Abstract
This pilot study sought to identify potential markers of improvement from pre-post treatment in response to computerized working memory (WM) training for youth (ages 8–18) with autism spectrum disorder (ASD) and comorbid intellectual disability (ID) in a single arm, pre-post design. Participants included 26 children with ASD and 18 with comorbid ASD and fragile × syndrome (ASD+FXS). Analyses were adjusted for age and IQ. The ASD group demonstrated greater improvement on WM training relative to the ASD+FXS group. Participants improved on WM and far transfer outcomes, however, there were no significant group differences in improvement except for repetitive behavior. Higher hyperactivity/impulsivity ratings predicted lower performance on visuospatial WM. Findings suggest cognitive training may be beneficial for youth with ASD and ID, warranting further exploration.
Keywords: computerized training, Cogmed, cognitive training, autism, fragile × syndrome
Autism spectrum disorder (ASD) is an early onset, neurodevelopmental disorder characterized by deficits in social communication and interaction, and restricted, repetitive patterns of behavior, interests, or activities (American Psychological Association, 2013). ASD has an overall prevalence of 1.47% of children in the United States (Baio et al., 2018), and is associated with numerous adverse functional outcomes, including impairment in academic performance (Miller et al., 2017), social relationships (Mendelson et al., 2016), and daily living skills (Bal et al., 2015).
Fragile × syndrome (FXS) is the leading single-gene cause of ASD, accounting for an estimated 1% to 6% of all cases of ASD (Muhle et al., 2004; Schaefer & Mendelsohn, 2008). FXS results from a full mutation, an expansion of more than 200 trinucleotide (CGG) repeats, in the fragile × mental retardation 1 (FMR1) gene at Xq27.3 (Oostra & Willemsen, 2003). The full mutation causes a diminished or absent production of FMR1 protein (FMRP), which plays a crucial role in brain development and functioning (Bassell & Warren, 2008). Consequently, individuals with FXS typically experience many cognitive, social, and linguistic deficits, including intellectual disability (ID), language impairment, and attention deficit-hyperactivity disorder (ADHD) related behaviors (Bailey et al., 2001; Roberts et al., 2007).
Deficits in executive functioning, a broad construct of higher order cognitive processes that enable goal directed behavior and novel problem solving (Baddeley, 2007; Miyake et al., 2000) are well-documented in both ASD (Craig et al., 2016) and FXS (Schmitt et al., 2019). Among the many executive function deficits experienced by individuals with ASD and FXS, working memory (WM) has received considerable attention. Accumulating evidence indicates that WM is largely impaired in ASD (Wang et al., 2017) and FXS (Baker et al., 2011), and is strongly related to critical functional outcomes, such as academic achievement (Alloway, 2009; Friedman et al., 2018; Swanson et al., 2009), and to behavioral and genetic components of ASD and FXS, respectively. For example, poor verbal WM is associated with greater problems in adaptive behavior and more restrictive and repetitive behavior in ASD (Kercood et al., 2014). Among individuals with FXS, WM has been shown to be significantly correlated with FMRP, even after accounting for mean parental IQ, quality of the home environment, and educational services (Dyer-Friedman et al., 2002). Significant correlations have also been found between FMRP expression and frontal lobe brain activity in regions involved in WM performance (Kwon et al., 2001). Collectively, these findings suggest that WM may be a critical target for intervention.
Treatment for ASD, and especially affected persons with below average intellectual ability levels, traditionally involves an individualized and intensive (e.g., 40 hr per week) one-on-one behavioral treatment program (i.e., Applied Behavior Analysis). This form of intervention has the strongest evidence base (Roane et al., 2016; Weitlauf et al., 2014); however, with the rising prevalence of ASD (Baio et al., 2018), limited access to trained professionals, and the relatively high cost of service delivery, there is a need for additional and supplemental interventions. Similarly, investigation of additional treatment for FXS may be beneficial due to inconclusive evidence regarding the primary treatment (i.e., pharmacological intervention) for the disorder (Berry-Kravis et al., 2018).
Based on the findings discussed previously, one potential, supplemental treatment is computerized working memory training (CWMT). Cogmed is likely the most widely investigated CWMT program, with over 80 original, peer-reviewed research articles (Cogmed Claims and Evidence; https://www.cogmed.com/). Briefly, Cogmed involves at-home practice on memory span tasks that increase in difficulty as performance improves. Training is often completed on an iPad or Android tablet under supervision of a parent and is coupled with off-line coaching from a staff member. The premise behind CWMT is that repeated practice of WM will result in improvement in the neural systems that support WM (Sala & Gobet, 2017; Shipstead et al., 2012). By extension, these WM improvements are expected to transfer to other abilities that rely on the same neural networks (Simons et al., 2016). Compelling literature indicates that WM underlies inattentive and hyperactive/impulsive symptoms (Kofler et al., 2010; Rapport et al., 2009), social functioning (McQuade et al., 2013), and academic performance (Swanson & Alloway, 2012), and thus improvement in WM is expected to result in improvement in other cognitive and behavioral domains (Klingberg et al., 2005).
The evidence regarding CWMT in ASD has been mixed, with some studies indicating none to little improvement (de Vries et al., 2015; 2018) and another preliminary study suggesting some improvement (Kerns et al., 2017), though neither study involved the use of Cogmed’s version of CWMT or involved children with ID. CWMT-related improvements in WM measures (near-transfer effects) are well-documented across typically developing children (Sala & Gobet, 2017) and other populations, such as ADHD (Rapport et al., 2013). Participants significantly improve (e.g., recall more stimuli correct) on memory tasks that are similar or identical to the training tasks in CWMT (Sala et al., 2019), and improvements are maintained up to 3 to 6 months posttraining (Rapport et al., 2013). A recent meta-analysis examining Cogmed’s version of CWMT in children and adults with and without clinical disorders revealed small to medium effects in memory tasks (i.e., near transfer measures; Aksayli et al., 2019); however, improvement in other domains (far-transfer effects) are less consistent. For example, some investigations indicate CWMT-related improvement in simulated academic and academic domains (Green et al., 2012; Shinaver, 2014), whereas other studies have shown that CWMT improvements do not generalize to nonverbal and verbal reasoning, academic achievement, or other executive functions (Melby-Lervåg & Hulme, 2013; Rapport et al., 2013; Shipstead et al., 2012). These inconsistent findings beg the question of whether certain factors, genetic or behavioral, influence the efficacy of CWMT.
Individuals with ASD caused by a specific single gene (i.e., FMR1 mutation) may differ in treatment response compared to children with idiopathic ASD. Boys with FXS and comorbid ASD display less severe ASD symptoms, particularly in the social domain, relative to those with ASD without FXS (Abbeduto et al., 2019; Thurman et al., 2015), however, they exhibit poorer developmental outcomes, including weaker communication and adaptive behaviors, and greater cognitive impairment (Bailey et al., 2000). Few studies have investigated the effectiveness of CWMT in FXS (Hessl et al., 2019; Scott et al., 2020). A randomized controlled trial (RCT) of CWMT in children with FXS revealed improvements in WM, attention, and other executive functions with maintained improvements at 3 months follow up (Hessl et al., 2019). Improvement between adaptive and nonadaptive treatment conditions did not differ, indicating that increasing WM load by expanding span length did not provide added benefit. Although Hessl and colleagues provided evidence that CWMT can improve WM, attention, and other executive functions in children with FXS, it remains unknown whether the presence of the FMR1 mutation (FXS) may impact treatment response in children with ASD.
It is also possible that ADHD behavioral symptoms may serve as a behavioral marker for treatment response to cognitive training. A substantial portion of children with ASD (40%–70%; Lyall et al., 2017; Rommelse et al., 2010) and FXS (54%–59%; Sullivan et al., 2006) exhibit significant problems with attention, impulsivity, and excessive gross motor activity, which may exacerbate academic and social difficulties at home and at school. Although one study has demonstrated that ADHD symptoms negatively affected psychosocial treatment outcomes in children with ASD (Antshel et al., 2011), no study to our knowledge has investigated the effect of ADHD symptoms on CWMT training in children with ASD. It has been hypothesized that ADHD-related genes and behaviors affect the expression of the ASD phenotype (Yerys, 2009). Consistent with this hypothesis, greater ADHD symptoms have been shown to be associated with greater functional impairments in children with ASD, including poorer executive control, adaptive behavior, disruptive behavior and working memory (Yerys et al., 2009). Extant literature also indicates that higher rates of hyperactivity-impulsivity (Tillman et al., 2011) and inattentive (Neely et al., 2016; Rogers et al., 2011) symptoms are negatively correlated to WM performance. Furthermore, among children with comorbid ADHD and Learning Disorder, greater parent-reported ADHD symptoms are associated with lower CWMT-related WM improvement on WM training tasks (Gray et al., 2012). Taken together, extant literature suggests that greater ADHD symptoms may result in poorer treatment response to CWMT.
Current knowledge of CWMT in children with ASD is derived from samples of children with low average to high intellectual functioning, however, more than 90% of males with FXS (Hessl et al., 2009) and over a third of individuals with ASD (Baio et al., 2018; Ryland et al., 2014) have comorbid ID. Despite the large prevalence of ID among individuals with ASD, there is little known regarding successful intervention for this group.
This project aimed to (1) determine feasibility of Cogmed CWMT in children with ASD (with/without FXS) and ID and (2) examine preliminary intervention effects using a pre-post design. We recently investigated Cogmed CWMT feasibility and found high completion rates and positive parent satisfaction ratings in children with ASD and ID, which included all 26 children with idiopathic ASD from the current article (Benyakorn et al., 2018). Feasibility of CWMT within the FXS population was also previously examined (Au et al., 2014). Thus, the present report focuses on preliminary efficacy results.
The purpose of the present study is to (1) investigate pre-post change in WM abilities after Cogmed CWMT in children with ASD and accompanying ID and (2) examine differences in pre-post change between children with idiopathic ASD and FXS-related ASD. In addition, we explore pre-post changes in far transfer (non-WM) measures and the extent to which a behavioral marker, severity of ADHD symptoms, predicts pre-post change. Lastly, given the differences in duration, number and types of games, and difficulty level between the two Cogmed versions (JM, designed for preschool children and RM, designed for school age children), we compare near-transfer (WM) effects between Cogmed JM and RM collapsed across diagnostic groups.
