Abstract
Simple Summary
Colorectal cancer (CRC) is a significant cause of death worldwide. The inefficacy of the current treatment regimens is reflected in the frequent recurrence and emergence of a drug-resistant form of CRC. Numerous published reports from independent investigators around the globe have shown the great potential of natural products as a source of anti-CRC drug-leads with novel functions. Here, we have reviewed the literature on phenolic phytochemicals carrying anti-CRC activity in various in vivo models and analyzed their molecular basis of action to understand the implications of these findings in the future treatment and prevention of CRC.
Abstract
Colorectal cancer (CRC) is the third most diagnosed and second leading cause of cancer-related death worldwide. Limitations with existing treatment regimens have demanded the search for better treatment options. Different phytochemicals with promising anti-CRC activities have been reported, with the molecular mechanism of actions still emerging. This review aims to summarize recent progress on the study of natural phenolic compounds in ameliorating CRC using in vivo models. This review followed the guidelines of the Preferred Reporting Items for Systematic Reporting and Meta-Analysis. Information on the relevant topic was gathered by searching the PubMed, Scopus, ScienceDirect, and Web of Science databases using keywords, such as “colorectal cancer” AND “phenolic compounds”, “colorectal cancer” AND “polyphenol”, “colorectal cancer” AND “phenolic acids”, “colorectal cancer” AND “flavonoids”, “colorectal cancer” AND “stilbene”, and “colorectal cancer” AND “lignan” from the reputed peer-reviewed journals published over the last 20 years. Publications that incorporated in vivo experimental designs and produced statistically significant results were considered for this review. Many of these polyphenols demonstrate anti-CRC activities by inhibiting key cellular factors. This inhibition has been demonstrated by antiapoptotic effects, antiproliferative effects, or by upregulating factors responsible for cell cycle arrest or cell death in various in vivo CRC models. Numerous studies from independent laboratories have highlighted different plant phenolic compounds for their anti-CRC activities. While promising anti-CRC activity in many of these agents has created interest in this area, in-depth mechanistic and well-designed clinical studies are needed to support the therapeutic use of these compounds for the prevention and treatment of CRC.
Keywords: colorectal cancer, phenolic compounds, prevention, treatment, molecular mechanisms, in vivo
1. Introduction
The diagnosis of colorectal cancer (CRC) is a death sentence to many. CRC is the third most diagnosed and second leading cause of cancer mortality worldwide [1]. In the United States alone, there were 149,500 new cases and 52,980 deaths in 2021, with an estimated 151,030 new cases for 2022 [1]. Globally, there were 1.9 million new cases and 935,000 deaths in 2020 [2]. These numbers have risen since 2018, as at that time statistics were noted to be 1.8 million new cases and 861,000 deaths [3]. Analyses predicted the global CRC burden to rise by 60% to 2.2 million new cases and 1.1 million deaths by 2030 [3,4,5,6]. Rising cases are attributed to a more sedentary lifestyle and altered dietary habits, such as consuming processed foods, tobacco usage, and heavy alcohol consumption. India’s incidence of colon cancer in 2016 was estimated to be 63,000, with a sizeable interstate variation [7,8].
Since the implementation of a screening program in the United States in 1990, CRC incidence has consistently decreased in the population of those older than 50 years [9,10]. In contrast, CRC incidence has shown a significant and steady increase (2% per year) in the population of those less than 50 years of age, which is called young-onset CRC (yCRC) [9,11,12]. While yCRC comprises only 10% of total CRC incidence, 75% of yCRC incidence affects the population of those between 40 and 49 years of age [9,11,12,13,14,15]. A study undertaken between 1975 and 2010 predicted that yCRC would double by 2030 in the U.S. population of those younger than 35, indicating racial disparity [9,11,12,13,14,15].
Current treatment options available for colorectal cancer include laparoscopic surgery, resection, palliative, neoadjuvant chemotherapy, and radiotherapy [15,16,17,18,19,20,21,22]. Chemotherapy causes undesirable side effects. In addition to being frequently ineffective, current treatments are expensive.
Utilizing phytochemicals for cancer treatment and prevention has been a matter of serious discussion for decades [3,23]. Plants have been used to treat many diseases in traditional medicine and have been a forefront in alternative approach. Over 3000 plant species have anticancer activities, with thirty plant-derived compounds undergoing preclinical testing [5]. Anticancer activity in citrus fruits, allium vegetables, and medicinal plants has demonstrated preclinical success [5,8]. Secondary plant metabolites have been shown to decrease inflammation and increase apoptosis in addition to possessing antioxidant, anticarcinogenic, and antimetastatic properties [8,23,24]. The attraction to phytochemicals arises from relatively safer and cost-efficient natural products, and their consumption by humans is widespread [5]. While research is being conducted, often with promising results, only a limited number of natural compounds have been approved for clinical use, while the clinical application of many is hindered due to low bioavailability [5,23].
Numerous literature reviews and studies on natural compounds in CRC were dissected and sorted thoroughly for relevant and vital information. It was noted that very few articles reviewed CRC and the therapeutic prospects with polyphenols [25,26]. There is no review literature explaining all classes of phenolic compounds and their signaling pathways in contrast with CRC. We have also noted that few previous reviews have focused on using plant extracts and fractions rich in phenols and pure phenolic compounds [25,26]. Some have examined flavonoids and their effects on CRC [27,28,29,30,31,32,33,34,35,36], yet no such reviews consider other classes of phenolic compounds and their effects on CRC. In contrast, numerous reviews were dedicated to discussing the deadly disease of CRC, but did not examine natural products for its treatment. A few reviews that included CRC studied general nutrition and dietary effects, but the literature examined dietary products, such as calcium, fiber, processed meats, or medicinal plants, rather than plant phenolic compounds [37,38,39,40,41]. Furthermore, a review was noted to include the effects of phytochemicals on CRC, but only mentioned specific biochemical properties and pathways of cancer development [42]. In view of the aforementioned limitations, our present review is up-to-date and offers the most recent information compared to previously published works. In this review, we first evaluated pertinent literature to present the characteristics of CRC and identify common risk factors and current treatment options. Then, we evaluated various in vivo studies on different phenolic phytochemicals to understand the potential of these natural agents for CRC prevention and treatment. We hope these phenolic phytocompounds spark interest in conducting new studies to eventually aid in decreasing the prevalence and lowering the risk of CRC.
2. Risk Factors
Familial, hereditary, and lifestyle factors are independent risk factors for developing CRC [43]. Genetic syndromes comprise 20–30% of CRC cases and can be divided into non-polyposis and polyposis types (Table 1). Lynch syndrome, an alternate term for the non-polyposis syndrome, is an autosomal dominant disease associated with a defect in DNA mismatch repair genes, such as hMLH1, hMSH2, hMSH6, or hPMS2 [44,45]. This mutation results in microsatellite instability (MSI) regions, which is also associated with ~15% of sporadic CRC cases. As expected, individuals with MSI regions carry an increased risk for other cancers, such as endometrial carcinoma [44].
Table 1.
Genes involved in different CRC syndromes and associated clinical symptoms.
| Syndrome | Genetic Defects | Clinical Manifestations | References |
|---|---|---|---|
| Hereditary nonpolyposis cancer syndromes | |||
| Lynch syndrome | MLH1, MSH2, MSH6, MSH3, and PMS2 | Increased risk for CRC, (10–47%) depending on gene mutated; asymptomatic unless altered bowel habits, GI bleeding due to tumors/polyps occurs; increased risk for endometrial cancer; extracolonic manifestations are associated as Muir-Torre, Turcot. | [44,46,47] |
| Muir-Torre syndrome (HNPCC + Sebaceous gland malignancies) | MLH1, MSH2, MSH6, and PMS2 | Sebaceous skin tumor/keratoacanthoma and Lynch syndrome features. | [48,49] |
| Turcot syndrome type 1 (HNPCC with primary brain tumors) | MMR, MLH1, and PMS2 | Features of Lynch syndrome + primary brain tumors. | [50,51,52,53] |
| Hereditary polyposis colorectal cancers | |||
| Familial adenomatous polyposis (FAP) syndrome | APC | More than colorectal adenomatous polyps; 100% cancer risk | [50,54] |
| Turcot syndrome type II (FAP with Primary Brain tumors) | APC | FAP syndrome + primary brain tumors, medulloblastoma, glioblastoma, astrocytoma. | [50,51,52,53] |
| Gardner syndrome | APC | FAP syndrome+ extraintestinal manifestations of desmoid tumors; sebaceous cysts; osteomas of mandible, skull, fibromatosis, congenital hypertrophy of retinal pigment epithelium (CHRPE); adrenal adenomas. | [55,56] |
| Adenomatous polyposis syndromes | APC and MUTYH | Increased number of colorectal adenomas (10–100 s), serrated polyposis, mixed polyps; duodenal adenomas are common; 43–33% increased risk of CRC; increased thyroid nodules, adrenal lesions, jawbone cysts. | [50,57,58,59] |
| Juvenile polyposis coli | BMPR1A and SMAD4 | Multiple hamartomatous polyps in the GI tract- mainly colorectum; rectal bleeding due to polyps is a common presenting symptom; anemia due to bleeding is common; extracolonic manifestations hereditary hemorrhagic Telangiectasia (HHT) telangiectasias of buccal mucosa and skin, epistaxis, and anemia, with AV malformations; colorectal cancer risk 38.7% increased. | [60,61,62] |
| Peutz-Jeghers syndrome | STK11 | Mucocutaneous pigmentation; hamartomatous polyps; 39% increased risk for CRC. | [63,64] |
| Cowden syndrome (multiple hamartomasyndrome) | PTEN | Mucocutaneous lesions and macrocephaly; skin manifestations; uterine leiomyomas, ovarian cysts; multiple hamartomas on any organ; increased risk of breast, thyroid, renal, endometrial, and colorectal cancer; 9–16% risk of CRC.; increased risk for malignant melanomas; specific dysplastic gangliocytoma of the cerebellum; Lhermitte-Duclos disease is specific to Cowden disease. | [65,66] |
Abbreviations: MUTYH, mutY DNA glycosylase; STK11, serine/threonine kinase; 11SMAD4, mothers against decapentaplegic homolog 4; PTEN, phosphate and tensin homolog; BMPR1A, bone morphogenic protein receptor type 1A; MLH, MutL homolog; MSH, MutS homolog; MMR, mismatch repair.
Familial adenomatous polyposis syndrome (FAP), which is characterized by multiple polyp formations in the gastrointestinal tract, is caused by a germline mutation in the adenomatous polyposis coli (APC) gene [67,68,69]. Inheriting a polyposis syndrome can increase an individual’s risk of developing colon cancer up to 100% [70]. Furthermore, these patients carry the risk of developing other gastrointestinal cancers and desmoid tumors. MUTYH-associated polyposis (MAP), Peutz-Jeughers syndrome (STK11), Juvenile polyposis syndrome (SMAD4 and BMPR1A), hyperplastic polyposis (HPP), familial CRC (FCC) syndrome X, and Cowden syndrome (PTEN) are other polyposis syndromes that predispose individuals to an increased risk of developing CRC [50,71,72].
Chronic inflammatory bowel diseases, which encompass both ulcerative colitis and Crohn’s disease, predispose individuals to CRC [73]. Additionally, previous abdominopelvic radiation is a potent risk factor for CRC, especially for childhood cancer survivors [74]. Furthermore, individuals receiving prostate cancer-related radiation therapy are at a higher risk of developing rectal carcinoma, supporting previous radiation therapy as a risk factor for CRC [75]. Cystic fibrosis is also implicated in CRC, as there is a 5–10 times greater risk of acquiring CRC in these patients. As a result, they have a separate management for CRC screening, especially post-transplant [76].
Lifestyle patterns, such as smoking, consumption of alcohol, obesity, sedentary lifestyles, and chronic diseases, pose a potent overall risk of developing sporadic CRC [77,78,79]. A westernized diet, rich in processed foods and red meat and deficient in fruits, fiber, and leafy vegetables, can contribute to CRC development [16,80]. Conversely, consuming more vegetables, fruits, and fiber is protective against CRC. A meta-analysis has elucidated the risk of CRC with food’s dietary inflammatory index (DII). A higher DII correlating with a pro-inflammatory state increases CRC risk [81]. Numerous studies have explored the opposite end of the spectrum, examining anti-inflammatory foods and drugs for CRC chemoprevention and treatment. This is supported by a case-control meta-analysis where a higher intake of calcium, magnesium, and potassium lowered the occurrence of CRC [82].
The risk of CRC is low in vegetarians compared to meat eaters with an HR ratio of 0.49 [95% confidence interval (CI): 0.36 to 0.66], and 0.73 [95% CI: 0.54 to 0.99] when not adjusted and adjusted (for sociodemographic and lifestyle factors, multimorbidity, and body mass index) respectively. When CRC was subcategorized, the HR of 0.69 [95% CI: 0.48 to 0.99] for the colon and 0.43 [95% CI: 0.22 to 0.82] for the proximal colon was observed in vegetarians, which is much less compared to meat eaters [83]. Adherence to the Mediterranean diet was found to be associated with a low risk of rectal cancer with RR of 0.82 [95% CI: 0.71 to 0.95] for rectal cancer, 0.94 [95% CI: 0.87 to 1.02] for proximal colon cancer, and 0.91 [95% CI: 0.79 to 1.04] for distal colon cancer [84]. The unhealthy diet pattern is associated with CRC-specific mortality with RR/HR of 1.52 [95% CI: 1.13 to 2.06] [85]. The high intake of dietary calcium and magnesium is negatively associated with CRC risk with HR of 0.76 [95% CI: 0.72 to 0.80] and 0.80 [95% CI: 0.73 to 0.87], respectively. The higher intake of dietary heme, however, was positively correlated to colon cancer incidence with HR of 1.01 (95% CI: 0.82 to 1.19) and rectal cancer incidence with HR of 1.04 [95% CI: 0.67 to 1.42] [82]. The increase in DII score, and CRC are found to be positively associated with an overall increased risk of CRC by 40% with RR of 1.40 [95% CI: 1.26 to 1.55] [81]. Smoking and CRC shows a positive association with ever smoker versus never smoker, the pooled RR was 1.18 [95% CI: 1.11 to 1.25], and the pooled risk estimate was 1.25 [95% CI: 1.14 to 1.37] [77]. Alcohol consumption is also associated with an increased risk for CRC mortality. In comparison, the pooled RR was 1.03 [95% CI: 0.93 to 1.15] for any, 0.97 for light drinkers who consume ≤12.5 g of ethanol/day, 1.04 [95% CI: 0.94 to 1.16] for moderate drinkers who consume 12.6–49.9 g ethanol/day), 1.04 [95% CI: 0.94 to 1.16] for heavy drinking men (who consume ≥50 g ethanol/day), which is higher than heavy drinking women [pooled RR = 0.79 (95% CI: 0.40 to 1.54)] [78].
3. Pathogenesis
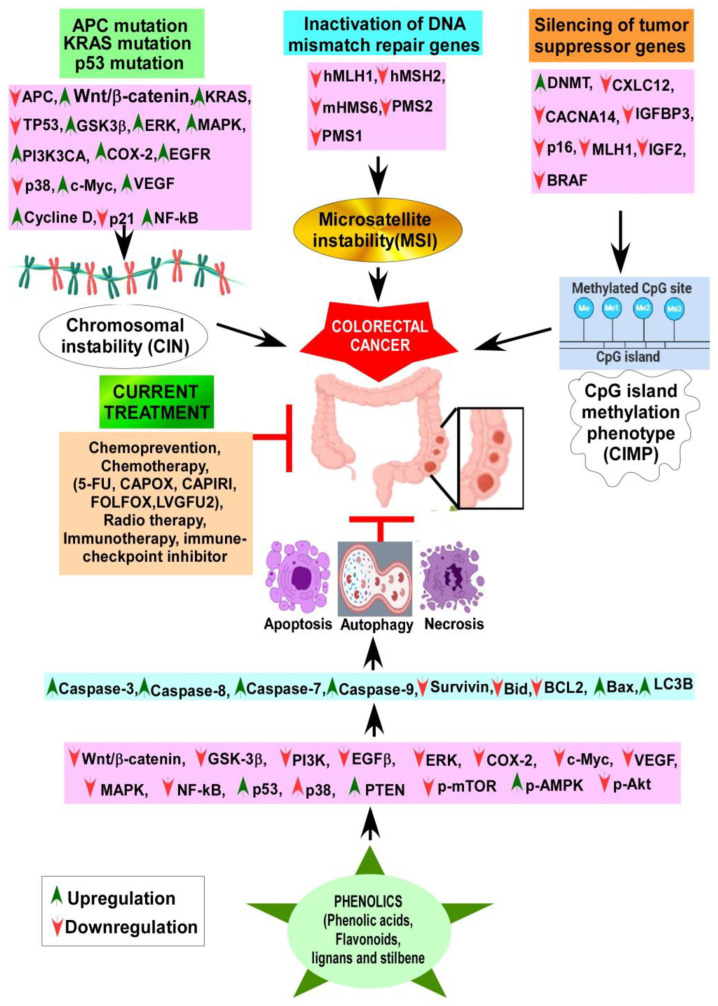
Overall, the pathogenesis of colon cancer involves three main pathways: the chromosomal instability (CIN)/classic adenoma-carcinoma sequence [86,87], the CpG island methylator phenotype (CIMP), and the microsatellite instability (MSI) pathway [88]. While these are separate pathways, there is potential overlap within them. Moreover, they involve the stepwise accumulation of multiple mutations, eventually leading to CRC development [89].
The classic adenoma-carcinoma sequence accounts for 65–70% of sporadic diseases commonly observed as left-sided CRCs [90]. This mechanism involves a dysfunctional/inactivated APC gene located on chromosome 5q21. APC, a “gatekeeper” of colonic neoplasia, has been implicated in familial adenomatous polyposis (FAP) syndrome. The onset of CRC is inevitable in a population with an inactivating mutation in both copies of the APC gene [91,92]. APC controls cell growth and differentiation through the Wnt/β-catenin signaling pathway. The Wnt pathway is an essential cellular signaling system by which several developmental events for embryological and tissue homeostasis occur, involving cellular proliferation and differentiation. Deregulation of the Wnt pathway can lead to the development of cancer. When the Wnt/β-catenin pathway is suppressed, there is a lower rate of cellular proliferation and fewer intestinal stem cells [93]. Activating mutations of Wnt/β-catenin leads to the pathogenesis of CRC. Over 90% of CRC cases carry mutations within this pathway [94]. It has been found that APC deletion/loss of function leads to CRC development, while restoring APC function can regress adenomas by reducing Wnt activity [93].
Apart from APC, there are other Wnt activating mutations, such as mutations in the CTNNB1 gene encoding β-catenin. R-spondins are another module of Wnt signal activators, which are associated with up to 10% of CRC mutations. Antagonism of RSPO3 with paclitaxel effectively targeted Wnt signaling in CRC [95]. A higher expression of ß-catenin in CRC cells is associated with a worse prognosis and advanced stage of the disease. Because of this, CRC metastasis was determined by the combined β-catenin odds ratio in the nucleus [96].
In the absence of APC function, β-catenin translocate to the nucleus. In cooperation with the DNA binding factor TCF, it promotes the growth of colonic epithelium via uncontrolled overexpression of its targets c-Myc and cyclin D1 [93]. Next, a mutation in KRAS contributes to molecular pathogenesis by promoting adenoma formation [97]. Finally, a mutation in p53 facilitates the progression of CRC [98]. Although important roles of p53 and KRAS were implied in the adenoma-carcinoma pathway, mouse knockout of APC develops carcinoma irrespective of its KRAS and p53 status, and re-introduction of APC restores cellular differentiation and normal crypt formation [43,93].
The microsatellite instability pathway occurs due to the inactivation of DNA mismatch repair genes, which includes ATPases hMSH2, hMSH6, hMSH3, hMLH1, hPMS2, hPMS1, and hMLH3, as involved in Lynch syndrome [99]. The MSI pathway is involved in roughly 15% of CRCs, 3% of which are Lynch syndrome while the rest are sporadic, mainly caused by MLH1 hypermethylation. Finally, the CpG island methylator phenotype (CIMP) is involved in silencing genes by hypermethylation of CpG islands on their promoters [100,101]. CIMP has been associated with older patients, female patients, and right-sided lesions with high MSI and BRAF mutations. CIMP is also associated with PI3K mutations but lacks KRAS and p53 mutations. A clearer insight and greater understanding of CIMP is required to better study the treatment and prevention of CRC [102].
4. Chemoprevention
Chemoprevention aims to intervene, prevent, suppress, and reverse the initiation and progression of carcinogenesis. It further attempts to decrease the recurrence of cancer through the usage of drugs, vitamins, and nutritional supplements [66]. Various agents, including nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin, and other agents, such as metformin, statins, minerals, and vitamins, have been previously studied for their chemopreventive benefits regarding CRC (Table 2). There is little doubt that a significant stride has been made into the unventured territories for the chemoprevention of CRC.
Table 2.
Various drugs alone and in combination tested for their effects on clinical CRC chemoprevention studies.
| Drugs | Study Design | Mechanism | Main Findings | References |
|---|---|---|---|---|
| Aspirin | Meta-analysis | COX-2 inhibition | There was a dose-dependent reduction in the risk of CR by aspirin. An aspirin dose of 75–100 mg/day reduced the risk by 10%, and 325 mg/day reduced the risk by 35% (Meta-analysis of 45 studies [RR = 0.73, 95% confidence interval (CI) 0.69–0.78]) | [103,104,105,106] |
| Non-aspirin NSAIDS | Meta-analysis | COX-2 inhibition | Data from 23 studies suggested using higher doses of non-aspirin NSAIDs in the general population aged 40 years or older reduced CRC risk, specifically for white women, for distal colon cancer. (Pooled ODDs ratio was 0.74 (0.67–0.81), I2 = 75.9%, p < 0.001.) | [107] |
| Sulindac+ DFMO | RCT | Sulindac inhibits COX-2 DFMO- irreversibly inhibits Ornithine decarboxylase (polyamine synthesis) |
Significant reduction of recurrent adenomas (12 vs. 41%, risk ratio 0.30), advanced adenomas (0.7 vs. 8.5%, risk ratio 0.09), and multiple adenomas (0.7 vs. 13.2%, risk ratio 0.06) | [108,109] |
| DFMO + Aspirin | RCT | Aspirin inhibits COX-2 DFMO inhibits polyamine synthesis Both combined may have a synergistic action. |
After one year of treatment, in the DFMO + aspirin arm vs. placebo, there was a significant reduction in rectal aberrant crypt foci (precursor of rectal carcinoma). (74% vs. 45%, p = 0.020). No statistically significant reduction of colorectal adenomas was observed. | [110] |
| Erlotinib + Sulindac | RCT | Erlotinib is an EGFR inhibitor; sulindac is a COX-2 inhibitor. | In 82 patients of familial adenomatous polyposis, Sulindac + Erlotinib was associated with a 69.4% decrease in those with an intact colorectum compared with placebo (95% CI, 28.8−109.2%; p = 0.009) | [111] |
| Celecoxib | Meta-analysis | Selective COX-2 inhibitor, more specific for inflammation, with fewer GI side effects. Celecoxib has higher cardiovascular mortality | 3 RCTs (involving 4420 patients) and 3 post-trial studies (2159) showed a significant reduction in the incidence of adenoma RR (0.67 [95% CI, 0.62–0.72] compared with placebo). There was an increased risk of cardiovascular mortality with twice dosing 400 mg celecoxib (RR 3.42 [95% CI, 1.56–7.46]). Once-a-day dosing did not show an increased CV risk. (1.01 [95% CI, 0.70–1.46]). | [112] |
| Clopidogrel | Case-control Study | Clopidogrel inhibits platelet aggregation via irreversible inhibition of the P2Y12 receptor | Clopidogrel decreased CRC risk in patients receiving treatment >1 year. (0.65% AOR; 95% CI, 0.55–0.78). Dual antiplatelet therapy (Clopidogrel aspirin) had the same effect as either drug is taken as monotherapy. | [113] |
| Metformin | Meta-analysis | Activates AMPK, inhibits mTOR pathway | Metformin users had a significantly lower incidence. CRC (RR 0.76, CI 0.69–0.84, p < 0.001) compared with non-metformin users. Further analysis on the overall survival of metastatic CRC patients revealed significantly higher survival rates in metformin users (HR 0.77, CI 0.68–0.87, p < 0.001). | [114] |
| UCDA | Cohort Study | Has antioxidant action. Prevents NF-κB and AP1 activity. Inhibits c-Myc |
Chronic liver disease patients with UCDA have a reduced risk of colorectal cancer. UDCA use was associated with a reduced risk of CRC (hazard ratio, 0.60; 95% confidence interval [CI], 0.39–0.92). | [115] |
| Statin | Meta-analysis |
3-HMGCOA reductase inhibitor decreases cholesterol synthesis. Antioxidant activity; shows pro-apoptotic effects on human CRC lines. Anti-inflammatory properties |
14 studies involving 130,994 patients. In terms of post-diagnosis statin uses, the pooled HR of all-cause mortality was 0.86 (95% CI, 0.76–0.98), and the pooled HR of CSM was 0.79 (95%CI, 0.70–0.89) (Cancer-Specific Mortality). | [116,117] |
| Menopausal hormone therapy (combined estrogen-progestin) | Nationwide Cohort Study (Norway) | Estrogens have been proposed to alter bile acid composition, modulate colonic transit. Decrease production of mitogenic insulin-like growth factor |
The current use of postmenopausal hormone therapy was associated with a decreased CRC risk. RR (for combined estrogen-progestin therapy) in oral formulations was 0.86 (95% CI 0.71 to 1.05) | [118] |
| Bisphosphonates | Meta-analysis | Inhibits osteoclastic bone resorption, Anti-apoptotic effect |
Meta-analysis of 34 studies and 4,508,261 participants. There was a significant reduction in the risk of CRC. (RR = 0.89, 95% CI: 0.81–0.98) | [119] |
Abbreviations: RCT, randomized control trial; RR, relative risk; HR, hazard ratio; OR, odds ratio, AOR, adjusted odds ratio; CI, confidence interval; DFMO, difluoromethylornithine; UCDA, ursodeoxycholic acid.
In CRC involving the APC/β-catenin pathway, cyclooxygenase-2 (COX-2) is often implicated in the early and later stages of the adenoma sequence, driving the formation into a carcinoma [120,121,122,123]. Furthermore, COX-2 overexpression produces vascular endothelial growth factor (VEGF), which promotes tumor angiogenesis [124,125]. Hence, by targeting COX-2, various studies have shown that NSAIDs, ranging from aspirin and sulindac to the more selective COX-2 inhibitors, such as celecoxib, have proven benefits in reducing disease risk [126,127]. In the 1990s, the U.S. Preventive Services Task Force recommended aspirin to prevent non-high-risk CRC [128,129,130].
Other drugs, such as metformin, showed promising effects in reducing the risk of CRC development. Recent meta-analyses showed that metformin could reduce CRC risk by 22% [131]. In an ongoing ASAMET trial for the tertiary prevention of stage I–III CRC, patients were administered low doses of aspirin combined with metformin for a potential synergistic chemo-preventive action [132]. Statins, a specific inhibitor of HMG-CoA reductase in the mevalonate synthesis pathway, have been recommended to lower serum lipid levels [133]. Statins were shown to reduce CRC alone and in combination with NSAIDS [134,135]. Further investigations on multiple agents, such as antioxidants, minerals, such as selenium, and vitamins, including A, C, E, and β-carotene, were previously believed to have benefits in decreasing the risk of CRC, yet they have yielded mixed results [130,136,137]. Studies on folate’s use to lower CRC risks also yielded mixed results [130]. Fiber, alcohol, monounsaturated fatty acids, polyunsaturated fatty acids, omega-3, omega-6, niacin, thiamine, riboflavin, vitamin B6, vitamin B12, zinc, magnesium, selenium, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, anthocyanin, flavonoids, garlic, ginger, onions, thyme, oregano, saffron, turmeric, rosemary, eugenol, caffeine, and tea have all demonstrated anti-inflammatory benefits, and therefore reduce the risk of CRC development [138,139]. A higher intake of dietary fiber, pertaining to whole grains, was associated with a lower CRC risk in men [140].
5. Treatment
CRC incidence and mortality have been efficiently controlled by the routine screening and removal of polyps through colonoscopy [141]. Surgery, chemotherapy, and immunotherapy are mainstay treatments for CRC; the stage of CRC progression in each patient determines an appropriate combination. The treatment of CRC depends upon the diagnosis through tumor/node/metastasis (TNM) staging of the lesion. Adjuvant chemotherapy with fluorouracil (5-FU) decreases death rates in patients with high-risk stage II colon cancer by 3–5% and 10–15% in stage III disease alone [142]. MSI/MMR protein levels determined by IHC aid in deciding the adjuvant therapy [143,144,145]. Furthermore, after primary tumor resection, TNM or immunoscore can be considered to assess the tumor recurrence risk [146].
Single-agent therapy with 5-FU or therapy with multiple agents composed of 5-FU and oxaliplatin (FOLFOX), 5-FU and irinotecan (FOXFIRI) (IRI), or capecitabine and oxaliplatin (CAPOX), capecitabine (CAP), and irinotecan (CAPIRI) as first line chemotherapy is recommended based on the sensitivity and the stage of the disease. In many cases, single-agent chemotherapies yielded better results than combination therapy, given the associated systemic toxicity and unsatisfying responses [147,148,149]. A combination of 5-FU or CAP with oxaliplatin (OX) is recommended for stage III CRC for three to six months. Patients with intermediate-risk stage II CRC are recommended either 5-FU or CAP, which are added to OX, if the patients are high risk (stage II), for three months [145]. The International Duration Evaluation of Adjuvant Chemotherapy (IDEA) collaboration helped investigate whether three or six months of adjuvant chemotherapy was necessary, as cumulative toxicity develops from fluoropyrimidines/oxaliplatin in the form of peripheral sensory neuropathy. Results show that the overall disease-free survival was similar at 74.6% and 75.5% for three months and six months, respectively. After three months of treatment, the overall sensory peripheral neuropathy reduced from 34% to 11%. However, per ESMO guidelines, stage III CRC should still be treated with six months of FOLFOX or CAPOX if the patient falls within the high-risk category. For patients who do not tolerate oxaliplatin, capecitabine, or LVGFU2 can be acceptable alternatives [145].
Various forms of supplemental targeted immunotherapies are considered to aid chemotherapy. Monoclonal antibodies are used to attack various potential genes, such as ERFR, VEGF, and PDL-1/PDL-1. Cetuximab, an anti-EGFR chimeric monoclonal antibody, and bevacizumab, an anti-VEGF chimeric monoclonal antibody, both of which prolong OS, were the first line targeted drugs approved by the United States Food and Drug Administration (FDA) in 2004 [150,151]. An immune checkpoint blocker α-PD1/PDL-1 antibody, in combination with chemo- and radiation therapy, was approved by the FDA for MSI-H and dMMR classes of CRCs for sustained progression-free survival [152]. Cetuximab yielded a positive outcome for CRC that did not respond to single-agent IRI or fluoropyrimidine therapy. Combining cetuximab with IRI, fluorocytidine, or OX delivered promising results [151,153]. EGFR (epidermal growth factor receptor) is overexpressed in various cancers to different extents, including 25–75% in CRC [154]. Cetuximab, once bound, results in the internalization and degradation of EGFR [111]. However, cetuximab was inactive in CRCs carrying the RAS (KRAS) mutation. Like EGFR, the VEGF level is also elevated in CRC, predicting a poor prognosis [155]. Along with an elevated VEGF level, increased vascular endothelial growth factor receptor (VEGFR) activity is found in adenomas, as well as in the metastatic stage of CRC [147,156]. While cetuximab is not suitable as a second line agent, bevacizumab is often an excellent choice.
