Abstract
Background: The various mechanisms involved in peripheral nerve regeneration, induced by exercise and electrical nerve stimulation, are still unclear. Objective: The aim of this review was to summarize the influence of physical exercise and/or electrical stimulation on peripheral nerve repair and regeneration and the variation of impact of intervention depending on timing, as well as kind and dosage of the intervention. A literature survey was conducted on PubMed, Scopus, and Web of Science, between February 2021 to July 2021, with an update in September 2022. Methodology: The literature search identified 101,386 articles with the keywords: “peripheral nerve” OR “neuropathy” AND “sprouting” OR “neuroapraxia” OR “axonotmesis” OR “neurotmesis” OR “muscle denervation” OR “denervated muscle” AND “rehabilitation” OR “physical activity” OR “physical exercise” OR “activity” OR “electrical stimulation”. A total of 60 publications were included. Eligible studies were focused on evaluating the process of nerve repair (biopsy, electromyographic parameters or biomarker outcomes) after electrical stimulation or physical exercise interventions on humans or animals with peripheral sensory or motor nerve injury. Synthesis: This study shows that the literature, especially regarding preclinical research, is mainly in agreement that an early physical program with active exercise and/or electrical stimulation promotes axonal regenerative responses and prevents maladaptive response. This was evaluated by means of changes in electrophysiological recordings of CMAPs for latency amplitude, and the sciatic functional index (SFI). Furthermore, this type of activity can cause an increase in weight and in muscle fiber diameter. Nevertheless, some detrimental effects of exercising and electrical stimulation too early after nerve repair were recorded. Conclusion: In most preclinical studies, peripheral neuropathy function was associated with improvements after physical exercise and electrical stimulation. For humans, too little research has been conducted on this topic to reach a complete conclusion. This research supports the need for future studies to test the validity of a possible rehabilitation treatment in humans in cases of peripheral neuropathy to help nerve sprouting.
Keywords: peripheral neuropathy, physical activity, exercise, nerve regeneration, humans, animals, nerve stimulation
1. Introduction
Peripheral neuropathy can result from damage to and dysfunctions of motor, sensory or autonomic peripheral nerves. Moreover, denervation and disuse lead to the loss of muscle mass [1]. Thus, peripheral neuropathies are highly disabling, negatively affecting mobility and reducing autonomy in the activities of daily living [2].
No specific rehabilitative therapies have been accepted as a standardized treatment for peripheral neuropathy-related symptoms [3]. For this reason, research in this field is very interesting. For example, treadmill training seems to promote locomotor recovery in mouse models with chronic spinal cord injury, influencing neurotrophin expression such as Brain-Derived Neurotrophic Factor (BDNF) [4]. Furthermore, therapeutic exercise and rehabilitative strategies have been proposed to influence axon regeneration in the peripheral nervous system [5] and prevent peripheral nerve dysfunction in animals [6,7]. Numerous types of physical exercise, such as passive range-of-motion exercises, active-assisted exercises, locomotion (gait, treadmill and running training), and electrostimulation (a direct stimulation of the muscle fiber with an electric charge) of denervated muscle, have been proposed as effective strategies for retarding muscle atrophy and improving contractility after reinnervation [6,8,9,10,11,12]. Moreover, these strategies seem to upregulate genes and kinases associated with neuronal plasticity in the spinal cord and skeletal muscle [13], such as the BDNF [7,10,13,14,15,16,17], especially in the presence of the glia maturation factor (GMF) [18], calcitonin gene-related peptide (CGRP) related axonal regeneration [19,20], GAP43 [16,21], and phospho-ERK1/2 protein [21] involved in the recovery of peripheral nerve injury.
Electrical stimulation is a type of physical therapy treatment used to accomplish various tasks in physical therapy. According to research on transplanted nerves, it accelerates the regeneration rate of reconstructed peripheral nerves [22,23]. For this reason, it is interesting to assess the response during the process of nerve repair in peripheral neuropathies.
The paucity of literature on the relationship between peripheral neuropathy biomarkers and physical exercise in humans makes it necessary to extend our research to animal studies to better understand the mechanisms of nerve regeneration related to exercise. The aim of this systematic review was to determine whether physical exercise and electrical stimulation play a role in peripheral nerve repair and regeneration, and especially to understand the mechanisms by which electrical stimulation and physical exercise influence recovery after peripheral neuropathies. Furthermore, the aim was to highlight the impact of these specific strategies, their timing, kind, and dosage, especially regarding when to start this treatment, immediately after injury or during nerve regeneration, among humans and animals with peripheral neuropathy or nerve injury.
2. Materials and Methods
2.1. Information Sources and Database Search
The search was conducted on the following medical electronic databases: PubMed, Scopus, and Web of Science. The reference list of related articles was also used to identify any other suitable documents. The search strategy was conducted from February 2021 to July 2021, with an update in September 2022. The search used the following terms and keywords: “peripheral nerve” OR “neuropathy” AND “sprouting” OR “neuroapraxia” OR “axonotmesis” OR “neurotmesis” OR “muscle denervation” OR “denervated muscle” AND “rehabilitation” OR “physical activity” OR “physical exercise” OR “activity” OR “electrical stimulation”.
The review protocol was registered in the PROSPERO database of systematic reviews (www.crd.york.ac.uk/Prospero with the registration number CRD42021248509, accessed on 8 July 2021).
2.2. Eligibility Criteria
Studies were included in this systematic review when they described the mechanisms linked to electrical stimulation and physical exercise that influenced the process of nerve repair in peripheral neuropathies. Moreover, the studies were included according to the Population, Intervention, Comparison, Outcome, and Study Design (PICOS) criteria that the authors followed: the participants were animals (rats, rabbits, snails) and adult humans with sensory and/or motor peripheral nerve injuries, or neuropathies or muscle denervation; intervention was based on physical exercises, electrical stimulation, rehabilitation therapy, and forced immobility; the comparators included hypomobility/immobility, or absence of nerve injury; the outcomes were represented by changes in electrophysiological values, muscle and axon characteristics (muscle weight, muscle fibers and axon diameter), biomarkers and sciatic functional index (SFI); study design was preclinical and clinical experimental studies. Articles about chemotherapy-induced peripheral neuropathy were not added because the particularity of the topic and the necessity of proper research about the mechanisms of nerve repair after neurotoxicity. Studies concerning motor neuron disease, and any remaining duplicates were excluded. Physical modalities such as ultrasound, laser, and transcutaneous electrical nerve stimulation (TENS) were excluded from the search for the existing review about their role on nerve repair [24,25,26]. Zotero software was used to manage the citations and auto-eliminate duplicates.
2.3. Selection Criteria and Data Extraction
To identify eligible studies, two authors independently conceptualized keywords, and came to a consensus on which keywords to use for the search; then, each author independently assessed the articles. For quality assurance, study research and data extraction were repeated during the last update in September 2022. In the event of conflicting opinions, a consensus was reached after discussion between the authors. The selected full texts were then reviewed and included in the systematic review according to the Preferred Reporting Items for Systemic Reviews and Meta-analyses (PRISMA) statement [27], and following the Population, Intervention, Comparison, Outcome, and Study Design (PICOS) criteria [28] (Table 1 and Table 2).
2.4. Quality of the Results and Risk of Bias
Two independent authors assessed the included studies for the quality of study outcome (Table 3), and the risk of bias with SYRCLE’s tool [29]. The risk of bias elements included sequence generation, baseline characteristics, allocation concealment, blinding of investigators, random outcome assessment, blinding of assessor, incomplete outcome data, selective outcome reporting, and other sources of bias (Table 4). Their quality was low, unclear, or high for risk for each study analyzed by the authors.
Table 1.
Physical exercise and instrumental therapy in peripheral neuropathy in animals: characteristics and outcomes of studies included in the systematic review.
| Authors, Publication Year | Study Design, Sample (Species; Body Weight) |
Groups | Peripheral Nerve Lesion | Intervention | Assessments, Parameters, Scales, Scores, Indices | Results |
|---|---|---|---|---|---|---|
| Ahlborn 2007 [30] | Randomized blinded study 16 female rats (C57BL/6J r) (weight unspecified) |
|
Femoral nerve | Low-frequency electrical stimulation at 20-Hz once for one hour after 1 week from injury | Video-based motion analysis that allowed the precise evaluation of muscle function during locomotion | Brief electrical stimulation of femoral nerve led to accelerated locomotor recovery with respect to control group at 12 weeks (p = 0.063) |
| Asensio-Pinilla 2009 [11] | Comparative preclinical experimental study 45 female rats (Sprague–Dawley) (250 ± 300 g) |
|
Sciatic nerve | Electrical stimulation (3 V, 0.1 ms at 20 Hz) and/or treadmill running (for 4 weeks, 5 m/min, 2 h daily) | Nerve conduction study: H reflex and algesimetry tests performed at 1, 3, 5, 7 and 9 weeks after surgery. Thermal nociception evaluated by a heat-radiation method using the plantar test | Combining electrical stimulation with treadmill significantly improved muscle reinnervation during the initial phase compared with running group (p < 0.05) |
| Boeltz 2013 [31] | Comparative preclinical experimental study 12 female rats (Sprague–Dawley and Lewis) (±250 g) |
|
Tibial nerve | Moderate daily treadmill 5 days/week for 2 weeks, at a slow speed (10 m/min), for 1 h/day, beginning 3 days after transection and surgical repair | Nerve conduction study: H reflexes, kinematic parameters (limb angle, length during locomotion), M-response latency | Moderate daily exercise applied immediately after nerve injury is sufficient to promote axon regeneration, and restore muscle reflexes (p < 0.009 vs. untrained |
| Brown 1979 [32] | Preclinical comparative experimental study (weight unspecified) |
|
Peroneal nerve | Direct stimulation 100 Hz for 0.5 s/30 s or 150 Hz for 0.5 s/10s |
Histological examination of nerve | Direct stimulation of a partially denervated muscle inhibits sprouting vs untreated muscles (p < 0.00003). |
| Brushart 2005 [33] | Randomized study 14 female rats (Sprangue-Dawley) (±250 g) |
|
Femoral cutaneous branch | A total of 1 h of 20 Hz electrical stimulation | Histological examination of nerve | Electrical stimulation is thus highly effective at altering the pathway choices made by regenerating sensory axons, both decreasing projections to muscle nerve (p = 0.0282) and increasing those to cutaneous nerve (p = 0.0008) |
| Cobianchi 2010 [34] | Randomized blinded study 60 male rats (CD1) (40–45 g) |
|
Sciatic nerve |
|
Sciatic nerve immunohistochemistry; Electronic Von Frey and Plantar test devices to measure mechanical and thermal, nociceptive withdrawal thresholds | Short-lasting treadmill running, by reducing the neuropathic pain symptoms and facilitating the regenerative processes of the injured nerve, has beneficial rehabilitative effects on the functional recovery after peripheral nerve injury reflexes compared to long-lasting groups (p < 0.05) |
| Cohan 1986 [35] | Comparative preclinical experimental study snails. A total of 143 growth cones (extension of a developing or regenerating neurite) from 21 neurons |
|
Peripheral nerves | Electrical activity 45 mV, 10 msec |
Rates of growth cone | Growth rates decreased from 12.5 ± 1.1 µm/hour before stimulation to 4.7 ± 1.4 µm/hour after stimulation (p < 0.002; 18 growth cones) |
