Abstract
Autism spectrum disorder (ASD) has become a critical public health issue that affects more than 78 million people. In many recent studies, the authors have demonstrated that equine-assisted activities and therapies (EAATs) can substantially improve the social and behavioral skills of children with ASD. However, the qualities of the studies differ, and some authors reached opposite conclusions. In this review, we systematically and objectively examined the effectiveness of EAATs for people with ASD, combining both qualitative and quantitative methods. We searched five databases (PubMed, Scopus, ERIC, ProQuest, and MEDLINE) and added relevant references, and we identified 25 articles for data extraction and analysis. According to our results, EAAT programs can substantially improve the social and behavioral functioning and language abilities of children with ASD. However, among the subdomains, the results were inconsistent. According to the meta-analyses, there were considerable improvements in the social cognition, communication, irritability, and hyperactivity domains, but not in the domains of social awareness, mannerisms, motivation, lethargy, stereotypy, or inappropriate speech. Moreover, there was a lack of sufficient comparative data to conclude that EAAT programs lead to substantial improvements in motor and sensory functioning. In addition, among the included studies, we noted the indicator of whether EAAT programs decreased parental stress and improved family functioning, and although there were four articles in which the researchers considered this aspect, we were unable to draw any conclusions because of the insufficient data and conflicting descriptive evidence. However, we need to consider the improvement in parental mental health as a factor in the effectiveness of this complementary intervention. We hope that in future studies, researchers will focus on family functioning and conduct more randomized controlled trials (RCTs) with blinded assessments using different scales and measures.
Keywords: equine-assisted activities and therapies, autism spectrum disorder, social and behavior function, family function
1. Introduction
1.1. ASD
Over the past half-century, the prevalence of ASD has dramatically increased [1]. Currently, ASD affects more than 78 million people worldwide, and it has become a critical public health issue [2]. ASD is a restricted, lifelong, innate, and complex neurodevelopmental disorder that hampers social interactions, cognitive functioning, and perceptual abilities, and it has a high incidence of associated mental retardation issues that can even cause individuals to die by suicide [3,4]. Individuals with ASD frequently exhibit a clinically heterogeneous and considerable proportion of uncontrollable repetitive patterns, emotional dysfunction, and reduced verbal and nonverbal communication during interactions, including less eye contact and body language [5]. Thus, people with ASD are more likely to struggle with multiple communicative and cognitive comorbidities that prevent them from strengthening relationships with others, which results in detrimental social relationships when compared with those of their typically developing peers or people with other psychopathologies [6,7].
Meanwhile, due to the difficulty of self-managing emotions, impaired self-regulation is a predisposition that is inherent in ASD [8] and that can lead to individuals with ASD experiencing more emotion-related problems in daily life than their non-autistic peers (e.g., symptoms of anxiety, depression, aggression, irritability, hyperactivity, rule-breaking, elopement, sensory processing, and sleep) [9,10,11]. Furthermore, individuals with ASD tend to be less adept at employing emotional strategies to self-manage emotion due to their difficulties forming and maintaining friendships, poor academic performances, and participation in social activities, which include experiences such as bullying and exclusion [12,13,14]. These core symptoms frequently persist after childhood and co-occur in adolescence and adulthood [15]. In particular, in a recent study, the authors revealed that there is an increased probability of additional co-occurring symptoms in children with autism, most typically attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and anxiety [16].
The difficulties that autistic people present also impact family members, particularly parents. According to a 2012 study, the parents of autistic children face more mental health difficulties than other parents [17]. Although parent perspectives are critical in understanding the impacts and processes of ASD interventions [18], parents struggle to choose from the many treatment options [19], which complicates the care for people with ASD.
1.2. Therapies for ASD
Therefore, the development of an effective treatment has been given priority in this area for individuals with ASD. In recent years, some researchers have shown that the genetic, immunological, environmental, and epigenetic factors are the most important factors in the development and progression of ASD [20]. Researchers try to look for effective and efficient approaches to treating ASD based on pathophysiology and syndromes, typically focusing on six primary areas: (1) sensory integration and sensory-based interventions; (2) relationship-based interactive interventions; (3) skill-based developmental programs; (4) social cognitive skills training; (5) parent-directed or parent-mediated approaches; (6) intensive behavioral interventions [2,21]. Following decades of research, conventional interventions have increasingly become concerned with fostering a positive environment for social engagement and self-regulation in people with ASD, attenuating the negative effects of autistic traits, and enhancing the quality of life and wellbeing in the ASD population [22]. Meanwhile, since ASD is a multifactorial disease, numerous treatment options have become available, and complementary and alternative medicine (CAM) may increasingly be used alongside classical medical practices to treat ASD [23,24,25]. Among the most popular and successful forms of CAM were swimming, music therapy, art therapy, and animal-assisted interventions (AAIs) [25].
1.3. Animal-Assisted Interventions (AAIs) for ASD
Under the influence of these studies, AAIs have entered the field of vision of researchers, and it is one of the most promising areas for remediating the core impairments of people with ASD [26,27]. AAIs include a variety of animals, such as dogs, horses, rabbits, dolphins, guinea pigs, and llamas, and incorporating animals into therapeutic treatments appears to effectively decrease the problematic behaviors and improve social communication for ASD populations [28]. In numerous emerging studies in recent years, researchers have laid the groundwork for the use of AAIs to assist individuals with ASD in regulating their emotions, improving their cognitive domains and social communication functioning, engaging in prosocial actions, and reducing maladaptive repetitive behaviors that are associated with stress [28,29,30,31,32,33].
1.4. EAATs for ASD
EAATs can help people with autism, cerebral palsy, intellectual disabilities, multiple sclerosis (MS), and post-traumatic stress disorder (PTSD), among other conditions [34,35]. Of all the animal-assisted therapies for ASD, the EAAT program is the most widely utilized [36]. In a 2018 study [37], the authors found that 10% of the parents of children with ASD have used therapies or interventions that include horses, which could be because EAATs have benefits that are different from those of other animal-assisted therapies. In three studies [38,39,40], the authors noted that the rhythmic movements of horseback riding can especially activate the vestibular systems of children with ASD, which can enhance their speech production and improve their learning outcomes. Riders have to actively manage their own body behaviors, which promotes their capacities for voluntary control and nonverbal communication. Another analysis revealed that the hippotherapy (HIP) exercises had a beneficial effect on postural control, interpersonal relationships, and adaptive behaviors [41]. Therefore, horses can offer people with ASD a special way of fostering positive social engagement [32]. Other researchers [28,40,42,43] have offered another insight: that the effects of horseback riding interventions might be optimally shaped by the relationship that forms over time between all humans and horses in groups, including a series of training steps and an accumulation of stimuli to elicit social interaction. From the same perspective, occupational therapists frequently employ “catalyst techniques” to increase arousal emotions, which contributes to behavioral and multisensory perception improvements [28,44,45]. In summary, because these interventions created upbeat and happy environments and provided multisensory stimulation, the benefits of horseback riding for people with ASD were enhanced. Equine-assisted interventions (EAIs) for ASD are rapidly increasing as a complementary therapy for ASD [32,36].
EAIs are programs that incorporate horses to provide rehabilitative and educational benefits to the participants [46]. EAIs are typically referred to as EAATs, and they include two main types of interventions: (1) equine-assisted therapies (EATs), which include hippotherapy (HIP) and equine-assisted psychotherapy (EAP), and equine-assisted activities (EAAs), which include therapeutic horseback riding (THR) [42]. Each method has a different specific therapeutic focus [47]. The fundamental and core idea behind THR is to engage people with ASD in horseback riding and nonriding activities (Barn activities, such as cleaning the barn, feeding horses, and watching the horses’ motions) with licensed instructors, counselors, or equestrians who teach them horsemanship skills that target improving their physical, behavioral, and prosocial health [38]. Hippotherapy utilizes occupational or physical therapy exercises by using the horse’s movements to improve the engagement of the sensory, neuromotor, and cognitive systems to improve the functional outcomes. Equine-assisted occupational therapy (EAOT), therapeutic riding (TR), and equine-facilitated learning (EFL) are a few of the other common terms. Notably, as opposed to EATs and HIP, equine-facilitated learning (EFL) is a distinct experiential learning technique that blends learning abilities and interaction with horses (ponies, miniature horses, donkeys, and mules) with individual therapy and emotional regulation to strengthen children’s awareness and control of their emotions, cognition, and behavior [48].
1.5. Relevance
In numerous studies, researchers have demonstrated that the participation of autistic people in therapeutic horseback riding programs improves their social interaction, socialization, and stereotyping behaviors. However, these therapies and interventions still need to be improved [29,49], and the extent to which these improvements occur, as well as whether the involvement duration and type of treatment have an impact on the treatment outcome, have not been thoroughly investigated [49]. Thus, in this review, we attempted to evaluate a number of aspects that influence how well the treatments work. Our primary objective was to use Sackett’s level of evidence, the Physiotherapy Evidence Database (PEDro) scale, and forest plots to evaluate the viability of the conclusions of all the included studies to better understand how EAATs affect the individual domains, such as the social, communicative, and behavioral abilities, as well as more the general functional outcomes, such as family functioning and quality of life. Our second objective was to assess whether the adjuvant therapy had a lasting effect after the horse intervention ended.
2. Methods
2.1. Search Protocol and Procedure
2.1.1. Eligibility Criteria
To build upon the detailed, exact, and comprehensive reviews, our methodology for this systematic literature review followed the meta-analyses (PRISMA 2020) guidelines [50] and PROSPERO registration requirements (CRD42022363685). We screened the studies based on the following search terms and their variants and synonyms: autism spectrum disorder and equine-assisted activities and therapies (see Appendix A.1 for details). Moreover, to ensure the inclusion of all apt and precise articles that fulfilled the requirements, two authors simultaneously independently screened the publications based on the same eligibility criteria (see Table 1 for details). After screening all the included articles, a third author independently compared the studies screened by the first two authors. If the two authors had different opinions, then the three authors simultaneously reviewed the full texts of the articles to determine whether they met the inclusion criteria. Although we did not include systematic reviews as one of the criteria for this review, we still evaluated the relevant systematic studies so as not to exclude any relevant studies from this review.
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria |
|---|
| (1) Article published in a journal with peer review; |
| (2) Publication in English language; |
| (3) Investigates the use of experimental or quasi-experimental designs in the reporting of the outcome data for the “EAATs” of “ASD”. |
| Exclusion Criteria |
| (1) Publication in languages other than English; |
| (2) Literature reviews (including systematic reviews), commentaries, etc.; |
| (3) Animal-assisted or pet-assisted therapies for ASD that used animals other than horses; |
| (4) No clear outcomes. |
In addition, the participants in the eligible studies must have had a diagnosis of ASD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or have previously been diagnosed with autism in a qualified hospital or medical center. If the study included patients other than those with ASD (such as those with ADHD or ID), then it met the inclusion criteria; if no autistic patients were included, then the study was not eligible. Under the same criteria, in the included studies, we allowed trials without control groups; however, these trials needed to include the participation of live horses and not virtual or robot horses (simulated horseback riding), which we excluded.
2.1.2. Information Sources
In the research domains, we searched all the articles from five electronic databases (PubMed, Scopus, ERIC, ProQuest, MEDLINE) that were related to psychology, health, education, physical training, clinical therapy, and neuroscience on 10 October 2022. We obtained the additional detailed supplementary information by utilizing the websites “connected papers” and “Google Scholar”. To find more specific articles, we also looked at the appropriate reference sections for any other relevant articles. Furthermore, we employed Review Manager 5 and Endnote X9 to archive and evaluate all the associated articles.
2.1.3. Study Selection
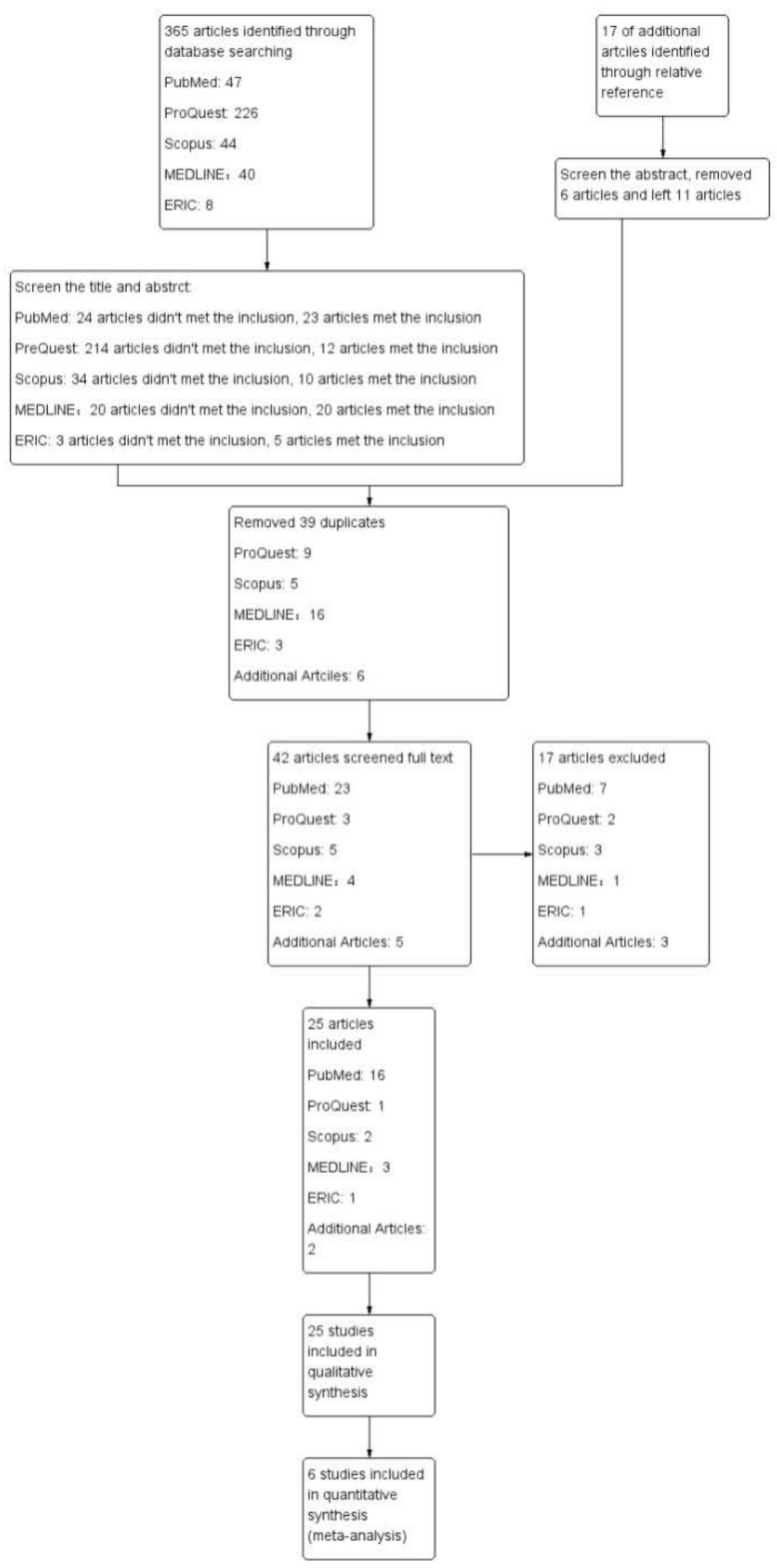
In the database searches that corresponded to the search terms, we identified 365 publications between 2009 and 2022 (see Figure 1 for details). After we filtered the title and abstract screenings, 70 articles met the inclusion criteria, and 295 articles did not. In the screening process, we included 11 additional relevant articles from the references. Then, we selected a total of 81 articles to meet the inclusion criteria. Of the 81 articles that were initially eligible, 39 were duplicates that we needed to eliminate, while we reviewed the remaining 42 in their entireties. A total of 17 articles did not meet the criteria, and so we excluded them from the analysis. We included the other 25 articles. Notably, 6 articles provided sufficient and reasonable raw data for this meta-analysis, while we used the remaining 19 articles for the qualitative synthesis, as they did not provide raw data or lacked sufficient data (Appendix A.2, Table A1).
