Abstract
Background
drug development and disease prevention of heart failure (HF) and atrial fibrillation (AF) are impeded by a lack of robust early-stage surrogates. We determined to what extent cardiac magnetic resonance (CMR) measurements act as surrogates for the development of HF or AF in healthy individuals.
Methods
Genetic data was sourced on the association with 22 atrial and ventricular CMR measurements. Mendelian randomization was used to determine CMR associations with atrial fibrillation (AF), heart failure (HF), non-ischemic cardiomyopathy (CMP), and dilated cardiomyopathy (DCM). Additionally, for the CMR surrogates of AF and HF, we explored their association with non-cardiac traits.
Results
In total we found that 9 CMR measures were associated with the development of HF, 7 with development of non-ischemic CMR, 6 with DCM, and 12 with AF. biventricular ejection fraction (EF), biventricular or end-systolic volumes (ESV) and left-ventricular (LV) end diastolic volume (EDV) were associated with all 4 cardiac outcomes. Increased LV-MVR (mass to volume ratio) affected HF (odds ratio (OR) 0.83, 95%CI 0.79; 0.88), and DCM (OR 0.26, 95%CI 0.20; 0.34. We were able to identify 9 CMR surrogates for HF and/or AF (including LV-MVR, biventricular EDV, ESV, and right-ventricular EF) which associated with non-cardiac traits such as blood pressure, lung function traits, BMI, cardioembolic stroke, and late-onset Alzheimer’s disease.
Conclusion
CMR measurements may act as surrogate endpoints for the development of HF (including non-ischemic CMP and DCM) or AF. Additionally, we show that changes in cardiac function and structure measured through CMR, may affect diseases of other organs leading to lung disease or late-onset Alzheimer’s disease.
Introduction
Heart failure (HF) and atrial fibrillation (AF) are major cardiac diseases that cause considerable burden in terms of health and economic costs, as well as mortality1–3. HF is a clinical syndrome secondary to dysfunction of the right ventricle (RV) or left ventricle (LV), while AF is defined by uncoordinated electrical activation and consequently ineffective contraction of the atria. Both diseases are intricately related and while the causative relationship between the two conditions has not been fully determined, it is clear these two syndromes frequently co-occur4.
Despite recent advances in medicines, for example offered by sodium-glucose co-transporter-2 inhibitors, drug development for cardiac disease suffers from high failure rates, often occurring during costly late-stage clinical testing5–7. Unlike with the cholesterol content on low-density lipoprotein for coronary heart disease, drug development for AF and HF is impeded by a lack of robust early-stage surrogates (or intermediates) for cardiac disease.
Cardiac magnetic resonance (CMR) imaging is the gold standard for quantification of atrial and ventricular function and morphology, and has become an integral diagnostic modality for cardiac diseases. It is however unclear to what extent CMR measurements act as surrogates for the development of cardiac disease in otherwise healthy individuals.
Both HF and AF are associated with multimorbidity including non-cardiac diseases, such as stroke, chronic kidney disease (CKD), diabetes mellitus, and neurological diseases such as Alzheimer’s disease (AD). Because HF and AF are clinical manifestations of underlying changes in cardiac function and structure, patients with similar diagnoses may vary considerably in underlying pathophysiology and disease progression. Unlike HF or AF diagnoses, CMR measurements directly reflect cardiac physiology, and therefore provide an opportunity to explore the effects changes in cardiac function and structure may elicit in other organs.
Recently, CMR measurements of thousands of subjects have been linked to genetic data and analysed through genome-wide analysis studies (GWAS). Aggregate data from GWAS, consisting of variant-specific point estimates and standard errors, can be used in Mendelian randomization analyses to ascertain the causal effects a CMR trait may have on disease. In the current manuscript, we leveraged data from three recent GWAS of CMR measurements of atrial and ventricular structure and function8, LV trabeculation morphology9, and left atrial (LA) volume10, jointly consisting of 22 measurements conducted in over 35,000 UK biobank (UKB) participants. These data were used to conduct Mendelian randomization analyses of the association between CMR traits and cardiac events, including HF, dilated cardiomyopathy (DCM), non-ischemic cardiomyopathy (CMP), and AF. Subsequently, we explored the association of CMR proxies for HF or AF with 20 clinically relevant non-cardiac traits.
