]) AND transit*[Title/Abstract]) OR ((duoden*[All Fields] OR intestin*[All Fields]) AND obstruct*[Title/Abstract]) OR (gut motilit*[Title/Abstract]) OR (gut dysmotilit*[Title/Abstract]) OR (gut transit*[Title/Abstract]) OR (colorectal[All Fields] OR intestine*[All Fields] OR colectom*[All Fields] OR ileostom*[All Fields] OR colonic*[All Fields] OR gynecologic*[All Fields] OR gynaecolog*[All Fields] OR auscultation*[All Fields] OR peristalis*[Title/Abstract]) OR ((postoperative[All Fields] OR post‐operative[All Fields]) AND ileus[Title/Abstract]))
Appendix 4. EMBASE (via Ovid)
1. (sham$ adj5 chew$).tw.
2. (gum$ adj5 chew$).tw.
3. (chew$ adj5 gum$).tw.
4. exp Chewing Gum/
5. (vagal$ adj5 cholinerg$ adj5 stimulation$).tw.
6. (cephalic‐vagal$ adj5 stimulation$).tw.
7. gum$.tw.
8. (sham$ adj5 feed$).tw.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. exp Gastrointestinal Motility/
11. exp Intestinal Obstruction/ or exp Intestine Pseudoobstruction/ or exp Ileus/ or exp Duodenum Obstruction/
12. exp Gastrointestinal Transit/
13. exp Peristalsis/
14. exp Auscultation/
15. ileus$.tw.
16. bowel$ function$.tw.
17. (intestin$ adj5 pseudo‐obstruct$).tw.
18. exp ileus/
19. ((postoperative or post‐operative) adj5 recover$).tw.
20. ((gastro‐intestinal or gastrointestinal) adj5 function$).tw.
21. ((gastrointestinal or gastro‐intestinal) adj5 dysmotilit$).tw.
22. ((gastrointestinal or gastro‐intestinal) adj5 motilit$).tw.
23. ((gastrointestinal or gastro‐intestinal) adj5 transit$).tw.
24. ((duoden$ or intestin$) adj5 obstruct$).tw.
25. (gut adj5 (motilit$ or dysmotilit$ or transit$)).tw.
26. (colorect$ or intestin$ or colectom$ or ileostom$ or colonic$ or gynecologic$ or gynaecolog$ or auscultation$ or peristalsis).tw.
27. ((postoperative or post‐operative) adj5 ileus).tw.
28. (bowel adj5 (motlit$ or dysmotilit$ or transit$)).ti,ab.
29. (colon adj5 (motilit$ or dysmotilit$ or transit$)).ti,ab.
30. (caesarean or cesarean or C‐section or cystectom$ or cholecystectom$ or appendectom$ or colon resection or bowel resection or surger$).tw.
31. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
32. 9 and 31
Appendix 5. CINAHL (via EBSCO)
S1. (MM "Chewing Gum")
S2. TI sham* N5 chew* OR AB sham* N5 chew*
S3. TI sham* N5 feed* OR AB sham* N5 feed*
S4. TI chew* N5 gum* OR AB chew* N5 gum*
S5. TI gum* OR AB gum*
S6. TI vagal* N5 cholinerg* N5 stimulat* OR AB vagal* N5 cholinerg* N5 stimulat*
S7. TI cephalic‐vagal* OR AB cephalic‐vagal*
S8. S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7
S9. (MM "Gastrointestinal Motility") OR (MM "Gastrointestinal Transit")
S10. (MM "Intestinal Obstruction") OR (MM "Intestinal Pseudo‐Obstruction") OR (MM "Gastric Outlet Obstruction")
S11. (MM "Gastrointestinal Transit")
S12. (MM "Auscultation")
S13. TI ileus* OR AB ileus*
S14. TI bowel* function* OR AB bowel* function*
S15. TI ( caesarean or cesarean or C‐section or cystectom* or cholecystectom* or appendectom* or colon resection or bowel resection or surger* or hospital stay ) OR AB ( caesarean or cesarean or C‐section or cystectom* or cholecystectom* or appendectom* or colon resection or bowel resection or surger* or hospital stay )
S16. TI intestinal N5 pseudo‐obstruct* OR AB intestinal N5 pseudo‐obstruct*
S17. TI ( (postoperative or post‐operative) N5 recover* ) OR AB ( (postoperative or post‐operative) N5 recover* )
S18. TI ( (gastrointestinal or gastro‐intestinal) N5 function* ) OR AB ( (gastrointestinal or gastro‐intestinal) N5 function* )
S19. TI ( (gastrointestinal or gastro‐intestinal) N5 dysmotilit* ) OR AB ( (gastrointestinal or gastro‐intestinal) N5 dysmotilit* )
S20. TI ( (gastrointestinal or gastro‐intestinal) N5 motilit* ) OR AB ( (gastrointestinal or gastro‐intestinal) N5 motilit* )
S21. TI ( (gastrointestinal or gastro‐intestinal) N5 transit* ) OR AB ( (gastrointestinal or gastro‐intestinal) N5 transit* )
S22. TI ( (duoden* or intestin*) N5 obstruct* ) OR AB ( (duoden* or intestin*) N5 obstruct* )
S23. TI ( gut N5 (motilit* or dysmotilit* or transit*) ) OR AB ( gut N5 (motilit* or dysmotilit* or transit*) )
S24. TI ( colorect* or intestin* or colectom* or ileostom* or colonic* or gynecologic* or gynaecolog* or auscultaion* or peristalsis ) OR AB ( colorect* or intestin* or colectom* or ileostom* or colonic* or gynecologic* or gynaecolog* or auscultaion* or peristalsis )
S25. TI ( (postoperative or post‐operative) N5 ileus* ) OR AB ( (postoperative or post‐operative) N5 ileus* )
S26. S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25
S27. S8 AND S26
Appendix 6. ISI Web of Science
Databases=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH Timespan=All years
#1. Topic=(post‐operative ileus or post operative ileus or postoperative ileus or post operative recover* or postoperative recover* or post‐operative recover*) OR Title=(post‐operative ileus or post operative ileus or postoperative ileus or post operative recover* or postoperative recover* or post‐operative recover*)
#2. Topic=(gut motilit* or gut NEAR motilit* or auscultation or peristalsis) OR Title=(gut motilit* or gut NEAR motilit* or auscultation or peristalsis)
#3. Topic=(gastrointestinal transit* or gastrointestinal NEAR transit* or duodenal obstruction or duodenal NEAR obstruction) OR Title=(gastrointestinal transit* or gastrointestinal NEAR transit* or duodenal obstruction or duodenal NEAR obstruction)
#4. Topic=(intestinal psuedo‐obstruction or gastrointestinal motilit* or gastrintestinal NEAR motilit*) OR Title=(intestinal psuedo‐obstruction or gastrointestinal motilit* or gastrintestinal NEAR motilit*)
#5. Topic=(ileus) OR Title=(ileus)
#6. Topic=(colorect* or intestin* or colectom* or ileostom* or colonic* or gynecologic* or gynaecologic or gynecolog* or gynaecolog* or caesarean or cesarean or C‐section or cystectom* or cholecystectom* or appendectom* or colon resection or surgery)
#7. Title=(colorect* or intestin* or colectom* or ileostom* or colonic* or gynecologic* or gynaecologic or gynecolog* or gynaecolog* or caesarean or cesarean or C‐section or cystectom* or cholecystectom* or appendectom* or colon resection or surgery)
#8. #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#9. Title=(chew* gum or gum* chew* or sham* chew* or sham* feed* or vagal NEAR cholernergic NEAR stimulat* or cephalic‐vagal stimulat*)
#10. Topic=(chew* gum or gum* chew* or sham* chew* or sham* feed* or vagal NEAR cholernergic NEAR stimulat* or cephalic‐vagal stimulat*)
#11. #10 OR #9
#12. #11 AND #8
Appendix 7. Adapted Risk of Bias Tool
RANDOM SEQUENCE GENERATION
|
LOW RISK The investigators describe a random component in the sequence generation process such as:
|
|
HIGH RISK The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
|
|
UNCLEAR RISK Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’ |
ALLOCATION CONCEALMENT
|
LOW RISK Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
|
HIGH RISK Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
|
UNCLEAR RISK Insufficient information to permit judgement of ‘Yes’ or ‘No’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
BLINDING OF PARTICIPANTS AND PERSONNEL
All HIGH RISK as impossible to blind participants
BLINDING OF OUTCOME ASSESSORS
|
LOW RISK Methods of blinding of outcome assessors described sufficiently and deemed adequate |
|
HIGH RISK Any one of the following:
|
|
UNCLEAR RISK Insufficient information to permit judgement of ‘Yes’ or ‘No’ ” |
INCOMPLETE OUTCOME DATA
|
LOW RISK Any one of the following:
|
|
HIGH RISK Any one of the following:
|
|
UNCLEAR RISK Any one of the following:
|
SELECTIVE OUTCOME REPORTING
|
LOW RISK The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
|
HIGH RISK Any one of the following:
|
|
UNCLEAR RISK Insufficient information to permit judgement of ‘Yes’ or ‘No’.
|
OTHER BIAS
LOW RISK
|
|
HIGH RISK There is at least one important risk of bias. For example, the study:
Sample size:
Baseline differences: large differences in any relevant baseline variable Differences in randomisation: non‐specified differences of greater than 10% in randomisation to each arm |
|
UNCLEAR RISK There may be a risk of bias, but there is either:
Sample size: no reported sample size calculation, but sample greater than 20 participants per arm |
Appendix 8. Main results: fixed‐effect model
| Outcome or Subgroup | Studies | Participants | Effect Estimate |
| 1.1 Time to first flatus [Hours] | 77 | 8293 | ‐9.08 [‐9.32, ‐8.84] |
| 1.1.1 Colorectal surgery | 22 | 1668 | ‐12.53 [‐13.86, ‐11.20] |
| 1.1.2 Caesarean section | 14 | 2401 | ‐7.21 [‐7.71, ‐6.71] |
| 1.1.3 Other surgery | 43 | 4224 | ‐9.52 [‐9.80, ‐9.24] |
| 1.2 Time to first bowel movement [Hours] | 62 | 7283 | ‐9.16 [‐9.41, ‐8.92] |
| 1.2.1 Colorectal surgery | 20 | 1470 | ‐17.61 [‐19.36, ‐15.87] |
| 1.2.2 Caesarean section | 11 | 2336 | ‐8.41 [‐8.95, ‐7.87] |
| 1.2.3 Other surgery | 33 | 3477 | ‐9.15 [‐9.43, ‐8.87] |
| 1.3 Length of hospital stay [Days] | 50 | 5278 | ‐0.24 [‐0.27, ‐0.20] |
| 1.3.1 Colorectal surgery | 18 | 1523 | ‐0.89 [‐1.15, ‐0.64] |
| 1.3.2 Caesarean section | 6 | 1239 | ‐0.16 [‐0.20, ‐0.12] |
| 1.3.3 Other surgery | 28 | 2516 | ‐0.74 [‐0.84, ‐0.64] |
| 1.4 Time to first bowel sounds [Hours] | 23 | 3981 | ‐4.26 [‐4.47, ‐4.05] |
| 1.4.1 Colorectal surgery | 2 | 291 | ‐3.31 [‐6.85, 0.23] |
| 1.4.2 Caesarean section | 10 | 2449 | ‐5.00 [‐5.29, ‐4.71] |
| 1.4.3 Other surgery | 11 | 1241 | ‐3.40 [‐3.71, ‐3.09] |
Appendix 9. Sensitivity Analysis 1 summary table: excluding studies with two high risks of bias
| Outcome or Subgroup | Studies | Participants | Effect Estimate (mean, 95% confidence interval) |
| 1.1 Time to first flatus [Hours] | 60 | 7202 | ‐10.15 [‐11.82, ‐8.48] |
| 1.1.1 Colorectal surgery | 15 | 1148 | ‐14.12 [‐19.80, ‐8.44] |
| 1.1.2 Caesarean section | 14 | 2401 | ‐7.92 [‐10.00, ‐5.84] |
| 1.1.3 Other surgery | 32 | 3653 | ‐9.88 [‐12.29, ‐7.48] |
| 1.2 Time to first bowel movement [Hours] | 50 | 6490 | ‐12.07 [‐14.01, ‐10.13] |
| 1.2.1 Colorectal surgery | 13 | 956 | ‐19.21 [‐28.20, ‐10.23] |
| 1.2.2 Caesarean section | 11 | 2336 | ‐9.06 [‐11.38, ‐6.74] |
| 1.2.3 Other surgery | 27 | 3198 | ‐11.78 [‐14.60, ‐8.97] |
| 1.3 Length of hospital stay [Days] | 38 | 4418 | ‐0.73 [‐0.89, ‐0.56] |
| 1.3.1 Colorectal surgery | 12 | 1042 | ‐1.32 [‐2.03, ‐0.62] |
| 1.3.2 Caesarean section | 6 | 1239 | ‐0.17 [‐0.30, ‐0.05] |
| 1.3.3 Other surgery | 21 | 2137 | ‐0.89 [‐1.20, ‐0.58] |
| 1.4 Time to first bowel sounds [Hours] | 23 | 3981 | ‐5.02 [‐6.38, ‐3.67] |
| 1.4.1 Colorectal surgery | 2 | 291 | ‐3.21 [‐7.04, 0.62] |
| 1.4.2 Caesarean section | 10 | 2449 | ‐4.35 [‐5.89, ‐2.81] |
| 1.4.3 Other surgery | 11 | 1241 | ‐6.25 [‐8.70, ‐3.79] |
Appendix 10. Sensitivity analysis 2 summary table: excluding studies that do not report complications
| Outcome or Subgroup | Studies | Participants | Effect Estimate (mean, 95% confidence interval) |
| 1.1 Time to first flatus [Hours] | 60 | 6760 | ‐9.95 [‐11.22, ‐8.69] |
| 1.1.1 Colorectal surgery | 20 | 1586 | ‐13.86 [‐18.71, ‐9.01] |
| 1.1.2 Caesarean section | 9 | 1791 | ‐6.20 [‐7.70, ‐4.71] |
| 1.1.3 Other surgery | 33 | 3383 | ‐9.28 [‐10.79, ‐7.78] |
| 1.2 Time to first bowel movement [Hours] | 50 | 6323 | ‐13.07 [‐15.29, ‐10.85] |
| 1.2.1 Colorectal surgery | 18 | 1388 | ‐20.01 [‐27.63, ‐12.40] |
| 1.2.2 Caesarean section | 7 | 1959 | ‐8.37 [‐12.23, ‐4.52] |
| 1.2.3 Other surgery | 27 | 2976 | ‐11.83 [‐14.76, ‐8.89] |
| 1.3 Length of hospital stay [Days] | 45 | 4847 | ‐0.65 [‐0.82, ‐0.49] |
| 1.3.1 Colorectal surgery | 17 | 1481 | ‐1.05 [‐1.72, ‐0.39] |
| 1.3.2 Caesarean section | 6 | 1239 | ‐0.17 [‐0.30, ‐0.05] |
| 1.3.3 Other surgery | 24 | 2127 | ‐0.81 [‐1.16, ‐0.45] |
| 1.4 Time to first bowel sounds [Hours] | 17 | 3358 | ‐5.46 [‐7.18, ‐3.74] |
| 1.4.1 Colorectal surgery | 2 | 291 | ‐3.21 [‐7.04, 0.62] |
| 1.4.2 Caesarean section | 6 | 1936 | ‐3.96 [‐6.32, ‐1.59] |
| 1.4.3 Other surgery | 9 | 1131 | ‐7.44 [‐10.64, ‐4.24] |
Appendix 11. Sensitivity analysis 3 summary table: excluding all estimated results
| Outcome or Subgroup | Studies | Participants | Effect Estimate (mean, 95% confidence interval) |
| 1.1 Time to first flatus [Hours] | 60 | 6385 | ‐10.66 [‐12.33, ‐8.99] |
| 1.1.1 Colorectal surgery | 16 | 1065 | ‐14.24 [‐19.38, ‐9.09] |
| 1.1.2 Caesarean section | 11 | 1819 | ‐7.77 [‐10.10, ‐5.43] |
| 1.1.3 Other surgery | 34 | 3501 | ‐10.53 [‐12.83, ‐8.23] |
| 1.2 Time to first bowel movement [Hours] | 48 | 5437 | ‐12.49 [‐14.51, ‐10.47] |
| 1.2.1 Colorectal surgery | 14 | 824 | ‐18.09 [‐27.40, ‐8.78] |
| 1.2.2 Caesarean section | 9 | 1716 | ‐9.45 [‐12.36, ‐6.54] |
| 1.2.3 Other surgery | 26 | 2897 | ‐12.44 [‐15.25, ‐9.62] |
| 1.3 Length of hospital stay [Days] | 36 | 3711 | ‐0.81 [‐0.98, ‐0.64] |
| 1.3.1 Colorectal surgery | 13 | 827 | ‐1.10 [‐1.81, ‐0.39] |
| 1.3.2 Caesarean section | 5 | 1189 | ‐0.21 [‐0.34, ‐0.09] |
| 1.3.3 Other surgery | 19 | 1695 | ‐1.01 [‐1.32, ‐0.71] |
| 1.4 Time to first bowel sounds [Hours] | 20 | 3236 | ‐5.17 [‐6.60, ‐3.74] |
| 1.4.1 Colorectal surgery | 1 | 155 | ‐4.40 [‐8.48, ‐0.32] |
| 1.4.2 Caesarean section | 9 | 1949 | ‐4.30 [‐5.96, ‐2.65] |
| 1.4.3 Other surgery | 10 | 1132 | ‐6.36 [‐8.90, ‐3.83] |
Appendix 12. Sensitivity analysis 4 summary table: applying less conservative estimated results
| Outcome or Subgroup | Studies | Participants | Effect Estimate (mean, 95% confidence interval) |
| 1.1 Time to first flatus [Hours] | 77 | 8293 | ‐10.30 [‐11.77, ‐8.84] |
| 1.1.1 Colorectal surgery | 22 | 1668 | ‐11.98 [‐16.62, ‐7.33] |
| 1.1.2 Caesarean section | 14 | 2401 | ‐7.90 [‐9.88, ‐5.91] |
| 1.1.3 Other surgery | 43 | 4224 | ‐10.49 [‐12.53, ‐8.45] |
| 1.2 Time to first bowel movement [Hours] | 62 | 7283 | ‐12.35 [‐14.12, ‐10.58] |
| 1.2.1 Colorectal surgery | 20 | 1470 | ‐17.91 [‐25.02, ‐10.80] |
| 1.2.2 Caesarean section | 11 | 2336 | ‐9.02 [‐11.23, ‐6.81] |
| 1.2.3 Other surgery | 33 | 3477 | ‐11.70 [‐14.22, ‐9.18] |
| 1.3 Length of hospital stay [Days] | 50 | 5278 | ‐0.64 [‐0.79, ‐0.49] |
| 1.3.1 Colorectal surgery | 18 | 1523 | ‐1.01 [‐1.59, ‐0.43] |
| 1.3.2 Caesarean section | 6 | 1239 | ‐0.17 [‐0.30, ‐0.05] |
| 1.3.3 Other surgery | 28 | 2516 | ‐0.69 [‐0.97, ‐0.41] |
| 1.4 Time to first bowel sounds [Hours] | 23 | 3981 | ‐5.00 [‐6.30, ‐3.70] |
| 1.4.1 Colorectal surgery | 2 | 291 | ‐3.21 [‐7.04, 0.62] |
| 1.4.2 Caesarean section | 10 | 2449 | ‐4.36 [‐5.81, ‐2.90] |
| 1.4.3 Other surgery | 11 | 1241 | ‐6.17 [‐8.55, ‐3.78] |
Appendix 13. Sensitivity analysis 5 summary table: ERAS studies
| Outcome or Subgroup | Studies | Participants | Effect Estimate (mean, 95% confidence interval) |
| 1.1 Time to first flatus [Hours] | 4 | 591 | ‐6.24 [‐15.44, 2.95] |
| 1.1.1 Colorectal surgery | 4 | 591 | ‐6.24 [‐15.44, 2.95] |
| 1.1.2 Caesarean section | 0 | 0 | Not estimable |
| 1.1.3 Other surgery | 0 | 0 | Not estimable |
| 1.2 Time to first bowel movement [Hours] | 4 | 634 | ‐21.08 [‐33.03, ‐9.14] |
| 1.2.1 Colorectal surgery | 4 | 634 | ‐21.08 [‐33.03, ‐9.14] |
| 1.2.2 Caesarean section | 0 | 0 | Not estimable |
| 1.2.3 Other surgery | 0 | 0 | Not estimable |
| 1.3 Length of hospital stay [Days] | 4 | 724 | 0.06 [‐0.42, 0.54] |
| 1.3.1 Colorectal surgery | 4 | 724 | 0.06 [‐0.42, 0.54] |
| 1.3.2 Caesarean section | 0 | 0 | Not estimable |
| 1.3.3 Other surgery | 0 | 0 | Not estimable |
| 1.4 Time to first bowel sounds [Hours] | 1 | 136 | 0.00 [‐7.12, 7.12] |
| 1.4.1 Colorectal surgery | 1 | 136 | 0.00 [‐7.12, 7.12] |
| 1.4.2 Caesarean section | 0 | 0 | Not estimable |
| 1.4.3 Other surgery | 0 | 0 | Not estimable |
Appendix 14. Meta‐regression summary table
Summary table for meta‐regression investigating the association between surgery type and the effectiveness of the intervention (caesarean section subgroup used as the reference group):
| Outcome or Subgroup | Unstandardised Coefficient | P value | 95% Confidence Interval |
| Time to First Flatus | |||
| Colorectal | ‐4.65 h | 0.067 | ‐9.62 to 0.34 |
| Other | ‐2.53 h | 0.224 | ‐6.63 to 1.57 |
| Constant | ‐8.06 h | < 0.001 | ‐11.54 to ‐4.57 |
| Time to First Bowel Movement | |||
| Colorectal | ‐8.67 h | 0.047 | ‐17.21 to ‐0.11 |
| Other | ‐3.25 h | 0.376 | ‐10.53 to 4.03 |
| Constant | ‐9.36 h | 0.003 | ‐15.52 to ‐3.20 |
| Length of Hospital Stay | |||
| Colorectal | ‐0.86 d | 0.026 | ‐1.62 to ‐0.11 |
| Other | ‐0.66 d | 0.045 | ‐1.30 to ‐0.01 |
| Constant | ‐0.15 d | 0.591 | ‐0.71 to 0.41 |
| Time to First Bowel Sounds | |||
| Colorectal | 1.54 h | 0.611 | ‐4.68 to 7.75 |
| Other | ‐1.61 h | 0.247 | ‐4.42 to 1.21 |
| Constant | ‐4.35 h | < 0.001 | ‐6.28 to ‐2.42 |
Summary table for meta‐regression investigating the association between risk of bias score and the effectiveness of the intervention (risk of bias score zero to three subgroup used as the reference group):
| Outcome or Subgroup | Unstandardised Coefficient | P value | 95% Confidence Interval |
| Time to First Flatus | |||
| Risk of Bias score 4 to 5 | 0.88 h | 0.688 | ‐3.47 to 5.23 |
| Risk of bias score 6 to 10 | ‐4.53 h | 0.107 | ‐10.05 to 0.99 |
| Constant | ‐10.28 h | < 0.001 | ‐14.1 to ‐6.43 |
| Time to First Bowel Movement | |||
| Risk of Bias score 4 to 5 | 4.10 h | 0.288 | ‐3.55 to 11.76 |
| Risk of bias score 6 to 10 | 4.68 h | 0.360 | ‐5.45 to 14.81 |
| Constant | ‐16.61 h | < 0.001 | ‐23.33 to ‐9.88 |
| Length of Hospital Stay | |||
| Risk of Bias score 4 to 5 | ‐0.24 d | 0.406 | ‐0.82 to 0.34 |
| Risk of bias score 6 to 10 | 0.21 d | 0.595 | ‐0.58 to 1.01 |
| Constant | ‐0.64 d | 0.007 | ‐1.10 to ‐0.19 |
| Time to First Bowel Sounds | |||
| Risk of Bias score 4 to 5 | 0.32 h | 0.836 | ‐2.89 to 3.53 |
| Risk of bias score 6 to 10 | 4.54 h | 0.153 | ‐1.83 to 10.92 |
| Constant | ‐5.44 h | 0.001 | ‐8.23 to ‐2.66 |
Summary table for mutually adjusted meta‐regression investigating the association between surgery type and risk of bias score on the effectiveness of the intervention (caesarean section subgroup and risk of bias score zero to three subgroups used as the reference groups):
| Outcome or Subgroup | Unstandardised Coefficient | P value | 95% Confidence Interval |
| Time to First Flatus | |||
| Colorectal | ‐3.87 h | 0.128 | ‐8.87 to 1.13 |
| Other | ‐1.48 h | 0.489 | ‐5.71 to 2.75 |
| Risk of Bias score 4 to 5 | 0.90 h | 0.681 | ‐3.45 to 5.25 |
| Risk of bias score 6 to 10 | ‐4.13 h | 0.153 | ‐9.81 to 1.56 |
| Constant | ‐8.70 h | < 0.001 | ‐13.37 to ‐4.03 |
| Time to First Bowel Movement | |||
| Colorectal | ‐8.89 h | 0.044 | ‐17.52 to ‐0.25 |
| Other | ‐3.96 h | 0.306 | ‐11.62 to 3.70 |
| Risk of Bias score 4 to 5 | 4.17 h | 0.272 | ‐3.34 to 11.68 |
| Risk of bias score 6 to 10 | 4.99 h | 0.340 | ‐5.38 to 15.36 |
| Constant | ‐12.41 h | 0.004 | ‐20.71 to ‐4.11 |
| Length of Hospital Stay | |||
| Colorectal | ‐0.86 d | 0.035 | ‐1.67 to ‐0.06 |
| Other | ‐0.79 d | 0.026 | ‐1.47 to ‐0.10 |
| Risk of Bias score 4 to 5 | ‐0.02 d | 0.938 | ‐0.60 to 0.56 |
| Risk of bias score 6 to 10 | 0.51 d | 0.213 | ‐0.30 to 1.31 |
| Constant | ‐0.14 d | 0.629 | ‐0.73 to 0.45 |
| Time to First Bowel Sounds | |||
| Colorectal | 2.33 h | 0.465 | ‐4.23 to 8.89 |
| Other | ‐2.22 h | 0.104 | ‐4.95 to 0.50 |
| Risk of Bias score 4 to 5 | 1.05 h | 0.521 | ‐2.32 to 4.42 |
| Risk of bias score 6 to 10 | 6.51 h | 0.047 | 0.11 to 12.92 |
| Constant | ‐5.19 h | 0.003 | ‐8.43 to ‐1.95 |
Appendix 15. Compliance
| Study | Attempts to monitor or improve compliance | Reports of compliance |
| Abd‐El‐Maeboud 2009 | Compliance was monitored by counting and recording the number of sticks remaining with the participant during recording of vital data observations postoperatively | Not reported |
| Abdollahi 2013 | Not reported | All gum‐chewing participants completed their course of gum chewing until bowel function |
| Atkinson 2014 | Participants in the gum chewing arm were asked to record when, and for how long, they chewed gum, to retain wrappers and the chewed pieces of gum, and to return any unused gum. A log book was provided for recording all gum‐chewing activities. Participants were asked to follow the gum chewing protocol for five days or until discharge; therefore, all data pertaining to compliance with the intervention were collected during their hospital stay. Participants in the control group were asked at the time of discharge if they chewed gum during their inpatient stay and, if so, how often | Not reported |
| Bonventre 2014 | The experimental treatments were administered by a nurse, and receipt was recorded on a specific form | Not reported |
| Choi 2011 | To ensure compliance, the study investigator checked and counted the number of pieces of chewed gum waste | Not reported |
| Choi 2014 | To ensure compliance, an investigator checked and calculated the number of pieces of chewed gum waste | Not reported |
| Crainic 2009 | Not reported | 4 participants were excluded for refusing to suck the hard candy (placebo group) or chew the gum |
| Ertas 2013 | The administration of therapy was implemented by nursing ward staff and recorded in the patients’ files | Not reported |
| Forrester 2014 | To ensure compliance, nurses were asked to administer the gum intervention as a medication 3 times a day in the morning (10:00 AM), afternoon (2:00 PM), and evening (6:00 PM), in the hope that this would provide a convenient time for nurses to remind participants about the importance of protocol compliance. If the participant was unable or unwilling to chew gum for 1 hour at the prescribed time (e.g. the participant was asleep or off the unit for a procedure, etc.), they were told that they could chew gum at any time prior to the next scheduled gum chewing time. Nurses documented participants’ gum chewing on a standardised data collection instrument | Not reported |
| Husslein 2013 | The chewing gums were separately packed in a cellophane bag and stapled onto cardboard. A total of 10 pieces of chewing gum were stapled on each piece of cardboard. Each chewing gum was numbered. Participants stopped gum chewing after first passage of flatus and returned the cardboard. From this the number of pieces of gum chewed could be calculated | Not reported |
| Jakkaew 2013 | To promote compliance, the gum was provided to participants by ward nurses at specific times | Not reported |
| Kafali 2010 | Not reported | All gum chewing participants completed their course of gum chewing |
| Lim 2013 | The administration of the therapy was implemented by ward nursing staff and recorded on a separate “masked” record in the patient's file | Not reported |
| Lu 2010a | Not reported | In this study, the compliance of gum chewing was very high, easy to handle and almost all participants were willing to accept |
| Matros 2006 | To ensure compliance, nurses were informed of the study and administered either the gum or bracelet (placebo) to participants as a medication at the pre‐specified times. Administration and receipt of the therapy was recorded by initialing a box on the medication administration record | Not reported |
| Ngowe 2010 | A member of the surgical team gave gum as soon as the participant became conscious. After giving each dose of chewing gum, the nurse noted this on the participant’s questionnaire. The time it was given and the chewing time could therefore be checked by the medical doctor | Not reported |
| Schuster 2006 | Participants’ nurses filled out a written log to record times of gum chewing | Not reported |
| Schweizer 2010 | The leading medical assistant and supervising nurse repeated instructions | Not reported |
| Wang 2011a | The researcher distributed the gum and monitored gum chewing | Not reported |
| Yang 2011 | Not reported | Pre‐school children generally like chewing gum because it is similar to bubble gum so they were more compliant in gum chewing and parents also helped |
| Yi 2013 | Not reported | Compliance to gum chewing was good |
| Zaghiyan 2013 | On each postoperative morning on postoperative days 1 to 7, participants were interviewed by one investigator to ensure adherence to the study protocol and to assess primary and secondary outcomes | Not reported |
Data and analyses
