Abstract
Purpose:
The U.S. Food and Drug Administration indications for cochlear implantation in children is currently 9 months of age and older for children with bilateral profound sensorineural hearing loss (SNHL). Studies have shown that earlier activation of a cochlear implant (CI) can lead to better spoken language outcomes. As auditory skills are a precursor to the development of spoken language, this study was developed to investigate the influence of age at CI activation on auditory skill acquisition in young children. A secondary aim was to describe the auditory skills of children implanted prior to 9 months of age as compared to children with older ages of activation.
Method:
Functional Listening Index (FLI) scores obtained during routine clinical visits were reviewed for 78 pediatric CI recipients with congenital bilateral profound hearing loss who were activated before 2 years of age. A linear mixed-effects model assessed the effect of age at CI activation on cumulative FLI scores over time.
Results:
There was a significant interaction between age at activation and chronological age at the time of evaluation, indicating that children with earlier access to sound achieved a greater number of auditory skills than those with later CI activations when measured at the same chronological age. Children activated before the age of 9 months approximated scores expected of children with typical hearing, whereas children activated between 9 and 24 months of age did not.
Conclusions:
Younger age at CI activation is associated with increased auditory skills over time. Children who undergo cochlear implantation and CI activation before 9 months achieve more auditory skills by 4 years of age than children who are activated at later ages. These data suggest that reducing the approved age at cochlear implantation for children with congenital bilateral profound SNHL may support optimal auditory skill acquisition.
Early identification of hearing loss and appropriate intervention is critical for spoken language development in children. The Joint Committee on Infant Hearing (JCIH) has recommended that children undergo a hearing screening by 1 month of age, diagnostic evaluation by 3 months of age, and appropriate intervention by 6 months of age. The most recent JCIH position statement recommends a shift to a 1–2–3 criteria, for those centers currently meeting 1–3–6 goals (JCIH, 2019). The Food and Drug Administration (FDA) has approved cochlear implantation in cases of bilateral profound sensorineural hearing loss (SNHL), though not until 9 months of age (U.S. FDA, 2020); putting these children behind the 1–3–6 timeline. Typically, children with bilateral profound SNHL are fit with hearing aids; unfortunately, amplification for acoustic thresholds exceeding 90 dB HL provide little improvement in performance. For individuals with profound SNHL, hearing aids do not compensate for distortions within the auditory system and are limited in their ability to provide access to a full range of sounds with sufficient audibility (Lesica, 2018). Thus, acoustic amplification for children with bilateral profound SNHL who are pursuing spoken language would not meet the definition of “appropriate intervention” set forth by the JCIH. Cochlear implantation is the most effective intervention for the development of listening and spoken language in children with bilateral profound SNHL (Ching et al., 2017; Chweya et al., 2021; Dettman et al., 2007, 2021; Geers, 2004, 2006; Karltorp et al., 2020; Niparko et al., 2010; Sharma et al., 2020).
There is longstanding evidence that a younger age at implantation is associated with better outcomes for pediatric cochlear implant (CI) recipients, which has triggered decreasing in the approved age at implantation. In 1997, researchers investigated speech perception outcomes in children using early multichannel electrode arrays. They compared children implanted between 2 and 4 years, 4 and 5 years, 5 and 8 years, and 8+ years of age and found better open set speech perception for children implanted before 5 years of age as compared to those implanted after 5 years (Fryauf-Bertschy et al., 1997). To describe the benefits of implantation before age 2 years, Waltzman et al. (2002) studied the surgical and speech perception outcomes of 11 children implanted between 14 and 23 months of age. They did not find increased surgical risk and descriptive comparisons to a previous study suggested that children implanted under age 2 years had better outcomes than their older counterparts. Downward movement of the age of implantation has continued, with studies indicating that implantation prior to 12 months of age is advantageous for language development (Leigh et al., 2013; Ruben, 2018; Tajudeen et al., 2010; Wie et al., 2020).
Some studies have challenged the “younger is better” notion (Holt & Svirsky, 2008), particularly when considering other factors such as device use, cognition, communication mode, and duration of use (Wie et al., 2007). Since those earlier years, however, the benefits of early implantation in young children have been demonstrated using language assessments, with CI users implanted at younger ages experiencing better outcomes, even when controlling for other factors, such as socioeconomic status, IQ, and maternal sensitivity (Ching et al., 2018; Leigh et al., 2013, 2016). In 2020, the FDA approved CIs for children down to 9 months of age (U.S. FDA, 2020); the first change in pediatric CI age recommendations in nearly 20 years (Park et al., 2021).
While the change in indications for cochlear implantation has moved closer in the direction of meeting JCIH recommendations there is a question of whether 9 months is an optimal limit. Delays in expressive spoken language have been found to be approximately equal to the length of auditory deprivation (Leigh et al., 2013, 2016). Early implantation and activation of the CI reduces the length of auditory deprivation and takes advantage of sensitive periods of auditory neural plasticity (Cardon et al., 2012). To meet JCIH guidelines for early intervention, cochlear implantation and subsequent activation of the CI must happen earlier than 9 months of age. Recent research shows that cochlear implantation is safe for younger children. Surgical complications in children under 12 months are no greater than children implanted at later ages (Miyamoto et al., 2017; Purcell et al., 2021; Sbeih et al., 2022). Implantation under 9 months of age has also been found to carry no age-specific risks (Deep et al., 2021; Karltorp et al., 2020).