Methods
Genetic data on CMR and cardiac traits
We leveraged aggregate data (i.e., point estimates and standard errors) from 3 GWAS of deep-learning derived CMR measurements conducted using UKB participants; please see the specific study references for details on the derivation methods. Ahlberg et al.10 provided measurements on LA volume (LA-V (max) and LA-V (min)), LA total emptying fraction (LA-TF), LA active emptying fraction (LA-AF), and passive emptying fraction (LA-PF) from 35,658 subjects. Genetic data on LV trabecular morphology (LV-TM), measured as a fractal dimension ratio, was available from Meyer et al.9 on 18,096 subjects. Schmidt et al8. provided (n: 36,548) data on LV and right-ventricular (RV) ejection fraction (EF), stroke volume (SV), peak filling rate (PFR), peak ejection rate (PER), end-diastolic or end-systolic volumes (EDV, ESV), LV end-diastolic mass (LV-EDM), the LV mass to volume ratio (LV-MVR), and biatrial PFR. All three GWAS excluded subjects with pre-existing cardiac conditions such as AF, HF, cardiomyopathies, myocardial infarction, or congenital heart disease.
GWAS data was sourced on the following cardiac outcomes: HF (52,496 cases)11, non-ischemic CMP (1,816 cases)12, DCM (2,719 cases)13, and AF (60,620 cases)14. The following 20 traits were used in the non-cardiac phenome-wide scan: stroke (subtypes), venous thromboembolism (VTE), abdominal aortic aneurysm (AAA), systolic/diastolic blood pressure (SBP/DBP), body mass index (BMI), diabetes (T2DM), glycated haemoglobin, c-reactive protein (CRP), forced expiratory volume (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF) CKD, estimated glomerular filtration rate (eGFR), AD, and late-onset AD; please see the data availability section for more detail.
Mendelian randomization analysis
Genetic instruments were selected from throughout the genome using an F-statistic > 24 and a minor allele frequency (MAF) of at least 0.01. Variants were clumped to a linkage disequilibrium (LD) R-squared threshold of 0.30, with residual LD modelled using a generalized least square (GLS) solution15 and a reference panel from a random sample of 5,000 of white British ancestry UKB participants; this following the source GWAS data excluding non-European ancestries to prevent bias through population stratification.
Mendelian randomization was conducted using the GLS implementation of the inverse-variance weighted (IVW) estimator, as well as with an Egger correction to protect against horizontal pleiotropy16. To further minimize the potential influence of horizontal pleiotropy, we excluded variants with a leverage of more than 3 times the mean or an outlier Chi-square statistic of statistic above 6.63, with the Q-statistic identifying possible remaining violations17. Finally, a model selection framework, proposed by Bowden et. al., was applied to select the most appropriate estimator (IVW or MR-Egger) for each individual exposure-outcome relation17,18.
Where appropriate, results were presented as odds ratio (OR, for binary traits) with 95% confidence interval (95%CI) or mean difference (MD, for continuous traits). Associations with cardiac outcomes were declared significant using the standard alpha of 0.05, with a multiplicity corrected alpha of 1.25×10−2 (correcting for the 20 non-cardiac traits) applied to the more exploratory phewas analysis. Under the null-hypothesis (i.e., where all results are false positives) p-values follow a standard uniform distribution. Hence, to identify CMR associations driven by multiplicity we performed Kolmogorov-Smirnov “KS”-tests19 evaluating the agreement of the empirical p-value distribution with the standard uniform distribution.
Results
Biventricular and atrial CMR associations with incident cardiac outcomes
Sourcing CMR measurements in people without pre-existing cardiac conditions we employed Mendelian randomization to determine their association with the development of cardiac events. Higher EF of both ventricles was associated with decreased risk of HF, non-ischemic CMP, and DCM; Fig. 1, Supplementary Table 1. Higher RV-PFR increased the risk of AF (OR 2.12, 95%CI 1.16; 3.89), while higher LV-PER decreased AF risk. Higher EDV and ESV of both ventricles protected against AF, while increasing the risk of HF, non-ischemic CMP, and DCM (excluding RV-EDV); Fig. 1. Higher LV-MVR decreased the risks of HF (OR 0.83, 95%CI 0.79; 0.88), and DCM (OR 0.26, 95%CI 0.20; 0.34). From the atrial traits presented in Fig. 2, higher LA-V (max) increased the risk of AF (OR 1.11 95%CI 1.00; 1.23), while LA-V (min) increased HF risk (OR 1.20 95%CI 1.04; 1.38), and LA-PFR decreased the risk of non-ischemic CMP (OR 0.80 95%CI 0.67; 0.95).