6. Literature Search Methodology
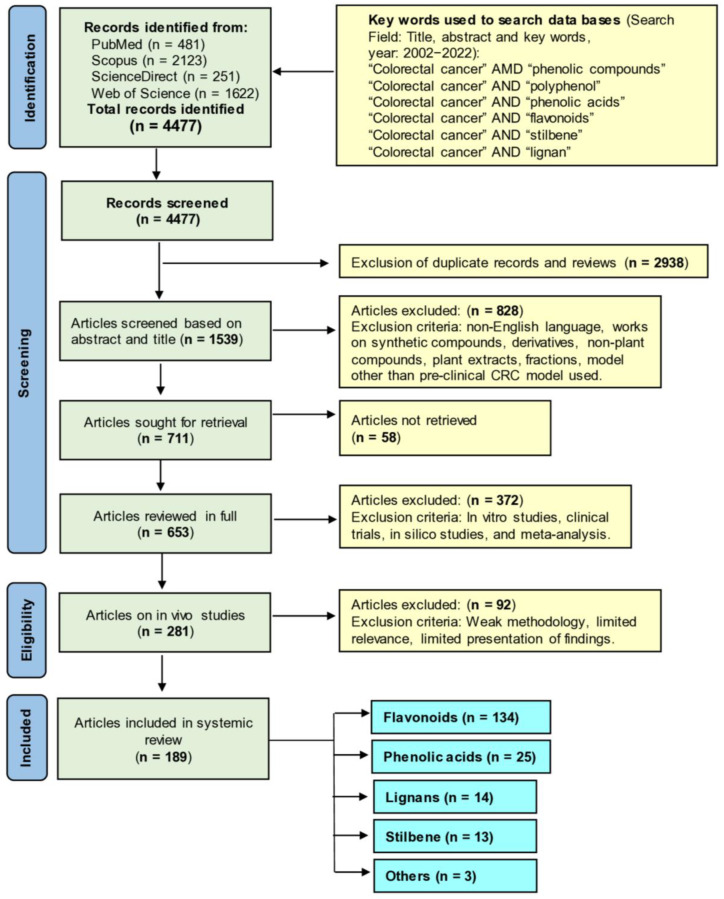
We have followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [157] for this work. Four scholarly databases, namely PubMed, Scopus, ScienceDirect, and Web of Science, were utilized to screen the literature for the last 20 years (2002 to 2022 November) by searching the title, abstract, and key words section with the key words “colorectal cancer” AND “phenolic compounds”, “colorectal cancer” AND “polyphenol”, “colorectal cancer” AND “phenolic acids”, “colorectal cancer” AND “flavonoids”, “colorectal cancer” AND “stilbene”, and “colorectal cancer” AND “lignan.” All search results were gathered, and duplicate files were removed. Next, literature was scanned based on title and abstract. Selected articles were then searched for retrieval. After reading the full articles, preclinical studies (in vivo animal models) with polyphenols were selected and incorporated. The methodology for literature search and study selection is depicted in Figure 1.
Figure 1.
The PRISMA flow chart summarizing the literature search. Here “n” represents the number of articles.
7. Phenolic Compounds with In Vivo Anti-CRC Activities
Plants synthesize phenolic compounds as secondary metabolites and carry multiple aromatic rings with two or more hydroxyl groups. Phenolic compounds carry a wide (~8000 different) variety of chemical structures. Based on chemical structures, phenolic compounds are divided into different classes, such as flavonoids (e.g., anthocyanidins, flavanols, flavanones, flavones, flavonols, and isoflavoniods) and non-flavonoids, including phenolic acids (e.g., hydroxycinnamic acids and hydroxybenzoic acids), coumarins, stilbenes, lignans, and tannins [158,159,160]. Significant sources of phenolic compounds are fruits and vegetables. Various phenolic compounds are known for their interesting pharmacological properties, including antioxidant, anti-inflammatory, neuroprotective, and anticancer properties [161,162].
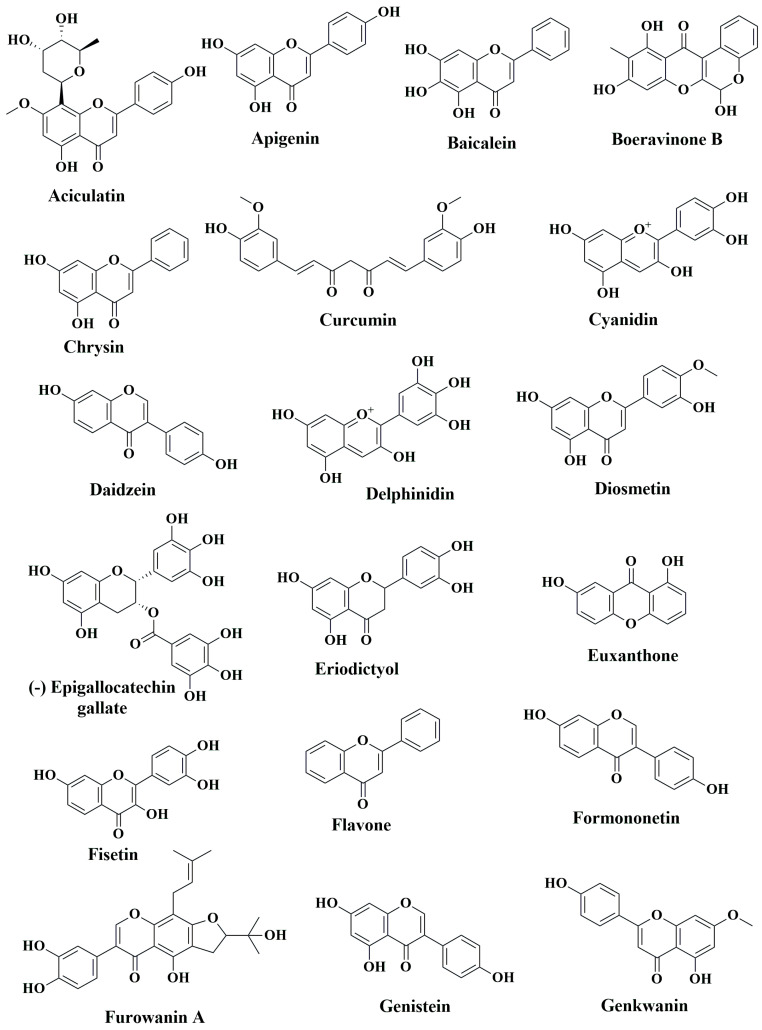
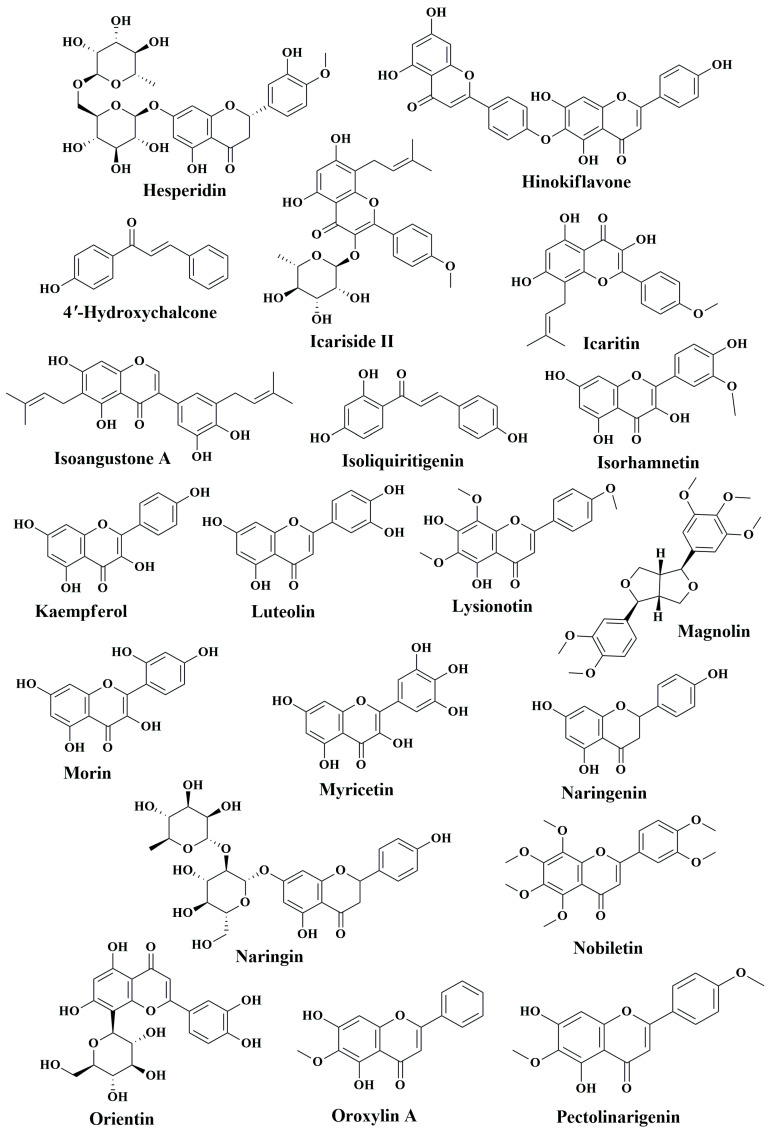
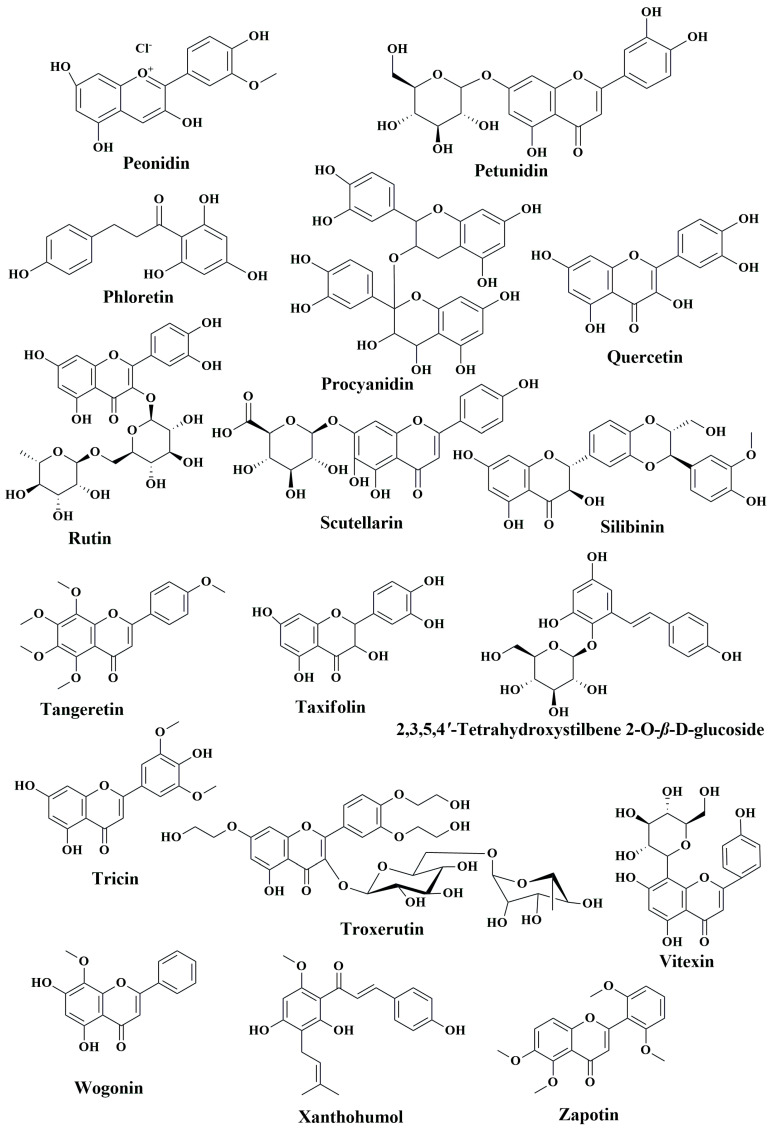
While western medicines have significant effects on CRC chemoprevention and treatment, extracts of numerous plants and plant products are still currently in use, as humanity has used plants for thousands of years as traditional or ethnic medicines for the prevention and treatment of various ailments, including cancer. The primary reasons for their popularity are fewer side effects, easy availability, and low cost compared to synthetic drugs [163,164,165]. Over the last several decades, steady progress has been achieved in identifying the bioactive secondary metabolites of plants, such as phenolic compounds, and understanding their mode of action to explain their health benefits [166,167,168,169]. In the following sections, we aim to summarize the anti-CRC effects of phenolic compounds based on animal studies. Table 3 describes the in vivo CRC activity of the compounds as revealed by our literature search as depicted in Figure 1. We have selected 16 relatively well-studied compounds to describe their anti-CRC activities in a greater detail in the following sections. The chemical structures of various classes of phenolic compounds with in vivo anti-CRC activities are presented in Figure 2, Figure 3, Figure 4 and Figure 5.
Table 3.
Anti-CRC effects and mechanisms of action of phenolic compounds based on in vivo studies.
| Phytocompound | Source | Animal Model Studied | Dose and Route of Administration | Mode of Action | Reference |
|---|---|---|---|---|---|
| Flavonoids | |||||
| 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside | Polygonum multiflorum Thunb | AOM-induced colon carcinogenesis in male F144 rats | Oral administration, 30, 150, 250 mg/kg | Decreased the number ofACF by 47–54%; suppressed tumor growth; downregulated NF-κB in nucleus and cytoplasm; downregulated CEA | [170] |
| 4′-hydroxychalcone | Herb, teas, and spices | APCMin/+ mice | Oral administration, 10 mg/kg | Reduced the incidences and size of adenomas; induced apoptosis; suppressed proliferation of polyps; downregulated Ki-67; downregulated c-Myc, Axin2 and CD44 gene expression | [171] |
| Aciculatin | Chrysopogon aciculatus | HCT116 induced tumor xenograft SCID mice | Intraperitoneal injection, 30 mg/kg | Suppressed tumor growth without losing weight; upregulated the expression of p53 and downregulated the expression of Ki-67; induced apoptosis; arrested cells in sub G1 phase | [172] |
| Apigenin | Parsley, wheat, onions, apples, and tea plants |
AOM-induced CF-1 mice and Min mice carrying mutant APC gene | Oral administration of 0.1% dietary apigenin | Reduced ACF formation and ODC activity | [173] |
| Male BALB/c-nu mice | Intraperitoneal injection, 20 mg/kg | Induced apoptosis of CRC cells; upregulated FADD expression and its phosphorylation | [174] | ||
| Male BALB/c-nu mice injected with SW480 cells | Route of administration not reported, 50 mg/kg | Elevated transgelin and downregulation of MMP-9 expression via reducing Akt phosphorylation at Ser473 and Thr308 | [175] | ||
| APCMin/+ mice | Oral gavage, 25 and 50 mg/kg | Reduced the number of polyps; induced of p53 activity | [176] | ||
| Nude BALB/c mice injected with HT-29 cells |
Subcutaneous injection, 35 mg/kg | Induced apoptosis; induced autophagy through inhibition mTOR/PI3K/Akt signaling pathway | [177] | ||
| SCID mice | Oral gavage, 25 mg/kg | Suppressed prosurvival regulators Mcl-1, Akt, and ERK | [178] | ||
| NEDD9 knock downed DLD1 cells mediated metastasis model in female athymic nude mice | Intraperitoneal injection, 20 mg/kg | Suppressed invasion, migration, and metastasis by downregulating overexpressed Neural precursor cells expressed NEDD9 | [179] | ||
| Baicalein | Scutellaria baicalensis Georgi | AMO and DSS induced colon tumor in male ICR mice | Oral administration, 1,5 and 10 mg/kg | Restored colon length; reduced tissue inflammation. | [180] |
| SW620 xenograft in BALB/c nude mice | Intraperitoneal injection, 50 mg/kg | Suppressed tumor growth by 55% without losing body weight | [181] | ||
| CT-26 derived tumor in female BALB/c mice | Intraperitoneal injection, 20 and 40 mg/kg | Reduced tumor growth rate; downregulated TLR4 and p-IκBα protein expression; inhibited NF-κB | [182] | ||
| HT-29 cell-induced tumor xenograft in male nude mice | Oral administration, 10 mg/kg | Suppressed tumor growth by 29.33% compared to the control group; induced apoptosis; upregulated p53 and p21 | [183] | ||
| DLD-1 tumor xenograft in BALB/c athymic nude mice |
Intragastric administration, 20 mg/kg | Suppression of tumor growth; inhibition of ERK phosphorylation; downregulation of MMP-2 and MMP-9 | [184] | ||
| HCT116 tumor xenograft in NSG immunodeficient mice | Intraperitoneal injection, 50 mg/kg | Suppressed tumorigenesis; inhibited colon cancer growth; induced apoptosis and senescence | [185] | ||
| HCT116 tumor xenograft in athymic BALB/c nude mice | Intraperitoneal injection, 80 mg/kg | Suppressed tumor growth; induced senescence; upregulated DEPP; activated Ras/Raf/MEK/ERK pathway | [186] | ||
| HT-29 tumor xenograft in nude mice | Intraperitoneal injection, 50 and 100 mg/kg | Suppressed tumor growth | [187] | ||
| HCT116 tumor xenograft in athymic BALB/c nude mice | Intraperitoneal injection, 100 and 200 mg/kg | Suppressed tumor growth; induced apoptosis; suppressed cancer stem cells; inhibited EMT and cyclin D1 | [188] | ||
| APCMin/+ mice | Oral administration, 30 mg/kg | Reduced tumor numbers; suppressed IL-1β, IL-2, IL-6, and IL-10 | [189] | ||
| HCT116 tumor xenograft in male BALB/c nude mice | Intraperitoneal injection, 100 mg/kg | Suppressed tumor growth; decreased circMYH9, mir761 and HDGF | [190] | ||
| Boeravinone B | Boerhaavia diffusa | DMH-induced CRC in Swiss albino Wistar rats | Intraperitoneal injection, 20 and 40 mg/kg | Decreased the number of tumor incidences; downregulated LPO; upregulated catalase, SOD and GSH; downregulated TNF-α, IL-1β, IL-6, COX-2, PGE2 and iNOS; upregulated levels of IL-4 and IL-10; down regulated MPO; downregulated the expression of GDI2 mRNA | [191] |
| Chrysin | Passiflora caerulea, Passiflora incarnata, Oroxylum indicum | AOM-induced ACF in male F344 rats | Dietary administration, 0.001% and 0.01% | Reduced mitotic index and increased apoptotic index; reduced the frequency of ACF | [192] |
| Male albino rats injected with DMH + DSS | Oral administration, 125 and 250 mg/kg | Reduced the level of CXCL1, AREG and MMP-9 | [193] | ||
| Curcumin | Curcuma longa | DSS-induced colitis in C57BL/6 mice | Oral consumption as dietary supplement, 0.6% | Reduced tumor incidences; inhibited nuclear translocation of β-catenin; downregulated TNF-α and interferon-γ; downregulated COX-2 and p53 | [194] |
| HCT116 tumor xenograft in female ICR SCID mice | Intragastric administration, 500 mg/kg | Suppressed tumor growth; inhibited proteasome; suppressed proliferation; induced apoptosis | [195] | ||
| AOM-DSS induced CRC in male C57BL/6 mice | Oral gavage, 500 mg/kg | Reduced CRC tumor number; downregulated IL-1β, IL-6, COX-2 and β-catenin; suppressed Axin2 by inhibiting Wnt/β-catenin pathway | [196] | ||
| AOM-induced colonic preneoplastic lesion in C57BL/KsJ-db/db obese mice | Dietary supplement, 0.2% and 2.0% | Inhibited colonic premalignant lesion | [197] | ||
| HCT116 tumor xenograft in athymic nu/nu nude mice | Oral administration, 1 g/kg | Enhanced the efficacy of radiation therapy; suppressed NF-κB activity and expression | [198] | ||
| Colo205 and LoVo tumor xenografts in athymic nu/nu mice | Tail vein injection, 40 mg/kg | Inhibited tumor growth; suppressed angiogenesis | [199] | ||
| AOM-induced colon carcinogenesis in Il10−/− mice | Oral administration, 1% | Reduced colon tumors | [200] | ||
| AOM/DSS-induced colitis in C5757BL/6 mice | Oral administration, 25 mg/kg | Suppressed colitis-associated colon cancer and reduced tumor number | [201] | ||
| Cyanidin | Blackberries (Rubus fruticosus) |
ApcMin/+ mice | Dietary supplementation, 0.03%, 0.1% or 0.3% | Reduced adenoma counts | [202] |
| Daidzein | Soybeans and soy-based products, and nuts |
Male albino rats injected with DMH + DSS | Oral administration, 5 and 10 mg/kg | Reduced the level of CXCL1, AREG and MMP-9 | [193] |
| Delphinidin | Berries, pomegranates, eggplant, roselle, and wine | Male BALB/c nude mice xenograft with luciferase-transfected DLD-1 cells | Intraperitoneal injection, 100 μM | Suppressed integrin/FAK nexus; elevated miR-204–3p levels | [203] |
| Diosmetin | Chamomile, parsley, rosemary, rooibos tea, green tea, and other plants fof the mint and citrus family (Lamiaceae) |
NCr nu/nu nude mice injected with HCT-116 cells | Oral administration, 50 and 100 mg/kg | Downregulated Bcl-2; upregulated Bax | [204] |
| EGCG | Camellia sinensis L. Ktze | SW837 xenograft in male BALB/c nude mice | Oral administration, 0.01% and 0.1% | Reduced tumor growth; inhibited phosphorylation of VEGFR-2, Akt and ERK | [205] |
| AOM-induced colonic premalignant lesions C57BL/KsJ-db/db mice | Oral administration, 0.01% and 0.1% | Decreased p-IGF-IR, p-GSK-3β, β-catenin, COX-2 and cyclin D1 in colonic mucosa; reduced IGF-I, insulin, triglyceride, cholesterol and leptin in serum | [206] | ||
| AOM-induced colonic carcinogenesis in ICR mice | Oral administration, 0.25% and 0.5% | Inhibited large ACF formation; inhibited iNOS and COX-2 | [207] | ||
| HCT116-SDCSCs tumor xenograft in athymic nude mice | Cells were pretreated, 100 μM | Suppressed tumor formation; downregulated Notch1, Bmi1, Suz12, and Ezh1; upregulated miR-34a, miR-145 and miR-200c | [208] | ||
| DMH-induced colon carcinogenesis in Wister rats | Oral administration, 0.2% | Inhibited ACF and induced apoptosis | [209] | ||
| DMH-induced CRC in male Wistar rats | Oral administration, 50, 100 and 200 mg/kg | Lowered ACF formation; reduced tumor volume | [210] | ||
| Eriodictyol | Eriodictyon californicum | DMH-induced colon carcinogenesis in male albino Wistar rats | Intragastrical administration, 200 mg/kg | Suppressed the number of polyps, ACF and lipid peroxidation levels; upregulated catalase, SOD, GPX, GST, GSH and GR | [211] |
| Euxanthone | Polygala caudata | HT-29 cells induced tumor in BALB/c nude mice | Intraperitoneal injection, 20 and 40 mg/kg | Suppressed tumor growth; induced apoptosis; upregulated Bax; downregulated Bcl-2; induced caspase-3 cleavage; downregulated CIP2A expression and upregulated PP2A | [212] |
| Fisetin | Strawberry, apple, persimmon, grapes, onion, and cucumber | AOM and DSS induced CAC in male BALB/c mice | Oral administration, 20 mg/kg | Suppressed dysplastic lesions; induced apoptosis in colonic tissue; downregulated Bcl-2 and STAT3 | [213] |
| FC1 mice, 3K1 mice, ApcMin/+ males, 3K1ApcMin/+ mice, B6 congenic strain, B6 FC13K1ApcMin/+ mice | Intraperitoneal injection, 1 mg/animal | Upregulated AMPK phosphorylation; suppressed PI3K/Akt/mTOR signaling | [214] | ||
| Male athymic nude mice | Oral administration, 400 and 800 mg/kg | Induced apoptosis, caspase-8 and cyt.; inhibited IGF1R and Akt | [215] | ||
| CT-26 tumor in BALB/c nude mice | Subcutaneous injection, 5 mg/kg | Suppressed oncoprotein securin in p53-independent fashion | [216] | ||
| BALB/c mice | Tail vein injection, 50 mg/kg | Inhibited programmed cell death and angiogenesis | [217] | ||
| HCT116 tumor xenograft in mice NOD/Shi-scid-IL2R gamma (null) (NOG) | Intraperitoneal injection, 30, 60 and 120 mg/kg | Suppressed tumor growth in a dose-dependent manner | [218] | ||
| Flavone | Fruits and vegetables | DMM-induced colon carcinogenesis in C57BL/6J mice | Subcutaneous injection, 15 and 400 mg/kg | Suppressed ACF formation and multiplicity | [219] |
| Formononetin | Astragalus membranaceus | Female BALB/c-nu/nu mice injected with HCT-116 cells | Intraperitoneal injection, 20 mg/kg | Decreased VEGF, MMP-2 and MMP-9 levels | [220] |
| Furowanin A | Millettia pachycarpa Benth | HT-29 tumor xenograft in male athymic BALB/c nude mice | Intraperitoneal injection, 20 and 40 mg/kg | Suppressed tumor growth, induced apoptosis and autophagy; upregulated cleaved caspase-3, LC3BII, Beclin and p27; downregulated Ki-67, pSTAT3, Mcl-1, p62, and cyclin D | [221] |
| Genistein | Genista tinctoria | DMH-induced colon cancer in Wistar rats | Oral administration, 2.5 mg/kg | Regulated tumor microenvironment; upregulated SOD, CAT, GPx, GR, vitamin A, vitamin C, vitamin E and GSH; activated NRF2 and HO-1; reduced expression of CD133, CD44 and β-catenin | [222] |
| AOM-induced colon cancer in Sprague-Dawley rats | Dietary supplementation, 140 mg/kg | Suppressed the expression of cyclin-D1 and c-Myc; decreased expression of Wnt5a, Sfrp1, Sfrp2, and Sfrp5; downregulated Wnt/ β-catenin pathway | [223] | ||
| HCT116 tumor xenograft in athymic BALB/c mice | Oral administration, 75 mg/kg | Didn’t inhibit tumor growth; suppress metastasis; downregulated MMP-2 and EGFR3 | [224] | ||
| Genkwanin | Dried flower buds of Daphne genkwa | APCMin/+ mice | Oral administration, 12.5 and 25 mg/kg | Inducted host defense; reduced proinflammatory cytokine levels | [225] |
| AOM/DSS-induced C57BL/6J mice | Oral administration, 22.5 mg/kg | Suppressed colon cancer growth by triggering tumor cell death; inhibited of pro-inflammatory cytokines | [226] | ||
| Hesperidin | Citrus fruits | AOM-induced Swiss albino mice | Oral administration, 25 mg/kg | Inhibited NF-κB, iNOS and COX-2; reduced cellular oxidative indicators and improved antioxidant status | [227] |
| AOM-induced male Swiss albino mice | Oral administration, 25 mg/kg | Inhibited the constitutively active Aurora-A driven PI3K/Akt/GSK-3 and mTOR; activated autophagy | [228] | ||
| AOM-induced male F344 rats | Oral administration, 1000 ppm | Inhibited ACF formation; reduced colonic mucosal ODC activity and polyamine levels in the blood | [229] | ||
| DMH-induced CRC in albino rats | Oral administration, 25 mg/kg | Elevated the expression of Smad4 and activin A | [230] | ||
| Hinokiflavone | Selaginella tamariscina, Juniperus phoenicea, and Rhus succedanea | CT-26 tumor in female BALB/c mice | Intraperitoneal injection, 25 and 50 mg/kg | Suppressed tumor growth and proliferation; induced apoptosis; downregulated Ki-67 and MMP-9 | [231] |
| Icariside II | Epimedi Herba | SW620 tumor xenograft in nude BALB/c mice | Intraperitoneal injection, 25 mg/kg | Suppressed tumor growth; induced apoptosis | [232] |
| Icaritin | Epimedium sp. | HT-29 tumor xenograft in male nude mice | Oral gavage, 10 mg/kg | Suppressed tumor growth and volume | [233] |
| Isoangustone A | Glycyrrhiza sp. | SW480 tumor xenograft in male BALB/c nu/nu mice | Intraperitoneal injection, 10 mg/kg | Suppressed tumor growth; induced autophagic cell death; upregulated phosphorylation of AMPK, ACC and LC3B-1 and II levels | [234] |
| Isoliquiritigenin | Glycyrrhiza glabra | AOM/DSS-induced colon carcinogenesis in male BALB/c mice | Intragastrical administration, 3, 15 and 75 mg/kg | Suppressed tumorigenesis; inhibited macrophage polarization; upregulated TNF-α, INF-γ and IL-12; downregulated TGF-β, IL-10 and IL-1 and COX-2 | [235] |
| Glycyrrhiza uralensis Fisher | AOM-treated colon carcinogenesis in 344 rats | Oral administration, 100 ppm dietary supplementation | Suppressed ACF formation; induced apoptosis | [236] | |
| Isorhamnetin | Opuntia ficus-indica | HT-29 RFP xenograft in immunosuppressed mice | Oral administration, dose not reported | Elevated cleaved caspase-9, Hdac11, and Bai1 proteins | [237] |
| FVB/N mice treated with AOM/DSS | Oral administration, dietary supplement, dose not reported | Inhibited nuclear translocation of β-catenin and c-Src stimulation; activated CSK | [238] | ||
| Kaempferol | Apple, tea, broccoli, and grapefruit | DMH-induced colorectal carcinogenesis in male Wistar rats | Oral administration, 200 mg/kg | Restored CAT, SOD, and GPx | [239] |
| DMH-induced colon carcinoma in male Sprague Dawley rats | Oral administration, 200 mg/kg | Reduced multiple plaque lesions and preneoplastic lesions | [240] | ||
| DMH-induced colitis in Sprague-Dawley albino rats | Oral administration, 200 mg/kg | Reduced multiplicity of the ACF; downregulated COX-2 and PCNA | [241] | ||
| Luteolin | Celery, parsley, broccoli, onion leaves, carrots, peppers, cabbages, and tea | DMH-induced carcinogenesis in male Wistar rats | Subcutaneous injection, 0.2 mg/kg | Reduced the number of tumor polyps and colon polyploids; decreased COX-2 level in blood and colonic tissue | [242] |
| AOM-induced CRC in male BALB/c mice | Oral administration, 1.2 mg/kg | Reduced the levels of alkaline phosphatase and lactate dehydrogenase; suppressed iNOS and COX-2 | [243] | ||
| AOM-induced CRC in male BALB/c mice | Oral administration, 1.2 mg/kg | Reduced cytochrome b5, cytochrome P450 and cytochrome b5; enhanced the expression of UDP-GT and GST in colonic tissue; upregulated Nrf2 | [244] | ||
| CT-26 mediated lung metastasis | Oral administration, 10 and 50 mg/kg | Suppressed lung nodules and nodule volume; inhibited MMP-9 expression | [245] | ||
| AOM-induced colon carcinogenesis in BALB/c mice | Oral administration, 1.2 mg/kg | Inhibited MMP-2 and MMP-9; downregulated γ-glutamyl transferase, 5′ nucleotidase, cathepsin D, and carcinoembryonic antigen | [246] | ||
| HT-29 tumor xenograft in BALB/c nude mice | Intragastric administration, 100 mg/kg | Suppressed CRC metastasis; upregulated miR-384; downregulated pleiotrophin expression | [247] | ||
| HT-29 tumor xenograft in BALB/c nude mice | Intraperitoneal injection, 50 mg/kg | Inhibited tumor growth; induced apoptosis | [248] | ||
| Lysionotin | Lysionotus pauciflorus Maxim | HCT116 tumor xenograft in athymic nude mice | Intraperitoneal injection, 20 mg/kg | Suppressed tumor growth; induced ferroptosis | [249] |
| Magnolin | Magnolia biondii | HCT116 tumor xenograft in female BALB/c athymic nude mice | Intraperitoneal injection, 20 mg/kg | Suppressed tumor growth; downregulated LIF, STAT3 and Mcl-1 | [250] |
| Morin | Old fustic (Chlorophora tinctoria) and osage orange (Maclura pomifera) |
Male athymic nude mice injected with HCT-116 cells | Intraperitoneal injection, 30 and 60 mg/kg | Inactivated NF-κB signaling | [251] |
| Male albino Wistar rats injected with DMH | Intraperitoneal injection, 30 and 60 mg/kg | Modulated tumor metabolism via β-cateinin/c-myc signaling, glycolysis and glutaminolysis pathways | [252] | ||
| Pirc rats (F344/NTac-Apc am1137) | Dietary supplementation, 50 mg/kg | Restored the sensitivity to apoptosis by inhibiting LMW-PTP | [253] | ||
| Male albino Wistar rats injected with DMH | Intragastric administration, 50 mg/kg | Reduced ACF formation; suppressed fecal and mucosal biotransformation enzymes | [254] | ||
| Male albino Wistar rats injected with DMH | Intragastric administration, 50 mg/kg | Inhibited NF-κB and inflammatory mediators; suppressed proapoptotic pathway | [255] | ||
| DMH-induced colon carcinogenesis in a male Wistar rats | Oral administration, 50 mg/kg | Reduced lipid hydroperoxides and CD; increased superoxide SOD, CAT, GST, GPx, GR; decreased GSH | [256] | ||
| Myricetin | Tea, barriers, fruits, vegetables | DMH-induced rat colon carcinogenesis | Dietary supplementation, 50, 100 and 200 mg/kg myricetin | Restored CAT, GPx and GSH | [257] |
| APCMin/+ C57BL/6J mice | Oral gavage, 100 mg/kg | Promoted apoptosis in adenomatous polyps; lowered IL-6 and PGE2; downregulated p38 MAPK/Akt/mTOR signaling pathway | [258] | ||
| AOM/DSS-induced in BALB/c mice | Oral gavage, 40 and 100 mg/kg | Inhibited the development of colorectal tumors and colorectal polyps; decreased the levels of TNF-, IL-1, IL-6, NF-κB, p-NF-κB, COX-2, PCNA, and cyclin D1 | [259] | ||
| AOM/DSS-induced colitis in C57BL/6 mice | Oral administration, 100 mg/kg | Decreased CSF/M-CSF, IL-6, and TNF-α in colonic mucosa; inhibited NF-κB/IL-6/STAT3 pathway | [260] | ||
| Naringenin | Oranges, lemons, and grapefruit | AOM-induced colon carcinogenesis in rats | Dietary supplement, 0.02% | Reduce the number of HMACF by 51% and the proliferative index by 32% | [261] |
| DSS-induced murine colitis model | Oral administration, 50 mg/kg | Decreased iNOS, ICAM-1, MCP-1, COX-2, TNF-α, and IL-6 transcript levels | [262] | ||
| HT-29 tumor xenograft in athymic NIH Swiss nude mice | Oral administration, 40 mg/kg | Suppressed tumor growth; inhibited COX-1 | [263] | ||
| Naringin | Oranges, lemons, and grapefruit | DMH-induced female Wistar rats | Oral gavage, 10, 100, 200 mg/kg | Reduced cell proliferation and tissue iron levels; upregulated antioxidant mineral levels | [264] |
