Abstract
Background:
Previous research finds a range of numbers impairments in Parkinsonian syndromes (PS), but has largely focused on how visuospatial impairments impact deficits in basic numerical processes (e.g., magnitude judgments, chunking). Differentiation between these basic functions and more complex numerical processes often utilized in everyday tasks may help elucidate neurocognitive and neuroanatomic bases of numbers deficits in PS.
Objective:
To test neurocognitive and neuroanatomic correlates of complex numerical processing in PS, we assessed number abilities, neuropsychological performance, and cortical thickness in progressive supranuclear palsy (PSP) and Lewy body spectrum disorders (LBSD).
Methods:
Fifty-six patients (LBSD = 35; PSP = 21) completed a Numbers Battery, including basic and complex numerical tasks. The Mini-Mental State Exam (MMSE), letter fluency (LF), and Judgment of Line Orientation (JOLO) assessed global, executive, and visuospatial functioning respectively. Mann-Whitney U tests compared neuropsychological testing and rank-transformed analysis of covariance (ANCOVA) compared numbers performance between groups while adjusting for demographic variables. Spearman’s and partial correlations related numbers performance to neuropsychological tasks. Neuroimaging assessed cortical thickness in disease groups and demographically-matched healthy controls.
Results:
PSP had worse complex numbers performance than LBSD (F = 6.06, p = 0.02) but similar basic numbers performance (F = 0.38, p > 0.1), covarying for MMSE and sex. Across syndromes, impaired complex numbers performance was linked to poor LF (rho = 0.34, p = 0.01) but not JOLO (rho = 0.23, p > 0.05). Imaging revealed significant frontal atrophy in PSP compared to controls, which was associated with worse LF and complex numbers performance.
Conclusion:
PSP demonstrated selective impairments in complex numbers processing compared to LBSD. This complex numerical deficit may relate to executive dysfunction and frontal atrophy.
Keywords: Cognitive decline, executive function, frontal lobe, neurodegenerative diseases, Parkinsonian disorders, progressive supranuclear palsy
INTRODUCTION
Numerical processing, such as the ability to quantify items and perform arithmetic, is needed in many daily activities and relies on multiple cognitive domains for appropriate execution, including visuospatial processing, cognitive flexibility, and attentional resources [1–3]. These abilities can be easily compromised in neurodegenerative disease patients yet remain understudied within Parkinsonian syndromes. While patients with Parkinsonian syndromes are characterized primarily by their motor deficits, they are still vulnerable to cognitive impairment [4–6] and poor numerical processing. Evidence suggests a broad range of numbers impairments within Parkinsonian syndromes: patients with 4-repeat tauopathies like progressive supranuclear palsy (PSP) demonstrate severe numbers impairment [7, 8], whereas patients with Lewy body spectrum disorders (LBSD) show relatively preserved number abilities [9, 10]. Still, the cognitive and neuroanatomic basis for these differences in numbers performance across syndromes, especially PSP, is not well understood.
One source of variation in numbers performance may be task requirements, as numerical processing has a multifactorial structure. ‘Basic’ numerical processing, such as quantifying clustered items [11] or adding/subtracting single-digit quantities [12], relies on a visuospatial component that is thought to contribute to magnitude judgments [1, 3]. This ‘basic’ process promotes pattern recognition and enables chunking of large quantities of nearby objects into smaller clusters [11, 13, 14] (e.g., quick recognition of four dots on a die without actual counting). Comparably, more ‘complex’ numerical processing, such as tallying disorganized items [11] or adding/subtracting double-digit quantities [12], relies on an executive component that may support the combinatorial aspect of numerical calculations [1, 2]. Both visual search tasks involving scattered stimuli as well as calculations incorporating double-digit numbers are complex number tasks that require the brain to hold and manipulate information [1–3]. These differences in ‘basic’ versus ‘complex’ processing demands suggest that patients may demonstrate preserved or compromised ability, depending on the type of numerical task employed.
Regional brain atrophy may also contribute in part to observed differences in numbers performance across Parkinsonian syndromes, as basic and complex numerosity tasks have different neuroanatomic links. Imaging research in healthy, unimpaired adults has shown that basic numbers tasks are linked to parietal lobe activity [3, 15] while more complex numbers tasks may require additional inputs from the frontal lobe, including the prefrontal cortex (PFC) [16] and the inferior frontal gyrus (IFG) [3, 12, 17, 18]. Likewise, neurodegenerative research in dementia patients has shown that frontal lobe disease is associated with difficulty understanding higher-order numerical quantifiers [19], and this deficit correlated with executive dysfunction [20]. Executive dysfunction has also been independently linked to frontal lobe atrophy, namely in the right IFG and insular cortex, in a variety of dementias [21–23]. Indeed, frontal lobe processing is responsible for executive functions [24], most of which are implicated in complex numbers performance. The PFC, and specifically the frontal gyrus, is responsible for manipulating information within executive control [25], which is a key requirement for higher-level visual search tasks and numerical calculations. Altogether, these findings suggest that executive functioning—a known behavioral metric of frontal lobe atrophy—may govern complex numerical processing.
Parkinsonian syndromes also have different atrophy patterns and vulnerable cognitive domains. PSP patients frequently show reduced cortical volume in frontal lobe regions, particularly the PFC, frontal gyri, insular cortex, and supplementary motor areas [26] as well as executive deficits [27]. By contrast, cognitive impairments in LBSD can often manifest as visuospatial deficits [28] and have been related more strongly to accumulating pathology [29, 30] and atrophy [31] in the temporal-parietal region. Given these differences in atrophy patterns and cognition [32, 33] between LBSD and PSP, the comparison of these two Parkinsonian syndromes may help elucidate the neuroanatomic and neurocognitive bases of numbers impairments. For example, PSP patients may be specifically vulnerable to complex numerical deficits due to their frontal lobe disease and executive dysfunction, despite relatively preserved parietal lobe mediated spatial-magnitude processing. By contrast, LBSD patients may show more preserved numerical processing for both basic and complex tasks given the relative sparing of frontal-executive components in their underlying disease. Because these Parkinsonian syndromes have a predominant motor dysfunction with relatively mild cognitive impairments, the comparison of PSP with LBSD might reduce the influence of confounding cognitive deficits on numbers performance that could be seen with more severe dementias like Alzheimer’s disease (AD).
Here, we compared numerical abilities in PSP and LBSD using a Numbers Battery and tested whether impaired letter fluency (LF), a marker of executive functioning [33], contributed to complex numbers deficits across patients with Parkinsonian syndromes. To control for poor performance due to basic numbers deficits and visuospatial dysfunction, the ‘Basic’ section of the Numbers Battery assessed simpler numerical abilities and Judgment of Line Orientation (JOLO) assessed visuospatial abilities. Finally, structural neuroimaging measured cortical thickness (CT) in PSP and LBSD relative to controls and related cortical thinning to complex numbers performance and executive functioning within PSP patients. We hypothesized that executive dysfunction [27] driven by frontal lobe atrophy [24] in PSP would contribute to impaired complex numerical processing, compared to LBSD patients [32, 33]. Because co-occurring AD pathology can contribute to cognitive deficits in Parkinsonian syndromes [29, 30, 34], we repeated results excluding patients with biomarker evidence indicative of AD co-pathology.
MATERIALS AND METHODS
Patients
Fifty-six patients with Parkinsonian syndromes, either PSP (n = 21) or LBSD (n = 35), were retrospectively selected from the University of Pennsylvania Integrated Neurodegenerative Disease Database (INDD) [35] as of July 29, 2020. Patients were followed and evaluated at the University of Pennsylvania’s Frontotemporal Degeneration Center (FTDC). To investigate the differences in numbers performance between clinical groups, inclusion criteria were completion of Complex and Basic subsections of the Numbers Battery and a clinical diagnosis of PSP [4] or LBSD, including dementia with Lewy bodies (DLB, n = 8) [5], Parkinson’s disease (PD, n = 7), Parkinson’s disease with mild cognitive impairment (PD-MCI, n = 17), and Parkinson’s disease dementia (PDD, n = 3) [6]. We included the full spectrum of PD, PD-MCI, and PDD patients because cognitive impairment, including mild executive dysfunction, has been shown in early PD [36]. All clinical diagnoses were confirmed to meet current diagnostic criteria [4–6] at consensus meetings.
