Abstract
Cadmium (Cd) is a toxic metal that accumulates in kidneys, especially in the proximal tubular epithelial cells, where virtually all proteins in the glomerular ultrafiltrate are reabsorbed. Here, we analyzed archived data on the estimated glomerular filtration rate (eGFR) and excretion rates of Cd (ECd), total protein (EProt), albumin (Ealb), β2-microglobulin (Eβ2M), and α1-microglobulin (Eα1M), which were recorded for residents of a Cd contamination area and a low-exposure control area of Thailand. Excretion of Cd and all proteins were normalized to creatinine clearance (Ccr) as ECd/Ccr and EProt/Ccr to correct for differences among subjects in the number of surviving nephrons. Low eGFR was defined as eGFR ≤ 60 mL/min/1.73 m2, while proteinuria was indicted by EPro/Ccr ≥ 20 mg/L of filtrate. EProt/Ccr varied directly with ECd/Ccr (β = 0.263, p < 0.001) and age (β = 0.252, p < 0.001). In contrast, eGFR values were inversely associated with ECd/Ccr (β = −0.266, p < 0.001) and age (β = −0.558, p < 0.001). At ECd/Ccr > 8.28 ng/L of filtrate, the prevalence odds ratios for proteinuria and low eGFR were increased 4.6- and 5.1-fold, respectively (p < 0.001 for both parameters). Thus, the eGFR and tubular protein retrieval were both simultaneously diminished by Cd exposure. Of interest, ECd/Ccr was more closely correlated with EProt/Ccr (r = 0.507), Eβ2M (r = 0.430), and Eα1M/Ccr (r = 0.364) than with EAlb/Ccr (r = 0.152). These data suggest that Cd may differentially reduce the ability of tubular epithelial cells to reclaim proteins, resulting in preferential reabsorption of albumin.
Keywords: albumin, albumin-to-creatinine ratio, α1-microglobulin, β2-microglobulin, cadmium, creatinine clearance, estimated glomerular filtration rate, protein reabsorption, tubulopathy, urine total protein
1. Introduction
Cadmium (Cd) is an environmental contaminant of continuing public health concern worldwide because it is detectable in most food types; as such, diet forms the main source of exposure in non-occupationally exposed and non-smoking populations [1,2]. Multiple organ systems, including kidneys [1,2], bone [3], liver [4,5] and the central nervous system [6], are susceptible to the toxicity of Cd, even at low body burdens. The cytotoxicity of Cd has been demonstrated in nearly all cell types, such as erythrocytes and the tubular epithelial cells of kidneys, which are known to actively accumulate Cd [1,2,7].
The pivotal role played by kidney tubular epithelial cells in reuptake and excretion of proteins is gaining support from recent research data [8,9,10,11,12,13]. An approximate 40–50 g of protein may reach the urinary space daily, and virtually all of it is reabsorbed [8,9,10,11,12,13]. The majority of protein in the glomerular ultrafiltrate is retrieved in the S1 sub-segment of the proximal tubule, where the receptor-mediated endocytosis involving the megalin/cubillin system is involved [8,9]. Protein reabsorption occurs also in the distal tubule and the collecting duct, where the process is mediated by the neutrophil gelatinase-associated lipocalin (NGAL)/lipocalin-2 receptor system [14,15,16].
The protein albumin, with a molecular weight of 66 kDa, is synthesized in the liver and ordinarily secreted into the circulation at a rate of 10–15 g per day [12,13]. Catabolism in muscle, the liver, and the kidney proximal tubular epithelial cells balance synthesis, and homeostasis is continued. In good health, the plasma concentration of albumin is between 3.5 g/dL and 5 g/dL, and the average half-life in plasma is 19 days [12,13]. Albumin is not normally filtered by glomeruli, due to its large molecular weight and its negative charge. By means of transcytosis through endothelial cells and podocyte foot processes, albumin enters the urinary space at a rate of 1–10 g per day [8,9,10,11,12,13,17].
Albuminuria is diagnosed when excretion of albumin, measured as albumin-to-creatinine ratio, rises to levels above 20 and 30 mg/g creatinine in men and women, respectively [18,19,20]. The persistence of albuminuria for at least three months is a diagnostic criterion of CKD. A progressive decrease in the eGFR below 60 mL/min/1.73 m2, termed low eGFR, is also a diagnostic criterion of CKD [18,19,20].
An elevated excretion of low-molecular-weight proteins, namely, retinol binding protein, β2-microglobulin (β2M), and α1-microglobulin (α1M), has been the most frequently investigated [1,2]. The protein β2M, with a molecular weight of 11,800 Da, is synthesized and shed by all nucleated cells in the body [21]. By virtue of its small mass, β2M is filtered freely by the glomeruli and is reabsorbed almost completely by the kidney’s tubular cells [22]. Cd has been shown to cause a reduction in the tubular maximum reabsorption of β2M [23], and increased β2M excretion has been used as an indicator of tubulopathy for many decades. However, our previous study showed that β2M excretion of 100–299, 300–999, and ≥ 1000 μg/g creatinine was associated with 4.7-, 6.2-, and 10.5-fold increases in the risk of an estimated glomerular filtration rate (eGFR) ≤ 60 mL/min/1.73 m2, which is commensurate with CKD [24]. These data suggest that an increased excretion of β2M above 300 µg/day, termed β2-microglobunuria, could be a consequence of Cd-induced tubulopathy in conjunction with nephron loss, evident from a reduction in the eGFR to 60 mL/min/1.73 m2 or below [24].
The present study aimed to evaluate the concurrent effects of Cd accumulation in kidneys on GFR reduction and excretion rates of proteins of high and low molecular weights, namely, total protein, albumin, β2M, and α1M. To enable an accurate assessment of these effects of Cd, we normalized excretion of Cd and all excreted proteins to creatinine clearance (Ccr). This Ccr-normalization corrects for differences in the number of surviving nephrons among study subjects, and it depicts an amount of a given chemical excreted per volume of filtrate, which is at least roughly related to the amount of the chemical excreted per nephron [25].
2. Results
2.1. Descriptive Characteristics of Study Subjects According to eGFR
Among 405 cohort participants, 190 (46.9%) were residents of a low-exposure area (Bangkok), and 215 (53.1%) persons lived in a high-exposure area of Mae Sot District, where Cd pollution was endemic (Table 1).
Table 1.
Descriptive characteristics of the study subjects according to eGFR levels.