| AOM/DSS-induce Male C57BL/6 mice | Oral gavage, 50 and 100 mg/kg | Suppressed ER stress-induced autophagy in colorectal mucosal cells | [265] | ||
| AOM-induced ACF in Sprague Dawley rats | Oral administration, 200 mg/kg | Reduced total number of ACF; suppressed proliferation; induced apoptosis; downregulated COX-2 and iNOS | [266] | ||
| Nobiletin | Peel of various Citrus fruits | AOM-DSS-induced colon carcinogenesis in male CD-1 mice | Oral, dietary supplement, 100 ppm | Reduced tumor incidences and multiplicity | [267] |
| Orientin | Ocimum sanctum | DMH-induced CRC in male Wister rats | Intraperitoneal injection, 10 mg/kg | Reduced NF-κB, TNF-α and IL-6; downregulated Ki-67 and PCNA; suppressed iNOS and COX-2 | [268] |
| DMH-induced CRC in male Wister rats | Intraperitoneal injection, 10 mg/kg | Suppressed ACF and crypt multiplicity; elevated the level of antioxidants; downregulated phase I enzymes and upregulated phase II enzymes | [269] | ||
| Oroxylin A | Scutellaria baicalensis | AOM-DSS induced CRC in C57BL/6 mice | Dietary supplementation, 50, 100 and 200 mg/kg | Suppressed tumor formation and colitis associated CRC; induced apoptosis; downregulated IL-6, IL-1β, p-STAT3, cyclin D, and Bcl-2; upregulated Bax | [270] |
| HCT116 tumor xenograft in male athymic BALB/c nude mice and AOM-DSS induced colon carcinogenesis in male C57BL/6 mice | Oral administration, 150 and 300 mg/kg | Suppressed carcinogenesis and primary colon cancer progression; reduced triglyceride; downregulated HIF1α, Srebp1, FASN, ADRP and FABP7; upregulated CPT1 | [271] | ||
| Pectolinarigenin | Cirsium chanroenicum | Murine CT26 CRC cells were introduced into BALB/C mice | Intraperitoneal injection, 25 and 50 mg/kg | Induced apoptotic death of cancer cells; suppression STAT3 | [272] |
| Peonidin | Sweet potato (Ipomoea batatas) | AOM-induced CF-1 mice | Dietary supplementation, 10 to 30% | Blocked cell cycle at the G1 phase; activated caspase-3 | [273] |
| Petunidin | Lycium ruthenicum | Nude mice | Intraperitoneal injection, 25 and 50 mg/kg | Induced ferroptosis via inhibiting SLC7A11 | [274] |
| Phloretin | Manchurian apricot | COLO 205 cells derived tumor in BALB/c nude mice | Route of administration not reported, 25 mg/kg | Inhibited tumor growth; upregulated p53, p21 and E-cadherin | [275] |
| Polyphenon E | AOM-induced colon carcinogenesis in F344 rats | Oral administration, 0.24% | Induced apoptosis; decreased eicosanoid, prostaglandin E2, and interleukin B4 in plasma; decreased nuclear β-catenin and increased expression of RXRα,β and γ in adenocarcinomas | [276] | |
| Procyanidin | Cider apple (Malus domestica) | AOM-induced Wistar rats | Oral administration, 0.01% | Suppressed protein kinase; down-regulated of polyamine production; stimulated caspase-3 | [277] |
| Male C57/BL6 mice transfected with CT26 cells | Oral gavage, 30 mg/kg | Reduced cellular oxidative stress through modulation of Nrf2/ARE signaling | [278] | ||
| Quercetin | Apples, nuts, cauliflower, cabbage, onions, grapes, berries, broccoli, citrus fruits, cherries, green tea, and coffee |
AOM-induced colon tumor in C57BL/6J male mice | Dietary supplementation, 0.5% | Induced apoptosis; upregulated CB1-R; downregulated STAT3 and p-STAT3; downregulated Bax/Bcl-2 ratio | [279] |
| Subcutaneous DLD-1 human colon tumor fragment implant in male athymic nu/nu mice | Intraperitoneal injection, 30 mg/kg | Enhanced radiosensitivity by inhibiting ATM-mediated signaling pathway | [280] | ||
| AOM-induced CRC in male weanling Sprague-Dawley rats | Dietary supplement, 4.5 g/kg | Reduced the number of crypts; inhibited proliferation; induced apoptosis; suppressed COX-1, COX-2 and iNOS | [281] | ||
| AOM/DSS induced colon carcinogenesis in C57BL/6J mice | Dietary supplementation, 30 mg/kg | Reduced number and size of colon tumors; suppressed inflammation; downregulated LOP, NO, SOD, G6PD, and GSH | [282] | ||
| CT-26 lung tumor metastasis in BALB/c mice | Intraperitoneal injection, 50 mg/kg | Suppressed lung metastasis; induced apoptosis | [283] | ||
| HT-29 tumor xenograft in BALB/c nude mice | Subcutaneous injection, 10 mg/kg | Enhanced radiosensitivity; inhibited Notch-1 signaling | [284] | ||
| Rutin | Buckwheat, Mez, Labisia pumila, Sophora japonica L., Schum, Canna indica L., and Ruta graveolens L. | SW480 cell-induced tumor xenograft | Intraperitoneal injection, 20 mg/kg | Suppressed tumor growth; decreased angiogenesis and VEGF levels | [285] |
| Scutellarin | Scutellaria barbata | AOM/DSS-induced male C57BL/6 mice | Intraperitoneal injection, 25, 50 and 100 mg/kg | Inhibited Wnt/β-catenin signal transduction | [286] |
| RKO cells were subcutaneously implanted into female nude mice | Intraperitoneal injection, 50, 150 and 300 mg/kg | Suppressed tumor growth and metastasis | [287] | ||
| AOM/DSS-induced mice | Intraperitoneal injection, 25, 50 and 100 mg/kg | Suppressed the Hedgehog signaling cascade | [288] | ||
| Silibinin | Silybum marianum | LoVo cell deposition on eight days old fertilized chicken egg | Route of administration not reported, 9.64 μg/mL | Decreased in VDI; upregulated Flt-1 gene | [289] |
| AOM-induced CRC in male Wistar rats | Intragastric intonation, 300 mg/kg | Suppressed preneoplastic lesion formation; activated apoptosis; registered sub G0/G1 cell cycle arrest; reduced MMP-7, IL-1β and TNF-α | [290] | ||
| Tangeretin | Peel of citrus fruits | HT-29 induced tumor xenograft in BALB/c nude mice | Route of administration not reported, 5 mg/kg |
Suppressed tumor growth | [291] |
| Taxifolin | Olive oil, grapes, citrus fruits, and onion | HCT116 tumor xenograft in athymic male nude mice | Intraperitoneal injection, 15 and 25 mg/kg | Suppressed tumor growth; induced apoptosis; inhibited cyclin D; degraded β-catenin; inhibited of Akt phosphorylation | [292] |
| Tricin | Rice bran, oats, barley, and wheat | Colon26-Luc colon tumor and lung metastasis model in BALB/c mice | Oral gavage, 19 and 37.5 mg/kg | Suppressed tumor growth; reduced metastasis incidence | [293] |
| AOM-DSS induced CRC in male Crj: CD-1 mice | Dietary supplement, 50 and 250 ppm | Restored colonic length; reduced number of incidences and multiplicity of adenomas and adenocarcinomas; downregulated PCNA and TNF-α | [294] | ||
| Troxerutin | Tea and coffee | DMH-induced colon carcinogenesis in male albino Wistar rats | Oral administration, 12.5, 25 and 50 mg/kg | Lowered ACF formation and crypt multiplicity; reduced cytochrome P450, cytochrome b5, cytochrome P4502E1, NADPH-cytochrome P450 reductase, and NADH-cytochrome b5 reductase and upregulates phase GST, DTD and UDPGT | [295] |
| Vitexin | Passionflower, bamboo leaves, pearl, and millet | HCT116 tumor xenograft in nude BALB/c mice | Oral administration, 25, 50 and 100 mg/kg | Suppressed tumor growth; increased phosphorylation of JNK; upregulated LC3 II and ApoL1 | [296] |
| HCT116DR tumor xenograft in female athymic BALB/c nude mice | Oral administration, 25 and 50 mg/kg | Suppressed tumor growth; induced apoptosis; downregulated HSP90, HSP70, HSP27, Atg7, Beclin-1, LC3 II and Bcl-2; upregulated Bax and PARP1; cleaved caspase-3 and caspase-9 | [297] | ||
| Wogonin | Scutellaria baicalensis, Scutellaria radix | AOM/DSS-induced colitis related colon cancer in C57BL/6 mice | Gastric intubation, 60 mg/kg | Decreased cell proliferation; lowered the expression and secretion of IL-6 and IL-1β and expression of NF-κB; increased Nrf2 nuclear translocation | [298] |
| AOM-DSS-induced CRC animal model in C57BL/6 mice | Route of administration not reported, 50 and 100 mg/kg | Reduced tumor multiplicity; reverted colon length to normal | [299] | ||
| SW480 induced tumor xenograft in BALB/c nude mice | Intraperitoneal injection, 2 mM | Downregulated of YAP-1 and IRF3; upregulated p-YAP | [300] | ||
| Xanthohumol | Humulus lupulus | AOM-induced colorectal carcinogenesis in male Sprague-Dawley rats | Oral gavage, 5 mg/kg | Suppressed tumor growth; induced apoptosis; suppressed COX-2 and iNOS | [301] |
| Zapotin | Tropical fruit zapote blanco (Casimiroa edulis) | AOM/DSS-induced female CF-1 mice | Intragastric administration, 5 and 10 mg/kg | Induced cell cycle arrest and apoptosis |
[302] |
| Phenolic acids | |||||
| Caffeic acid | Coffee, wine tea | CT-26 lung metastasis in BALB/c mice | Oral administration, 0.1 and 0.5 g/kg | Inhibited lung metastasis; suppressed MEK1, TOPK, and TAP-induced activation of AP1, NF-κB and ERK signaling; inhibited TAP, EGF and H-Ras induced neoplastic transformation | [303] |
| HCT116 tumor xenograft in NSG mice | Intraperitoneal injection, 10 mg/kg | Inhibited CSC growth and self-renewal by inhibition of PI3K/Akt signaling | [304] | ||
| HCT116 tumor xenograft in BALB/c AnN Foxn-1 nude mice | Oral administration, 50 nmol/kg | Inhibited PI3K/Akt/mTOR pathway; suppressed MMP-9, cyclin D1, Cdk4, cyclin E, PCNA, FASN c-Myc, and N-cadherin expression; upregulated p21 | [305] | ||
| HT-29 tumor xenograft in BALB/c nude mice | Intragastric administration, CAPE (10 mg/kg); CAPE-pNO2 5, (10 and 20 mg/kg) | Inhibited tumor growth and VEGF expression; upregulated p53, p27, p21, cyt. c, and cleaved caspase-3; downregulated procaspase-3, Cdk2, and c-Myc; | [306] | ||
| HCT116 tumor xenograft in nude mice | Oral administration, 0.2 and 2 mg/kg | Suppressed tumor growth; displayed cell cycle arrest in S phase and autophagic cell death | [307] | ||
| Chlorogenic acid | Apple, betel, coffee beans, kiwi, grapes, eggplant, pear, plum, potato, and tea | MAM acetate-induced carcinogenesis hamsters | Oral administration, 0.025% dietary supplement | Reduced colon tumor incidences; registered antioxidative effect; inhibited the activity of microsomal enzyme | [308] |
| AOM-induced ACF in colon of male F344 rats | Oral administration, 0.025% dietary supplement | Reduced ACF formation and growth | [309] | ||
| Ellagic acid | AOM-induced colon tumors in rats | Oral administration, 250, 2500 and 5000 ppm | Inhibited the incidence of adenocarcinomas in the small intestine | [310] | |
| DMH-induced colon cancer in rats | Oral administration, 60 mg/kg | Lowered the frequency of ACF and lipid peroxidation; increased the activity of CAT, SOD, GPx, GR and GST; restored the levels of vitamin C, vitamin E and GSH | [311] | ||
| DMH-induced colon cancer in Wistar albino rats | Oral administration, 60 mg/kg | Inhibited NF-κB, iNOS, COX-2, TNF-α and IL-6; restored the levels 5′-ND, γ-GT, CEA, AFP and LDH | [312] | ||
| DMH-induced colon cancer in rats | Oral administration, 60 mg/kg | Inhibited PI3K-p58 activation; downregulated Akt and Bcl-2; upregulated Bax | [313] | ||
| DMH-induced colorectal cancer in rats | Oral administration, 60 mg/kg | Inhibited ACF formation; increased the activity of CAT, SOD, GPx, and GR; inhibited ODC expression through inhibition of c-MYC | [314] | ||
| DMH-induced colon cancer in male Laca mice | Oral administration, 10 mg/kg | Restored colon membrane alterations | [315] | ||
| Ferulic acid | Rice, wheat, pineapple, grains, and peanuts | AOM-induced colon cancer in male Fischer 344 rats | Oral administration, 250 ppm and 500 ppm | Reduced number and size of adenomas; increased the activity of GST and QR | [316] |
| AOM-induced colon carcinogenesis in F344 rats | Dietary supplement of 3-(4′-geranyloxy-3-methoxyphenyl)-2propenoate (geranylated derivative of ferulic acid) 0.1% and 0.2% | Decreased the number of ACF | [317] | ||
| Gallic acid | Barriers and pomegranates | DMH-induced colon cancer in male Wister rats | Oral administration, 50 mg/kg | Reduced lipid peroxidation, LOOH, CD, SOD, CAT, GSH, GR and GPx; reduced ascorbic acid and tocopherol levels | [318] |
| SW480 induced tumor xenograft in NOD SCID gamma NSG mice | Intraperitoneal injection, 200 mg/kg | Exerted antitumor activity mediated by interaction with G-quadruplexes | [319] | ||
| DSS-induced acute colitis in C57BL/6 mice | Oral administration, 5 and 25 mg/kg | Suppressed acute colitis; inhibited phosphorylation of STAT3 | [320] | ||
| HCT116 and HT-29 tumor xenografts in BALB/c nude mice | Intraperitoneal injection, 80 mg/kg | Suppressed p-SRC, p-EGFR, p-Akt and p-STAT3 | [321] | ||
| Ulcerative colitis in rats | Oral administration, 10 mg/kg | Suppressed colon cancer; induced ferroptosis | [322] | ||
| DMH-induced colon cancer in male albino Wister rats | Oral administration, 50 mg/kg | Elevated the activity of cytochrome P450, cytochrome b5, GST, DT-diaphorase and γ-GT | [323] | ||
| Geraniin | Phyllanthus amarus | SW480 tumor xenograft in nude mice | Oral administration, 10, 20 and 40 mg/kg | Suppressed tumor growth; induced apoptosis; inhibited phosphorylation of PI3K and Akt | [324] |
| p-Coumaric acid | Mushrooms, apples, pears, barriers, oranges, and beans | DMH-induced colon carcinogenesis in male albino Wistar rats | Intragastric intubation, 100 mg/kg | Reduced ACF, DACF, MDF and BCAC | [325] |
| Syringic acid | Olives, dates, pumpkins, grapes, and palms | DSS-induced mice | Oral administration, 25 mg/kg | Decreased the level of iNOS, COX-2, TNF-α, IL-1β and IL-6; reduced activation and accumulation of p-STAT-3 by decreasing expression of p65-NF-κB | [326] |
| DMH-induced colorectal cancer in male rats | Oral administration, 50 mg/kg | Reduced tumor incidences, tumor volume and average number of tumors | [327] | ||
| Lignans | |||||
| Arctigenin | Arctium lappa, Forsythia suspensa. | CT-26 cells derived lung metastasis model in BALB/c mice | Oral gavage, 50 mg/kg | Reduced the number of lung nodules; induced apoptosis in lung tissue; inhibited EMT in lung tissue; induced cleavage of caspase-3, caspase-9, and PARP; downregulated Bcl-2 and Bcl-xL; upregulated Bax | [328] |
| Daurinol | Haplophyllum dauricum | HCT116 tumor xenograft in athymic BALB/c (Slc/nu) nude mice | Oral administration, 5 and 10 mg/kg | Suppressed tumor growth; upregulated p-Chk1(Ser345)/Chk1 | [329] |
| Dehydrodiisoeugenol | Myristica fragrans Houtt | HCT116, zsw480, and patient-derived xenograft in female NOD/SCID mice | Intraperitoneal injection, 40 mg/kg | Suppressed tumor growth by inducing ER stress; upregulated BiP, PERK, and IRE1α | [330] |
| Gomisin A | Schisandra chinesis | CT-26 induced lung metastasis in female BALB/c mice | Intraperitoneal injection, 50 mg/kg | Suppressed lung metastasis; reduced the number of lung nodules; increased phosphorylation of AMPK and p38 in lung tissue | [331] |
| Honokiol | Magnolia grandiflora | SW620 tumor xenograft in female athymic BALB/c nude mice nu/nu | Intragastric administration, 50 mg/kg | Inhibited proliferation of CRC; upregulated TGF-β1 and p53 by upregulating BMP7 | [332] |
| Justicidin A | Justicia procumbens | HT-29 tumor xenograft in NOD-SCID mice | Oral administration, 6.2 mg/kg | Suppressed tumor growth; induced autophagy in tumor tissue; induced apoptosis | [333] |
| Magnolol | Magnolia officinalis | CT-26 and HT-29 tumor in BALB/c and Cg-Foxn1nu/CrlNarl nude mice respectively | Route of administration not reported, 50 and 100 mg/kg | Inhibited tumor growth; induced apoptosis; upregulation of Fas, Fas-L, cleaved caspase-3, cleaved caspase-9 and cleaved caspase-8; downregulated NF-κB, PKCδ, ERK, Akt, C-FLIP, and MCL-1; inhibition of PKCδ-NF-κB signaling | [334] |
| HCT116 tumor xenograft in female BLB/c nude mice | Intraperitoneal injection, 5 mg/kg | Suppressed tumor growth without showing any toxicity | [335] | ||
| Schisandrin B | Schisandra chinensis, Schisandra propinqua, and Schisandra rubriflora | AOM-DSS-induced CRC in C57BL/6 mice | Oral administration, 3.7–30 mg/kg | Suppressed SIRT1 | [336] |
| Secoisolariciresinol | Fitzroya cupressoides and Crossosoma bigelovii | HCT116 tumor xenograft in male BALB/c nude mice | Route of administration not reported, 50, 100 and 200 mg/kg | Inhibited tumor growth; induced pyroptosis; downregulated Ki-67; upregulated N-GSDMD | [337] |
| DSS-induced colitis in mice | Dietary supplementation, 200 mg/kg | Suppressed tumor growth; reduced IL-1β, IL-18, TNF-α and NLRP1 | [338] | ||
| Sesaminol | Sesamum indicum | Ethanol-induced CRC in male C57BL/6NCr mice | Oral administration, 2.5 mg/mice | Reduced colonic lesions; downregulated iNOS and CYP2E1; lowered TNF-α, IL-6, MCP-1 and NF-κB levels; increased cell adhesion by upregulation of ZO-1, occludin and cladulin-1 | [339] |
| Tracheloside | Carthamus tinctorious L. (safflower) | CT-26 lung metastasis in BALB/c mice | Oral administration, 25 and 50 mg/kg | Suppressed lung metastasis; induced apoptosis; upregulated E-cadherin RNA; downregulated N-cadherin, vimentin, snail and twist RNA | [340] |
| Vitexin | Vitex negudo | HCT116 tumor xenograft in female nu/nu mice | Intraperitoneal injection, 40 mg/kg | Inhibited tumor growth and lowered tumor volume; upregulated PUMA and p53; induced PUMA-mediated apoptosis | [341] |
| Stilbenes | |||||
| Piceatannol | Red and white grapes | AOM/DSS-induced colon tumor in C57BL/6J mice | Oral administration, 5 and 12.5 mg/kg | Decreased tumor number and size; decreased Ki-67- and COX-2-positive cell number; downregulated MCP1 and PD1 | [342] |
| Polydatin | Picea sitchensis | Caco-2 tumor xenograft in C57BL/6 mice | Subcutaneously into the tumor, 150 mg/kg | Suppressed tumor growth; upregulated miR-382; downregulated PD-L1 | [343] |
| Pterostilbene | Blueberries and cranberries | AOM-induced colonic ACF preneoplastic lesions and adenomas in male ICR mice | Oral administration, 50 or 250 ppm | Reduced ACF and adenoma formation; induced apoptosis; downregulated iNOS and COX-2; inhibited Wnt/β-catenin signaling through suppressing phosphorylation of GSK3β; inhibited VEGF, cyclin D, MMPs, Ras, PI3K/Akt and EGFR | [344] |
| AOM-induced colon tumors in F344 rats | Oral administration, 40 ppm | Reduced the proliferation of non-metastatic adenomas; downregulated IL-1β, IL-4, TNF-α, PCNA, β-catenin and cyclin D and p-NF-κB/p65 | [345] | ||
| AOM-induced colon tumor in male BALB/c mice | Oral administration, 50 or 250 ppm | Reduced NF-κB through inhibition of PKC-β phosphorylation; downregulated iNOS, COX-2 and aldose reductase; upregulated HO-1, GR and Nrf2 signaling | [346] | ||
| CL187 transplantation model in BALB/c nude mice | Intraperitoneal injection, 25, 50, 100 and 200 mg/kg | Inhibited Top1-mediated DNA damage repair pathway | [347] | ||
| AOM-induced colonic ACF preneoplastic lesions in F344 rats | Oral administration, 40 ppm | Inhibited ACF formation; blocked cell proliferation and iNOS | [348] | ||
| Resveratrol | Grapes, blueberries, raspberries, mulberries, and peanuts | LoVo cell-mediated metastasis model in mice | Intragastric administration, 50, 100 and 150 mg/kg | Inhibited metastasis; decreased tumor size; suppressed TGF-β1/Smad pathway; downregulated Snail and vimentin; upregulated E-cadherin expression | [349] |
| APCCKO/Krasmut mice | Dietary supplementation, 150 ppm and 300 ppm | Suppressed tumor formation; reduced tumor size; downregulated Kras expression | [350] | ||
| DSS-induced colon carcinogenesis in rats | Oral administration, 60 mg/kg | Reduced ACF and MDF | [351] | ||
| HCT116 tumor xenograft in ICR SCID mice | Oral administration, 150 mg/kg | Suppressed tumor growth; induced apoptosis; inhibited NF-κB |
[352] | ||
| COLO250-luc tumor xenograft in athymic mice | Injection in tumor, 6 μg/implant | Suppressed tumor growth | [353] | ||
| HT-29 tumor xenograft in BALB/c nu/nu mice | Intragastric administration, 480, 960 and 1920 mg/kg | Suppressed VEGF-mediated angiogenesis | [354] | ||
| Miscellaneous compounds | |||||
| Oleuropein | Olives (Olea europaea) | AOM-induced CRC in female A/J mice | Dietary supplementation, 125 mg/kg | Suppressed preneoplastic lesions; lowered tumor incidences; prevented DNA damage | [355] |
| Thymol | Thymus vulgaris L. | HCT116 tumor xenograft and lung metastasis model in BALB/c nude mice | Intraperitoneal injection, 75 and 150 mg/kg | Induced apoptosis; upregulated E-cadherin; downregulated N-cadherin; suppressed lung metastasis by inhibiting Wnt/β-catenin pathway | [356] |
| Verbascoside | Genus, Cistanche | HCT116 tumor xenograft in BALB/c nude mice | Tail vein injection, 20, 40, and 80 mg/kg | Upregulated HIPK2, p53 and Bax; downregulation Bcl-2 | [357] |
Abbreviation: ACC, acetyl CoA carboxylase; ACF, aberrant crypt foci; AFP, α-fetoprotein; AOM, azoxymethane; APC, adenomatous polyposis coli; BAX, B-cell lymphoma 2 associated x protein; BCAC, β-catenin accumulated crypts; BCL-2, B-cell lymphoma 2; BID, BH3 interacting-domain death agonist; CAC, colitis-associated colorectal cancer; CAT, catalase; CEA, carcinoembryonic antigen; CD, conjugated dienes; CIP2A, cancerous inhibitor of PP2A; c-MYC, cellular myelocytomatosis oncogene; COX-2, cyclooxygenase-2; CRC, colorectal cancer; CSK, C-terminal Src kinase; DACF, dysplastic aberrant crypt foci; DMH, 1,2-dimethylhydrazine; DNMT, DNA methyltransferase; EGCG, (-) epigallocatechin gallate; EGF-β, epidermal growth factor-β; EGFR, epidermal growth factor receptor; ERK, extracellular-signal-regulated kinase; FADD, Fas-associated protein with death domain; Flt-1, fms-like tyrosine kinase-1; GPx, glutathione peroxidase; GR, glutathione reductase; GSK-3β, glycogen synthase kinase-3β; GSH, glutathione; GST, glutathione S-transferase; γ-GT, γ-glutamyl transpeptidase; HMACF, high multiplicity aberrant crypt foci; IGF2, insulin like growth factor 2; IGFBP3, insulin like growth factor binding protein 3; IL-6, interleukin 6; iNOS, inducible nitric oxide synthase; KRAS, Kirsten rat sarcoma viral oncogene homolog; LC3b, light chain 3B of microtubule-associated proteins 1A/1B; LDH, lactate dehydrogenase; LMW-PTP, low molecular weight protein tyro-sine phosphatase; MAM, methyl azoxymethane; MAPK, mitogen-activated protein kinase; MDF, mucin-depleted foci; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; 5′-ND, 5′-nucleotidase; NEDD9, developmentally downregulated 9; NF-κβ, nuclear factor-κβ; Nrf-2, nuclear factor erythroid -2 related factor; ODC, ornithine decarboxylase; PCNA, proliferating cell nuclear antigen; PI3K, phosphoinositide 3-kinase; PP2A, protein phosphatase 2A; PTEN, phosphatase and TENsin homolog deleted on chromosome 10; QR, quinone reductase; SCID, severe combined immunodeficient; SIRT, Sirtuin 1; SOD, superoxide dismutase; STAT3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor-α; Top1, topoisomerase 1; VDI, vascular density index; VEGF, vascular endothelial growth factor; γ-GT, γ-glutamyl transpeptidase.
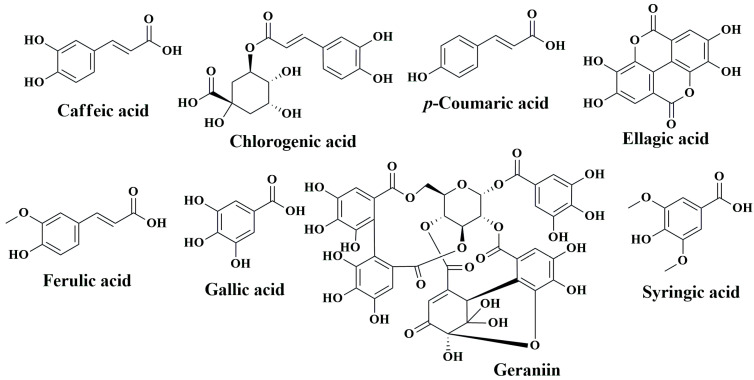
Figure 2.
Chemical structures of flavonoids with in vivo anti-CRC activities.
Figure 3.
Chemical structures of phenolic acids with in vivo anti-CRC activities.
Figure 4.
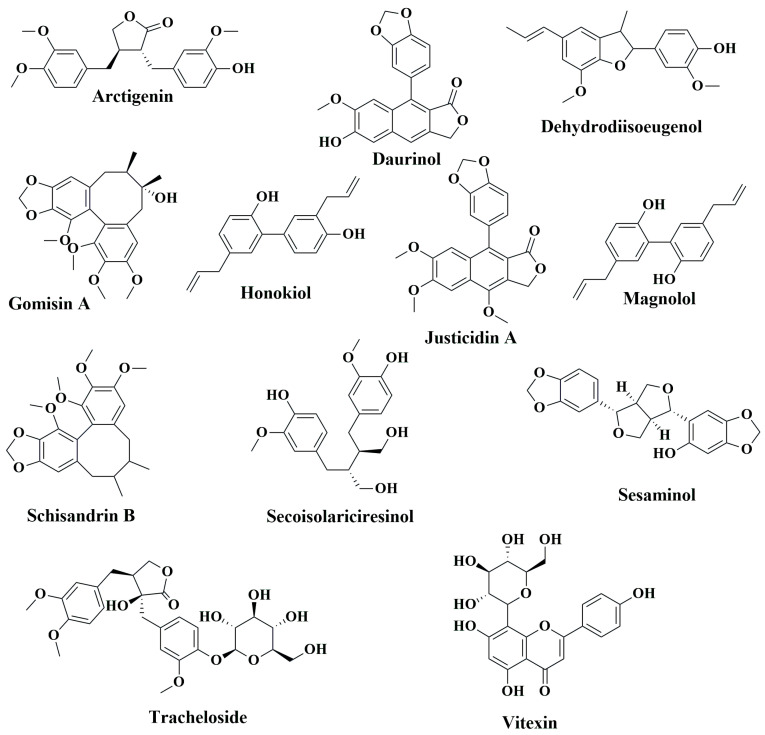
Chemical structures of lignans with in vivo anti-CRC activities.
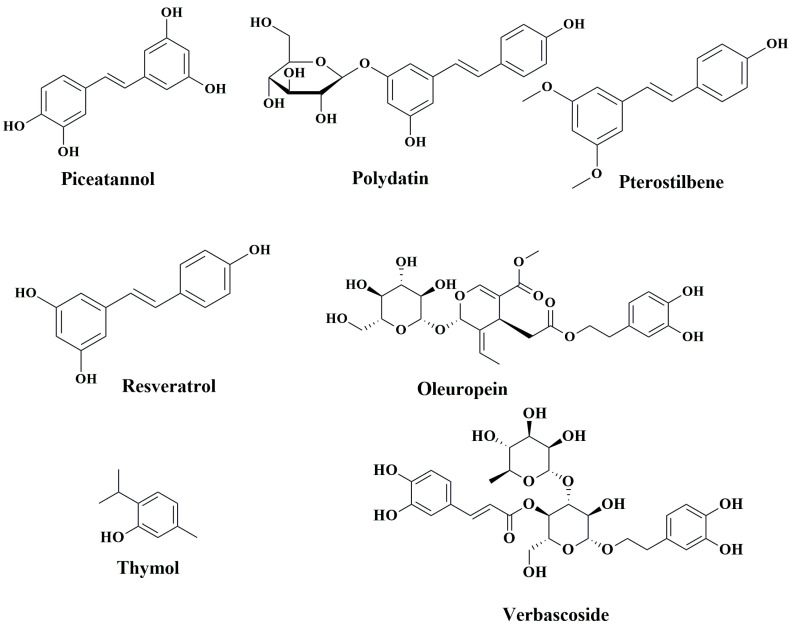
Figure 5.
