Abstract
Simple Summary
In the present work, we describe the satellite DNA families that occur in the genomes of two snakes from different families: Daboia russelii (Viperidae) and Pantherophis guttatus (Colubridae). We show high conservation of nucleotide sequences and chromosomal localizations of these satellites, despite the widespread view that such genomic elements evolve very rapidly.
Abstract
Repetitive DNA sequences constitute a sizeable portion of animal genomes, and tandemly organized satellite DNAs are a major part of them. They are usually located in constitutive heterochromatin clusters in or near the centromeres or telomeres, and less frequently in the interstitial parts of chromosome arms. They are also frequently accumulated in sex chromosomes. The function of these clusters is to sustain the architecture of the chromosomes and the nucleus, and to regulate chromosome behavior during mitosis and meiosis. The study of satellite DNA diversity is important for understanding sex chromosome evolution, interspecific hybridization, and speciation. In this work, we identified four satellite DNA families in the genomes of two snakes from different families: Daboia russelii (Viperidae) and Pantherophis guttatus (Colubridae) and determine their chromosomal localization. We found that one family is localized in the centromeres of both species, whereas the others form clusters in certain chromosomes or subsets of chromosomes. BLAST with snake genome assemblies showed the conservation of such clusters, as well as a subtle presence of the satellites in the interspersed manner outside the clusters. Overall, our results show high conservation of satellite DNA in snakes and confirm the “library” model of satellite DNA evolution.
Keywords: Serpentes, Colubridae, Viperidae, sex chromosomes, repetitive DNA, centromere
1. Introduction
Repetitive DNA sequences are a key component of eukaryotic genomes. There are several types of repeats, classified by their structure and sub-chromosomal localization. Interspersed and tandem repeats are recognized by their genomic organization. Interspersed repeats can be located in various regions of the genome, whereas tandem repeats are mostly organized into clusters in specific segments of chromosomes [1]. Satellite DNA sequences (satDNA) are among the most abundant types of tandem repeats. They are usually located in the C-positive heterochromatic blocks at centromeres, as well as in the pericentromeric, subtelomeric, and, more rarely, interstitial chromosomal regions [2]. Every eukaryotic genome usually contains several families of satDNAs, with each family having its specific localization. For example, centromeric heterochromatin is typically composed of the special centromeric satellite, whereas the pericentromeric heterochromatin blocks harbor the satellites of other families [3]. Some satDNA families and subfamilies occur at similar positions in all chromosomes (e.g., pan-centromeric repeats), whereas others are accumulated on a subset of chromosomes or even one specific chromosome (for example, a sex chromosome) [4]. Specific satellite families spread inside chromosomes and between chromosomes by means of ectopic recombination, gene conversion, and transposition with mobile genetic elements (TEs) [5,6,7].
Since satDNAs do not encode proteins, they were once viewed as “selfish”, “junk DNA”, and “genomic parasites”. However, there is a growing body of evidence that satDNA clusters are technical elements of chromosomes that participate in regulating their structure and behavior during the cell cycle, i.e., condensation, decondensation, kinetochore formation, and meiotic pairing [8,9,10]. Depending on their function, satDNAs differ in their degree of conservation. While certain families are species-specific, others can be characteristic for the whole genus or taxonomic family [11,12,13]. It has been hypothesized that satDNA divergence may contribute to the constrained meiotic chromosome pairing in hybrids, thus directly affecting speciation [14]. This makes satDNA an important marker to study phylogenetics, genome evolution, and genome function in diverse animal groups.
In reptiles, satDNAs are poorly studied. A notable exception is the lizard family Lacertidae, in which numerous satellites have been identified and extensively studied [15,16,17,18,19]. Two satellites have been identified in Scincidae [20,21], and two satellite families have been found in Varanidae [22,23]. Four types of satDNAs are known from the Chinese softshell turtle (Pelodiscus sinensis) [24]. Recently, a high conservation of tandem repetitive DNAs has been demonstrated in crocodilians [25]. Snakes comprise nearly half of the total squamate diversity; however, data on their satDNAs are scarce. Four families of satDNAs were found in different snake species. The PFL-MspI satellite was isolated from Protobothrops flavoviridis (Crotalinae, Viperidae), located in the centromeric regions of its chromosomes. This satDNA is shared at least by Gloydius blomhoffi from the same subfamily Crotalinae, as shown by FISH and slot blot hybridization. The slot blot analysis did not reveal this satellite even in Bitis arietans (Viperinae, Viperidae), a member of the same family. The PBI-MspI satellite was found in Python bivittatus, P. molurus, and Boa constrictor by FISH and slot blot, indicating the conservation of this satellite at least at the Henophidia level. Lastly, the PBI-DdeI satellite was initially identified as a major centromeric satellite in P. bivittatus, whereas FISH and slot blot failed to detect this satellite in any other genus [26]. However, later the PBI-DdeI was found in a wide set of diverse snake species using PCR. In Naja kaouthia, this repeat was accumulated in the W chromosome [27]. Apparently, sequence divergence and/or low copy number may impede the detection of a satDNA by hybridization methods. Another repetitive sequence, BamHI-B4, is specific to the terminal part of the homolog of the Anolis chromosome 6 (ZZ/ZW chromosome in Caenophidia and XX/XY chromosome in Python) and is conserved in pythons, colubrids, and pit vipers [28].