The significant benefits of CI use in the development of language have been observed for children implanted under 9 months of age as compared to children implanted between 9 and 12 months of age (Chweya et al., 2021; Deep et al., 2021; Hoff et al., 2019; Miyamoto et al., 2017; Roland et al., 2009). For instance, Chweya et al. (2021) compared language outcomes between children implanted before 9 months of age, between 9 and 11 months of age, and between 12 and 36 months of age. Children who were implanted under 9 months of age had significantly better receptive and expressive language skills than those in the 9- to 11-month group (Chweya et al., 2021). Karltorp et al. (2020) found that children who received their first CI at 5–8 months of age had similar expressive and receptive language outcomes on the Reynell Development Language Scales as children with typical hearing by 4 years of age. Children who received their first CI at 9–12 months of age were on average 9 months behind children with typical hearing for expressive and receptive language (Karltorp et al., 2020). Recently, Dettman et al. (2021) reported findings from a longitudinal study that evaluated language outcomes in children who received their CI prior to 9 months of age. Consistent with Karltorp et al.'s (2020) findings, children who received their CI prior to 9 months of age scored within the range of children with typical hearing. The authors noted a significant effect of the age at CI activation for language performance. While these studies offer compelling evidence regarding language and communication outcomes, little is known about the effects of age at implantation on early auditory skill development.
Children implanted in infancy are not able to participate in speech perception testing for several years after they receive access to sound, yet they continue to learn about sound in their environments. Auditory skills are the precursor to the development of spoken language and have been shown to predict speech perception and production abilities (Tait et al., 2007). Before young children can participate in speech perception testing, audiologists and speech-language pathologists (SLPs) can use checklists of auditory skill development. Current commercially available instruments used to measure performance with CIs in the youngest population include the Infant-Toddler Meaningful Auditory Integration Scale (IT-MAIS; Zimmerman-Phillips et al., 1997), the LittlEARS Auditory Questionnaire (LEAQ; Coninx et al., 2009), and the Auditory Skills Checklist (ASC; Meinzen-Derr et al., 2007). Tracking auditory skill development allows the clinician to measure how listening skills are progressing prior to the development of spoken language, as demonstrated in the Childhood Development After Cochlear Implantation study (Eisenberg et al., 2006). The authors of the Pediatric Minimum Speech Test Battery suggest using the ASC or the LEAQ; however, they acknowledge that children may approach ceiling performance on the LEAQ before 2 years of age (Uhler et al., 2017).
The more recently developed Functional Listening Index (FLI), developed by The Shepherd Centre, is a 60-item scale that tracks the development of auditory skills from birth through 5 years of age for six categories: sound awareness, associating sound with meaning, comprehending simple spoken language, comprehending language in different listening conditions, listening through discourse and narratives, and advanced open listening set (Davis et al., 2015). While somewhat hierarchical in nature, skills in each category overlap in expected time course of development. In general, children with typical hearing achieve sound awareness by 3 months, begin associating sound with meaning at 4 months, start to understand simple spoken language by 7 months, begin to comprehend language in different conditions at 10 months, begin to listen through discourse and narratives at 15 months, and start to understand advanced open listening sets at 3 years of age (HearingFirst, 2022). The FLI tracks many of the same skills as the ASC but moves through more advanced open set listening skills and may be more sensitive to subtle differences in auditory development than other scales (Davis et al., 2015; Meinzen-Derr et al., 2007). The FLI is given over multiple time points, building on previous scores at every administration. At each visit, the examiner probes each of the 60 items that have not yet been achieved. If the child is performing that skill, their current age is recorded. An age of acquisition is recorded for each of the 60 items; hence a cumulative score can be calculated for the age of the child at each test administration by adding the number of skills that have been achieved by that test point. The FLI can help clinicians observe progress in audition before the child has developed spoken language skills, allowing for earlier intervention if necessary. This intervention may consist of CI mapping changes, changes to speech therapy programs, more frequent audiology visits, or additional referrals (Kosaner et al., 2013).
Few studies have investigated the impact of age at implantation on the development of auditory skills. Tait et al. (2007) studied 99 implanted children and found that auditory skills were best in those implanted between 1 and 2 years of age. Using the IT-MAIS, a study of Mandarin speaking children found a positive correlation between age and auditory skills in children implanted between ages 1 and 3 years (Long et al., 2018). McConkey Robbins et al. (2004) studied 107 children implanted between 1 and 3 years of age, also with the IT-MAIS, and found that children implanted at younger age acquired auditory skills more like their peers with typical hearing (McConkey Robbins et al., 2004). Notably, all the children in that study underwent cochlear implantation by 12 months of age or later. One study with a relatively small number of participants used the LEAQ to compare the auditory skills of children implanted under 9 months of age to the skills of children implanted at 9–12 or over 12 months of age. While the same study found the youngest age group achieved better language outcomes, they did not see a significant difference in auditory skills (Chweya et al., 2021). There is a need to understand auditory skill development as a function of age at CI activation with a tool that assesses as broad a range of skills (Hagr et al., 2015) and includes children implanted under 9 months of age. The purpose of this study was to investigate the influence of age at CI activation on the acquisition of auditory skills. Of particular interest was the comparison of children activated before and after 9 months of age. It was hypothesized that age at CI activation would influence the number of auditory skills a child would be expected to achieve as they aged. Specifically, we expected to see that children with congenital bilateral profound SNHL who began to listen with a CI before 9 months of age would acquire a greater number of auditory skills at a younger age than children activated between 9 and 23 months.
Method
This retrospective review was approved by the institutional review board at the University of North Carolina at Chapel Hill. A clinical database query included typically developing children with congenital bilateral profound SNHL who received a CI and had access to sound by 2 years of age. Participants were excluded if English was not the primary language of the home. Pure-tone thresholds were obtained prior to implantation via age-appropriate behavioral methods (visual reinforcement audiometry or conditioned play audiometry) using insert earphones. Participants were implanted at the study site between January of 2014 and April of 2021. All were bilateral CI users by the final data collection point. Six-hundred thirty-two test points were included for 78 children activated between 0.6 and 1.9 years of age (M = 1.1 ± 0.3 years). The youngest participant was implanted at 7 months and 8 days of age. Participants were tested an average of 8 times across the study period (SD = ± 2.8).