Figure 1. Forest plot of Mendelian randomization estimates of biventricular CMR associations with the onset of HF and AF.
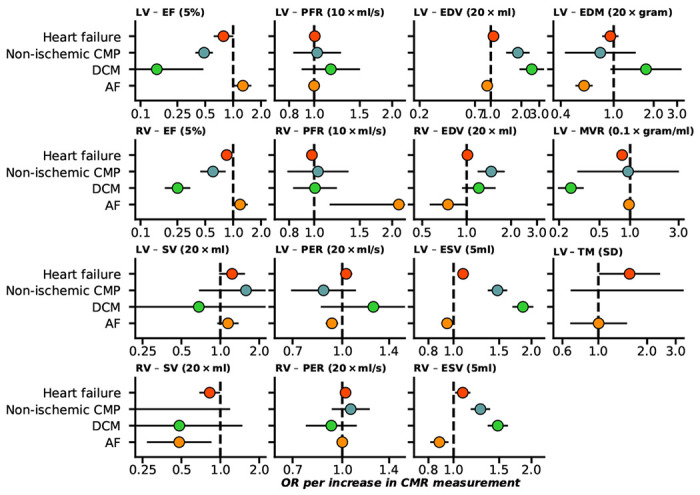
n.b. Point estimates reflect odds ratios (OR) with 95% confidence intervals presented as horizontal line segments. LV: left-ventricle, RV: right-ventricle, EF: ejection fraction, SV: stroke volume, PFR: peak filling rate, PER: peak ejection rate, EDV/ESV: diastolic or systolic volumes, EDM: end diastolic mass, MVR: mass to volume ratio, TM: trabecular morphology. Outcome data was available on heart failure (52,496 cases), DCM (dilated cardiomyopathy, 2,719 cases), non-ischemic CMP(1,816 cases), and AF (atrial fibrillation, 60,620 cases).
Figure 2. Forest plot of Mendelian randomization estimates of atrial CMR associations with the onset of HF and AF.
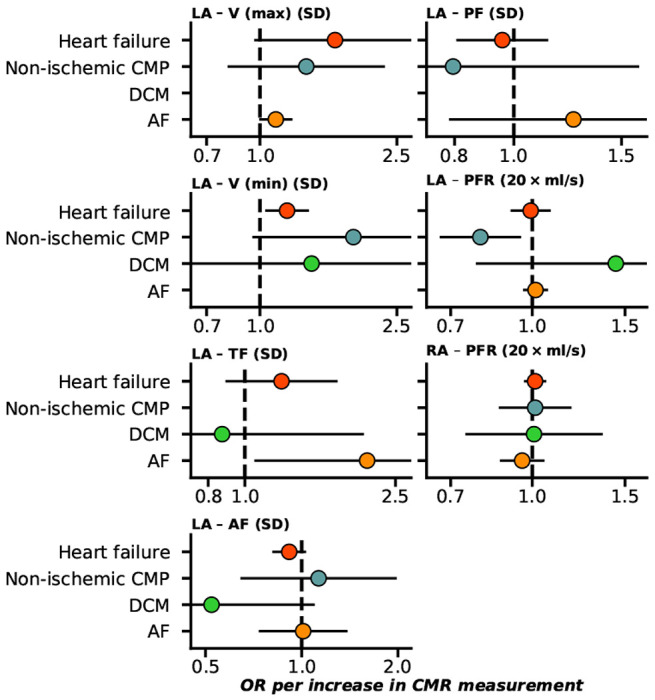
n.b. Point estimates reflect odds ratios (OR) with 95% confidence intervals presented as horizontal line segments. RA: right-atrial, LA: left-atrial, V (max): maximum volume, V (min): minimum volume, TF: total emptying fraction, AF: active emptying fraction, PF: passive emptying fraction, PFR: peak filling rate. Outcome data was available on heart failure (52,496 cases), DCM (dilated cardiomyopathy, 2,719 cases non-ischemic CMP(1,816 cases), and AF (atrial fibrillation, 60,620 cases).
