PURPOSE
Frequent aspirin use has been associated with reduced ovarian cancer risk, but no study has comprehensively assessed for effect modification. We leveraged harmonized, individual-level data from 17 studies to examine the association between frequent aspirin use and ovarian cancer risk, overall and across subgroups of women with other ovarian cancer risk factors.
METHODS
Nine cohort studies from the Ovarian Cancer Cohort Consortium (n = 2,600 cases) and eight case-control studies from the Ovarian Cancer Association Consortium (n = 5,726 cases) were included. We used Cox regression and logistic regression to assess study-specific associations between frequent aspirin use (≥ 6 days/week) and ovarian cancer risk and combined study-specific estimates using random-effects meta-analysis. We conducted analyses within subgroups defined by individual ovarian cancer risk factors (endometriosis, obesity, family history of breast/ovarian cancer, nulliparity, oral contraceptive use, and tubal ligation) and by number of risk factors (0, 1, and ≥ 2).
RESULTS
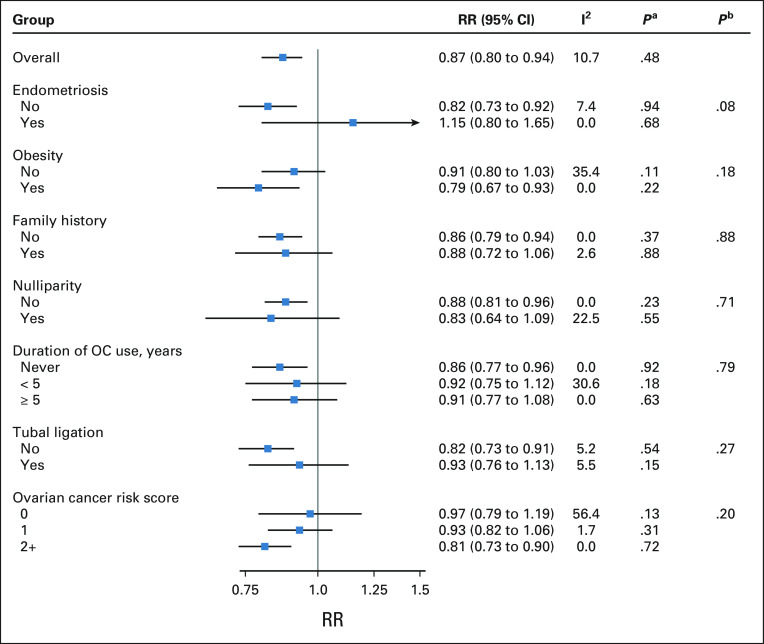
Overall, frequent aspirin use was associated with a 13% reduction in ovarian cancer risk (95% CI, 6 to 20), with no significant heterogeneity by study design (P = .48) or histotype (P = .60). Although no association was observed among women with endometriosis, consistent risk reductions were observed among all other subgroups defined by ovarian cancer risk factors (relative risks ranging from 0.79 to 0.93, all P-heterogeneity > .05), including women with ≥ 2 risk factors (relative risk, 0.81; 95% CI, 0.73 to 0.90).
CONCLUSION
This study, the largest to-date on aspirin use and ovarian cancer, provides evidence that frequent aspirin use is associated with lower ovarian cancer risk regardless of the presence of most other ovarian cancer risk factors. Risk reductions were also observed among women with multiple risk factors, providing proof of principle that chemoprevention programs with frequent aspirin use could target higher-risk subgroups.
INTRODUCTION
Ovarian cancer is the most fatal gynecologic cancer, largely because of nonspecific symptom presentation and lack of early detection strategies.1 Chemoprevention holds promise but remains an understudied paradigm to reduce ovarian cancer burden.2 Chronic inflammation likely plays a key role in ovarian carcinogenesis,3 as factors associated with epithelial disruption from ovulation,4,5 inflammation-related exposures such as endometriosis and pelvic inflammatory disease,6,7 and circulating biomarkers of inflammation8,9 are associated with ovarian cancer risk. Anti-inflammatory medications such as aspirin may lower risk of ovarian cancer development via inhibition of the cyclooxygenase enzymes, leading to decreased synthesis of inflammatory mediators, or via cyclooxygenase-independent pathways including inhibition of Wnt/β-catenin and NF-κβ.10
CONTEXT
Key Objective
To determine whether the association between frequent aspirin use and ovarian cancer risk is modified by established ovarian cancer risk factors (endometriosis, obesity, family history of breast/ovarian cancer, parity, oral contraceptive use, and tubal ligation).
Knowledge Generated
In combined analyses of individual participant data from 17 study populations, frequent aspirin use was associated with a 13% reduction in ovarian cancer risk overall. Consistent risk reductions were observed across most subgroups of women with other ovarian cancer risk factors, with the exception of endometriosis. Among women with two or more risk factors, frequent aspirin use was associated with a 19% reduction in ovarian cancer risk.
Relevance
This study confirms the association of frequent aspirin use with decreased risk of ovarian cancer. The use of aspirin for ovarian cancer chemoprevention may best be targeted to higher-risk women with two or more ovarian cancer risk factors, to maximize the population-level benefit/risk ratio.
A growing body of evidence supports a role of aspirin in reducing ovarian cancer risk. Pooled secondary analyses of randomized controlled trials of aspirin for cardiovascular disease prevention have noted a decreased risk of female reproductive cancers with ≥ 3 years of aspirin use, although too few ovarian cancer cases were diagnosed in these trial populations to draw inferences for ovarian cancer specifically.11 In the observational setting, individual study results have been mixed,12-23 but meta-analyses24 and pooled analyses of cohort25 and case-control26 studies have found that aspirin may reduce ovarian cancer risk by 10%-20%, particularly when used frequently (ie, daily or almost daily).
However, although aspirin use appears to be one of the few modifiable protective factors for ovarian cancer, population-wide chemoprevention programs are generally considered infeasible because of the low incidence of ovarian cancer and the known risk of bleeding conferred by frequent aspirin use.27 Instead, such programs will likely need to focus on subgroups of women at elevated risk of ovarian cancer.28 Established factors that increase ovarian cancer risk include a family history of breast or ovarian cancer, endometriosis, and obesity, whereas factors that decrease risk include parity, oral contraceptive use, and tubal ligation. Whether frequent aspirin use reduces risk of ovarian cancer among subgroups of women defined by these risk factors is unknown, and extremely large, well-powered studies are needed.
In this study, we leveraged harmonized, individual-level data from two ovarian cancer consortia that previously reported on frequent aspirin and ovarian cancer risk25,26 to comprehensively assess this association across key subgroups of interest. By meta-analyzing results from these 17 studies, we aimed to test for the consistency of the association across study design and personal characteristics and provide the most precise estimates of the aspirin-ovarian cancer association to date.
METHODS
Study Populations
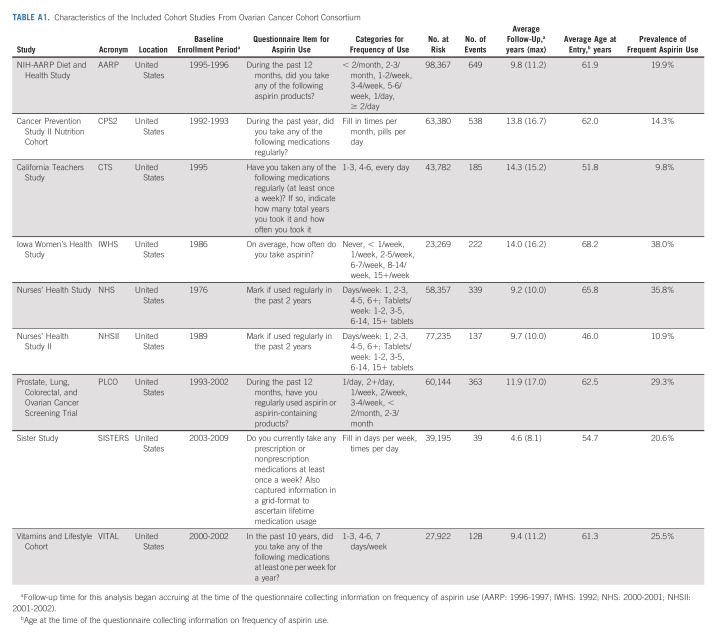
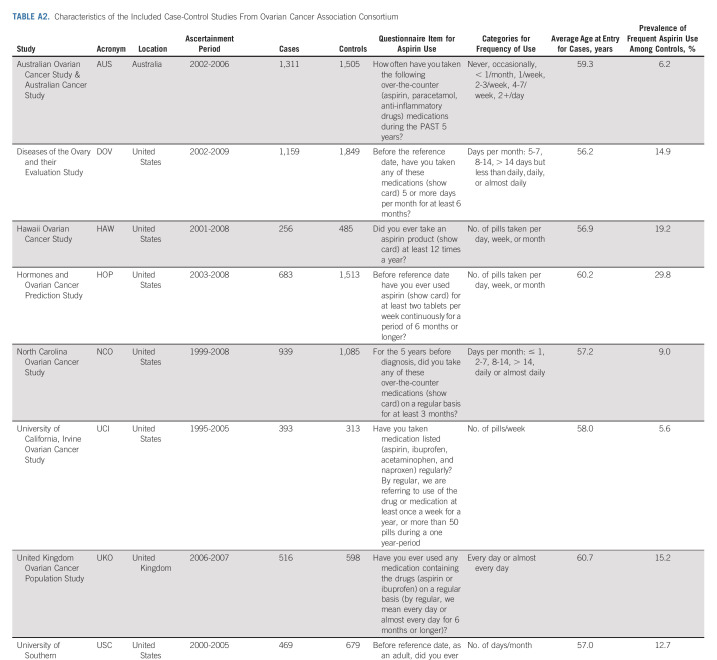
We analyzed individual-level data from prospective cohort studies from the Ovarian Cancer Cohort Consortium (OC3)29 and population-based case-control studies from the Ovarian Cancer Association Consortium (OCAC). Studies were included if they collected information on frequency of aspirin use; this resulted in the inclusion of nine cohort and eight case-control studies, a subset of the studies included in previous aspirin analyses from these consortia.25,26 The cohort studies (NIH-AARP Diet and Health Study,16,30 Cancer Prevention Study II Nutrition Cohort,31,32 California Teachers Study,33 Iowa Women's Health Study,19 Nurses' Health Study,18 Nurses' Health Study II,18 Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial,34 Sister Study,35 and Vitamins and Lifestyle Cohort36,37) were all US-based cohorts; our analysis included women from these cohorts with at least one intact ovary, no history of cancer at baseline, and nonmissing age and frequency of aspirin use. The case-control studies (Australian Ovarian Cancer Study,22 Diseases of the Ovary and their Evaluation Study,23,38 Hawaii Ovarian Cancer Study,39,40 Hormones and Ovarian Cancer Prediction Study,41 North Carolina Ovarian Cancer Study,42,43 University of California, Irvine Ovarian Cancer Study,44 United Kingdom Ovarian Cancer Population Study,45 University of Southern California, and Study of Lifestyle and Women's Health46) were from the United States, United Kingdom, and Australia.