Methods
Participants
Participants included 44 children from 8 to 18 years of age with ASD, including 26 diagnosed with ASD and 18 diagnosed with comorbid ASD and FXS. Recruitment for children with idiopathic ASD was conducted through University of California (UC) Davis MIND Institute’s Subject Tracking System, flyers located at the local clinic, Alta California regional center, and advertisements placed in websites and local newspapers. Recruitment for children with ASD and comorbid FXS (ASD+FXS) was conducted through the Fragile × Center at the UC Davis MIND Institute. All parents and children provided their informed consent/assent prior to participating in the study, and approval from the UC Davis Institutional Review Board was obtained prior to the onset of data collection. Inclusion criteria were below average IQ (FSIQ < 85); normal or corrected to normal vision and hearing; ability to pass three-span Cogmed demo tasks; English speaking; and parental agreement to maintain adherence to the training schedule and to not alter other treatments during the study. The exclusion criteria were significant brain trauma, previous Cogmed training, and significant medical or severe behavioral problems that would interfere with the study. Inclusion and exclusion criteria were based on parental report, with the exception of ability to complete Cogmed demo tasks, which was verified online by the researchers, and IQ (discussed in the following sections).
Intellectual functioning was determined by current or previous testing (administered within the past 3 years) using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003) or Stanford-Binet Intelligence Scales, Fifth Edition (SB-5; Roid, 2003). The verbal and nonverbal routing subtests of the SB-5 were administered during the baseline visit to estimate the abbreviated IQ (ABIQ) for participants without recent testing.
To verify ASD diagnoses, all participants were required to provide a copy of a psychological report indicating a diagnoses of ASD using gold standard assessments, the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Gotham et al., 2006) or the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al., 2003).To confirm that participants continued to meet criteria for ASD, they were required to have a total score greater than 15 on Social Communication Questionnaire Lifetime (SCQ; Berument et al., 1999). FXS was confirmed by genetic testing documentation indicating FMR1 full mutation.
Procedure
After inclusion criteria were met via a phone screen, a researcher travelled to the family’s home to obtain consent/assent, assess baseline and intellectual functioning, and determine the appropriate version of Cogmed (JM vs. RM). Of note, one participant elected to come to the MIND Institute for assessments. Participants completed 5 to 6 weeks of Cogmed training and one week later, researchers re-administered the same test battery (with the exception of SB-5 routing subtests) to assess training effects.
Intervention
All participants were instructed to complete five web-based Cogmed Working Memory Training sessions per week for 5 weeks, for a total of 25 training sessions, as indicated by the Cogmed protocol (www.Cogmed.com). There were two difficulty levels for participants, which were determined during the initial assessment by the researcher. Cogmed JM is designed for preschool children, whereas Cogmed RM is designed for school-aged typically developing children. Those who were able to complete 9 of the 11 Cogmed RM games were assigned to Cogmed RM, and the remainder were assigned to Cogmed JM.
Cogmed JM training involves completing three of the seven JM games, whereas the RM training involved the completion of eight of the 10 RM games, with games automatically rotated in each session. As such, each Cogmed JM training session lasts approximately 15 min and Cogmed RM training sessions lasts approximately 30 min. Cogmed JM is based on an amusement park theme and consists of visuospatial memory training tasks. For example, one JM task involves users being presented with bumper cars that move around the screen and light up one at a time, and are then instructed to recall the order in which the cars lit up by clicking/touching the cars on the screen. Four of the seven JM tasks involve only the storage of visual information (pool, hotel, roll-ercoaster, twister), two involve both manipulating and storage of visual information (ferris wheel, bumper cars), and one involves the storage of visual and auditory information (wheel of animals). Cogmed RM is based on a robot theme and consist of tasks that are more complex than JM, involving rotating displays, moving targets, reverse sequence tasks, numeric information to recall, and delayed responses. In addition, the RM version includes verbal WM span tasks (e.g., user is presented digits verbally on a robot and asked to recall these digits in reverse order using a visual number pad).
Both versions are adaptive; the difficulty gradually increases after correct trials and decreased after incorrect trials. Both versions emit auditory and visual feedback after each trial to indicate success or failure at the task. After the completion of each training session, Cogmed JM users receive a virtual fish for their digital aquarium, and Cogmed RM users play a racing game as a reward. For added motivation, users receive a sticker to add to their reward chart after each session, and families decided on daily, weekly, and full training completion rewards.
Participants were trained either on the Cogmed tablet app (n = 31; use of finger for item responses), with tablets provided as necessary, or on the Cogmed website (n = 13; use of PC with a mouse for item responses).Participants were allowed to choose whether they wanted to use a tablet or the computer, depending on availability and familiarity with equipment; however, during the course of the study, the research group received funding for tablets, which allowed for participants to borrow a tablet if they chose to. Each training session was conducted at home in a location with limited distractions and parental supervision.
As per the Cogmed protocol, participants were provided with a Cogmed coach from the research team staff and parents served as training aides. For each participant, the staff member who served as the coach was different than the researcher who collected baseline data. At the beginning of the session, the coaches explained the expectations and goals for CWMT, established a reward system, and planned the training (e.g., what days/times to train). The coach also established a set time once a week for Cogmed coaching calls to ensure that the participant was doing their training as planned and that the training plan (e.g., reward system) was working, and to encourage and reinforce both the participant and parent. These coaches had online access to participants’ frequency of use and performance on Cogmed tasks to track progress and provide feedback as necessary. As training aides, parents were instructed to (a) sit near their child during training and have the screen within view; (b) advise their child to take a break if they showed signs of frustration or missed three trials in a row; (c) ensure their child is not cheating (e.g., writing down the numbers, saying the numbers out loud, tilting their head to better see the moving exercises, missing trials in an attempt to complete the day’s training faster); and (d) be encouraging and praise their child’s effort.
Measures
Cogmed Performance
Cogmed automatically computes three global indices of performance: Start Index, Max Index, and Improvement Index. The Start Index is based on results from day 2 to 3, and the Max index is a mean of the three best successful trials on the two best training days. The Improvement Index is the difference between the Max Index and the Start Index. In addition, an average maximum span for each daily training session was also calculated by averaging the maximum number of items recalled across all games.
Near-Transfer (WM) Measures
Leiter-R Spatial Memory Subtest.
The Leiter-Revised (Leiter-R; Roid & Miller, 1997) measures nonverbal intelligence. The Spatial Memory subtest was used to assess visual WM. An array of familiar items was visually presented in a matrix for 10 s and then removed, after which the participant was instructed to place cards of the previously shown items in the correct locations on a blank matrix. The subtest has 20 items and starts and ends with a single picture in a two-box matrix and eight pictures in a 12-box matrix, respectively. The assignment is terminated after six errors one after the other. The total items correct was used as an outcome measure.
Stanford Binet 5 Block Span Subtest.
The Stanford Binet 5 (SB-5; Roid, 2003) Block Span subtest was also used to assess visual WM. Examiners tapped blocks in a particular order, and participants were instructed to recall the pattern by tapping the blocks in the same order. The subtest has a total of 30 items and was discontinued after two consecutive errors. To allow more range for lower functioning individuals, five additional easier items were created and added to the subtest. The total correct trials was used as an outcome measure.
WISC-IV Digit Span Backward Subtest.
The Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003) Digit Span Backward subtest was used to assess auditory WM. In the Digit Span Backward subtest, the participant is asked to recall the numbers in reverse order. The list-length starts with two digits (four items) and increases by one digit every two items. There is a total of 16 items and testing is discontinued as soon as the child demonstrates less than perfect recall of both same-length items. The total correct trials was used as an outcome measure.
Exploratory Far-Transfer (Non-WM) Measures
Restricted Academic Situations Task.
The Restricted Academic Situations Task (RAST) was used to measure on-task behavior during performance on an academic task. This measure is sensitive to medication effects in ADHD and has been used in studies of children with comorbid ADHD and intellectual disability (Fischer & Newby, 1998; Handen et al., 1998) and has detected improvement associated with CWMT in a randomized controlled trial in ADHD as well (Green et al., 2012). The assessment is sensitive to inattention and hyperactive behaviors and does not appear to lead to practice effects (Green et al., 2012; Grizenko et al., 2004). The RAST provides information regarding the frequency and duration of off-task behaviors in the following five domains: (a) off-task, (b) out-of-seat, (c) fidgets, (d) vocalizes, and (e) plays with object. RAST sessions were video recorded and blind observers quantified the off-task behaviors from the video recordings.
First, the child was presented with an array of toys, instructed to select the toys of greatest interest and then instructed to remain seated and play independently. After 5 min, the researcher moved the toys aside, but within arm’s reach, and introduced a paper-based shape-matching task of moderate difficulty level. If the participant correctly responded on five problems in under 15 s, the researcher presented the advanced matching worksheet. If the participant incorrectly responded on three or more problems or took more than 30 s to complete the first five problems on the moderate matching worksheet, the researcher presented the easy matching worksheet. Once the appropriate level (easy, moderate, hard) was chosen, the researcher instructed the child to continue completing the matching worksheet for 10 min. Before leaving the table, the researcher instructed the child not to leave his or her seat or to touch any of the toys.
Observers recorded the occurrence (yes/no) of the following behaviors (i.e., partial-interval time sampling procedure) within consecutive 15 s intervals: (a) off-task (looks away from paper), (b) out-of-seat (leaves chair), (c) fidgets (repetitive purposeless motion), (d) vocalizes, and (e) plays with object (touches any object in the room unrelated to the task). For each behavior, the number of intervals in which the behavior occurred was used in analyses. To account for slight variations in the number of coded intervals (typically 30) across videos, the number of intervals was log-transformed and entered into the analytic model as an offset. For descriptive analyses, the number of 15 s intervals with an occurrence of each off-task behavior was converted to a percentage of time intervals spent engaging in the off-task behavior. Raters were trained by an experienced RAST coder on how to code the behaviors on the RAST. Then 20% of the RAST data were scored by a second trained RAST coder to verify inter-observer reliability in the RAST scoring. Interobserver reliability was assessed by calculating percent agreement between the two raters for each clip (agreements/ [agreements disagreements] × 100). The average percent + agreement between the two raters was very high: (a) off-task (94%), (b) plays with object (94%), (c) out-of-seat (99%), (d) fidgets (84%), and (e) vocalizes (95%). The five RAST behavior outcomes were analyzed separately because each behavior is assumed to reflect a different construct. For example, fidgeting behavior is indicative of hyperactivity, whereas off-task behavior reflects inattentiveness or distractibility.