Comparison 1. Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first flatus | 77 | 8293 | Mean Difference (IV, Random, 95% CI) | ‐10.43 [‐11.94, ‐8.92] |
| 1.1 Colorectal surgery | 22 | 1668 | Mean Difference (IV, Random, 95% CI) | ‐12.46 [‐17.17, ‐7.76] |
| 1.2 Caesarean section | 14 | 2401 | Mean Difference (IV, Random, 95% CI) | ‐7.92 [‐10.00, ‐5.84] |
| 1.3 Other surgery | 43 | 4224 | Mean Difference (IV, Random, 95% CI) | ‐10.57 [‐12.68, ‐8.47] |
| 2 Time to first bowel movement | 62 | 7282 | Mean Difference (IV, Random, 95% CI) | ‐12.66 [‐14.48, ‐10.85] |
| 2.1 Colorectal surgery | 20 | 1469 | Mean Difference (IV, Random, 95% CI) | ‐18.09 [‐25.32, ‐10.85] |
| 2.2 Caesarean section | 11 | 2336 | Mean Difference (IV, Random, 95% CI) | ‐9.06 [‐11.38, ‐6.74] |
| 2.3 Other surgery | 33 | 3477 | Mean Difference (IV, Random, 95% CI) | ‐12.27 [‐14.85, ‐9.69] |
| 3 Length of hospital stay | 50 | 5278 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.84, ‐0.53] |
| 3.1 Colorectal surgery | 18 | 1523 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.61, ‐0.41] |
| 3.2 Caesarean section | 6 | 1239 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.05] |
| 3.3 Other surgery | 28 | 2516 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.11, ‐0.51] |
| 4 Time to first bowel sounds | 23 | 3981 | Mean Difference (IV, Random, 95% CI) | ‐5.02 [‐6.38, ‐3.67] |
| 4.1 Colorectal surgery | 2 | 291 | Mean Difference (IV, Random, 95% CI) | ‐3.21 [‐7.04, 0.62] |
| 4.2 Caesarean section | 10 | 2449 | Mean Difference (IV, Random, 95% CI) | ‐4.35 [‐5.89, ‐2.81] |
| 4.3 Other surgery | 11 | 1241 | Mean Difference (IV, Random, 95% CI) | ‐6.25 [‐8.70, ‐3.79] |
| 5 Complications ‐ Nausea and Vomiting [Frequency] | Other data | No numeric data | ||
| 5.1 Colorectal surgery | Other data | No numeric data | ||
| 5.2 Caesarean section | Other data | No numeric data | ||
| 5.3 Other surgery | Other data | No numeric data | ||
| 6 Complications ‐ Mortality [Frequency] | Other data | No numeric data | ||
| 6.1 Colorectal surgery | Other data | No numeric data | ||
| 6.2 Other surgery | Other data | No numeric data | ||
| 7 Complications ‐ Infection [Frequency] | Other data | No numeric data | ||
| 7.1 Colorectal surgery | Other data | No numeric data | ||
| 7.2 Caesarean section | Other data | No numeric data | ||
| 7.3 Other surgery | Other data | No numeric data | ||
| 8 Complications ‐ Readmissions [Frequency] | Other data | No numeric data | ||
| 8.1 Colorectal surgery | Other data | No numeric data | ||
| 8.3 Other surgery | Other data | No numeric data | ||
| 9 Complications ‐ Other [Frequency] | Other data | No numeric data | ||
| 9.1 Colorectal surgery | Other data | No numeric data | ||
| 9.2 Caesarean section | Other data | No numeric data | ||
| 9.3 Other surgery | Other data | No numeric data | ||
| 10 Complications related to the intervention [Frequency] | Other data | No numeric data | ||
| 10.1 Colorectal surgery | Other data | No numeric data | ||
| 10.3 Other surgery | Other data | No numeric data | ||
| 11 Tolerability of gum | Other data | No numeric data | ||
| 11.1 Colorectal surgery | Other data | No numeric data | ||
| 11.2 Caesarean section | Other data | No numeric data | ||
| 11.3 Other surgery | Other data | No numeric data | ||
| 12 Cost | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd‐El‐Maeboud 2009.
| Methods | Randomised controlled trial Study conducted July 2006 to January 2007 |
|
| Participants | 200 participants undergoing elective caesarean section under general anaesthesia Mean age: 26.2 ± 4.1 y (intervention group), 26.4 ± 4.6 y (control group) Females |
|
| Interventions | Intervention group: chewed 1 stick of commercially available sugar‐less gum (Samarah Foods, Cairo, Egypt) for 15 min every 2 h during day time, from 2 h after surgery (performed in the early morning) until passage of flatus occurred as oral intake of clear fluids and soft foods were allowed. Same postoperative rehabilitation programme as the control group Control group: were not given anything by mouth postoperatively after caesarean section. Participants were allowed to sip small amounts of water only 12 h postoperatively |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup Study funded by the authors Study conducted in Egypt |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation plan |
| Allocation concealment (selection bias) | Unclear risk | Each enrolled participant was allocated the next available number on the concealed sequence – no information provided about concealment process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. Personnel were not blinded, as the authors state that the nature of the study did not permit blinding |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | 'The nature of this study did not allow blinding after application of the assigned intervention postoperatively' |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | 'The nature of this study did not allow blinding after application of the assigned intervention postoperatively' |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | High risk | 'The nature of this study did not allow blinding after application of the assigned intervention postoperatively' |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | High risk | 'The nature of this study did not allow blinding after application of the assigned intervention postoperatively' |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | 'The nature of this study did not allow blinding after application of the assigned intervention postoperatively' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the protocol were reported |
| Other bias | Low risk | No baseline imbalances between groups Small difference of 7% in the number of participants randomised to each group Sample size met the calculated sample size requirement |
Abdollahi 2013.
| Methods | Randomised controlled trial Study conducted in 2009 |
|
| Participants | 46 participants aged over 15 y who underwent appendectomy or cholecystectomy Male:Female 17:6 (intervention group), 17:6 (control group) |
|
| Interventions | Intervention group: chewed gum 3 times for 20 min at 4, 10 and 18 h after regaining consciousness Control group: did not receive any special treatment |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup Within each treatment arm, participants were reported in subgroups based on surgery type: appendectomy or cholecystectomy No information provided about sources of funding 2011 articles translated from Farsi Study conducted in Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Assessed using 2‐hourly interviews. Study stated as double‐blind, but participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Assessed using 2‐hourly interviews. Study stated as double‐blind, but participants cannot be adequately blinded |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Bowel sounds were monitored every 2 h with a stethoscope. Study stated as double‐blind, but no further information provided about blinding of investigators |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Akhlaghi 2008.
| Methods | Randomised controlled trial Study conducted over one year |
|
| Participants | 400 participants who underwent elective caesarean section Mean age: 27.3 y (intervention group), 26.6 y (control group) Females |
|
| Interventions | Intervention group: chewed gum (free from sugar and flavours) for 45 min at 8:00 AM, 14:00 PM, 20:00 PM immediately after regaining consciousness Control group: participants’ diet started the day after the operation if intestinal movement and gas passage had started |
|
| Outcomes | Time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup No information on when the chewing gum intervention was stopped, and if it was implemented on each postoperative day No information provided about sources of funding Article translated from Farsi Study conducted in Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | High risk | Participants reported bowel sounds. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Time to first flatus stated as an outcome in the publication but not reported. Abdominal distention and postoperative length of ileus are reported, but not pre‐specified in the publication as outcomes. Tolerability of gum reported but not pre‐specified as an outcome in the publication |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Asao 2002.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 19 participants who had elective laparoscopic colectomy for colorectal cancer Mean age and range: 58.6 ± 9.1 y (41 to 71 y) (intervention group), 60.6 ± 6.0 y (52 to 74 y) (control group) Male:Female 7:3 (intervention group), 6:3 (control group) |
|
| Interventions | Intervention group: chewed commercially available sugar‐less gum (Kanebo Foods, Tokyo, Japan) 3 times a day, from the first postoperative morning until the day participants began oral intake (oral intake began on the first morning after passage of flatus) Control group: received the same postoperative rehabilitation programme for ambulation as the intervention group, excluding gum chewing |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'colorectal surgery' subgroup This work was supported, in part, by Grant‐in‐Aid for Scientific Research (B) No. 13557095 from the Japan Society for the Promotion of Science Study conducted in Japan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | High risk | No baseline imbalances between groups No sample size calculation. Small sample size of less than 20 participants per arm |
Askarpour 2009.
| Methods | Randomised controlled trial Study conducted July 2006 to February 2007 |
|
| Participants | 97 participants randomised to 4 groups, 48 including just the gum chewing and control groups. Participants underwent uncomplicated cholecystectomy Mean age and range: 46.54 ± 7.66 y (intervention group), 46.03 ± 10.6 y (control group), 29 to 72 y (overall) Gender: 32% males, 68% females |
|
| Interventions | Intervention group: chewed gum for 30 min 3 times a day, starting from 6 h after surgery Control group: nil by mouth |
|
| Outcomes | Time to first bowel movement, length of hospital stay, time to first bowel sounds, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information on when the chewing gum intervention was stopped 2 additional groups: intervention – laxative initiated 6 h after surgery; intervention – early peroral feeding initiated 6 h after surgery with liquid and then regular diet No information provided about sources of funding Study conducted in Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | After extubation, bowel sounds were examined every 6 h by an experienced physician with a Littmann stethoscope. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Outcomes not pre‐specified. No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Atkinson 2014.
| Methods | Multicentre randomised controlled trial Study conducted October 2010 to August 2013 |
|
| Participants | 412 participants randomised, 400 analysed. Participants were scheduled to have elective colorectal resection due to colorectal neoplasia (invasive cancer or benign dysplasia), diverticular disease, or ulcerative colitis Mean age and range: 65.4 ± 14.2 y (intervention group), 66.8 ± 11.6 y (control group), 20 to 95 (overall) Male:Female 109:87 (intervention group), 115:82 (control group), 57% male and 43% female (overall) 96% white ethnicity (overall) |
|
| Interventions | Intervention: chewed gum for at least 10 min 4 times a day at times equivalent to drug dispensing rounds (approximately 6:00 to 7:00 AM, 12:00 PM, 6:00 PM and 10:00 PM) from the first postoperative morning for 5 days (or until discharge, whichever came first), plus usual care (ERAS) Control: usual care only (ERAS) |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup 5 sites located at Bristol, Nottingham, Plymouth, Torbay and Yeovil, UK Data published as an abstract and poster Further information provided by authors This study was funded by the National Institute for Health Research; the UH Bristol, as sponsor, was responsible for financial management of the study Time to first flatus, time to first bowel movement and time to first bowel sounds were only recorded for the first 5 postoperative days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation, stratified by hospital site and pathology of disease |
| Allocation concealment (selection bias) | Low risk | Full concealment of allocation using an Access database (unpublished information) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. Ward nurses were trained not to inform the surgical team about participants' treatment allocation. Participants were requested not to disclose their treatment allocation to their surgical team. Ward staff were not blinded (unpublished information) |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Low risk | Ths surgical team made the decision to discharge participants. Ward nurses were trained not to inform the surgical team about participants' treatment allocation. Participants were requested not to disclose their treatment allocation to their surgical team |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | High risk | Data collected by an unblinded study nurse (unpublished information) |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | Data collected by an unblinded study nurse (unpublished information) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 10% missing data for time to first flatus, time to first bowel movement and time to first bowel sounds |
| Selective reporting (reporting bias) | Unclear risk | Tolerability of gum and cost pre‐specified as outcomes in the protocol, but not reported. Data only presented as a conference abstract and poster; authors have explained that these outcomes will be published in the full manuscript |
| Other bias | Low risk | No baseline imbalances between groups At analysis the sample size was within 10% of the calculated sample size requirement (protocol states that 200 participants were required; 204 and 208 were randomised, and 198 and 202 intervention and control participants respectively were analysed) |
Bahena‐Aponte 2010.
| Methods | Randomised controlled trial Study conducted January 2007 to December 2008 |
|
| Participants | 32 participants who had undergone an elective open left hemicolectomy or who had an end to end anastomosis of colon to colon in 2 planes with manual suture and those who required a loop colostomy (for malignancy) Mean age: 55.6 ± 38 y (intervention group), 56.6 ± 10.6 y (control group) Male:Female 11:5 (intervention group), 9:7 (control group) |
|
| Interventions | Intervention group: chewed sugar‐free gum every 8 h for 30 min each time (without interrupting the night’s sleep), from immediately postoperatively (within 24 h) until participants tolerated oral intake Control group: received standard postoperative care, such as care of surgical wounds, early assisted mobilisation, inspiratory exercises, compression stockings for pelvic organs, gastric mucosa protection, NSAIDs, and prophylactic antibiotics (ceftriaxone and metronidazole), except the chewing gum |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about sources of funding Article translated from Spanish Study conducted in Mexico |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Every group had a daily review to find out their outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Every group had a daily review to find out their outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | High risk | No baseline imbalances between groups No sample size calculation. Small sample size of less than 20 participants per arm |
Bonventre 2014.
| Methods | Randomised controlled trial Study conducted April 2010 to December 2012 |
|
| Participants | 360 participants randomised to 5 groups, 144 including just the intervention and control group. Participants were undergoing abdominal surgery Mean age and range: 56.6 ± 18.0 y, 15 to 88 y (intervention group), 61.4 ± 20.5 y, 14 to 91 y (control group) Male:Female 30:42 (intervention group), 22:50 (control group) |
|
| Interventions | Intervention group: chewed a sugar‐free peppermint flavoured gum (ingredients included sorbitol, gum base, mannitol, glycerol, maltitol, aspartame, acesulfame potassium, softeners, and natural and artificial flavours) for 30 min 3 times a day (8:00 AM, 4:00 PM and 8:00 PM), starting from 6 h postoperatively Control group: standard therapy For each type of surgery, participants received the same postoperative care regimen, including NSAID pain control if necessary, removal of nasogastric tube, and early ambulation (first postoperative day) |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Within each treatment arm, participants were reported in subgroups based on surgery type: videolaparoscopic cholecystectomy, colorectal surgery, Hartmann procedure, gastric surgery Study subgroups were allocated to the review 'colorectal surgery' and 'other surgery' subgroups 3 additional groups (each of 30 participants) not included in this review. Interventions were:
Additional unpublished data regarding first interquartiles and ranges, and clarifying third interquartiles, were provided by authors No information provided about sources of funding Study conducted in Italy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to intervention type by a draw (unpublished information) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. In order to ensure blinding of the study's investigators and surgical team, participants were instructed during enrolment not to inform the surgeon, nurse or research team to which group they had been randomised |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were instructed to record the exact time of flatus and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were instructed to record the exact time of flatus and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Low risk | Doctors reporting outcomes were unaware to which treatment arm participants had been allocated. In order to ensure blinding of the study's investigators and surgical team, participants were instructed during enrolment not to inform the surgeon, nurse or research team to which group they had been randomised. No clinical rounds were made by the investigating team during the administration of treatment |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Low risk | Doctors reporting outcomes were unaware to which treatment arm participants had been allocated. In order to ensure blinding of the study's investigators and surgical team, participants were instructed during enrolment not to inform the surgeon, nurse or research team to which group they had been randomised. No clinical rounds were made by the investigating team during the administration of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Complications and tolerability of gum were reported but not pre‐specified as outcomes in the protocol |
| Other bias | Low risk | No baseline imbalances Calculated sample size requirement met |
Cabrera 2012.
| Methods | Randomised controlled trial Study conducted 1st September 2004 to 1st February 2005 |
|
| Participants | 34 participants who had a penetrating wound in the abdomen with gastrointestinal lesion, caused by knives and fireweapons Male:Female 28:6 |
|
| Interventions | Intervention group: chewed gum for an 1 h 3 times a day, from 6 h postoperatively Control group: no gum |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information on when the chewing gum intervention was stopped No sources of support Article translated from Spanish Study conducted in Argentina |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence was generated by order of hospital admission – participants corresponding to odd numbers were allocated to the control group, participants corresponding to even numbers were allocated to the study group |
| Allocation concealment (selection bias) | High risk | Participants were allocated alternately to each group based on hospital admission order |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Abdominal distension only partially reported |
| Other bias | High risk | No information provided about baseline imbalances between groups No sample size calculation. Small sample size of less than 20 participants per arm |
Cao 2008.
| Methods | Randomised controlled trial Study conducted March 2006 to December 2007 |
|
| Participants | 115 participants who underwent colorectal cancer resection surgery Mean age and range: 58 y (overall), 32 to 66 y (overall) Male:Female 62:53 (overall) |
|
| Interventions | Intervention group: chewed gum for 15 min 3 times a day, from 12 to 24 h postoperatively until first flatus Control group: same perioperative management as the intervention group except for chewing gum |
|
| Outcomes | Time to first flatus, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated based on date and time of admission |
| Allocation concealment (selection bias) | Unclear risk | No information No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate not mentioned, unclear if all randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Chen 2010.
| Methods | Randomised controlled trial Study conducted October 2008 to June 2009 |
|
| Participants | 130 participants who underwent gastrointestinal resection Mean age and range: 52.09 ± 9.67 y (10 to 76 y) (intervention group), 50.86 ± 8.56 y (14 to 70 y) (control group) Male:Female 36:28 (intervention group), 39:27 (control group) |
|
| Interventions | Intervention group: chewed 2 to 3 pieces of Wrigley’s gum for 15 to 20 min 3 times a day (morning, midday and at night), from the first postoperative day until passage of first flatus. Those who had a dry mouth were allowed to chew gum an additional time Control group: same perioperative management as the intervention group (early ambulation) except for chewing gum |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay | |
| Notes | Allocated to the 'other surgery' subgroup Assumption that results have been published the wrong way around, based on corresponding text No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate not mentioned, unclear if all randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Chen 2011.
| Methods | Randomised controlled trial Study conducted December 2009 to June 2010 |
|
| Participants | 178 participants who underwent common bile duct extortion surgery under epidural anaesthesia Age: 24 aged 25 to 40 y, 48 aged 40 to 60 y, 18 aged > 60 y (intervention group); 20 aged 25 to 40 y, 59 aged 40 to 60 y, 10 aged > 60 y (control group) Male: Female 40:50 (intervention group), 40:48 (control group) |
|
| Interventions | Intervention group: chewed a piece of gum for 20 min 4 times a day, from 6 h after anaesthesia had worn off until first flatus Control group: early ambulation (after 6 h postoperatively participants were instructed to turn their bodies from side to side at 2 h intervals and exercise their limbs at 2 h intervals for 5 to 10 min. On the second postoperative day, participants got out of bed and moved about with assistance) |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Chen 2012.
| Methods | Randomised controlled trial Study conducted October 2011 to January 2012 |
|
| Participants | 80 participants who had gastric resection Age range: 36 to 64 y (overall) Male:Female 57:23 |
|
| Interventions | Intervention group: chewed gum for 15 min 3 times a day, from the first postoperative day until postoperative exhaust; also the same care as the control group Control group: usual postoperative care, including fasting until postoperative exhaust, turning around in bed every 2 h after vital signs are stabilised, moving limbs in bed under staff instructions for 5 min 3 times a day, and starting to practise how to get off the bed 48 h postoperatively |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Choi 2011.