| Parameters | All Subjects n = 405 |
eGFR a, mL/min/1.73 m2 | p | ||
|---|---|---|---|---|---|
| >90, n = 207 | 61–90, n = 147 | ≤60, n = 51 | |||
| Low-exposure controls (%) | 46.9 | 84.1 | 10.9 | 0 | <0.001 |
| Females (%) | 51.4 | 48.3 | 56.5 | 49.0 | 0.299 |
| Smoking (%) | 45.9 | 32.9 | 56.5 | 68.6 | <0.001 |
| Diabetes (%) | 2.7 | 0 | 4.8 | 7.8 | 0.001 |
| Hypertension (%) | 13.8 | 2.9 | 19.7 | 41.2 | <0.001 |
| Age, years | 44.6 ± 16.2 | 33.2 ± 9.9 | 53.2 ± 11.5 | 65.6 ± 10.6 | <0.001 |
| eGFR, mL/min/1.73 m2 | 87.3 ± 23.3 | 106.0 ± 10.1 | 75.5 ± 8.2 | 45.4 ± 11.3 | <0.001 |
| Plasma creatinine, mg/dL | 0.98 ± 0.29 | 0.84 ± 0.1392 | 0.98 ± 0.14 | 1.50 ± 0.44 | <0.001 |
| Urine creatinine, mg/dL | 106 ± 68 | 92 ± 68 | 116 ± 62 | 135 ± 69 | <0.001 |
| Plasma total protein, g/dL | 8.04 ± 0.45 | 8.05 ± 0.45 | 7.93 ± 0.47 | − | 0.337 |
| Plasma albumin, g/dL | 4.97 ± 0.30 | 4.97± 0.31 | 4.98 ± 0.28 | − | 0.861 |
| Urine protein, mg/L | 47.18 ± 150.6 | 5.42 ± 10.33 | 50.83 ± 137.3 | 206.2 ± 307.7 | <0.001 |
| Urine protein ≥ 20 mg/L (%) | 28.6 | 3.9 | 45.6 | 80.4 | <0.001 |
| Urine Cd, µg/L | 6.54 ± 10.59 | 2.24 ± 8.38 | 9.46 ± 8.22 | 15.6 ± 15.3 | <0.001 |
| Normalized to Ecr as Ex/Ecr b | |||||
| EProt/Ecr, mg/g creatinine | 43.76 ± 132.57 | 6.52 ± 11.58 | 52.36 ± 141.78 | 170 ± 246 | <0.001 |
| EProt/Ecr ≥ 100 mg/g creatinine (%) | 8.6 | 0.5 | 8.8 | 41.2 | <0.001 |
| ECd/Ecr, µg/g creatinine | 5.81 ± 7.64 | 2.14 ± 5.43 | 8.95 ± 7.06 | 11.69 ± 9.20 | <0.001 |
| Normalized to Ccr as Ex/Ccr c | |||||
| (EProt/Ccr) × 100, mg/L filtrate | 60.2 ± 236.5 | 5.32 ± 9.00 | 51 ± 134 | 310 ± 568 | <0.001 |
| (EProt/Ccr) × 100 ≥ 20 mg/L (%) | 30.6 | 3.9 | 49 | 86.3 | <0.001 |
| (ECd/Ccr) × 100, µg/L filtrate | 6.21 ± 9.00 | 1.70 ± 4.55 | 8.67 ± 6.85 | 17.44 ± 14.17 | <0.001 |
n, number of subjects; eGFR, estimated glomerular filtration rate; Ex, excretion of x; cr, creatinine; Ccr, creatinine clearance; Prot, protein; Cd, cadmium; a eGFR, was determined by equations of the Chronic Kidney Disease Epidemiology Collaboration [20]. b Ex/Ecr = [x]u/[cr]u; c Ex/Ccr = [x]u[cr]p/[cr]u, where x = Prot or Cd [25]. Data for all continuous variables are arithmetic means ± standard deviation (SD). Data for plasma protein and plasma albumin are from 190 subjects of the low-exposure control group. Data for all other variables are from all subjects (n = 405). For all tests, p ≤ 0.05 identifies statistical significance, determined by Pearson chi-square test for % differences and by Kruskal–Wallis test for mean differences across three eGFR subsets.
The overall mean age was 44.6 years. Of the total participants, 45.9% were smokers, including those who had quit less than 10 years ago. The percentages of females, hypertension, and diabetes were 51.4%, 13.8%, and 2.7%, respectively. The overall % of subjects with proteinuria, defined as urine protein ≥ 20 mg/L was 28.6%. The % of proteinuria was 30.6% when protein excretion (EProt) was normalized to Ccr and EProt/Ccr × 100 ≥ 20 mg/L was a cutoff value.
By eGFR stratification, 207 (51.1%), 147 (36.3%), and 51 (12.6%) had eGFR values > 90, 61–90, and ≤60, mL/min/1.73 m2, respectively. For simplicity, the eGFR values > 90, 61–90, and ≤60 mL/min/1.73 m2 were referred to as high, moderate, and low, respectively. The distributions of men and women across these three eGFR groups were similar. The low-eGFR group was the oldest, with the highest % of smoking, diabetes, and hypertension.
The % of proteinuria (urine protein ≥ 20 mg/L) in the low-, moderate-, and high-eGFR groups were 80.4%, 45.6%, and 3.9%, respectively (p < 0.001). The corresponding percentages of proteinuria were 86.3%, 49%, and 3.9% when (EProt/Ccr) × 100 ≥ 20 mg/L filtrate was defined as proteinuria.
Mean plasma creatinine, mean urine creatinine, mean urine protein, and mean urine Cd concentrations all were highest, middle, and lowest in the low-, moderate-, and high-eGFR groups, respectively (p < 0.001). Mean plasma total protein and mean plasma albumin in the high- and moderate-eGFR groups did not differ. Mean values of ECd/Ecr and EProt/Ecr were highest, middle, and lowest in the low-, moderate-, and high-eGFR groups, as were mean values of ECd/Ccr and EProt/Ccr (p < 0.001).
2.2. Predictors of Protein Excretion
Table 2 provides results of the multiple regression modeling of protein excretion as log[(EProt/Ccr × 105) where Cd excretion was incorporated as log[(ECd/Ccr × 105) along with other five independent variables (age, diabetes, sex, hypertension, and smoking).
Table 2.
Multiple regression analyses to evaluate strength of association of log[(EProt/Ccr × 105) with log log[(ECd/Ccr × 105) and other independent variables.
| Independent Variables/ Factors |
Urinary Excretion of Protein a | |||||
|---|---|---|---|---|---|---|
| All subjects, n = 405 | Males, n = 197 | Females, n = 208 | ||||
| β b | p | β | p | β | p | |
| Age, years | 0.263 | <0.001 | 0.222 | 0.028 | 0.260 | 0.011 |
| Log [(ECd/Ccr) × 105], µg/L filtrate | 0.252 | <0.001 | 0.376 | <0.001 | 0.179 | 0.050 |
| Diabetes | −0.039 | 0.353 | 0.012 | 0.831 | −0.097 | 0.114 |
| Sex | 0.078 | 0.107 | − | − | − | − |
| Hypertension | −0.065 | 0.152 | −0.116 | 0.069 | −0.002 | 0.974 |
| Smoking | −0.075 | 0.150 | 0.007 | 0.911 | −0.152 | 0.040 |
| Adjusted R2 | 0.306 | <0.001 | 0.371 | <0.001 | 0.259 | <0.001 |
n, number of subjects; a urinary excretion of protein as log[(EProt/Ccr) ×105]; b β, standardized regression coefficients. Coding: female = 1, male = 2, hypertension = 1, normotension = 2, smoker = 1, non-smoker = 2. Data were generated from regression model analyses relating EProt to six independent variables (first column) in all subjects, males, and females. For all tests, p-values < 0.05 indicate a statistical significance association. β coefficients indicate the strength of association of EProt and independent variables. Adjusted R2 indicates the proportion of the variation in EProt attributable to all six independent variables.
Age, ECd/Ccr, diabetes, sex, hypertension, and smoking together accounted for 30.6%, 37%, and 25.9% of total variation in EProt/Ccr among all subjects (p < 0.001), men (p < 0.001), and women (p < 0.001), respectively. Age and ECd/Ccr were independently associated with EProt/Ccr in men and women. A positive association of EProt/Ccr and ECd/Ccr was stronger in men (β = 0.376, p < 0.001) than women (β = 0.179, p = 0.050).
An additional regression analysis of EProt/Ccr was undertaken to assess a potential influence of plasma protein/albumin levels using data from 190 subjects of the low-exposure group. Plasma protein, plasma albumin, age, log [(ECd/Ccr) × 105], sex, and smoking altogether did not explain the EProt/Ccr variability (adjusted R2 = 0.008, p = 0.281). Associations of EProt/Ccr with plasma protein, plasma albumin, and other independent variables were all not significant (p > 0.05).
