Abstract
Background: Patients with adolescent idiopathic scoliosis (AIS) are found to have a lower level of physical activity, and may have reduced exercise capacity due to spinal deformity. Previous study showed the benefits of high-intensity interval training (HIIT), named E-Fit, which is specifically designed for patients with AIS to improve musculoskeletal health and psychological well-being. To optimize the beneficial effects of training, the current study aimed to investigate the appropriate exercise intensity and metabolic demand in patients with AIS when performing E-Fit. Methods: In all, 22 female subjects, 10 diagnosed with AIS and 12 gender-matched healthy controls, aged between 10 and 16 years, were recruited. Subjects were instructed to perform two trials of a seven min E-Fit. Breath-by-breath gas exchange parameters including oxygen consumption (VO2), heart rate (HR) and the rate of perceived exertion (PRE) were measured during exercise. Demographic data and clinical features of AIS and body composition were obtained. Metabolic demand between AIS and control groups was compared using MANOVA with covariates adjustment. Results: Patients with AIS had an earlier onset of menarche (p = 0.01), higher visceral adipose tissue (p = 0.04) and percentage body fat (p = 0.03) as compared to controls. Patients with AIS showed a significantly higher adjusted means of VO2 average in both the first (p = 0.014) and second trials (p = 0.011) of E-Fit. The adjusted mean of the highest measured VO2 was higher than healthy controls and reached statistical significance in the second trial (p = 0.004). Both the AIS and control group exercised at a similar percentage of VO2 peak (64.26% vs. 64.60%). Conclusion: Patients with AIS showed higher oxygen consumption during E-Fit than heathy controls, which might indicate a higher metabolic cost. Patients with AIS could carry out exercise at a moderate exercise intensity similar to that of healthy controls, but special considerations in designing an exercise program, such as frequent rest intervals, would be useful to avoid fatigue among patients with AIS.
Keywords: adolescent idiopathic scoliosis, exercise, high-intensity interval training, metabolic cost, oxygen consumption
1. Introduction
Adolescent idiopathic scoliosis (AIS) is a three-dimensional deformity of the spine, mostly occurring in females between the age of 10 and 16 years, with a prevalence of 1–4% worldwide [1,2]. Complex spinal deformity in AIS is thought to be associated with the differential development of the musculoskeletal system [3], which could influence the cardiorespiratory system, leading to a lower physical capacity even in patients with mild curve [4,5,6]. Reduced physical capacity in patients with mild-to-moderate AIS could result in an increase in energy expenditure for performing physical activities, and might have a detrimental effect on pubertal development carrying over into adulthood [7,8].
The developmental deformation of the spine and ribcage in AIS could influence cardiorespiratory functions. Recent study revealed that patients with moderate AIS had an imbalance growth between the sternum and thoracic spinal column [9], with shallow and flat chest ribcage rotation [10,11]. The decreased mobility in the spine and ribcage of AIS affects the ability of the chest wall, abdomen, and diaphragm, to expand when breathing [12]. These restrictions could cause an increase in breathing frequency and a decrease in oxygen consumption [13]. Apart from structural abnormalities, chronic physical deconditioning from the lack of regular exercise, reduced ventilatory capacity, and generalized muscle dysfunction, could lead to a loss of exercise capacity [4,14,15,16,17]. Patients with moderate AIS exhibited respiratory muscle weakness and impaired breathing pattern, even during walking [17]. Nutritional status, physical activity level, and systemic inflammation were thought to attribute to generalized deranged muscle functions in patients with AIS [16]. Patients with AIS who engaged in regular aerobic exercises were shown to have significant improvement in pulmonary functions [6] and aerobic capacity [18]. In patients with severe AIS, who had curves that progressed up to surgical thresholds [19], recent study showed that combining resistance and aerobic training improved functional exercise capacity and pulmonary functions, more than a training regimen with aerobic training alone.
An E-Fit exercise intervention (E-Fit), which is a high-intensity interval training (HIIT) specifically designed for patients with AIS, was developed in order to promote habitual exercise. E-Fit comprises of whole-body aerobic and resistance exercises, with frequent rest intervals. Previous study showed that E-Fit could provide physical benefits on bone mineral density and muscle endurance, as well as the psychosocial benefits of improving sport participation, and self-image in patients with AIS [20]. Metabolic stress induced by exercise intervention can vary between individuals and therefore it is important to determine the appropriate exercise intensity, which could result in better adherence and effectiveness [21]. This prospective case-control study aimed to determine the metabolic demand of E-Fit by evaluating the cardiovascular responses in patients with AIS, compared with healthy controls.
2. Materials and Methods
2.1. Subjects
Twenty-two female subjects between the ages of 10 and 16 were recruited in this study. 10 subjects who had a diagnosis of AIS confirmed by clinical examination and standard standing posteroanterior radiograph of the whole spine, Cobb angle of ≥15°, without prior treatment were recruited from a local hospital. Exclusion criteria included: Cobb angle of ≥40°; scoliosis with any known aetiology, endocrine and connective tissue abnormalities; heart condition or other diseases that could affect the safety of exercise; eating disorders or gastrointestinal malabsorption disorders; and currently taking medication that affects bone or muscle metabolism. Twelve healthy female adolescent subjects matched in age without spinal deformity were recruited as controls. Written informed consent was obtained from all subjects and their guardians before enrolment. The study was approved by the local ethics committees in compliance with the Helsinki Declaration.
