Abstract
Fertilization of eggs from the African clawed frog Xenopus laevis is characterized by an increase in cytosolic calcium, a phenomenon that is also observed in other vertebrates such as mammals and birds. During fertilization in mammals and birds, the transfer of the soluble PLCζ from sperm into the egg is thought to trigger the release of calcium from the endoplasmic reticulum (ER). Injecting sperm extracts into eggs reproduces this effect, reinforcing the hypothesis that a sperm factor is responsible for calcium release and egg activation. Remarkably, this occurs even when sperm extracts from X. laevis are injected into mouse eggs, suggesting that mammals and X. laevis share a sperm factor. However, X. laevis lacks an annotated PLCZ1 gene, which encodes the PLCζ enzyme. In this study, we attempted to determine whether sperm from X. laevis express an unannotated PLCZ1 ortholog. We identified PLCZ1 orthologs in 11 amphibian species, including 5 that had not been previously characterized, but did not find any in either X. laevis or the closely related Xenopus tropicalis. Additionally, we performed RNA sequencing on testes obtained from adult X. laevis males and did not identify potential PLCZ1 orthologs in our dataset or in previously collected ones. These findings suggest that PLCZ1 may have been lost in the Xenopus lineage and raise the question of how fertilization triggers calcium release and egg activation in these species.
Background
For most animals, fertilization triggers a rapid increase in the calcium of the egg cytoplasm. This elevated calcium then initiates polyspermy blocks, which prevent multiple sperm from entering an already fertilized egg, and embryonic development (Denninger et al., 2014; Swann and Lai, 2016). The sperm-derived enzyme phospholipase C zeta (PLCζ) is proposed to signal this process in mammals (Nomikos et al., 2017; Saunders et al., 2002) and birds (Coward et al., 2005) by increasing the egg inositol trisphosphate (IP3). This increase in IP3 then triggers calcium release from the endoplasmic reticulum (ER) (Bedford-Guaus et al., 2011; Cox et al., 2002; Ito et al., 2008; Mizushima et al., 2014; Mizushima et al., 2007; Ross et al., 2008; Saunders et al., 2002; Yoneda et al., 2006). It is not yet known whether other animals use PLCζ in the same way.
We sought to determine if the African clawed frog (Xenopus laevis) uses PLCζ in fertilization. Fertilization of X. laevis eggs increases cytoplasmic calcium (Grey et al., 1982; Kline, 1988), which then activates a chloride channel called TMEM16A to depolarize the membrane and initiate the fast block to polyspermy (Wozniak et al., 2018a). The initiation of the fast block to polyspermy requires PLC activation (Wozniak et al., 2018b), and we previously explored a role for egg PLCs in signaling the fast block. X. laevis eggs express three PLC isoforms (Plcg1, Plcb1, and Plcb3) (Komondor et al., 2022). However, we found that the signaling pathways that typically activate these isoforms are not involved in the fast block (Komondor et al., 2022). We therefore considered the possibility that X. laevis sperm donates PLCζ to the egg, as occurs in mammals and birds. The observation that injecting extracts from X. laevis sperm into mouse eggs leads to an elevation of cytoplasmic calcium comparable to fertilization in mouse eggs supports the idea that PLCζ may be involved in fertilization in X. laevis (Dong et al., 2000). However, no PLCζ-encoding gene (PLCZ1) has been identified in the genomes of X. laevis or the closely related species X. tropicalis.
Results
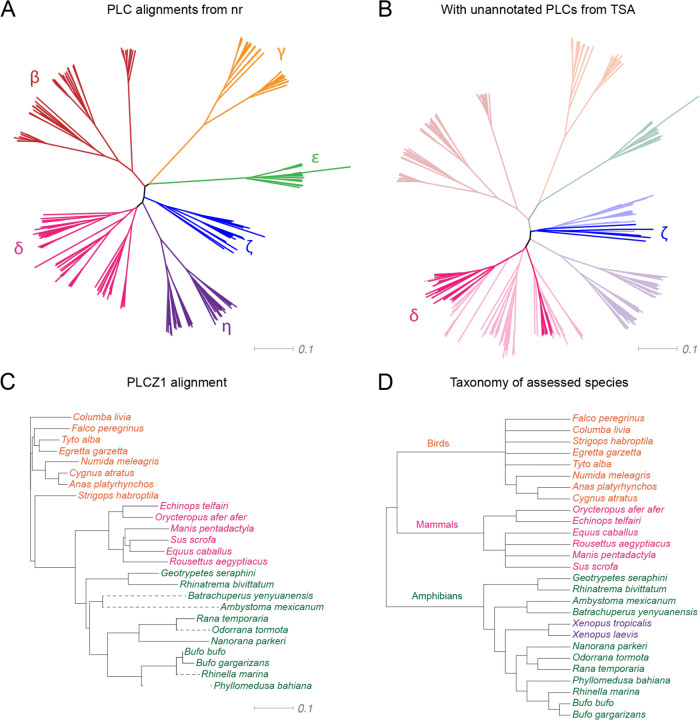
At the outset of our study, we conducted a search for PLCζ in amphibians. The class Amphibia is comprised of three orders: tailless frogs and toads (Anura), newts and salamanders (Urodela), and limbless caecilians (Gymnophiona). Using blastp with mouse Plcz1 as our query against the non-redundant sequence database (nr), we identified PLCZ1 orthologs in six unique amphibian species: the Gaboon caecilian (Geotrypetes seraphini), two-lined caecilian (Rhinatrema bivittatum), common frog (Rana temporaria), Tibetan frog (Nanorana parkeri), common toad (Bufo bufo), and the Asiatic toad (Bufo gargarizans) (Fig. 1a). These putative PLCZ1 orthologs, along with other PLC isoforms found in the same species, were included in an alignment with PLCs from representative species including mammals, birds, reptiles, and lungfish, which served as an outgroup (Fig. 1b). The annotated PLCZ1 orthologs were found to cluster with other PLCZ1 isoforms in the alignment (Fig. 1c), supporting their classification as PLCζ isozymes.
