ABSTRACT
Ozanimod is an oral sphingosine-1-phosphate receptor modulator. Although it can be an effective drug for the induction and maintenance of remission in patients with moderately to severely active ulcerative colitis, there have been a few reported cases of various malignancies after exposure to this small molecule. We describe a unique case of biopsy-proven Kaposi sarcoma of the skin and colon in a patient with biologic-resistant ulcerative colitis after treatment with ozanimod for 2 months. Given the potential risk of malignancy associated with this agent, physicians should be aware of this rare adverse event.
INTRODUCTION
Kaposi sarcoma (KS) is a vascular tumor that develops after infection with the KS-associated herpesvirus, also known as human herpesvirus-8 (HHV-8).1 HHV-8 must be present for development of KS; however, the presence of HHV-8 alone is not enough to cause KS; a weakened immune system is necessary for KS development. There are 4 different subtypes: classic (sporadic), endemic (African), epidemic (acquired immunodeficiency syndrome–related), and iatrogenic. The iatrogenic form commonly occurs in organ transplant patients and patients on long-term immunosuppressive drugs.1,2 Ozanimod is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist approved for the treatment of ulcerative colitis (UC) in May 2021.3–5 We report a case of a patient with UC who was initiated on ozanimod after failing multiple other drugs to treat his symptoms and subsequently developed biopsy-proven colonic and dermatologic KS, after receiving ozanimod for 2 months.
CASE REPORT
A 64-year-old man with a medical history of human papillomavirus–induced anal canal dysplasia and UC presented to Gastroenterology Clinic in May 2022 with 3 months of bloody diarrhea (>15 bowel movements per day), urgency, and abdominal cramping. He had been previously diagnosed with UC 9 years earlier, with exposure to mesalamine, infliximab, golimumab, vedolizumab, and ustekinumab. Of note, repeated tests for human immunodeficiency virus were negative. His last flexible sigmoidoscopy had been in October 2021, which showed patchy severe inflammation characterized by ulcerations, erythema, and friability in the rectosigmoid (Mayo endoscopic subscore = 3), with biopsies showing severe chronic active colitis. He was started on ozanimod (induction dose: 0.23 mg once daily on days 1–4, 0.46 mg once daily days 5–7, and then 0.92 mg once daily on day 8 and thereafter) in March 2022 and 40 mg of prednisone. After induction, he developed a partial response, with a 50% reduction in bowel movements and resolution of all other symptoms aside from mild urgency.
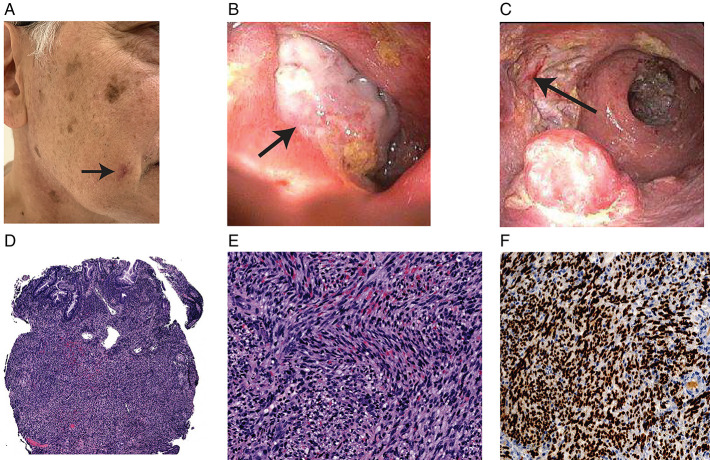
In May 2022, he presented to Dermatology Clinic for evaluation of a skin lesion on his right cheek that had developed over the past few months (Figure 1). Biopsies of the lesion revealed KS.
Figure 1.
(A) Skin lesion (arrow) on the right cheek with biopsies returning positive for Kaposi sarcoma. (B) Large ulcer (arrow) (>2-cm) deeply cratered ulcer noted in the sigmoid colon, biopsy-positive for Kaposi sarcoma. (C) Large (>2-cm) deeply cratered ulcer (arrow) noted in rectum, biopsy-positive for Kaposi sarcoma. (D) Low-power view shows an infiltrative spindle cell proliferation occupies the colonic mucosa suggestive of KS (H&E 40×, rectal ulcer biopsy). (E) Higher-power view shows the tumor cells with uniform spindled nuclei and extensive red blood cell extravasation, suggestive of KS (H&E 200×, rectal ulcer biopsy). (F) IHC stain for human herpesvirus-8 shows diffusely and strongly nuclear staining in the spindled tumor cells, suggestive of KS (IHC, 200×, rectal ulcer biopsy). H&E, hematoxylin and eosin; IHC, immunohistochemical; KS, Kaposi sarcoma.
After the diagnosis of dermatologic KS in May 2022, he was tapered off prednisone and ozanimod after having been on both drugs for 3 months. He redeveloped increased bowel frequency, urgency, tenesmus, and rectal bleeding. He was started on 9 mg of oral budesonide. He had a colonoscopy in June 2022, which demonstrated severe inflammation in the left colon with large, deeply cratered ulcers measuring >2 cm that were not previously seen on his sigmoidoscopy 6 months earlier (Figure 1). Biopsies of the colon revealed severely active chronic inflammation, with biopsies of the ulcers demonstrating KS (Figure 1).
DISCUSSION
Ozanimod was first developed to treat patients with multiple sclerosis (MS) and was approved to treat UC in May 2, 2021.6 Among MS patients, efficacy and safety trials of ozanimod reported a low incidence of herpesvirus infections ranging from 0.2% to 1.1%.7,8 Seven malignancies were reported, including breast cancer, keratoacanthoma, and basal cell carcinoma. KS was not observed in these trials. In a phase 3 clinical trial for efficacy and safety of ozanimod in treating UC, cancer was diagnosed in 8 participants, including basal cell carcinoma, colorectal adenocarcinoma, and breast cancer.3 However, there were no reports of KS.