The clinical protocol at the study site is for SLPs and audiologists to document patients' auditory skills using the FLI during mapping appointments up to 5 years of age. Responses are based on clinicians' observations during the appointment, reports from early interventionists, and parent/guardian report. Chronological age and cumulative number of FLI skills are recorded at each visit. There are opportunities to record FLI data at approximately eight visits over the first year of CI use and twice a year thereafter. For this study, clinical data were sparse after the age of 4 years; therefore, the reviewed data were those obtained between activation and 4 years of age.
Data Analysis
A linear mixed-effects model evaluated the main effects and associated interactions of age at test interval and age at activation (AgeCIA) on the cumulative FLI score using R statistical software (R Core Team, 2022) with participant as a random factor. Each participant had multiple points included in the analysis as they were assessed during follow-up appointments as they aged. Since a logarithmic relationship was noted, age at test point was transformed using a log2 transform prior to analysis (log2Age).
Results
Descriptive patient data are presented in Table 1, and a summary of continuous patient factors in Table 2. Device wear time is described in the table as hearing hours percentage, as described by Park et al. (2019).
Table 1.
Demographic summary of study patients.
| Variable | < 9 mo (n = 16) n (% of group) |
9–11 mo (n = 19) n (% of group) |
12–17 mo (n = 34) n (% of group) |
18–23 mo (n = 9) n (% of group) |
|---|---|---|---|---|
| Etiology | ||||
| cCMV | 0 (0%) | 2 (11%) | 4 (12%) | 0 (0%) |
| Connexin 26 | 5 (31%) | 6 (39%) | 5 (15%) | 2 (22%) |
| Malformation | 0 (0%) | 0 (0%) | 2 (6%) | 0 (0%) |
| Other genetic | 0 (0%) | 2 (11%) | 1 (3%) | 0 (0%) |
| Pendred Syndrome | 0 (0%) | 0 (0%) | 1 (3%) | 2 (22%) |
| Prematurity complications | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) |
| Suspected hereditary | 3 (19%) | 1 (5%) | 1 (3%) | 1 (11%) |
| Unknown | 8 (50%) | 6 (32%) | 16 (47%) | 3 (33%) |
| Usher's Syndrome | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Waardenburg Syndrome | 0 (0%) | 1 (5%) | 3 (9%) | 0 (0%) |
| Manufacturer | ||||
| Advanced Bionics | 1 (6%) | 1 (5%) | 2 (6%) | 0 (0%) |
| MED-EL | 12 (75%) | 6 (32%) | 13 (38%) | 2 (22%) |
| Cochlear | 3 (19%) | 12 (63%) | 19 (56%) | 7 (78%) |
| Race/ethnicity | ||||
| African American | 3 (19%) | 0 (0%) | 8 (24%) | 2 (22%) |
| Asian | 1 (6%) | 3 (16%) | 0 (0%) | 0 (0%) |
| Hispanic | 2 (13%) | 1 (5%) | 4 (12%) | 3 (33%) |
| Mixed race | 1 (6%) | 1 (5%) | 4 (12%) | 1 (11%) |
| Native American | 0 (0%) | 1 (5%) | 1 (3%) | 0 (0%) |
| Other | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) |
| White | 9 (56%) | 13 (68%) | 16 (47%) | 3 (33%) |
| Surgery setting | ||||
| Simultaneous | 13 (81%) | 13 (68%) | 8 (24%) | 2 (23%) |
| Sequential | 3 (19%) | 6 (32%) | 26 (76%) | 7 (77%) |
Note. mo = months; cCMV = congenital cytomegalovirus.
Table 2.
Continuous demographic factors of the study patients.
| Variable | < 9 mo | 9–11 mo | 12–17 mo | 18–23 mo |
|---|---|---|---|---|
| Age at first implant activation (Y) | ||||
| M (± SD) | 0.75 (± 0.05) | 0.93 (± 0.06) | 1.16 (± 0.11) | 1.89 (± 0.09) |
| Min | 0.65 | 0.84 | 1.00 | 1.52 |
| Max | 0.83 | 0.98 | 1.38 | 2.00 |
| Interdevice interval (Y) | ||||
| M (± SD) | 0.05 (± 0.11) | 0.12 (± 0.18) | 9.69 (± 0.92) | 0.66 (± 0.50) |
| Min | 0.00 | 0.00 | 0.00 | 0.00 |
| Max | 0.28 | 0.52 | 1.53 | 1.30 |
| Hearing hours percentage (%) | ||||
| M (± SD) | 54.84 (± 25.96) | 59.92 (± 24.78) | 66.18 (± 25.30) | 72.67 (± 22.55) |
| Min | 0.00 | 0.00 | 0.00 | 16.89 |
| Max | 107.73 | 103.56 | 114.51 | 105.76 |
| Age at test point (Y) | ||||
| M (± SD) | 1.28 (± 0.68) | 1.69 (± 0.79) | 1.97 (± 0.77) | 2.61 (± 0.70) |
| Min | 0.65 | 0.84 | 1.06 | 1.78 |
| Max | 3.95 | 3.98 | 3.99 | 4.00 |
| Average number of test points (Y) | ||||
| M (± SD) | 6.31 (± 2.57) | 8.84 (± 2.92) | 8.65 (± 2.55) | 7.67 (± 1.89) |
| Min | 3 | 3 | 3 | 4 |
| Max | 12 | 16 | 13 | 10 |
Note. mo = months; Y = years; SD = standard deviation.
The results of the linear mixed model are presented in Table 3. There was a significant interaction between AgeCIA and log2Age on FLI scores, F(1, 540) = 11.63, p < .001), indicating that age at implantation impacted a child's FLI score at each test point. Results suggest that children who were activated at younger ages achieved higher cumulative FLI scores as chronological age increased than those who gained access to sound at older ages. The model resulted in the equation below:
| (1) |
| (2) |
Table 3.