All participating studies obtained institutional review board approval at their respective institutions. Participants provided written informed consent or implicit consent through return of study questionnaires. The coordinating centers for OC3 (Brigham and Women's Hospital, Harvard T. H. Chan School of Public Health) and OCAC (Duke University) received institutional review board approval from their institutions and participating registries as required for data acquisition, pooling, and harmonization.
Study Variables
Given previous findings that frequent aspirin use was most strongly associated with ovarian cancer risk,25,26 our primary exposure was frequent aspirin use, which was self-reported in all included studies (Appendix Tables A1 and A2, online only). Frequent aspirin use was harmonized across the study populations to indicate aspirin use for ≥ 6 days/week or ≥ 28 days/month and for a duration of ≥ 6 months. Frequent aspirin use was defined irrespective of dose, as few studies collected data on aspirin dose. Women who reported less frequent or no aspirin use were combined to form the reference group. Other covariates were centrally harmonized at the coordinating centers of OC3 and OCAC.6,29,47-49 For the cohort studies, aspirin use and other covariates were assessed at enrollment or at a subsequent questionnaire cycle, which then became the baseline for this analysis. For the case-control studies, covariates were ascertained at enrollment.
Our primary outcome was invasive epithelial ovarian, fallopian tube, or peritoneal cancer. In the cohort studies, cases were identified through linkage to cancer registries or medical chart review.29 Nonepithelial tumors and tumors of low malignant potential/borderline were excluded. Case ascertainment for the case-control studies included linkage to cancer registries or hospital registries, surgical treatment centers, gynecologic oncology centers, physician offices, and/or pathology databases.47 We also examined associations for the most common ovarian cancer histotypes, including high-grade serous, mucinous, endometrioid, clear cell, and other epithelial tumors. Very few low-grade serous tumors were observed in these study populations; these tumors were consequently excluded from histotype-specific analyses.
Statistical Analysis
For each cohort study, hazard ratios (HRs) and 95% CIs comparing frequent aspirin use to nonfrequent use were calculated using Cox proportional hazards regression. Women entered the analysis at age at study entry and contributed person-time until first diagnosis of ovarian cancer, death, or end of follow-up. Models were adjusted for baseline age, number of full-term births (none, one, two, three, or ≥ four), duration of oral contraception use (never, ≤ 1, > 1-5, > 5-10, or > 10 years), duration of menopausal hormone therapy use (premenopausal, never, ≤ 5, > 5-10, or > 10 years), and body mass index (BMI, < 20, 20 to < 25, 25 to < 30, 30 to < 35, or ≥ 35 kg/m2). For each case-control study, odds ratios (ORs) and 95% CIs were calculated using logistic regression, adjusting for the same covariates. Study-specific HRs and ORs were calculated overall as well as for subgroups defined by age at baseline (cohort studies) or diagnosis/index date (case-control studies; < 50, 50-59, 60-69, or ≥ 70 years), history of endometriosis (yes or no), obesity (BMI ≥ 30 or < 30 kg/m2), parity (parous or nulliparous), family history of breast or ovarian cancer (yes or no), duration of oral contraceptive use (never, < 5, or ≥ 5 years), tubal ligation (yes or no), and nonaspirin nonsteroidal anti-inflammatory drug (NSAID) use (yes or no). Study-specific effect estimates, overall and for each subgroup, were combined using random effects meta-analysis to generate summary relative risks (RRs).
We also calculated RRs within subgroups defined by an ovarian cancer risk score (range, 0-6, categorized as 0, 1, and ≥ 2), with each ovarian cancer risk factor (endometriosis, obesity, nulliparity, family history of breast or ovarian cancer, no oral contraceptive use, and no tubal ligation) contributing one point to this score. Before using this score, we confirmed that the risk score was positively associated with ovarian cancer risk (RR for a score of 1 v 0: 1.20, 95% CI, 1.10 to 1.30; RR for a score of ≥ 2 v 0: 1.78, 95% CI, 1.64 to 1.94). Risk score analyses were adjusted for age and duration of menopausal hormone therapy use.
To examine associations by ovarian cancer histotype, we conducted competing risks Cox regression using an augmented data approach with the pooled cohort data,50 and polytomous logistic regression with the pooled case-control data,51,52 adjusting for study and the same covariates as above. We conducted pooled instead of study-specific analyses because of the smaller number of events by histotype. The results from the cohort and case-control analyses were combined using random effects meta-analysis.
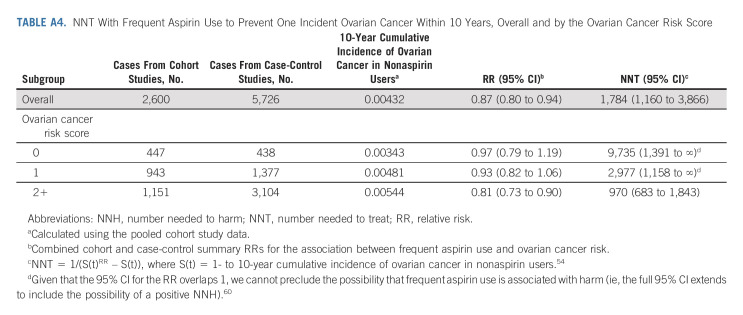
We examined heterogeneity in effect estimates by study, study design, subgroup, and histotype using Cochran's Q tests.53 The number needed to treat (NNT) to prevent one ovarian cancer was calculated using the observed 10-year absolute risk of ovarian cancer among nonaspirin users in the cohort studies and the combined cohort and case-control summary RRs.54 All statistical tests were two-sided, and P values < .05 were considered statistically significant. Study-specific and pooled analyses were conducted in SAS 9.4, meta-analyses were conducted using the meta command in Stata 16, and figures were generated in R 4.0.2.
RESULTS
In the nine cohort studies, there were 491,651 women at risk. The mean age at baseline ranged from 46.0 to 68.2 years, mean follow-up ranged from 4.6 to 14.3 years, and the prevalence of frequent aspirin use ranged from 9.8% to 38%. During follow-up, 2,600 women were diagnosed with incident ovarian cancer (56% high-grade serous, 2% low-grade serous, 9% endometrioid, 5% clear cell, 4% mucinous, and 23% other/unknown epithelial). Across the eight case-control studies, there were 5,726 cases (54% high-grade serous, 4% low-grade serous, 15% endometrioid, 9% clear cell, 5% mucinous, and 13% other/unknown epithelial) and 8,027 controls. The median age of the cases ranged from 56.2 to 60.7 years, and the prevalence of frequent aspirin use ranged from 5.6% to 29.8%. Additional characteristics of the study populations are described in Appendix Tables A1 and A2.