Figure 1.
Identification of amphibian PLCζ isozymes. a Phylogram displaying the alignment of annotated PLC isoforms from representative mammals, birds, and amphibians. b Alignment of unannotated PLCZ1 homologs from the amphibian transcriptome shotgun assembly (TSA) dataset. Reference sequences from (a) are included. c Phylogram representing the alignment of PLCZ1 orthologs (branches indicated with dashed lines) identified in (b) with annotated sequences from the non-redundant nucleotide database (solid branches). d Cladogram of species from (c); Xenopus species (for which no PLCZ1 orthologs could be identified) are indicated in purple.
To expand our search for PLCZ1 isozymes in amphibians, we used tblastn with Plcz1 from the Tibetan frog (Nanorana parkeri) as our query against the TSA database. This analysis identified both PLCZ1 and PLCD1–4 orthologs. We included these sequences in an alignment with PLCs from a variety of representative species, which clearly identified five additional PLCZ1 orthologs: the Yenyuan stream salamander (Batrachuperus yenyuanensis), axolotl (Ambystoma mexicanum), concave-eared torrent frog (Odorrana tormota), cane toad (Rhinella marina), and the neotropical leaf-frog (Phyllomedusa bahiana) (Fig. 1b). These additional sequences also grouped with other PLCZ1 orthologs from amphibian species in a way that matched the taxonomic classification of the assessed species (as shown in Figs. 1c&d).
We conducted transcriptomic analysis to determine if an unannotated PLCZ1 ortholog is expressed in X. laevis. In mammals, the PLCζ protein is exclusively found in sperm (Session et al., 2016), but mature sperm are transcriptionally quiescent (Kierszenbaum and Tres, 1975) and not suitable for RNA-seq. We therefore performed RNA-seq on whole testes, recognizing that not all RNA present in the testes represents proteins found in sperm, but RNA that encode sperm proteins should be present in the testes. We obtained RNA from the testes of three individual adult male X. laevis frogs and performed RNA-seq on these three samples. Of the contiguous RNA-seq reads, 48–51% were aligned to the X. laevis genome. We observed at least one transcript in one testis from an individual frog for 33,377 genes. We performed gene ontology (GO) analysis on 6,000 of the most highly expressed genes among these testis-derived transcripts. Of these, 48.3% were identified with GO biological process terms, many of which were important for general cell maintenance (e.g. translation, protein folding, cell cycle). Some were also involved in specialized processes such as spermatogenesis. Our overall results were highly correlated (Fig. S1) and generally matched related datasets (Fig. 2). In our dataset, we identified several essential sperm transcripts, including deleted in azoospermia-like (dazl) (Houston and King, 2000) and the testes-specific histone (h1–10) (Oikawa et al., 2020; Shechter et al., 2009). Other enriched transcripts, such as astl2b, rflcii, and ribc1, are known to be specifically expressed in X. laevis testis (Session et al., 2016) or as proteins in sperm (Teperek et al., 2014). Some of these transcripts, including rflcii, cfh, and tubb4b, have also been shown to be required for fertility in other species (Table 1) (Agarwal et al., 2016; Ferlin et al., 2012; Maccarinelli et al., 2017; Sakaue et al., 2010).
Figure 2.
