Abstract
We have created a federated database for genome studies of Magnaporthe grisea, the causal agent of rice blast disease, by integrating end sequence data from BAC clones, genetic marker data and BAC contig assembly data. A library of 9216 BAC clones providing >25-fold coverage of the entire genome was end sequenced and fingerprinted by HindIII digestion. The Image/FPC software package was then used to generate an assembly of 188 contigs covering >95% of the genome. The database contains the results of this assembly integrated with hybridization data of genetic markers to the BAC library. AceDB was used for the core database engine and a MySQL relational database, populated with numerical representations of BAC clones within FPC contigs, was used to create appropriately scaled images. The database is being used to facilitate sequencing efforts. The database also allows researchers mapping known genes or other sequences of interest, rapid and easy access to the fundamental organization of the M.grisea genome. This database, MagnaportheDB, can be accessed on the web at http://www.cals.ncsu.edu/fungal_genomics/mgdatabase/int.htm.
INTRODUCTION
The ascomycetous fungus Magnaporthe grisea (anamorph Pyricularia grisea) causes rice blast, one of the most important diseases of rice, the staple food for half of the world’s population (1,2). Once a problem of moderate significance, crop losses associated with this disease have been magnified in recent times with the intensification of rice production. We can expect increasing pressure from this disease as crop production is enhanced to meet the demands of a growing world population. Genetic resistance has been and continues to be the major method of disease control for blast. However, as with many diseases, M.grisea is able to rapidly overcome host resistance (3). Fungal plant pathogens, including M.grisea, impact more than food security. In addition to affecting the food supply directly through reduction in yield, they also affect food quality. The wholesale destruction of large fields can affect ecosystem function and have devastating effects on local economies.
Since there are many pathogens worthy of investigation, and given our limited pool of resources, the most reasonable research approach is to fully understand a ‘model’ pathogenic organism and apply that knowledge to design control strategies for other related pathogens. A candidate ‘model’ organism should have the following characteristics:
1. It should use an infection strategy that is evolutionarily related to other pathogens.
2. It should have a relatively small genome size.
3. It should be economically important.
4. It should be relatively well studied and amenable to molecular and classical genetic experimentation.
Magnaporthe grisea is ideal as a ‘model organism’ to represent plant pathogenic filamentous fungi (4,5). The fungus has a small genome size (40 Mb) making it an excellent target for whole genome sequencing (6,7). Decades of intense investigation have resulted in extensive genetic maps and a well established transformation system (8,9). In addition to being representative of many phytopathogenic fungi, M.grisea is closely related to other prominent non-pathogenic model fungi, such as Neurospora crassa and Aspergillus nidulans (10).
The strategy we initially chose for our genome sequencing project was to create a BAC library, sequence the ends, and then use fingerprinting analysis to create a physical framework spanning the entire genome, from which a minimum tiling path of BAC clones would be deduced for sequencing. BAC end sequence and fingerprinting data were obtained from a large insert (∼130 kb) HindIII BAC library containing 9216 clones prepared from rice-infecting strain 70-15 using the BAC vector pBACwich. This library provides >25-fold coverage of the rice blast genome (11). Additional information can be found via the links shown in Table 1.
Table 1. Links to further information.
| Description of rice blast project | http://www.cals.ncsu.edu/fungal_genomics/fromMagna/project_riceblast.html |
| BAC end sequencing and physical mapping | http://www.cals.ncsu.edu/fungal_genomics/fromMagna/BAC_ENDS.html |
| Status of BACS in the minimum tiling path which have been sequenced | http://www.cals.ncsu.edu/fungal_genomics/fromMagna/current_bacs.html |
| Blast against BAC end sequences | http://fungi.cals.ncsu.edu/tdsblastscript.html |
| Database Description: technical details and Scriptable access instructions | http://www.cals.ncsu.edu/fungal_genomics/mgdatabase/magnaporthedb.htm |
| CVS Repository: downloadable scripts and tools used in the FGL Bioinformatics Lab | http://fungi.cals.ncsu.edu/cgi-bin/cvsweb.cgi/ |
DATABASE DESCRIPTION
We have created an integrated physical and genetic map that interfaces with a searchable database containing end sequence data from the BAC clones, genetic marker data and FPC contig assembly data that represent the majority of the rice blast genome. The database contains:
1. A HindIII digestion profile ‘fingerprint’ of each BAC clone and FPC contig assemblies.
2. Nucleotide sequence data and analysis of BAC ends.
3. Genetic marker data generated previously and assembled into a genetic map at the University of Wisconsin (9) (I. Yap and S. McCouch; http://ascus.plbr.cornell.edu/blastdb/).