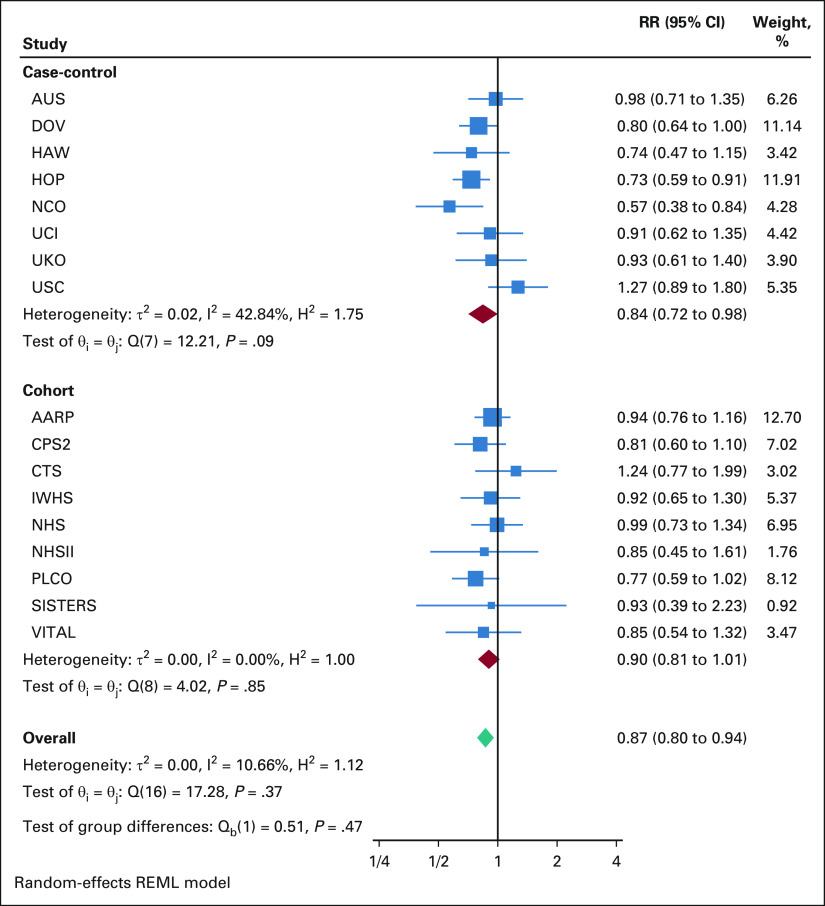
Overall, frequent aspirin use was associated with a 10% reduction in ovarian cancer risk in the cohort studies (HR, 0.90; 95% CI, 0.81 to 1.01) and a 16% reduced risk in the case-control studies (OR, 0.84; 95%, CI, 0.72 to 0.98; Appendix Fig A1 [online only]). Meta-analyzing the cohort and case-control studies yielded an overall summary RR of 0.87 (95% CI, 0.80 to 0.94), with no difference between the cohort and case-control study results (P-heterogeneity = .48).
Using the combined cohort and case-control data, when we examined associations within subgroups defined by factors that increase ovarian cancer risk, we observed possible effect modification by history of endometriosis (Fig 1). Among women without endometriosis, frequent aspirin use was associated with reduced ovarian cancer risk (RR, 0.82; 95% CI, 0.73 to 0.92), whereas no risk reduction was observed among women with endometriosis (RR, 1.15; 95% CI, 0.80 to 1.65; P-heterogeneity = .08). However, the CI for the latter effect estimate was large because of the small number of women with endometriosis (prevalence range, 1%-9% in the cohort studies, 3%-11% among OCAC controls). Frequent aspirin use was associated with lower ovarian cancer risk regardless of obesity, although the association was slightly stronger among obese women (RR, 0.79; 95% CI, 0.67 to 0.93) than among nonobese women (RR, 0.91; 95% CI, 0.80 to 1.03; P-heterogeneity = .18). Associations were similar across strata of family history of breast or ovarian cancer (RR, 0.86; 95% CI, 0.79 to 0.94 for women without a family history, RR, 0.88; 95% CI, 0.72 to 1.06 for women with a family history, P-heterogeneity = .88).
FIG 1.
Summary RRs for the association between frequent aspirin use and ovarian cancer risk in OC3 and OCAC, overall and by key subgroups of interest. Number of studies included in subgroup-specific meta-analyses: endometriosis (n = 11), obesity (n = 16), family history of breast/ovarian cancer (n = 15 for no/ n = 16 for yes), parity (n = 17), duration of OC use (n = 16), tubal ligation (n = 14), and risk score (n = 17). Models were adjusted for age, parity, duration of oral contraceptive use, duration of menopausal hormone therapy use, and BMI. Models stratified by risk score were adjusted for age and duration of menopausal hormone therapy use. Participants with missing data on these covariates (< 10% for all covariates except duration of menopausal hormone therapy use) were retained in the models using missing indicators. We also conducted a complete case analysis and the results were unchanged. aP value for heterogeneity by study design. bP value for heterogeneity by subgroup. BMI, body mass index; OC, oral contraceptive; OC3, Ovarian Cancer Cohort Consortium; OCAC, Ovarian Cancer Association Consortium; RR, relative risk.
Consistent risk reductions were observed within subgroups defined by protective factors for ovarian cancer, including parity (P-heterogeneity = .71), duration of oral contraceptive use (P-heterogeneity = .79), and tubal ligation (P-heterogeneity = .27, Fig 1). There was also no effect modification by nonaspirin NSAID use (RR, 0.86; 95% CI, 0.77 to 0.95 for no NSAID use, RR, 0.86; 95% CI, 0.75 to 0.98 for NSAID use, P-heterogeneity = .95).
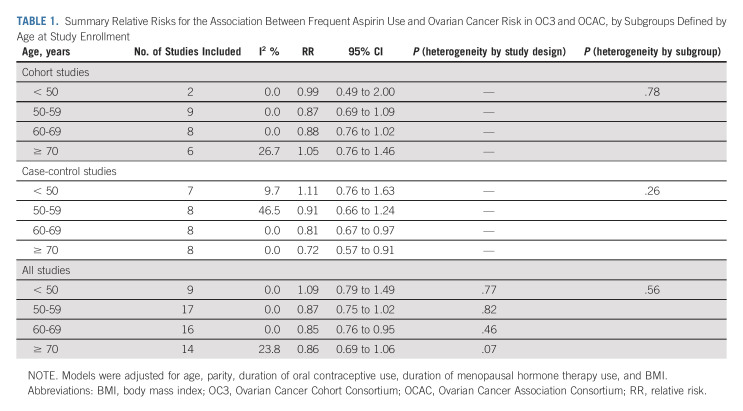
We did not observe effect modification by age at enrollment in the cohort (P-heterogeneity = .78) or case-control (P-heterogeneity = .26) studies (Table 1). However, in the case-control studies, there was possible strengthening of the association with age, with the strongest inverse association observed among women age 70 years or older at diagnosis/enrollment (OR, 0.72; 95% CI, 0.57 to 0.91).
TABLE 1.