2.2. Baseline Measurements
Anthropometric measurements were measured with standard stadiometry techniques. Sexual maturity was assessed by self-reported onset of menarche and standard Tanner Scale using the validated protocols [22]. For AIS group, skeletal age was measured with Thumb Ossification Composite Index (TOCI) (stage 1–8 with higher values representing more mature bone) using hand X-ray [23]. Body composition parameters, including fat-free mass (FFM), body fat percentage (BF%) and visceral adipose tissue (VAT), were examined using the bioelectrical impedance analysis (BIA) (InBody 720; Biospace Co., Ltd.; Seoul, Republic of Korea). The average physical activity level in the past year was assessed using a self-administered and validated 1-item questionnaire, The Chinese University of Hong Kong: Physical Activity Rating for Children and Youth (CUHK-PARCY) [24].
2.3. Testing Procedures
Subjects in both groups were instructed about the E-Fit exercises. The room for obtaining outcome measures had an ambient temperature between 22–24 °C with approximately 50% humidity. Each subject was required to undergo a submaximal treadmill exercise testing and performed two trials of E-Fit. Each trial of E-Fit lasted for around 7 min in total and included 12 short and intense exercises. Selected E-Fit exercises in each trial had included a mix of aerobic and resistance exercises involving large muscle groups of the whole body, such as elbow-to-knee, arm walk, triceps dips, bridging, squat with weight and squat thrust. Subjects were given 10 min rest period between each testing. A 50% of heart rate reserve (HRR) was first determined by maximal heart rate (HRmax) defined as 200 beats per minute (bpm), and resting heart rate (HRrest) measured after a rest period of 10 min.
Prior to the submaximal treadmill exercise testing, subjects were first instructed to have a warm-up and then fast walk at a self-selected pace to obtain a heart rate (HR) of 50% HRR within a 3 min time. The main part of the exercise testing involved normal walking at comfortable pace for 3 min, fast walking for another 3 min, then fast walking at the self-selected pace for 6 min until a steady HR was reached, with the HR of 5th and 6th min not exceeding 6 bpm. Subjects proceeded to cool down and walk at a comfortable pace for 5 min until HR returning below 90 bpm. Subjects were seated immediately after completing the exercises and measurements were continued for a further 2 min.
2.4. Outcome Measures of Oxygen Consumption (VO2), Heart Rate (HR) and Rate of Perceived Exertion (RPE)
A telemetric portable gas analyzer system (Cosmed K5; Cosmed Srl, Rome, Italy) was utilized to obtain breath-by-breath measurement of cardiorespiratory and gas exchange parameters to analyze oxygen consumption (VO2) expressed as mL/kg/min during the E-Fit [25]. Heart rate (HR) was measured continuously in conjunction with the gas analyzer system using a chest strap (Garmin HRM3-SS; Garmin, Olathe, KS, USA). Breath-by-breath values were averaged over 30 s interval of each movement element. The system was calibrated for volumes and gas exchange composition using reference gases of known concentrations. Respiratory gas measurements were taken during the submaximal exercise testing using a motorised treadmill (GK2200; Mobility Research, Tempe, AZ, USA) and two trials of E-Fit. Modified Borg Scale was used to assess the subjective rating of perceived exertion at the beginning and immediately after the end of each E-Fit trial. It has shown to be a reliable indicator for exercise exertion with a good accuracy in children older than 9 years of age [26,27].
2.5. Statistical Analysis
Independent t-test and chi-square test were used to compare the demographic data and baseline outcome variables between AIS and healthy control groups. Multivariate analysis of variance (MANOVA) was performed to compare the metabolic demand of E-Fit between groups. Potential confounders such as onset of menarche, VAT, BF% and resting VO2 were included in the model where appropriate. SPSS statistic software (version 26; SPSS Inc., Chicago, IL, USA) was used for statistical analyses. p-value of < 0.05 was adopted as the general level of significance. Forty female adolescents aged between 11 and 14 years with AIS were targeted and recruited from an out-patient scoliosis clinic in a local hospital. All subjects would be included if they were female AIS patients who were newly diagnosed with AIS by standard standing long X-ray examinations, with Cobb angle larger or equal to 15°, without prior treatment for their AIS and have been cleared for physical activity by their doctors. Subjects were excluded if they had: (i) Cobb angle larger or equal to 40°; (ii) scoliosis with any known aetiologies, such as congenital, neuromuscular, metabolic and skeletal dysplasia; (iii) known endocrine and connective tissue abnormalities; (iv) known heart condition or other diseases that could affect the safety of exercise; (v) eating disorders or gastrointestinal malabsorption disorders; and (vi) currently taking medications that affecting their bone or muscle metabolism. All subjects signed a consent form in the presence of their parents after thorough explanation by the attending doctor.