Effects of Alzheimer’s disease
Due to the frequent presence and known interactions of AD co-pathology with cognition and clinical presentation in LBSD [29, 30] and PSP [34], we repeated statistical analyses on numbers and neuropsychological data using either autopsy (n = 4) or biomarker data (n = 40) to exclude patients with evidence of AD pathology. Of our original cohort of 56 patients, we excluded all 4 patients (n = 2 PSP, n = 2 LBSD) with a secondary neuropathological diagnosis of AD at autopsy, 7 patients (n = 3 PSP, n = 4 LBSD) with cerebrospinal fluid biomarkers indicative of amyloid pathology (Aβ42 < 192) [37], and 1 patient (LBSD) with positive PET amyloid imaging. We also removed 12 patients (n = 3 PSP, n = 9 LBSD) with no available biomarker data since we were unable to render an assumption about underlying pathology for these cases. Thirty-two total patients (n = 13 PSP, n = 19 LBSD) were used in our pathologically “pure” sub-analyses.
Numbers assessment
To assess numbers performance in Parkinsonian syndromes, we retrospectively selected data from a Numbers Battery as previously described [11, 38]. To summarize, the battery comprised two main tasks: arithmetic calculations and numeral-dot array matching tasks. The ‘Complex’ arithmetic section included 16 double-digit problems, 8 addition and 8 subtraction tasks (16 + 17; 25 – 17); the ‘Basic’ arithmetic section was organized similarly but focused on single-digit mathematical problems (i.e., 5 + 3; 7 − 2). The numeral-dot array matching tasks involved two 16-trial blocks presented in multiple-choice format: matching a dot array to Arabic numeral choices (D-to-N) and matching an Arabic numeral to dot array choices (N-to-D). For the ‘Complex’ sections, six items were presented as randomly distributed dot arrays, and for the ‘Basic’ sections, six items were presented as spatially templated dot arrays that facilitated chunking [13], all with a 5–10 cardinality range. In addition to the basic and complex sections, each D-to-N and N-to-D task also had a 4-item ‘Subitizing’ section, which refers to the ability to automatically recognize a small number of objects (cardinality ≤ 4) without counting [39]. Subitizing scores were used as a screening tool to ensure preserved numerical perception and patient’s understanding of the task; results were not included in final analyses. Individual task scores were then combined to derive a total ‘Basic’ (6-item templated dot arrays; 8-item single-digit arithmetic; maximum = 28) and a total ‘Complex’ (6-item randomly distributed dot arrays; 8-item double-digit arithmetic; maximum = 28) score. All tasks were administered in an untimed, “paper-and-pencil” fashion.
Neuropsychological testing
To test the association between numbers performance and visuospatial and executive functioning across Parkinsonian syndromes, we also selected available neuropsychological data collected at the closest timepoint within 12 months of the Numbers Battery. The 15-item JOLO (n = 54), which involved matching a given line to a visually arranged template of lines at different angles, was used to assess visuospatial functioning [40]. Executive functioning was assessed using LF (n = 54), i.e., the total number of unique words produced beginning with the letters ‘F’, ‘A’, and ‘S’, given this task’s previous use in differentiating executive deficits between parkinsonian syndromes [33]; patients were given one minute for each letter [41]. The Mini-Mental State Examination (MMSE, n = 55) total score was used as a measure of global cognition [42]. Not all patients performed all neuropsychological measures for various random reasons (i.e., intercurrent disease, difficulty testing remotely during COVID). All standardized instructions for neuropsychological exams were administered to participants by trained research personnel.
Statistical analyses
Shapiro-Wilk tests indicated that Numbers Battery data, neuropsychological scores, and most demographic variables were not normally distributed (p < 0.05). A chi-squared test compared the categorical variable of sex, and Mann-Whitney U tests compared education, age at Numbers Battery test date, and neuropsychological scores between LBSD and PSP groups (Table 1).
Table 1.
Patient demographics and neuropsychological testing for LBSD and PSP
| LBSD | PSP | p | |
|---|---|---|---|
| (n = 35) | (n = 21) | ||
| Demographic data | |||
| Sex, na,c | |||
| Male | 25 (71%) | 9 (43%) | 0.07 |
| Female | 10 (29%) | 12 (57%) | |
| Education, yb,d | 16.0 [3.0] | 16.0 [6.0] | 0.5 |
| Age at test, yb,d | 70.0 [9.5] | 67.0 [8.0] | 0.2 |
| Neuropsychological data | |||
| MMSEb,d | N = 34 | N = 21 | 0.01* |
| (max = 30) | 28.5 [3.0] | 27.0 [2.0] | |
| JOLOb,d | N = 35 | N = 19 | 0.06 |
| (max = 15) | 12.0 [5.0] | 10.0 [4.0] | |
| LFb,d | N = 35 | N = 19 | < 0.00001* |
| (word count) | 40.0 [16.0] | 18.0 [17.0] |
Data reported as count (%).
Data reported as median [interquartile range].
p value reflects χ2 test estimate.
p value reflects U test estimate.
LBSD, Lewy body spectrum disorders; PSP, progressive supranuclear palsy; Age at test, age at Numbers Battery test date; MMSE, Mini-Mental State Examination; JOLO, Judgment of Line Orientation; LF, Letter fluency.
Analyses of covariance (ANCOVA) tested between-group comparisons on Basic and Complex numbers performance, with sex [43] and global cognition (MMSE) included as covariates to account for possible effects on numbers scores. To perform these non-parametric comparisons, we rank-transformed the Numbers Battery and neuropsychological data [44, 45]. Spearman’s and partial Spearman’s correlations related Basic and Complex numbers scores to LF and JOLO.
All analyses were performed using R (version 1.2.5033) and used a two-tailed test with alpha = 0.05.
Imaging analyses
Imaging patient cohort
Of our final cohort of patients, 42 (75%) had MRI data available within 12 months of the Numbers Battery test date. Quality control excluded 6 of those patients with poor scan data due to either participant motion or scanner artifact; 36 total patients (n = 15 PSP, n = 21 LBSD) were included in imaging analyses. This disease imaging subset was comparable to the full patient cohort on all demographic variables (p > 0.1) as determined by Mann-Whitney U tests. An imaging control group (CTRL) was selected from a convenience sample to be age-, sex-, and education-matched to the LBSD and PSP patient cohorts (n = 36) using the R programming package MatchIt [46]. Healthy controls were judged to be cognitively normal based on self-reported medical history and a MMSE score > 27 [42]. The healthy control group did not significantly differ from LBSD or PSP in any demographic variables (all p > 0.1) except for MMSE (p < 0.05) (Table 2). These controls were only used for CT comparisons to identify regions of atrophy in disease groups; there were no numerosity data or other neuropsychological variables of interest available on these subjects,
Table 2.
Imaging disease cohort and matched control demographics
| LBSD | PSP | CTRL | |
|---|---|---|---|
| (n = 21) | (n = 15) | (n = 36) | |
| Sex, na | |||
| Male | 15 (71%) | 5 (33%) | 20 (56%) |
| Female | 6 (29%) | 10 (67%) | 16 (44%) |
| Education, yb | 16.0 [4.0] | 16.0 [6.0] | 16.0 [3.0] |
| Age at MRI, yb | 68.0 [7.0] | 66.0 [8.0] | 66.5 [10.5] |
| MMSEb | 28.0 [3.0]c | 27.0 [2.5]c | 29.0 [2.0] |
Data reported as count (%).
Data reported as median [interquartile range].
U test shows significant difference from control group (p < 0.05).
LBSD, Lewy body spectrum disorders; PSP, progressive supranuclear palsy; CTRL, control; MMSE, Mini-Mental State Examination.
Neuroimaging acquisition and preprocessing
This subset of study participants underwent a high-resolution research quality T1-weighted magnetization prepared rapid gradient echo (MPRAGE) scan on a 3.0 Tesla SIEMENS TIM Trio scanner using the following parameters:
3.0 Tesla SIEMENS TIM Trio scanner, 8 channel head coil, axial plane with repetition time = 1620 ms, echo time = 3.09 ms, slice thickness = 1.0 mm, flip angle = 15 degrees, matrix = 256 × 192, in-plane resolution 0.9766 × 0.9766 mm (n = 29 CTRL, n = 6 PSP, n = 13 LBSD).
3.0 Tesla SIEMENS Prisma scanner, 64 channel head coil, sagittal plane with repetition time 2400 ms, echo time = 1.96 ms, flip angle = 8 degrees, matrix = 320 × 320, slice thickness = 0.8mm, in-plane resolution = 0.8 × 0.8 mm. This protocol used the mean-squared combination of four echoes for the T1 weighted image (n = 6 CTRL, n = 3 PSP).
