Abstract
(1) Background: Although gastritis has been associated with multiple etiologies, in pediatrics the main etiology is idiopathic. Many studies have reported mild-to-severe gastritis Helicobacter pylori (H. pylori) as an etiological factor. We evaluated the distribution of the infection with H. pylori by age, gender and place of living; (2) Methods: A retrospective study was conducted over a period of 3 years, over a cohort of 1757 patients of both sexes, aged between 1 and 18 years, admitted to a regional gastroenterology center in Iasi, Romania, with clinical signs of gastritis which underwent upper gastrointestinal endoscopy. The research was based on the analysis of data from patient observation charts and hospital discharge tickets, as well as endoscopy result registers; (3) Results: Out of the 1757 children, in 30.8% of cases the H. pylori infection was present. Out of them, 26.8% were males and 73.2% females. The average age of children with an H. pylori infection was higher (14.1 + 2.8 DS), compared with children without H. pylori (12.8 + 3.7 SD), an average difference of 1.3 years (95% confidence interval 0.96 to 1.66; p < 0.001). By place of living, children with H. pylori infection were from urban areas at 24.7% and from rural areas at 75.3%; (4) Conclusions: H. pylori infection incidence is still high in children, especially in teenagers, so extensive prevention and treatment programs are needed.
Keywords: gastritis, H. pylori, child, endoscopy, epidemiology
1. Introduction
Acute gastritis is a term that covers a wide spectrum of entities that induce inflammatory changes in the gastric mucosa. Some etiologies share the same general clinical presentation, but they differ histologically [1]. Some conditions (Helicobacter pylori (H. pylori), inflammatory bowel disease, allergic gastroenteritis) that injure the gastric mucosa can lead to inflammation. Thus, gastritis, as suggested by the suffix -itis, is characterized by the presence of inflammatory cells [2]. The inflammation of the gastric and/or duodenal mucosa is the end result of an imbalance between defensive and aggressive mucosal factors. The degree of inflammation and the presence of this imbalance may subsequently result in varying degrees of gastritis and/or ulceration of the mucosa [3].
Although gastritis has been associated with multiple etiologies, in pediatrics the main etiology is idiopathic. Many studies have reported mild-to-severe gastritis H. pylori as an etiological factor. [4].
H. pylori is a Gram-negative microaerophilic bacterium which colonizes the gastric mucosa generally in childhood and can determine chronic active gastritis, peptic ulcer disease, gastric cancer and mucosa-associated lymphoid tissue lymphoma later on during adulthood [5]. Its transmission route is still partially unclear, but the infections occur as a result of direct human-to-human transmission or environmental contamination [6]. The increased number of siblings, the education level of the parents, the water sources and garbage collection are also known to be representing important risk factors for the H. pylori infection among the pediatric population [7].
It is known that the rate of infection with H. pylori reaches a percentage of 50% of the total population and approximately one-third of all children around the world with a high prevalence in low-income countries and in the absence of sanitary conditions, the incidence of infection being severely influenced by the socioeconomic status [8]. In their review, Zabala et al. made an examination of the data from seven cohort studies and showed that the rate of the infection with H. pylori in healthy children under 5 years of age remained between 20% and 40% in high-income countries, whereas in the upper-middle income ones, the infection rates variated between 30% and 50%. These data suggest the importance of the country of birth concerning the prevalence of the infection [9]. Additionally, Venneman et al. found in their complex review that in Europe the H. pylori infection reached its highest rates in Eastern and Southern Europe which represent, as well, the regions with the highest stomach cancer incidence rates in the European Union [10]. Evidently, the clinical outcome of an H. pylori infection depends on multiple favorizing circumstances such as the virulence factors or the host gastric mucosal factors [11].
Clearly, the importance of an early diagnostic of the H. pylori infection is undeniable as it can prevent complications in adulthood and implicitly the apparition of gastric cancer [12].
In children, the guidelines recommend that the diagnosis of H. pylori infection be based on positive culture or gastritis with H. pylori on histopathology with at least one other positive test based on biopsy [13]. The authors of a recent study from Iraq regarding detection of H. pylori infection by invasive and non-invasive techniques in adults concluded markedly the role of real-time PCR as more sensitive and accurate than other diagnostic methods because it offers several advantages over culture [14].
Once diagnosed, an H. pylori infection has to be eradicated, but the efficacy of the regimen consisting of a standard triple therapy involving antibiotics as amoxicillin, clarithromycin and metronidazole along with a proton pump inhibitor seems to be decreasing lately due to H. pylori-resistant strains [15]. Thus, eradicating H. pylori infection is starting to become a challenge for all the pediatricians all around the world [16].
As for the epidemiological aspects of the treatment, the results of the EuroPedHP Registry 2013 to 2016 showed that the primary antibiotic resistance rates may vary significantly across the geographical regions and that they can also be correlated with the migrant status [17]. In the present study, we aimed to evaluate the cases’ distribution based on sex, age and environmental sources of the H. pylori infection. Although its prevalence seems to decrease lately, H. pylori infection remains an important public health problem in Romania, and epidemiological studies on its impact among the pediatric population in our country are limited. We evaluated the data from a certain region of Romania, namely the northeast of the country, trying to identify the particularities of H. pylori infection among children in relation to their age, gender and environment of origin.