3. Results
3.1. Group Comparison
Ten patients with AIS and 12 age-matched healthy controls with a mean age of 12.83 ± 2.09 years old completed the study. Both groups had similar anthropometric measurements, but the AIS group had an earlier onset of menarche (p = 0.014), higher visceral adipose tissue (p = 0.037) and BF% (p = 0.027). Both groups did not show any statistical significance in physical activity level (Table 1).
Table 1.
Basic characteristics between AIS and control groups.
| AIS (n = 10) | Control (n = 12) | p-Value | |
|---|---|---|---|
| Age (yr) a | 13.71 ± 2.04 | 12.11 ± 1.92 | 0.080 |
| Anthropometric measurements | |||
| Body weight (kg) b | 44.38 ± 6.67 | 42.00 ± 8.39 | 0.477 |
| Body height (cm) b | 154.57 ± 7.14 | 152.67 ± 7.96 | 0.565 |
| Sitting height (cm) a | 78.50 ± 16.07 | 86.33 ± 14.63 | 0.668 |
| Arm span (cm) b | 153.90 ± 9.40 | 153.98 ± 7.33 | 0.446 |
| BMI (kg/m2) a | 18.61 ± 1.27 | 17.86 ± 2.12 | 0.467 |
| BMI by arm span a | 18.63 ± 1.40 | 18.31 ± 2.15 | 0.684 |
| Maturity | |||
| Tanner scale—breast development (1–5) c | 3.20 (3, 4) | 2.25 (2, 3) | 0.312 |
| Tanner scale—pubic hair development (1–5) c | 3.10 (3, 4) | 1.92 (1, 3) | 0.155 |
| Menarche onset (months) a | 22.90 ± 15.28 | 7.83 ± 16.28 | 0.014 * |
| Curve Features | |||
| Cobb angle (˚) | 24.50 ± 6.38 | N/A | N/A |
| TOCI (stage 1–8) | 5.60 ± 1.71 | N/A | N/A |
| Body Composition b | |||
| Fat free mass (kg) | 32.69 ± 3.97 | 33.32 ± 5.53 | 0.768 |
| Skeletal muscle mass (kg) | 17.18 ± 2.25 | 17.49 ± 3.39 | 0.806 |
| Visceral adipose tissue (cm2) | 52.67 ± 18.28 | 38.19 ± 11.89 | 0.037 * |
| BF% | 24.78 ± 4.16 | 19.99 ± 5.10 | 0.027 * |
| Physical Activity Level | |||
| CUHK-PARCY c | 4.00 (3, 6) | 5.33 (6, 8) | 0.698 |
The values are presented as mean ± standard deviation or median (range) as appropriate. a: Student’s t-test was used on data with normal distribution. b: Mann-Whitney U test was used on data which is not normally distributed. c: Chi-square test was used for the dichotomous data. Abbreviations: BMI = body mass index; TOCI = thumb ossification composite index; BF% = body fat percentage; CUHK-PARCY = The Chinese University of Hong Kong: Physical Activity Rating for Children and Youth. * p ≤ 0.05.
3.2. Metabolic Demand between AIS and Healthy Controls
The resting HR, 50% HRR and predicted VO2 peak in both groups did not show significant difference, however, the AIS group had a statistically significant lower resting VO2 at 3.19 mL/kg/min (p = 0.045) than the control group at 4.16 mL/kg/min (Table 2).
Table 2.
Baseline metabolic demand parameters between AIS and control groups.
| AIS (n = 10) | Control (n = 12) | p-Value | |
|---|---|---|---|
| Resting HR (bpm) | 89.10 ± 11.41 | 89.75 ± 9.54 | 0.190 |
| 50% HRR (bpm) | 144.55 ± 5.70 | 143.67 ± 3.87 | 0.367 |
| Resting VO2 (mL/kg/min) | 3.19 ± 0.54 | 4.16 ± 1.03 | 0.045 * |
| Predicted VO2 peak (mL/kg/min) | 57.43 ± 14.14 | 56.65 ± 11.57 | 0.353 |
The values are presented as mean ± standard deviation. Covariates: onset of menarche, body fat percentage and visceral adipose tissue. Abbreviations: HR = heart rate; 50% HRR = 50% heart rate reserve; VO2 = oxygen consumption. * p ≤ 0.05.