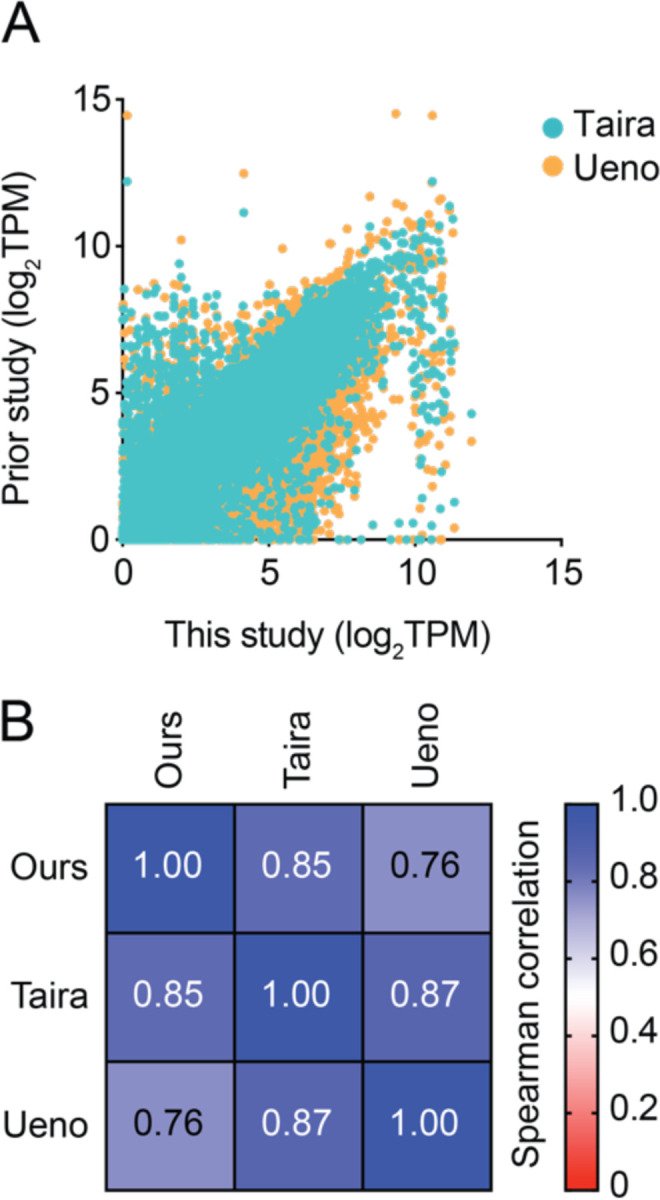
Comparison of our RNA-seq data with two other published datasets. a Plot showing transcripts per million (TPM) for matched transcripts between this study (X-axis) and two datasets in a previous study (Session et al., 2016). b Array representing the Spearman rank correlation between each dataset in (a).
Table 1.
Comparative gene expression in testes of X. laevis. Expression of testis-associated genes between our averaged datasets and the Taira dataset (Session et al. 2016).
| Gene | This study | Taira (2016) |
|---|---|---|
| ASTL2b.L | 11.81 | 12.62 |
| DAZL.L | 10.21 | 10.62 |
| RFLCII.S | 12.55 | 11.09 |
| CFH.L | 9.18 | 9.92 |
| H1–10.S | 6.76 | 9.77 |
| TUBB4B.L | 10.20 | 9.27 |
| RIBC1.S | 8.29 | 9.86 |
| SYCP3.L | 8.01 | 9.04 |
| SYNGR4.L | 8.46 | 9.26 |
| PBK.L | 9.53 | 10.12 |
| HORMAD1.S | 7.84 | 8.59 |
| ODF3.S | 8.22 | 7.90 |
To identify sequences in the unmapped RNA that may belong to a PLCZ1 gene, we ran a blastx search of the reads that hadn’t been matched to any known genes against a database of annotated PLCZ1 sequences from mammals and amphibians. This search identified 360 reads that had a very low E-value (less than 10−6) and covered a specific region (residues 170–654) of a representative PLCZ1 protein sequence from the neotropical leaf frog, Phyllomedusa bahiana. However, when we used a blastn search to compare these reads to the amphibian nr database, we discovered that most of them matched to PLCD4 or other isoforms (Supporting files). This makes sense in that PLCζ is the only PLC isoform that lacks a pleckstrin homology (PH) domain but is otherwise like PLCδ (Fig. 3). Our results suggest that the unmapped reads in our dataset arose from incomplete contigs of annotated genes.
Figure 3.
Domain architecture of PLC isoforms PLCδ and PLCζ. Schematic representation of the domain structures: pleckstin homology (PH) domain, EF hands, X and Y catalytic domains, and the C-terminal C2 domain.