Classical “wet” methods of satDNA isolation include the analysis of genomic fragments in gradient centrifugation and the digestion of genomic DNA with restriction enzymes, while a range of bioinformatic approaches have recently been suggested to search for tandemly arranged DNAs in genomic data. In the present work, we used the Tandem Repeat Analyzer software (TAREAN) [29] to identify satellite repeats in two species of snakes, Daboia russelii (Viperinae, Viperidae) and Pantherophis guttatus (Colubridae), from short genomic reads. This software de novo identifies tandem organized satellite repeats from raw Illumina reads of a genomic sample. We studied their chromosomal localization using FISH and analyzed the cross-species conservation using BLAST on the available snake genome assemblies. The genome assemblies of Vipera latastei (Viperinae, Viperidae) (rVipLat1.pri) and V. ursinii (rVipUrs1.1) were used for quantitative and localization analysis, since they have the best assembled repeat clusters among the available assemblies of snakes.
2. Materials and Methods
2.1. Cell Line Establishment and Karyotype Analysis
The P. guttatus and D. russelii cells were grown from fibroblasts obtained from the Cambridge Resource Center for Comparative Genomics, Department of Veterinary Medicine, UK. The cell cultures were provided to the Institute of Molecular and Cellular Biology, SB RAS, Russia for joint research. The cell lines of P. guttatus and D. russelii were deposited in the IMCB SB RAS cell bank (“The general collection of cell cultures”, 0310-2016-0002). Chromosome suspensions from the cell cultures were obtained in the Laboratory of Comparative Genomics, IMCB SB RAS, Novosibirsk, Russia, as described previously [30,31].
2.2. Repetitive DNA Identification
DNA sequencing data were downloaded from the NCBI SRA database (accession number SRR5506741 for D. russelii genomic reads and SRR9596755 for P. guttatus) and used for the identification of tandemly arranged repeats. Filtering by quality and adapter trimming was performed using fastp 0.23.2 [32] with the parameters “--detect_adapter_for_pe -5 -3 -r -l 75”. Trimmed reads were used in the analysis with the TAREAN 2.3.7 tool [29], which identified clusters of the most abundant tandemly arranged repeats. NCBI BLAST [33] was used to compare consensus tandem repeat sequences with available genome assemblies. RepBase was used to compare consensus tandem repeat sequences with available described repeat sequences [34]
2.3. Fluorescence In Situ Hybridization (FISH)
DNA of P. guttatus and D. russelii was extracted from the cell cultures using the standard phenol–chloroform technique. Primers for PCR amplification and labeling of seven probes were designed with PrimerQuestTool [35] (Table 1). PCR amplification was performed as described earlier [36]. Labeling was performed using PCR by incorporation of biotin-dUTP and digoxigenin-dUTP (Sigma, Darmstadt, Germany). FISH was performed in accordance with previously published protocols [37]. Images were captured using the VideoTest-FISH software (Imicrotec, New York, NY, USA) with a JenOptic charge-coupled device (CCD) camera (Jena, Germany) mounted on an Olympus BX53 microscope (Shinjuku, Japan). All images were processed in Adobe PhotoShop 2021 (Adobe, San Jose, CA, USA).
Table 1.
Primers used to amplify satDNA in the current study.
| Satellite | Primer Sequences |
|---|---|
| PGU-Sat-1 | F 5’–TTTCAAGTACGAGCTTTCCC–3’ R 5’–GCTGAATTGAGCCCTACTG–3’ |
| PGU-Sat-2 | F 5’–GACACCAGGATGAGTTTCAG–3’ R 5’–TCCTGACCGTGGAGTAAA–3’ |
| PGU-Sat-3 | F 5’–CTTCCTCGGGCAGCAAA–3’ R 5’–GTAACAACGGATGCTAGAATGT–3’ |
| DRU-Sat-1 | F 5’–CCCGCCTGACCGAAGACC–3’ R 5’–GAGCTCTATCTGCAACGGG–3’ |
| DRU-Sat-2 | F 5’–ACCCCGAATCTCATTCTGGC–3’ R 5’–TCCTGATGCCGGGGTCAG–3’ |
| DRU-Sat-3 | F 5’–TTGTGTTTCTGGATCAATAACC–3’ R 5’–GCCTTTCCTGTATAATCCAAA–3’ |
| DRU-Sat-5 | F 5’–CAGAGCTGCTGGGAAGTG–3’ R 5’–GAGATCAATGAGGACCCCA–3’ |
3. Results
3.1. Tandem Repeat Identification
The TAREAN analysis revealed four high-confidence satellite repeats in the genome of D. russelii and three high-confidence satellite repeats in the genome of P. guttatus, which were named DRU-Sat-1, DRU-Sat-2, DRU-Sat-3, DRU-Sat-5, PGU-Sat-1, PGU-Sat-2, and PGU-Sat-3, respectively (Table 2). The satellites DRU-Sat-1 and PGU-Sat-1 were found to belong to the same family, while the satellites DRU-Sat-2, PGU-Sat-2, and PGU-Sat-3 belonged to another family. Interestingly, DRU-Sat-2 and PGU-Sat-2 shared a high level of similarity and were more distantly related to PGU-Sat-3 (Table 3, File S1).
Table 2.
Putative satellites revealed by TAREAN in the genomes of Daboia russelii and Pantherophis guttatus.
| Sattelite Name | Monomer Size (bp) | Genome Proportion, % | GC | Species | Accession Number |
|---|---|---|---|---|---|
| Content, % | |||||
| DRU-Sat-1 | 168 | 0.3 | 42.3 | Daboia russelii (Russell’s viper) | OP820475 |
| DRU-Sat-2 | 170 | 0.13 | 35.9 | —//— | OP820476 |
| DRU-Sat-3 | 64 | 0.012 | 37.5 | —//— | OP820477 |
| DRU-Sat-5 | 147 | 0.025 | 44.9 | —//— | OP820478 |
| PGU-Sat-1 | 167 | 0.31 | 42.5 | Pantherophis guttatus | OP820479 |
| (Corn snake) | |||||
| PGU-Sat-2 | 187 | 0.085 | 37.4 | —//— | OP820480 |
| PGU-Sat-3 | 169 | 0.043 | 39.1 | —//— | OP820481 |
Table 3.