In general, we found that the incidence of cardiac disease was determined by biventricular and atrial changes in function and structure (Figs. 1–3). For example both HF and AF were affected by atrial as well as ventricular changes.
Figure 3. CMR association counts with cardiac and non-cardiac traits, and Kolmogorov-Smimov test results for multiplicity.
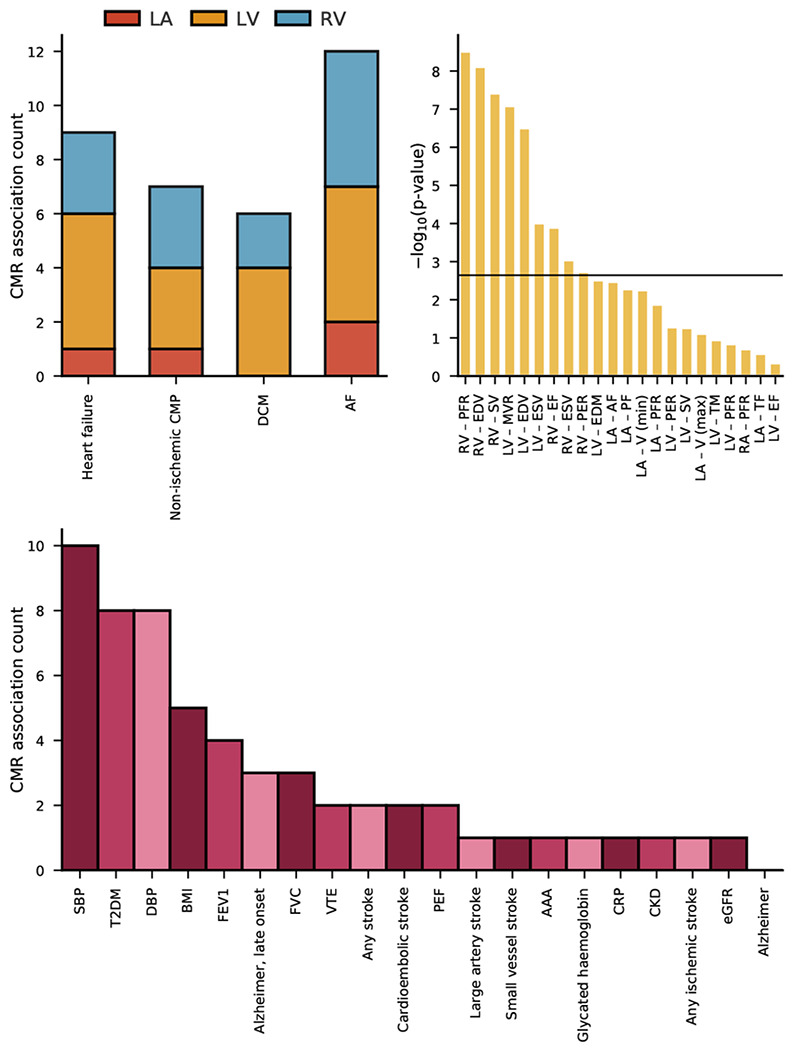
n.b The top left bar chart represents the counts of significant Mendelian randomization CMR effects grouped by chamber. The top right bar chart represent the −log10(p-value) of Kolmogorov-Smirnov tests, were a significant result indicates the phewas results are unlikely driven by multiple testing. The horizontal line indicates the significance threshold of 0.05/20. The bottom bar chart represents the counts of significant Mendelian randomization CMR effects on the considered phewas traits. The following abbreviations were used, LV: left-ventricle, RV: right-ventricle, RA: right-atrial, LA: right-atrial, HCM: hypertrophic cardiomyopathy, DCM: dilated cardiomyopathy, AF: atrial fibrillation, T2DM: type 2 diabetes, CKD: chronic kidney disease, VTE: venous thromboembolism, AAA: abdominal aortic aneurysm, SBP/DBP: systolic/diastolic blood pressure, BMI: body mass index, CRP: c-reactive protein, FVC: forced vital capacity, FEV1: forced expiratory volume, PEF: peak expiratory flow, eGFR: estimated glomerular filtration rate, HbA1c: glycated haemoglobin.