| De Moraes 2018 [36] | Randomized study 30 male rats (BALB/c) (200– 300 g) |
|
Sciatic nerve | Swimming daily sessions in a glass tank with a 30 cm depth, water temperature at 32 ± 0.5 °C | Histological examination of nerve | Moderate swimming was a therapeutic resource for nerve regeneration. Nerve area and minimum diameter were significantly lower (p < 0.05) compared to control group |
| Einsiedel 1994 [37] | Comparative preclinical experimental study 32 rats (Sprague–Dawley) (400 g) |
|
Sciatic nerve | Treadmill walking for 1.5 h/day and after 14 days walking a least 1 km/day | Fatigue index, vulnerability index, mean peak tetanic force, innervation ratio, time to peak of isometric twitch; twitch force; maximal tetanic force; axonal conduction velocity, weight muscle, CNAP, CSA | Increased motoneuron activity induced by treadmill walking is an important factor in determining the rate of motoneuron sprouting compared to unoperated animals (p <0.05) |
| Eisen 1973 [38] | Comparative preclinical experimental study n.22 rats (thy-1-YFP-H) (275 g) |
|
Sciatic nerve | Contralateral immobilization 0–6 weeks | Histological examination of nerve: mean number of fibers/nerves, axon and fiber diameter | The differences in fiber diameter of nerves from limbs of non-immobilized animals compared to immobilized limbs and contralateral limbs are significant (p < 0.001) |
| English 2007 [39] | Comparative preclinical experimental study n.6 rats (NT-4/5 knockout) (weight unspecified) |
|
Sciatic nerve | One-hour application of electrical stimulation at 20 Hz at the time of surgical repair | Immunohisto-fluorescence | Electrical stimulation enhances axons regeneration in cut peripheral nerves, independent of neurotrophin, but dependent on stimulation of trkB. Among electrical stimulation neurons, a significant increase in the proportion of neurons as immunoreactive to BDNF was recorded compared to untreated group (p < 0.01) |
| Florence 2001 [40] | Comparative preclinical experimental study n.6 monkeys (Macaca radiata) (weight unspecified) |
|
Median nerve | Rehabilitation involving sensory retraining | Electrophysiological mapping studies | In the monkeys reared without sensory enrichment during recovery, there were significantly larger and multiple fields compared to the sensory enriched monkeys (p < 0.05) |
| Gardiner 1984 [41] | Comparative preclinical experimental study n.49 rats (female Sprague–Dawley) (weight unspecified) |
|
Sciatic nerve | Treadmill immediately after nerve injury for 10 weeks for 1 h, at a speed of 26.8 m/min and an inclination of 15% | Body and muscle weights, twitch and tetanic contraction | Physical exercise enhances short-term sprouting of fast muscle motoneurons compared with untreated group (p < 0.05) |
| Gardiner 1986 [42] | Randomized study n.50 female rats (Sprague–Dawley) (180–200 g) |
|
Sciatic nerve | Daily program of increased activity, including grid climbing and voluntary wheel exercise, for 14 days, denervation at 5th day. | CSA, maximum tetanic tension | Muscle tetanic tension of partially denervated muscles in partial denervation of one hindlimb group and partial denervation of one hindlimb plus daily exercise were significantly (p < 0.05) larger than L5-evoked tension in left control muscles, and significantly (p < 0.05) lower than L4 plus L5-evoked control tension |
| Geremia 2006 [16] | Comparative preclinical experimental study n.76 female rats (Sprague–Dawley) (220–240 g) |
|
Femoral nerves | 20 Hz continuous electrical stimulation immediately after surgical repair | Hybridization quantification and analysis; immunohistochemistry | Electrical stimulation of 1 h led to a significant increase in DRG neurons regenerating into cutaneous and muscle branches, significantly increased the numbers of neurons that regenerated axons compared with the other group (p < 0.05), increased expression of GAP-43 in the regenerating neurons and of BDNF compared with the other group (p < 0.001) |
| Gomez-Pinilla 2002 [13] | Randomized study 10 male rats (Sprague–Dawley) (weight unspecified) |
|
Peripheral neuropathy | Wheel running for 7 days | Levels of neurotrophins and neurotropic factors: BDNF and its signal transduction receptor (trkB) | Voluntary exercise increased the expression of several molecules associated with the action of BDNF on synaptic function and neurite outgrowth in the lumbar region of the spinal cord and soleus muscle comparing sedentary group (p < 0.01) |
| Herbison 1973 [43] | Comparative preclinical experimental study 15 female rats (Wistar) (200 g) |
|
Sciatic nerve | Overwork (induced in soleus and plantaris by tenotomy of synergistic muscles) after 72 h, 1, 2, 3, 4, 6 weeks from denervation | Muscle weights, protein content, and conduction latencies, fiber diameters | Overwork within the period of reinnervation may be more beneficial than when initiated before this event. In the Group of tenotomy 3 weeks after denervation, the muscle weights, absolute amount of sarcoplasmic, myofibrillar, and stromal proteins and Type I and II fiber diameters of the soleus and plantaris were greater (p < 0.05) than control values |
| Herbison 1974 [44] | Comparative preclinical experimental study 40 rats (Wistar) (200–215 g) |
|
Sciatic nerve | Swimming 3–4 weeks after denervation for one or two hours each day for 3–4 weeks | Histological examination of nerve: fiber diameter and composition of fiber types in reinnervating soleus and plantaris muscles, total proteins | Intense swimming (2 h every day) does not enhance the repair of reinnervation muscle, and a high workload may be hazardous in the early phase of reinnervation. Moreover, Group C total protein concentration was significantly lower (p < 005) than that of the remaining four groups |
| Herbison 1980 [45] | Comparative preclinical experimental study 21 groups of female rats (Winstar) (200–225 g) |
|
Sciatic nerve | Treadmill 27m/min at 35% grade and bilateral cast immobilization I hind limbs were started 2–3 weeks after sciatic nerve crush 5 with a current of 15–20 V |
Isometric twitch/tension, time to peak tension. | The extremes of activity or inactivity retarded, but did not prevent, the recovery of the slow more than the fast muscle during reinnervation. Moreover, the tetanic tensions of the crush-denerveted muscles were significantly different from the crush-denervated control values at 6 weeks after crush; the soleus was 20.6% less (p < 0.05) and the plantaris was 23.5% greater (p < 0.01) than the crush-denervated control values |
| Herbison 1986 [46] | Comparative preclinical experimental study n.30 female rats (Wistar) (200–225 g) |
|
Sciatic nerve | Electrical stimulation 4 ms, 2–4 mA current distributed at 10 pulses per second stimulated for 2, 4, 8 h per day, 5 days per week for 6 weeks | Electric study, muscle weight, twitch and tetanic tension, fiber area, contraction time | Chronic stimulation of intact axons of partially denervated muscle increases the muscle weight and tension of the electrically stimulated muscle. Moreover, the weights of the muscles of the bilateral partial nerve section groups compared with their respective normal control muscles showed significant atrophy (p < 0.01) |
| Hines 1942 [47] | Comparative preclinical experimental study Albino rats (weight unspecified) |
|
Tibial nerve | Forced activity | Muscle creatine content, isometric tension, muscle weight | Activity improves the rate and extent of the recovery from peripheral nerve paralysis. After 21 days, there was no difference in the strength and weight of the muscle from the animals in the immobilized groups (p > 0.05) |
| Huang 2012 [17] | Randomized blinded study n.140 young male rats (Sprague–Dawley) (weight unspecified) |
|
Sciatic nerve | Intermittent electrical stimulation (3 V, 20 Hz) every two days for 8 times, assessed at 4, 8, 12 weeks Direct current or alternating current |
Expression of regeneration-associated genes, SFI, modified Sticky Tape Test, morphometric analysis, atrophy, protein levels | Electrical stimulation accelerates nerve regeneration and promotes functional recovery. Moreover, the protein levels of S-100, BDNF, P0 and Par-3 were significantly upregulated in the conductive scaffold + electrical stimulation group compared to non-conductive scaffold + electrical stimulation group (p < 0.05) |
| Ilha 2008 [48] | Randomized study n.37 male rats (Wistar) (280–330 g) |
|
Sciatic nerve | Endurance on a treadmill (from 20 to 60′ in 4 weeks) + warmup (running for 5′) 5 sessions per week, once a day during 5 weeks + Resistance Exercise (Climbing a 1 m-long ladder) A total of 3 sessions per week with 48 to 72 h of rest between sessions for 5 weeks |
Histological and Morphometric Nerve Studies, SFI | Endurance exercise improves sciatic nerve regeneration and resistance exercise. Endurance-resistance training may delay functional recovery and does not alter sciatic nerve fiber regeneration. Moreover, the SFI values of experimental groups were significantly lower than those of the control group (p < 0.001) |
| Irintchev 1990 [49] | Comparative preclinical experimental study n.15 rats (C57Bl/6J) (300 g) |
|
Sciatic nerve | Running 7–8 weeks in wheels (5.8 km + 1.5 S.D.) during the time of denervation and reinnervation period | Tetanic muscle force, muscle weight | Tetanic muscle force reached on average 72% of contralateral muscles after 5–10 months, (p < 0.01) and 87% of unoperated animals after 10 months (p < 0.05) |
| Irintchev 1991 [50] | Comparative preclinical experimental study n.10 female rats (NMRI) (weight unspecified) |
|
Sciatic nerve | Running wheels 39 weeks, 14–18 weeks after the nerve injury | Tetanic muscle force, isometric contraction measurements, muscle weight | Physical exercise during progressive muscle atrophy is effective and has significant and enduring impact on muscle recovery after reinnervation There were highly significant effects of exercise on nerve damage (p = 6–7% for the F-ratio) |
| Jaweed 1982 [51] | Comparative preclinical experimental study n.60 female rats (Wistar) (200–225 g) |
|
Sciatic nerve | Low-frequency electrical stimulation (2- to 4-mA pulses at 4 ms duration) at 10 Hz continuously 8 h daily for 10, 15, 20, 25, 30 days | Isometric twitch contraction, muscle weight | The effectiveness of long-term (200 to 240 h) direct, low frequency (10 Hz) electrical stimulation. In normal muscle, 25 and 30 days of electrical stimulation produced significant results (p < 0.05) |
| Kao 2013 [20] | Randomized study n.50 diabetic male rats (Sprague–Dawley) (250–300 g) |
|
Sciatic nerve | Percutaneous electrical stimulation 1 mA at 0, 2, 20, or 200 Hz, started after 1 week from the injury for 3 weeks | Morphometric analysis of axonal regeneration and remyelination; electrophysiological recordings of CMAPs for the conductive velocity, peak amplitude, area, latency 4 weeks postoperatively: macrophage density, CGRP area ratio | High-frequency electric stimulation could be necessary to heal the diabetic peripheral nerve. Larger nerve conductive velocity, amplitudes, and areas of the MAPs and shorter latencies were seen as the frequency of electrical stimulation was increased, where the differences of all of these parameters between the electrical stimulation groups at 0 Hz and 200 Hz reached significance at p < 0.05 |
| Kim 1998 [52] | Randomized study n.33 rabbits (New Zealand white) (2000–2500 g) |
|
Medial popliteal nerve | Continuous passive motion for 14 days | Nerve conduction study at 100 days (nerve conduction velocity, fiber density, and diameter, weight of soleus) | Continuous passive motion after nerve repair induces regeneration. The mean nerve conduction velocity was significantly lower in the two treatment groups than in the control groups (p = 0.0001) |
| Liao 2017 [19] | Randomized study (species and weight unspecified) |
|
Sciatic nerve | Swimming | Axon regeneration, electrophysiological parameters, muscular weights, macrophage infiltration, CGRP | Moderate swimming significantly improved CGRP-related axonal regeneration. Total nerve regeneration area of the swam group (10 min/3 times/week) was significantly elevated to approximately two-fold more than that of the sedentary control group (p < 0.05) |
| López-Álvarez 2015 [53] | Randomized blinded study rats (Sprague–Dawley) (240–300 g) |
|
Sciatic nerve | A total of 1 h running, starting at a locomotion speed of 10 cm/s and increasing 2 cm/s every 5 min, until a maximal speed of 32 cm/s | Thermal and mechanical thresholds Sensory PGP-IR fibers, BDNF, GAP43, NGF expression, CGRP neurons in the L3 DRG and KCC2 dephosphorylation in the dorsal horn, microglial activation | Recodification of spontaneous neural activity after peripheral nerve injury by specific graded intensity exercises may be a potent neurorehabilitation tool to prevent neuropathic pain. The expression of BDNF in microglia was greatly increased in untrained injured rats after sciatic nerve lesion compared to that of the untrained group (p < 0.0001) |