Figure 1.
Flow diagram.
3. Data Extraction and Evaluation
3.1. Data Items
Two members of the research team used Excel-2212 (Microsoft, Redmond, Washington State, United States of America) and Review Manager 5.4.1 software (Cochrane, London, United Kingdom) to collect the following data: article data (first author, publication year, journal name, and country); participant data (sample demographics: age, gender, geographical information); sample features (diagnosis severity, diagnostic measure, other therapies, horseback riding experience); interventional and controlled characteristic data (handler accreditation, duration and session of program, terminology, comparison condition); outcome data (scales and subscales, scale validity, statistics including mean, standard deviation (SD), p-value, effect size) (see Table 2 and Table 3 for details).
3.2. Risk of Bias for Evaluation
We evaluated the internal validity and applicability of the RCTs and controlled clinical trials (CCTs) for the systematic review using the PEDro scale [51,52], which has 11 items. As usual, we considered PEDro scores from 6 to 10 to be high quality, scores from 4 to 5 to be fair quality, and scores of equal to or less than 3 to be low quality (see Table 4 for details). In addition, we used Sackett’s level of evidence [49], which can be used to sort individual studies into five levels of evidence, from Level I to Level V, for more accurate and reliable evaluations. RCTs are the best kind of evidence (Level 1), indicating clear relationships or conclusions. The lowest degree of evidence (Level 5) contains some single-case reports, narrative statements, or studies that do not indicate clear relationships or results (see Table 5 for details). We also looked at the “Risk of Bias Table” from Cochrane to evaluate the bias risk in these studies by looking up the criteria for each study and giving them a rating of low, high, or unclear in terms of risk (see Figure 2 for details).
Figure 2.
Risk of bias summary [25,28,30,38,40,42,43,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70].
Table 2.
Study characteristics.
| Basic Article Information | Sample Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Demographics | Diagnoses and Measures | ||||||||||
| First Author, Year, Journal |
Type of Trial | Reported Inclusion/ Exclusion of Participants (Y/N) |
Final Sample of Participants (E, C) |
Age (Range, Mean, SD) |
Gender (Male/Female) |
IQ (SD) | Participant Eligibility Criteria | Past/Recent EAI Experience |
Screening Instrument for Participants | Diagnoses | Diagnostic Measure |
| Peters, B. C., 2022, [43], J Autism Dev Disord |
RCTs | Y |
n = 21 E(OTEE): 12 C: 9 |
6–13 THR: (-), 8.68 ± 2.09 RA: (-), 9.45 ± 1.62 |
Male: 16 Female: 5 THR: 10/2 CT: 6/3 |
NVIQ ≥ 55 | (1) Aged 6–13 years old; (2) SCQ score ≥ 15; (3) Meets clinical cutoffs for ASD on ADOS, ADOS-2, or SRS-2; (4) Leiter-3 score ≥ 55; (5) Combined irritability and hyperactivity score ≥ 25 |
No EAA experience during previous 6 months. | SCQ ADOS-2 Leiter-3 |
ASD | HCC ABC-C SRS-2 GAS PEDI-CAT ASD |
| Zoccante, L., 2021, [25], J Clin Med |
Pre–post design | Y |
n = 15 Level 1: 7 Level 2: 6 Level 3: 2 |
7–15, 9.8 ± 2.2 | Male: 13 Female: 2 |
(-) | ASD participants without: (1) Critical medical illness; (2) Previous experience with horses; (3) Distressed behavior. |
N | ADOS-2 DSM-5 |
ASD ADHD: 9 |
VABS-2 DCDQ’07 PSI-SF IEMS |
| Zhao, M., 2021, [40], Int J Environ Res Public Health |
RCTs | Y |
n = 61 E(THR): 31 C(RA): 30 |
6–12; THR: (-), 7.06 ± 1.50 RA: (-), 7.13 ± 1.36 |
Male: 44 Female: 17 THR: 21/10 RA: 23/7 |
(-) | Children diagnosed with ASD, aged 6–12 years old. | (-) | DSM-5 | ASD | SSIS-RS; ABLLS-R |
| Peters, B. C., 2020, [28], OTJR (Thorofare N J) |
SCED | Y | n = 6 | 6–13 | (-) | NVIQ ≥ 55 on Leiter-3 | (1) Aged 6–13 years old; (2) Diagnosed with ASD on SCQ (≥15); (3) NVIQ ≥ 55 on Leiter-3; (4) Combined score ≥ 11 on irritability and hyperactivity subscales of ABC-C; (5) Meets physical, mental, and emotional standards set forth by PATH Intl. |
No THR experience during previous 6 months | SCQ ADOS Leiter-3 |
ASD | VAS |
| Kalmbach, D., 2020, [53], Occup Ther Health Care |
Explanatory sequential design | (-) | n = 4 | 8–13 | Male: 4 Female: 0 |
NVIQ (M: 100, SD: 15) | (-) | (-) | ABAS | ASD | Semistructured interviews |
| Ozyurt, Gonca., 2020, [54], Montenegrin Journal of Sports Science and Medicine |
RCTs | (-) |
n = 24 E(EAA): 12 C: 12 |
4–12, 6.77 ± 1.3 EAA: 6.75 ± 0.7 C: 6.7 ± 0.64 |
Male: 17 Female: 7 EAA: 8/4 C: 9/3 |
(-) | (-) | No previous experience with equine-assisted activities. | (-) | ASD | CGAS FAD SCQ |
| Kwon, S., 2019, [55], Ann Rehabil Med |
More group control | Y |
n = 29 E(THR): 18 C(CT): 11 |
6–11 THR: 8.2 ± 1.7 CT: 7.5 ± 1.1 |
Male: 16 Female: 13 THR: 11/7 CT: 5/6 |
(-) | (1) Diagnosis of ASD or ID; (2) Aged 6–13 years old; (3) Body weight < 35 kg; height < 150 cm; (4) Understanding of simple instructions; (5) Appropriate physical development for rehabilitative horseback riding; (6) Informed consent from legal guardian. |
N | (-) | ASD: 19 ID: 10 |
REVT PRES K-ABC-2 BSID-2 Luria Model |
| Pan, Z., 2018, [56], Front Vet Sci |
RCTs | Y |
n = 16 E(THR): 8 C(BA): 8 |
6–16 THR: 11.88 ± 2.45 BA: 9.80 ± 2.82 |
Male: 13 Female: 3 THR: 6/2 BA: 7/1 |
THR: 102.88 ± 16.28 BA: 100.25 ± 29.26 |
(1) Aged 6–16 years; diagnosis of ASD; (2) Combined total score of > 11 on irritability and stereotype subscales of ABC-C; (3) NVIQ score of ≥ 40 by Leiter-3. |
(-) | SCQ ADOS-2 ABC-C Leiter-3 |
ASD CPD: 12 PM: 9 PD: 1 MD: 3 AD: 8 ADHD: 7 LD: 1 |
SALT SRS ABC-C Saliva cortisol |
| Gabriels, R. L., 2018, [57], Front Vet Sci |
RCTs | Y |
n = 64 E(THR): 36 C(BA): 28 |
6–16 | (-) | NVIQ 85 or > 85 | 6 months | (-) | ASD | ABC-C SRS SALT |
|
| Tan, V. X., 2018, [58], J Autism Dev Disord |
Case design | (-) | n = 6 | 3–14 | Male: 1 Female: 5 |
NVIQ: 40 and 56 | (-) | 8 months–5 years | (-) | ASD | IPA |
| Harris, A., 2017, [59], Int J Environ Res Public Health |
More group control | Y |
n = 26 E(HR): 12 C: 14 |
6.08–9.33, 7.5 ± 10.57 HR: 8.2 ± 10.56 C: 7 ± 3.95 |
Male: 22 Female: 4 |
(-) | Excluded: (1) Not wearing helmet; (2) Known history of treating animals; (3) Fear or dislike of animals. |
Four children in intervention group had more than 2–3 years. |
Test in Social Communication Clinic | ASD | CARS2 ABC-C MOPI |
| Anderson, S., 2016, [60], J Autism Dev Disord |
Case–control | Y | n = 15 | 5–16, 10 [3.8] | Male: 11 Female: 4 |
(-) | (1) Diagnosis of ASD; (2) No previous experience with horses. |
N | DSM | ASD (27%) ADHD (20%) HSID (53%) |
VABS ASQ EQ/SQ |
| Borgi, M., 2016, [61], J Autism Dev Disord |
RCTs | Y |
n = 28: E(EAT): 15 C: 13 |
6–12, 8.6 ± 1.7 EAT: 9.2 ± 1.8 CG: 8.0 ± 1.5 |
(-) | IQ > 70 EAT: 98.3 ± 16.2 CG: 92.8 ± 19.9 |
(1) Diagnosis of ASD, aged 6–12 years; (2) IQ > 70 on WISC-III; (3) Lack of previous THR experience. |
Lack of previous therapeutic riding experience. | (-) | ASD | VABS TOL |
| Gabriels, R. L., 2015, [30], J Am Acad Child Adolesc Psychiatry |
RCTs | Y |
n = 116 E(THR): 58 C(BA): 58 |
6–16; THR: (-), 10.5 ± 3.2 BA: (-), 10.0 ± 2.7 |
Male: 101 Female: 15 THR: 49/9 BA: 52/6 |
NVIQ: THR: 86.7 [25.5] BA: 86.1 [22.7] |
Leiter-R nonverbal IQ cutoff ≥ 40; SCQ-ASD screening cutoff ≥ 15; ABC-C score ≥ 11. |
No more than two hours of EAAT within past six months. | SCQ ADOS-2 ABC-C Leiter-3 |
ASD MD AD ADHD, LD SD |
PPVT-4 SALT SRS BOT-2 SIPT VABS ABC-C |
| Steiner, H., 2015, [62], Acta Physiol Hung |
Control design | (-) |
n = 26 E(THR): 13 C(PT): 13 |
10–13 | Male: 12 Female: 14 THR: 6/7 PT: 6/7 |
(-) | (-) | (-) | (-) | ASD | APAS PAC-test |
| Holm, M. B., 2014, [63], J Autism Dev Disord |
Single-case–control | Y | n = 3 | 6–8 | Male: 3 Female: 0 |
(-) | (1) Diagnosis of ASD; (2) Aged 5–13 years old; (3) Available to participant in intervention; (4) Parental agreement. |
Approximately one year. | (-) | ASD | KTEA-2 ABC-C CARS SRS SP-CQ |
| Lanning, B. A., 2014, [42], J Autism Dev Disord |
Control design | Y |
n = 18 * E(EAA): 10 C(SC): 8 |
4–15 EAA: 4–15, 7.5 ± 3.2 C: 5–14, 9.8 ± 3.2 |
Male: 21 Female: 4 EAA: 9/4 C: 12 |
(-) | (1) Diagnosis of ASD from physician or therapist; (2) Parental agreement; (3) No participation in EAA 6 months prior to start of study. |
No EAA experiences during previous 6 months. | (-) | ASD | PedsQL CHQ |
| Ward, S. C., 2013, [38], J Autism Dev Disord |
Quasi-experimental | Y | n = 21 | 8.1 | Male: 15 Female: 6 |
(-) | (1) Meeting criteria for autism according to DSM IV-TR; (2) Qualified for services in public school division. |
Thirteen participants with no TR experience. | DSM IV-TR CAB-T |
ASD | GARS-2 SPSC |
| Jenkins, Sarah R., 2013, [64], Research in Autism Spectrum Disorders |
Multiple baseline SCED | Y |
n = 7 THR: 4 C: 3 |
6–14, 9.5 | Male: 6 Female: 1 |
(-) | (1) No prior exposure to THR or hippotherapy; (2) Residing within 30 miles of primary research site. |
No THR or HIP experience. | VABS-2 | ASD TC Verbal and Motor Apraxia |
CBC BOT-2 |
| Ghorban, Hemati, 2013, [65], Journal of education and learning |
Pre–post design | Y | n = 6 | 6–12 | Male: 1 Female: 5 |
(-) | Meeting criteria for DSM-IV-TR. | (-) | (-) | ASD | TSSA |
| Gabriels, Robin L., 2012, [66], Research in Autism Spectrum Disorders |
Waitlist control and pre–post design | Y |
n = 42 THR: 26 C: 16 |
6–16, 8.7 E: 5–16, 8.6 C: 6–14, 8.8 |
Male: 36 Female: 6 E: 21/5 C: 15/1 |
NVIQ range of 44–139, Mean: 95.2 | (1) Chronological ages of 6–16 years; (2) Diagnosis of autistic or Asperger’s disorder; (3) Combined score of at least 11 on ABC-C. |
No THR experience within past three years. | ABC-C SCQ ADOS Leiter-R |
ASD Asperger’s disorder Seizures |
ABC-C BOT-2 SIPT VABS-2 |
| Tabares, C., 2012, [67], Neurochemical Journal |
Pre–post design | (-) | n = 8 | 8–16 | Male: 8 | (-) | (-) | (-) | (-) | ASD | ELISA |
| Janet K Kern., 2011, [68], Alternative Therapies in Health and Medicine |
Pre–post design | Y | n = 24 | 3–12, 7.8 ± 2.9 | Male: 18 Female: 6 |
(-) | (1) Between 3 and 12 years of age; (2) Primary diagnosis of ASD; (3) CARS score ≥ 30; (4) No previous participation in equine-assisted activities. |
No previous EAA participation. | CARS | ASD | CARS Timberlawn Parent–Child Interaction Scales SP QLES-Q TSS |
| Bass, M. M., 2009, [69], J Autism Dev Disord |
Case–control | Y |
n = 34 E: 19 C: 15 |
5–10 E: 6.95 ± 1.67 C: 7.73 ± 1.65 |
Male: 29 Female: 5 E: 17/2 C: 12/3 |
(-) | (1) Meeting criteria for DSM-IV-TR. | No EAA experience. | DSM-IV-TR SSSS |
ASD Asperger’s |
SRS SP |
| Taylor, Renee R., 2009, [70], Occupational Therapy in Mental Health |
SCED | (-) | n = 3 | 4–6 | (-) | (-) | (1) Aged 4–6 years; (2) No other medical or psychiatric diagnoses. |
(-) | (-) | ASD | PVQ |
Table chronologically sorted and abbreviations alphabetically ordered as follows: (-): not reported; Y: yes; N: no; E: experimental group; C: control group; n/a: not applicable; n: number; BA: barn activity; Type of Trial: SCED: single-case experimental design; Final sample of participants: n = 18 *, initial sample = 25; BA: barn activities; CT: conventional therapy; EAAs: equine-assisted activities; EAT: equine-assisted therapy; HR: horseback riding; OTEE: occupational therapy in equine environment; PT: physical therapy; RA: regular activity; SC: social circle; THR: therapeutic horseback riding; Participant eligibility criteria: ABC-C: Aberrant Behavior Checklist—Community; NVIQ: nonverbal IQ; PATH Intl.: Professional Association of Therapeutic Horsemanship International; WISC-Ⅲ: Wechsler Intelligence Scale for Children III; Screening Instruments: ABAS: Adaptive Behavior Assessment System; ABC-C: Aberrant Behavior Checklist—Community; ADOS: Autism Diagnostic Observation Schedule; CAB-T: Clinical Assessment Battery Teacher Rating Form; CARS: Childhood Autism Rating Scale; DSM: Diagnostic and Statistical Manual of Mental Disorders; Leiter: Leiter International Performance Scale; SCQ: Social Communication Questionnaire; SSSS: Stone’s Social Skills Scale; VABS: Vineland Adaptive Behavior Scale; Diagnosis: AD: anxiety disorder; ADHD: attention-deficit/hyperactivity disorder; CPD: community psychiatric diagnoses; HSID: hypersensitivity and sensory integration disorder; ID: intellectual disability; LD: learning disability; MD: mood disorder; PD: psychotic disorder; SD: seizure disorder; TC: tuberous sclerosis; Diagnostic Measure: ABLLS-R: Assessment of Basic Language and Learning Skills—Revised; APAS: Ariel Performance Analysis System; BOT: Bruininks–Oseretsky Test of Motor Proficiency; BSID-2: Cognitive Domain of Bayley Scales of Infant Development 2; CBC: Child Behavior Checklist; CGAS: Children’s Global Assessment Scale; CHQ: Child Health Questionnaire; DCDQ’07: Developmental Coordination Disorder Questionnaire, as revised in 2007; ELISA: competitive enzyme immune essay method; EQ/SQ: empathizing quotient/systemizing quotient; FAD: McMaster Family Assessment Device; GARS-2: Gilliam Autism Rating Scale 2; GAS: Goal Attainment Scale; HCC: hair cortisol content; IEMS: interaction emotions motor skills; IPA: interpretive phenomenological analysis; K-ABC-2: Kaufman Assessment Battery for Children 2; KTEA-2: Kaufman Test of Educational Achievement—Second Edition; PAC-test: Pedagogical Analysis and Curriculum; PEDI-CAT ASD: Pediatric Evaluation of Disability Inventory Computer Adaptive Test, Autism Spectrum Disorder Module; MOPI: observational measure of child’s engagement; PedsQL: Pediatric Quality of Life 4.0 Generic Core Scales; PPVT-4: Peabody Picture Vocabulary Test, Fourth Edition; PRES: Preschool Receptive–Expressive Language Scale; PSI-SF: Parenting Stress Index—Short Form; PVQ: Pediatric Volitional Questionnaire; QLES-Q: Quality of Life Enjoyment and Satisfaction Questionnaire; REVT: Receptive and Expressive Vocabulary Test; SALT: Systematic Analysis of Language Transcripts; SIPT: Sensory Integration and Praxis Test; SP: Sensory Profile; SPSC: Sensory Profile School Companion; SRS: Social Responsiveness Scale; SSIS-RS: Social Skills Improvement System Rating Scale; TSIF: Test of Sensory Integration Function; TOL: Tower of London; TSS: Treatment Satisfaction Survey; TSSA: Triad Social Skills Assessment; VAS: Visual Analog Scale.