The p-distances between the consensus sequences of the PGU-Sat-2/PGU-Sat-3/DRU-Sat-2 family.
| PGU-Sat-2 | PGU-Sat-3 | DRU-Sat-2 | |
|---|---|---|---|
| PGU-Sat-2 | - | —//— | —//— |
| PGU-Sat-3 | 0.278 | - | —//— |
| DRU-Sat-2 | 0.262 | 0.226 | - |
3.2. FISH Analysis
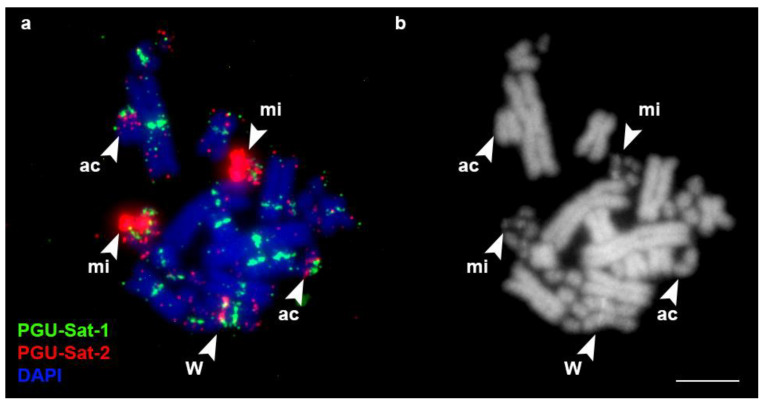
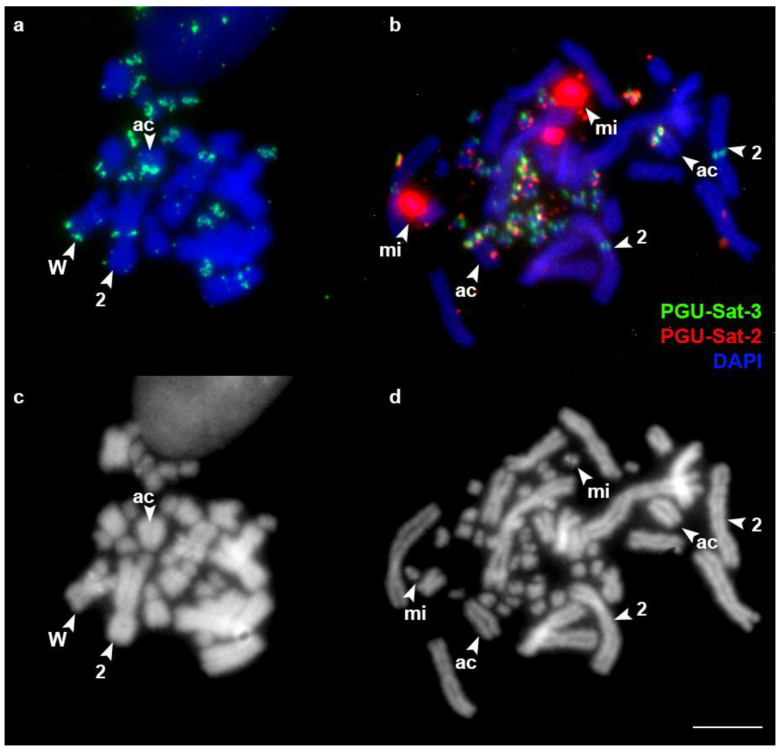
The karyotypes of the studied specimens comprised 36 chromosomes (eight pairs of macrochromosomes and 10 pairs of microchromosomes) with pairs of heteromorphic Z and W chromosomes. This is a typical snake karyotype that corresponds to the previously described karyotypes of these species [38,39]. In P. guttatus, the satellite PGU-Sat-1 was localized in the centromeric regions of macrochromosomes and in several microchromosomes. It was also localized in the DAPI-positive interstitial band of the W chromosome (Figure 1). The PGU-Sat-2 and PGU-Sat-3 satellite types, despite belonging to the same family, showed strikingly different chromosomal localizations. The PGU-Sat-2 satellite was mapped to the same DAPI-positive band in the W chromosome and in the pericentromeric region of one small acrocentric macrochromosome. It was also present in certain pairs of microchromosomes, being extensively amplified in one pair (Figure 1, Figure 2 and Figure S1). The PGU-Sat-3 satellite tended to be localized in microchromosomes, but not in all pairs. It was colocalized with PGU-Sat-2 in the pericentromeric region of one small acrocentric macrochromosome, and it was also present in the pericentromeric region of the q-arm of the chromosome 2 and in the terminal region of the p-arm of the W chromosome (Figure 2).
Figure 1.
Localization of the satellites PGU-Sat-1 and PGU-Sat-2 in the chromosomes of P. guttatus. mi: the PGU-Sat-2 bearing microchromosome; ac: the acrocentric macrochromosome with both PGU-Sat-1 and PGU-Sat-2 signals; W: the W chromosome. (a) Merged image; (b) DAPI channel. Scale bar: 10 μm.
Figure 2.