2.3. Predictors of eGFR Deterioration
Table 3 provides results of multiple regression models of eGFR in which Cd excretion as log[(ECd/Ccr × 105) was incorporated together with five over independent variables (age, diabetes, sex, hypertension, and smoking).
Table 3.
Multiple regression analyses to determine strength of association of eGFR with log[(ECd/Ccr × 105) and other independent variables.
| Independent Variables/ Factors |
eGFR, mL/min/1.73 m2 a | |||||
|---|---|---|---|---|---|---|
| All Subjects, n = 405 | Males, n = 197 | Females, n = 208 | ||||
| β b | p | β | p | β | p | |
| Age, years | −0.558 | <0.001 | −0.603 | <0.001 | −0.504 | <0.001 |
| Log [(ECd/Ccr) × 105], µg/L filtrate | −0.266 | <0.001 | −0.178 | 0.012 | −0.334 | <0.001 |
| Diabetes | 0.034 | 0.256 | 0.048 | 0.246 | 0.033 | 0.441 |
| Sex | −0.049 | 0.155 | − | − | − | − |
| Hypertension | 0.084 | 0.010 | 0.158 | 0.001 | 0.012 | 0.790 |
| Smoking | −0.043 | 0.247 | −0.061 | 0.168 | 0.016 | 0.750 |
| Adjusted R2 | 0.650 | <0.001 | 0.681 | <0.001 | 0.635 | <0.001 |
n, number of subjects; a eGFR was determined by equations of the Chronic Kidney Disease Epidemiology Collaboration [20]; b β, standardized regression coefficients. Coding: female = 1, male = 2, hypertension = 1, normotension = 2, smoker = 1, non-smoker = 2. Data were generated from regression model analyses relating eGFR to six independent variables (first column) in all subjects, males, and females. ECd was as log [(ECd/Ccr) × 105]. For all tests, p-values < 0.05 indicate a statistical significance association. β coefficients indicate strength of association of eGFR and independent variables. Adjusted R2 indicates the proportion of the variation in eGFR attributable to all six independent variables.
Age, ECd/Ccr, diabetes, sex, hypertension, and smoking together accounted for 65%, 68%, and 63.5% of total variation in eGFR among all subjects (p < 0.001), men (p < 0.001), and women (p < 0.001), respectively. Lower eGFR values were associated with older age (β = −0.558, p < 0.001) and higher ECd/Ccr (β = −0.266, p < 0.001). An inverse relationship between eGFR and ECd/Ccr Cd excretion rates in women (β = −0.334, p < 0.001) was stronger than men (β = −0.178, p = 0.012).
2.4. Prevalence Odds Ratios for Proteinuria and Low eGFR in Relation to Cadmium Exposure
Table 4 provides results of logistic regression modeling where the prevalence odds ratios (POR) for proteinuria and low eGFR were determined for subjects of three Cd exposure groups. The independent variables included age, diabetes, sex, hypertension, and smoking.
Table 4.
Prevalence odds ratios for proteinuria and low eGFR in relation to cadmium excretion and other variables.
| Parameters | Number of Subjects | Proteinuria a | Low eGFR b | ||
|---|---|---|---|---|---|
| POR (95% CI) | p | POR (95% CI) | p | ||
| Age, years | 405 | 0.923 (0.897, 0.949) | <0.001 | 0.888 (0.854, 0.924) | <0.001 |
| Diabetes | 11 | 0.726 (0.181, 2.916) | 0.652 | 0.582 (0.119, 2.861) | 0.506 |
| Sex (females) | 208 | 1.030 (0.539, 1.971) | 0.928 | 0.775 (0.336, 1.787) | 0.550 |
| Hypertension | 56 | 0.498 (0.244, 1.017) | 0.055 | 0.363 (0.159, 0.826) | 0.016 |
| Smoking | 186 | 0.778 (0.398, 1.520) | 0.462 | 1.271 (0.523, 3.092) | 0.597 |
| ECd/Ccr × 100, µg/L filtrate | |||||
| 0.04–2.71 | 203 | Referent | |||
| 2.72–8.28 | 102 | 1.252 (0.670, 2.341) | 0.482 | 4.579 (1.116, 18.79) | 0.035 |
| 8.29–63 | 100 | 4.575 (1.880, 11.13) | 0.001 | 5.109 (2.093, 12.47) | <0.001 |
POR, prevalence odds ratio; CI, confidence interval. Coding: female = 1, male = 2, hypertensive = 1, normotensive = 2, smoker = 1, non-smoker = 2. a Proteinuria was defined as (EProt/Ccr) × 100 ≥ 20 mg/L filtrate; b low eGFR was defined as estimated GFR ≤ 60 mL/min/1.73 m2. Data were generated from logistic regression analyses relating POR for proteinuria and low eGFR to a set of six independent variables (first column). For all tests, p-values ≤ 0.05 indicate a statistically significant association of POR with a given independent variable. Arithmetic means (SD) of (ECd/Ccr) × 100 ranges: 0.04–2.71, 2.72–8.28, and 8.29–63 µg/L filtrate were 0.59 (0.54), 5.40 (1.71), and 18.5 (10.5) µg/L filtrate, respectively.
The POR for proteinuria was lower at younger ages (POR = 0.923, p < 0.001), as was the POR for low eGFR (POR = 0.888, p < 0.001). The POR for proteinuria was increased 4.6-fold in those with (ECd/Ccr) × 100 > 8.28 µg/L filtrate (p < 0.001). Normotension was associated with a 64% decrease in POR for low eGFR (p = 0.016). The effect of hypertension on the POR for proteinuria was insignificant (p = 0.055). A dose–effect relationship was seen between POR for low eGFR and ECd/Ccr; the POR for low eGFR was increased 4.6-fold in those with (ECd/Ccr) × 100 ranging between 2.72 and 8.28 µg/L filtrate (p = 0.035), and the POR for low eGFR rose to 5.1 at ECd/Ccr) × 100 > 8.28 µg/L filtrate (p < 0.001).
2.5. Excretion Rates of Various Proteins and Cadmum in the High-Exposure Group
We analyzed the urine composition of 215 subjects who resided in a Cd-contaminated area in an attempt to quantify the influence of the number of surviving nephrons and Cd exposure. By eGFR stratification, there were 33 (15.3%), 131 (61%), and 51 (23.7%) who had eGFR > 90, 61–90, and ≤60 mL/min/1.73 m2, respectively (Table 5).
Table 5.
Comparing excretion rates of various proteins and cadmium in the high-exposure group stratified by eGFR levels.