Table 3.
Intervention characteristics.
| First Author, Year |
Intervention Time | Intervention Information | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (Weeks) |
Session Frequency (Per Week) |
Session Time (min) |
Total Therapy Time (Minutes) |
Test at Pre-, Interim, and Postintervention |
Terminology | Intervention Format (I or G) |
Intervention Provider Accreditation |
Other Therapy | Setting | Clients/Caregivers/ Animals |
Interventional Components |
Control Group Condition | |
| Peters, B. C., 2022, [43] |
10 | (-) | 60 | (-) | At baseline and after intervention. | OTEE | G | AOTA, AHA, PATH | (-) | Riding center | Five occupational therapists, instructors, volunteers, leaders, side walkers, and horses. | (1) Greetings; (2) Activities with horses; (3) Goodbyes and caregiver debriefing. |
Waitlist/OTGE |
| Zoccante, L., 2021, [25] |
20 | 1 | 45 | 900; 50% individual sessions; 50% couple sessions. |
Before and after 20 individual sessions. | EAAT | I/G | ASD① | Amateur Sports | Horse valley | One veterinarian, one horse assistant, two healthcare professionals, three horses. | Grooming, activities on ground, activities on horse | n/a |
| Zhao, M., 2021, [40] |
16 | 2 | ≈60 | ≈1920 | One week prior to intervention; at 8th interim week; after 16-week postintervention. |
THR | G | IETC① | (-) | Outdoor and indoor arenas | Instructors, volunteers, and horses. | (1) Warm-up activities; (2) Instruction in riding skills and horsemanship skills; (3) THR exercises and activities; (4) Cool-down and reward activities. |
RA |
| Peters, B. C., 2020, [28] |
10 | (-) | 45–60 | (-) | (-) | OTEE/HIP | I | PATH/AHA | School SLP, School OT | Riding center | Two occupational therapists, volunteers, leaders, side walkers. | (1) Greetings; (2) Ground and mounted activities; (3) Parent debriefing and goodbyes. |
n/a |
| Kalmbach, D., 2020, [53] |
10 | 1 | 45–60 | 450–600 | 1: From four to six weeks after intervention; 2: Six months after intervention. |
OTEE | I/G | AOTA | SLP, PT, OT | Riding arena | Three researchers, occupational therapists, side walkers, volunteers, horses. | (1) Premounting segment; (2) Mounted segment; (3) Postmounting segment. |
(-) |
| Ozyurt, Gonca., 2020, [54] |
8 | 1 | 60 | 480 | Pretesting and post-testing. | EAA | G | PATH | RT, special education | Riding center | Clinicians, educators, occupational therapist, physical therapist, therapeutic riding instructor, speech and language therapists, pediatrician, horses. | (1) Preparation and warmup; (2) Grooming and feeding; (3) Mounting and dismounting; (4) Horsemanship activities; (5) Finishing. |
RT |
| Kwon, S., 2019, [55] |
8 | 1 | 30 | 240 | Before and after intervention. | THR | G | (-) | CT | Riding center | Riders, instructors, national licenses, leaders, side walkers, and horses. | (1) Stretching exercises; (2) Riding skills and riding; (3) Interaction with horses (such as brushing, feeding, putting stickers on them). |
CT |
| Pan, Z., 2018, [56] |
10 | (-) | 45 | (-) | SALT: within one month pre- and postintervention; SRS: within 1 month pre- and postintervention; ABC-C: after 10-week intervention; SCDC: before each THR session and 20 min following each session. |
THR | G | PATH | (-) | Riding center | Research staff, trained volunteers, horses. | (1) Saliva collection; (2) Sitting with a volunteer; (3) Starting group; (4) Reviewing group schedules; (5) Warm-up exercises; (6) Lesson and activity; (7) Cool-down exercises; (8) THR group dismount and thanking horses, all groups, and volunteers; (9) Drawing activity at table (20 min); (10) Saliva collection. |
BA |
| Gabriels, R. L., 2018, [57] |
10 | 1 | 45 | 450 | (1) Baseline assessment; (2) Postintervention assessment; (3) 6-month postintervention follow-up assessment. |
THR | G | PATH | Same as in Gabriels R. L. (2015) | Riding center | THR: leader, side walker, instructors, and horses; BA: volunteers. |
Same as in Gabriels R. L. (2015). | BA |
| Tan, V. X., 2018, [58] |
at least 4 | 1 | (-) | (-) | After intervention | EAI: 5 TR: 1 |
I | (-) | SOT physiotherapy ST SST |
(-) | Mental health professional | (-) | n/a |
| Harris, A., 2017, [59] |
First Class: 7 Second Class: 5 |
1 | 45 | 225–315 | Before and after intervention over approximately 7 weeks; MOPI at end of each session. |
THR | G | BHS | Speech and language therapy; CPI/T; OT. |
Horse-riding facility | Instructors, side walkers, minorities, teaching staff, volunteers, and horses. | (1) Preparation and mounting; (2) Riding skills and exercises; (3) Stretching exercises; (4) Thanking instructors and horses. |
Waitlist |
| Anderson, S., 2016, [60] |
5 | 1 | 180 | 900 | First and last days of EAA intervention. | THR | G/I | BHS RDA |
N | Horse center | Instructors, volunteers, and horses. | (1) Health and safety briefing; (2) Parental self-assessments and interviews; (3) Horsemanship activities, including grooming, leading, and mucking out; (4) Therapeutic riding. |
Horsemanship/ stable management |
| Borgi, M., 2016, [61] |
6 months | 1 | 60–70 | 1500–1750 | 30 days before EAT sessions and 6 months after intervention. | EAT | G | FISE | CT and SA | Riding center | Instructors, expert veterinarian, 20 horses. | (1) Grooming and hand-walking horses; (2) Horseback riding; (3) Closure, feeding and saying goodbye to horses and group. |
Waitlist |
| Gabriels, R. L., 2015, [30] |
10 | 1 | 45 | 450 | ABC-C and SRS measures assessed 1 month pre- and postintervention. |
THR | G | PATH | Psychotropic medicine |
Riding center | Leaders, volunteers, instructors. | Warm up; therapeutic riding skills (mounting, halting, steering, running, trotting); horsemanship skills (how to lead and care for horse); cool down. |
BA |
| Steiner, H., 2015, [62] |
(-) | (-) | 30 | (-) | Before and after one month of therapy; after three-month break (without therapy). | THR | G | (-) | Pedagogical sessions | (-) | Leader, assistants, horses. | (1) Warm-up exercise of stretching on horseback while horses not moving; (2) Horseback riding. |
PT |
| Holm, M. B., 2014, [63] |
Phase A: 4 Phase B: (-) Phase A’: (-) |
Phase A: 1 Phase B: 1, 3 or 5 Phase A’: 1 |
30–45 | (-) | 1-month postintervention. | THR | I | NARHA | Medical therapeutics |
Riding center | Walkers, leader, instructors, horses. | (1) Grooming, emphasizing touch, naming of parts, and following instructor; (2) Riding session. |
THR dose (1 time/week) |
| Lanning, B. A., 2014, [42] |
12 | 1 | ≈60 | ≈720 | 3, 6, 9, and 12 weeks. | EAA | G | PATH | (-) | Riding center | Psychology student trainees, occupational therapist, physical therapist, pediatrician or family physician, instructors, side walkers, horses. | (1) Basic safety lessons; (2) Grooming lessons; (3) Riding activities. |
SC |
| Ward, S. C., 2013, [38] |
6-week TR 6-week break 4-week TR 6-week break 8-week TR |
(-) | 40–45 | (-) | Prior to intervention and Weeks 6, 16, 23, 26, and 30. | TR | G | PATH | Speech services OT: 1 PT: 1 |
Cori Sikich Therapeutic Riding Center | Coordinator, instructor, leader, two side walkers, rider pai, and horses. | (1) Orientation; (2) Mounting and riding; (3) Riding skills; (4) Closure. |
(-) |
| Jenkins, Sarah R., 2013, [64] |
9 | 1 | 60 | 540 | Before, weekly during THR, and after 9-week therapy program. | THR | G | PATH | (-) | Horse arena | Leader, side walker, instructor. | Creating lesson plans based on each rider’s skill level and acquisition of target horsemanship skills. | Waitlist |
| Ghorban, Hemati., 2013, [65] |
4 | 2 | 45 | 360 | Before and after intervention. | THR | G | (-) | (-) | Horseback riding | Trainers, parents, teachers. | (1) Familiarity stage; (2) Practices; (3) Riding skills; (4) End of riding stage. |
n/a |
| Gabriels, Robin L., 2012, [66] |
10 | 1 | 60 | 600 | Within one month of THR and one month after THR. | THR | G | PATH | Psychoactive medications | Riding center | Clinical psychologist, instructor, occupational therapist, volunteers. | (1) Putting on riding helmets; (2) Sitting and waiting on bench; (3) Mounting horses; (4) THR activities; (5) Dismounting horses; (6) Grooming horses; (7) Putting away equipment. |
Waitlist |
| Tabares, C., 2012, [67] |
4 | 1 | (-) | (-) | Before and after hippotherapy sessions. | HIP | I | AZE | (-) | Equestrian center | (-) | (1) Making contact with animals; (2) Mounting horses; (3) Exercise ring; (4) Dismounting horses; (5) Saying goodbye. |
n/a |
| Janet K Kern., 2011, [68] |
24 | 1 | 60 | 1440 | (1) Before beginning 3–6-month waiting period; (2) Before starting riding treatment; (3) After 3 months; (4) Within 6 months of riding. |
EAA | G | (-) | (-) | Riding center | Parents, caregivers, health professionals, instructor, horses. | Leading, grooming, and tacking responsibilities. | n/a |
| Bass, M. M., 2009, [69] |
12 | 1 | 60 | ≈720 | Pretesting and post-testing. | THR | G | (-) | CT: 11 | Good Hope Equestrian Training Center | Instructors, side walkers, volunteers, horses. | (1) Mounting and dismounting; (2) Warm-up exercises to stretch bodies; (3) Riding skills; (4) Mounted games; (5) Horsemanship activities. |
Waitlist |
| Taylor, Renee R., 2009, [70] |
16 | 1 | 45 | 720 | Before, during, and after hippotherapy. | HIP | I | (-) | None | Riding facility | Occupational therapists, pediatric physical therapist, leader, side walkers. | (1) Donning of helmets and mounting preparation; (2) Riding and dismounting. |
n/a |
Table chronologically sorted and abbreviations alphabetically ordered as follows: I: individuals; G: group; C: controlled; AC: activity control; SCDC: saliva collection and determination of cortisol; Terminology: EAATs: equine-assisted activities and therapies; EAAs: equine-assisted activities; EAI: equine-assisted intervention; EAT: equine-assisted therapy; HIP: hippotherapy (equine-assisted occupational or physical therapy); OTEE: occupational therapy in equine environment; THR: therapeutic horseback riding; TR: therapeutic riding; HR: horse riding; Other Therapy: CPI/T: continued previous intervention/therapy; CT: conventional therapy; OT: occupational therapy; RT: regular therapy; SA: scholastic assistance; SLP: speech–language pathology; SOT: speech and occupational therapy; SST: Samoans sound therapy; ST: speech therapy; Intervention Provider Accreditation: AHA: American Hippotherapy Association; AOTA: American Occupational Therapy Association; ASD①: Associazione Sportiva Dilettantistica; AZE: Association of Zootherapy of Extremadura; BHS: British Horse Society; IETC①: International Equestrian Training Center, China; IFES: Italian Federation of Equestrian Sports; NARHA: North American Riding for the Handicapped Association; PATH: Professional Association of Therapeutic Horsemanship International; RDA: Riding for Disabled; Control Group Condition: OTGE: occupational therapy garden environment; SC: social circle, providing educational and recreational activities.
Table 4.
PEDro scoring for RCTs.
| First Author, Year |
Specified Eligibility Criteria |
Random Subject Allocation |
Allocation Concealment |
Baseline Similarity of Groups |
Blinding of Subjects |
Blinding of Therapies |
Blinding of Assessors |
Measures of Outcomes |
Intention to Treat |
Between-Group Comparisons |
Point and Variability Measures |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peters, B. C., 2022, [43] | Y | Y | (-) | Y | (-) | (-) | Y | Y | Y | Y | Y | 7 |
| Zhao, M., 2021, [40] | Y | Y | (-) | Y | (-) | (-) | N | Y | Y | Y | Y | 6 |
| Ozyurt, Gonca., 2020, [54] | N | Y | (-) | (-) | (-) | (-) | Y | Y | Y | Y | Y | 5 |
| Pan, Z., 2018, 55] | Y | Y | (-) | Y | (-) | (-) | Y | Y | Y | Y | Y | 7 |
| Gabriels, R. L., 2018, [57] | Y | Y | (-) | Y | (-) | N | Y | Y | Y | Y | Y | 7 |
| Borgi, M., 2016, [61] | Y | Y | (-) | Y | (-) | (-) | (-) | Y | Y | Y | Y | 6 |
| Gabriels, R. L., 2015, [30] | Y | Y | (-) | Y | (-) | N | N | Y | Y | Y | Y | 6 |
| Bass, M. M., 2009, [69] | Y | Y | (-) | Y | (-) | (-) | (-) | Y | Y | Y | Y | 6 |
Scores: 6–10 (high quality); 4–5 (fair quality); (-), not report; Y: yes; N: no.
Table 5.