Associations of cardiac function and structure with non-cardiac traits
We next explored whether changes in cardiac function and structure could be associated with non-cardiac traits. We found that CMR traits were frequently associated with blood pressure, incidence T2DM, BMI, late onset AD (after an age of 65 years), lung function measurements, and cardioembolic stroke (Fig. 3, Supplementary Table 2).
Prioritizing results that were unlikely driven by multiple testing (Figs. 3–4), we identified 9 CMR surrogates for HF and/or AF that were associated with non-cardiac traits: LV-MVR, LV-EDM, biventricular EDV, RV-SV, RV-ESV, RV-EF, RV-PFR, and LV-ESV.
Figure 4. A targeted Mendelian randomization phenome-wide scan comparing the effect of changes in cardiac function and structure to that of a HF or AF diagnosis.
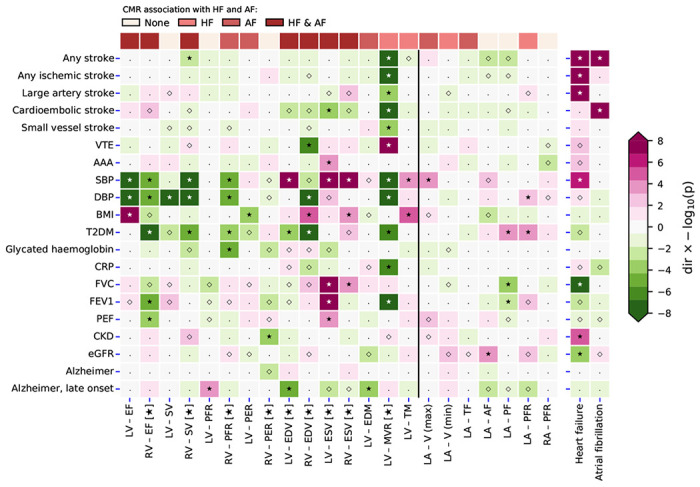
N.b. P-values passing the 0.05 threshold are indicated by an open diamond with stars indicating results passing a threshold of 1.25×10−2. Cells were coloured by effect direction times −log10(p-value); where p-values were truncated at 8 for display purposes. CMR measurements that passed the Kolmogorov-Smirnov test for multiplicity are indicated with a star, with results of non-indicated CMR measurements more likely driven by multiplicity. The following abbreviations were used, LV: left-ventricle, RV: right-ventricle, RA: right-atrial, LA: right-atrial, EF: ejection fraction, SV: stroke volume, PFR: peak filling rate, PER: peak ejection rate, EDV/ESV: diastolic or systolic volumes, EDM: end diastolic mass, MVR: mass to volume ratio, TM: trabecular morphology V (max): maximum volume, V (min): minimum volume, TF: total emptying fraction, AF: active emptying fraction, PF: passive emptying fraction, AF: atrial fibrillation, T2DM: type 2 diabetes, CKD: chronic kidney disease, VTE: venous thromboembolism, AAA: abdominal aortic aneurysm, SBP/DBP: systolic/diastolic blood pressure, BMI: body mass index, CRP: c-reactive protein, FVC: forced vital capacity, FEV1: forced expiratory volume, PEF: peak expiratory flow, eGFR: estimated glomerular filtration rate, HbA1c: glycated haemoglobin.
For example, we observed that aside from LV-EDM, all the aforementioned CMR measures of cardiac structure or function associated with blood pressure (Fig. 4). We additionally observed that increased LV-MVR (OR 0.63, 95%CI 0.55; 0.73) decreased the risk of cardioembolic stroke. LV-EDV and LV-EDM were associated with a decreased risk of late-onset AD (Fig. 4): OR 0.82 (95%CI 0.75; 0.90) and OR 0.68 (95%CI 0.55; 0.84), respectively. BMI was affected by changes in RV-EDV and RV-ESV, and T2DM risk was driven by RV-EF, RV-SV, RV-PFR, biventricular EDV, and LV-MVR. The lung function measurements (FVC, FEV1, PEF) were predominantly affected by RV-EF and LV-ESV. LV-MVR was a particularly important measure, associating with all stroke types, VTE, SBP, DPB, T2DM, CRP, and FEV1; Fig. 4.