3.0 Tesla SIEMENS TIM Trio scanner, 64 channel head coil, sagittal plane with repetition time = 2300 ms, echo time = 2.95 ms, slice thickness = 1.2 mm, flip angle = 9 degrees, matrix = 256 × 240, in-plane resolution = 1.05 × 1.05 mm (n = 1 CTRL, n = 5 PSP, n = 7 LBSD).
3.0 Tesla SIEMENS TIM Trio scanner, 8 channel head coil, axial plane with repetition time = 1800 ms, echo time = 3.8 ms, slice thickness = 1.0 mm, flip angle = 9 degrees, matrix = 256 × 256, in-plane resolution 1.0 × 1.0 mm (n = 1 LBSD).
3.0 Tesla SIEMENS Prisma scanner, 8 channel head coil, sagittal plane with repetition time 1620 ms, echo time = 4.53 ms, flip angle = 15 degrees, matrix = 265 × 192, slice thickness = 1.0 mm, in-plane resolution = 0.9766 × 0.9766 mm (n = 1 PSP).
Because protocols #4 and #5 were represented by just one scan each, we combined protocol #4 with #3 as well as protocol #5 with #1 due to similarity in flip angle parameters in order reduce variance when controlling for these different T1 protocols. After combining protocols, we were ultimately left with three ‘scanning parameter’ variables, which were coded as dummy variables and included as covariates in CT and regression analyses below.
Image processing to generate voxel-wise CT estimates was performed using Advanced Normalization Tools (ANTs) as previously described [47]. A brief summary is as follows: N4 bias correction in the T1 image reduced potential field bias [48]. A template-based method extracted the brain from the skull and used a symmetric diffeomorphic algorithm to register images to the Open Access Series of Imaging Studies (OASIS) template [49], and the brain was segmented into six tissue classes (cortical gray matter, subcortical gray matter, white matter, cerebrospinal fluid, brainstem, and cerebellum) using a combination of probabilistic tissue mapping and established priors [50]. CT maps were then generated and warped to the Montreal Neurological Institute 152 (MNI-152) template space [51] for subsequent analysis. Images were smoothed by a 2- sigma kernel prior to analysis.
Cortical thickness (CT) comparisons
Atrophied regions in disease groups were found via whole-brain voxel-wise t-tests of CT. All CT comparisons were performed using the FMRIB Software Laboratory (FSL) randomise program [52]; significant voxels were calculated using non-parametric statistics with 10,000 permutations of input data. FSL’s threshold-free cluster enhancement (TFCE) algorithm [53] was applied for all analyses. Disease groups (PSP, LBSD) were compared directly to one another, as well as independently to the imaging control group; all CT analyses controlled for T1 scanner parameters. Results are presented with an a priori threshold of family wise error (FWE) corrected p-value <0.05.
Regressions
Regressions relating voxel-wise CT to LF and complex numbers performance within the PSP group alone controlling for sex, age, and T1 scanner parameters were also performed using the FSL randomise program [52]. We included sex and age as covariates because CT is known to differ by these variables [54, 55]. Because our study focuses on numeric deficits in PSP, we tested the regressions specifically in brain regions where PSP showed significant atrophy relative to controls. As above, significant voxels were calculated using non-parametric statistics with 10,000 permutations of input data. Because we assess atrophied regions of overlap associated with both LF and complex numbers performance in a small population, we report clusters surviving a lenient uncorrected threshold of p < 0.05 and a minimum of 15 adjacent voxels.
All procedures were performed with written informed consent from all patients in compliance with human subject research and under guidelines approved by the Institutional Review Board at the University of Pennsylvania.
RESULTS
Demographics and neuropsychological performance
Demographic characteristics of LBSD and PSP are described in Table 1. The two clinical groups did not significantly differ in education, sex distribution, or age at Numbers Battery testing. Table 1 also summarizes comparisons of neuropsychological scores between groups. PSP patients performed worse than LBSD patients on MMSE and LF, while both groups performed similarly on JOLO.
Numbers performance and correlation with executive function
PSP patients performed significantly worse on Complex numbers tasks than LBSD patients (F(2, 51) = 6.06, p = 0.02) (Fig. 1A); sex and MMSE covariates had no effect on the model (p > 0.1). As a comparison measure, we assessed Basic numbers scores and found no difference in performance between clinical groups (p > 0.1) (Fig. 1B). We also compared numbers performance across clinical phenotypes (i.e., PD, PD-MCI, PDD, DLB) within the LBSD group and by sex [43] and found no differences (all p > 0.1). Subitizing scores, or tasks with object cardinality ≤ 4 that allows for instinctive recognition [39], were used as a control measure to ensure that baseline numerical perception was intact and patients could perform simple tasks. Nearly all patients (n = 55) had a perfect subitizing score except for 1 LBSD patient that scored 7 out of 8.
Fig. 1.
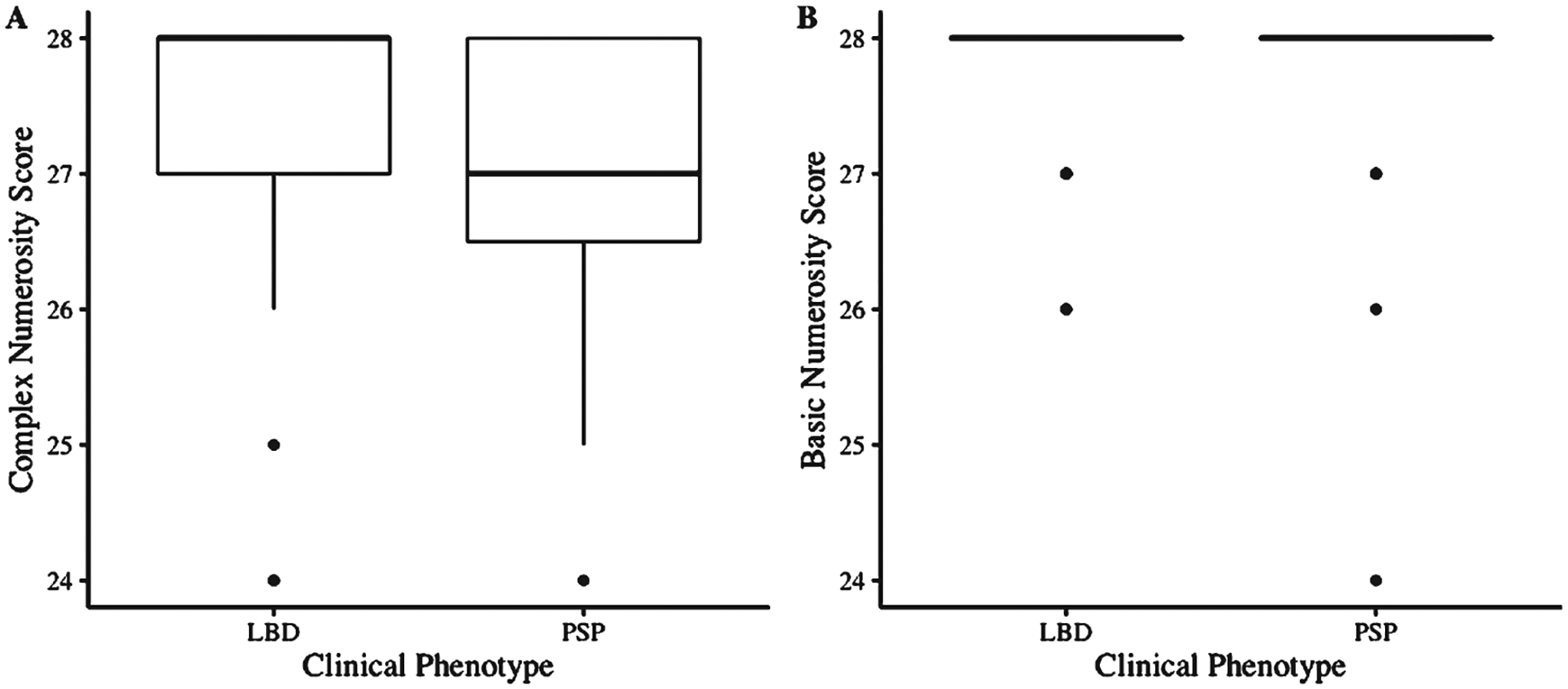
Complex and Basic numbers performance between LBSD and PSP patients. PSP performs worse than LBSD on Complex numbers tasks (A) but not Basic numbers tasks (B). Boxplots illustrate the median, interquartile range, and range of scores for each group independently.