Trial quality.
| First Author, Year |
Study Methods | C-Group Allocation |
Report: Past/ Recent Riding Experience |
Report: Screening of Riding Center |
Report: Treatment Fidelity/ Integrity |
PEDro Score |
Level of Evidence |
|---|---|---|---|---|---|---|---|
| Peters, B. C., 2022, [43] | RCTs | Randomized | Y | Y | Y | 7 | I |
| Zoccante, L., 2021, [25] | Pre–post design | n/a | Y | Y | (-) | n/a | III |
| Zhao, M., 2021, [40] | RCTs | Randomized | (-) | Y | (-) | 6 | I |
| Peters, B. C., 2020, [28] | SCED | n/a | Y | Y | Y | n/a | IV |
| Kalmbach, D., 2020, [53] | Explanatory sequential design | n/a | (-) | Y | Y | n/a | IV |
| Ozyurt, Gonca., 2020, [54] | RCTs | Randomized | Y | Y | Y | 5 | II |
| Kwon, S., 2019, [55] | More group control | Nonrandomized but testing of baseline similarity | N | Y | Y | n/a | II |
| Pan, Z., 2018, [56] | RCTs | Randomized | (-) | Y | Y | 7 | I |
| Gabriels, R. L., 2018, [57] | RCTs | Randomized | (-) | Y | Y | 7 | I |
| Tan, V. X., 2018, [58] | Case design | n/a | Y | (-) | (-) | n/a | V |
| Harris, A., 2017, [59] | More group control | Nonrandomized but testing of baseline similarity | Y | Y | Y | n/a | II |
| Anderson, S., 2016, [60] | Pre–post design | Nonrandomized but testing of baseline similarity | Y | Y | Y | n/a | III |
| Borgi, M., 2016, [61] | RCTs | Randomized | Y | Y | Y | 6 | I |
| Gabriels, R. L., 2015, [30] | RCTs | Randomized | (-) | Y | Y | 7 | I |
| Steiner, H., 2015, [62] | Control design | Randomized | (-) | (-) | (-) | n/a | III |
| Holm, M. B., 2014, [63] | SCED | n/a | Y | Y | Y | n/a | IV |
| Lanning, B. A., 2014, [42] | Control design | Randomized | Y | Y | (-) | n/a | III |
| Ward, S. C., 2013, [38] | Quasi experimental interrupted-time-series design | Based on public classroom assignment and testing of baseline similarity | Y | Y | Y | n/a | III |
| Jenkins, Sarah R., 2013, [64] | SCED | Nonrandomized but testing of baseline similarity | N | (-) | Y | n/a | IV |
| Ghorban, Hemati., 2013, [65] | Pre–post design | n/a | (-) | (-) | (-) | n/a | IV |
| Gabriels, Robin L., 2012, [66] | Waitlist control and pre–post design | Testing of baseline similarity | Y | Y | Y | n/a | II |
| Tabares, C., 2012, [67] | Pre–post design | n/a | (-) | Y | Y | n/a | IV |
| Janet K Kern., 2011, [68] | Pre–post design | n/a | Y | (-) | Y | n/a | IV |
| Bass, M. M., 2009, [69] | RCTs | Randomized | Y | Y | (-) | 6 | I |
| Taylor, Renee R., 2009, [70] | SCED | n/a | (-) | (-) | Y | n/a | IV |
4. Results
4.1. Description of Study
After careful screening and analysis, we gathered 25 eligible articles between 2009 and 2022: 2009 (n = 2); 2021 (n = 1); 2012 (n = 2); 2013 (n = 3); 2014 (n = 2); 2015 (n = 2); 2016 (n = 2); 2017 (n = 1); 2018 (n = 3); 2019 (n = 1); 2020 (n = 3); 2021 (n = 2); 2022 (n = 1). Researchers have conducted 25 studies in 10 countries, performing most of them in the United States (n = 14) [28,38,42,43,53,56,57,62,63,64,66,68,69,70]. They performed the others in the following countries: the United Kingdom (n = 2) [59,60]; Italy (n = 2) [25,61]; China (n = 1) [40]; Iran (n = 1) [65]; Australia (n = 1) [58]; Spain (n = 1) [67]; Hungary (n = 1) [62]; Turkey (n = 1) [54]; Korea (n = 1) [55].
4.2. Sample Characteristics
Individuals with ASD
Of all the included studies, the range of participants with ASD was 3–116, producing a total final sample size of 623, with an average sample size of 25. The largest sample included 115 participants [30], while the smallest sample included only three people [63,70]. The median sample size was 21, and 12 studies (48%) had sample intervals that focused on 15–29 participants. Meanwhile, the researchers reported the age of the demographic factors in 25 studies. The age range was 3–16 years old, and in four studies, the authors did not note the gender factor [25,47,56,60]. In almost all the studies (84%, n = 21), there were more male participants than female participants, which is consistent with the age and gender differences in the pathology of autism. In addition, in two trials, the ratios of male-to-female patients were approximately equal [55,62], and in two studies, the number of females was higher than the number of males [58,65]. Other than age and gender, researchers reported the nonverbal intelligence quotient in nine studies [25,27,37,49,52,53,56,60,69]. Additionally, in eight studies, the authors reported that all patients diagnosed with autism had also been diagnosed with other disorders or conditions, such as ADHD, ID, hypersensitivity and sensory integration disorder (HSID), learning disability (LD), seizure disorder (SD), etc. (see Table 2 for details).
4.3. Intervention Characteristics
4.3.1. Screening Criteria and Instrument
In a total of 19 of 25 articles (76%), the authors reported the participant eligibility criteria; however, in six articles, they did not specify the criteria in detail for the population inclusion in the trials. Among the inclusion criteria, in 18 studies (72%), the authors specifically indicate whether the participants had previously participated in EAAT programs or had horseback riding experience. Although in no studies do the authors clearly point out whether the riding experience affected the experimental treatment effect, it is a key factor that can be explored to determine whether EAATs have lasting therapeutic effects, and we hope that researchers will test this hypothesis in future trials.
The researchers most frequently used the Autism Diagnostic Observation Schedule (ADOS) (n = 6), Social Communication Questionnaire (SCQ) (n = 5), Leiter International Performance Scale (Leiter) (n = 5), and DSM (n = 5) during the participant screening phase. In the remaining studies, the authors used screening scales such as the Aberrant Behavior Checklist—Community (ABC-C), Adaptive Behavior Assessment System (ABAS), Clinical Assessment Battery Teacher Rating Form (CAB-T), Vineland Adaptive Behavior Scale (VABS), Childhood Autism Rating Scale (CARS), and Stone’s Social Skills Scale (SSSS). (See Table 2 for details).
4.3.2. Intervention Dose
In addition to the abovementioned factors, the trial session, frequency, and total time were also paramount. Of the twenty-five included studies, eight studies lacked data on the total length of the trials, while the authors reported the durations in the remaining 17 (68%) trials. The total durations of the programs ranged from 240 to 1920 min. The average length of the trials was approximately 756 min, with a median time of 600 min, and 11 studies had trials from 450 to 900 min. Whereas the shortest trial was 4 weeks and the longest was 25 weeks, with a mean of 11 weeks and median of 10 weeks, seven of the twenty-two trials (32%) in which the authors reported the program weeks had set-up times of 10 weeks (see Table 3 for details).
4.3.3. Terminology
In these studies, the authors use different intervention terminologies, and the most used is THR (n = 13, 52%), while the remaining authors use terms such as occupational therapy in an equine environment (OTEE) (n = 3), EAA (n = 3), HIP (n = 2), EAT (n = 1), EAI (n = 1), TR (n = 1), and EAAT (n = 1). Although the names of the terminologies are different, the treatments that they describe primarily include the same training stages: warming up (health and safety briefings, stretching exercises, and so on), riding and horsemanship skills (mounting, halting, steering, running, trotting, brushing, feeding, and putting stickers on their horses), and cool-down and reward activities (thanking instructors and horses) (see Table 3 for details).
4.4. Study Methods and Trial Quality
Eight of the included articles were RCTs, and all but one of them (of fair quality) were of high quality. Notwithstanding, we noticed that in all eight RCTs, the authors did not report the allocation concealment or participant blinding because it was difficult to conceal the trial groupings from the participants in such trials. Additionally, the authors of 10 of the 25 studies that were included in this review randomly allocated the control groups to the experimental groups, whereas in the remaining nine studies, the authors did not have control groups and generally used within-group pre and post designs. A total of five of these nine trials were designed for individuals as opposed to groups. A total of 11 studies (44%) scored at Level II or higher when we assessed all 25 studies using Sackett’s level of evidence, which further indicated the high dependability of the studies used in this review (see Table 4 and Table 5 for details). To thoroughly analyze the trustworthiness of this systematic review, we also utilized the Cochrane risk-of-bias tool to evaluate three different types of risk (low risk, unclear risk, and high risk) for the six subdomains: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting. None of the 25 studies were low risk, and performance and detection biases were the most common (see Figure 2 for details), which is consistent with the nature of such trials, and which made it challenging to double-blind the participants and assessors, revealing an improvement direction for future trials, in which researchers should aim to blind the assessors.
5. Outcome Measures and Effects
5.1. Outcome Measures
In only five of the twenty-five research studies do the authors report the intragroup effects. Authors report both the inter- and intragroup effects in a total of thirteen studies, and seven studies lack sufficient data to identify the effects. In numerous studies in this review, the authors evaluated the EAAT impact on multiple ability categories using a collection of subjective and objective measurements, including standardized tests graded by experts or parents, qualitative observational measures, physiological indicators, etc. Specifically, in 15 of the 25 studies, the authors employed caregiver-rated measures, such as the Social Responsiveness Scale (SRS), ABC-C, Sensory Profile (SP), Assessment of Basic Language and Learning Skills—Revised (ABLLS-R), Pediatric Volitional Questionnaire (PVQ), Triad Social Skills Assessment (TSSA), VABS, and ABAS. In six studies, the authors utilized standardized tests administered by trained clinicians or experts, such as the CARS, Sensory Integration and Praxis Test (SIPT), and Bruininks–Oseretsky Test of Motor Proficiency (BOT). In three studies, the authors used semi-structured interviews and observational measures to analyze the performance improvements during the training sessions. In three studies, the authors incorporated physiological parameters, such as the salivary hormone levels, and in one study, they evaluated the EAAT effects using a computer-assisted methodology (see Table 3, Table 6 and Table 7 for details).
5.2. Severity of ASD
The key criterion that we utilized to assess the effectiveness of the intervention was the improvement in the ASD severity. In this comprehensive review, in three studies that reported the ASD severity effects, the authors used the following four distinctive standardized measures: (1) the CARS; (2) the autism spectrum quotient (ASQ); (3) the Beck Depression Inventory (BDI); (4) the Children’s Global Assessment Scale (CGAS). They also used an additional qualitative observational scale: interpretive phenomenological analysis (IPA).
In a 2011 study [68] in which the authors used the CARS, they discovered that the EAAT program reduced the ASD severity in the participants. The findings are consistent with those of studies conducted in 2013 and 2017 in which the authors used the CARS [38,59], as well as with those of studies [54] in which the authors used the CGAS. Using IPA observed by parents, in a 2018 study, the authors illustrated the EAI impact on autism from a qualitative perspective, revealing that the EAI improved the broad aspects of overall psychosocial functioning [58] and for which the psychological and emotional satisfactions were consistent with those of the previous 2011 and 2014 studies [42,71] but slightly different from those of another previous study [64]. Overall, there is only limited evidence that EAAT programs are successful at reducing the autism severity in patients given the paucity of adequate primary data (see Table 6 for details).
Table 6.
Study results.