| Love 2002 [54] | Comparative preclinical experimental study n. 9 rats (species and weight unspecified) |
|
Tibial nerve | Electrical stimulation of 12 mA for 7 days (20 or 100Hz) | Fluorescent Measurements of Tension and Motor Unit Size; Fluorescent Labeling |
Muscle stimulation reduces sprouting by removing the means by which sprouts navigate to denervated end plates, i.e., terminal Schwann cells bridges. The number of end plates reinnervated by nodal sprouts was 19 ± 8% in sham-stimulated muscles and 14 ± 3% in stimulated muscles (p < 0.18) |
| Marqueste 2003 [6] | Randomized study n.36 rats (Sprague–Dawley) (weight unspecified) |
|
Peroneal nerve | Biphasic electro-myo-stimulation and exercise 5 days/week for 10 weeks | Monopolar tungsten electrode, CNAP | Chronic muscle electrostimulation partially favors the recovery of muscles, rehabilitation by treadmill running also efficiently induced a better functional muscle afferent recovery. When twitches were induced by muscle stimulation, CT significantly (p < 0.01 and p < 0.001) decreased in the LS and LSE groups |
| Marqueste 2006 [55] | Randomized study n.56 female rats (Sprague–Dawley) (300–350 g) |
|
Peroneal nerve | Monophasic or biphasic electro-myo-stimulation from 4 Hz to 75 Hz for 10 weeks | Muscle weight, Twitch characteristics, fatigue index, protein | Muscle electrostimulation following denervation and reinnervation tends to restore size (muscle atrophy was reduced in LSEm and absent in LSEb groups) and functional and histochemical properties during reinnervation better than unstimulated muscle. p < 0.001 indicated that the fatigue index significantly differed from that of controlled rats |
| Martins 2011 [56] | Randomized blinded study n.56 rats (Wistar) (250–280 g) |
|
Sciatic nerve | A total of 15 sessions every day of joint mobilization | Morphological analysis and immunoreactivity of CD11b/c and GFAP, SFI | Mobilization produces an anti-hyperalgesia effect and peripheral nerve regeneration. Mechanical and thermal hyperalgesia and motor performance deficit were detected in the Crush + Anesthesia group (p < 0.001), which was significantly decreased after joint mobilization (p < 0.001). In the morphological analysis, the Crush + Anesthesia group presented reduced myelin sheath thickness (p < 0.05), but the joint mobilization group presented enhanced myelin sheath thickness (p < 0.05) Peripheral nerve injury increased the immunoreactivity for CD11b/c and GFAP in the spinal cord (p < 0.05), and joint mobilization markedly reduced CD11b/c and GFAP immunoreactivity (p < 0.01) |
| Martins 2017 [57] | Blinded study n.40 male rats (swiss) (250–300 g) |
|
Sciatic nerve | Treadmill for 30 min at a speed of 6, 10, or 14 m/min with–16° slope, 5 days per week, over 8 weeks. Exercises began on the second post-operative week | Grip strength test, SFI | Exercised groups presented less neuropathic pain-like behavior and better functional recovery than non-exercised groups. Biochemically, exercise reduced TNF-α in the muscle and increased sciatic nerve IGF-1 levels in sciatic nerve crush. SFI value of the regular eccentric exercise groups were significantly better than those of the crush non-exercise group (p < 0.05) |
| Michel 1989 [58] | Comparative preclinical experimental study 70 female rats (Sprague–Dawley) (200–220 g) |
|
Sciatic nerve | Overload for 37 days | Body and plantaris weight, cross-sectional area, half-relaxation time, and maximum tetanic tension, fatigue index | Neuromuscular adaptation in response to compensatory overload does not favor the functional recovery from a partial denervation lesion. Significant main overload and partial denervation effects and interactions (p < 0.05) of groups compared with the control group |
| Molteni 2004 [7] | Blinded study n.12 rats (species and weight unspecified) |
|
Sciatic nerve | A total of 3 or 7 days of exercise | Analysis of regeneration; immunofluorescence; isolation of RNA and Real-Time Quantitative RT-PCR | Voluntary enhanced regrowth of axons after nerve injury. Differences in length of 3 and 7 days vs. 0 and 7 days vs. 3 days were statistically significant (p < 0.0001 and p < 0.01, respectively) |
| Pachter 1989 [8] | Comparative preclinical experimental study n.18 rats (Wistar) (weight unspecified) |
|
Peroneal nerve | A total of 4 days (2 h/day) of physical exercise | Isometric contractile properties, endplate ultrastructure | Denervated muscles exercised 4 days before reinnervation can preserve the structure of the endplate, enhance reinnervation and sprouting at the endplates after 11 days of denervation. The postsynaptic area and endplate were decreased compared with the control group (p < 0.05) |
| Sabatier 2008 [12] | Comparative preclinical experimental study n.19 rats (Thy-1-YFP-H) (weight unspecified) |
|
Sciatic nerve | Two weeks of treadmill, 5 days per week for 2 weeks | Tissue Harvesting and Microscopy | Treadmill exercise enhances axon regeneration in the peripheral nervous system. The sprouting index was significantly increased in all high-intensity groups (p ≤ 0.05) |
| Sarikcioglu 2001 [59] | Comparative preclinical experimental study n.36 rabbits (weight unspecified) |
|
Sciatic nerves | Swam 10 min/day for 10 days in a pool at 37 °C tap water | HRP neuro-histochemistry and modified Pal-Weigert methods | Exercise is effective for axonal regeneration in the 4th regeneration week. There was no myelinated fiber in the sedentary group, and there was a significant difference between exercise trained and sedentary groups (p < 0.05) |
| Seburn 1996 [60] | Randomized study n.73 male rats (Sprague–Dawley) (250–300 g) |
|
Popliteal nerve | Running | Motor unit tetanic force | Daily locomotor activity can enhance the tension-generating capacity of chronically enlarged motor units compared to sedentary group (p < 0.05) |
| Seo 2006 [61] | Randomized blinded study n.160 male Sprague–Dawley rats (220–240 g) |
|
Sciatic nerve | Treadmill walking for 10 min/day for 2 days prior to sciatic nerve injury | SFI, Western blotting and immunofluorescence staining | Treadmill promoted axonal regeneration. Differences in SFI values among the groups were statistically significant (p < 0.003) |
| Seo 2009 [21] | Randomized blinded study 108 male rats (Sprague–Dawley) (200–220 g) |
|
Sciatic nerve |
|
Levels of neurotrophins and neurotropic factors: expression levels of GAP-43 mRNA | Increased ERK1/2 activity in Schwann cells may play an important role in treadmill-mediated enhancement of axonal regeneration in the injured peripheral nerve. Protein levels in the treadmill groups were significantly higher than in sedentary controls (p < 0.01) |
| Sinis 2008 [62] | Randomized blinded study n.48 female rats (Fast Blue) (175–200 g) |
|
Facial nerve, median nerve | Manual stimulation | Restoration of grasping force, degree of collateral axonal branching, pattern of reinnervation of the motor endplates, index of axonal branching | Manual stimulation is beneficial in motor nerve injury, not in mixed nerves. It did not influence the degree of axonal sprouting or the extent of poly-innervation of motor endplates (p < 0.05) |
| Skouras 2009 [63] | Comparative preclinical experimental study n.64 female rats (Wistar) (175–200 g) |
|
Facial nerve |
|
Video-based motion analysis, analysis of vibrissae motor performance | Electrical stimulation did not improve functional outcome and failed to reduce the proportion of poly-innervated motor end-plates. By contrast, manual stimulation restored normal whisking function and reduced poly-innervation (p < 0.05). |
| Sobral 2008 [64] | Comparative preclinical experimental study n.20 male rats (Wistar) (229.05 ± 18.02 g) |
|
Sciatic nerve | Running at a speed=8m/min, inclination = 0%, 30 min/day, for 14 days. DEC group started exercise 24 h after the nerve injury. DCE group started on the 14th day after the injury, assessment at 7th, 14th, 21st and 28th days after the operation |
Axon and fiber diameter, myelin thickness, SFI | The treadmill exercise, during the immediate and late phase of nerve regeneration after crushing the sciatic nerve of rats, did not influence axonal budding, degree of maturation of the regenerated fibers or the functionality of the reinnervated muscles. The number of regenerated axons in denervated + cage + exercise groups was greater than in the others (p < 0.05) |
| Soucy 2013 [65] | Randomized blinded study n.8 female rats (Sprague–Dawley) (135–155 g) |
|
Sciatic nerve | Voluntary Motor Activity 9 days of daily handling and mild treadmill exercise. A total of 60 min at a speed of 30 m/min, at a 5% incline |
Histological examination of nerve: regeneration rate of axons | Increased activity has no effect on axon regeneration rate, but may be detrimental to the reinnervation process. Significant effect of exercise, crush, and interaction (p < 0.05) were detected at force integral index measured at force integral index measured at frequencies of 100–400 Hz |
| Tam 2001 [66] | Randomized blinded study n.55 female rats (Sprague–Dawley) (180–200 g) |
|
Sciatic nerves |
|
Electrophysiological and histochemical examination, CSA | Increased neuromuscular activity is not recommended as rehabilitation immediately after motoneuron injury or in the early stages of motoneuron disease. MU twitch forces were significantly larger than those of the control (p < 0.001). The shift in the MU twitch force distributions was much less but significant (p < 0.01) for moderately denervated muscles |
| Teodori 2011 [67] | Randomized study n.20 male rats (Wistar) (220 ± 12 g) |
|
Sciatic nerve | Swimming | SFI, axon number and diameter, fiber diameter and numbers, myelin thickness | After 30 days, the number of axons in CS1 and CS14 was lower than in C (p < 0.01). The diameter of axons and nerve fibers was larger in CS1 (p < 0.01) and CS14 (p < 0.05) than in C, and myelin sheath thickness was lower in all crushed groups (p < 0.05). There was no functional difference between CS1 and CS14 (p > 0.05) |
| Udina 2010 [68] | Comparative preclinical experimental study n.30 female rats (Sprague–Dawley) (weight unspecified) |
|
Sciatic nerve |
|
Nerve conduction study: Latency, M amplitude, H/M ratio, MEP/M ratio | Exercise increases trophic factor release to act on regenerating axons and to modulate central neuronal plasticity. MEP/M amplitude ratio during follow-up in rats untreated and treated with passive and active exercise for gastrocnemius, tibialis anterior, and plantar muscles is significantly different than the untreated group (p < 0.05). |
| Van Meeteren-Wiegant 1997 [69] | Randomized blinded study n.20 rats (Wistar) (200–220 g) |
|
Sciatic nerve | Locomotor activity in the open field | SFI, withdrawal reflex | Existence of a relationship between individual behavioral characteristics and sensory recovery of nerve function following crush lesion in rats. Recovery of motor function revealed no significant differences between both groups, whereas recovery of sensory function in active rats was significantly more rapid than that of the low active rats (p = 0.01) |
| van Meeteren– Gispen 1997 [70] | Randomized blinded study n.20 male rats (Wistar) (140–160 g) |
|
Sciatic nerve | Physical exercises | SFI, electrophysiologic findings: motor nerve conduction velocity | Beneficial effects of 24 days of exercise after crush persist in the late phase of peripheral nerve recovery. The motor nerve conduction velocity, as measured in the late phase of recovery, was significantly better in the trained group than in the control group (p < 0.01). |
| Werning 1991 [71] | Comparative preclinical experimental study n.30 male rats (CBA/J) (weight unspecified) |
|
- | Motor-driven treadmill, for total period of 9 h (3 x 3 h) with 30 min rest periods in between, 14 m min−1, slope of 6 degrees | Histological examination of nerve: chronic signs of damage: split fibers, central nuclei | The incidence of sprouting was significantly elevated 3–21 days after a single exercise (p < 0.01), and more so after repeated running (p < 0.01) |
Years, y; Sensory Amplitude, SNAP; Motor Amplitude, CMAP; Physical Activity Scale for the Elderly, PASE; Nerve Action Potential Amplitude, CNAP; Repetitions, reps; Extracellular signal-regulated Kinase ½, ERK1/2; Sciatic Nerve Function Index, SFI; Cross-Sectional Area, CSA; Grams, g; Growth-associated Protein 43, GAP-43; Brain-Derived Neurotrophic Factor, BDNF; Calcitonin Gene-Related Peptide, CGRP; Glial fibrillary acidic protein, GFAP.
Table 2.