Spearman’s correlation tested the relationship between neuropsychological performance and numbers impairments across the entire patient cohort. We found a positive correlation between LF and Complex numbers performance (rho = 0.34, p = 0.01) (Fig. 2), but no correlation between LF and Basic numbers tasks (p > 0.1). When accounting for the effects of JOLO, the relationship between Complex numbers performance and LF was weaker (rho = 0.25, p = 0.07). We also tested if the association with Complex numbers performance was specific to LF and found no correlation between Complex scores and JOLO (rho = 0.23, p = 0.09) or MMSE (rho = 0.26, p = 0.06).
Fig. 2.
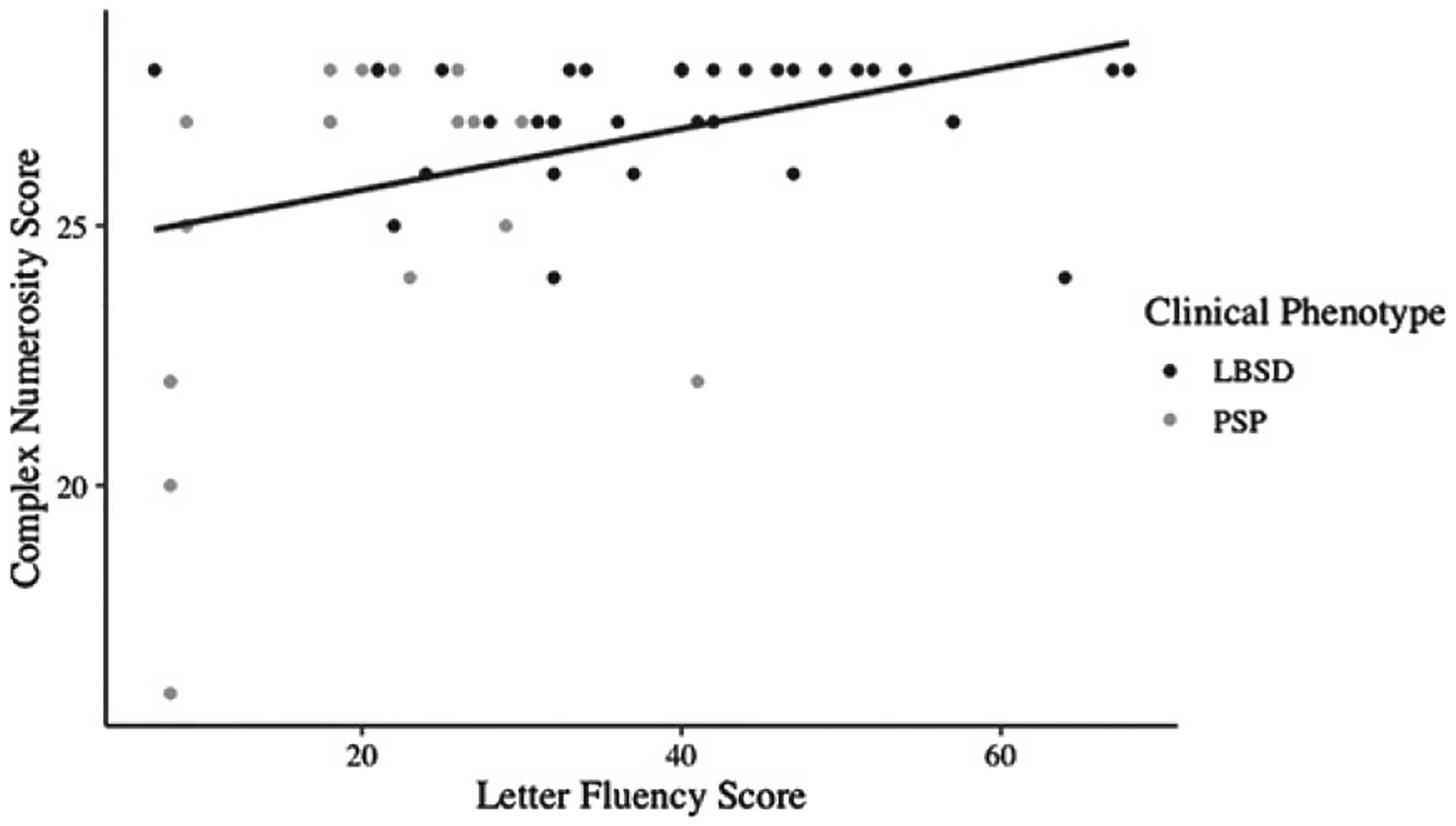
Complex numbers tasks correlate with LF performance. Scatterplot depicts individual patients’ Complex numbers task scores plotted against Letter Fluency (LF) scores with a line of best fit. Datapoints are colored by clinical phenotype of LBSD (black) versus PSP (grey).
Excluding patients with AD biomarkers
Analyses were repeated excluding the 24 patients with evidence of AD co-pathology; all main findings were consistent, showing that clinical Parkinsonian syndromes differed in LF (U = 185, p < 0.001) and MMSE (U = 179, p = 0.03) but not JOLO (p > 0.1). PSP again performed worse compared to LBSD (F = 7.76, p < 0.01), covarying for sex and MMSE (both p > 0.1). Clinical groups did not differ in Basic numbers task scores (p > 0.1). Spearman’s correlation also found a positive relationship between Complex numbers performance and LF (rho = 0.40, p = 0.03) but not JOLO (p > 0.1).
Imaging analyses
Analysis of CT in PSP patients relative to controls showed significant atrophy in the bilateral frontal and temporal cortices, especially the superior and inferior frontal gyri and insular cortices (Fig. 3A). PSP patients also showed significant atrophy in the bilateral PFC relative to LBSD patients (Fig. 3B). There were no regions of reduced CT in LBSD relative to PSP, nor relative to controls. Finally, we related both complex numerical processing and LF to CT within the PSP patient group. Regression analysis revealed lower complex numbers scores associated with atrophy of the bilateral PFC, IFG, and pre-motor+motor cortices while poor LF performance related to atrophy in the right IFG, insular cortex, and frontal fields and bilateral PFC (Fig. 3C, Table 3). Atrophied regions of overlap related to both LF and complex numbers impairments centered on the left anterior PFC and right insular cortex.
Fig. 3.
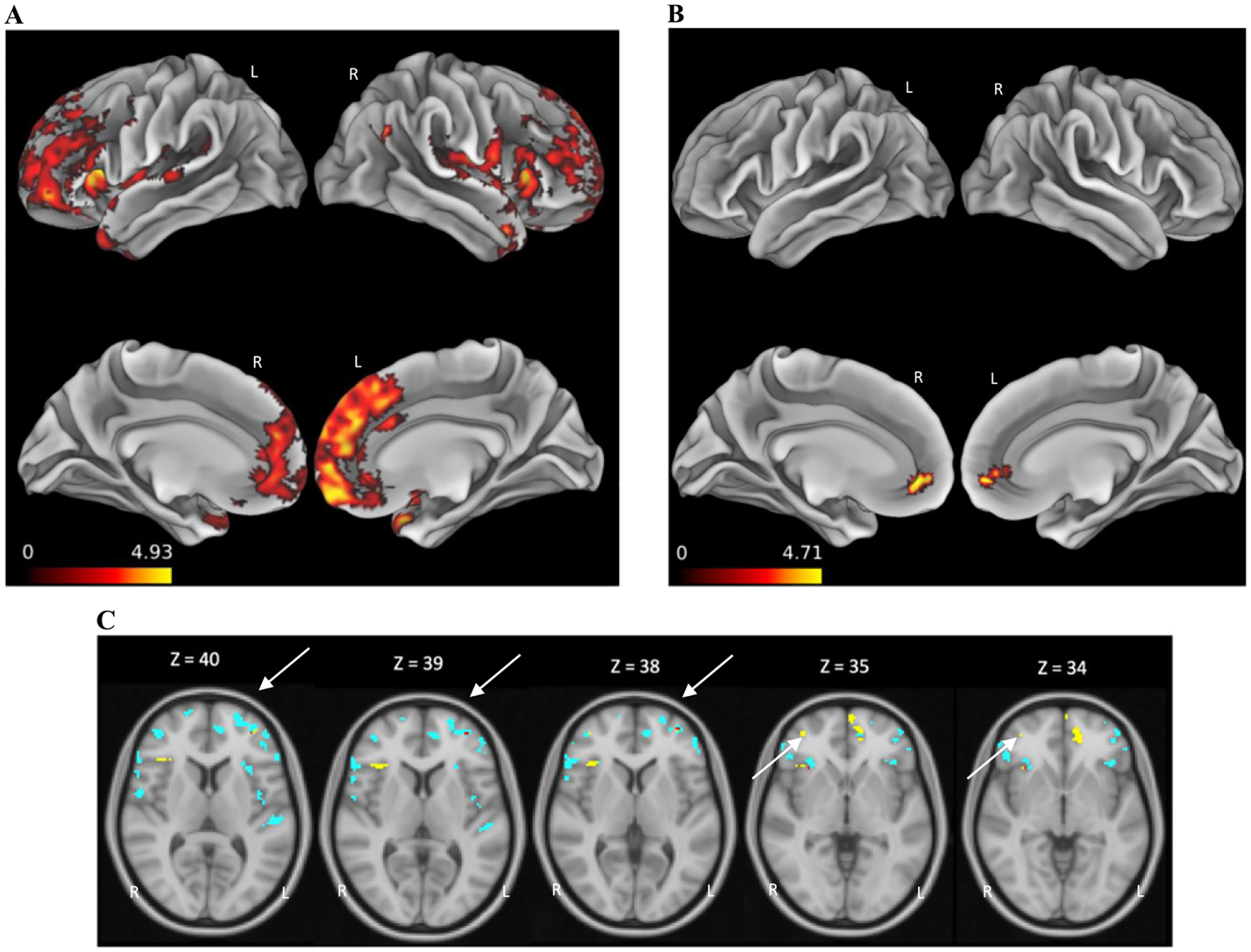
Regions of cortical thinning. Regions of cortical thinning (colored areas) in patients with PSP relative to healthy controls (A) and LBSD (B), and regions related to complex numbers scores (blue), LF (yellow), and both complex numbers scores and LF (red; overlap indicated by arrows) within PSP patient cohort (C).