| Parameters | All Subjects n = 215 |
eGFR a, mL/min/1.73 m2 | p | ||
|---|---|---|---|---|---|
| >90, n = 33 | 61–90, n = 131 | ≤60, n = 51 | |||
| Age, years | 57.0 ± 11.1 | 49.4 ± 9.4 | 55.6 ± 9.6 | 65.6 ± 10.6 | <0.001 |
| BMI, kg/m2 | 21.4 ± 3.6 | 21.2 ± 3.2 | 21.3 ± 3.5 | 21.7 ± 4.3 | 0.822 |
| eGFR, mL/min/1.73 m2 | 71.6 ± 19.4 | 100.4 ± 8.3 | 74.6 ± 8.2 | 45.4 ± 11.3 | <0.001 |
| Plasma creatinine, mg/dL | 1.07 ± 0.35 | 0.79 ± 0.13 | 0.98 ± 0.14 | 1.50 ± 0.44 | <0.001 |
| Urine creatinine, mg/dL | 118.4 ± 62.2 | 99.1 ± 53.1 | 116.8 ± 60.2 | 135.2 ± 69.4 | 0.054 |
| Plasma to urine creatinine ratio | 0.0125 ± 0.0096 | 0.0116 ± 0.0097 | 0.0118 ± 0.0094 | 0.0148 ± 0.0098 | 0.034 |
| Urine Cd, µg/L | 11.85 ± 12.28 | 11.18 ± 18.70 | 10.56 ± 8.05 | 15.61 ± 15.31 | 0.079 |
| Urine β2M, mg/L | 4.92 ± 17.43 | 0.20 ± 0.36 | 1.18 ± 4.02 | 17.57 ± 32.31 | <0.001 |
| Urine α1M, mg/L | 13.09 ± 18.68 | 5.66 ± 6.17 | 8.37 ± 7.91 | 30.04 ± 30.31 | <0.001 |
| Urine albumin, mg/L | 25.57 ± 70.59 | 7.62 ± 7.29 | 22.64 ± 76.57 | 44.72 ± 73.74 | <0.001 |
| Urine protein, mg/L | 85.4 ± 199.1 | 14.9 ± 22.6 | 56.2 ± 144.6 | 206.2 ± 307.7 | <0.001 |
| Normalized to Ecr as Ex/Ecr b | |||||
| ECd/Ecr, µg/g creatinine | 10.43 ± 8.02 | 10.26 ± 10.35 | 9.98 ± 6.79 | 11.69 ± 9.20 | 0.641 |
| Eβ2M/Ecr, mg/g creatinine | 4.87 ± 16.55 | 0.23 ± 0.37 | 1.66 ± 9.72 | 16.13 ± 27.49 | <0.001 |
| Eα1M/Ecr, mg/g creatinine | 11.34 ± 15.00 | 5.78 ± 4.95 | 7.53 ± 6.30 | 24.72 ± 24.57 | <0.001 |
| EAlb/Ecr, mg/g creatinine | 23.21 ± 55.07 | 10.47 ± 15.68 | 20.71 ± 59.50 | 37.88 ± 57.23 | <0.001 |
| EProt/Ecr, mg/g creatinine | 78.25 ± 174.96 | 16.73 ± 24.54 | 57.98 ± 149.26 | 170.13 ± 246.01 | <0.001 |
| Eβ2M/Ecr, µg/g creatinine (%) | |||||
| <100 | 36.7 | 51.5 | 43.5 | 9.8 | <0.001 |
| 100–999 | 37.7 | 42.4 | 42.0 | 23.5 | <0.001 |
| 1000 | 25.6 | 6.1 | 14.5 | 66.7 | <0.001 |
| EAlb/Ecr, mg/g creatinine (%) | |||||
| <30 | 84.2 | 93.9 | 88.5 | 66.7 | <0.001 |
| 30–300 | 15.3 | 0.1 | 10.7 | 33.3 | <0.001 |
| >300 | 0.5 | 0 | 0.8 | 0 | 0.017 |
| Normalized to Ccr as Ex/Ccr c | |||||
| (ECd/Ccr) × 100, µg/L filtrate | 11.27 ± 9.89 | 8.10 ± 9.06 | 9.67 ± 6.60 | 17.44 ± 14.17 | <0.001 |
| (Eβ2M/Ccr) × 100, mg/L filtrate | 7.74 ± 29.06 | 0.18 ± 0.28 | 1.82 ± 11.58 | 27.82 ± 52.20 | <0.001 |
| (Eα1M/Ccr) × 100, mg/L filtrate | 15.00 ± 28.25 | 4.46 ± 3.59 | 7.45 ± 6.63 | 41.20 ± 48.68 | <0.001 |
| (EAlb/Ccr) × 100, mg/L filtrate | 29.06 ± 75.93 | 7.50 ± 9.83 | 20.23 ± 56.82 | 65.68 ± 119.75 | <0.001 |
| (EProt/Ccr) × 100, mg/L filtrate | 109.9 ± 316.8 | 13.0 ± 19.1 | 56.3 ± 141.0 | 310.2 ± 568.2 | <0.001 |
n, number of subjects; eGFR, estimated glomerular filtration rate; Ex, excretion of x; cr, creatinine; Ccr, creatinine clearance; Prot, protein; Cd, cadmium; a eGFR was determined by equations of the Chronic Kidney Disease Epidemiology Collaboration [20]. b Ex/Ecr = [x]u/[cr]u; c Ex/Ccr = [x]u[cr]p/[cr]u, where x = Prot or Cd [25]. Data for all continuous variables are arithmetic means ± standard deviation (SD). For all tests, p ≤ 0.05 identifies statistical significance, determined by Kruskal–Wallis test for mean differences across three eGFR ranges.
Relative to the high-eGFR group, the excretion of creatinine tended to rise in the moderate- and the low-eGFR groups (p = 0.054), while the urinary Cd concentrations (µg/L) in three eGFR groups showed no variation (p = 0.646) (Table 5). Consequently, the Cd body burdens of subjects in the three eGFR groups were not distinguishable (p = 0.079).
Distinctively, differences in body burden of Cd were apparent when ECd was normalized to Ccr; the mean ECd/Ccr was highest, middle, and lowest in the low-, moderate-, and high-eGFR groups (p < 0.001). As expected, those in the low-eGFR group excreted β2M, α1M, albumin, total protein, and Cd at the highest rates.
The % of Eβ2M/Ecr ≥ 1000 µg/g creatinine were 25.6%, and more than half (66.7%) of those with such a high Eβ2M excretion were in the low eGFR group. The % of Eβ2M/Ecr ≥ 1000 µg/g creatinine was lowest, middle, and highest in the high-, moderate-, and low-eGFR groups, respectively (p < 0.001).
Only one subject (0.5%) had EAlb/Ecr > 300 mg/g creatinine. EAlb/Ecr values in the majority of subjects (84.2%) were < 30 mg/g creatinine. Only 15.3% had microalbuminuria (EAlb/Ecr values of 30–300 mg/g creatinine). The % of those with microalbuminuria was lowest, middle, and highest in the high-, moderate-, and low-eGFR groups, respectively (p < 0.001).
2.6. Correlation Analysis of Age, BMI, and Chemical Constiuents of Urine
We next undertook a correlation analysis of nine parameters that included age, BMI, excretion of β2M, α1M, albumin, total protein, and Cd (Table 6).
Table 6.
The Pearson correlation analysis of chemical compositions of urine samples from 215 residents of a high-exposure area.
| Variables | Age | BMI | ECd/Ccr | EProt/Ccr | EAlb/Ccr | Eβ2M/Ccr | Eα1M/Ccr |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| BMI | −0.248 *** | ||||||
| ECd/Ccr | 0.778 *** | −0.050 | |||||
| EProt/Ccr | 0.516 *** | −0.133 | 0.507 *** | ||||
| EAlb/Ccr | 0.320 *** | −0.120 | 0.152 * | 0.546 *** | |||
| Eβ2M/Ccr | 0.421 *** | −0.184 ** | 0.430 *** | 0.508 *** | 0.537 *** | ||
| Eα1M/Ccr | 0.368 *** | −0.164 * | 0.364 *** | 0.508 *** | 0.653 *** | 0.825 *** | |
| Creatinine | 0.152 ** | 0.084 | 0.169 ** | 0.217 *** | −0.024 | −0.067 | 0.015 |
Numbers are Pearson’s correlation coefficients. * p = 0.016–0.026, ** p = 0.001–0.007, *** p < 0.001.
With the exception of BMI, age correlated positively with all other variables. BMI was negatively correlated with age, Eβ2M/Ccr, and Eα1M/cr. The excretion of creatinine correlated positively with age, ECd/Ccr, and EProt/Ccr. This parameter did not correlate with EAlb/Ccr, Eβ2M, or Eα1M/Ccr. ECd/Ccr was more closely correlated with EProt/Ccr (r = 0.507), Eβ2M (r = 0.430), and Eα1M/Ccr (r = 0.364) than with EAlb/Ccr.