2. Materials and Methods
A retrospective study was carried out over a period of 3 years on a cohort of 1757 patients of both sexes, aged between 1 and 18 years, mainly hospitalized in the Gastroenterology Pediatric Clinic, but also in the other clinics of the Emergency Hospital for Children “St. Maria” in Iasi, Romania, with symptoms suggestive of gastritis or gastroduodenal ulcer such as upper abdominal pain, abdominal distension, dyspepsia, nausea or vomiting, in which superior digestive endoscopy (SDE) was performed. Some of them had a positive fecal H. pylori antigen in ambulatory. Our hospital is the only one in the northeast of Romania where SDEs can be performed, so our results reflect the epidemiological situation of the pediatric population suffering from this disease in this area with great accuracy.
The main criterion for inclusion in the study was the definite diagnosis of the disease by performing SDE with biopsies taken from the gastric and/or duodenal mucosa. Intravenous sedation was given and standard upper gastrointestinal endoscopy, using the Olympus and Pentax video pediatric gastro-duodenoscopes, was performed to identify the macroscopic changes. General anesthesia in children aged below 10 years of age was used. We obtained 2 biopsies from the antrum, 2 biopsies from the corpus for the histopathological evaluation and 1 biopsy from antrum for the rapid urease test [18,19]. The gastritis was graded according to Houston-updated Sydney system: absent inflammation (Grade 0), mild inflammation (Grade 1), moderate inflammation (Grade 2) and severe inflammation (Grade 3).
During this period, 2042 SDEs were conducted, out of which we excluded 256 SDEs that were performed for verifying the response to therapy rather than for initial diagnosis purposes. Out of the 1786 children for which SDE was performed for diagnosis, we excluded another 29 children who did not have complete data in the observation files. The study was conducted on a final number of 1757 patients.
The research was based on the analysis of data from hospital discharge tickets, patient observation charts and endoscopy result registers. The data regarding the batch considered for the study were organized into a table structure containing a number of 90 category variables and 2 continuous variables. The processing of these data was performed using the SPSS 17.0 platform as well as Excel 2016 software. Chi Squared was calculated to asses association of independent values. Cox regression models adjusting for patient age, sex and area of origin were used characterize the relationship in pediatric patients with gastritis and the probability of testing HP-positive.
All patient’s caregivers have given written informed consent and the “St. Mary” Children Emergency Hospital Ethics Committee’s approval was obtained for publishing this study (31490/29 October 2021).
3. Results
We evaluated 1757 patients, out of which 1210 were females and 547 males, whereas 1114 of them originated from rural areas and 643 patients were from urban areas.
Of the 1757 children diagnosed in our study with various forms of gastritis and/or gastroduodenal ulcers, 542 of them (30.8%) had an associated H. pylori infection, while the other 1215 (69.2%) did not have the infection at the time of diagnosis. All 542 children were confirmed with H. pylori infection by endoscopy with biopsies, all of whom underwent rapid urease testing (RUT).
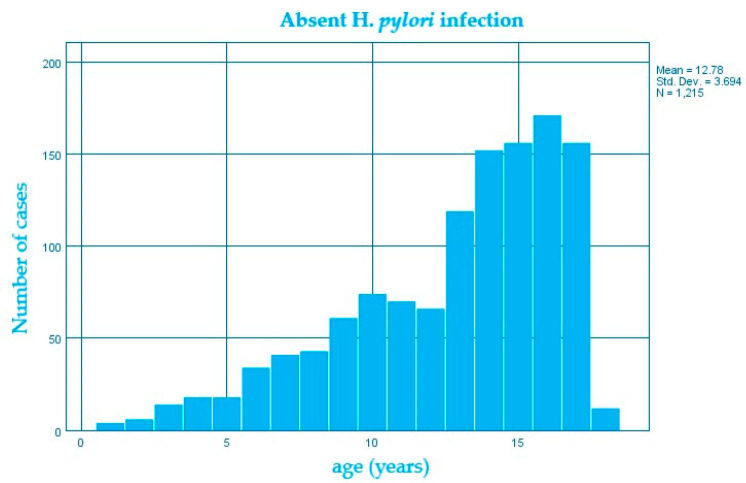
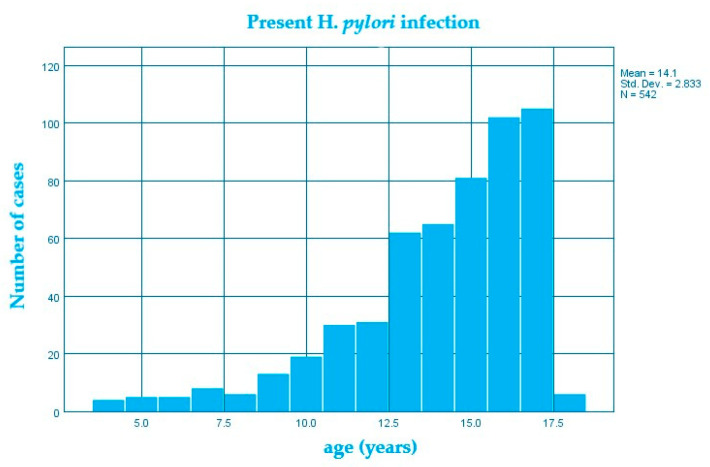
In the studied group, the average age of children who had an H. pylori infection was higher (14.1 ± 2.8 DS) than in those without an H. pylori infection (12.8 ± 3.7 DS) (Figure 1 and Figure 2), the average difference being 1.3 years; confidence interval 95% 0.96–1.66; p < 0.0001 (Table 1).
Figure 1.
Structure of the group of patients without H. pylori infection by age (years).
Figure 2.
Structure of the group of patients with H. pylori infection by age (years).
Table 1.