| First Author, Year |
Tested Domains/Variables | Type of Effect (B/W) |
Type of Trial | Reported Measures/Variables Showing Substantial Improvement |
Reported Measures/Variables Showing No Substantial Improvement | Results | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Group (M, SD/SE) | Intervention Group (M, SD/SE) | Control Group (M, SD/SE) | Magnitude of Reported ES | ||||||
| Peters, B. C., 2022, [43] |
Self-regulation, social communication, and social play. | B/W | Qualitative and quantitative research | Improved goal attainment and social motivation and decreased irritability. | Social communication, hyperactivity. | GAS: Primary goals: IG: 2.00, CG: 2.00; averages of goals: IG: 2.00, CG: 2.00 ABC: Irritability: IG: 14.65 (6.99), CG: 17.68 (4.69) SRS-2: Social motivation: 69.85 (9.39), CG: 74.67 (8.20) |
GAS: Primary goal: 0.75 (1.45) Average of all goals: 0.39 (1.13) ABC: Irritability: 12.00 (5.89) SRS-2: Social motivation: 66.75 (12.39) |
GAS: Primary goal: 0.00 (1.22) Average of all goals: −0.48 (1.03) ABC: Irritability: 15.53 (6.84) SRS-2: Social motivation: 71.00 (7.86) |
GAS: Primary goal: p < 0.001 Average of all goals: p < 0.001 ABC: Irritability: p = 0.040 SRS-2: Social motivation: p = 0.033 |
| Zoccante, L., 2021, [25] |
Adaptive behavior, neuromotor function, and parent–child interaction. |
W | Qualitative research | Greater adaptive behavior and coordination with increasing complexity of positive behavioral support. | Reduced parental distress. | Vineland-II: Communication: 48.1, SE [6.5] Socialization: 55.5, SE [4.9] Daily living skills: 60.5, SE [5.0] Motor skills: 66.9, SE [8.3] PSI-SF: Total score: 86.4, SE [4.3] Parental distress: 30.5, SE [2.4] PCDI: 25.7, SE [1.4] Difficult child: 29.6, SE [1.7] DCDQ’07: Total score: 37.5, SE [2.4] |
Vineland-II: Communication: 57.5, SE [6.4] Socialization: 63.0, SE [5.4] Daily living skills: 72.5, SE [5.2] Motor skills: 83.6, SE [6.9] PSI-SF: Total score: 87.7, SE [6.0] Parental distress: 30.1, SE [2.6] PCDI: 26.2, SE [1.9] Difficult child: 32.4, SE [1.9] DCDQ’07: Total score: 40.2, SE [2.1] |
n/a | Vineland-II: Communication: EAAT p < 0.001 Socialization: EAAT p < 0.001 Daily living skills: EAAT p = 0.01 Motor skills: EAAT p < 0.001 PSI-SF: Total score: p = 0.67 Parental distress: p = 0.69 PCDI: p = 0.62 Difficult child: p = 0.03 DCDQ’07: Total score: p = 0.01 |
| Zhao, M., 2021, [40] |
Social behavior change; communication skills. |
B/W | Qualitative research | Overall social interaction, communication, responsibility, and self-control. | (-) | SISS: Social skills: IG: 44.68 ± 7.48, CG: 44.27 ± 4.31 Subdomains: Communication: IG: 6.71 ± 1.77, CG: 7.03 ± 1.54 Cooperation: IG: 7.55 ± 1.77, CG: 7.50 ± 1.41 Assertion: IG: 4.90 ± 1.58, CG: 4.63 ± 1.10 Responsibility: IG: 6.23 ± 1.23, CG: 5.87 ± 1.01 Empathy: IG: 5.42 ± 1.29, CG: 5.70 ± 1.02 Engagement: IG: 6.65 ± 1.45, CG: 6.47 ± 1.14 Self-control: IG: 7.23 ± 1.73, CG: 7.07 ± 1.53 ABLLS-R: Social interaction scores: IG: 24.03 ± 3.38, CG: 24.13 ± 3.59 |
SISS: Social skills: Interim: 48.26 ± 6.51, Post: 50.87 ± 6.47 Subdomains: Communication: Interim: 7.74 ± 1.55, Post: 8.48 ± 1.86 Cooperation: Interim: 7.97 ± 1.66, Post: 8.16 ± 1.73 Assertion: Interim: 5.23 ± 1.52, Post: 5.71 ± 1.47 Responsibility: Interim: 6.74 ± 1.21, Post: 7.00 ± 1.24 Empathy: Interim: 5.68 ± 1.19, Post: 5.90 ± 1.27 Engagement: Interim: 7.52 ± 1.36, Post: 7.68 ± 1.51 Self-control: Interim: 7.39 ± 1.75, Post: 7.94 ± 1.55 ABLLS-R: Social interaction scores: Interim: 27.74 ± 2.66, Post: 33.84 ± 4.00 |
SISS: Social skills: Interim: 45.13 ± 4.67, Post: 45.43 ± 5.08 Subdomains: Communication: Interim: 7.17 ± 1.53 Post: 7.27 ± 1.46 Cooperation: Interim: 7.57 ± 1.30 Post: 7.63 ± 1.22 Assertion: Interim: 4.80 ± 1.19, Post: 5.07 ± 1.39 Responsibility: Interim: 6.33 ± 1.21, Post: 6.13 ± 1.17 Empathy: Interim: 5.60 ± 1.10, Post: 5.53 ± 1.17 Engagement: Interim: 6.90 ± 1.09, Post: 7.03 ± 1.19 Self-control: Interim: 6.77 ± 1.55, Post: 6.77 ± 1.55 ABLLS-R: Social interaction scores: Interim: 25.60 ± 3.52 Post: 25.87 ± 3.05 |
SISS: Social skills: Compared with CG: p < 0.05 Compared with pretest: p < 0.01 ABLLS-R: Social interaction scores: Compared with CG: p < 0.01 Compared with pretest: p < 0.05 or p < 0.01 |
| Peters, B. C., 2020, [28] |
Performance goals, behavior, and social functioning. | n/a | Qualitative research | Four participants reported improvements in irritability and hyperactivity. | Two participants reported improvements in irritability and hyperactivity. | n/a | n/a | n/a | n/a |
| Kalmbach, D., 2020, [53] |
Parental perspectives on child’s experience of occupational therapy (one) and its impact on child’s (two) and family’s daily lives (three). | n/a | Qualitative research | Occupational performance, social motivation, social communication, and self-regulation. | (-) | n/a | n/a | n/a | n/a |
| Ozyurt, Gonca., 2020, [54] |
Social functioning, autistic behaviors, family functioning, and clinical severity. | B/W | Qualitative research | ASD severity and improvements in maternal mental health and family functioning. | Responsiveness and general functions. | CGAS: 57 ± 9.24 FAD: Communication: 2.5 ± 0.52 Role subscale: 2.31 ± 0.59 Involvement: 2.38 ± 0.58 Behavioral control: 2.23 ± 0.55 SCQ: 19.92 ± 4.12 BDI: 18.5 ± 6.31 |
CGAS: 61.83 ± 11.47 FAD: Communication: 2.2 ± 0.59 Role subscale: 1.88 ± 0.38 Involvement: 1.93 ± 0.59 Behavioral control: 1.93 ± 0.38 SCQ: 18.25 ± 3.70 BDI: 16.25 ± 5.46 |
FAD: Involvement: 2.42 ± 0.56 Behavioral control: 2.35 ± 0.47 |
CGAS: p = 0.0004 FAD: Communication: p = 0.001 Responsiveness: p > 0.05 Involvement: p = 0.01 Behavioral control: p = 0.01 General functions: p > 0.05 SCQ: p = 0.002 BDI: p = 0.0001 |
| Kwon, S., 2019, [55] |
Language function; cognitive function; intelligence and achievement. |
W | Qualitative research | Significant improvements in receptive and expressive language and cognitive functions. | (-) | REVT Reception: IG: 17.44 ± 19.97, CG: 13.82 ± 18.81 BSID of Cognitive Domain: IG: 130.38 ± 21.87, CG: 136.00 ± 19.51 |
REVT Reception: 20.11 ± 20.84 BSID of Cognitive Domain: 133.69 ± 23.29 |
REVT Reception: 15.27 ± 18.12 BSID of Cognitive Domain: 138.33 ± 20.20 |
All domains statistically significant (p > 0.05) |
| Pan, Z., 2018, [56] |
Adaptive skills and aberrant and social behaviors. | B/W | Qualitative research | Significant improvements in hyperactivity, social awareness, irritability, and communication behaviors. | Words or new words spoken. | ABC-C: Hyperactivity: IG 20.86 (12.13), CG 17.33 (4.46) SRS: Social awareness: IG 15.43 (3.95), CG 12.29 (2.56) Social communication: IG 41.00 (9.33), CG 29.29 (9.83) |
ABC-C: Hyperactivity: 16.00 (8.64) SRS: Social awareness: 11.29 (1.38) Social communication: 34.57 (3.95) |
ABC-C: Hyperactivity: 24.33 (6.02) SRS: Social awareness: 13.57 (4.12) Social communication: 31.29 (10.98) |
ABC-C: Hyperactivity: p = 0.04 SRS: Social awareness: p = 0.01 Social communication: p = 0.03 |
| Gabriels, R. L., 2018, [57] |
Irritability, hyperactivity, social and communication behaviors. | B/W | Qualitative research | Reduction in irritability behavior Significant improvement in social and communication behaviors. |
(-) | Irritability: IG 15.86 (9.52), CG 14.43 (8.69) Hyperactivity: IG 20.75 (20.71), CG 20.71 (20.75) |
Irritability: 9.00 (8.08) Hyperactivity: 13.28 (17.07) |
Irritability: 11.96 (9.29) Hyperactivity: 17.07 (13.28) |
Irritability: p < 0.02; after 6 months: p = 0.52 Hyperactivity: p = 0.08; after 6 months: p = 0.2 |
| Tan, V. X., 2018, [58] |
Psychosocial outcomes. | n/a | Qualitative research | Self-concept, emotional wellbeing, self-regulatory ability, social benefits. | (-) | n/a | n/a | n/a | n/a |
| Harris, A., 2017, [59] |
Symptomology and social functioning. | B/W | Qualitative research | Significant reduction in ASD symptom severity and hyperactivity. | Irritability, lethargy, stereotype, inappropriate speech. | CARS-2: IG 40.95 (6.07), CG 42.61 (7.52) ABC-C: Hyperactivity: IG 26.30 (10.73), CG 21 (1.07) |
CARS-2: 40.05 (5.57) ABC-C: Hyperactivity: 22.30 (9.67) |
CARS-2: 42.61 (7.52) ABC-C: Hyperactivity: 21 (11) |
CARS-2: p = 0.013, ES = 0.5 ABC-C: Hyperactivity: p = 0.009, ES = 0.518 |
| Anderson, S., 2016, [60] |
Social functioning; behavior skills. |
B/W | Qualitative research | Increasing empathy and reduction in maladaptive behaviors. | Communicative, socialization, systemizing quotient. | ||||
| Borgi, M., 2016, [58] |
Adaptive and executive functioning. | B/W | Qualitative research | Social and executive functioning. | (-) | VABS: Socialization: p = 0.034 Motor skills: p = 0.021 TOL: Planning time: p = 0.026 |
|||
| Gabriels, R. L., 2015, [30] |
Self-regulation; socialization; communication; adaptive, and motor behaviors. |
B/W | Qualitative and quantitative research | Significant improvement in irritability and hyperactivity; social cognition and communication; total number of words and new words. |
Lethargy/social withdrawal, stereotyping, inappropriate speech, social awareness, social motivation, autistic mannerism. | ABC: Irritability: IG 16.0 (9.84), CG 16.1 (9.80) Hyperactivity: IG 21.9 (10.7), CG 21.0 (9.69) SRS: Social cognition: IG 20.3 (5.63), CG 19.3 (5.58) Social communication IG 36.8 (10.04), CG 33.9 (8.84) SALT: Number of different words used: IG 104.6 (58.45), CG 119.1 (64.55) Number of words used: IG 219.2 (132.19), CG 277.6 (171.53) |
ABC: Irritability: 9.5 (7.98) Hyperactivity: 14.3 (9.66) SRS: Social cognition: 17.6 (5.55) Social communication 30.2 (8.75) SALT: Number of different words used: 116.7 (66.00) Number of words used: 253.7 (154.62) |
ABC: Irritability: 13.6 (10.08) Hyperactivity: 18.4 (10.26) SRS: Social cognition: 19.1 (5.64) Social communication 33.6 (11.38) SALT: Number of different words used: 118.4 (62.75) Number of words used: 270.5 (162.88) |
Regulation of irritability: ES = 0.5, p = 0.002 Hyperactivity: ES = 0.53, p = 0.001 Social cognition: ES = 0.41, p = 0.05 Social communication: ES = 0.63, p = 0.003 Total number of words: ES = 0.54, p = 0.01 New words: ES = 0.54, p = 0.01 |
| Steiner, H., 2015, [62] |
Communication, self-care, motor skills, and socialization. | B/W | Qualitative and quantitative research | Communication, self-care, motor skills, and socialization. | |||||
| Holm, M. B., 2014, [63] |
Parent-nominated target behaviors. |
W | Qualitative research | Positive behaviors to be increased included eye contact, verbalization, and naming of people/items. |
|||||
| Lanning, B. A., 2014, [42] |
Social and emotional functioning. | B/W | Qualitative research | Physical, emotional, and social functioning. | Emotional and social functioning. | ||||
| Ward, S. C., 2013, [38] |
Social communication and sensory processing skills. | W | Qualitative and quantitative research | Social interaction, sensory processing, and decreased severity of symptoms associated with ASD. | (-) | ||||
| Jenkins, Sarah R., 2013, [64] |
Behavior | n/a | Qualitative and quantitative research | N | No clinically significant effects on mood, off-task behavior, problem behavior, compliance, or language. | n/a | n/a | n/a | n/a |
| Ghorban, Hemati., 2013, [65] |
Social skills | W | Quantitative research | Initiating interactions and substantially maintaining interactions. | Responding interaction | n/a | n/a | n/a | Total score of social skills: sig. = 0.04 Subtest: Affective understanding/perspective taking and initiating interaction: sig. = 0.01 Maintaining interaction: sig. = 0.003 |
| Gabriels, Robin L., 2012, [66] |
Self-regulation, adaptive living skills, motor skills. | B/W | Quantitative research | Self-regulation behaviors | Receptive language | ABC-C: Irritability 20.2 ± 8.9 Lethargy 12.4 ± 7.7 Stereotypy 6 ± 4.2 Hyperactivity 23.7 ± 9.9 Adaptive skills: Raw social score 104.9 ± 29.9 Raw communication score 143.6 ± 24.9 Raw daily score 110.6 ± 35.1 Adaptive total score 75.5 ± 10.4 Motor skills BOT: 2 45.5 ± 15.5 SIPT: verbal score: 16 ± 7.2 SIPT: postural score: 19.5 ± 7.4 |
ABC-C: Irritability: 12.9 ± 8.5 Lethargy: 6.3 ± 7.1 Stereotypy: 3.3 ± 3.5 Hyperactivity: 17.1 ± 11.6 Adaptive skills: Raw social score: 113.2 ± 27.4 Raw communication score: 149 ± 24.8 Raw daily score: 117.4 ± 32.6 Adaptive total score: 79.2 ± 11.3 Motor skills BOT 2: 53.4 ± 15.2 SIPT: verbal score: 18.8 ± 7 SIPT: postural score: 22.9 ± 7.1 |
ABC-C: Irritability, lethargy, stereotypy, hyperactivity: p < 0.001 Adaptive skills: Raw social score: p = 0.016 Raw communication score: p = 0.035 Raw daily score: p = 0.011 Adaptive total score: p < 0.001 Motor skills BOT 2: p < 0.001 SIPT: verbal score: p < 0.001 SIPT: postural score: p = 0.009 |
|
| Tabares, C., 2012, [67] |
Hormonal changes | n/a | Quantitative research | Decreased salivary cortisol levels and increased progesterone. | (-) | Hormone cortisol: pre-hippotherapy: 2.79 ± 0.52 ng/mL Hormone progesterone: pre-hippotherapy: 28.63 ± 12.81 pg/mL |
Hormone cortisol: post-hippotherapy: 4.015 ± 1.59 ng/mL The rest of the post-hippotherapy sessions: 2.23 ± 0.75 ng/mL Hormone progesterone: post-hippotherapy: 51.59 ± 33.11 pg/mL The rest of the sessions: 26.03 ± 11.98 pg/mL |
n/a | All domains statistically significant (p ≤ 0.05) |
| Janet K Kern., 2011, [68] |
Severity of autism symptoms, parent–child interactions. |
W | Quantitative research | Severity of autism symptoms. | Parent–child interactions. |
||||
| Bass, M. M., 2009, [69] |
Social functioning | B/W | Qualitative and quantitative research | Social function, greater sensory seeking, sensory sensitivity, social motivation, less inattention, distractibility, and sedentary behaviors. | Social cognition and social awareness. | ||||
| Taylor, Renee R., 2009, [70] |
Motivation | n/a | Qualitative research | Volition | (-) | n/a | n/a | n/a | n/a |
Abbreviation: B: between-group effect; W: within-group effect; CI: confidence interval; IG: intervention group; CG: control group.
Table 7.
Assessor types.
| First Author, Year |
Type of Assessment |
Information Sources | Blinding of Assessors |
Raters/Informants/ | |||
|---|---|---|---|---|---|---|---|
| Authors/ Research Staff |
Parent/ Caregiver |
Staff/Instructor at Horse Center | Independent Raters |
||||
| Peters, B. C., 2022, [43] | Expert and parent questionnaires/semi structured interviews/physiological measures | Parents, occupational therapists, authors | Partly Blind | Y | OTs | OTs | |
| Zoccante, L., 2021, [25] | Parent questionnaires | Caregiver, clinical psychologist | (-) | Clinical psychologist | |||
| Zhao, M., 2021, [40] | Parent/expert questionnaires | Teachers at training center and parents | Nonblind | Y | Y | ||
| Peters, B. C., 2020, [28] | Parent/expert questionnaires/visual analog scale | Parents | Nonblind | Y | |||
| Kalmbach, D., 2020, [53] | Semi structured interviews | Parents | Nonblind | Y | Y | ||
| Ozyurt, Gonca., 2020, [54] | Clinical and parent questionnaires | Parents and educators | Blind | Y | Clinician/educator | ||
| Kwon, S., 2019, [55] | Expert questionnaires/Luria model, battery | Speech and occupational therapists | (-) | Speech and occupational therapists | |||
| Pan, Z., 2018, [55] | Expert and parent questionnaires/saliva sample test | Caregiver, study personnel, speech therapist |
Partly Blind | Y | Y | Speech therapist | |
| Gabriels, R. L., 2018, [57] |
Parent/expert questionnaires | Caregiver, speech therapist | Partly Blind | Y | Speech therapist | ||
| Tan, V. X., 2018, [58] |
Semi structured interviews | Parents | Nonblind | Y | |||
| Harris, A., 2017, [59] |
Expert questionnaires | School teaching staff | (-) | School teaching staff | |||
| Anderson, S., 2016, [60] |
Parent-reported questionnaires and semi structured tests | Parents | (-) | Y | |||
| Borgi, M., 2016, [61] |
Interviews | Parents | Blind | Y | |||
| Gabriels, R. L., 2015, [30] |
Parent/expert questionnaires | Study personnel, speech therapist, caregiver | Blinding to study personnel Unblinded caregiver questionnaires |
Y | Y | ||
| Steiner, H., 2015, [62] |
APAS equipment/special test | Authors | Nonblind | Y | |||
| Holm, M. B., 2014, [63] |
Parent evaluation/ caregiver questionnaire/ videotaped and observed measures |
Parents/caregiver/ leader |
Nonblind | Y | Y | Y | |
| Lanning, B. A., 2014, [42] |
Parent questionnaires | Parents/children | Nonblind | Y | |||
| Ward, S. C., 2013, [38] |
Parent rating/questionnaires | School group teacher and coordinator | Blind | School teacher | |||
| Jenkins, Sarah R., 2013, [64] |
Observational assessment/parent questionnaires | Parents/observers/teachers | (-) | Y | |||
| Ghorban, Hemati., 2013, [65] |
Parent questionnaires | Parent or teacher | (-) | Y | |||
| Gabriels, Robin L., 2012, [66] |
Parent questionnaires | Parents/legal guardians, graduate student research assistants, occupational therapists | Nonblind | Y | Y | Y | |
| Tabares, C., 2012, [67] |
Laboratory methods | Research staff | Nonblind | Y | |||
| Janet K Kern., 2011, [68] |
Parent- and clinician-rated measures | Research assistant/parents | Blind | Y | Research assistant | ||
| Bass, M. M., 2009, [69] |
Social and communication skills | Parents or teachers | Blind | Y | School teacher | ||
| Taylor, Renee R., 2009, [70] |
Observational assessment tool | OTgs | Blind | Y | OTgs | ||
Abbreviations: (-): not reported; Y: yes; N: no; OTs: occupational therapies; OTgs: occupational therapy graduate students; APAS: Ariel Performance Analysis System.