Summary Relative Risks for the Association Between Frequent Aspirin Use and Ovarian Cancer Risk in OC3 and OCAC, by Subgroups Defined by Age at Study Enrollment
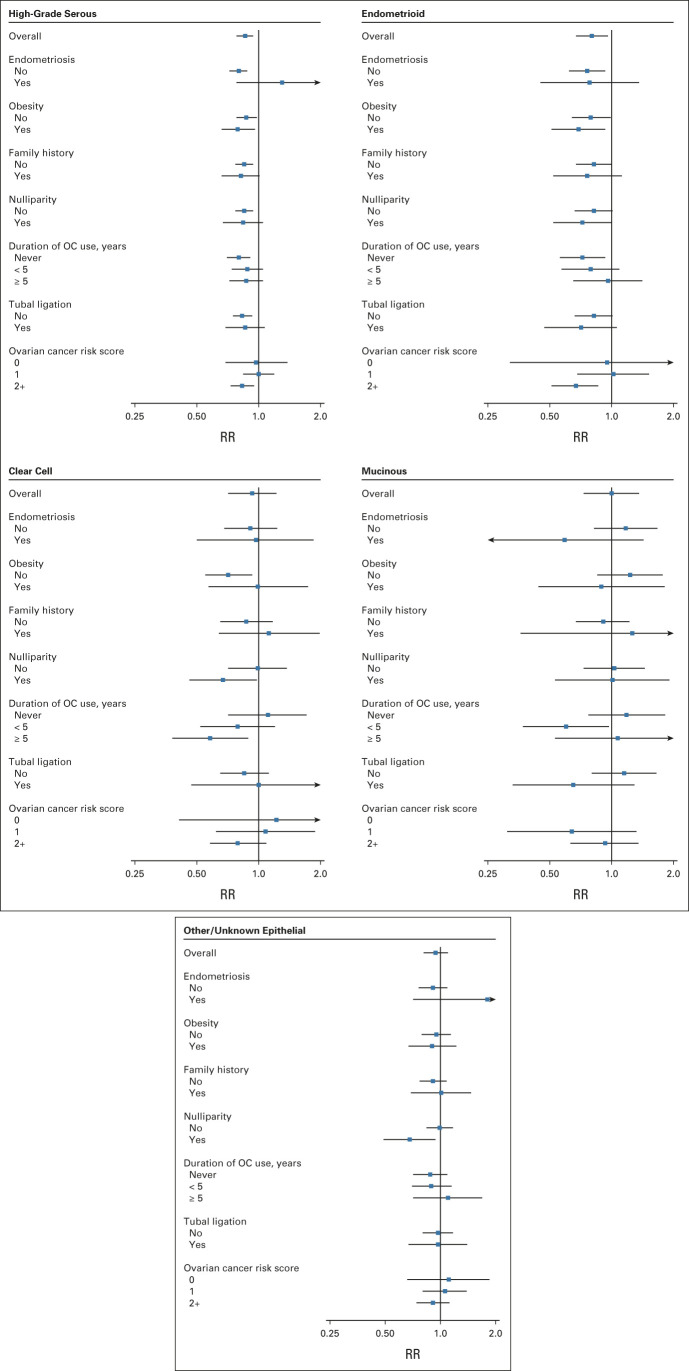
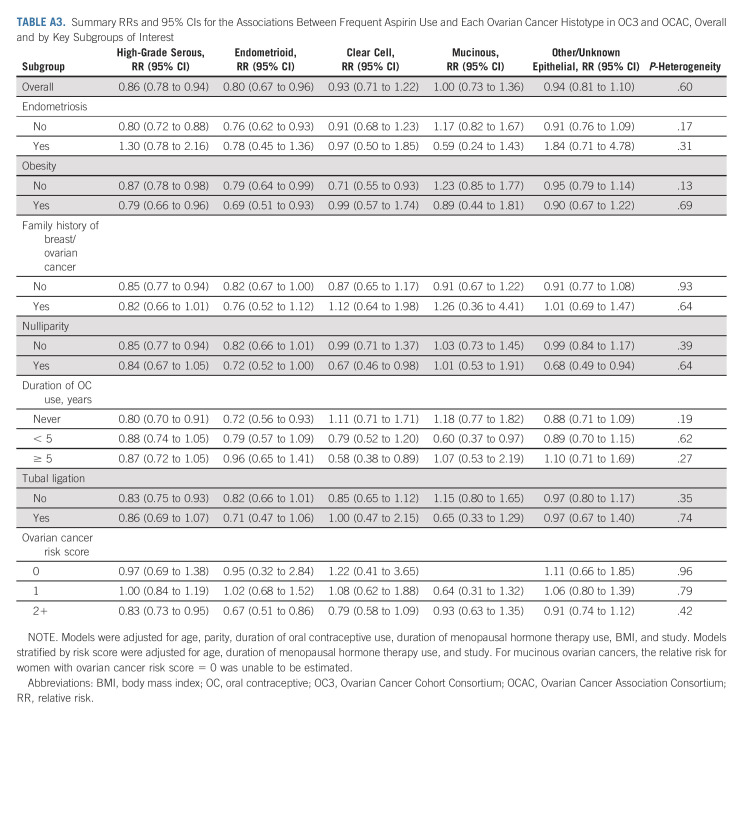
In general, associations with frequent aspirin use were similar for all ovarian cancer histotypes (Fig 2, Appendix Table A3, online only). Risk reductions were particularly robust for high-grade serous ovarian cancer, both overall (RR, 0.86; 95% CI, 0.78 to 0.94) and across subgroups defined by ovarian cancer risk factors. For women with endometriosis, although there was no association between frequent aspirin use and ovarian cancer overall, there was suggestion of an inverse association with endometrioid ovarian cancer, the histotype most strongly associated with endometriosis.
FIG 2.
Summary RRs for the associations between frequent aspirin use and each ovarian cancer histotype in OC3 and OCAC, overall and by key subgroups of interest. Tests for heterogeneity in the association across ovarian cancer histotypes: overall (P = .60), no endometriosis (P = .17), endometriosis (P = .31), no obesity (P = .13), obesity (P = .69), no family history of breast/ovarian cancer (P = .93), family history of breast/ovarian cancer (P = .64), parous (P = .39), nulliparous (P = .64), no OC use (P = .19), < 5 years of OC use (P = .62), 5+ years of OC use (P = .27), no tubal ligation (P = .35), tubal ligation (P = .74), ovarian cancer risk score = 0 (P = .96), ovarian cancer risk score = 1 (P = .79), and ovarian cancer risk score ≥ 2 (P = .42). Models were adjusted for age, parity, duration of oral contraceptive use, duration of menopausal hormone therapy use, BMI, and study. Models stratified by risk score were adjusted for age, duration of menopausal hormone therapy use, and study. For mucinous ovarian cancers, the RR for women with ovarian cancer risk score = 0 was unable to be estimated. BMI, body mass index; OC, oral contraceptive; OC3, Ovarian Cancer Cohort Consortium; OCAC, Ovarian Cancer Association Consortium; RR, relative risk.
In the cohort studies, 21% of women had none of the six ovarian cancer risk factors, 42% had one risk factor, and 37% had ≥ two. In the case-control studies, the corresponding percentages of women with zero, one, and ≥ two risk factors were 8%, 28%, and 64% for cases, and 12%, 37%, and 51% for controls. In analyses stratified by the number of risk factors (ie, the ovarian cancer risk score), frequent aspirin use was inversely associated with ovarian cancer risk among women at higher risk of ovarian cancer because of the presence of ≥ two risk factors (RR, 0.81; 95% CI, 0.73 to 0.90, Fig 1). The protective association for these higher-risk women was consistent across histotypes (Fig 2, Appendix Table A3, P-heterogeneity = .42). Among the higher-risk women, the NNT to prevent one ovarian cancer within 10 years was 970 (95% CI, 683 to 1,843; Appendix Table A4, online only). By contrast, the NNT for all women in the study population regardless of risk score was 1784 (95% CI, 1,160 to 3,866).
DISCUSSION
In this analysis of data from two ovarian cancer consortia, frequent aspirin use was associated with a 13% reduction in ovarian cancer risk overall. A similar risk reduction was observed for high-grade serous ovarian cancer, the most common and one of the most fatal histotypes, which is important because most established risk factors are more weakly associated with high-grade serous ovarian cancers.6 The consistency of the frequent aspirin use-ovarian cancer association across the individual case-control and cohort study populations was notable and provides strong support for a beneficial effect of frequent aspirin use on ovarian cancer risk.
Importantly, our study also found that established ovarian cancer risk factors do not modify the association between frequent aspirin use and ovarian cancer risk. There was a suggestion of effect modification by endometriosis, with an inverse association observed for women without but not with self-reported endometriosis, but this was likely driven by the small number of women with endometriosis and the limited power to detect associations within this subgroup. Additionally, there was no effect modification by endometriosis for endometrioid or clear cell tumors, the two specific histotypes for which endometriosis is a risk factor.6
Risk reductions associated with frequent aspirin use were otherwise consistent across subgroups defined by factors that increase (obesity and family history of breast/ovarian cancer) and decrease (parity, oral contraceptive use, and tubal ligation) ovarian cancer risk. The lack of effect modification by adiposity is particularly notable, given that other studies have reported aspirin to be more weakly associated with reduced cardiovascular disease and colorectal cancer risk55,56 and more strongly associated with reduced endometrial cancer risk57 among obese individuals; this could suggest that aspirin's mechanism of action for preventing cardiovascular disease and these other cancers may differ from that preventing ovarian cancer.
There was possible effect modification by the ovarian cancer risk score, with a null association observed among women with zero ovarian cancer risk factors. However, the results for women with zero risk factors were inconclusive, given the small number of cases and heterogeneity in the study-specific results for this subgroup. More critically, we observed a clear inverse association between frequent aspirin use and ovarian cancer among women with multiple ovarian cancer risk factors. These results are important, given that any implementation of aspirin use for ovarian cancer chemoprevention will likely need to focus on specific high-risk subgroups.28 Our study suggests that frequent aspirin use is protective among women at increased risk of ovarian cancer because of the presence of established epidemiologic risk factors, with a lower NNT among women with ≥ 2 risk factors, and that targeting chemoprevention programs to women with epidemiologic risk factors may thus be a viable strategy.