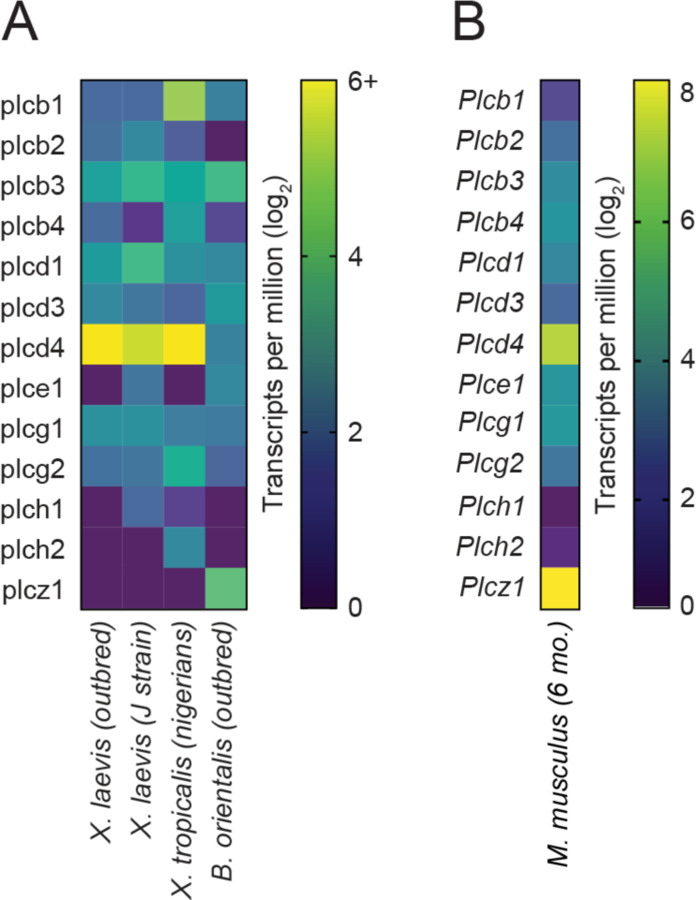
We searched the RNA-seq dataset for transcripts encoding PLC isoforms and found transcripts for PLCβ, PLCδ, and PLCγ isoforms (Fig. 4a). Of these, the plcd4 transcript was the most abundant. plcd4 was also the most abundant PLC transcript in testis RNA-seq datasets from the inbred X. laevis J strain (Session et al., 2016) and the X. tropicalis Nigerian strain, both of which lacked PLCZ1. By comparison, testes from the Oriental fire-bellied toad Bombina orientalis contained plcz1 transcripts but had substantially reduced levels of plcd4 compared to Xenopus (Fig. 4a). By comparison, data obtained from adult (6 months old) mouse testis revealed that Plcz1 was the most abundant transcript, followed by Plcd4 (Fig. 4b) (Huang et al., 2021).
Figure 4. Heatmap of PLC expression in anuran and mouse testis.
a Heatmaps of the expression levels (shown as log2 transformed transcripts per million) of annotated PLC genes from the indicated animals. The X. laevis (outbred) data obtained here, X. laevis J strain from GSE73419 (Session et al., 2016), X. tropicalis from GSM5230669 (unpublished), and B. orientalis from GSE163874 (unpublished). b Expression levels of PLC isoforms in the testis of adult (6 month) mice (GSE181426) (Huang et al., 2021).
Discussion
As in other species, fertilization in X. laevis triggers an elevation of cytosolic calcium in the egg (Runft et al., 2002). This calcium is released from the ER by a pathway that requires PLC (Runft et al., 1999). Several mechanisms have been proposed to explain the trigger that initiates this process. In mammals and birds, the release of PLCζ from the sperm into the egg during fertilization is thought to trigger the increase in calcium in the egg cytoplasm (Swann and Lai, 2016).
Because of the role that it plays in mammalian and avian fertilization, we were surprised to find that there was no annotated ortholog of PLCZ1 in X. laevis. This prompted us to investigate whether other amphibians possess this enzyme. In our search, we used both nucleotide and transcriptome shotgun assembly (TSA) databases, using an amphibian PLCZ1 ortholog as a query to increase our chances of finding a match. We were ultimately able to identify PLCZ1 orthologs in 11 amphibian species (Fig. 1a–b). Although this represents a small portion of the amphibian transcriptomes available in the TSA database, the lack of PLCZ1 in the TSA search does not necessarily mean that it is not expressed in the associated species, as many amphibian transcriptome datasets do not include testes, where PLCζ is known to be expressed.
We aligned the amino acid sequences of the PLCZ1 protein in amphibians to those of the same protein in other animals, as well as to the sequences of other PLC subtypes, including the beta, gamma, delta, epsilon, and eta isoforms. This comparison included species with PLCζ enzymes with a verified role in fertilization, such as mammals and birds. By aligning the sequences of all PLC isoforms from each species, we found that the isoforms tended to group together primarily by subtype (Fig. 1a–b) and within subtype by order (Fig. 1c). This pattern of grouping by order is also reflected in the taxonomic classification of the species we examined (Fig. 1d). With the exceptions of X. laevis and X. tropicalis, every amphibian in our dataset transcribed a PLCZ1 ortholog. These results suggest that PLCZ1 may have been lost in the Xenopus lineage.