Results of the linear mixed-effects model.
| Variable | Estimate | SE | T value | p value |
|---|---|---|---|---|
| (Intercept) | 32.32 | 2.32 | 13.93 | < .001 |
| AgeCIA | −28.32 | 2.20 | −12.86 | < .001 |
| log2Age | 18.71 | 1.46 | 12.80 | < .001 |
| AgeCIA*log2Age | 4.70 | 1.38 | 3.41 | < .001 |
Note. AgeCIA = age at cochlear implant activation; log2Age = log2 transformed age at test; SE = standard error.
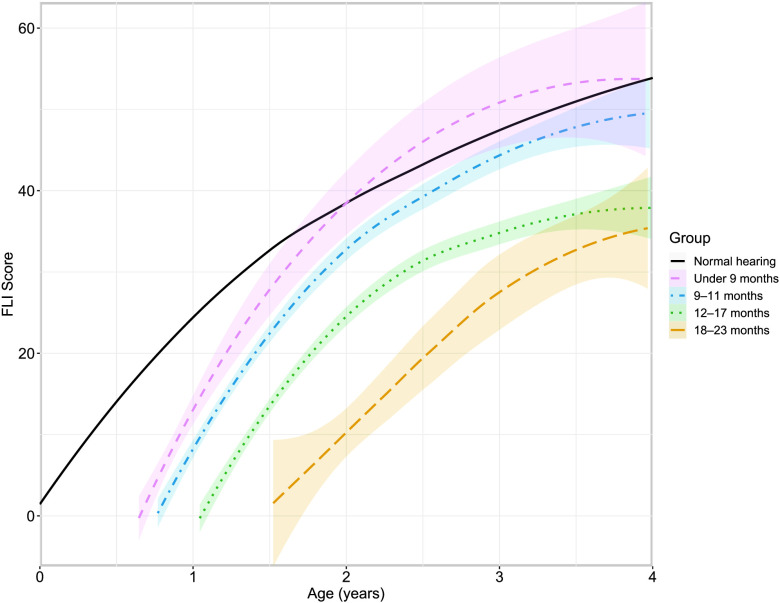
Figure 1 plots the FLI scores over time as a function of age at CI activation in comparison to the expected trajectory for children with typical hearing reported by Davis et al. (2015). Data were separated into groups based on age at activation (i.e., < 9 months, 9–11 months, 12–17 months, and 18–23 months), with 16, 19, 34, and nine patients, respectively. This grouping aligns with changes in candidacy criteria over time (Park et al., 2021). We were unable to statistically compare groups due to the large difference in numbers of patients; however, visualization of the data suggests that children implanted before 9 months of age approximate the auditory skills of children with typical hearing by 2 years of age, whereas children implanted at older ages remained delayed compared to children with typical hearing.
Figure 1.
Regression curves for Functional Listening Index (FLI) scores by age at testing grouped by age at cochlear implant activation as defined in the legend. Shaded regions represent the 95% confidence interval. The typical hearing trajectory is based on clinical knowledge of auditory skill development (Davis et al., 2015).
The model equation was used to estimate the expected cumulative FLI score based on age at CI activation and chronological age at each test point. Table 4 lists the predicted FLI scores and number of expected skills for the age at CI activation groups at different chronological ages. These findings suggest that children activated at a younger age accumulate more FLI skills over time as compared to children whose CI is activated at an older age. Table 5 shows the clinical correlate of the predicted FLI score (Davis et al., 2015).
Table 4.
Predicted Functional Listening Index (FLI) scores for the age at cochlear implant activation groups at different chronological ages.
| Group | Age 1 Y [log2Age = 0] | Age 2 Y [log2Age = 1] | Age 3 Y [log2Age = 1.58] | Age 4 Y [log2Age = 2] |
|---|---|---|---|---|
| AgeCIA = 7 M | 16 (26) | 37 (38) | 49 (47) | 58 (54) |
| AgeCIA = 10 M | 8 (26) | 31 (38) | 44 (47) | 54 (54) |
| AgeCIA = 13 M | 26 (38) | 39 (47) | 49 (54) | |
| AgeCIA = 16 M | 19 (38) | 34 (47) | 44 (54) | |
| AgeCIA = 19 M | 14 (38) | 29 (47) | 40 (54) | |
| AgeCIA = 22 M | 8 (38) | 24 (47) | 35 (54) |
Note. The expected number of FLI skills based on typical development is presented in parentheses. Y = years; log2Age = log2 transformed age at test; AgeCIA = age at cochlear implant activation; M = months.
Table 5.
Typical clinical correlates of the skills represented by the predicted Functional Listening Index scores in Table 4 (Davis et al., 2015).
| Group | Age 1 Y [log2Age = 0] | Age 2 Y [log2Age = 1] | Age 3 Y [log2Age = 1.58] | Age 4 Y [log2Age = 2] |
|---|---|---|---|---|
| AgeCIA = 7 M | Responds to name in quiet | Selects two items through listening alone | Accurately repeats highly predictable sentences | Repeats a 6–10 word sentence from a digital voice—unfamiliar topic and vocabulary |
| AgeCIA = 10 M | Responds to music | Responds appropriately to everyday requests without context | Answers simple questions about a known topic | Has 2–3 appropriate conversational turns with a familiar speaker |
| AgeCIA = 13 M | Selects one item through listening alone | Demonstrates “overhearing” | Accurately repeats highly predictable sentences | |
| AgeCIA = 16 M | Recognizes names of immediate family members | Identifies familiar songs from a digital signal | Answers simple questions about a known topic | |
| AgeCIA = 19 M | Discriminates between angry and friendly tones | Imitates words heard | Selects 3 items through listening alone | |
| AgeCIA = 22 M | Responds to music | Responds to name in background noise | Imitates 2-word utterances |
Note. Y = years; log2Age = log2 transformed age at test; AgeCIA = age at cochlear implant activation; M = months.