Comparison to HF and AF effects on non-cardiac traits
Next, as comparison we leveraged genetic instruments with a clinical diagnosis of HF or AF, and performed Mendelian randomization to determine the causal effects HF or AF had on non-cardiac traits. HF increased the risk of any stroke, any ischemic stroke, as well as large artery stroke, and chronic kidney disease, while decreasing FVC and increasing SBP (Fig. 4, Supplementary Table 3). AF diagnosis increased the risk of any stroke, and cardioembolic stroke (OR 2.13 95%CI 1.35; 3.34).
Discussion
In the current manuscript we employed Mendelian randomization combined with CMR measurements in participants without pre-existing cardiac disease and identified surrogate outcomes for the onset of HF (52,496 cases) and AF (60,620 cases). We show that biventricular EF, biventricular ESV, and LV-ESV are associated with de novo development of HF, non-ischemic CMP, DCM, and/or AF. Importantly, we found that the development of HF or AF is not exclusively driven by any single ventricular or atrial measurement, but is determined by combinations of changes in atrial and ventricular function and structure.
In total, we identified 9 CMR measures associated with the development of HF, 7 with development of non-ischemic CMP, 6 with DCM, and 12 with AF. This indicates that CMR measurements can be used to monitor disease occurrence and help identify high-risk patients in need of preventative measures. Additionally, our findings imply that CMR measurement might be used as surrogate endpoints in early clinical studies, which can assist in prioritizing compounds for confirmatory outcome trials.
We explored the phenotypic effects that changes in cardiac function and structure may have on non-cardiac traits (Fig. 3–4), identifying 9 CMR surrogates (5 RV, and 4 LV CMR measurements) for HF or AF that also associated with non-cardiac traits. We observed a strong association of these CMR measurements with SBP and DBP, confirming the well-established relation between HF and blood pressure20. Similarly, we found that RV-EF, biventricular ESV and LV-MVMR were associated with lung function measurements, recapitulating know associations between cardiac disease and chronic obstructive pulmonary disease (COPD). Increased LV-EDV and LV-EDM were both associated with lower risk of AF as well as lower risk of late-onset AD, indicating a shared aetiology between both diseases. The Mendelian randomization analysis of AF on AD did not find a similar association, highlighting the increase in power offered by using quantitative exposure compared to a binary exposure.
Interestingly, LV-EF was not strongly associated with the development of non-cardiac disease. Instead, we observed a strong association of LV-MVR with 11 non-cardiac traits (Fig. 4), including 5 stroke subtypes, VTE, CRP, and FEV1. This suggests that while LV-EF has important diagnostic implications for HF, a broader consideration of CMR measurements might provide further information relevant for risk mitigation of diseases often co-occurring in people at high risk of developing HF or AF. This is further highlighted by our finding that 5 of the 9 CMR measurement associated with non-cardiac traits were RV, supporting the need to for a more holistic consideration changes in cardiac function and structure may have on disease risk.
The study has a number of limitations that deserve consideration. First, while we sourced genetic associations with CMR measurement taken from subjects without pre-existing cardiac conditions, a proportion of subjects may have had undiagnosed disease. The UKB however represents a relatively healthy subset of the UK population, likely minimizing the number of individuals with latent disease. Second, our choice of CMR measurement was limited by the publicly available data, for example preventing us from exploring the association between ratio of measure (such as PEF/EDV or PFR/EDV) not available in the original results. Third, while Mendelian randomization is robust against bias through reverse causality and confounding bias, it critically assumes the absence of horizontal pleiotropy, where the genetic variant only affects the outcome through its association with the CMR measurement. In the current analysis, we performed automatic model selection to decide between an IVW or more robust MR-egger models, and additionally removed potentially pleiotropic variants through the identification and removal of outliers and high leverage points. Fourth, due to its protection against reverse causation, Mendelian randomization results are naturally imbued with a clear directionality of association. In the current analyses this means that the observed Mendelian randomization estimates proxy the effects underlying changes in cardiac function or morphology may have on the considered outcomes. Finally, the conducted Mendelian randomization analyses implicitly assess a linear trend between CMR and outcome. In the presence of non-linearity, the presented Mendelian randomization estimates represent a population average effect which may not necessarily apply to any single individual, but often offers a reasonably approximation. While non-linear Mendelian randomization methods have been developed25,26, these require access to individual participant data which, even for UKB sized data, only offer a fraction of the disease cases we have been able to leverage here.