Conclusions
Since the Xenopus species examined in our study lack a PLCζ isozyme, how fertilization activates PLC to trigger calcium release from the ER in these frogs is yet to be determined. Several sperm factors other than PLCζ have been proposed to initiate this process, including citrate synthase (Harada et al., 2011; Harada et al., 2007) and post-acrosomal WWP-domain-binding protein (PAWP) (Wu et al., 2007). For Xenopus, it’s possible that a sperm derived protein does not signal an increase of the cytoplasmic calcium in the egg. For example, another possibility is the “receptor model” of egg activation, in which a signaling cascade is activated by the binding of a sperm surface ligand to an egg receptor (Jaffe, 1990). X. laevis eggs express a PLCγ isoform (Plcg1), which can be activated by phosphorylation of a critical residue in the active site (Kadamur and Ross, 2013), as well as PLCβ isoforms (Plcb1 and Plcb3), which are activated by GPCRs (Rhee, 2001; Smrcka et al., 1991). However, fertilization in X. laevis does not cause observable phosphorylation of the critical tyrosine residue of Plcg1, and inhibitors of either PLCγ or PLCβ pathway had no effect on fertilization (Komondor et al., 2022). Another alternative explanation is the “calcium bomb” hypothesis, which proposes that a burst of calcium is introduced to the egg by the sperm itself, resulting in egg activation and calcium release from the ER (Jaffe, 1983). In other model systems, introducing calcium alone was not sufficient to produce calcium release, indicating that other factors are required (Swann and Ozil, 1994). Whether calcium alone is sufficient to initiate the polyspermy blocks and embryonic development in Xenopus eggs is yet to be determinized. Further research is needed to fully understand the mechanisms behind activation of Xenopus eggs.
Methods
Retrieval of amphibian PLCZ1 sequences
We searched the BLAST non-redundant protein sequence database for amphibian PLCZ1 orthologs using the protein sequence of mouse Plcz1 as a query. We retrieved annotated orthologs from six amphibian species and included them, along with sequences encoding other PLC isoforms (beta, gamma, delta, epsilon, and eta) in an alignment of representative PLC sequences from amphibians, birds, and mammals using Kalign (Lassmann, 2020). We used the neighbor-joining phylogenetic output from this alignment to generate an unrooted tree with Dendroscope (Huson and Scornavacca, 2012).
We expanded our search for PLCZ1 orthologs by performing a reverse translated tblastn search using the sequence of the Nanorana parkerii PLCZ1 as a query against the amphibian transcriptome shotgun assembly (TSA) database. We retrieved more than 100 sequences of possible PLCZ1 orthologs, which we translated using getorf (EMBOSS), selecting the longest open reading frames from each transcript. We aligned these sequences with the representative PLC sequences that we had previously obtained from amphibians, birds, and mammals to distinguish genuine PLCZ1 orthologs from hits that encoded other PLC subtypes. Through this analysis, we identified genes encoding PLCZ1 in 5 additional amphibian species. Finally, we used InterProScan (Nomikos et al., 2005) to validate the potential PLCZ1 orthologs that we had identified by looking for the absence of a pleckstrin homology (PH) domain, which is a defining feature of PLCζ enzymes.
Animals
All animal procedures were conducted using acceptable standards of humane animal care and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. X. laevis adults were obtained commercially (Nasco) and kept in a controlled environment with a 12-hour light/dark cycle at 20°C.
RNA isolation
To obtain RNA, we euthanized sexually mature X. laevis males by immersing them in a solution of tricaine-S (3.6 g/L, pH 7.4) for 30 minutes. We then removed the testes and carefully cleaned them to remove fat and vascular tissue. We prepared tissue for RNA isolation by freezing it with liquid nitrogen and grinding it into a powder with a mortar and pestle. We then isolated RNA following instructions provided by RNeasy and QIAshreddder kits (QIAgen).
RNA-seq library preparation and data acquisition
Before preparing the RNA-seq library, we first determined the integrity of the RNA using electrophoresis and measured its concentration by Qubit (Life Technologies). We then used Illumina TruSeq mRNA kit (Illumina) with modified protocol: SuperScript IV (Invitrogen) was used for first strand synthesis, the library amplified with 10 cycles of PCR, and the amplified library was cleaned up with 35 µL AMPureXP beads (Beckman Coulter). The library was sequenced with 75 bp paired-end mRNA reads on an Illumina NextSeq500 platform with a Mid Output 150 flowcell (Illumina).
RNA-seq analysis
Sequencing reads were uploaded to the public server at usegalaxy.org (Afgan et al., 2018). The reads were then aligned to Xenopus laevis genome (version 10.1) using HISAT2 (Galaxy version 2.2.1+galaxy0) with default settings for paired reads. Next, aligned fragments were mapped and quantified with featureCounts (Galaxy version 2.0.1+galaxy2) (Liao et al., 2014) using the Xenbase gene model as a reference.