To our knowledge, this study is the largest to date on aspirin and ovarian cancer risk and the first to examine effect modification by a comprehensive set of ovarian cancer risk factors. Previous studies of aspirin, examined alone or combined with other NSAIDs, have also reported no effect modification by BMI,17,18 parity,12,16,18,46 or oral contraceptive use,16,18,46 but these individual studies were only powered to detect very strong differences. One study observed a possible stronger association between daily aspirin use and ovarian cancer risk with increasing BMI,58 a trend that was mirrored in our study, although our study suggests that frequent aspirin may still be modestly protective among nonobese women. Our study confirms and expands upon these prior studies by combining the existing observational data, which facilitated a well-powered analysis, even among subgroups. Access to the individual-level data from each study allowed us to apply a standardized analytic approach, assess associations by histotype, and focus specifically on frequent aspirin use, the pattern of use that appears most protective against ovarian cancer.25,26
Although we combined results across study design, such pooling was necessary to obtain sufficient power to test for effect modification. Moreover, formal comparison of the cohort and case-control results revealed no meaningful or statistically significant differences. There may have been some bias because of the use of observational data, but research has found that observational studies of aspirin and cancer can recapitulate randomized controlled trial findings when there is detailed recording of aspirin use and careful adjustment for confounders.59 We were unable to examine associations specifically for low-dose aspirin, which has been more strongly associated with reduced ovarian cancer risk in prior studies,13,26 but frequent aspirin use was highly correlated with low-dose use in the studies with dosage information available (ρ = 0.97 in OC3, ρ = 0.82 in OCAC controls). We did not have data on indication for aspirin use or age at initiation of use, both of which require further study. We did not look at associations among women at increased risk of ovarian cancer because of common or rare genetic variants as genetic data were not available for all included studies; whether aspirin reduces risk among women with highly penetrant mutations (ie, BRCA1/BRCA2 or Lynch syndrome) will require examination in specialized study populations. Finally, when calculating the NNT, we were unable to incorporate associations for precise durations of aspirin use because of the use of observational data. The NNT also does not account for the known risks associated with frequent aspirin use, and further research on the net benefits and harms is needed.
In conclusion, this study, the largest to date on frequent aspirin use and ovarian cancer, supports a 13% reduction in ovarian cancer risk with frequent aspirin use, with a 14% reduction for high-grade serous carcinoma, the most common and one of the more lethal histotypes. Similar risk reductions were observed across subgroups defined by established epidemiologic risk factors, and no subgroup experienced a significant increased risk with aspirin use. These results suggest that primary prevention of ovarian cancer is an added benefit of frequent aspirin use that could be incorporated into composite risk-benefit calculations. Given that women with elevated ovarian cancer risk because of epidemiologic risk factors also benefit and that the NNT to prevent one ovarian cancer is lower for higher-risk women, future work should explore how chemoprevention programs with aspirin could complement existing preventive strategies, which are currently limited to women with the highest risk (ie, prophylactic salpingo-oophorectomy for BRCA1/2 carriers) and target additional high-risk subgroups to maximize population-level impact and minimize risks.
ACKNOWLEDGMENT
The authors would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data. The authors would like to acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, as the home of the Nurses' Health Study. The collection of cancer incidence data used in the California Teachers Study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention's National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The opinions, findings, and conclusions expressed herein are those of the author(s) and do not necessarily reflect the official views of the State of California, Department of Public Health, the National Cancer Institute, the National Institutes of Health, the Centers for Disease Control and Prevention or their contractors and subcontractors, or the regents of the University of California, or any of its programs.
The Australian Ovarian Cancer Study Management Group (D. Bowtell, G. Chenevix-Trench, A. deFazio, D. Gertig, A. Green, and P. Webb) and Australian Cancer Study investigators (A. Green, P. Parsons, N. Hayward, P. Webb, and D. Whiteman) thank all the clinical and scientific collaborators (see http://www.aocstudy.org/) and the women for their contribution. Some of this work was undertaken at University College London Hospital/University College London, which received a proportion of funding from the Department of Health's National Institutes for Health Research Biomedical Research Centre funding scheme. The authors particularly thank I. Jacobs, M. Widschwendter, E. Wozniak, A. Ryan, J. Ford, and N. Balogun for their contribution to the study. Support for title page creation and format was provided by AuthorArranger, a tool developed at the National Cancer Institute. AOCS Study Group members are listed at Appendix 1 (online only).
APPENDIX 1. AOCS STUDY GROUP
Management Group: D. Bowtell (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Department of Pathology, University of Melbourne, Parkville, Victoria, Australia; Sir Peter MacCallum Cancer Centre Department of Oncology, University of Melbourne, Parkville, Victoria, Australia; Department of Biochemistry and Molecular Biology, University of Melbourne, Parkville, Victoria, Australia; Ovarian Cancer Action Research Centre, Department of Surgery and Cancer, Imperial College London, London, England, United Kingdom), G. Chenevix-Trench (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), A. Green (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), P. Webb (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), A. DeFazio (Centre for Cancer Research, The Westmead Institute for Medical Research, Sydney, New South Wales, Australia; The University of Sydney, Sydney, New South Wales, Australia; Department of Gynecological Oncology, Westmead Hospital, Sydney, New South Wales, Australia), D. Gertig (Melbourne School of Population and Global Health, University of Melbourne, Parkville, Victoria, Australia)
Project and Data Managers: N. Traficante (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Sir Peter MacCallum Cancer Centre Department of Oncology, University of Melbourne, Parkville, Victoria, Australia), S. Fereday (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Sir Peter MacCallum Cancer Centre Department of Oncology, University of Melbourne, Parkville, Victoria, Australia), S. Moore (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), J. Hung (Centre for Cancer Research, The Westmead Institute for Medical Research, Sydney, New South Wales, Australia), K. Harrap (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), T. Sadkowsky (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), N. Pandeya (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), Research Nurses and Assistants: M Malt (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), A. Mellon (John Hunter Hospital, Lookout Road, New Lambton, New South Wales, Australia), R. Robertson (John Hunter Hospital, Lookout Road, New Lambton, New South Wales, Australia), T. Vanden Bergh (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), M. Jones (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), P. Mackenzie (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), J. Maidens (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia), K. Nattress (Royal Prince Alfred Hospital, Missenden Road, Camperdown, New South Wales, Australia), Y.E. Chiew (Centre for Cancer Research, The Westmead Institute for Medical Research, Sydney, New South Wales, Australia), A. Stenlake (Department of Gynecological Oncology, Westmead Hospital, Sydney, New South Wales, Australia), H. Sullivan (Department of Gynecological Oncology, Westmead Hospital, Sydney, New South Wales, Australia), B. Alexander (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), P. Ashover (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), S. Brown (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), T. Corrish (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), L. Green (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), L. Jackman (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), K. Ferguson (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), K. Martin (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), A. Martyn (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), B. Ranieri (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), J. White (Royal Adelaide Hospital, North Terrace, Adelaide, South Australia, Australia), V. Jayde (Royal Hobart Hospital, 48 Liverpool St, Hobart, Tasmania, Australia), P. Mamers (Monash Medical Centre, Clayton, Victoria, Australia), L. Bowes (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), L. Galletta (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), D. Giles (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), J. Hendley (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), K. Alsop (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), T. Schmidt (Western Australian Research Tissue Network (WARTN), St John of God Pathology, Osborne Park, Western Australia, Australia), H. Shirley (Western Australian Research Tissue Network (WARTN), St John of God Pathology, Osborne Park, Western Australia, Australia), C. Ball (Women and Infant's Research Foundation, King Edward Memorial Hospital, Subiaco, Western Australia, Australia), C. Young (Women and Infant's Research Foundation, King Edward Memorial Hospital, Subiaco, Western Australia, Australia), S. Viduka (Western Australian Research Tissue Network (WARTN), St John of God Pathology, Osborne Park, Western Australia, Australia), Hoa Tran (Western Australian Research Tissue Network [WARTN], St John of God Pathology, Osborne Park, Western Australia, Australia), Sanela Bilic (Western Australian Research Tissue Network (WARTN), St John of God Pathology, Osborne Park, Western Australia, Australia), Lydia Glavinas (Western Australian Research Tissue Network (WARTN), St John of God Pathology, Osborne Park, Western Australia, Australia), Julia Brooks (St John of God Hospital, Subiaco, Western Australia, Australia), Clinical and Scientific Collaborators: R. Stuart-Harris (Canberra Hospital, Garran, Australian Capitol Territory, Australia), F. Kirsten (Bankstown Cancer Centre, Bankstown Hospital, Bankstown, New South Wales, Australia), J. Rutovitz (Northern Haematology & Oncology Group, Integrated Cancer Centre, Wahroonga, New South Wales, Australia), P. Clingan (Illawarra Shoalhaven Local Health District, Wollongong Hospital, Wollongong, New South Wales, Australia), A. Glasgow (Illawarra Shoalhaven Local Health District, Wollongong Hospital, Wollongong, New South Wales, Australia), A. Proietto (John Hunter Hospital, Lookout Road, New Lambton, New South Wales, Australia), S. Braye (John Hunter Hospital, Lookout Road, New Lambton, New South Wales, Australia), G. Otton (John Hunter Hospital, Lookout Road, New Lambton, New South Wales, Australia), J. Shannon (Nepean Hospital, Kingswood, New South Wales, Australia), T. Bonaventura (Newcastle Mater Misericordiae Hospital, Waratah, New South Wales, Australia), J. Stewart (Newcastle Mater Misericordiae Hospital, Waratah, New South Wales, Australia), S. Begbie (Port Macquarie Base Hospital, Port Macquarie, New South Wales, Australia) M. Friedlander (Prince of Wales Clinical School, University of New South Wales, New South Wales, Australia), D. Bell (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia), S. Baron- Hay (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia), A. Ferrier (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia) (dec), G. Gard (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia), D. Nevell (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia), N. Pavlakis (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia), S. Valmadre (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia), B. Young (Royal North Shore Hospital, Reserve Road, St Leonards, New South Wales, Australia), C. Camaris (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), R. Crouch (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), L. Edwards (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), N. Hacker (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), D. Marsden (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), G. Robertson (Royal Hospital for Women, Barker Street, Randwick, New South Wales, Australia), P. Beale (Royal Prince Alfred Hospital, Missenden Road, Camperdown, New South Wales, Australia), J. Beith (Royal Prince Alfred Hospital, Missenden Road, Camperdown, New South Wales, Australia), J. Carter (Royal Prince Alfred Hospital, Missenden Road, Camperdown, New South Wales, Australia), C. Dalrymple (Royal Prince Alfred Hospital, Missenden Road, Camperdown, New South Wales, Australia), R. Houghton (Royal Prince Alfred Hospital, Missenden Road, Camperdown, New South Wales, Australia), P. Russell (Royal Prince Alfred Hospital, Missenden Road, Camperdown, New South Wales, Australia), M. Links (St George Hospital, Kogarah, New South Wales, Australia), J. Grygiel (St Vincent's Hospital, Darlinghurst, New South Wales, Australia), J. Hill (Wagga Wagga Base Hospital, Wagga Wagga, New South Wales, Australia), A. Brand (The University of Sydney, Sydney, New South Wales, Australia; Crown Princess Mary Cancer Centre, Westmead Hospital, Sydney, New South Wales, Australia), K. Byth (Crown Princess Mary Cancer Centre, Westmead Hospital, Sydney, New South Wales, Australia), R. Jaworski (Department of Pathology, Westmead Clinical School, Westmead Hospital, The University of Sydney, New South Wales, Australia), P. Harnett (The University of Sydney, Sydney, New South Wales, Australia; Crown Princess Mary Cancer Centre, Westmead Hospital, Sydney, New South Wales, Australia), R. Sharma (The University of Sydney, Sydney, New South Wales, Australia; Department of Pathology, Westmead Clinical School, Westmead Hospital, The University of Sydney, New South Wales, Australia), G. Wain (Crown Princess Mary Cancer Centre, Westmead Hospital, Sydney, New South Wales, Australia), B. Ward (Mater Misericordiae Hospital, South Brisbane, Queensland, Australia), D. Papadimos (Mater Misericordiae Hospital, South Brisbane, Queensland, Australia), A. Crandon (The Royal Brisbane and Women's Hospital, Herston, Queensland, Australia), M. Cummings (The Royal Brisbane and Women's Hospital, Herston, Queensland, Australia), K. Horwood (The Royal Brisbane and Women's Hospital, Herston, Queensland, Australia), A. Obermair (The Royal Brisbane and Women's Hospital, Herston, Queensland, Australia), L. Perrin (The Royal Brisbane and Women's Hospital, Herston, Queensland, Australia), D. Wyld (The Royal Brisbane and Women's Hospital, Herston, Queensland, Australia), J. Nicklin (The Royal Brisbane and Women's Hospital, Herston, Queensland, Australia; Wesley Hospital, 451 Auchenflower, Queensland, Australia), M. Davy (Royal Adelaide Hospital, North Terrace, Adelaide, South Australia, Australia), M.K. Oehler (Royal Adelaide Hospital, North Terrace, Adelaide, South Australia, Australia), C. Hall (Royal Adelaide Hospital, North Terrace, Adelaide, South Australia, Australia), T. Dodd (Royal Adelaide Hospital, North Terrace, Adelaide, South Australia, Australia), T. Healy (Burnside Hospital, Toorak Gardens, South Australia, Australia), K. Pittman (Burnside Hospital, Toorak Gardens, South Australia, Australia), D. Henderson (Burnside Hospital, Toorak Gardens, South Australia, Australia), J. Miller (Queen Elizabeth Hospital, Woodville South, South Australia, Australia), J. Pierdes (Queen Elizabeth Hospital, Woodville South, South Australia, Australia), P. Blomfield (Royal Hobart Hospital, 48 Liverpool St, Hobart, Tasmania, Australia), D. Challis (Royal Hobart Hospital, 48 Liverpool St, Hobart, Tasmania, Australia), R. McIntosh (Royal Hobart Hospital, 48 Liverpool St, Hobart, Tasmania, Australia), A. Parker (Royal Hobart Hospital, Hobart, Tasmania, Australia), B. Brown (Freemasons Hospital, 20 East Melbourne, Victoria, Australia), R. Rome (Freemasons Hospital, Victoria, Australia), D. Allen (Mercy Hospital for Women, Heidelberg, Victoria, Australia), P. Grant (Mercy Hospital for Women, Heidelberg, Victoria, Australia), S. Hyde (Mercy Hospital for Women, Heidelberg, Victoria, Australia), R. Laurie (Mercy Hospital for Women, Heidelberg, Victoria, Australia), M. Robbie (Mercy Hospital for Women, Heidelberg, Victoria, Australia), D. Healy (Monash Medical Centre, Clayton, Victoria, Australia), T. Jobling (Monash Medical Centre, Clayton, Victoria, Australia), T. Manolitsas (Monash Medical Centre, Clayton, Victoria, Australia), J. McNealage (Monash Medical Centre, Clayton, Victoria, Australia), P. Rogers (Monash Medical Centre, Clayton, Victoria, Australia), B. Susil (Monash Medical Centre, 246 Clayton Rd, Clayton, Victoria, Australia), E. Sumithran (Monash Medical Centre, Clayton, Victoria, Australia), I. Simpson (Monash Medical Centre, 246 Clayton Rd, Clayton, Victoria, Australia), K. Phillips (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), D. Rischin (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), S. Fox (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), D. Johnson (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), S. Lade (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), M. Loughrey (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), N. O'Callaghan (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), W. Murray (Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia), P. Waring (Department of Pathology, University of Melbourne, Parkville, Victoria, Australia), V. Billson (The Royal Women's Hospital, Parkville, Victoria, Australia), J. Pyman (The Royal Women's Hospital, Parkville, Victoria, Australia), D. Neesham (The Royal Women's Hospital, Parkville, Victoria, Australia), M. Quinn (The Royal Women's Hospital, Parkville, Victoria, Australia), C. Underhill (Border Medical Oncology, Wodonga, Victoria, Australia), R. Bell (Andrew Love Cancer Centre, Geelong, Victoria, Australia), LF Ng (Ballarat Base Hospital, Ballarat, Victoria, Australia), R. Blum (Bendigo Health Care Group, Bendigo, Victoria, Australia), V. Ganju (Peninsula Health, Frankston, Victoria, Australia), I. Hammond (Women and Infant's Research Foundation, King Edward Memorial Hospital, Subiaco, Western Australia, Australia), Y. Leung (Women and Infant's Research Foundation, King Edward Memorial Hospital, Subiaco, Western Australia, Australia), A. McCartney (Women and Infant's Research Foundation, King Edward Memorial Hospital, Subiaco, Western Australia, Australia) (dec), M. Buck (Mount Hospital, Perth, Western Australia, Australia), I. Haviv (Faculty of Medicine, Bar-Ilan University, Safed, Israel), D. Purdie (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), D. Whiteman (QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia), N. Zeps (Western Australian Research Tissue Network (WARTN), St John of God Pathology, Osborne Park, Western Australia, Australia)
FIG A1.
Meta-analysis of the overall associationa between frequent aspirin use and ovarian cancer risk in OC3 and OCAC. aAdjusted for age, parity, duration of oral contraceptive use, duration of menopausal hormone therapy use, and BMI. AARP, NIH-AARP Diet and Health Study; AUS, Australian Ovarian Cancer Study & Australian Cancer Study; BMI, body mass index; CPS2, Cancer Prevention Study II Nutrition Cohort; CTS, California Teachers Study, DOV, Diseases of the Ovary and their Evaluation Study; HAW, Hawaii Ovarian Cancer Study; HOP, Hormones and Ovarian Cancer Prediction Study; IWHS, Iowa Women's Health Study; NCO, North Carolina Ovarian Cancer Study; NHS, Nurses' Health Study; NHSII, Nurses' Health Study II; OC3, Ovarian Cancer Cohort Consortium; OCAC, Ovarian Cancer Association Consortium; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; RR, relative risk; SISTERS, Sister Study; UCI, University of California, Irvine Ovarian Cancer Study; UKO, United Kingdom Ovarian Cancer Population Study; USC, University of Southern California, Study of Lifestyle and Women’s Health; VITAL, Vitamins and Lifestyle Cohort.
TABLE A1.
Characteristics of the Included Cohort Studies From Ovarian Cancer Cohort Consortium
TABLE A2.
Characteristics of the Included Case-Control Studies From Ovarian Cancer Association Consortium
TABLE A3.
Summary RRs and 95% CIs for the Associations Between Frequent Aspirin Use and Each Ovarian Cancer Histotype in OC3 and OCAC, Overall and by Key Subgroups of Interest
TABLE A4.