Discussion
The results of this study indicate that earlier age of CI activation is associated with accumulation of a greater quantity of auditory skills at a younger chronological age. Children implanted and activated before 9 months of age experienced a rapid growth of auditory skills that reached the expected performance of children with typical hearing by 2 years of age. Children implanted at 9 months and older also experienced an improvement in auditory skills, though average performance did not converge with children with typical hearing. For example, the model shows that a child who begins listening with a CI by 7 months of age is predicted to have a FLI score of 16 by 12 months of age. Clinically, that would correlate to consistently responding to their name in quiet (see Tables 4 and 5). A child who undergoes implantation at the FDA-approved criterion (i.e., 9 months) and is activated at 10 months of age is predicted to have a FLI score of 8 by 12 months of age. This child would just be beginning to react to music, not yet attaching meaning to sound as the child activated at 7 months of age does.
Auditory skill acquisition also appears to stagnate for children activated at 12 months of age or later. For example, a child activated by 10 months of age is predicted to have accumulated eight skills within the 2 months they have access to sound. The same can be said for a child activated at 22 months of age. However, after an additional year of use, the child activated at 10 months of age would be predicted to have accumulated 23 additional skills while the child activated at 22 months would only have accumulated 16 more skills over the same period. Clinically speaking, this would mean that the child activated by 10 months would be able to respond appropriately to everyday requests without context after 14 months of listening, while the child activated later would have only recently begun responding to their name in the presence of background noise after the same period of CI use. As this study included data up until 4 years of age, it is possible that children in the older age groups eventually reach the auditory skill achievements of typical hearing children beyond the age of 4 years. Dunn et al. (2014) found that the impact of age at implantation diminishes over time with respect to higher order language skills, and it is possible that the same happens for auditory skills. The long-term impacts of age at implantation on auditory skills have not yet been investigated.
Mixed outcomes have been reported from the few studies that have investigated the development of auditory skills in children undergoing implantation as infants. Similar to findings in this study, Waltzman and Roland (2005) found a younger age of implantation resulted in better auditory outcomes. The authors used the IT-MAIS to assess auditory skills in children who were implanted before 12 months of age. Of the 18 participants, three received their CI prior to 9 months of age, with the youngest implanted at 6 months of age. Although some improvement in scores was attributed to maturational effects, significant improvements were made in all categories at by 6-month postactivation visit and were maintained at the 12-month visit (Waltzman & Roland, 2005). Chweya et al. (2021) did not find implantation under 9 months of age to be associated with a greater benefit to auditory skill acquisition, as the LEAQ showed no significant differences in auditory skills as compared to older age groups (Chweya et al., 2021). This may be reflective of the limitations of the questionnaire regarding children reaching ceiling effects early (Uhler et al., 2017).
Outcomes for children implanted in infancy are typically reported based on language development; however, auditory development forms the basis for spoken communication. Standardized language measures generally do not address a young child's responses to sounds and phonemes. With the exception of a few items in the Preschool Language Scales–Fifth Edition, no language measures address a child's responses to sounds in their environment (Zimmerman et al., 2011). These are foundational skills achieved before children develop comprehension or use of actual vocabulary and language. There are no current language measures that assess responses to Ling Six sounds, whispered sounds, suprasegmental information (e.g., Learning to Listen Sounds, songs/nursery rhymes, tone of voice), sound/object associations, localization, responses to speech in noise, or other related auditory skills; questionnaires and checklists are generally used. As younger children are implanted, it is important to have developmentally appropriate measures in place. The inclusion of auditory skill development measures can allow for assessment of progress in younger CI recipients. Tracking auditory skills can also be a useful counseling tool for parents. Acknowledging progress in their child's listening abilities is a powerful counseling tool to motivate parents prior to hearing their child's first words. The results of this study can be used to support parental consideration of early implantation, showing that even before the age of 2 years, earlier implantation results in better development of audition; the building blocks for spoken language.
This was a clinically focused, small study that did have limitations. There are several factors aside from age at implantation that are known to influence outcomes and were not considered here. Further research in this area may compare broader family demographic data such as maternal education, socioeconomic status, enrollment in early intervention, and linguistic environment to determine impacts on auditory skill acquisition (Arjmandi et al., 2021; Ching, 2015; Geers & Brenner, 2003). The FLI has been updated by The Shepherd Centre since this study was initiated (Davis et al., 2022), and validation of the new measure is ongoing (ClinicalTrials.gov, 2021; The Shepherd Centre, 2018). The version used in this study is not standardized or normed. Additionally, we were not able to directly compare group outcomes statistically. Controlled studies with more subjects will contribute to establishing normative results to accurately compare auditory skill growth.
Current FDA indications require children with profound hearing loss to wait longer than JCIH recommendation of 6 months for appropriate intervention. Previous studies have clearly established that CI use is the most beneficial treatment toward the goal of listening and spoken language for children with bilateral profound hearing loss (Ching et al., 2017, 2018; Colletti et al., 2011; Dettman et al., 2007; Hayes et al., 2009; Leigh et al., 2013; Miyamoto et al., 2008; Niparko et al., 2010; Sharma et al., 2020). The present data indicate that earlier age at implantation provides benefit regarding auditory skill development in children with congenital bilateral profound SNHL.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Award UL1TR002489. M.T.D., M.E.R., and L.R.P. are supported by a research grant provided to their university by MED-EL Corporation. K.D.B. serves on the Surgical Advisory Boards for MED-EL Corporation and Advanced Bionics Corporation and is a consultant for Cochlear Corporation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors acknowledge the Shepherd Centre's work in developing the Functional Listening Index. They also thank the speech-language pathologists and audiologists at the Children's Cochlear Implant Center at the University of North Carolina for their diligence in collecting the data used in this study.