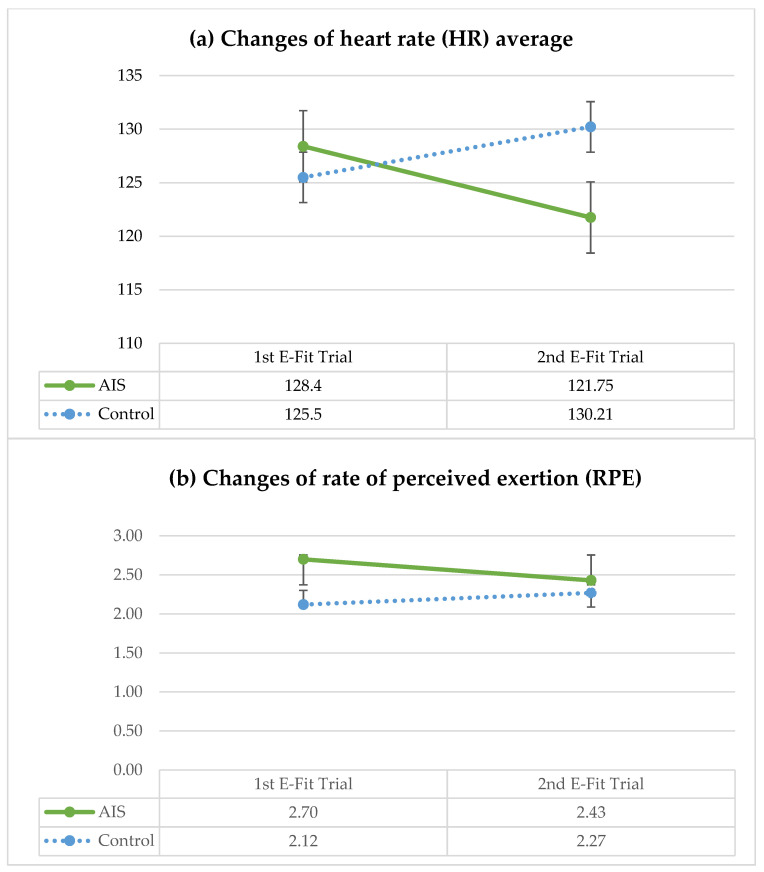
During E-Fit, the AIS group also showed significant higher adjusted means of VO2 average in the first trial (20.27 mL/kg/min, p = 0.033). The adjusted mean of the highest measured VO2 was numerically higher than healthy controls, reaching statistical significance in the second trial (37.46 mL/kg/min vs. 33.87 mL/kg/min, p = 0.010) with a marginally significant overall-between effect (p = 0.055). However, the AIS group was observed to have a significantly lower VO2 average than the healthy controls in the second trial (19.45 mL/kg/min vs. 19.48 mL/kg/min, p = 0.022) with a reducing trend in HR peak, HR average and RPE. The overall within-group effect of RPE was statistically different (p = 0.016) with the AIS group showing a significant decline in RPE score from 2.85 to 2.55 (adjusted mean of 2.70 to 2.43) towards the second trial of E-Fit. Both the AIS and control groups exercised at a similar percentage of VO2 peak (62.85% to 64.23% vs. 62.44% to 65.94%) in both trials. The BP and SpO2 at pre-test (measured after a 10 min rest period before each trial) and post-test also showed no significant difference between the groups. The metabolic demand parameters between the AIS and control group when performing E-Fit are presented in Table 3.
Table 3.
Mean, adjusted mean, standard deviation and standard errors for metabolic demand between AIS and control groups when performing E-Fit trials.
| AIS (n = 10) | Control (n = 12) | p-Value a | ||||
|---|---|---|---|---|---|---|
| M ± SD | Madj ± SE | M ± SD | Madj ± SE | |||
| HR peak | 1st E-Fit Trial | 152.30 ± 10.64 | 149.85 ± 3.96 | 145.58 ± 12.44 | 147.63 ± 3.55 | 0.204 |
| 2nd E-Fit Trial | 148.90 ± 17.01 | 143.78 ± 5.25 | 146.17 ± 14.01 | 150.44 ± 4.71 | 0.324 | |
| HR average | 1st E-Fit Trial | 131.20 ± 9.31 | 128.4 ± 3.13 | 125.50 ± 10.21 | 127.83 ± 2.81 | 0.093 |
| 2nd E-Fit Trial | 126.60 ± 11.65 | 121.75 ± 3.66 | 126.17 ± 11.67 | 130.21 ± 3.27 | 0.129 | |
| Highest measured VO2 | 1st E-Fit Trial | 34.65 ± 7.18 | 36.85 ± 2.14 | 34.38 ± 6.12 | 32.54 ± 1.92 | 0.186 |
| 2nd E-Fit Trial | 35.24 ± 5.35 | 37.46 ± 1.20 | 35.72 ± 3.94 | 33.87 ± 1.08 | 0.010 * | |
| VO2 average | 1st E-Fit Trial | 19.03 ± 3.14 | 20.27 ± 0.79 | 19.66 ± 2.46 | 18.63 ± 0.71 | 0.033 * |
| 2nd E-Fit Trial | 18.73 ± 2.57 | 19.45 ± 0.56 | 20.08 ± 1.17 | 19.48 ± 0.51 | 0.022 * | |
| %VO2 peak | 1st E-Fit Trial | 62.89 ± 16.97 | 62.85 ± 5.50 | 62.41 ± 13.98 | 62.44 ± 4.93 | 0.548 |
| 2nd E-Fit Trial | 64.98 ± 19.05 | 64.23 ± 6.06 | 65.32 ± 14.08 | 65.94 ± 5.43 | 0.692 | |
| RPE | 1st E-Fit Trial | 2.85 ± 0.35 | 2.70 ± 0.42 | 2.00 ± 0.36 | 2.12 ± 0.38 | 0.236 |
| 2nd E-Fit Trial | 2.55 ± 0.40 | 2.43 ± 0.36 | 2.17 ± 0.29 | 2.27 ± 0.32 | 0.143 | |
| Overall within-group effect of RPE: p = 0.016 * | ||||||
The values are presented as mean ± standard deviation or adjusted mean (standard error) as appropriate. a: p-value of adjusted mean. Covariates: onset of menarche, body fat percentage, visceral adipose tissue, and resting oxygen consumption. Abbreviations: HR = heart rate; VO2 = oxygen consumption; RPE = rate of perceived exertion. * p ≤ 0.05.