Average age of diagnosis of infection with H. pylori.
| Age (Years) | Frequency Total | H. pylori Negative | H. pylori Positive | Pearson Chi Square | Likelihood Ratio | p Value |
|---|---|---|---|---|---|---|
| 1 | 4 (0.2%) | 4 (100%) | 0 | 61.7 | 72.33 | p < 0.0001 |
| 2 | 6 (0.3%) | 6 (100%) | 0 | |||
| 3 | 14 (0.8%) | 14 (100%) | 0 | |||
| 4 | 22 (1.3%) | 18 (81.8%) | 4 (18.2%) | |||
| 5 | 23 (1.3%) | 18 (78.3%) | 5 (21.7%) | |||
| 6 | 39 (2.2%) | 34 (87.2%) | 5 (12.8%) | |||
| 7 | 49 (2.8%) | 41 (83.7%) | 8 (16.3%) | |||
| 8 | 49 (2.8%) | 43 (87.8%) | 6 (12.2%) | |||
| 9 | 74 (4.2%) | 61 (82.4%) | 13 (17.6%) | |||
| 10 | 93 (5.3%) | 74 (79.6%) | 19 (20.4%) | |||
| 11 | 100 (5.7%) | 70 (70%) | 30 (30%) | |||
| 12 | 97 (5.5%) | 66 (68%) | 31 (32%) | |||
| 13 | 181 (10.3%) | 119 (65.7%) | 62 (34.3%) | |||
| 14 | 217 (12.4%) | 152 (70%) | 65 (30%) | |||
| 15 | 238 (13.5%) | 157 (66%) | 81 (34%) | |||
| 16 | 273 (15.5%) | 171 (62.6%) | 102 (37.4%) | |||
| 17 | 260 (14.8%) | 152 (59.6%) | 105 (40.4%) | |||
| 18 | 18 (1%) | 12 (66.7%) | 6 (33.3%) | |||
| Total | 1757 (100%) | 1215 (69.2%) | 542 (30.8%) |
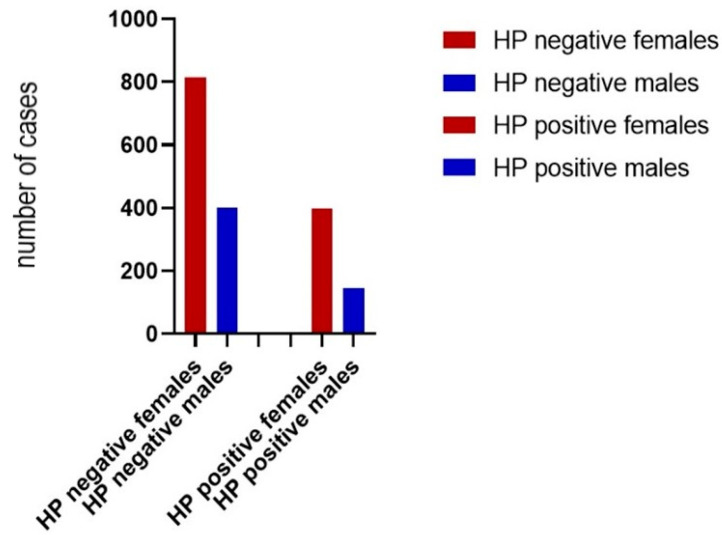
In the studied batch, the female gender represented approximately two-thirds of the entire batch with a percentage of 68.9% (1210 female children), compared to one-third represented by the male gender with a percentage of 31.1% (547 children). The distribution of children with an H. pylori infection according to the gender variable revealed a frequency of 26.6% for boys and a frequency of 32.8% for girls (Figure 3). From the statistical analysis, it was concluded that there was a significant difference of this association (χ2, p = 0.009) (Table 2). The possibility for females displaying gastritis with H. pylori is 1.34 times higher as compared to males (OR = 1.34).
Figure 3.
Structure of the batch of patients with or without H. pylori infection by gender.
Table 2.
Estimated parameters in testing the association between H. pylori infection and the gender variable.
| Female | Male | Pearson Chi Square | Likelihood Ratio | p Value | |
|---|---|---|---|---|---|
| HP positive | 397 (32.81%) | 145 (26.51%) | 7.01 | 7.13 | p = 0.008 |
| HP negative | 813 (67.19%) | 402 (73.49%) | |||
| Total | 1210 (100%) | 547 (100%) |
The first column represents females with H. pylori infection (32.81%) and without H. pylori infection (67.19%). The second column describes males with HP infection (26.51%) and without HP infection (73.49%). Chi Squared test of independence was performed and showed there was significant association between gender and H. pylori infection (χ2 = 7.01, p = 0.008).