Pervasive Developmental Disorder Behavior Inventory.
The Pervasive Developmental Disorder Behavior Inventory (PDDBI) is a reliable and valid assessment tool designed to monitor parent-rated treatment outcome in children diagnosed with a pervasive developmental disorder (PDD; Cohen & Sudhalter 2005). The PDDBI has high interrater reliability and factor analyses confirmed it has good construct validity (Cohen et al., 2003). We used two subscales from the PDDBI, the repetitive, ritualistic, and pragmatic problems (REPRIT) scale and the expressive social communication abilities (EXSCA) scale.
Exploratory ADHD Symptoms
Conners 3rd Edition–Parent.
The Conners 3rd Edition–Parent (Conners 3-P; Conners, 2008) includes 99 items and is used to obtain parent-rated observations about their child/adolescent’s behavior. The Inattention and Hyperactivity/Impulsivity Content scaled scores were used to assess ADHD symptoms. The Conners 3-P shows sensitivity to medication effects on ADHD symptoms in children with FXS (Torrioli et al., 2008) and ASD (Pearson et al., 2013) and has well-established psychometric properties (Gallant, 2007). T scores greater or equal to 65 are considered within the clinically concern range.
Statistical Analysis
Group differences in demographic and clinical characteristics and Cogmed performance were assessed using chi-square (or Fisher’s exact test, as appropriate) for categorical variables and nonparametric Wilcoxon rank-sum test for continuous variables. Analyses were conducted within a generalized linear mixed-effects model framework (McCulloch et al., 2008) because it can accommodate both dependent variables that are normally distributed (WM measures, PDDBI subscales) and counts (RAST variables). This approach uses all available data, accounts for the correlated structure of the data due to repeated assessments over time and produces valid inference under the assumption that data are missing at random. WM and PDDBI measures were analyzed as normally distributed (using identity link and a normal variance function) and the RAST behaviors were analyzed as counts (using a log link and negative binomial variance function to model the number of intervals with occurrence of the respective behavior). To account for slight variations in the number of intervals coded across participants, the number of intervals was log-transformed and entered into the negative binomial models as an offset. The core models included fixed effects for group (ASD, ASD+FXS), time (Pre-, Post-), age, IQ (FSIQ or ABIQ), and a random effect for child to account for the within-child dependence. Interactions between group and time were also tested but they were removed from the reported models unless they contributed significantly to the models. Residual analyses and graphical diagnostics demonstrated model assumptions were adequately met.
Because our goal was to investigate whether severity of ADHD symptoms was associated with response to training, we calculated Spearman’s rank correlations between baseline scaled scores on the Conners 3-P and change (the difference between post- and pretraining scores) on outcome measures that children significantly improved from pre- to posttraining. Because our sample was predominantly male (as expected in ASD), we conducted a sensitivity analyses by excluding the girls from the sample and rerunning the models for WM. Finally, we explored differences in version (JM vs. RM) by conducting another series of mixed-effects models for WM measures. These models included fixed effects for version (JM, RM), time (Pre-, Post-), age, IQ (FSIQ or ABIQ), and a random effect for child to account for the within-child dependence. All analyses were implemented using SAS Version 9.4 (SAS Institute Inc., Cary, NC). All tests were two-sided, and p-values < 0.05 were considered statistically significant.
Results
Demographic and Clinical Characteristics of Participants at Baseline
Table 1 presents summary demographic and clinical characteristics for the two groups. Out of the 44 total participants, only one participant (from the ASD group) did not complete the training (discontinued after training session 16 due to technical problems). The ASD and ASD+FXS groups did not differ significantly by gender, race, income, ethnicity, or current enrollment in therapy. It is important to note, however, that the ASD+FXS group, recruited from throughout the United States, consisted of mostly (88%) White and middle class (82% above 50k income range) participants. In contrast, the ASD sample was commensurate with the racial composition of the Sacramento geographic area according to the U.S. 2019 Census report, and included 56% White, 12% Black or African American, 20% Asian, and 12% multiracial or other races. Regarding ethnicity, the ASD group had a slightly higher percentage (32%) of Hispanic or Latinx than the ASD+FXS (25%) group. The ASD group had a roughly even distribution of participants across income categories (32% with < 50k, 32% with 50k–100k, and 36% with > 100k). The two groups had similar levels of ADHD symptoms, with the majority of individuals with inattentive (88% in ASD, 89% in ASD+FXS) and hyperactivity/impulsivity (83% in ASD, 78% in ASD+FXS) scaled scores in the clinical concern range. The ASD group was significantly younger (p < 0.05) and had higher IQ (p < 0.05). Thus, age and IQ were used as covariates in the subsequent analyses. The ASD+FXS group also included a significantly greater number of participants with actively prescribed stimulant (44%; p < 0.01) and antidepressant medication (44%, p < 0.05) than the ASD group (4% and 12%, respectively).
Table 1.
Demographic and Clinical Characteristics of Participants at Baseline
| Characteristic | ASD (n = 26) | ASD + FXS (n = 18) | P-valuea |
|---|---|---|---|
| Age (years), mean (SD) | 11.1 (2.4) | 13.4 (3.3) | 0.01 |
| Gender, n (%) | 1.0 | ||
| Female | 5 (19%) | 4 (22%) | |
| Male | 21 (81%) | 14 (78%) | |
| Race, n (%) | 0.20 | ||
| Black | 3 (12%) | 0 (0%) | |
| White | 14 (56%) | 15 (88%) | |
| Asian | 5 (20%) | 2 (12%) | |
| Other | 3 (12%) | 0 (0%) | |
| Income, n (%) | 0.60 | ||
| <50k | 8 (32%) | 3 (19%) | |
| $50k-100k | 8 (32%) | 7 (44%) | |
| >$100k | 9 (36%) | 6 (38%) | |
| Ethnicity, n (%) | 0.72 | ||
| Hispanic or Latinx | 6 (32%) | 4 (25%) | |
| Not Hispanic or Latinx | 13 (68%) | 12 (75%) | |
| IQ, mean (SD) | 65.4 (13.7) | 55.9 (10.4) | 0.04 |
| Current therapy, n (%) | |||
| ABA | 11 (42%) | 4 (24%) | 0.21 |
| Occupational therapy | 15 (58%) | 6 (35%) | 0.15 |
| Physical therapy | 4 (15%) | 4 (24%) | 0.69 |
| Speech therapy | 21 (81%) | 13 (76%) | 1.0 |
| Psychotropic Medication, n (%) | |||
| ADHD Stimulant | 1 (4%) | 8 (44%) | 0.002 |
| ADHD Nonstimulant | 4 (16%) | 2 (11%) | 1.0 |
| Antidepressant | 3 (12%) | 8 (44%) | 0.03 |
| Antipsychotic | 5 (19%) | 3 (17%) | 1.0 |
| Anti-seizure | 3 (12%) | 0 (0%) | 0.26 |
| Other medications | 2 (8%) | 1 (6%) | 1.0 |
| Conners 3-P Content Scaled Scoresb | |||
| Inattention T-score, mean (SD) | 74.8 (11.0) | 80.1 (9.7) | 0.12 |
| Clinical concern range, n (%) | 21 (88%) | 16 (89%) | 1.00 |
| Hyperactivity/Impulsivity T-score, mean (SD) | 76.1 (12.9) | 76.3 (14.4) | 0.86 |
| Clinical concern range, n (%) | 20 (83%) | 14 (78%) | 0.71 |
Note. ASD = Autism Spectrum Disorder; FXS = Fragile × syndrome; SD = standard deviation; ABA = applied behavioral analysis; ADHD Attention Deficit-Hyperactivity Disorder; Conners 3-P= The Conners 3rd Edition–Parent; Clinical concern range = T-scores ≥ 65.
Group differences were tested using Wilcoxon rank-sum test for continuous variables and chi-square or Fisher’s exact test (when appropriate) for categorical variables.
Data missing for 2 children in the ASD group.
Cogmed Working Memory Training
There were no significant differences between groups in training platform, total number of Cogmed sessions per week, Cogmed version (JM vs. RM), or total number of training days (see Table 2). Statistical comparisons showed that the ASD group started training with a higher Start Index (p < 0.001), completed training with a higher Max Index (p < 0.001) and demonstrated a higher Index of Improvement relative to the ASD+FXS group (p = 0.02; see Table 2). Daily Cogmed data were available on 23 ASD and 16 ASD+FXS children. Data for four participants were not available from the Cogmed/Pearson Corporation and data from one participant was unusable due to the participant not following directions. As depicted in Figure 1, the ASD participants started training with an average maximum span length of 4.0, 95% CI [3.6,4.3], and improved to an average maximum span of 5.5, 95% CI [5.2, 5.9], at the end training. The ASD+FXS participants started training with an average maximum span length of 3.3, 95% CI [3.0, 3.5], and improved to an average maximum span of 4.1, 95% CI [3.5, 4.7], at the end of training. As illustrated in Figure 1, training gains after 20 sessions in both groups tended to stabilize or decline.
Table 2.
Cogmed Training Platform and Performance
| Cogmed Variable | ASD (n = 26) | ASD + FXS (n = 18) | P-valuea |
|---|---|---|---|
| Cogmed training device, n (%) | 0.83 | ||
| Tablet | 18 (69%) | 13 (72%) | |
| PC | 8 (31%) | 5 (28%) | |
| Cogmed version, n (%) | 0.32 | ||
| JM | 15 (58%) | 13 (72%) | |
| RM | 11 (42%) | 5 (28%) | |
| Cogmed sessions per week, mean (SD) | 4.8 (1.3) | 4.2 (1.2) | 0.06 |
| Total Training Days, mean (SD) | 24.6 (2.8) | 23.9 (2.2) | 0.22 |
| Active Training Time Per Day (min), mean (SD) | 23.5 (11.0) | 19.4 (6.8) | 0.42 |
| Start Index, mean (SD) | 57.2 (10.8) | 40.9 (8.3) | < 0.001 |
| Max Indexb, mean (SD) | 78.7 (11.1) | 57.4 (10.8) | < 0.001 |
| Index Improvementb, mean (SD) | 21.7 (7.1) | 16.4 (5.8) | 0.02 |
Note. ASD = Autism Spectrum Disorder; FXS = Fragile × syndrome; SD = standard deviation; JM = Cogmed for preschool-aged children; RM Cogmed for school-aged children.