BLAST search for unmapped PLCZ1
Command line BLASTx was used to search unmapped RNA-seq reads against a database containing annotated PLCZ1 protein sequences from Mus musculus (AAI06769.1), Homo sapiens (AAI25068.1), Bufo gararizans (XP_044137439.1), Bufo bufo (XP_040291278.1), Rhinatrema bivittatum (XP_029453199.1), and Nanorana parkeri (XP_018417886.1). We used an E value cutoff of 10−6, which produced 360 reads. We then aligned these reads to the nucleotide sequence of PLCZ1 from Phyllomedusa bahiana, which produced a noncontiguous alignment covering residues 170–654 of PLCZ1. To verify that these reads were not attributable to other PLC isoforms, we performed a multiple query BLAST search with these reads against amphibian sequences from the non-redundant nucleotide (nt) database.
Comparative transcriptome analysis
To compare transcript levels, we took the average of three individual experiments and reported transcript abundance as the number of transcripts per million. We deposited this data with the NCBI Gene Expression Omnibus (Edgar et al., 2002), which can be accessed using accession number GSE224304. To ensure that our data could be compared to previously existing data, we matched the transcriptome of our dataset against a GEO dataset for X. laevis (J strain) (GSE73419) (Session et al., 2016). We identified 13,277 genes to use for direct comparison, which we then ranked and compared using Spearman’s rank correlation in Prism (Graphpad).
To compare the expression of PLC subtypes in the testes of different organisms, we obtained GEO data for Xenopus laevis (J strain) (GSE73419), Xenopus tropicalis (nigerians) (GSM5230669), Bombina orientalis (GSE163874), and Mus musculus (GSE181426). We processed the expression data using a log2 transformation and created a heatmap using Prism (Graphpad) to visualize the interspecies comparison.
Supplementary Material
Acknowledgements
We thank D. Summerville for excellent technical assistance, and M.T. Lee for stimulating conversations.
Funding
This work was supported by National Institute for Health grant 1R01GM125638 to A.E.C., a Mellon Foundation fellowship to R.E.B., and a CMRF grant from the University of Pittsburgh to J.C.R. This project used the University of Pittsburgh HSCRF Genomics Research Core, RRID: SCR_018301 RNA-sequencing.
List of abbreviations
- PLC
phospholipase C
- IP3
inositol triphosphate
- ER
endoplasmic reticulum
- TMEM16A
TransMEMbrane 16A
- TSA
transcriptome shotgun assembly
- nr
non-redundant sequence database
- RNA-seq
RNA sequencing
Footnotes
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Gene Expression Omnibus, which can be accessed using accession number GSE224304.
Competing Interest
The authors declare no competing interests.
References
- Afgan E., Baker D., Batut B., van den Beek M., Bouvier D., Čech M., Chilton J., Clements D., Coraor N., Grüning B.A., Guerler A., Hillman-Jackson J., Hiltemann S., Jalili V., Rasche H., Soranzo N., Goecks J., Taylor J., Nekrutenko A., Blankenberg D., 2018. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research 46, W537–W544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Sharma R., Durairajanayagam D., Cui Z., Ayaz A., Gupta S., Willard B., Gopalan B., Sabanegh E., 2016. Spermatozoa protein alterations in infertile men with bilateral varicocele. Asian J Androl 18, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford-Guaus S.J., McPartlin L.A., Xie J., Westmiller S.L., Buffone M.G., Roberson M.S., 2011. Molecular cloning and characterization of phospholipase C zeta in equine sperm and testis reveals species-specific differences in expression of catalytically active protein. Biol Reprod 85, 78–88. [DOI] [PubMed] [Google Scholar]
- Coward K., Ponting C.P., Chang H.Y., Hibbitt O., Savolainen P., Jones K.T., Parrington J., 2005. Phospholipase Cz, the trigger of egg activation in mammals, is present in a non-mammalian species. Reproduction 130, 157–163. [DOI] [PubMed] [Google Scholar]
- Cox L.J., Larman M.G., Saunders C.M., Hashimoto K., Swann K., Lai F.A., 2002. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 124, 611–623. [DOI] [PubMed] [Google Scholar]