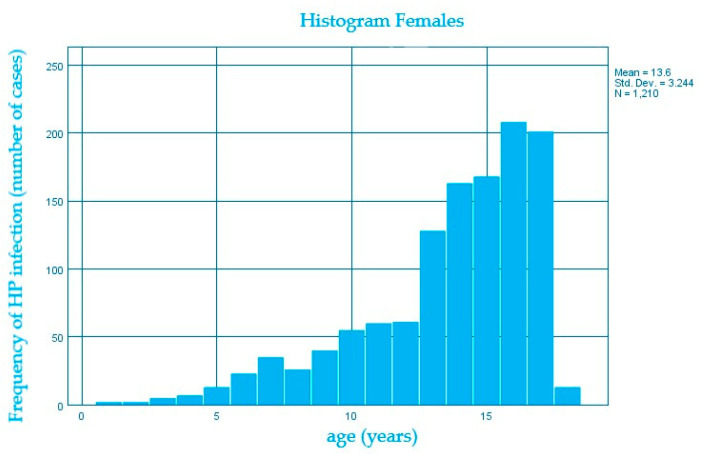
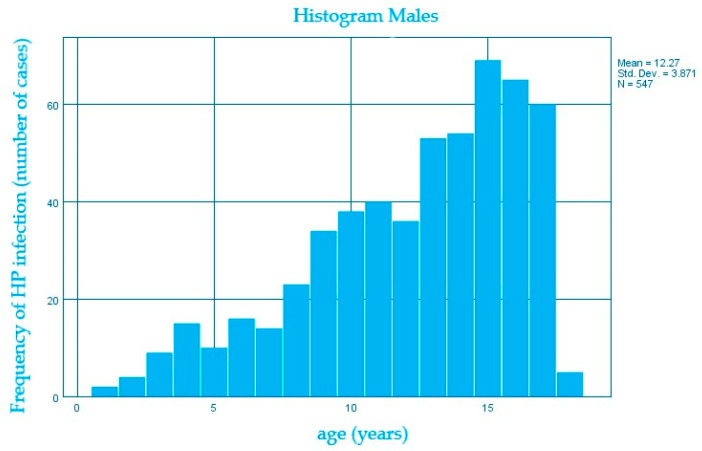
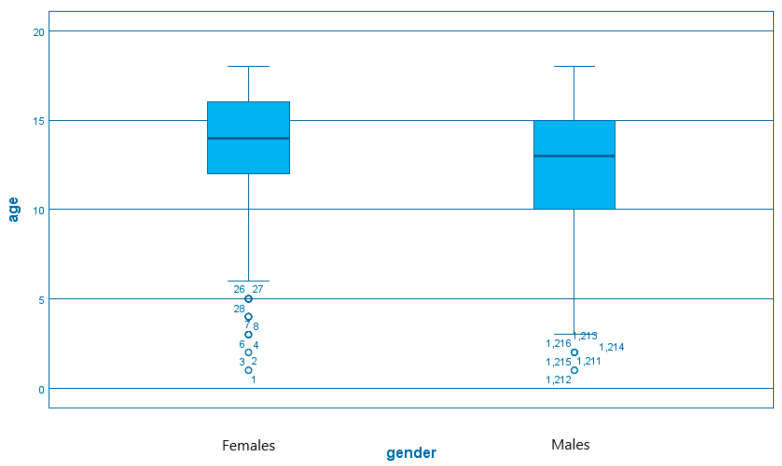
We also evaluated the distribution of the H. pylori infection according to age within both genders and we noticed that the number of cases increases along with the median age of 13.6 years for females compared to 12.2 years for males, the average difference being 1.4 years. (Figure 4 and Figure 5)
Figure 4.
Distribution of H. pylori infection (number of cases) according to age (years) in females.
Figure 5.
Distribution of H. pylori infection (number of cases) according to age (years) in males.
A direct comparison between the median age of debut of the Helicobacter pylori infection for males and females demonstrates similarities between both genders (Figure 6).
Figure 6.
The distribution of age (years) within gender variables in H. pylori infection.
According to patients’ backgrounds, out of the total number of 1757 patients, we observed that 1114 of them (63.4%) originated from the rural areas, whereas 643 of them originated from urban areas with a percentage of 36.6%. (Table 3). From the statistical analysis, it was concluded that there was a powerfully significant difference of this association (χ2; p < 0.0001) (Table 3). The presence of gastritis with H. pylori in patients from rural environments is 2.2 times higher than in patients from urban environments (OR = 2.2).
Table 3.
Estimated parameters in testing the association between H. pylori infection and the origin variable.
|
Rural
(Number/Percentage) |
Urban
(Number/Percentage) |
Pearson Chi Square | Likelihood Ratio | p Value | |
| HP positive | 408 (36.6%) | 134 (20.8%) | 47.62 | 49.38 | p < 0.0001 |
| HP negative | 706 (63.4%) | 509 (79.2%) | |||
| Total | 1114 (100%) | 643 (100%) |
The first column represents patients originating from rural environment with H. pylori infection (36.6%) and without H. pylori infection (63.4%). The second column describes children originating from the urban areas with HP infection (20.8%) and without HP infection (79.2%). Chi Squared test of independence was performed and showed there was significant association between environment of origin and H. pylori infection (χ2 = 47.62, p = 0.0001).
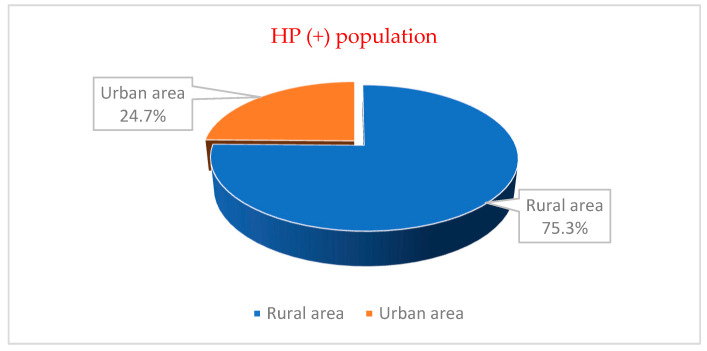
The distribution of children with an H. pylori infection according to the origin variable showed a frequency of 24.7% in the urban environment and a frequency of 75.3% in the rural environment (Figure 7).
Figure 7.
The structure of the group of patients according to the place of living for H. pylori present.
Logistic regression was used to characterize the relationship between age, gender and living conditions (stratified as urban/rural area) for pediatric gastritis patients and the probability of testing H. pylori-positive. The most parsimonious model included only age; the results from the model indicate an increased risk of H. pylori infection mirroring the aging process. A more complex model included age and living conditions with a similar log likelihood ratio suggesting an increased risk of H. pylori infection associated with rural living conditions—odds ratio ~2. A summary of the logistic regression results is shown in Table 4. Adding gender to a third model was considered but the result was not statistically significant (p = 0.34) (Wald).