Among three proteins quantified, β2M and α1M represented low-molecular-weight proteins. Albumin represented a high-molecular-weight protein, while urinary total protein was a measure of all proteins. As expected, EPro/Ccr was highly correlated with all three proteins, β2M, α1M, and albumin (r = 0.508 − 0.546).
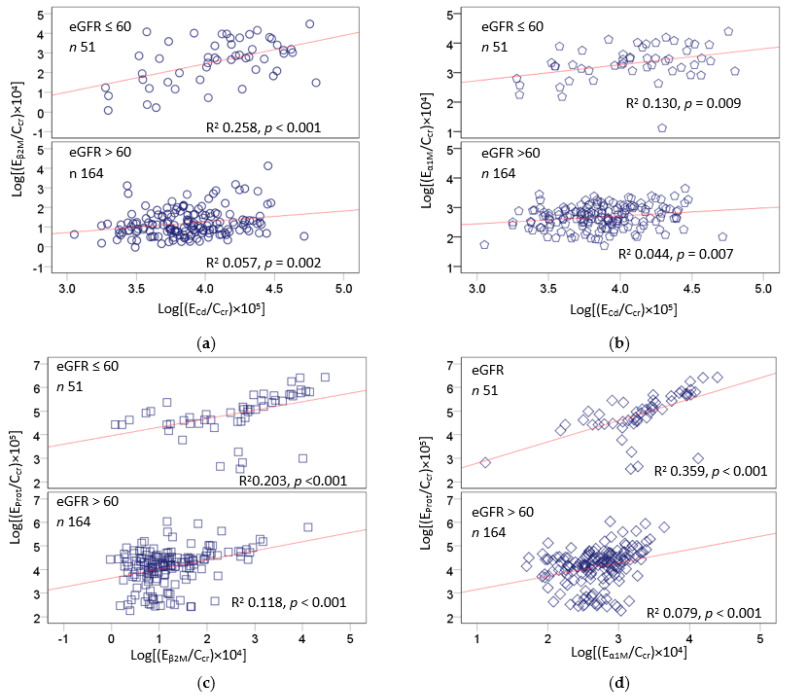
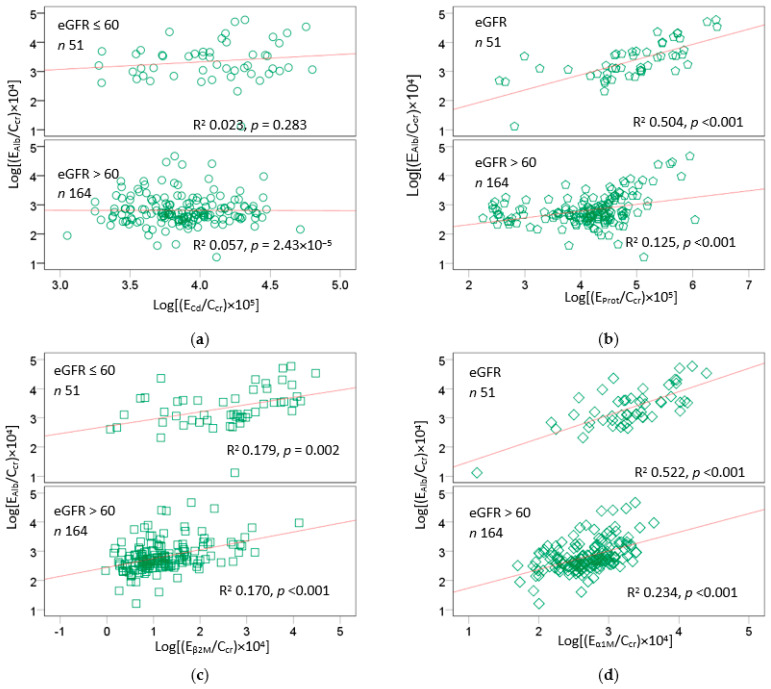
Figure 1 and Figure 2 provide scatterplots showing differential effects of Cd exposure on protein excretion in subsets of subjects stratified by nephron mass, based on eGFR > 60 and ≤60 mL/min/1.73 m2.
Figure 1.
Effects of cadmium exposure and nephron mass on excretion of β2-microglobulin and α1-microglobulin. Scatterplot (a) relates log[(EβM/Ccr) × 104] to log[(ECd/Ccr) × 105]. Scatterplot (b) relates log[(Eα1M/Ccr) × 104] to log[(ECd/Ccr) × 105]. Scatterplot (c) relates log[(EProt/Ccr) × 105] to log[(Eβ2M/Ccr) × 104]. Scatterplot (d) relates log[(EProt/Ccr) × 105] to log[(Eα1M/Ccr) × 104] (d). Units of Eβ2M/Ccr, Eα1M/Ccr, and EProt/Ccr are mg/L of filtrate, and the unit of ECd/Ccr is µg/L of filtrate. Coefficients of determination (R2) and p-values are provided for all scatterplots.
Figure 2.
Effects of cadmium exposure and nephron mass on excretion of albumin. Scatterplot (a) relates log[(EAlb/Ccr) × 104] to log[(ECd/Ccr) × 105]. Scatterplot (b) relates log[(EAlb/Ccr) × 104] to log[(EProt/Ccr) × 105]. Scatterplot (c) relates log[(EAlb/Ccr) × 104] to log[(Eβ2M/Ccr) × 104]. Scatterplot (d) relates log[(EAlb/Ccr) × 104] to log[(Eα1M/Ccr) × 104]. Units of EAlb/Ccr, Eβ2M/Ccr, and Eα1M/Ccr are mg/L of filtrate, and the unit of ECd/Ccr is µg/L of filtrate. Coefficients of determination (R2) and p-values are provided for all scatterplots.
Stratification of subjects by eGFR uncovered differences in renal handling of albumin vs. β2M and α1M in response to Cd. Higher Eβ2M and Eα1M values were associated with higher ECd in both eGFR groups (Figure 1a,b). The associations of Eβ2M, Eα1M, and ECd all were stronger in the low-eGFR group than the high-eGFR group. In comparison, the relationship between EAlb and ECd was insignificant in both eGFR groups (Figure 2a). An association between EAlb and EProt was particularly strong in the low-eGFR group (Figure 2b), as were the associations of EAlb and Eα1M (Figure 2d) and of EProt and Eα1M (Figure 1d). In comparison, the relationships between EAlb and Eβ2M were similar in the two eGFR groups (Figure 2c), as were the relationships between EProt and Eβ2M (Figure 1c).
3. Discussion
A defective tubular reabsorption of the low-molecular-weight filterable protein β2M, referred to as tubular proteinuria, has been the most frequently reported adverse effect of environmental exposure to Cd. Analytical and biochemical epidemiologic research dissecting Cd-induced proteinuria is lacking. As demonstrated in the present study, mechanistic insights into proteinuria following long-term exposure to Cd and its accumulation in tubular epithelial cells of kidneys emerged when excretion rates of various proteins and Cd were normalized to creatinine (Ccr). Ccr-normalization corrects for differences among subjects in number of surviving nephrons. A conventional method of normalization of the rate of excretion of a given substance to creatinine excretion (Ecr) corrects for urine dilution, but it introduces high variance into the dataset, because Ecr is not related to Cd exposure nor the function of kidneys. This has led to erroneous data interpretations in the past.