Table 3.
Summary of CT associated with complex number scores, LF, and regions of overlap in PSP
| CT | Complex number scores | ||||||
|---|---|---|---|---|---|---|
| CT in PSP | BA | x | y | z | k | t score |
| Left Anterior PFC | 10 | −30 | 46 | 14 | 207 | 4.99 |
| Left Dorsolateral PFC | 46 | −48 | 44 | 4 | 194 | 4.86 |
| Left Anterior PFC | 10 | −12 | 52 | 12 | 96 | 4.99 |
| Left Frontal Pole Cortex | NA | −46 | 46 | −16 | 85 | 6.07 |
| Right Anterior PFC | 10 | 44 | 46 | 8 | 69 | 4.77 |
| Right Premotor + Supp. Motor Areas | 6 | 58 | −2 | 8 | 63 | 2.98 |
| Left Primary Auditory Cortex | 41 | −58 | −20 | 8 | 51 | 5.71 |
| Left Primary Motor Cortex | 4 | −38 | −6 | 12 | 50 | 3.60 |
| Left Broca – Pars Triangularis (IFG) | 45 | −32 | 18 | 12 | 48 | 5.42 |
| Right Broca – Pars Triangularis (IFG) | 45 | 58 | 24 | 6 | 44 | 3.72 |
| Right Anterior PFC | 10 | 16 | 64 | 10 | 42 | 3.48 |
| Left Premotor+Supp. Motor Areas | 6 | −50 | 4 | 34 | 40 | 2.99 |
| Right Orbitofrontal Cortex | 11 | 30 | 22 | −2 | 36 | 4.33 |
| Right Orbitofrontal Cortex | 11 | 2 | 44 | −20 | 35 | 3.13 |
| Left Broca – Pars Triangularis (IFG) | 45 | −56 | 28 | 12 | 31 | 4.74 |
| Right Broca – Pars Opercularis (IFG) | 44 | 60 | 14 | 10 | 30 | 3.55 |
| Right Frontal Eye Fields | 8 | 6 | 30 | 38 | 28 | 3.77 |
| Right Pars Orbitalis (IFG) | 47 | 48 | 32 | −6 | 24 | 2.64 |
| Left Anterior PFC | 10 | −38 | 50 | −12 | 24 | 2.88 |
| Left Pars Orbitalis (IFG) | 47 | −36 | 36 | −10 | 24 | 3.66 |
| Left Pars Orbitalis (IFG) | 47 | −40 | 28 | −4 | 21 | 2.16 |
| Left Anterior PFC | 10 | −14 | 62 | 28 | 17 | 3.12 |
| Right Dorsolateral PFC | 46 | 54 | 40 | 0 | 15 | 6.40 |
| CT | LF | ||||||
| CT in PSP | BA | x | y | z | k | t score |
| Left Anterior PFC | 10 | −10 | 50 | −6 | 75 | 5.52 |
| Right Broca – Pars Triangularis (IFG) | 45 | 38 | 26 | 4 | 50 | 4.48 |
| Right Anterior PFC | 10 | 38 | 38 | 18 | 46 | 4.21 |
| Right Insular Cortex | 13 | 36 | −10 | 18 | 42 | 4.76 |
| Left Anterior PFC | 10 | −2 | 66 | −10 | 31 | 4.18 |
| Right Frontal Eye Fields | 8 | 8 | 24 | 38 | 19 | 3.83 |
| Right Anterior PFC | 10 | 36 | 50 | 0 | 18 | 3.36 |
| Left Dorsolateral PFC | 46 | −36 | 48 | 8 | 18 | 3.07 |
| Left Anterior PFC | 10 | −4 | 58 | 28 | 17 | 3.73 |
| CT | Complex number scores + LF regions of overlap | ||||||
| CT in PSP | BA | x | y | z | k | t score |
| Left Anterior PFC | 10 | −36 | 54 | 4 | 4 | 3.14 |
| Right Insular Cortex | 13 | 32 | 22 | −2 | 2 | 1.94 |
| Left Anterior PFC | 10 | −34 | 48 | 8 | 1 | 2.71 |
CT, cortical thickness; PSP, progressive supranuclear palsy; LF, Letter fluency; BA, Brodmann area; x,y,z, MNI coordinates; k, cluster extent; NA, region outside of defined BA.
DISCUSSION
In the present study, we investigated numerical processing in Parkinsonian syndromes and related numbers performance to neurocognitive and neuroanatomic outcomes. We found that PSP patients are selectively impaired in complex numerical processing, showing greater difficulty with double-digit arithmetic and tallying disorganized dot arrays, but not basic numerical processing. In comparison, LBSD patients showed preserved computation on both basic and complex numerical tasks relative to PSP patients. PSP patients were also more impaired on LF, and across all Parkinsonian syndrome patients, more severe complex numbers deficits correlated with lower LF scores. Likewise, PSP patients demonstrated widespread frontal lobe atrophy compared with healthy controls, and this atrophy was related to greater complex numbers and LF deficits. Together, these findings suggest that poor complex numbers ability in PSP may be due in part to greater executive dysfunction and frontal lobe atrophy, compared with LBSD patients. Our results emphasize the multifactorial structure of numerical processing, underscoring the presence of a frontal-executive component which supports computation and combinatorial calculations. These findings highlight an understudied numbers deficit in Parkinsonian syndromes and may have important implications on the clinical assessment and functional management of numbers impairments in Parkinsonian syndromes.
Previous research assessing numerical processes in Parkinsonian syndromes is limited, and the few existing studies have found variable levels of impairment. Case reports of patients with clinical features of PSP suggest that some initial presentations involve notable deficits in mathematical ability [7, 8]. Other studies focused on LBSD have found no significant differences in arithmetic between PD patients and healthy elderly controls [9, 10], even when executive functions like set shifting and cognitive redirection were employed [9]. Still, these studies all use different measures of numerical ability, and they fail to compare numerical processing across different syndromes or relate numbers performance to specific neuroanatomic outcomes. Here, we expand on this previous literature to directly compare numerical abilities in PSP and LBSD, and we identify a neuroanatomic basis for complex numbers impairment in the frontal lobe.