| Methods | Randomised controlled trial Study conducted July 2007 to September 2009 |
|
| Participants | 62 participants randomised, 60 included who underwent radical cystectomy with pelvic lymphadenectomy for muscle invasive or high risk uncontrolled superficial bladder cancer Mean age: 63.5 ± 4.5 y (intervention group), 64.5 ± 8.8 y (control group) |
|
| Interventions | Intervention group: participants chewed sugar‐free gum for 30 min 3 times daily at 10:00 AM, 3:00 PM and 8:00 PM until passage of flatus and diet was advanced per judgment of the surgical team Control group: same evidence‐based protocol of perioperative management, except for chewing gum |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup Subgroups reported by surgery type (open and robot‐assisted) No information provided about sources of funding Study conducted in Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomised by an investigator 'under no restrictions by chewing gum in the nature of the alternative randomisation sequence considering the sample size in each group' |
| Allocation concealment (selection bias) | High risk | Alternative randomisation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. During the study, participants were instructed not to tell the surgical team member to which group they had been enrolled. The primary surgical team did not make clinical rounds around specified treatment times of 10:00 AM, 3:00 PM and 8:00 PM |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | To accurately monitor the recovery of bowel function, all participants were instructed to notify the nurses or study investigator when a bowel related event occurred. Immediately after they passed either gas or a bowel movement, the outcomes were recorded by the nurses or study investigator and counted to the nearest whole hour. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | To accurately monitor the recovery of bowel function, all participants were instructed to notify the nurses or study investigator when a bowel related event occurred. Immediately after they passed either gas or a bowel movement, the outcomes were recorded by the nurses or study investigator and counted to the nearest whole hour. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | The primary surgical team did not make clinical rounds around specified treatment times of 10:00 AM, 3:00 PM and 8:00 PM. The study investigator did not participate in the clinical rounds. Participants were instructed not to inform surgical team members of their treatment allocation. Unclear if the same investigator that checked gum also recorded outcomes |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | The primary surgical team did not make clinical rounds around specified treatment times of 10:00 AM, 3:00 PM and 8:00 PM. The study investigator did not participate in the clinical rounds. Participants were instructed not to inform surgical team members of their treatment allocation. Unclear if the same investigator that checked gum also recorded outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data |
| Selective reporting (reporting bias) | High risk | Tolerance of gum stated in the publication as an outcome, but not reported. No protocol available |
| Other bias | Low risk | No baseline imbalances between groups At analysis the sample size was within 10% of the calculated sample size requirement (64 participants were needed, 62 were randomised and 60 analysed) |
Choi 2014.
| Methods | Randomised controlled trial Study conducted January 2010 to February 2012 |
|
| Participants | 40 participants randomised, 37 included who had radical retropubic prostatectomy for localised prostate cancer Mean age: 66.3 ± 8.5 y (intervention group), 65.3 ± 5.2 y (control group) Males |
|
| Interventions | Intervention group: participants chewed sugar‐free gum for 30 min 3 times daily at 10:00 AM, 3:00 PM and 8:00 PM until passage of flatus and diet was advanced per judgment of the surgical team Control group: no information provided |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information on when the chewing gum intervention started This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011‐0020128) Study conducted in Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomised by an investigator |
| Allocation concealment (selection bias) | High risk | Alternative randomisation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. Participants were kept in a concealed status. During the study, participants were instructed not to tell the surgical team member to which group they had been enrolled. The primary surgical team did not make clinical rounds during the specified treatment times of 10:00 AM, 3:00 PM and 8:00 PM |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | To monitor bowel recovery, all participants were instructed to inform the study investigator or the nurses about their status. Flatus or a bowel movement was recorded instantly as an outcome, and counted to the nearest hour. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | To monitor bowel recovery, all participants were instructed to inform the study investigator or the nurses about their status. Flatus or a bowel movement was recorded instantly as an outcome, and counted to the nearest hour. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | The primary surgical team did not make clinical rounds around specified treatment times of 10:00 AM, 3:00 PM and 8:00 PM. The study investigator did not participate in the clinical rounds. Participants were instructed not to inform surgical team members of their treatment allocation. Unclear if same investigator that checked gum also recorded outcomes |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | The primary surgical team did not make clinical rounds around specified treatment times of 10:00 AM, 3:00 PM and 8:00 PM. The study investigator did not participate in the clinical rounds. Participants were instructed not to inform surgical team members of their treatment allocation. Unclear if same investigator that checked gum also recorded outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data |
| Selective reporting (reporting bias) | High risk | Tolerance of gum and specific complications (e.g. symptomatic infectious colitis) stated as outcomes in the publication, but not reported. No protocol available |
| Other bias | High risk | No baseline imbalances between groups No results presented for sample size calculation. Small study as less than 20 per arm |
Chou 2006.
| Methods | Randomised controlled trial Study conducted January to December 2005 |
|
| Participants | 26 participants undergoing D2 subtotal gastrectomy Mean age: 50.14 ± 9.96 y (intervention group), 51.95 ± 9.91 y (control group) Male:Female 7:6 (intervention group), 8:5 (control group |
|
| Interventions | Intervention group: chewed commercially available sugar‐free gum for 5 min 4 times a day (9:00 AM, 12:00 AM, 5:00 PM, 9:00 PM) from the first postoperative day until first passage of stool Control group: no gum chewing |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum, cost | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Study conducted in Taiwan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sequential randomised card‐pull design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Cost reported but not pre‐specified as an outcome measure in the publication |
| Other bias | High risk | No baseline imbalances between groups No sample size calculation. Small study as less than 20 participants per arm |
Chuamor 2014.
| Methods | Randomised controlled trial Study conducted July 2010 to June 2011 |
|
| Participants | 128 participants randomised who underwent abdominal surgery for benign gynaecological diseases Mean age: 43.5 ± 7.1 y (intervention group), 43.7 ± 9.3 y (control group) Females |
|
| Interventions | Intervention group: chewed gum for 15 min 3 times a day for 3 days, plus standard postoperative care Control group: standard postoperative care |
|
| Outcomes | Time to first flatus, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup No information on when the chewing gum intervention started or stopped No information provided about sources of funding Study conducted in Thailand |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation was by simple randomisation. Experiment codes were produced using a computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Codes were individually contained in sealed opaque envelopes, which were sequentially numbered then chronologically opened after identification of an eligible individual |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | The participants’ postoperative progress was assessed by an independent investigator (investigator A) who was blinded to the assigned treatment. Investigator A also provided the gum, so unlikely to be adequately blinded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | The participants’ postoperative progress was assessed by an independent investigator (investigator A) who was blinded to the assigned treatment. The number of bowel movements was assessed at 12 and 24 h postoperatively and at 2:00 PM for 3 days. Investigator A also provided the gum, so unlikely to be adequately blinded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | High risk | The participants’ postoperative progress was assessed by an independent investigator (investigator A) who was blinded to the assigned treatment. Investigator A also provided the gum, so unlikely to be adequately blinded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | The participants’ postoperative progress was assessed by an independent investigator (investigator A) who was blinded to the assigned treatment. Investigator A also provided the gum, so unlikely to be adequately blinded. Participants are unable to be adequately blinded with an intervention of this nature |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate not mentioned, unclear if all randomised participants analysed |
| Selective reporting (reporting bias) | High risk | Number of bowel movements at 12 and 24 h postoperatively, and at 2:00 PM for 3 days was stated as assessed, but no results are presented |
| Other bias | High risk | Significant difference in blood loss between groups (P = 0.011) Sample size met calculated sample size requirement |
Crainic 2009.
| Methods | Randomised controlled trial Study conducted over 14 months |
|
| Participants | 97 enrolled, 66 included randomised to 3 groups, 44 including just the intervention and control group Participants underwent colectomy Mean age, SEM and range: 58.7 ± 1.8 y, 22 to 85 y (all 3 groups) Gender: 40% males, 60% females (all 3 groups) |
|
| Interventions | Intervention group: chewed 1 stick of sugar‐less gum (Extra Sugarless Gum, Wrigley Jr. Company, Chicago, IL) for 30 min 3 times a day, from within 24 h until first bowel movement Control group: not given any gastrointestinal stimulant |
|
| Outcomes | Time to first flatus, time to first bowel movement, tolerability of gum | |
| Notes | Allocated to the 'colorectal surgery' subgroup Additional group of 22 participants not included in this review – intervention: sucking on hard candy until dissolved 3 times a day until first bowel movement Subgroups also reported for open and laparoscopic surgery types No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Every 24 h an investigator asked participants if the passage of flatus or bowel movement had occurred within the last day. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Every 24 h an investigator asked participants if the passage of flatus or bowel movement had occurred within the last day. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Greater than 10% missing data, as there was an attrition rate of 31 of 97 the randomised participants due to various reasons |
| Selective reporting (reporting bias) | High risk | Length of hospital stay stated as an outcome, but reported incompletely. Tolerability of gum reported but not pre‐specified as an outcome in the publication |
| Other bias | Low risk | No information about baseline imbalances Number of participants remaining after exclusions exactly met number required from sample size calculation |
Ertas 2013.
| Methods | Randomised controlled trial Study conducted 21st January 2012 to 20th April 2013 |
|
| Participants | 152 participants randomised, 149 included who were preparing for complete surgical staging for malignant gynecologic disease such as endometrial cancer, cervix cancer and ovarian cancer Mean age: 52.7 ± 11.2 y (intervention group), 55.4 ± 10.1 y (control group) Female |
|
| Interventions | Intervention group: chewed sugar‐free peppermint‐flavoured chewing gum for 30 min 3 times a day, from the first postoperative day until return of bowel function Control group: same evidence‐based protocol of perioperative management for both groups, all participants received the same postoperative care regimen |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup Additional unpublished data regarding specific statistical tests used for each variable were provided by the authors (not reported in this review) Study conducted in Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code using the blocked randomisation method |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. Personnel were not blinded as the authors state that the nature of the study did not permit complete blinding |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | To be able to precisely monitor the recovery of bowel function, participants were instructed to notify ward nurses or investigators immediately after the first passage of flatus or a bowel movement and defaecation. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | To be able to precisely monitor the recovery of bowel function, participants were instructed to notify ward nurses or investigators immediately after the first passage of flatus or a bowel movement and defaecation. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | An outcome assessor who was blinded to study allocation evaluated symptoms and signs of ileus. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | An outcome assessor who was blinded to study allocation evaluated symptoms and signs of ileus. Blinding of staff not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data. Less than 10% difference in dropout between groups |
| Selective reporting (reporting bias) | High risk | Did not report gastrointestinal disturbance as stated in the protocol (nausea, abdominal cramping, abdominal distension, vomiting). Reported ileus symptoms and tolerability of gum, which were not stated as outcomes in the protocol |
| Other bias | Low risk | No baseline imbalances between groups Sample size met the calculated sample size requirement |
Fan 2009.
| Methods | Randomised controlled trial Study conducted October 2008 to April 2009 |
|
| Participants | 42 participants who had radical resection (open surgery) for bowel cancer. Cancer was diagnosed using endoscopic biopsy, chest x‐ray, ultrasound of the abdomen (colour Doppler) or CT scan (no distant metastasis) Mean age: 47.6 ± 16.5 y (intervention group), 49.7 ± 13.2 y (control group) Male:Female 14:7 (intervention group), 13:8 (control group) |
|
| Interventions | Intervention group: asked to chew 1 piece of xylitol sugar‐less gum for 30 min in the morning, midday and at night, from the first postoperative day until they were asked to stop fasting (food was introduced after recovery of gut function). Each piece of chewing gum weighed about 1.5 g Control group: same perioperative management except for chewing gum |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate not mentioned, unclear if all randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Forrester 2014.
| Methods | Multicentre randomised controlled trial No information provided about duration of study |
|
| Participants | 47 participants randomised to 3 groups, 31 including just the intervention and control group. Participants underwent open or laparoscopic sigmoid colectomy Mean age: 55.8 y (intervention group), 63.3 y (control group) Gender: 25.4% males, 84.6% females (intervention group), 38.9% males, 61.1% females (control group) |
|
| Interventions | Intervention group: standard postoperative care and participants chewed 1 to 4 sticks of sugar‐less gum (Orbit brand sugar‐free gum in a flavour of their choice) for at least 1 h at least 3 times a day in the morning (10:00 AM), afternoon (2:00 PM), and evening (6:00 PM) from the first postoperative morning or after removal of the nasogastric tube. The number of sticks of gum each participant chewed was determined by participant preference (i.e. if the gum lost its flavour, the participant might have chosen to refresh with a replacement stick of gum). If the participant was unable or unwilling to chew gum for 1 h at the prescribed time (e.g. the participant was asleep or off the unit for a procedure, etc.), they were told that they could chew gum at any time prior to the next scheduled gum chewing time Control group: standard postoperative care, including removal of the nasogastric tube and early ambulation. Diets consisted of nothing by mouth with ice chips only until the first passage of flatus. After flatus, diet was advanced at the discretion of the surgical team |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about location of sites No information provided on when the gum chewing intervention stopped Additional group of 16 participants not included in this review – intervention: an attention control (silicone‐adhesive patch applied to the deltoid region of the upper arm). Administered as a medication 3 times a day in the morning (10:00 AM), afternoon (2:00 PM), and evening (6:00 PM) This study was supported by a grant from the Center for Clinical Investigation of the Wound Ostomy and Continence Nurses Society, and published in the Journal of Wound Ostomy & Continence Nursing Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on randomisation process |
| Allocation concealment (selection bias) | Low risk | Sequential randomised card‐pull design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | All participants in all study groups were instructed to immediately report to their nurse: first flatus, first bowel movement, and return of appetite (self‐report of hunger). All study data were recorded by nurses’ data on a standardised data collection instrument designed specifically for our study. Participants and nurses completed a log that documented the following: times of gum chewing (treatment group only), application of attention control intervention patch (control group only) time of first flatus, first bowel movement, and return of appetite (self‐report of hunger) and tolerance of first solid food in days and h. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | All participants in all study groups were instructed to immediately report to their nurse: first flatus, first bowel movement, and return of appetite (self‐report of hunger). All study data were recorded by nurses’ data on a standardised data collection instrument designed specifically for our study. Participants and nurses completed a log that documented the following: times of gum chewing (treatment group only), application of attention control intervention patch (control group only) time of first flatus, first bowel movement, and return of appetite (self‐report of hunger) and tolerance of first solid food in days and hours. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Length of hospital stay was recorded. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Operative and postoperative data were recorded. Blinding of staff not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate of greater than 10% of participants due to use of alvimopan. More than 10% missing data for bowel movement for remaining participants |
| Selective reporting (reporting bias) | High risk | Complications pre‐specified in the publication as an outcome measure, but no details provided nor information on which groups these occurred in |
| Other bias | High risk | No baseline imbalances between groups ‘The inclusion of 90 participants in our study would have guaranteed sufficient statistical power analysis to test the hypothesis and make inferences regarding the generalisability of study findings.’ – only included 47 participants in total, does not state how many participants needed for a 2‐arm trial. At analysis the sample size was more than 10% below the calculated sample size requirement |
Garshasbi 2011.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 500 participants who underwent caesarean section Females |
|
| Interventions | Intervention group: chewed gum for at least half an hour 3 times a day, from straight after surgery until regular diet was initiated Control group: no information provided |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup Study published as an abstract No information provided about sources of funding Study conducted in Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Unclear who reported bowel sounds |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No information provided about a sample size calculation, reasonable sample size as at least 20 participants per arm |
Ghafouri 2008.
| Methods | Randomised controlled trial Study conducted 2006 to 2007 |
|
| Participants | 50 participants undergoing elective upper gastrointestinal surgery Mean age and range: 62.6 ± 14.6 y (intervention group), 60.5 ± 14.8 y (control group); 61.68 ± 14.45 y (overall) , 25 to 104 y (overall) Gender: 66% males, 34% females |
|
| Interventions | Intervention group: chewed sugar‐free gum (Orbit) for 1 h 3 times a day, from the first postoperative morning until participants were allowed to take solid food. Similar postoperative care to the control group Control group: standard care (including chest physiotherapy and early mobilisation). Nil by mouth until passage of first flatus, then the nasogastric tube was removed and participants were fed with liquids if tolerated. Participants were allowed solid food following first defaecation |
|
| Outcomes | Time to first flatus, time to first bowel movement, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Farsi Study conducted in Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Recorded by nurses blind to participants' arm allocation. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Recorded by nurses blind to participants' arm allocation. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Complications stated in the publication as assessed, but not reported. Tolerabilty of gum reported but not pre‐specified in the publication as an outcome measure |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Gong 2011.
| Methods | Randomised controlled trial Study conducted June 2009 to November 2010 |
|
| Participants | 120 participants undergoing gastrointestinal surgery Mean age: 52.32 y (intervention group), 54.21 y (control group) Male:Female 38:22 (intervention group), 36:44 (control group) |
|
| Interventions | Intervention group: chewed gum for 20 min 3 times a day (early, middle and late), after recovery from anaesthesia until flatus and bloating had disappeared Control group: routine postoperative care (including standing up and early activities) |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Guangqing 2011.
| Methods | Randomised controlled trial Study conducted October 2008 to December 2009 |
|
| Participants | 160 participants who had minimally invasive gynaecological surgery Mean age and range: 34.74 ± 6.90 y (21 to 46 y) (intervention group), 34.26 ± 8.33 y (23 to 48 y) (control group) Females |
|
| Interventions | Intervention group: chewed 2 slices of sugar‐free chewing gum for 10 to 15 min, 4 times a day. If participants felt thirsty or had a dry mouth, they chewed gum once more. Participants also had normal care combined with early function training. Control group: normal care combined with early function training. Specific methods were used like lying flat without pillow postoperatively until return of steady blood pressure, then moving to a semi‐reclined position. Family members or nurses could help participants turn their bodies once every 2 h. Medical staff instructed participants about upper and lower limb joints exercises like stretching, flexing and rotating inwards and outwards for 3 min 3 times a day. Within 24 to 48 h postoperatively, participants should have been assisted to sit up and practise getting off the bed. After 48 h postoperatively, participants should have increased their exercise levels and tried to complete daily tasks themselves |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were observed. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were observed. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Participants were observed. Blinding of staff not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Han 2011.
| Methods | Randomised controlled trial Study conducted August to October 2010 |
|
| Participants | 300 participants randomised, 291 participants included who had elective uterine fibroid surgery Mean age: 36.42 ± 6.18 y (intervention group), 37.25 ± 7.16 y (control group) Female |
|
| Interventions | Intervention group: chewed mint‐flavoured xylitol gum for 15 min at 3 h intervals during the daytime only, from 4 h after surgery until first flatus. Participants started drinking water 12 h postoperatively. Liquid food was provided after first bowel sounds, and soft food after first flatus. Early ambulation was also encouraged Control group: same perioperative management except for gum chewing |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque and sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. The surgeons who performed the surgery were blinded |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were asked to self‐record time to first bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were asked to self‐record time to first bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | A blinded specialist nurse recorded observations. No further information on blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | A blinded specialist nurse checked every hour for bowel sounds. No further information on blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | A nurse asked participants every hour if they had complications. Participants are unable to be adequately blinded with an intervention of this nature |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data. Less than 10% difference in dropout between groups |
| Selective reporting (reporting bias) | High risk | Gum tolerability stated as recorded, but not reported. No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Hirayama 2006.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 24 participants who underwent elective open surgery for colorectal cancer Mean age: 55.6 ± 12.0 y (intervention group), 60.6 ± 15.2 y (control group) Male:Female 5:5 (intervention group), 8:6 (control group) |
|
| Interventions | Intervention group: chewed commercial sugar‐less gum (Kanabe FOODS, Tokyo; contained 32.3% xylitol as a sweetener, no glucose, fructose, sucrose, nor lipids; each piece contained 37 kcal and weighed 3.1 g) for 30 min 3 times a day during each meal time, from the first postoperative morning Control group: no gum intake per os. Had the same medical care for all participants apart from gum serving. Similar preoperative protocols for all participants |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information on when the chewing gum intervention was stopped This study was supported in part by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Sicence and Technology of Japan Study conducted in Japan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Precisely recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Precisely recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on dropouts or withdrawals. Assumed to include 24 participants as stated in the Methods, but Abstract states 22 participants |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | High risk | No baseline imbalances between groups No sample size calculation. Small study as less than 20 participants per arm. Difference of 17% in number of participants randomised to each group |
Huang 2012a.
| Methods | Randomised controlled trial Study conducted May 2010 to April 2011 |
|
| Participants | 60 participants randomised to 3 groups, 40 including just the intervention and control group. Participants were undergoing gastrointestinal surgery under general anaesthesia Mean age and range: 65.24 ± 3.21 y, 60 to 73 y (overall) Male:Female 38:22 |
|
| Interventions | Intervention group: chewed 2 pieces of gum for 15 to 20 min 3 times a day, from 8 to 12 h after surgery until first flatus Control group: standard care |
|
| Outcomes | Time to first flatus, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup Additional group of 20 participants not included in this review – intervention: early rehabilitation comprising exercises for early ambulation (e.g. stretches, lying on the side, getting out of bed, walking) and sphincter exercises No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Observations recorded. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Huang 2012b.
| Methods | Randomised controlled trial Study conducted March 2009 to March 2010 |
|
| Participants | 60 participants who had laparoscopic appendectomy Mean age: 28.10 ± 5.37 y (intervention group), 28.20 ± 4.61 y (control group) Male:Female 27:3 (intervention group), 28:2 (control group) |
|
| Interventions | Intervention group: chewed Wrigley’s doublemint gum for 15 to 20 min 3 times a day, from 1 h after surgery until bowel exhaustion Control group: started early exercise in bed after the anaesthetic has passed. They flipped over every 2 h. When healthy enough they would get off the bed to exercise to promote intestinal peristalsis. Other than that, the care management in the 2 groups were the same |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Complications partially reported, but not pre‐specified as an outcome. No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Husslein 2013.
| Methods | Randomised controlled trial Study conducted July 2011 to August 2012 |
|
| Participants | 180 participants randomised, 179 included who were undergoing laparoscopic surgery for benign gynaecologic conditions under general anaesthesia Median age and range: 40 y, 21 to 75 (intervention group), 42 y, 19 to 74 y (control group) Female |
|
| Interventions | Intervention group: started chewing a commercially available sugar‐less gum for 15 min every 2 h, from 2 h postoperatively until passage of first flatus. Same diet progression as the control group Control group: standard care and did not chew gum. Participants could start oral intake of fluids, soft and solid foods when bowel sounds were first notices (at earliest 6 h postoperatively) |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Study conducted in Austria |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation plan using 1:1 randomisation |
| Allocation concealment (selection bias) | Unclear risk | Participants were allocated the next available number in the concealed sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants informed nursing staff when outcomes occurred. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants informed nursing staff when outcomes occurred. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Low risk | Observer blinding was achieved as a result of partition of gum chewing (starting 2 h postoperatively, every 2 h) and examination times (starting 3 h postoperatively, every 2 h). Participants and nursing staff were educated to keep the group allocation secret. The cardboard including the chewing gum was at all times hidden from the research team by being placed in the participants' personal bedside locker |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Low risk | 2 members of the research team checked for bowel sounds on each participant using a standard stethoscope every 2 h beginning 3 h postoperatively until first bowel sounds were noticed. Observer blinding was achieved as a result of partition of gum chewing (every 2 h, starting 2 h postoperatively) and examination times (every 2 h, starting 3 h postoperatively). Participants and nursing staff were educated to keep the group allocation secret. The cardboard including the chewing gum was at all times hidden from the research team by being placed in the participants' personal bedside locker |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | The complication reported was dry mouth. Participants are unable to be adequately blinded with an intervention of this nature |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data |
| Selective reporting (reporting bias) | High risk | Bowel sounds reported, but not pre‐specified as an outcome measure in the protocol |
| Other bias | Low risk | No baseline imbalances between groups Sample size met the calculated sample size requirement Difference of 5% in number of participants randomised to each group |
Jakkaew 2013.