4. Hybridization of genetic markers to the BAC library, which physically anchors FPC contigs onto one of the seven chromosomes in the M.grisea genome.
5. Sequence information for GenBank sequences with significant similarity to M.grisea BAC end sequences.
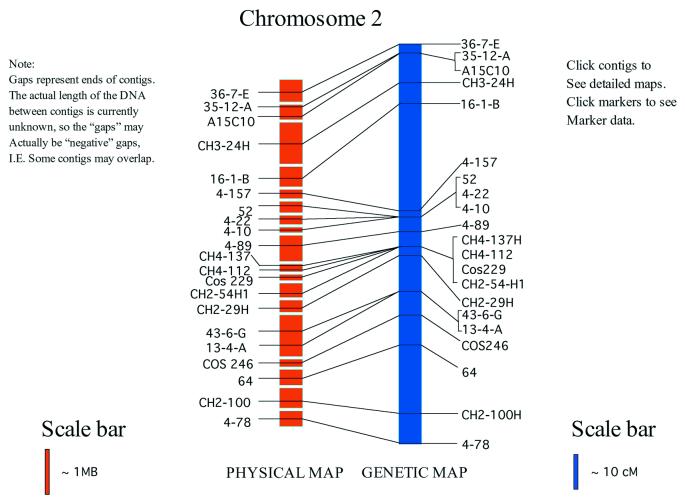
We are using this database as a foundation for generating annotated sequence data of chromosome 7 (4.2 Mb) and to ‘fill in the gaps’ in the parts of the genome which will remain to be sequenced following whole genome shotgun sequencing. Table 2 contains summary statistics of the data contained in the database. The combined physical and genetic map shown in Figure 1 is an image map whose links provide a ‘gateway’ to the database used to view M.grisea BAC end sequence data, FPC assembled BAC contigs and marker data. The database engine is AceDB, originally developed for use with the Caenorhabditis elegans sequencing project (12). Its native graphical libraries have made it a de facto standard for database engines for medium-sized sequencing projects. Figure 2 shows a graphical representation of an example of an FPC contig presented by the database.
Table 2. MagnaportheDB summary statistics.
| Number of fingerprinted BAC clones | 7338 | ||||||
| Number of M.grisea BAC end sequences | 17684 | ||||||
| Number of TBLASTX hits with significant (E-value < 1 × 10–10) homology to a BAC end sequence | 1609 | ||||||
| Number of contigs from the fingerprint assembly | 188 | ||||||
| Number of markers in the database | 188 | ||||||
| Number of anchored contigs by chromosome | I | II | III | IV | V | VI | VII |
| 13 | 11 | 12 | 14 | 6 | 14 | 8 |
Figure 1.
Chromosome 2 of M.grisea. Integrated physical and genetic map. All integrated M.grisea chromosome maps serve as gateways to the federated database.
Figure 2.
FPC contig 31 as displayed by AceDB. Clones are listed in the same order as they occur in the contig. More information about each clone can be obtained by clicking on the clone name.
DATABASE INFRASTRUCTURE
The Image/FPC package, available from the Sanger Centre was used to analyze the gels generated in the BAC fingerprinting project and to construct contigs (13). A MySQL database was used to tabulate FPC data. A perl script reads the database. A program written in C and known as ‘The Fly’ (http://www.w3perl.com/fly/) was used to actually generate graphics in gif format to represent the physical and genetic maps of the chromosomes. The graphical maps of the contigs were generated by the native graphics capability in AceDB. AceBrowser (14) was used to display the AceDB graphics over the web. The Sitedefs.pm module in AceBrowser was modified to interface with both MySQL and AceDB. The HTML web pages are served via a Dell PowerEdge 3500 running Redhat Linux 7.0 and the Apache webserver.