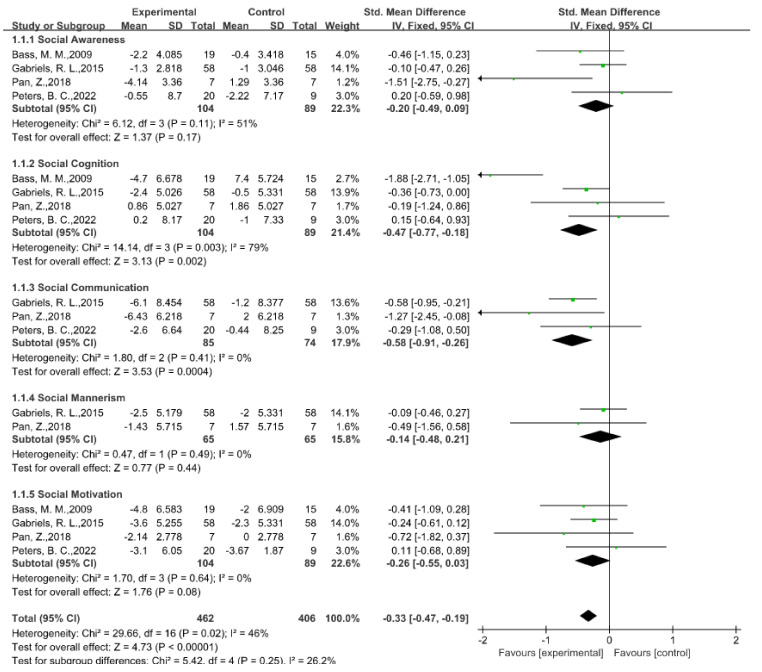
5.3. Social Functioning
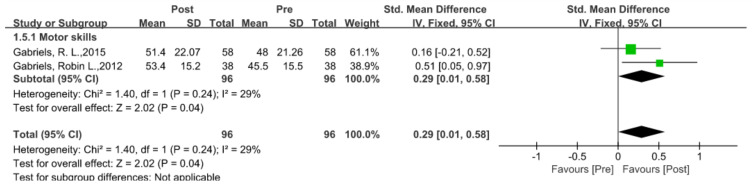
The social communication domain was the most crucial aspect of the generally reported autism impairment, with a total of 13 studies (52%) in which the authors describe the social and communication abilities following EAAT programs, using seven different standardized measures rated by parents or caregivers. In all the reported testing in the social domains, in five studies, the authors found considerable improvements in the overall SRS scores; however, the findings differed among the SRS subscales. More specifically, we analyzed the effects of EAAT programs on the social communication symptoms connected to ASD from four studies in which the authors provide exact data using the SRS [27,37,50,53]. According to the meta-analysis results, the EAATs more significantly improved the social functioning of the children with ASD than that of those in the control group (SMD = −0.33, 95% CI [−0.47, −0.19], p < 0.00001) (see Figure 3 for details). Among the five subdomains, social cognition (SMD = −0.47, 95% CI [−0.77, −0.18], p = 0.002) and social communication (SMD = −0.58, 95% CI [−0.91, −0.26], p = 0.0004) had significant improvements after the participants experienced EAAT programs; however, social awareness (SMD = −0.20, 95% CI [−0.49, 0.09], p = 0.17), social mannerisms (SMD = −0.14, 95% CI [−0.48, 0.21], p = 0.44), and social motivation (SMD = −0.26, 95% CI [−0.55, 0.03], p = 0.08) had no statistically significant differences. The findings of the meta-analysis are in line with those of earlier studies in which the authors found that the parents believed that their children were more driven to complete daily tasks and got along better with others after participating in the EAATs [47,63,69]. However, the outcomes of the subdomain improvements reported across the trials were not totally uniform in all the research. For instance, in a 2018 study with 14 samples [56], the authors found that EAAT substantially enhanced the social awareness domain in autistic children; however, based on the forest plot and according to the outcomes of the other three trials, it was not effective at enhancing the social awareness domain. In the studies on subdomains such as social cognition, the authors also report different results, which constitutes heterogeneity in the subdomains and overall.
The remaining six scales used to assess the social communication domain level include the SCQ, ABLLS-R, PVQ, CARS, TSSA, and Social Skills Improvement System (SSIS) rating scales. In a 2020 study [54] in which the authors utilized the SCQ, they demonstrated that the experimental group exhibited a considerable improvement in the social domain, while the control group had no substantial changes after 8 weeks of EAA. This was followed by a study in 2021 [40] in which the authors utilized the ABLLS-R and SSIS. They revealed substantial improvements in the social communication domain in the experimental group compared with the conventional group over 16 weeks. These findings, presented using multiple scales, were also largely compatible with the results displayed in the forest plot, which indicates that, in general, EAAT programs improve the social communication domains of individuals with ASD; however, the aspect and improvement degrees of the EAAT on the participants differed based on numerous parameters.
5.4. Language Ability
As another area of high impairment for individuals with autism, in four studies, the authors made use of four different standard assessments and reported improvements in the language skills of individuals with autism after trials. These four measures included the following: the Receptive and Expressive Vocabulary Test (REVT) (n = 1) [55]; the Preschool Receptive–Expressive Language Scale (PRES) (n = 1) [55]; the Systematic Analysis of Language Transcripts (SALT) (n = 3) [27,53,56]; the Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4) (n = 1) [30].
Following the extraction of the raw data from a meta-analysis of two trials using the SALT, the experimental group’s language abilities had significantly improved compared with those of the comparison group following EAAT programs (SMD = 0.52, 95% CI [0.27, 0.77], p < 0.0001) (see Figure 4 for details). Although the 2018 trial [56] did not reveal significant gains in the two subdomains, the number of different words domain and number of words domain, according to another follow-up experiment performed in 2018 [57], there were substantial improvements in the two subdomains, and the authors also offer some evidence of the lasting effects of EAAT programs on enhancing the language abilities of people with autism, as they again measured the results with the SALT six months later and revealed non-substantial declines in the two subdomains. In another 2019 study in which the authors employed the REVT [55], they found considerable gains in the receptive linguistic knowledge. Overall, there is limited evidence to suggest that EAAT programs improve the language skills of individuals with ASD.
Figure 4.
Figure 3.
5.5. Behavioral Regulation
In a 2014 study, the authors found that self-regulation in children was linked to improvements in the executive function, sensory processing, and emotion regulation, all of which led to behavior problems [71]. Therefore, measuring and assessing the self-regulation and behavior of individuals with autism is one of the universal criteria for measuring ASD severity. In this review, we used seven different measurement tests to determine the behavioral regulation scores. The most common measurement standards for measuring the intervention effects on people with ASD are the ABC-C (n = 9) and VABS (n = 5). The five remaining scales and instruments that researchers used to measure behavioral competence are as follows: the ABAS-3 (n = 1) [28]; the Autism Spectrum Disorder Module (PEDI-CAT ASD) (n = 1) [43]; the Interaction Emotions and Motor Skills observational scale (IEMS) (n = 1) [25]; the Child Health Questionnaire (CHQ) (n = 1) [42]; the Child Behavior Checklist (CBC) (n = 1) [64]; physiological measures using salivary or hair cortisol (n = 3) [37,53,58]. These tools have high interverbal consistencies, good reliabilities, and well-established validities.
Specifically, five studies in which the authors used the ABC-C [27,37,53,56,59] provided us with sufficient and reliable data to conclude that the behavioral competence of individuals with ASD significantly improved after EAAT programs compared with the control group (SMD = −0.30, 95% CI [−0.42, −0.17], p < 0.00001) (see Figure 5 for details). Based on the subdomain analyses, the irritability (SMD = −0.44, 95% CI [−0.70, −0.19], p = 0.0007) and hyperactivity (SMD = −0.43, 95% CI [−0.69, −0.17], p = 0.001) had statistically significant improvements; however, there were no significant improvements in terms of lethargy (SMD = −0.21, 95% CI [−0.53, 0.11], p = 0.19), stereotypy (SMD = −0.04, 95% CI [−0.36, 0.27], p = 0.79), or inappropriate speech (SMD = −0.20, 95% CI [−0.52, 0.12], p = 0.21).
Figure 5.
Forest plot of behavioral function using ABC-C [30,43,56,57,59].
The remaining four studies [27,49,61,62] in which the authors used the VABS lacked controlled trials; however, they do report pre- and post-trial data. We also used the fixed-effects model with 95% confidence intervals that the participants’ behavioral competency would substantially increase following the EAAT programs (SMD = 0.22, 95% CI [0.09, 0.35], p = 0.0008) (see Figure 6 for details). Participants also demonstrated appreciable improvements in the socialization subdomain following EAATs (SMD = 0.27, 95% CI [0.03, 0.52], p = 0.03). However, the data do not indicate any appreciable improvement for the other three domains: communication (SMD = 0.18, 95% CI [−0.06, 0.43], p = 0.15); daily living skills (SMD = 0.20, 95% CI [−0.06, 0.46], p = 0.13); adaptive behavior (SMD = 0.24, 95% CI [−0.06, 0.54], p = 0.11).
Figure 6.
Forest plot of behavioral functioning (pre-post design) using VABS [25,30,60,66].
The results of the meta-analysis are basically consistent with the conclusions reported in previous studies. In five previous studies [25,36,49,52,55], the authors found that EAATs led to considerable improvements in self-control and decreases in negative behaviors in the experimental groups. However, in a 2013 [64] study in which the authors used direct observation to measure the effects, they found that a 9-week THR program did not substantially improve the mood, behavior, or communication abilities. Overall, there were enough data to conclude that EAAT programs enhance the behavioral abilities of ASD populations, despite the varied reports among the subfields.
5.6. Motor and Sensory Functions
In recent evidence-based investigations, the authors reveal that individuals with ASD exhibit motor and sensory deficits throughout their lifespans [49,72]. In nine studies, the authors implemented six different standard measures to assess the impacts of the EAAT programs on the motor and sensory functions of individuals with autism. Of these standard measures, researchers most frequently use the SP (n = 4) [25,50,51,55], BOT-2 (n = 2) [30,66], and SIPT (n = 2) [30,66]. The remaining scales include the CGAS (n = 1) [54], Developmental Coordination Disorder Questionnaire’07 (DCDQ’07) (n = 1) [25], and Sensory Profile School Companion (SPSC) (n = 1) [38]. In two studies [30,66], the authors employed the SIPT and BOT-2, which indicated changes in the motor and sensory functioning of children with ASD following their participation in EAATs; however, there was only one study in which the authors used a control group [30]. Thus, we compared the pre- and post-changes, revealing that the patients with autism who participated in EAAT programs appeared to have experienced significant improvements in their motor (SMD = 0.29, 95% CI [0.01, 0.58], p = 0.04) (see Figure 7 for details) and sensory (SMD = 0.29, 95% CI [0.10, 0.48], p = 0.003) abilities (see Figure 8 for details). Nevertheless, among the subcomponents, the verbal function did not significantly improve (SMD = 0.21, 95% CI [−0.07, 0.50], p = 0.14). Only the postural subdomain improved (SMD = 0.36, 95% CI [0.10, 0.62], p = 0.007). In three previous investigations [33,62,64], the authors confirmed the findings of the meta-analysis and demonstrated that the motor and sensory functioning domains substantially improved from the pre- to post-test in the experimental groups. However, according to another two studies [63,69], the EAAT programs had no clinical effects for ASD in this area. Overall, because only two articles provided enough data for the meta-analysis, there was only limited evidence that patients with autism who participate in EAAT programs have substantially improved outcomes in their motor and sensory functioning.
Figure 7.
Forest plot of motor function (pre-post comparison) using BOT-2 [30,66].
Figure 8.
Forest plot of sensory function (pre-post comparison) using SIPT [30,66].
5.7. Cognitive and Executive Functions
Although social cognition and executive functioning are not regularly examined features of ASD, in two articles in this review, the authors employed three different scales to evaluate the effects of the EAAT program on individuals with autism in these two domains: (1) the Kaufman Assessment Battery for Children II (K-ABC-II) (n = 1) [55]; (2) the Bayley Scales of Infant Development II (BSID-II) (n = 1) [55]; (3) the Tower of London (TOL) (n = 1) [61]. In a 2019 study [55], the authors indicated that the cognitive domains of the THR group substantially improved following an 8-week intervention compared with those preintervention; however, there was no statistically significant difference between the THR group and control group receiving conventional therapy. In a 2016 study [61], the authors found that the planning time for the problem-solving test decreased following the intervention, which likely indicated that EAT can improve the executive function in ASD patients. However, there was insufficient evidence from the two studies to conclude that EAAT training can substantially enhance the social cognitive and executive domains.
5.8. Family Functioning
In the previous literature, the authors highlight the importance of parental engagement and improved caregiver–child connections to enhancing the intervention outcomes [73], and consequently, family functioning is an essential indicator for assessing ASD [74]. In this review, in four out of twenty-five studies, the authors reported the family function outcomes using two different standardized assessments: (1) the Parenting Stress Index—Short Form (PSI-SF) (n = 1) [25] and (2) the McMaster Family Assessment Device (FAD) (n = 1) [54]. In two previous qualitative studies, the authors demonstrated that the EAAT programs were beneficial to family activities for children with autism, resulting in reduced parental stress [53,58]. In a 2020 study [54], the authors confirm the prior finding and offer preliminary evidence that EAAT with children with ASD has considerable benefits for family functioning; however, in a 2021 study [25], the authors suggest that EAAT did not reduce the parent distress after the intervention. Reports on the effects of EAAT programs on the families or parents of people with autism are still rare. While in three articles the authors preliminarily suggest that parental stress may be reduced by EAAT programs due to their therapeutic efficacy, in another article, the authors suggest the opposite. As a result, this evidence was limited, and we hope that researchers will quantitatively examine this crucial factor in future studies.
5.9. Other Skills
In addition to the above indicators, in eight studies, the authors report other helpful metrics that were hard to attribute to the above subdomains but nevertheless provided reliable qualitative or quantitative results. In four studies [37,53,58,59], the authors used laboratory methods that assessed the participant interaction and physiological state, as well as saliva or hair collection to measure the amount of cortisol present, with three out of the four studies providing data from baseline and after the intervention. According to the analysis, the data from the existing studies support that EAAT programs can significantly reduce the cortisol levels in participants (SMD = 0.53, 95% CI [0.02, 1.05], p = 0.04) (see Figure 9 for details). However, in a 2017 study [59], the authors report that there was no evidence to indicate a substantial improvement after the intervention. In conclusion, due to a lack of additional experimental and compared evidence, the veracity and reliability of the conclusion that EAAT programs can substantially reduce the cortisol levels in participants with ASD need to be questioned.
Figure 9.
The other assessment methods included semi structured interviews, such as the Canadian Occupational Performance Measure (COPM) (n = 1) [28] and Goal Attainment Scaling (GAS) (n = 1) [43], which revealed that, after receiving EAATs, the participants were more effective at achieving their stated performance goals. The result was similar to that of a previous 2015 study [62], in which the authors used the Pedagogical Analysis and Curriculum (PAC) test. Additionally, the authors used the empathizing–systemizing quotients (EQ/SQ) (n = 1) [60], Ariel Performance Analysis System (APAS) (n = 1) [62], and Pediatric Quality of Life (PedsQL) 4.0 generic score scales (n = 1) [42] to evaluate the different aspects of the effects after the EAAT programs. In a 2018 study, the authors noted the advantages of ecopsychology following EAI for both the parents and children [58].