As shown in Table 5, the exposure levels of Cd, measured as ECd/Ecr among groups of residents of a high-exposure area, did not differ statistically (p = 0.641); subjects in all three eGFR groups excreted a similarly high ECd/Ecr of 10 µg/g creatinine. As a result of the indistinguishable body burden, a dose–effect relationship between excretion of various proteins and a Cd exposure measure could not be established.
In comparison, Cd exposure, measured as ECd/Ccr, increased from 8.1 to 9.7 and then 17 µg/L of filtrate in subjects with high, moderate, and low eGFR, respectively (p < 0.001). Excretion rates of total protein, Alb, β2M, and α1M all increased in parallel to ECd/Ccr increment. In effect, a clear dose–response between ECd/Ccr and excretion rates of total protein, Alb, β2M, and α1M was evident.
A further analysis of the relationship of the exposure measure (ECd/Ccr) and excretion rates of total protein, Alb, β2M, and α1M, shown in Figure 1 and Figure 2, provided further evidence for preferential reabsorption of albumin. An association between EAlb and ECd was absent in both low- and high-eGFR groups (Figure 2a). This finding may be interpreted to suggest that most Alb is reabsorbed, consistent with a current view that albumin is reabsorbed almost completely and that a fraction of it is returned to the systemic circulation [10,11,12,13,14,15].
In an experimental study, Cd was found to disable the cubilin/megalin receptor system of albumin endocytosis, leading to albuminuria [26]. In addition, Cd diminished expression of megalin and ClC5 channels [27]. Cd may also increase glomerular permeability to albumin, as shown in another study, where a non-cytotoxic concentration of Cd (1 µM) increased the permeability of human renal glomerular endothelial cells in monolayers and caused the redistribution of the adherens junction proteins vascular endothelial-cadherin and β-catenin [28,29].
Of concern, CKD is a progressive syndrome with high morbidity and mortality and affects 8% to 16% of the world’s population, with increasing incidence worldwide [30,31,32,33]. Here, we have shown that Cd simultaneously increased risks of low eGFR and proteinuria 4- to 5-fold (Table 4). Indeed, associations between Cd exposure and low eGFR and albuminuria were repeatedly observed among participants in the U.S. National Health and Nutrition Examination Survey (NHANES) undertaken between 1999 and 2016 [34,35,36,37]. Increased Ealb was associated with a urinary Cd level as low as 0.22 μg/L in a study of participants in NHANES 2009–2012, aged ≥20 years [38], while an increase in risk of CKD among NHANES 1999–2006 was associated with ECd/Ecr values ≥ 1 µg/g creatinine [34]. In a Spanish population study, a urinary Cd excretion (ECd) of 0.27 µg/g creatinine was associated with an increase in the risk of albuminuria by 58% [39]. In another study, ECd > 1.72 µg/g creatinine was associated with elevated Ealb in Shanghai residents [40]. In a systematic and meta-analysis of pooled data from 28 studies, Cd exposure was found to increase the risk of proteinuria by 48% [41]. The pathogenesis of Cd-induced proteinuria, especially in low environmental Cd exposure conditions, requires further study, especially in experimentation, given that the glomerular and tubular causes of albuminuria may not be distinguishable in epidemiologic studies.
The results of the present study have strongly implicated Cd exposure as a risk factor for CKD. Minimization/avoidance of Cd exposure from smoking and habitual consumption of foods containing high levels of the metal are warranted. Kidney fibrosis after chronic exposure to Cd has been demonstratable in experimental studies [42,43]. Evidence from the synchrotron imaging of metals in human kidney tissue samples is in line with Cd-induced fibrosis [44]. The degree of tubular atrophy was positively associated with the level of Cd accumulation in a histopathological examination of kidney biopsies from healthy kidney transplant donors [45]. A prospective cohort study of Japanese residents in an area similarly polluted by Cd reported a 49% increase in mortality from kidney failure among women after adjustment for potential confounding factors [46].
4. Materials and Methods
4.1. Participants
We assembled data from 405 persons (197 men and 208 women) who participated in the larger population-based studies undertaken in a low-exposure area (Bangkok), and an endemic area of Cd contamination in the Mae Sot District, Tak Province, Thailand [47]. The Institutional Ethical Committee, Chiang Mai University and the Mae Sot Hospital Ethical Committee approved the study protocol. At the time of recruitment, all participants had lived at their current addresses for at least 30 years, and all gave informed consent prior to their participation. Exclusion criteria were pregnancy, breastfeeding, a history of metal work, and a hospital record or physician’s diagnosis of an advanced chronic disease. Smoking, diabetes, hypertension, regular use of medications, educational level, occupation, and family health history were ascertained by questionnaire. Diabetes was defined as fasting plasma glucose levels ≥ 126 mg/dL or a physician’s prescription of anti-diabetic medications. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, a physician’s diagnosis, or prescription of anti-hypertensive medications.
4.2. Collection and Analysis of Blood and Urine Samples
Simultaneous blood and urine sampling is required to normalize ECd to Ccr. Accordingly, second morning urine samples were collected after an overnight fast, and whole blood samples were obtained within 3 h after the urine sampling. Aliquots of urine, whole blood, and plasma were stored at −20 °C or −80 °C for later analysis. The assay for urine and plasma concentrations of creatinine ([cr]u and [cr]p) was based on the Jaffe reaction. The assay of β2M in urine ([β2M]u) was based on the latex immunoagglutination method (LX test, Eiken 2MGII; Eiken and Shionogi Co., Tokyo, Japan). The assay of urinary albumin ([alb]u) was based on a turbidimetric method (UniCel® DxC800 Synchron system, Beckman Coulter, Fullerton, CA, USA).
For the Bangkok group, the urinary concentration of Cd ([Cd]u) was determined by inductively coupled plasma mass spectrometry (ICP/MS, Agilent 7500, Agilent Technologies, Santa Clara, CA, USA) because it had the high sensitivity required to measure very low Cd concentrations. Multi-element standards (EM Science, EM Industries, Inc., Newark, NJ, USA) were used to calibrate the Cd analyses. The accuracy and precision of those analyses were ascertained with reference urine (Lyphochek®, Bio-Rad, Sydney, Australia). The low limit of detection (LOD) of urine Cd, calculated as 3 times the standard deviation of blank measurements, was 0.05 µg/L. The Cd concentration assigned to samples with Cd below the detection limit was the detection limit divided by the square root of 2 [48].
For the Mae Sot group, [Cd]u was determined with atomic absorption spectrophotometry (Shimadzu Model AA-6300, Kyoto, Japan). Urine standard reference material No. 2670 (National Institute of Standards, Washington, DC, USA) was used for quality assurance and control purposes. The low limit of detection of Cd quantitation, defined as 3 times the standard deviation of blank measurements, was 0.06 µg/L. None of the urine samples contained [Cd]u below the detection limit.
4.3. Estimated Glomerular Filtration Rates and CKD Stratified by the KDIGO Categories
The GFR is the product of nephron number and mean single nephron GFR, and, in theory, GFR is indicative of nephron function [19,20,49]. In practice, the GFR is estimated from established Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations and is reported as eGFR [20,49].
Male eGFR = 141 × [plasma creatinine/0.9]Y × 0.993age, where Y = −0.411 if [cr]p ≤ 0.9 mg/dL and Y = −1.209 if [cr]p > 0.9 mg/dL. Female eGFR = 144 × [plasma creatinine/0.7]Y × 0.993age, where Y = −0.329 if [cr]p ≤ 0.7 mg/dL and Y = −1.209 if [cr]p > 0.7 mg/dL.