| Methods | Randomised controlled trial Study conducted September 2010 to December 2010 |
|
| Participants | 50 participants undergoing caesarean section Mean age: 29.48 ± 5.91 y (intervention group), 31.20 ± 6.33 y (control group) Female |
|
| Interventions | Intervention group: same feeding protocol as controls. Participants chewed 2 tablets of artificial fresh mint‐flavoured sugar‐less gum (Lotte Xylitol, Thai Lotte Co., Ltd., Chonburi, Thailand) for 30 min 4 times a day (morning, noon, evening, and before bed time) from regaining consciousness and normal vital signs until the first passage of flatus. For those who were allowed to receive diet but had not had first passage of flatus, they were asked to continue gum chewing for 30 min before each meal and at bed time until the first passage of flatus Control group: participants were fed according to conventional feeding protocol without gum chewing. According to the conventional feeding protocol, participants were not given anything by mouth after surgery until at least 2 of the following signs of bowel function recovery, the presence of bowel sound, the feeling of hunger, and the passage of flatus or defaecation, were evidenced. Then sips of water were allowed. Subsequently, the feeding schedule proceeded to liquid diet for the next meal. Soft diet was given on the next day given good tolerance to the liquid diet. Once the passage of flatus occurred, diet was advanced to regular diet |
|
| Outcomes | Time to first flatus, length of hospital stay, complications | |
| Notes | Allocated to the 'caesarean section' subgroup Study funded by the Faculty of Medicine, Chiang Mai University and the National Research University Project under Thailand’s Office of the Higher Education Commission Study conducted in Thailand |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by computer‐based program. Randomisation was stratified according to type of anaesthesia (regional and general) |
| Allocation concealment (selection bias) | Low risk | Central telephone assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were instructed to notify ward nurses or investigators immediately after first passage of flatus and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were instructed to notify ward nurses or investigators immediately after first passage of flatus and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | No information on blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | The symptoms and signs of gastrointestinal disturbance were evaluated daily by the outcome assessor who was blinded to the study allocation. No further information on blinding of staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results using an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Pneumonia, would infection and lung atelectasis pre‐specified in the publication as outcomes but not reported |
| Other bias | Low risk | No baseline imbalances between groups Sample size met the calculated sample size requirement |
Jernigan 2014.
| Methods | Randomised controlled trial Study conducted 1st December 2010 to 29th February 2012 |
|
| Participants | 109 participants undergoing gynaecologic surgery via an exploratory laparotomy Mean age and range: 42.8 ± 8.7 y (intervention group), 42.1 ± 10.6 y (control group), 17 to 76 y (overall) Females |
|
| Interventions | Intervention group: asked to chew Wrigley’s Sugar‐free Extra Spearmint gum (William Wrigley Jr Company, Peoria, IL, USA) for 15 min every 4 h whilst awake (vital signs were checked every 4 h, which participants were asked to use as a prompt) Control group: routine care |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup Additional information provided through author correspondence No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (1:1) using a random number generator (http://stattrek.com/Tables/Random.aspx), although 1:1 randomisation not achieved due to early halting of the study |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes (unpublished information) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. Participants and providers were not masked to group assignment |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Individuals reviewing charts were blinded. No further detail provided for blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | Complications reported by participants and staff. Participants are unable to be adequately blinded with an intervention of this nature |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 10% missing data for time to first flatus and time to first bowel movement |
| Selective reporting (reporting bias) | High risk | Time to first bowel movement reported but not pre‐specified in protocol |
| Other bias | High risk | Baseline differences in BMI, ethnicity and use of epidural (results for postoperative ileus adjusted for difference in epidural anaesthesia) At analysis the sample size was more than 10% below the calculated sample size requirement, as the study was halted early following an interim analyses demonstrating a significant decrease in postoperative ileus in the intervention group, and because low response rates indicated that meeting the assigned numbers for the primary outcome (time to first flatus) would not be feasible. Requirement was 63 participants in each group, with a goal of 132 participants recruited. 109 participants were recruited. |
Jin 2010.
| Methods | Randomised controlled trial Study conducted January to October 2008 |
|
| Participants | 88 participants undergoing kidney resection | |
| Interventions | Intervention group: chewed 1 piece of xylitol gum for 10 min 4 times a day, from 2 h after recovery from general anaesthesia until resumption of normal diet Control group: normal postoperative management |
|
| Outcomes | Time to first flatus, complications | |
| Notes | Allocated to the 'other surgery' subgroup Only part of this publication could be sourced No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Stated as ‘randomly divided’, but also that participants were allocated according to their operation time |
| Allocation concealment (selection bias) | High risk | Stated as ‘randomly divided’, but also that participants were allocated according to their operation time |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Kafali 2010.
| Methods | Randomised controlled trial Study conducted 1st November 2007 to 30th September 2008 |
|
| Participants | 157 participants randomised, 150 included who underwent caesarean section Mean age: 29.3 ± 3.8 y (intervention group), 29.2 ± 4.8 y (control group) Females |
|
| Interventions | Intervention group: chewed 1 stick of sugar‐less gum for 15 min the initial time, then for 1 h 3 times a day, starting 2 h postoperatively. Same early oral hydration and ambulation protocols as the control group Control group: oral fluids initiated within 6 h after surgery (irrespective of bowel sounds), participants were encouraged to increase oral intake to ensure a minimum of 500 ml intake within the first 24 h. Solid food was allowed after 24 h on detection of bowel sounds. In participants without bowel sounds, solid oral feeds were postponed until bowel sounds. Both groups received 3 litres of intravenous fluid 12 h postoperatively |
|
| Outcomes | Time to first flatus, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup No information on when the chewing gum intervention was stopped No information provided about sources of funding Study conducted in Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential randomised card‐pull design |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Outcomes were recorded following examination by the participants' assistant at specific times. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Outcomes were recorded following examination by the participants' assistant at specific times. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Outcomes were recorded following examination by the participants' assistant at specific times. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data, less than 10% difference in dropouts between groups |
| Selective reporting (reporting bias) | High risk | Bowel movement pre‐specified in Methods as an outcome but no results presented. Results presented for bowel sounds but not pre‐specified as an outcome. Tolerability of gum reported but not pre‐specified as an outcome in the publication |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Ledari 2012.
| Methods | Randomised controlled trial Study conducted June 2010 to March 2011 |
|
| Participants | 110 participants randomised, 100 included who were scheduled for caesarean section with local anaesthesia (spinal) Mean age: 27.9 ± 6.4 y (intervention group), 28.5 ± 6.2 y (control group) Female |
|
| Interventions | Intervention group: chewed sugar‐free gum (commercially available sugar‐free gum ‐ Wrigley Company, Poland) for at least 1 h 3 times daily from 6 h postoperatively (after recovery of anaesthesia) until being discharged Control group: the postoperative feeding regime was standardised for all the women |
|
| Outcomes | Time to first flatus, time to first bowel movement, time to first bowel sounds, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup No information provided about sources of funding Study conducted in Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence from a statistics program |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were regularly followed up until discharge, and recorded time to first bowel sounds, flatus, feeling of hunger and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were regularly followed up until discharge, and recorded time to first bowel sounds, flatus, feeling of hunger and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | High risk | Participants were regularly followed up until discharge, and recorded time to first bowel sounds, flatus, feeling of hunger and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Documented. Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate of 10 of 110 randomised participants due to the surgeon's decision. Unclear to which group these participants were initially randomised |
| Selective reporting (reporting bias) | High risk | Complications stated as an outcome in the publication but not reported |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Lee 2004.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 64 participants undergoing gynaecologic abdominal laparotomy | |
| Interventions | Intervention group: chewed gum 3 times a day, from the first postoperative morning until passage of flatus Control group: no information provided |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup Study published as an abstract No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals or missing data not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Li 2007a.
| Methods | Randomised controlled trial Study April 2005 to April 2006 |
|
| Participants | 120 individuals undergoing gastrointestinal surgery Mean age and range: 56 y, 38 to 75 y (intervention group), 59 y, 41 to 75 (control group) Male:Female 33:27 (intervention group), 35:25 (control group) |
|
| Interventions | Intervention group: chewed xylitol gum 3 times a day (morning, afternoon and at night) for 15 min each time, after moistening mouths and lips, from 24 h postoperatively until passage of flatus. Participants were allowed to chew gum an additional time if they experienced dry mouth Control group: same perioperative management as intervention group except chewing gum. Participants were provided cotton balls soaked in saline solution to maintain oral hygiene (same duration and frequency as intervention group) |
|
| Outcomes | Complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated based on hospital bed number |
| Allocation concealment (selection bias) | High risk | Sequence generated based on hospital bed number |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | All outcomes pre‐specified in the publication were reported. No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Li 2012a.
| Methods | Randomised controlled trial Study conducted April 2009 to April 2012 |
|
| Participants | 156 participants undergoing abdominal surgery Mean age and range: 49.6 ± 7.3 y, 15 to 72 y (overall) Male:Female 95:61 (overall) |
|
| Interventions | Intervention group: chewed sugar‐free gum for 15 to 20 min 3 times a day (early, middle and late), from 1 h after awakening from anaesthesia until resumption of diet. Also usual care management and early recovery training Control group: participants started doing the usual recovery routine training (such as early exercises) when they returned to the wards from theatre. After vital signs were stable, participants turned over every 2 h and carried out a suitable amount of limb exercise once to twice a day. If participants were well enough they would walk slowly for 5 min. On the second day participants exercised off the bed |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Unclear who reported time to first bowel sounds |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Outcomes not pre‐specified. No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Li 2012b.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 73 participants undergoing surgery for colon cancer Mean age and range: 54.3 ± 7.62 y, 41 to 72 y (intervention group), 56.2 ± 8.97 y, 43 to 76 y (control group) Male:Female 21:16 (intervention group), 18:13 (control group) |
|
| Interventions | Intervention group: chewed 2 to 3 pieces of xylitol sugar‐free gum for 15 to 20 min each time from 8 h after surgery until bowel exhaustion Control group: same postoperative care as intervention group (regular postoperative care, gastrointestinal decompression, no food or water until recovery of gastrointestinal function, after which diet would be provided) |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information on how many times a day participants chewed gum No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All outcomes pre‐specified in the publication reported. No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Liang 2007.
| Methods | Randomised controlled trial Study conducted January to June 2006 |
|
| Participants | 120 participants undergoing caesarean section Age range: 21 to 38 y (overall) Females |
|
| Interventions | Intervention group: chewed xylitol sugar‐less gum for 15 min at 2 h intervals, up to 3 times from immediately after surgery Control group: no information provided |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup No information on whether gum was chewed daily, and at what point the intervention was stopped No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Stated that bowel sounds were listened for, but no results presented |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Lim 2013.
| Methods | Randomised controlled trial Study conducted June 2008 to March 2011 |
|
| Participants | 168 participants randomised, 157 included who were undergoing colorectal resectional surgery for any indication Mean age and range: 63 y, 19 to 83 y (intervention group), 62 y, 32 to 88 y (control group) Male:Female 47:30 (intervention group), 48:32 (control group) |
|
| Interventions | Intervention group: chewed sorbitol‐free gum for 15 min 4 times a day at 8:00 AM, 12:00 PM, 4:00 PM, and 8:00 postoperatively. Also the established ERAS programme applied to the control group too Control group: cared for using an established ERAS programme including avoidance of mechanical bowel preparation for all resections not involving defunctioning stomas, preoperative immunonutrition (Impact, Nestle, Australia), no nasogastric tubes, avoidance of urinary catheters for most colectomies, with early removal for anterior resection, avoidance of drains, preoperative and intraoperative warming (Bair Hugger, AugustineMedical, Eden Prairie, MN), high flow oxygen for at least 6 h postoperatively, early mobilisation, and early commencement of diet |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information on when the chewing gum intervention started or was stopped An additional arm for upper gastrointestinal surgery had been planned to produce 3 overall groups for the study: open colorectal surgery, laparoscopic colorectal surgery and upper gastrointestinal surgery. This third group was cancelled due to problems with surgical equipoise and recruitment. This did not affect the sample size calculation Subgroups reported within each study group for laparoscopic and open surgery Additional numerical data for LOHS provided by authors No financial support was taken for this project (internally or externally) from any organisation or institution Study conducted in Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, in blocks of 10, without stratification |
| Allocation concealment (selection bias) | Unclear risk | Concealment was performed by using numbered opaque envelopes, kept at a central location, and opened sequentially, at the commencement of surgery. Unclear if sealed. Randomisation, opening of envelopes, and allocation were all performed by a third party not involved with clinical care or follow‐up |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. The ward nurses were not able to be blinded. All other clinicians and investigators were blinded. This was achieved by providing a concealed universal trial chart in the participants’ bed notes, allowing ward nurses to know which participants to administer gum to, while preventing access to treating surgeons and other clinicians |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants completed a questionnaire. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants completed a questionnaire. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Low risk | An independent investigator collected data at discharge. Nurses were aware of which participants to dispense gum to through use of a chart which was concealed in participants' notes, preventing other medical staff from identifying to which group participants had been allocated. Participants were taught to conceal to which arm they had been allocated, by not chewing gum in the presence of clinicians. Participants were also provided with containers in which to dispose of gum |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Low risk | An independent investigator collected data at discharge. Nurses were aware of which participants to dispense gum to through use of a chart which was concealed in participants' notes, preventing other medical staff from identifying to which group participants had been allocated. Participants were taught to conceal to which arm they had been allocated, by not chewing gum in the presence of clinicians. Participants were also provided with containers in which to dispose of gum |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis described as intention‐to‐treat, but only 157 of the 168 participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Time to flatus reported but not pre‐specified as an outcome in the protocol. Wound dehiscence and prolonged ileus pre‐specified in the protocol as outcomes but no results presented. Tolerability of gum reported but not pre‐specified in the protocol |
| Other bias | Low risk | No baseline imbalances At analysis the sample size was within 10% of the calculated sample size requirement (80 were required in each arm. 83 and 85 were randomised, and 77 and 80 were analysed in the intervention and control groups respectively) |
Lu 2010a.
| Methods | Randomised controlled trial Study conducted March 2000 to May 2009 |
|
| Participants | 60 participants randomised to 3 groups, 40 including just the intervention and control group. Participants had been admitted for bladder “transitional cell” cancer. All participants had been examined by cystoscope and diagnosed that their tumour stage was T2 Mean age: 65.8 y (intervention group), 64.3 y (control group) Male:Female 17:3 (intervention group), 18:2 (control group) |
|
| Interventions | Intervention group: participants were required to chew sugar‐free xylitol‐containing chewing gum for 0.5 h, 3 times a day. Same standardised postoperative treatment programme as the control group Control group: received the usual postoperative treatment with no exception. Postoperatively participants were given venous proton pump inhibitors or H2 antagonists to prevent ulcer formation. If bowel sound was present after surgery, nasogastric tubes could be removed. Fluid diet could be given to participants who were able to pass wind postoperatively. Normal diet could be resumed when participants were able to pass normal stools, and they can be discharged from the hospital |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information about when the chewing gum intervention started or was stopped Additional group of 20 participants not included in this review – intervention: participants were asked to massage their stomach Assumed that results have been published the wrong way around, based on accompanying text No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Recorded each day by a doctor. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Recorded each day by a doctor. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | High risk | Wound condition stated as an outcome in the publication but not reported |
| Other bias | Unclear risk | No baseline imbalances between groups No information provided about a sample size calculation |
Lu 2010b.
| Methods | Randomised controlled trial Study conducted June 2009 to May 2010 |
|
| Participants | 97 participants who underwent caesarean section Mean age range: 20 to 35 y (overall) Females |
|
| Interventions | Intervention group: chewed 1 to 2 pieces of gum for 30 to 40 min at 2 h intervals, from 2 h postoperatively until first flatus. Participants had the same perioperative care as the control group. After chewing gum, participants were provided with traditional Chinese medicinal food (containing radish (daikon), astralagus, tangerine peel, lean pork, chicken essence and salt) Control group: participants were given intravenous fluid, anti‐infective drugs if needed, and were observed for uterine contractions. Participants lay flat on the bed without a pillow for 6 h. After first flatus, participants were provided with semi‐solid food, and gradually introduced to solid food. After 12 h postoperatively, participants were asked to change to a reclining position |
|
| Outcomes | Time to first flatus, time to first bowel movement | |
| Notes | Allocated to the 'caesarean section' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Outcomes not pre‐specified in publication |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Lu 2011.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 109 participants who underwent laparoscopic gynaecological surgery Female |
|
| Interventions | Intervention group: gum chewing group Control group: non‐gum chewing group |
|
| Outcomes | Time to first flatus, length of hospital stay, time to first bowel sounds, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Study published as an abstract Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not reported |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No information provided about baseline imbalances between groups No information provided about a sample size calculation. Reasonable sample size as at least 20 participants per arm |
Luo 2010.
| Methods | Randomised controlled trial Study conducted January to November 2009 |
|
| Participants | 300 participants undergoing caesarean section Mean age: 26.3 ± 3.2 y (intervention group), 27.5 ± 3.6 y (control group) Females |
|
| Interventions | Intervention group: chewed 2 to 4 pieces of sugar‐less gum for 10 to 15 min 4 times a day, from 2 h after surgery until first flatus Control group: standard care. Participants were asked to fast for 6 h after surgery. Semi‐solid food was introduced after that but participants were not allowed to have sweet food and milk. Normal feeding was introduced after first flatus |
|
| Outcomes | Time to first flatus, time to first bowel movement, time to first bowel sounds, complications | |
| Notes | Allocated to the 'caesarean section' subgroup Study funded by the ShenZhen City, Luohu District Science and Technology Grant [2008] 37 Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Observations recorded. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Marwah 2012.
| Methods | Randomised controlled trial Study conducted May 2006 to December 2009 |
|
| Participants | 100 participants undergoing relaparotomy for elective small intestinal anastomosis for the closure of a stoma made earlier Mean age and range: 36.90 ± 15.97y , 10 to 75 y (intervention group), 39.94 ± 15.75 y, 16 to 70 y (control group) Male:Female 32:18 (intervention group), 36:14 (control group) |
|
| Interventions | Intervention group: chewed commercially available sugar‐free gum (Orbit) for 1 h 3 times a day, from 6 h after surgery until passage of flatus. Control group: kept nil orally in the postoperative period until passage of flatus. For both groups, nasogastric tubes were removed after passage of flatus and oral allowed thereafter |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Study conducted in India |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants drew slips |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | All cases were monitored and recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | All cases were monitored and recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | All cases were monitored and recorded. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | All cases were monitored and recorded. Blinding of staff not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Results for frequency of nausea/vomiting were reported as a separate outcome from complications, but not pre‐specified in the publication. Abdominal distension was stated as recorded, but no results were presented. Tolerability of gum reported but not pre‐specified in the publication |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Matros 2006.
| Methods | Randomised controlled trial Study conducted April 2003 to June 2004 |
|
| Participants | 66 participants randomised to 3 groups, 43 including just the intervention and control group. Participants were undergoing elective partial colectomy Mean age: 62 ± 14 y (intervention group), 58 ± 15 y (control group) Gender: 36% males, 64% females (intervention group), 57% males, 43% females (control group) Ethnicity: Caucasian 95%, non‐Caucasian 5% (intervention group), Caucasian 90%, non‐Caucasian 10% (control group) |
|
| Interventions | Intervention group: chewed sugar‐free peppermint‐flavoured gum (ingredients included sorbitol, gum base, glycerol, mannitol, natural and artificial flavours, maltitol, aspartame, softeners, acesulfame potassium gum) for 45 min 3 times daily at 9:00 AM, 4:00 PM and 8:00 PM until passage of flatus. Received the same postoperative care regimen as the control group. Control group: received epidural analgesia when not contraindicated, removal of the nasogastric tube on the first postoperative morning, and early ambulation. Diet consisted of sips of water up to 30 ml per hour for participants assigned to standard of care and the placebo and active therapy arms until first passage of flatus. After flatus, diet was advanced at the discretion of the surgical team |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about on when the chewing gum intervention started Additional group of 23 participants not included in this review – intervention: acupressure wrist bracelet, worn at the same times as when gum was chewed by the intervention group Unpublished data in the form of means and standard deviations were provided by the authors No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. Participants were stratified according to type of colectomy performed (low anterior resection, hemicolectomy, abdominoperineal resection, end colostomy reversal, segmental resection) |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was carried out at the pharmacy, unclear if adequate concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. To ensure blinding of the surgical team, participants were instructed during enrolment not to inform the surgeon and surgical team to which group they were randomised. In addition, the primary surgical team did not make clinical rounds during the specified treatment times of 9:00 AM, 4:00 PM, and 8:00 PM. To conceal the bracelet or gum, participants stored these items inside the bedside drawer when not in use |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were instructed to notify nurses or investigators as soon as flatus or bowel movement was passed. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were instructed to notify nurses or investigators as soon as flatus or bowel movement was passed. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Low risk | A blinded study nurse or investigator recorded outcomes daily to the nearest hour. Participants were taught not to reveal to the surgeon, surgical team, or research nurse to which arm they had been allocated. Clinical rounds were not made at the times of treatment (9:00 AM, 4:00 PM and 8:00 PM). Participants in the intervention and placebo arms stored the gum and bracelet in a bedside drawer |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Low risk | A blinded study nurse or investigator recorded outcomes daily to the nearest hour. Participants were taught not to reveal to the surgeon, surgical team, or research nurse to which arm they had been allocated. Clinical rounds were not made at the times of treatment (9:00 AM, 4:00 PM and 8:00 PM). Participants in the intervention and placebo arms stored the gum and bracelet in a bedside drawer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results only included 49 of 66 participants across the 3 study groups for bowel movement ‐ not stated which groups these participants belonged to, greater than 10% missing data (participants were not required to have a bowel movement before discharge) |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Low risk | No baseline imbalances At analysis the sample size was within 10% of the calculated sample size requirement (22 participants were required in each arm. 22 and 21 participants were randomised to the intervention and control arms respectively) |
McCormick 2005.
| Methods | Multicentre randomised controlled trial No information provided about duration of study |
|
| Participants | 102 participants undergoing elective colon resection (unpublished information) Mean age: 61 ± 14 y (in both groups) (unpublished information) |
|
| Interventions | Intervention group: chewed 1 stick of gum for 15 min 4 times a day Control group: sips of clear liquid |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'colorectal surgery' subgroup 3 sites located at the University of Texas Southwestern Medical Center, Dallas; Western Pennsylvania Hospital, Pittsburgh; and Presbyterian Hospital, Dallas Results presented as laparoscopic and open surgery subgroups as well as overall intervention and control groups Study published as an abstract Published abstract presents data for only 88 participants Additional press release, unpublished presentation and table of results provided by authors No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation (unpublished information) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results (unpublished information) |
| Selective reporting (reporting bias) | High risk | Data for time to first bowel sounds, nausea and vomiting presented graphically ‐ no numerical data provided (unpublished information) |
| Other bias | High risk | No baseline differences (unpublished information) No sample size calculation. Reasonable sample size as at least 20 participants per arm. Difference of 35% in number of participants randomised to each group |
Ngowe 2010.
| Methods | Randomised controlled trial Study started in January 2006 |
|
| Participants | 46 participants undergoing open appendectomy Mean age: 42.4 ± 8.6 y (intervention group), 43.7 ± 10.0 y (control group) Male:Female 13:10 in each group |
|
| Interventions | Intervention group: chewed sugar‐less chewing gum for 30 min 3 times a day (morning, afternoon and evening), from as soon as participants regained consciousness until bowel function resumed. Same postoperative feeding regime as control group Control group: feeding started after passage of first flatus, beginning with fluids on the first day, followed the next day by a semi‐fluid diet to reach the normal diet on the third day |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Study conducted in Cameroon |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Simple randomisation by allocating the first participant to the intervention group and the next to the control group, and repeating for the whole sample |
| Allocation concealment (selection bias) | High risk | Alternate allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Postoperative findings were recorded every day on the participant's questionnaire. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Postoperative findings were recorded every day on the participant's questionnaire. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | Postoperative findings were recorded every day on the participant's questionnaire. Participants are unable to be adequately blinded with an intervention of this nature |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | Tolerability of gum not pre‐specified in publication ‐ unclear if this affects risk of bias |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Park 2009.
| Methods | Randomised controlled trial Study conducted April to December 2007 |
|
| Participants | 20 participants who underwent abdominal surgery Mean age and range: 59.7 ± 11.1 y, 35 to 75 y (intervention group), 52.0 ± 10.5 y, 37 to 70 y (control group) Male:Female 6:4 (intervention group), 5:5 (control group) |
|
| Interventions | Intervention group: chewed a piece of chewing gum (commonly available xylitol chewing gum of which ingredients include xylitol, gum base and artificial flavour) for 30 min 3 times daily, from the first day after the surgery until they started their first oral intake of food Control group: no information provided |
|
| Outcomes | Time to first flatus, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Korean Study conducted in Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Accurately recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Stated as 62 individuals screened and 38 refused. This should leave 24, but only 20 were randomised and analysed – 4 participants unaccounted for, greater than 10% missing data |
| Selective reporting (reporting bias) | High risk | Length of hospital stay and tolerability of gum reported, but not pre‐specified as outcomes in the publication |
| Other bias | High risk | Stated that there are no baseline imbalances, but the calculated P value for difference in age between groups is 0.02 No sample size calculation. Small study as less than 20 participants per arm |
Pilehvarzadeh 2014.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 50 participants who underwent cholecystectomy Mean age: 56.6 ± 13.9 y (intervention group), 56.2 ± 15.7 y (control group) Male:Female 12:12 (intervention group), 14:12 (control group) |
|
| Interventions | Intervention group: chewed sugar‐free gum (Wrigley, Orbit) 3 times a day for 20 min each time, between recovery and the onset of oral feeding Control group: similar nursing and care as the intervention group |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Farsi Study conducted in Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Every 2 h a blinded trained nurse recorded passage of flatus and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Every 2 h a blinded trained nurse recorded passage of flatus and bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | No further information on blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Every 2 h bowel sounds were recorded by a blinded general practitioner. No further information on blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate not mentioned, unclear if all randomised participants were analysed |
| Selective reporting (reporting bias) | High risk | Length of hospital stay not pre‐specified in the publication |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Qiao 2011.