Group differences were tested=using Wilcoxon rank-sum test for continuous variables and chi-square test for categorical variables (i.e., Cogmed training device and version).
Data from 1 child in ASD group was unusable due to the participant not following directions.
Figure 1.
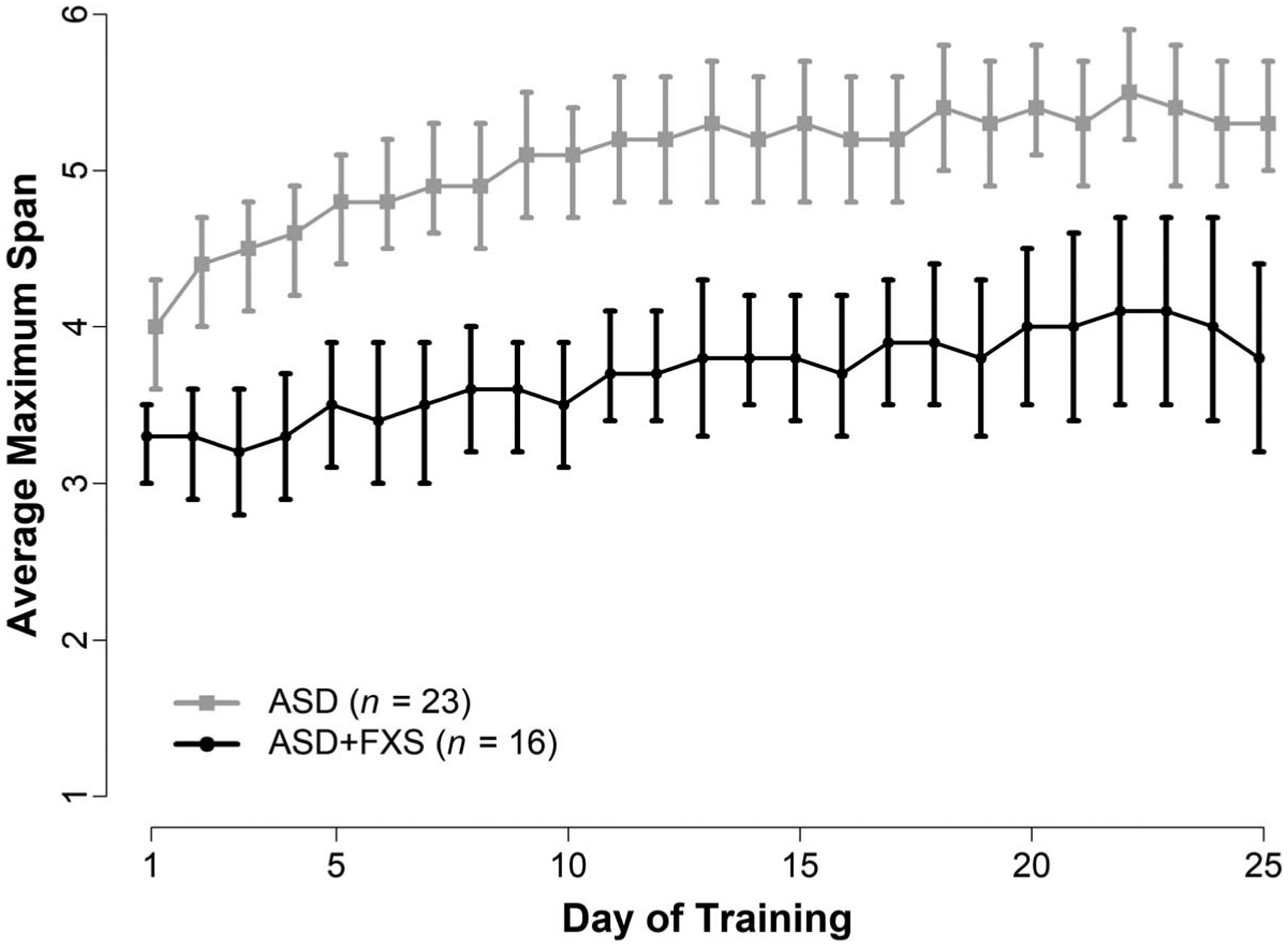
Cogmed Average Maximum Span Across Training by Diagnostic Group
Note. Average maximum working memory span length (and 95% confidence intervals) across training for participants with autism spectrum disorders (ASD) and autism spectrum disorders and fragile × (ASD+FXS).
Near-Transfer (WM) Effects
Participants significantly improved from pre- to posttraining on all measures of WM (SB-5 Block Span, Leiter-Revised Spatial Memory scale, and the WISC Digit Span Backward Subtest; see Table 3). Although the ASD group tended to have better WM outcomes, after controlling for age and IQ, there was no significant effect of group. Interactions between group and time for all near-transfer measures were tested, but none reached statistical significance, indicating that there was no difference in WM improvement between the two groups. Missing data on the near-transfer (WM) measures was minimal (only one child in the ASD+FXS group had missing postintervention Leiter-Revised Spatial Memory data and one child in the ASD group had missing pre and postintervention WISC Digit Span Backward Subtest data).
Table 3.
Summary for the Pre- and Postintervention Performance for the Two Groups
| Outcome Measure | ASD (n = 26) | ASD+FXS (n = 18) | Post- vs. Pre- Differencea | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-M (SD) | Post-M (SD) | Effect Sizeb | Pre-M (SD) | Post-M (SD) | Effect Sizeb | Estimate (95% Cl) | P-value | |
| Near Transfer (WM) Measures | ||||||||
| Stanford Binet 5 Block Span | 11.5 (6.3) | 14.6 (5.8) | 0.50 | 7.6 (4.6) | 9.7 (4.9) | 0.47 | 2.40 [1.45, 3.36] | <0.001 |
| Leiter-Revised Spatial Memoryc | 21.9 (16.4) | 26.1 (20.2) | 0.26 | 14.9 (8.3) | 18.1 (9.9) | 0.38 | 3.34 [0.69, 5.98] | 0.01 |
| WISCd Digit Span Backward | 3.5 (2.8) | 4.1 (2.7) | 0.20 | 2.1 (2.0) | 2.4 (2.1) | 0.17 | 0.38 [0.09, 0.68] | 0.01 |
| Far Transfer (non-WM) Measures | ||||||||
| PDDBI Score | ||||||||
| REPRITe | 72.6 (34.2) | 62.5 (33.8) | −0.30 | 42.4 (19.2) | 46.0 (22.1) | 0.19 | −10.1 [−16.5, −3.7] | 0.003 |
| 51.9 (13.9) −0.29 | ||||||||
| EXSCAf | 140.1 (36.3) | 145.1 (37.1) | 0.14 | 155.1 (32.6) | 140.6 (28.6) | −0.44 | 1.51 [−5.19, 8.22] | 0.65 |
| RASTg | ||||||||
| Percent Intervals Off-Task | 35.9 (32.1) | 25.6 (32.7) | −0.32 | 43.4 (37.3) | 35.7 (34.1) | −0.20 | −0.44 [−0.77, −0.10] | 0.01 |
| Percent Intervals Fidgeting | 14.6 (22.1) | 19.7 (28.7) | 0.23 | 39.4 (16.2) | 37.0 (28.0) | −0.15 | 0.03 [−0.42, 0.47] | 0.91 |
| Percent Intervals Vocalizing | 38.8 (29.6) | 43.8 (36.3) | 0.17 | 41.2 (30.4) | 39.0 (37.3) | −0.07 | 0.01 [−0.26, 0.27] | 0.96 |
| Percent Intervals Play Object | 19.1 (30.3) | 17.1 (28.5) | −0.07 | 17.4 (33.4) | 24.2 (35.5) | 0.20 | 0.00 [−0.59, 0.59] | 1.00 |
| Percent Intervals Out of Seat | 7.0 (13.7) | 1.1 (3.0) | −0.43 | 13.2 (26.5) | 2.8 (8.3) | −0.39 | −1.66 [−2.72, −0.59] | 0.003 |
Note. ASD = Autism Spectrum Disorder; FXS = Fragile × Syndrome; SD = Standard Deviation; WISC = Wechsler Intelligence Scale for Children; PDDBI = Pervasive Developmental Disorder Behavior Inventory; REPRIT = Repetitive, Ritualistic, and Pragmatic Problems Composite; EXSCA = Expressive Social Communication Abilities Composite; RAST = Restricted Academic Situations Task.
Estimated differences and p-values from mixed effect linear or negative binomial (for the RAST variables) models that included fixed effects for group (ASD, ASD+FXS), time (Pre-, Post-), IQ, and age and a random effect for child. Log transformed number of coded intervals was used as an offset in the RAST models. Interactions between group and time were also tested, but only reached statistical significance for PDDBI REPRIT scale. For this variable, the reported confidence interval represents the difference in ASD group. For the ASD+FXS group, the estimated difference is 1.9, 95% CI [−8.2, 12.10], p=0.70.
Because the intervention may affect SD of postmeasurements, the effect size was calculated as Glass’s Δ for within-subjects design, (i.e., Δ=Mdiff/SDpre, where Mdiff is the mean of the difference scores [Postintervention–Preintervention] and SDpre is the SD of the Preintervention scores). Data missing for:
1 child in the ASD+FXS group postintervention;
1 child in ASD group at both times;
2 children in ASD group and 7 children in ASD+FXS at both times;
2 children in ASD group and 8 children in ASD+FXS preintervention and 2 children in ASD group and 7 children in ASDþFXS postintervention;
9 children in ASD+FXS group at both times.
Sensitivity analyses were conducted to examine whether near-transfer (WM) outcomes were affected when excluding girls from the analyses. Findings revealed similar magnitude of improvement to the primary analyses for SB-5 Block Span, 2.8, 95% CI [1.4, 4.2] vs. 2.4 points, 95% CI [1.5,3.4], and Leiter-Revised Spatial Memory, 2.9, 95% CI [0.04, 5.9] vs. 3.3 points, 95% CI [0.69, 6.0], tasks when girls were excluded and included, respectively. In contrast, the improvement in the WISC Digit Span Backwards Subtest was diminished when girls were excluded, 0.22 points, 95% CI [−0.13, 0.59] vs. 0.38 points, 95% CI [0.09,0.68]. This suggests that gender likely has only a modest impact on the SB-5 Block Span and Leiter-Revised Spatial Memory tasks, but may have a greater influence on the WISC Digit Span Backwards, such that girls may improve more on the latter task relative to boys.