Chemical structures of stilbenes and miscellaneous compounds with in vivo anti-CRC activities.
7.1. Flavonoids
7.1.1. Baicalin
Baicalin (molecular weight: 446.4 g/mol), conjointly called baicalein 7-O-glucuronide and 7-D-glucuronic acid-5, 6-dihydroxyflavone or known by its chemical name, 5, 6 dihydroxy-4-oxo-2phenyl-chromen-7-yl) oxy-3, 4, 5-trihydeoxytetrahydropyran-2-carboxylic acid, is a glycosyloxyflavone. It is a key component of a variety of traditional medicine preparations, consisting of Sho-Saiko-To, Yangkun pills, Kushen decoction, and Shuanghuanglian injections. Scutellariae radix, Scutellaria planipes, Scutellaria rehderiana, and Scutellaria scandens are only a few of the Scutellaria species that contain the compound baicalin, which is extensively distributed throughout the genus [358].
Baicalein suppressed AOM/DSS-induced colon tumors in mice and induced apoptotic cell death. Baicalein suppressed inflammation by PARP1-mediated NF-κB inhibition [180]. A dose of 50 mg/kg baicalin suppressed the growth of highly metastatic SW620 tumor xenograft in BALB/c nude mice [181]. Baicalin inhibited the TLR4/NF-κB signaling and significantly suppressed CT-26 tumor growth, migration, and invasion. Anti-tumor immunity was also enhanced by an increase in CD4+ and CD8+ T cells in CT-26 tumors [182]. Baicalein treatment induced apoptosis in a p53-mediated Akt-dependent manner and suppressed HT-29 tumor xenograft [183]. In another study, baicalein suppressed MMP-2 and MMP-9 and inhibited DLD1 tumor growth and metastatic effects by inhibiting phosphorylation of ERK [184].
Dou et al. [185] showed that baicalein and baicalin can significantly inhibit the growth of HCT116 tumor xenograft by inducing tumor cell apoptosis and senescence through inhibiting the telomerase reverse transcriptase. It has also been hypothesized that the control of colon cancer cell apoptosis and senescence is caused by the MAPK, ERK, and p38 signaling pathways. Wang et al. [186] verified that baicalin application increased the expression of DEPP and triggered its downstream target Ras/Raf/MEK/ERK and p16INK4A/Rb pathways by serving as an antioxidant, resulting in senescence in colon carcinoma cells in HCT116 tumor model in BALB/c athymic nude mice. It was revealed that baicalin inhibited the HT-29 xenograft tumor in nude mice by suppressing c-Myc as the driver of miRNAs responsible for oncogenic development (oncomiRs). These findings demonstrated an association of c-Myc in baicalin-mediated inhibition of colon cancer growth [187]. In orthotopically transplanted tumors of CRC cells in BALB/c nude mice, baicalin administration lowered the levels of marker proteins for cell cycle, EMT, and stemness [188].
Wang et al. [189] observed that the baicalein therapy dramatically decreased tumor numbers in the small intestine and colon of ApcMin/+ mice. Furthermore, reduced levels of inflammatory cytokines, such IL-1, IL-2, IL-6, G-CSF, and GM-CSF B, in this mouse tumor model suggested that baicalein’s anti-CRC action was mediated by reducing gut inflammation. Baicalin treatment suppressed HCT116 tumor xenograft growth by downregulation of CircMYH9 and HDGF, and upregulation of miR-761 [190].
7.1.2. Curcumin
Curcumin, with the chemical name (1E, 6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione(diferuloylmethane), is a hydrophobic polyphenol derived from the roots of a well-known Indian spice, turmeric (Curcuma longa). Consumption of turmeric is believed to provide protection from numerous ailments, including CRC [359,360,361,362]. Anti-CRC activities of curcumin were demonstrated by several independent groups. Curcumin reduced putative precursor colonic lesions, e.g., aberrant crypt foci (ACF), through suppressing the levels of proinflammatory cytokines, such as TNF-α and IL-6, and proinflammatory mediators, such as COX-2, in obese and diabetic (db/db) mice [197]. Adiponectin plays an important anti-inflammatory role in the gut [363,364,365]. Curcumin increased the adiponectin level in both AOM-treated and untreated C57BL/KsJ-db/db (db/db) mice [197]. Leptin levels are directly proportional to body fat. High serum leptin levels can cause inflammation-mediated CRC [366,367]. Curcumin was able to reduce the body fat content along with serum leptin levels, and thus reduce the severity of CRC. This study also observed AMPK activation and COX-2 inhibition in those animals [197].
Curcumin reduced DSS-induced ACF and β-catenin accumulation. Due to its anti-inflammatory properties, curcumin suppressed pro-inflammatory cytokines and COX-2 and iNOS in DSS-induced colonic tissue [194]. Curcumin suppressed the growth of HCT116 tumor xenograft in ICR SCID mice. Curcumin treatment led to proteasome inhibition and induction of apoptosis which, in turn, suppressed the HCT116 tumor growth [195]. In another study, curcumin inhibited AOM/DSS-induced tumorigenesis in mice. Curcumin also downregulated Axin2 and exerted its anticancer activity by Axin2 mediated inhibition of the Wnt/β-catenin pathway [196].
Curcumin was found to inhibit HCT116-induced xenografts in male nude mice, along with suppressing NF-κB regulated genes, including Bcl-2, c-FLIP, IAP1, and survivin. It further cleaved procaspase-3 and procaspase-9. Curcumin pretreatment sensitized the tumor xenograft to γ-radiation and suppressed NF-κB activity by inhibiting the binding of NF-κB to its response element on its target genes, thus minimizing invasion, migration, and angiogenesis. Curcumin ameliorated the γ-radiation mediated increase of cellular proinflammatory mediator COX-2 and c-Myc in a HCT116 xenograft tumor model [198,199].
Furthermore, curcumin was found to modulate gut microbiome habitat in AOM-injected IL10-/-mice and was implicated in the function of anti-inflammation and the maintenance of gut homeostasis. The aberrant cytoplasmic and nuclear localization of β-catenin in AOM-treated wild-type and AOM/Il-10-/-mice was significantly reduced by curcumin treatment [200].
Curcumin enhanced the anti-CRC activity of capecitabine in HCT116 tumor xenografts in male athymic nu/nu mice through the induction of apoptosis and inhibition of angiogenesis, invasion, and metastatic factors, such as VEGF, ICAM-1, and MMP-9, and CXCR4. Inhibition of COX-2 and cell cycle progression mediators, cyclin D1 and c-Myc, was also observed in the curcumin-treated animals. The anti-CRC effects of liposomal curcumin alone and combined with oxaliplatin were tested on CRC xenografts induced by Colo205 and LoVo cells in athymic nu/nu mice. The combination therapy showed efficient tumor growth inhibition by apoptosis (PARP-1 cleavage). Liposomal curcumin also inhibited angiogenesis in consistence with the inhibition of VEGF, CD31, and IL-8 expression [201]. Phytosomal curcumin was tested for its ameliorative effects on an AOM/DSS model of colitis-associated CRC alone and in combination with 5-FU in in vivo. Curcumin, alone and in combination, functioned through modulating Wnt/β-catenin signaling and E-cadherin activities. Curcumin administered by oral gavage and in combination with 5-FU significantly inhibited GSK3 α/β and cyclin D1 expression. Curcumin was shown to reduce oxidative stress induced ACF and colon injuries induced by AOM/DSS by upregulating endogenous antioxidative enzymes, such as superoxide dismutase (SOD), catalase (CAT), thiolase, and inducing autophagy by upregulating beclin1 [200].
7.1.3. Catechins
Catechins are a group of polyphenols abundantly present in tea, cocoa, berries, grapes, and apples. Catechins have a myriad of health benefits, and their anticancer properties have been extensively studied [368,369]. Kim et al. [370] examined the effects of green tea polyphenol (GTP) dosage on DSS-induced acute colitis and DMH and DSS-induced colon cancer developed in male ICR mice. GTP contained 70% of total catechins, half of which were EGCG and 3% being caffeine. This study showed that a specific dosage of GTP was effective in ameliorating the carcinogenic effect of DSS/DMH. The basis of this activity was implicated in the antioxidant properties of GTP. If the dosage was higher or lower than the effective dose, GTP was ineffective. This is potentially due to a loss of, or insufficient, antioxidant properties. Depending on the treatment conditions, GTFP can exhibit antioxidant or pro-oxidant properties [371].
The anticancer effect of EGCG was also tested on azoxymethane (AOM)-induced male C57BL/KsJ-db/db (db/db) mice. EGCG caused a significant reduction in the levels of IGF-IR, phospho-IGF-IR, phospho-GSK-3β, β-catenin, COX-2, and cyclin D1. There was also an increase in serum IGFBP3 and a decrease in serum IGF-I, insulin, triglyceride, cholesterol, and leptin in the treated mice [206].
Zhong et al. [207] investigated the acetylated-EGCG activity against protumorigenic inflammatory mediators in AOM-mediated colitis-induced CRC in a male mouse model. Acetylated-EGCG inhibited the expression of pro-tumorigenic inflammatory mediators, such as inducible nitric oxide synthase (iNOS) and COX-2. iNOS is one of the enzymes that remain in ACF and causes the continuous formation of nitric oxide (NO), leading to the promotion of tumorigenesis [372,373,374]. Furthermore, COX-2 converts arachidonate to prostaglandin E2. A sustained overexpression of prostaglandin E2 in the tissues may lead to epithelial cell cancers, including CRC [207,375,376].
EGCG showed the antistemness and chemosensitizing effects on xenograft tumors of HCT116 spheroid-derived cancer stem cells in male nude mice. EGCG inhibited CRC stemness and sensitized 5-FU-resistant HCT116 cells. EGCG suppressed stemness markers, such as Notch-1, and upregulated the expression of tumor suppressive miRNAs, including miR34a, miR200c, and miR-145 [208].
Another study demonstrated the effects of green tea catechins alone and in combination with curcumin on DMH-induced colon cancer in male Wistar rats [209]. A 32-week-long dietary treatment with curcumin, green tea catechins, and their combination showed a significant reduction in the number of colorectal aberrant cryptic foci in these animals. Notably, the combinatorial treatment had a greater effect than that with either of the compounds acting alone. A significant decrease in the proliferation index and an increase in the apoptotic index were reported in the groups treated with a combination of the compounds, compared to the mock-treated group or those receiving only DMH [209].
The anticancer effect of polyphenol E (PPE) was tested on AOM-treated F344 rats. PPE is a standardized GTP mixture containing 65% EGCG and other catechins. After AOM treatment, the animals were given a 20% high-fat diet, with or without 0.24% PPE for 34 weeks. PPE treatment resulted in a significant reduction in tumor size and the number of tumors in these animals. PPE was shown to decrease nuclear β-catenin levels, induce apoptosis, and increase the levels of RXR-α, RXR-β and RXR-γ expression in adenocarcinomas. This was accompanied by the lowering of proinflammatory eicosanoids, prostaglandin E2, and leukotriene B4 in the plasma [276].
7.1.4. Fisetin
Fisetin is a hydroxy flavone under the subgroup of flavonoid found in various fruits and vegetables, such as strawberry, apple, persimmon, grapes, onion, and cucumber. In an AOM/DSS-induced colitis associated CRC model in BALB/c mice, fisetin suppressed dysplastic lesions through inducing apoptosis in the colonic tissue along with downregulation of Bcl-2 and STAT3, and upregulation of cleaved-caspase-3 and BAX. Fisetin treatment restored the level of enzymatic (SOD, CAT, GPx, and GR) and non-enzymatic (vitamin E, and vitamin C) antioxidants in DMH-induced colonic tissue back to normal [213].
Fisetin treatment resulted in activation of AMPKα and inhibition of PI3K/Akt/mTOR signaling pathway along with decreased expression of PI3K, reduced Akt phosphorylation in PIK3CA mutants. In FC13K1ApcMin/+ mice, fisetin decreased the occurrence of colonic tumor incidences. In combination with 5-FU, fisetin reduced the overall colonic tumor incidences [214].
Fisetin inhibited growth of LoVo tumor xenograft in athymic nude mouse model. Mechanistic study revealed that fisetin acted by inducing apoptosis in tumor tissue through activation of caspase-8 and increased cyt. c expression. In the tumor tissue of treated animals, inhibition of IGF1R and Akt activation was observed [215].
Although CT-26 tumor growth was suppressed upon the intratumoral injection of fisetin, HCT116 tumors were not sensitive to the similar treatment where a combination of radiation with fisetin was more effective. Fisetin suppressed the oncoprotein securin in CT-26 tumor in a p53-independent fashion, but securin null HCT116 tumors are more sensitive to fisetin treatment [216].
Fisetin suppressed HCT116 induced tumor growth in NOD/Shi-scid-IL2R gamma (null) (NOG) mice in a dose-dependent manner compared to control group [218]. Another study showed that due to poor water solubility, the fisetin micelles, composed of poly(ethylene glycol)-poly(ε-caprolactone), i.e., MPEG-PCL, are more efficient antitumor agents over free fisetin as tested in CT-26 tumor model. MPEG-PCL showed enhanced inhibition of angiogenesis through inducing apoptotic cell death [217].
7.1.5. Genistein
Genistein, a naturally occurring isoflavone, was first isolated from Genista tinctoria. Its anticancer properties have been extensively studied [377]. Sekar et al. [222] examined genistein’s role in regulating the tumor microenvironment in DMH-induced colon cancer in Wistar rats. This study revealed that genistein could regulate enzymatic (SOD, CAT, GPx, and GR) and non-enzymatic (vitamin E, vitamin C, vitamin A, and GSH) antioxidants in DMH-induced colonic tissue environments. It was found that the loss of mucin secretion in DMH-induced animals was restored by genistein. There was also a reduction of mast cell population and collagen deposition in genistein-treated animals compared to mock-treated animals. Argyrophilic nuclear organizer region and proliferating cell nuclear antigen, two prognostic markers, were decreased by genistein in DMH-treated rats. Genistein activated NRF2 and its downstream target, heme oxygenase-1, and alleviated DMH-induced oxidative stress. Higher expression of colonic stem cell markers, such as CD133, CD44, and β-catenin, was found to be reduced by genistein in DMH-treated animals [222].
It was shown that oral administration of genistein to mice carrying orthotopically implanted human CRC did not inhibit tumor growth. However, it did show inhibition of distant metastasis formation at a dose non-toxic to the animals. Subsequent biochemical analyses showed genistein-mediated downregulation of matrix metalloproteinase-2 (MMP-2) and FMS-related tyrosine kinase 4, also known as vascular endothelial growth factor receptor 3, suggesting its inhibitory role against neoangiogenesis in mouse tumors [224].
Chen et al. [378] conducted a study in which clinical signatures of the anti-CRC activity of genistein were tested in clinical samples of plasma, tumor tissue samples, and standard tissue samples isolated from patients. The expression of miR-95, serum glucocorticoid kinase 1 (SGK1), Bcl-2, and Erk1 was highly elevated in samples of CRC compared to the normal samples. Furthermore, genistein could sensitize CRC SW620 cells to apoptosis with increased LDH content in a concentration-dependent manner, accompanied by downregulation of endogenous miR-95, SGK1, and Erk1 activities [378].
Zhang et al. [223] studied the effect of genistein on AOM-induced colon carcinogenesis in male Sprague Dawley rats. The animals were given a control diet, soya protein isolate (SPI), and a genistein diet orally, starting from gestation to 13 weeks of age. Pre-AOM treatment analysis was performed by taking samples at seven weeks of age, and the remaining rats were AOM-treated at this time for six weeks for analysis. Compared to the control group, AOM injections did not cause aberrant nuclear accumulation of β-catenin in SPI and genistein-treated groups. Moreover, SPI and genistein suppressed the expression of cyclin-D1 and c-Myc. In addition, the expression of Wnt signaling genes (Wnt5a, Sfrp1, Sfrp2, Sfrp5) was decreased to a level comparable to that of pre-AOM treatment by SPI and genistein. Furthermore, the rats fed SPI and genistein had lower numbers of total aberrant crypts, which correlated with the reduction in Wnt/β-catenin signaling. Genistein also lowered the number of ACF [223].
The first clinical study to assess the safety and tolerability of genistein in combination with chemotherapy for the treatment of metastatic CRC was conducted by Pivota et al. [379]. Patients diagnosed with metastatic CRC but not previously treated were administered FOLFOX or FOLFOX-bevacizumab. Genistein (60 mg/day) was given orally for seven days every two weeks. Treatment was started four days before chemotherapy and continued through days one through three of infusion chemotherapy. In this trial, thirteen patients received combinatorial treatment. Treatment with genistein alone resulted in mild side effects, such as headaches, nausea, and hot flashes, with one subject experiencing grade 3 hypertension. There was no increase in chemotherapy-related adverse events when genistein was added to FOLFLOX. The best overall response rate for the genistein supplementation of the chemotherapy regimen was 61.5%. The median progression-free survival of the same study was 11.5 months [379].
7.1.6. Kaempferol
Kaempferol, a dietary flavanol found in many plants, including apple, tea, broccoli, and grapefruit, has been demonstrated to carry antitumor effects based on preclinical studies [380]. Nirmala et al. [239] demonstrated the beneficial effects of orally administered kaempferol with intravenous irinotecan in 1,2-dimethyl hydrazine (DMH)-induced colorectal carcinoma in male Wistar rats. In the kaempferol-fed animal groups, levels of DMH-induced erythrocyte lysate levels and decreased liver thiobarbituric acid reactive substances. Levels of several antioxidant enzymes, such as catalase, superoxide dismutase, and glutathione peroxidase, were recovered, and the most successful effects were achieved at a dose of 200 mg/kg body weight of kaempferol (which is comparable to irinotecan).
The combined effect of fluoxetine, an antidepressant drug, and kaempferol in alleviation of DMH-induced colon carcinoma in male Sprague Dawley rats was also analyzed. Compared to fluoxetine and kaempferol alone, combined treatment of these two agents caused greater reduction in multiple plaque lesions and preneoplastic lesions in the colonic tissues. This combinatorial treatment also reduced tissue concentration of malondialdehyde and NO. Both serum and tissue β-catenin levels were significantly decreased by the combinatorial treatment. There was also a significant increase in serum and tissue caspase-3 levels. PCNA and COX-2 positive cells in the colon of animals receiving the combinatorial treatment were lower when compared to fluoxetine and kaempferol treatments alone [240].
Hassanein et al. [241] studied the effect of sulindac in combination with either EGCG or kaempferol in DMH-induced colon carcinogenesis in male Sprague Dawley rats. The combinations of EGCG and kaempferol with sulindac, a nonsteroidal anti-inflammatory drug, caused great enhancement of sulindac’s antioxidant, anti-inflammatory, antiproliferative, and apoptotic activities. Sulindac combined with both compounds caused a decrease in thiobarbituric acid-reactive substance, tissue NO, and both serum and tissue β-catenin. Downregulation of PCNA and COX-2 and a decrease in the number of ACF caused by DMH administration were also noted [241].
7.1.7. Luteolin
Luteolin (3′,4′,5,7-tetrahydroxyflavone) was discovered in different fruits, vegetables, and medicinal herbs. Plants rich in luteolin are used for treating various ailments, such as hypertension, inflammation, and cancer in Chinese traditional medicine [381,382]. The anti-CRC activity, as well as the anti-angiogenic, anti-invasive, and antimetastatic effects of luteolin were studied using AOM-induced colitis models of male BALB/c mice. Upregulation of γ-glutamyl transferase (GGT), found in a number of human neoplasms, facilitates neoplastic progression and metastasis [246,383]. GGT and 5′-nucleotidase (5′ND) were inhibited in AOM-treated mice by luteolin. Furthermore, luteolin reduced other tumor markers in AOM-treated animals, such as cathepsin-D and carcinoembryonic antigen (CEA), which are correlated with poor prognosis [246]. Luteolin inhibited invasion and metastasis by reducing the expression of MMP-2 and MMP-9 along with enhancing expression of tissue inhibitor metalloproteinases 2 (TIMP-2) [246]. Mast cells were associated with enhanced angiogenesis and tumor malignancy [384]. It was found that luteolin also decreased giant mast cell and total mast cell populations in AOM-treated mice, compared to AOM-induced control animals [246].
Luteolin reduced the number and size polyps of DSS-treated mice. Upon luteolin treatment, DSS-induced oxidative stress, level of carcinoembryonic antigen and COX-2 were decreased in colonic tissue [242]. In another study, luteolin was shown to suppress AOM-induced CRC by downregulating iNOS and COX-2 expression level [243]. Luteolin also suppressed AOM-induced CRC by activating Nrf2/Keap1 pathway [244].
Luteolin inhibited HT29 xenograft’s growth in nude mice by an activity consistent with modulation of miR-384/pleiotrophin axis [247]. miR384 expression was found to be downregulated in the majority (83%) of CRC biopsy samples, correlating with the invasiveness and migratory abilities of CRC [385]. Pleiotrophin plays a major role in angiogenesis through upregulation of VEGF in CRC [386]. Luteolin treatment of HT-29 cell-induced xenograft tumor developed in female nude BALB/c mice efficiently suppressed the migration of CRC cells from the spleen to the liver and metastasis through upregulation of miR-384/pleiotrophin axis. Luteolin upregulated the expression of miR-384, which, by targeting pleiotrophin expression, inhibited the expression of MMP-2, MMP-3, MMP-9, MMP-16, as well as invasion and metastasis of CRC [247]. Luteolin, in synergy with adenovirus CD55-TRAIL, inhibited HT-29 xenografts in female BALB/c nude mice through increasing the apoptotic activity [248].
In another study, luteolin showed antimetastatic activity against CT-26 lung metastasis by downregulating MMP-2 and MMP-9. MEK and Akt phosphorylation was suppressed by the inhibition of Raf and PI3K by luteolin [245].
7.1.8. Myricetin
Myricetin (3,3′,4′,5,5′,7-hexahydroxyflavone), a naturally occurring flavonoid pigment, is typically present in fruits, herbs, and nuts. The presence of three hydroxyl groups at 3-′, 4-′, and 5′-carbon positions makes myricetin unique from other flavanols [387]. Studies by Nirmala and Ramachandran [257] showed the efficacy of myricetin on 1,2-dimethylhydrazine-induced rat colon carcinogenesis. They demonstrated that myricetin administration reduced the incidence of tumor-bearing rats and tumors in total. Furthermore, myricetin supplementation dramatically decreased intestinal tumorigenesis developed in adenomatous polyposis coli multiple intestinal neoplasia (APCMin/+) mice. Additionally, myricetin treatment improved the antioxidant enzymes, including catalase, glutathione peroxidase, and GSH, in a dose-dependent manner [257].
Li et al. [258] assessed the effectiveness of myricetin against intestinal tumorigenesis in adenomatous polyposis coli multiple intestinal neoplasia (APCMin/+) mice. Promoting apoptosis in adenomatous polyps, myricetin-fed APCMin/+ mice grew fewer, smaller polyps and did not appear to experience negative side effects. By modifying the GSK-3 and Wnt/-catenin pathways, lowering the levels of the proinflammatory cytokines IL-6 and PGE2, and downregulating the phosphorylated p38 MAPK/Akt/mTOR signaling pathway, myricetin prevents the growth of intestinal tumors [258].
AOM/DSS-induced mice were used by Zhang et al. [259] to test myricetin’s effectiveness against chronic colonic inflammation and inflammation-driven carcinogenesis. Myricetin significantly decreased the levels of inflammatory factors, such as TNF-, IL-1, IL-6, NF-B, p-NF-B, COX-2, PCNA, and cyclin D1, to inhibit the development of colorectal tumors and shrink colorectal polyps [259].
M10, a new derivative of myricetin, was tested by Wang et al. [205] to show that M10 inhibits robust endoplasmic reticulum (ER) stress-induced autophagy in inflamed colonic mucosal cells of AOM/DSS-induced mice model. The decreased levels of proinflammatory mediators, such CSF/M-CSF, IL-6, and TNF-α, in colonic mucosa and the prevention of the NF-κB/IL-6/STAT3 pathway, were shown to be associated with the antitumor activity [260].
7.1.9. Naringenin
Naringenin, a flavonoid found mostly in citrus fruits and vegetables with no taste or color, carries antioxidant, anti-inflammatory, antiviral, antimicrobial, and antitumor properties [388]. In addition, naringenin was found to reduce the number of high multiplicity aberrant crypt foci (HMACF) by 51% and the proliferative index by 32% in an AOM-induced rat model. Here, naringenin was implied to prevent CRC through decreasing proliferation and increasing apoptosis of luminal surface colonocytes [261].
Naringenin inhibited a dextran sulfate sodium (DSS)-induced murine colitis model. The inhibitory action was correlated with the inhibition of iNOS, ICAM-1, MCP-1, COX-2, TNF-α, and IL-6 transcript levels. The decrease in TNF-α and IL-6 levels was consistent with the suppression of TLR4 mRNA and protein in the colon mucosa. LPS-induced nuclear translocation of p65/RelA was also inhibited by naringenin in RAW264.7 cells, suggesting its action through TLR4 inhibition [262].
6-C-(E-phenylethenyl)-naringenin (6CEPN) inhibited anchorage independent growth of CRC cells, as well as in a CRC-induced xenograft in a dose-dependent manner through the inhibition of COX-1, an underlying cause of malignant character of CRC cells [263].
Naringin was shown to reduce tumor size and growth of AMO or DSS-induced CRC model in C57BL/6 mice by suppressing ER stress-induced autophagy in colorectal mucosal cells [265]. Another study showed naringin-mediated inhibition of tumor cell proliferation and AOM-induced CRC through inducing apoptosis in an AOM-injected Sprague-Dawley rat model [266,389].
7.1.10. Quercetin
Quercetin (3,4,5,7-pentahydroxyflavone), a polyphenolic flavonoid, was isolated from vegetables, fruits, grain, seeds, and tea [282,390]. Quercetin was shown to carry various pharmacological properties, including anticancer properties. It was further found to be effective against AOM/DSS-mediated colitis induced CRC and showed a decrease in mucin-depleted foci and aberrant crypt foci development [391]. In addition, quercetin treatment was shown to efficiently reduce AOM/DSS-induced inflammation, a major cause of colon carcinogenesis [282,392,393]. In another study, quercetin was found to restore leukocyte levels lost by AOM/DSS treatment. It was also noted that quercetin efficiently downregulated various oxidative stress-related markers, such as lipid peroxide (LPO), NO, SOD, glucose-6-phosphate (G6PD), and glutathione (GSH), explaining its role in neutralizing inflammation. The metabolic profiling of sera demonstrated the effect of quercetin through the downregulation of biomarkers that are upregulated in AOM/DSS-treated mice [282].
In a metastatic cancer model induced in BALB/c mice by CT-26 cells, quercetin was shown to be effective through inducing the intrinsic pathway of apoptosis, along with upregulating the p-38 MAPK pathway. Notably, quercetin function was correlated with modulation of the EMT markers, such as downregulation of N-cadherin, snail, MMP-2, and MMP-9, while E-cadherin, TIMP-1, and TIMP-2 were upregulated [283].
Quercetin augmented radio-sensitization of CRC cells observed in HT-29 tumor xenografts through induction of apoptosis. Combining quercetin with a low dosage of 5Gy radiation effectively suppressed CRC cell proliferation with little toxicity towards normal colonic epithelial cells, CCD-18Co. The combinational therapy was found to target cancer stem cells, as suggested by the reduction of cancer stemness factors, such as DCLK-1, CD24, Lgr5, CD29, and CD44, and the colonosphere formation. The proportion of CD133+ cells also decreased in DLD-1 and HT-29 cells under combinatorial treatments [284].
Li et al. [284] further observed that the combinational therapy of ionizing radiation and quercetin targets the notch-signaling pathway through the downregulation of γ-secretase. The combinational therapy of ionizing radiation and quercetin effectively reduced the expression of γ-secretase complex components nicastrin, PEN2, APH1, presenilin-1, and presenilin-2, which suppressed notch cleavage and thus notch signaling. The combination therapy also inhibited the expression of Jagged-1 and cleaved Notch-1 protein levels [284].
Quercetin induced antiproliferative activity and proapoptotic effects are mediated by the upregulation of cannabinoid receptor-1 (CB1-R) in AOM-treated mice. The downregulation of STAT3 and pSTAT3 was also observed [279].
When radiation therapy was used with quercetin treatment, it suppressed the tumor size of the DLD1 tumor xenograft in athymic nude mice, indicating that quercetin enhanced the radiosensitivity of DLD1 tumors [280].
7.1.11. Rutin
Rutin, a glycosidic derivative of quercetin, is also known as quercetin-3-O-rutinoside or vitamin-P. It is known to carry antimicrobial, antifungal, anti-inflammatory, anticancer, and antiallergic properties, with poor solubility in water [394]. Rutin naturally occurs in various plants, including buckwheat, Mez, Labisia pumila, Sophora japonica L., Schum, Canna indica L., and Ruta graveolens L. [395,396]. In a dose-dependent manner, rutin suppressed SW480 cell-induced tumor growth in a tumor xenograft model without affecting the organ or body weight. In the same model, rutin was shown to enhance mean survival time by 50 days and suppressed angiogenesis through decreasing the serum VEGF level [285].
7.1.12. Tangeretin
Fruits and vegetables contain a wide variety of flavonoids. Citrus fruit flavonoids exhibit various biological effects, such as anticancer and antitumor properties. For example, tangeretin, a polymethoxylated (5,6,7,8,4′-pentamethoxyflavone) flavone, is predominant in the peel of citrus fruits and is thought to operate as a natural resistance factor against pathogenic fungus. In addition, tangeretin has been demonstrated to have several biological properties, including the capacity to suppress cancer cell growth [397].
Bao et al. [291] sought to create a nano-system that included tangeretin (TAGE) and atorvastatin (ATST) and was embellished with RGD (cyclized arginine-glycine-aspartic acid sequences) to treat colon cancer. To assess the anticancer effects of these two drugs on colon cancer cells and in female BALB/c mice harboring cancer models, these researchers produced ATST and TAGE combination nanosystems; RGD-ATST/TAGE CNPs. Results indicated that the RGD-decorated nano-system was more hazardous to HT-29 cells than the undecorated nano-system and that the weight ratio of ATST to TAGE, at which the highest synergism was seen, was 1:1. The integrated nano-systems had a high in vivo biodistribution in the tumor site and effectively reduced in vivo tumor development without significantly harming the treated mice’s primary organs and tissues [291].
7.1.13. Wogonin
The medicinal plant Scutellaria baicalensis and the traditional Chinese medicine of Huang-Qin (Scutellaria radix) include a significant active monoflavonoid called wogonin (5,7-dihydroxy-8-methoxyflavone). Wogonin has many therapeutic possibilities, including anti-inflammatory and anticancer effects. It has also been observed to inhibit the development of several types of cancer cells with excellent specificity between normal cells and cancer cells [398,399].
To study wogonin’s role in colitis-associated colorectal cancer (CAC), Yao et al. [298] developed the AOM/DSS-induced C57BL/6 mice paradigm. They discovered that wogonin markedly reduced the prevalence of tumors and prevented the growth of colorectal adenomas by lowering the expression and secretion of IL-6 and IL-1β, as well as decreasing the cell proliferation and expression of NF-κB in adenomas and adjacent tissues. Further, it increased Nrf2 nuclear translocation in those same tissues [298].
Feng et al. [299] evaluated wogonin’s anti-colon cancer effect in an AOM-DSS-induced CRC animal model. They discovered that wogonin decreased tumor abundance and kept colon length within normal range without adversely affecting other organs. In addition, wogonin administration inhibited the SW480 cell-induced xenograft growth in BALB/c mice. Another study, by You et al. [300], further examined the effects of wogonin in mice with colon cancer. Treatment with wogonin abrogated the survival and metastasis properties of colon cancer cells in vivo. A detailed analysis revealed that wogonin-mediated upregulation of p-YAP1 level was responsible for the observed anti-colon cancer effect. This suggested the involvement of the Hippo signaling pathway in the process.
7.2. Phenolic Acids
7.2.1. Caffeic Acid
Caffeic acid (3,4-dihydroxycinnamic acid) is a nonflavonoid catechol with potent antioxidant properties. It is found in almost all plants as an intermediate in the lignin biosynthesis pathway. The prime source of caffeic acid is coffee. Caffeic acid possesses various pharmacological properties, such as antioxidant, anti-inflammatory, anticancer, and neuroprotective effects [400]. Caffeic acid, by direct interaction, inhibited MEK1 and TOPK activity in an ATP non-competitive manner. Kang et al. [303] conducted experiments using caffeic acid on a mouse tumor model. It demonstrated action by inhibition of ERK and p90RSK activation. Caffeic acid suppressed the TPA-induced activation of AP1, NF-κB, and ERK signaling, and thus neoplastic transformation induced by TPA, EGF, and H-Ras. Through inhibition of ERK functions, caffeic acid inhibited lung metastasis of CT-26 cells. This study also indicated the usefulness of caffeic acid in reducing ERK activity in patient tumor samples.