4. Discussion
This was the first study to investigate the metabolic demand of patients with AIS performing E-Fit, which is an exercise intervention utilizing HIIT. HIIT has been shown to be a feasible, time efficient and efficacious strategy for improving physical and psychological well-being in healthy adolescents [28] and patients with AIS [20]. The significantly lower resting VO2 in the AIS group found in this study indicates that patients had a lower resting metabolic demand in comparison to healthy controls. Multiple factors were shown to be related to a lower resting metabolic demand, including higher body fat, lower bone density, reduced skeletal muscle, nutritional status and the quality of sleep [29,30,31]; these factors were also associated with AIS [32]. The cause of lower resting VO2 found in the AIS group of this study is unclear, but they were found to have a higher VAT and BF%.
Both the AIS and control groups showed no difference in the predicted VO2 peak, which could imply both groups showed similar peak aerobic capacity. This finding was in accordance with those reported by Czaprowski et al., that the maximum oxygen consumption calculated based on a submaximal physical test did not differ between patients with mild AIS with a Cobb angle of less than 25°, and controls, during a cycle ergometer test [8]. Bas et al. [33] and Leech et al. [34] also found similar maximum oxygen consumption between patients with mild AIS and healthy controls. In addition, both the AIS and control groups exercised at a moderate-intensity level, with a similar percentage of VO2 peak (62.85% to 64.23% vs. 62.44% to 65.94%) in both trials. The comparable predicted VO2 peak in both groups indicated that there was no exercise limitation in patients with AIS, in comparison to healthy controls. E-Fit also seemed to have an appropriate and adequate training intensity for both groups, at a moderate level.
The AIS group showed a numerically higher adjusted mean of the highest measured VO2 and reached statistically between-group significance in the second trial, indicating that they demonstrated higher oxygen consumption and increased metabolic cost when performing E-Fit. Mahaudens et al. also concluded that patients with mild AIS had an increased metabolic cost by around 30%, and a decrease in muscle efficiency even in a common activity such as walking [35]. They suggested that the increased physical effort exerted in patients with AIS was not only due to a mechanical disorder but also inefficient muscle or muscular dysfunction. Kearon et al. reported that patients with AIS showed reduced strength in both respiratory and limb muscles, which was associated with a decrease in calculated lean mass [15]. Martínez-Llorens et al. also found patients with AIS had generalized muscle dysfunction including respiratory muscles, upper and lower limb muscles on both sides, which contributed to reduce exercise capacity [16]; however, the causes of muscle dysfunction were not fully understood. Several factors, such as nutritional status, physical activity level, reduced bone density, melatonin-level related to sleep quality or systemic inflammation, could influence the systemic mechanisms of muscle function [3,16,36].
It is worth noting that the AIS group showed a drop in HR peak, HR average and RPE in the second E-Fit trial (Figure 1). They had a significantly higher adjusted mean of the highest measured VO2, but a significantly lower adjusted mean of VO2 compared with the healthy controls. This finding and observation suggested that patients with AIS might be showing signs of fatigue during the second E-Fit trial. Some patients with AIS were observed to have slowed down and it became more difficult for them to follow the exercise rhythm, despite similar verbal cueing given to both groups. Previous studies also reported high scores of perceived exertions in patients with AIS during exercise [6,37]. Patients with AIS were observed to have shallow breathing, increased breathing frequency and an altered breathing pattern of disproportion between inspiration and expiration [8,13]. Shneerson et al. found patients with AIS, on average, hyperventilated by 20% more than healthy controls [38]. Decreased cardiovascular fitness in patients with AIS could affect tidal volume, leading to an increase in the respiratory rate, compensating for a larger oxygen demand [39]. Apart from an observed reduction in spinal and chest wall mobility, this could be due to the weakness of respiratory muscles and chronic deconditioning [8,17]. We postulated that the increased physical effort and reduced exercise capacity observed in the current study were likely due to muscle dysfunction, such as reduced overall muscle strength, rather than cardiorespiratory restriction.
Figure 1.
Changes of: (a) heart rate (HR) average; and (b) rate of perceived exertion (RPE), between AIS and control groups in both E-Fit trials.
Systematic training, such as E-Fit, could induce metabolic and structural adaptations to improve the function and endurance of respiratory muscles [40,41]. Although exercise training may have a limited effect on the maximum pulmonary function, it can reduce the feeling of breathlessness and improve the ability to sustain high levels of submaximal ventilation during exercise [18]. Special considerations should be made when designing an exercise program for patients with AIS. For example, slower exercise rhythm with larger movements, more frequent rest intervals and adding more cueing could help prevent early fatigability, and sustain exercises at an adequate intensity and duration.