In conclusion, we have identified biventricular and left-atrial CMR measurements that may act as surrogate endpoints for future cardiac events, including heart failure, cardiomyopathies, and atrial fibrillation. We additionally show that changes in cardiac function and structure may affect other organs, resulting in diseases such as COPD and late-onset Alzheimer’s.
Acknowledgement
This research has been conducted using the UK Biobank Resource under Application Numbers 24711, 12113, and 44972. The authors are grateful to UK Biobank participants. UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government, and the Northwest Regional Development Agency. It has also had funding from the Welsh Assembly Government and the British Heart Foundation.
Funding and role of funding sources
AFS is supported by BHF grants PG/18/5033837, PG/22/10989, and the UCL BHF Research Accelerator AA/18/6/34223. CF and AFS received additional support from the National Institute for Health Research University College London Hospitals Biomedical Research Centre. JvS is supported by Dutch Heart Foundation grant 03-004-2019-T045. ATR is supported by the Cardiovascular Research Initiative of the Netherlands Heart Foundation (CVON 2015-12 eDETECT) and ZonMW Off Road. This work was supported by grant [R01 LM010098] from the National Institutes of Health (USA) and by EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking BigData@Heart grant n° 116074, as well as by the UKRI/NIHR Multimorbidity fund Mechanism and Therapeutics Research Collaborative MR/V033867/1 and the Rosetrees Trust.
Guarantor
AFS performed the presented analyses. AFS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest statements
AFS and FWA have received Servier funding for unrelated work.
Footnotes
Code availability
Analyses were conducted using Python v3.7.10 (for GNU Linux), Pandas v1.3.5, Numpy v1.20.3, and Matplotlib 3.3.2. Scripts and data necessary to generate the illustrations have been deposited: XX.
Prior postings and presentations
A preprint version of this manuscript has been deposited on medrxiv: .
Contributor Information
Amand Schmidt, University College London.
Chris Finan, University College London.
Mimount Bourfiss, University College London.
Birgitta Velthuis, University College London.
Esther Puyol-Antón, University College London.
Abdulrahman Alasiri, UMC Utrecht.
Bram Ruijsink, University College London.
Folkert Asselbergs, UCL.
Anneline ter Riele, UMCU.
Jessica van Setten, UMCU.
Data availability
Genetic data for the phewas of non-cardiac traits was sourced for stroke (subtypes) from MEGASTROKE (http://www.megastroke.org/index.html), venous thromboembolism and abdominal aortic aneurysm from (https://www.globalbiobankmeta.org/), blood pressure from Evangelou et.al. (https://www.nature.com/articles/s41588-018-0205-x), glycemic traits, and lung function measurement were sourced from (http://www.nealelab.is/uk-biobank); type 2 diabetes from DIAGRAM (http://diagram-consortium.org/index.html); BMI from GAINT (https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files); CRP from (https://www.ebi.ac.uk/gwas/publications/30388399); the CKDGen consortium provided GWAS associations on estimated glomerular filtration rate (eGFR), and chronic kidney disease (http://ckdgen.imbi.uni-freiburg.de/); Alzheimer’s disease data was sourced from Jansen et.al. (https://ctg.cncr.nl/software/summary_statistics) and Kunkle et.al. (https://www.nature.com/articles/s41588-019-0358-2); Lewy body dementia from (https://www.ebi.ac.uk/gwas/publications/33589841);
References
- 1.Taylor C. J. et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ 364, 1223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee D. S. et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail 4, 36–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khayatt B. M. et al. Paradoxical impact of socioeconomic factors on outcome of atrial fibrillation in Europe: trends in incidence and mortality from atrial fibrillation. European Heart Journal 42, 847–857(2021). [DOI] [PubMed] [Google Scholar]
- 4.Anter E., Jessup M. & Callans D. J. Atrial Fibrillation and Heart Failure. Circulation 119, 2516–2525 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Redfield M. M. et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med 373, 2314–2324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheorghiade M. et al. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 314, 2251–2262 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Fordyce C. B. et al. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol 65, 1567–1582 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A. et al. Druggable proteins influencing cardiac structure and function: implications for heart failure therapies and cancer related cardiotoxicity. Preprint at 10.21203/rs.3.rs-1305500/v1 (2022). [DOI] [PMC free article] [PubMed]