Exploratory Analyses Assessing Far-Transfer (Non-WM) Effects
In exploratory analyses we examined improvements from pre- to posttraining scores on far-transfer (non-WM) measures (Table 3). For the REPRIT scale of the PDDBI, there was a significant group × time interaction (p =0.049), such that parents of ASD reported significantly lower levels of repetitive, ritualistic, and pragmatic problems at posttraining relative to pretraining, while there was no difference between post- and pretraining for the parents of ASD+FXS children. In contrast, participants in either group did not significantly improve on Expressive Social Communication Abilities (EXSCA) of the PDDBI.
In addition, participants demonstrated a significant reduction from pre- to posttraining in off-task and out-of-seat behavior on the RAST, a simulated classroom task. There were no significant differences from pre- to posttraining on fidgeting, vocalizing, and playing with objects, with participants maintaining the observed pretraining levels.
There was a significant main effect of group in off-task and fidgeting behavior, such that the ASD+FXS group spent more time off-task and fidgeting. Interactions between group and time did not reach statistical significance for any far-transfer (non-WM) measures other than the REPRIT scale of the PDDBI.
It is important to note that data on both PDDBI and RAST were missing for several participants, particularly in the ASD+FXS group (7–9 children in the ASD+FXS group vs. 2 children in the ASD group were missing PDDBI or RAST data, see note in Table 3 for details). Families in the ASD+FXS study were participating in another, primary FXS study, during the testing session and those additional assessments were frequently prioritized by the study team to reduce assessment burden on the parents and child, when necessary. Missing RAST task data was also sometimes due to child fatigue or inability to perform the RAST tasks, the child moving out of the camera range, or the testing session extending beyond what was the available time for the family.
Exploratory Analyses Assessing Associations of Post-and Pretraining Scores With Severity of ADHD Symptoms
A total of 12 Spearman’s rank correlations were calculated to examine the association between baseline ADHD symptoms (Inattentive and Hyperactivity/Impulsivity T-Scores) and change (the difference between post- and pretraining scores) on the six outcome measures that children significantly improved from pre- to posttraining (SB-5 Block Span, Leiter-R Spatial Memory Task, WISC-IV Digits Backwards; REPRIT scale of the PDDBI, RAST-out of seat, RAST off-task). This analysis revealed a significant correlation between baseline Hyperactivity/Impulsivity T Scores and change in scores from pre- to posttraining on the SB-5 Block Span (Spearman’s ρ = −0.46, p = 0.002), indicating that those with greater baseline hyperactivity/impulsivity symptoms demonstrate less improvement on a visuospatial WM task. No other ADHD-type symptom ratings were associated with response to training (all p > 0.05).
RM Vs. JM
Additional analyses were conducted to compare CWMT performance and treatment outcomes in near-transfer (WM) measures between children enrolled in Cogmed JM and those enrolled in Cogmed RM. As depicted in Figure 2, children enrolled the Cogmed RM started CWMT at higher average maximum span relative to those enrolled in Cogmed JM. The participants enrolled in Cogmed JM participants started training with an average maximum span length of 3.5, 95% C: [3.2, 3.8], and improved to an average maximum span of 4.6, 95% CI [4.1, 5.1], at the end training. The participants enrolled in Cogmed RM started training with an average maximum span length of 4.0, 95% CI [3.6, 4.4], and improved to an average maximum span of 5.2, 95% CI [4.5, 5.8], at the end training. However, the overall trajectories of learning appear to be parallel for the two versions, suggesting children in the JM and RM group had similar improvements in CWMT performance. Children in both versions showed medium effect size improvements in the SB-5 Block Span task and small-medium effect size improvements in Leiter-R Spatial Memory tasks (see Table 4). In contrast, children enrolled in the RM version demonstrated medium magnitude improvements (Glass’s Δ = 0.53) in the WISC Digit Span Backwards Task, whereas children in the JM group did not improve (Δ = 0.03). The results of the linear mixed-effects models confirmed that the improvement for SB-5 Block Span and Leiter-Revised Spatial Memory were similar for JM and RM. However, a significant interaction between Cogmed version and time was detected for the WISC Digit Span Backwards, such that only children in the RM version improved from pre- to posttraining.
Figure 2.
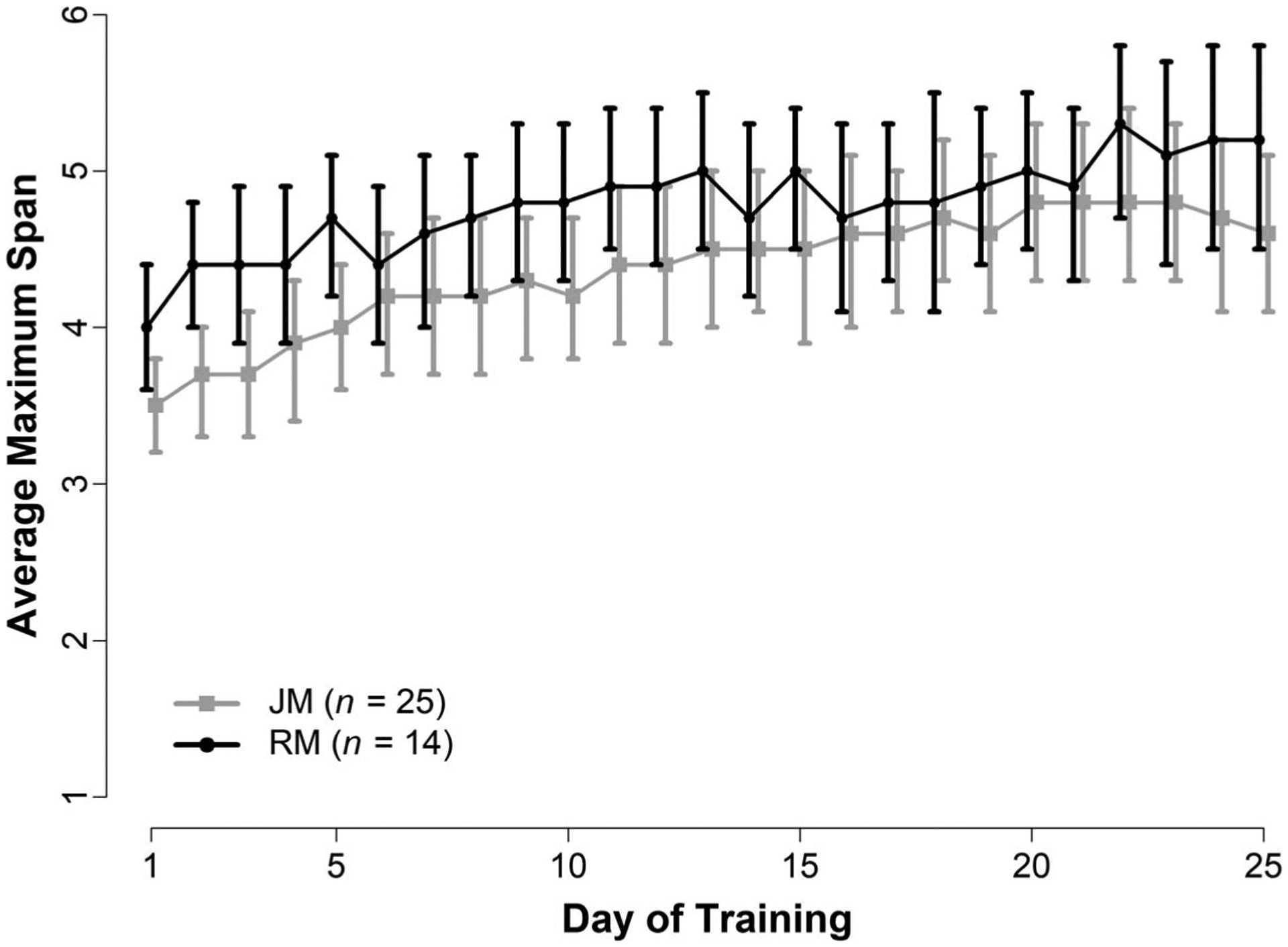
Cogmed Average Maximum Span Across Training by Cogmed Version
Note. Average maximum working memory span length (and 95% confidence intervals) across training for participants enrolled in Cogmed JM and Cogmed RM. JM = Cogmed for preschool-aged children; RM = Cogmed for school-aged children.
Table 4.
Summary for the Pre- and Postintervention Performance on Near Transfer (WM) Measures for the Two Cogmed Versions
| Outcome Measure | JM (n = 28) | RM (n = 16) | Post- vs. Pre- Differencea | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-M (SD) | Post-M (SD) | Effect Sizeb | Pre-M (SD) | Post-M (SD) | Effect Sizeb | Estimate (95% CI) | P-value | |
| Near-Transfer (WM) Measures | ||||||||
| Stanford Binet 5 Block Span | 7.5 (5.7) | 10.3 (5.6) | 0.49 | 14.1 (3.6) | 16.8 (3.6) | 0.75 | 2.75 [1.54, 3.96] | < 0.001 |
| Leiter-Revised Spatial Memoryc | 15.6 (13.3) | 25.1 (13.4) | 0.16 | 17.8 (14.6) | 31.6 (18.3) | 0.48 | 3.51 [0.97, 6.04] | 0.008 |
| WISCd Digit Span Backward | 2.0 (2.4) | 2.1 (2.3) | 0.03 | 4.4 (2.1) | 5.6 (1.2) | 0.53 | 1.13 [0.38, 1.87] | 0.006 |
Note. WM = Working memory; JM = Cogmed for preschool-aged children; RM Cogmed for school-aged children; SD = Standard Deviation; WISC = Wechsler Intelligence Scale for Children.
Estimated differences and p-values from mixed effect linear models that included fixed effects for version (JM, RM), time (Pre-, Post), IQ, and age and a random effect for child. Interactions between version and time were also tested, but only reached statistical significance for WISC Digit Span Backward. For this variable, the reported confidence interval represents the difference for children using the RM version. For children using the JM version, the estimated difference is 0.07, 95% CI [−0.26, 0.41], p = 0.66.