A limitation of this current study was that we only recruited a small sample of female subjects, of single ethnicity, with mild-to-moderate curve severity. Future studies could recruit more subjects with different ethnicity, a wider age range, various curve types and severity, for stratification analysis. The physical activity level between the AIS and control groups was assessed using a self-reported questionnaire, which is useful to differentiate the different levels of physical activity in our subjects, and to identify subjects with a high level of physical activity at baseline. However, self-reported questionnaires rely on subjects’ recall ability and can be less robust in assessing in detail the subject’s physical effort and energy expenditure. Further, the VO2 max was not measured directly in the current study; however, a submaximal treadmill exercise test was utilized to predict the peak of oxygen consumption. Submaximal tests, including the measurement of respiratory gas exchange, are widely accepted in the evaluation of physical capacity in children and adolescents, with or without AIS [7,15,42]. Since this study focused on the metabolic demand of patients with AIS performing E-Fit, assessments on respiratory functions and muscle strength were not included. The relationships between other possible underlying causes, such as respiratory functions or muscle functions, that might influence the reduced exercise capacity as observed in the current study, were beyond the scope of this study but are worthy of consideration when further studies are planned in this regard.
5. Conclusions
The current study indicated that patients with mild-to-moderate AIS had higher oxygen consumption and metabolic cost during E-Fit, as compared with healthy controls. Patients with AIS could carry out exercise at a moderate exercise intensity level, similar to that of healthy controls, but special considerations in designing the exercise program would be useful to avoid early fatigability. Further studies are warranted to investigate idiopathic scoliosis at various stages of the disease for defining the applicability of E-Fit for those with early onset, or those with curves reaching surgical threshold, and for evaluating the relationship between cardiorespiratory and muscle functions, to unravel the underlying causes of limited exercise capacity in patients with AIS.
Acknowledgments
The authors would like to acknowledge Tung Wah College, Hong Kong SAR, China, for the financial support to this project. We would like to express our appreciation to all subjects who participated in this study, and their parents for the authorization.
Author Contributions
Conceptualization, R.W.-L.L. and S.S.-C.H.; data curation, R.W.-L.L.; formal analysis, R.W.-L.L. and R.L.-C.K.; funding acquisition, R.W.-L.L.; investigation, R.W.-L.L.; methodology, R.W.-L.L., S.S.-C.H. and T.-P.L.; project administration, R.W.-L.L.; resources, J.C.-Y.C., S.S.-C.H. and T.-P.L.; supervision, J.C.-Y.C., S.S.-C.H. and T.-P.L.; writing—original draft, R.W.-L.L.; writing—review and editing, R.W.-L.L., R.L.-C.K., J.C.-Y.C., S.S.-C.H. and T.-P.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and ICH-GCP with ethical approval obtained from Clinical Research Ethics Committee of the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Committee (Ref. 2020.498) and Research Ethics Committee of the Tung Wah College (REC2021083).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues of the subjects.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by School Research Grant, Tung Wah College, Hong Kong SAR, China (Project number: 2019-02-52-SRG190201).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cheng J.C., Castelein R.M., Chu W.C., Danielsson A.J., Dobbs M.B., Grivas T.B., Gurnett C.A., Luk K.D., Moreau A., Newton P.O., et al. Adolescent idiopathic scoliosis. Nat. Rev. Dis. Prim. 2015;1:15030. doi: 10.1038/nrdp.2015.30. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein S.L., Dolan L.A., Cheng J.C.Y., Danielsson A., Morcuende J.A. Adolescent idiopathic scoliosis. Lancet. 2008;371:1527–1537. doi: 10.1016/S0140-6736(08)60658-3. [DOI] [PubMed] [Google Scholar]
- 3.Smit T.H. Adolescent idiopathic scoliosis: The mechanobiology of differential growth. JOR Spine. 2020;3:e1115. doi: 10.1002/jsp2.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrios C., Pérez-Encinas C., Maruenda J.I., Laguía M. Significant Ventilatory Functional Restriction in Adolescents With Mild or Moderate Scoliosis During Maximal Exercise Tolerance Test. Spine. 2005;30:1610–1615. doi: 10.1097/01.brs.0000169447.55556.01. [DOI] [PubMed] [Google Scholar]
- 5.Koumbourlis A.C. Scoliosis and the respiratory system. Paediatr. Respir. Rev. 2006;7:152–160. doi: 10.1016/j.prrv.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Dos Santos Alves V.L., Stirbulov R., Avanzi O. Impact of a physical rehabilitation program on the respiratory function of adolescents with idiopathic scoliosis. Chest. 2006;130:500–505. doi: 10.1016/S0012-3692(15)51867-9. [DOI] [PubMed] [Google Scholar]