Caffeic acid effectively inhibited cancer stem cells (CSC) and reduced radiation-induced sphere formation of CD133+ and CD44+ CSC in two patient-derived tumor xenograft (PDTX) models of human CRC in immune-suppressed mice. In vivo, the radiation-induced elevation of PI3K/Akt signaling pathway was also suppressed by caffeic acid. In caffeic acid-treated xenograft samples, the abundance of CD133+ and CD44+ subpopulations of CSC cells were decreased. In addition, CD44+ and CD133+ cells of CRC lost their ability for self-renewal, migration, and CSC-like properties due to caffeic acid in a PDTX xenograft model. Inhibition of PI3K/Akt signaling was described as a significant mode of action caffeic acid in inhibiting CSC proliferation [304].
Both caffeic acid phenethyl ester (CAPE) and caffeic acid phenylpropyl ester (CAPPE) could inhibit HCT116 cell-induced tumor xenograft in immune-compromised mice through inhibition of PI3K/Akt and inactivation of mTORC1 by AMPK activation. Treatment with CAPE and CAPPE reduced the MMP-9 level at a non-hepatotoxic concentration. In addition, CAPE and CAPPE suppressed expression of cyclin D1, Cdk4, cyclin E, c-Myc, and N-cadherin, and upregulated p21 in vivo. Expression of tumor biomarkers, such as PCNA and FASN, was also suppressed by CAPE and CAPPE in tumor tissue [305].
CAPE and caffeic acid p-nitro-phenethyl ester (CAPE-pNO2) upregulated the levels of p53, p27, p21, cytochrome c (cyt. C), and cleaved caspase-3, but downregulated procaspase-3, Cdk2, and c-Myc in HT-29 tumor xenograft in mice. There was a dose-dependent inhibition of tumor growth and VEGF expression by these compounds, with no visible toxicity to normal cells [306].
Consumption of decyl caffeic acid inhibited tumor growth in mice with a HCT116-induced tumor xenograft. The mechanism of action involved the induction of cell cycle arrest at the S phase as well as autophagy [307].
7.2.2. Gallic Acid
Gallic acid (3,4,5-trihydoxy benzoic acid) is a naturally occurring polyhydroxy phenolic acid found as an active compound in various fruits, nuts, food compounds, vegetables, and numerous plants, such as green chicory, grapes, blackberries, raspberries, blueberries, and strawberries. Gallic acid is well known for its antimicrobial, antioxidant, anti-inflammatory, and anticancer potential [401,402]. In a dose-dependent manner, gallic acid was shown to inhibit DSS-induced colitis in mice through the inhibition of STAT3 phosphorylation [320]. This inhibitory mechanism includes reduced proinflammatory mediators Th1, TNF-α, and IL-6, and chemokines, such as KC and MCP-1 [320].
In another study, the inhibitory effects of gallic acid were tested in HCT116 and HT29 cells and tumor xenografts in BALB/c mice. The function of pro-oncogenic factors, such as Src, STAT3, EGFR, and Akt, along with key players in the apoptosis pathway were analyzed. The results demonstrated inhibition of STAT3 and Akt by inhibiting Src and EGFR functions. Furthermore, net enhancement of the cleaved caspase-3 and caspase-9 suggested the involvement of apoptosis as the mechanism behind cell death [321].
Gallic acid was shown to ameliorate ulcerative colitis-associated CRC induced in rats by TNBS treatment by modulating ferroptosis, an iron-dependent process of cellular necrosis [322]. Gene expression profiling interactive analysis (GEPIA) and bioinformatics analysis identified significant involvement of ferroptosis-related genes in CRC prognosis. This analysis indicated that eight ferroptosis-related genes are involved in cell survival. This docking study suggested that gallic acid could induce ferroptosis by modulating some of these genes [322].
7.3. Stilbenes
Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene), a stilbenoid that can be found in peanuts, skin of red grapes, and blueberries, has been studied for its potential anticancer properties [403,404]. Saud et al. [350] used a mouse model with a knocked-out APC locus, and Kras activated specifically in the distant colon to study the effect of resveratrol on sporadic CRC. The mice received a diet supplemented with resveratrol (150 or 300 ppm) before the appearance of tumors. This resulted in a 60% inhibition of tumor production and loss of Kras expression in 40% of mice that developed tumors. Oral administration of resveratrol for tumor bearing mice resulted in complete tumor remission in 33% of mice and a decrease in tumor size in 97% of the remaining mice. Upregulation of miR-96, a negative regulator of Kras expression, in non-tumoral and tumoral colonic tissues suggested that resveratrol exerted its anti-CRC effects by downregulating Kras expression [350]. Alfaras et al. [351] examined the effects of oral administration of trans-resveratrol on DMH-induced precancerous colonic lesions in male Sprague-Dawley rats. This resulted in the reduction of aberrant cryptic foci by 52% and mucin depleted foci by 45% in the colon. In colonic contents, dihydroresveratrol was the most abundant compound detected, followed by trans-resveratrol and its derivatives [351]. Synergistic effects of resveratrol and curcumin on CRC were studied by Majumdar et al. [352].
One study analyzed the effects of resveratrol and its PLGA-chitosan based nanoformulation in animal models (both xenograft and orthotopic) of colon cancer. Both the compound and its nanoformulation caused an appreciable decrease in tumor growth and hemoglobin percentages of tumor mass, signifying reduced angiogenesis with nanoformulation exhibiting more bioavailability and functional efficacy than [353]. Resveratrol combined with ginkgetin, a phytochemical obtained from Ginkgo biloba, exhibited a synergistic effect in suppressing VEGF-induced endothelial cell proliferation, migration, invasion, and tube formation in HT29 cell-induced xenografts in mice. When administered together, these two compounds demonstrated a synergistic antitumor effect with 5-FU, causing a reduction in micro vessel density of the tumors. Furthermore, the combinatorial treatment relieved the 5-FU-induced inflammatory response by lowering the expression of COX-2 and inflammatory cytokines [354]. Resveratrol also suppressed TGF-β1/Smad signaling, downregulated Snail and vimentin, and upregulated E-cadherin expression, which in turn inhibited EMT [349].
8. Phenolics in Clinical Trials for CRC Treatment
Many of the compounds discussed here, such as curcumin, resveratrol, EGCG, genistein, and fisetin, entered into different phases of clinical trials. Curcumin, the most studied phytochemical in both preclinical and clinical studies, has been tested for its effectiveness as an anti-inflammatory agent as well as its potential in prevention, management and therapy of different cancer types, including CRC [405]. The anticancer potential of resveratrol has been documented through studying its efficacy, safety, and pharmacokinetics in more than 244 clinical trials, with additional clinical trials currently being carried out by independent groups [406,407]. Although the clinical utility of resveratrol is well documented, the rapid metabolism and poor bioavailability have limited its therapeutic use [406,408]. Clinical trials on green tea extract containing EGCG as the major active component were conducted, demonstrating the good tolerance of the agent with no significant advantage of its inclusion between the placebo and the treated groups [409]. The efficacy of flavonoid fisetin supplementation on the inflammatory status and MMP levels was tested in small groups of CRC patients, while several markers were measured to assess its therapeutic efficacy, treatment with this polyphenol primarily resulted in the significant changes in IL-8 concentrations compared to the placebo group [410]. The safety and tolerability of genistein in combination with a chemotherapy agent in metastatic CRC were studied in a clinical trial with a small group of patients receiving FOLFOX or FOLFOX-bevacizumab. The results demonstrated the safety and tolerability of the treatment with notable efficacy [379]. While the results of these studies are encouraging, additional studies are needed to assess the long-term use of these phytochemicals in the clinic.
9. Conclusions and Future Perspectives
CRC is the third most diagnosed and second leading cause of cancer-related death worldwide. According to recent statistics, CRC claims close to a million lives, which is about half of the population it affects globally every year. Although the CRC death rate has declined due to routine screening and early detection, CRC incidence is predicted to be doubled by the end of this decade due to various reasons, demanding an urgent need to overcome the limitations of current treatment strategies, including the development of alternative therapy regimens. This review aims to present a detailed account of the recent advances in studies on various phenolic phytochemicals with anti-CRC activities demonstrated in animal experiments with the underlying molecular basis of their actions (summarized in Table 3).
As discussed here, the phytochemicals were reported to act through inhibiting hallmarks of various CRC attributes, such as the potential of cell growth and proliferation, self-renewal, invasion, migration, and angiogenesis through inducing apoptosis, ferroptosis, and autophagy-mediated cell death pathways (Figure 6). These activities involved the modulation of various pathways, such as the levels of proinflammatory cytokines and chemokines (IL-1, IL-6, ICAM-I, TNF, COX-2, iNOS, KC, and MCP1), oxidative stress markers and pathways (SOD, catalase, thiolase, glutathione peroxidase, GSH and Keap1/NRF2/GSK-3β/HO-1), cell cycle regulators (cyclin D1, cyclin E, and CDK 4/6), apoptotic/autophagy regulators (p21, p53, caspase-3, caspase-9, Bax, Bcl-2, Bak, and Beclin1), proliferative signaling pathways regulators (PI3K/Akt/mTOR/AMPK, Wnt/β-catenin, MAPK-p38, ERK, MEK, and c-Myc), regulators of invasion, migration, metastasis, and angiogenesis (Notch1, STAT-3, VEGF, CD31, MMP-2, MMP-3, MMP-9, MMP-16, EGFR, Twist1, Vimentin, FMS-related tyrosine kinase 4, endothelial growth receptor-3, Snail, N-cadherin, E-cadherin, TIMP-1, and TIMP-2), stemness (CD133, CD44, ALDH1, CD29, DCLK-1, and LGR5) and expression of tumor suppressive miRNAs (miR34a, miR200c, and miR145). The downregulation of COX-2 levels can be achieved upon treatment with EGCG [206], curcumin [194,197], kaempferol [239], luteolin [242,243], myricetin [259], naringenin [262], piceatannol [342], pterostilbene [344], syringic acid [326], boeravinone B [191], hesperidin [227], isoliquiritigenin [235], orientin [268], quercetin [281], and xanthohumol [301]. Caffeic acid suppressed TPA-induced activation of AP1 and NF-κB signaling [303]. Many phytophenols can induce an antioxidant response, such as EGCG, gallic acid, boeravinone B, eriodyctyol, luteolin, and morin. Caffeic acid phenethyl ester and caffeic acid phenylpropyl ester-induced mTOR inhibition through the activation of AMPK [305]. Isoangustone A upregulated AMPK phosphorylation in vivo [234]. Pterostilbene inhibited EGFR in an AOM-induced colonic adenomas in mice [344].
Figure 6.
Genetic and molecular basis of colorectal cancer along with the current treatment strategies where potentials of phenolic compounds were indicated. CIN, MSI and CIMP are the prime factors in CRC development. Besides the currently available chemotherapeutic treatment strategies, different polyphenols are reported to induce CRC cell death by apoptosis and/or autophagy and/or necrosis.
There is increasing evidence in favor of the idea that diet can influence the intestinal microbiome and thus the risk of CRC. Diets rich in fruits and vegetables can be associated with gut microbiome rich in Prevotella compared with Bacteroides associated with good colonic health while the consumption of diet with low plant-based food rich in processed food led to the opposite effects [411,412]. Diets rich in plant-based nutraceuticals could regulate host immune and inflammatory behavior and thus gut homeostasis through modulating the composition and functionality of the gut microbiome [413]. Therefore, CRC incidence and progression can be reduced by modulating gut microbiome by careful choice of diet and phytochemicals which could be a promising and efficient way to reduce the burden of CRC [413]. Gut microbiota can digest dietary phytochemicals by their unique ability to produce short chain fatty acids, such as butyric acid, with anti-inflammatory and antineoplastic activity [414]. Phenolic phytochemicals have served us as an important source of novel drugs/leads. While the studies discussed here provided encouraging results, several issues are needed to be considered to get a step closer to the end users, such as:
-
1.
Apparently, the functions of many phytochemicals are limited by their poor solubility, absorption, and bioavailability. Encapsulation by nano-formulation as well as chemical derivatization of the compound could resolve this issue.
-
2.
Some cases reproducing the activity observed in preclinical animal models into the clinic/human could be challenging due to several factors. Success in this endeavor requires careful optimization in administered doses to assess functional synergy, if any, with anti-CRC regimens used in the clinic. Once positive results are obtained in the preclinical settings, testing the validity of the finding, such as safety and efficacy, in clinical trials with appropriate controls will be important to move further.
-
3.
It is reasonable to think that a phenolic compound showing very weak and toxic activity can yield desirable effect when combined with another phytochemical. Therefore, a careful combination of selected polyphenols can yield unique anti-CRC activity. It is important to clearly determine the maximum tolerable dose of a phytochemical to better understand its therapeutic efficacy alone or in combination with another phytochemical or drug.
-
4.
Once a phenolic compound with unique anti-CRC activity is identified, it would be important to develop strategies to synthesize the compound in the laboratory, given the very low abundance of a secondary metabolite in the plants. A detailed understanding of the pharmacophore responsible for the observed function should be helpful for chemical synthesis or semi-synthesis, and cellular target identification of the compound. Given the structural complexity of the plant secondary metabolites, it is often a major challenge for natural product chemists and medicinal chemists to solve. Ideally, the simultaneous engagement of experts from interdisciplinary areas, such as ethnopharmacology, molecular biology, biochemistry, natural product chemistry, medicinal chemistry, bioinformatics, and pharmacology, will be necessary to achieve progress in real-time in harvesting the full potential of natural products as the source of novel drug leads.
Abbreviations
| 5-FU | 5-fluorouracil |
| 5′ND | 5-nucleotidase |
| 6CEPN | 6-C-(E-phenylethyl)-naringenin |
| ACF | aberrant crypt foci |
| AOM | azoxymethane |
| APC | adenomatous polyposis coli |
| ATST | atorvastatin |
| BAX | B-cell lymphoma 2-associated x protein |
| BCL-2 | B-cell lymphoma 2 |
| BID | BH3 interacting-domain death agonist |
| BRAF-B | rapidly accelerated fibrosarcoma/murine sarcoma viral oncogene homolog B |
| CAC | colitis-associated colorectal cancer |
| CACNA14 | voltage-dependent P/Q type calcium channel subunit alpha1A |
| CAP | capecitabine |
| CAPE, | caffeic acid phenethyl ester |
| CAPPE | caffeic acid phenylpropyl ester |
| CAPE-pNO2 | caffeic acid p-nitro-phenylethyl ester |
| CAPIRI | capecitabine and irinotecan |
| CAPOX | capecitabine and oxaliplatin |
| CEA | carcinoembryonic antigen |
| CIMP | CpG island methylation phenotype |
| CIN | chromosomal instability |
| CLXC12 | C–X–C chemokine 12 |
| COX-2 | cyclooxygenase-2 |
| CRC | colorectal cancer |
| CSC | cancer stem cell |
| cyt. c | cytochrome c |
| DII | dietary inflammatory index |
| Dkk | Dickkopf |
| DMH | dimethyl hydrazine |
| DNMT | DNA methyltransferase |
| DSS | dextran sulphate sodium |
| Dvl | Discevelled |
| EGF-β | epidermal growth factor-β |
| EGFR | epidermal growth factor receptor |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| FAP | familial adenomatous polyposis syndrome |
| FDA | Federal Drug Administration |
| FOLFOX | 5-FU and oxaliplatin |
| FOXFIRI | 5-FU and irinotecan |
| FZD | Frizzled receptor |
| G6PD | glucose-6-phosphate dehydrogenase |
| GEPIA | gene expression profiling interactive analysis |
| GGT | γ-glutamyl transferase |
| GSH | glutathione |
| GSK-3β | glycogen synthase kinase-3β |
| GTP | green tea polyphenol |
| HMACF | high multiplicity aberrant crypt foci |
| hMLH1 | human MutL homolog 1 |
| HPP | hyperplastic polyposis |
| IDEA | International Duration Evaluation of Adjuvant Chemotherapy |
| IGF-2 | insulin like growth factor-2 |
| IGFBP3 | insulin like growth factor-binding protein 3 |
| iNOS | inducible nitric oxide synthase |
| IRI | irinotecan |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LC3B | light chain 3B of microtubule-associated proteins 1A/1B |
| LPO | lipid peroxide |
| MAP | MUTYG- associated polyposis |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| MSI | microsatellite instability |
| mTOR | mammalian target of rapamycin |
| NF-κβ | nuclear factor-κβ |
| NO | nitric oxide |
| NSAID | nonsteroidal anti-inflammatory drug |
| oncomiRs | oncogenic miRNAs |
| OPE | orange peel extract |
| OX | oxaliplatin |
| PDTX | patient-derived tumor xenograft |
| PI3K | phosphoinositide 3-kinase |
| PPE | polyphenol E |
| PTEN | phosphatase and tensin homolog deleted on chromosome 10 |
| SEER | surveillance epidemiology and end results |
| SGK1 | serum glucocorticoid kinase 1 |
| Skip | Ski-interacting protein |
| SOD | superoxide dismutase |
| SPI | soya protein isolate |
| TAGE | tangeretin |
| TIMP | tissue inhibitor metalloproteinase |
| TNM | tumor/node/metastasis |
| TRPV1 | transient receptor potential vanilloid 1 |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| YAP | yes-associated protein |
| yCRC | young-onset colorectal cancer |
Author Contributions
Conceptualization, M.P.; methodology, S.D. and S.P.; investigation, S.D., S.P., A.B. (Ashish Bhattacharjee) and S.C.M.; data curation, S.D. and S.P.; writing—original draft preparation, S.D., S.P., K.P., A.M. (Anirban Manna), J.H., V.K.N., C.M., N.C. and A.B. (Anupam Bishayee); writing—review and editing, M.P., K.P., N.C. and A.B. (Anupam Bishayee); visualization, S.G., A.M. (Arijit Mondal) and S.B.; supervision, M.P. and A.B. (Anupam Bishayee); project administration, M.P. and A.B. (Anupam Bishayee), funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Funding Statement
This research was funded by the Science and Engineering Research Board, Department of Biotechnology (DBT), and Department of Science and Technology (DST) to M.P. S.D. is a University Grant Commission (UGC) Senior Research Fellow (SRF), A.M. (Anirban Manna) is a DBT SRF, and C.M. is a DST Inspire Fellow. S.G. was an SRF of the Council of Scientific and Industrial Research.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 5.Haque A., Brazeau D., Amin A.R. Perspectives on natural compounds in chemoprevention and treatment of cancer: An update with new promising compounds. Eur. J. Cancer. 2021;149:165–183. doi: 10.1016/j.ejca.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swetha M., Keerthana C., Rayginia T.P., Anto R.J. Cancer chemoprevention: A strategic approach using phytochemicals. Front. Pharmacol. 2021;12:809308. doi: 10.3389/fphar.2021.809308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhillon P.K., Mathur P., Nandakumar A., Fitzmaurice C., Kumar G.A., Mehrotra R., Shukla D., Rath G., Gupta P.C., Swaminathan R. The burden of cancers and their variations across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Oncol. 2018;19:1289–1306. doi: 10.1016/S1470-2045(18)30447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhari A.S., Mandave P.C., Deshpande M., Ranjekar P., Prakash O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020;10:1614. doi: 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel R.L., Miller K.D., Goding Sauer A., Fedewa S.A., Butterly L.F., Anderson J.C., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 10.Ranjan A., Ramachandran S., Gupta N., Kaushik I., Wright S., Srivastava S., Das H., Srivastava S., Prasad S., Srivastava S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019;20:4981. doi: 10.3390/ijms20204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G., Barzi A., Jemal A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 12.Mauri G., Sartore-Bianchi A., Russo A.G., Marsoni S., Bardelli A., Siena S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019;13:109–131. doi: 10.1002/1878-0261.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy C.C., Lund J.L., Sandler R.S. Young-onset colorectal cancer: Earlier diagnoses or increasing disease burden? Gastroenterology. 2017;152:1809–1812.e1803. doi: 10.1053/j.gastro.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.You Y.N., Xing Y., Feig B.W., Chang G.J., Cormier J.N. Young-onset colorectal cancer: Is it time to pay attention? Arch. Intern. Med. 2012;172:287–289. doi: 10.1001/archinternmed.2011.602. [DOI] [PubMed] [Google Scholar]
- 15.Holowatyj A.N., Ruterbusch J.J., Rozek L.S., Cote M.L., Stoffel E.M. Racial/ethnic disparities in survival among patients with young-onset colorectal cancer. J. Clin. Oncol. 2016;34:2148. doi: 10.1200/JCO.2015.65.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thanikachalam K., Khan G. Colorectal cancer and nutrition. Nutrients. 2019;11:164. doi: 10.3390/nu11010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka S., Oka S., Chayama K. Colorectal endoscopic submucosal dissection: Present status and future perspective, including its differentiation from endoscopic mucosal resection. J. Gastroenterol. 2008;43:641–651. doi: 10.1007/s00535-008-2223-4. [DOI] [PubMed] [Google Scholar]
- 18.Chakedis J., Schmidt C.R. Surgical treatment of metastatic colorectal cancer. Surg. Oncol. Clin. 2018;27:377–399. doi: 10.1016/j.soc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Mojtahedi Z., Koo J.S., Yoo J., Kim P., Kang H.-T., Hwang J., Joo M.K., Shen J.J. Palliative care and life-sustaining/local procedures in colorectal Cancer in the United States hospitals: A ten-year perspective. Cancer Manag. Res. 2021;13:7569–7577. doi: 10.2147/CMAR.S330448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend A., Price T., Karapetis C. Selective internal radiation therapy for liver metastases from colorectal cancer. Cochrane Database Syst. Rev. 2009:CD007045. doi: 10.1002/14651858.CD007045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanani A., Veen T., Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br. J. Surg. 2021;108:1417–1425. doi: 10.1093/bjs/znab342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loughrey M.B. Neoadjuvant immunotherapy and colorectal cancer treatment: Implications for the primary role of surgery. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2022;24:1460–1461. doi: 10.1111/codi.16416. [DOI] [PubMed] [Google Scholar]
- 23.Aiello P., Sharghi M., Mansourkhani S.M., Ardekan A.P., Jouybari L., Daraei N., Peiro K., Mohamadian S., Rezaei M., Heidari M. Medicinal plants in the prevention and treatment of colon cancer. Oxidative Med. Cell. Longev. 2019;2019:2075614. doi: 10.1155/2019/2075614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muppala S. Phytochemicals Targeting Colorectal Cancer Growth and Metastasis. Crit. Rev. Oncog. 2020;25:141–149. doi: 10.1615/CritRevOncog.2020035039. [DOI] [PubMed] [Google Scholar]
- 25.Sain A., Sahu S., Naskar D. Potential of Olive oil and its phenolic compounds as therapeutic intervention against colorectal cancer: A comprehensive review. Br. J. Nutr. 2021;128:1257–1273. doi: 10.1017/S0007114521002919. [DOI] [PubMed] [Google Scholar]
- 26.Cueva C., Silva M., Pinillos I., Bartolomé B., Moreno-Arribas M.V. Interplay between dietary polyphenols and oral and gut microbiota in the development of colorectal cancer. Nutrients. 2020;12:625. doi: 10.3390/nu12030625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews L. Dietary flavonoids for the prevention of colorectal cancer. Clin. J. Oncol. Nurs. 2013;17:671–673. doi: 10.1188/13.CJON.671-672. [DOI] [PubMed] [Google Scholar]
- 28.Woo H.D., Kim J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013;19:1011. doi: 10.3748/wjg.v19.i7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kocic B., Kitic D., Brankovic S. Dietary flavonoid intake and colorectal cancer risk: Evidence from human population studies. J. BUON. 2013;18:34–43. [PubMed] [Google Scholar]
- 30.Jin H., Leng Q., Li C. Dietary flavonoid for preventing colorectal neoplasms. Cochrane Database Syst. Rev. 2012:CD009350. doi: 10.1002/14651858.CD009350.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han S., Cao Y., Guo T., Lin Q., Luo F. Targeting lncRNA/Wnt axis by flavonoids: A promising therapeutic approach for colorectal cancer. Phytother. Res. 2022;36:4024–4040. doi: 10.1002/ptr.7550. [DOI] [PubMed] [Google Scholar]
- 32.Pereira-Wilson C. Can dietary flavonoids be useful in the personalized treatment of colorectal cancer? World J. Gastrointest. Oncol. 2022;14:1115. doi: 10.4251/wjgo.v14.i6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández J., Silván B., Entrialgo-Cadierno R., Villar C.J., Capasso R., Uranga J.A., Lombó F., Abalo R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021;143:112241. doi: 10.1016/j.biopha.2021.112241. [DOI] [PubMed] [Google Scholar]
- 34.Afshari K., Haddadi N.S., Haj-Mirzaian A., Farzaei M.H., Rohani M.M., Akramian F., Naseri R., Sureda A., Ghanaatian N., Abdolghaffari A.H. Natural flavonoids for the prevention of colon cancer: A comprehensive review of preclinical and clinical studies. J. Cell. Physiol. 2019;234:21519–21546. doi: 10.1002/jcp.28777. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Zhang T., Chen G.Y. Flavonoids and colorectal cancer prevention. Antioxidants. 2018;7:187. doi: 10.3390/antiox7120187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koosha S., Alshawsh M.A., Looi C.Y., Seyedan A., Mohamed Z. An association map on the effect of flavonoids on the signaling pathways in colorectal cancer. Int. J. Med. Sci. 2016;13:374. doi: 10.7150/ijms.14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potter J.D. Nutrition and colorectal cancer. Cancer Causes Control. 1996;7:127–146. doi: 10.1007/BF00115644. [DOI] [PubMed] [Google Scholar]
- 38.Martinez M.E., Willett W.C. Calcium, vitamin D, and colorectal cancer: A review of the epidemiologic evidence. Cancer Epidemiol. Biomark. Prev. 1998;7:163–168. [PubMed] [Google Scholar]
- 39.Terry P., Giovannucci E., Michels K.B., Bergkvist L., Hansen H., Holmberg L., Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J. Natl. Cancer Inst. 2001;93:525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 40.Santarelli R.L., Pierre F., Corpet D.E. Processed meat and colorectal cancer: A review of epidemiologic and experimental evidence. Nutr. Cancer. 2008;60:131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benarba B., Pandiella A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed. Pharmacother. 2018;107:408–423. doi: 10.1016/j.biopha.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 42.La Vecchia S., Sebastián C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev.Biol. 2020;98:63–70. doi: 10.1016/j.semcdb.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Keum N., Giovannucci E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 44.Lynch H.T., Smyrk T.C., Watson P., Lanspa S.J., Lynch J.F., Lynch P.M., Cavalieri R.J., Boland C.R. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: An updated review. Gastroenterology. 1993;104:1535–1549. doi: 10.1016/0016-5085(93)90368-M. [DOI] [PubMed] [Google Scholar]
- 45.Niessen R.C., Berends M.J., Wu Y., Sijmons R.H., Hollema H., Ligtenberg M.J., de Walle H.E., de Vries E.G., Karrenbeld A., Buys C.H. Identification of mismatch repair gene mutations in young patients with colorectal cancer and in patients with multiple tumours associated with hereditary non-polyposis colorectal cancer. Gut. 2006;55:1781–1788. doi: 10.1136/gut.2005.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seppälä T., Latchford A., Negoi I., Sampaio Soares A., Jimenez-Rodriguez R., Sánchez-Guillén L., Evans D., Ryan N., Crosbie E., Dominguez-Valentin M. European guidelines from the EHTG and ESCP for Lynch syndrome: An updated third edition of the Mallorca guidelines based on gene and gender. Br. J. Surg. 2021;108:484–498. doi: 10.1002/bjs.11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Møller P., Seppälä T.T., Bernstein I., Holinski-Feder E., Sala P., Evans D.G., Lindblom A., Macrae F., Blanco I., Sijmons R.H. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut. 2018;67:1306–1316. doi: 10.1136/gutjnl-2017-314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ponti G., Manfredini M., Tomasi A., Pellacani G. Muir–Torre Syndrome and founder mismatch repair gene mutations: A long gone historical genetic challenge. Gene. 2016;589:127–132. doi: 10.1016/j.gene.2015.06.078. [DOI] [PubMed] [Google Scholar]
- 49.Grzybowski A., Jablonska S. Muir–Torre Syndrome—Is It Really a New Syndrome? Am. J. Dermatopathol. 2009;31:799–802. doi: 10.1097/DAD.0b013e3181ba6c56. [DOI] [PubMed] [Google Scholar]
- 50.Kidambi T.D., Kohli D.R., Samadder N.J., Singh A. Hereditary polyposis syndromes. Curr. Treat. Options Gastroenterol. 2019;17:650–665. doi: 10.1007/s11938-019-00251-4. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton S.R., Liu B., Parsons R.E., Papadopoulos N., Jen J., Powell S.M., Krush A.J., Berk T., Cohen Z., Tetu B. The molecular basis of Turcot’s syndrome. N. Engl. J. Med. 1995;332:839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 52.Rutz H.P., de Tribolet N., Calmes J.M., Chapuis G. Long-time survival of a patient with glioblastoma and Turcot’s syndrome: Case report. J. Neurosurg. 1991;74:813–815. doi: 10.3171/jns.1991.74.5.0813. [DOI] [PubMed] [Google Scholar]
- 53.Haggar F.A., Boushey R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galiatsatos P., Foulkes W.D. Familial adenomatous polyposis. Off. J. Am. Coll. Gastroenterol. ACG. 2006;101:385–398. doi: 10.1111/j.1572-0241.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 55.Coffin C.M., Hornick J.L., Zhou H., Fletcher C.D. Gardner fibroma: A clinicopathologic and immunohistochemical analysis of 45 patients with 57 fibromas. Am. J. Surg. Pathol. 2007;31:410–416. doi: 10.1097/01.pas.0000213348.65014.0a. [DOI] [PubMed] [Google Scholar]