Because the intervention may affect SD of postmeasurements, the effect size was calculated as Glass’s Δ for within-subjects design, (i.e., Δ = Mdiff/SDpre, where Mdiff is the mean of the difference scores [Postintervention–Preintervention] and SDpre is the SD of the Preintervention scores. Data missing for:
1 child using the JM version postintervention;
1 child using the JM version at both times.
Discussion
We recently demonstrated that CWMT is a feasible treatment modality for children with ASD and ID (Benyakorn et al., 2018), however, in this report we expanded the aims beyond feasibility, and (1) investigated pre-post change in WM abilities after CWMT in children with ASD and accompanying ID and (2) compared pre-post change between children with idiopathic ASD and FXS-related autism. We also explored whether pre-post change in WM measures extended to far transfer (non-WM) measures, whether a behavioral marker or degree of ADHD symptoms predicted pre-post change, and whether there were differences in near-transfer (WM) effects between Cogmed versions. These findings attempt to facilitate a personalized health approach and identify potential baseline factors (i.e., idiopathic ASD vs. FXS+ASD; ADHD severity) that could predict training response.
Individuals demonstrated significant improvement across all WM measures from pre- to posttraining, consistent with the well-documented evidence of near-transfer effects of CWMT (Rapport et al., 2013; Sala & Gobet, 2017). This information is critical given the substantial WM deficits in ASD (Wang et al., 2017) and FXS (Baker et al., 2011) and the well-established role of WM in many functional outcomes (Alloway, 2009; Friedman et al., 2018; Swanson et al., 2009). Our data did not support significant group differences (ASD vs. ASD+FXS) in improvement on near transfer (WM) measures, wherein both groups showed similar rates of improvement from pre- to posttraining. The absence of group differences in improvement may be due to our small sample and insufficient power to detect an interaction between group and time. Due to the low prevalence of FXS in the population it was a challenge to recruit a larger sample size than included in this study. Research participants were recruited from throughout the United States in order to meet our sample size requirements for the FXS group in this pilot study.
Results indicated the Cogmed measures of daily average maximum span from the ASD+FXS group started lower than the ASD group, and even at the highest average daily maximum span, did not reach the average daily starting span for the ASD group. Gains were seen immediately for the ASD group and gradually increased over the days, whereas the group with FXS showed several days with stabilization and even decrements in the daily maximum span length.
A recent publication (Hessl et al., 2019) using a blinded RCT design with a relatively large sample (n = 100), demonstrated modest WM improvement in both the adaptive and nonadaptive (low dose) of Cogmed in children with FXS. The effect size in our pilot is larger than that reported in Hessl et al (2019) for the measures used in both studies (SB-5 Block Span, Leiter Spatial Memory). Improvements for several other outcomes were also found in the present study, which were not examined in the Hessl study, suggesting that there may be improvement in other domains, and thus a larger trial is warranted. Data from the Hessl et al. (2019) trial indicate that in a FXS population there are likely to be subgroups that have the capacity to progress, and that these individuals have the best potential for clinical improvement. This suggests that subgroups of participants could be reliably identified according to dimensions such as training quality, difficulty, accuracy, response time, and response time variability (Scott et al., 2020). Within a similar vein, results from our sensitivity analyses reveal that girls may benefit more from CWMT. Given the known gender differences in cognitive, behavioral, and functional domains in both ASD (Ferri et al., 2018) and FXS+ASD populations (Bartholomay et al., 2019), investigations with larger samples of female participants are suggested to either control for gender and/or examine gender differences in treatment outcomes.
In contrast to prior literature indicating that ADHD symptoms negatively impact treatment outcomes from other interventions (Antshel et al., 2011), ADHD symptoms did not significantly correlate with near- or far-transfer effects, with the exception of one visuospatial WM task (SB-5 Block Span). Results showed that those with greater baseline hyperactivity-impulsivity symptoms demonstrated less improvement on the SB-5 Block Span, consistent with other studies in children with ADHD. For example, Gray and colleagues (2012) demonstrated that those who showed the least improvement on WM training tasks at school had greater parent-reported ADHD symptoms. It may be possible that ADHD symptoms were associated with pre- to postchange on SB-5 Block Span and not WISC Digit Span Backwards because of the limited range of the change in WISC Digit Span Backwards scores from pre-to posttraining. For example, additional analyses showed that those in the JM version did not improve on WISC Digit Span Backwards, which is not surprising given evidence that WISC Digit Span Backwards measures the ability to store and manipulate information and there are less tasks in the JM version that target these processes. It is also possible that greater ADHD symptoms were associated with less improvement on SB-5 Block Span and not Leiter Spatial Memory test because the Block Span task involves greater WM load. For example, although both the SB-5 Block Span and Leiter Memory Subtest required participants to recall visually presented information, the SB-5 Block Span task had more possible answers (e.g., red and yellow rows) to select from and is therefore more difficult. Also, it is possible that the ADHD measure, the Conners’ Parent Rating Scale, is not the most sensitive measure for a population with ID as many of the items may not reflect the typical situation of some with ID. Future studies might consider using a measure such as the Aberrant Behavior Checklist (Aman et al., 2020) or the Scale of Attention in Intellectual Disability (SAID; Freeman et al., 2015).
Importantly, exploratory analyses revealed that participants demonstrated positive effects across other domains of behavior (far-transfer effects) beyond WM measures. Results showed a decrease in off-task and out-of-seat behavior during the RAST. These findings are in contrast to the absence of far transfer effects indicated by prior literature, however, a subsequent “review of reviews” contradicts some of the previous criticisms of CWMT (Shinaver et al., 2014). Shinaver and colleagues conclude that WM training consistently leads to improvement in attention and shows promising benefits in academic domains. Similarly, our findings of far-transfer effects are also consistent with a CWMT study in children with ADHD (Green et al., 2012), in which reductions in out-of-seat and off-task behavior was reported in this same simulated classroom task. The RAST may have greater ecological validity than other standardized behavior rating scales or other laboratory cognitive tasks. It allows for objective behavioral ratings of sustained attention and repetitive academic work in the presence of distractors with minimal supervision, similar to homework time or independent study time in the classroom. We also found a positive effect of CWMT on the REPRIT scale of the PDDBI, which suggests that the effects of the training may transfer to challenging behaviors associated with ASD. However, because the PDDBI uses parent report data, we cannot rule out the possibility that the observed pre-post change may be due to expectancy effects. As such, these findings will need to be replicated in a well-powered, blinded randomized controlled study using multiple measures of ASD symptoms and related behaviors to determine that changes were not merely due to practice or expectancy effects.
Despite the novel contributions of the present study (the number of objective measures used to assess near and far transfer effects, inclusion of children with ID, inclusion of children with FXS), several limitations warrant consideration. This project was funded by a pilot grant initiative under the Department of Defense Autism Research Program to support early-stage research and excluded the funding of RCTs. Independent replication with larger samples will be particularly important in accurately predicting who will respond to treatment, especially because the present findings (e.g., effect sizes) justify a larger RCT. Randomized control studies with a control condition are necessary to determine the efficacy of training, continuously challenging, the WM system. Comparison of the nonadaptive version of CWMT may not be necessary for the ASD+FXS group, considering improvement between adaptive and nonadaptive control conditions do not differ in this population (Hessl et al., 2019) and in groups with ID, the nonadaptive control condition is relatively challenging. A better control condition might be engaging in games with stimuli that require less of a demand on executive functioning, than the nonadaptive version of CWMT is in the ID population. Although we cannot completely rule out practice effects on the WM measures, we suspect these would be low in this population.
A substantial number of participants in our study were prescribed stimulant and nonstimulant medication used to treat ADHD, particularly in the ASD+FXS group, which may indicate that the ASD+FXS group had more severe cognitive and/or behavior problems. However, both groups had significant ADHD symptoms (no significant between-group differences), suggesting the difference in medication usage may be more associated with standard treatment regimens specific to the disorder. Future studies should recruit larger samples to investigate the effect of ADHD medication on CWMT-related performance and/or examine whether it affects the relationship between ADHD symptoms and training outcomes. We were also unable to examine the influence of both Cogmed version (i.e., JM vs. RM) and group (ASD vs. ASD+FXS) due to our small sample size. For example,there were only 5 children with ASD+FXS enrolled in the RM version. Future studies should recruit large enough samples to examine either RM or JM versions only.
Relatively high rates of missingness were present in the ASD+FXS group for PDDBI and RAST measures. Although we suspect that the data loss was largely due to logistic error (researchers not prioritizing these measures), if there is a relationship between the propensity of a data point to be missing and its values, this may have skewed the results. Subsequent research with cognitive training should also ensure to target WM abilities and other executive functions beyond WM. Studies that integrate virtual reality into the computer training may also facilitate generalization to real world functioning beyond what we found in this study. Lastly, although our sample appropriately reflects the ASD population in regard to race/ethnicity and socioeconomic status, which is a strength of the study, it disproportionately reflects the middle class White population in the ASD+FXS group. Although we acknowledge the difficulty in recruiting patients from the ASD+FXS group due to the rarity of the disorder, future studies should make focused efforts on recruiting ASD+FXS participants across all race, ethnic, and income groups.
In sum, our preliminary findings indicate that CWMT in children with ASD and ID may result in benefits in cognitive (i.e., WM) and behavioral (i.e., repetitive behavior, off-task behavior) outcomes, and that hyperactivity-impulsivity symptoms and the presence of FXS may complicate treatment response. Despite the cognitive and behavioral factors associated with this population that may have impeded treatment, our previous report of feasibility (Benyakorn, et al., 2018) and findings of the present study, suggest that CWMT and likely other digital interventions are potential treatment modalities for children with ASD and comorbid ID. Given the scarcity of treatment options to improve cognition in children with ID, we encourage future investigation and development of digital/computerized interventions for this population.