Table 4.
Logistic regression model including age, living conditions and H. pylori infection status for pediatric patient with gastritis.
| B | S.E. | Wald | df | Sig. | OR | 95% CI for OR | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Age (years) | 0.11 | 0.02 | 44.25 | 1 | 0.000 | 1.12 | 1.08 | 1.16 |
| Urban area | 39.05 | 1 | 0.000 | |||||
| Rural area | 0.73 | 0.12 | 39.05 | 1 | 0.000 | 2.08 | 1.65 | 2.61 |
| Constant | −2.84 | .25 | 126.09 | 1 | 0.000 | 0.06 |
B = beta coefficient; S.E. = standard error; df = degrees of freedom; Sig. = statistical significance; OR = odds ratio; CI = confidence interval.
4. Discussion
The results of our analysis confirm that almost one-third of the children enrolled in the study have a H. pylori infection but unfortunately, in the Romanian medical literature the data regarding this subject are not updated and comparisons are difficult to be made within our country’s territory. However, at the national level, the prevalence of H. pylori infection seems to be decreasing lately.
Usually, an H. pylori infection is acquired during childhood and persists as chronic gastritis if the organism is not eradicated. With the progress of gastritis over the years, the gastric mucosa undergoes a series of changes that can lead to glandular atrophy, intestinal metaplasia and with increased risk of gastric dysplasia and carcinoma [1,20,21]. On the other hand, a recent study conducted in China identified an inverse relationship between H. pylori and asthma, indicating that this infection may represent a protective factor for asthma (OR 1.887–2.008, p < 0.05) [22]. This affirmation is supported by the results of a meta-analysis by Chen et al. who observed the same inverse association between the CagA(+) strains of H. pylori and the risk of childhood asthma (OR = 0.58; CI, 0.35–0.96, p = 0.034) [23].
In the United States, the prevalence of gastritis with H. pylori in children appears to be age-dependent. Below the age of 5, few cases are reported but prevalence increases with age, becoming the most common cause of gastritis in adolescents [1,2,24].
The authors of a recent systematic review and meta-analysis regarding global prevalence of pediatric H. pylori infection reported that this was present in 32.3% of children and it was higher in low-income and middle-income countries than in high-income countries (43.2% vs. 21.7%). Additionally, the prevalence of infection was higher in older children than in younger ones (41.6% in 13–18-year-olds; 33.9% in 7–12-year-olds; 26.0% in 0–6-year-olds). H. pylori infection in children was associated with lower economic status, more children, room sharing, no access to a sewage system, having parents infected with H. pylori, drinking non-treated water and adolescents [25].
It has been suggested that gastric pathology (gastritis and ulcer) has become more prevalent in Western countries in the nineteenth century due to a change in the epidemiology of the H. pylori infection [24]. Starting from this hypothesis, from environmental changes (which can cause changes in the gastritis pattern) and from current nutrition, we have studied some aspects of gastritis and ulcers in children.
The gastric pathology associated with H. pylori infection was more common among adolescents, with 17-year-olds registering the highest frequency with an average age of 14.1 ± 2.8 DS. This distribution could be explained by adolescents’ diet consisting of fast food, carbonated juices, alcohol and coffee, which can exacerbate the symptoms of gastritis with H. pylori [25,26]. Their better compliance with digestive endoscopy with the possibility of clear diagnosis could also contribute to the deviation of the frequency to the adult age. There are studies that reported spontaneous elimination of the infection with age, thus explaining the lower prevalence of infection at age 10 [27]. Some authors also argue that the lower prevalence of infection in adolescence age could be explained by an increased attention to health problems in this age group and the use of antibiotics for other infectious diseases [28,29]. In our case, we cannot support these hypotheses, as the prevalence of infection increased in relation to age. The low prevalence at the age of 18 cannot be taken into account, given the lower number of children we have examined, since at this age most patients with similar symptoms resorted to adult gastroenterology exams.
In our study, the female sex was affected in 68.9% of cases (1210 girls out of a total of 1757) and 32.8% had the bacteria present. This may result from the fact that girls give more importance to the symptoms they have, thus increasing the addressability to the doctor. In contrast with our results, Ibrahim et al. show in their meta-analysis that the H. pylori infection was more frequent in males than in females (102 studies, OR = 1.06, 95%CI: 1.01, 1.12, I2 = 43.7%) [30]. Other studies did not find statistical differences between females and males [31,32,33].
According to the place of living, our statistical analysis showed that there is a higher prevalence of H. pylori infection among the children originating from the rural environments with a percentage of 63.35% in northeast Romania. Our data are in agreement with the results obtained by Melit et al. in their study conducted on 137 patients from Romania where the H. pylori infection rate was more important in the rural environment than in the urban areas (p = 0.0089) [34]. However, our results are not entirely consistent with a similar analysis made recently in a center in the northwest part of Romania which proved that there were no statistically significant differences between the prevalence of H. pylori infection in the rural areas (42.29%) versus the urban environment (39.75%) (p = 0.6) [35].
The higher frequency of infection in the rural environment could be explained by the lower socio-economic level and by the larger families in this environment, which promotes the spread of the bacterium. Several studies have reported a higher prevalence of the infection in large families [36,37].
More than 50% of the world’s population is infected with H. pylori, which is almost always acquired in the first 5 years of life [38]. In developed countries, the prevalence varies between 1.2% and 12.2% [39,40]. In developing countries, the prevalence is higher. In Indian children, the prevalence of H. pylori infection was reported to be 45% [41]. In Bolivia, seroprevalence at age 9 was 70% and in Alaska 69% [40,41].