| Methods | Randomised controlled trial Study conducted September 2009 to November 2010 |
|
| Participants | 40 participants who had gastric cancer surgery, liver cancer surgery or spleen resection surgery | |
| Interventions | Intervention group: chewed xylitol gum for 10 min 3 times a day, from the first postoperative day until they stopped fasting (after bowel exhaustion). Same care method as control group Control group: after participants’ vital signs had stabilised, they would turn over and exercise their limbs every 2 h. From the second postoperative day, participants could get off their bed to exercise. They fasted until bowel exhaustion |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observed. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observed. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Observed. Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | High risk | Outcomes reported incompletely in the publication. No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups. No sample size calculation Reasonable sample size as at least 20 participants per arm |
Qiu 2006.
| Methods | Randomised controlled trial Study conducted February 2005 to March 2006 |
|
| Participants | 128 participants undergoing gynaecological surgery Average age and range: 52.09 y, 30 to 76 y (intervention group); 50.86 y, 24 to 70 y (control group) Females |
|
| Interventions | Intervention group: chewed 5 to 10 pieces of Wrigleys doublemint per day, from 1 h postoperatively until flatulence Control group: no information provided |
|
| Outcomes | Time to first flatus, time to first bowel movement, time to first bowel sounds, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Abdominal pain and complications reported, but not pre‐specified as outcomes |
| Other bias | Unclear risk | No baseline imbalances between groups. No sample size calculation Reasonable sample size as at least 20 participants per arm |
Quah 2006.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 38 participants undergoing elective resection for left‐sided colorectal cancer Mean age: 67 ± 9.7 y (intervention group), 68 ± 10.1 y (control group) Male:Female 13:6 (intervention group), 12:7 (control group) |
|
| Interventions | Intervention group: chewed gum (commercially available sugar‐free gum (Wrigley, Plymouth, UK)) for at least 5 min 3 times daily, from the first postoperative morning until the oral intake of a solid diet. Same postoperative feeding regime as control group Control group: 30 to 60 ml of water per day was allowed from the first postoperative day until the first passage of flatus. On passing flatus, fluids as tolerated were allowed. Participants were allowed to progress to a solid diet after the passage of faeces |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about sources of funding Study conducted in the UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelope randomisation was performed by a computer‐generated code using the blocked randomisation method |
| Allocation concealment (selection bias) | Low risk | Consecutive opening of sequentially numbered, opaque,sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants' postoperative progress was assessed by a blinded independent specialist colorectal nurse practitioner. Participants are unable to be adequately blinded with an intervention of this nature. Authors report that for participants with a stoma, first passage of flatus or formed liquid stools into the stoma bag was recorded (this may therefore have been reported by staff rather than participants). Outcome assessment still deemed as high risk, as 45% of participants did not have a stoma |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants' postoperative progress was assessed by a blinded independent specialist colorectal nurse practitioner. Participants are unable to be adequately blinded with an intervention of this nature. Authors report that for participants with a stoma, first passage of flatus or formed liquid stools into the stoma bag was recorded (this may therefore have been reported by staff rather than participants). Outcome assessment still deemed as high risk, as 45% of participants did not have a stoma |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Length of hospital stay was documented. No further information on blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Participants' postoperative progress was assessed by a blinded independent specialist colorectal nurse practitioner. No further information on blinding of staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Abdominal pain and complications reported, but not pre‐specified as outcomes |
| Other bias | Unclear risk | No baseline imbalances between groups. No sample size calculation Reasonable sample size as at least 20 participants per arm |
Rashad 2013.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 60 participants who had caesarean section Females |
|
| Interventions | Intervention group: chewed 1 stick of sugar‐less gum for 30 min 3 times a day, from as soon as they were awake and had returned to the ward from the operating theatre Control group: followed the postoperative routine |
|
| Outcomes | Time to first flatus, time to first bowel movement, time to first bowel sounds | |
| Notes | Allocated to the 'caesarean section' subgroup No information on when the chewing gum intervention was stopped No information provided about sources of funding Study conducted in Saudi Arabia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were instructed to report passage of flatus or bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were instructed to report passage of flatus or bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Participants were examined with a stethoscope every 4 h. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | High risk | Baseline imbalance between groups' operative time No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Ray 2008.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 106 participants who underwent laparotomy for benign or malignant gynaecological disease | |
| Interventions | Intervention group: chewed sugar‐less gum for 30 min 3 times a day (even with a nasogastric tube), from the first postoperative day. Same diet advancement as the control group Control group: traditional management. Clear liquids on postoperative day 1 with diets advanced as tolerated. Participants requiring nasogastric tube placement immediately postoperatively were allowed nothing by mouth until the tube was removed, with similar diet advancement |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup Study published as an abstract No information about when the chewing gum intervention was stopped No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No information provided about baseline imbalances between groups No information provided about a sample size calculation. Reasonable sample size as at least 20 participants per arm |
Ren 2010.
| Methods | Randomised controlled trial Study conducted January to December 2012 |
|
| Participants | 200 participants randomised, 166 were included who underwent laparoscopic cholecystectomy Age range: 18 to 65 y (overall) |
|
| Interventions | Intervention group: chewed sugar‐less gum for 30 min at breakfast, lunch and dinner from the first postoperative day until passage of flatus. Also same perioperative management as intervention group Control group: standard care |
|
| Outcomes | Time to first flatus | |
| Notes | Allocated to the 'other surgery' subgroup Study published twice Study funded by the Wuxi Bureau of Health Foundation (Grant no. MX0805) Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 10% missing data |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Safdari‐Dehcheshmehi 2011.
| Methods | Randomised controlled trial Study conducted March to September 2007 |
|
| Participants | 120 participants undergoing elective caesarean section Mean age and range: 25.63 ± 4.53 y, 17 to 38 y Females |
|
| Interventions | Intervention group: chewed sugar‐free gum (manufactured by Saghez sazi Kurdestan, Iran) for 15 min 4 times daily, for 1 day as soon as they recovered from anaesthesia Control group: received routine postoperative dietary regimen and were fed with sweet liquid 12 h postoperatively |
|
| Outcomes | Time to first flatus, time to first bowel movement, time to first bowel sounds, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup Additional group of 60 participants not included in this review – intervention: early oral feeding – participants were fed with fruit juice 4 h postoperatively; if participants tolerated a liquid diet, they were placed on soft diet and then regular food Outcomes reported included both time to first bowel movement and time to first defaecation; definitions are not provided. Values for time to first defaecation have been used in this review, as values for time to first bowel movement occur before the reported values for time to first flatus No information provided about sources of funding Article translated from Farsi Study conducted in Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants and companions were taught to record the time of first flatus and bowel movement on a check list. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants and companions were taught to record the time of first flatus and bowel movement on a check list. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | A blinded nurse research assistant listened for bowel sounds. No further information on blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Nausea and vomiting stated as outcomes in the protocol, but not reported. Gum tolerance reported but not pre‐specified as an outcome in the protocol |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Satij 2006.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 32 participants who underwent caesarean section Mean age: 27.1 ± 6.5 y (intervention group), 28.4 ± 6.0 y (control group) (unpublished information) Females |
|
| Interventions | Intervention group: chewed gum 3 times a day, from as soon as they had recovered from anaesthesia until passage of flatus or defaecation Control group: no information provided |
|
| Outcomes | Time to first flatus, complications, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup Study published as an abstract Additional unpublished information in the form of presentation slides provided by authors Time to return of bowel function is reported, which was considered to be time to first flatus or defaecation. Results for this outcome therefore cannot be included in the meta‐analyses of this review No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation (unpublished information) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results (unpublished information) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No baseline imbalances between groups (unpublished information) No sample size calculation. Small study as less than 20 participants per arm |
Schluender 2005.
| Methods | Randomised controlled trial Study conducted January to October 2003 |
|
| Participants | 29 participants randomised, 28 were included who had elective colon resection Male:Female 12:16 |
|
| Interventions | Intervention group: chewed sugar‐less gum for at least half an hour 3 times a day, from postoperative day 1 throughout their hospital stay Control group: no information provided |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup Study published as a poster abstract Results presented in subgroups of open and laparoscopic surgery Methods explain that pain management included morphine participant controlled anaesthesia, feeding was surgeon dependant and bowel movements were not a prerequisite for discharge. Assumed that this relates to both groups No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. The operating surgeon was blinded to allocation |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Operating surgeon blinded to treatment arm allocation. Unclear who reported length of hospital stay. No further information on blinding of staff |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No information provided about a sample size calculation |
Schuster 2006.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 34 participants who were scheduled for elective sigmoid colon resection for recurrent diverticular disease or cancer Mean age: 60 ± 61 y (intervention group), 63 ± 8.5 y (control group) Male:Female 11:6 (intervention group), 12:5 (control group) |
|
| Interventions | Intervention group: chewed sugar‐less gum (1 stick) 3 times daily in the morning, afternoon, and evening, from the first postoperative morning until bowel function. Same mobilisation and postoperative pain control as control group Control group: mobilisation began on the first postoperative day. Participants had either postop epidural analgesia or subcutaneous local anaesthetic infusion pumps with patient‐controlled analgesia with morphine sulphate. Type of postoperative analgesia was chosen by the attending surgeons’ practice |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on randomisation process |
| Allocation concealment (selection bias) | Low risk | Sequential randomised card‐pull design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Nurses completed a written log. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Nurses completed a written log. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Postoperative findings were recorded. Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Tolerability of gum reported but not pre‐specified ‐ unclear if this affects risk of bias |
| Other bias | High risk | No baseline imbalances between groups No sample size calculation. Small study as less than 20 participants per arm |
Schweizer 2010.
| Methods | Randomised controlled trial Study conducted January 2007 to December 2008 |
|
| Participants | 105 participants undergoing abdominal surgery Mean age: 59.8 ± 15.2 y (intervention group), 64 ± 13 y (control group), 62 ± 14.2 y (overall) (unpublished information) Male:Female 27:23 (intervention group), 25:30 (control group) (unpublished information) |
|
| Interventions | Intervention group: chewed at least 3 portions per day of peppermint flavoured Cadbury sugar‐free gum for 15 min. Identical treatment in terms of food to the control group (unpublished information) Control group: diet was determined by spontaneous mentioning of appetence by the participant, presence of bowel sounds, passage of flatus and bowel movements (unpublished information) |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Subgroup analyses performed based on operation type (cholecystectomy, stomach/small intestine, colon, prostatectomy) Study subgroups were allocated to the review 'colorectal surgery' and 'other surgery' subgroups Study published as an abstract No numerical results provided in published abstract Unpublished information provided by the authors in the form of 2 student abstracts and 1 student thesis. Student thesis and 1 abstract translated from German No information provided about sources of funding Study conducted in Switzerland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Appears that participants were allocated to the control group due to denture use or if they did not want to chew gum (unpublished information) |
| Allocation concealment (selection bias) | High risk | Participants who refused to be in the test group could be in the control group. The control group included 12 participants who could not be in the test group due to denture/partial denture use, and 6 participants who refused gum |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were questioned (unpublished information). Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were questioned (unpublished information). Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results (unpublished information) |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication and unpublished material reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Shang 2010.
| Methods | Randomised controlled trial Study conducted February to May 2008 |
|
| Participants | 388 participants were randomised, 386 included. Participants were undergoing caesarean delivery under spinal anaesthesia Mean age and range: 29.4 ± 5.4 y (intervention group), 29.9 ± 6.4 y (control); 19 to 44 y (overall) Female |
|
| Interventions | Intervention group: chewed sugar‐free peppermint‐flavoured gum for at least half an hour, 3 times a day from immediately after returning to the ward from the operating theatre, until passage of first stool Control group: kept nil‐by‐mouth from immediately after returning to the ward from the operating theatre, until passage of flatus |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup No information provided about sources of funding Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelope randomisation was performed by a computer‐generated code using the blocked randomisation method |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. The obstetricians involved in intraoperative care were blinded to assignment. Participants were taught not to reveal to the surgeon, surgical team, nurse or investigators to which arm they had been randomised. Participants kept gum in the bedside drawer to conceal it |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants either told nurses or investigators, or wrote down on a piece of paper, when they passed flatus or a bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants either told nurses or investigators, or wrote down on a piece of paper, when they passed flatus or a bowel movement. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Low risk | Participants were taught not to reveal to the surgeon, surgical team, nurse or investigators to which arm they had been randomised. Participants kept gum in the bedside drawer to conceal it |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Low risk | Investigators checked for bowel sounds 5 times daily. Participants were taught not to reveal to the surgeon, surgical team, nurse or investigators to which arm they had been randomised. Participants kept gum in the bedside drawer to conceal it |
| Blinding of outcome assessment (detection bias) ‐ complications | Low risk | Outcomes were recorded daily in a blinded fashion by Investigator C. Participants were taught not to reveal to the surgeon, surgical team, nurse or investigators to which arm they had been randomised. Participants kept gum in the bedside drawer to conceal it |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups Sample size does not meet calculated sample size requirement of 6192 – unclear if this calculation is incorrect |
Sun 2005.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 348 participants who underwent abdominal surgery Male:Female 75:95 (intervention group), 74:100 (control group) |
|
| Interventions | Intervention group: chewed 1 to 2 pieces of sugar‐less gum for 5 to 10 min at 3 h intervals, from when anaesthesia had worn off until first flatus/bowel movement. Also standard care Control group: standard care (participants lay flat on the bed without a pillow after surgery until blood pressure had stabilised. They were then instructed to turn their body at 2 h intervals, and perform upper and lower joint exercises for 3 min 3 times a day. 24 to 48 h postoperatively, participants were encouraged to sit up and get out of bed. Participants were asked to increase ambulation gradually after 48 h postoperatively) |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Outcomes not pre‐specified |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Tan 2011.
| Methods | Randomised controlled trial Study conducted January 2010 to May 2011 |
|
| Participants | 120 participants who underwent gynaecological surgery Age range: 18 to 54 y (overall) Females |
|
| Interventions | Intervention group: chewed 2 to 3 pieces of gum for 5 to 10 min at 2 h intervals, after surgery until first flatus/bowel movement Control group: standard perioperative care; early ambulation |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Terzioglu 2013.
| Methods | Randomised controlled trial Study conducted 15th April to 18th October 2011 |
|
| Participants | 240 participants randomised to 8 groups, 60 including just the intervention and control group. Participants were undergoing abdominal gynaecological surgery for benign disorders under general anaesthesia Age: 25 (83.3%) aged ≤ 50 y and 5 (16.7%) aged > 50 y (intervention group), 19 (63.3%) aged ≤ 50 y and 11 (36.7%) aged > 50 y (control group) Females |
|
| Interventions | Intervention group: chewed sugar‐less gum for 15 to 20 min once in every 2 h after the operation. Intervention ceased between 12:00 AM and 8:00 AM Control group: no chewing gum, no early oral hydration, no early mobilisation. Participants were mobilised in the first 8 h and given 3000 ml intravenous fluid in the first 24 h. Oral liquids were started after passage of flatus |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about when the chewing gum intervention started and stopped 6 additional groups (each of 30 participants) not included in this review – intervention: combinations of chewing gum, early oral hydration (participants were allowed to drink 45 to 50 ml water between the 2 and 4 hours postoperatively; subsequently, 100 ml water was allowed every hour. Liquid was given freely once bowel sounds were heard and gas discharged) and early mobilisation (participants were mobilised 4 hours postoperatively after sitting for a period of 10 min in bed to prevent hypotension; participants were told to walk 5 to 10m once every 2 h at times when they felt able) No information provided about sources of funding Study conducted in Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Data were collected through data collection and participant inspection forms. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Data were collected through data collection and participant inspection forms. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ reported as a baseline characteristic in a categorical fashion, but not as an outcome measure |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Data were collected through data collection and participant inspection forms. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Tian 2013.
| Methods | Randomised controlled trial Study conducted May 2010 to November 2011 |
|
| Participants | 100 participants who underwent sphincter‐preserving surgery Mean age and range: 52.09 ± 9.67 y, 38 to 76 y (intervention group); 53.86 ± 8.56 y, 41 to 78 y (control group) Male:Female 29:21 (intervention group), 27:23 (control group) |
|
| Interventions | Intervention group: chewed 2 to 3 pieces of ‘Extra’ sugar‐less gum for 15 to 20 min 4 to 5 times per day, from 2 to 4 h after surgery until first flatus or first bowel movement Control group: standard care (fasting, gastrointestinal decompression, oral rehydration solution, antibiotics, sufficient energy intake) |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Wang 2008.
| Methods | Randomised controlled trial Study conducted January 2006 to September 2007 |
|
| Participants | 230 participants who underwent laparoscopic cholecystectomy Male:Female 100:15 (intervention group), 102:13 (control group) |
|
| Interventions | Intervention group: chewed 1 piece of gum for 5 to 10 min at 4 h intervals, from 1 h after the anaesthesia had worn off until first flatus. Participants gargled tepid water to moisten lips and mouth before chewing gum Control group: early ambulation – participants were asked to lie on their side every 1 to 2 h. At 6 h postoperatively, participants were instructed to exercise their limbs every 2 h (for 5 to 10 min) and walk for 5 min with assistance. On postoperative day 2 participants were encouraged to get out of bed and walk without assistance |
|
| Outcomes | Time to first flatus, time to first bowel movement, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Wang 2009a.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 100 participants who underwent emergency abdominal surgery Age range: 12 to 68 y (intervention group), 10 to 64 y (control group) Male:Female 20:30 (intervention group), 18:32 (control group) |
|
| Interventions | Intervention group: chewed ‘Wrigley gum for 10 to 15 min 3 times a day, from 8 h after surgery until first flatus Control group: standard care |
|
| Outcomes | Time to first flatus | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Outcomes not pre‐specified |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Wang 2011a.
| Methods | Randomised controlled trial Study conducted January to July 2010 |
|
| Participants | 160 participants randomised, 155 were included who had surgical treatment for rectal cancer Mean age: 55.63 ± 13.24 y (intervention group), 52.59 ± 11.32 y (control group) Male:Female 52:26 (intervention group), 49:28 (control group) |
|
| Interventions | Intervention group: chewed gum for 15 min every 4 h in the day time (no gum was chewed in the night), from 6 h postoperatively until the first postoperative exhaustion. Same diet advancement as control group Control group: did not chew gum postoperatively. Started drinking water after the first postoperative bowel sound, started fluid diet after the first postoperative exhaustion |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, time to first bowel sounds, complications, tolerability of gum | |
| Notes | Allocated to the 'colorectal surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated by the computer |
| Allocation concealment (selection bias) | Unclear risk | Participants’ names placed in sealed envelopes during the randomisation allocation – unclear if sequential and opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were questioned by a blinded doctor regarding outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were questioned by a blinded doctor regarding outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | Participants were questioned by a blinded doctor regarding outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% dropout rate, and less than 10% difference in dropouts between groups |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Wang 2011b.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 300 participants randomised, 234 were included who had a caesarean section Mean age: 25.9 ± 5.0 y (intervention group), 26.7 ± 4.2 y (control group) Females |
|
| Interventions | Intervention group: chewed 1 xylitol sugar‐less gum for 15 min at 2 h intervals, from 2 h after surgery during the day time until first flatus. Same perioperative management as the control group Control group: no food/beverage through the mouth, water or liquid feed was provided after first bowel sound |
|
| Outcomes | Time to first flatus, time to first bowel sounds | |
| Notes | Allocated to the 'caesarean section' subgroup Study funded by the Wuxi Bureau of Health Foundation (Grant no. MX0805) Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observed recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Observations recorded. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Greater than 10% missing data |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Watson 2008.
| Methods | Randomised controlled trial Study conducted February to July 2007 |
|
| Participants | 57 participants over the age of 18 undergoing segmental, partial or sub‐total colonic or rectal resection for malignant or benign disease were randomised, 53 analysed (unpublished information) Mean age: 70.62 ± 16.97 y (intervention group), 69.22 ± 13.35 y (control group) (unpublished information) Male:Female 15 (54%):13 (46%) (intervention group), 12 (41%):17 (58%) (control group) (unpublished information) |
|
| Interventions | Intervention group: usual care (which followed an enhanced recovery protocol) and chewed sugar‐free commercially available chewing gum for 30 min 3 times a day from the first postoperative morning until day of discharge (unpublished information) Control group: usual care (which followed an enhanced recovery protocol) (unpublished information) |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'colorectal surgery' subgroup Study published as an abstract Unpublished manuscript provided by authors No information provided about sources of funding Study conducted in the UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to receive gum or usual care. Treatment assignments were randomised in short blocks of varying length and stratified (laparoscopic surgery or open surgery) (unpublished information) |
| Allocation concealment (selection bias) | Low risk | Assignments were generated and sealed inside consecutively numbered opaque envelopes by a third party. Those recruiting participants were blind to the allocation sequence until after recruitment (unpublished information) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. Nurses were taught to help blinding by not revealing allocation to surgeons |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Direct participant questioning (unpublished information). Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Direct participant questioning (unpublished information). Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Low risk | Data were collected from patient case notes. Investigators were not aware of treatment allocation. Participants were asked not to inform data collectors to which group they had been allocated (unpublished information) |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Low risk | Data were collected from patient case notes. Investigators were not aware of treatment allocation. Participants were asked not to inform data collectors to which group they had been allocated (unpublished information) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data due to dropouts (unpublished information) |
| Selective reporting (reporting bias) | High risk | Bowel sounds examinations conducted (as a measure of ileus), but not reported (unpublished information) |
| Other bias | High risk | Baseline differences between groups in BMI, stoma creation and primary method of postoperative pain relief (results for time to first bowel movement adjusted for these and still significantly different between groups) (unpublished information) At analysis the sample size was within 10% of the calculated sample size requirement (unpublished information). 54 participants were required, 53 were analysed |
Webster 2007.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 33 participants undergoing laparoscopic urologic procedures (prostatectomy or renal surgery) Mean age: 55 ± 9.7 y Gender: 79% males, 21% females |
|
| Interventions | Intervention group: chewed gum immediately for 1 h 3 times a day alongside postoperative standard care Control group: standard postoperative care |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay | |
| Notes | Allocated to the 'other surgery' subgroup Study published as an abstract No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants completed self‐report forms to record outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants completed self‐report forms to record outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No information provided about a sample size calculation |
Yang 2011.
| Methods | Randomised controlled trial Study conducted March 2008 to February 2010 |
|
| Participants | 40 participants undergoing appendectomy Average age and range: 5.0 y, 3 to 7 y Male:Female 27:13 |
|
| Interventions | Intervention group: chewed 1 piece of sugar‐free gum for 15 to 30 min 3 times a day, from 8 h postoperatively until intestinal peristalsis was restored and the children started eating again. Same care management as control group Control group: usual care management ‐ children were instructed to move their limbs after 6 h postoperatively for 5 to 10 min every 4 h. After 24 h postoperatively, the children could get off their bed to exercise under supervision |
|
| Outcomes | Time to first flatus | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Noted. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Yi 2013.
| Methods | Randomised controlled trial Study conducted December 2010 to March 2012 |
|
| Participants | 126 participants undergoing common bile duct exploration surgery Mean age: 61.3 y (intervention group), 58.9 y (control group) Male:Female 32:34 (intervention group), 27:33 (control group) |
|
| Interventions | Intervention group: chewed 3 pieces of gum for 30 min 4 times a day, from 6 h after the anaesthesia had worn off until first flatus Control group: early ambulation (participants exercised limbs 6 h after the anaesthesia had worn off); postoperative day 1 abdomen area massaged for 10 to 15 min 4 times a day; postoperative day 2 participants got out of bed and moved about with assistance |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | A nurse recorded outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | A nurse recorded outcomes. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | A nurse recorded outcomes. Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | A nurse recorded outcomes. Blinding of staff not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Bowel sounds reported in the publication as recorded, but no results presented |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Zaghiyan 2013.
| Methods | Randomised controlled trial Study conducted August 2010 to March 2012 |
|
| Participants | 127 participants randomised, 114 were included who underwent colorectal surgery Mean age: 42.1 ± 15.8 y (intervention group), 48.8 ± 18.6 y (control group) Male:Female 33:21 (intervention group), 34:26 (control group) |
|
| Interventions | Intervention group: chewed sugared chewing gum (Wrigley’s Juicy Fruit) for 45 min 3 times a day, on postoperative days 1 to 7 (continued chewing gum as per protocol if discharged before postoperative day 7). Also enrolled in an ERAS programme. Control group: no intervention, enrolled in an ERAS programme and instructed not to chew gum |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup Subgroup analyses performed based on age and operation time No information provided about sources of funding Study conducted in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned via an online program (www.randomizer.org) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. Personnel were not blinded as this was a non‐blinded study |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants were interviewed by investigators to assess primary and secondary outcomes. Study stated as non‐blinded |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants were interviewed by investigators to assess primary and secondary outcomes. Study stated as non‐blinded |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | High risk | Study stated as non‐blinded |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | High risk | Study stated as non‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Greater than 10% difference in dropout rate between groups (11 intervention participants, 2 control participants) |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the protocol were reported |
| Other bias | High risk | Baseline imbalance between groups in age and operative time At analysis the sample size was within 10% of the calculated sample size requirement (required 57 per arm; 65 and 62 were enrolled, 54 and 60 were analysed in the intervention and control groups respectively) |
Zamora 2012.