Funding Statement
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Award UL1TR002489. M.T.D., M.E.R., and L.R.P. are supported by a research grant provided to their university by MED-EL Corporation. K.D.B. serves on the Surgical Advisory Boards for MED-EL Corporation and Advanced Bionics Corporation and is a consultant for Cochlear Corporation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Arjmandi, M. K. , Houston, D. , & Dilley, L. C. (2021). Variability in quantity and quality of early linguistic experience in children with cochlear implants: Evidence from analysis of natural auditory environments. Ear and Hearing, 43(2), 685–698. https://doi.org/10.1097/AUD.0000000000001136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon, G. , Campbell, J. , & Sharma, A. (2012). Plasticity in the developing auditory cortex: Evidence from children with sensorineural hearing loss and auditory neuropathy spectrum disorder. Journal of the American Academy of Audiology, 23(6), 396–411. https://doi.org/10.3766/jaaa.23.6.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching, T. Y. C. (2015). Is early intervention effective in improving spoken language outcomes of children with congenital hearing loss? American Journal of Audiology, 24(3), 345–348. https://doi.org/10.1044/2015_AJA-15-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching, T. Y. C. , Dillon, H. , Button, L. , Seeto, M. , Van Buynder, P. , Marnane, V. , Cupples, L. , & Leigh, G. (2017). Age at intervention for permanent hearing loss and 5-year language outcomes. Pediatrics, 140(3), e20164274. https://doi.org/10.1542/peds.2016-4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching, T. Y. C. , Dillon, H. , Leigh, G. , & Cupples, L. (2018). Learning from the Longitudinal Outcomes of Children with Hearing Impairment (LOCHI) study: Summary of 5-year findings and implications. International Journal of Audiology, 57(Suppl. 2), S105–S111. https://doi.org/10.1080/14992027.2017.1385865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chweya, C. M. , May, M. M. , DeJong, M. D. , Baas, B. S. , Lohse, C. M. , Driscoll, C. L. W. , & Carlson, M. L. (2021). Language and audiological outcomes among infants implanted before 9 and 12 months of age versus older children: A continuum of benefit associated with cochlear implantation at successively younger ages. Otology & Neurotology, 42(5), 686–693. https://doi.org/10.1097/MAO.0000000000003011 [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. (2021). FLI normative study. https://clinicaltrials.gov/ct2/show/study/NCT03410017
- Colletti, L. , Mandalà, M. , Zoccante, L. , Shannon, R. V. , & Colletti, V. (2011). Infants versus older children fitted with cochlear implants: Performance over 10 years. International Journal of Pediatric Otorhinolaryngology, 75(4), 504–509. https://doi.org/10.1016/j.ijporl.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Coninx, F. , Weichbold, V. , Tsiakpini, L. , Autrique, E. , Bescond, G. , Tamas, L. , Compernol, A. , Georgescu, M. , Koroleva, I. , Le Maner-Idrissi, G. , Liang, W. , Madell, J. , Mikić, B. , Obrycka, A. , Pankowska, A. , Pascu, A. , Popescu, R. , Radulescu, L. , Rauhamäki, T. , … Brachmaier, J. (2009). Validation of the LittlEARS® Auditory Questionnaire in children with normal hearing. International Journal of Pediatric Otorhinolaryngology, 73(12), 1761–1768. https://doi.org/10.1016/j.ijporl.2009.09.036 [DOI] [PubMed] [Google Scholar]
- Davis, A. , Harrison, E. , & Cowan, R. (2022). The Feasibility of the Functional Listening Index—Paediatric (FLI-P®) for young children with hearing loss. Journal of Clinical Medicine, 11(10), 2764. https://doi.org/10.3390/jcm11102764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, A. , Neal, K. , Abrahams, Y. , & Hansen, A. (2015). Using the Functional Listening Index following cochlear implantation in young children: The precursor to speech and language [Paper presentation] . CI 2015 Symposium. https://www.acialliance.org/resource/resmgr/CI2015_PPT/functional_Listening_Index_N.pdf [Google Scholar]
- Deep, N. L. , Purcell, P. L. , Gordon, K. A. , Papsin, B. C. , Roland, J. T. , & Waltzman, S. B. (2021). Cochlear implantation in infants: Evidence of safety. Trends in Hearing, 25. https://doi.org/10.1177/23312165211014695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettman, S. , Choo, D. , Au, A. , Luu, A. , & Dowell, R. (2021). Speech perception and language outcomes for infants receiving cochlear implants before or after 9 months of age: Use of category-based aggregation of data in an unselected pediatric cohort. Journal of Speech, Language, and Hearing Research, 64(3), 1023–1039. https://doi.org/10.1044/2020_JSLHR-20-00228 [DOI] [PubMed] [Google Scholar]
- Dettman, S. J. , Pinder, D. , Briggs, R. J. S. , Dowell, R. C. , & Leigh, J. R. (2007). Communication development in children who receive the cochlear implant younger than 12 months: Risks versus benefits. Ear and Hearing, 28(2), 11S–18S. https://doi.org/10.1097/AUD.0b013e31803153f8 [DOI] [PubMed] [Google Scholar]