Ozanimod, an agonist for the sphingosine-1-phosphate (S1P) receptor, may be implicated in cancer onset because of its role in cell proliferation and migration leading to increased tumor viability.9–11 The immunosuppression conferred by ozanimod may lead to a reduction in tumor surveillance.12 In addition, the increased incidence of herpesvirus infections with ozanimod may theoretically predispose to herpesvirus-related malignancies, including KS.
KS has been previously observed in patients with UC treated with tofacitinib, vedolizumab, and azathioprine.13–15 Patients treated with tofacitinib or azathioprine presented with KS tumors on the skin, whereas the patient treated with vedolizumab was reported to have colonic KS. The incidence of colonic KS in HIV-negative patients is rare, but inflammatory bowel disease patients treated with steroids or cyclosporine have been reported to develop colonic KS.16,17 KS has also been observed in patients with MS on fingolimod, a different S1p modulator.18 However, to the best of our knowledge, the present case is the first reported case of KS in a patient on ozanimod, with multiple lesions developing in the colon and on the skin. The large ulcers in the patient's colon may have driven his symptoms, including cramping, tenesmus, bloody stools, and diarrhea. The patient's history of drug exposures to multiple biologic classes also likely increased his susceptibility to KS.
Currently, the patient has discontinued all immunosuppressive therapy. He is undergoing Mohs surgery for the KS facial lesion and will be started on doxorubicin every 2 weeks for colonic KS. A colectomy may be considered if the colonic KS does not respond to chemotherapy.
As with other immunosuppressives, patients on ozanimod—or those who have been on multiple immunosuppressive medications—are at risk of the development of rare malignancies. New-onset malignancies should be considered among patients with UC who are maintained on immunosuppressive therapies, including ozanimod.
DISCLOSURES
Author contributions: All authors were involved in the collection of data and drafting of the manuscript. S. Jangi is the article guarantor.
Financial disclosure: S. Jangi is supported by an NIH/NCATS 1KL2TR002545.
Informed consent was obtained for this case report.
Contributor Information
Sarah Sandlow, Email: sarah.sandlow@tufts.edu.
Puja Rai, Email: prai@tuftsmedicalcenter.org.
Ann Shum, Email: ashum@tuftsmedicalcenter.org.
Hannah Chen, Email: hchen@tuftsmedicalcenter.org.
Jeffrey Arnold, Email: jarnold@tuftsmedicalcenter.org.
REFERENCES
- 1.Reddy NA, Mays SR, Pacha O. Kaposi's sarcoma in the immunosuppressed. J Immunother Precis Oncol. 2019;2(3):74–8. [Google Scholar]
- 2.Bishop BN, Lynch DT. Kaposi sarcoma. In: StatPearls. StatPearls Publishing: Treasure Island, FL, 2022 [PubMed] [Google Scholar]
- 3.Sandborn WJ, Feagan BG, Wolf DC, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374(18):1754–62. [DOI] [PubMed] [Google Scholar]
- 4.Sandborn WJ, Feagan BG, D'Haens G, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385(14):1280–91. [DOI] [PubMed] [Google Scholar]
- 5.Sabino J, Verstockt B, Vermeire S, Ferrante M. New biologics and small molecules in inflammatory bowel disease: An update. Ther Adv Gastroenterol. 2019;12:1756284819853208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb YN. Ozanimod: First approval. Drugs. 2020;80(8):841–8. [DOI] [PubMed] [Google Scholar]
- 7.Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): A multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18(11):1009–20. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JA, Comi G, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): A multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18(11):1021–33. [DOI] [PubMed] [Google Scholar]
- 9.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12(9):688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10(7):489–503. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Yuan Y, Lin W, Zhong H, Xu K, Qi X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019;19(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleuren GJ, Gorter A, Kuppen PJK. Immune surveillance. Encyclopedia Immunol. 1998:1243–7. [Google Scholar]
- 13.Wetwittayakhlang P, Golovics PA, Afif W, Bessissow T, Lakatos PL. Tofacitinib-associated iatrogenic Kaposi sarcoma in a patient with ulcerative colitis. ACG Case Rep J. 2021;8(11):e00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papa V, Giustiniani MC, Lopetuso LR, Papa A. Human herpesvirus 8-associated colonic Kaposi's sarcoma during vedolizumab treatment in ulcerative colitis: A case report and review of the literature. BMC Gastroenterol. 2020;20(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cetın B, Büyükberber S, Yilmaz IB, Yildiz R, Coşkun U, Beneklı M. Kaposi's sarcoma in patients with ulcerative colitis receiving immunosuppressive drugs: Report of a case. Turkish J Gastroenterol. 2011;22(6):621–5. [DOI] [PubMed] [Google Scholar]
- 16.Clarke LM, Chawla K, Tabbara N, et al. Cyclosporine-induced Kaposi sarcoma in a patient with ulcerative colitis. ACG Case Rep J. 2021;8(5):e00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Peláez M, Fernández-García MS, Gutiérrez-Corral N, et al. Kaposi's sarcoma: An opportunistic infection by human herpesvirus-8 in ulcerative colitis. J Crohns Colitis. 2010;4(5):586–90. [DOI] [PubMed] [Google Scholar]
- 18.Comi G, Hartung HP, Bakshi R, et al. Benefit-risk profile of sphingosine-1-phosphate receptor modulators in relapsing and secondary progressive multiple sclerosis. Drugs. 2017;77:1755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]