| Methods | Randomised controlled trial Study conducted August to December 2010 |
|
| Participants | 53 participants who had caesarean section under regional anaesthesia Female |
|
| Interventions | Intervention group: given 2 pellets of sugar‐less gum to be chewed for 15 min at 12 h postoperatively, then advanced to sips of clear liquids at 16 h postoperatively. 2 pellets of gum (2.8 g) contained isomalt, sorbitol, gumbase, mannitol, flavour, soybean lecithin, gum Arabic, aspartame, titanium dioxide, glycerin, carnauba wax, antioxidant bht. Same diet at 24 h and development of solid diet as control group (unpublished information) Control group: “nothing per orem” or “nothing per mouth” for 16 h post operation then advanced to sips of clear liquids. Soft boiled egg, tea and crackers were given after 24 h postoperatively. Soft diet was ordered to be given once with passage of flatus and regular diet once with bowel movement or once stool is passed (unpublished information) |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, tolerability of gum | |
| Notes | Allocated to the 'caesarean section' subgroup Study published as an oral presentation abstract Additional unpublished information provided by authors No information provided about sources of funding Study conducted in the Philippines |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated according to a computer‐generated randomisation list (Random Number Generator, Microsoft Excel) (unpublished information) |
| Allocation concealment (selection bias) | High risk | None (unpublished information) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants recorded passage of flatus and first bowel movement in a diary (unpublished information). Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Participants recorded passage of flatus and first bowel movement in a diary (unpublished information). Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results (unpublished information) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No baseline imbalances between groups (unpublished information) Sample size met calculated sample size requirement (recruited a 2:1 ratio for control group compared to intervention group) (unpublished information) |
Zhang 2008.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 18 participants who had gastrointestinal surgery Mean age: 8.61 ± 3.42 y (intervention group), 7.39 ± 4.07 y (control group) Male:Female 7:2 (intervention group), 7:2 (control group) |
|
| Interventions | Intervention group: chewed sugar‐less gum 3 times a day (morning, afternoon and evening), from the first postoperative morning until began oral intake (oral feeding started after first flatus) Control group: No information provided |
|
| Outcomes | Time to first flatus, complications | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Sound of bowel peristalsis was detected as proof of return of bowel movement, but not reported as an outcome |
| Other bias | High risk | No baseline imbalances between groups No sample size calculation. Small study as less than 20 participants per arm |
Zhao 2008.
| Methods | Randomised controlled trial Study conducted April 2006 to December 2007 |
|
| Participants | 34 participants who had open intestinal resection and anastomosis Mean age: 8.59 ± 2.87 y (intervention group), 7.88 ± 3.45 y (control group) Male:Female 13:4 (intervention group), 13:4 (control group) |
|
| Interventions | Intervention group: chewed xylitol sugar‐less gum (chewing gum weighed about 1.5g) for 30 min in the morning, midday and at night, from the first postoperative morning until began oral intake, from postoperative day 1 until food they were asked to stop fasting (food was introduced when regained gut function) Control group: same perioperative management as the intervention group, except chewing gum |
|
| Outcomes | Time to first flatus | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Article directly extracted from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Observations recorded. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | NA ‐ not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | High risk | Bowel sounds were observed as an indication of gastrointestinal motility (used as a marker to start feeding participants), but not reported as an outcome. Time to flatus only partially reported |
| Other bias | High risk | No baseline imbalances between groups No sample size calculation. Small study as less than 20 participants per arm |
Zhong 2009.
| Methods | Randomised controlled trial Study conducted January to October 2008 |
|
| Participants | 180 participants randomised to 3 groups, 120 including just the intervention and control group. Participants were undergoing surgery for colorectal cancer | |
| Interventions | Intervention group: chewed gum for 5 to 25 min 3 times a day from 12 h after surgery Control group: same treatment and postoperative care as the intervention group, but did not carry out any chewing action |
|
| Outcomes | Time to first flatus, length of hospital stay, complications | |
| Notes | Allocated to the 'colorectal surgery' subgroup Additional group of 60 participants not included in this review – intervention: participants chewed green tea leaves for 5 to 15 min 3 times a day from 12 h after surgery No information provided about when the gum chewing intervention stopped No information provided about sources of funding Article translated from Chinese Study conducted in China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Introspective randomised contrasting approach used |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. No reports of attempts to blind personnel |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications. Blinding of staff not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | All outcomes pre‐specified in the publication reported. No protocol available |
| Other bias | Unclear risk | No baseline imbalances between groups No sample size calculation. Reasonable sample size as at least 20 participants per arm |
Çavuşoğlu 2009.
| Methods | Randomised controlled trial Study conducted June 2006 to March 2008 |
|
| Participants | 30 participants undergoing intestinal resection Mean age and range: 7.23 ± 3.56 y, 3 to 14 y (intervention group), 7.00 ± 3.31 y, 3 to 13 y (control group) Male:Female 6:9 (intervention group), 12:3 (control group) |
|
| Interventions | Intervention group: chewed 1 stick of sugar‐less gum (Falim) for 1 h 3 times a day, from the first postoperative day until first bowel movement Control group: groups had the same postoperative care regimen as the control group |
|
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, complications, cost | |
| Notes | Allocated to the 'other surgery' subgroup No information provided about sources of funding Study conducted in Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants are unable to be adequately blinded with an intervention of this nature. The surgeons were blinded to study group |
| Blinding of outcome assessment (detection bias) ‐ time to first flatus | High risk | Log kept by residents in clinic. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel movement | High risk | Log kept by residents in clinic. Participants are unable to be adequately blinded with an intervention of this nature |
| Blinding of outcome assessment (detection bias) ‐ length of hospital stay | Unclear risk | Blinding of staff not discussed |
| Blinding of outcome assessment (detection bias) ‐ time to first bowel sounds | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessment (detection bias) ‐ complications | Unclear risk | Unclear who reported complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes in publication reported, no protocol available |
| Other bias | Low risk | No baseline imbalances between groups Sample size met the calculated sample size requirement |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alcántara 2010 | Article cannot be sourced |
| Alper 2006 | Article cannot be sourced |
| Anon 2006a | Article cannot be sourced |
| Anon 2006b | Not a randomised controlled trial |
| Anon 2006c | Not a randomised controlled trial |
| Anon 2008 | Not a randomised controlled trial |
| Apostolopoulos 2008 | Intervention not postoperative |
| Chathongyot 2010 | Not a randomised controlled trial |
| Darvall 2011 | Not a randomised controlled trial |
| Duluklu 2012 | Article cannot be sourced |
| Harma 2009 | Not a randomised controlled trial |
| Hwang 2013 | Not a randomised controlled trial |
| Keenahan 2014 | Not a randomised controlled trial |
| Kim 2010 | Not a randomised controlled trial |
| Li 2007b | Article cannot be sourced |
| Nimarta 2013 | Not a randomised controlled trial |
| Slim 2014 | Not a randomised controlled trial |
| Starly 2009 | Article cannot be sourced |
| Svarta 2012 | Intervention not postoperative |
| Takagi 2012 | Not a randomised controlled trial |
| Utli 2013 | Not a randomised controlled trial |
| Wang 2003 | Article cannot be sourced |
| Wang 2009b | Article cannot be sourced |
Characteristics of ongoing studies [ordered by study ID]
Abd‐El‐Maeboud 2010.
| Trial name or title | Postoperative gum chewing and the return of bowel motility after elective caesarean section under regional anaesthesia: a prospective randomised controlled trial |
| Methods | Prospective randomised controlled trial |
| Participants | 48 females aged 16 to 45 y, set for planned elective caesarean section under regional anaesthesia, providing written and signed informed consent by the participant to participate in the study Ain Shams University Hospitals, Egypt |
| Interventions | Chewing 1 stick of sugar‐less non‐sweetened gum (Samarah Foods, Cairo, Egypt) for 15 min every 2 h after surgery until the passage of flatus or bowel movement compared to traditional care (with clear liquids and soft foods allowed after passage of flatus and regular bowel movement) |
| Outcomes | Time to first bowel sounds, time to first flatus, time to first bowel movement, time to hospital discharge, tolerance of gum chewing, postoperative complications (including febrile morbidity, re‐operation, blood transfusion, postoperative ileus, hospital readmission), occurrence of mild ileus symptoms/postoperative paralytic ileus |
| Starting date | February 2010 |
| Contact information | Prof Karim Abd‐El‐Maeboud 2 Mobarak Str., Off Asmaa Fahmy, Ard El‐Golf, Heliopolis |
| Notes | Identifier: ISRCTN83008008 Complete/Not recruiting |
Andersson 2011.
| Trial name or title | Effekt av tuggummituggande mot postoperativt ileus hos patienter som genomgått pankreaskirurgi [Swedish] |
| Methods | Prospective randomised controlled trial |
| Participants | 50 individuals scheduled for open pancreatic surgery for malignancy |
| Interventions | Chewing gum for 45 min 4 times a day (8:00 AM, 12:00 PM, 5:00 PM and 8:00 PM) plus normal postoperative care from return to the ward until discharge, compared to normal postoperative care |
| Outcomes | Time to first flatus, time to first bowel movement, length of hospital stay, hunger, satiety, food and drink intake, gastrointestinal symptoms related to gum chewing, experience of gum chewing |
| Starting date | May 2011 |
| Contact information | Thomas Andersson Sahlgrenska universitetssjukhuset avd 31 |
| Notes | Identifier: VGFOUGSB‐181811 Completed |
Charoenkwan 2011.
| Trial name or title | Effects of gum chewing on recovery of bowel function following abdominal surgery for endometrial and ovarian cancer |
| Methods | Double blind 2‐arm randomised controlled trial |
| Participants | 220 females aged 18 to 80 y, undergoing staging or cytoreductive surgery for primary endometrial or ovarian cancer Maharaj Nakorn Chiang Mai hospital, Thailand |
| Interventions | Gum chewing (30 min 4 times a day at the usual time of meal, until the first flatus) in addition to conventional postoperative feeding schedule, compared to conventional postoperative feeding schedule |
| Outcomes | Time to first flatus, incidence and severity of postoperative nausea, vomiting, and abdominal discomfort, incidence of postoperative complications, time to first regular diet, time to first defaecation, postoperative analgesics requirement, hospital stay, participants' satisfaction |
| Starting date | July 2011 |
| Contact information | Dr Kittipat Charoenkwan kicharoe@med.cmu.ac.th |
| Notes | Identifier: NCT01389986 Ongoing |
Clark 2008.
| Trial name or title | Prevention of ileus after gynaecologic surgery using chewing gum |
| Methods | Randomised controlled trial |
| Participants | 400 females aged at least 18 y undergoing surgery for any gynaecologic procedure which the peritoneum is entered and general anaesthesia is administered Aultman Health Foundation, Ohio, USA |
| Interventions | Standard postoperative care with clear liquid diet as tolerated plus chewing gum (Extra Winterfresh) every 8 h for 30 minute chewing intervals, compared to standard postoperative care with clear liquid diet as tolerated |
| Outcomes | Incidence of ileus (until ileus formation or first postoperative flatus) |
| Starting date | April 2008 |
| Contact information | Aultman Health Foundation, Canton, Ohio, United States, 44710 |
| Notes | Identifier: NCT00831246 Complete |
Fakari 2011.
| Trial name or title | The effect of chewing sugar‐free gum on bowel function after cesarean section |
| Methods | Single‐blind randomised controlled trial |
| Participants | 92 females aged 18 to 35 y, parity 1 to 4, undergoing an uncomplicated and non‐emergency cesarean section with normal infant health during the operation Maternity Bennet Huda, Iran |
| Interventions | Chewing sugar‐free gum 3 times a day at 8:00 AM, 2:00 PM and 8:00 PM for 1 h, compared to normal diet and regular routine surgical care |
| Outcomes | Time to first bowel movement |
| Starting date | March 2011 |
| Contact information | Farzaneh Rashidi Fakari rashidif66@yahoo.com |
| Notes | Identifier: IRCT2012082610661N1 Complete |
Huang 2014.
| Trial name or title | Randomized Controlled Trial of Chewing Gum on Postoperative Patients' Gastrointestinal Function Recovery |
| Methods | Unblinded randomised controlled trial |
| Participants | 100 adults aged 18 to 85 who are undergoing gastrointestinal surgery and well‐conscious Tongji Hospital, Shanghai |
| Interventions | Chewing gum |
| Outcomes | Time to first flatus, time to first defaecation, operation duration, date of residence, cost of residence |
| Starting date | March 2014 |
| Contact information | Huang Qi hqhq007@hotmail.com |
| Notes | Identifier: ChiCTR‐TRC‐14004287 Ongoing |
Hulme 2011.
| Trial name or title | In patients undergoing elective open abdominal surgery, does chewing gum reduce postoperative complications compared to standard postoperative care? |
| Methods | Randomised controlled trial |
| Participants | 150 individuals aged at least 18 y, undergoing elective open abdominal surgery Wairau Hospital, New Zealand |
| Interventions | Chewing gum (single piece of sugar‐free) 3 times a day (breakfast, lunch, dinner) for at least 30 min from the first postoperative mealtime until discharge, compared to standard postoperative management (usually includes early (first postoperative day) and ongoing mobilisation, sips only of water until flatus then light diet as tolerated, analgesia as required, antiemetics as required, indwelling urinary catheter out as soon as mobilising, supportive intravenous fluids until sufficient fluid intake, bulking laxatives or codeine depending on bowel motion consistency, stoma nurse training if applicable, thromboprophylaxis, treatment of complications e.g. pneumonia, urinary tract infections, maintaining euvolaemia, wound care) |
| Outcomes | Time to first flatus, time to first bowel motion, nausea, pain |
| Starting date | December 2011 |
| Contact information | Dr Katherine Hulme kat_hulme@hotmail.com |
| Notes | Identifier: ACTRN12611001277932 Ongoing |
Lopez 2012.
| Trial name or title | Chewing gum use to Reduce postoperative ileus in paediatric patients |
| Methods | Double‐blind randomised controlled trial |
| Participants | 40 children aged 5 to 18 y undergoing gastrointestinal surgery Hospital San Jose Tec de Monterrey, Mexico |
| Interventions | Chewing gum and standard care compared to standard care only |
| Outcomes | Length of postoperative hospital stay, time to first flatus, time to first bowel motion, time to oral intake tolerance |
| Starting date | April 2012 |
| Contact information | Dr Gabriela Lopez Instituto Tecnologico y de Estudios Superiores de Monterey |
| Notes | Identifier: NCT01583452 Complete |
Lv 2011.
| Trial name or title | Gum chewing stimulates bowel motility in patients undergoing laparoscopic gynaecologic surgery. A prospective randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | 120 females undergoing laparoscopic gynaecologic surgery West China Second University Hospital, China |
| Interventions | Chewing gum compared to no chewing gum |
| Outcomes | Time to first flatus, length of hospital stay, time to first defaecation, time to first bowel sounds |
| Starting date | January 2011 |
| Contact information | Dr Donghao Lv dr.devinlv@gmail.com |
| Notes | Identifier: ChiCTR‐TRC‐11001325 Complete |
Manpunya 2011.
| Trial name or title | Effects of gum chewing on recovery of bowel function following benign gynaecologic surgery |
| Methods | Double blind 2‐arm randomised controlled trial |
| Participants | 124 females aged 18 to 80 y undergoing abdominal surgery for benign gynaecologic conditions Maharaj Nakorn Chiang Mai hospital, Thailand |
| Interventions | Chewing sugar‐free and calcium‐free gum for 30 min 4 times a day at the usual time of meal until first flatus in addition to conventional postoperative feeding schedule, compared to conventional postoperative feeding schedule |
| Outcomes | Time to first flatus, incidence and severity of postoperative nausea, vomiting and abdominal discomfort, Incidence of postoperative complications, time to first regular diet, time to first defaecation, hospital stay, participants’ satisfaction |
| Starting date | July 2011 |
| Contact information | Manatswee Manopunya manatsawee.m@hotmail.com |
| Notes | Identifier: NCT01394094 Ongoing |
Prakinoff 2009.
| Trial name or title | The effect of gum chewing on postoperative ileus |
| Methods | Single‐blind 3‐arm RCT |
| Participants | 60 children aged 6 to 18 y who have undergone appendectomy for perforated appendicitis Brenner Children's Hospital, North Carolina, USA |
| Interventions | Chewing gum after surgery for 20 min 4 times a day, compared to motion sickness wristband or usual postoperative care |
| Outcomes | Time to resolution of postoperative ileus |
| Starting date | April 2009 |
| Contact information | Dr Thomas Pranikoff tpraniko@wfubmc.edu, tpraniko@wakehealth.edu |
| Notes | Identifier: NCT00879294 Ongoing |
Ryu 2013.
| Trial name or title | Effect of sham feeding on postoperative ileus after elective liver transplantation |
| Methods | Randomised controlled trial |
| Participants | 70 individuals aged 18 to 70 y, undergoing elective liver transplantation surgery Seoul National University Hospital, Republic of Korea |
| Interventions | Chewing 2 tablets of sugar‐free xylitol gum for 15 min 3 times a day (morning, afternoon and evening) from the first postoperative morning until passage of flatus, compared to routine care during nil per os |
| Outcomes | Time to first flatus, percentage of target calories, length of intensive care unit stay, length of hospital stay |
| Starting date | October 2013 |
| Contact information | Dr Ho Geol Ryu hogeol@gmail.com |
| Notes | Identifier: NCT01956643 Ongoing |
Sabo 2012.
| Trial name or title | The effect of gum chewing on bowel motility in postoperative colon resection patients |
| Methods | Non‐blinded 2‐arm RCT |
| Participants | 80 English‐speaking participants aged at least 18 to 100 y, having had an open or laparoscopic colon resection United Hospital, Minnesota, USA |
| Interventions | Chewing of mint flavoured sugar‐less gum for 10 to 20 min 3 times a day following colon resection, compared to no gum chewing |
| Outcomes | Time to first flatus, time to first bowel movement, length of stay |
| Starting date | June 2012 |
| Contact information | Julie A Sabo julie.sabo@hcmed.org |
| Notes | Identifier: NCT01613274 Complete |
van Leersum 2012.
| Trial name or title | Kauwgom studie [Dutch] |
| Methods | Multicentre, single‐blinded, randomised controlled trial |
| Participants | 2000 participants aged at least 18 y, undergoing a planned laparotomy for surgical or gynaecological indications or a planned laparoscopic intestinal resection 8 centres |
| Interventions | Standard postoperative care and chewing gum (sugar‐less Stimorol) 3 times a day for 30 min, compared to standard postoperative care (includes an epidural catheter for 48 h, followed by standard pain medication (e.g. paracetamol and opioids in a standard scheme), removal of the gastric tube directly after surgery if possible, early ambulation, introduction and advancement of wish‐diet starting the day after surgery or as soon as tolerated) |
| Outcomes | Postoperative length of hospital stay, complication rate (infectious, non‐infectious), time to first flatus, time to first bowel movement, pain perception and diet tolerance |
| Starting date | February 2011 |
| Contact information | N.J. van Leersum nvanleersum@gmail.com |
| Notes | Identifier: NTR2594 Ongoing Preliminary results: 730 participants included so far. Chewing gum reduces the time to flatus and faeces, reduces complications related to ileus and shorten hospital stay after elective abdominal surgery |
Weiss 2012.
| Trial name or title | Does nicotine gum enhance bowel recovery after colorectal surgery? |
| Methods | Single‐blind 3‐arm randomised controlled trial |
| Participants | 300 participants aged 18 to 85 y, due to undergo small and/or large partial bowel resection via laparotomy or laparoscopy Cleveland Clinic Florida, USA |
| Interventions | Nicotine gum compared to regular chewing gum (both to chew 3 times a day until discharge or 7 days, whichever comes first) or no intervention |
| Outcomes | Time to first bowel movement or flatus, length of postoperative hospital stay, vomiting, nasogastric tube (re)insertions |
| Starting date | August 2012 |
| Contact information | Dr Karla Arancibia arancik@ccf.org Dr Jorge Canedo canedoj@ccf.org |
| Notes | Identifier: NCT01662115 Ongoing |
Williams 2010.
| Trial name or title | Interventions to decrease the impact of postoperative ileus after liver transplant or resection surgery |
| Methods | Double‐blind 3‐arm randomised controlled trial |
| Participants | 100 English‐speaking participants aged at least 19 y who have had a liver transplant or liver resection surgery Nebraska Medical Center, Nebraska, USA |
| Interventions | Standard therapy and chewing sugar‐free gum compared to standard therapy and acupressure bracelet or standard therapy alone (stool softener) |
| Outcomes | Time to first bowel movement, length of hospital stay |
| Starting date | September 2010 |
| Contact information | Laurel Williams University of Nebraska Medical Center |
| Notes | Identifier: NCT01156129 Ongoing |
Differences between protocol and review
Studies in which the intervention consisted of gum in combination with another intervention were not considered. Frequency of complications were reported rather than incidence, as stated in the protocol.
We did not state in the protocol that we planned to search Google Scholar every two weeks up to page 20 with various combinations of key terms such as “gum, ileus”, “gum, bowel” and “gum, gastrointestinal”, or that we would contact authors for information on references from their reference lists if we could not access or identify them ourselves.
Both CP and RP resolved inconsistency between review authors regarding articles for full‐text reading. Three authors (VS, GH and RP) extracted data for 20% of studies to ensure accurate data extraction, and for some studies ROB was assessed by these three authors to ensure consistent categorisations.
We stated in the protocol that we would use the ROB tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); however we developed a more detailed tool tailored to this review, based on the criteria provided by Cochrane.
In the protocol we did not state that we would do post‐hoc meta‐analyses of continuous outcomes using a fixed‐effect model.
In the protocol we stated that we would use the I2 measurement to assess degree of statistical heterogeneity; in the review we also visually inspected forest plots and used the Chi2 measurement (cut off of P < 0.01), and stated that 50% would be used as a cut off I2 value for significant heterogeneity.
We assessed all of our outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) protocol. In the protocol we stated that we would do a further subgroup analysis to assess the effect of the intervention in studies that applied an ERAS protocol compared to those that did not; in the review we explored use of CG in an ERAS context using a sensitivity analysis instead.
In the protocol we stated that we would only use meta‐regression to look at the association between surgical site and extent of effect, and whether this was a source of heterogeneity. In the review, we also looked at the association between ROB score and extent of effect, and considered whether either variable explained heterogeneity between studies.
Contributions of authors
VS: First reviewer to hand search literature, select studies, extract data, assess quality of trials, manage the data and write the review
GH: Second reviewer to select studies, extract data and assess quality of trials
RP: Second reviewer to conduct electronic search, extract data, assess quality of trials, check 21% of data for included studies, help write the Methods section of the review, provide general advice on the review and comments on drafts
CA: Checked 13.5% of data for included studies, provided general advice on the review and comments on drafts
ARN: Checked 12% of data for included studies, provided general advice on the review and comments on drafts
CP: Checked 20% of data for included studies, provided statistical and analytical advice for the review, helped write the Methods section of the review, provided general advice on the review and comments on drafts
ST: Checked 10% of data for included studies, provided general advice on the review and comments on drafts
HKA: Checked 10% of data for included studies, provided general advice on the review and comments on drafts
SJL: Checked 13.5% of data for included studies, provided clinical advice for the review, provided general advice on the review and comments on drafts
Sources of support
Internal sources
-
NIHR Biomedical Research Unit in Nutrition, Diet and Lifestyle, Bristol, UK.
This research is funded by the National Institute for Health Research (NIHR) Bristol Nutrition Biomedical Research Unit. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health
External sources
-
University of Bristol, UK.
Provided support in the form of a PhD scholarship for VS
Declarations of interest
CA, ARN, SJL and ST were involved in one of the trials included in this review (Atkinson 2014). CP was involved in the main analyses of this trial, and VS is involved in secondary analysis of data from this trial.
GH, RP and HKA: No conflict of interests.