Physical exercise in peripheral neuropathy in humans: characteristics and outcomes of studies included in the systematic review.
| Authors, Year | Study Design |
Sample Size, y | Neuropathy Characteristics | Rehabilitation and Instrumental Physical Therapy | Outcomes Measure | Results |
|---|---|---|---|---|---|---|
| Gordon 2009 [72] | Randomized control trial | A total of 21 subjects: 8 males, 13 females; 56 ± 17 y
|
Post-surgical median nerve compression | A total of 1 h 20 Hz of bipolar FES | Nerve conduction studies: MUNE and NCS; Purdue Pegboard Test, Semmes Weinstein Monofilaments, Levine’s Self-Assessment Questionnaire | The stimulation group had significant axonal regeneration 6–8 months after surgery when the MUNE increased to 290 ± 140 motor units from 150 ± 62 MU at baseline (p < 0.05). In comparison, MUNE did not significantly improve in the control group (pN0.2). Terminal motor latency significantly accelerated in the stimulation group but not the control group (p > 0.1). |
| Inoue 2011 [73] | Controlled experimental study | A total of 7 subjects, elderly men
|
Peripheral peroneal: n.5, axillary: n.1, ulnar: n.1 neuropathies | Self-guided rehabilitation | EMG, AROM for ankle and great toe | Complete functional recovery was observed in neurapraxia and partially in axonotmesis, and others showed reinnervation. An EMG examination revealed fibrillation potential (denervation potential) and reinnervation potential |
| Kluding 2012 [74] | Randomized blinded control trial | A total of 17 subjects 8 males/9 females; 58.4 ± 5.98 y; (duration of diabetes 12.4 ± 12.2 y) |
Diabetic peripheral neuropathy | In all, a 10-week exercise program with aerobic and strengthening exercises | Skin biopsy, QST of vibratory detection threshold, cooling detection threshold, and heat/pain threshold VAS, MNSI, IENF | Significant reduction in pain (p = 0.05), neuropathic symptoms (p = 0.01), and increased intraepidermal nerve fiber branching (p = 0.008) from a proximal skin biopsy were noted following intervention |
| Lange-Maia 2016 [75] | Controlled experimental study | A total of 328 subjects, old men, 78.8 ± 4.7 y |
Peripheral neuropathy | A total of 7 days of walking, strenuous moderate and light activities, strengthening exercises, lawn work and gardening occupational activities | Automated neurodiagnostic instrument motor and sensory latency, nerve function, CMAP, SNAP, F-wave latency PASE | Improvement in peripheral neuropathy was modestly associated with daily vigorous physical exercise in older men. Better motor latency was associated with higher PASE scores (p < 0.01) |
| Mennen 2002 [76] | Controlled experimental study | A total of 56 subjects 52 males, 4 females - |
Brachial plexus, ulnaris, medianous, radialis, digitalis, popliteus nerve lesion | Rehabilitation including sensory re-training and motor contraction exercises | EMG, MRC | End-to-side nerve suture and rehabilitation restores function, replace nerve grafting |
| Piccinini 2020 [77] | Randomized blinded control trial | A total of 38 subjects, 21 males, 17 females 37 ± 21 y |
Interosseous, abductor digiti, extensor digitorum communis, brachioradialis, vastus lateralis and medialis, biceps femoris, gastrocnemius, tibialis anterior, peroneus longus |
FES 150 ms, 1 Hz, 0.5 mA | MRC, strength with dynamometer, fibrillation potentials | FES improved in terms of clinical and neurophysiological parameters (MRC: p < 0.001 after FES) |
| Wong 2015 [78] | Randomized blinded Control Trial | A total of 36 subjects, 38.3 ± 39.3
|
Post-surgery after complete digital nerve transection | FES 1 h, 20 Hz | DASH, pressure threshold and quantitative small-fiber sensory testing |
Post-surgical FES enhanced sensory reinnervation in patients who sustained complete digital nerve transection. Although there was a trend of greater functional improvements in the ES group, it was not statistically significant (p > 0.01) |
Years, y; Sensory Amplitude, SNAP; Motor Amplitude, CMAP; Physical Activity Scale for the Elderly, PASE; Visual Analogue Scale, VAS; Michigan Neuropathy Screening Instrument, MNSI; Intraepidermal Nerve Fiber, IENF; Nerve Conduction Studies, NCS; Quantitative Sensory Testing, QST; Functional Electrical Stimulation, FES; Motor unit number estimation, MUNE; Disability of Arm, Shoulder and Hand questionnaire, DASH; Electromyography, EMG; Low-level laser therapy, LLLT.
Table 3.
Quality of evidence of studies.
| Quality Assessment | Summary of Findings | Quality of Evidence | ||||
|---|---|---|---|---|---|---|
| N° of Studies | Limitations | Inconsistency | Indirectness | Publication Bias | Characteristics of | |
| 60 studies | No significant limitations | No serious inconsistency | No serious indirectness | Unlikely | Population: humans or animals with neuropathic impairment Intervention: physical exercise or electrical stimulation Comparison: untreated group or treated with other therapy program Outcomes: changes in electrodiagnostic, muscle characteristics, specific indices and scales |
Moderate–High |
Table 4.
Risk of bias summary for each included study.
| Authors, Year | Random Sequence Generation | Allocation Concealment | Blinding Participants | Blinding of Outcome Assessment | Incomplete Data | Selective Reporting | Other Bias | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Ahlborn 2007 [30] | + | + | + | + | + | + | + | Low risk |
| Asensio-Pinilla 2009 [11] | - | - | - | - | + | + | + | Moderate risk |
| Boeltz 2013 [31] | - | - | - | - | + | + | + | Moderate risk |
| Brown 1979 [32] | - | - | - | - | - | + | + | High risk |
| Brushart 2005 [33] | + | + | - | - | + | + | + | Low risk |
| Cobianchi 2010 [34] | + | + | + | + | + | + | + | Low risk |
| Cohan 1986 [35] | - | - | - | - | + | + | + | Moderate risk |
| De Moraes 2018 [36] | + | + | - | - | + | + | + | Low risk |
| Einsiedel 1994 [37] | - | - | - | - | + | + | + | Moderate risk |
| Eisen 1973 [38] | - | - | - | - | + | + | + | Moderate risk |
| English 2007 [39] | - | - | - | - | + | + | + | Moderate risk |
| Florence 2001 [40] | - | - | - | - | + | + | + | Moderate risk |
| Gardiner 1984 [41] | - | - | - | - | + | + | + | Moderate risk |
| Gardiner 1986 [42] | + | + | - | - | + | + | + | Low risk |
| Geremia 2006 [16] | - | - | - | - | + | + | + | Moderate risk |
| Gordon 2009 [72] | + | + | - | - | + | + | + | Low risk |
| Gomez-Pinilla 2002 [13] | + | + | - | - | + | + | + | Low risk |
| Herbison 1973 [43] | - | - | - | - | + | + | + | Moderate risk |
| Herbison 1974 [44] | - | - | - | - | + | + | + | Moderate risk |
| Herbison 1980 [45] | - | - | - | - | + | + | + | Moderate risk |
| Herbison 1986 [46] | - | - | - | - | + | + | + | Moderate risk |
| Hines 1942 [47] | - | - | - | - | + | + | + | Moderate risk |
| Huang 2012 [17] | + | + | + | + | + | + | + | Low risk |
| Ilha 2008 [48] | + | + | - | - | + | + | + | Low risk |
| Inoue 2011 [73] | - | - | - | - | + | + | + | Moderate risk |
| Irintchev 1990 [49] | - | - | - | - | + | + | + | Moderate risk |
| Irintchev 1991 [50] | - | - | - | - | + | + | + | Moderate risk |
| Jaweed 1982 [51] | - | - | - | - | + | + | + | Moderate risk |
| Kao 2013 [20] | + | + | - | - | + | + | + | Low risk |
| Kim 1998 [52] | + | + | - | - | + | + | + | Low risk |
| Kluding 2012 [74] | + | + | + | + | + | + | + | Low risk |
| Lange-Maia 2016 [75] | - | - | - | - | + | + | + | Moderate risk |
| Liao 2017 [19] | + | + | - | - | - | + | + | Moderate risk |
| López-Álvarez 2015 [53] | + | + | + | + | + | + | + | Low risk |
| Love 2002 [54] | - | - | - | - | - | + | + | High risk |
| Marqueste 2003 [6] | + | + | - | - | + | + | + | Moderate risk |
| Marqueste 2006 [55] | + | + | - | - | + | + | + | Moderate risk |
| Martins 2011 [56] | + | + | + | + | + | + | + | Low risk |
| Martins 2017 [57] | - | - | + | + | + | + | + | Low risk |
| Mennen 2002 [76] | - | - | - | - | + | + | + | Moderate risk |
| Michel 1989 [58] | - | - | - | - | + | + | + | Moderate risk |
| Molteni 2004 [7] | - | - | + | + | - | + | + | Moderate risk |
| Pachter 1989 [8] | - | - | - | - | + | + | + | Moderate risk |
| Piccinini 2020 [77] | + | + | + | + | + | + | + | Low risk |
| Sabatier 2008 [12] | - | - | - | - | + | + | + | Moderate risk |
| Sarikcioglu 2001 [59] | - | - | - | - | + | + | + | Moderate risk |
| Seburn 1996 [60] | + | + | - | - | + | + | + | Low risk |
| Seo 2006 [61] | + | + | + | + | + | + | + | Low risk |
| Seo 2009 [21] | + | + | + | + | + | + | + | Low risk |
| Sinis 2008 [62] | + | + | + | + | + | + | + | Low risk |
| Skouras 2009 [63] | - | - | - | - | + | + | + | Moderate risk |
| Sobral 2008 [64] | - | - | - | - | + | + | + | Moderate risk |
| Soucy 2013 [65] | + | + | + | - | + | + | + | Moderate risk |
| Tam 2001 [66] | + | + | + | + | + | + | + | Low risk |
| Teodori 2011 [67] | + | + | - | - | + | + | + | Low risk |
| Udina 2010 [68] | - | - | - | - | + | + | + | Moderate risk |
| Van Meeteren-Wiegant 1997 [69] | + | + | + | + | + | + | + | Low risk |
| van Meeteren–Gispen1997 [70] | + | + | + | + | + | + | + | Low risk |
| Werning 1991 [71] | - | - | - | - | + | + | + | Moderate risk |
| Wong 2015 [78] | + | + | + | + | + | + | + | Low risk |
+ indicates reporting in full with low risk of bias; - indicates no reporting with high risk of bias.
3. Results
3.1. Description of the Studies
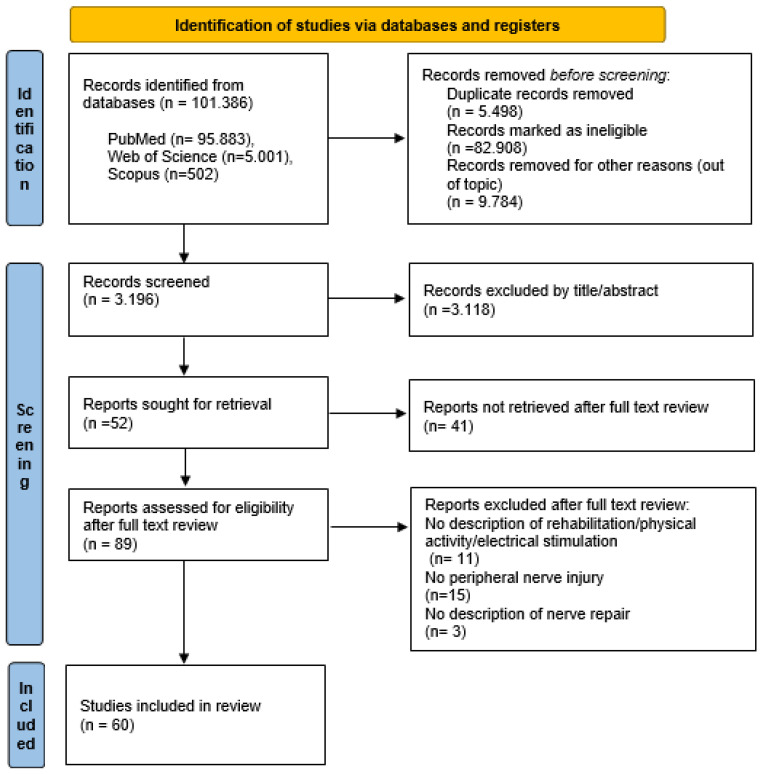
The literature search identified 101,386 articles. The authors reviewed the titles and abstracts, and 89 papers were selected for full text screening; suitability of inclusion in the study was independently assessed. Sixty publications met the inclusion criteria and were included in the systematic review. At the end of the search process, 29 articles were excluded for the following reasons: 15 did not consider peripheral nerve injuries, 11 did not describe any physical exercise, and 3 did not describe the nerve repair process. The number of studies produced at each stage of the search is shown in Figure 1. The sample characteristics and the design details of each study are shown in Table 1 for animals and Table 2 for humans. The evaluation of the quality of the studies is presented in Table 3, the assessment of risk of bias is shown in Table 4.
Figure 1.
Flow chart of the process of initial literature search and extraction of studies meeting the inclusion criteria.