- 56.Kiessling P., Dowling E., Huang Y., Ho M.L., Balakrishnan K., Weigel B.J., Highsmith W.E., Niu Z., Schimmenti L.A. Identification of aggressive Gardner syndrome phenotype associated with a de novo APC variant, c. 4666dup. Mol. Case Stud. 2019;5:a003640. doi: 10.1101/mcs.a003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutcliffe E.G., Thompson A.B., Stettner A.R., Marshall M.L., Roberts M.E., Susswein L.R., Wang Y., Klein R.T., Hruska K.S., Solomon B.D. Multi-gene panel testing confirms phenotypic variability in MUTYH-Associated Polyposis. Fam. Cancer. 2019;18:203–209. doi: 10.1007/s10689-018-00116-2. [DOI] [PubMed] [Google Scholar]
- 58.Curia M.C., Catalano T., Aceto G.M. MUTYH: Not just polyposis. World J. Clin. Oncol. 2020;11:428. doi: 10.5306/wjco.v11.i7.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogt S., Jones N., Christian D., Engel C., Nielsen M., Kaufmann A., Steinke V., Vasen H.F., Propping P., Sampson J.R. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology. 2009;137:1976–1985.e1910. doi: 10.1053/j.gastro.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 60.Calva-Cerqueira D., Chinnathambi S., Pechman B., Bair J., Larsen-Haidle J., Howe J. The rate of germline mutations and large deletions of SMAD4 and BMPR1A in juvenile polyposis. Clin. Genet. 2009;75:79–85. doi: 10.1111/j.1399-0004.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 61.Gallione C.J., Repetto G.M., Legius E., Rustgi A.K., Schelley S.L., Tejpar S., Mitchell G., Drouin É., Westermann C.J., Marchuk D.A. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4) Lancet. 2004;363:852–859. doi: 10.1016/S0140-6736(04)15732-2. [DOI] [PubMed] [Google Scholar]
- 62.Brosens L.A., Van Hattem A., Hylind L.M., Iacobuzio-Donahue C., Romans K.E., Axilbund J., Cruz-Correa M., Tersmette A.C., Offerhaus G.J.A., Giardiello F.M. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56:965–967. doi: 10.1136/gut.2006.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kopacova M., Tacheci I., Rejchrt S., Bures J. Peutz-Jeghers syndrome: Diagnostic and therapeutic approach. World J. Gastroenterol. 2009;15:5397. doi: 10.3748/wjg.15.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westerman A.M., Entius M.M., De Baar E., Boor P.P., Koole R., Van Velthuysen M.L.F., Offerhaus G.J.A., Lindhout D., De Rooij F.W., Wilson J.P. Peutz-Jeghers syndrome: 78-year follow-up of the original family. Lancet. 1999;353:1211–1215. doi: 10.1016/S0140-6736(98)08018-0. [DOI] [PubMed] [Google Scholar]
- 65.Smerdel M.P., Skytte A.-B., Jelsig A.M., Ebbehøj E., Stochholm K. Revised Danish guidelines for the cancer surveillance of patients with Cowden Syndrome. Eur. J. Med. Genet. 2020;63:103873. doi: 10.1016/j.ejmg.2020.103873. [DOI] [PubMed] [Google Scholar]
- 66.Lloyd K.M., Dennis M. Cowden’s disease: A possible new symptom complex with multiple system involvement. Ann. Intern. Med. 1963;58:136–142. doi: 10.7326/0003-4819-58-1-136. [DOI] [PubMed] [Google Scholar]
- 67.Miyaki M., Konishi M., Kikuchi-Yanoshita R., Enomoto M., Igari T., Tanaka K., Muraoka M., Takahashi H., Amada Y., Fukayama M. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994;54:3011–3020. [PubMed] [Google Scholar]
- 68.Lamlum H., Ilyas M., Rowan A., Clark S., Johnson V., Bell J., Frayling I., Efstathiou J., Pack K., Payne S. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: A new facet to Knudson’s’ two-hit’hypothesis. Nat. Med. 1999;5:1071–1075. doi: 10.1038/12511. [DOI] [PubMed] [Google Scholar]
- 69.Fodde R., Smits R., Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 70.Kanth P., Grimmett J., Champine M., Burt R., Samadder J.N. Hereditary colorectal polyposis and cancer syndromes: A primer on diagnosis and management. Off. J. Am. Coll. Gastroenterol. ACG. 2017;112:1509–1525. doi: 10.1038/ajg.2017.212. [DOI] [PubMed] [Google Scholar]
- 71.Mokarram P., Albokashy M., Zarghooni M., Moosavi M.A., Sepehri Z., Chen Q.M., Hudecki A., Sargazi A., Alizadeh J., Moghadam A.R. New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets. Autophagy. 2017;13:781–819. doi: 10.1080/15548627.2017.1290751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jasperson K.W., Tuohy T.M., Neklason D.W., Burt R.W. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potack J., Itzkowitz S.H. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008;2:61. doi: 10.5009/gnl.2008.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armenian S.H., Robison L.L. Childhood cancer survivorship: An update on evolving paradigms for understanding pathogenesis and screening for therapy-related late effects. Curr. Opin. Pediatr. 2013;25:16. doi: 10.1097/MOP.0b013e32835b0b6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baxter N.N., Goldwasser M.A., Paszat L.F., Saskin R., Urbach D.R., Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann. Intern. Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 76.Hadjiliadis D., Khoruts A., Zauber A.G., Hempstead S.E., Maisonneuve P., Lowenfels A.B., Braid A.L., Cullina J., Daggett A., Fink A. Cystic fibrosis colorectal cancer screening consensus recommendations. Gastroenterology. 2018;154:736–745.e714. doi: 10.1053/j.gastro.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Botteri E., Iodice S., Bagnardi V., Raimondi S., Lowenfels A.B., Maisonneuve P. Smoking and colorectal cancer: A meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 78.Cai S., Li Y., Ding Y., Chen K., Jin M. Alcohol drinking and the risk of colorectal cancer death: A meta-analysis. Eur. J. Cancer Prev. 2014;23:532–539. doi: 10.1097/CEJ.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 79.Kyrgiou M., Kalliala I., Markozannes G., Gunter M.J., Paraskevaidis E., Gabra H., Martin-Hirsch P., Tsilidis K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong M.C., Ding H., Wang J., Chan P.S., Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intest. Res. 2019;17:317. doi: 10.5217/ir.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shivappa N., Godos J., Hébert J.R., Wirth M.D., Piuri G., Speciani A.F., Grosso G. Dietary inflammatory index and colorectal cancer risk—A meta-analysis. Nutrients. 2017;9:1043. doi: 10.3390/nu9091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng Y., Sun J., Yu J., Wang C., Su J. Dietary intakes of calcium, iron, magnesium, and potassium elements and the risk of colorectal cancer: A meta-analysis. Biol. Trace Elem. Res. 2019;189:325–335. doi: 10.1007/s12011-018-1474-z. [DOI] [PubMed] [Google Scholar]
- 83.Parra-Soto S., Ahumada D., Petermann-Rocha F., Boonpoor J., Gallegos J.L., Anderson J., Sharp L., Malcomson F.C., Livingstone K.M., Mathers J.C. Association of meat, vegetarian, pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer: Findings from the UK Biobank prospective cohort study and meta-analysis. BMC Med. 2022;20:1–16. doi: 10.1186/s12916-022-02257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhong Y., Zhu Y., Li Q., Wang F., Ge X., Zhou G., Miao L. Association between Mediterranean diet adherence and colorectal cancer: A dose-response meta-analysis. Am. J. Clin. Nutr. 2020;111:1214–1225. doi: 10.1093/ajcn/nqaa083. [DOI] [PubMed] [Google Scholar]
- 85.Hoang T., Kim H., Kim J. Dietary intake in association with all-cause mortality and colorectal cancer mortality among colorectal cancer survivors: A systematic review and meta-analysis of prospective studies. Cancers. 2020;12:3391. doi: 10.3390/cancers12113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cho K.R., Vogelstein B. Genetic alterations in the adenoma–carcinoma sequence. Cancer. 1992;70:1727–1731. doi: 10.1002/1097-0142(19920915)70:4+<1727::AID-CNCR2820701613>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 87.Cho K.R., Vogelstein B. Suppressor gene alterations in the colorectal adenoma-carcinoma sequence. J. Cell. Biochem. 1992;50:137–141. doi: 10.1002/jcb.240501124. [DOI] [PubMed] [Google Scholar]
- 88.Tariq K., Ghias K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol. Med. 2016;13:120. doi: 10.20892/j.issn.2095-3941.2015.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hermsen M., Postma C., Baak J., Weiss M., Rapallo A., Sciutto A., Roemen G., Arends J.W., Williams R., Giaretti W. Colorectal adenoma to carcinoma progression follows multiple pathways of chromosomal instability. Gastroenterology. 2002;123:1109–1119. doi: 10.1053/gast.2002.36051. [DOI] [PubMed] [Google Scholar]
- 90.Baran B., Ozupek N.M., Tetik N.Y., Acar E., Bekcioglu O., Baskin Y. Difference between left-sided and right-sided colorectal cancer: A focused review of literature. Gastroenterol. Res. 2018;11:264. doi: 10.14740/gr1062w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gryfe R., Bapat B., Gallinger S., Swallow C., Redston M., Couture J. Molecular biology of colorectal cancer. Curr. Probl. Cancer. 1997;21:233–299. doi: 10.1016/S0147-0272(97)80003-7. [DOI] [PubMed] [Google Scholar]
- 92.Levy D.B., Smith K.J., Beazer-Barclay Y., Hamilton S.R., Vogelstein B., Kinzler K.W. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 1994;54:5953–5958. [PubMed] [Google Scholar]
- 93.Dow L.E., O’Rourke K.P., Simon J., Tschaharganeh D.F., van Es J.H., Clevers H., Lowe S.W. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell. 2015;161:1539–1552. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fischer M.M., Yeung V.P., Cattaruzza F., Hussein R., Yen W.-C., Murriel C., Evans J.W., O’Young G., Brunner A.L., Wang M. RSPO3 antagonism inhibits growth and tumorigenicity in colorectal tumors harboring common Wnt pathway mutations. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-15704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Z., He X., Jia M., Liu Y., Qu D., Wu D., Wu P., Ni C., Zhang Z., Ye J. β-catenin overexpression in the nucleus predicts progress disease and unfavourable survival in colorectal cancer: A meta-analysis. PLoS ONE. 2013;8:e63854. doi: 10.1371/journal.pone.0063854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grady W.M., Markowitz S.D. Genetic and epigenetic alterations in colon cancer. Annu. Rev. Genom. Hum. Genet. 2002;3:101–128. doi: 10.1146/annurev.genom.3.022502.103043. [DOI] [PubMed] [Google Scholar]
- 98.Armaghany T., Wilson J.D., Chu Q., Mills G. Genetic alterations in colorectal cancer. Gastrointest. Cancer Res. 2012;5:19. [PMC free article] [PubMed] [Google Scholar]
- 99.Lynch H.T., Boland C.R., Gong G., Shaw T.G., Lynch P.M., Fodde R., Lynch J.F., de la Chapelle A. Phenotypic and genotypic heterogeneity in the Lynch syndrome: Diagnostic, surveillance and management implications. Eur. J. Hum. Genet. 2006;14:390–402. doi: 10.1038/sj.ejhg.5201584. [DOI] [PubMed] [Google Scholar]
- 100.Toyota M., Ahuja N., Ohe-Toyota M., Herman J.G., Baylin S.B., Issa J.-P.J. CpG island methylator phenotype in colorectal cancer. Proc. Natl. Acad. Sci. USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weisenberger D.J., Siegmund K.D., Campan M., Young J., Long T.I., Faasse M.A., Kang G.H., Widschwendter M., Weener D., Buchanan D. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 102.Advani S.M., Advani P., DeSantis S.M., Brown D., VonVille H.M., Lam M., Loree J.M., Sarshekeh A.M., Bressler J., Lopez D.S. Clinical, pathological, and molecular characteristics of CpG island methylator phenotype in colorectal cancer: A systematic review and meta-analysis. Transl. Oncol. 2018;11:1188–1201. doi: 10.1016/j.tranon.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bosetti C., Santucci C., Gallus S., Martinetti M., La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: An updated meta-analysis through 2019. Ann. Oncol. 2020;31:558–568. doi: 10.1016/j.annonc.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 104.Cao Y., Nishihara R., Qian Z.R., Song M., Mima K., Inamura K., Nowak J.A., Drew D.A., Lochhead P., Nosho K. Regular aspirin use associates with lower risk of colorectal cancers with low numbers of tumor-infiltrating lymphocytes. Gastroenterology. 2016;151:879–892.e874. doi: 10.1053/j.gastro.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan A.T., Ogino S., Fuchs C.S. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 106.Rothwell P.M., Wilson M., Elwin C.-E., Norrving B., Algra A., Warlow C.P., Meade T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 107.Tomić T., Domínguez-López S., Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: A systematic review and meta-analysis. Cancer Epidemiol. 2019;58:52–62. doi: 10.1016/j.canep.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 108.Meyskens F.L., McLaren C.E., Pelot D., Fujikawa-Brooks S., Carpenter P.M., Hawk E., Kelloff G., Lawson M.J., Kidao J., McCracken J. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev. Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomasset S., Berry D.P., Cai H., West K., Marczylo T.H., Marsden D., Brown K., Dennison A., Garcea G., Miller A. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. 2009;2:625–633. doi: 10.1158/1940-6207.CAPR-08-0201. [DOI] [PubMed] [Google Scholar]
- 110.Sinicrope F.A., Velamala P.R., Song L.M.W.K., Viggiano T.R., Bruining D.H., Rajan E., Gostout C.J., Kraichely R.E., Buttar N.S., Schroeder K.W. Efficacy of difluoromethylornithine and aspirin for treatment of adenomas and aberrant crypt foci in patients with prior advanced colorectal neoplasms. Cancer Prev. Res. 2019;12:821–830. doi: 10.1158/1940-6207.CAPR-19-0167. [DOI] [PubMed] [Google Scholar]
- 111.Samadder N.J., Kuwada S.K., Boucher K.M., Byrne K., Kanth P., Samowitz W., Jones D., Tavtigian S.V., Westover M., Berry T. Association of sulindac and erlotinib vs placebo with colorectal neoplasia in familial adenomatous polyposis: Secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:671–677. doi: 10.1001/jamaoncol.2017.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Veettil S.K., Nathisuwan S., Ching S.M., Jinatongthai P., Lim K.G., Kew S.T., Chaiyakunapruk N. Efficacy and safety of celecoxib on the incidence of recurrent colorectal adenomas: A systematic review and meta-analysis. Cancer Manag. Res. 2019;11:561. doi: 10.2147/CMAR.S180261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodríguez-Miguel A., García-Rodríguez L.A., Gil M., Montoya H., Rodríguez-Martín S., de Abajo F.J. Clopidogrel and low-dose aspirin, alone or together, reduce risk of colorectal cancer. Clin. Gastroenterol. Hepatol. 2019;17:2024–2033.e2022. doi: 10.1016/j.cgh.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 114.Ng C.-A.W., Jiang A.A., Toh E.M.S., Ng C.H., Ong Z.H., Peng S., Tham H.Y., Sundar R., Chong C.S., Khoo C.M. Metformin and colorectal cancer: A systematic review, meta-analysis and meta-regression. Int. J. Color. Dis. 2020;35:1501–1512. doi: 10.1007/s00384-020-03676-x. [DOI] [PubMed] [Google Scholar]
- 115.Huang W.-K., Hsu H.-C., Liu J.-R., Yang T.-S., Chen J.-S., Chang J.W.-C., Lin Y.-C., Yu K.-H., Kuo C.-F., See L.-C. The association of ursodeoxycholic acid use with colorectal cancer risk: A nationwide cohort study. Medicine. 2016;95:e2980. doi: 10.1097/MD.0000000000002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y., He X., Ding Y.e., Chen H., Sun L. Statin uses and mortality in colorectal cancer patients: An updated systematic review and meta-analysis. Cancer Med. 2019;8:3305–3313. doi: 10.1002/cam4.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barbalata C.I., Tefas L.R., Achim M., Tomuta I., Porfire A.S. Statins in risk-reduction and treatment of cancer. World J. Clin. Oncol. 2020;11:573. doi: 10.5306/wjco.v11.i8.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Botteri E., Støer N.C., Sakshaug S., Graff-Iversen S., Vangen S., Hofvind S., De Lange T., Bagnardi V., Ursin G., Weiderpass E. Menopausal hormone therapy and colorectal cancer: A linkage between nationwide registries in Norway. BMJ Open. 2017;7:e017639. doi: 10.1136/bmjopen-2017-017639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Y.-Y., Gao L.-J., Zhang Y.-X., Liu S.-J., Cheng S., Liu Y.-P., Jia C.-X. Bisphosphonates and risk of cancers: A systematic review and meta-analysis. Br. J. Cancer. 2020;123:1570–1581. doi: 10.1038/s41416-020-01043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eberhart C.E., Coffey R.J., Radhika A., Giardiello F.M., Ferrenbach S., Dubois R.N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 121.Oshima M., Dinchuk J.E., Kargman S.L., Oshima H., Hancock B., Kwong E., Trzaskos J.M., Evans J.F., Taketo M.M. Suppression of intestinal polyposis in ApcΔ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/S0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 122.Castellone M.D., Teramoto H., Williams B.O., Druey K.M., Gutkind J.S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-ß-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 123.Wang D., DuBois R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Masferrer J.L., Leahy K.M., Koki A.T., Zweifel B.S., Settle S.L., Woerner B.M., Edwards D.A., Flickinger A.G., Moore R.J., Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 125.Seno H., Oshima M., Ishikawa T.-O., Oshima H., Takaku K., Chiba T., Narumiya S., Taketo M.M. Cyclooxygenase 2-and prostaglandin E2 receptor EP2-dependent angiogenesis in ApcΔ716 mouse intestinal polyps. Cancer Res. 2002;62:506–511. [PubMed] [Google Scholar]
- 126.Nan H., Hutter C.M., Lin Y., Jacobs E.J., Ulrich C.M., White E., Baron J.A., Berndt S.I., Brenner H., Butterbach K. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 2015;313:1133–1142. doi: 10.1001/jama.2015.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arber N., Eagle C.J., Spicak J., Rácz I., Dite P., Hajer J., Zavoral M., Lechuga M.J., Gerletti P., Tang J. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 128.Dehmer S.P., Maciosek M.V., Flottemesch T.J., LaFrance A.B., Whitlock E.P. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: A decision analysis for the US Preventive Services Task Force. Ann. Intern. Med. 2016;164:777–786. doi: 10.7326/M15-2129. [DOI] [PubMed] [Google Scholar]
- 129.Chubak J., Whitlock E.P., Williams S.B., Kamineni A., Burda B.U., Buist D.S., Anderson M.L. Aspirin for the prevention of cancer incidence and mortality: Systematic evidence reviews for the US Preventive Services Task Force. Ann. Intern. Med. 2016;164:814–825. doi: 10.7326/M15-2117. [DOI] [PubMed] [Google Scholar]
- 130.Katona B.W., Weiss J.M. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158:368–388. doi: 10.1053/j.gastro.2019.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu F., Yan L., Wang Z., Lu Y., Chu Y., Li X., Liu Y., Rui D., Nie S., Xiang H. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Oncotarget. 2017;8:16017. doi: 10.18632/oncotarget.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Petrera M., Paleari L., Clavarezza M., Puntoni M., Caviglia S., Briata I.M., Oppezzi M., Mislej E.M., Stabuc B., Gnant M. The ASAMET trial: A randomized, phase II, double-blind, placebo-controlled, multicenter, 2× 2 factorial biomarker study of tertiary prevention with low-dose aspirin and metformin in stage I-III colorectal cancer patients. BMC Cancer. 2018;18:1–9. doi: 10.1186/s12885-018-5126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chan K.K., Oza A.M., Siu L.L. The statins as anticancer agents. Clin. Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 134.Teraoka N., Mutoh M., Takasu S., Ueno T., Yamamoto M., Sugimura T., Wakabayashi K. Inhibition of intestinal polyp formation by pitavastatin, a HMG-CoA reductase inhibitor. Cancer Prev. Res. 2011;4:445–453. doi: 10.1158/1940-6207.CAPR-10-0028. [DOI] [PubMed] [Google Scholar]
- 135.Suh N., Reddy B.S., DeCastro A., Paul S., Lee H.J., Smolarek A.K., So J.Y., Simi B., Wang C.X., Janakiram N.B. Combination of atorvastatin with sulindac or naproxen profoundly inhibits colonic adenocarcinomas by suppressing the p65/β-catenin/cyclin D1 signaling pathway in rats. Cancer Prev. Res. 2011;4:1895–1902. doi: 10.1158/1940-6207.CAPR-11-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malila N., Virtamo J., Virtanen M., Albanes D., Tangrea J.A., Huttunen J.K. The effect of α-tocopherol and β-carotene supplementation on colorectal adenomas in middle-aged male smokers. Cancer Epidemiol. Prev. Biomark. 1999;8:489–493. [PubMed] [Google Scholar]
- 137.Papaioannou D., Cooper K., Carroll C., Hind D., Squires H., Tappenden P., Logan R. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: A systematic review and meta-analysis. Color. Dis. 2011;13:1085–1099. doi: 10.1111/j.1463-1318.2010.02289.x. [DOI] [PubMed] [Google Scholar]
- 138.Yusof A.S., Isa Z.M., Shah S.A. Dietary patterns and risk of colorectal cancer: A systematic review of cohort studies (2000–2011) Asian Pac. J. Cancer Prev. 2012;13:4713–4717. doi: 10.7314/APJCP.2012.13.9.4713. [DOI] [PubMed] [Google Scholar]
- 139.Vanio H., Bianchini F. IARC Handbooks of Cancer Prevention. IARC Press; Lyon, France: 2002. Weight Control and Physical Activity. [Google Scholar]
- 140.He X., Wu K., Zhang X., Nishihara R., Cao Y., Fuchs C.S., Giovannucci E.L., Ogino S., Chan A.T., Song M. Dietary intake of fiber, whole grains and risk of colorectal cancer: An updated analysis according to food sources, tumor location and molecular subtypes in two large US cohorts. Int. J. Cancer. 2019;145:3040–3051. doi: 10.1002/ijc.32382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brenner H., Chang–Claude J., Jansen L., Knebel P., Stock C., Hoffmeister M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146:709–717. doi: 10.1053/j.gastro.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 142.Wells K.O., Hawkins A.T., Krishnamurthy D.M., Dharmarajan S., Glasgow S.C., Hunt S.R., Mutch M.G., Wise P., Silviera M.L. Omission of adjuvant chemotherapy is associated with increased mortality in patients with T3N0 colon cancer with inadequate lymph node harvest. Dis. Colon Rectum. 2017;60:15–21. doi: 10.1097/DCR.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 143.Ribic C.M., Sargent D.J., Moore M.J., Thibodeau S.N., French A.J., Goldberg R.M., Hamilton S.R., Laurent-Puig P., Gryfe R., Shepherd L.E. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sargent D.J., Marsoni S., Monges G., Thibodeau S.N., Labianca R., Hamilton S.R., French A.J., Kabat B., Foster N.R., Torri V. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010;28:3219. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Argilés G., Tabernero J., Labianca R., Hochhauser D., Salazar R., Iveson T., Laurent-Puig P., Quirke P., Yoshino T., Taieb J. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1291–1305. doi: 10.1016/j.annonc.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 146.Angell H.K., Bruni D., Barrett J.C., Herbst R., Galon J. The immunoscore: Colon cancer and beyond. Clin. Cancer Res. 2020;26:332–339. doi: 10.1158/1078-0432.CCR-18-1851. [DOI] [PubMed] [Google Scholar]
- 147.Xie Y.-H., Chen Y.-X., Fang J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020;5:1–30. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Falcone A., Ricci S., Brunetti I., Pfanner E., Allegrini G., Barbara C., Crinò L., Benedetti G., Evangelista W., Fanchini L. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 149.Souglakos J., Androulakis N., Syrigos K., Polyzos A., Ziras N., Athanasiadis A., Kakolyris S., Tsousis S., Kouroussis C., Vamvakas L. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): A multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br. J. Cancer. 2006;94:798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 151.Mendelsohn J., Prewett M., Rockwell P., Goldstein N.I. CCR 20th anniversary commentary: A chimeric antibody, C225, inhibits EGFR activation and tumor growth. Clin. Cancer Res. 2015;21:227–229. doi: 10.1158/1078-0432.CCR-14-2491. [DOI] [PubMed] [Google Scholar]
- 152.Oliveira A.F., Bretes L., Furtado I. Review of PD-1/PD-L1 inhibitors in metastatic dMMR/MSI-H colorectal cancer. Front. Oncol. 2019;9:396. doi: 10.3389/fonc.2019.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jonker D.J., O’Callaghan C.J., Karapetis C.S., Zalcberg J.R., Tu D., Au H.-J., Berry S.R., Krahn M., Price T., Simes R.J. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 154.Roskoski R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 155.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti-and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guba M., Seeliger H., Kleespies A., Jauch K.-W., Bruns C. Vascular endothelial growth factor in colorectal cancer. Int. J. Color. Dis. 2004;19:510–517. doi: 10.1007/s00384-003-0576-y. [DOI] [PubMed] [Google Scholar]
- 157.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;88:105906. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Paulo Farias D., de Araujo F.F., Neri-Numa I.A., Pastore G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021;145:110383. doi: 10.1016/j.foodres.2021.110383. [DOI] [PubMed] [Google Scholar]
- 159.Tsimogiannis D., Oreopoulou V. Classification of phenolic compounds in plants. Polyphen. Plants (Second Ed.) 2019:263–284. doi: 10.1016/B978-0-12-813768-0.00026-8. [DOI] [Google Scholar]
- 160.Vuolo M.M., Lima V.S., Junior M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. Bioact. Compd. 2019:33–50. doi: 10.1016/B978-0-12-814774-0.00002-5. [DOI] [Google Scholar]
- 161.Kiruthiga C., Devi K.P., Nabavi S.M., Bishayee A. Autophagy: A potential therapeutic target of polyphenols in hepatocellular carcinoma. Cancers. 2020;12:562. doi: 10.3390/cancers12030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Darvesh A.S., Carroll R.T., Bishayee A., Geldenhuys W.J., Van der Schyf C.J. Oxidative stress and Alzheimer’s disease: Dietary polyphenols as potential therapeutic agents. Expert Rev. Neurother. 2010;10:729–745. doi: 10.1586/ern.10.42. [DOI] [PubMed] [Google Scholar]
- 163.Karimi A., Majlesi M., Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015;4:27. [PMC free article] [PubMed] [Google Scholar]
- 164.Samec M., Liskova A., Koklesova L., Samuel S.M., Zhai K., Buhrmann C., Varghese E., Abotaleb M., Qaradakhi T., Zulli A. Flavonoids against the Warburg phenotype—Concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. Epma J. 2020;11:377–398. doi: 10.1007/s13167-020-00217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jain A., Madu C.O., Lu Y. Phytochemicals in chemoprevention: A cost-effective complementary approach. J. Cancer. 2021;12:3686. doi: 10.7150/jca.57776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rajamanickam S., Agarwal R. Natural products and colon cancer: Current status and future prospects. Drug Dev. Res. 2008;69:460–471. doi: 10.1002/ddr.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bishayee A., Sethi G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016;40:1–3. doi: 10.1016/j.semcancer.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 168.Huang X.-M., Yang Z.-J., Xie Q., Zhang Z.-K., Zhang H., Ma J.-Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019;117:109142. doi: 10.1016/j.biopha.2019.109142. [DOI] [PubMed] [Google Scholar]
- 169.Atanasov A.G., Zotchev S.B., Dirsch V.M., Supuran C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin C.-L., Jeng J.-H., Wu C.-C., Hsieh S.-L., Huang G.-C., Leung W., Lee C.-T., Chen C.-Y., Lee C.-H. Chemopreventive potential of 2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-D-glucoside on The formation of aberrant crypt foci in azoxymethane-induced colorectal cancer in rats. BioMed Res. Int. 2017;2017:3634915. doi: 10.1155/2017/3634915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen Q., Lei J., Zhou J., Ma S., Huang Q., Ge B. Chemopreventive effect of 4′-hydroxychalcone on intestinal tumorigenesis in Apc Min mice Corrigendum in/10.3892/ol. 2021.12741. Oncol. Lett. 2021;21:213. doi: 10.3892/ol.2021.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lai C.-Y., Tsai A.-C., Chen M.-C., Chang L.-H., Sun H.-L., Chang Y.-L., Chen C.-C., Teng C.-M., Pan S.-L. Aciculatin induces p53-dependent apoptosis via MDM2 depletion in human cancer cells in vitro and in vivo. PLoS ONE. 2012;7:e42192. doi: 10.1371/journal.pone.0042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Au A., Li B., Wang W., Roy H., Koehler K., Birt D. Effect of dietary apigenin on colonic ornithine decarboxylase activity, aberrant crypt foci formation, and tumorigenesis in different experimental models. Nutr. Cancer. 2006;54:243–251. doi: 10.1207/s15327914nc5402_11. [DOI] [PubMed] [Google Scholar]
- 174.Wang Q.R., Yao X.Q., Wen G., Fan Q., Li Y.-J., Fu X.Q., Li C.K., Sun X.G. Apigenin suppresses the growth of colorectal cancer xenografts via phosphorylation and up-regulated FADD expression. Oncol. Lett. 2011;2:43–47. doi: 10.3892/ol.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chunhua L., Donglan L., Xiuqiong F., Lihua Z., Qin F., Yawei L., Liang Z., Ge W., Linlin J., Ping Z. Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. J. Nutr. Biochem. 2013;24:1766–1775. doi: 10.1016/j.jnutbio.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 176.Zhong Y., Krisanapun C., Lee S.-H., Nualsanit T., Sams C., Peungvicha P., Baek S.J. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer. 2010;46:3365–3374. doi: 10.1016/j.ejca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen X., Xu H., Yu X., Wang X., Zhu X., Xu X. Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. Buon. 2019;24:488–493. [PubMed] [Google Scholar]
- 178.Shao H., Jing K., Mahmoud E., Huang H., Fang X., Yu C. Apigenin sensitizes colon cancer cells to antitumor activity of ABT-263. Mol. Cancer Ther. 2013;12:2640–2650. doi: 10.1158/1535-7163.MCT-13-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dai J., Van Wie P.G., Fai L.Y., Kim D., Wang L., Poyil P., Luo J., Zhang Z. Downregulation of NEDD9 by apigenin suppresses migration, invasion, and metastasis of colorectal cancer cells. Toxicol. Appl. Pharmacol. 2016;311:106–112. doi: 10.1016/j.taap.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim D.H., Hossain M.A., Kang Y.J., Jang J.Y., Lee Y.J., Im E., Yoon J.-H., Kim H.S., Chung H.Y., Kim N.D. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int. J. Oncol. 2013;43:1652–1658. doi: 10.3892/ijo.2013.2086. [DOI] [PubMed] [Google Scholar]
- 181.Chen W.-C., Kuo T.-H., Tzeng Y.-S., Tsai Y.-C. Baicalin induces apoptosis in SW620 human colorectal carcinoma cells in vitro and suppresses tumor growth in vivo. Molecules. 2012;17:3844–3857. doi: 10.3390/molecules17043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Song L., Zhu S., Liu C., Zhang Q., Liang X. Baicalin triggers apoptosis, inhibits migration, and enhances anti-tumor immunity in colorectal cancer via TLR4/NF-κB signaling pathway. J. Food Biochem. 2022;46:e13703. doi: 10.1111/jfbc.13703. [DOI] [PubMed] [Google Scholar]
- 183.Kim S.-J., Kim H.-J., Kim H.-R., Lee S.-H., Cho S.-D., Choi C.-S., Nam J.-S., Jung J.-Y. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol. Med. Rep. 2012;6:1443–1449. doi: 10.3892/mmr.2012.1085. [DOI] [PubMed] [Google Scholar]
- 184.Chai Y., Xu J., Yan B. The anti-metastatic effect of baicalein on colorectal cancer. Oncol. Rep. 2017;37:2317–2323. doi: 10.3892/or.2017.5437. [DOI] [PubMed] [Google Scholar]