- Dunn, C. C. , Walker, E. A. , Oleson, J. , Kenworthy, M. , Van Voorst, T. , Tomblin, J. B. , & Gantz, B. J. (2014). Longitudinal speech perception and language performance in pediatric cochlear implant users: The effect of age at implantation. Ear and Hearing, 35(2), 148–160. https://doi.org/10.1097/AUD.0b013e3182a4a8f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg, L. S. , Johnson, K. C. , Martinez, A. S. , Cokely, C. G. , Tobey, E. A. , Quittner, A. L. , Fink, N. E. , Wang, N. Y. , Niparko, J. K. , & CDaCI Investgative Team. (2006). Speech recognition at 1-year follow-up in the childhood development after cochlear implantation study: Methods and preliminary findings. Audiology & Neurotology, 11(4), 259–268. https://doi.org/10.1159/000093302 [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy, H. , Tyler, R. S. , Kelsay, D. M. , Gantz, B. J. , & Woodworth, G. G. (1997). Cochlear implant use by prelingually deafened children: The influences of age at implant and length of device use. Journal of Speech, Language, and Hearing Research, 40(1), 183–199. https://doi.org/10.1044/jslhr.4001.183 [DOI] [PubMed] [Google Scholar]
- Geers, A. E. (2004). Speech, language, and reading skills after early cochlear implantation. Archives of Otolaryngology—Head & Neck Surgery, 130(5), 634–638. https://doi.org/10.1001/archotol.130.5.634 [DOI] [PubMed] [Google Scholar]
- Geers, A. E. (2006). Factors influencing spoken language outcomes in children following early cochlear implantation. Advances in Oto-Rhino-Laryngology, 64, 50–65. https://doi.org/10.1159/000094644 [DOI] [PubMed] [Google Scholar]
- Geers, A. E. , & Brenner, C. (2003). Background and educational characteristics of prelingually deaf children implanted by five years of age. Ear and Hearing, 24(1), 2S–14S. https://doi.org/10.1097/01.AUD.0000051685.19171.BD [DOI] [PubMed] [Google Scholar]
- Hagr, A. , Garadat, S. N. , Al-Momani, M. , Alsabellha, R. M. , & Almuhawas, F. A. (2015). Feasibility of one-day activation in cochlear implant recipients. International Journal of Audiology, 54(5), 323–328. https://doi.org/10.3109/14992027.2014.996824 [DOI] [PubMed] [Google Scholar]
- Hayes, H. , Geers, A. E. , Treiman, R. , & Moog, J. S. (2009). Receptive vocabulary development in deaf children with cochlear implants: Achievement in an intensive auditory-oral educational setting. Ear and Hearing, 30(1), 128–135. https://doi.org/10.1097/AUD.0b013e3181926524 [DOI] [PubMed] [Google Scholar]
- HearingFirst. (2022). Development milestones: Birth to 8 years. https://www.hearingfirst.org/
- Hoff, S. , Ryan, M. , Thomas, D. , Tournis, E. , Kenny, H. , Hajduk, J. , & Young, N. M. (2019). Safety and effectiveness of cochlear implantation of young children, including those with complicating conditions. Otology & Neurotology, 40(4), 454–463. https://doi.org/10.1097/MAO.0000000000002156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, R. F. , & Svirsky, M. A. (2008). An exploratory look at pediatric cochlear implantation: Is earliest always best? Ear and Hearing, 29(4), 492–511. https://doi.org/10.1097/AUD.0b013e31816c409f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing. (2019). Year 2019 Position Statement: Principles and guidelines for early hearing detection and intervention programs. Journal of Early Hearing Detection and Intervention , 4(2), 1–44. https://doi.org/10.15142/fptk-b748 [PubMed] [Google Scholar]
- Karltorp, E. , Eklöf, M. , Östlund, E. , Asp, F. , Tideholm, B. , & Löfkvist, U. (2020). Cochlear implants before 9 months of age led to more natural spoken language development without increased surgical risks. Acta Paediatrica, 109(2), 332–341. https://doi.org/10.1111/apa.14954 [DOI] [PubMed] [Google Scholar]
- Kosaner, J. , Sonuguler, S. , Olgun, L. , & Amann, E. (2013). Young cochlear implant users' auditory development as measured and monitored by the LittlEARS® Auditory Questionnaire: A Turkish experience. International Journal of Pediatric Otorhinolaryngology, 77(8), 1359–1363. https://doi.org/10.1016/j.ijporl.2013.05.036 [DOI] [PubMed] [Google Scholar]
- Leigh, J. , Dettman, S. , Dowell, R. , & Briggs, R. (2013). Communication development in children who receive a cochlear implant by 12 months of age. Otology & Neurotology, 34(3), 443–450. https://doi.org/10.1097/MAO.0b013e3182814d2c [DOI] [PubMed] [Google Scholar]
- Leigh, J. R. , Dettman, S. J. , & Dowell, R. C. (2016). Evidence-based guidelines for recommending cochlear implantation for young children: Audiological criteria and optimizing age at implantation. International Journal of Audiology, 55(Suppl. 2), S9–S18. https://doi.org/10.3109/14992027.2016.1157268 [DOI] [PubMed] [Google Scholar]
- Lesica, N. A. (2018). Why do hearing aids fail to restore normal auditory perception? Trends in Neurosciences, 41(4), 174–185. https://doi.org/10.1016/j.tins.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, Y. , Liu, H. , Li, Y. , Jin, X. , Zhou, Y. , Li, J. , Zheng, Z. , Liu, P. , Zhao, Y. , Zheng, J. , Zhang, J. , Chen, M. , Hao, J. , Yang, Y. , & Liu, W. (2018). Early auditory skills development in Mandarin speaking children after bilateral cochlear implantation. International Journal of Pediatric Otorhinolaryngology, 114, 153–158. https://doi.org/10.1016/j.ijporl.2018.08.039 [DOI] [PubMed] [Google Scholar]
- McConkey Robbins, A. , Koch, D. B. , Osberger, M. J. , Zimmerman-Phillips, S. , & Kishon-Rabin, L. (2004). Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Archives of Otolaryngology—Head & Neck Surgery, 130(5), 570–574. https://doi.org/10.1001/archotol.130.5.570 [DOI] [PubMed] [Google Scholar]
- Meinzen-Derr, J. , Wiley, S. , Creighton, J. , & Choo, D. (2007). Auditory skills checklist: Clinical tool for monitoring functional auditory skill development in young children with cochlear implants. The Annals of Otology, Rhinology & Laryngology, 116(11), 812–818. https://doi.org/10.1177/000348940711601104 [DOI] [PubMed] [Google Scholar]