Edited (no change to conclusions)
References
References to studies included in this review
Abd‐El‐Maeboud 2009 {published data only}
- Abd‐El‐Maeboud KHI, Ibrahim MI, Shalaby DAA, Fikry MF. Gum chewing stimulates early return of bowel motility after caesarean section. British Journal of Obstetrics and Gynaecology 2009;116(10):1334‐9. [DOI] [PubMed] [Google Scholar]
Abdollahi 2013 {published data only}
- Abdollahi AA, Yazdi K, Behnampour N, Niazi M. Effect of gum‐chewing on the movement of intestines after abdominal resection and length of hospital stay. International Journal of Hospital Research 2013;2(3):127‐32. [Google Scholar]
- Abdollahi AA, Yazdi Kh, Behnampour N, Niazi M. The effect of chewing gum on bowel movements after appendectomy [Farsi] [تاثیر جویدن آدامس برروي حرکات دودي دستگاه گوارش بعد از عمل جراحیآپا ندکتومی]. Arak Medical University Journal 2011;13(4):38‐43. [Google Scholar]
- Yazdi K, Abdollahi AA, Behnampour N, Niazi M, Arya B, Azadrah M. Effect of chewing gum on the bowel motility after cholecystectomy [Farsi] [تاثير جويدن آدامس بر روي حركات دودي دستگاه گوارش بعد از عمل كولهسيستكتومي]. Zahedan Journal of Research in Medical Sciences 2011;13(3):20‐4. [Google Scholar]
Akhlaghi 2008 {published data only}
- Akhlaghi F, Pourjavad M, Mansouri A, Tara F, Vahedian M. Effect of gum chewing on prevention of post cesarean ileus [Farsi] [اثر جويدن آدامس در پيشگيري از ايلئوس پس از سزارين]. Journal of Faculty of Nursing and Midwifery 2008;14(2):35‐40. [Google Scholar]
Asao 2002 {published data only}
- Allen G. Evidence for practice. Effect of gum chewing on postoperative ileus. AORN Journal 2003;77(2):461. [Google Scholar]
- Asao T, Kuwano H, Nakamura J, Morinaga N, Hirayama I, Ide M. Gum chewing enhances early recovery from postoperative ileus after laparoscopic colectomy. Journal of the American College of Surgeons 2002;195(1):30‐2. [DOI] [PubMed] [Google Scholar]
Askarpour 2009 {published data only (unpublished sought but not used)}
- Askarpour S, Shoushtari M, Saadati M. Study of the effect of early feeding, chewing gums, and laxative on ileus in patients who underwent open cholecystectomy. The Internet Journal of Surgery 2009;22(2). [Google Scholar]
Atkinson 2014 {published and unpublished data}
- Atkinson C, Penfold C, Ness A, Longman R, Thomas S, Hollingworth W, Leary S, Lewis S. A randomised trial of chewing gum to reduce post‐operative ileus. https://b‐com.mci‐group.com/Abstract/Statistics/AbstractStatisticsViewPage.aspx?AbstractID=222972 [Accessed 13/08/2014].
Bahena‐Aponte 2010 {published data only}
- Bahena‐Aponte JA, Cárdenas‐Lailson E, Chávez‐Tapia N, Flores‐Gama F. Usefulness of chewing gum for the resolution of postoperative ileus in left colon resections [Spanish] [Utilidad de la goma de mascar para la resolución del íleo posoperatorio en resecciones de colon izquierdo]. Revista de Gastroenterologia de Mexico 2010;4(75):369‐73. [PubMed] [Google Scholar]
Bonventre 2014 {published and unpublished data}
- Bonventre S, Inviati A, Paola V, Morreale P, Giovanni S, Carlo P, Schifano D, Frazzetta G, Gulotta G, Scerrino G. Evaluating the efficacy of current treatments for reducing postoperative ileus: a randomized clinical trial in a single center. Minerva Chirurgica 2014;69(1):47‐55. [PubMed] [Google Scholar]
Cabrera 2012 {published data only}
- Cabrera GTO, Justiniano K, Herrera L, Ortiz MV, Vargas VC. Effectiveness of chewing gum in restoring intestinal transit for postoperative paralytic ileus: a prospective randomised study [Spanish] [Eficacia de la goma de mascar en el restablecimiento del tránsito intestinal por íleo paralitico: un estudio prospectivo y aleatorio]. Panamerican Journal of Trauma, Critical Care & Emergency Surgery 2012;1(3):193‐7. [Google Scholar]
Cao 2008 {published data only}
- Cao R, Chen Y, Chen, H, Zhu X, Kang Z. Colorectal cancer patients chewing gum on intestinal function [Chinese]. Today Nurse 2008;11:45‐6. [Google Scholar]
Çavuşoğlu 2009 {published data only}
- Çavuşoğlu YH, Azılı MN, Karaman A, Aslan MK, Karaman I, Erdoğan D, Tütün O. Does gum chewing reduce postoperative ileus after intestinal resection in children? A prospective randomized controlled trial. European Journal of Pediatric Surgery 2009;19(3):171‐3. [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen Y, Ma C, Lu R, Yao H, Peng N. Early postoperative abdominal chewing gum to promote the recovery of gastrointestinal function [Chinese]. Nursing Journal of Chinese People's Liberation Army 2010;27(16):1275‐6. [Google Scholar]
Chen 2011 {published data only}
- Chen L, Xie S, Xiao Y, Shan Y. Early chewing gum on the bile duct exploration postoperative recovery of bowel movements [Chinese]. Journal of Nurses Training 2011;26(11):1046‐7. [Google Scholar]
Chen 2012 {published data only}
- Chen X, Sun D. Influence of chewing gum on recovery of gastrointestinal function after gastric resection [Chinese]. West China Medical Journal 2012;24(10):2003‐4. [Google Scholar]
Choi 2011 {published data only (unpublished sought but not used)}
- Choi H, Kang SH, Yoon DK, Kang SG, Ko HY, Moon DG, Park JY, Joo KJ, Cheon J. Chewing gum has a stimulatory effect on bowel motility in patients after open or robotic radical cystectomy for bladder cancer: a prospective randomized comparative study. Urology 2011;77(4):884‐90. [DOI] [PubMed] [Google Scholar]
Choi 2014 {published data only}
- Choi H, Kim JH, Park JY, Ham BK, Shim JS, Bae JH. Gum chewing promotes bowel motility after a radical retropubic prostatectomy. Asia‐Pacific Journal of Clinical Oncology 2014;10:53‐9. [DOI] [PubMed] [Google Scholar]
Chou 2006 {published data only}
- Chou SJ, Lin CH, Hsieh HF, Yu JC, Chen TW, Chan DC. Gum chewing in patients with subtotal gastrectomy. Chirurgische Gastroenterologie 2006;22(4):269‐71. [Google Scholar]
Chuamor 2014 {published data only (unpublished sought but not used)}
- Chuamor BNK, Thongdonjuy BNJ. Effectiveness of standard nursing care with gum chewing to reduce bowel ileus in post‐operative gynecologic patients: randomized controlled trials. Siriraj Medical Journal 2014;66(2):33‐8. [Google Scholar]
Crainic 2009 {published data only}
- Crainic C, Erickson K, Gardner J, Haberman S, Patten P, Thomas P, Hays V. Comparison of methods to facilitate postoperative bowel function. MEDSURG Nursing 2009;18(4):235‐8. [PubMed] [Google Scholar]
Ertas 2013 {published data only (unpublished sought but not used)}
- Ertas IE, Gungorduk K, Ozdemir A, Solmaz U, Dogan A, Yildirim Y. Influence of gum chewing on postoperative bowel activity after complete staging surgery for gynecological malignancies: A randomized controlled trial. Gynecologic Oncology 2013;131(1):118‐22. [DOI] [PubMed] [Google Scholar]
Fan 2009 {published data only}
- Fan Q, Geng X, Chen H, Yu J, Hu M, Yi J. Effects of chewing gum on recovery of gastrointestinal motility in patients undergoing total resection of colorectal cancer [Chinese]. Medical Journal of National Defending Forces in Southwest China 2009;19(12):1240‐1. [Google Scholar]
Forrester 2014 {published data only (unpublished sought but not used)}
- Doyle‐Munoz J, Forrester DA, McTigue, T, D'Andrea S, Natale‐Ryan A. The efficacy of gum chewing in reducing postoperative ileus. Journal of Wound Ostomy and Continence Nursing 2012;39(3):S1‐2. [DOI] [PubMed] [Google Scholar]
- Forrester DA, Doyle‐Munoz J, McTigue T, D’Andrea S, Natale‐Ryan A. The efficacy of gum chewing in reducing postoperative ileus: a multisite randomized controlled trial. Journal of Wound Ostomy and Continence Nursing 2014;41(3):1‐6. [DOI] [PubMed] [Google Scholar]
Garshasbi 2011 {published data only (unpublished sought but not used)}
- Garshasbi A, Behboudi S. The effect of gum chewing on postoperative ileus after cesarean section. Society for Obstetric Anesthesia and Perinatology (SOAP) 42nd Annual Meeting 2011;[http://soap.org/abstracts‐uploads‐spring‐2011/1011411033345The_effect_of_Gum_c.pdf]:Accessed 13 March 2014. [Google Scholar]
Ghafouri 2008 {published data only}
- Ghafouri A, Soroush AR, Moini N, Hedayat A, Khorgami Zh. The efficacy of sugar free gum chewing after upper GI tract operation on ileus: a clinical trial [Farsi] [بررسي اثرات جويدن آدام سهاي بدون قند در بهبود ايلئوس بعد از عمل جراحيلولة گوارش فوقاني: كارآزمايي باليني]. Iranian Journal of Surgery 2008;16(1):79‐84. [Google Scholar]
Gong 2011 {published data only}
- Gong L. Chewing gum on gastrointestinal surgery patients after bowel function [Chinese]. Chinese Journal of Aesthetic Medicine 2011;20(Z2):325‐6. [Google Scholar]
Guangqing 2011 {published data only}
- Guangqing Y, Xiaomei Y, Chunlan W, et al. Influence of chewing gum on gastrointestinal function of postoperative patients in obstetrics and gynecology department after undergoing minimally invasive surgery [Chinese]. Chinese Nursing Research 2011;25(2A):311‐2. [Google Scholar]
Han 2011 {published data only}
- Han Z, Zhao H, Liu C, Jin Q. Randomized controlled study on the role of chewing gum in bowel function recovery for patients with leiomyoma after surgery [Chinese]. China Medical Herald 2011;28(8):37‐9. [Google Scholar]
Hirayama 2006 {published data only}
- Hirayama I, Suzuki M, Ide M, Asao T, Kuwano H. Gum‐chewing stimulates bowel motility after surgery for colorectal cancer. Hepato‐gastroenterology 2006;53(68):206‐8. [PubMed] [Google Scholar]
Huang 2012a {published data only}
- Huang Y, Ling Y, Huang Q, Huang M. Influence of chewing gum on recovery of gastrointestinal function in elder patients after gastrointestinal operation [Chinese]. Chinese Journal of Misdiagnostics 2012;12(17):4500‐2. [Google Scholar]
- Huang Y, Ling Y, Huang Q, Huang M. Three different methods of care for elderly postoperative gastrointestinal function recovery of gastrointestinal comparison [Chinese]. Journal of Youjiang Medical University for Nationalities 2012;34(3):442‐4. [Google Scholar]
Huang 2012b {published data only}
- Huang W, Yu X. Influence of gum chewing on gastrointestinal function of patients after undergoing laparoscopic appendectomy [Chinese]. Chinese Nursing Research 2012;26(23):2153‐4. [Google Scholar]
Husslein 2013 {published data only}
- Husslein H, Franz M, Gutschi M, Worda C, Polterauer S, Leipold H. Postoperative gum chewing after gynecologic laparoscopic surgery: a randomized controlled trial. Obstetrics & Gynecology 2013;122(1):85‐90. [DOI] [PubMed] [Google Scholar]
Jakkaew 2013 {published data only}
- Jakkaew B. Effects of gum chewing on recovery of bowel function following cesarean section: a randomized controlled trial. Archives of Gynecology and Obstetrics 2013;288(2):255‐60. [DOI] [PubMed] [Google Scholar]
Jernigan 2014 {published and unpublished data}
- Jernigan AM, Chen CCG, Sewell C. A randomized trial of chewing gum to prevent postoperative ileus after laparotomy for benign gynecologic surgery. International Journal of Gynecology and Obstetrics 2014;127(3):279‐82. [DOI] [PubMed] [Google Scholar]
Jin 2010 {published data only}
- Jin Q, Huang J, Songlin Y, Wang C, Yin X. Chewing gum for kidney patients after recovery of gastrointestinal function [Chinese]. Chinese Journal of Modern Nursing 2010;16(23):2833‐4. [Google Scholar]
Kafali 2010 {published data only}
- Kafali H, Duvan CI, Gözdemir E, Simavli S, Onaran Y, Keskin E. Influence of gum chewing on postoperative bowel activity after cesarean section. Gynecol Obstet Invest 2010;69(2):84‐7. [DOI] [PubMed] [Google Scholar]
Ledari 2012 {published data only}
- Ledari FM, Barat S, Delavar MA. Chewing gums has stimulatory effects on bowel function in patients undergoing cesarean section: a randomized controlled trial. Bosnian Journal of Basic Medical Sciences 2012;12(4):265‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledari FM, Barat S, Delavar MA, Banihosini SZ, Khafri S. Chewing sugar‐free gum reduces ileus after cesarean section in nulliparous women: a randomized clinical trial. Iranian Red Crescent Medical Journal 2013;15(4):330‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2004 {published data only (unpublished sought but not used)}
- Lee DDK, Runowicz, CD, Chambers JT, Langer O. Efficacy of gum chewing in improving the recovery of bowel function after major gynecologic surgery. Obstetrics and Gynecology 2004;103(4):21S. [Google Scholar]
Li 2007a {published data only}
- Li L, Cai L. Observation on effect of chewing gum in mouth care for patients after underwent gastrointestinal operation [Chinese]. Chinese Nursing Research 2007;21(5):417‐8. [Google Scholar]
Li 2012a {published data only}
- Li M. Chewing gum can promote the recovery of intestinal function [Chinese]. Chinese Manipulation & Rehabilitation Medicine 2012;3(11):89‐90. [Google Scholar]
Li 2012b {published data only}
- Li Y. Chewing gum on the impact of early postoperative gastrointestinal function in patients with colon cancer [Chinese]. World Health Digest 2012;41:69‐70. [Google Scholar]
Liang 2007 {published data only}
- Liang J, Gao T, Han W, Zhang Y, Liu S, Dai Q. The clinical observation of enhancing recovery of gastrointestinal function after cesarean section by gum chewing [Chinese]. Journal of Tongji University (Medical Science) 2007;28(2):81‐3. [Google Scholar]
Lim 2013 {published and unpublished data}
- Lim P, Morris OJ, Nolan G, Moore S, Draganic B, Smith SR. Sham feeding with chewing gum after elective colorectal resectional surgery: a randomized clinical trial. Annals of Surgery 2013;257(6):1016‐24. [DOI] [PubMed] [Google Scholar]
- Smith SR, Lim P, Draganic B. Effect of gum chewing on gastrointestinal recovery after laparoscopic colorectal resectional surgery: a prospective randomized clinical trial. ANZ Journal of Surgery 2010;80(S1):A17. [Google Scholar]
Lu 2010a {published data only}
- Lu Q, Wu W, Yang L, Zhao X, Zhong C, Wang Y, Hu S. The effect of gum chewing on bowel motility in patients after radical cystectomy with urinary diversion [Chinese]. China Journal of Modern Medicine 2010;21(10):1255‐7. [Google Scholar]
Lu 2010b {published data only}
- Lu L, Zhao A. "False eat" diet promote cesarean section with recovery of gastrointestinal function and clinical studies lactation [Chinese]. Journal of Nurses Training 2010;25(23):2158‐9. [Google Scholar]
Lu 2011 {published data only (unpublished sought but not used)}
- Lu D, Liu Q, Shi G. Gum chewing stimulates early return of bowel motility after gynecologic laparoscopic surgery. Fertility and Sterility 2011;96(3):S32. [Google Scholar]
Luo 2010 {published data only}
- Luo S, Wu C, Yang X, Lei L, Deng H, Li H. Effect of chewing gum after cesarean section on restoration of gastrointestinal function [Chinese]. China Journal of Modern Nursing 2010;16(24):2948‐9. [Google Scholar]
Marwah 2012 {published data only}
- Marwah S, Singla S, Tinna P. Role of gum chewing on the duration of postoperative ileus following ileostomy closure done for typhoid ileal perforation: a prospective randomized trial. Saudi Journal of Gastroenterology 2012;18(2):111‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matros 2006 {published and unpublished data}
- Matros E, Rocha F, Zinner M, Wang J, Ashley S, Breen E, Soybel D, Shoji B, Burgess A, Bleday R, Kuntz R, Whang E. Does gum chewing ameliorate postoperative ileus? Results of a prospective, randomized, placebo‐controlled trial. Journal of the American College of Surgeons 2006;202(5):773‐8. [DOI] [PubMed] [Google Scholar]
- Rocha FG, Matros E, Ashley SW, Breen E, Shoji BT, Soybel DI, Zinner MJ, Bleday R, Whang EE. Does gum chewing ameliorate postoperative ileus? Results of a prospective, randomized placebo‐controlled trial. Gastroenterology 2005;128(4):A800. [DOI] [PubMed] [Google Scholar]
McCormick 2005 {published and unpublished data}
- Anon. Chewing gum aids recovery after colon resection. OR Manager 2005;21(12):11. [Google Scholar]
- Garvin, R, McCormick JT, Read TE, Papasavas PK, Caushaj PF. Gum chewing accelerates recovery of bowel function after intestinal resection: Multi‐institutional prospective randomized trial. American Journal of Gastroenterology 2005;100(9):S269. [Google Scholar]
- McCormick JT. Gum in the postoperative setting: something to chew on. Diseases of the Colon & Rectum 2013;56(3):273‐4. [DOI] [PubMed] [Google Scholar]
- McCormick JT, Garvin R, Caushaj P, Simmang C, Gregorcyk S, Huber P, Odom C, Downs M, Read T, Papaconstantinou H. The effects of gum‐chewing on bowel function and hospital stay after laparoscopic vs open colectomy: a multi‐institution prospective randomized trial. Journal of the American College of Surgeons 2005;201(3):S66‐7. [Google Scholar]
Ngowe 2010 {published data only}
- Ngowe MN, Eyenga VC, Kengne BH, Bahebeck J, Sosso AM. Chewing gum reduces postoperative ileus after open appendectomy. Acta Chirurgica Belgica 2010;111(2):195‐9. [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park SY, Chung M. Can gum chewing reduce postoperative ileus after open abdominal surgery? [Korean] [복부 수술 후 껌을 이용한 장폐색의 단축]. Journal of the Korean Surgical Society 2009;77(5):206‐9. [Google Scholar]
Pilehvarzadeh 2014 {published data only}
- Pilehvarzadeh M, Shamsi A, Salari S, Rafeti F, Rezaii H, Ebadi A. Effect of gum chewing in the reduction of paralytic ileus following cholecystectomy [Farsi] [تأثير جويدن آدامس در بهبود ايليوس پارالتيك بعد از كله سيستكتومي]. Journal of Qazvin University of Medical Sciences 2014;17(6):24‐9. [Google Scholar]
Qiao 2011 {published data only}
- Qiao J. Clinical observation of chewing gum in promoting the recovery of gastrointestinal function after gastrointestinal disease surgery [Chinese]. Journal of Clinical Medicine in Practice 2011;15(14):45‐6. [Google Scholar]
Qiu 2006 {published data only}
- Qiu F, Ren X. Chewing gum after abdominal surgery in gynecologic patients with gastrointestinal function recovery application [Chinese]. Modern Nursing 2006;12(17):1624‐5. [Google Scholar]
Quah 2006 {published data only}
- Quah HM, Samad A, Neathey AJ, Hay DJ, Maw A. Does gum chewing reduce postoperative ileus following open colectomy for left‐sided colon and rectal cancer? A prospective randomized controlled trial. Colorectal Disease 2006;8(1):64‐70. [DOI] [PubMed] [Google Scholar]
Rashad 2013 {published data only}
- Rashad WAE, Yousef SAAL. Effect of sugarless gum chewing on intestinal movement after cesarean section. Life Science Journal 2013;10(4):3257‐61. [Google Scholar]
Ray 2008 {published data only (unpublished sought but not used)}
- Ray KL, Estes JM, Huh WK. Prospective, randomized trial of postoperative gum‐chewing and return of bowel function in gynecology. Obstetrics and Gynecology 2008;111(4):7S‐8S. [Google Scholar]
Ren 2010 {published data only}
- Ren Y, Qin X. Effect of chewing gum on the time of anal exhaust after laparoscopic cholecystectomy [Chinese]. Journal of Qilu Nursing 2010;16(20):4‐5. [Google Scholar]
- Ren Y, Qin X, Dai X. Effect of chewing gum on gastrointestinal function after laparoscopic abdominal surgery [Chinese]. Chinese Journal of Practical Nursing 2010;26(9B):68‐70. [Google Scholar]
Safdari‐Dehcheshmehi 2011 {published data only}
- Safdari‐Dehcheshmehi F, Salehian T, Parvin N, Akbari N. Comparison of the effects of gum chewing with those of early initiation of oral feeding and routine regimen on recovery of bowel function in primiparous women after cesarean section [Farsi] [بررسي اثر جويدن آدامس در مقايسه با رژيم مايعات زودرس و رژيم روتين در برگشتحركات روده اي پس از عمل سزارين انتخابي در زنان نخست زا]. Scientific Journal of Kurdistan University of Medical Sciences 2011;16(2):9‐15. [Google Scholar]
Satij 2006 {published and unpublished data}
- Satij B, Cohen SA. Evaluation of gum chewing on the return of bowel function in cesarean‐delivery patients. Obstetrics and Gynecology 2006;107(4):10S. [Google Scholar]
Schluender 2005 {published data only (unpublished sought but not used)}
- Schluender SS, Gurland BHG, Divino CD, Horovitz JH, Adler HA, Chernobelsky LC, Macura JM, Wasserman HW, Ahmad SA. Gum chewing does not enhance the return of bowel function in patients undergoing elective colon resection in a randomized blinded pilot study. Colorectal Disease 2005;95(Suppl 1):92. [Google Scholar]
Schuster 2006 {published data only}
- Anon. Chewing gum after colectomy. American Journal of Nursing 2006;106(9):72JK‐L. [Google Scholar]
- Anon. Gum chewing reduces ileus after colectomy. OR Manager 2006;22(4):32. [Google Scholar]
- Anon. Postop bowel function: chewing gum may speed recovery. Nursing 2006;36(5):34. [Google Scholar]
- Schuster R, Grewal N, Greaney GC, Waxman K. Gum chewing reduces ileus after elective open sigmoid colectomy. Archives of Surgery 2006;14(2):174‐6. [DOI] [PubMed] [Google Scholar]
- Steurer J. Gum chewing promotes the intestinal function after sigmoid resection: Comment [German] [Kaugummi kauen fordert die darmfunktion nach sigmaresektion]. Schweizerische Rundschau fur Medizin ‐ Praxis 2006;95(50):1987‐8. [Google Scholar]
- Wind J, Busch ORC. Gum chewing leads to a faster recovery from open sigmoid colectomy. [Dutch] [Kauwen van kauwgom leidt tot een sneller herstel na open sigmoidresecties]. Nederlands Tijdschrift voor Geneeskunde 2006;150(30):1699. [Google Scholar]
Schweizer 2010 {published and unpublished data}
- Schweizer W, Häne R. Sham‐feeding of patients with chewing gum after abdominal operations. British Journal of Surgery 2010;97(S3):8. [Google Scholar]
Shang 2010 {published data only}
- Shang H, Yang Y, Tong X, Zhang L, Fang A, Hong L. Gum chewing slightly enhances early recovery from postoperative ileus after cesarean section: results of a prospective, randomized, controlled trial. American journal of perinatology 2010;27(5):387‐91. [DOI] [PubMed] [Google Scholar]
Sun 2005 {published data only}
- Sun L, Gong S, Gong H, Zhang Y. Chewing gum used in abdominal surgery and postoperative care observation [Chinese]. Today Nurse 2005;7:11‐2. [Google Scholar]
Tan 2011 {published data only}
- Tan Y, Tang X. Chewing gum after abdominal surgery for gynecological promote recovery of gastrointestinal function [Chinese]. Today Nurse 2011;10:47‐8. [Google Scholar]
Terzioglu 2013 {published data only (unpublished sought but not used)}
- Terzioglu F, Simsek S, Karac, K, Sariince N, Altunsoy P, Salman MC. Multimodal interventions (chewing gum, early oral hydration and early mobilisation) on the intestinal motility following abdominal gynaecologic surgery. Journal of Clinical Nursing 2013;22:1917‐25. [DOI] [PubMed] [Google Scholar]
Tian 2013 {published data only}
- Tian M. Effect of chewing gums on gastrointestinal function of rectal cancer patients undergoing surgical operations [Chinese]. Modern Clinical Nursing 2013;12(2):45‐7. [Google Scholar]
Wang 2008 {published data only}
- Wang X, Liao H, Gao L, Ma Y, Zhang L. Chewing gum on recovery of gastrointestinal function after abdominal surgery impact [Chinese]. Journal of Nurses Training 2008;23(10):938‐9. [Google Scholar]
Wang 2009a {published data only}
- Wang H. Chewing gum to promote recovery of bowel movement after abdominal surgery observation [Chinese]. Journal of Qiqihar Medicine 2009;30(4):492. [Google Scholar]
Wang 2011a {published data only}
- Wang S, Hou Y, Dong S. A randomized controlled trial of chewing gum to promote postoperative bowel recovery for patients with rectal cancer [Chinese]. Sichuan Medical Journal 2011;32(12):1956‐8. [Google Scholar]
Wang 2011b {published data only}
- Wang X, Ren Y, Qin X, Dai X. Influence of sham feeding on motilin and evacuating time after accepting cesarean section [Chinese]. Chinese Nursing Research 2011;25(8):682‐3. [Google Scholar]
Watson 2008 {published and unpublished data}
- Watson H, Griffiths P, Lamparelli M, Watson M. Does chewing (gum) aid recovery after bowel resection? A randomized controlled trial (RCT). Colorectal Disease 2008;10(Suppl. 1):6. [Google Scholar]
Webster 2007 {published data only (unpublished sought but not used)}
- Webster B, Corcoran A, Smaldone M, Morrisroe S, Stockton B, Jackman S, Averch T. Does gum chewing reduce postoperative ileus following urologic laparoscopy? A prospective randomized controlled trial. Journal of Endourology 2007;21(Supp 1):A137. [Google Scholar]
Yang 2011 {published data only}
- Yang H. Influence of gum chewing on recovery of intestinal motility after ecphyadectomy surgery in children. Journal of Nursing Science 2011;26(6):23. [Google Scholar]
Yi 2013 {published data only}
- Yi Y, Guo H, Xian W, Chen Y. Bile duct exploration postoperative recovery chewing gum promote peristalsis clinical observation [Chinese]. Laboratory Medicine and Clinic 2013;10(10):1270‐1. [Google Scholar]
Zaghiyan 2013 {published data only}
- Zaghiyan K, Felder S, Ovsepyan G, Murrell Z, Sokol T, Moore B, Fleshner P. A prospective randomized controlled trial of sugared chewing gum on gastrointestinal recovery after major colorectal surgery in patients managed with early enteral feeding. Diseases of the Colon & Rectum 2013;56(3):328‐35. [DOI] [PubMed] [Google Scholar]
Zamora 2012 {published and unpublished data}
- Zamora BBB, Kalalo RE. Gum chewing versus traditional feeding on the early return of bowel motility after cesarean delivery: A prospective randomized controlled trial. International Journal of Gynecology and Obstetrics 2012;119:S525. [Google Scholar]
Zhang 2008 {published data only}
- Zhang Q, Zhao P. Influence of gum chewing on return of gastrointestinal function after gastric abdominal surgery in children. European Journal of Pediatric Surgery 2008;18(1):44‐6. [DOI] [PubMed] [Google Scholar]
Zhao 2008 {published data only}
- Zhao P, Zhang Q. The influence of chewing gum on the return of intestinal motility after abdominal surgery in children and its mechanism of action [Chinese]. Journal of Clinical Pediatric Surgery 2008;7(3):24‐6. [Google Scholar]
Zhong 2009 {published data only}
- Zhong Z, Ye F, Lin J. A study on how chewing action promotes gastrointestinal functions recovery after colorectal cancer surgery. Chinese Journal of Gastrointestinal Surgery 2009;12(6):632‐3. [Google Scholar]
References to studies excluded from this review
Alcántara 2010 {published data only}
- Alcántara B, Luis A. Benefit of chewing gum vs metoclopramide in the prevention of postoperative ileus ofter laparotomy [Spanish] [Beneficio de la goma de mascar vs metoclopramide en la prevención del íleo postoperatorio de laparotomía]. Revista Venezolana de Cirugía 2010;63(1):32‐41. [Google Scholar]
Alper 2006 {published data only}
- Alper BS. Evidence‐based medicine. Gum chewing may reduce postoperative ileus. Clinical Advisor for Nurse Practitioners 2006;9(7):141. [Google Scholar]
Anon 2006a {published data only}
- Anon. Gum chewing may speed colon surgery recovery. Mayo Clinic Health Letter 2006;24(9):4. [PubMed] [Google Scholar]
Anon 2006b {published data only}
- Anon. By gum, it might be good for you. Recent studies show that gum chewing may speed recovery from bowel surgery. Harvard Health Letter 2006;31(10):5. [PubMed] [Google Scholar]
Anon 2006c {published data only}
- Anon. Medical matters. Gum chewing speeds surgery recovery. Consumer Reports on Health 2006;18(12):7. [Google Scholar]
Anon 2008 {published data only}
- Anon. Chewing gum could change practice. Gastrointestinal Nursing 2008;6(2):6. [Google Scholar]
Apostolopoulos 2008 {published data only}
- Apostolopoulos P, Kalantzis C, Gralnek IM, Liatsos C, Tsironis C, Kalantzis, N. Clinical trial: effectiveness of chewing‐gum in accelerating capsule endoscopy transit time ‐ a prospective randomized, controlled pilot study. Alimentary Pharmacology and Therapeutics 2008;28(4):405‐11. [DOI] [PubMed] [Google Scholar]
Chathongyot 2010 {published data only}
- Chathongyot N, Thongkhruea C, Borwornpadungkitti S, Predanon C. Effect of gum chewing to reduce postoperative ileus following anatrophic nephrolithotomy or nephrectomy [Thai] [ผลของการเคี้ยวหมากฝรั่งต่อการลดอาการท้องอืดในผู้ป่วยผ่าตัด Anatrophic Nephrolithotomy หรือ Nephrectomy]. Journal of Health Science 2010;19(3):457‐66. [Google Scholar]
Darvall 2011 {published data only}
- Darvall JN, Simmons SW, Leslie K. Acceptability of chewing gum for postoperative nausea and vomiting prevention in high risk patients: a pilot study. Anaesthesia and Intensive Care 2011;39(3):515‐6. [PubMed] [Google Scholar]
Duluklu 2012 {unpublished data only}
- Duluklu B. The role of gum chewing early return of intestinal functions after left column and/or rectal surgery intestinal functions [Turkish] [Sol kolon velveya rectum cerrahisi sonrası bağırsak fonksiyonlarının başlamasında sakız çiğnemenin rolü]. Unpublished surgical nursing masters thesis, Ankara, Turkey 2012.