One group of Parkinsonian syndromes not included in this study are patients with corticobasal syndrome (CBS) where acalculia is often documented [11, 19, 20, 38, 56]. Indeed, research shows that CBS patients are impaired when judging target numerosities [56] and quantifiers [19, 20] with both large and small cardinalities, especially small numeral-dot array stimuli [38]. Another study, which utilized the same Numbers Battery as described here, found CBS patients to be impaired on basic arithmetic calculations [11] and identified magnitude and chunking effects [13, 14], with arithmetic and numeral-dot array performance correlating to number cardinality and spatial distribution of dots, respectively. However, none of these studies in CBS distinguish ‘basic’ numerical processing from more ‘complex’ numerical processing. While the aforementioned study linked magnitude and chunking effects (i.e., basic number knowledge) to parietal lobe activity and visuospatial functioning [11], no existing studies have directly explored a frontal-executive component that may be implicated in more ‘complex’ numbers tasks [3] within neurodegenerative Parkinsonian syndrome patients.
Our results here suggest that larger-cardinality arithmetic and spatially disorganized dot arrays may rely on executive functioning mediated by frontal lobe regions, especially the IFG. These findings are supported by a broad body of neurocognitive and neuroanatomic evidence in many populations. Research in children suggests that executive functioning—a cognitive domain that relies on the frontal lobe region of the brain [24]—is implicated in the development of mathematics proficiency [2]. Likewise, studies in dementia populations known to affect the frontal lobe (i.e., frontotemporal dementia) have shown that impaired higher-order numerical quantification is associated with poor executive performance [20]. Neuroimaging research in healthy older adults supports the role of the frontal lobe in complex number tasks, as fMRI studies have found growing frontal lobe activation dependent on number task complexity [3, 12, 16]. Other healthy control studies [17, 18] have similarly linked mathematical processing to frontal-executive brain activation, particularly the IFG. Indeed, results of the present study show increased atrophy in the superior and inferior frontal gyri in PSP patients relative to healthy controls. Atrophy of the IFG was also associated with both LF scores and complex numbers deficits, thus supporting the role of the IFG in complex number abilities like arithmetic and computation. Future work is needed to disentangle how specific cognitive impairments and spreading disease contribute to selective deficits in different types of number abilities, like logical quantifiers, change calculations, and time relationships.
In addition to the IFG, we also found other brain regions associated with complex numbers scores and LF, such as the PFC, frontal eye fields, right insular cortex, and premotor and motor cortices. Previous neuroimaging research in humans [57] and non-human primates [58] has suggested that the PFC is heavily involved in visual search tasks via the frontal eye fields. The search tasks described in these previous studies are similar to the D-to-N and N-to-D tasks employed in the present study, thus supporting the role of the PFC and frontal eye fields in complex numbers performance given known associations between task difficulty and increased frontal lobe activation [3, 12, 16]. The involvement of the right insular cortex in complex numerical processing is less apparent but may be related to the stimulus-switching nature of N-to-D and D-to-N tasks, as previous literature suggests that the right fronto-insular cortex plays a key role in alternating between stimulus modalities [59]. The relationship between complex numerosity and motor cortices also remains unclear but may be due in part to the brain regions’ role in mental syllabary as patients perform more advanced calculations and tally greater numbers of items in their mind [60]. Future work should continue to explore the implications of other brain regions like the temporo-parietal cortex that may play a role in the spatial-magnitude component of numerical processing in Parkinsonian syndrome patients.
Despite these widespread frontal atrophy findings, our regression results suggest that a particular subregion, namely the left PFC, may contribute to executive and complex numerical deficits specifically in PSP. Indeed, functional neuroimaging studies in primates have shown that PFC neurons are heavily involved in numerical encoding and quantitative judgments [61]. Additionally, developmental studies have shown that the right PFC is implicated in overlearned, automated processes, whereas the left PFC is involved in more complex, demanding processes [62]. Together, this evidence lends support to our findings relating complex numerosity and executive functioning to CT of the left PFC. Moreover, previous aging studies have found that older adults require even greater activation in the left PFC during arithmetic tasks than younger adults [18], potentially due to significant age-related cortical reductions in the PFC [25]. In the present study, we found greater mesiofrontal PFC atrophy in PSP specifically, which is supported by previous literature [26], and this atrophy related to complex numerical deficits and executive dysfunction. The increased frontal atrophy and enhanced executive impairments seen in PSP may require these patients to recruit additional attentional resources via the left PFC when performing complex numerical tasks. Future studies should continue to analyze brain regions that might contribute to differences in complex numerical processing among Parkinsonian movement disorders, both cross-sectionally and longitudinally.
Another potential, but understudied, source of variation in numbers performance across Parkinsonian syndromes is the pathological heterogeneity of syndromes. LBSD is typically related to the accumulation of alpha-synuclein protein [63], while CBS and PSP are both associated with 4-repeat (4R) tauopathy [7]. However, none of these clinicopatho-logical correlations are perfect: CBS is associated with heterogeneous underlying pathology including AD neuropathologic change, TDP-43 proteinopathy, and 3R and 4R tauopathies [64], and AD pathology is frequently concomitant with alpha-synuclein and 4R tau in LBSD and PSP, respectively [29, 65]. Due to small numbers of patients with autopsy data (4 of 56), we were unable to test directly how heterogeneous pathologies across syndromes interact with numbers performance. Despite this limitation, reliable biomarkers for AD pathology exist [66], which allowed us to test effects of coincident AD pathology in patients without autopsy data. Previous studies have found evidence of interactions between AD pathology and cognition in Parkinsonian syndromes [29, 30]; thus, we examined the possibility that AD co-pathology may be a confounding factor in numbers deficits. We performed a sub-analysis removing patients with known AD biomarkers; overall results remained stable, suggesting that aggregation of AD pathology was not an explanatory factor for numbers deficits in this study. These null findings may be partly explained by the fact that AD pathology tends to accumulate in temporal and hippocampal regions [67], while we find that the frontal lobe is critical to complex numbers performance. Future work should explore how numerical processing in Parkinsonian syndromes relates to pathology type and accumulation, especially in the frontal lobe.
Altogether, these findings highlight the potential clinical utility of numbers assessment in Parkinsonian syndromes and AD and related dementias. Numerical tests assessing complex numbers performance may aid diagnoses associated with executive dysfunction, such that greater impairments may be indicative of phenotypes classically associated with more frontal disease (i.e., PSP). These tests might also serve as a prognostic tool to predict functional impairments in common numerical tasks (e.g., financial management), so future studies should explore whether complex numbers deficits and specific atrophy patterns differentially influence instrumental activities of daily living across phenotypes. Given pathological heterogeneities, these tests may also be useful when patients with concomitant AD pathology present with Parkinsonian phenotypes. Even in non-Parkinsonian phenotypes, complex numerical tests may help differentiate frontal dementias (i.e., FTD) from other dementias with more widespread atrophy patterns (i.e., AD). Future work should extend the present findings to non-Parkinsonian syndromes to confirm the clinical utility of complex numerical assessment across all AD and related dementias.
Limitations of this study include the retrospective nature of available data and limited testing of numerical abilities. While these data attempt to relate numerical processing to other cognitive domains and CT, the lack of completely harmonized neuropsychological and MRI data prevented testing of more specific cognitive factors (e.g., attention, cognitive flexibility) and added excess variance to our CT analyses through the T1 protocol covariate. Because we focused on numerical deficits specifically in PSP, our regression analyses may have been underpowered due to limited sample size (n = 15). Although we found atrophied regions of overlap for LF and complex numerosity in PSP that corroborates previous literature [57–59], we report these results with a lenient cluster extent threshold and uncorrected p-value so the possibility for Type I error does exist. The overlapping voxel sizes are also very small which may reduce meaningful interpretation. Future efforts should focus on collecting prospective, harmonized, longitudinal datasets with MRI in a larger sample to better understand how declining numbers ability in Parkinsonian syndromes relates to spreading atrophy.
Second, we delivered a battery of tasks, each of which probed a different aspect of numerical processing (i.e., arithmetic, numeral-dot array matching) with somewhat distinct neural correlates. Indeed, numerical-dot arrays may rely more on the posterior visuospatial network, while arithmetic may rely on the intraparietal sulcus (IPS) [68], with both tasks recruiting executive resources from the frontal lobe as complexity increases. If so, there may be an interaction between task type and complexity. The small number of trials for each task type in this study precluded this investigation, so future studies should test specific numerical domains individually with a greater number of trials to explore this possibility. Moreover, while we hypothesized that both clinical groups were preserved in basic numerical processing, there are other computational abilities not probed by this battery (e.g., spatial reasoning, calculating change, general quantifier knowledge), and thus subtle deficits in other ‘basic’ and ‘complex’ numerical tasks may have been undetected.