NNT With Frequent Aspirin Use to Prevent One Incident Ovarian Cancer Within 10 Years, Overall and by the Ovarian Cancer Risk Score
Alpa V. Patel
Research Funding: GRAIL (Inst)
Joellen M. Schildkraut
Patents, Royalties, Other Intellectual Property: Patent related treatment of PAD (peripheral arterial disease)(I)
Andrew Berchuck
Honoraria: Clovis Oncology, Merck, Tesaro, Premier Research
Research Funding: Novadaq Technologies
Travel, Accommodations, Expenses: Merck
Andrew T. Chan
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, Bayer
Research Funding: Zoe Ltd, Pfizer
Celeste Leigh Pearce
Stock and Other Ownership Interests: SAVA, ANVS
Honoraria: ViiV Healthcare (I)
Consulting or Advisory Role: ViiV Healthcare (I)
Speakers' Bureau: ViiV Healthcare (I)
Research Funding: CytoDyn (I)
Usha Menon
Stock and Other Ownership Interests: Abcodia
Research Funding: Abcodia (Inst)
Patents, Royalties, Other Intellectual Property: Patent no: EP10178345.4 for Breast Cancer Diagnostics
Uncompensated Relationships: ILOF (Inst), Dana-Farber Cancer Institute (Inst), RNA Guardian (Inst), Micronoma (Inst)
Penelope M. Webb
Research Funding: AstraZeneca (Inst)
Shelley S. Tworoger
Research Funding: BMS (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This is a US Government work. There are no restrictions on its use.
SUPPORT
This study was funded by US Department of Defense Ovarian Cancer Research Program (W81XWH-19-1-0346). The OC3 received support from the US Department of Defense Ovarian Cancer Research Program (W81XWH-12-1-0561, W81XWH-19-1-0346) and the Intramural Research Program of the National Cancer Institute at the National Institutes of Health. The Ovarian Cancer Association Consortium was supported by a grant from the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith. The Australian Ovarian Cancer Study and Australian Cancer Study were funded by the US Army Medical Research and Material Command (DAMD17-01-1-0729), National Health and Medical Research Council of Australia (199600, 400413), Cancer Councils of New South Wales, Victoria, Queensland, South Australia, and Tasmania, Cancer Foundation of Western Australia. All aspects of the Cancer Prevention Study II were funded by the Intramural Research Program of the American Cancer Society and by the National Cancer Institute at the National Institutes of Health Intramural Research Program. The California Teachers Study and the research reported in this publication were supported by National Cancer Institute at the National Institutes of Health (grant numbers U01 CA199277, P30 CA033572, P30 CA023100, UM1 CA164917, and R01 CA077398). The Diseases of the Ovary and their Evaluation Study was funded by R01 CA112523 and R01 CA87538. The Hawaii Ovarian Cancer Case–Control Study was funded by R01 CA58598, N01 CN55424, and N01 PC67001. The Hormones and Ovarian Cancer Prediction Study was funded by R01 CA95023 and Department of Defense (DOD) grant DAMD17-02-1-0669. The Iowa Women's Health Study was funded by the National Cancer Institute at the National Institutes of Health (R01 CA39742). The North Carolina Ovarian Cancer Study was funded by R01 CA76016 and the DOD grant DAMD17-02-1-0666. The Nurses' Health Study and Nurses' Health Study II were funded by the National Cancer Institute at the National Institutes of Health (UM1 CA186107, P01 CA87969, R01 CA49449, U01 CA176726, and R01 CA67262). The NIH-AARP Diet and Health Study was funded by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health. PLCO was funded through contracts administered by the Division of Cancer Prevention at the National Cancer Institute, National Institutes of Health, with support from the National Cancer Institute Intramural Research Program. The Sister Study was funded by Intramural Research Program of the National Institute of Environmental Health Sciences at the National Institutes of Health (Z01ES044005). The University of California, Irvine Ovarian Cancer Study was funded by R01 CA58860, R01 CA92044, PSA 042205, and the Lon V Smith Foundation grant LVS-39420. The United Kingdom Ovarian Cancer Population Study was funded by Cancer Research UK, the Eve Appeal, and the OAK Foundation. The University of Southern California, Study of Lifestyle and Women's Health was funded by R01 CA17054, R01 CA14089, R01 CA61132, N01-PC-67010, P01 CA17054, California Cancer Research Program (00-01389V-20170, R03 CA113148, R03 CA115195, N01 CN25403), and California Cancer Research Program (2II0200; USC). The Vitamins and Lifestyle Study was funded by the National Cancer Institute and Office of Dietary Supplements at the National Institutes of Health (K05 CA154337).
AUTHOR CONTRIBUTIONS
Conception and design: Nicolas Wentzensen, Hoda Anton-Culver, Argyrios Ziogas, Shelley S. Tworoger, Britton Trabert
Financial support: Francesmary Modugno, Britton Trabert
Administrative support: Mary K. Townsend, Andrew Berchuck, Shelley S. Tworoger, Britton Trabert
Provision of study materials or patients: Lauren R. Teras, Jennifer A. Doherty, Marc T. Goodman, Francesmary Modugno, Kim Robien, Joellen M. Schildkraut, Andrew T. Chan, Nicolas Wentzensen, Dale P. Sandler, Hoda Anton-Culver, Susan J. Ramus, Celeste Leigh Pearce, Anna H. Wu, Ulrike Peters, Penelope M. Webb, Shelley S. Tworoger, Britton Trabert
Collection and assembly of data: Mary K. Townsend, Susan J. Jordan, Alpa V. Patel, Lauren R. Teras, Marc T. Goodman, Yurii B. Shvetsov, Francesmary Modugno, Kirsten B. Moysich, Kim Robien, Joellen M. Schildkraut, Andrew Berchuck, Andrew T. Chan, Nicolas Wentzensen, Patricia Hartge, Dale P. Sandler, Hoda Anton-Culver, Argyrios Ziogas, Usha Menon, Susan J. Ramus, Celeste Leigh Pearce, Anna H. Wu, Emily White, Ulrike Peters, Penelope M. Webb, Shelley S. Tworoger, Britton Trabert
Data analysis and interpretation: Lauren M. Hurwitz, Susan J. Jordan, Jennifer A. Doherty, Holly R. Harris, Marc T. Goodman, Kim Robien, Anna Prizment, Andrew Berchuck, Renée T. Fortner, Andrew T. Chan, Nicolas Wentzensen, Patricia Hartge, Hoda Anton-Culver, Argyrios Ziogas, Ulrike Peters, Penelope M. Webb, Shelley S. Tworoger, Britton Trabert
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Modification of the Association Between Frequent Aspirin Use and Ovarian Cancer Risk: A Meta-Analysis Using Individual-Level Data From Two Ovarian Cancer Consortia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alpa V. Patel
Research Funding: GRAIL (Inst)
Joellen M. Schildkraut
Patents, Royalties, Other Intellectual Property: Patent related treatment of PAD (peripheral arterial disease)(I)
Andrew Berchuck
Honoraria: Clovis Oncology, Merck, Tesaro, Premier Research
Research Funding: Novadaq Technologies
Travel, Accommodations, Expenses: Merck
Andrew T. Chan
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, Bayer
Research Funding: Zoe Ltd, Pfizer
Celeste Leigh Pearce
Stock and Other Ownership Interests: SAVA, ANVS
Honoraria: ViiV Healthcare (I)
Consulting or Advisory Role: ViiV Healthcare (I)
Speakers' Bureau: ViiV Healthcare (I)
Research Funding: CytoDyn (I)
Usha Menon
Stock and Other Ownership Interests: Abcodia
Research Funding: Abcodia (Inst)
Patents, Royalties, Other Intellectual Property: Patent no: EP10178345.4 for Breast Cancer Diagnostics
Uncompensated Relationships: ILOF (Inst), Dana-Farber Cancer Institute (Inst), RNA Guardian (Inst), Micronoma (Inst)
Penelope M. Webb
Research Funding: AstraZeneca (Inst)
Shelley S. Tworoger
Research Funding: BMS (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2021. Atlanta, GA: 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html [Google Scholar]
- 2. Kathawala RJ, Kudelka A, Rigas B. The chemoprevention of ovarian cancer: The need and the options. Curr Pharmacol Rep. 2018;4:250–260. doi: 10.1007/s40495-018-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 4. Fathalla MF. Incessant ovulation—A factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 5. Moorman PG, Schildkraut JM, Calingaert B, et al. Ovulation and ovarian cancer: A comparison of two methods for calculating lifetime ovulatory cycles (United States) Cancer Causes Control. 2002;13:807–811. doi: 10.1023/a:1020678100977. [DOI] [PubMed] [Google Scholar]
- 6. Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: An analysis from the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34:2888–2898. doi: 10.1200/JCO.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Z, Zeng F, Yuan J, et al. Pelvic inflammatory disease and the risk of ovarian cancer: A meta-analysis. Cancer Causes Control. 2017;28:415–428. doi: 10.1007/s10552-017-0873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poole EM, Lee IM, Ridker PM, et al. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor α receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256–1264. doi: 10.1093/aje/kwt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trabert B, Pinto L, Hartge P, et al. Pre-diagnostic serum levels of inflammation markers and risk of ovarian cancer in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Gynecol Oncol. 2014;135:297–304. doi: 10.1016/j.ygyno.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joharatnam-Hogan N, Cafferty FH, Macnair A, et al. The role of aspirin in the prevention of ovarian, endometrial and cervical cancers. Womens Health (Lond) 2020;16:1745506520961710. doi: 10.1177/1745506520961710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: Analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 12. Baandrup L, Kjaer SK, Olsen JH, et al. Low-dose aspirin use and the risk of ovarian cancer in Denmark. Ann Oncol. 2015;26:787–792. doi: 10.1093/annonc/mdu578. [DOI] [PubMed] [Google Scholar]