Localization of the satellites PGU-Sat-2 and PGU-Sat-3 in the chromosomes of P. guttatus. mi: the PGU-Sat-2 bearing microchromosome; ac: the acrocentric macrochromosome with both PGU-Sat-2 and PGU-Sat-3 signals; 2: chromosome 2; W: the W chromosome. (a,b) Merged images; (c,d) DAPI channel. Scale bar: 10 μm.
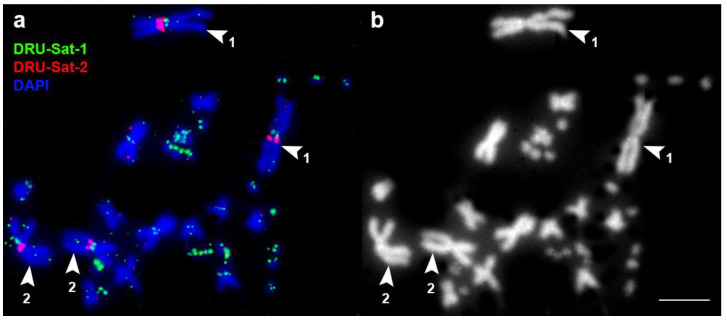
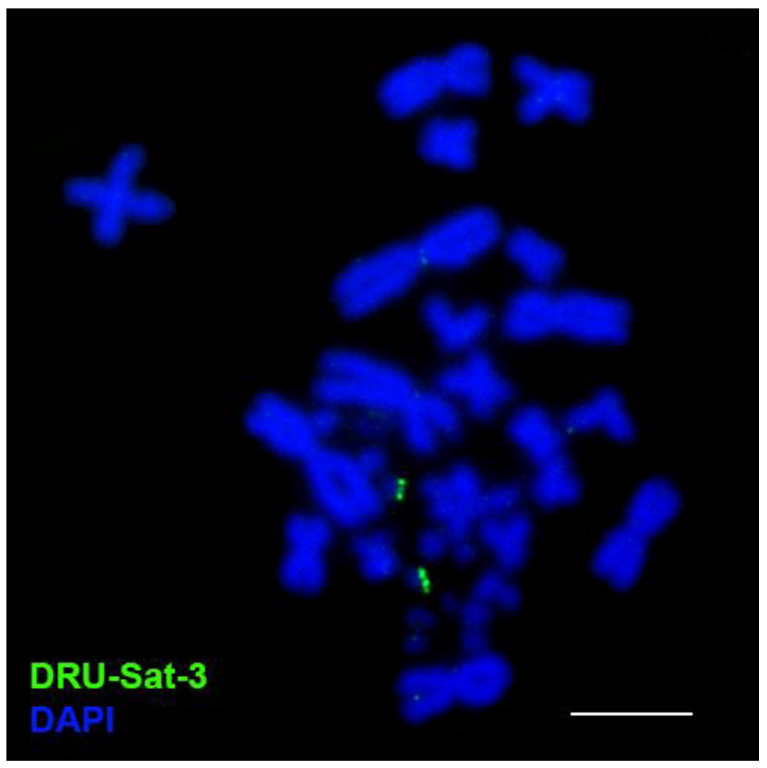
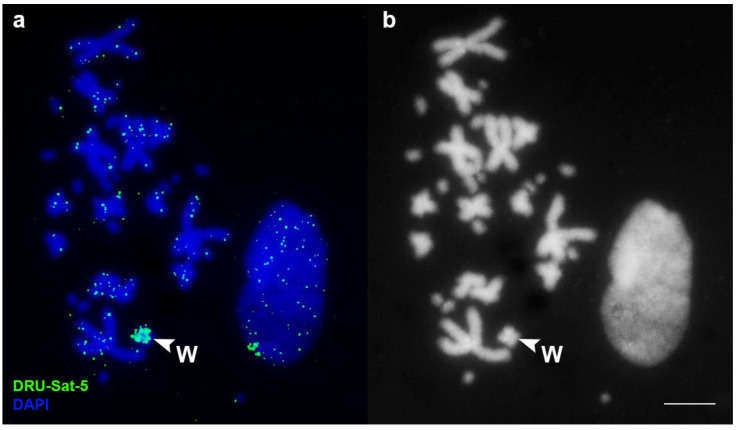
In D. russelii, the satellite DRU-Sat-1 was localized in the centromeric areas of all chromosomes. The satellite DRU-Sat-2 was localized in the p-arm of the chromosome 1 and the q-arm of chromosome 2 (Figure 3). The satellite DRU-Sat-3 was chromosome-specific and showed a band in one pair of microchromosomes (Figure 4). The satellite DRU-Sat-5 was amplified throughout the whole length of the W chromosome (Figure 5).
Figure 3.
Localization of the satellites DRU-Sat-1 and DRU-Sat-2 in the chromosomes of D. russelii. 1: chromosome 1; 2: chromosome 2. (a) Merged image; (b) DAPI channel. Scale bar: 10 μm.
Figure 4.
Localization of the satellite DRU-Sat-3 in the chromosomes of D. russelii. Scale: 10 μm.
Figure 5.
Localization of the satellite DRU-Sat-5 in the chromosomes of D. russelii. W: the W chromosome. (a) Merged image; (b) DAPI channel. Scale bar: 10 μm.