For dichotomous comparisons, CKD was defined as eGFR ≤ 60 mL/min/1.73 m2 or ACR > 30 mg/g creatinine. The KDIGO categories of CKD stages 1, 2, 3a, 3b, 4, and 5 corresponded to eGFR of 90–119, 60–89, 45–59, 30–44, 15–29, and <15 mL/min/1.73 m2, respectively. The KDIGO classification of ACR < 30, 30–300, and >300 mg/g creatinine corresponded to normal albumin excretion, microalbuminuria, and a severely elevated albumin excretion [19].
4.4. Normalization of ECd to Ecr and Ccr
Ex was normalized to Ecr as [x]u/[cr]u, where x = Cd; [x]u = urine concentration of x (mass/volume); and [cr]u = urine creatinine concentration (mg/dL). The ratio [x]u/[cr]u was expressed in μg/g of creatinine.
Ex was normalized to Ccr as Ex/Ccr = [x]u[cr]p/[cr]u, where x = Cd; [x]u = urine concentration of x (mass/volume); [cr]p = plasma creatinine concentration (mg/dL); and [cr]u = urine creatinine concentration (mg/dL). Ex/Ccr was expressed as the excretion of x per volume of filtrate [25].
4.5. Statistical Analysis
Data were analyzed with IBM SPSS Statistics 21 (IBM Inc., New York, NY, USA). The one-sample Kolmogorov–Smirnov test was used to identify departures of continuous variables from a normal distribution, and a logarithmic transformation was applied to variables that showed rightward skewing before they were subjected to parametric statistical analysis. The Kruskal–Wallis test was used to assess differences in means among three exposure groups. The chi-square test was used to determine differences in percentage and prevalence data. To determine strength of association of excretion rates of total protein and eGFR with independent variables, the multiple linear regression model analysis was used. The multivariable logistic regression analysis was used to determine the prevalence odds ratio (POR) for albuminuria and CKD in relation to six independent variables: age, gender, diabetes, smoking, hypertension, and Cd exposure, measured as Cd excretion (ECd).
5. Conclusions
The conventional method for adjusting excretion rates of Cd and proteins of low and high molecular weight, namely, β2M, α1M, albumin, and total protein, to creatinine excretion understate the severity of the nephrotoxicity of Cd. The body burden of Cd among residents of an area affected by Cd contamination is indistinguishable when the urinary Cd excretion levels are adjusted to creatinine excretion, thereby nullifying a dose–response relationship analysis. In comparison, normalization of excreted Cd, β2M, α1M, albumin, and total protein to creatinine clearance enables a dose–effect analysis and provides, for the first time, evidence that proteinuria after chronic Cd exposure is a manifestation of Cd-induced tubulopathy and Cd-induced nephron loss. The results of a dose–effect analysis also provide evidence that albumin is preferentially reabsorbed.
Acknowledgments
This work was supported with resources from the Kidney Disease Research Collaborative, Translational Research Institute, and the Department of Nephrology, Princess Alexandra Hospital.
Author Contributions
Conceptualization, S.S.; methodology, S.S.; formal analysis, S.S.; investigation, S.S.; resources, D.A.V. and G.C.G.; writing—original draft preparation, S.S.; writing—review and editing, D.A.V. and G.C.G.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study analyzed archived data taken from published reports [47]. Ethical review and approval were not applicable.
Informed Consent Statement
Written informed consent was obtained from all participants.
Data Availability Statement
All data are contained within this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Satarug S., Gobe G.C., Vesey D.A. Multiple targets of toxicity in environmental exposure to low-dose cadmium. Toxics. 2022;10:472. doi: 10.3390/toxics10080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satarug S., Vesey D.A., Gobe G.C., Phelps K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023 doi: 10.1007/s00204-022-03432-w. [DOI] [PubMed] [Google Scholar]
- 3.Browar A.W., Leavitt L.L., Prozialeck W.C., Edwards J.R. Levels of cadmium in human mandibular bone. Toxics. 2019;7:31. doi: 10.3390/toxics7020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branca J.J.V., Fiorillo C., Carrino D., Paternostro F., Taddei N., Gulisano M., Pacini A., Becatti M. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants. 2020;9:492. doi: 10.3390/antiox9060492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satarug S. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr. Drug Metab. 2012;13:257–271. doi: 10.2174/138920012799320446. [DOI] [PubMed] [Google Scholar]
- 6.Skipper A., Sims J.N., Yedjou C.G., Tchounwou P.B. Cadmium chloride induces DNA damage and apoptosis of human liver carcinoma cells via oxidative stress. Int. J. Environ. Res. Public Health. 2016;13:88. doi: 10.3390/ijerph13010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demchenkov E.L., Nagdalian A.A., Budkevich R.O., Oboturova N.P., Okolelova A.I. Usage of atomic force microscopy for detection of the damaging effect of CdCl2 on red blood cells membrane. Ecotoxicol. Environ. Saf. 2021;208:111683. doi: 10.1016/j.ecoenv.2020.111683. [DOI] [PubMed] [Google Scholar]
- 8.Molitoris B.A., Sandoval R.M., Yadav S.P.S., Wagner M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022;102:1625–1667. doi: 10.1152/physrev.00014.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshbach M.L., Weisz O.A. Receptor-mediated endocytosis in the proximal tubule. Annu. Rev. Physiol. 2017;79:425–448. doi: 10.1146/annurev-physiol-022516-034234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bökenkamp A. Proteinuria-take a closer look! Pediatr. Nephrol. 2020;35:533–541. doi: 10.1007/s00467-019-04454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comper W.D., Vuchkova J., McCarthy K.J. New insights into proteinuria/albuminuria. Front. Physiol. 2022;13:991756. doi: 10.3389/fphys.2022.991756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gburek J., Konopska B., Gołąb K. Renal handling of albumin-from early findings to current concepts. Int. J. Mol. Sci. 2021;22:5809. doi: 10.3390/ijms22115809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benzing T., Salant D. Insights into glomerular filtration and albuminuria. N. Engl. J. Med. 2021;384:1437–1446. doi: 10.1056/NEJMra1808786. [DOI] [PubMed] [Google Scholar]
- 14.Edwards A., Long K.R., Baty C.J., Shipman K.E., Weisz O.A. Modelling normal and nephrotic axial uptake of albumin and other filtered proteins along the proximal tubule. J. Physiol. 2022;600:1933–1952. doi: 10.1113/JP282885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langelueddecke C., Roussa E., Fenton R.A., Wolff N.A., Lee W.K., Thévenod F. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 2012;287:159–169. doi: 10.1074/jbc.M111.308296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dizi E., Hasler U., Nlandu-Khodo S., Fila M., Roth I., Ernandez T., Doucet A., Martin P.Y., Feraille E., de Seigneux S. Albuminuria induces a proinflammatory and profibrotic response in cortical collecting ducts via the 24p3 receptor. Am. J. Physiol. Renal Physiol. 2013;305:F1053–F1063. doi: 10.1152/ajprenal.00006.2013. [DOI] [PubMed] [Google Scholar]
- 17.Castrop H., Schießl I.M. Novel routes of albumin passage across the glomerular filtration barrier. Acta Physiol. 2017;219:544–553. doi: 10.1111/apha.12760. [DOI] [PubMed] [Google Scholar]