In Romania, the authors of a retrospective study conducted in Cluj-Napoca, on 194 children, reported the general prevalence of H. pylori infection as 36.6% [42]. In another study in Romania, in Targu Mures, conducted on 1041 children aged from 2 to 18 years, the prevalence was similar, of 33.05% [43].
In our study, we found a prevalence of H. pylori infection of 30.85%, similar to that reported in the other Romanian studies. The prevalence of the bacteria was roughly the same over the three years of study.
Initially, the specific guidelines for eradicating H. pylori infection were limited to peptic ulcer, but in 1997, the “Digestive Health Initiative” (DHI) during the “International Update Conference on H. pylori” extended the recommendations for testing and treating H. pylori [44]. Thus, the recommendations for H. pylori treatment are as follows: in the presence of H. pylori-associated peptic ulcer, treating H. pylori infection in the absence of peptic ulcer in children with dyspeptic symptoms may be considered, a “test and treat” strategy is not recommended in children based on non-invasive methods, in children infected with H. pylori who have a first-degree relative with gastric cancer, treatment may be recommended and the monitoring of antibiotic resistance rates of H. pylori strains in children and adolescents is recommended in different countries and geographical areas [45].
For the diagnosis, we performed SDE with biopsies taken from the gastric and/or duodenal mucosa such as in the recommendation of the new guidelines. The guidelines recommended that the initial diagnosis of H. pylori infection should not be based on noninvasive tests. A positive bacterial culture or H. pylori gastritis on histopathology with at least one other positive test such as rapid urease test, or molecular-based assays (polymerase chain reaction or fluorescent in situ hybridization) are necessary [13].
H. pylori has been shown to be highly resistant to clarithromycin in both children and adults, which is why studies at all ages recommend testing for antimicrobial susceptibility in H. pylori using molecular biopsy-based techniques, such as real-time PCR [13,46]. In our center, this test is not available and we considered it a limitation for our study. This is added to the unavailable laboratory investigations due to the fluctuation of existing funds (the study of vacA and cagA strains of H. pylori, the analysis of H. pylori antigen from fecal samples throughout the study period) and the impossibility of long-term follow-up of patients due to their very large number, but also to the lack of cooperation on their part.
5. Conclusions
The infection rate with H. pylori is still high in children, especially in teenagers, so extensive prevention and treatment programs are needed. An early diagnosis can significantly minimize complications during adulthood and, undoubtedly, can present an important impact on the socio-economic status in Romania.
Author Contributions
Conceptualization, A.L. and V.V.L.; methodology, I.C.M. and E.T.; software, A.T.C.; formal analysis, A.T.C. and B.S.; investigation, A.L., A.L.C. and V.V.L.; data curation, A.T.C.; writing—original draft preparation, A.L., A.L.C., I.M.S. and V.V.L.; writing—review and editing, I.C.M., E.T., A.T.C., C.G., B.S. and S.F.; visualization, I.M.S. and B.S.; supervision, I.C.M. and C.G.; project administration, S.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the “St. Mary” Children Emergency Hospital, Iasi, Romania (31490/29 October 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sarah El-Nakeep B.S. Anand Acute Gastritis. Medscape 2021. [(accessed on 30 August 2022)]. Available online: https://emedicine.medscape.com/article/175909-overview?reg=1.
- 2.Dohil R.D., Hassall E., Jevon G., Dimmick J. Gastritis and Gastropathy of Childhood. J. Pediatr. Gastroenterol. Nutr. 1999;29:378–394. doi: 10.1097/00005176-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Blecker U., Mehta D.I., Gold B.D. Pediatric gastritis and peptic ulcer disease. Indian J. Pediatr. 1999;66:725–733. doi: 10.1007/BF02726263. [DOI] [PubMed] [Google Scholar]
- 4.Gold B.D., Colletti R.B., Abbott M., Czinn S.J., Elitsur Y., Hassall E., Macarthur C., Snyder J., Sherman P.M. Medical position statement: The north American society for pediatric gastroenterology and nutrition: Helicobacter pylori infection in children: Recommendations for diagnosis and treatment. J. Pediatr. Gastroenterol. Nutr. 2000;31:490–497. doi: 10.1097/00005176-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 6.Rothenbacher D., Bode G., Berg G., Knayer U., Gonser T., Adler G., Brenner H. Helicobacter Pylori among preschool children and their parents: Evidence of parent-child transmission. J. Infect. Dis. 1999;179:398–402. doi: 10.1086/314595. [DOI] [PubMed] [Google Scholar]
- 7.Jafri W., Yakoob J., Abid S., Siddiqui S., Awan S., Nizami S.Q. Helicobacter pylori infection in children: Population-based age specific prevalence and risk factors in a developing country. Acta Paediatr. 2010;99:279–282. doi: 10.1111/j.1651-2227.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- 8.Okuda M., Lin Y., Kikuchi S. Helicobacter pylori Infection in Children and Adolescents. Adv. Exp. Med. Biol. 2019;1149:107–120. doi: 10.1007/5584_2019_361. [DOI] [PubMed] [Google Scholar]