3.3. BLAST Analysis
Even though none of the detected satellites were found in the nr/nt NCBI database by BLAST, we found that the DRU-Sat-1/PGU-Sat-1 satellite belongs to the same family as PFL-MspI, which was described earlier [26,27]. We did not reveal homology between the other detected satDNAs and any of the previously described snake repetitive elements. However, we detected all the satellite families found in this work in the RefSeq genomes of other snakes by BLAST. The DRU-Sat-1/PGU-Sat-1, DRU-Sat-2/PGU-Sat-2/PGU-Sat-3 and DRU-Sat-3 satellites were found in various higher snakes, namely, Protobothrops mucrosquamatus, Crotalus tigris (Crotalinae, Viperidae), Notechis scutatus, Pseudonaja textilis (Elapidae), Thamnophis sirtalis, and Thamnophis elegans (Colubridae). Interestingly, BLAST revealed the DRU-Sat-3 satellite in Pantherophis guttatus, whereas TAREAN did not. The DRU-Sat-5 satellite was not found in any genome assemblies except those of Viperidae.
The alignment of DRU-Sat-1/PGU-Sat-1 to the genome assembly of V. latastei revealed its high copy number in all chromosome scaffolds except 15 and 17 (from 8877 in scaffold 3 to 358 in the scaffold Z), with predominantly medial localization, possibly corresponding to the centromere. The percentage identity between DRU-Sat-1/PGU-Sat-1 and the V. latastei sequences did not vary between the scaffolds and was between 95% and 97% for DRU-Sat-1.
The DRU-Sat-2/PGU-Sat-2/PGU-Sat-3 satellite was present in the scaffolds 1–3, Z, and 5–10, being the most abundant in scaffolds 2, 3, and 5. The copy numbers were 17,561, 2923, and 2522, respectively, in contrast to 101 in scaffold 1, where it was the second most abundant. In scaffolds 2, 3, and 5, this satellite was accumulated in clusters surrounding the centromere, possibly corresponding to the pericentromeric C bands. The copies in scaffolds 2, 3, and 5 had higher similarity to DRU-Sat-2 than the copies located in the scaffolds where this satellite was less abundant (percent of identity 91.86–94.08% versus 71.97–90.8%).
The satellite DRU-Sat-3 was present in 983 copies in scaffold 16 and was clustered in the subterminal position of the p-arm, if the DRU-Sat-1/PGU-Sat-1 cluster is considered as the centromere. Scaffold 2, where it was the second most abundant, harbored only 12 copies. The copies located in scaffold 16 had up to 96.88% identity with DRU-Sat-3, whereas the copies from other scaffolds had 75.86–93.1% identity. In the assembly of V. ursinii, scaffold 15 with 459 copies and similar cluster localization was the only scaffold where DRU-Sat-3 was found.
Lastly, satellite DRU-Sat-5 showed a small copy number in the genome assembly of V. latastei (up to 117 in the scaffold 1) and did not show any clustering. The W chromosome was not present in the assembly. In the assembly of V. ursinii, DRU-Sat-5 was accumulated in the W chromosome (1998 copies), whereas, in the autosomes, it had no more than 15 copies per chromosome.
4. Discussion
The satDNAs DRU-Sat-1/PGU-Sat-1, DRU-Sat-2/PGU-Sat-2/PGU-Sat-3, and DRU-Sat-3 are found in a wide range of higher snake genomes, which means that they originated at least in the common ancestor of Viperidae and Colubridae at ~42 MYA [40]. In contrast, DRU-Sat-5 is apparently younger as it is restricted to the Viperidae. Since it is present in both Viperinae and Crotalinae, its estimated age is therefore around 31 MY [40]. Previously, a more ancient snake satDNA, PBI-DdeI, which is shared by Henophidia and Caenophidia, was described [27]. In most species, it has a low copy number and probably lacks the tandem organization pattern; therefore, it is detectable only by PCR and BLAST with good-quality genome assemblies, and not by FISH and slot blot [26,27]. This satellite was also not detected by TAREAN in our work, although it is probably present in the genomes of the species studied, since TAREAN detects only highly repeated and tandemly organized elements. Possibly, PBI-DdeI is dispersed and low-copy in the genomes of D. russelii and P. guttatus. These findings challenge the common conception that satDNAs evolve very rapidly and are usually restricted to one species or a narrow phylogenetic clade, since the “recent appearance” may in fact mean a “recent rise in copy number” of an ancient satellite [41]. According to the concept known as the “library” model of satDNA evolution, animal genomes usually contain many diverse families of satDNAs (the “library”), only a few of which are highly amplified. During phylogenesis and speciation, the “library” experiences dynamic evolution, with satDNA families rising and decreasing in copy number, which leads to contrasting satDNA profiles in related species despite the qualitative conservation of the satDNA repertoires [11].
PGU-Sat-2 strongly indicates a pair of microchromosomes and may represent a considerable part of this chromosomes content. This is in contrast to avian microchromosomes, which are usually gene-rich and heterochromatin-poor. The revealed accumulation makes the PGU-Sat-2 probe a convenient tool for microchromosome identification.
The distribution of BLAST hits of the detected satellites in the genome assemblies of V. latastei and V. ursinii was similar to that observed in the FISH results for D. russelii. Specifically, DRU-Sat-1/PGU-Sat-1, which belong to the same family as the previously described PFL-MspI satellite, represent a centromeric repeat, DRU-Sat-2/PGU-Sat-2/PGU-Sat-3 is located in the pericentromeric clusters in a subset of macrochromosomes, DRU-Sat-3 is accumulated in one pair of microchromosomes, and DRU-Sat-5 is accumulated in the W chromosome. This result indicates that this satellite landscape at least predates the divergence between Vipera and Daboia, which occurred around 15 MYA [40]. We suppose that PCR for the DRU-Sat-5 marker may serve as a molecular sexing method for at least Vipera and Daboia. It should be further tested in other species of Viperidae.