- Miyamoto, R. T. , Colson, B. , Henning, S. , & Pisoni, D. (2017). Cochlear implantation in infants below 12 months of age. World Journal of Otorhinolaryngology-Head and Neck Surgery, 3(4), 214–218. https://doi.org/10.1016/j.wjorl.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, R. T. , Hay-McCutcheon, M. J. , Kirk, K. I. , Houston, D. M. , & Bergeson-Dana, T. (2008). Language skills of profoundly deaf children who received cochlear implants under 12 months of age: A preliminary study. Acta Oto-Laryngologica, 128(4), 373–377. https://doi.org/10.1080/00016480701785012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko, J. K. , Tobey, E. A. , Thal, D. J. , Eisenberg, L. S. , Wang, N.-Y. , Quittner, A. L. , Fink, N. E. , & CDaCI Investigative Team. (2010). Spoken language development in children following cochlear implantation. The Journal of the American Medical Association, 303(15), 1498–1506. https://doi.org/10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, L. R. , Gagnon, E. B. , & Brown, K. D. (2021). The limitations of FDA criteria: Inconsistencies with clinical practice, findings, and adult criteria as a barrier to pediatric implantation. Seminars in Hearing, 42(04), 373–380. https://doi.org/10.1055/s-0041-1739370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, L. R. , Gagnon, E. B. , Thompson, E. , & Brown, K. D. (2019). Age at full-time use predicts language outcomes better than age of surgery in children who use cochlear implants. American Journal of Audiology, 28(4), 986–992. https://doi.org/10.1044/2019_AJA-19-0073 [DOI] [PubMed] [Google Scholar]
- Purcell, P. L. , Deep, N. L. , Waltzman, S. B. , Roland, J. T. , Cushing, S. L. , Papsin, B. C. , & Gordon, K. A. (2021). Cochlear implantation in infants: Why and how. Trends in Hearing, 25. https://doi.org/10.1177/23312165211031751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
- Roland, J. T. , Cosetti, M. , Wang, K. H. , Immerman, S. , & Waltzman, S. B. (2009). Cochlear implantation in the very young child: Long-term safety and efficacy. The Laryngoscope, 119(11), 2205–2210. https://doi.org/10.1002/lary.20489 [DOI] [PubMed] [Google Scholar]
- Ruben, R. J. (2018). Language development in the pediatric cochlear implant patient. Laryngoscope Investigative Otolaryngology, 3(3), 209–213. https://doi.org/10.1002/lio2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbeih, F. , Bouzaher, M. H. , Appachi, S. , Schwartz, S. , Cohen, M. S. , Carvalho, D. , Yoon, P. , Liu, Y. C. C. , & Anne, S. (2022). Safety of cochlear implantation in children 12 months or younger: Systematic review and meta-analysis. Otolaryngology—Head and Neck Surgery. Advance online publication. https://doi.org/10.1177/01945998211067741 [DOI] [PubMed] [Google Scholar]
- Sharma, S. D. , Cushing, S. L. , Papsin, B. C. , & Gordon, K. A. (2020). Hearing and speech benefits of cochlear implantation in children: A review of the literature. International Journal of Pediatric Otorhinolaryngology, 133, 109984. https://doi.org/10.1016/j.ijporl.2020.109984 [DOI] [PubMed] [Google Scholar]
- Tait, M. E. , Nikolopoulos, T. P. , & Lutman, M. E. (2007). Age at implantation and development of vocal and auditory preverbal skills in implanted deaf children. International Journal of Pediatric Otorhinolaryngology, 71(4), 603–610. https://doi.org/10.1016/j.ijporl.2006.12.010 [DOI] [PubMed] [Google Scholar]
- Tajudeen, B. A. , Waltzman, S. B. , Jethanamest, D. , & Svirsky, M. A. (2010). Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otology & Neurotology, 31(8), 1254–1260. https://doi.org/10.1097/MAO.0b013e3181f2f475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Shepherd Centre. (2018). FLI-P® | Hearhub. https://hearhub.org/home/features/tools/fli-p/
- Uhler, K. , Warner-Czyz, A. , Gifford, R. , & PMSTB Working Group. (2017). Pediatric minimum speech test battery. Journal of the American Academy of Audiology, 28(3), 232–247. https://doi.org/10.3766/jaaa.15123 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. (2020). Nucleus 24 cochlear implant system PMA supplement approval letter. U.S. FDA Device Approvals, Denials and Clearances. [Google Scholar]
- Waltzman, S. B. , Cohen, N. L. , Green, J. , & Roland, J. T. (2002). Long-term effects of cochlear implants in children. Otolaryngology–Head & Neck Surgery, 126(5), 505–511. https://doi.org/10.1067/mhn.2002.124472 [DOI] [PubMed] [Google Scholar]
- Waltzman, S. B. , & Roland, J. T. (2005). Cochlear implantation in children younger than 12 months. Pediatrics, 116(4), e487–e493. https://doi.org/10.1542/peds.2005-0282 [DOI] [PubMed] [Google Scholar]
- Wie, O. B. , Falkenberg, E.-S. , Tvete, O. , & Tomblin, B. (2007). Children with a cochlear implant: Characteristics and determinants of speech recognition, speech-recognition growth rate, and speech production. International Journal of Audiology, 46(5), 232–243. https://doi.org/10.1080/14992020601182891 [DOI] [PubMed] [Google Scholar]
- Wie, O. B. , Torkildsen, J. v. K. , Schauber, S. , Busch, T. , & Litovsky, R. (2020). Long-term language development in children with early simultaneous bilateral cochlear implants. Ear and Hearing, 41(5), 1294–1305. https://doi.org/10.1097/AUD.0000000000000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, I. L. , Steiner, V. G. , & Pond, R. E. (2011). Preschool Language Scale (5th ed.). APA PsycTests. [Google Scholar]
- Zimmerman-Phillips, S. , Osberger, M. J. , & Robbins, A. M. (1997). Infant-Toddler Meaningful Auditory Integration Scale. Advanced Bionics Corporation. [Google Scholar]