3.2. Variations of Study Characteristics across the Studies and Risk of Bias
As reported in Table 1 and Table 2, most of the studies were on animals, especially rats, and 7 articles regarded human participants.
The paucity of significant positive results made an evaluation for humans incomplete and uncertain; however, the study on humans is worthy of further investigation as a subject that is still little debated and for which it is necessary to stimulate more interest.
There was a large variation among the studies concerning the proposed activity, regarding the type, duration, and start of intervention from nerve repair, assessed parameters and evaluations.
To reduce the risk of bias, the results were considered separately for humans and animals. Nevertheless, most preclinical studies used different animals, in number, type, age, and weight. Moreover, the included studies used different treatments, regarding timing, duration, type of exercises, and intensity of electrical stimulation. Because of the heterogeneity of the therapeutic strategies, a quantitative analysis of the results from the different interventions and the start of treatment to understand the best strategy was not possible.
3.3. Outcome Measurements
Electrophysiological parameters, Semmes–Weinstein monofilaments, contractions, and characteristics of muscle (i.e., weight), SFI, skin, nerve or muscle biopsy were used to evaluate nerve damage and repair processes.
In humans, electromyography (EMG) was used to reveal nerve lesions [73,76,77]. The electrophysiological recordings of nerve conduction studies focused on amplitude, latency [75], and unit number estimation (MUNE) [72]. The use of Semmes–Weinstein monofilaments showed the reinnervation of receptors that confer tactile function [78]. A proximal skin biopsy showed intraepidermal nerve fiber branching [74].
In animal research, nerve conduction studies were also used [6,11,19,31,40,52], especially for the analysis of nerve conduction velocity [37,70] and compound muscle action potentials (CMAPs) [17,20,43,68]. Nociceptive withdrawal thresholds [34,79] and thermal and mechanical thresholds [53] were also documented. Furthermore, muscles reacted to physical exercise after the nerve repair process with an increase in weight [19,42,43,46,47,49,50,51,55,58] and fiber diameter [38,43,44,52,64,67]. Tetanic tension was used to assess nerve repair [37,41,42,46,49,50,58,60] as well as isometric contraction [8,37,45,46,47,50,51]. Additionally, the analysis of SFI was another interesting parameter used [17,48,56,57,61,64,67,69,70]. Furthermore, histological nerve examination [32,33,34,35,36,38,44,65,71], in particular immunohisto-fluorescence [7,39,54,61] and immunohistochemistry [8,12,16,59,66], were diagnostic parameters. Likewise, the levels of neurotrophins and neurotropic factors were considered as parameters of nerve repair [7,12,53], in particular the expression of BDNF, its signal transduction receptor (trkB), and molecules associated with its action [7,10,13,14,34,39,53,65,79,80], the levels of GAP-43 mRNA [21,62], NGF [79], and hosphor-ERK1/2 protein [21]. Lastly, functional recovery was assessed by video-based motion analysis that precisely evaluated muscle function during locomotion [30,63]. This complexity of mechanisms involved in axon regeneration is shown in Table 5.
Table 5.
Mechanisms of action of controlled physical exercise on nerve repair and regeneration.
| Kind of Exercise | Action of Exercise on Nerve Repair | In-Depth Features | References |
|---|---|---|---|
| Modulator of the Neurotrophins | |||
| Physical exercise | Increment of neurotrophin levels | For regenerating neurons | [7,14,34,79] |
| Increment of neurotrophin levels such as BDNF | For the survival and regeneration of injured axons | [7,14,39] | |
| Induction of GMF and BDNF | GMF could be necessary for exercise induction of BDNF and could promote neuroprotection through BDNF production | [18] | |
| High-intensity physical exercise | Reduction both in early hyperalgesia, decreasing the production of NGF in the skin and in sensory neurons, and late hyperalgesia related to reinnervation by regenerating nerve fibers | Early hyperalgesia is associated with collateral sprouting of intact nerve fibers | [79] |
| High-intensity physical exercise | Reduction in BDNF at the level of microglia and dorsal root ganglia | Modulation of neurotrophin mechanisms that regulate growth and excitability of sensory neurons after peripheral nerve injury | [53] |
| Treadmill running | Hyperalgesic responses are strongly dependent on NGF | The early reduction in hyperalgesia is likely associated with the reduction in local NGF production | [79] |
| Low intensity, but not high intensity | Low-intensity, but not high-intensity treadmill increased neurite outgrowth of dorsal root ganglion (DRG) sensory neurons and potentiated Schwann cell proliferation | Treadmill elevated levels of GAP-43 mRNA and protein, and hosphor-ERK1/2 protein in the injured sciatic nerves | [21] |
| Voluntary exercise | Increase in axonal regeneration through a neurotrophin-dependent mechanism and neurite outgrowth | Increase in expression of several molecules associated with the action of BDNF on synaptic function | [13,65,80] |
| Voluntary exercise | Sensory ganglia from the 3- and 7-day-exercised animals contained higher brain-derived neurotrophic factor, neurotrophin 3, synapsin I, and GAP43 mRNA levels than those from sedentary animals | Increase in axonal regeneration after 3–7 days of exercises through a neurotrophin-dependent mechanism | [7] |
| Voluntary exercise | Modulation of neurotrophin signal | Regulating the growth of sensory neurons | [7] |
| Regular eccentric exercise | Reduction in TNF-α in the muscle and increase in IGF-1 in nerve. Activation of serotoninergic and noradrenergic systems (descending pain inhibitory systems) improved morphological nerve regeneration |
In sciatic nerve crush-subjected animals: reduced mechanical and cold hyperalgesia accelerated motor functional recovery |
[57] |
| Electrical stimulation and exercises | Increase in BDNF and trkB expression | Increase in the expression of BDNF and trkB mRNA in regenerating femoral motoneurons | [10] |
| Brief electrical stimulation | Decrease in dorsal root ganglion neurons regenerating into cutaneous and muscle branches, increase in numbers of neurons that regenerated axons, and the expression of GAP-43 mRNA in the regenerating neurons and of BDNF | - | [16] |
| Electrical stimulation | Up-regulation of S-100, BDNF, Par-3 | - | [17] |
| Swimming exercises | Increase nerve repair-associated makers, and calcitonin gene-related peptide (CGRP) | - | [19] |
| Sprouting | |||
| High intensity | Inhibition of denervation and induction of early collateral sprouting | Hampering of longer duration nerve regeneration | [79] |
| Increase in axonal outgrowth | |||
| Prolonged treadmill exercise | Promotion of enlargement of fast-fatigable and fast–intermediate motor units | At the level of partially denervated gastrocnemius | [37] |
| Electrical stimulation | FES-induced acceleration of axon regeneration in post-surgical carpal tunnel syndrome |
Improved MUNE, motor units, terminal motor latency, sensory nerve conduction values | [72] |
| Regulation of neuronal cotransporters | |||
| High-intensity exercises | Prevention of NKCC1/KCC2 deregulation | It is a nerve injury-dependent mechanism of central disinhibition | [53] |
Growth-Associated Protein 43, GAP-43; Brain-Derived Neurotrophic Factor, BDNF; Nerve Growth Factor, NGF; Na-K-2Cl Cotransporter isoform 1, NKCC1; K-Cl Cotransporter isoform 2, KCC2; Motor unit number estimation, MUNE.
3.4. Electrical Stimulation and Physical Exercise: Intervention Characteristics and Effects on Peripheral Neuropathy
Electrostimulation of denervated muscle and locomotion exercises were shown to be effective strategies in retarding muscle atrophy and improving contractile response after reinnervation [6].
Most of the animal studies used a low-frequency electrical stimulation at 20 Hz [11,16,17,30,33,39,54,63,66] or at 10 Hz [20,35,46,51], other studies used a variable frequency, at 0, 2, 20, or 200 Hz [20], at 100 or 150 Hz [32], at 20 or 100 Hz [54], and from 4 Hz (200 ls) to 75 Hz [6,55].
In humans, functional electrical stimulation at 20 Hz was proposed by two authors [72,78], and at 1 Hz by only one author [77].
In regard to the kind of proposed exercise, most of the animal studies used the treadmill [12,31,37,41,45,57,61,68,71], high speed exercise (running) [34,49,50,53,60,64,66], swimming [19,36,44,59,67], voluntary locomotor activity [7,13,42,65,69,70], overwork [43,47,58], endurance and resistance exercises [48], isometric exercises [8], sensory retraining [40], manual stimulation [62], continuous passive motion [52], joint mobilization [56], and constraint therapy [38].
In humans, rehabilitation was proposed by four authors, with self-guided exercises [73], aerobic and strengthening exercises and task-oriented activities [74,75], sensory re-training and motor contraction exercises [76], with few positive results.
In animals, a total of nine studies measured the role of exercise or electrical implications applied early in the process of nerve repair, that is, immediately after nerve injuries, until 3 weeks after denervation, and before the period of reinnervation or during the early stage of reinnervation [44]. These studies supported both positive effects after electrical stimulation [17,20] and physical exercise [43,67,68]; however, there were also negative effects after exercise [66], especially when too intense [44], with a high variability of results [48,64]. Conversely, a total of six studies measured the effects of exercise or electrical stimulation applied during late stage reinnervation, that is, 4 weeks after denervation [44]. In the late stage, only one study documented positive effects after electrical stimulation [17]. Regarding physical exercise, a total of four studies reported positive effects [67,69,70], and two reported negative [48] and no effects [64] at late stage reinnervation. In regard to these articles, a total of six were of high quality according to SYRCLE’s risk of bias tool [17,20,48,67,69,70]. No data were presented about exercising early after nerve repair in humans.
Concerning duration, Cobianchi et al. [34] considered short-lasting exercises, when they lasted 1 h a day for not more than 5 days, and long-lasting training of 1 h a day for more than 5 days. On the one hand, short-lasting running had beneficial rehabilitative effects on functional recovery after peripheral nerve injury [34]. On the other hand, a prolonged electrical stimulation and long-lasting exercise had detrimental effects on peripheral nerve regeneration and neuropathic pain [11]. Of these articles, it is important to consider that one was of high quality according to SYRCLE’s risk of bias tool [34]. No data were presented about the timing of interventions on humans.
In conclusion, regarding the physical exercise described above, several types have been proposed in experimental nerve injury models; however, there have been conflicting results regarding their effectiveness on axonal regeneration. No standardized therapy has been applied, with differences especially in the choice of intensity, duration, and timing of programs. Despite this heterogenicity, this research showed that the literature agrees in affirming that especially early exercise programs promoted an axonal regenerative response and prevented maladaptive responses in animals.
Even if no studies in humans showed a clear relationship between rehabilitation and axon regeneration, certainly specific physical exercise avoids secondary injuries, such as contractures, disuse atrophy, stasis edema, and pain.
3.5. Voluntary, Forced, and Passive Exercises
Regarding the kind of exercise, the literature proposes voluntary, forced, and passive exercises.
In animal models, forced (an external source guided the exercise) and passive range-of-motion exercises had a standardized load of training on the basis of the study protocol, while for physical exercise, based on voluntary activities of animals, the research studied the response to the type of exercise. Passive exercise of the denervated muscle before reinnervation preserved a healthy structure and enhanced regeneration and reinnervation [8,52,68]. Combined rehabilitation of motor and sensory functions by passive and active physical exercise improved the coordination of sensory–motor tasks and restored adequate circuitry at the spinal level [68]. Moreover, both passive and active exercises promoted the regeneration of axons of distal nerves, as well as the reduction in the hyper-excitability of spinal reflexes after nerve injury [68]. However, only one article was of high quality according to SYRCLE’s risk of bias tool [52].
In humans, the proposed exercises included stretching to warm up, aerobic or strengthening exercises, cardiovascular training with body recumbent steppers, upright cycle, recumbent cycle, and treadmill training. Strength training included abdominal curls, biceps curls, chest presses, lat pulldowns, leg extensions, seated leg curls, seated rows, shoulder presses, squats, and triceps presses [74], sensory re-training and motor contraction exercises [76]. Suggested intense activities included walking, jogging, swimming, singles tennis, or moderate activities included golf without a cart, doubles tennis or light activities (e.g., golf with a cart, shuffleboard), muscle strengthening exercises, lawn work and gardening, occupational activities that include walking or standing, caring for another person, home repairs, and housework [75]. Only one article proposed self-guided rehabilitation, with exercises that were carried out at home [73].
All these studies on humans described positive effects regarding functional recovery, improvement of symptoms and neurophysiological parameters.