Acknowledgments
We thank Steven Riley, Hilary Sisson, Ryan Shickman, Yingratana A. McLennan, Cindy Johnston for testing the research participants, Jaewon Kim, Samantha Schauer, Mellisa Villacarte for assistance with data collection, entry and scoring, Connie Ha and Letitia Pirau for assistance in scoring the videotapes, Andrea Schneider, PhD for contributing to coaching the participants, and the families and participants for permitting us to test in their homes. We thank the Pearson Corporation for donating Cogmed user licenses for research and the Cogmed team (Kathryn Ralph, Stina Soderqvist, and James Meurer) for technical support. The Pearson Corporation had no role in the conduct, analyses, or reporting of the study. Funding and support for this work were provided by the Faculty of Medicine, Srinakharinwirot University, the Department of Defense through a pilot award (AR120332 to Schweitzer), Merck Foundation (Hessl), and National Institutes of Health (P50MH106438).
Subject tracking and data management for this project were supported by the MIND Institute Intellectual and Developmental Disabilities Research Center (P50HD103526) and the National Center for Advancing Translational Sciences, National Institutes of Health, through award UL1 TR001860, respectively.
Contributor Information
Catrina A. Calub, University of California, Davis;.
Songpoom Benyakorn, Srinakharinwirot University, Bangkok, Thailand;.
Shuai Sun, University of California, Davis..
Ana-Maria Iosif, University of California, Davis..
Lauren H. Boyle, University of California, Davis.
Marjorie Solomon, University of California, Davis..
David Hessl, University of California, Davis..
Julie B. Schweitzer, University of California, Davis.
References
- Abbeduto L, Thurman AJ, McDuffie A, Klusek J, Feigles RT, Ted Brown W, Harvey DJ, Adayev T, LaFauci G, Dobkins C, & Roberts JE (2019). ASD comorbidity in fragile × syndrome: Symptom profile and predictors of symptom severity in adolescent and young adult males. Journal of Autism and Developmental Disorders, 49(3), 960–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksayli ND, Sala G, & Gobet F (2019). The cognitive and academic benefits of Cogmed: A meta-analysis. Educational Research Review, 27, 229–243. [Google Scholar]
- Alloway TP (2009). Working Memory, but Not IQ, Predicts Subsequent Learning in Children With Learning Difficulties. European Journal of Psychological Assessment, 25(2), 92–98. [Google Scholar]
- Aman MG, Norris M, Kaat AJ, Andrews H, Choo T-H, Chen C, Wheeler A, Bann C, & Erickson C (2020). Factor structure of the aberrant behavior checklist in individuals with fragile × syndrome: Clarifications and future guidance. Journal of Child and Adolescent Psychopharmacology, 30(8), 512–521. 10.1089/cap.2019.0177 [DOI] [PubMed] [Google Scholar]
- American Psychological Association. (2013). The Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (5th ed.). American Psychiatric Publishing. [Google Scholar]
- Antshel KM, Polacek C, McMahon M, Dygert K, Spenceley L, Dygert L, Miller L, & Faisal F (2011). Comorbid ADHD and anxiety affect social skills group intervention treatment efficacy in children with autism spectrum disorders. Journal of Developmental and Behavioral Pediatrics, 32(6), 439–446. 10.1097/DBP.0b013e318222355d [DOI] [PubMed] [Google Scholar]
- Au J, Berkowitz-Sutherland L, Schneider A, Schweitzer JB, Hessl D, & Hagerman R (2014). A feasibility trial of Cogmed working memory training in fragile × syndrome. Journal of Pediatric Genetics, 3(3), 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (2007). Working memory, thought, and action (Vol. 45). Oxford University Press. [Google Scholar]
- Bailey DB, Hatton DD, Mesibov G, Ament N, & Skinner M (2000). Early development, temperament, and functional impairment in autism and fragile × syndrome. Journal of Autism and Developmental Disorders, 30(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Skinner M, & Mesibov G (2001). Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile × syndrome. Journal of Autism and Developmental Disorders, 31(2), 165–174. [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee LC, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Van Naarden Braun K, & Dowling NF (2018). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. MMWR Surveillance Summaries, 67(6), 1–23. 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S, Hooper S, Skinner M, Hatton D, Schaaf J, Ornstein P, & Bailey D (2011). Working memory subsystems and task complexity in young boys with fragile × syndrome. Journal of Intellectual Disability Research, 55(1), 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal VH, Kim S-H, Cheong D, & Lord C (2015). Daily living skills in individuals with autism spectrum disorder from 2 to 21 years of age. Autism, 19(7), 774–784. 10.1177/1362361315575840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay KL, Lee CH, Bruno JL, Lightbody AA, & Reiss AL (2019). Closing the gender gap in fragile × syndrome: Review of females with fragile × syndrome and preliminary research findings. Brain Sciences, 9(1), 1–14. 10.3390/brainsci9010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, & Warren ST (2008). Fragile × syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron, 60(2), 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyakorn S, Calub CA, Riley SJ, Schneider A, Iosif AM, Solomon M, Hessl D, & Schweitzer JB (2018). Computerized cognitive training in children with autism and intellectual disabilities: Feasibility and satisfaction study. Journal of Medical Internet Research Mental Health, 5(2), e40. 10.2196/mental.9564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis EM, Lindemann L, Jnch AE, Apostol G, Bear MF, Carpenter RL, Crawley JN, Curie A, Des Portes V, Hossain F, & Jacquemont S (2018). Drug development for neurodevelopmental disorders: Lessons learned from fragile × syndrome. Nature Reviews Drug Discovery, 17(4), 280–299. 10.1038/nrd.2017.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, & Bailey A (1999). Autism screening questionnaire: Diagnostic validity. The British Journal of Psychiatry, 175(5), 444–451. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Schmidt-Lackner S, Romanczyk R, & Sudhalter V (2003). The PDD Behavior Inventory: A rating scale for assessing response to intervention in children with pervasive developmental disorder. Journal of Autism and Developmental Disorders, 33(1), 31–45. [DOI] [PubMed] [Google Scholar]
- Cohen IL, & Sudhalter V (2005). PDD Behavior Inventory (PDDBI) Psychological Assessment Resources. [Google Scholar]
- Conners CK (2008). Conners Third Edition (Conners 3). Western Psychological Services. [Google Scholar]
- Craig F, Margari F, Legrottaglie AR, Palumbi R, De Giambattista C, & Margari L (2016). A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatric Disease and Treatment, 12, 1191–1202. 10.2147/NDT.S104620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M, Prins PJ, Schmand BA, & Geurts HM (2015). Working memory and cognitive flexibility-training for children with an autism spectrum disorder: A randomized controlled trial. Journal of Child Psychology and Psychiatry, 56(5), 566–576. [DOI] [PubMed] [Google Scholar]
- de Vries M, Verdam MG, Prins PJ, Schmand BA, & Geurts HM (2018). Exploring possible predictors and moderators of an executive function training for children with an autism spectrum disorder. Autism, 22(4), 440–449. [DOI] [PubMed] [Google Scholar]
- Dyer-Friedman J, Glaser B, Hessl D, Johnston C, Huffman LC, Taylor A, Wisbeck J, & Reiss AL (2002). Genetic and environmental influences on the cognitive outcomes of children with fragile × syndrome. Journal of the American Academy of Child and Adolescent Psychiatry, 41(3), 237–244. 10.1097/00004583-200203000-00002 [DOI] [PubMed] [Google Scholar]
- Ferri SL, Abel T, & Brodkin ES (2018). Sex differences in autism spectrum disorder: A review. Current Psychiatry Reports, 20(2), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, & Newby RF (1998). Use of the restricted academic task in ADHD dose-response relationships. Journal of Learning Disabilities, 31(6), 608–612. 10.1177/002221949803100611 [DOI] [PubMed] [Google Scholar]
- Freeman NC, Gray KM, Taffe JR, & Cornish KM (2015). Development of a new attention rating scale for children with intellectual disability: The Scale of Attention in Intellectual Disability (SAID). American Journal on Intellectual and Developmental Disabilities, 120(2), 91–109. [DOI] [PubMed] [Google Scholar]
- Friedman LM, Rapport MD, Orban SA, Eckrich SJ, & Calub CA (2018). Applied problem solving in children with ADHD: The mediating roles of working memory and mathematical calculation. Journal of Abnormal Child Psychology, 46(3), 491–504. 10.1007/s10802-017-0312-7 [DOI] [PubMed] [Google Scholar]
- Gallant S (2007, August 17–20). Psychometric properties of the Conners 3rd Edition [Poster presentation]. Annual meeting of the American Psychological Association, San Francisco, CA [Google Scholar]
- Gotham K, Risi S, Pickles A, & Lord C (2006). The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37(4), 613–627. 10.1007/s10803-006-0280-1 [DOI] [PubMed] [Google Scholar]
- Gray S, Chaban P, Martinussen R, Goldberg R, Gotlieb H, Kronitz R, Hockenberry M, & Tannock R (2012). Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: A randomized controlled trial. Journal of Child Psychology and Psychiatry, 53(12), 1277–1284. [DOI] [PubMed] [Google Scholar]
- Green CT, Long DL, Green D, Iosif A-M, Dixon JF, Miller MR, Fassbender C, & Schweitzer JB (2012). Will working memory training generalize to improve off-task behavior in children with attention-deficit/hyperactivity disorder? Neurotherapeutics, 9(3), 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grizenko N, Lachance M, Collard V, Lageix P, Baron C, Ben Amor L, Ter Stepanian M, Mbekou V, Schwartz G, Bellingham J, & Joober R (2004). Sensitivity of tests to assess improvement in ADHD symptomatology. The Canadian Child and Adolescent Psychiatry Review 13(2), 36–39. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2538631/ [PMC free article] [PubMed] [Google Scholar]
- Handen BL, McAuliffe S, Janosky J, Feldman H, & Breaux AM (1998). A playroom observation procedure to assess children with mental retardation and ADHD. Journal of Abnormal Child Psychology, 26(4), 269–277. 10.1023/a:1022654417460 [DOI] [PubMed] [Google Scholar]