- Denninger P., Bleckmann A., Lausser A., Vogler F., Ott T., Ehrhardt D.W., Frommer W.B., Sprunck S., Dresselhaus T., Grossmann G., 2014. Male-female communication triggers calcium signatures during fertilization in Arabidopsis. Nat Commun 5, 4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.B., Tang T.S., Sun F.Z., 2000. Xenopus and chicken sperm contain a cytosolic soluble protein factor which can trigger calcium oscillations in mouse eggs. Biochem Biophys Res Commun 268, 947–951. [DOI] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., Lash A.E., 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlin A., Menegazzo M., Gianesello L., Selice R., Foresta C., 2012. Effect of relaxin on human sperm functions. J Androl 33, 474–482. [DOI] [PubMed] [Google Scholar]
- Grey R.D., Bastiani M.J., Webb D.J., Schertel E.R., 1982. An electrical block is required to prevent polyspermy in eggs fertilized by natural mating of Xenopus laevis. Dev Biol 89, 475–484. [DOI] [PubMed] [Google Scholar]
- Harada Y., Kawazoe M., Eto Y., Ueno S., Iwao Y., 2011. The Ca2+ increase by the sperm factor in physiologically polyspermic newt fertilization: its signaling mechanism in egg cytoplasm and the species-specificity. Dev Biol 351, 266–276. [DOI] [PubMed] [Google Scholar]
- Harada Y., Matsumoto T., Hirahara S., Nakashima A., Ueno S., Oda S., Miyazaki S., Iwao Y., 2007. Characterization of a sperm factor for egg activation at fertilization of the newt Cynops pyrrhogaster. Dev Biol 306, 797–808. [DOI] [PubMed] [Google Scholar]
- Houston D.W., King M.L., 2000. A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development 127, 447–456. [DOI] [PubMed] [Google Scholar]
- Huang Y., Li X., Sun X., Yao J., Gao F., Wang Z., Hu J., Wang Z., Ouyang B., Tu X., Zou X., Liu W., Lu M., Deng C., Yang Q., Xie Y., 2021. Anatomical Transcriptome Atlas of the Male Mouse Reproductive System During Aging. Front Cell Dev Biol 9, 782824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D.H., Scornavacca C., 2012. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Systematic biology 61, 1061–1067. [DOI] [PubMed] [Google Scholar]
- Ito M., Shikano T., Oda S., Horiguchi T., Tanimoto S., Awaji T., Mitani H., Miyazaki S., 2008. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod 78, 1081–1090. [DOI] [PubMed] [Google Scholar]
- Jaffe L.A., 1990. First messengers at fertilization. J Reprod Fertil Suppl 42, 107–116. [PubMed] [Google Scholar]
- Jaffe L.F., 1983. Sources of calcium in egg activation: a review and hypothesis. Dev Biol 99, 265–276. [DOI] [PubMed] [Google Scholar]
- Kadamur G., Ross E.M., 2013. Mammalian phospholipase C. Annu Rev Physiol 75, 127–154. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum A., Tres L.L., 1975. Structural and transcriptional features of the mouse spermatid genome. The Journal of cell biology 65, 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D., 1988. Calcium-dependent events at fertilization of the frog egg: injection of a calcium buffer blocks ion channel opening, exocytosis, and formation of pronuclei. Dev Biol 126, 346–361. [DOI] [PubMed] [Google Scholar]
- Komondor K.M., Bainbridge R.E., Sharp K.G., Rosenbaum J.C., Carlson A.E., 2022. TMEM16A activation for the fast block to polyspermy in the African clawed frog does not require conventional activation of egg PLCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann T., 2020. Kalign 3: multiple sequence alignment of large datasets. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Smyth G.K., Shi W., 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Maccarinelli F., Regoni M., Carmona F., Poli M., Meyron-Holtz E.G., Arosio P., 2017. Mitochondrial ferritin deficiency reduces male fertility in mice. Reprod Fertil Dev 29, 2005–2010. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Hiyama G., Shiba K., Inaba K., Dohra H., Ono T., Shimada K., Sasanami T., 2014. The birth of quail chicks after intracytoplasmic sperm injection. Development 141, 3799–3806. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Takagi S., Ono T., Atsumi Y., Tsukada A., Saito N., Shimada K., 2007. Possible role of calcium on oocyte development after intracytoplasmic sperm injection in quail (Coturnix japonica). J Exp Zool A Ecol Genet Physiol 307, 647–653. [DOI] [PubMed] [Google Scholar]
- Nomikos M., Blayney L.M., Larman M.G., Campbell K., Rossbach A., Saunders C.M., Swann K., Lai F.A., 2005. Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem 280, 31011–31018. [DOI] [PubMed] [Google Scholar]
- Nomikos M., Kashir J., Lai F.A., 2017. The role and mechanism of action of sperm PLC-zeta in mammalian fertilisation. Biochem J 474, 3659–3673. [DOI] [PubMed] [Google Scholar]