3.6. Intensity of Physical Exercise and Recovery of Nerve Function
Concerning intensity, in animals, exercise was considered high when it lasted 2 h a day [44], moderate at 20 min/3 times/week [19], and low intensity was considered as a very undemanding load (i.e., 8 m/min of treadmill) [21].
Low-intensity physical exercise was described as positive in nerve regeneration [61]; indeed, it potentiated Schwann cell proliferation in regenerating the sciatic nerve in rats [61]. Sabatier et al. [12] showed that low-volume intermittent exercise in very small quantities, instead of continuous exercise at higher volume, enhanced axonal outgrowth.
Moreover, positive effects of nerve repair were also attributed to moderate intensity physical exercise [5,11,12,19,36], enhancing the regeneration of injured axons with differences related to sex [5,11].
Ten studies evaluated high-intensity exercise [34,49,50,53,60,64,66] and overwork activities [43,47,58]. Some described positive effects, except five articles that described negative effects [44,58,66], and two articles that described no effects [49,64].
Furthermore, the progressive increase in training intensity was described as positive in nerve regeneration processes by five articles [12,34,37,53].
Concerning the regeneration process, while continuous moderate or intermittent high-intensity physical exercise was able to promote axonal elongation after allograft repair, even if without increasing the sprouting index [12], a progressive increase in volume and/or intensity of exercise was an important factor in determining the rate of motoneuron sprouting [37] and was associated with neurotrophin upregulation [12]. Moreover, an early and progressive increase in training intensity reduced neuropathic pain [34,79] and prevented neurotrophin-mediated hyperexcitability of peripheral injured nerves [53] and hyperreflexia, as shown by the reduction in the facilitation of the monosynaptic H reflex [11] and the early reappearance of the H reflex with increased amplitude after exercise [31]. On the other hand, it was hypothesized that due to a feedback mechanism, large doses of BDNF inhibited axon regeneration [81]. Sprouting was inhibited in vivo by increased neuromuscular activity associated with wheel running in rats [66]. In fact, some evidence showed that muscle stimulation dramatically reduced the terminal sprouting that normally occurred following partial denervation [32,54]. Regarding increased daily neuromuscular activity preceding partial denervation, this did not lead to motoneuron sprouting responses of slow muscle, such as the soleus, but enhanced short-term sprouting of fast muscle motoneurons, such as the plantaris muscle [41]. This contrasted with the absence of enhancement of sprouting in rats subjected to daily exercises following partial denervation of the plantaris muscle [42].
Intense and prolonged physical exercise reduced the mechanical hyperalgesia of the sciatic nerve after its regeneration [53]. On the other hand, as reported, manual stimulation for injuries of mixed nerves [62] and electrical stimulation [63], especially if prolonged [11], did not improve functional outcomes. A prolonged electrical stimulation or an intense locomotion exercise had detrimental effects on peripheral nerve regeneration and neuropathic pain [11,34]. However, six articles were of high quality according to SYRCLE’s risk of bias tool [34,42,53,60,61,66].
Shared results of a progressive and high level of physical exercise were unclear.
In humans, concerning the load of exercise, the improvement in peripheral neuropathy was modestly associated with daily vigorous physical exercise [75].
4. Discussion
4.1. Summary of Collected Data
To our knowledge, this is the first systematic review looking at the role of activity-based rehabilitation and electrical stimulation in the processes of spontaneous or surgical nerve repair secondary to peripheral neuropathies. The effectiveness of activity-based training, rehabilitation and electrical stimulation on peripheral nerve regeneration and muscle reinnervation is controversial. The lack of guidelines regarding the type of exercises, duration, and intensity makes the results heterogeneous and not easily comparable. Furthermore, in most studies, the involved processes of nerve repair are not the principal focus.
This review presents important clinical considerations: the effectiveness of different physical exercises and electrical stimulations to improve the recovery of peripheral nerve injury, the possibility of application in humans and the detrimental effects of exercise too early after nerve injury. Indeed, it proposes promising patterns for further investigation, comparing (1) the outcomes and kind of rehabilitation proposed early and late in the process of nerve repair, (2) short- and long-lasting exercises, (3) different intensity (from high to low), and (4) progressive versus sudden high load.
4.2. When to Start Treatment
The effectiveness of physical exercise could be related to the time of the start of activity. In fact, it has been shown that starting exercises for denervated muscles early, immediately after injury, contributes to accelerating nerve regeneration and synaptic elimination [67]. In particular, early progressive resistance exercises in the presence of axonal dysfunction could enhance spontaneous neurologic recovery [82], and physical exercise during the denervation period could cause more positive effects compared to performing exercises during reinnervation and the recovery period [49]. Moreover, treadmill training during the early and late phases of nerve regeneration after crushing the sciatic nerve of rats did not influence axonal budding, degree of maturation of regenerated fibers or the functionality of the reinnervated muscles [64]. However, according to a few authors, exercise seems to be effective starting from the 4th regeneration week after nerve injury and not before [59].
According to other authors, increased neuromuscular activity was not recommended immediately after motoneuron injury or in the early stages of motoneuron disease [66]. Additionally, high workload, over-training and overuse proved to interfere with peripheral nerve recovery, especially in the early phase of recovery [44]. Indeed, an excessively early start of treatment (before the period of reinnervation) and intense physical exercise had detrimental effects [44]. On the contrary, long-lasting [34], high-intensity training, overload, and overwork, especially at the onset of reinnervation [43], or increased neuromuscular activity in extensively denervated muscles [66] represented an unphysiological stimulus that interfered with normal anatomical and biochemical recovery [44,66].
4.3. Specific Physical Programs and Conflicting Views in the Literature
Regarding the kind of proposed exercise, passive and active exercise is effective in nerve repair processes [8,52,56,68], including voluntary exercise [7]. Combined therapies for motor and sensory recovery with passive or active exercise programs could improve the coordination of sensory–motor tasks and restore an adequate circuitry at the spinal level [68].
Specific exercises such as eccentric [57], endurance [48], and sensory rehabilitation [40] are described as effective in nerve regeneration processes. In particular, regular eccentric exercise improves morphological nerve regeneration, reduces mechanical and cold hyperalgesia, and accelerates motor functional recovery [57]. Sensory rehabilitation [40,76], especially with intensive programs of sensory rehabilitation after regeneration, can be used to improve even sensory perception [40].
On the contrary, resistance exercises and immobilization seem to delay functional recovery [38,47,48]. Too many heterogeneous results were recorded for manual stimulation [62,63]. In particular, resistance exercise or the combination of resistance and endurance training may delay functional recovery, but do not alter sciatic nerve regeneration, while moderate endurance exercise improves nerve regeneration [48].
Immobilization seems to have no detrimental effects on the count of number of fibers from a limb compared to contralateral active limb [38], but it delays recovery, probably because of a reduction in muscle regeneration rate rather than because of an influence on nerve regeneration [47].
4.4. Electrical Stimulation and Conflicting Views in the Literature
Concerning electrical stimulation, positive effects in the nerve repair process were recorded in both animal [6,16,20,33,35,39,46,55] and human research [72,78]. The findings indicate that it is efficient for maintaining muscle weight, but other parameters, such as twitch characteristics, fatigue index, mechanosensitivity, and metabosensitivity, are totally restored when an animal performs a running exercise during the rehabilitation period [6,55]. High-frequency electrical stimulation has been shown to exert benefits in diabetic peripheral neuropathies [20]. Brief electrical stimulation leads to accelerated locomotor recovery [30] and facilitates reinnervation [33,72,78]. The chronic and prolonged stimulation of intact axons of partially denervated muscles enhances muscle recovery [6,55] and increases muscle weight and the tension of the electrically stimulated muscle [46]. Moreover, electrical stimulation of the facial nerve did not improve the functional outcome nor reduce aberrant regeneration after facial nerve reconstruction in rats [63].
The only positive effect of this treatment on the facial nerve was a transient improvement of protraction velocity between 1 and 3 months after surgical reconstruction [63].
Acute electrical stimulation appears to exert some transient benefit in small animal laboratory models following injury to mixed peripheral nerves; thus, this intervention does not confer any long-term benefit following injury to a purely motor nerve.
In humans, clinical use of direct current electrical stimulation of acupuncture needles together with self-guided rehabilitation for motor function recovery after neurapraxia and axonotmesis was suggested for peripheral nerve damage with positive results [73].
4.5. Electrophysiological Parameters, Muscle Characteristics, and Neurotrophic Mechanisms
The electrophysiological changes related to the start of treatment, secondary to physical exercise and direct stimulation, included CMAP latency and amplitude both in rats and in humans, nerve conduction velocity in humans, SFI, muscle fiber and axon diameter in animals to highlight the differences, the possibility of recovery or detrimental effects related to rehabilitation of nerve repair processes after injury.
In humans, when EMG reveals complete nerve lesions, the reinnervation is generally unlikely to happen after electrical stimulation [77]. Furthermore, it is important to highlight that it is possible that the improvement of the parameters was partially explained by the natural history of the disease [77]. In particular, amplitude and latency are indicators of different types of peripheral nerve damage, with worse amplitude indicative of axonal degeneration, while latency, a component of conduction velocity, is a sign of demyelination [75]. Nevertheless, in the early stage, an absent EMG response did not prove failure of nerve growth or re-innervation due to smaller axons, which need to be myelinated and that lasted for a long time (up to three years) [76]. Axonal regeneration is quantified using motor unit number estimation (MUNE) and sensory and motor nerve conduction studies [72]. Semmes–Weinstein monofilaments show reinnervation of receptors that confer tactile function [78]. A proximal skin biopsy shows intraepidermal nerve fiber branching [74].
In regard to the start of treatment, the studies on electrophysiological changes described encouraging results when rehabilitation was proposed early. Indeed, when therapeutic interventions started early (immediately or within the first weeks after nerve injury), the literature revealed changes in electrophysiological recordings of CMAPs for latency.
When therapeutic strategies started early, the SFI in rats showed increased values [64,67] until the first week after nerve injury, while decreased values were observed until the second and the third weeks, indicating significant functional loss [48,64,67]. Highly variable results were recorded when the start of treatment was not until the fourth week, reporting both decreased [17,48] and increased values [64,67,69,70]. Physical exercise significantly improved the value of SFI when it started early, within 2–3 weeks from nerve injury.
In animals, muscles reacted to physical exercise with an increase in weight, as well described for the plantaris [41,42,58] and the soleus muscle [49]. On the other hand, no effect of training was shown in muscles atrophied after denervation [37,44]. Stimulation for a brief period (three minutes a day) greatly retarded muscle atrophy prior to reinnervation and accelerated the recovery of muscle weight and strength subsequent to reinnervation. This was documented for gastrocnemius muscles [47] and chronic stimulation (for six to ten weeks) of intact axons of partially denervated muscle increased muscle weight, as documented for the soleus muscle [46,55]. Furthermore, a considerable increase in diameter of myelinated axons after electrical stimulation was shown [17,64,67].
The regenerative mechanisms were connected to the production of neurotrophic factors. Collateral sprouting and axonal elongation of undamaged axons occurred in response to denervation due to local production of neurotrophic and neurotropic factors [53]. The levels of neurotrophins were related to physical exercise; they enhance axon elongation [12]. Regarding the type of training, voluntary exercise, even brief [7], increased axonal regeneration through a neurotrophin-dependent mechanism and neurite outgrowth [7,13,80].
4.6. The Rehabilitative Point of View and Implications for Humans
Too many differences exist between animals and humans, i.e., genetics, habits, adherence to treatment, and comorbidities that could influence outcomes. For example, exercise could modify the natural history of diabetic peripheral neuropathy; for this reason, human studies must be encouraged. Indeed, in people with diabetic peripheral neuropathy, a short-term intervention with a supervised aerobic and resistance exercise program seems to exert beneficial effects on pain and neuropathic symptoms [72].
At the same time, a study by Lange-Maia et al. [75] showed that in elderly humans, a reduction in discomfort related to peripheral neuropathies was associated with higher levels of self-reported physical exercise and more daily minutes of objectively measured vigorous activity. In an interesting report, at peripheral nerve testing, sensory amplitude (SNAP), indicative of axonal degeneration, was associated with objective vigorous physical exercise [75], while distal motor latency, a component of conduction velocity related to demyelination, was associated with self-reported physical exercise [75]. Thus, a relationship between a better motor nerve function and higher levels of physical exercise was proposed [75]. Moreover, the extent of the damage dictates the need to proceed with appropriate strengthening programs [82].