- 7.Teoh O.H., Trachsel D., Mei-Zahav M., Selvadurai H. Exercise testing in children with lung diseases. Paediatr. Respir. Rev. 2009;10:99–104. doi: 10.1016/j.prrv.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Czaprowski D., Kotwicki T., Biernat R., Urniaż J., Ronikier A. Physical capacity of girls with mild and moderate idiopathic scoliosis: Influence of the size, length and number of curvatures. Eur. Spine J. 2012;21:1099–1105. doi: 10.1007/s00586-011-2068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B., Tan Q., Chen H., Luo F., Xu M., Zhao J., Liu P., Sun X., Su N., Zhang D., et al. Imbalanced development of anterior and posterior thorax is a causative factor triggering scoliosis. J. Orthop. Transl. 2019;17:103–111. doi: 10.1016/j.jot.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi T., Harimaya K., Matsumoto Y., Iwamoto Y. Aortic location and flat chest in scoliosis: A prospective study. Fukuoka Igaku Zasshi. 2011;102:14–19. [PubMed] [Google Scholar]
- 11.Doi T., Matsumoto Y., Tono O., Tarukado K., Harimaya K., Okada S., Kubota K., Hayashida M., Iwamoto Y. A shallow chest correlates with the aortic position in the normal spine: Features resembling those observed in structural scoliosis. Scoliosis. 2014;9:14. doi: 10.1186/1748-7161-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leong J.C., Lu W.W., Luk K.D., Karlberg E.M. Kinematics of the chest cage and spine during breathing in healthy individuals and in patients with adolescent idiopathic scoliosis. Spine. 1999;24:1310–1315. doi: 10.1097/00007632-199907010-00007. [DOI] [PubMed] [Google Scholar]
- 13.Coast J., Cline C. The effect of chest wall restriction on exercise capacity. Respirology. 2004;9:197–203. doi: 10.1111/j.1440-1843.2004.00559.x. [DOI] [PubMed] [Google Scholar]
- 14.Kesten S., Garfinkel S.K., Wright T., Rebuck A.S. Impaired exercise capacity in adults with moderate scoliosis. Chest. 1991;99:663–666. doi: 10.1378/chest.99.3.663. [DOI] [PubMed] [Google Scholar]
- 15.Kearon C., Viviani G.R., Killian K.J. Factors influencing work capacity in adolescent idiopathic thoracic scoliosis. Am. Rev. Respir. Dis. 1993;148:295–303. doi: 10.1164/ajrccm/148.2.295. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Llorens J., Ramírez M., Colomina M.J., Bagó J., Molina A., Cáceres E., Gea J. Muscle dysfunction and exercise limitation in adolescent idiopathic scoliosis. Eur. Respir. J. 2010;36:393–400. doi: 10.1183/09031936.00025509. [DOI] [PubMed] [Google Scholar]
- 17.Sperandio E.F., Alexandre A.S., Yi L.C., Poletto P.R., Gotfryd A.O., Vidotto M.C., Dourado V.Z. Functional aerobic exercise capacity limitation in adolescent idiopathic scoliosis. Spine J. 2014;14:2366–2372. doi: 10.1016/j.spinee.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Athanasopoulos S., Paxinos T., Tsafantakis E., Zachariou K., Chatziconstantinou S. The effect of aerobic training in girls with idiopathic scoliosis. Scand. J. Med. Sci. Sport. 1999;9:36–40. doi: 10.1111/j.1600-0838.1999.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 19.Xavier V.B., Avanzi O., de Carvalho B.D.M.C., Alves V.L.d.S. Combined aerobic and resistance training improves respiratory and exercise outcomes more than aerobic training in adolescents with idiopathic scoliosis: A randomised trial. J. Physiother. 2020;66:33–38. doi: 10.1016/j.jphys.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Lau R.W.-L., Cheuk K.-Y., Ng B.K.-W., Tam E.M.-S., Hung A.L.-H., Cheng J.C.-Y., Hui S.S.-C., Lam T.-P. Effects of a Home-Based Exercise Intervention (E-Fit) on Bone Density, Muscle Function, and Quality of Life in Girls with Adolescent Idiopathic Scoliosis (AIS): A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health. 2021;18:10899. doi: 10.3390/ijerph182010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biddle S.J., Batterham A.M. High-intensity interval exercise training for public health: A big HIT or shall we HIT it on the head? Int. J. Behav. Nutr. Phys. Act. 2015;12:95. doi: 10.1186/s12966-015-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng J.C.Y., Leung S.S.F., Lau J. Anthropometric measurements and body proportions among Chinese children. Clin. Orthop. Relat. Res. 1996;323:22–30. doi: 10.1097/00003086-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hung A.L.H., Chau W.W., Shi B., Chow S.K., Yu F.Y.P., Lam T.P., Ng B.K.W., Qiu Y., Cheng J.C.Y. Thumb Ossification Composite Index (TOCI) for Predicting Peripubertal Skeletal Maturity and Peak Height Velocity in Idiopathic Scoliosis: A Validation Study of Premenarchal Girls with Adolescent Idiopathic Scoliosis Followed Longitudinally Until Skeletal Maturity. J. Bone Jt. Surg. Am. 2017;99:1438–1446. doi: 10.2106/JBJS.16.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong A.P., Choi K.C., Li A.M., Hui S.S., Chan M.H., Wing Y.K., Ma R.C., Lam C.W., Lau J.T., So W.Y., et al. Association between physical activity and cardiovascular risk in Chinese youth independent of age and pubertal stage. BMC Public Health. 2010;10:303. doi: 10.1186/1471-2458-10-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinnington H.C., Wong P., Tay J., Green D., Dawson B. The level of accuracy and agreement in measures of FEO2, FECO2 and VE between the cosmed K4b2 portable, respiratory gas analysis system and a metabolic cart. J. Sci. Med. Sport. 2001;4:324–335. doi: 10.1016/S1440-2440(01)80041-4. [DOI] [PubMed] [Google Scholar]