- 185.Dou J., Wang Z., Ma L., Peng B., Mao K., Li C., Su M., Zhou C., Peng G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget. 2018;9:20089. doi: 10.18632/oncotarget.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Z., Ma L., Su M., Zhou Y., Mao K., Li C., Peng G., Zhou C., Shen B., Dou J. Baicalin induces cellular senescence in human colon cancer cells via upregulation of DEPP and the activation of Ras/Raf/MEK/ERK signaling. Cell Death Dis. 2018;9:217. doi: 10.1038/s41419-017-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tao Y., Zhan S., Wang Y., Zhou G., Liang H., Chen X., Shen H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018;8:14477. doi: 10.1038/s41598-018-32734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang B., Bai H., Sa Y., Zhu P., Liu P. Inhibiting EMT, stemness and cell cycle involved in baicalin-induced growth inhibition and apoptosis in colorectal cancer cells. J. Cancer. 2020;11:2303. doi: 10.7150/jca.37242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang C.-Z., Zhang C.-F., Luo Y., Yao H., Yu C., Chen L., Yuan J., Huang W.-H., Wan J.-Y., Zeng J. Baicalein, an enteric microbial metabolite, suppresses gut inflammation and cancer progression in Apc Min/+ mice. Clin. Transl. Oncol. 2020;22:1013–1022. doi: 10.1007/s12094-019-02225-5. [DOI] [PubMed] [Google Scholar]
- 190.Zhang W., Liu Q., Luo L., Song J., Han K., Liu R., Gong Y., Guo X. Use Chou’s 5-steps rule to study how Baicalin suppresses the malignant phenotypes and induces the apoptosis of colorectal cancer cells. Arch. Biochem. Biophys. 2021;705:108919. doi: 10.1016/j.abb.2021.108919. [DOI] [PubMed] [Google Scholar]
- 191.Zhou C., Ou W., Xu Q., Lin L., Xu F., Chen R., Miao H. Chemoprotective effect of boeravinone B against 1, 2-dimethyl hydrazine induced colorectal cancer in rats via suppression of oxidative stress and inflammatory reaction. J. Gastrointest. Oncol. 2022;13:1832–1841. doi: 10.21037/jgo-22-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Miyamoto S., Kohno H., Suzuki R., Sugie S., Murakami A., Ohigashi H., Tanaka T. Preventive effects of chrysin on the development of azoxymethane-induced colonic aberrant crypt foci in rats. Oncol. Rep. 2006;15:1169–1173. doi: 10.3892/or.15.5.1169. [DOI] [PubMed] [Google Scholar]
- 193.Salama A.A., Allam R.M. Promising targets of chrysin and daidzein in colorectal cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharmacol. 2021;892:173763. doi: 10.1016/j.ejphar.2020.173763. [DOI] [PubMed] [Google Scholar]
- 194.Villegas I., Sánchez-Fidalgo S., de la Lastra C.A. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Mol. Nutr. Food Res. 2011;55:259–267. doi: 10.1002/mnfr.201000225. [DOI] [PubMed] [Google Scholar]
- 195.Milacic V., Banerjee S., Landis-Piwowar K.R., Sarkar F.H., Majumdar A.P., Dou Q.P. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hao J., Dai X., Gao J., Li Y., Hou Z., Chang Z., Wang Y. Curcumin suppresses colorectal tumorigenesis via the Wnt/β-catenin signaling pathway by downregulating Axin2. Oncol. Lett. 2021;21:186. doi: 10.3892/ol.2021.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kubota M., Shimizu M., Sakai H., Yasuda Y., Terakura D., Baba A., Ohno T., Tsurumi H., Tanaka T., Moriwaki H. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr. Cancer. 2012;64:72–79. doi: 10.1080/01635581.2012.630554. [DOI] [PubMed] [Google Scholar]
- 198.Kunnumakkara A.B., Diagaradjane P., Guha S., Deorukhkar A., Shentu S., Aggarwal B.B., Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to γ-radiation by targeting nuclear factor-κB–regulated gene products. Clin. Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 199.Li L., Ahmed B., Mehta K., Kurzrock R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007;6:1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 200.McFadden R.-M.T., Larmonier C.B., Shehab K.W., Midura-Kiela M., Ramalingam R., Harrison C.A., Besselsen D.G., Chase J.H., Caporaso J.G., Jobin C. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel Dis. 2015;21:2483–2494. doi: 10.1097/MIB.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Marjaneh R.M., Rahmani F., Hassanian S.M., Rezaei N., Hashemzehi M., Bahrami A., Ariakia F., Fiuji H., Sahebkar A., Avan A. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J. Cell. Physiol. 2018;233:6785–6798. doi: 10.1002/jcp.26538. [DOI] [PubMed] [Google Scholar]
- 202.Cooke D., Schwarz M., Boocock D., Winterhalter P., Steward W.P., Gescher A.J., Marczylo T.H. Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the ApcMin mouse model of intestinal carcinogenesis—Relationship with tissue anthocyanin levels. Int. J. Cancer. 2006;119:2213–2220. doi: 10.1002/ijc.22090. [DOI] [PubMed] [Google Scholar]
- 203.Huang C.-C., Hung C.-H., Hung T.-W., Lin Y.-C., Wang C.-J., Kao S.-H. Dietary delphinidin inhibits human colorectal cancer metastasis associating with upregulation of miR-204-3p and suppression of the integrin/FAK axis. Sci. Rep. 2019;9:18954. doi: 10.1038/s41598-019-55505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Koosha S., Mohamed Z., Sinniah A., Alshawsh M.A. Evaluation of anti-tumorigenic effects of diosmetin against human colon cancer xenografts in athymic nude mice. Molecules. 2019;24:2522. doi: 10.3390/molecules24142522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shimizu M., Shirakami Y., Sakai H., Yasuda Y., Kubota M., Adachi S., Tsurumi H., Hara Y., Moriwaki H. (−)-Epigallocatechin gallate inhibits growth and activation of the VEGF/VEGFR axis in human colorectal cancer cells. Chem.-Biol. Interact. 2010;185:247–252. doi: 10.1016/j.cbi.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 206.Shimizu M., Shirakami Y., Sakai H., Adachi S., Hata K., Hirose Y., Tsurumi H., Tanaka T., Moriwaki H. (−)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev. Res. 2008;1:298–304. doi: 10.1158/1940-6207.CAPR-08-0045. [DOI] [PubMed] [Google Scholar]
- 207.Zhong Y., Chiou Y.-S., Pan M.-H., Ho C.-T., Shahidi F. Protective effects of epigallocatechin gallate (EGCG) derivatives on azoxymethane-induced colonic carcinogenesis in mice. J. Funct. Foods. 2012;4:323–330. doi: 10.1016/j.jff.2011.12.011. [DOI] [Google Scholar]
- 208.Toden S., Tran H.-M., Tovar-Camargo O.A., Okugawa Y., Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. 2016;7:16158. doi: 10.18632/oncotarget.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xu G., Ren G., Xu X., Yuan H., Wang Z., Kang L., Yu W., Tian K. Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food Chem. Toxicol. 2010;48:390–395. doi: 10.1016/j.fct.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 210.Wang Y., Jin H.-Y., Fang M.-Z., Wang X.-F., Chen H., Huang S.-L., Kong D.-S., Li M., Zhang X., Sun Y. Epigallocatechin gallate inhibits dimethylhydrazine-induced colorectal cancer in rats. World J. Gastroenterol. 2020;26:2064. doi: 10.3748/wjg.v26.i17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mariyappan P., Kalaiyarasu T., Manju V. Effect of eriodictyol on preneoplastic lesions, oxidative stress and bacterial enzymes in 1, 2-dimethyl hydrazine-induced colon carcinogenesis. Toxicol. Res. 2017;6:678–692. doi: 10.1039/C7TX00074J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang N., Zhou F., Guo J., Zhu H., Luo S., Cao J. Euxanthone suppresses tumor growth and metastasis in colorectal cancer via targeting CIP2A/PP2A pathway. Life Sci. 2018;209:498–506. doi: 10.1016/j.lfs.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 213.Kunchari Kalaimathi S., Sudhandiran G. Fisetin ameolirates the azoxymethane and dextran sodium sulfate induced colitis associated colorectal cancer. Int. J. Pharm. Clin. Res. 2016;8:551–560. [Google Scholar]
- 214.Khan N., Jajeh F., Eberhardt E.L., Miller D.D., Albrecht D.M., Van Doorn R., Hruby M.D., Maresh M.E., Clipson L., Mukhtar H. Fisetin and 5-fluorouracil: Effective combination for PIK3CA-mutant colorectal cancer. Int. J. Cancer. 2019;145:3022–3032. doi: 10.1002/ijc.32367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jeng L.B., Kumar Velmurugan B., Chen M.C., Hsu H.H., Ho T.J., Day C.H., Lin Y.M., Padma V.V., Tu C.C., Huang C.Y. Fisetin mediated apoptotic cell death in parental and Oxaliplatin/irinotecan resistant colorectal cancer cells in vitro and in vivo. J. Cell. Physiol. 2018;233:7134–7142. doi: 10.1002/jcp.26532. [DOI] [PubMed] [Google Scholar]
- 216.Leu J.D., Wang B.S., Chiu S.J., Chan C.Y., Chen C.C., Chen F.D., Avirmed S., Lee Y.J. Combining fisetin and ionizing radiation suppresses the growth of mammalian colorectal cancers in xenograft tumor models. Oncol. Lett. 2016;12:4975–4982. doi: 10.3892/ol.2016.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen Y., Wu Q., Song L., He T., Li Y., Li L., Su W., Liu L., Qian Z., Gong C. Polymeric micelles encapsulating fisetin improve the therapeutic effect in colon cancer. ACS Appl. Mater. Interfaces. 2015;7:534–542. doi: 10.1021/am5066893. [DOI] [PubMed] [Google Scholar]
- 218.Li L., Wang M., Yang H., Li Y., Huang X., Guo J., Liu Z. Fisetin Inhibits Trypsin Activity and Suppresses the Growth of Colorectal Cancer in Vitro and in Vivo. Nat. Prod. Commun. 2022;17:1934578X221115511. doi: 10.1177/1934578X221115511. [DOI] [Google Scholar]
- 219.Winkelmann I., Diehl D., Oesterle D., Daniel H., Wenzel U. The suppression of aberrant crypt multiplicity in colonic tissue of 1, 2-dimethylhydrazine-treated C57BL/6J mice by dietary flavone is associated with an increased expression of Krebs cycle enzymes. Carcinogenesis. 2007;28:1446–1454. doi: 10.1093/carcin/bgm040. [DOI] [PubMed] [Google Scholar]
- 220.Auyeung K.K.-W., Law P.-C., Ko J.K.-S. Novel anti-angiogenic effects of formononetin in human colon cancer cells and tumor xenograft. Oncol. Rep. 2012;28:2188–2194. doi: 10.3892/or.2012.2056. [DOI] [PubMed] [Google Scholar]
- 221.Ma Z., Bao X., Gu J. Furowanin A-induced autophagy alleviates apoptosis and promotes cell cycle arrest via inactivation STAT3/Mcl-1 axis in colorectal cancer. Life Sci. 2019;218:47–57. doi: 10.1016/j.lfs.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 222.Sekar V., Anandasadagopan S.K., Ganapasam S. Genistein regulates tumor microenvironment and exhibits anticancer effect in dimethyl hydrazine-induced experimental colon carcinogenesis. Biofactors. 2016;42:623–637. doi: 10.1002/biof.1298. [DOI] [PubMed] [Google Scholar]
- 223.Zhang Y., Li Q., Zhou D., Chen H. Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of WNT/β-catenin signalling and reduces colon pre-neoplasia in rats. Br. J. Nutr. 2013;109:33–42. doi: 10.1017/S0007114512000876. [DOI] [PubMed] [Google Scholar]
- 224.Xiao X., Liu Z., Wang R., Wang J., Zhang S., Cai X., Wu K., Bergan R.C., Xu L., Fan D. Genistein suppresses FLT4 and inhibits human colorectal cancer metastasis. Oncotarget. 2015;6:3225. doi: 10.18632/oncotarget.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang X., Song Z.-J., He X., Zhang R.-Q., Zhang C.-F., Li F., Wang C.-Z., Yuan C.-S. Antitumor and immunomodulatory activity of genkwanin on colorectal cancer in the APCMin/+ mice. Int. Immunopharmacol. 2015;29:701–707. doi: 10.1016/j.intimp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 226.Yin H.-F., Yin C.-M., Ouyang T., Sun S.-D., Chen W.-G., Yang X.-L., He X., Zhang C.-F. Self-Nanoemulsifying Drug Delivery System of Genkwanin: A Novel Approach for Anti-Colitis-Associated Colorectal Cancer. Drug Des. Dev. Ther. 2021;15:557. doi: 10.2147/DDDT.S292417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Saiprasad G., Chitra P., Manikandan R., Sudhandiran G. Hesperidin alleviates oxidative stress and downregulates the expressions of proliferative and inflammatory markers in azoxymethane-induced experimental colon carcinogenesis in mice. Inflamm. Res. 2013;62:425–440. doi: 10.1007/s00011-013-0595-2. [DOI] [PubMed] [Google Scholar]
- 228.Saiprasad G., Chitra P., Manikandan R., Sudhandiran G. Hesperidin induces apoptosis and triggers autophagic markers through inhibition of Aurora-A mediated phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and glycogen synthase kinase-3 beta signalling cascades in experimental colon carcinogenesis. Eur. J. Cancer. 2014;50:2489–2507. doi: 10.1016/j.ejca.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 229.Tanaka T., Makita H., Kawabata K., Mori H., Kakumoto M., Satoh K., Hara A., Sumida T., Tanaka T., Ogawa H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis. 1997;18:957–965. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- 230.El-Deek S.E., Abd-Elghaffar S.K., Hna R.S., Mohamed H.G., El-Deek H.E. Effect of hesperidin against induced colon cancer in rats: Impact of Smad4 and activin a signaling pathway. Nutr. Cancer. 2022;74:697–714. doi: 10.1080/01635581.2021.1907424. [DOI] [PubMed] [Google Scholar]
- 231.Zhou J., Zhao R., Ye T., Yang S., Li Y., Yang F., Wang G., Xie Y., Li Q. Antitumor activity in colorectal cancer induced by hinokiflavone. J. Gastroenterol. Hepatol. 2019;34:1571–1580. doi: 10.1111/jgh.14581. [DOI] [PubMed] [Google Scholar]
- 232.Shi C.-J., Li S.-Y., Shen C.-H., Pan F.-F., Deng L.-Q., Fu W.-M., Wang J.-Y., Zhang J.-F. Icariside II suppressed tumorigenesis by epigenetically regulating the circβ-catenin-Wnt/β-catenin axis in colorectal cancer. Bioorganic Chem. 2022;124:105800. doi: 10.1016/j.bioorg.2022.105800. [DOI] [PubMed] [Google Scholar]
- 233.Zhou C., Gu J., Zhang G., Dong D., Yang Q., Chen M.-B., Xu D. AMPK-autophagy inhibition sensitizes icaritin-induced anti-colorectal cancer cell activity. Oncotarget. 2017;8:14736. doi: 10.18632/oncotarget.14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tang S., Cai S., Ji S., Yan X., Zhang W., Qiao X., Zhang H., Ye M., Yu S. Isoangustone A induces autophagic cell death in colorectal cancer cells by activating AMPK signaling. Fitoterapia. 2021;152:104935. doi: 10.1016/j.fitote.2021.104935. [DOI] [PubMed] [Google Scholar]
- 235.Zhao H., Zhang X., Chen X., Li Y., Ke Z., Tang T., Chai H., Guo A.M., Chen H., Yang J. Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE2 and IL-6. Toxicol. Appl. Pharmacol. 2014;279:311–321. doi: 10.1016/j.taap.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 236.Takahashi T., Takasuka N., Iigo M., Baba M., Nishino H., Tsuda H., Okuyama T. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004;95:448–453. doi: 10.1111/j.1349-7006.2004.tb03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Antunes-Ricardo M., Guardado-Félix D., Rocha-Pizaña M., Garza-Martínez J., Acevedo-Pacheco L., Gutiérrez-Uribe J., Villela-Castrejón J., López-Pacheco F., Serna-Saldívar S. Opuntia ficus-indica Extract and Isorhamnetin-3-O-Glucosyl-Rhamnoside Diminish Tumor Growth of Colon Cancer Cells Xenografted in Immune-Suppressed Mice through the Activation of Apoptosis Intrinsic Pathway. Plant Foods Hum. Nutr. 2021;76:434–441. doi: 10.1007/s11130-021-00934-3. [DOI] [PubMed] [Google Scholar]
- 238.Saud S.M., Young M.R., Jones-Hall Y.L., Ileva L., Evbuomwan M.O., Wise J., Colburn N.H., Kim Y.S., Bobe G. Chemopreventive Activity of Plant Flavonoid Isorhamnetin in Colorectal Cancer Is Mediated by Oncogenic Src and β-CateninIsorhamnetin-Induced CSK Inhibits Colorectal Cancer. Cancer Res. 2013;73:5473–5484. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nirmala P., Ramanathan M. Effect of kaempferol on lipid peroxidation and antioxidant status in 1, 2-dimethyl hydrazine induced colorectal carcinoma in rats. Eur. J. Pharmacol. 2011;654:75–79. doi: 10.1016/j.ejphar.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 240.Hassan E.S., Hassanein N.M., Ahmed H.M.S. Probing the chemoprevention potential of the antidepressant fluoxetine combined with epigallocatechin gallate or kaempferol in rats with induced early stage colon carcinogenesis. J. Pharmacol. Sci. 2021;145:29–41. doi: 10.1016/j.jphs.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 241.Hassanein N.M., Hassan E.S., Hegab A.M., Elahl H.M.S. Chemopreventive effect of sulindac in combination with epigallocatechin gallate or kaempferol against 1, 2-dimethyl hydrazine-induced preneoplastic lesions in rats: A comparative study. J. Biochem. Mol. Toxicol. 2018;32:e22198. doi: 10.1002/jbt.22198. [DOI] [PubMed] [Google Scholar]
- 242.Osman N.H., Said U.Z., El-Waseef A.M., Ahmed E.S. Luteolin supplementation adjacent to aspirin treatment reduced dimethylhydrazine-induced experimental colon carcinogenesis in rats. Tumor Biol. 2015;36:1179–1190. doi: 10.1007/s13277-014-2678-2. [DOI] [PubMed] [Google Scholar]
- 243.Pandurangan A.K., Kumar S.A.S., Dharmalingam P., Ganapasam S. Luteolin, a bioflavonoid inhibits azoxymethane-induced colon carcinogenesis: Involvement of iNOS and COX-2. Pharmacogn. Mag. 2014;10:S306. doi: 10.4103/0973-1296.133285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pandurangan A.K., Ananda Sadagopan S.K., Dharmalingam P., Ganapasam S. Luteolin, a bioflavonoid inhibits Azoxymethane-induced colorectal cancer through activation of Nrf2 signaling. Toxicol. Mech. Methods. 2014;24:13–20. doi: 10.3109/15376516.2013.843111. [DOI] [PubMed] [Google Scholar]
- 245.Kim H.Y., Jung S.K., Byun S., Son J.E., Oh M.H., Lee J., Kang M.J., Heo Y.S., Lee K.W., Lee H.J. Raf and PI3K are the molecular targets for the anti-metastatic effect of Luteolin. Phytother. Res. 2013;27:1481–1488. doi: 10.1002/ptr.4888. [DOI] [PubMed] [Google Scholar]
- 246.Pandurangan A., Dharmalingam P., Sadagopan S., Ganapasam S. Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Hum. Exp. Toxicol. 2014;33:1176–1185. doi: 10.1177/0960327114522502. [DOI] [PubMed] [Google Scholar]
- 247.Yao Y., Rao C., Zheng G., Wang S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol. Rep. 2019;42:131–141. doi: 10.3892/or.2019.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xiao B., Qin Y., Ying C., Ma B., Wang B., Long F., Wang R., Fang L., Wang Y. Combination of oncolytic adenovirus and luteolin exerts synergistic antitumor effects in colorectal cancer cells and a mouse model. Mol. Med. Rep. 2017;16:9375–9382. doi: 10.3892/mmr.2017.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gao Z., Jiang J., Hou L., Ji F. Lysionotin Induces Ferroptosis to Suppress Development of Colorectal Cancer via Promoting Nrf2 Degradation. Oxidative Med. Cell. Longev. 2022;2022:1366957. doi: 10.1155/2022/1366957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yu H., Yin S., Zhou S., Shao Y., Sun J., Pang X., Han L., Zhang Y., Gao X., Jin C. Magnolin promotes autophagy and cell cycle arrest via blocking LIF/Stat3/Mcl-1 axis in human colorectal cancers. Cell Death Dis. 2018;9:702. doi: 10.1038/s41419-018-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chen R., Zhang L. Morin inhibits colorectal tumor growth through inhibition of NF-κB signaling pathway. Immunopharmacol. Immunotoxicol. 2019;41:622–629. doi: 10.1080/08923973.2019.1688344. [DOI] [PubMed] [Google Scholar]
- 252.Sharma S.H., Thulasingam S., Chellappan D.R., Chinnaswamy P., Nagarajan S. Morin and Esculetin supplementation modulates c-myc induced energy metabolism and attenuates neoplastic changes in rats challenged with the procarcinogen 1, 2-dimethylhydrazine. Eur. J. Pharmacol. 2017;796:20–31. doi: 10.1016/j.ejphar.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 253.Lori G., Paoli P., Femia A.P., Pranzini E., Caselli A., Tortora K., Romagnoli A., Raugei G., Caderni G. Morin-dependent inhibition of low molecular weight protein tyrosine phosphatase (LMW-PTP) restores sensitivity to apoptosis during colon carcinogenesis: Studies in vitro and in vivo, in an Apc-driven model of colon cancer. Mol. Carcinog. 2019;58:686–698. doi: 10.1002/mc.22962. [DOI] [PubMed] [Google Scholar]
- 254.Vennila S., Nalini N. Modifying effects of morin on the development of aberrant crypt foci and bacterial enzymes in experimental colon cancer. Food Chem. Toxicol. 2009;47:309–315. doi: 10.1016/j.fct.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 255.Sharma S.H., Kumar J.S., Chellappan D.R., Nagarajan S. Molecular chemoprevention by morin–A plant flavonoid that targets nuclear factor kappa B in experimental colon cancer. Biomed. Pharmacother. 2018;100:367–373. doi: 10.1016/j.biopha.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 256.Sreedharan V., Venkatachalam K.K., Namasivayam N. Effect of morin on tissue lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Investig. New Drugs. 2009;27:21–30. doi: 10.1007/s10637-008-9136-1. [DOI] [PubMed] [Google Scholar]
- 257.Nirmala P., Ramanathan M. Effect of myricetin on 1, 2 dimethylhydrazine induced rat colon carcinogenesis. J. Exp. Ther. Oncol. 2011;9:101–108. [PubMed] [Google Scholar]
- 258.Li Y., Cui S.-X., Sun S.-Y., Shi W.-N., Song Z.-Y., Wang S.-Q., Yu X.-F., Gao Z.-H., Qu X.-J. Chemoprevention of intestinal tumorigenesis by the natural dietary flavonoid myricetin in APCMin/+ mice. Oncotarget. 2016;7:60446. doi: 10.18632/oncotarget.11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang M.-J., Su H., Yan J.-Y., Li N., Song Z.-Y., Wang H.-J., Huo L.-G., Wang F., Ji W.-S., Qu X.-J. Chemopreventive effect of Myricetin, a natural occurring compound, on colonic chronic inflammation and inflammation-driven tumorigenesis in mice. Biomed. Pharmacother. 2018;97:1131–1137. doi: 10.1016/j.biopha.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 260.Wang F., Song Z.-Y., Qu X.-J., Li F., Zhang L., Li W.-B., Cui S.-X. M10, a novel derivative of Myricetin, prevents ulcerative colitis and colorectal tumor through attenuating robust endoplasmic reticulum stress. Carcinogenesis. 2018;39:889–899. doi: 10.1093/carcin/bgy057. [DOI] [PubMed] [Google Scholar]
- 261.Leonardi T., Vanamala J., Taddeo S.S., Davidson L.A., Murphy M.E., Patil B.S., Wang N., Carroll R.J., Chapkin R.S., Lupton J.R. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp. Biol. Med. 2010;235:710–717. doi: 10.1258/ebm.2010.009359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Dou W., Zhang J., Sun A., Zhang E., Ding L., Mukherjee S., Wei X., Chou G., Wang Z.-T., Mani S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013;110:599–608. doi: 10.1017/S0007114512005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Li H., Zhu F., Chen H., Cheng K.W., Zykova T., Oi N., Lubet R.A., Bode A.M., Wang M., Dong Z. 6-C-(E-phenylethenyl)-Naringenin Suppresses Colorectal Cancer Growth by Inhibiting Cyclooxygenase-16CEPN Suppresses Colon Cancer by Inhibiting COX-1. Cancer Res. 2014;74:243–252. doi: 10.1158/0008-5472.CAN-13-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sequetto P.L., Oliveira T.T., Maldonado I.R., Augusto L.E.F., Mello V.J., Pizziolo V.R., Almeida M.R., Silva M.E., Novaes R.D. Naringin accelerates the regression of pre-neoplastic lesions and the colorectal structural reorganization in a murine model of chemical carcinogenesis. Food Chem. Toxicol. 2014;64:200–209. doi: 10.1016/j.fct.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 265.Zhang Y.-S., Wang F., Cui S.-X., Qu X.-J. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol. Ther. 2018;19:735–744. doi: 10.1080/15384047.2018.1453971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Vanamala J., Leonardi T., Patil B.S., Taddeo S.S., Murphy M.E., Pike L.M., Chapkin R.S., Lupton J.R., Turner N.D. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis. 2006;27:1257–1265. doi: 10.1093/carcin/bgi318. [DOI] [PubMed] [Google Scholar]
- 267.Miyamoto S., Yasui Y., Tanaka T., Ohigashi H., Murakami A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis. 2008;29:1057–1063. doi: 10.1093/carcin/bgn080. [DOI] [PubMed] [Google Scholar]
- 268.Thangaraj K., Vaiyapuri M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-κB mediated inflammatory response in 1, 2-dimethylhydrazine induced colorectal carcinogenesis. Biomed. Pharmacother. 2017;96:1253–1266. doi: 10.1016/j.biopha.2017.11.088. [DOI] [PubMed] [Google Scholar]
- 269.Thangaraj K., Natesan K., Palani M., Vaiyapuri M. Orientin, a flavanoid, mitigates 1, 2 dimethylhydrazine-induced colorectal lesions in Wistar rats fed a high-fat diet. Toxicol. Rep. 2018;5:977–987. doi: 10.1016/j.toxrep.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yang X., Zhang F., Wang Y., Cai M., Wang Q., Guo Q., Li Z., Hu R. Oroxylin A inhibits colitis-associated carcinogenesis through modulating the IL-6/STAT3 signaling pathway. Inflamm. Bowel Dis. 2013;19:1990–2000. doi: 10.1097/MIB.0b013e318293c5e0. [DOI] [PubMed] [Google Scholar]
- 271.Ni T., He Z., Dai Y., Yao J., Guo Q., Wei L. Oroxylin A suppresses the development and growth of colorectal cancer through reprogram of HIF1α-modulated fatty acid metabolism. Cell Death Dis. 2017;8:e2865. doi: 10.1038/cddis.2017.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gan C., Li Y., Yu Y., Yu X., Liu H., Zhang Q., Yin W., Yu L., Ye T. Natural product pectolinarigenin exhibits potent anti-metastatic activity in colorectal carcinoma cells in vitro and in vivo. Bioorganic Med. Chem. 2019;27:115089. doi: 10.1016/j.bmc.2019.115089. [DOI] [PubMed] [Google Scholar]
- 273.Lim S., Xu J., Kim J., Chen T.Y., Su X., Standard J., Carey E., Griffin J., Herndon B., Katz B. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013;57:1908–1917. doi: 10.1002/mnfr.201300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Han L., Yan Y., Fan M., Gao S., Zhang L., Xiong X., Li R., Xiao X., Wang X., Ni L. Pt3R5G inhibits colon cancer cell proliferation through inducing ferroptosis by down-regulating SLC7A11. Life Sci. 2022;306:120859. doi: 10.1016/j.lfs.2022.120859. [DOI] [PubMed] [Google Scholar]
- 275.Lin S.-T., Tu S.-H., Yang P.-S., Hsu S.-P., Lee W.-H., Ho C.-T., Wu C.-H., Lai Y.-H., Chen M.-Y., Chen L.-C. Apple polyphenol phloretin inhibits colorectal cancer cell growth via inhibition of the type 2 glucose transporter and activation of p53-mediated signaling. J. Agric. Food Chem. 2016;64:6826–6837. doi: 10.1021/acs.jafc.6b02861. [DOI] [PubMed] [Google Scholar]
- 276.Hao X., Xiao H., Ju J., Lee M.-J., Lambert J.D., Yang C.S. Green tea polyphenols inhibit colorectal tumorigenesis in azoxymethane-treated F344 rats. Nutr. Cancer. 2017;69:623–631. doi: 10.1080/01635581.2017.1295088. [DOI] [PubMed] [Google Scholar]
- 277.Gossé F., Guyot S., Roussi S., Lobstein A., Fischer B., Seiler N., Raul F. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis. 2005;26:1291–1295. doi: 10.1093/carcin/bgi074. [DOI] [PubMed] [Google Scholar]
- 278.Zhu X., Tian X., Yang M., Yu Y., Zhou Y., Gao Y., Zhang L., Li Z., Xiao Y., Moses R.E. Procyanidin B2 promotes intestinal injury repair and attenuates colitis-associated tumorigenesis via suppression of oxidative stress in mice. Antioxid. Redox Signal. 2021;35:75–92. doi: 10.1089/ars.2019.7911. [DOI] [PubMed] [Google Scholar]
- 279.Tutino V., De Nunzio V., Tafaro A., Bianco G., Gigante I., Scavo M.P., D’ALESSANDRO R., Refolo M.G., Messa C., Caruso M.G. Cannabinoid receptor-1 up-regulation in azoxymethane (AOM)-treated mice after dietary treatment with quercetin. Anticancer Res. 2018;38:4485–4491. doi: 10.21873/anticanres.12752. [DOI] [PubMed] [Google Scholar]
- 280.Lin C., Yu Y., Zhao H.-g., Yang A., Yan H., Cui Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother. Oncol. 2012;104:395–400. doi: 10.1016/j.radonc.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 281.Warren C.A., Paulhill K.J., Davidson L.A., Lupton J.R., Taddeo S.S., Hong M.Y., Carroll R.J., Chapkin R.S., Turner N.D. Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J. Nutr. 2009;139:101–105. doi: 10.3945/jn.108.096271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lin R., Piao M., Song Y., Liu C. Quercetin suppresses AOM/DSS-induced colon carcinogenesis through its anti-inflammation effects in mice. J. Immunol. Res. 2020;2020:9242601. doi: 10.1155/2020/9242601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kee J.-Y., Han Y.-H., Kim D.-S., Mun J.-G., Park J., Jeong M.-Y., Um J.-Y., Hong S.-H. Inhibitory effect of quercetin on colorectal lung metastasis through inducing apoptosis, and suppression of metastatic ability. Phytomedicine. 2016;23:1680–1690. doi: 10.1016/j.phymed.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 284.Li Y., Wang Z., Jin J., Zhu S.-X., He G.-Q., Li S.-H., Wang J., Cai Y. Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. Biochem. Biophys. Res. Commun. 2020;523:947–953. doi: 10.1016/j.bbrc.2020.01.048. [DOI] [PubMed] [Google Scholar]
- 285.Alonso-Castro A.J., Domínguez F., García-Carrancá A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch. Med. Res. 2013;44:346–351. doi: 10.1016/j.arcmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 286.Zeng S., Chen L., Sun Q., Zhao H., Yang H., Ren S., Liu M., Meng X., Xu H. Scutellarin ameliorates colitis-associated colorectal cancer by suppressing Wnt/β-catenin signaling cascade. Eur. J. Pharmacol. 2021;906:174253. doi: 10.1016/j.ejphar.2021.174253. [DOI] [PubMed] [Google Scholar]
- 287.Xiong L.-L., Du R.-L., Xue L.-L., Jiang Y., Huang J., Chen L., Liu J., Wang T.-H. Anti-colorectal cancer effects of scutellarin revealed by genomic and proteomic analysis. Chin. Med. 2020;15:1–5. doi: 10.1186/s13020-020-00307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zeng S., Tan L., Sun Q., Chen L., Zhao H., Liu M., Yang H., Ren S., Ming T., Tang S. Suppression of colitis-associated colorectal cancer by scutellarin through inhibiting Hedgehog signaling pathway activity. Phytomedicine. 2022;98:153972. doi: 10.1016/j.phymed.2022.153972. [DOI] [PubMed] [Google Scholar]
- 289.Yang S.-H., Lin J.-K., Huang C.-J., Chen W.-S., Li S.-Y., Chiu J.-H. Silibinin inhibits angiogenesis via Flt-1, but not KDR, receptor up-regulation1. J. Surg. Res. 2005;128:140–146. doi: 10.1016/j.jss.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 290.Kauntz H., Bousserouel S., Gosse F., Marescaux J., Raul F. Silibinin, a natural flavonoid, modulates the early expression of chemoprevention biomarkers in a preclinical model of colon carcinogenesis. Int. J. Oncol. 2012;41:849–854. doi: 10.3892/ijo.2012.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bao H., Zheng N., Li Z., Zhi Y. Synergistic effect of tangeretin and atorvastatin for colon cancer combination therapy: Targeted delivery of these dual drugs using RGD Peptide decorated nanocarriers. Drug Des. Dev. Ther. 2020;14:3057. doi: 10.2147/DDDT.S256636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Razak S., Afsar T., Ullah A., Almajwal A., Alkholief M., Alshamsan A., Jahan S. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway. BMC Cancer. 2018;18:1–18. doi: 10.1186/s12885-018-4959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yue G.G.-L., Gao S., Lee J.K.-M., Chan Y.-Y., Wong E.C.-W., Zheng T., Li X.-X., Shaw P.-C., Simmonds M.S., Lau C.B.-S. A natural flavone tricin from grains can alleviate tumor growth and lung metastasis in colorectal tumor mice. Molecules. 2020;25:3730. doi: 10.3390/molecules25163730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Oyama T., Yasui Y., Sugie S., Koketsu M., Watanabe K., Tanaka T. Dietary tricin suppresses inflammation-related colon carcinogenesis in male Crj: CD-1 mice. Cancer Prev. Res. 2009;2:1031–1038. doi: 10.1158/1940-6207.CAPR-09-0061. [DOI] [PubMed] [Google Scholar]