5.10. Persistence of Intervention Effect
Although in many studies, the authors report the considerable intervention effects of EAAT in many areas for individuals with autism, in only a few trials have the authors examined whether these effects were sustained. The sustainability of the intervention effects is a key factor in assessing the overall effectiveness of the intervention. In one study [38], the authors reveal that EAATs led to short-term improvements; however, the children’s behavior returned to baseline once the intervention ended. However, in another study [75], the authors discovered sustained behavioral gains even after the equine intervention was completed. Overall, the inconsistent information on the long-term benefits of EAATs makes it difficult to draw solid conclusions about the persistence of the therapeutic effects after interventions in ASD.
6. Discussion
Summary of Results
The purpose of conducting this review was to use systematic review and meta-analysis techniques to evaluate the effectiveness of EAAT as a supplementary treatment for individuals with ASD. In our review, we combined qualitative and quantitative methodologies to assess two main aspects: (1) the social and communication functioning and (2) self-regulation functioning, and we objectively assessed the effectiveness of EAAT as an adjunctive therapy for ASD.
We selected 25 articles from 382, and of the 25 included studies, sixteen trials included control groups, and nine trials did not. For the study quality, we used the Sackett level of evidence, PEDro, and Cochrane’s “Risk of Bias Table”. According to the results, 11 of the 25 studies had qualities of Ⅰ and Ⅱ, only one study had a quality of Ⅴ, and the remaining 13 studies had qualities of Ⅲ and Ⅳ. Seven of the eight RCTs included in this study had scores of 6 or above, and one study had a score of 5. However, of all the included trials, we only assessed ten studies that were blinded or partially blinded, and in future trials, researchers should be blinded to ensure the accuracy of the measurements.
For most of the trials in all 25 included articles, the authors report the EAAT effects on multiple subsystems in ASD. As shown in Table 7, the social and behavioral domains were the most assessed for ASD, and while it appeared from the review that the EAAT programs substantially improved the social and behavioral skills of the participants with autism, some researchers reached the opposite conclusion, especially in terms of the subdomains, such as social awareness, social mannerisms, and social motivation, for which the meta-analysis revealed no substantial improvements. Moreover, there were no substantial improvements in the lethargy and stereotypes subdomains. The possible reasons for this were bias due to the length of the treatment, differences in the sites, or small samples. Although we did not have sufficient evidence on the program duration that provides the best treatment outcome for participants, according to a 2020 study [28], adolescents need weeks or months to learn new skills and change their behaviors, which suggests that interventions should be longer than 5 weeks. Similarly, according to the meta-analyses, EAAT programs substantially improve the skills in individuals with autism; however, in only two studies do the authors provide enough raw data that researchers should consider the EAAT program effects on language skills in individuals with autism in subsequent studies whenever possible. In the remaining four meta-analyses, because the authors did not set up control groups or obtain data from control groups in the original experiments, although their results indicate that EAAT programs substantially improve the motor and perceptual function and reduce the cortisol levels in patients with autism, this conclusion needs to be supported by future trials in which the researchers include control group data.
7. Conclusions
In this review, we utilized systematic review and meta-analysis methodologies to explore the findings of 25 studies on the impact of EAAT programs on individuals with ASD. The included studies provided us with sufficient data to draw the conclusion that EAAT programs substantially improve the social and behavioral functions in people with ASD, which is broadly in line with other research findings. The results also indicate substantial improvements in the language abilities and motor and sensory functioning. However, in only 11 studies did the authors achieve a quality rating of Ⅱ or higher, while the qualities of the remaining 14 studies still need to be improved, especially in terms of the following: the lack of control conditions; small sample sizes; unknown inclusion criteria; inability to randomly assign experimental and control groups; inability to set up control groups; dependence on parental assessment, single-assessment methods, etc. In addition, the effect assessment of the study should be stretched over a longer period, considering whether various aspects of the participants’ functioning are maintained after a considerable period. We hope that researchers will provide more evidence in the future.
Appendix A
Appendix A.1. Database Search Terms
(“Autism Spectrum Disorder” or “Autism Spectrum Disorders” or “Autistic Spectrum Disorder” or “Autistic Spectrum Disorders” or “Disorder, Autistic Spectrum” or “ASD” or “ASDs”) AND (“Equine-Assisted Therapy” or “Equine Assisted Therapy” or “Equine-Assisted Therapies” or “Therapy, Equine Assisted” or “Equine-assisted activities” or “Equine Assisted Psychotherapy” or “Equine Assisted Psychotherapy” or “Equine Assisted Psychotherapies” or “Hippotherapy” or “Recreational Horseback Riding Therapy” or “Horseback Riding Therapy” or “Horseback Riding Therapies” or “Therapy, Horseback Riding” or “Equine Facilitated Therapy” or “Horse-riding”).
Appendix A.2. List of Excluded Articles after Screening Full Texts
Table A1.
Excluded Studies.
| First Author, Year |
Details | Reason for Exclusion |
|---|---|---|
| B. Cailtlin Peters, 2022 | Therapeutic Horseback Riding Social Functioning Outcomes in Youth with Autism Spectrum Disorder. Frontiers in Pediatrics, 10, 884054. | Secondary mediation analysis of previous publication |
| Maresca G, 2022 | Hippotherapy in neurodevelopmental disorders: a narrative review focusing on cognitive and behavioral outcomes. Appl Neuropsychic Child, 11(3):553–560. | Narrative review |
| Mureșanu IA, 2022 |
Evaluation of post-traumatic stress disorder (PTSD) and related comorbidities in clinical studies. J Med Life, 15(4):436–442. | PTSD and without equine-assisted therapy |
| Solgi M, 2022 |
Challenging Case: The Role of Genetic Testing in Complex Autism. J Dev Behav Pediatr, 43(1):60–62. | Nonequine therapy |
| Chinatsu Hayashibara, 2022 | The Potential and Effects of Equine-Assisted Activities in a Day Care Center for Children and Adolescents with Developmental Disorders. Occupational Therapy in Mental Health, DOI: 10.1080/0164212X.2022.2069200 | Not designed for ASD |
| Steffanie Burk, 2022 |
Therapeutic Riding or Mindfulness: Comparative Effectiveness of Two Recreational Therapy Interventions for Adolescents with Autism. Journal of Autism and Developmental Disorders, 52, p2438–2462. | With other therapy |
| Laura Contalbrigo, 2021 | Equine-Assisted Interventions (EAIs) for Children with Autism Spectrum Disorders (ASD): Behavioural and Physiological Indices of Stress in Domestic Horses (Equus caballus) during Riding Sessions. Animals, 11(6), 1562. |
Experiment designed to consider horse welfare |
| Claire C. St. Peter, 2021 | Comparing training methods to improve volunteer skills during therapeutic horseback riding: A randomized control trial. Journal of Applied Behavior Analysis, 54(3), 1157–1174. https://doi.org/10.1002/jaba.823 | Not designed for ASD |
| Portaro S, 2020 | Can Individuals with Down Syndrome Benefit from Hippotherapy? An Exploratory Study on Gait and Balance. Dev Neurorehabil, 23(6):337–342. | Individuals with Down syndrome |
| Lovrić, R., 2020 | Parental perception of changes in basic life needs of children with disabilities after six months of therapeutic horseback riding: A qualitative study. International Journal of Environmental Research and Public Health, 17(4), 1213. | Not designed for ASD |
| Temple Grandin, 2019 | Case Study: How Horses Helped a Teenager with Autism Make Friends and Learn How to Work. International journal of Environmental Research and Public Health, 16(13), 2325. | Case study |
| Shelef A, 2019 | Equine Assisted Therapy for Patients with Post Traumatic Stress Disorder: A Case Series Study. Mil Med, 184(9–10):394–399. | Post-traumatic stress disorder and case study |
| Chevalier C., 2019 | Autism and therapeutic mediation with bareback horse-riding. Increasing the quality of paternal holding and the emergence of the body ego. Neuropsychiatrie de l’Enfance et de l’Adolescence, 67 (1), 25–33. 10.1016/j.neurenf.2018.07.007 | Published in language other than English |
| Ogrinc M, 2018 | Horseback riding therapy for a deafblind individual enabled by a haptic interface. Assist Technol, 30(3):143–150. | Nonequine therapy |
| Guérin NA, 2018 | Reliability and Validity Assessment of the Observation of Human-Animal Interaction for Research (OHAIRE) Behavior Coding Tool. Front Vet Sci, 5:268. | Nonequine therapy |
| Vanessa Xue-Ling Tan, 2018 | Parent Perceptions of Psychosocial Outcomes of Equine-Assisted Interventions for Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders volume 48, pages759–769. | Commentary |
| Jessie D. Petty, 2017 | Therapeutic Horseback Riding Crossover Effects of Attachment Behaviors with Family Pets in a Sample of Children with Autism Spectrum Disorder. Int. J. Environ. Res. Public Health, 14(3), 256. | Experiment designed to consider horse welfare |
| Gonca Ozyurt, 2017 | The effect of therapeutic horseback riding for children diagnosed with autism spectrum disorder on autistic symptoms and the quality of life. ALPHA PSYCHIATRY, 18(6), 630–636. | Published in language other than English |
| Cerino S, 2016 | Equine-Assisted Intervention in a child diagnosed with autism spectrum disorder: a case report. Riv Psichiatr, 51(6):270–274. | Case report |
| Roslyn Malcolm, 2016 | ‘It just opens up their world’: autism, empathy, and the therapeutic effects of equine interactions. Medical Anthropology, 25(2), 220–234. | Interview and commentary |
| Tuba Tulay Koca, 2015 | What is hippotherapy? The indications and effectiveness of hippotherapy. North Clinics of Istanb, 2(3), 247–252. | Commentary |
| Jurecka A, 2014 | Attenuated adenylosuccinate lyase deficiency: a report of one case and a review of the literature. Neuropediatrics, 45(1):50–5. | Nonequine therapy |
| Bánszky N, 2012 | The psychiatric aspects of animal assisted therapy. Psychiatr Hung, 27(3):180–90. | Animal-assisted and psychiatric aspects |
| Wuang YP, 2010 | The effectiveness of simulated developmental horse-riding program in children with autism. Adapt Phys Activ Q, 27(2):113–26. | Simulated developmental horse-riding program |
| Memishevikj, H., 2010 | The effects of equine-assisted therapy in improving the psychosocial functioning of children with autism. The Journal of Special Education and Rehabilitation, 11(3), 57–67. | Publication in Russian and English |
| Hiromi KEINO, 2009 | Psycho-educational Horseback Riding to Facilitate Communication Ability of Children with Pervasive Developmental Disorders. Journal of Equine Science, 20(4), 79–88. | Not designed for ASD |
Author Contributions
Conceptualization, N.X.; Methodology, N.X. and K.S.; Software, N.X., X.H., B.L. and J.Q.; Validation, N.X., K.S. and S.K.; Formal analysis, N.X. and S.K.; Resources, N.X. and B.L.; Data curation, N.X., K.S. and S.K.; Original draft preparation, N.X.; Writing, N.X.; Supervision, S.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chiarotti F., Venerosi A. Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates Since 2014. Brain Sci. 2020;10:274. doi: 10.3390/brainsci10050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lord C., Charman T., Havdahl A., Carbone P., Anagnostou E., Boyd B., Carr T., de Vries P.J., Dissanayake C., Divan G., et al. The Lancet Commission on the future of care and clinical research in autism. Lancet. 2021;399:271–334. doi: 10.1016/S0140-6736(21)01541-5. [DOI] [PubMed] [Google Scholar]
- 3.Happé F., Frith U. Annual Research Review: Looking back to look forward—Changes in the concept of autism and implications for future research. J. Child Psychol. Psychiatry. 2020;61:218–232. doi: 10.1111/jcpp.13176. [DOI] [PubMed] [Google Scholar]
- 4.Mundy P., Newell L. Attention, Joint Attention, and Social Cognition. Curr. Dir. Psychol. Sci. 2007;16:269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruinsma Y., Koegel R.L., Koegel L.K. Joint Attention and Children with Autism: A Review of the Literature. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:169–175. doi: 10.1002/mrdd.20036. [DOI] [PubMed] [Google Scholar]
- 6.Ee D., Hwang Y.I., Reppermund S., Srasuebkul P., Trollor J.N., Foley K.-R., Arnold S.R. Loneliness in Adults on the Autism Spectrum. Autism Adulthood. 2019;1:182–193. doi: 10.1089/aut.2018.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matson J.L., Wilkins J., Macken J. The Relationship of Challenging Behaviors to Severity and Symptoms of Autism Spectrum Disorders. J. Ment. Health Res. Intellect. Disabil. 2008;2:29–44. doi: 10.1080/19315860802611415. [DOI] [Google Scholar]
- 8.Cai R.Y., Richdale A.L., Uljarević M., Dissanayake C., Samson A.C. Emotion regulation in autism spectrum disorder: Where we are and where we need to go. Autism Res. 2018;11:962–978. doi: 10.1002/aur.1968. [DOI] [PubMed] [Google Scholar]
- 9.Operto F., Smirni D., Scuoppo C., Padovano C., Vivenzio V., Quatrosi G., Carotenuto M., Precenzano F., Pastorino G. Neuropsychological Profile, Emotional/Behavioral Problems, and Parental Stress in Children with Neurodevelopmental Disorders. Brain Sci. 2021;11:584. doi: 10.3390/brainsci11050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazefsky C.A., Borue X., Day T.N., Minshew N.J. Emotion Regulation Patterns in Adolescents with High-Functioning Autism Spectrum Disorder: Comparison to Typically Developing Adolescents and Association with Psy-chiatric Symptoms. Autism Res. 2014;7:344–354. doi: 10.1002/aur.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabriels R.L., Agnew J.A., Miller L.J., Gralla J., Pan Z., Goldson E., Hooks E., Ledbette J.C., Dinkins J.P. Is There a Relationship between Restricted, Repetitive, Stereotyped Behaviors and Interests and Abnormal Sensory Response in Children with Autism Spectrum Disorders? Res. Autism Spectr. Disord. 2008;2:660–670. doi: 10.1016/j.rasd.2008.02.002. [DOI] [Google Scholar]
- 12.Jahromi L.B., Bryce C.I., Swanson J. The Importance of Self-Regulation for the School and Peer Engagement of Children with High-Functioning Autism. Res. Autism Spectr. Disord. 2013;7:235–246. doi: 10.1016/j.rasd.2012.08.012. [DOI] [Google Scholar]
- 13.Pellicano L., Bölte S., Stahmer A. The current illusion of educational inclusion. Autism. 2018;22:386–387. doi: 10.1177/1362361318766166. [DOI] [PubMed] [Google Scholar]
- 14.Nader-Grosbois N., Mazzone S. Emotion Regulation, Personality and Social Adjustment in Children with Autism Spectrum Disorders. Psychology. 2014;5:18. doi: 10.4236/psych.2014.515182. [DOI] [Google Scholar]