Harma 2009 {published data only}
- Harma MI, Barut A, Arikan II, Harma M. Gum‐chewing speeds return of first bowel sounds but not first defecation after cesarean section. Anatolian Journal of Obstetrics & Gynecology 2009;1:1‐3. [Google Scholar]
Hwang 2013 {published data only}
- Hwang DY, Kim HY, Kim JH, Lee IG, Kim JK, Oh ST, Lee YS. Effect of gum chewing on the recovery from laparoscopic colorectal cancer surgery. Annals of Coloproctology 2013;29(6):248‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keenahan 2014 {published data only}
- Keenahan, M. Does gum chewing prevent postoperative paralytic ileus?. Nursing 2014;44(6):1‐2. [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim SS, Lee EN, Kim HS, Kim MK, Lee KS, Nam HJ, Kim MY. Effects of gum‐chewing on the recovery of bowel motility and length of hospital stay after surgery for colorectal cancer [Korean]. Journal of Korean Oncology Nursing 2010;10(2):191‐8. [Google Scholar]
Li 2007b {published data only}
- Li L, Cai L, Xia W, Even Y. Postoperative gastrointestinal diseases chewing gum effect observed [Chinese]. Shandong Medical Journal 2007;47(6). [Google Scholar]
Nimarta 2013 {published data only}
- Nimarta NVS, Shruti RG. Effectiveness of chewing gum on bowel motility among the patients who have undergone abdominal surgery. Nursing and Midwifery Research Journal 2013;9(3):108‐17. [Google Scholar]
Slim 2014 {published data only}
- Slim K, Demartines N, Fearon KC, Lobo DN, Ramirez J, Scott M, Ljungqvist O. Beyond ERAS?. Colorectal Disease 2014;16(3):219‐20. [DOI] [PubMed] [Google Scholar]
Starly 2009 {unpublished data only}
- Starly DJ. Bubble gum chewing helps in early return of bowel function after abdominal surgery. Unpublished surgical nursing masters dissertation, Bangalore, India 2009.
Svarta 2012 {published data only}
- Svarta S, Ou G, Enns RA. The effect of chewing gum on small bowel transit time: a prospective randomized trial. Gastrointestinal Endoscopy 2012;75(4):124. [DOI] [PubMed] [Google Scholar]
Takagi 2012 {published data only}
- Takagi K, Teshima H, Arinaga K, Yoshikawa K, Hori H, Kashikie, H, Nakamura K. Gum chewing enhances early recovery of bowel function following transperitoneal abdominal aortic surgery. Surgery Today 2012;42(8):759‐64. [DOI] [PubMed] [Google Scholar]
Utli 2013 {published data only}
- Utli H, Caliskan N. The effect of chewing gum on post‐cesarean intestine functions [Turkish] [SAKIZ ÇİĞNEMENİN SEZARYEN SONRASI BAĞIRSAK FONKSİYONLARINA ETKİSİ]. Anatolian Journal of Clinical Investigation 2013;7(4):215‐21. [Google Scholar]
Wang 2003 {published data only}
- Wang L. Chewing gum to promote recovery of gastrointestinal function after abdominal surgery clinical observation [Chinese]. Journal of Huaihai Medicine 2003;21(5):378‐9. [Google Scholar]
Wang 2009b {unpublished data only}
- Wang K. Chewing gum in the recovery of gastrointestinal function after caesarean section’s role [Chinese]. Unpublished masters dissertation, Shanghai, China 2009.
References to ongoing studies
Abd‐El‐Maeboud 2010 {published data only}
- Abd‐El‐Maeboud K. Post‐operative gum chewing and the return of bowel motility after elective caesarean section under regional anaesthesia: a prospective randomised controlled trial. http://www.controlled‐trials.com/ISRCTN83008008 [Accessed 23/07/2014].
Andersson 2011 {published data only}
- Andersson T. Effect of gum chewing on postoperative ileus in patients undergoing pancreativ surgery [Effekt av tuggummituggande mot postoperativt ileus hos patienter som genomgått pankreaskirurgi [Swedish]]. http://www.researchweb.org/is/sverige/document/78631 [Accessed 23/07/2014].
Charoenkwan 2011 {published data only}
- Charoenkwan K. Effects of gum chewing on recovery of bowel function following abdominal surgery for endometrial and ovarian cancer. http://clinicaltrials.gov/show/NCT01389986 [Accessed 23/07/2014].
Clark 2008 {published data only}
- Clark JM, Hopkins M. Prevention of ileus after gynaecologic surgery using chewing gum. http://clinicaltrials.gov/show/NCT00831246 [Accessed 23/07/2014].
Fakari 2011 {published data only}
- Fakari FR, Abasi Z. The effect of chewing sugar‐free gum on bowel function after cesarean section. http://www.irct.ir/searchresult.php?id=10661&number=1 [Accessed 23/07/2014].
Huang 2014 {published data only}
- Huang Q, Wang J. Randomized Controlled Trial of Chewing Gum on Postoperative Patients' Gastrointestinal Function Recovery. http://www.chictr.org/en/proj/show.aspx?proj=7736 [Accessed 23/07/2014].
Hulme 2011 {published data only}
- Hulme K. In patients undergoing elective open abdominal surgery, does chewing gum reduce postoperative complications compared to standard postoperative care?. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611001277932 [Accessed 23/07/2014].
Lopez 2012 {published data only}
- Lopez G. Chewing gum use to Reduce post‐operative ileus in pediatric patients. http://clinicaltrials.gov/show/NCT01583452 [Accessed 23/07/2014].
Lv 2011 {published data only}
- Lv D, Liu Q. Gum chewing stimulates bowel motility in patients undergoing laparoscopic gynecologic surgery. A prospective randomized controlled trial. http://www.chictr.org/en/proj/show.aspx?proj=81 [Accessed 23/07/2014].
Manpunya 2011 {published data only}
- Manopunya M. Effects of gum chewing on recovery of bowel function following benign gynecologic surgery. http://clinicaltrials.gov/show/NCT01394094 [Accessed 23/07/2014].
Prakinoff 2009 {published data only}
- Pranikoff T. The effect of gum chewing on postoperative ileus. http://clinicaltrials.gov/ct2/show/study/NCT00879294 [Accessed 23/07/2014].
Ryu 2013 {published data only}
- Ryu HG. Effect of sham feeding on postoperative ileus after elective liver transplantation. http://clinicaltrials.gov/show/NCT01956643 [Accessed 23/07/2014].
Sabo 2012 {published data only}
- Sabo JA. The effect of gum chewing on bowel motility in post‐operative colon resection patients. http://clinicaltrials.gov/show/NCT01613274 [Accessed 23/07/2014].
van Leersum 2012 {published data only}
- Leersum NJ. Chewing gum study [Dutch] [Kauwgomstudie]. https://kauwgomstudie‐nl.firstfind.nl [Accessed 23/07/214].
- Leersum NJ. Gum chewing and postoperative ileus. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2594 [Accessed 23/07/2014].
- Leersum NJ, Bonsing BA, Kroon HM, Sijp JR, Weel V. Chewing gum to prevent postoperative ileus [Dutch] [Kauwgom kauwen ter preventie van postoperatieve ileus]. Nederlands Tijdschrift voor Geneeskunde 2012;156(22):A4794. [PubMed] [Google Scholar]
Weiss 2012 {published data only}
- Weiss E, Arancibia K, Canedo J. Does nicotine gum enhance bowel recovery after colorectal surgery?. http://clinicaltrials.gov/show/NCT01662115 [Accessed 23/07/2014].
Williams 2010 {published data only}
- Williams L. Interventions to decrease the impact of post‐operative ileus after liver transplant or resection surgery. http://clinicaltrials.gov/show/NCT01156129 [Accessed 23/07/2014].
Additional references
Barletta 2014
- Barletta JF, Senagore AJ. Reducing the burden of postoperative ileus: evaluating and implementing an evidence‐based strategy. World Journal of Surgery 2014;38(8):1966‐77. [DOI] [PubMed] [Google Scholar]
Basaran 2009
- Basaran M, Pitkin RM. Gum chewing to prevent postoperative ileus. Anatolian Journal of Obstetrics & Gynecology 2009;1(2):1‐3. [Google Scholar]
Bashankaev 2009
- Bashankaev B, Daniel M, Khaikin M, Wexner SD. Postoperative ileus: an algorithm for prevention and management. Pharmacy Practice News 2009;36(10):71‐8. [Google Scholar]
Behm 2003
- Behm B, Stollman N. Postoperative ileus: etiologies and interventions. Clinical Gastroenterology and Hepatology 2003;1(2):71‐80. [DOI] [PubMed] [Google Scholar]
Belghazi 2012
- Belghazi L, Skalli S, Bombail M, Nouvel M, Faudel A, Parat S, Rioufol C. Does chewing gum present therapeutic effects on bowel motility after abdominal surgery?. International Journal of Clinical Pharmacy 2012;34:241. [Google Scholar]
Chan 2007
- Chan MKY, Law WL. Use of chewing gum in reducing postoperative ileus after elective colorectal resection: a systematic review. Diseases of the Colon & Rectum 2007;50(12):2149‐57. [DOI] [PubMed] [Google Scholar]
Chang 2002
- Chang SS, Cookson MS, Baumgartner RG, Wells N, Smith JA Jr. Analysis of early complications after radical cystectomy: results of a collaborative care pathway. Journal of Urology 2002;167(5):2012‐6. [PubMed] [Google Scholar]
Craciunas 2014
- Craciunas L, Sajid MS, Ahmed AS. Chewing gum in preventing postoperative ileusin women undergoing caesarean section:a systematic review and meta‐analysis ofrandomised controlled trials. BJOG: an international journal of obstetrics and gynaecology 2014;121(7):793‐800. [DOI] [PubMed] [Google Scholar]
Endo 2014
- Endo M, Hori M, Ozaki H, Oikawa T, Hanawa T. Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti‐inflammatory action through nicotinic acetylcholine receptors. Journal of Gastroenterology 2014;49(6):1026‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fanning 2011
- Fanning J, Hojat R. Safety and efficacy of immediate postoperative feeding and bowel stimulation to prevent ileus after major gynecologic surgical procedures. The Journal of the American Osteopathic Association 2011;111(8):469‐72. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2009
- Fitzgerald JE, Ahmed I. Systematic review and meta‐analysis of chewing‐gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World Journal of Surgery 2009;33(12):2557‐66. [DOI] [PubMed] [Google Scholar]
Fujii 2014
- Fujii S, Ishibe A, Ota M, Yamagishi S, Watanabe K, Watanabe K, Kanazawa A, Ichikawa Y, Oba M, Morita S, Hashiguchi Y, Kunisaki C, Endo I. Short‐term results of a randomized study between laparoscopic and open surgery in elderly colorectal cancer patients. Surgical endoscopy 2014;28(2):466‐76. [DOI] [PubMed] [Google Scholar]
Garcia 2008
- Garcia MK, Skibber JM, Rodriguez‐Bigas MA, Chang DZ, Feig BW, Bisanz AK, Palmer JL, Cohen L, Chiang JS. Acupuncture to prevent prolonged postoperative ileus: a randomized controlled trial. Medical Acupuncture 2008;20(2):83‐8. [Google Scholar]
Gervaz 2006
- Gervaz P, Bucher P, Scheiwiller A, Mugnier‐Konrad B, Morel P. The duration of postoperative ileus after elective colectomy is correlated to surgical specialization. International Journal of Colorectal Disease 2006;21(6):542‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Ho 2014
- Ho YM, Smith SR, Pockney P, Lim P, Attia J. A meta‐analysis on the effect of sham feeding following colectomy: should gum chewing be included in enhanced recovery after surgery protocols?. Diseases of the Colon & Rectum 2014;57(1):115‐26. [DOI] [PubMed] [Google Scholar]
Holte 2000
- Holte K, Kehlet H. Postoperative ileus: a preventable event. British Journal of Surgery 2000;87(11):1480‐93. [DOI] [PubMed] [Google Scholar]
Hosono 2006
- Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta‐analysis of short‐term outcomes after laparoscopy‐assisted distal gastrectomy. World Journal of Gastroenteroly 2006;12(47):7676‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, bxk+2and the size of a sample. BMC Medical Research Methodology 2005;5(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2009
- Johnson MD, Walsh RM. Current therapies to shorten postoperative ileus. Cleveland Clinic Journal of Medicine 2009;76(11):641‐8. [DOI] [PubMed] [Google Scholar]
Kouba 2007
- Kouba EJ, Wallen EM, Pruthi RS. Gum chewing stimulates bowel motility in patients undergoing radical cystectomy with urinary diversion. Urology 2007;70(6):1053‐6. [DOI] [PubMed] [Google Scholar]
Kronberg 2011
- Kronberg U, Kiran RP, Soliman MS, Hammel JP, Galway U, Coffey JC, Fazio VW. A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Annals of Surgery 2011;253(1):78‐81. [DOI] [PubMed] [Google Scholar]
Le Blanc‐Louvry 2002
- Blanc‐Louvry I, Costaglioli B, Boulon C, Leroi AM, Ducrotte P. Does mechanical massage of the abdominal wall after colectomy reduce postoperative pain and shorten the duration of ileus? Results of a randomized study. Journal of Gastrointestinal Surgery 2002;6(1):43‐9. [DOI] [PubMed] [Google Scholar]
Lewis 2001
- Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta‐analysis of controlled trials. British Medical Journal 2001;323(7316):773‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013
- Li S, Liu YQ, Peng QL, Xie, L, Wang J, Qin X. Chewing gum reduces postoperative ileus following abdominal surgery: a meta‐analysis of 17 randomized controlled trials. Journal of Gastroenterology and Hepatology 2013;28(7):1122‐32. [DOI] [PubMed] [Google Scholar]
Livingston 1990
- Livingston EH, Passaro EP. Postoperative ileus. Digestive Diseases and Sciences 1990;35(1):121‐32. [DOI] [PubMed] [Google Scholar]
Massey 2010
- Massey RL. A randomized trial of rocking‐chair motion on the effect of postoperative ileus duration in patients with cancer recovering from abdominal surgery. Applied Nursing Research 2010;23(2):59‐64. [DOI] [PubMed] [Google Scholar]
Müller 2012
- Müller SA1=, Rahbari NN, Schneider F, Warschkow R, Simon T, Frankenberg M, Bork U, Weitz J, Schmied BM, Büchler MW. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. British Journal of Surgery 2012;99(11):1530‐8. [DOI] [PubMed] [Google Scholar]
Noble 2009
- Noble EJ, Harris R, Hosie KB, Thomas S, Lewis SJ. Gum chewing reduces postoperative ileus? A systematic review and meta‐analysis. International Journal of Surgery 2009;7(2):100‐5. [DOI] [PubMed] [Google Scholar]
Parnaby 2009
- Parnaby CN, MacDonald AJ, Jenkins JT. Sham feed or sham? A meta‐analysis of randomized clinicaltrials assessing the effect of gum chewing on gut functionafter elective colorectal surgery. International Journal of Colorectal Disease 2009;24(5):585‐92. [DOI] [PubMed] [Google Scholar]
Purkayastha 2008
- Purkayastha S, Tilney HS, Darzi AW, Tekkis PP. Meta‐analysis of randomized studies evaluating chewing gum to enhance postoperative recovery following colectomy. Archives of Surgery 2008;143(8):788‐93. [DOI] [PubMed] [Google Scholar]
Smith 2014
- Smith S. Sham feeding with gum following caesarian section: something to chew on. BJOG: an international journal of obstetrics and gynaecology 2014;121(7):799. [Google Scholar]
StataCorp 2013 [Computer program]
- TX: StataCorp LP. StataCorp. Version Stata Statistical Software: Release 13. College Station: TX: StataCorp LP, 2013.
Story 2009
- Story SK, Chamberlain RS. A comprehensive review of evidence‐based strategies to prevent and treat postoperative ileus. Digestive Surgery 2009;26(4):265‐75. [DOI] [PubMed] [Google Scholar]
Svatek 2010
- Svatek RS, Fisher MB, Williams MB, Matin SF, Kamat AM, Grossman HB, Nogueras‐Gonzalez GM, Urbauer DL, Dinney CP. Age and body mass index are independent risk factors for the development of postoperative paralytic ileus after radical cystectomy. Urology 2010;76(6):1419‐24. [DOI] [PubMed] [Google Scholar]
Tan 2006
- Tan EK, Cornish J, Darzi AW, Tekkis PP. Meta‐analysis: alvimopan vs. placebo in the treatment ofpost‐operative ileus. Alimentary Pharmacology & Therapeutics 2006;25(1):47‐57. [DOI] [PubMed] [Google Scholar]
Tandeter 2009
- Tandeter H. Hypothesis: hexitols in chewing gum may play a role in reducing postoperative ileus. Medical Hypotheses 2009;72(1):39‐40. [DOI] [PubMed] [Google Scholar]
Tu 2014
- Tu C, Tsai C, Tsai C, Huang T, Cheng S, Liu T. Postoperative ileus in the elderly. International Journal of Gerontology 2014;8(1):1‐5. [Google Scholar]
Vasquez 2009
- Vasquez W, Hernandez AV, Garcia‐Sabrido JL. Is gum chewing useful for ileus after elective colorectal surgery? A systematic review and meta‐analysis of randomized clinical trials. Journal of Gastrointestinal Surgery 2009;13(4):649‐56. [DOI] [PubMed] [Google Scholar]
Vather 2013
- Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. Journal of Gastrointestinal Surgery 2013;17(5):962‐72. [DOI] [PubMed] [Google Scholar]
Warren 2011
- Warren J, Bhalla V, Cresci G. Postoperative diet advancement: surgical dogma vs evidence‐based medicine. Nutrition in Clinical Practice 2011;26(2):115‐25. [DOI] [PubMed] [Google Scholar]
Yin 2013
- Yin Z, Sun J, Liu, T, Zhu Y, Peng SL, Wang J. Gum chewing: another simple potential method for more rapid improvement of postoperative gastrointestinal function. Digestion 2013;87(2):67‐74. [DOI] [PubMed] [Google Scholar]
Yuan 2011
- Yuan Y, Zhao H, He J, Gong D. Chewing gum in promoting bowel recovery after cesarean section: a systematic review. 中国循证医学杂志 2011;11(4):427‐32. [Google Scholar]
Zhu 2014
- Zhu YP, Wang WJ, Zhang SL, Dai B, Ye DW. Effects of gum chewing on postoperative bowel motility after caesarean section: a meta‐analysisof randomised controlled trials. BJOG: an international journal of obstetrics and gynaecology 2014;121(7):787‐92. [DOI] [PubMed] [Google Scholar]
Zhuang 2014
- Zhuang CL, Chen WZ, Yu Z. Should gum chewing be included in enhanced recovery after surgery programs for colorectal surgery?. Diseases of the Colon & Rectum 2014;57(6):E361‐2. [DOI] [PubMed] [Google Scholar]