AVAILABILITY
The database can be accessed via the International Rice Blast Genome Consortium homepage: http://www.riceblast.org. Curator: Stan Martin, stan_l_martin@ncsu.edu.
FUTURE DEVELOPMENT
The database will be expanded to include additional sequencing information and annotation. Every effort is being made to ensure that the annotation taxonomy is compliant with the Gene Ontology Consortium (15) schema to ensure universal accessibility. New modules are being developed that will include functional data derived from microarrays, as well as data on mutant phenotypes.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported in part by grants from the National Science Foundation, the USDA and Syngenta Corporation.
REFERENCES
- 1.Ou S.H. (1985) Rice Diseases, 2nd edn. Commonwealth Mycological Institute, Kew, UK, pp. 1–380.
- 2.Zeigler R.S., Leong,S.A. and Teng,P.S. (1994) Rice Blast Disease. CAB International, Wallingford, pp. 1–626.
- 3.Bonman J.M. and MacKill,D.J. (1988) Durable resistance to rice blast disease. Oryza, 25, 103–110. [Google Scholar]
- 4.Valent B. (1990) Rice blast as a model system for plant pathology. Phytopathology , 80, 33–36. [Google Scholar]
- 5.Dean R.A. (1997) Signal pathways and appressorium morphogenesis. Annu. Rev. Phytopathol., 35, 211–234. [DOI] [PubMed] [Google Scholar]
- 6.Orbach M.J., Chumley,F.G. and Valent,B. (1996) Electrophoretic karyotypes of Magnaporthe grisea pathogens of diverse grasses. Mol. Plant Microbe Interact., 9, 261–271. [Google Scholar]
- 7.Zhu H., Blackmon,B., Sasinowski,M. and Dean,R.A. (1999) Physical map and organization of chromosome 7 in the rice blast fungus, Magnaporthe grisea. Genome Res., 9, 739–750. [PMC free article] [PubMed] [Google Scholar]
- 8.Leung H.U., Lehtinen,R. Karjalainen,R., Skinner,D.,Tooley,P., Leong,S.A. and Ellingboe,A. (1990) Transformation of the rice blast fungus Magnaporthe grisea to hygromycin B resistance. Curr. Genet., 17, 409–411. [DOI] [PubMed] [Google Scholar]
- 9.Nitta N., Farman,M.L. and Leong,S.A. (1997) Genome organization of Magnaporthe grisea: integration of genetic maps, clustering of transposable elements and identification of genome duplications and rearrangements. Theor. Appl. Gen., 95, 20–32. [Google Scholar]
- 10.Taylor J.W., Bowman,B.H., Berbee,M.L. and White,T.J. (1993) Fungal model organisms: phylogenetics of Saccharomyces, Aspergillus, and Neurospora.Syst. Biol., 42, 440–457. [Google Scholar]
- 11.Zhu H., Choi,S., Johnston,A.K., Wing,R.A. and Dean,R.A. (1997) A large insert (130 kbp) bacterial artificial chromosome library of the rice blast fungus Magnaporthe grisea: genome analysis, contig assembly and gene cloning. Fungal Genet. Biol., 21, 337–347. [DOI] [PubMed] [Google Scholar]
- 12.Stein L., Sternberg,P., Durbin,R., Thierry-Mieg,J. and Spieth,J. (2001) WormBase: network access to the genome and biology of Caenorhabditis elegans. Nucleic Acids Res., 29, 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soderlund C., Longden I. and Mott,R. (1997) FPC: A system for building contigs from restriction fingerprinted clones. Comp. Appl. Biosci., 13, 523–535. [DOI] [PubMed] [Google Scholar]
- 14.Stein L. and Thierry-Mieg,J. (1998) Scriptable access to the Caenorhabditis elegans genome sequence and other ACEDB databases. Genome Res., 8, 1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blake J. (2001) The gene ontology consortium creating the gene ontology resource: design and implementation. Genome Res., 11, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]