Third, we used clinical diagnosis to define patients as “LBSD” or “PSP,” but few patients had available autopsy data. Although we were able to screen for amyloid biomarkers, we could not confirm neuropathological diagnoses as alpha-synuclein or 4R tauopathy, nor could we directly assess for other co-pathologies, such as amyloid and TDP-43, which may influence cognitive decline in the aging brain [69]. Even so, our sub-analysis removing patients with known AD biomarkers ensured AD pathology was likely not confounding results [29]. Moreover, we included PD, PD-MCI, PDD, and DLB within our ‘LBSD’ patient grouping. Although these phenotypes are all associated with underlying alpha-synuclein pathology, their atrophy patterns and domains and severity of cognitive impairment can differ [4–6]; thus, these differences may have created heterogeneity within the LBSD group and obscured our findings. Future studies should investigate longitudinal numerical abilities in autopsy- or neuroimaging-defined cohorts to explore potential effects of pathology and regional brain atrophy across all Parkinsonian syndrome phenotypes.
Finally, since our neuroimaging analyses did not find appreciable atrophy in the LBSD group, the greater complex numbers impairment seen in PSP could have been attributed to more general impairment from a more advanced stage of disease in comparison to LBSD. To account for this potential confound, we included MMSE as a measure of global disease severity in our ANCOVA analyses. Furthermore, atrophy patterns in PSP relative to LBSD (Fig. 3B) were more restricted in comparison to the widespread differences seen between PSP and controls (Fig. 3A); therefore, it is possible that LBSD patients may have had subtle frontal atrophy that did not withstand robust FWE corrections. Future work should relate numbers performance and atrophy patterns to more comprehensive assessments such as the PSP Rating Scale (PSPRS) or Unified Parkinson’s Disease Rating Scale (UPDRS) that include motor components.
In conclusion, we show that complex numerical processing has a relatively specific association with executive dysfunction and frontal lobe atrophy in Parkinsonian syndromes. We also show that PSP is more impaired than LBSD on complex numbers tasks and executive functioning, but not basic numbers tasks or visuospatial functioning. Exploring complex numbers tasks in relation to executive functioning emphasizes the compound nature of numerical processing, underlining the presence of both spatial-magnitude and executive components. Accounting for different mechanisms of numerical processing will have important implications for functional assessment and cognitive therapies in neurodegenerative disease patients.
ACKNOWLEDGMENTS
Data contributed by current project center on Alpha-synuclein Strains in Alzheimer Disease & Related Dementias (U19 AG062418, Trojanowski JQ-PI) and Spreading Tau Pathology in Non-Amnestic Alzheimer’s Disease (R01-AG054519, Grossman M) and Clinical Core (P01-AG066597, Irwin DJ) at the Perelman School of Medicine at the University of Pennsylvania. We also thank all our patients and caregivers for their participation in our research.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5327r2).
Statistical Analysis conducted by Erica Howard, BS, Samantha Ballinger, BS, Nikolas G. Kinney, MS, Jeffrey S. Phillips, PhD and Katheryn A.Q. Cousins, PhD, Perelman School of Medicine at the University of Pennsylvania.
REFERENCES
- [1].Vuokko E, Niemivirta M, Helenius P (2013) Cortical activation patterns during subitizing and counting. Brain Res 1497, 40–52. [DOI] [PubMed] [Google Scholar]
- [2].Cragg L, Gilmore C (2014) Skills underlying mathematics: The role of executive function in the development of mathematics proficiency. Trends Neurosci Educ 3, 63–68. [Google Scholar]
- [3].Dormal V, Dormal G, Joassin F, Pesenti M (2012) A common right fronto-parietal network for numerosity and duration processing: An fMRI study. Hum Brain Mapp 33, 1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I; Movement Disorder Society-endorsed PSP Study Group (2017) Clinical diagnosis of progressive supranuclear palsy: The Movement Disorder Society criteria. Mov Disord 32, 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30, 1591–1601. [DOI] [PubMed] [Google Scholar]
- [7].Katsuse O, Iseki E, Arai T, Akiyama H, Togo T, Uchikado H, Kato M, de Silva R, Lees A, Kosaka K (2003) 4-repeat tauopathy sharing pathological and biochemical features of corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 106, 251–260. [DOI] [PubMed] [Google Scholar]
- [8].Sakamoto R, Tschuiya K, Mimura M (2010) Clinical heterogeneity in progressive supranuclear palsy: Problems of clinical diagnostic criteria of NINDS-SPSP in a retrospective study of seven Japanese autopsy cases. Neuropathol 30, 24–35. [DOI] [PubMed] [Google Scholar]
- [9].Zamarian L, Visani P, Delazer M, Seppi K, Mair KJ, Diem A, Poewe W, Benke T (2006) Parkinson’s disease and arithmetics: The role of executive functions. J Neurol Sci 248, 124–130. [DOI] [PubMed] [Google Scholar]
- [10].Dormal V, Grade S, Mormont E, Pesenti M (2012) Dissociation between numerosity and duration processing in aging and early Parkinson’s disease. Neuropsychologia 50, 2365–2370. [DOI] [PubMed] [Google Scholar]
- [11].Spotorno N, McMillan CT, Powers JP, Clark R, Grossman M (2014) Counting or chunking? Mathematical and heuristic abilities in patients with corticobasal syndrome and posterior cortical atrophy. Neuropsychologia 64, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fehr T, Code C, Herrmann M (2007) Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI-BOLD activation. Brain Res 1172, 93–102. [DOI] [PubMed] [Google Scholar]
- [13].Mathy F, Feldman J (2012) What’s magic about magic numbers? Chunking and data compression in short-term memory. Cognition 122, 346–62. [DOI] [PubMed] [Google Scholar]
- [14].Feigenson L, Halberda J (2004) Infants chunk object arrays into sets of individuals. Cognition 91, 173–90. [DOI] [PubMed] [Google Scholar]
- [15].Harvey BM, Klein BP, Petridou N, Dumoulin SO (2013) Topographic representation of numerosity in the human parietal cortex. Science 341, 1123–1126. [DOI] [PubMed] [Google Scholar]
- [16].Piazza M, Pinel P, Le Bihan D, Dehaene S (2007) A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron 53, 292–305. [DOI] [PubMed] [Google Scholar]
- [17].Wang K, Banich MT, Reineberg AE, Leopold DR, Will-cutt EG, Cutting LE, Del Tufo SN, Thompson LA, Opfer J, Kanayet FJ, Lu ZL, Petrill SA (2020) Left posterior prefrontal regions support domain-general executive processes needed for both reading and math. J Neuropsychol 14, 467–495. [DOI] [PubMed] [Google Scholar]
- [18].Hinault T, Larcher K, Bherer L, Courtney SM, Dagher A (2019) Age-related differences in the structural and effective connectivity of cognitive control: A combined FMRI and DTI study of mental arithmetic. Neurobiol Aging 82, 30–39. [DOI] [PubMed] [Google Scholar]
- [19].McMillan CT, Clark R, Moore P, Grossman M (2006) Quantifier comprehension in corticobasal degeneration. Brain Cogn 62, 250–60. [DOI] [PubMed] [Google Scholar]
- [20].Morgan B, Gross RG, Clark R, Dreyfuss M, Boller A, Camp E, Liang TW, Avants B, McMillan CT, Grossman M (2011) Some is not enough: Quantifier comprehension in corticobasal syndrome and behavioral variant frontotemporal dementia. Neuropsychologia 49, 3532–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tartaglia MC, Zhang Y, Racine C, Laluz V, Neuhaus J, Chao L, Kramer J, Rosen H, Miller B, Weiner M (2012) Executive dysfunction in frontotemporal dementia is related to abnormalities in frontal white matter tracts. J Neurol 259, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zheng D, Sun H, Dong X, Liu B, Xu Y, Chen S, Song L, Zhang H, Wang X (2014) Executive dysfunction and gray matter atrophy in amnestic mild cognitive impairment. Neurobiol Aging 35, 548–555. [DOI] [PubMed] [Google Scholar]
- [23].Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J (2005) Executive dysfunction in early stages of Huntingdon’s disease is associated with striatal and insular atrophy: A neuropsychological and voxel-based morphometry study. J Neurol Sci 239, 11–19. [DOI] [PubMed] [Google Scholar]
- [24].Stuss DT (2011) Functions of the frontal lobes: Relation to executive functions. J Int Neuropsychol Soc 17, 759–765. [DOI] [PubMed] [Google Scholar]
- [25].Nissim NR, O’Shea AM, Bryant V, Porges EC, Cohen R, Woods AJ (2017) Frontal structural neural correlates of working memory performance in older adults. Front Aging Neurosci 8, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brenneis C, Seppi K, Schocke M, Benke T, Wenning GK, Poewe W (2004) Voxel-based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 75, 246–249. [PMC free article] [PubMed] [Google Scholar]