- Oikawa M., Simeone A., Hormanseder E., Teperek M., Gaggioli V., O’Doherty A., Falk E., Sporniak M., D’Santos C., Franklin V.N.R., Kishore K., Bradshaw C.R., Keane D., Freour T., David L., Grzybowski A.T., Ruthenburg A.J., Gurdon J., Jullien J., 2020. Epigenetic homogeneity in histone methylation underlies sperm programming for embryonic transcription. Nat Commun 11, 3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.G., 2001. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70, 281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P.J., Beyhan Z., Iager A.E., Yoon S.Y., Malcuit C., Schellander K., Fissore R.A., Cibelli J.B., 2008. Parthenogenetic activation of bovine oocytes using bovine and murine phospholipase C zeta. BMC Dev Biol 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft L.L., Jaffe L.A., Mehlmann L.M., 2002. Egg activation at fertilization: where it all begins. Dev Biol 245, 237–254. [DOI] [PubMed] [Google Scholar]
- Runft L.L., Watras J., Jaffe L.A., 1999. Calcium release at fertilization of Xenopus eggs requires type I IP3 receptors, but not SH2 domain-mediated activation of PLCγ or Gq-mediated activation of PLCβ. Dev Biol 214, 399–411. [DOI] [PubMed] [Google Scholar]
- Sakaue T., Takeuchi K., Maeda T., Yamamoto Y., Nishi K., Ohkubo I., 2010. Factor H in porcine seminal plasma protects sperm against complement attack in genital tracts. J Biol Chem 285, 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C.M., Larman M.G., Parrington J., Cox L.J., Royse J., Blayney L.M., Swann K., Lai F.A., 2002. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129, 3533–3544. [DOI] [PubMed] [Google Scholar]
- Session A.M., Uno Y., Kwon T., Chapman J.A., Toyoda A., Takahashi S., Fukui A., Hikosaka A., Suzuki A., Kondo M., van Heeringen S.J., Quigley I., Heinz S., Ogino H., Ochi H., Hellsten U., Lyons J.B., Simakov O., Putnam N., Stites J., Kuroki Y., Tanaka T., Michiue T., Watanabe M., Bogdanovic O., Lister R., Georgiou G., Paranjpe S.S., van Kruijsbergen I., Shu S., Carlson J., Kinoshita T., Ohta Y., Mawaribuchi S., Jenkins J., Grimwood J., Schmutz J., Mitros T., Mozaffari S.V., Suzuki Y., Haramoto Y., Yamamoto T.S., Takagi C., Heald R., Miller K., Haudenschild C., Kitzman J., Nakayama T., Izutsu Y., Robert J., Fortriede J., Burns K., Lotay V., Karimi K., Yasuoka Y., Dichmann D.S., Flajnik M.F., Houston D.W., Shendure J., DuPasquier L., Vize P.D., Zorn A.M., Ito M., Marcotte E.M., Wallingford J.B., Ito Y., Asashima M., Ueno N., Matsuda Y., Veenstra G.J., Fujiyama A., Harland R.M., Taira M., Rokhsar D.S., 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter D., Nicklay J.J., Chitta R.K., Shabanowitz J., Hunt D.F., Allis C.D., 2009. Analysis of histones in Xenopus laevis. I. A distinct index of enriched variants and modifications exists in each cell type and is remodeled during developmental transitions. J Biol Chem 284, 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka A.V., Hepler J.R., Brown K.O., Sternweis P.C., 1991. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science 251, 804–807. [DOI] [PubMed] [Google Scholar]
- Swann K., Lai F.A., 2016. Egg Activation at Fertilization by a Soluble Sperm Protein. Physiol Rev 96, 127–149. [DOI] [PubMed] [Google Scholar]
- Swann K., Ozil J.P., 1994. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol 152, 183–222. [DOI] [PubMed] [Google Scholar]
- Teperek M., Miyamoto K., Simeone A., Feret R., Deery M.J., Gurdon J.B., Jullien J., 2014. Sperm and Spermatids Contain Different Proteins and Bind Distinct Egg Factors, International Journal of Molecular Sciences, pp. 16719–16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak K.L., Phelps W.A., Tembo M., Lee M.T., Carlson A.E., 2018a. The TMEM16A channel mediates the fast polyspermy block in Xenopus laevis. J Gen Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak K.L., Tembo M., Phelps W.A., Lee M.T., Carlson A.E., 2018b. PLC and IP3-evoked Ca(2+) release initiate the fast block to polyspermy in Xenopus laevis eggs. J Gen Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A.T., Sutovsky P., Manandhar G., Xu W., Katayama M., Day B.N., Park K.W., Yi Y.J., Xi Y.W., Prather R.S., Oko R., 2007. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem 282, 12164–12175. [DOI] [PubMed] [Google Scholar]
- Yoneda A., Kashima M., Yoshida S., Terada K., Nakagawa S., Sakamoto A., Hayakawa K., Suzuki K., Ueda J., Watanabe T., 2006. Molecular cloning, testicular postnatal expression, and oocyte-activating potential of porcine phospholipase Czeta. Reproduction 132, 393–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.