5. Conclusions
In this work, we described four satellite DNA families in snake genomes, and revealed their chromosomal localization using FISH and BLAST in chromosome-level genome assemblies. Three of these four families are completely novel. We show that three families are conserved in Colubridae and Viperidae, whereas one is characteristic for Viperidae. In two satellite families, the pattern of chromosomal localization is conserved in both Colubridae and Viperidae, and, in two families, it is conserved in Daboia and Vipera. Our results indicate that, despite the common opinion that satellite DNA evolves extremely quickly and is usually species- or genus-specific, ancient repeat families are not rare. This corroborates the “library” model of the satellite DNA evolution, which supposes that diverse types of satellites may coexist in the genome, and that the common view of their very rapid appearance and disappearance may be due to their changes in copy number.
Acknowledgments
Computational resources were provided by the ELIXIR-CZ project (LM2015047), part of the international ELIXIR infrastructure.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani13030334/s1: File S1. FASTA alignment file showing sequence similarity of DRU-Sat-2, PGU-Sat-2, and PGU-Sat-3. Localization of the satellite PGU-Sat-2 (red) in the chromosomes of P. guttatus with reduced exposure time to show the signal in the PGU-Sat-2-bearing microchromosome in more detail. (a) Merged image; (b) DAPI channel. Scale bar: 10 μm.
Author Contributions
Conceptualization, V.T.; methodology, V.T., D.P. and A.L.; software, D.P.; validation, A.R., D.P. and V.T.; formal analysis, D.P. and A.R.; investigation, A.R.; resources, V.T. and M.F.-S.; data curation, D.P.; writing—original draft preparation, A.L.; writing—review and editing, A.L., V.T., A.R., D.P. and M.F.-S.; visualization, A.R. and A.L.; supervision, V.T.; project administration, V.T.; funding acquisition, V.T. All authors read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The consensus sequences of the identified satDNAs are deposited in GenBank.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by RFBR grant No 19-54-26017 and by the Ministry of Science and Higher Education of the Russian Federation grant number FWNR-2022-0015.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Biscotti M.A., Olmo E., Heslop-Harrison J.S. Repetitive DNA in Eukaryotic Genomes. Chromosome Res. 2015;23:415–420. doi: 10.1007/s10577-015-9499-z. [DOI] [PubMed] [Google Scholar]
- 2.Garrido-Ramos M.A. Satellite DNA: An Evolving Topic. Genes. 2017;8:230. doi: 10.3390/genes8090230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plohl M., Luchetti A., Meštrović N., Mantovani B. Satellite DNAs between Selfishness and Functionality: Structure, Genomics and Evolution of Tandem Repeats in Centromeric (Hetero) Chromatin. Gene. 2008;409:72–82. doi: 10.1016/j.gene.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Navajas-Pérez R., de La Herrán R., Jamilena M., Lozano R., Rejón C.R., Rejón M.R., Garrido-Ramos M.A. Reduced Rates of Sequence Evolution of Y-Linked Satellite DNA in Rumex (Polygonaceae) J. Mol. Evol. 2005;60:391–399. doi: 10.1007/s00239-004-0199-0. [DOI] [PubMed] [Google Scholar]
- 5.Meštrović N., Mravinac B., Pavlek M., Vojvoda-Zeljko T., Šatović E., Plohl M. Structural and Functional Liaisons between Transposable Elements and Satellite DNAs. Chromosome Res. 2015;23:583–596. doi: 10.1007/s10577-015-9483-7. [DOI] [PubMed] [Google Scholar]
- 6.Šatović E., Plohl M. Tandem Repeat-Containing MITEs in the Clam Donax Trunculus. Genome Biol. Evol. 2013;5:2549–2559. doi: 10.1093/gbe/evt202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satović E., Vojvoda Zeljko T., Luchetti A., Mantovani B., Plohl M. Adjacent Sequences Disclose Potential for Intra-Genomic Dispersal of Satellite DNA Repeats and Suggest a Complex Network with Transposable Elements. BMC Genom. 2016;17:997. doi: 10.1186/s12864-016-3347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagannathan M., Cummings R., Yamashita Y.M. A Conserved Function for Pericentromeric Satellite DNA. Elife. 2018;7:e34122. doi: 10.7554/eLife.34122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spangenberg V., Losev M., Volkhin I., Smirnova S., Nikitin P., Kolomiets O. DNA Environment of Centromeres and Non-Homologous Chromosomes Interactions in Mouse. Cells. 2021;10:3375. doi: 10.3390/cells10123375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biscotti M.A., Canapa A., Forconi M., Olmo E., Barucca M. Transcription of Tandemly Repetitive DNA: Functional Roles. Chromosome Res. 2015;23:463–477. doi: 10.1007/s10577-015-9494-4. [DOI] [PubMed] [Google Scholar]