- 13. Barnard ME, Poole EM, Curhan GC, et al. Association of analgesic use with risk of ovarian cancer in the Nurses' Health Studies. JAMA Oncol. 2018;4:1675–1682. doi: 10.1001/jamaoncol.2018.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brasky TM, Liu J, White E, et al. Non-steroidal anti-inflammatory drugs and cancer risk in women: Results from the Women's Health Initiative. Int J Cancer. 2014;135:1869–1883. doi: 10.1002/ijc.28823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lacey JV, Jr, Sherman ME, Hartge P, et al. Medication use and risk of ovarian carcinoma: A prospective study. Int J Cancer. 2004;108:281–286. doi: 10.1002/ijc.11538. [DOI] [PubMed] [Google Scholar]
- 16. Murphy MA, Trabert B, Yang HP, et al. Non-steroidal anti-inflammatory drug use and ovarian cancer risk: Findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control. 2012;23:1839–1852. doi: 10.1007/s10552-012-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peres LC, Camacho F, Abbott SE, et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Br J Cancer. 2016;114:819–825. doi: 10.1038/bjc.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinheiro SP, Tworoger SS, Cramer DW, et al. Use of nonsteroidal antiinflammatory agents and incidence of ovarian cancer in 2 large prospective cohorts. Am J Epidemiol. 2009;169:1378–1387. doi: 10.1093/aje/kwp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prizment AE, Folsom AR, Anderson KE. Nonsteroidal anti-inflammatory drugs and risk for ovarian and endometrial cancers in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:435–442. doi: 10.1158/1055-9965.EPI-09-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Setiawan VW, Matsuno RK, Lurie G, et al. Use of nonsteroidal anti-inflammatory drugs and risk of ovarian and endometrial cancer: The multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2012;21:1441–1449. doi: 10.1158/1055-9965.EPI-12-0390-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hurwitz LM, Pinsky PF, Huang WY, et al. Aspirin use and ovarian cancer risk using extended follow-up of the PLCO Cancer Screening Trial. Gynecol Oncol. 2020;159:522–526. doi: 10.1016/j.ygyno.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Merritt MA, Green AC, Nagle CM, et al. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122:170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 23. Hannibal CG, Rossing MA, Wicklund KG, et al. Analgesic drug use and risk of epithelial ovarian cancer. Am J Epidemiol. 2008;167:1430–1437. doi: 10.1093/aje/kwn082. [DOI] [PubMed] [Google Scholar]
- 24. Zhang D, Bai B, Xi Y, et al. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol Oncol. 2016;142:368–377. doi: 10.1016/j.ygyno.2016.04.543. [DOI] [PubMed] [Google Scholar]
- 25. Trabert B, Poole EM, White E, et al. Analgesic use and ovarian cancer risk: An analysis in the Ovarian Cancer Cohort Consortium. J Natl Cancer Inst. 2019;111:137–145. doi: 10.1093/jnci/djy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: A pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431. doi: 10.1093/jnci/djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826–835. doi: 10.7326/M15-2112. [DOI] [PubMed] [Google Scholar]
- 28. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909–927. doi: 10.1097/AOG.0000000000002580. [DOI] [PubMed] [Google Scholar]
- 29. Townsend MK, Trabert B, Fortner RT, et al. Cohort profile: The Ovarian Cancer Cohort Consortium (OC3) Int J Epidemiol. 2022;51:e73–e86. doi: 10.1093/ije/dyab211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : The National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 31. Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: Rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 32. Jacobs EJ, Thun MJ, Connell CJ, et al. Aspirin and other nonsteroidal anti-inflammatory drugs and breast cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:261–264. [PubMed] [Google Scholar]
- 33. Bernstein L, Allen M, Anton-Culver H, et al. High breast cancer incidence rates among California teachers: Results from the California Teachers Study (United States) Cancer Causes Control. 2002;13:625–635. doi: 10.1023/a:1019552126105. [DOI] [PubMed] [Google Scholar]
- 34. Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 35. Kim S, Shore DL, Wilson LE, et al. Lifetime use of nonsteroidal anti-inflammatory drugs and breast cancer risk: Results from a prospective study of women with a sister with breast cancer. BMC Cancer. 2015;15:960. doi: 10.1186/s12885-015-1979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White E, Patterson RE, Kristal AR, et al. VITamins and Lifestyle cohort study: Study design and characteristics of supplement users. Am J Epidemiol. 2004;159:83–93. doi: 10.1093/aje/kwh010. [DOI] [PubMed] [Google Scholar]
- 37. Ready A, Velicer CM, McTiernan A, et al. NSAID use and breast cancer risk in the VITAL cohort. Breast Cancer Res Treat. 2008;109:533–543. doi: 10.1007/s10549-007-9665-x. [DOI] [PubMed] [Google Scholar]
- 38. Rossing MA, Cushing-Haugen KL, Wicklund KG, et al. Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2548–2556. doi: 10.1158/1055-9965.EPI-07-0550. [DOI] [PubMed] [Google Scholar]
- 39. Goodman MT, Lurie G, Thompson PJ, et al. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15:1055–1060. doi: 10.1677/ERC-08-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lurie G, Wilkens LR, Thompson PJ, et al. Combined oral contraceptive use and epithelial ovarian cancer risk: Time-related effects. Epidemiology. 2008;19:237–243. doi: 10.1097/EDE.0b013e31816334c5. [DOI] [PubMed] [Google Scholar]
- 41. Ness RB, Dodge RC, Edwards RP, et al. Contraception methods, beyond oral contraceptives and tubal ligation, and risk of ovarian cancer. Ann Epidemiol. 2011;21:188–196. doi: 10.1016/j.annepidem.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moorman PG, Calingaert B, Palmieri RT, et al. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am J Epidemiol. 2008;167:1059–1069. doi: 10.1093/aje/kwn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schildkraut JM, Iversen ES, Wilson MA, et al. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;5:e10061. doi: 10.1371/journal.pone.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ziogas A, Gildea M, Cohen P, et al. Cancer risk estimates for family members of a population-based family registry for breast and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:103–111. [PubMed] [Google Scholar]
- 45. Balogun N, Gentry-Maharaj A, Wozniak EL, et al. Recruitment of newly diagnosed ovarian cancer patients proved challenging in a multicentre biobanking study. J Clin Epidemiol. 2011;64:525–530. doi: 10.1016/j.jclinepi.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 46. Wu AH, Pearce CL, Tseng CC, et al. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124:1409–1415. doi: 10.1002/ijc.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pearce CL, Templeman C, Rossing MA, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olsen CM, Nagle CM, Whiteman DC, et al. Obesity and risk of ovarian cancer subtypes: Evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20:251–262. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sieh W, Salvador S, McGuire V, et al. Tubal ligation and risk of ovarian cancer subtypes: A pooled analysis of case-control studies. Int J Epidemiol. 2013;42:579–589. doi: 10.1093/ije/dyt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 51. Dubin N, Pasternack BS. Risk assessment for case-control subgroups by polychotomous logistic regression. Am J Epidemiol. 1986;123:1101–1117. doi: 10.1093/oxfordjournals.aje.a114338. [DOI] [PubMed] [Google Scholar]
- 52. Zabor EC, Begg CB. A comparison of statistical methods for the study of etiologic heterogeneity. Stat Med. 2017;36:4050–4060. doi: 10.1002/sim.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borenstein M, Hedges LV, Higgins JPT, et al. Subgroup Analyses, Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons; 2009. [Google Scholar]
- 54. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ardeshna D, Khare S, Jagadish PS, et al. The dilemma of aspirin resistance in obese patients. Ann Transl Med. 2019;7:404. doi: 10.21037/atm.2019.07.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet. 2018;392:387–399. doi: 10.1016/S0140-6736(18)31133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Webb PM, Na R, Weiderpass E, et al. Use of aspirin, other nonsteroidal anti-inflammatory drugs and acetaminophen and risk of endometrial cancer: The Epidemiology of Endometrial Cancer Consortium. Ann Oncol. 2019;30:310–316. doi: 10.1093/annonc/mdy541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hurwitz LM, Michels KA, Cook MB, et al. Associations between daily aspirin use and cancer risk across strata of major cancer risk factors in two large U.S. cohorts. Cancer Causes Control. 2020;26:020–01357. doi: 10.1007/s10552-020-01357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 60. Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]