- 18.Soveri I., Berg U.B., Björk J., Elinder C.G., Grubb A., Mejare I., Sterner G., Bäck S.E., SBU GFR Review Group Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014;64:411–424. doi: 10.1053/j.ajkd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Murton M., Goff-Leggett D., Bobrowska A., Garcia Sanchez J.J., James G., Wittbrodt E., Nolan S., Sörstadius E., Pecoits-Filho R., Tuttle K. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: A Systematic review. Adv. Ther. 2021;38:180–200. doi: 10.1007/s12325-020-01568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey A.S., Becker C., Inker L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA. 2015;313:837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Argyropoulos C.P., Chen S.S., Ng Y.H., Roumelioti M.E., Shaffi K., Singh P.P., Tzamaloukas A.H. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front. Med. 2017;4:73. doi: 10.3389/fmed.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portman R.J., Kissane J.M., Robson A.M. Use of B2-microglobulin to diagnose tubulo-interstitial renal lesions in children. Kidney Int. 1986;30:91–98. doi: 10.1038/ki.1986.156. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier C., Nguyen-Simonnet H., Vincent C., Revillard J.-P., Pellet M.V. Renal tubular absorption of beta 2 micro-globulin. Kidney Int. 1984;26:170–175. doi: 10.1038/ki.1984.151. [DOI] [PubMed] [Google Scholar]
- 24.Satarug S., Vesey D.A., Nishijo M., Ruangyuttikarn W., Gobe G.C. The inverse association of glomerular function and urinary β2-MG excretion and its implications for cadmium health risk assessment. Environ. Res. 2019;173:40–47. doi: 10.1016/j.envres.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Phelps K.R., Gosmanova E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020;94:43–49. doi: 10.5414/CN110056. [DOI] [PubMed] [Google Scholar]
- 26.Santoyo-Sánchez M.P., Pedraza-Chaverri J., Molina-Jijón E., Arreola-Mendoza L., Rodríguez-Muñoz R., Barbier O.C. Impaired endocytosis in proximal tubule from subchronic exposure to cadmium involves angiotensin II type 1 and cubilin receptors. BMC Nephrol. 2013;14:211. doi: 10.1186/1471-2369-14-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gena P., Calamita G., Guggino W.B. Cadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cells. Environ. Health Perspect. 2010;118:1551–1556. doi: 10.1289/ehp.0901874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L., Dong F., Xu D., Du L., Yan S., Hu H., Lobe C.G., Yi F., Kapron C.M., Liu J. Short-term, low-dose cadmium exposure induces hyperpermeability in human renal glomerular endothelial cells. J. Appl. Toxicol. 2016;36:257–265. doi: 10.1002/jat.3168. [DOI] [PubMed] [Google Scholar]
- 29.Li Z., Jiang L., Tao T., Su W., Guo Y., Yu H., Qin J. Assessment of cadmium-induced nephrotoxicity using a kidney-on-a-chip device. Toxicol. Res. 2017;6:372–380. doi: 10.1039/C6TX00417B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols G.A., Déruaz-Luyet A., Brodovicz K.G., Kimes T.M., Rosales A.G., Hauske S.J. Kidney disease progression and all-cause mortality across estimated glomerular filtration rate and albuminuria categories among patients with vs. without type 2 diabetes. BMC Nephrol. 2020;21:167. doi: 10.1186/s12882-020-01792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George C., Mogueo A., Okpechi I., Echouffo-Tcheugui J.B., Kengne A.P. Chronic kidney disease in low-income to middle-income countries: Case Increased Screening. BMJ Glob Health. 2017;2:e000256. doi: 10.1136/bmjgh-2016-000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George C., Echouffo-Tcheugui J.B., Jaar B.G., Okpechi I.G., Kengne A.P. The need for screening, early diagnosis, and prediction of chronic kidney disease in people with diabetes in low- and middle-income countries-a review of the current literature. BMC Med. 2022;20:247. doi: 10.1186/s12916-022-02438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K., Jafar T.H., Nitsch D., Neuen B.L., Perkovic V. Chronic kidney disease. Lancet. 2021;398:786–802. doi: 10.1016/S0140-6736(21)00519-5. [DOI] [PubMed] [Google Scholar]
- 34.Ferraro P.M., Costanzi S., Naticchia A., Sturniolo A., Gambaro G. Low level exposure to cadmium increases the risk of chronic kidney disease: Analysis of the NHANES 1999–2006. BMC Publ. Health. 2010;10:304. doi: 10.1186/1471-2458-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navas-Acien A., Tellez-Plaza M., Guallar E., Muntner P., Silbergeld E., Jaar B., Weaver V. Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am. J. Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y.S., Ho W.C., Caffrey J.L., Sonawane B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014;134:33–38. doi: 10.1016/j.envres.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Madrigal J.M., Ricardo A.C., Persky V., Turyk M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2018;169:180–188. doi: 10.1016/j.envres.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X.J., Wang J.J., Mao J.H., Shu Q., Du L.Z. Relationships of cadmium, lead, and mercury levels with albuminuria in US adults: Results from the National Health and Nutrition Examination Survey Database, 2009–2012. Am. J. Epidemiol. 2019;188:1281–1287. doi: 10.1093/aje/kwz070. [DOI] [PubMed] [Google Scholar]
- 39.Grau-Perez M., Pichler G., Galan-Chilet I., Briongos-Figuero L.S., Rentero-Garrido P., Lopez-Izquierdo R., Navas-Acien A., Weaver V., García-Barrera T., Gomez-Ariza J.L., et al. Urine cadmium levels and albuminuria in a general population from Spain: A gene-environment interaction analysis. Environ. Int. 2017;106:27–36. doi: 10.1016/j.envint.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Feng X., Zhou R., Jiang Q., Wang Y., Chen C. Analysis of cadmium accumulation in community adults and its correlation with low-grade albuminuria. Sci. Total Environ. 2022;834:155210. doi: 10.1016/j.scitotenv.2022.155210. [DOI] [PubMed] [Google Scholar]
- 41.Jalili C., Kazemi M., Cheng H., Mohammadi H., Babaei A., Taheri E., Moradi S. Associations between exposure to heavy metals and the risk of chronic kidney disease: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2021;51:165–182. doi: 10.1080/10408444.2021.1891196. [DOI] [PubMed] [Google Scholar]
- 42.Thijssen S., Lambrichts I., Maringwa J., Van Kerkhove E. Changes in expression of fibrotic markers and histopathological alterations in kidneys of mice chronically exposed to low and high Cd doses. Toxicology. 2007;238:200–210. doi: 10.1016/j.tox.2007.06.087. [DOI] [PubMed] [Google Scholar]
- 43.Liang L., Huang K., Yuan W., Liu L., Zou F., Wang G. Dysregulations of miR-503-5p and Wnt/β-catenin pathway coordinate in mediating cadmium-induced kidney fibrosis. Ecotoxicol. Environ. Saf. 2021;224:112667. doi: 10.1016/j.ecoenv.2021.112667. [DOI] [PubMed] [Google Scholar]
- 44.Gobe G.C., Mott S.A., de Jonge M., Hoy W.E. Heavy metal imaging in fibrotic human kidney tissue using the synchrotron X-ray fluorescence microprobe. Transl. Androl. Urol. 2019;8:S184–S191. doi: 10.21037/tau.2019.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barregard L., Sallsten G., Lundh T., Mölne J. Low-level exposure to lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ. Res. 2022;211:113119. doi: 10.1016/j.envres.2022.113119. [DOI] [PubMed] [Google Scholar]
- 46.Nishijo M., Nogawa K., Suwazono Y., Kido T., Sakurai M., Nakagawa H. Lifetime cadmium exposure and mortality for renal diseases in residents of the cadmium-polluted Kakehashi River Basin in Japan. Toxics. 2020;8:81. doi: 10.3390/toxics8040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satarug S., Swaddiwudhipong W., Ruangyuttikarn W., Nishijo M., Ruiz P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013;121:531–536. doi: 10.1289/ehp.1104769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hornung R.W., Reed L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5:46–51. doi: 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 49.White C.A., Allen C.M., Akbari A., Collier C.P., Holland D.C., Day A.G., Knoll G.A. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin. Chim. Acta. 2019;488:189–195. doi: 10.1016/j.cca.2018.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within this article.