- 9.Meyer H. V. et al. Genetic and functional insights into the fractal structure of the heart. Nature 584, 589–594(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahlberg G. et al. Genome-wide association study identifies 18 novel loci associated with left atrial volume and function. European Heart Journal 42, 4523–4534 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu K.-H. H. et al. Polygenic risk score from a multi-ancestry GWAS uncovers susceptibility of heart failure. 2021.12.06.21267389 Preprint at 10.1101/2021.12.06.21267389 (2021). [DOI] [Google Scholar]
- 12.Aragam Krishna G. et al. Phenotypic Refinement of Heart Failure in a National Biobank Facilitates Genetic Discovery. Circulation 139, 489–501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garnier S. et al. Genome-wide association analysis in dilated cardiomyopathy reveals two new players in systolic heart failure on chromosomes 3p25.1 and 22q11.23. Eur Heart J 42, 2000–2011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen J. B. et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet 50, 1234–1239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess S., Zuber V, Valdes-Marquez E., Sun B. B. & Hopewell J. C. Mendelian randomization with fine-mapped genetic data: Choosing from large numbers of correlated instrumental variables. Genetic Epidemiology 41, 714–725 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowden J., Smith G. D. & Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. International Journal of Epidemiology 44, 512–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden J. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Statistics in medicine 36, 1783–1802 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowden J. et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol 47, 1264–1278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storey J. D. A direct approach to false discovery rates. Journal of the Royal Statistical Society Series B: Statistical Methodology 64, 479–498 (2002). [Google Scholar]
- 20.Gheorghiade M. et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 296, 2217–2226 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Kamel H. & Healey J. S. Cardioembolic Stroke. Circulation Research 120, 514–526 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khurshid S. et al. Atrial Fibrillation Risk and Discrimination of Cardioembolic From Noncardioembolic Stroke. Stroke 51, 1396–1403 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Widya R. L. et al. Right Ventricular Involvement in Diabetic Cardiomyopathy. Diabetes Care 36, 457–462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeCarli C. The link between blood pressure and Alzheimer’s disease. The Lancet Neurology 20, 878–879 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Silverwood R. J. et al. Testing for non-linear causal effects using a binary genotype in a Mendelian randomization study: application to alcohol and cardiovascular traits. Int J Epidemiol 43, 1781–1790 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staley J. R. & Burgess S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol 41, 341–352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlay S. M. et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation (2019) doi: 10.1161/CIR.0000000000000691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Genetic data for the phewas of non-cardiac traits was sourced for stroke (subtypes) from MEGASTROKE (http://www.megastroke.org/index.html), venous thromboembolism and abdominal aortic aneurysm from (https://www.globalbiobankmeta.org/), blood pressure from Evangelou et.al. (https://www.nature.com/articles/s41588-018-0205-x), glycemic traits, and lung function measurement were sourced from (http://www.nealelab.is/uk-biobank); type 2 diabetes from DIAGRAM (http://diagram-consortium.org/index.html); BMI from GAINT (https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files); CRP from (https://www.ebi.ac.uk/gwas/publications/30388399); the CKDGen consortium provided GWAS associations on estimated glomerular filtration rate (eGFR), and chronic kidney disease (http://ckdgen.imbi.uni-freiburg.de/); Alzheimer’s disease data was sourced from Jansen et.al. (https://ctg.cncr.nl/software/summary_statistics) and Kunkle et.al. (https://www.nature.com/articles/s41588-019-0358-2); Lewy body dementia from (https://www.ebi.ac.uk/gwas/publications/33589841);