- 9.Zabala Torrres B., Lucero Y., Lagomarcino A.J., Orellana-Manzano A., George S., Torres J.P., O’Ryan M. Review: Prevalence and dynamics of Helicobacter pylori infection during childhood. Helicobacter. 2017;22:e12399. doi: 10.1111/hel.12399. [DOI] [PubMed] [Google Scholar]
- 10.Venneman K., Huybrechts I., Gunter M.J., Vandendaele L., Herrero R., Van Herck K. The epidemiology of Helicobacter pylori infection in Europe and the impact of lifestyle on its natural evolution toward stomach cancer after infection: A systematic review. Helicobacter. 2018;23:e12483. doi: 10.1111/hel.12483. [DOI] [PubMed] [Google Scholar]
- 11.Sgouros S.N., Bergele C. Clinical outcome of patients with Helicobacter pylori infection: The bug, the host, or the environment? Postgrad. Med. J. 2006;82:338–342. doi: 10.1136/pgmj.2005.038273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mărginean C.O., Meliț L.E., Săsăran M.O. Traditional and Modern Diagnostic Approaches in Diagnosing Pediatric Helicobacter pylori Infection. Children. 2022;9:994. doi: 10.3390/children9070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones N.L., Koletzko S., Goodman K., Bontems P., Cadranel S., Casswall T., Czinn S., Gold B.D., Guarner J., Elitsur Y., et al. Joint ESPGHAN/NASPGHAN Guidelines for the Management of Helicobacter pylori in Children and Adolescents (Update 2016) J. Pediatr. Gastroenterol. Nutr. 2017;64:991–1003. doi: 10.1097/MPG.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 14.Hussein R.A., Al-Ouqaili M.T.S., Majeed Y.H. Detection of Helicobacter Pylori infection by invasive and non-invasive techniques in patients with gastrointestinal diseases from Iraq: A validation study. PLoS ONE. 2021;16:e0256393. doi: 10.1371/journal.pone.0256393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agudo S., Alarcón T., Urruzuno P., Martínez M.J., López-Brea M. Detection of Helicobacter pylori and Clarithro-mycin Resistance in Gastric Biopsies of Pediatric Patients by Using a Commercially Available Real-Time Polymerase Chain Reaction after NucliSens Semiautomated DNA Extraction. Diagn. Microbiol. Infect. Dis. 2010;67:213–219. doi: 10.1016/j.diagmicrobio.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Meliț L.E., Mărginean C.O., Săsăran M.O. The Challenges of Eradicating Pediatric Helicobacter Pylori Infection in the Era of Probiotics. Children. 2022;9:795. doi: 10.3390/children9060795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kori M., Le Thi T.G., Werkstetter K., Sustmann A., Bontems P., Lopes A.I., Oleastro M., Iwanczak B., Kalach N., Misak Z., et al. Helicobacter pylori Infection in Pediatric Patients Living in Europe: Results of the EuroPedHP Registry 2013 to 2016. J. Pediatr. Gastroenterol. Nutr. 2020;71:476–483. doi: 10.1097/MPG.0000000000002816. [DOI] [PubMed] [Google Scholar]
- 18.Lupu A., Miron I.C., Cianga A.L., Cernomaz A.T., Lupu V.V., Munteanu D., Ghica D.C., Fotea S. The Relationship between Anemia and Helicobacter Pylori Infection in Children. Children. 2022;9:1324. doi: 10.3390/children9091324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupu A., Miron I.C., Cianga A.L., Cernomaz A.T., Lupu V.V., Gavrilovici C., Stârcea I.M., Tarca E., Ghica D.C., Fotea S. The Prevalence of Liver Cytolysis in Children with Helicobacter pylori Infection. Children. 2022;9:1498. doi: 10.3390/children9101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uemura N., Okamoto S., Yamamoto S., Matsumura N., Yamaguchi S., Yamakido M., Taniyama K., Sasaki N., Schlemper R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 21.Asaka M., Sugiyama T., Nobuta A., Kato M., Takeda H., Graham D.Y. Atrophic gastritis and intestinal metaplasia in Japan: Results of a large multicenter study. Helicobacter. 2001;6:294–299. doi: 10.1046/j.1523-5378.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang D., Chen Y., Ding Y., Tu J. Inverse association between Helicobacter pylori infection and childhood asthma in a physical examination population: A cross-sectional study in Chongqing, China. BMC Pediatr. 2022;22:615. doi: 10.1186/s12887-022-03682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Zhan X., Wang D. Association between Helicobacter pylori and risk of childhood asthma: A meta-analysis of 18 observational studies. J. Asthma. 2022;59:890–900. doi: 10.1080/02770903.2021.1892752. [DOI] [PubMed] [Google Scholar]
- 24.Snyder J.D., Hard S.C., Thorne G.M., Hirsch B.Z., Antonioli D.A. Primary antral gastritis in young American children. Low prevalence of Helicobacter pylori infections. Dig. Dis. Sci. 1994;39:1859–1863. doi: 10.1007/BF02088115. [DOI] [PubMed] [Google Scholar]
- 25.Yuan C., Adeloye D., Luk T.T., Huang L., He Y., Xu Y., Ye X., Yi Q., Song P., Rudan I., et al. The global prevalence of and factors associated with Helicobacter pylori infection in children: A systematic review and meta-analysis. Lancet Child Adolesc. Health. 2022;6:185–194. doi: 10.1016/S2352-4642(21)00400-4. [DOI] [PubMed] [Google Scholar]
- 26.Drăgan F., Lupu V.V., Pallag A., Barz C., Fodor K. Rational consumption of nutrients at school-aged children. IOP Conf. Ser. Mater. Sci. Eng. 2017;200:012063. doi: 10.1088/1757-899X/200/1/012063. [DOI] [Google Scholar]