- 295.Vinothkumar R., Kumar R.V., Sudha M., Viswanathan P., Balasubramanian T., Nalini N. Modulatory effect of troxerutin on biotransforming enzymes and preneoplasic lesions induced by 1, 2-dimethylhydrazine in rat colon carcinogenesis. Exp. Mol. Pathol. 2014;96:15–26. doi: 10.1016/j.yexmp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 296.Bhardwaj M., Paul S., Jakhar R., Khan I., Kang J.I., Kim H.M., Yun J.W., Lee S.-J., Cho H.J., Lee H.G. Vitexin confers HSF-1 mediated autophagic cell death by activating JNK and ApoL1 in colorectal carcinoma cells. Oncotarget. 2017;8:112426. doi: 10.18632/oncotarget.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Bhardwaj M., Cho H.J., Paul S., Jakhar R., Khan I., Lee S.-J., Kim B.-Y., Krishnan M., Khaket T.P., Lee H.G. Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells. Oncotarget. 2018;9:3278. doi: 10.18632/oncotarget.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Yao J., Zhao L., Zhao Q., Zhao Y., Sun Y., Zhang Y., Miao H., You Q., Hu R., Guo Q. NF-κB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014;5:e1283. doi: 10.1038/cddis.2014.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Feng Q., Wang H., Pang J., Ji L., Han J., Wang Y., Qi X., Liu Z., Lu L. Prevention of wogonin on colorectal cancer tumorigenesis by regulating p53 nuclear translocation. Front. Pharmacol. 2018;9:1356. doi: 10.3389/fphar.2018.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.You W., Di A., Zhang L., Zhao G. Effects of wogonin on the growth and metastasis of colon cancer through the Hippo signaling pathway. Bioengineered. 2022;13:2586–2597. doi: 10.1080/21655979.2021.2019173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Liu H., Zhang L., Li G., Gao Z. Xanthohumol protects against Azoxymethane-induced colorectal cancer in Sprague-Dawley rats. Environ. Toxicol. 2020;35:136–144. doi: 10.1002/tox.22849. [DOI] [PubMed] [Google Scholar]
- 302.Murillo G., Hirschelman W.H., Ito A., Moriarty R.M., Kinghorn A.D., Pezzuto J.M., Mehta R.G. Zapotin, a phytochemical present in a Mexican fruit, prevents colon carcinogenesis. Nutr. Cancer. 2007;57:28–37. doi: 10.1080/01635580701268097. [DOI] [PubMed] [Google Scholar]
- 303.Kang N.J., Lee K.W., Kim B.H., Bode A.M., Lee H.-J., Heo Y.-S., Boardman L., Limburg P., Lee H.J., Dong Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis. 2011;32:921–928. doi: 10.1093/carcin/bgr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Park S.-R., Kim S.-R., Hong I.-S., Lee H.-Y. A novel therapeutic approach for colorectal cancer stem cells: Blocking the PI3K/Akt signaling axis with caffeic acid. Front. Cell Dev. Biol. 2020;8:585987. doi: 10.3389/fcell.2020.585987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chiang E.-P.I., Tsai S.-Y., Kuo Y.-H., Pai M.-H., Chiu H.-L., Rodriguez R.L., Tang F.-Y. Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the PI3-K/Akt and AMPK signaling pathways. PLoS ONE. 2014;9:e99631. doi: 10.1371/journal.pone.0099631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tang H., Yao X., Yao C., Zhao X., Zuo H., Li Z. Anti-colon cancer effect of caffeic acid p-nitro-phenethyl ester in vitro and in vivo and detection of its metabolites. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-07953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chen C., Kuo Y.-H., Lin C.-C., Chao C.-Y., Pai M.-H., Chiang E.-P.I., Tang F.-Y. Decyl caffeic acid inhibits the proliferation of colorectal cancer cells in an autophagy-dependent manner in vitro and in vivo. PLoS ONE. 2020;15:e0232832. doi: 10.1371/journal.pone.0232832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tanaka T., Nishikawa A., Shima H., Sugie S., Shinoda T., Yoshimi N., Iwata H., Mori H. Inhibitory effects of chlorogenic acid, reserpine, polyprenoic acid (E-5166), or coffee on hepatocarcinogenesis in rats and hamsters. Basic Life Sci. 1990;52:429–440. doi: 10.1007/978-1-4615-9561-8_45. [DOI] [PubMed] [Google Scholar]
- 309.Morishita Y., Yoshimi N., Kawabata K., Matsunaga K., Sugie S., Tanaka T., Mori H. Regressive effects of various chemopreventive agents on azoxymethane-induced aberrant crypt foci in the rat colon. Jpn. J. Cancer Res. 1997;88:815–820. doi: 10.1111/j.1349-7006.1997.tb00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rao C.V., Tokumo K., Rigotty J., Zang E., Kelloff G., Reddy B.S. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, α-difluoromethylornithine, 16α-fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer Res. 1991;51:4528–4534. [PubMed] [Google Scholar]
- 311.Umesalma S., Sudhandiran G. Chemomodulation of the antioxidative enzymes and peroxidative damage in the colon of 1, 2-dimethyl hydrazine-induced rats by ellagicacid. Phytother. Res. 2010;24:S114–S119. doi: 10.1002/ptr.2962. [DOI] [PubMed] [Google Scholar]
- 312.Umesalma S., Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2010;107:650–655. doi: 10.1111/j.1742-7843.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 313.Umesalma S., Sudhandiran G. Ellagic acid prevents rat colon carcinogenesis induced by 1, 2 dimethyl hydrazine through inhibition of AKT-phosphoinositide-3 kinase pathway. Eur. J. Pharmacol. 2011;660:249–258. doi: 10.1016/j.ejphar.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 314.Kumar K.N., Raja S.B., Vidhya N., Devaraj S.N. Ellagic acid modulates antioxidant status, ornithine decarboxylase expression, and aberrant crypt foci progression in 1, 2-dimethylhydrazine-instigated colon preneoplastic lesions in rats. J. Agric. Food Chem. 2012;60:3665–3672. doi: 10.1021/jf204128z. [DOI] [PubMed] [Google Scholar]
- 315.Goyal Y., Koul A., Ranawat P. Ellagic acid modulates cisplatin toxicity in DMH induced colorectal cancer: Studies on membrane alterations. Biochem. Biophys. Rep. 2022;31:101319. doi: 10.1016/j.bbrep.2022.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kawabata K., Yamamoto T., Hara A., Shimizu M., Yamada Y., Matsunaga K., Tanaka T., Mori H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/S0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 317.Han B.S., Park C.B., Takasuka N., Naito A., Sekine K., Nomura E., Taniguchi H., Tsuno T., Tsuda H. A Ferulic Acid Derivative, Ethyl 3-(4′-Geranyloxy-3-methoxyphenyl)-2-propenoate, as a New Candidate Chemopreventive Agent for Colon Carcinogenesis in the Rat. Jpn. J. Cancer Res. 2001;92:404–409. doi: 10.1111/j.1349-7006.2001.tb01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Giftson J.S., Jayanthi S., Nalini N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1, 2-dimethyl hydrazine induced rat colon carcinogenesis. Investig. New Drugs. 2010;28:251–259. doi: 10.1007/s10637-009-9241-9. [DOI] [PubMed] [Google Scholar]
- 319.Sanchez-Martin V., Plaza-Calonge M.d.C., Soriano-Lerma A., Ortiz-Gonzalez M., Linde-Rodriguez A., Perez-Carrasco V., Ramirez-Macias I., Cuadros M., Gutierrez-Fernandez J., Murciano-Calles J. Gallic Acid: A Natural Phenolic Compound Exerting Antitumoral Activities in Colorectal Cancer via Interaction with G-Quadruplexes. Cancers. 2022;14:2648. doi: 10.3390/cancers14112648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Jeong J.H., Kim E.Y., Choi H.J., Chung T.W., Kim K.J., Kim S.Y., Ha K.T. Gallic acid inhibits STAT3 phosphorylation and alleviates DDS-induced colitis via regulating cytokine production. J. Physiol. Pathol. Korean Med. 2016;30:338–346. doi: 10.15188/kjopp.2016.10.30.5.338. [DOI] [Google Scholar]
- 321.Lin X., Wang G., Liu P., Han L., Wang T., Chen K., Gao Y. Gallic acid suppresses colon cancer proliferation by inhibiting SRC and EGFR phosphorylation. Exp. Ther. Med. 2021;21:1–11. doi: 10.3892/etm.2020.9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hong Z., Tang P., Liu B., Ran C., Yuan C., Zhang Y., Lu Y., Duan X., Yang Y., Wu H. Ferroptosis-related genes for overall survival prediction in patients with colorectal cancer can be inhibited by gallic acid. Int. J. Biol. Sci. 2021;17:942. doi: 10.7150/ijbs.57164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Senapathy J.G., Jayanthi S., Viswanathan P., Umadevi P., Nalini N. Effect of gallic acid on xenobiotic metabolizing enzymes in 1, 2-dimethyl hydrazine induced colon carcinogenesis in Wistar rats–a chemopreventive approach. Food Chem. Toxicol. 2011;49:887–892. doi: 10.1016/j.fct.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 324.Zhou L.-A., Liu T.-B., Lü H.-N. Geraniin inhibits proliferation and induces apoptosis through inhibition of phosphatidylinositol 3-kinase/Akt pathway in human colorectal cancer in vitro and in vivo. Anti-Cancer Drugs. 2020;31:575–582. doi: 10.1097/CAD.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 325.Sharma S.H., Chellappan D.R., Chinnaswamy P., Nagarajan S. Protective effect of p-coumaric acid against 1, 2 dimethylhydrazine induced colonic preneoplastic lesions in experimental rats. Biomed. Pharmacother. 2017;94:577–588. doi: 10.1016/j.biopha.2017.07.146. [DOI] [PubMed] [Google Scholar]
- 326.Fang W., Zhu S., Niu Z., Yin Y. The protective effect of syringic acid on dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Dev. Res. 2019;80:731–740. doi: 10.1002/ddr.21524. [DOI] [PubMed] [Google Scholar]
- 327.Mihanfar A., Darband S.G., Sadighparvar S., Kaviani M., Mirza-Aghazadeh-Attari M., Yousefi B., Majidinia M. In vitro and in vivo anticancer effects of syringic acid on colorectal cancer: Possible mechanistic view. Chem.-Biol. Interact. 2021;337:109337. doi: 10.1016/j.cbi.2020.109337. [DOI] [PubMed] [Google Scholar]
- 328.Han Y.-H., Kee J.-Y., Kim D.-S., Mun J.-g., Jeong M.-Y., Park S.-H., Choi B.-M., Park S.-J., Kim H.-J., Um J.-Y. Arctigenin inhibits lung metastasis of colorectal cancer by regulating cell viability and metastatic phenotypes. Molecules. 2016;21:1135. doi: 10.3390/molecules21091135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Kang K., Oh S.H., Yun J.H., Jho E.H., Kang J.-H., Batsuren D., Tunsag J., Park K.H., Kim M., Nho C.W. A novel topoisomerase inhibitor, daurinol, suppresses growth of HCT116 cells with low hematological toxicity compared to etoposide. Neoplasia. 2011;13:1043–1057. doi: 10.1593/neo.11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Li C., Zhang K., Pan G., Ji H., Li C., Wang X., Hu X., Liu R., Deng L., Wang Y. Dehydrodiisoeugenol inhibits colorectal cancer growth by endoplasmic reticulum stress-induced autophagic pathways. J. Exp. Clin. Cancer Res. 2021;40:1–15. doi: 10.1186/s13046-021-01915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kee J.-Y., Han Y.-H., Mun J.-G., Park S.-H., Jeon H.D., Hong S.-H. Gomisin a suppresses colorectal lung metastasis by inducing AMPK/P38-mediated apoptosis and decreasing metastatic abilities of colorectal cancer cells. Front. Pharmacol. 2018;9:986. doi: 10.3389/fphar.2018.00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Li Q., Ma Y., Liu X.L., Mu L., He B.C., Wu K., Sun W.J. Anti-proliferative effect of honokiol on SW620 cells through upregulating BMP7 expression via the TGF-β1/p53 signaling pathway. Oncol. Rep. 2020;44:2093–2107. doi: 10.3892/or.2020.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Won S.J., Yen C.H., Liu H.S., Wu S.Y., Lan S.H., Jiang-Shieh Y.F., Lin C.N., Su C.L. Justicidin A-induced autophagy flux enhances apoptosis of human colorectal cancer cells via class III PI3K and Atg5 pathway. J. Cell. Physiol. 2015;230:930–946. doi: 10.1002/jcp.24825. [DOI] [PubMed] [Google Scholar]
- 334.Su C.-M., Weng Y.-S., Kuan L.-Y., Chen J.-H., Hsu F.-T. Suppression of PKCδ/NF-κB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated with Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo. Int. J. Mol. Sci. 2020;21:3527. doi: 10.3390/ijms21103527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kang Y.-J., Park H.J., Chung H.-J., Min H.-Y., Park E.J., Lee M.A., Shin Y., Lee S.K. Wnt/β-catenin signaling mediates the antitumor activity of magnolol in colorectal cancer cells. Mol. Pharmacol. 2012;82:168–177. doi: 10.1124/mol.112.078535. [DOI] [PubMed] [Google Scholar]
- 336.Pu Z., Zhang W., Wang M., Xu M., Xie H., Zhao J. Schisandrin B Attenuates colitis-associated colorectal cancer through SIRT1 linked SMURF2 signaling. Am. J. Chin. Med. 2021;49:1773–1789. doi: 10.1142/S0192415X21500841. [DOI] [PubMed] [Google Scholar]
- 337.Chen T., Wang Z., Zhong J., Zhang L., Zhang H., Zhang D., Xu X., Zhong X., Wang J., Li H. Secoisolariciresinol diglucoside induces pyroptosis by activating caspase-1 to cleave GSDMD in colorectal cancer cells. Drug Dev. Res. 2022;83:1152–1166. doi: 10.1002/ddr.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wang Z., Chen T., Yang C., Bao T., Yang X., He F., Zhang Y., Zhu L., Chen H., Rong S. Secoisolariciresinol diglucoside suppresses Dextran sulfate sodium salt-induced colitis through inhibiting NLRP1 inflammasome. Int. Immunopharmacol. 2020;78:105931. doi: 10.1016/j.intimp.2019.105931. [DOI] [PubMed] [Google Scholar]
- 339.Ohira H., Oikawa D., Kurokawa Y., Aoki Y., Omura A., Kiyomoto K., Nakagawa W., Mamoto R., Fujioka Y., Nakayama T. Suppression of colonic oxidative stress caused by chronic ethanol administration and attenuation of ethanol-induced colitis and gut leakiness by oral administration of sesaminol in mice. Food Funct. 2022;13:9285–9298. doi: 10.1039/D1FO04120G. [DOI] [PubMed] [Google Scholar]
- 340.Shin M.-K., Jeon Y.-D., Hong S.-H., Kang S.-H., Kee J.-Y., Jin J.-S. In vivo and In vitro effects of Tracheloside on colorectal cancer cell proliferation and metastasis. Antioxidants. 2021;10:513. doi: 10.3390/antiox10040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Chen J., Zhong J., Liu Y., Huang Y., Luo F., Zhou Y., Pan X., Cao S., Zhang L., Zhang Y. Purified vitexin compound 1, a new neolignan isolated compound, promotes PUMA-dependent apoptosis in colorectal cancer. Cancer Med. 2018;7:6158–6169. doi: 10.1002/cam4.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kimura Y. Long-term oral administration of piceatannol (3, 5, 3′, 4′-tetrahydroxystilbene) attenuates colon tumor growth induced by azoxymethane plus dextran sulfate sodium in C57BL/6J mice. Nutr. Cancer. 2022;74:2184–2195. doi: 10.1080/01635581.2021.1985532. [DOI] [PubMed] [Google Scholar]
- 343.Jin Y., Zhan X., Zhang B., Chen Y., Liu C., Yu L. Polydatin exerts an antitumor effect through regulating the miR-382/PD-L1 axis in colorectal cancer. Cancer Biother. Radiopharm. 2020;35:83–91. doi: 10.1089/cbr.2019.2999. [DOI] [PubMed] [Google Scholar]
- 344.Chiou Y.-S., Tsai M.-L., Wang Y.-J., Cheng A.-C., Lai W.-M., Badmaev V., Ho C.-T., Pan M.-H. Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxymethane-treated mice. J. Agric. Food Chem. 2010;58:8833–8841. doi: 10.1021/jf101571z. [DOI] [PubMed] [Google Scholar]
- 345.Paul S., DeCastro A.J., Lee H.J., Smolarek A.K., So J.Y., Simi B., Wang C.X., Zhou R., Rimando A.M., Suh N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the β-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis. 2010;31:1272–1278. doi: 10.1093/carcin/bgq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Chiou Y.-S., Tsai M.-L., Nagabhushanam K., Wang Y.-J., Wu C.-H., Ho C.-T., Pan M.-H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- 347.Zhang Y., Li Y., Sun C., Chen X., Han L., Wang T., Liu J., Chen X., Zhao D. Effect of pterostilbene, a natural derivative of resveratrol, in the treatment of colorectal cancer through Top1/Tdp1-mediated DNA repair pathway. Cancers. 2021;13:4002. doi: 10.3390/cancers13164002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Suh N., Paul S., Hao X., Simi B., Xiao H., Rimando A.M., Reddy B.S. Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin. Cancer Res. 2007;13:350–355. doi: 10.1158/1078-0432.CCR-06-1528. [DOI] [PubMed] [Google Scholar]
- 349.Ji Q., Liu X., Han Z., Zhou L., Sui H., Yan L., Jiang H., Ren J., Cai J., Li Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15:1–12. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Saud S.M., Li W., Morris N.L., Matter M.S., Colburn N.H., Kim Y.S., Young M.R. Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic Kras expression. Carcinogenesis. 2014;35:2778–2786. doi: 10.1093/carcin/bgu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Alfaras I., Juan M.E., Planas J.M. trans-Resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats. J. Agric. Food Chem. 2010;58:8104–8110. doi: 10.1021/jf100702x. [DOI] [PubMed] [Google Scholar]
- 352.Majumdar A.P., Banerjee S., Nautiyal J., Patel B.B., Patel V., Du J., Yu Y., Elliott A.A., Levi E., Sarkar F.H. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer. 2009;61:544–553. doi: 10.1080/01635580902752262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sudha T., El-Far A.H., Mousa D.S., Mousa S.A. Resveratrol and its nanoformulation attenuate growth and the angiogenesis of xenograft and orthotopic colon cancer models. Molecules. 2020;25:1412. doi: 10.3390/molecules25061412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Hu W.-H., Chan G.K.-L., Duan R., Wang H.-Y., Kong X.-P., Dong T.T.-X., Tsim K.W.-K. Synergy of ginkgetin and resveratrol in suppressing VEGF-induced angiogenesis: A therapy in treating colorectal cancer. Cancers. 2019;11:1828. doi: 10.3390/cancers11121828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sepporta M.V., Fuccelli R., Rosignoli P., Ricci G., Servili M., Fabiani R. Oleuropein prevents Azoxymethane-induced Colon crypt dysplasia and leukocytes DNA damage in a/J mice. J. Med. Food. 2016;19:983–989. doi: 10.1089/jmf.2016.0026. [DOI] [PubMed] [Google Scholar]
- 356.Zeng Q., Che Y., Zhang Y., Chen M., Guo Q., Zhang W. Thymol Isolated from Thymus vulgaris L. inhibits colorectal cancer cell growth and metastasis by suppressing the Wnt/β-catenin pathway. Drug Des. Dev. Ther. 2020;14:2535. doi: 10.2147/DDDT.S254218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Zhou L., Feng Y., Jin Y., Liu X., Sui H., Chai N., Chen X., Liu N., Ji Q., Wang Y. Verbascoside promotes apoptosis by regulating HIPK2–p53 signaling in human colorectal cancer. BMC Cancer. 2014;14:1–11. doi: 10.1186/1471-2407-14-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Singh S., Meena A., Luqman S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021;164:105387. doi: 10.1016/j.phrs.2020.105387. [DOI] [PubMed] [Google Scholar]
- 359.Patel V.B., Misra S., Patel B.B., Majumdar A.P. Colorectal cancer: Chemopreventive role of curcumin and resveratrol. Nutr. Cancer. 2010;62:958–967. doi: 10.1080/01635581.2010.510259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Prasad S., Aggarwal B.B. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2011. Turmeric, the golden spice; pp. 263–288. [Google Scholar]
- 361.Wang Y., Bu C., Wu K., Wang R., Wang J. Curcumin protects the pancreas from acute pancreatitis via the mitogen-activated protein kinase signaling pathway. Mol. Med. Rep. 2019;20:3027–3034. doi: 10.3892/mmr.2019.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Mishra S., Palanivelu K. Thread Rating. Ann. Indian Acad. Neurol. 2008;11:13–19. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zorena K., Jachimowicz-Duda O., Ślęzak D., Robakowska M., Mrugacz M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int. J. Mol. Sci. 2020;21:3570. doi: 10.3390/ijms21103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Achari A.E., Jain S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017;18:1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Weidinger C., Ziegler J.F., Letizia M., Schmidt F., Siegmund B. Adipokines and their role in intestinal inflammation. Front. Immunol. 2018;9:1974. doi: 10.3389/fimmu.2018.01974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kelesidis T., Kelesidis I., Chou S., Mantzoros C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010;152:93–100. doi: 10.7326/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Iikuni N., Kwan Lam Q.L., Lu L., Matarese G., Cava A.L. Leptin and inflammation. Curr. Immunol. Rev. 2008;4:70–79. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Aggarwal V., Tuli H.S., Tania M., Srivastava S., Ritzer E.E., Pandey A., Aggarwal D., Barwal T.S., Jain A., Kaur G. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin. Cancer Biol. 2022;80:256–275. doi: 10.1016/j.semcancer.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 369.Musial C., Kuban-Jankowska A., Gorska-Ponikowska M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020;21:1744. doi: 10.3390/ijms21051744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Kim J.S., Kim J.-M., Jeong-Ja O., Jeon B.S. Inhibition of inducible nitric oxide synthase expression and cell death by (−)-epigallocatechin-3-gallate, a green tea catechin, in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. J. Clin. Neurosci. 2010;17:1165–1168. doi: 10.1016/j.jocn.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 371.Kim M., Murakami A., Miyamoto S., Tanaka T., Ohigashi H. The modifying effects of green tea polyphenols on acute colitis and inflammation-associated colon carcinogenesis in male ICR mice. Biofactors. 2010;36:43–51. doi: 10.1002/biof.69. [DOI] [PubMed] [Google Scholar]
- 372.De Oliveira G.A., Cheng R.Y., Ridnour L.A., Basudhar D., Somasundaram V., McVicar D.W., Monteiro H.P., Wink D.A. Inducible nitric oxide synthase in the carcinogenesis of gastrointestinal cancers. Antioxid. Redox Signal. 2017;26:1059–1077. doi: 10.1089/ars.2016.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Cianchi F., Cortesini C., Fantappiè O., Messerini L., Schiavone N., Vannacci A., Nistri S., Sardi I., Baroni G., Marzocca C. Inducible nitric oxide synthase expression in human colorectal cancer: Correlation with tumor angiogenesis. Am. J. Pathol. 2003;162:793–801. doi: 10.1016/S0002-9440(10)63876-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Mandal P. Molecular signature of nitric oxide on major cancer hallmarks of colorectal carcinoma. Inflammopharmacology. 2018;26:331–336. doi: 10.1007/s10787-017-0435-z. [DOI] [PubMed] [Google Scholar]
- 375.Sheng J., Sun H., Yu F.-B., Li B., Zhang Y., Zhu Y.-T. The role of cyclooxygenase-2 in colorectal cancer. Int. J. Med. Sci. 2020;17:1095. doi: 10.7150/ijms.44439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Greenhough A., Smartt H.J., Moore A.E., Roberts H.R., Williams A.C., Paraskeva C., Kaidi A. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 377.Tuli H.S., Tuorkey M.J., Thakral F., Sak K., Kumar M., Sharma A.K., Sharma U., Jain A., Aggarwal V., Bishayee A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019;10:1336. doi: 10.3389/fphar.2019.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Chen X., Gu J., Wu Y., Liang P., Shen M., Xi J., Qin J. Clinical characteristics of colorectal cancer patients and anti-neoplasm activity of genistein. Biomed. Pharmacother. 2020;124:109835. doi: 10.1016/j.biopha.2020.109835. [DOI] [PubMed] [Google Scholar]
- 379.Pintova S., Dharmupari S., Moshier E., Zubizarreta N., Ang C., Holcombe R.F. Genistein combined with FOLFOX or FOLFOX–Bevacizumab for the treatment of metastatic colorectal cancer: Phase I/II pilot study. Cancer Chemother. Pharmacol. 2019;84:591–598. doi: 10.1007/s00280-019-03886-3. [DOI] [PubMed] [Google Scholar]
- 380.Felice M.R., Maugeri A., De Sarro G., Navarra M., Barreca D. Molecular Pathways Involved in the Anti-Cancer Activity of Flavonols: A Focus on Myricetin and Kaempferol. Int. J. Mol. Sci. 2022;23:4411. doi: 10.3390/ijms23084411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Lin Y., Shi R., Wang X., Shen H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ganai S.A., Sheikh F.A., Baba Z.A., Mir M.A., Mantoo M.A., Yatoo M.A. Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother. Res. 2021;35:3509–3532. doi: 10.1002/ptr.7044. [DOI] [PubMed] [Google Scholar]
- 383.Xiao Y., Yang H., Lu J., Li D., Xu C., Risch H.A. Serum gamma-glutamyltransferase and the overall survival of metastatic pancreatic cancer. BMC Cancer. 2019;19:1–7. doi: 10.1186/s12885-019-6250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Ribatti D., Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 385.Wang Y.-X., Chen Y.-R., Liu S.-S., Ye Y.-P., Jiao H.-L., Wang S.-Y., Xiao Z.-Y., Wei W.-T., Qiu J.-F., Liang L. MiR-384 inhibits human colorectal cancer metastasis by targeting KRAS and CDC42. Oncotarget. 2016;7:84826. doi: 10.18632/oncotarget.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Kong Y., Bai P.-S., Nan K.-J., Sun H., Chen N.-Z., Qi X.-G. Pleiotrophin is a potential colorectal cancer prognostic factor that promotes VEGF expression and induces angiogenesis in colorectal cancer. Int. J. Color. Dis. 2012;27:287–298. doi: 10.1007/s00384-011-1344-z. [DOI] [PubMed] [Google Scholar]
- 387.Zhu M.-L., Zhang P.-M., Jiang M., Yu S.-W., Wang L. Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement. Med. Ther. 2020;20:1–9. doi: 10.1186/s12906-020-02965-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Rauf A., Shariati M.A., Imran M., Bashir K., Khan S.A., Mitra S., Emran T.B., Badalova K., Uddin M., Mubarak M.S. Comprehensive review on naringenin and naringin polyphenols as a potent anticancer agent. Environ. Sci. Pollut. Res. 2022;29:31025–31041. doi: 10.1007/s11356-022-18754-6. [DOI] [PubMed] [Google Scholar]
- 389.Ghanbari-Movahed M., Jackson G., Farzaei M.H., Bishayee A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin against Human Malignancies. Front. Pharmacol. 2021;12:250. doi: 10.3389/fphar.2021.639840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Gao R., Wang L., Yang Y., Ni J., Zhao L., Dong S., Guo M. Simultaneous determination of oleanolic acid, ursolic acid, quercetin and apigenin in Swertia mussotii Franch by capillary zone electrophoresis with running buffer modifier. Biomed. Chromatogr. 2015;29:402–409. doi: 10.1002/bmc.3290. [DOI] [PubMed] [Google Scholar]
- 391.Khan F., Niaz K., Maqbool F., Ismail Hassan F., Abdollahi M., Nagulapalli Venkata K.C., Nabavi S.M., Bishayee A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients. 2016;8:529. doi: 10.3390/nu8090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Terzić J., Grivennikov S., Karin E., Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 393.Long A.G., Lundsmith E.T., Hamilton K.E. Inflammation and colorectal cancer. Curr. Color. Cancer Rep. 2017;13:341–351. doi: 10.1007/s11888-017-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Nouri Z., Fakhri S., Nouri K., Wallace C.E., Farzaei M.H., Bishayee A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers. 2020;12:2276. doi: 10.3390/cancers12082276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chua L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013;150:805–817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 396.Negahdari R., Bohlouli S., Sharifi S., Maleki Dizaj S., Rahbar Saadat Y., Khezri K., Jafari S., Ahmadian E., Gorbani Jahandizi N., Raeesi S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021;35:1719–1738. doi: 10.1002/ptr.6904. [DOI] [PubMed] [Google Scholar]
- 397.Pan M.-H., Chen W.-J., Lin-Shiau S.-Y., Ho C.-T., Lin J.-K. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677–1684. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- 398.He L., Lu N., Dai Q., Zhao Y., Zhao L., Wang H., Li Z., You Q., Guo Q. Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells. Toxicology. 2013;312:36–47. doi: 10.1016/j.tox.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 399.Tan H., Li X., Yang W.-H., Kang Y. A flavone, Wogonin from Scutellaria baicalensis inhibits the proliferation of human colorectal cancer cells by inducing of autophagy, apoptosis and G2/M cell cycle arrest via modulating the PI3K/AKT and STAT3 signalling pathways. J. BUON. 2019;24:1143–1149. [PubMed] [Google Scholar]
- 400.Alam M., Ahmed S., Elasbali A.M., Adnan M., Alam S., Hassan M.I., Pasupuleti V.R. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 2022;12:860508. doi: 10.3389/fonc.2022.860508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Banerjee N., Kim H., Krenek K., Talcott S.T., Mertens-Talcott S.U. Mango polyphenolics suppressed tumor growth in breast cancer xenografts in mice: Role of the PI3K/AKT pathway and associated microRNAs. Nutr. Res. 2015;35:744–751. doi: 10.1016/j.nutres.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 402.Boghossian S., Hawash A. Chemoprevention in colorectal cancer-where we stand and what we have learned from twenty year’s experience. Surgeon. 2012;10:43–52. doi: 10.1016/j.surge.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 403.Ko J.-H., Sethi G., Um J.-Y., Shanmugam M.K., Arfuso F., Kumar A.P., Bishayee A., Ahn K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017;18:2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Bishayee A. Cancer Prevention and Treatment with Resveratrol: From Rodent Studies to Clinical TrialsResveratrol and Cancer: In vivo and Clinical Studies. Cancer Prev. Res. 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 405.Arslan A.K.K., Uzunhisarcıklı E., Yerer M.B., Bishayee A. The golden spice curcumin in cancer: A perspective on finalized clinical trials during the last 10 years. J. Cancer Res. Ther. 2022;18:19–26. doi: 10.4103/jcrt.JCRT_1017_20. [DOI] [PubMed] [Google Scholar]
- 406.Singh A.P., Singh R., Verma S.S., Rai V., Kaschula C.H., Maiti P., Gupta S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019;39:1851–1891. doi: 10.1002/med.21565. [DOI] [PubMed] [Google Scholar]
- 407.Wu X.-Y., Zhai J., Huan X.-K., Xu W.-W., Tian J., Farhood B. A Systematic Review of the Therapeutic Potential of Resveratrol During Colorectal Cancer Chemotherapy. Mini Rev. Med. Chem. 2022 doi: 10.2174/1389557522666220907145153. [DOI] [PubMed] [Google Scholar]
- 408.Ma Z., Wang N., He H., Tang X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release. 2019;316:359–380. doi: 10.1016/j.jconrel.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 409.Seufferlein T., Ettrich T.J., Menzler S., Messmann H., Kleber G., Zipprich A., Frank-Gleich S., Algül H., Metter K., Odemar F. Green tea extract to prevent colorectal adenomas, results of a randomized, placebo-controlled clinical trial. Off. J. Am. Coll. Gastroenterol. ACG. 2022;117:884–894. doi: 10.14309/ajg.0000000000001706. [DOI] [PubMed] [Google Scholar]
- 410.Farsad-Naeimi A., Alizadeh M., Esfahani A., Aminabad E.D. Effect of fisetin supplementation on inflammatory factors and matrix metalloproteinase enzymes in colorectal cancer patients. Food Funct. 2018;9:2025–2031. doi: 10.1039/C7FO01898C. [DOI] [PubMed] [Google Scholar]
- 411.Ganesan K., Jayachandran M., Xu B. Diet-derived phytochemicals targeting colon cancer stem cells and microbiota in colorectal cancer. Int. J. Mol. Sci. 2020;21:3976. doi: 10.3390/ijms21113976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Greiner A.K., Papineni R.V., Umar S. Chemoprevention in gastrointestinal physiology and disease. Natural products and microbiome. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014;307:G1–G15. doi: 10.1152/ajpgi.00044.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Dacrema M., Ali A., Ullah H., Khan A., Di Minno A., Xiao J., Martins A.M.C., Daglia M. Spice-Derived Bioactive Compounds Confer Colorectal Cancer Prevention via Modulation of Gut Microbiota. Cancers. 2022;14:5682. doi: 10.3390/cancers14225682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.O’keefe S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016;13:691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]