The timeliness, the choice of the type of rehabilitative exercises, and symptomatology are essential for successful treatment. Moreover, there is evidence that the recovery of sensory and motor nerves is not identical. Furthermore, individual differences in recovery of function after peripheral nerve damage could be related to the origin of the peripheral neuropathy, pre-existing biological traits, hormonal balance, and behavioral characteristics [69]. For this reason, rehabilitative programs should be personalized.
5. Limitations
Differences in the kind and number of animals with respect to humans, as well as the types and duration of interventions, may affect the results of the present review. Different typologies of animals, mostly rats of different species, but also rabbits and snails result in the samples being very heterogeneous. Furthermore, human subjects vary in age and their comorbidities are not always sufficiently described. Moreover, few studies examined the voluntary activities of animals, thus a standardized control of the intensity, duration, and pattern of training is missing compared to organized and controlled passive or active activity programs. Indeed, when animals are free to move, their exercises are not comparable to a measurable and preventive chosen training. Furthermore, the type of exercise used in the various studies is very different. Indeed, active, passive, or controlled exercises are proposed, with different intensity (high, moderate, or low) as well as duration and timing (early or late starting). A similar heterogeneity is present for electrical stimulation: it is mostly used at 20 Hz, but the frequency is also variable in some articles. This heterogeneity did not allow a quantitative analysis of data, but it suggests new promising research to understand the ways in which exercise and electrostimulation can influence nerve repair.
6. Conclusions
In most preclinical studies, peripheral neuropathy function was associated with improvements due to physical exercise and electrical stimulation. For humans, limited research has been conducted on this topic to reach a complete evaluation. Certainly, it would be desirable to determine an effective therapy for peripheral neuropathy in humans. Unfortunately, physical exercise often has no effects on the course of peripheral neuropathies; in fact, in rats, both detrimental and beneficial effects have been described. Thus, this study shows that the literature is inclined to propose the thesis that an early physical program with active exercise and/or electrical stimulation promotes an axonal regenerative response and prevents maladaptive responses, with changes in electrophysiological recordings.
The degree to which the types of activity or the duration of exercise could influence outcomes is unclear. In humans, only a few studies with patients with diabetes indicate that exercise could reduce the symptoms related to neuropathies; however, robust trials are necessary to understand whether controlled physical exercise and consequent specific rehabilitation plays a role in regeneration and repair of injured nerves and denervated muscle.
This study intends to spur new research to explore the possible mechanisms of activity-based rehabilitation on peripheral neuropathies, as well as immediate and long-term effects. Moreover, our findings support the need for future research to test the validity of a possible rehabilitation treatment in cases of peripheral neuropathy to help maintain functional independence.
Author Contributions
Conceptualization M.V. and R.C.; methodology M.V and R.C.; validation, M.V., V.P., G.T., I.P., D.S., G.M. and G.L.M.; writing—original draft preparation, R.C.; writing—review and editing, M.V.; visualization, M.V., V.P., G.T., I.P, D.S., G.M. and G.L.M.; supervision, M.V. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors have no conflict of interest relevant to this article.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Küllmer K., Sievers K.W., Reimers C.D., Rompe J.-D., Müller-Felber W., Nägele M., Harland U. Changes of sonographic, magnetic resonance tomographic, electromyographic, and histopathologic findings within a 2-month period of examinations after experimental muscle denervation. Arch. Orthop. Trauma Surg. 1998;117:228–234. doi: 10.1007/s004020050234. [DOI] [PubMed] [Google Scholar]
- 2.Win M.M.T.M., Fukai K., Nyunt H.H., Hyodo Y., Linn K.Z. Prevalence of peripheral neuropathy and its impact on activities of daily living in people with type 2 diabetes mellitus. Nurs. Health Sci. 2019;21:445–453. doi: 10.1111/nhs.12618. [DOI] [PubMed] [Google Scholar]
- 3.Maugeri G., D’Agata V., Trovato B., Roggio F., Castorina A., Vecchio M., Di Rosa M., Musumeci G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon. 2021;7:e08281. doi: 10.1016/j.heliyon.2021.e08281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibata T., Tashiro S., Shinozaki M., Hashimoto S., Matsumoto M., Nakamura M., Okano H., Nagoshi N. Treadmill training based on the overload principle promotes locomotor recovery in a mouse model of chronic spinal cord injury. Exp. Neurol. 2021;345:113834. doi: 10.1016/j.expneurol.2021.113834. [DOI] [PubMed] [Google Scholar]
- 5.English A.W., Wilhelm J.C., Sabatier M.J. Enhancing recovery from peripheral nerve injury using treadmill training. Ann. Anat. Anat. Anz. 2011;193:354–361. doi: 10.1016/j.aanat.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marqueste T., Alliez J.-R., Alluin O., Jammes Y., Decherchi P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J. Appl. Physiol. 2004;96:1988–1995. doi: 10.1152/japplphysiol.00775.2003. [DOI] [PubMed] [Google Scholar]
- 7.Molteni R., Zheng J.-Q., Ying Z., Gomez-Pinilla F., Twiss J.L. Voluntary exercise increases axonal regeneration from sensory neurons. Proc. Natl. Acad. Sci. USA. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pachter B.R., Eberstein A. Passive Exercise and Reinnervation of the Rat Denervated Extensor Digitorum Longus Muscle after Nerve Crush. Am. J. Phys. Med. Rehabilitation. 1989;68:179–182. doi: 10.1097/00002060-198908000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Udina E., Cobianchi S., Allodi I., Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann. Anat.-Anat. Anz. 2011;193:347–353. doi: 10.1016/j.aanat.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 10.A Al-Majed A., Brushart T.M., Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000;12:4381–4390. [PubMed] [Google Scholar]
- 11.Asensio-Pinilla E., Udina E., Jaramillo J., Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Sabatier M.J., Redmon N., Schwartz G., English A.W. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez-Pinilla F., Ying Z., Roy R.R., Molteni R., Edgerton V.R. Voluntary Exercise Induces a BDNF-Mediated Mechanism That Promotes Neuroplasticity. J. Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 14.Ying Z., Roy R.R., Edgerton V.R., Gómez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Pinilla F., Ying Z., Opazo P., Roy R.R., Edgerton V.R. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur. J. Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 16.Geremia N.M., Gordon T., Brushart T.M., Al-Majed A.A., Verge V.M. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp. Neurol. 2007;205:347–359. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Huang J., Lu L., Zhang J., Hu X., Zhang Y., Liang W., Wu S., Luo Z. Electrical Stimulation to Conductive Scaffold Promotes Axonal Regeneration and Remyelination in a Rat Model of Large Nerve Defect. PLoS ONE. 2012;7:e39526. doi: 10.1371/journal.pone.0039526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaheer A., Haas J.T., Reyes C., Mathur S.N., Yang B., Lim R. GMF-Knockout Mice are Unable to Induce Brain-Derived Neurotrophic Factor after Exercise. Neurochem. Res. 2006;31:579–584. doi: 10.1007/s11064-006-9049-3. [DOI] [PubMed] [Google Scholar]
- 19.Liao C.-F., Yang T.-Y., Chen Y.-H., Yao C.-H., Way T.-D., Chen Y.-S. Effects of swimming exercise on nerve regeneration in a rat sciatic nerve transection model. Biomed. Pharmacother. 2017;7:3. doi: 10.1051/bmdcn/2017070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao C.-H., Chen J.-J.J., Hsu Y.-M., Bau D.-T., Yao C.-H., Chen Y.-S. High-Frequency Electrical Stimulation Can Be a Complementary Therapy to Promote Nerve Regeneration in Diabetic Rats. PLoS ONE. 2013;8:e79078. doi: 10.1371/journal.pone.0079078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo T.B., Oh M.-J., You B.-G., Kwon K.-B., Chang I.-A., Yoon J.-H., Lee C.Y., Namgung U. ERK1/2-Mediated Schwann Cell Proliferation in the Regenerating Sciatic Nerve by Treadmill Training. J. Neurotrauma. 2009;26:1733–1744. doi: 10.1089/neu.2008.0711. [DOI] [PubMed] [Google Scholar]
- 22.Zhong S., Zhang Z., Su H., Li C., Lin Y., Lu W., Jiang Z., Yang L. Efficacy of Biological and Physical Enhancement on Targeted Muscle Reinnervation. Cyborg Bionic Syst. 2022;2022:9759265. doi: 10.34133/2022/9759265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haastert-Talini K., Grothe C. Electrical Stimulation for Promoting Peripheral Nerve Regeneration. Int. Rev. Neurobiol. 2013;109:111–124. doi: 10.1016/b978-0-12-420045-6.00005-5. [DOI] [PubMed] [Google Scholar]
- 24.Acheta J., Stephens S.B.Z., Belin S., Poitelon Y. Therapeutic Low-Intensity Ultrasound for Peripheral Nerve Regeneration–A Schwann Cell Perspective. Front. Cell Neurosci. 2022;15:812588. doi: 10.3389/fncel.2021.812588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosso M.P.D.O., Buchaim D.V., Junior G.M.R., Andreo J.C., Pomini K.T., Buchaim R.L. Low-Level Laser Therapy (LLLT) Improves the Repair Process of Peripheral Nerve Injuries: A Mini Review. Int. J. Neurorehabilit. 2017;4:260. doi: 10.4172/2376-0281.1000260. [DOI] [Google Scholar]
- 26.Alarcón J.B., Chuhuaicura P.B., Sluka K.A., Vance C.G., Fazan V.P.S., Godoy K.A., Fuentes R.E., Dias F.J. Transcutaneous Electrical Nerve Stimulation in Nerve Regeneration: A Systematic Review of In Vivo Animal Model Studies. Neuromodulation: Technol. Neural Interface. 2022;25:1248–1258. doi: 10.1016/j.neurom.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J.S., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011;343:889–893. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahlborn P., Schachner M., Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp. Neurol. 2007;208:137–144. doi: 10.1016/j.expneurol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Boeltz T., Ireland M., Mathis K., Nicolini J., Poplavski K., Rose S.J., Wilson E., English A.W. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J. Neurophysiol. 2013;109:2645–2657. doi: 10.1152/jn.00946.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown M.C., Holland R.L. A central role for denervated tissues in causing nerve sprouting. Nature. 1979;282:724–726. doi: 10.1038/282724a0. [DOI] [PubMed] [Google Scholar]
- 33.Brushart T.M., Jari R., Verge V., Rohde C., Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp. Neurol. 2005;194:221–229. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Cobianchi S., Marinelli S., Florenzano F., Pavone F., Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience. 2010;168:273–287. doi: 10.1016/j.neuroscience.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 35.Cohan C.S., Kater S.B. Suppression of Neurite Elongation and Growth Cone Motility by Electrical Activity. Science. 1986;232:1638–1640. doi: 10.1126/science.3715470. [DOI] [PubMed] [Google Scholar]
- 36.de Moraes A.A., de Almeida C.A.S., Lucas G., Thomazini J.A., DeMaman A.S. Effect of swimming training on nerve morphological recovery after compressive injury. Neurol. Res. 2018;40:955–962. doi: 10.1080/01616412.2018.1504180. [DOI] [PubMed] [Google Scholar]