- [27].Gerstenecker A, Mast B, Duff K, Ferman TJ, Litvan I (2013) Executive dysfunction is the primary cognitive impairment in progressive supranuclear palsy. Arch Clin Neuropsychol 28, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargallo N, Tolosa E (2009) Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Mov Disord 24, 1193–1199. [DOI] [PubMed] [Google Scholar]
- [29].Howard E, Irwin DJ, Rascovsky K, Nevler N, Shellikeri S, Tropea TF, Spindler M, Deik A, Chen-Plotkin A, Siderowf A, Dahodwala N, Weintraub D, Shaw LM, Trojanowski JQ, Vaishnavi SN, Wolk DA, Mechanic-Hamilton D, Morley JF, Duda JE, Grossman M, Cousins KAQ (2021) Cognitive profile and markers of Alzheimer disease-type pathology in patient with Lewy body dementias. Neurology 96, e1855–e1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Coughlin D, Xie SX, Liang M, Williams A, Peterson C, Weintraub D, McMillan CT, Wolk DA, Akhtar RS, Hurtig HI, Branch Coslett H, Hamilton RH, Siderowf AD, Duda JE, Rascovsky K, Lee EB, Lee VM, Grossman M, Trojanowski JQ, Irwin DJ (2018) Cognitive and pathological influences of tau pathology in Lewy Body disorders. Ann Neurol 85, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tam CW, Burton EJ, McKeith IG, Burn DJ, O’Brien JT (2005) Temporal lobe atrophy on MRI in Parkinson disease with dementia: A comparison with Alzheimer disease and dementia with Lewy bodies. Neurology 64, 861–865. [DOI] [PubMed] [Google Scholar]
- [32].Sulena, Gupta D, Sharma AK, Kumar N (2017) Clinical profile of cognitive decline in patients with Parkinson’s disease, progressive supranuclear palsy, and multiple system atrophy. J Neurosci Rural Pract 8, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lange KW, Tucha O, Alders GL, Preier M, Csoti I, Merz B, Mark G, Herting B, Fornadi F, Reichmann H, Vieregge P, Reiners K, Becker G, Naumann M (2003) Differentiation of parkinsonian syndromes according to differences in executive functions. J Neural Transm (Vienna) 110, 983–995. [DOI] [PubMed] [Google Scholar]
- [34].Jecmenica Lukic M, Kurz C, Respondek G, Grau-Rivera O, Compta Y, Gelpi E, Troakes C; Barcelona Brain Bank collaborative group, the MDS-endorsed PSP study group, van Swieten JC, Giese A, Roeber S, Arzberger T, Höglinger G (2020) Copathology in progressive supranuclear palsy: Does it matter? Mov Disord 35, 984–993. [DOI] [PubMed] [Google Scholar]
- [35].Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, Xie SX, McBride J, Wood EM, Schuck T, Irwin DJ, Gross RG, Hurtig H, McCluskey L, Elman L, Karlawish J, Schellenberg G, Chen-Plotkin A, Wolk D, Grossman M, Arnold SE, Shaw LM, Lee VM, Trojanowski JQ (2014) A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement 10, 477.e1–484.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Watson GS, Leverenz JB (2010) Profile of cognitive impairment in Parkinson’s disease. Brain Pathol 20, 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ; Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s Disease Neuroimaging subjects. Ann Neurol 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Halpern C, Clark R, Moore P, Cross K, Grossman M (2007) Too much to count on: Impaired very small numbers in corticobasal degeneration. Brain Cogn 64, 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Simon TJ, Vaishnavi S (1996) Subitizing and counting depend on different attentional mechanisms: Evidence from visual enumeration in afterimages. Percept Psychophys 58, 915–926. [DOI] [PubMed] [Google Scholar]
- [40].Qualls CE, Bliwise NG, Stringer AY (2000) Short forms of the Benton Judgment of Line Orientation Test: Development and psychometric properties. Arch Clin Neuropsychol 15, 159–163. [PubMed] [Google Scholar]
- [41].Benton AL, Hamsher K, Sivan AB (1994) Multilingual Aphasia Examination. AJA Associates, Iowa City, IA. [Google Scholar]
- [42].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 1289–198. [DOI] [PubMed] [Google Scholar]
- [43].Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, Gernsbacher MA (2007) The science of sex differences in science and mathematics. Psychol Sci Public Interest 8, 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen T, Tang W, Lu Y, Tu X (2014) Rank regression: An alternative regression approach for data with outliers. Shanghai Arch Psychiatry 26, 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Conover WJ, Iman RL (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 35, 124–129. [Google Scholar]
- [46].Ho DE, Imai K, King G, Stuart EA (2007) Matching as non-parametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 15, 199–236. [Google Scholar]
- [47].Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, Kandel BM, van Strien N, Stone JR, Gee JC, Avants BB (2015) Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage 99, 166–179. [DOI] [PubMed] [Google Scholar]
- [48].Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC (2010) N4ITK: Improved N3 bias correction. IEEE Trans Med Imaging 29, 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL (2007) Open Access Series of Imaging Studies (OASIS): Cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci 19, 1498–1507. [DOI] [PubMed] [Google Scholar]
- [50].Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC (2011) An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics 9, 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mazziotta JC, Toga AW, Evans AC, Fox PT, Lancaster JL (1995) Digital brain atlases. Trends Neurosci 18, 210–211. [DOI] [PubMed] [Google Scholar]
- [52].Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Smith SM, Nichols TE (2009) Threhold-free cluster enhancement: Addressing problems of smoothing, threshold dependence, and localization in cluster inference. Neuroimage 44, 83–98. [DOI] [PubMed] [Google Scholar]
- [54].Hurtz S, Woo E, Kebets V, Green AE, Zoumalan C, Wang B, Ringman JM, Thompson PM, Apostolova LG (2014) Age effects on cortical thickness in cognitively normal elderly individuals. Dement Geriatr Cogn Disord Extra 4, 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW (2007) Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 17, 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Koss S, Clark R, Vesely L, Weinstein J, Powers C, Richmond L, Farag C, Gross R, Liang TW, Grossman M (2010) Numerosity impairment in corticobasal syndrome. Neuropsychology 24, 476–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3, 201–215. [DOI] [PubMed] [Google Scholar]
- [58].Paneri S, Gregoriou GG (2017) Top-down control of visual attention by the prefrontal cortex – functional specialization and long-range interactions. Front Neurosci 11, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sridharan D, Levitin DJ, Menon V (2008) A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Levelt WJM, Roelofs A, Meyer AS (1999) A theory of lexical access in speech production. Behav Brain Sci 22, 1–38. [DOI] [PubMed] [Google Scholar]
- [61].Nieder A, Freedman DJ, Miller EK (2002) Representation of the quantity of visual items in the primate prefrontal cortex. Science 297, 1708–1711. [DOI] [PubMed] [Google Scholar]
- [62].Arsalidou M, Pawliw-Levac M, Sadeghi M, Pascual-Leone J (2018) Brain areas associated with numbers and calculations in children: Meta-analyses of fMRI studies. Devel Cog Neurosci 30, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim WS, Kagedal K, Halliday GM (2014) Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Ther 6, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hu WT, Rippon GW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Josephs KA (2009) Alzheimer’s disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord 24, 1375–1379. [DOI] [PubMed] [Google Scholar]
- [65].Keith-Rokosh J, Ang LC (2008) Progressive supranuclear palsy: A review of co-existing neurodegeneration. Can J Neurol Sci 35, 602–608. [DOI] [PubMed] [Google Scholar]
- [66].Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R; Contributors (2018) NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [68].Artemenko C, Soltanlou M, Dresler T, Ehlis AC, Nuerk HC (2018) The neural correlates of arithmetic difficulty depend on mathematical ability: Evidence from combined fNIRS and ERP. Brain Struc Funct 223, 2561–2574. [DOI] [PubMed] [Google Scholar]
- [69].Shih YH, Tu LH, Chang TY, Ganesan K, Chang WW, Chang PS, Fang YS, Lin YT, Jin LW, Chen YR (2020) TDP-43 interacts with amyloid-B, inhibits fibrillization, and worsens pathology in a model of Alzheimer’s disease. Nature Commun 11, 5950. [DOI] [PMC free article] [PubMed] [Google Scholar]