- 27.Rothenbacher D., Bode G., Brenner H. Dynamics of Helicobacter pylori infection in early childhood in a high-risk group living in Germany: Loss of infection higher than acquisition. Aliment. Pharmacol. Ther. 2002;16:1663–1668. doi: 10.1046/j.1365-2036.2002.01330.x. [DOI] [PubMed] [Google Scholar]
- 28.Hestvik E., Tylleskar T., Kaddu-Mulindwa D.H., Ndeezi G., Grahnquist L., Olafsdottir E., Tumwine J.K. Helicobacter pylori in apparently healthy children aged 0-12 years in urban Kampala, Uganda: A community-based cross-sectional survey. BMC Gastroenterol. 2010;10:62. doi: 10.1186/1471-230X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broussard C.S., Goodman K.J., Phillips C.V., Smith M.A., Fischbach L.A., Day R.S., Aragaki C.C. Antibiotics taken for other illnesses and spontaneous clearance of Helicobacter pylori infection in children. Pharmacoepidemiol. Drug Saf. 2009;18:722–729. doi: 10.1002/pds.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim A., Morais S., Ferro A., Lunet N., Peleteiro B. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: Systematic review and meta-analysis of 244 studies. Dig. Liver Dis. 2017;49:742–749. doi: 10.1016/j.dld.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Torres J., Pérez-Pérez G., Goodman K.J., Atherton J.C., Gold B.D., Harris P.R., Madrazo-de la Garza A., Guarner J., Muñoz O. A comprehensive review of the natural history of Helicobacter pylori infection in children. Arch. Med. Res. 2000;31:431–469. doi: 10.1016/S0188-4409(00)00099-0. [DOI] [PubMed] [Google Scholar]
- 32.Malaty H.M., El-Kasabany A., Graham D.Y., Miller C.C., Reddy S.G., Srinivasan S.R., Yamaoka Y., Berenson G.S. Age at acquisition of Helicobacter pylori infection: A follow-up study from infancy to adulthood. Lancet. 2002;359:931–935. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- 33.Soltani J., Amirzadeh J., Nahedi S., Shahsavari S. Prevalence of Helicobacter Pylori Infection in Children, a Population-Based Cross-Sectional Study in West Iran. Iran J. Pediatr. 2013;23:13–18. [PMC free article] [PubMed] [Google Scholar]
- 34.Meliţ L.E., Mărginean M.O., Mocan S., Mărginean C.O. The Usefulness of Inflammatory Biomarkers in Diagnosing Child and Adolescent’s Gastritis: STROBE Compliant Article. Medicine. 2019;98:e16188. doi: 10.1097/MD.0000000000016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corojan A.L., Dumitrașcu D.L., Ciobanca P., Leucuta D.C. Prevalence of Helicobacter pylori infection among dyspeptic patients in Northwestern Romania: A decreasing epidemiological trend in the last 30 years. Exp. Ther. Med. 2020;20:3488–3492. doi: 10.3892/etm.2020.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos I.S., Boccio J., Santos A.S., Valle N.C., Halal C.S., Bachilli M.C., Lopes R.D. Prevalence of Helicobacter pylori infection and associated factors among adults in Southern Brazil: A population-based cross-sectional study. BMC Public Health. 2005;5:118. doi: 10.1186/1471-2458-5-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda M., Kikuchi S., Kasugai T., Shunichi T., Miyake C. Helicobacter pylori risk associated with childhood home environment. Cancer Sci. 2003;94:914–918. doi: 10.1111/j.1349-7006.2003.tb01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suerbaum S., Michetti P. Helicobacter pylori infection. N. Engl. Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 39.Mourad-Baars P.E., Verspaget H.W., Mertens B.J., Luisa Merain M. Low prevalence of Helicobacter pylori infection in young children in the Netherlands. Eur. J. Gastroenterol. Hepatol. 2007;19:213–216. doi: 10.1097/MEG.0b013e328011050f. [DOI] [PubMed] [Google Scholar]
- 40.Rajindrajith S., Devanarayana N.M., de Silva H.J. Helicobacter Pylori Infection in Children. Saudi J. Gastroenterol. 2009;15:86–94. doi: 10.4103/1319-3767.48964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold B.J. Helicobacter pylori infection in children. Curr. Prob. Pediatr. 2001;31:247–266. doi: 10.1067/mps.2001.118485. [DOI] [PubMed] [Google Scholar]
- 42.Slăvescu K.C., Șarban C., Pîrvan A., Gheban D., Mărgescu C., Miu N. Prevalența infecției cu Helicobacter pylori la copiii cu gastrită și ulcer gastro-duodenal în nord-vestul și centrul României. Clujul Med. 2012;85:456–461. [Google Scholar]
- 43.Mărginean O., Pitea A.M., Brînzaniuc K. Helicobacter pylori Gastritis in Children—Assessment of Resistance to Treatment on the Casuistry of the Ist Pediatric Clinic Tîrgu Mures. AMM. 2015;21:487–490. [Google Scholar]
- 44.Akiva J.M., Anand B.S. Chronic Gastritis Treatment & Management. Medscape 2019/176156. [(accessed on 30 August 2022)]. Available online: https://emedicine.medscape.com/article/176156-treatmentlast.
- 45.Kalach N., Bontems P., Cadranel S. Advances in the treatment of Helicobacter pylori infection in children. Ann. Gastroenterol. 2015;28:10–18. [PMC free article] [PubMed] [Google Scholar]
- 46.Hussein R.A., Al-Ouqaili M.T.S., Majeed Y.H. Detection of clarithromycin resistance and 23SrRNA point mutations in clinical isolates of Helicobacter pylori isolates: Phenotypic and molecular methods. Saudi J. Biol. Sci. 2022;29:513–520. doi: 10.1016/j.sjbs.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.