- Hessl D, Nguyen DV, Green C, Chavez A, Tassone F, Hagerman RJ, Senturk D, Schneider A, Lightbody A, Reiss AL & Hall S (2009). A solution to limitations of cognitive testing in children with intellectual disabilities: The case of fragile × syndrome. Journal of Neurodevelopmental Disorders, 1(1), 33–45. 10.1007/s11689-008-9001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Schweitzer JB, Nguyen DV, McLennan YA, Johnston C, Shickman R, & Chen Y (2019). Cognitive training for children and adolescents with fragile × syndrome: A randomized controlled trial of Cogmed. Journal of Neurodevelopmental Disorders, 11(1), 1–14. 10.1186/s11689-019-9264-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kercood S, Grskovic JA, Banda D, & Begeske J (2014). Working memory and autism: A review of literature. Research in Autism Spectrum Disorders, 8(10), 1316–1332. [Google Scholar]
- Kerns KA, Macoun S, MacSween J, Pei J, & Hutchison M (2017). Attention and working memory training: A feasibility study in children with neurodevelopmental disorders. Applied Neuropsychology: Child, 6(2), 120–137. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, & Westerberg H (2005). Computerized training of working memory in children with ADHD—A randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry, 44(2), 177–186. 10.1097/00004583-200502000-00010 [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, & Raiker JS (2010). ADHD and working memory: The impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. Journal of Abnormal Child Psychology, 38(2), 149–161. [DOI] [PubMed] [Google Scholar]
- Kwon H, Menon V, Eliez S, Warsofsky IS, White CD, Dyer-Friedman JJ, Taylor AK, Glover GH & Reiss AL (2001). Functional neuroanatomy of visuospatial working memory in fragile × syndrome: Relation to behavioral and molecular measures. American Journal of Psychiatry, 158(7), 1040–1051. 10.1176/appi.ajp.158.7.1040 [DOI] [PubMed] [Google Scholar]
- Lyall K, Schweitzer JB, Schmidt RJ, Hertz-Picciotto I, & Solomon M (2017). Inattention and hyperactivity in association with autism spectrum disorders in the CHARGE study. Research in Autism Spectrum Disorders, 35, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR, & Neuhaus JM (2008). Generalized, linear, and mixed models (Vol. 279). Wiley. [Google Scholar]
- McQuade JD, Murray-Close D, Shoulberg EK, & Hoza B (2013). Working memory and social functioning in children. Journal of Experimental Child Psychology, 115(3), 422–435. [DOI] [PubMed] [Google Scholar]
- Melby-Lervåg M, & Hulme C (2013). Is working memory training effective? A meta-analytic review. Developmental Psychology, 49(2), 270–291. [DOI] [PubMed] [Google Scholar]
- Mendelson JL, Gates JA, & Lerner MD (2016). Friendship in school-age boys with autism spectrum disorders: A meta-analytic summary and developmental, process-based model. Psychological Bulletin, 142(6), 601–622. 10.1037/bul0000041 [DOI] [PubMed] [Google Scholar]
- Miller LE, Burke JD, Troyb E, Knoch K, Herlihy LE, & Fein DA (2017). Preschool predictors of school-age academic achievement in autism spectrum disorder. The Clinical Neuropsychologist, 31(2), 382–403. 10.1080/13854046.2016.1225665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitiive Psycholgy, 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, & Rapin I (2004). The genetics of autism. Pediatrics, 113(5), e472–e486. [DOI] [PubMed] [Google Scholar]
- Neely RJ, Green JL, Sciberras E, Hazell P, & Anderson V (2016). Relationship between executive functioning and symptoms of attention-deficit/hyperactivity disorder and autism spectrum disorder in 6–8 year old children. Journal of Autism and Developmental Disorders, 46(10), 3270–3280. [DOI] [PubMed] [Google Scholar]
- Oostra BA, & Willemsen R (2003). A fragile balance: FMR1 expression levels. Human Molecular Genetics, 12 Spec No 2, R249–257. 10.1093/hmg/ddg298 [DOI] [PubMed] [Google Scholar]
- Pearson DA, Santos CW, Aman MG, Arnold LE, Casat CD, Mansour R, Lane DM, Loveland KA, Bukstein OG, Jerger SW, & Jerger SW (2013). Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. Journal of Child and Adolescent Psychopharmacology, 23(5), 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, & Alderson RM (2009). Hyperactivity in boys with attention-deficit/hyperactivity disorder (ADHD): A ubiquitous core symptom or manifestation of working memory deficits? Journal of Abnormal Child Psychology, 37(4), 521–534 [DOI] [PubMed] [Google Scholar]
- Rapport MD, Orban SA, Kofler MJ, & Friedman LM (2013). Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clinical Psychology Review, 33(8), 1237–1252. [DOI] [PubMed] [Google Scholar]
- Roane HS, Fisher WW, & Carr JE (2016). Applied behavior analysis as treatment for autism spectrum disorder. Journal of Pediatrics, 175, 27–32. 10.1016/j.jpeds.2016.04.023 [DOI] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LAH, Hatton DD, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile × syndrome. Journal of Autism and Developmental Disorders, 37(9), 1748–1760. [DOI] [PubMed] [Google Scholar]
- Rogers M, Hwang H, Toplak M, Weiss M, & Tannock R (2011). Inattention, working memory, and academic achievement in adolescents referred for attention deficit/hyperactivity disorder (ADHD). Child Neuropsychology, 17(5), 444–458. 10.1080/09297049.2010.544648 [DOI] [PubMed] [Google Scholar]
- Roid G, & Miller L (1997). Leiter International Performance Scale-Revised. Stoelting. [Google Scholar]
- Roid GH (2003). Stanford-Binet Intelligence Scales–Fifth Edition. Riverside Publishing. [Google Scholar]
- Rommelse NN, Franke B, Geurts HM, Hartman CA, & Buitelaar JK (2010). Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disor der. European Child and Adolescent Psychiatry, 19(3), 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, & Lord C (2003). Autism Diagnostic Interview-Revised Manual. Western Psychological Services. [Google Scholar]
- Ryland HK, Hysing M, Posserud M-B, Gillberg C, & Lundervold AJ (2014). Autistic features in school age children: IQ and gender effects in a population-based cohort. Research in Autism Spectrum Disorders, 8(3), 266–274. [Google Scholar]
- Sala G, Aksayli ND, Tatlidil KS, Tatsumi T, Gondo Y, Gobet F, Zwaan R, & Verkoeijen P (2019). Near and far transfer in cognitive training: A second-order meta-analysis. Collabra Psychology, 5(1), 1–22. 10.1525/collabra.203 [DOI] [Google Scholar]
- Sala G, & Gobet F (2017). Working memory training in typically developing children: A meta-analysis of the available evidence. Developmental Psychology, 53(4), 671–685. [DOI] [PubMed] [Google Scholar]
- Schaefer GB, & Mendelsohn NJ (2008). Clinical genetics evaluation in identifying the etiology of autism spectrum disorders. Genetics in Medicine, 10(4), 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LM, Shaffer RC, Hessl D, & Erickson C (2019). Executive function in fragile × syndrome: A systematic review. Brain Sciences, 9(1), 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H, Harvey DJ, Li Y, McLennan YA, Johnston CK, Shickman R, Piven J, Schweitzer JB, & Hessl D (2020). Cognitive training deep dive: The impact of child, training behavior and environmental factors within a controlled trial of Cogmed for fragile × syndrome. Brain Sciences, 10(10), 1–14. 10.3390/brainsci10100671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinaver CS, Entwistle PC, & Söderqvist S (2014). Cogmed WM training: Reviewing the reviews. Applied Neuropsychology: Child, 3(3), 163–172. [DOI] [PubMed] [Google Scholar]
- Shipstead Z, Redick TS, & Engle RW (2012). Is working memory training effective? Psychological Bulletin, 138(4), 628–654. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, & Stine-Morrow EA (2016). Do “brain-training” programs work? Psychological Science in the Public Interest, 17(3), 103–186. 10.1177/1529100616661983 [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, & Bailey D Jr. (2006). ADHD symptoms in children with FXS. American Journal of Medical Genetics Part A, 140A(21), 2275–2288. 10.1002/ajmg.a.31388 [DOI] [PubMed] [Google Scholar]
- Swanson HL, & Alloway TP (2012). Working memory, learning, and academic achievement. In Harrisss KR, Graham S, Urdan T, McCormick CB, Sinatra GM, & Sweller J (Eds.), APA educational psychology handbook, Vol 1: Theories, constructs, and critical issues (pp. 327–366). American Psychological Association. 10.1037/13273-012 [DOI] [Google Scholar]
- Swanson HL, Xinhua Z, & Jerman O (2009). Working memory, short-term memory, and reading disabilities: A selective meta-analysis of the literature. Journal of Learning Disabilities, 42(3), 260–287. 10.1177/0022219409331958 [DOI] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Kover ST, Hagerman RJ, & Abbeduto L (2015). Autism symptomatology in boys with fragile × syndrome: A cross sectional developmental trajectories comparison with nonsyndromic autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(9), 2816–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman C, Eninger L, Forssman L, & Bohlin G (2011). The relation between working memory components and ADHD symptoms from a developmental perspective. Developmental Neuropsychology, 36(2), 181–198. [DOI] [PubMed] [Google Scholar]
- Torrioli MG, Vernacotola S, Peruzzi L, Tabolacci E, Mila M, Militerni R, Musumeci S, Ramos FJ, Frontera M, Sorge G, Marzullo E, Romeo G, Vallee L, Veneselli E, Cocchi E, Garbarino E, Moscato U, Chiurazzi P, D’Iddio S, Calvani M, …Neri G (2008). A double-blind, parallel, multicenter comparison of L-acetylcarnitine with placebo on the attention deficit hyperactivity disorder in fragile × syndrome boys. American Journal of Medical Genetics Part A, 146A(7), 803–812. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, & Kenworthy L (2009). Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research, 2(6), 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang Y. -b., Liu L. -l., Cui J. -f., Wang J, Shum DH, van Amelsvoort T & Chan RC (2017). A meta-analysis of working memory impairments in autism spectrum disorders. Neuropsychology Review, 27(1), 46–61. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children. Psychological Corporation. [Google Scholar]
- Weckstein SM, Weckstein EJ, Parker CD, & Westerman MW (2017). A retrospective chart analysis with follow-up of cogmed working memory training in children and adolescents with autism spectrum disorder. Medical Science Monitor Basic Research, 23, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf AS, McPheeters ML, Peters B, Sathe N, Travis R, Aiello R, Williamson E, Veenstra-VanderWeele J, Krishnaswami S, Jerome R, & Warren Z (2014). AHRQ comparative effectiveness reviews. In Therapies for children with autism spectrum disorder: Behavioral interventions update. Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]