- 26.Borg G.A. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982;14:377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Hommerding P.X., Donadio M.V., Paim T.F., Marostica P.J. The Borg scale is accurate in children and adolescents older than 9 years with cystic fibrosis. Respir. Care. 2010;55:729–733. [PubMed] [Google Scholar]
- 28.Costigan S.A., Eather N., Plotnikoff R.C., Hillman C.H., Lubans D.R. High-Intensity Interval Training for Cognitive and Mental Health in Adolescents. Med. Sci. Sport. Exerc. 2016;48:1985–1993. doi: 10.1249/MSS.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman B.A., Warren M.P., Dominguez J.E., Wang J., Heymsfield S.B., Pierson R.N. Bone Density and Amenorrhea in Ballet Dancers Are Related to a Decreased Resting Metabolic Rate and Lower Leptin Levels. J. Clin. Endocrinol. Metab. 2002;87:2777–2783. doi: 10.1210/jcem.87.6.8565. [DOI] [PubMed] [Google Scholar]
- 30.Hohenadel M.G., Hollstein T., Thearle M., Reinhardt M., Piaggi P., Salbe A.D., Krakoff J. A low resting metabolic rate in late childhood is associated with weight gain in adolescence. Metabolism. 2019;93:68–74. doi: 10.1016/j.metabol.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurado-Fasoli L., Mochon-Benguigui S., Castillo M.J., Amaro-Gahete F.J. Association between sleep quality and time with energy metabolism in sedentary adults. Sci. Rep. 2020;10:4598. doi: 10.1038/s41598-020-61493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe T.G., Edgar M., Margulies J.Y., Miller N.H., Raso V.J., Reinker K.A., Rivard C.H. Etiology of idiopathic scoliosis: Current trends in research. J. Bone Jt. Surg. Am. 2000;82:1157–1168. doi: 10.2106/00004623-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Bas P., Romagnoli M., Gomez-Cabrera M.C., Bas J.L., Aura J.V., Franco N., Bas T. Beneficial effects of aerobic training in adolescent patients with moderate idiopathic scoliosis. Eur. Spine J. 2011;20:415–419. doi: 10.1007/s00586-011-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leech J.A., Ernst P., Rogala E.J., Gurr J., Gordon I., Becklake M.R. Cardiorespiratory status in relation to mild deformity in adolescent idiopathic scoliosis. J. Pediatr. 1985;106:143–149. doi: 10.1016/S0022-3476(85)80487-X. [DOI] [PubMed] [Google Scholar]
- 35.Mahaudens P., Detrembleur C., Mousny M., Banse X. Gait in adolescent idiopathic scoliosis: Energy cost analysis. Eur. Spine J. 2009;18:1160–1168. doi: 10.1007/s00586-009-1002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machida M., Dubousset J., Imamura Y., Miyashita Y., Yamada T., Kimura J. Melatonin. A possible role in pathogenesis of adolescent idiopathic scoliosis. Spine. 1996;21:1147–1152. doi: 10.1097/00007632-199605150-00005. [DOI] [PubMed] [Google Scholar]
- 37.Alves V.L., Avanzi O. Objective assessment of the cardiorespiratory function of adolescents with idiopathic scoliosis through the six-minute walk test. Spine. 2009;34:E926–E929. doi: 10.1097/BRS.0b013e3181afd1b2. [DOI] [PubMed] [Google Scholar]
- 38.Shneerson J.M. Pulmonary artery pressure in thoracic scoliosis during and after exercise while breathing air and pure oxygen. Thorax. 1978;33:747–754. doi: 10.1136/thx.33.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiRocco P.J., Vaccaro P. Cardiopulmonary functioning in adolescent patients with mild idiopathic scoliosis. Arch. Phys. Med. Rehabil. 1988;69:198–201. [PubMed] [Google Scholar]
- 40.Low W.D., Chew E.C., Kung L.S., Hsu L.C., Leong J.C. Ultrastructures of nerve fibers and muscle spindles in adolescent idiopathic scoliosis. Clin. Orthop. Relat. Res. 1983;174:217–221. doi: 10.1097/00003086-198304000-00032. [DOI] [PubMed] [Google Scholar]
- 41.Grinton S., Powers S.K., Lawler J., Criswell D., Dodd S., Edwards W. Endurance training-induced increases in expiratory muscle oxidative capacity. Med. Sci. Sport. Exerc. 1992;24:551–555. doi: 10.1249/00005768-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Takken T., Bongers B.C., van Brussel M., Haapala E.A., Hulzebos E.H.J. Cardiopulmonary Exercise Testing in Pediatrics. Ann. Am. Thorac. Soc. 2017;14:S123–S128. doi: 10.1513/AnnalsATS.201611-912FR. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues of the subjects.