- 37.Einsiedel L.J., Luff A.R. Activity and motor unit size in partially denervated rat medial gastrocnemius. J. Appl. Physiol. 1994;76:2663–2671. doi: 10.1152/jappl.1994.76.6.2663. [DOI] [PubMed] [Google Scholar]
- 38.Eisen A.A., Carpenter S., Karpati G., Bellavance A. The effect of muscle hyper- and hypoactivity upon fibre diameters of intact and regenerating nerves. J. Neurol. Sci. 1973;20:457–469. doi: 10.1016/0022-510X(73)90176-7. [DOI] [PubMed] [Google Scholar]
- 39.English A.W., Schwartz G., Meador W., Sabatier M.J., Mulligan A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev. Neurobiol. 2007;67:158–172. doi: 10.1002/dneu.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Florence S.L., Boydston L.A., Hackett T.A., Lachoff H.T., Strata F., Niblock M.M. Sensory enrichment after peripheral nerve injury restores cortical, not thalamic, receptive field organization. Eur. J. Neurosci. 2001;13:1755–1766. doi: 10.1046/j.0953-816x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- 41.Gardiner P., Michel R., Iadeluca G. Previous exercise training influences functional sprouting of rat hindlimb motoneurons in response to partial denervation. Neurosci. Lett. 1984;45:123–127. doi: 10.1016/0304-3940(84)90086-7. [DOI] [PubMed] [Google Scholar]
- 42.Gardiner P.F., Faltus R.E. Contractile responses of rat plantaris muscles following partial denervation, and the influence of daily exercise. 1986, 406, 51–56. Pflügers Arch. 1986;406:51–56. doi: 10.1007/BF00582952. [DOI] [PubMed] [Google Scholar]
- 43.Herbison G.J., Jaweed M., Ditunno J.F., Scott C.M. Effect of overwork during reinnervation of rat muscle. Exp. Neurol. 1973;41:1–14. doi: 10.1016/0014-4886(73)90176-3. [DOI] [PubMed] [Google Scholar]
- 44.Herbison G.J., Jaweed M.M., Ditunno J.F. Effect of swimming on reinnervation of rat skeletal muscle. J. Neurol. Neurosurg. Psychiatry. 1974;37:1247–1251. doi: 10.1136/jnnp.37.11.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbison G.J., Jaweed M.M., Ditunno J.F. Effect of Activity and Inactivity on Reinnervating of Skeletal Muscle Contarctility. Exp. Neurol. 1980;70:498–506. doi: 10.1016/0014-4886(80)90176-4. [DOI] [PubMed] [Google Scholar]
- 46.Herbison G.J., Jaweed M.M., Ditunno J.F. Electrical stimulation of sciatic nerve of rats after partial denervation of soleus muscle. Arch. Phys. Med. Rehabilitation. 1986;67:79–83. doi: 10.1016/0003-9993(86)90104-8. [DOI] [PubMed] [Google Scholar]
- 47.Hines H.M. Effects of Immobilization and Activity on Neuromuscular Regeneration. JAMA. 1942;120:515–517. doi: 10.1001/jama.1942.02830420023006. [DOI] [Google Scholar]
- 48.Ilha J., Araujo R.T., Malysz T., Hermel E.E.S., Rigon P., Xavier L.L., Achaval M. Endurance and Resistance Exercise Training Programs Elicit Specific Effects on Sciatic Nerve Regeneration After Experimental Traumatic Lesion in Rats. Neurorehabilit. Neural Repair. 2007;22:355–366. doi: 10.1177/1545968307313502. [DOI] [PubMed] [Google Scholar]
- 49.Irintchev A., Draguhn A., Wernig A. Reinnervation and recovery of mouse soleus muscle after long-term denervation. Neuroscience. 1990;39:231–243. doi: 10.1016/0306-4522(90)90236-W. [DOI] [PubMed] [Google Scholar]
- 50.Irintchev A., Carmody J., Werning A. Effects on recovery of soleus and extensor digitorum longus muscles of prolonged wheel running during a period of repeated nerve damage. Neuroscience. 1991;44:515–519. doi: 10.1016/0306-4522(91)90074-X. [DOI] [PubMed] [Google Scholar]
- 51.Jaweed M., Herbison G.J., Ditunno J.F. Direct electrical stimulation of rat soleus during denervation-reinnervation. Exp. Neurol. 1982;75:589–599. doi: 10.1016/0014-4886(82)90027-9. [DOI] [PubMed] [Google Scholar]
- 52.Kim H.K.W., Kerr R.G., Turley C.B., Evans P.J., Jay V., Salter R.B. The Effects of Postoperative Continuous Passive Motion on Peripheral Nerve Repair and Regeneration. J. Hand Surg. 1998;23:594–597. doi: 10.1016/S0266-7681(98)80008-9. [DOI] [PubMed] [Google Scholar]
- 53.López-Álvarez V.M., Modol L., Navarro X., Cobianchi S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain. 2015;156:1812–1825. doi: 10.1097/j.pain.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 54.Love F.M., Son Y.-J., Thompson W.J. Activity alters muscle reinnervation and terminal sprouting by reducing the number of schwann cell pathways that grow to link synaptic sites. J. Neurobiol. 2003;54:566–576. doi: 10.1002/neu.10191. [DOI] [PubMed] [Google Scholar]
- 55.Marqueste T., Decherchi P., Desplanches D., Favier R., Grelot L., Jammes Y. Chronic electrostimulation after nerve repair by self-anastomosis: Effects on the size, the mechanical, histochemical and biochemical muscle properties. Acta Neuropathol. 2006;111:589–600. doi: 10.1007/s00401-006-0035-2. [DOI] [PubMed] [Google Scholar]
- 56.Martins D.F., Mazzardo-Martins L., Gadotti V.M., Nascimento F.P., Lima D.A., Speckhann B., Favretto G.A., Bobinski F., Cargnin-Ferreira E., Bressan E., et al. Ankle joint mobilization reduces axonotmesis-induced neuropathic pain and glial activation in the spinal cord and enhances nerve regeneration in rats. Pain. 2011;152:2653–2661. doi: 10.1016/j.pain.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 57.Martins D.F., Martins T.C., Batisti A.P., Leonel L.D.S., Bobinski F., Belmonte L.A.O., Mazzardo-Martins L., Cargnin-Ferreira E., Santos A.R.S. Long-Term Regular Eccentric Exercise Decreases Neuropathic Pain-like Behavior and Improves Motor Functional Recovery in an Axonotmesis Mouse Model: The Role of Insulin-like Growth Factor-1. Mol. Neurobiol. 2017;55:6155–6168. doi: 10.1007/s12035-017-0829-3. [DOI] [PubMed] [Google Scholar]
- 58.Michel R.N., Gardiner P.F. Influence of overload on recovery of rat plantaris from partial denervation. J. Appl. Physiol. 1989;66:732–740. doi: 10.1152/jappl.1989.66.2.732. [DOI] [PubMed] [Google Scholar]
- 59.Sarikcioglu L., Oguz N. Exercise Training and Axonal Regeneration After Sciatic Nerve Injury. Int. J. Neurosci. 2001;109:173–177. doi: 10.3109/00207450108986533. [DOI] [PubMed] [Google Scholar]
- 60.Seburn K.L., Gardiner P.F. Properties of sprouted rat motor units: Effects of period of enlargement and activity level. Muscle Nerve. 1996;19:1100–1109. doi: 10.1002/(SICI)1097-4598(199609)19:9<1100::AID-MUS4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Seo T.B., Han I.S., Yoon J.-H., Hong K.-E., Yoon S.-J., Namgung U. Involvement of Cdc2 in Axonal Regeneration Enhanced by Exercise Training in Rats. Med. Sci. Sports Exerc. 2006;38:1267–1276. doi: 10.1249/01.mss.0000227311.00976.68. [DOI] [PubMed] [Google Scholar]
- 62.Sinis N., Guntinas-Lichius O., Irintchev A., Skouras E., Kuerten S., Pavlov S.P., Schaller H.E., Dunlop S.A., Angelov D.N. Manual stimulation of forearm muscles does not improve recovery of motor function after injury to a mixed peripheral nerve. Med. Sci. Sports Exerc. 2006;38:1267–1276. doi: 10.1007/s00221-007-1174-y. [DOI] [PubMed] [Google Scholar]
- 63.Skouras E., Merkel D., Grosheva M., Angelova S.K., Schiffer G., Thelen U., Kaidoglou K., Sinis N., Igelmund P., Dunlop S.A., et al. Manual stimulation, but not acute electrical stimulation prior to reconstructive surgery, improves functional recovery after facial nerve injury in rats. Restor. Neurol. Neurosci. 2009;27:237–251. doi: 10.3233/RNN-2009-0474. [DOI] [PubMed] [Google Scholar]
- 64.Sobral L.L., Oliviera L.S., Takeda S.Y.M., Somazz M.C., Montebelo M.I.L., Teodori R.M. Immediate versus later exercises for rat sciatic nerve regeneration after axonotmesis: Histomorphometric and functional analyses. Rev. Bras. Fisioter. 2008;12:311–316. doi: 10.1590/S1413-35552008000400010. [DOI] [Google Scholar]
- 65.Soucy M., Seburn K., Gardiner P. Is Increased Voluntary Motor Activity Beneficial or Detrimental During the Period of Motor Nerve Regeneration/Reinnervation? Can. J. Appl. Physiol. 1996;21:218–224. doi: 10.1139/h96-018. [DOI] [PubMed] [Google Scholar]
- 66.Tam S.L., Archibald V., Jassar B., Tyreman N., Gordon T. Increased Neuromuscular Activity Reduces Sprouting in Partially Denervated Muscles. J. Neurosci. 2001;21:654–667. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teodori R.M., Betini J., de Oliveira L.S., Sobral L.L., Takeda S.Y.M., Montebelo M.I.D.L. Swimming Exercise in the Acute or Late Phase after Sciatic Nerve Crush Accelerates Nerve Regeneration. Neural Plast. 2011;2011:783901. doi: 10.1155/2011/783901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Udina E., Puigdemasa A., Navarro X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve. 2011;43:500–509. doi: 10.1002/mus.21912. [DOI] [PubMed] [Google Scholar]
- 69.van Meeteren N., Brakkee J., Helders P., Croiset G., Gispen W., Wiegant V. Recovery of function after sciatic nerve crush lesion in rats selected for diverging locomotor activity in the open field. Neurosci. Lett. 1997;238:131–134. doi: 10.1016/S0304-3940(97)00870-7. [DOI] [PubMed] [Google Scholar]
- 70.van Meeteren N.L., Brakkee J.H., Hamers F.P., Helders P.J., Gispen W.H. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch. Phys. Med. Rehabilitation. 1997;78:70–77. doi: 10.1016/S0003-9993(97)90013-7. [DOI] [PubMed] [Google Scholar]
- 71.Wernig A., Salvini T.F., Irintchev A. Axonal sprouting and changes in fibre types after running-induced muscle damage. J. Neurocytol. 1991;20:903–913. doi: 10.1007/BF01190468. [DOI] [PubMed] [Google Scholar]
- 72.Gordon T., Amirjani N., Edwards D.C., Chan K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010;223:192–202. doi: 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Inoue M., Katsumi Y., Itoi M., Hojo T., Nakajima M., Ohashi S., Oi Y., Kitakoji H. Direct Current Electrical Stimulation of Acupuncture Needles for Peripheral Nerve Regeneration: An Exploratory Case Series. Acupunct. Med. 2011;29:88–93. doi: 10.1136/aim.2010.003046. [DOI] [PubMed] [Google Scholar]
- 74.Kluding P.M., Pasnoor M., Singh R., Jernigan S., Farmer K., Rucker J., Sharma N.K., Wright D.E. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J. Diabetes its Complicat. 2012;26:424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lange-Maia B.S., Cauley J.A., Newman A.B., Boudreau R.M., Jakicic J.M., Glynn N.W., Zivkovic S., Dam T.-T.L., Caserotti P., Cawthon P.M., et al. Sensorimotor Peripheral Nerve Function and Physical Activity in Older Men. J. Aging Phys. Act. 2016;24:559–566. doi: 10.1123/japa.2015-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mennen U. End-to-side Nerve Suture in Clinical Practice. Hand Surg. 2003;8:33–42. doi: 10.1142/S0218810403001352. [DOI] [PubMed] [Google Scholar]
- 77.Piccinini G., Cuccagna C., Caliandro P., Coraci D., Germanotta M., Bs C.P., Padua L. Efficacy of electrical stimulation of denervated muscle: A multicenter, double-blind, randomized clinical trial. Muscle Nerve. 2020;61:773–778. doi: 10.1002/mus.26880. [DOI] [PubMed] [Google Scholar]
- 78.Wong J.N., Olson J.L., Morhart M.J., Chan K.M. Electrical stimulation enhances sensory recovery: A randomized controlled trial. Ann. Neurol. 2015;77:996–1006. doi: 10.1002/ana.24397. [DOI] [PubMed] [Google Scholar]
- 79.Cobianchi S., de Cruz J., Navarro X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp. Neurol. 2014;255:1–11. doi: 10.1016/j.expneurol.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 80.Ying Z., Roy R.R., Edgerton V., Gómez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987:93–99. doi: 10.1016/S0006-8993(03)03258-X. [DOI] [PubMed] [Google Scholar]
- 81.Boyd J.G., Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur. J. Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 82.Lolis A.M., Falsone S., Beric A. Handbook of Clinical Neurology. Volume 158. Elsevier; Amsterdam, The Netherlands: 2018. Common peripheral nerve injuries in sport: Diagnosis and management; pp. 401–419. [DOI] [PubMed] [Google Scholar]