- 11.Meštrović N., Plohl M., Mravinac B., Ugarković D. Evolution of Satellite DNAs from the Genus Palorus—Experimental Evidence for the “library” Hypothesis. Mol. Biol. Evol. 1998;15:1062–1068. doi: 10.1093/oxfordjournals.molbev.a026005. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann L., Venanzetti F., Sbordoni V. Characterization of a Species-Specific Satellite DNA Family of Dolichopoda Schiavazzii (Orthoptera, Rhaphidophoridae) Cave Crickets. J. Mol. Evol. 1994;39:274–281. doi: 10.1007/BF00160151. [DOI] [PubMed] [Google Scholar]
- 13.Mravinac B., Plohl M., Ugarković D. Preservation and High Sequence Conservation of Satellite DNAs Suggest Functional Constraints. J. Mol. Evol. 2005;61:542–550. doi: 10.1007/s00239-004-0342-y. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez F.R., de La Herrán R., Navajas-Pérez R., Cano-Roldán B., Sola-Campoy P.J., García-Zea J.A., Rejón C.R. Centromeric Satellite DNA in Flatfish (Order Pleuronectiformes) and Its Relation to Speciation Processes. J. Hered. 2017;108:217–222. doi: 10.1093/jhered/esw076. [DOI] [PubMed] [Google Scholar]
- 15.Capriglione T., Cardone A., Odierna G., Olmo E. Evolution of a Centromeric Satellite DNA and Phylogeny of Lacertid Lizards. Comp. Biochem. Physiol. Part B Comp. Biochem. 1991;100:641–645. doi: 10.1016/0305-0491(91)90233-4. [DOI] [PubMed] [Google Scholar]
- 16.Ciobanu D., Grechko V.v., Darevsky I.S., Kramerov D.A. New Satellite DNA in Lacerta s. str. Lizards (Sauria: Lacertidae): Evolutionary Pathways and Phylogenetic Impact. J. Exp. Zool. B Mol. Dev. Evol. 2004;302B:505–516. doi: 10.1002/jez.b.21014. [DOI] [PubMed] [Google Scholar]
- 17.Giovannotti M., Nisi Cerioni P., Rojo V., Olmo E., Slimani T., Splendiani A., Caputo Barucchi V. Characterization of a Satellite DNA in the Genera Lacerta and Timon (Reptilia, Lacertidae) and Its Role in the Differentiation of the W Chromosome. J. Exp. Zool. B Mol. Dev. Evol. 2018;330:83–95. doi: 10.1002/jez.b.22790. [DOI] [PubMed] [Google Scholar]
- 18.Giovannotti M., S’Khifa A., Nisi Cerioni P., Splendiani A., Slimani T., Fioravanti T., Olmo E., Caputo Barucchi V. Isolation and Characterization of Two Satellite DNAs in Atlantolacerta andreanskyi (Werner, 1929) (Reptilia, Lacertidae) J. Exp. Zool. B Mol. Dev. Evol. 2020;334:178–191. doi: 10.1002/jez.b.22937. [DOI] [PubMed] [Google Scholar]
- 19.Rojo V., Martínez-Lage A., Giovannotti M., González-Tizón A.M., Cerioni P.N., Barucchi V.C., Galán P., Olmo E., Naveira H. Evolutionary Dynamics of Two Satellite DNA Families in Rock Lizards of the Genus Iberolacerta (Squamata, Lacertidae): Different Histories but Common Traits. Chromosome Res. 2015;23:441–461. doi: 10.1007/s10577-015-9489-1. [DOI] [PubMed] [Google Scholar]
- 20.Giovannotti M., Nisi Cerioni P., Caputo V., Olmo E. Characterisation of a GC-Rich Telomeric Satellite DNA in Eumeces schneideri Daudin (Reptilia, Scincidae) Cytogenet. Genome Res. 2009;125:272–278. doi: 10.1159/000235933. [DOI] [PubMed] [Google Scholar]
- 21.Giovannotti M., Cerioni P.N., Splendiani A., Ruggeri P., Olmo E., Barucchi V.C. Slow Evolving Satellite DNAs: The Case of a Centromeric Satellite in Chalcides ocellatus (Forskål, 1775) (Reptilia, Scincidae) Amphib. Reptil. 2013;34:401–411. doi: 10.1163/15685381-00002905. [DOI] [Google Scholar]
- 22.Chaiprasertsri N., Uno Y., Peyachoknagul S., Prakhongcheep O., Baicharoen S., Charernsuk S., Nishida C., Matsuda Y., Koga A., Srikulnath K. Highly Species-Specific Centromeric Repetitive DNA Sequences in Lizards: Molecular Cytogenetic Characterization of a Novel Family of Satellite DNA Sequences Isolated from the Water Monitor Lizard (Varanus salvator macromaculatus, Platynota) J. Hered. 2013;104:798–806. doi: 10.1093/jhered/est061. [DOI] [PubMed] [Google Scholar]
- 23.Prakhongcheep O., Thapana W., Suntronpong A., Singchat W., Pattanatanang K., Phatcharakullawarawat R., Muangmai N., Peyachoknagul S., Matsubara K., Ezaz T., et al. Lack of Satellite DNA Species-Specific Homogenization and Relationship to Chromosomal Rearrangements in Monitor Lizards (Varanidae, Squamata) BMC Evol. Biol. 2017;17:193. doi: 10.1186/s12862-017-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada K., Nishida-Umehara C., Matsuda Y. Molecular and Cytogenetic Characterization of Site-Specific Repetitive DNA Sequences in the Chinese Soft-Shelled Turtle (Pelodiscus sinensis, Trionychidae) Chromosome Res. 2005;13:33–46. doi: 10.1007/s10577-005-2351-0. [DOI] [PubMed] [Google Scholar]
- 25.Romanenko S.A., Prokopov D.Y., Proskuryakova A.A., Davletshina G.I., Tupikin A.E., Kasai F., Ferguson-Smith M.A., Trifonov V.A. The Cytogenetic Map of the Nile Crocodile (Crocodylus niloticus, Crocodylidae, Reptilia) with Fluorescence In Situ Localization of Major Repetitive DNAs. Int. J. Mol. Sci. 2022;23:13063. doi: 10.3390/ijms232113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsubara K., Uno Y., Srikulnath K., Seki R., Nishida C., Matsuda Y. Molecular Cloning and Characterization of Satellite DNA Sequences from Constitutive Heterochromatin of the Habu Snake (Protobothrops flavoviridis, Viperidae) and the Burmese Python (Python bivittatus, Pythonidae) Chromosoma. 2015;124:529–539. doi: 10.1007/s00412-015-0529-6. [DOI] [PubMed] [Google Scholar]