- 15.Lecavalier L., Leone S., Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J. Intellect. Disabil. Res. 2006;50:172–183. doi: 10.1111/j.1365-2788.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- 16.Brookman-Frazee L., Stadnick N., Chlebowski C., Baker-Ericzén M., Ganger W. Characterizing psychiatric comorbidity in children with autism spectrum disorder receiving publicly funded mental health services. Autism. 2017;22:938–952. doi: 10.1177/1362361317712650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend E.A. Boundaries and Bridges to Adult Mental Health: Critical Occupational and Capabilities Perspectives of Justice. J. Occup. Sci. 2012;19:8–24. doi: 10.1080/14427591.2011.639723. [DOI] [Google Scholar]
- 18.Stadnick N.A., Drahota A., Brookman-Frazee L. Parent Perspectives of an Evidence-Based Intervention for Children with Autism Served in Community Mental Health Clinics. J. Child Fam. Stud. 2012;22:414–422. doi: 10.1007/s10826-012-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant N., Rodger S., Hoffmann T. Intervention decision-making processes and information preferences of parents of children with autism spectrum disorders. Child Care Health Dev. 2015;42:125–134. doi: 10.1111/cch.12296. [DOI] [PubMed] [Google Scholar]
- 20.Marotta R., Risoleo M.C., Messina G., Parisi L., Carotenuto M., Vetri L., Roccella M. The Neurochemistry of Autism. Brain Sci. 2020;10:163. doi: 10.3390/brainsci10030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case-Smith J., Arbesman M. Evidence-Based Review of Interventions for Autism Used in or of Relevance to Occupational Therapy. Am. J. Occup. Ther. 2008;62:416–429. doi: 10.5014/ajot.62.4.416. [DOI] [PubMed] [Google Scholar]
- 22.Den Houting J. Neurodiversity: An Insider’s Perspective. Autism. 2019;23:271–273. doi: 10.1177/1362361318820762. [DOI] [PubMed] [Google Scholar]
- 23.Volkmar F., Siegel M., Woodbury-Smith M., King B., McCracken J., State M. Practice Parameter for the Assessment and Treatment of Children and Adolescents with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:237–257. doi: 10.1016/j.jaac.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Frye R.E., Rossignol D.A. Identification and Treatment of Pathophysiological Comorbidities of Autism Spectrum Disorder to Achieve Optimal Outcomes. Clin. Med. Insights Pediatr. 2016;10:CMPed.S38337. doi: 10.4137/CMPed.S38337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoccante L., Marconi M., Ciceri M., Gagliardoni S., Gozzi L., Sabaini S., Di Gennaro G., Colizzi M. Effectiveness of Equine-Assisted Activities and Therapies for Improving Adaptive Behavior and Motor Function in Autism Spectrum Disorder. J. Clin. Med. 2021;10:1726. doi: 10.3390/jcm10081726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito L., McCune S., Griffin J.A., Maholmes V. Directions in Human-Animal Interaction Research: Child Development, Health, and Therapeutic Interventions. Child Dev. Perspect. 2011;5:205–211. doi: 10.1111/j.1750-8606.2011.00175.x. [DOI] [Google Scholar]
- 27.Zhao M., Chen S., You Y., Wang Y., Zhang Y. Effects of Therapeutic Horseback Riding Program on Social and Communication Skills in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health. 2022;19:2656. doi: 10.3390/ijerph192114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters B.C., Wood W., Hepburn S., Bundy A. Pilot Study: Occupational Therapy in an Equine Environment for Youth with Autism. OTJR Occup. Particip. Health. 2020;40:190–202. doi: 10.1177/1539449220912723. [DOI] [PubMed] [Google Scholar]
- 29.Peters B.C.M., Wood W. Autism and Equine-Assisted Interventions: A Systematic Mapping Review. J. Autism Dev. Disord. 2017;47:3220–3242. doi: 10.1007/s10803-017-3219-9. [DOI] [PubMed] [Google Scholar]
- 30.Gabriels R.L., Pan Z., Dechant B., Agnew J.A., Brim N., Mesibov G. Randomized Controlled Trial of Therapeutic Horseback Riding in Children and Adolescents with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:541–549. doi: 10.1016/j.jaac.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters B.C., Pan Z., Christensen H., Gabriels R.L. Self-Regulation Mediates Therapeutic Horseback Riding Social Functioning Outcomes in Youth with Autism Spectrum Disorder. Front. Pediatr. 2022;10:884054. doi: 10.3389/fped.2022.884054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Haire M.E. Animal-Assisted Intervention for Autism Spectrum Disorder: A Systematic Literature Review. J. Autism Dev. Disord. 2013;43:1606–1622. doi: 10.1007/s10803-012-1707-5. [DOI] [PubMed] [Google Scholar]
- 33.Viau R., Arsenault-Lapierre G., Fecteau S., Champagne N., Walker C.-D., Lupien S. Effect of service dogs on salivary cortisol secretion in autistic children. Psychoneuroendocrinology. 2010;35:1187–1193. doi: 10.1016/j.psyneuen.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Mueller M.K., McCullough L. Effects of Equine-Facilitated Psychotherapy on Post-Traumatic Stress Symptoms in Youth. J. Child Fam. Stud. 2017;26:1164–1172. doi: 10.1007/s10826-016-0648-6. [DOI] [Google Scholar]
- 35.Pálsdóttir A.M., Gudmundsson M., Grahn P. Equine-Assisted Intervention to Improve Perceived Value of Everyday Occupations and Quality of Life in People with Lifelong Neurological Disorders: A Prospective Controlled Study. Int. J. Environ. Res. Public Health. 2020;17:2431. doi: 10.3390/ijerph17072431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Haire M. Research on Animal-Assisted Intervention and Autism Spectrum Disorder, 2012–2015. Appl. Dev. Sci. 2017;21:200–216. doi: 10.1080/10888691.2016.1243988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindly O.J., Thorburn S., Heisler K., Reyes N.M., Zuckerman K.E. Parents’ Use of Complementary Health Approaches for Young Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017;48:1803–1818. doi: 10.1007/s10803-017-3432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward S.C., Whalon K., Rusnak K., Wendell K., Paschall N. The Association Between Therapeutic Horseback Riding and the Social Communication and Sensory Reactions of Children with Autism. J. Autism Dev. Disord. 2013;43:2190–2198. doi: 10.1007/s10803-013-1773-3. [DOI] [PubMed] [Google Scholar]
- 39.Katz-Nave G., Adini Y., Hetzroni O.E., Bonneh Y.S. Sequence Learning in Minimally Verbal Children with ASD and the Beneficial Effect of Vestibular Stimulation. Autism Res. 2020;13:320–337. doi: 10.1002/aur.2237. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M., Chen S., You Y., Wang Y., Zhang Y. Effects of a Therapeutic Horseback Riding Program on Social Interaction and Communication in Children with Autism. Int. J. Environ. Res. Public Health. 2021;18:2656. doi: 10.3390/ijerph18052656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdman P., Miller D., Jacobson S. The Impact of an Equine Facilitated Learning Program on Youth with Autism Spectrum Disorder. Hum.-Anim. Interact. Bull. 2015;1:21–36. doi: 10.1079/hai.2015.0011. [DOI] [Google Scholar]
- 42.Lanning B.A., Baier M.E.M., Ivey-Hatz J., Krenek N., Tubbs J.D. Effects of Equine Assisted Activities on Autism Spectrum Disorder. J. Autism Dev. Disord. 2014;44:1897–1907. doi: 10.1007/s10803-014-2062-5. [DOI] [PubMed] [Google Scholar]
- 43.Peters B.C., Wood W., Hepburn S., Moody E.J. Preliminary Efficacy of Occupational Therapy in an Equine Environment for Youth with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022;52:4114–4128. doi: 10.1007/s10803-021-05278-0. [DOI] [PubMed] [Google Scholar]
- 44.Tomchek S., Koenig K.P., Arbesman M., Lieberman D. Occupational Therapy Interventions for Adolescents with Autism Spectrum Disorder. Am. J. Occup. Ther. 2017;71:7101395010p1–7101395010p3. doi: 10.5014/ajot.2017.711003. [DOI] [PubMed] [Google Scholar]
- 45.Martini R., Cramm H., Egan M., Sikora L. Scoping Review of Self-Regulation: What Are Occupational Therapists Talking About? Am. J. Occup. Ther. 2016;70:7006290010p1–7006290010p15. doi: 10.5014/ajot.2016.020362. [DOI] [PubMed] [Google Scholar]
- 46.Kendall E., Maujean A., Pepping C.A., Downes M., Lakhani A., Byrne J., Macfarlane K. A systematic review of the efficacy of equine-assisted interventions on psychological outcomes. Eur. J. Psychother. Couns. 2015;17:57–79. doi: 10.1080/13642537.2014.996169. [DOI] [Google Scholar]
- 47.Lentini J.A., Knox M.S. Equine-Facilitated Psychotherapy with Children and Adolescents: An Update and Literature Review. J. Creat. Ment. Health. 2015;10:278–305. doi: 10.1080/15401383.2015.1023916. [DOI] [Google Scholar]
- 48.Pendry P., Carr A.M., Smith A.N., Roeter S.M. Improving Adolescent Social Competence and Behavior: A Randomized Trial of an 11-Week Equine Facilitated Learning Prevention Program. J. Prim. Prev. 2014;35:281–293. doi: 10.1007/s10935-014-0350-7. [DOI] [PubMed] [Google Scholar]
- 49.Srinivasan S.M., Cavagnino D.T., Bhat A.N. Effects of Equine Therapy on Individuals with Autism Spectrum Disorder: A Systematic Review. Rev. J. Autism Dev. Disord. 2018;5:156–175. doi: 10.1007/s40489-018-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;89:105906. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moseley A.M., Herbert R., Sherrington C., Maher C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro) Aust. J. Physiother. 2002;48:43–49. doi: 10.1016/S0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 52.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 53.Kalmbach D., Wood W., Peters B.C. Parental Perspectives of Occupational Therapy in an Equine Environment for Children with Autism Spectrum Disorder. Occup. Ther. Health Care. 2020;34:230–252. doi: 10.1080/07380577.2020.1751903. [DOI] [PubMed] [Google Scholar]
- 54.Ozyurt G., Ozcan K., Elikucuk C.D., Odek U., Akpınar S. Equine Assisted Activities Have Positive Effects on Children with Autism Spectrum Disorder and Family Functioning. Montenegrin J. Sports Sci. Med. 2020;9:51–58. doi: 10.26773/mjssm.200909. [DOI] [Google Scholar]
- 55.Kwon S., Sung I.Y., Ko E.J., Kim H.S. Effects of Therapeutic Horseback Riding on Cognition and Language in Children with autism Spectrum Disorder or Intellectual Disability: A Preliminary Study. Ann. Rehabil. Med. 2019;43:279–288. doi: 10.5535/arm.2019.43.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan Z., Granger D.A., Guérin N.A., Shoffner A., Gabriels R.L. Replication Pilot Trial of Therapeutic Horseback Riding and Cortisol Collection with Children on the Autism Spectrum. Front. Vet. Sci. 2018;5:312. doi: 10.3389/fvets.2018.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabriels R.L., Pan Z., Guérin N.A., DeChant B., Mesibov G. Long-Term Effect of Therapeutic Horseback Riding in Youth with Autism Spectrum Disorder: A Randomized Trial. Front. Vet. Sci. 2018;5:156. doi: 10.3389/fvets.2018.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan V.X.-L., Simmonds J.G. Parent Perceptions of Psychosocial Outcomes of Equine-Assisted Interventions for Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018;48:759–769. doi: 10.1007/s10803-017-3399-3. [DOI] [PubMed] [Google Scholar]
- 59.Harris A., Williams J.M. The Impact of a Horse Riding Intervention on the Social Functioning of Children with Autism Spectrum Disorder. Int. J. Environ. Res. Public Health. 2017;14:776. doi: 10.3390/ijerph14070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson S., Meints K. Brief Report: The Effects of Equine-Assisted Activities on the Social Functioning in Children and Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016;46:3344–3352. doi: 10.1007/s10803-016-2869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borgi M., Loliva D., Cerino S., Chiarotti F., Venerosi A., Bramini M., Nonnis E., Marcelli M., Vinti C., De Santis C., et al. Effectiveness of a Standardized Equine-Assisted Therapy Program for Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016;46:1–9. doi: 10.1007/s10803-015-2530-6. [DOI] [PubMed] [Google Scholar]
- 62.Steiner H., Kertesz Z. Effects of therapeutic horse riding on gait cycle parameters and some aspects of behavior of children with autism. Acta Physiol. Hung. 2015;102:324–335. doi: 10.1556/036.102.2015.3.10. [DOI] [PubMed] [Google Scholar]
- 63.Holm M.B., Baird J.M., Kim Y.J., Rajora K.B., D’Silva D., Podolinsky L., Mazefsky C., Minshew N. Therapeutic Horseback Riding Outcomes of Parent-Identified Goals for Children with Autism Spectrum Disorder: An ABA’ Multiple Case Design Examining Dosing and Generalization to the Home and Community. J. Autism Dev. Disord. 2014;44:937–947. doi: 10.1007/s10803-013-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenkins S.R., Reed F.D.D. An experimental analysis of the effects of therapeutic horseback riding on the behavior of children with autism. Res. Autism Spectr. Disord. 2013;7:721–740. doi: 10.1016/j.rasd.2013.02.008. [DOI] [Google Scholar]
- 65.Ghorban H., Sedigheh R.D., Marzieh G., Yaghoob G. Effectiveness of Therapeutic Horseback Riding on Social Skills of Children with Autism Spectrum Disorder in Shiraz, Iran. J. Educ. Learn. 2013;2:79–84. doi: 10.5539/jel.v2n3p79. [DOI] [Google Scholar]
- 66.Gabriels R.L., Agnew J.A., Holt K.D., Shoffner A., Zhaoxing P., Ruzzano S., Clayton G.H., Mesibov G. Pilot study measuring the effects of therapeutic horseback riding on school-age children and adolescents with autism spectrum disorders. Res. Autism Spectr. Disord. 2012;6:578–588. doi: 10.1016/j.rasd.2011.09.007. [DOI] [Google Scholar]
- 67.Tabares C., Vicente F., Sanchez S., Aparicio A., Alejo S., Cubero J. Quantification of hormonal changes by effects of hippotherapy in the autistic population. Neurochem. J. 2012;6:311–316. doi: 10.1134/S1819712412040125. [DOI] [Google Scholar]
- 68.Kern J.K., Fletcher C.L., Garver C.R., Mehta J.A., Grannemann B.D., Knox K.R., Richardson T.A., Trivedi M.H. Prospective trial of equine-assisted activities in autism spectrum disorder. Altern. Ther. Health Med. 2011;17:14–20. [PubMed] [Google Scholar]
- 69.Bass M.M., Duchowny C.A., Llabre M.M. The Effect of Therapeutic Horseback Riding on Social Functioning in Children with Autism. J. Autism Dev. Disord. 2009;39:1261–1267. doi: 10.1007/s10803-009-0734-3. [DOI] [PubMed] [Google Scholar]
- 70.Taylor R.R., Kielhofner G., Smith C., Butler S., Cahill S.M., Ciukaj M.D., Gehman M. Volitional Change in Children with Autism: A Single-Case Design Study of the Impact of Hippotherapy on Motivation. Occup. Ther. Ment. Health. 2009;25:192–200. doi: 10.1080/01642120902859287. [DOI] [Google Scholar]
- 71.Kendall E., Maujean A., Pepping C.A., Wright J.J. Hypotheses about the Psychological Benefits of Horses. Explore. 2014;10:81–87. doi: 10.1016/j.explore.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Bedford R., Pickles A., Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Res. 2016;9:993–1001. doi: 10.1002/aur.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bratton S.C., Ray D.C., Edwards N.A., Landreth G. Child-Centered Play Therapy (Ccpt): Theory, Re-search, and Practice/Kindzentrierte Spieltherapie (Ccpt): Theorie, Forschung, Praxis/Terapia De Juego Centrada En El Niño (Tjcn): Teoría, Investigación Y Práctica/La Thérapie Par Le Jeu Centrée Sur L’enfant: Théorie, Recherche Et Pratique/Lu-doterapia Centrada Na Criança (Ltcc): Teoria, Investigação E Prática. Pers.-Cent. Exp. Psychother. 2009;8:266–281. [Google Scholar]
- 74.Karst J.S., Van Hecke A.V. Parent and Family Impact of Autism Spectrum Disorders: A Review and Proposed Model for Intervention Evaluation. Clin. Child Fam. Psychol. Rev. 2012;15:247–277. doi: 10.1007/s10567-012-0119-6. [DOI] [PubMed] [Google Scholar]
- 75.Wuang Y.-P., Wang C.-C., Huang M.H., Su C.-Y. The Effectiveness of Simulated Develop-mental Horse-Riding Program in Children with Autism. Adapt. Phys. Act. Q. 2010;27:113–126. doi: 10.1123/apaq.27.2.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be directed to the corresponding author.