- 27.Thongchum R., Singchat W., Laopichienpong N., Tawichasri P., Kraichak E., Prakhongcheep O., Sillapaprayoon S., Muangmai N., Baicharoen S., Suntrarachun S., et al. Diversity of PBI-DdeI Satellite DNA in Snakes Correlates with Rapid Independent Evolution and Different Functional Roles. Sci. Rep. 2019;9:15459. doi: 10.1038/s41598-019-51863-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsubara K., Tarui H., Toriba M., Yamada K., Nishida-Umehara C., Agata K., Matsuda Y. Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc. Natl. Acad. Sci. USA. 2006;103:18190–18195. doi: 10.1073/pnas.0605274103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novák P., Robledillo L.Á., Koblížková A., Vrbová I., Neumann P., Macas J. TAREAN: A Computational Tool for Identification and Characterization of Satellite DNA from Unassembled Short Reads. Nucleic Acids Res. 2017;45:e111. doi: 10.1093/nar/gkx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graphodatsky A.S., Yang F., Serdukova N., Perelman P., Zhdanova N.S., Ferguson-Smith M.A. Dog Chromosome-Specific Paints Reveal Evolutionary Inter- and Intrachromosomal Rearrangements in the American Mink and Human. Cytogenet. Genome Res. 2000;90:275–278. doi: 10.1159/000056788. [DOI] [PubMed] [Google Scholar]
- 31.Yang F., O’Brien P.C.M., Milne B.S., Graphodatsky A.S., Solanky N., Trifonov V., Rens W., Sargan D., Ferguson-Smith M.A. A Complete Comparative Chromosome Map for the Dog, Red Fox, and Human and Its Integration with Canine Genetic Maps. Genomics. 1999;62:189–202. doi: 10.1006/geno.1999.5989. [DOI] [PubMed] [Google Scholar]
- 32.Chen S., Zhou Y., Chen Y., Gu J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson M., Zaretskaya I., Raytselis Y., Merezhuk Y., McGinnis S., Madden T.L. NCBI BLAST: A Better Web Interface. Nucleic Acids Res. 2008;36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao W., Kojima K.K., Kohany O. Repbase Update, a Database of Repetitive Elements in Eukaryotic Genomes. Mob DNA. 2015;6:11. doi: 10.1186/s13100-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owczarzy R., Tataurov A.v., Wu Y., Manthey J.A., McQuisten K.A., Almabrazi H.G., Pedersen K.F., Lin Y., Garretson J., McEntaggart N.O., et al. IDT SciTools: A Suite for Analysis and Design of Nucleic Acid Oligomers. Nucleic Acids Res. 2008;36:W163–W169. doi: 10.1093/nar/gkn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biltueva L.S., Prokopov D.Y., Makunin A.I., Komissarov A.S., Kudryavtseva A.v., Lemskaya N.A., Vorobieva N.V., Serdyukova N.A., Romanenko S.A., Gladkikh O.L., et al. Genomic Organization and Physical Mapping of Tandemly Arranged Repetitive DNAs in Sterlet (Acipenser ruthenus) Cytogenet. Genome Res. 2017;152:148–157. doi: 10.1159/000479472. [DOI] [PubMed] [Google Scholar]
- 37.Romanenko S.A., Biltueva L.S., Serdyukova N.A., Kulemzina A.I., Beklemisheva V.R., Gladkikh O.L., Lemskaya N.A., Interesova E.A., Korentovich M.A., Vorobieva N.v., et al. Segmental Paleotetraploidy Revealed in Sterlet (Acipenser ruthenus) Genome by Chromosome Painting. Mol. Cytogenet. 2015;8:90. doi: 10.1186/s13039-015-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singchat W., Ahmad S.F., Sillapaprayoon S., Muangmai N., Duengkae P., Peyachoknagul S., O’Connor R.E., Griffin D.K., Srikulnath K. Partial amniote sex chromosomal linkage homologies shared on snake W sex chromosomes support the ancestral super-sex chromosome evolution in amniotes. Front. Genet. 2020;11:948. doi: 10.3389/fgene.2020.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augstenová B., Mazzoleni S., Kratochvíl L., Rovatsos M. Evolutionary dynamics of the W chromosome in caenophidian snakes. Genes. 2017;9:5. doi: 10.3390/genes9010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaher H., Murphy R.W., Arredondo J.C., Graboski R., Machado-Filho P.R., Mahlow K., Montingelli G.G., Quadros A.B., Orlov N.L., Wilkinson M., et al. Large-Scale Molecular Phylogeny, Morphology, Divergence-Time Estimation, and the Fossil Record of Advanced Caenophidian Snakes (Squamata: Serpentes) PLoS One. 2019;14:e0216148. doi: 10.1371/journal.pone.0216148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Ruano F.J., López-León M.D., Cabrero J., Camacho J.P.M. High-Throughput Analysis of the Satellitome Illuminates Satellite DNA Evolution. Sci. Rep. 2016;6:28333. doi: 10.1038/srep28333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The consensus sequences of the identified satDNAs are deposited in GenBank.